Alzheimer's disease--input of vitamin D with mEmantine assay (AD-IDEA trial): study protocol for a randomized controlled trial
- PMID: 22014101
- PMCID: PMC3212921
- DOI: 10.1186/1745-6215-12-230
Alzheimer's disease--input of vitamin D with mEmantine assay (AD-IDEA trial): study protocol for a randomized controlled trial
Abstract
Background: Current treatments for Alzheimer's disease and related disorders (ADRD) are symptomatic and can only temporarily slow down ADRD. Future possibilities of care rely on multi-target drugs therapies that address simultaneously several pathophysiological processes leading to neurodegeneration. We hypothesized that the combination of memantine with vitamin D could be neuroprotective in ADRD, thereby limiting neuronal loss and cognitive decline. The aim of this trial is to compare the effect after 24 weeks of the oral intake of vitamin D3 (cholecalciferol) with the effect of a placebo on the change of cognitive performance in patients suffering from moderate ADRD and receiving memantine.
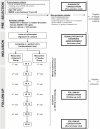
Methods: The AD-IDEA Trial is a unicentre, double-blind, randomized, placebo-controlled, intent-to-treat, superiority trial. Patients aged 60 years and older presenting with moderate ADRD (i.e., Mini-Mental State Examination [MMSE] score between 10-20), hypovitaminosis D (i.e., serum 25-hydroxyvitamin D [25OHD] < 30 ng/mL), normocalcemia (i.e., serum calcium < 2.65 mmol/L) and receiving no antidementia treatment at time of inclusion are being recruited. All participants receive memantine 20 mg once daily -titrated in 5 mg increments over 4 weeks- and each one is randomized to one of the two treatment options: either cholecalciferol (one 100,000 IU drinking vial every 4 weeks) or placebo (administered at the same pace). One hundred and twenty participants are being recruited and treatment continues for 24 weeks. Primary outcome measure is change in cognitive performance using Alzheimer's Disease Assessment Scale-cognition score. Secondary outcomes are changes in other cognitive scores (MMSE, Frontal Assessment Battery, Trail Making Test parts A and B), change in functional performance (Activities of Daily Living scale, and 4-item Instrumental Activities of Daily Living scale), posture and gait (Timed Up & Go, Five Time Sit-to-Stand, spatio-temporal analysis of walking), as well as the between-groups comparison of compliance to treatment and tolerance. These outcomes are assessed at baseline, 12 and 24 weeks, together with the serum concentrations of 25OHD, calcium and parathyroid hormone.
Discussion: The combination of memantine plus vitamin D may represent a new multi-target therapeutic class for the treatment of ADRD. The AD-IDEA Trial seeks to provide evidence on its efficacy in limiting cognitive and functional declines in ADRD.
Trial registration: ClinicalTrials.gov number, NCT01409694.
Figures
Similar articles
-
Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial.JAMA. 2014 Jan 1;311(1):33-44. doi: 10.1001/jama.2013.282834. JAMA. 2014. PMID: 24381967 Free PMC article. Clinical Trial.
-
Memantine treatment in patients with mild to moderate Alzheimer's disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial.Curr Alzheimer Res. 2008 Feb;5(1):83-9. doi: 10.2174/156720508783884576. Curr Alzheimer Res. 2008. PMID: 18288936 Clinical Trial.
-
Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial.JAMA. 2004 Jan 21;291(3):317-24. doi: 10.1001/jama.291.3.317. JAMA. 2004. PMID: 14734594 Clinical Trial.
-
Memantine for dementia.Cochrane Database Syst Rev. 2003;(3):CD003154. doi: 10.1002/14651858.CD003154. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2004 Oct 18;(4):CD003154. doi: 10.1002/14651858.CD003154.pub2 PMID: 12917950 Updated. Review.
-
Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Nov. Report No.: 14-05198-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Nov. Report No.: 14-05198-EF-1. PMID: 24354019 Free Books & Documents. Review.
Cited by
-
The Vitamin D Receptor as a Potential Target for the Treatment of Age-Related Neurodegenerative Diseases Such as Alzheimer's and Parkinson's Diseases: A Narrative Review.Cells. 2023 Feb 19;12(4):660. doi: 10.3390/cells12040660. Cells. 2023. PMID: 36831327 Free PMC article. Review.
-
The Efficacy of Vitamin D Supplementation in Patients With Alzheimer's Disease in Preventing Cognitive Decline: A Systematic Review.Cureus. 2022 Nov 20;14(11):e31710. doi: 10.7759/cureus.31710. eCollection 2022 Nov. Cureus. 2022. PMID: 36569670 Free PMC article. Review.
-
Naturally Occurring Antioxidant Therapy in Alzheimer's Disease.Antioxidants (Basel). 2022 Jan 23;11(2):213. doi: 10.3390/antiox11020213. Antioxidants (Basel). 2022. PMID: 35204096 Free PMC article. Review.
-
Vitamins in Alzheimer's Disease-Review of the Latest Reports.Nutrients. 2020 Nov 11;12(11):3458. doi: 10.3390/nu12113458. Nutrients. 2020. PMID: 33187212 Free PMC article. Review.
-
Focus on 1,25-Dihydroxyvitamin D3 in the Peripheral Nervous System.Front Neurosci. 2019 Apr 12;13:348. doi: 10.3389/fnins.2019.00348. eCollection 2019. Front Neurosci. 2019. PMID: 31031586 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical