Magnetoencephalography
- PMID: 24647614
- PMCID: PMC4174130
- DOI: 10.1136/practneurol-2013-000768
Magnetoencephalography
Keywords: BRAIN MAPPING; NEUROPHYSIOLOGY, EXPT.
Figures
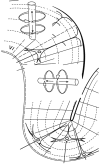



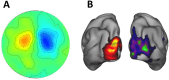
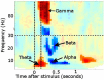
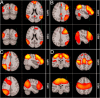
Similar articles
-
Magnetoencephalography in Cognitive Neuroscience: A Primer.Neuron. 2019 Oct 23;104(2):189-204. doi: 10.1016/j.neuron.2019.07.001. Neuron. 2019. PMID: 31647893 Review.
-
Magnetoencephalography and magnetic source imaging. Capabilities and limitations.Neuroimaging Clin N Am. 1995 May;5(2):227-49. Neuroimaging Clin N Am. 1995. PMID: 7640886 Review.
-
[Magnetoencephalography (MEG) and functional brain mapping].Rinsho Shinkeigaku. 1994 Dec;34(12):1253-4. Rinsho Shinkeigaku. 1994. PMID: 7774127 Review. Japanese. No abstract available.
-
A new method for magnetoencephalography: a three-dimensional magnetometer-spatial filter system.Neuroscience. 1999;91(2):405-15. doi: 10.1016/s0306-4522(98)00629-0. Neuroscience. 1999. PMID: 10365998
-
Neuroimaging studies of bilingual expressive language representation in the brain: potential applications for magnetoencephalography.Neurosci Bull. 2012 Dec;28(6):759-64. doi: 10.1007/s12264-012-1278-7. Epub 2012 Nov 3. Neurosci Bull. 2012. PMID: 23124647 Free PMC article. Review.
Cited by
-
A Transmissive Theory of Brain Function: Implications for Health, Disease, and Consciousness.NeuroSci. 2022 Aug 9;3(3):440-456. doi: 10.3390/neurosci3030032. eCollection 2022 Sep. NeuroSci. 2022. PMID: 39483436 Free PMC article.
-
Neuroimaging to Facilitate Clinical Trials in Huntington's Disease: Current Opinion from the EHDN Imaging Working Group.J Huntingtons Dis. 2024;13(2):163-199. doi: 10.3233/JHD-240016. J Huntingtons Dis. 2024. PMID: 38788082 Free PMC article. Review.
-
The cortical neurophysiological signature of amyotrophic lateral sclerosis.Brain Commun. 2024 May 13;6(3):fcae164. doi: 10.1093/braincomms/fcae164. eCollection 2024. Brain Commun. 2024. PMID: 38779353 Free PMC article.
-
From bench to bedside: Overview of magnetoencephalography in basic principle, signal processing, source localization and clinical applications.Neuroimage Clin. 2024;42:103608. doi: 10.1016/j.nicl.2024.103608. Epub 2024 Apr 20. Neuroimage Clin. 2024. PMID: 38653131 Free PMC article. Review.
-
Eavesdropping on Tinnitus Using MEG: Lessons Learned and Future Perspectives.J Assoc Res Otolaryngol. 2023 Dec;24(6):531-547. doi: 10.1007/s10162-023-00916-z. Epub 2023 Nov 28. J Assoc Res Otolaryngol. 2023. PMID: 38015287 Free PMC article. Review.
References
-
- Buzsaki G. Rhythms of the Brain. USA: OUP, 2011
-
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci 2005;9:474–80 - PubMed
-
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci 2001;5:16–25 - PubMed
-
- Fernando H, Lopes da Silva MEG: an introduction to methods. eds: Hansen, Kringelback & Salmelin. USA: OUP, 2010:1–23, figure 1.3 from p6
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

