Experience with irinotecan for the treatment of malignant glioma
- PMID: 18784279
- PMCID: PMC2718962
- DOI: 10.1215/15228517-2008-075
Experience with irinotecan for the treatment of malignant glioma
Abstract
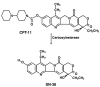
Malignant glioma is the most commonly occurring primary malignant brain tumor. It is difficult to treat and is usually associated with an inexorable, rapidly fatal clinical course. Chemotherapy, radiotherapy, and surgical excision are core components in the management of malignant glioma. However, chemotherapy, even with the most active regimens currently available, achieves only modest improvement in overall survival. Novel agents and new approaches to therapy are required to improve clinical outcomes. Irinotecan, a first-line treatment for metastatic colorectal cancer and an agent with high activity against solid tumors of the gastrointestinal tract, is an inhibitor of topoisomerase I, a critical enzyme needed for DNA transcription. Irinotecan crosses the blood-brain barrier and, in preclinical investigations, has demonstrated cytotoxic activity against central nervous system tumor xenografts. Its antitumor activity has also been demonstrated against glioblastoma cells with multidrug resistance. Studies in adult and pediatric patients with recurrent, intractable malignant glioma have evaluated irinotecan as monotherapy and in combination with other agents, including temozolomide, carmustine, thalidomide, and bevacizumab. Studies of irinotecan in combination with other medications, particularly temozolomide and bevacizumab, have yielded promising results. Irinotecan monotherapy has demonstrated efficacy; however, its efficacy appears to be enhanced when used in combination with other chemotherapeutic agents. When administered concurrently with enzyme-inducing antiepileptic drugs, the dosage must be increased to compensate for enhanced cytochrome CY3A4/5 enzyme activity. Toxicities associated with irinotecan have been manageable; the most important dose-limiting toxicities are neutropenia and diarrhea. Irinotecan-based chemotherapy of malignant glioma merits further study.
Figures
Similar articles
-
The emerging role of irinotecan (CPT-11) in the treatment of malignant glioma in brain tumors.Cancer. 2003 May 1;97(9 Suppl):2359-62. doi: 10.1002/cncr.11305. Cancer. 2003. PMID: 12712457 Review.
-
Phase I clinical and pharmacokinetic study of irinotecan in adults with recurrent malignant glioma.Clin Cancer Res. 2003 Aug 1;9(8):2940-9. Clin Cancer Res. 2003. PMID: 12912940 Clinical Trial.
-
Irinotecan in the treatment of glioma patients: current and future studies of the North Central Cancer Treatment Group.Cancer. 2003 May 1;97(9 Suppl):2352-8. doi: 10.1002/cncr.11304. Cancer. 2003. PMID: 12712456
-
Irinotecan: promising activity in the treatment of malignant glioma.Oncology (Williston Park). 2003 May;17(5 Suppl 5):9-14. Oncology (Williston Park). 2003. PMID: 12800600 Review.
-
Phase II study of irinotecan (CPT-11) in children with high-risk malignant brain tumors: the Duke experience.Neuro Oncol. 2002 Apr;4(2):102-8. doi: 10.1093/neuonc/4.2.109. Neuro Oncol. 2002. PMID: 11916501 Free PMC article. Clinical Trial.
Cited by
-
Local Delivery of Irinotecan to Recurrent GBM Patients at Reoperation Offers a Safe Route of Administration.Cancers (Basel). 2024 Aug 29;16(17):3008. doi: 10.3390/cancers16173008. Cancers (Basel). 2024. PMID: 39272866 Free PMC article.
-
Preclinical evidence for the use of anti-Trop-2 antibody-drug conjugate Sacituzumab govitecan in cerebral metastasized castration-resistant prostate cancer.Cancer Med. 2024 Jun;13(12):e7320. doi: 10.1002/cam4.7320. Cancer Med. 2024. PMID: 38895886 Free PMC article.
-
CPT-11 mitigates autoimmune diseases by suppressing effector T cells without affecting long-term anti-tumor immunity.Cell Death Discov. 2024 May 4;10(1):218. doi: 10.1038/s41420-024-01983-8. Cell Death Discov. 2024. PMID: 38704362 Free PMC article.
-
Local administration of irinotecan using an implantable drug delivery device stops high-grade glioma tumor recurrence in a glioblastoma tumor model.Drug Deliv Transl Res. 2024 Nov;14(11):3070-3088. doi: 10.1007/s13346-024-01524-x. Epub 2024 Feb 6. Drug Deliv Transl Res. 2024. PMID: 38319555 Free PMC article.
-
Systemic Therapy for Metastatic Triple Negative Breast Cancer: Current Treatments and Future Directions.Cancers (Basel). 2023 Jul 26;15(15):3801. doi: 10.3390/cancers15153801. Cancers (Basel). 2023. PMID: 37568617 Free PMC article. Review.
References
-
- Reardon DA, Wen PY. Therapeutic advances in the treatment of glioblastoma: rationale and potential role of targeted agents. Oncologist. 2006;11:152–164. - PubMed
-
- Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977–2000. Cancer. 2004;101:2293–2299. - PubMed
-
- CBTRUS. Statistical Report: Primary Brain Tumors in the United States, 1998–2002. Central Brain Tumor Registry of the United States. Available at http://www.cbtrusorg/reports//2005-2006/2006reportpdf2006.
-
- American Cancer Society. Cancer Facts and Figures 2006. Atlanta: American Cancer Society; 2006. Available at http://www.Cancer.org.
-
- Grossman SA, Batara JF. Current management of glioblastoma multiforme. Semin Oncol. 2004;31:635–644. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


