Oxidation in rheumatoid arthritis
- PMID: 15535839
- PMCID: PMC1064874
- DOI: 10.1186/ar1447
Oxidation in rheumatoid arthritis
Abstract
Oxygen metabolism has an important role in the pathogenesis of rheumatoid arthritis. Reactive oxygen species (ROS) produced in the course of cellular oxidative phosphorylation, and by activated phagocytic cells during oxidative bursts, exceed the physiological buffering capacity and result in oxidative stress. The excessive production of ROS can damage protein, lipids, nucleic acids, and matrix components. They also serve as important intracellular signaling molecules that amplify the synovial inflammatory-proliferative response. Repetitive cycles of hypoxia and reoxygenation associated with changes in synovial perfusion are postulated to activate hypoxia-inducible factor-1alpha and nuclear factor-kappaB, two key transcription factors that are regulated by changes in cellular oxygenation and cytokine stimulation, and that in turn orchestrate the expression of a spectrum of genes critical to the persistence of synovitis. An understanding of the complex interactions involved in these pathways might allow the development of novel therapeutic strategies for rheumatoid arthritis.
Figures


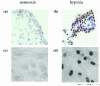
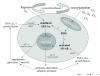
Similar articles
-
Redox signalling and the inflammatory response in rheumatoid arthritis.Clin Exp Immunol. 2008 Jun;152(3):415-22. doi: 10.1111/j.1365-2249.2008.03634.x. Epub 2008 Apr 16. Clin Exp Immunol. 2008. PMID: 18422737 Free PMC article. Review.
-
Amplification of the synovial inflammatory response through activation of mitogen-activated protein kinases and nuclear factor kappaB using ligation of CD40 on CD14+ synovial cells from patients with rheumatoid arthritis.Arthritis Rheum. 2004 Jul;50(7):2167-77. doi: 10.1002/art.20340. Arthritis Rheum. 2004. PMID: 15248214
-
Hypoxia and angiogenesis in rheumatoid arthritis.Curr Opin Rheumatol. 2005 May;17(3):293-8. doi: 10.1097/01.bor.0000155361.83990.5b. Curr Opin Rheumatol. 2005. PMID: 15838239 Review.
-
The contribution of hypoxia-reperfusion injury to inflammatory synovitis: the influence of reactive oxygen intermediates on the transcriptional control of inflammation.Ann N Y Acad Sci. 1994 Jun 17;723:308-17. Ann N Y Acad Sci. 1994. PMID: 8030874 Review. No abstract available.
-
Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis.Chem Biol Interact. 2018 Feb 1;281:121-136. doi: 10.1016/j.cbi.2017.12.024. Epub 2017 Dec 16. Chem Biol Interact. 2018. PMID: 29258867 Review.
Cited by
-
NF-κB Signaling Pathway in Rheumatoid Arthritis: Mechanisms and Therapeutic Potential.Mol Neurobiol. 2024 Nov 19. doi: 10.1007/s12035-024-04634-2. Online ahead of print. Mol Neurobiol. 2024. PMID: 39560902 Review.
-
Sera from Rheumatoid Arthritis Patients Induce Oxidative Stress and Pro-Angiogenic and Profibrotic Phenotypes in Human Endothelial Cells.J Clin Med. 2024 Oct 3;13(19):5913. doi: 10.3390/jcm13195913. J Clin Med. 2024. PMID: 39407973 Free PMC article.
-
Navel orange peel ethanolic extract and naringin ameliorate CFA-induced arthritis in Wistar rats through their modulatory effects on Th1/Th2/Th17 cytokines and oxidative stress.Am J Transl Res. 2024 Sep 15;16(9):4696-4713. doi: 10.62347/OEHX5202. eCollection 2024. Am J Transl Res. 2024. PMID: 39398602 Free PMC article.
-
Mitochondria: a breakthrough in combating rheumatoid arthritis.Front Med (Lausanne). 2024 Aug 5;11:1439182. doi: 10.3389/fmed.2024.1439182. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39161412 Free PMC article.
-
Exploring Therapeutic Potential of Catalase: Strategies in Disease Prevention and Management.Biomolecules. 2024 Jun 14;14(6):697. doi: 10.3390/biom14060697. Biomolecules. 2024. PMID: 38927099 Free PMC article. Review.
References
-
- Lotz M. Neuropeptides, free radicals and nitric oxide. Rheumatology. 2003:135–146. - PubMed
-
- van de Loo FA, Bennink MB, Arntz OJ, Smeets RL, Lubberts E, Joosten LA, van Lent PL, Coenen-de Roo CJ, Cuzzocrea S, Segal BH, et al. Deficiency of NADPH oxidase components p47phox and gp91phox caused granulomatous synovitis and increased connective tissue destruction in experimental arthritis models. Am J Pathol. 2003;163:1525–1537. - PMC - PubMed
-
- van der Veen RC, Dietlin TA, Hofman FM, Pen L, Segal BH, Holland SM. Superoxide prevents nitric oxide-mediated suppression of helper T lymphocytes: decreased autoimmune encephalomyelitis in nicotinamide adenine dinucleotide phosphate oxidase knockout mice. J Immunol. 2000;164:5177–5183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


