Systematic Review with Network Meta-Analysis: Comparative Efficacy and Safety of Combination Therapy with Angiotensin II Receptor Blockers and Amlodipine in Asian Hypertensive Patients
- PMID: 31827918
- PMCID: PMC6885253
- DOI: 10.1155/2019/9516279
Systematic Review with Network Meta-Analysis: Comparative Efficacy and Safety of Combination Therapy with Angiotensin II Receptor Blockers and Amlodipine in Asian Hypertensive Patients
Abstract
Background: Hypertension (HTN) is the leading risk factor for cardiovascular mortality globally. The WHO estimates a 60% increase in Asian HTN patients between 2000 and 2025. Numerous studies have compared safety and efficacy between antihypertensive classes, but in-class comparisons of angiotensin II receptor blockers (ARBs) in combination therapy (CT) (fixed-dose combination or dual combination) with a calcium channel blocker (CCB) are lacking in Asia.
Objective: To compare the efficacy and safety of the various ARB-amlodipine CTs and amlodipine (AML) monotherapy for treatment of HTN in Asian population.
Methods: A systematic literature review sourced Asian randomized controlled trials (RCTs) from PubMed and Cochrane Libraries to inform a network meta-analysis (NMA). We considered the ARB-AML CT. The primary efficacy and safety endpoints were short-term (8-12 weeks) treatment response and treatment-emergent adverse events (TEAEs), respectively. AML monotherapy was used as a comparator to allow for indirect treatment effect estimation in the absence of direct RCTs evidence comparing the different ARB-AML CTs.
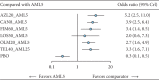
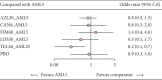
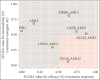
Results: The analysis included 1198 Asian HTN patients from seven studies involving six ARB-AML CTs: azilsartan (AZL), candesartan (CAN), fimasartan (FIM), losartan (LOS), olmesartan (OLM), and telmisartan (TEL). Compared to AML monotherapy, CT of AZL-AML had five times greater odds of prompting a treatment response (OR 5.2, 95% CI: 2.5, 11.2), while CAN-AML had 3.9 (95% CI: 2.5, 6.4), FIM-AML had 3.4 (95% CI: 1.4, 8.5), TEL-AML had 3.3 (95% CI: 1.6, 7.1), OLM-AML had 2.7 (95% CI: 1.6, 5.0), and LOS-AML had 2.0 (95% CI: 0.6, 7.3). All ARB-AML CTs had safety profiles comparable to AML monotherapy except TEL-AML, which had significantly lower odds of TEAEs (0.26 (95% CI: 0.087, 0.70)).
Conclusion: This study suggests that all ARB-AML CTs compared favorably to AML monotherapy regarding short-term treatment response in uncomplicated HTN patients of Asian origin. AZL-AML prompted the most favorable treatment response. Safety profiles among the ARB-AML CTs were largely comparable. Due to the limited study size and small number of trials (direct evidence), our findings should best be interpreted as an exploratory effort importance to inform future research direction.
Copyright © 2019 Dae Wook Lee et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Meta-analysis of the efficacy and safety of adding an angiotensin receptor blocker (ARB) to a calcium channel blocker (CCB) following ineffective CCB monotherapy.J Thorac Dis. 2015 Dec;7(12):2243-52. doi: 10.3978/j.issn.2072-1439.2015.12.39. J Thorac Dis. 2015. PMID: 26793346 Free PMC article.
-
Efficacy of amlodipine/olmesartan ± hydrochlorothiazide in patients uncontrolled on prior calcium channel blocker or angiotensin II receptor blocker monotherapy.Adv Ther. 2012 Jun;29(6):508-23. doi: 10.1007/s12325-012-0030-z. Epub 2012 Jul 4. Adv Ther. 2012. PMID: 22773358 Clinical Trial.
-
Rationale, design and patient baseline characteristics of OlmeSartan and calcium antagonists randomized (OSCAR) study: a study comparing the incidence of cardiovascular events between high-dose angiotensin II receptor blocker (ARB) monotherapy and combination therapy of ARB with calcium channel blocker in Japanese elderly high-risk hypertensive patients (ClinicalTrials. gov no. NCT00134160).Hypertens Res. 2009 Jul;32(7):575-80. doi: 10.1038/hr.2009.60. Epub 2009 May 15. Hypertens Res. 2009. PMID: 19444280 Clinical Trial.
-
Antihypertensive efficacy of olmesartan medoxomil or valsartan in combination with amlodipine: a review of factorial-design studies.Curr Med Res Opin. 2009 Jan;25(1):177-85. doi: 10.1185/03007990802597456. Curr Med Res Opin. 2009. PMID: 19210150 Review.
-
Blood pressure control with angiotensin receptor blocker-based three-drug combinations: key trials.Adv Ther. 2012 May;29(5):401-15. doi: 10.1007/s12325-012-0019-7. Epub 2012 May 17. Adv Ther. 2012. PMID: 22610686 Review.
Cited by
-
Management of Hypertension in the Asia-Pacific Region: A Structured Review.Am J Cardiovasc Drugs. 2024 Mar;24(2):141-170. doi: 10.1007/s40256-023-00625-1. Epub 2024 Feb 8. Am J Cardiovasc Drugs. 2024. PMID: 38332411 Free PMC article. Review.
-
Efficacy and safety of olmesartan medoxomil-amlodipine besylate tablet in Chinese patients with essential hypertension: A prospective, single-arm, multi-center, real-world study.J Clin Hypertens (Greenwich). 2024 Jan;26(1):5-16. doi: 10.1111/jch.14700. Epub 2023 Sep 4. J Clin Hypertens (Greenwich). 2024. PMID: 37667532 Free PMC article.
-
Comparison of the Ameliorating Effects of Valsartan and Amlodipine on Vascular Endothelial Dysfunction and Oxidative Stress in Elderly Patients with Type H Hypertension.Evid Based Complement Alternat Med. 2022 Aug 8;2022:5054511. doi: 10.1155/2022/5054511. eCollection 2022. Evid Based Complement Alternat Med. 2022. Retraction in: Evid Based Complement Alternat Med. 2023 Jun 21;2023:9858540. doi: 10.1155/2023/9858540 PMID: 35979006 Free PMC article. Retracted.
References
-
- World Health Organization. A Global Brief on Hypertension: Silent Killer, Global Public Health Crisis: World Health Day 2013. Geneva, Switzerland: World Health Organization; 2013.
-
- Kim K. I., Chang H. J., Cho Y. S., et al. Current status and characteristics of hypertension control in community resident elderly Korean people: data from a Korean longitudinal study on health and aging (KLoSHa study) Hypertension Research. 2008;31(1):97–105. doi: 10.1291/hypres.31.97. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials


