A new hypothesis for foregut and heart tube formation based on differential growth and actomyosin contraction
- PMID: 28526751
- PMCID: PMC5536863
- DOI: 10.1242/dev.145193
A new hypothesis for foregut and heart tube formation based on differential growth and actomyosin contraction
Abstract
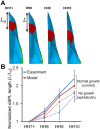
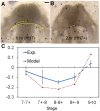
For decades, it was commonly thought that the bilateral heart fields in the early embryo fold directly towards the midline, where they meet and fuse to create the primitive heart tube. Recent studies have challenged this view, however, suggesting that the heart fields fold diagonally. As early foregut and heart tube morphogenesis are intimately related, this finding also raises questions concerning the traditional view of foregut formation. Here, we combine experiments on chick embryos with computational modeling to explore a new hypothesis for the physical mechanisms of heart tube and foregut formation. According to our hypothesis, differential anisotropic growth between mesoderm and endoderm drives diagonal folding. Then, active contraction along the anterior intestinal portal generates tension to elongate the foregut and heart tube. We test this hypothesis using biochemical perturbations of cell proliferation and contractility, as well as computational modeling based on nonlinear elasticity theory including growth and contraction. The present results generally support the view that differential growth and actomyosin contraction drive formation of the foregut and heart tube in the early chick embryo.
Keywords: Biomechanics; Computational model; Contraction; Growth; Morphogenesis.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



 , see
, see 


 ,
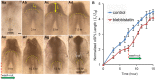
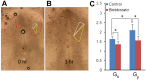
,  ; d=pipette diameter) at stages HH8 (n=6) and HH9 (n=5) (two-way ANOVA, *P<0.01). (D) Experimental elastic stretch ratios in the FG for stage HH8+ embryos cultured from stage HH8 onwards in control media (n=7) and media containing 50 μM aphidicolin (n=8). Inhibition of cell proliferation resulted in increased FG tension in the axial direction during Phase 2 (two-way ANOVA, *P<0.01). AIP, anterior intestinal portal; NT, neural tube. Scale bars: 50 μm (A″); 100 μm (B); 200 μm (A′,B′).
; d=pipette diameter) at stages HH8 (n=6) and HH9 (n=5) (two-way ANOVA, *P<0.01). (D) Experimental elastic stretch ratios in the FG for stage HH8+ embryos cultured from stage HH8 onwards in control media (n=7) and media containing 50 μM aphidicolin (n=8). Inhibition of cell proliferation resulted in increased FG tension in the axial direction during Phase 2 (two-way ANOVA, *P<0.01). AIP, anterior intestinal portal; NT, neural tube. Scale bars: 50 μm (A″); 100 μm (B); 200 μm (A′,B′).
Similar articles
-
Not just inductive: a crucial mechanical role for the endoderm during heart tube assembly.Development. 2012 May;139(9):1680-90. doi: 10.1242/dev.073486. Development. 2012. PMID: 22492358 Free PMC article.
-
Hensen's node gives rise to the ventral midline of the foregut: implications for organizing head and heart development.Dev Biol. 2003 Jan 15;253(2):175-88. doi: 10.1016/s0012-1606(02)00024-6. Dev Biol. 2003. PMID: 12645923
-
The heart tube forms and elongates through dynamic cell rearrangement coordinated with foregut extension.Development. 2018 Mar 29;145(7):dev152488. doi: 10.1242/dev.152488. Development. 2018. PMID: 29490984 Free PMC article.
-
Formation of the primitive myo- and endocardial tubes in the chicken embryo.J Mol Cell Cardiol. 1989 Feb;21(2):123-37. doi: 10.1016/0022-2828(89)90856-0. J Mol Cell Cardiol. 1989. PMID: 2664188 Review.
-
Antero-posterior patterning of the vertebrate digestive tract: 40 years after Nicole Le Douarin's PhD thesis.Int J Dev Biol. 2005;49(2-3):335-47. doi: 10.1387/ijdb.041946ag. Int J Dev Biol. 2005. PMID: 15906249 Review.
Cited by
-
Elongation of the nascent avian foregut requires coordination of intrinsic and extrinsic cell behaviors.bioRxiv [Preprint]. 2024 Nov 1:2024.10.31.621372. doi: 10.1101/2024.10.31.621372. bioRxiv. 2024. PMID: 39554178 Free PMC article. Preprint.
-
Integrating mechanical cues with engineered platforms to explore cardiopulmonary development and disease.iScience. 2023 Nov 15;26(12):108472. doi: 10.1016/j.isci.2023.108472. eCollection 2023 Dec 15. iScience. 2023. PMID: 38077130 Free PMC article. Review.
-
Allometrically scaling tissue forces drive pathological foreign-body responses to implants via Rac2-activated myeloid cells.Nat Biomed Eng. 2023 Nov;7(11):1419-1436. doi: 10.1038/s41551-023-01091-5. Epub 2023 Sep 25. Nat Biomed Eng. 2023. PMID: 37749310 Free PMC article.
-
A chemo-mechanical model of endoderm movements driving elongation of the amniote hindgut.bioRxiv [Preprint]. 2023 May 18:2023.05.18.541363. doi: 10.1101/2023.05.18.541363. bioRxiv. 2023. Update in: Development. 2023 Nov 15;150(22):dev202010. doi: 10.1242/dev.202010 PMID: 37292966 Free PMC article. Updated. Preprint.
-
Heart in a dish - choosing the right in vitro model.Dis Model Mech. 2023 May 1;16(5):dmm049961. doi: 10.1242/dmm.049961. Epub 2023 Feb 24. Dis Model Mech. 2023. PMID: 36825553 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials


