Abstract
Hibernation is an extreme physiological challenge for the brown bear (Ursus arctos) in which metabolism is based mainly on lipids. The study objective was to compare plasma lipids in hibernating and active free‐ranging brown bears and relate them to arterial histopathology. Blood was drawn from seven immobilized free‐ranging brown bears (three females, 2–3 years old) during hibernation in February and from the same bears while active in June and analyzed by enzymatic and automated hematology methods within 48 hours of sampling. Left anterior descending coronary arteries and aortic arches from 12 bears (six females, 1.5–12 years old) killed in hunting were examined by histopathology. Total plasma cholesterol decreased from hibernation to the active period (11.08 ± 1.04 mmol/L vs. 7.89 ± 1.96 mmol/L, P= 0.0028) as did triglyceride (3.16 ± 0.62 mmol/L vs. 1.44 ± 0.27 mmol/L, P= 0.00012) and LDL cholesterol (4.30 ± 0.71 mmol/L vs. 2.02 ± 1.03 mmol/L, P= 0.0075), whereas HDL cholesterol was unchanged. No atherosclerosis, fatty streaks, foam cell infiltration, or inflammation were seen in any arterial samples. Brown bears tolerate elevated cholesterol levels, obesity, physical inactivity, and circulatory slow flow during hibernation without signs of atherosclerosis. This species might serve as a reverse translational model for atherosclerosis resistance. Clin Trans Sci 2012; Volume 5: 269–272
Keywords: apolipoproteins, cholesterol, hibernation physiology, triglycerides
Introduction
Hibernation is an extreme physiological challenge for the brown bear. For 5–7 months of the year metabolism is based mainly on lipids from stored fat while protein synthesis is reduced. The dependency on lipid metabolism during hibernation increases plasma lipids as documented in two American black bears (Ursus americanus) and one Asiatic black bear (Ursus thibetanus), 1 four American black bears 2 and two brown bears 3 —all kept in captivity. A recent study compared plasma lipids in seven captive and nine free‐ranging adult American black bears and although no sampling was done during hibernation, lipid concentrations were found to vary significantly among populations of black bears. 4 Despite the lack of information from free‐ranging bears, the overall conclusions from these previous studies are that not only are plasma lipid levels raised during hibernation, lipids are also generally elevated compared to human values.
In humans, plasma lipid concentrations and atherosclerosis are associated, as is also the case for various mammal models of atherosclerosis. 5 , 6 , 7 , 8 We have only been able to find one published account on atherosclerosis in bears, a case report of cerebrovascular atherosclerosis in an aged grizzly bear (Ursus arctos horribilis). 9
The objective of this study was to ascertain plasma concentrations of lipids in free‐ranging brown bears during hibernation and in the same bears while active. To establish whether the presumably high plasma concentrations of lipids cause atherosclerotic fatty streaks and plaque building we related the biochemical findings to coronary and aortic histopathology from bears shot in Sweden during the legal hunting season.
Methods
Material
Blood samples were collected from free‐ranging brown bears during hibernation (February 2010) and during their active period in summer (June 2010). The bears were immobilized in the den during February and from a helicopter during June by darting with a mixture of tiletamine‐zolazepam and medetomidine. 10 Blood was drawn from the jugular vein as described previously. 11 Only already radio‐collared bears were studied to engage the bear at the denning site with the highest precision possible. The experimental design of recaptuing provides controls for both internal and external effects (body and external temperature, feeding status, metabolic rate) in accordance with recommendations by Carey et al. 12 The study of bears was approved by the Swedish Ethical Committee on animal research (C212/9). All procedures described were in compliance with Swedish laws and regulations.
From bears shot during hunting during late summer and early fall of 2010 we collected myocardial specimens containing the left descending anterior coronary artery (LAD) and aortic arches within 3 hours from the killing of the animals. The tissue samples were immediately stored in formalin for histopathologic examination.
Biochemical analyses
Plasma from blood, collected in Li‐Heparin tubes (Vacuette®, Greiner Bio‐one, Kremsmünster, Austria) were used to measure the concentrations of total cholesterol, triglycerides, low‐density lipoprotein (LDL) cholesterol and high‐density lipoprotein (HDL) cholesterol. The levels were measured by enzymatic methods using VITROS 5.1 FS Chemistry systems instrument (Ortho‐clinical diagnostics, Rochester, NY, USA) according to the manufacturer’s instructions. Briefly, total cholesterol, triglyceride and HDL cholesterol were analyzed by multilayer film dry‐slide chemistry with colorimetric detection. 13 Lipoprotein analysis of LDL was measured in a two‐step reaction sequence, i.e., enzymatic–colorimetric reaction. 13 , 14
Hematocrit concentration was determined in EDTA whole blood (Vacuette®, Greiner Bio‐one), by an automated hematology analyzer (XE‐5000, Sysmex Corporation, Kobe, Japan).
Histopathologic examination
Tissue was fixed in buffered formalin and paraffin‐embedded. All LAD specimens for histological analysis were taken within 2–3 cm from the LAD ostium and all aortic specimens were sections of the arch. Representative paraffin blocks were cut in sections with an approximate thickness of 3 μm. On all specimens haematoxylin‐eosin, elastic van Gieson, and trichrome reactions were performed.
Statistics
Values are presented as mean ± standard deviation (s.d.). A paired t‐test was used for the statistical comparison between plasma lipid levels during hibernation and during active state. Differences were considered statistically significant when P < 0.05.
Results
Blood samples were collected from seven free‐ranging brown bears, (three females and four males, 2–3 years old). Material for histological investigation was obtained from 12 brown bears (six females, six males), between 1.5 and 12 years old (mean 4.6 ± 0.7 years), weighing 101.6 ± 14.5 kg. Samples from these bears shot during hunting were collected between 1.5 and 7 hours post mortem (mean 3.0 ± 0.4 hours).
Total plasma cholesterol decreased in all bears from hibernation to the active summer period (11.08 ± 1.04 mmol/L vs. 7.89 ± 1.96 mmol/L, P= 0.0028, Figure 1A ) as did triglyceride (3.16 ± 0.62 mmol/L vs. 1.44 ± 0.27 mmol/L, P= 0.00012, Figure 1D ) and LDL cholesterol (4.30 ± 0.71 mmol/L vs. 2.02 ± 1.03 mmol/L, P= 0.0075, Figure 1B ). There was no statistically significant difference between the two conditions regarding HDL cholesterol (4.78 ± 0.59 mmol/L vs. 4.95 ± 0.51 mmol/L, P= 0.18, Figure 1C ). Blood hematocrit was higher during hibernation (56.8% vs. 45.0%, P= 0.00013).
Figure 1.
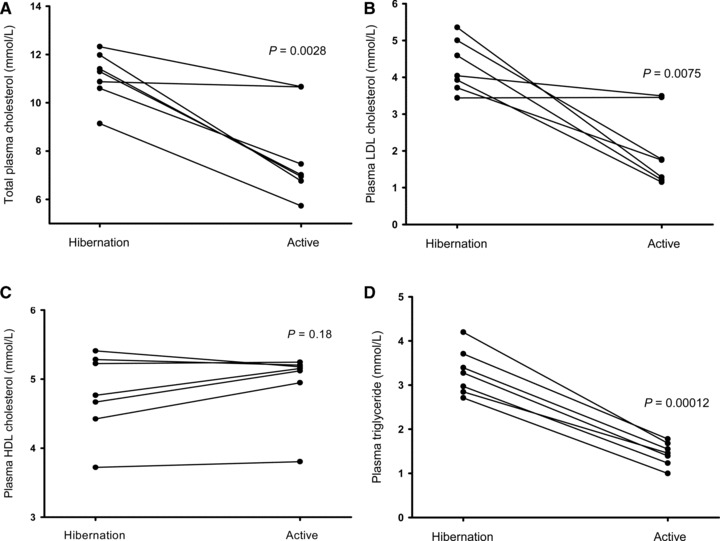
Individual total plasma cholesterol levels of seven free‐ranging brown bears during hibernation in February and when active in June (A). Analogous graphic presentations of LDL‐cholesterol (B), HDL‐cholesterol (C) and triglycerides (D).
Aortas were examined from 11 animals. The average wall thickness was around 5 mm with slight tapering toward the aortic arch; there was a definite thinning when reaching neck arteries, the thickness of those were 2–3 mm. Left anterior descending coronary artery sections were obtained from all 12 bears. The wall thickness was about 1–1.5 mm.
Histology
Aortas consisted of the typical three vessel wall layers, internal, medial, and adventitial. No atherosclerosis, fatty streaks, foam cell infiltration, or inflammation were seen ( Figure 2 ). The internal layer was of quite normal appearance compared with human aortas. The media was thick and with considerably more smooth muscle cells compared with human histology. It is evident that groupings of smooth muscle cells running obliquely to the long axis of the aorta is more pronounced in bears than in humans. The elastic laminae and fibers appeared normal and uninterrupted and the histological appearance of the adventitia did not differ from human equivalents.
Figure 2.
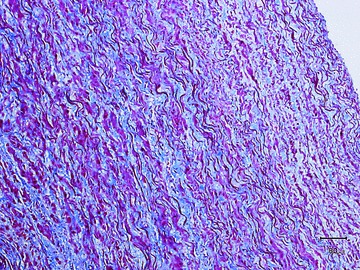
Section of the aortic arch (Mason trichrome stain) from an 8‐year‐old male brown bear weighing 238 kg and shot during hunting. Red color denotes smooth muscle, blue is collagen tissue, and distinct dark fibers signify elastic lamellae. No atherosclerotic changes were found.
The left anterior descending coronary artery was free of atherosclerotic changes and had the morphology of a muscular artery as is also found in nonatherosclerotic healthy humans ( Figure 3 ).
Figure 3.
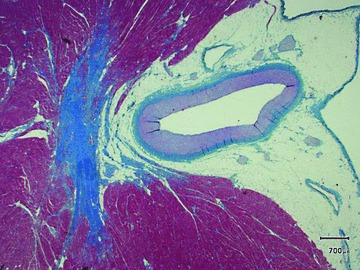
Section of the left anterior descending coronary artery (muscular type of artery) from a 4‐year‐old female brown bear weighing 101 kg and shot during hunting (staining as in Figure 1). No atherosclerotic changes were found.
Discussion
This study represents the first systematic comparison of plasma lipids in hibernating and active free‐ranging brown bears and the first description of aorta and coronary artery histopathology in this species. Our main findings were that brown bear plasma lipids are elevated during hibernation compared with active state and that cholesterol levels generally are much higher than normal human values. Furthermore, bears up to 12 years old show no signs of atherosclerosis—not even fatty streaks.
The most probable source of increased plasma lipids is from stored triglycerides deposited in adipose tissue. Lipid oxidation of this fat is the primary fuel in bears during hibernation and the increase in cholesterol and triglycerides may be viewed as an alteration in the metabolism of lipoproteins responsible for clearance of these lipids. 2 Hematocrits were higher during hibernation and hemoconcentration likely contributed to the increase in cholesterol but can only explain part of the rise in this parameter.
Previous studies have documented increasing cholesterol levels in captive American black bears during hibernation. 1 , 2 Nelson et al. measured cholesterol in plasma and found levels in the same range as we (total cholesterol of 13.4 mmol/L in winter and 9.6 mmol/L in summer) (1). In contrast, Hissa et al. reported higher cholesterol values during summer than winter in two captive brown bears. 3 Although Frank et al. measured cholesterol in wild American black bears they did not include hibernation sampling. 4 Chauhan et al. 2 analyzed serum and not plasma and used a different analysis technology (thin layer chromatography) thus hindering direct comparisons with our findings. Despite considerable variation in food patterns and physical activity between free‐ranging and captive bears, the findings of the present study corroborate that brown bear plasma lipids are elevated during hibernation and that the levels generally are considerably higher than what is normally found in humans. The bears in this study had very high plasma concentrations of HDL cholesterol compared to humans. Although low HDL concentrations are associated with increased risk of cardiovascular disease in humans, recent findings question the protective role of high HDL levels. 15
Although seasonal variations and inactivity influence lipid levels, this is not confined to hibernators. In healthy humans total cholesterol peaks in December–January although the total seasonal variation is around 0.1 mmol/L only with changes in plasma volume accounting for much of the variation. 16 Two bed rest studies in humans, one lasting 35 days 17 and one lasting 20 days 18 both documented an HDL cholesterol decrease and a triglycerides increase in relation to bed rest. However, these changes are far from the drastic alterations in the same variables in brown bears. Although sedentary life style and high cholesterol levels are powerful risk factors for cardiovascular disease in humans, our histopathological findings indicate that brown bears are relatively resistant to atherosclerosis. This observation seems even more remarkable when considering that during hibernation heart rate, and thus blood flow, is reduced dramatically. In captive grizzly bears average resting heart rate is reduced from 84 to 17 beats per minute from active state to hibernation despite unchanged stroke volume. 19 When blood flow is reduced fluid shear stress is also typically reduced, and low shear stress in blood vessels is related to the forming of atherosclerotic plaques. 20 But another risk factor in the atherosclerotic process—oxidative stress—is increased in hibernating American black bears 21 and adds to the picture of bears as unique biological models.
The instrument and methods used in this study are routinely used for measuring blood levels of total cholesterol, LDL, HDL, and triglycerides in human samples. To the best of our knowledge, the methods have not yet been validated for use in bears. However, in veterinary clinics identical analyses, using the same instruments and reagents, are routinely performed in a wide variety of animal species (e.g., horse, monkey, and lizard). Our methodology for measuring LDL cholesterol is not a routine veterinary technique, but is also an enzymatic colormetric method, which must be considered reliable irrespective of species. It is a limitation to our study that plasma lipid measurements were done in one group of bears whereas histopathology was investigated in another group. However, the last group was older, thus increasing the chance of detecting atherosclerotic changes. As cholesterol levels rise with age in humans 22 we have no reason to believe that cholesterol levels were lower in the bears shot during hunting. One could argue that the histopathological examinations were done in bears too young to develop atherosclerosis. However, brown bears typically reach reproductive maturity before the age of 5 years and rarely live to after the age of 25. 23 Human data supports that early findings of atherosclerosis can be present at a very young age. 24 Had we also measured additional fatty acids and lipoprotein lipase (lipogenic) or hormone‐sensitive lipase (lipolytic) we could have speculated in the causes of raised cholesterol in bears, but this was outside our scope.
Conclusion
We conclude that brown bear total cholesterol and triglyceride levels peak during hibernation and are considerably higher than human levels. Despite this and regardless of exposure to other factors constituting risks for development of cardiovascular disease in humans (obesity, physical inactivity, and circulatory slow flow during hibernation) we found no signs of atherosclerosis in brown bears. We hypothesize that brown bear resistance to atherosclerosis may serve as a biological model for prevention of cardiovascular disease in humans. Future studies must determine the biological mechanisms behind this resistance and whether for example the inflammatory or antioxidant defense systems, cholesterol recycling, endocrinological changes, or perhaps even how bears handle mental stress could improve our understanding.
Sources of Funding
This study was supported by a researcher network grant from NordForsk (an organization under the Nordic Council of Ministers. Project number 44042).
Conflict of Interests
The authors declare that they have no financial, personal, or professional associations that could be perceived as interfering with the objectivity of the study.
Acknowledgments
We thank the Scandinavian Brown Bear Research Project for excellent collaboration and the accredited Clinical Chemical Laboratory at Örebro University Hospital, for help with hematology and biochemical analysis. We wish to explain our gratitude to Sven Brunberg for organization, logistics and capture of bears, to Johan Josefsson for handling of blood in the field, to Christina Gustafsson for technical assistance with blood sample analysis, and to Bente Wormstrup and Mette Skov Mikkelsen for preparation of histological specimens.
The work presented here was carried out in collaboration between all authors. KA, BS, and OF defined the research subject. ALE and JMA designed and supervised capture and fieldwork. ALE carried out and supervised sampling of bears shot during hunting. BS carried out blood sample laboratory experiments and UB designed and carried out histologypathology. KA and OF analyzed the data, interpreted the results and wrote the paper. All authors have contributed to, seen and approved the manuscript.
References
- 1. Nelson RA, Wahner HW, Jones JD, Ellefson RD, Zollman PE. Metabolism of bears before, during, and after winter sleep. Am J Physiol. 1973; 224(2): 491–496. [DOI] [PubMed] [Google Scholar]
- 2. Chauhan V, Sheikh A, Chauhan A, Tsiouris J, Malik M, Vaughan M. Changes during hibernation in different phospholipid and free and esterified cholesterol serum levels in black bears. Biochimie. 2002; 84(10): 1031–1034. [DOI] [PubMed] [Google Scholar]
- 3. Hissa R. Physiology of the European brown bear (Ursus arctos arctos). Ann Zool Fennici. 1997; 34: 267–287. [Google Scholar]
- 4. Frank N, Elliott SB, Allin SB, Ramsay EC. Blood lipid concentrations and lipoprotein patterns in captive and wild American black bears (Ursus americanus). Am J Vet Res. 2006; 67(2): 335–341. [DOI] [PubMed] [Google Scholar]
- 5. Torii R, Shiomi M, Ito T, Yamada S, Eguchi Y, Ikeda N. Cholesterol‐fed ovariectomized monkeys are good animal models for human atherosclerosis of postmenopausal women. Primates. 2003; 44(3): 247–252. [DOI] [PubMed] [Google Scholar]
- 6. Buja LM, Kita T, Goldstein JL, Watanabe Y, Brown MS. Cellular pathology of progressive atherosclerosis in the WHHL rabbit. An animal model of familial hypercholesterolemia. Arteriosclerosis. 1983; 3(1): 87–101. [DOI] [PubMed] [Google Scholar]
- 7. Rapacz J, Hasler‐Rapacz J, Taylor KM, Checovich WJ, Attie AD. Lipoprotein mutations in pigs are associated with elevated plasma cholesterol and atherosclerosis. Science. 1986; 234(4783): 1573–1577. [DOI] [PubMed] [Google Scholar]
- 8. Rogers KA, Karnovsky MJ. Dietary fish oil enhances monocyte adhesion and fatty streak formation in the hypercholesterolemic rat. Am J Pathol. 1988; 132(2): 382–388. [PMC free article] [PubMed] [Google Scholar]
- 9. Miller AD, McDonough S. Interthalamic hematoma secondary to cerebrovascular atherosclerosis in an aged grizzly bear (Ursus arctos horribilis) with primary cardiac schwannoma. J Zoo Wildl Med. 2008; 39(4): 659–662. [DOI] [PubMed] [Google Scholar]
- 10. Kreeger TJ, Arnemo JM. Handbook of Wildlife Chemical Immobilization. 3rd edn Laramie , Wyoming , USA : International Wildlife Veterinary Services; 2007: 165–166. [Google Scholar]
- 11. Frobert O, Christensen K, Fahlman A, Brunberg S, Josefsson J, Sarndahl E, Swenson JE, Arnemo JM. Platelet function in brown bear (Ursus arctos) compared to man. Thromb J. 2010; 8: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003; 83(4): 1153–1181. [DOI] [PubMed] [Google Scholar]
- 13. Duvillard L, Gambert P. Evaluation of the VITROS chemistry products dHDL slides for direct measurement of high density lipoprotein cholesterol. Clin Chim Acta. 2006; 366(1–2): 130–136. [DOI] [PubMed] [Google Scholar]
- 14. Yip PM, Chan MK, Nelken J, Lepage N, Brotea G, Adeli K. Pediatric reference intervals for lipids and apolipoproteins on the VITROS 5,1 FS Chemistry System. Clin Biochem. 2006; 39(10): 978–983. [DOI] [PubMed] [Google Scholar]
- 15. Keidar S, Bogner I, Gamliel‐Lazarovich A, Leiba R, Fuhrman B, Kouperberg E. High plasma high‐density lipoprotein levels, very low cardiovascular risk profile, and subclinical carotid atherosclerosis in postmenopausal women. J Clin Lipidol. 2009; 3(5): 345–350. [DOI] [PubMed] [Google Scholar]
- 16. Ockene IS, Chiriboga DE, Stanek EJ III, Harmatz MG, Nicolosi R, Saperia G, Well AD, Freedson P, Merriam PA, Reed G, et al. Seasonal variation in serum cholesterol levels: treatment implications and possible mechanisms. Arch Intern Med. 2004; 164(8): 863–870. [DOI] [PubMed] [Google Scholar]
- 17. Mazzucco S, Agostini F, Mangogna A, Cattin L, Biolo G. Prolonged inactivity up‐regulates cholesteryl ester transfer protein independently of body fat changes in humans. J Clin Endocrinol Metab. 2010; 95(5): 2508–2512. [DOI] [PubMed] [Google Scholar]
- 18. Yanagibori R, Suzuki Y, Kawakubo K, Kondo K, Iwamoto T, Itakura H, Makita Y, Sekiguchi C, Gunji A, Kondou K. The effects of 20 days bed rest on serum lipids and lipoprotein concentrations in healthy young subjects. J Gravit Physiol. 1997; 4(1): S82–S90. [PubMed] [Google Scholar]
- 19. Barrows ND, Nelson OL, Robbins CT, Rourke BC. Increased cardiac alpha‐myosin heavy chain in left atria and decreased myocardial insulin‐like growth factor (IGF‐I) expression accompany low heart rate in hibernating grizzly bears. Physiol Biochem Zool. 2010; 84(1): 1–17. [DOI] [PubMed] [Google Scholar]
- 20. Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985; 5(3): 293–302. [DOI] [PubMed] [Google Scholar]
- 21. Chauhan VP, Tsiouris JA, Chauhan A, Sheikh AM, Brown WT, Vaughan M. Increased oxidative stress and decreased activities of Ca(2+)/Mg(2+)‐ATPase and Na(+)/K(+)‐ATPase in the red blood cells of the hibernating black bear. Life Sci. May 31 2002; 71(2): 153–161. [DOI] [PubMed] [Google Scholar]
- 22. Connelly PW, MacLean DR, Horlick L, O’Connor B, Petrasovits A, Little JA. Plasma lipids and lipoproteins and the prevalence of risk for coronary heart disease in Canadian adults. Canadian Heart Health Surveys Research Group. CMAJ. Jun 1 1992; 146(11): 1977–1987. [PMC free article] [PubMed] [Google Scholar]
- 23. Schwarz CC, Keating KA, Reynolds HV, Barnes VG, Sellers RA, Swenson JE, Miller SD, McLellan BN, Keay J, McCann R, et al. Reproductive maturation and senescence in the female brown bear. Ursus. 2003; 14(2): 109–119. [Google Scholar]
- 24. Enos WF, Holmes RH, Beyer J. Coronary disease among United States soldiers killed in action in Korea. JAMA.Jul 181953; 152(12): 1090–1093. [DOI] [PubMed] [Google Scholar]



