Tumors as organs: complex tissues that interface with the entire organism
- PMID: 20627072
- PMCID: PMC2905377
- DOI: 10.1016/j.devcel.2010.05.012
Tumors as organs: complex tissues that interface with the entire organism
Abstract
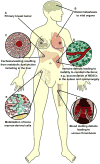
Solid tumors are not simply clones of cancer cells. Instead, they are abnormal organs composed of multiple cell types and extracellular matrix. Some aspects of tumor development resemble processes seen in developing organs, whereas others are more akin to tissue remodeling. Some microenvironments, particularly those associated with tissue injury, are favorable for progression of mutant cells, whereas others restrict it. Cancer cells can also instruct surrounding tissues to undergo changes that promote malignancy. Understanding the complex ways in which cancer cells interact with their surroundings, both locally in the tumor organ and systemically in the body as a whole, has implications for effective cancer prevention and therapy.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
[Role of the stroma in the initiation and progression of tumors].Orv Hetil. 2015 Nov 8;156(45):1816-23. doi: 10.1556/650.2015.30294. Orv Hetil. 2015. PMID: 26522855 Review. Hungarian.
-
Microenvironmental regulation of tumor progression and metastasis.Nat Med. 2013 Nov;19(11):1423-37. doi: 10.1038/nm.3394. Nat Med. 2013. PMID: 24202395 Free PMC article. Review.
-
Stroma-derived factor (SDF-1/CXCL12) and human tumor pathogenesis.Am J Physiol Cell Physiol. 2007 Mar;292(3):C987-95. doi: 10.1152/ajpcell.00406.2006. Epub 2006 Aug 30. Am J Physiol Cell Physiol. 2007. PMID: 16943240 Review.
-
Can Targeting Stroma Pave the Way to Enhanced Antitumor Immunity and Immunotherapy of Solid Tumors?Cancer Immunol Res. 2016 Apr;4(4):269-78. doi: 10.1158/2326-6066.CIR-16-0011. Cancer Immunol Res. 2016. PMID: 27036971 Free PMC article. Review.
-
Migratory neighbors and distant invaders: tumor-associated niche cells.Genes Dev. 2008 Mar 1;22(5):559-74. doi: 10.1101/gad.1636908. Genes Dev. 2008. PMID: 18316475 Free PMC article. Review.
Cited by
-
The heterogeneity of NOTCH1 to tumor immune infiltration in pan-cancer.Sci Rep. 2024 Nov 14;14(1):28071. doi: 10.1038/s41598-024-79883-1. Sci Rep. 2024. PMID: 39543218 Free PMC article.
-
Temporal recording of mammalian development and precancer.Nature. 2024 Oct;634(8036):1187-1195. doi: 10.1038/s41586-024-07954-4. Epub 2024 Oct 30. Nature. 2024. PMID: 39478207 Free PMC article.
-
A multi-modal single-cell and spatial expression map of metastatic breast cancer biopsies across clinicopathological features.Nat Med. 2024 Nov;30(11):3236-3249. doi: 10.1038/s41591-024-03215-z. Epub 2024 Oct 30. Nat Med. 2024. PMID: 39478111 Free PMC article.
-
Lactylation in cancer: Mechanisms in tumour biology and therapeutic potentials.Clin Transl Med. 2024 Nov;14(11):e70070. doi: 10.1002/ctm2.70070. Clin Transl Med. 2024. PMID: 39456119 Free PMC article. Review.
-
Development of a nomogram-based model incorporating radiomic features from follow-up longitudinal lung CT images to distinguish invasive adenocarcinoma from benign lesions: a retrospective study.BMC Pulm Med. 2024 Oct 26;24(1):534. doi: 10.1186/s12890-024-03360-8. BMC Pulm Med. 2024. PMID: 39455958 Free PMC article.
References
-
- Anderson AR, Weaver AM, Cummings PT, Quaranta V. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell. 2006;127:905–915. - PubMed
-
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. - PubMed
-
- Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14:53–65. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


