Abstract
The 70-kb virulence plasmid enables Yersinia spp. (Yersinia pestis, Y. pseudotuberculosis, and Y. enterocolitica) to survive and multiply in the lymphoid tissues of their host. It encodes the Yop virulon, an integrated system allowing extracellular bacteria to disarm the cells involved in the immune response, to disrupt their communications, or even to induce their apoptosis by the injection of bacterial effector proteins. This system consists of the Yop proteins and their dedicated type III secretion apparatus, called Ysc. The Ysc apparatus is composed of some 25 proteins including a secretin. Most of the Yops fall into two groups. Some of them are the intracellular effectors (YopE, YopH, YpkA/YopO, YopP/YopJ, YopM, and YopT), while the others (YopB, YopD, and LcrV) form the translocation apparatus that is deployed at the bacterial surface to deliver the effectors into the eukaryotic cells, across their plasma membrane. Yop secretion is triggered by contact with eukaryotic cells and controlled by proteins of the virulon including YopN, TyeA, and LcrG, which are thought to form a plug complex closing the bacterial secretion channel. The proper operation of the system also requires small individual chaperones, called the Syc proteins, in the bacterial cytosol. Transcription of the genes is controlled both by temperature and by the activity of the secretion apparatus. The virulence plasmid of Y. enterocolitica and Y. pseudotuberculosis also encodes the adhesin YadA. The virulence plasmid contains some evolutionary remnants including, in Y. enterocolitica, an operon encoding resistance to arsenic compounds.
Invasive pathogenic bacteria have in common the capacity to overcome the defense mechanisms of their animal host and to proliferate in its tissues. They each have their own life-style and target organs and cause a variety of symptoms and diseases, which suggested the existence of great diversity among the bacterial virulence strategies. However, recent data contradict this view and reveal the existence of major virulence mechanisms in various pathogenic bacteria. One is the release of A-B toxins as exemplified by Bordetella pertussis and Bacillus anthracis. Another was discovered more recently in a number of bacterial pathogens. By this mechanism, sometimes referred to as type III, extracellular bacteria that are in close contact with a eukaryotic cell deliver bacterial proteins into the cytosol of this cell. The Yop system of Yersinia spp., which we describe in this review, represents an archetype for this new mechanism. The other animal pathogens with related systems are Salmonella spp., Shigella spp., enteropathogenic Escherichia coli (EPEC), Pseudomonas aeruginosa, Chlamydia psittaci (165), and Bordetella spp. (383a). Related systems are also found in the plant pathogens that elicit the so-called hypersensitive response, such as Erwinia amylovora, Pseudomonas syringae, Xanthomonas campestris, and Ralstonia solanacearum (for reviews, see references 4 and 351). The literature on all the type III systems is now so abundant that an exhaustive description could no longer fit in one review. This review is thus specifically dedicated to the Yersinia type III system. However, homologs of the various Yersinia proteins in the other bacteria are mentioned and even described when appropriate. To integrate the Yop virulon in the general context of cross talk between bacterial pathogens and their host, the reader may refer to broader reviews (94, 95, 107). More information on Yersinia virulence in general is also available in recent reviews (51, 255). Less exhaustive reviews dealing with the type III system (201, 352) or, more specifically, the Yop virulon (75, 98, 335–337) are also available.
Yersinia Life-Style
The genus Yersinia includes three species that are pathogenic for rodents and humans; Yersinia pestis causes plague, Yersinia pseudotuberculosis causes mesenteric adenitis and septicemia, and Yersinia enterocolitica, the most prevalent in humans, causes gastrointestinal syndromes ranging from an acute enteritis to mesenteric lymphadenitis (76). Y. pestis is generally inoculated by a flea bite, while the two others are food-borne pathogens. In spite of these differences in the infection routes, all three have a common tropism for lymphoid tissues and a common capacity to resist the nonspecific immune response, in particular phagocytosis and killing by macrophages and polymorphonuclear leukocytes (PMNs). Y. pestis and Y. pseudotuberculosis are natural rodent pathogens. Although this does not seem to be the case for Y. enterocolitica, experimental infection of mice reproduces some of the symptoms observed in humans, in particular those related to invasion of the lymphoid tissues. After orogastric inoculation of mice, Y. enterocolitica selectively invades the Peyer’s patches via M cells (15, 131, 140). This invasion leads to an enormous recruitment of PMNs, formation of microabscesses comprising extracellular Yersinia, and, finally, complete destruction of the cytoarchitecture of the Peyer’s patches. Later, abscesses appear in mesenteric lymph nodes, suggesting that Y. enterocolitica disseminates via the lymphatic vessels (15). Anatomopathological examination of mice experimentally infected with Y. pseudotuberculosis also concluded that these bacteria are largely extracellular (309). In accordance with these in vivo observations, Yersinia manifests some resistance to phagocytosis in vitro, both by macrophages (87, 281) and by PMNs (53, 65, 291, 361). Once they are phagocytosed, Y. pseudotuberculosis and Y. enterocolitica generally do not survive. These observations led to the concept that Y. pseudotuberculosis and Y. enterocolitica are extracellular pathogens and that their survival strategy basically consists in avoiding the nonspecific immune response. Y. pestis has the same capacity as the other Yersinia spp. to resist phagocytosis. However, if it has been phagocytosed, it probably has a better capacity to resist killing. Early work by Straley (333, 334) showed that indeed Y. pestis can grow in the phagolysosome of cultured murine resident peritoneal macrophages. The reason for this capacity is not clearly established, but it does not depend on the type III system.
From Ca2+ Dependency to a Comprehensive View of the System
It has been known since the mid-1950s that Y. pestis is unable to grow at 37°C in Ca2+-deprived media (157). It has also been known for decades that this unusual property can be lost and that its loss correlates with a loss of virulence. This Ca2+ dependency phenotype offered an extraordinary clue to the pathogenicity arsenal because nonvirulent mutants could be easily detected and even selected for. It appeared that virulence and Ca2+ dependency are encoded by a 70-kb plasmid (112, 390), sometimes called pYV (200). Under conditions of growth restriction, this plasmid governs the synthesis of a set of about 12 proteins called Yops (for “Yersinia outer membrane proteins”), which were originally designated by a letter, a number, or their molecular weight, according to the authors (42, 44, 73, 74, 97, 100, 110, 220, 256, 267, 330, 331, 377). The LcrV protein, an antigen of Y. pestis that had already been discovered in the mid-1950s (53), turned out to be one of these Yops (97, 239, 331). Most of the yop genes have been identified and sequenced, and they appeared to be almost identical in the three species. A uniform nomenclature has been introduced for YopB, YopD, YopE, YopH, YopM, and LcrV. YopN is sometimes still called LcrE (360). A few other Yops do not benefit from a common nomenclature because they were discovered or characterized more recently: YopO, YopP, and YopQ in Y. enterocolitica (74, 229) are called YpkA, YopJ, and YopK, respectively, in Y. pseudotuberculosis (111). The YopJ nomenclature is also used in Y. pestis (330). YopR (8) turned out to be the product of yscH. A Y. pestis YopL has been mentioned (329, 332), but its gene has not yet been identified and sequenced and it is not known whether it corresponds to a Yop described in Y. enterocolitica or Y. pseudotuberculosis. Finally, YopT was described only very recently (170). The “S” has been skipped to avoid confusion with Yop in the plural.
Although initially described as outer membrane proteins, the Yops could also be recovered from the culture supernatant (149, 151), and it was later found that they were actually secreted proteins (229). Their secretion occurs by a new pathway (now called type III) and requires a specific apparatus (called Ysc for “Yop secretion”), which is also encoded by the pYV plasmid (228, 229).
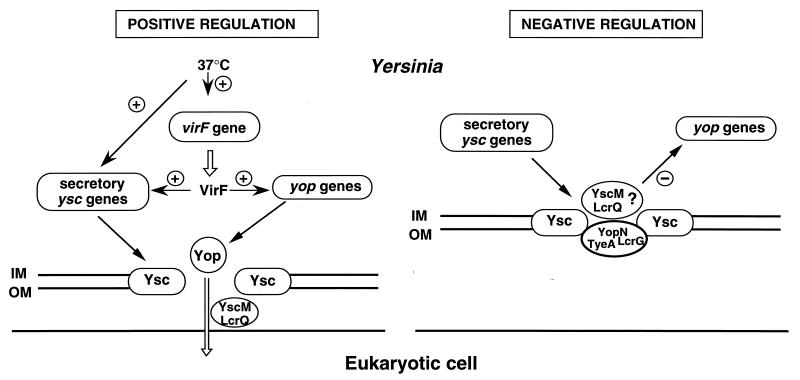
To trigger Yop secretion in vitro, Yersinia is generally grown at 28°C in a medium depleted of Ca2+ and then transferred to 37°C. Ca2+ depletion (or contact with a eukaryotic cell [see below]) and temperature both control transcription of the yop genes. The best-characterized regulator is VirF (LcrF in Y. pestis and Y. pseudotuberculosis), a transcriptional activator of the AraC family (72). It controls transcription of most of the genes involved in Yop synthesis and secretion (199).
Genetic analysis indicated that most of the Yop proteins are essential for virulence. In particular, YopE turned out to be responsible for a cytotoxic activity (282) that had been described earlier (119, 266). YopH was found to inhibit the phagocytosis of bacteria by macrophages (281) and later was shown to be a protein tyrosine phosphatase (PTPase) related to eukaryotic counterparts (132). However, three observations were enigmatic: (i) Yops form large and insoluble aggregates in the culture medium, which is unusual for virulence effectors; (ii) YopE has no toxic activity on its own (119, 287); and (iii) what would be the role of an extracellular PTPase?
A major advance occurred when Rosqvist et al. (283) showed that Yop preparations elicit a cytotoxic response when microinjected into HeLa cells, indicating that the target of the YopE protein was intracellular. A yopD mutant was unable to affect HeLa cells, while a preparation of Yops secreted by the very same mutant was cytotoxic when microinjected into the cytosol of HeLa cells. Rosqvist et al. logically concluded from this that the YopD protein should play a role in translocating the YopE protein across the plasma membrane of the eukaryotic target cell to reach the cytosolic compartment (283).
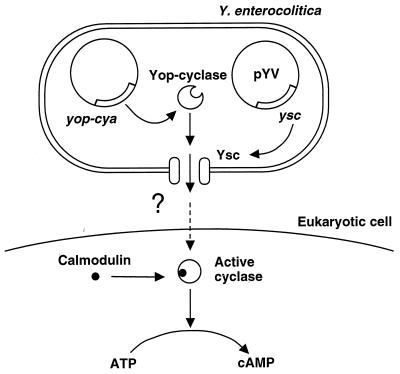
The evidence for YopD-mediated translocation of the YopE protein was essentially genetic. In 1994, this elegant hypothesis was confirmed by two different approaches. The first was based on immunofluorescence and confocal laser-scanning microscopy examinations. Rosqvist et al. (285) showed that the YopE protein appeared in the cytosol of HeLa cells infected with wild-type Y. pseudotuberculosis. In contrast, when cells were infected with a mutant strain of Y. pseudotuberculosis unable to produce YopD, YopE was no longer internalized, showing that the YopD protein was essential for the translocation of YopE across the target cell membrane (285). The second approach was based on a reporter enzyme strategy introduced by Sory and Cornelis (321) (Fig. 1). The reporter system consisted of the calmodulin-activated adenylate cyclase domain (called Cya) of the Bordetella pertussis cyclolysin (118). The rationale was as follows: the Yop-Cya hybrid enzyme introduced into the cytosol of eukaryotic cells would produce cyclic AMP (cAMP) while the intrabacterial Yop-Cya hybrid would not, because of the absence of calmodulin in the bacterial cytoplasm. Since the catalytic domain of cyclolysin is unable to enter eukaryotic cells by itself, accumulation of cAMP would essentially reflect Yop internalization. Infection of HeLa cells with recombinant Y. enterocolitica producing a hybrid YopE-Cya protein resulted in a marked increase in the level of cAMP even when internalization of the bacteria themselves was prevented by cytochalasin D. Infection with a Y. enterocolitica mutant unable to produce both the YopD and YopB proteins did not lead to cAMP accumulation, confirming the involvement of YopD and/or YopB in translocation of the YopE protein across eukaryotic membranes (321).
FIG. 1.
The Yop-Cya reporter strategy used to study translocation of Yop proteins into the cytosol of eukaryotic cells. Reprinted from reference 321 with permission of the publisher.
In light of these results, a coherent model could be established. According to this model (Fig. 2), the Yops form two distinct groups of proteins. Some Yops are intracellular effectors delivered inside eukaryotic cells by extracellular Yersinia organisms adhering at the cell surface, while other Yops (translocator Yops) form a delivery apparatus. This model is now largely supported by a number of other results that will be presented in this review. Among others, it is supported by immunological observations. While antigens processed in phagocytic vacuoles of phagocytes are cleaved and presented by major histocompatibility complex class II molecules, epitope 249-257 of YopH produced by Y. enterocolitica during a mouse infection is presented by major histocompatibility complex class I molecules, like cytosolic proteins (328).
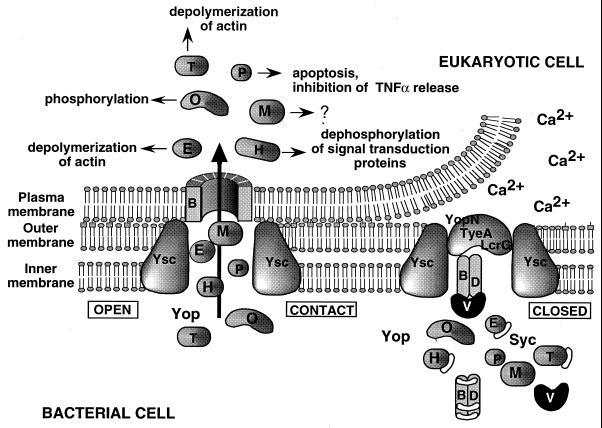
FIG. 2.
A tentative model for the interaction between Yersinia and a macrophage. When Yersinia is placed at 37°C in a rich environment, the Ysc secretion apparatus is installed and a stock of Yop proteins is synthesized. Some of these proteins are capped with their specific Syc chaperones, which presumably prevent premature associations. As long as there is no contact with a eukaryotic cell, the YopN-TyeA-LcrG plug blocks the Ysc secretion channel. Upon Ca2+ depletion or contact with the eukaryotic target cell, the secretion channel opens and the YopB translocator inserts in the eukaryotic cell with the help of YopD and LcrV. The Yop effectors (YopE, YopH, YopM, YopO/YpkA, YopP/YopJ, and YopT) are then transported through the secretion channel and translocated across the plasma membrane, guided by the translocators. YopE and YopT act on the cytoskeleton, while YopP/YopJ induces apoptosis and inhibits the release of TNF-α.
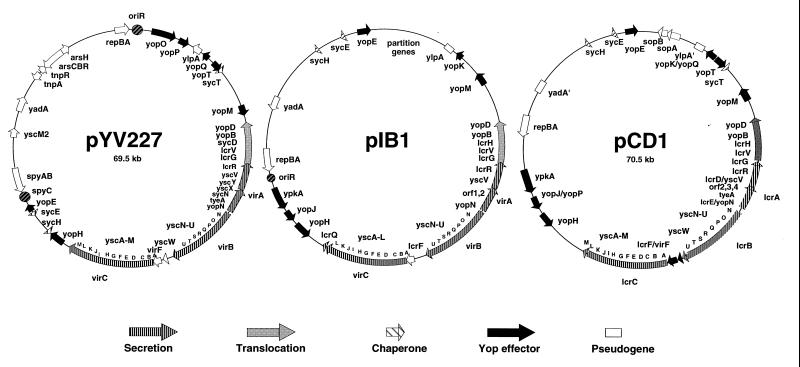
The virulence plasmid thus encodes an integrated antihost system allowing the delivery of a set of effector Yops into the cytosol of eukaryotic cells by a delivery apparatus and a specialized secretion system. The virulence plasmid has now been completely sequenced in Y. enterocolitica W22703 (pYV227) (171) and in Y. pestis KIM (pCD1) (165a, 257a). Most of the sequence of plasmid piB1 from Y. pseudotuberculosis YPIII is also available. The genetic maps are given in Fig. 3. About 50 genes are involved in virulence, and they occupy three-quarters of the plasmid. A total of 35 genes encoding the secretion and translocation machineries form a continuous block flanked on both sides by more dispersed genes encoding effectors and their chaperones.
FIG. 3.
The genetic maps of pYV227 from Y. enterocolitica W227 (serotype O:9) (redrawn from reference 170), pIB1 from Y. pseudotuberculosis YPIII (redrawn from reference 259), and pCD1 from Y. pestis KIM (redrawn from references 165a and 257a). Note that none of these maps is complete. For pCD1, the plasmid has been sequenced twice (165a, 257a) and only the genes that are identified in the two sequences or in one sequence and in Y. enterocolitica are shown. Plasmid pYV227 has also been completely sequenced (171), but only the genes described in this review are included here. Shading of the genes has been done on the basis of the data presented in this review.
We first review the effects of this virulence apparatus on eukaryotic cells and then analyze in detail the fate of the Yops, from secretion to delivery and action in eukaryotic cells. We then describe the adhesin YadA and, finally, review the genetic aspects, regulation of gene expression, and plasmid organization.
EFFECTS ON HOST CELLS
Macrophages
Macrophages are part of the first line of defense against invading organisms, and several elements of the virulon allow Yersinia to circumvent the microbicidal action of these phagocytes. Upon interaction with macrophages, Yersinia has the capacity to impair phagocytosis, to inhibit the respiratory burst, to trigger apoptosis, and to suppress the normal release of tumor necrosis factor alpha (TNF-α). Each of these four aspects is treated individually below.
Inhibition of phagocytosis.
One of the simplest ways to resist killing by macrophages is to avoid being ingested. It has been known for a long time that Yersinia spp. are endowed with the capacity to resist phagocytosis by macrophages and that this property depends on the presence of the pYV plasmid (58, 59).
Working in vitro with Y. pseudotuberculosis and resident mouse peritoneal macrophages, Rosqvist et al. (281) showed by a double-immunofluorescence technique (153) that a strain unable to express YopH has a reduced ability to resist phagocytosis. The ability to resist phagocytosis could be complemented in trans by introduction of a plasmid carrying only the yopH gene, demonstrating that YopH is indeed involved in the antiphagocytic effect. However, mutation of yopH did not completely abolish the resistance to phagocytosis. Macrophages phagocytosed 80% of yopH mutant bacteria, in comparison to 95% of pYV− and 35% of pYV+ bacteria. This intermediate level of phagocytosis inhibition by the yopH mutant suggested that another virulence factor was involved in this phenomenon. This second factor turned out to be YopE, since a double yopH yopE mutant showed the same low level of phagocytosis resistance as a plasmid-cured strain (282). YopE and YopH thus act in concert to enable Yersinia to inhibit its own uptake by macrophages and hence to proliferate in the Peyer’s patches as extracellular microcolonies (140).
Fällman et al. (87) undertook more detailed study of the uptake of Y. pseudotuberculosis by the macrophage-like cell line J774A.1. Both nonopsonized bacteria and bacteria opsonized with rabbit anti-Yersinia immunoglobulin G were able to inhibit their uptake by J774A.1 macrophages, indicating that Yersinia can resist specific uptake via Fc receptors. Pretreatment of J774A.1 cells with wild-type bacteria prevented the uptake of nonrelated prey (immunoglobulin G-opsonized yeast particles), while preincubation with mutants impaired in resistance to phagocytosis had no effect; the Yersinia antiphagocytic effect thus involves the blocking of a general phagocytic mechanism and is not restricted to the uptake of Yersinia organisms themselves (87). Further studies with J774A.1 macrophages suggested that YopH, in addition to inducing an overall dephosphorylation of host cell proteins (34, 36, 128, 147), is able to interfere with early tyrosine phosphorylation signals that occur in the cell during phagocytosis. Andersson et al. (11) showed that exposure of J774A.1 macrophages to yopH mutant Y. pseudotuberculosis resulted in a transient increase in tyrosine phosphorylation of a number of proteins, including paxillin, which is known to be tyrosine phosphorylated upon Fc receptor-mediated signaling associated with phagocytosis in macrophages (129). This transient tyrosine kinase activity, which probably constitutes part of an early phagocytic signal, was impaired by yopH+ bacteria (11). Recently, two eukaryotic cell proteins, focal adhesion kinase (FAK) and p130CAP, have been identified as YopH targets in epithelial cells (see below); this activity of YopH results in disruption of the focal adhesion structures and correlates with an impaired ability of the target cell to carry on the invasion-mediated internalization of the bacteria (31, 258). The role of FAK and p130CAP in phagocytic cells remains to be elucidated.
Inhibition of the respiratory burst.
It was suspected for a long time that Yersinia interferes with the normal respiratory burst of macrophages, since the oxidative burst occurring after interaction with Y. pestis is much lower than that seen after phagocytosis of E. coli (59). More recently, Hartland et al. (147) infected bone marrow-derived macrophages with various Y. enterocolitica mutant strains before stimulation of the respiratory burst by the addition of zymosan, which triggers the CR3 receptor. They measured the intensity of the respiratory burst by assaying the amount of reduced cytochrome c produced during the generation of O2− (127). This showed that Y. enterocolitica also has the capacity to inhibit the respiratory burst and that this capacity depends on the pYV plasmid. Loss of the effectors YopE, YopH, and YopO/YpkA did not affect this capacity, but loss of the translocator YopD did. This property thus probably depends on an effector different from YopE, YopH, and YopO.
Since tyrosine phosphorylation is an important component of the signaling pathways responsible for the activation of the macrophage respiratory burst, Green et al. (128) investigated the possible link between the YopH tyrosine phosphatase activity and the inhibition of the respiratory burst. They infected bone marrow-derived macrophages with Y. enterocolitica and monitored both tyrosine phosphorylation and respiratory burst in response to zymosan (127). Infection with pYV+ Y. enterocolitica suppressed both phenomena. However, loss of YopH abolished the suppressive effect on tyrosine phosphorylation but not on the respiratory burst. This observation agrees with those of Hartland et al. (147) and confirms that the inhibition of the zymosan-induced macrophage respiratory burst by Y. enterocolitica involves a plasmid-encoded virulence protein other than YopH, possibly in addition to YopH.
However, these conclusions differ from those of Bliska and Black (33), who showed that YopH is responsible for the inhibition of the Fc receptor-mediated oxidative burst in macrophages infected by Y. pseudotuberculosis. The reasons for these discrepancies are not known, but it is important to note that the experimental procedures used in the two studies are different and thus difficult to compare. Indeed, the pathways used to trigger the respiratory burst involved either the macrophage complement receptors or the Fc receptors; in addition, the type of macrophages, the Yersinia species, and the quantification methods were different (33, 82, 127, 128, 147).
In conclusion, Yersinia spp. are able to impair the oxidative burst of the macrophages, and so far, the only Yop effector protein that has been shown to be involved in the phenomenon is YopH. However, the role of YopH in the inhibition of the respiratory burst remains a matter of debate, since the detection of this role depends on the pathway used to trigger the respiratory burst.
Induction of apoptosis.
In 1986, Goguen et al. (119) reported that Y. pestis and Y. pseudotuberculosis have a cytotoxic effect on the mouse macrophage cell lines IC21 and P388D1 as well as on mouse resident peritoneal macrophages. They observed that cells infected with a wild-type strain change shape, acquire a granular aspect, and detach easily from the culture dish. This effect, which was dependent on the presence of the pYV plasmid, evokes apoptosis, although it was not described as such at that time. Recently, three groups, two working with Y. enterocolitica (232, 290) and one working with Y. pseudotuberculosis (237), showed that Yersinia triggers apoptosis of cultured macrophages. Infected macrophages displayed general features of apoptosis, such as membrane blebbing (apoptotic body formation), cellular shrinkage (232, 290), and DNA fragmentation (Fig. 4). Infection of macrophages with secretion and translocation mutants of Y. enterocolitica did not lead to apoptosis, showing that a translocated Yop effector is involved. Screening of a library of yop mutants showed that the YopE cytotoxin is not involved and identified YopP as the effector responsible for apoptosis (232) (Fig. 4). In an independent study, Monack et al. (237) came to the conclusion that YopJ, the Y. pseudotuberculosis homolog of YopP, is required for the induction of the cell death process. The phenomenon displays some cell specificity, since epithelial cells (232, 237, 290) and fibroblasts (237) do not undergo apoptosis upon infection with Yersinia. The mechanism by which Yersinia induces macrophage apoptosis remains to be elucidated, but it parallels that used by cytotoxic T lymphocytes to kill their target cells; cytotoxic T cells inject granzyme B into the cytosol of their target cells, thereby inducing apoptosis (308). One of the virulence functions of Yersinia organisms thus appears to mimic a physiological process of their host.
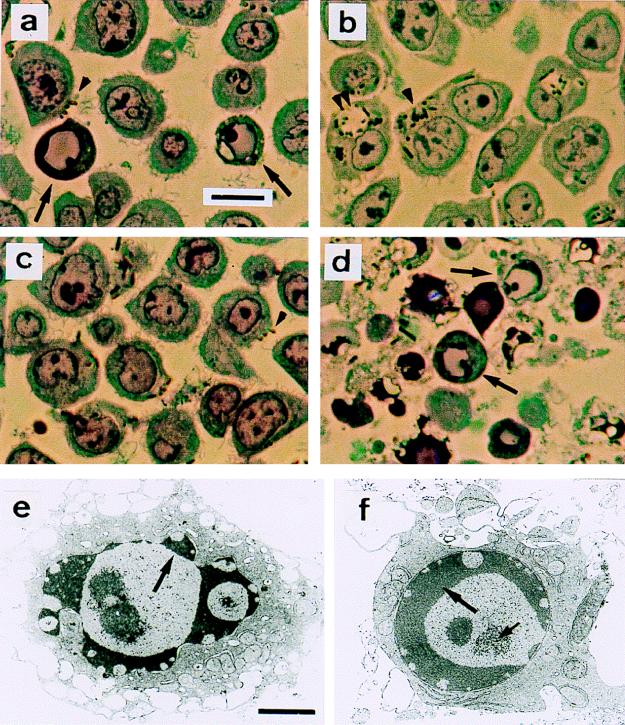
FIG. 4.
YopP-induced apoptosis. Semithin sections were stained with toluidine blue and examined by light microscopy (a to d). (a) Wild-type Y. enterocolitica E40. Apoptotic nuclei (arrows) and cell surface-associated bacteria (brown particles, arrowhead) are visible. (b) yscN secretion mutant. No apoptotic cells are detected. Internalized bacteria either in tight (single arrowhead) or spacious (double arrowhead) phagosomes are abundant. (c) yopP effector mutant. No apoptotic cells are detected. Bacteria are seen at the cell surface (arrowhead). (d) yopP+++ (yopP cloned in a multicopy vector). Apoptotic cells are visible (arrows). (e and f) Ultrastructural analysis of cells infected with yopP+++ from panel d is shown. Typical features of apoptosis include (i) peripheral chromatin condensation in crescents, except in the vicinity of nuclear pores (large arrows); (ii) bulging of nuclear crescents into the cytoplasm (best seen in panel e); and (iii) appearance of central clusters of small particles of unknown nature, typical of apoptosis (small arrow in panel f). Nuclear and plasma membrane alterations contrast with a good ultrastructural preservation of cytoplasm, particularly of endoplasmic reticulum and mitochondria. Bars, 10 μm (a to d) and 2 μm (e and f). Reprinted from reference 232 with permission of the publisher.
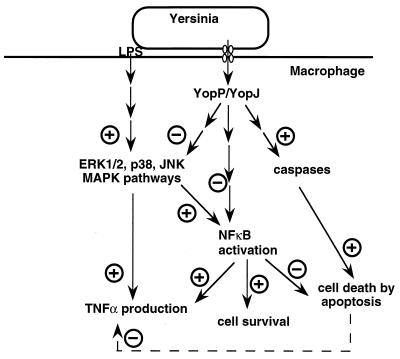
Although apoptosis is obvious in vitro, its physiopathological role is not yet clear. A yopP/yopJ mutant is not affected in virulence, at least in a mouse model (111, 330), and moreover, the induction of apoptosis by Yersinia has never been shown in vivo. The only known element is that in Y. enterocolitica-infected mice there is an increased number of apoptotic cells in the infected Peyer’s patches (15). It remains to be demonstrated if this effect is a consequence of a general degradation of the infected tissues or is due to the action of YopP/YopJ. However, the induction of apoptosis of target cells is undoubtedly a master strategy used by several invasive pathogens (for reviews, see references 394 and 395). Yersinia spp. are not the only pathogens endowed with a type III system that have been found to induce apoptosis. Apoptosis induction has also been reported for Shigella (393) and Salmonella (60, 238). For Shigella, it has been shown that apoptosis is mediated by IpaB (392), which binds to and activates the interleukin-1β (IL-1β)-converting enzyme (caspase 1) (62, 158, 346). SipB, the Salmonella homolog of IpaB, is likely to induce apoptosis (60, 236) by a similar mechanism (236, 391). The mechanism by which Yersinia induces apoptosis is probably different. First, Yersinia induces apoptosis from the outside of the host cell (232), which is different from what has been described for Shigella (393). Second, YopB, which is the Yersinia counterpart of IpaB and SipB, is not the effector of the phenomenon, although it is indirectly involved in the induction of apoptosis through its role of translocator. Third, an inhibitor of caspase 1 does not prevent Yersinia-induced apoptosis (290). However, a broad-spectrum caspase inhibitor blocks the completion but not the onset of Yersinia-induced apoptosis, suggesting that Yersinia might initiate apoptosis at a level upstream from caspases (290). Recently, Ruckdeschel et al. (288) showed that Y. enterocolitica inhibits activation of the transcription factor NF-κB in murine J774A.1 and peritoneal macrophages; analysis of different Y. enterocolitica mutants revealed a striking correlation between the abilities to inhibit NF-κB activation and to trigger apoptosis. Several reports showed that apoptosis can be prevented by the expression of NF-κB, suggesting that the induction of NF-κB may be part of a survival mechanism (21, 23, 215, 341, 349, 350, 364). These results suggest that Yersinia could trigger apoptosis by suppressing the cellular activation of NF-κB (288) (Fig. 5).
FIG. 5.
Model showing the effects of Yersinia spp. on the macrophage intracellular cascades. Lipopolysaccharide (LPS) activates the ERK1/2, JNK, and p38 MAPK pathways, leading to increased TNF-α production. Activated MAPKs can lead to NF-κB activation; activated NF-κB can, in turn, enhance TNF-α transcription. Translocated YopP/YopJ induces macrophage apoptosis by a mechanism involving caspase activation. It also downregulates MAPKs and impairs NF-κB activation, two effects that could explain the YopP/YopJ-induced reduction of TNF-α production. See the text for details and references.
Inhibition of TNF-α and IFN-γ release.
TNF-α is a proinflammatory cytokine that plays a central role in the development of the immune and inflammatory responses to infection. Secreted mainly by macrophages, TNF-α acts on various cell types involved in the host defense mechanisms. It stimulates both macrophage and PMN microbicidal activity and acts on natural killer cells together with IL-12 to provoke the release of gamma interferon (IFN-γ), which further increases the microbicidal activity of macrophages. Moreover, TNF-α induces expression of adhesion molecules on endothelial cells and is chemotactic for monocytes, thus contributing to the amplification of the inflammatory response (for a review, see reference 356). The importance of the cytokines TNF-α and IFN-γ in the host immune response against a Yersinia infection was first illustrated by the fact that treatment of mice with antibodies directed against TNF-α or IFN-γ exacerbates infection by Y. enterocolitica (16). Moreover, an immunohistological study showed that administration of anti-TNF-α antibodies to mice before and after orogastric infection with Y. enterocolitica leads to complete destruction of Peyer’s patches and to a dramatic increase of bacterial counts in Peyer’s patches, mesenteric lymph nodes, and spleen, even though phagocytes were normally recruited in Peyer’s patches and mesenteric lymph nodes (17). This suggests that TNF-α plays an essential role in the local host defense mechanism in the intestinal tissues, possibly by activating phagocytes (17).
Interestingly, the levels of TNF-α and IFN-γ in mice infected with wild-type Y. pestis are much lower than those observed in mice infected with a pYV− strain, suggesting that the pYV plasmid encodes a factor suppressing TNF-α and IFN-γ synthesis (240). Further studies with Y. pestis suggested a role for LcrV in this process, based on the observations that passive immunization with anti-LcrV antibodies or active immunization with purified protein A-LcrV hybrid protein protected mice against lethal doses of Y. pestis (240, 241). Another group working with mouse peritoneal macrophages and Y. enterocolitica confirmed the suppressive effect of virulent Yersinia on TNF-α release and claimed that YopB was responsible for this phenomenon (26). More recently, Ruckdeschel et al. (289), working with the mouse monocyte-macrophage cell line J774A.1 and Y. enterocolitica, showed that a functional type III secretion machinery is required for the phenomenon to occur and suggested a correlation between this inhibition of TNF-α release and inhibition of the ERK1/2, p38, and JNK mitogen-activated protein kinase (MAPK) activities. Several reports already described a link between MAPK activation and TNF-α production (202, 203, 273, 280, 347, 388). It has been shown recently both for Y. enterocolitica (39) and for Y. pseudotuberculosis (251) that the Yersinia-induced inhibition of TNF-α release requires not only the type III secretion apparatus but also a functional Yop translocation apparatus and the effector YopP (Y. enterocolitica)/YopJ (Y. pseudotuberculosis). No other translocated effector seem to be involved in the phenomenon (39, 251). In addition, a strain secreting only YopB, YopD, YopN, YopE, YopH, and LcrV does not impair TNF-α release in vitro, indicating that these proteins are not, or at least not solely, responsible for the phenomenon (289). Taken together, these results suggest that YopB and LcrV presumably act indirectly as part of the translocation machinery required to deliver YopP/YopJ inside the macrophages (41, 138, 294). However, it must be added here that Brubaker and collegues provided evidence for a direct immunosuppressive effect of purified LcrV injected into mice (241). Thus, although LcrV is undoubtedly an element of the virulon and as such is required for the intracellular delivery of effectors, it may also act on its own as a protein released during the infection. The same could apply to YopB (26, 52a).
In agreement with the results of Ruckdeschel et al. (289), YopP/YopJ is also involved in the inhibition of the ERK2, p38, and JNK MAPK activities in infected macrophages (39, 251), but its actual target and mechanism of action remain unknown. It is noteworthy that YopP/YopJ is also involved in the triggering of apoptosis (see above), and it may well be that the two phenomena are linked. The link between apoptosis and MAPK activation is not clear, but the Y. enterocolitica-induced inhibition of NF-κB activation mentioned previously (288) is correlated not only to the induction of apoptosis but also to the inhibition of TNF-α production (288). One can thus speculate that YopP/YopJ could act upstream or at the junction of cascades leading to apoptosis on one hand and to inhibition of TNF-α on the other hand; alternatively, the initial role of YopP/YopJ could be to induce the death of the macrophage by triggering apoptosis, thereby impairing the synthesis and release of TNF-α (Fig. 5).
Inhibition of TNF-α production is not only encountered during Yersinia infection of macrophages. Other bacteria such as Brucella spp. (55), Listeria monocytogenes (78), Bacillus anthracis (163), and Mycobacterium avium (297) also possess the capacity to disturb the normal cytokine production. In Brucella spp., inhibition of TNF-α expression is due to the release of a specific, protease-sensitive bacterial factor (54). Parasites such as Leishmania donovani (79) and viruses (122, 317) also interfere with TNF-α production, showing that this defense mechanism is widely used by pathogens.
Polymorphonuclear Leukocytes
PMNs constitute the second group of professional phagocytes that are encountered by Yersinia bacteria invading the lymphoid tissues of their host. The interaction between Yersinia and PMNs has been studied for more than a decade, essentially with human PMNs and Y. enterocolitica.
Resistance to phagocytosis and killing.
The interaction between Y. enterocolitica and PMNs was first studied by monitoring the luminol-enhanced chemiluminescence (CL) response (211), which is a measure of the intensity of the oxidative burst (82). A pYV+ Y. enterocolitica strain grown at 37°C (Yop-inducing conditions) induced four- to sixfold less CL than did the same strain grown at 25°C or a plasmidless, isogenic strain grown at either temperature. This demonstrated for the first time the involvement of pYV-encoded proteins in the inhibition of the PMN oxidative burst (211). Since the CL response is a sensitive, indirect measure of the degree of phagocytosis in human neutrophils (126), this also suggested that Y. enterocolitica may resist phagocytosis by PMNs. Indeed, Lian et al. (210) showed that wild-type pYV+ bacteria are resistant to phagocytosis by PMNs while pYV− bacteria are not. This effect was seen not only in vitro but also in vivo; after intradermal inoculation into rabbits, histological examination of the inflammatory lesions by light or electron microscopy revealed that numerous bacteria of the pYV− strain were located intracellularly in vacuoles of PMNs and mononuclear cells while pYV+ bacteria were extracellular and surrounded by inflammatory cells without being phagocytosed (209).
To identify the pYV gene(s) encoding these capacities to inhibit the oxidative burst and to resist phagocytic uptake by PMNs, China et al. (65) tested the CL response of PMNs to various well-characterized Y. enterocolitica pYV mutants opsonized with normal human serum (NHS). They came to the conclusion that the YadA outer membrane protein, also encoded by the pYV plasmid (see below), is involved in both inhibition of the CL response and resistance to phagocytosis. The mechanism by which Y. enterocolitica resists phagocytosis could involve a reduction of complement-mediated opsonization due to the YadA protein (65). YadA binds complement factor H (66) and thus reduces opsonization by C3b molecules (66), and there is a correlation between the lack of an oxidative burst and the reduction of opsonization by C3b molecules (344).
Experiments carried out with human PMNs and various Y. enterocolitica strains opsonized with rabbit immune serum instead of NHS confirmed that plasmid-bearing bacteria resist phagocytosis and killing by PMNs while plasmid-cured bacteria are readily ingested and killed by these cells (361). However, under the latter conditions, YadA did not play a major role; Y. enterocolitica mutants expressing YadA but lacking Yops were ingested by PMNs to the same extent as were pYV-cured bacteria, while mutants lacking YadA but secreting Yops were poorly ingested. Thus, in the presence of anti-Yersinia antibodies and complement, some Yop rather than YadA is responsible for the inhibition of phagocytosis of Y. enterocolitica by human PMNs. This difference in the observed mechanisms probably results from different opsonization conditions and possibly from different uptake mechanisms.
The differential contribution of YadA and Yops to evasion of the antibacterial activities of PMNs (oxidative burst, phagocytosis, killing) was further studied by Ruckdeschel et al. in an attempt to clarify the situation (291). It could be concluded that in the presence of NHS, (i) the YadA protein is essential for the protection of Y. enterocolitica from PMNs, since a yadA mutant induces a CL response stronger than that induced by the wild-type strain; (ii) that expression of YadA alone does not have any effect, since a secretion mutant that still produces YadA induced the same CL response as a pYV− strain; (iii) YopH also plays an important role, since a strain affected in YopH secretion (sycH mutant) was highly susceptible to phagocytosis and killing by PMNs; (iv) the strain impaired in YopH secretion also failed to inhibit a secondary zymosan-induced CL response, indicating that YopH is also involved in the oxidative burst inhibition; and (v) YopE is also involved, since a strain producing both YopE and YopH was more efficient in reducing the oxidative burst and in preventing phagocytosis and killing than a strain producing YopH only. Taken together, these results indicate that YopH, YopE, and YadA act in concert to resist antibacterial activities of PMNs under opsonizing conditions with NHS. The hypothesis of Ruckdeschel et al. (291) is that the adhesin YadA favors the adherence of bacteria to PMNs and that inhibition of the bactericidal functions is caused predominantly by YopH and, to a certain extent, also by YopE.
Resistance to antimicrobial peptides.
As described above, pYV+ Y. enterocolitica strains impede to some extent their phagocytosis by PMNs. However, when ingested, most of the pYV+ bacteria are not killed whereas pYV− bacteria are killed almost instantly (86, 362), implying that plasmid-encoded factors can interfere with the killing mechanisms. These involve oxygen-dependent mechanisms (oxidative burst) and oxygen-independent mechanisms, which include acidification of the phagosome and attack by antimicrobial polypeptides. Antimicrobial polypeptides present in azurophilic granules of human granulocytes include bactericidal permeability-increasing protein, cathepsin G, elastase, proteinase 3, azurocidin, lysozyme, and defensins. These antimicrobial polypeptides are released into the phagolysosome through fusion of cytoplasmic granules with the phagosomes. Using a gel overlay assay (205), Visser et al. (362) showed that pYV− Y. enterocolitica strains are more susceptible to these granule-antimicrobial polypeptides than are wild-type Yersinia strains. Similarly, a yadA mutant was also more sensitive than wild-type bacteria, and introduction of a plasmid encoding only YadA in a pYV− strain restored, at least partially, the bacterial protection against the microbicidal activity of the granule extracts. YadA is thus involved in the resistance of Y. enterocolitica to the antimicrobial activity of polypeptides from human granulocytes, although the involvement of other plasmid-encoded factors could not be completely ruled out (362).
Epithelial Cells
The cell types that are the actual targets of the Yop effector proteins in vivo are not known at the moment, and although macrophages and PMNs are obvious in vivo targets, one can speculate that endothelial cells and epithelial cells of the gastrointestinal tract may also be targets of the Yop virulon. Endothelial cells play an important role in the development of the immune and inflammatory responses, by recruiting PMNs through expression of adhesion molecules. Epithelial cells not only constitute a barrier against bacterial invasion but also synthesize and secrete a number of cytokines.
Cytotoxicity.
HeLa cells have been very important in the discovery of injection of Yop effectors inside eukaryotic cells by extracellular adhering bacteria (137, 259, 285, 321). HEp-2 cells (267, 358, 359) and HeLa cells (287) are very sensitive to the cytotoxic effect of YopE. This cytotoxic effect consists in rounding up of the cells and detachment from the extracellular matrix (119, 282). Rosqvist et al. (283) showed that the YopE-induced cytotoxicity is due to disruption of the actin microfilament structures of the target cell and that this effect is mediated by intracellularly located YopE. In addition to YopE, three other Yops, namely YopH, YopO, and YopT, have a cytotoxic effect on cultured epithelial cells (see “Yop effectors and their targets” for details).
Cytokine response.
The cytokine response of epithelial cells to Yersinia infection has been investigated by using the HEp-2 human laryngeal epithelium cell line (13) and various human colon epithelial cell lines (181, 303). The capacity of HEp-2 cells to release cytokines is modified upon Yersinia infection, and although these cells do not originate from the gastrointestinal tract, this observation suggests that epithelial cells may participate in the modulation of the immune response against infection by Yersinia via the release of cytokines (13). In agreement with this idea, infection of monolayers of human colon epithelial cells (T84, HT29, and Caco-2) with invasive bacteria, including Y. enterocolitica, results in the coordinate expression and upregulation of a specific array of four proinflammatory cytokines, namely, IL-8, monocyte chemotactic protein-1, granulocyte-macrophage colony-stimulating factor, and TNF-α, as assessed by mRNA levels and cytokine secretion (181). The same cytokines, as well as IL-6, are also expressed by freshly isolated human colon epithelial cells and upregulated upon infection with invasive bacteria including Y. enterocolitica (181). These cytokines play a role in the initiation or amplification of the inflammatory response; IL-8 and monocyte chemotactic protein-1 act as potent chemoattractants and activators of neutrophils and monocytes, respectively; TNF-α activates neutrophils and mononuclear phagocytes, while granulocyte-macrophage colony-stimulating factor prolongs the survival of neutrophils and monocytes and increases the response of those cells to other proinflammatory stimuli, which can further amplify the inflammatory response. Colon epithelial cells thus appear to be programmed to provide a set of chemotactic and activating signals to adjacent and underlying immune and inflammatory cells in the earliest phases after microbial infection (181). Interestingly, virulent Y. enterocolitica strains induce a significantly lower level of IL-8 secretion by T84 cells than do nonvirulent Y. enterocolitica strains and the YopB and YopD proteins are required for this suppressive effect (303). It is easily conceivable that this effect favors Yersinia, especially during the early phase of infection, by delaying a massive influx of PMNs into the site of infection.
YOP SECRETION
Yop Secretion Pathway
Discovery of Yop secretion.
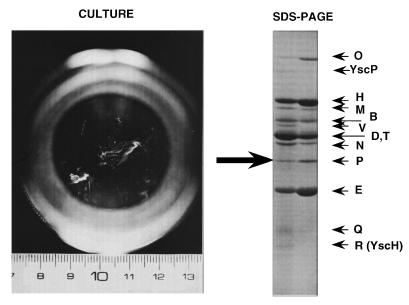
The Yops were initially described as outer membrane proteins (44, 267, 331). Later, Heesemann et al. (149, 151) showed that Yops could also be recovered from the culture supernatant. Some of the Yops (LcrV, YopM, YopQ/YopK, and YopR) are soluble in the culture supernatant, but others (YopH, YopE, YopO/YpkA, YopB, YopD, YopP/YopJ, and YopN/LcrE) have a propensity to aggregate as visible filaments (229) (Fig. 6). This led Michiels et al. (229) to question the outer membrane localization of the Yops. These authors studied the kinetics of transcription and appearance of the Yops in the different compartments and observed the following. (i) Yops are detected first in the supernatant and later in the membrane fraction. (ii) The appearance of Yops in the membrane fraction is concomitant with the decrease of the corresponding protein in the supernatant. (iii) Disappearance of the less soluble Yops from the supernatant is not a consequence of degradation. (iv) There is a correlation between the propensity of a given Yop to aggregate in the supernatant and the presence of that Yop in the membrane fraction. (v) Yops still accumulate in the membrane fraction after 3 h of induction, whereas transcription of the yop genes at that time is dramatically reduced. (vi) Yops are separated from the cell fraction upon treatment with hydrophobic agents such as xylene or hexadecane, whereas chromosome-encoded integral membrane proteins and YadA are not. On the basis of these observations, Michiels et al. (229) concluded that Yops are not membrane-anchored proteins but true secreted proteins that copurify with membranes when they are prepared as centrifugation pellets. The name YOP, introduced by the group of Wolf-Watz (44) for yersinia outer membrane protein, could thus be questioned, but it is so popular that it was decided, during the Keystone 1990 meeting on Yersinia, to keep it but to write it Yop(s) rather than YOP(s) to indicate that it is not a set of initials. The name “Yersinia outer proteins” fits with the acronym but is not particularly elegant.
FIG. 6.
Yops secreted by Y. enterocolitica W22703. Bacteria were grown at 28°C in a conical flask (seen from the top) containing oxalated brain heart infusion and then transferred to 37°C. The photograph showing the Yop filaments was taken 4 h after the temperature shift. SDS-PAGE of the filaments (right lane) and of Yops precipitated from the supernatant by ammonium sulfate (left lane) is shown on the right. Adapted from reference 229.
In vitro and in rich Ca2+-deprived medium, Yops are very abundant. Michiels et al. (229) calculated that 107 Y. enterocolitica W22703 cells secrete 1 μg of Yops, which corresponds roughly to 20% of the total bacterial proteins. A peculiarity of Y. pestis must be mentioned here: in the supernatant of Y. pestis cultures, Yops are rapidly degraded by the membrane-associated Pla protease, which is encoded by a small bacteriocinogenic plasmid (318, 319). YopM, YopN/LcrE, and LcrV are relatively resistant to this proteolysis (208, 329).
No classical signal sequence is cleaved off.
When Bölin and Wolf-Watz (46) and Michiels and Cornelis (226) sequenced the yopH gene (then known as yop2b and yop51), they noticed that the N-terminal end of the predicted YopH protein does not resemble a typical signal sequence. In 1990, Michiels et al. (229) determined the sequence of the N terminus of the secreted YopH and found the same sequence as that deduced from translation of the 5′ end of the gene, including the terminal methionine. Reisner and Straley (274) showed that the 13 N-terminal residues of YopM are also identical to those deduced from the nucleic sequence. The same observation was made later for YopN by Forsberg et al. (99): residues 2 to 9 obtained by the Edman degradation procedure were those encoded by codons 2 to 9. Håkansson et al. (136) reported the same for YopD. Finally, the 7 N-terminal residues of YpkA/YopO and the 11 N-terminal residues of YopJ/YopP have also been found to match the translated nucleic sequence (111). Hence, secretion of YopH, YopN, YopP/YopJ, YpkA/YopO and YopD occurs without removal of an N-terminal signal sequence. This presumably also applies to the other Yops. Indeed, no typical signal was found in the sequence of YopE (100, 229), YopQ (229), YopM (208), LcrV (25), YopB (136), YopR (8), or YopT (170).
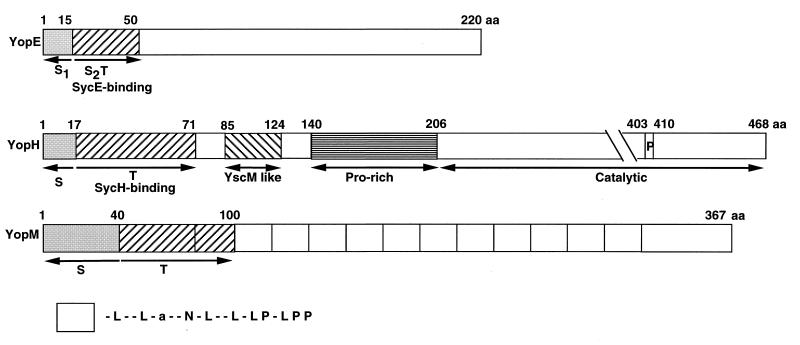
The N-terminal (or 5′ mRNA) secretion signal.
Analysis of the secretion of hybrid proteins composed of the N terminus of YopH or YopE and various prokaryotic or even eukaryotic proteins indicated that the information necessary for Yop secretion is nevertheless contained in the N terminus (227, 321–323). The minimal region shown to be sufficient for secretion of YopH was gradually reduced from 48 residues in a YopH-PhoA hybrid (227) to 17 residues in a YopH-Cya hybrid (320). Similarly, the minimal sequence required for secretion of YopE was reduced to 15 residues by gradual deletions of YopE-Cya hybrids (320) and later even to 11 residues, still by the same approach (300). By analysis of translational fusions to neomycin phosphotransferase (Npt), Anderson and Schneewind (10) localized the YopN secretion signal in the first 15 codons of the gene. The minimal domain of YopM sufficient for secretion of YopM-Cya was found to be shorter than 40 residues (41). For YopO/YpkA and YopP/YopJ, it is shorter than 77 and 43 residues, respectively (324).
There is no similarity between the secretion domains of the Yops with respect to amino acid sequence, hydrophobicity profile, distribution of charged residues, or prediction of secondary structure, which suggested recognition of a conformational motif of the nascent protein (227). To explain that proteins with no common signal could be recruited by the same secretion apparatus, Wattiau and Cornelis (366) suggested that the Syc chaperones (discussed below) could serve as pilots. However, this hypothesis was questioned when it appeared that YopE could be secreted even if its chaperone-binding domain had been deleted (106, 376). It was then concluded that secretion was dependent only on the short N-terminal signal, but secretion of a Yop lacking only this N-terminal signal had never been tested.
A systematic mutagenesis of the secretion signal by Anderson and Schneewind (10) led to doubts about this signal being of purely proteic nature. No point mutation could be identified that specifically abolished the secretion of YopE or YopN. Moreover, frameshift mutations that completely altered the peptide sequences of the signals also failed to prevent secretion. Anderson and Schneewind (10) concluded that the signal that leads to the secretion of Yops could be in the mRNA rather than in the peptide sequence. However, some point mutations in the YopE signal do abolish Yop secretion (300).
A second secretion signal?
The experiments of Sory et al. (320) demonstrated that the first 15 codons of YopE contain a signal that is sufficient to promote secretion in rich culture medium. They did not show that this N-terminal signal is absolutely required for YopE secretion. To address this question, Cheng et al. (63) deleted codons 2 to 15 and monitored secretion of the hybrid YopE-Npt. They observed that 10% of the hybrid proteins deprived of the N-terminal secretion signal were still secreted in M9 medium supplemented with 1% Casamino Acids. They inferred that there is a second secretion signal and showed that this second, weaker secretion signal corresponds to the SycE-binding site (see below). Not surprisingly, it is functional only in the presence of the SycE chaperone (63), rejuvenating the pilot hypothesis of Wattiau and Cornelis (366) for SycE. As discussed below, this second signal, binding the chaperone, is required for translocation of YopE into eukaryotic cells (204a, 320).
What has been shown for YopE might also apply to YopH, since it also has a chaperone (365) (see below). However, this should be checked, because some older observations suggest that the N-terminal signal sequence is absolutely required for secretion. Indeed, Michiels and Cornelis (227) replaced the first six codons of a truncated YopH by 12 codons of lacZ′ and did not observe secretion.
Conclusion.
There are two different signals driving the export of YopE by the type III secretion apparatus. The first is the structure of the 5′ mRNA, and the second, built into the protein, uses the chaperone as a pilot. The same could apply to the effectors YopH and YopT. Some other effector Yops do not seem to have a chaperone, in which case they would be recognized only by their N- or 5′-terminal signal. Finally, it must be stressed that we know less about secretion of the translocators. No signal sequence is removed from YopB, YopD, and LcrV, but their secretion signal has not yet been identified. Some observations tend to suggest that secretion of YopB and YopD could proceed by a mechanism slightly different from that used by the effector Yops. First, LcrV appears to be necessary for secretion of YopB and YopD (294). Second, mutations in some genes such as virG (7), yscF (8), or yscM/lcrQ (275, 327) lead to phenotypes in which YopB, YopD, and LcrV are secreted differently from the other Yops.
Ysc Secretion Apparatus
In 1991, Michiels et al. (228) established that, like the Yops, the Yop secretion apparatus is encoded by the pYV plasmid itself and in particular by genes that they called ysc (for “Yop secretion”). Some of these genes had previously been considered regulatory genes; this misinterpretation can be explained by the fact that there is a strong regulatory feedback that blocks Yop synthesis as soon as secretion is compromised (see “Regulation of transcription of the virulon genes,” below).
The ysc genes are contained in four contiguous loci that were initially called virA, virB, virG, and virC (for “virulence”) in Y. enterocolitica (73) (Fig. 3). lcrD (for “low calcium response”), initially described in Y. pestis (263), turned out later to be one of these secretion genes (265), and it will probably be called yscV in the future. In total, 28 genes have been identified within these loci. Knockout mutants have been constructed for most but not all of them. The information available on these genes and their products is detailed in the next paragraphs and summarized in Table 1. For the sake of clarity, the four loci are treated separately.
TABLE 1.
Ysc secretion apparatus
| Protein | Size (kDa) | Featuresa | Localization in bacteriaa | Role in Yop secretiona | Reference(s) |
|---|---|---|---|---|---|
| YscA | 3.8 | Hydrophobic C-terminal domain | Unknown | − | 228, 296 |
| YscB | 15.4 | Unknown | NT | 228 | |
| YscC | 67.1 | Signal sequence of 26 residues; member of the secretin family; forms pores of 200 Å with a central channel of 50 Å | OM | + | 194, 228 |
| YscD | 46.7 | Hydrophobic domain (aa 120–130) | IM | + | 228, 265 |
| YscE | 7.4 | Hydrophobic C-terminal domain | Unknown | + | 8, 228 |
| YscF | 9.4 | Unknown | + | 8, 228 | |
| YscG | 12.9 | Hydrophobic N-terminal domain | C/M | + | 8, 228, 265 |
| YopR | 18.3 | Encoded by yscH | Secreted | − | 8, 228 |
| YscI | 12.6 | Unknown | + | 8, 228 | |
| YscJ | 27.0 | Lipoprotein; hydrophobic C-terminal domain followed by 3 positively charged residues; previously called YlpB | Unknown | + | 8, 64, 228 |
| YscK | 23.9 | One hydrophobic domain | Unknown | + | 8, 228 |
| YscL | 24.9 | Unknown | + | 228, 326 | |
| YscM1/LcrQ | 12.3 | Resembles YscM2 and YopH | Secreted | − | 8, 228, 327 |
| YscN | 47.8 | Contains Walker box A and B; putative ATPase | IM/C | + | 24, 93, 375 |
| YscO | 19.0 | Secreted | + | 24, 93, 252 | |
| YscP | 50.4 | Secreted | + | 24, 93, 252a, 326a | |
| YscQ | 34.4 | Unknown | + | 24, 93 | |
| YscR | 24.4 | Four transmembrane domains and a large central cytoplasmic region | IM | + | 24, 93 |
| YscS | 9.6 | Two putative transmembrane domains | Unknown (probably IM) | + | 24, 93, 261 |
| YscT | 28.4 | Six putative transmembrane domains | Unknown (probably IM) | NT | 24, 93 |
| YscU | 40.3 | Four transmembrane domains at the N terminus with a large cytoplasmic C-terminal region | IM | + | 9, 24, 93 |
| YscX | 13.6 | Unknown | + | 170a | |
| YscY | 13.1 | Unknown | + | 170a | |
| LcrD/YscV | 77.8 | Eight potential transmembrane domains; hydrophobic N-terminal half; hydrophilic C terminus predicted to protrude into the cytoplasm | IM | + | 263 |
| VirG/YscW | 14.6 | Lipoprotein ancillary to YscC secretin | Probably OM | + (YopB, YopD, LcrV) | 7 |
IM, inner membrane; OM, outer membrane; C, cytosolic; M, membrane-associated protein; NT, not tested; +, required for secretion; −, not required for secretion; aa, amino acids.
YscC secretin and other products of the virC operon.
The virC locus of Y. enterocolitica consists of a large operon, yscABCDEFGHIJKLM, encoding 13 proteins (228, 327). Parts of the virC locus have also been analyzed in Y. pestis (134) and Y. pseudotuberculosis (275), where the counterparts of yscH, yscI, yscJ, yscK, yscL, and yscM have been initially called lcrP, lcrO, lcrKa, lcrKb, lcrKc, and lcrQ, respectively. Apart from yscM1, which is called lcrQ in Y. pseudotuberculosis (275), the ysc nomenclature has now been adopted in the three species. Nonpolar mutations in yscC, yscD, yscE, yscF, yscG, yscI, yscJ, yscK, and yscL completely abolish Yop secretion (8, 265). In contrast, nonpolar yscA, yscH, and yscM mutants are not impaired in Yop secretion (8, 296).
yscC encodes an outer membrane protein (194, 228, 265) that belongs to the family of secretins, a group of outer membrane proteins involved in the transport of various macromolecules and filamentous phages across the outer membrane (113, 212b, 292). All the secretins have a conserved domain in the C terminus, whereas the N-terminal domains are conserved only between proteins of related systems (113). Several members of this family (61, 142, 212a, 242, 307), including YscC (265), form large multimers. Koster et al. (194) showed that the 600-kDa very stable YscC complex forms a ring-shaped structure with an external diameter of about 200 Å and an apparent central pore of about 50 Å. As a matter of comparison, the PIV secretin of phage f1 has an internal diameter of about 80 Å, allowing the passage of the filamentous capsid with a diameter of 65 Å (212b). Lipoprotein VirG (7), described below, is required for efficient targeting of the YscC complex to the outer membrane (194), a situation reminiscent of that of secretin PulD and lipoprotein PulS (143).
Relatively little is known about the other proteins of the virC operon that are required for secretion. YscB is a 15.4-kDa protein which has neither a putative signal sequence nor a hydrophobic domain (228). YscD is an inner membrane protein (265). Complete inactivation of yscF abolishes Yop secretion. However, truncation of YscF reduces the secretion only of YopB and YopD and not that of the other Yops, suggesting that YopB and YopD are secreted via a slightly different mechanism (see the previous section) or that secretion of YopB and YopD is more sensitive to small alterations in the secretion machinery (8). YscJ is a 27.0-kDa lipoprotein (228). YscL has no obvious membrane-spanning domain, but it could be membrane associated (228).
yscH encodes the 18.3-kDa secreted protein that was called YopR (8). YopR is not required for secretion of the other Yops, but it could be involved in pathogenesis, since the 50% lethal dose of the yscH mutant was 10-fold higher than that of the wild-type strain (8).
Finally, yscM, the last gene of the virC operon, is not required for Yop secretion but is involved in the feedback inhibition of Yop synthesis (8, 327) (see below).
The order of the 13 genes is the same in the three Yersinia species (134, 228, 275), but there could be minor differences in their transcriptional organization. In Y. enterocolitica, the virC locus consists of only one large operon extending from yscA to yscM (8, 228, 327), while primer extension analysis suggested the existence of a Ca2+-regulated promoter within yscF in Y. pestis (134). lcrQ in Y. pseudotuberculosis has been reported to be monocistronic (275).
Several proteins encoded by the virC operon have sequence homology to components of other type III secretion systems and also to proteins involved in the assembly of flagella (Table 2). Homologs to all the proteins encoded by the virC operon, except YscA and YscM/LcrQ, have been identified in P. aeruginosa (for a review, see reference 102). YscC and YscJ have counterparts in the Shigella, Salmonella, and EPEC type III secretion systems. Homologs to YscF have been identified in Shigella and Salmonella spp. The identity between YscJ and YscF and the corresponding genes in the Shigella system, MxiJ and MxiH, is 26 and 24%, respectively, but the genes from the two species are not functionally interchangeable (8). For a more complete review of type III secretion homologs, see references 4, 201, and 352.
TABLE 2.
Homologs to the Ysc proteins
| Yersinia protein | Homolog in:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Shigella spp.a |
Salmonella spp.
|
EPECd | Pseudomonas aeruginosae | Chlamydia psittacif | Phytopathogenic bacteria (HR reaction)g | Rhizobium spp.h | Flagellai | ||
| SPI Ib | SPI IIc | ||||||||
| YscA | |||||||||
| YscB | PscB | ||||||||
| YscC | MxiD | InvG | EscC | PscC | HrcC | ||||
| YscD | EscD | PscD | HrpQ* | ||||||
| YscE | PscE | ||||||||
| YscF | MxiH | PrgI | SsaH | EscF | PscF | ||||
| YscG | PscG | ||||||||
| YscH | PscH | ||||||||
| YscI | PscI | ||||||||
| YscJ | MxiJ | PrgK | SsaJ | EscJ | PscJ | HrcJ | NolT | FliF | |
| YscK | PscK | ||||||||
| YscL | SsaK | PscL | HrpF** | ||||||
| YscM | |||||||||
| YscN | Spa47/SpaL | SpaL/InvC | SsaN | EscN | PscN | HrcN | HrcN | FliI | |
| YscO | Spa13 | SpaM/InvI | SsaO | PscO | FliJ | ||||
| YscP | Spa32 | SpaN/InvJ | |||||||
| YscQ | Spa33/SpaO | SpaO/InvK | SsaQ | HrcQ | HrcQ | FliN/Y | |||
| YscR | Spa24/SpaP | SpaP/InvL | SsaR | EscR | HrcR | HrcR | FliP | ||
| YscS | Spa9/SpaQ | SpaQ/InvM | SsaS | EscS | HrcS | HrcS | FliQ | ||
| YscT | Spa29/SpaR | SpaR/InvN | SsaT | EscT | HrcT | HrcT | FliR | ||
| YscU | Spa40/SpaS | SpaS | SsaU | EscU | Cds1 | HrcU | HrcU | FlhB | |
| YscX | Pcr3 | ||||||||
| YscY | Pcr4 | ||||||||
| LcrD/YscV | MxiA | InvA | SsaV | EscV | Cds2 | HrcV/HrpI | FlhA/FlbF | ||
| VirG/YscW | ExsB | ||||||||
VirG/YscW lipoprotein.
virG is a small, monocistronic gene situated immediately upstream from the virC operon and downstream from the regulatory gene virF (7) (Fig. 3). It encodes a polypeptide of 131 amino acids with a predicted molecular mass of 14.7 kDa and a calculated isoelectric point of 11.1. The signal sequence of VirG ends with Leu-Xaa-Gly-Cys, a motif characteristic of the processing site of lipoproteins. While attempting to show that VirG is a lipoprotein that can be labelled by [3H]palmitate, Allaoui et al. (7) encountered the difficulty that gram-negative bacteria produce several lipoproteins in the range of 10 to 30 kDa. To circumvent this, they labeled three strains containing different virG-phoA gene fusions and detected the larger VirG-PhoA hybrid proteins among the proteins labelled with [3H]palmitate. VirG is thus a small lipoprotein. Allaoui et al. (7) constructed a nonpolar virG mutant and observed that secretion of some Yops, in particular YopB, YopD and LcrV, was severely impaired. The function of VirG became more clear when the YscC secretin was characterized by Koster et al. (194). It appeared that VirG is required for proper insertion of YscC in the outer membrane, but more work is needed for an understanding of its exact function (194). The correlation between the role of VirG in the installation of the secretin and the requirement of VirG for secretion of YopB, YopD, and LcrV suggests that these Yops could be the most bulky ones to be transported through the YscC channel. Since lipoprotein VirG belongs to the Ysc secretion apparatus, we suggest that it be renamed YscW.
VirG/YscW shows extensive similarity (26.2% identity in a 126-amino-acid overlap) to ExsB, a 137-amino-acid putative polypeptide from P. aeruginosa, encoded by a trans-regulatory locus controlling exoenzyme S synthesis (ExoS) (103). However, exsB does not seem to be expressed in P. aeruginosa (122a).
Products of the virB operon.
The virB operon consists of eight genes, yscN to yscU (24, 93). Among the proteins encoded by these genes, YscN, YscR, and YscU are the best characterized so far. YscN is a 47.8-kDa protein with ATP-binding motifs (Walker boxes A and B) resembling the β catalytic subunit of F0F1 proton translocase and related ATPases. A pYV derivative encoding an YscN protein deprived of Walker box A is impaired in Yop secretion, indicating that YscN is a necessary component of the secretion machinery and possibly acts as an energizer (375). It has not been shown that YscN is an ATPase, but this was shown for InvC, the YscN homolog in S. typhimurium (83). It is thus reasonable to assume that YscN acts as an ATPase. YscR is a 24.4-kDa inner membrane protein with four transmembrane regions and a large central hydrophilic domain, as suggested by the analysis of yscR-phoA translational gene fusions (93). The 40.3-kDa YscU is a second inner membrane protein with four transmembrane segments anchoring a large cytoplasmic C-terminal domain (9). Not surprisingly, mutations in yscR and yscU abolish Yop secretion (9, 93). Interestingly, the products of yscO (251) and yscP (252a, 326a) are secreted by the Ysc apparatus under low-Ca2+ conditions. YscO (251) is required for secretion of all the Yops, while YscP (252a) is required for normal secretion of some Yops. Less is known about YscQ (93) and YscS (261), but they have been shown to be required for Yop secretion. Finally, the importance of YscT has not been determined.
The virB locus as a whole is remarkably well conserved in other type III secretion systems such as those of Shigella spp., Salmonella spp., EPEC, P. aeruginosa, Chlamydia psittaci, Rhizobium spp., and phytopathogenic bacteria (Table 2). The degree of identity varies between 20 and 50% for each individual protein, but every gene is found at the same relative position. There is also a significant homology between the products of the virB operon and proteins implicated in the building of the flagellum in various species (Table 2). The virB locus is thus the most highly conserved part of the type III secretion machinery.
virA locus: yopN, tyeA, sycN, yscXY, lcrD/yscV, and lcrR.
The virA locus encodes first YopN, TyeA, and SycN, which are described later in this review. The next two genes, yscX and yscY, encode small proteins that are required for Yop secretion (170a). The next gene, lcrD/yscV, encodes a 77-kDa inner membrane protein that is required for Yop secretion (263, 264). LcrD/YscV is the archetype of a family of proteins encountered in every known type III system (Table 2). The predicted overall secondary structure of these proteins is quite well conserved and consists of a hydrophobic N terminus with six to eight potential transmembrane domains and a hydrophilic C terminus protruding into the cytoplasm. All the members of this family can be aligned over the entire length of the amino acid sequence, with the highest degree of homology occurring in the N terminus (108, 263, 264). At least some of the members have interchangeable functions. For instance, MxiA from Shigella is able to complement the eukaryotic cell entry defect of a Salmonella invA mutant, but LcrD/YscV from Yersinia cannot. However, a chimeric protein consisting of the N-terminal part of LcrD/YscV and the C-terminal end of InvA can replace InvA, suggesting that the C-terminal end of these proteins may determine the specificity for each of the secretory systems (115).
lcrD forms an operon with lcrR. The latter gene encodes a hydrophilic, basic protein of 16.4 kDa whose function remains unknown (22).
Conclusion.
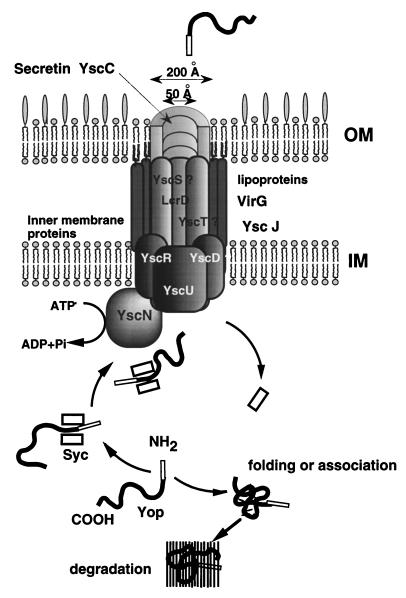
Secretion of the Yop proteins requires a complex secretion machinery made of at least 28 proteins. These proteins, especially those encoded by the virB operon, are quite well conserved in the type III secretion system of plant and animal pathogens. Four proteins, LcrD, YscD/YscV, YscR, and YscU, span the inner membrane. The YscC secretin forms a large pore in the outer membrane, presumably stabilized by lipoprotein VirG. The YscN ATPase energizes the transfer of Yops, but the proton motive force could also be involved (Fig. 7).
FIG. 7.
Model for Yop secretion. OM, outer membrane; IM, inner membrane.
Syc Cytosolic Chaperones, SycE, SycH, SycT, SycN, and SycD
Discovery of the Yersinia chaperones.
Genetic analysis of the yopE region of Y. enterocolitica, Y. pseudotuberculosis, and Y. pestis revealed the existence of a conserved 620-bp region upstream of the yopE gene (101). This region contains a 130-codon open reading frame encoding a 14.7-kDa protein, transcribed divergently from the yopE gene. A mutant with an insertion in this region showed a reduced intracellular level of YopE protein as compared to the wild type (101), which suggested that this locus plays a regulatory role and inspired the name yerA, for “yopE-regulating gene A” (101). In 1993, Wattiau and Cornelis (366) observed that the counterpart of YerA in Y. enterocolitica is required for YopE secretion but not for its synthesis and that it binds specifically to YopE. Hence, they concluded that it is a chaperone specific for YopE, and they called it SycE (for “specific Yop chaperone E”).
The discovery of SycE prompted a search for other chaperones (365). A Y. enterocolitica gene located immediately downstream of yopH and transcribed divergently encodes a putative protein of 141 residues with a calculated molecular mass of 15.7 kDa, an acidic pI (4.88), and no classical N-terminal signal sequence. Its sequence does not resemble that of SycE, but its predicted physicochemical properties (pI, hydrophobicity, and hydrophobic moment) and secondary structures (α-helices) strikingly evoke those of SycE/YerA. This made it a likely candidate to be the YopH chaperone, and it was called SycH. In agreement with this hypothesis, SycH turned out to be necessary for the secretion of YopH and to bind specifically to YopH (365).
SycT (170), the chaperone of YopT, has 69.7% similarity to SycE/YerA and 23% similarity to SycH. It has the same properties as the other chaperones (pI of 4.40, molecular mass of 15.1 kDa), it is necessary for efficient secretion of the YopT protein (see below), and it binds to YopT (170).
SycN, encoded next to YopN and TyeA, appears to be a chaperone of the same family, dedicated to YopN (170a).
The SycD protein is encoded by the lcrGV sycD yopBD operon (25, 270). It was first described as a regulatory gene (271), which explains its designation, LcrH (for “low-calcium-response gene H”) in Y. pestis and Y. pseudotuberculosis. Indeed, an lcrH/sycD mutant of Y. pestis showed only limited growth at 37°C when Ca2+ was present (271), while the parental Y. pestis strain showed full growth in these conditions (271). Moreover, Price and Straley (271) showed that LcrH/SycD is required for normal Yop expression. Bergman et al. (25) also assigned a negative control function to LcrH/SycD because they observed that overproduction of LcrH/SycD in polar insertion lcrV and lcrH/sycD mutants resulted in the loss of Ca2+ dependency and in a downregulation of the expression of the lcrGV sycD yopBD operon and of yopE. However, on the basis of its gene location, size, and pI, Wattiau et al. (365) considered that SycD/LcrH could be a specific chaperone serving YopB and/or YopD. They constructed a nonpolar sycD/lcrH mutant, and from its analysis, they concluded that SycD is required for YopB and YopD secretion (365). It was also shown that SycD binds to YopD (365) and to YopB (243), thus acting as a bivalent chaperone. In the absence of SycD, YopD and YopB are less detectable inside the bacterial cell (243, 365). SycD thus appears to be a chaperone, which does not exclude the possibility that it also plays a regulatory role but that this regulatory role is an indirect consequence of its role in YopB and YopD secretion.
Finally, a gene upstream from yopO/ypkA encodes a putative protein (called ORF 155) that has clear characteristics of a Syc chaperone (171). It is orphan up till now.
In summary, six chaperones have been identified so far. In Y. enterocolitica, their genes are located close to the gene encoding the corresponding Yop. In Y. pseudotuberculosis, however, the sycH gene has been separated from yopH by some genetic rearrangement (259) (Fig. 3). No chaperone has been described so far for YopM, YopO/YpkA, YopP/YopJ, YopQ/YopK, YopR, and LcrV. Although putative chaperone ORF 155 is still orphan, it seems reasonable to believe that not every Yop has a chaperone.
Common properties of the Syc chaperones.
Although the Syc chaperones seem to play a common role in protein secretion, they are distantly or even not related in terms of amino acid sequence. However, they have some common features: an acidic pI, a size in the range of 15 to 19 kDa, and a C-terminal amphiphilic α-helix (Table 3). SycE and SycH possess a conserved “leucine repeat” motif in this α-helix structure, where most of the hydrophobic residues, essentially leucines, are present on the same side of the helix. A consensus sequence was derived by Wattiau et al. (368) from the alignment of this conserved leucine repeat of SycE and SycH and their homologs (LLWxRxPLxxxxxxxLxxxLExLVxxAExL) (Table 3) (see below). The C-terminal potential amphiphilic α-helix of SycD, however, does not include a leucine repeat.
TABLE 3.
Syc cytosolic chaperones
| Protein | Size
|
pI | C-terminal domain | Role | Relevant similarities | Reference(s) | |
|---|---|---|---|---|---|---|---|
| aaa | kDa | ||||||
| SycD/LcrH | 168 | 19.0 | 4.53 | Amphipathic α-helix | Needed for secretion of YopB and YopD; no specific binding domain | IpgC (Shigella), SicA (Salmonella), PcrH (P. aeruginosa) | 183, 225, 243, 365, 379 |
| SycE/YerA | 130 | 14.7 | 4.55 | Amphipathic α-helix, Leu repeat | Needed for secretion of YopE; binds to aa 15–50; pilot for YopE | SycT, ORF1 (P. aeruginosa), Scc1 (C. psittaci) | 63, 106, 165, 170, 366, 376 |
| SycH | 141 | 15.7 | 4.88 | Amphipathic α-helix, Leu repeat | Needed for secretion of YopH; binds to aa 20–70 | OrfU (EPEC) | 179, 365 |
| SycT | 130 | 15.1 | 4.40 | Amphipathic α-helix | Needed for secretion of YopT | SycE | 170 |
| SycN | 123 | 13.6 | 5.2 | Amphipathic α-helix | Needed for secretion of YopN | Pcr2 (P. aeruginosa) | 170a |
| ORF155 | 155 | 17.2 | 4.5 | Amphipathic α-helix | Unknown | ORF1 (Y. pseudotuberculosis), ORF1 (P. syringae) | 4a, 171 |
aa, amino acids.
Role of SycE and SycH.
The three chaperones SycE/YerA, SycH, and SycD/LcrH were first thought to constitute a single family of new protein chaperones (365), but this hypothesis appears questionable today. SycE and SycH appear to play related roles, and they could belong to the same new family, but SycD/LcrH could be quite different. Therefore, they are discussed separately. However, the role of SycT is not discussed, since it has not yet been completely analyzed.
The Syc chaperones were first thought to be necessary mainly for the secretion of their cognate Yop, and Wattiau and Cornelis hypothesized that they could act as some kind of secretion pilots to drive nascent Yops to the secretion machinery (365, 366). This hypothesis was first questioned by Frithz-Lindsten et al. (106), who showed by confocal microscopy that when a double yerA/sycE yopD mutant strain infects HeLa cells, YopE localizes at discrete spots at the zone of contact between the bacterium and the HeLa cell, as it does in yopD mutants (285). This showed that YerA/SycE is not required for the targeting of YopE to these translocation sites. Moreover, residual secretion of YopE or YopH was observed in the absence of SycE/YerA or SycH, respectively (365, 366). However, these observations were made in the presence of the other chaperone, and one could hypothesize that this second chaperone could partially take over from the missing one. Woestyn et al. (376) then mapped the regions of YopE and YopH that bind to the cognate chaperone (see below) and showed that hybrid YopE-Cya or YopH-Cya proteins devoid of the SycE or SycH chaperone-binding site are normally secreted in the presence and in the absence of both chaperones. Similarly, YopH devoid of its chaperone-binding site is normally secreted in the presence and in the absence of SycH. This suggested that SycE/YerA and SycH are probably not targeting factors. However, at that time, no experiments were done with YopE and YopH deprived of their N-terminal secretion signal. Later, Cheng et al. (63) deleted the N-terminal domain from YopE and showed that YopE could still be secreted but only if SycE was present. As we have seen before, this indicated that SycE/YerA can act as a kind of secretion pilot, as initially suggested by Wattiau and Cornelis (366). This is probably not the only role for the Syc chaperones.
YopH and YopE have a discrete domain (residues 15 to 50 for YopE and residues 20 to 70 for YopH) that is specifically required for their translocation into eukaryotic cells (320) (see “Translocation signal on Yop effectors”). Woestyn et al. (376) showed that the Syc-binding domain is unique for both chaperones and that the very same region of YopE and YopH is required for translocation. In addition, they showed that in a sycH mutant, YopH secretion is more efficient in the absence of YopB and YopD than in their presence (376). This result suggests that SycH could prevent the association of YopH with YopB and/or YopD, but this hypothesis still awaits a confirmation.
SycE/YerA also plays an antidegradation role, since the half-life of YopE is longer in wild-type bacteria than in sycE/yerA mutant bacteria (63, 106). SycH does not play such a clear antidegradation role, since YopH can be detected in the cytosol of sycH mutant bacteria (365).
Finally, SycE/YerA and SycH could also play an antifolding role, to maintain the Yop proteins in a conformation which is adequate for secretion.
In summary, it appears that the SycE and SycH chaperones fulfill several roles concerning YopE and YopH stability, secretion, and possibly conformation and interaction between translocators and effectors. One could imagine that the chaperone first acts as a secretion pilot, leading the Yop protein to the secretion locus and simultaneously preventing premature association with the translocators. By binding the Yop, the Syc chaperone could ensure the stability and proper conformation of the protein. At the moment of secretion, the chaperone is released from the partner Yop and the translocation domain would be free to interact with the translocation machinery, which then leads the Yop to the host cell cytoplasm.
Role of SycD/LcrH.
As mentioned above, it was first thought that SycD/LcrH was a regulatory protein, involved in Ca2+ responsiveness (25, 271). Now it appears that SycD is a chaperone for the YopD and YopB proteins and that its presumed regulatory role is probably indirect. However, this chaperone is somewhat different from SycE and SycH in terms of its role and the way it behaves. First, it serves two Yops rather than one; second, it serves translocators whereas SycE and SycH serve effector Yops. Third, SycD binds to several domains on YopB (243) whereas SycE and SycH bind their cognate Yop at a unique site. The last property is similar to that of SecB, a molecular chaperone in E. coli which is dedicated to the export of newly synthesized proteins (196) and which also has multiple binding sites on these proteins, such as the maltose-binding protein (191).
YopB and YopD are less detectable inside Yersinia in the absence of SycD than in its presence (243, 365), while YopE and YopH can still be detected in the bacterial cytosol in the absence of their cognate chaperone. This suggests that in the absence of their chaperone, the hydrophobic YopB and YopD proteins could be toxic for the bacterium and are thus probably degraded by a bacterial “housekeeping” protease. This hypothesis is supported by the work of Neyt and Cornelis (243), who showed that overexpression of YopB in E. coli leads to cell lysis and death whereas overexpression of YopB in the presence of the SycD chaperone does not. This protective role of SycD is unprecedented for Syc chaperones, but it is not necessarily the only role of SycD/LcrH. SycD resembles the IpgC chaperone from Shigella flexneri, which has been shown to prevent the association between its cognate Ipas, namely, IpaB and IpaC (225). The similarity (51%) between IpgC and SycD/LcrH suggests that SycD could play a similar role and would thus prevent the intrabacterial association of YopB and YopD. Neyt and Cornelis (243) investigated this hypothesis by testing the ability of YopB and YopD to associate in the presence of SycD. Surprisingly, they observed that YopB and YopD, bound to SycD, can still associate. In agreement with this, they observed that YopB and YopD are already associated in the bacterial cell in the presence of SycD (243).
Homologs of the Syc chaperones in other species.
As mentioned above, SycD/LcrH is related to the Shigella chaperone IpgC (previously called IppI) (298), which binds IpaB and IpaC independently, preventing the formation of a complex between the two Ipa proteins in the bacterial cytoplasm (225). Although SycD/LcrH is 51.0% similar and 26.5% identical to IpgC, we have seen that their roles could be different. IpgC is 57% similar to SicA (Salmonella invasion chaperone A) (183). SycD/LcrH also has a homolog in the P. aeruginosa Psp system (PcrH, 75% similarity) (379) and in the EPEC Esp system (CesD, 67% similarity) (363a).
The SycE/YerA chaperone is homologous to open reading frame 1 (ORF1) from P. aeruginosa (44% identity). ORF1 is adjacent to the gene encoding the ADP-ribosyltransferase ExoS (see below) (105), and its importance is controversial. Yahr et al. (378) showed that the secretion of ExoS expressed from a plasmid in P. aeruginosa PA103 does not require ORF1. However, when the exoS gene is expressed in Yersinia (see below), the absence of an intact ORF1 results in a 10-fold reduction in the secretion of ExoS (105), which suggests that ORF1 serves a similar function in the secretion of ExoS to that of SycE for YopE. SycE/YerA also has a homolog in Chlamydia psittaci, which is called Scc1 (54% similarity) (165).
The SycH chaperone has a homolog called OrfU in EPEC (179). The similarity is rather weak (19.8% identity), but the C-terminal amphipathic helix seems to be conserved. This protein is encoded by a gene adjacent to eaeA, which encodes intimin. It is unlikely that this putative chaperone serves intimin, because intimin is exported by the Sec pathway (121), but we would like to hypothesize that it serves the protein Tir, which is encoded by a gene located upstream of orfU. Transfer of Tir to the host cell is dependent on the type III secretion apparatus (189).
Conclusion.
In conclusion, the Syc chaperones probably constitute two different families. SycE/YerA, SycH, and presumably SycT appear to be both secretion/translocation pilots and anti-association factors, while SycD/LcrH is more likely to play a protective role covering the YopB-YopD cytoplasmic association until secretion. Concerning the antidegradation role of the Syc chaperones, it is unclear whether the Yops are degraded in the absence of their chaperone because they are not secreted or because the chaperone plays a direct antidegradation role.
DELIVERY OF EFFECTOR YOPS INTO EUKARYOTIC CELLS
Translocation across the Eukaryotic Cell Plasma Membrane
Identification of intracellular effectors.
As discussed above, translocation of YopE across the eukaryotic cell membrane was demonstrated in 1994 by two different approaches: confocal microscopy (285) and the Yop-Cya reporter enzyme strategy (321). The same methods were applied to demonstrate translocation of YopH and YopM across the plasma membrane of epithelial cells and macrophages (41, 259, 320).
Delivery into eukaryotic cells of less abundant Yop proteins such as YopO/YpkA, YopP/YopJ, and YopT turned out to be more difficult to monitor. Håkansson et al. (137) constructed a mutant of Y. pseudotuberculosis that was unable to produce the more abundant Yop effectors (YopE, YopH, and YopM) as well as YopK (see below), and this strain allowed the visualization of translocation of YpkA/YopO into HeLa cells by confocal microscopy (88, 137, 161). A similar multiple-yop mutant of Y. enterocolitica was constructed (39), and this mutant allowed the demonstration of the delivery of YopO-Cya, YopP-Cya, and YopT-Cya into macrophages (170, 172, 324), by a system dependent on both YopD and YopB.
Translocation requires adherence of living bacteria to their target.
Only Y. pseudotuberculosis producing Inv can deliver Yops into HeLa cells (282). However, the presence of Inv is not an absolute requirement, since centrifuged bacteria producing YadA but not Inv still translocate YopH through the plasma membrane of HeLa cells (259). Surprisingly, in vivo, the Inv protein is not required for the full virulence of Y. pseudotuberculosis.
In Y. enterocolitica, the YadA adhesin is more important than Inv for the delivery of YopE into HeLa cells (321), but adherence is still a requirement for efficient injection of Yops. A double yadA inv mutant is indeed devoid of any cytotoxicity (the most sensitive indicator of translocation) in epithelial cells (47). However, either YadA or Inv will suffice to initiate the contact between Y. enterocolitica and epithelial cells to allow subsequent translocation.
Goguen et al. had already noticed in 1986 (119) that only live Y. pestis bacteria could induce a cytotoxic effect in macrophages. Moreover, Y. pestis, which is unable to adhere to HeLa cells, was also inefficient in inducing cytotoxicity in this cell type. However, introduction of a gene encoding either the invasin (Inv)—a large outer membrane protein binding β1-integrins (206, 308)—or YadA conferred on Y. pestis the ability to induce cytotoxicity in HeLa cells (282). Thus, like Y. pseudotuberculosis and Y. enterocolitica, Y. pestis needs to adhere before it can deliver its effector Yops. However, the main element needed for this adherence has not been identified.
The pH 6 antigen of Y. pestis (213, 214), the pH 6 antigen of Y. pseudotuberculosis (381) and its counterpart, the Myf fibrillae of Y. enterocolitica (175), could promote adherence (381). However, synthesis of the pH 6 antigen requires an acidic environment, and the lack of the pH 6 antigen causes only a relatively small decrease in virulence of Y. pestis in a systemic challenge, in contrast to a yopE or yopH mutation. In this context, it is quite surprising that Y. pestis produces neither Inv nor YadA (286, 310).
Translocation is the feat of extracellular bacteria.
Rosqvist et al. (282) demonstrated that selective killing of extracellular bacteria by gentamicin inhibits cytotoxicity. Killing of extracellular bacteria also prevents translocation of YopH across the plasma membrane of eukaryotic cells, indicating that translocation occurs not from intracellular bacteria but, rather, from extracellular bacteria (259). In agreement with this, Sory and Cornelis (321) showed that the use of cytochalasin D, which inhibits the entry of bacteria into cells, does not greatly affect the amount of YopE that is internalized. Moreover, the same authors showed that Y. enterocolitica that is unable to synthesize Inv and thus is unable to invade HeLa cells delivers YopE-Cya almost as efficiently as wild-type bacteria do (321). It thus appears that intracellular bacteria do not translocate Yops across the endosomal membrane, which is in good agreement with the observation that intracellular bacteria produce only small amounts of Yops (282).
Influence of de novo protein synthesis on translocation.
It is difficult to clearly report on the role of de novo protein synthesis in translocation, because the experimental conditions used to address this question were highly variable. Protein synthesis was inhibited with chloramphenicol or gentamicin, and translocation was monitored by measuring cytotoxicity, phagocytosis, or even the oxidative burst. As described above, the addition of gentamicin during incubation of eukaryotic cells with Y. pseudotuberculosis impaired the YopE-mediated cytotoxicity (282) as well as the inhibition of phagocytosis caused by YopH (87). However, some of the YopH-mediated antiphagocytic activity could still be observed when bacteria were preincubated under conditions favorable for Yop production (37°C in the absence of Ca2+) (87). Also, when Y. pestis or Y. pseudotuberculosis was preincubated at 37°C in the presence of Ca2+ (nonpermissive for Yop secretion) before their contact with macrophages, cytotoxicity or an inhibition of the oxidative burst occurred even in the presence of chloramphenicol (33, 119). In contrast, no cytotoxicity was observed in the presence of chloramphenicol when bacteria were preincubated at 28°C. This suggests that bacteria grown at 37°C are inherently able to deliver Yops inside eukaryotic cells whereas bacteria grown at 28°C require some incubation under conditions that permit Yop protein synthesis. The secretion apparatus and the elements of the translocation apparatus to be deployed are probably present after incubation at 37°C, in any medium, and some Yops await translocation. A signal then settles the secretion and translocation systems, and the delivery of the Yops is amplified by a specific enhancement of Yop synthesis (see “Regulation of transcription of the virulon genes,” below). Depending on the conditions used to incubate the bacteria and to the phenomenon recorded (inhibition of phagocytosis, cytotoxicity, etc.), a variable lag phase will thus be observed before the effect on eukaryotic cells is recorded.
Directionality of secretion.
When Yop secretion is triggered by eukaryotic cells, it is “polarized” in the sense that the majority of the Yop effector molecules produced are directed into the cytosol of the eukaryotic cell, not to the outside environment (285). The term “polarization” is perhaps confusing, because it may suggest that it occurs at the pole of the bacterium, which has never been shown. To avoid this, one might say that Yop translocation is “oriented” or “directional.” Whatever the term, there is some discrepancy in the degree of “directionality.” Using various Cya reporters, Boland et al. (41) observed that roughly only half of the amount of Yops secreted by Y. enterocolitica is directed into cultured macrophages. In contrast, Persson et al. (259) reported that Y. pseudotuberculosis delivers more than 99% of the YopH-associated PTPase activity into HeLa cells. Whether the discrepancy originates from the bacterium or from the system used for the study is not known. As discussed below, YopN, TyeA, and LcrG are involved in this “contact-oriented” phenomenon (41, 98, 172, 259, 285, 295).
Translocation signal on Yop effectors.
Taking advantage of the Yop-Cya strategy, Sory et al. (320) identified a domain required for the internalization of YopE and YopH into murine PU5-1.8 macrophages. Starting from hybrids that were readily translocated, they engineered gradual deletions into the yop gene, starting from a restriction site at the hinge between yopE or yopH and cyaA. Internalization into macrophages, revealed by cAMP production, required the N-terminal 50 amino acids of YopE and the N-terminal 71 amino acids of YopH. Sory et al. (320) concluded that YopE and YopH are modular proteins composed of a secretion domain, a translocation domain, and an effector domain (Fig. 8). As discussed above, this translocation domain corresponds to the Syc-binding domain (376). The identification of a domain specifically required for translocation does not imply that secretion and translocation are uncoupled. Indeed, the fact that delivery occurs only when translocators and effectors are synthesized by the same bacterium suggests that translocation immediately follows secretion (320).
FIG. 8.
Schematic representation of YopE, YopH, and YopM. S1, first secretion domain; S2/T, second secretion domain and translocation domain. The catalytic domain of YopH includes the P-loop (P). The LRR motifs (193) in YopM are represented by open boxes, and their composition is given below.
The same experiments, carried out on YopE from Y. pseudotuberculosis, confirmed these observations (300). However, there is a difference in the results. While YopE-Cya hybrids containing between 15 and 47 amino acids of YopE were shown to be secreted by Y. enterocolitica (320), YopE-Cya hybrids containing between 11 and 75 residues were exported by Y. pseudotuberculosis but remained attached to the bacterial surface, which suggested to the authors that there is a “release domain” in YopE (300). The reason for the discrepancy between these two reports is not known. It could be due to a slight difference between Y. enterocolitica and Y. pseudotuberculosis, but this seems to be rather unlikely.
More recently, Lee et al. (204a) showed that YopE residues 1 to 100 are necessary and sufficient for the targeting of hybrid neomycin phosphotransferase. As expected, SycE was required for this targeting (204a).
The domain required for translocation of the other effectors has also been shown to reside in the N-terminal domain. For YopM, it is localized within the first 100 residues and extends further than the first 41 residues (41). For YopO/YpkA and YopP/YopJ, the signal is localized within the 77 N-terminal residues and the 99 N-terminal residues, respectively (172, 324). For YopT, it is within the 124 N-terminal residues (170).
Conclusion.
So far, six effector proteins, YopE, YopH, YpkA/YopO, YopM, YopP/YopJ, and YopT, are known to be translocated across the eukaryotic membrane by a directional process (41, 137, 170, 172, 259, 285, 320, 321, 324). The YopN plug also appears to be translocated (204a). The process requires bacteria that are alive and endowed with the capacity to adhere to eukaryotic cells. It requires protein synthesis if bacteria were grown at low temperature before their contact with eukaryotic cells. If bacteria were incubated at 37°C, they can probably deliver a load of presynthesized effectors, but if Yop synthesis resumes, larger amounts of Yops will be delivered. Finally, the phenomenon is oriented in the sense that most of the production is delivered into cells and not secreted in the culture supernatant, but the degree of orientation—or leakiness—remains a matter of debate.
Delivery Apparatus
Among the 12 secreted Yops, only 2, YopB and YopD, have hydrophobic domains (136), suggesting that they could interact with membranes. They are encoded by the same large lcrGV sycD yopBD operon (25, 239, 270), which also encodes LcrG, LcrV, and SycD/LcrH, the chaperone of YopB and YopD (see above). As discussed in detail in this section, the whole lcrGV sycD yopBD operon is involved in translocation. The information is also summarized in Table 4.
TABLE 4.
Yop proteins involved in translocation
| Protein | Size (kDa) | Structural featuresa | Role(s) | Relevant similarities | References |
|---|---|---|---|---|---|
| YopB | 41.8 | Central hydrophobic domains (aa 168–208 and aa 224–258); coiled coils (aa 103–165 and aa 330–385) | Needed for translocation; needed for contact hemolysis; hypothetical constituent of a pore | RTX toxin (LktA, HlyA, …); IpaB (S. flexneri); PopB (P. aeruginosa); YopD; EspB (EPEC); SipB (S. typhimurium) | 41, 136, 138, 146, 183, 190, 224, 321, 379 |
| YopD | 33.3 | Central hydrophobic domain (aa 122–152); coiled coils (aa 249–292); C-terminal amphipathic α-helix | Needed for translocation | PopD (P. aeruginosa); YopB | 41, 136, 259, 321, 379 |
| LcrV | 37.2 | Polymorphism | Required for extrusion of YopB and YopD | PcrV (P. aeruginosa) | 270, 276, 294, 379 |
| YopK/YopQ | 20.8 | None | Controls translocation by modulating the size of the YopB-induced pore | None | 161, 162 |
aa, amino acids.
YopD.
Early on, different observations pointed to the importance of YopD in translocation. First, a yopD mutant was unable to induce cytotoxicity in HeLa cells whereas a preparation of Yops obtained from the same strain induced cytotoxicity after microinjection (283). Second, when HeLa cells were infected with a yopD mutant and studied by immunofluorescence techniques, no YopE was found in the cytosol (285). Third, a polar yopB insertion mutant was unable to translocate a YopE-Cya hybrid into HeLa cells (321). These observations led to the conclusion that YopD is required for translocation of YopE across the eukaryotic cell membrane. Independently, Hartland et al. (147) observed that a yopD mutant also has a markedly reduced ability to inhibit the respiratory burst, to disrupt actin filaments, and to resist phagocytosis. This pleiotropic effect indicated that YopD was involved in the translocation of a number of Yop effectors, not just YopE. The importance of YopD in translocation of the effectors YopE, YopH, YopM, YopO/YpkA, and YopP/YopJ was then shown directly using the Cya reporter enzyme system (41, 320, 324) or confocal microscopy (259, 285, 324).
YopD consists of 306 amino acids and has a molecular mass of 33.3 kDa and a neutral pI (136). Analysis of YopD with the Lupas algorithm (217) suggests the presence of a domain that could form coiled coils (structures commonly involved in protein-protein interactions) spanning residues 249 to 292. The same domain could also form an amphipathic helix. The hydropathy analysis identifies a 31-amino-acid hydrophobic region in the middle of YopD (136). The Eisenberg plot analysis (84) suggests that YopD is a transmembrane protein (136). YopD has a homolog in P. aeruginosa, PopD, which is 43% identical (379).
YopB.
Transcomplementation of a yopBD mutant with the yopD gene alone was not sufficient to restore the ability of the bacteria to cause cytotoxicity or tyrosine dephosphorylation in cultured cells or virulence in mice (146). However, transcomplementation with both the yopB and yopD genes restored these functions, indicating that YopB also plays a role in translocation. Recent analysis of nonpolar yopB and yopD mutants showed that YopB is also individually required for translocation of the effectors across the eukaryotic cell plasma membrane (41, 138).
YopB is a 401-residue protein with a molecular mass of 41.8 kDa and a neutral pI (136). Analysis of YopB with the Lupas algorithm (217) predicts the presence of two putative coiled coils, spanning residues 103 to 165 and residues 330 to 385. The central part of YopB contains two hydrophobic regions, separated by only 15 amino acids, and, as with YopD, the Eisenberg plot of YopB suggests that it is a transmembrane protein (84, 136). YopB has a moderate level of similarity to proteins of the RTX family of alpha-hemolysins and leukotoxins such as LktA of Pasteurella haemolytica (338) and HlyA of E. coli (27, 90). The homology between YopB and the RTX proteins is limited to the hydrophobic regions. Since, in the RTX proteins, these hydrophobic regions are believed to be involved in disrupting the target cell membrane (371), it seems logical to assume that they play the same role in YopB. The fact that YopB resembles proteins of the pore-forming toxins of the RTX family suggests that the translocation apparatus could be some kind of a pore, where YopB would be the main element. The observation of Håkansson et al. (138) that Yersinia has a YopB- and contact-dependent lytic activity on sheep erythrocytes supports this hypothesis. Moreover, purified YopB has the ability to disrupt lipid bilayers (138). This YopB-dependent lytic activity is higher when the effector yop genes are deleted, suggesting that the pore is normally filled with effectors during contact (138). The presence of sugar molecules of a given size in the medium can inhibit YopB-mediated sheep erythrocyte lysis, which allowed an approximate determination of the size of the putative pore: since dextran 4 has an inhibitory effect while raffinose has no significant effect, the inner diameter of the pore would be between 12 and 35 Å. YopB also has a homolog in Shigella, namely, IpaB. Interestingly, IpaB, which also contains two possible transmembrane regions, is implicated in the entry of Shigella into epithelial cells, but its exact role in this process is not known (223). YopB also shows a moderate level of homology to YopD, and it has a homolog in P. aeruginosa, called PopB (379).
Interaction between YopB and YopD.
The fact that YopB and YopD are both hydrophobic proteins needed for translocation suggests that they could associate at some stage to fulfill their function. This idea is reinforced by the presence of hypothetical coiled coils in both proteins. In good agreement with this hypothesis, YopB and YopD appear to be associated in the bacterium prior to their secretion (243). In an attempt to localize the domain of YopB that is involved in this interaction with YopD, Neyt and Cornelis (243) analyzed the capacity of a set of truncated YopB proteins to bind to YopD. The outcome of this analysis is that the binding does not occur at one precise site on YopB but, rather, at different sites along the protein. These observations suggest that YopB and YopD could insert together in the eukaryotic membrane and that the putative pore described above could consist of YopB and YopD, but this has not been shown yet. Until now, the pore has been neither purified nor observed on eukaryotic target cells by electron microscopy.
Role of YopQ/YopK.
Yop translocation through the putative pore seems to be controlled by the 20.8-kDa YopK/YopQ (162). A yopK mutant of Y. pseudotuberculosis indeed delivers more YopE and YopH into HeLa cells than does the wild-type strain, whereas a strain overexpressing YopK is impaired in translocation. Overproduction of YopK also leads to a reduction of the YopB-dependent lytic effect on infected HeLa cells and sheep erythrocytes, probably by influencing the size of the pore, as shown by the protective effect of different-sized sugars (161).
Role of LcrV.
The lcrGV sycD yopBD operon also encodes the LcrV protein, known since the mid-1950s as a protective antigen against plague (53). Unlike YopB and YopD, this Yop exhibits a certain degree of polymorphism; in particular, the region between amino acids 225 and 232 appears to be hypervariable (276). LcrV has been described as a regulatory protein involved in the calcium response, since a mutant with an in-frame deletion mutation in lcrV was found to be Ca2+ independent and downregulated in transcription of yop genes (25, 257, 269, 312, 336). However, recent data from Sarker et al. (294) indicate that LcrV could be a functional element of the translocation apparatus, since an entire deletion of the lcrV gene abolishes the secretion of LcrV, YopB, and YopD but has no effect on the secretion of the other Yops. The lack of secretion of YopB and YopD is not due to a lack of transcription or translation or to proteolysis, which indicates that LcrV is specifically involved in the release of YopB and YopD. Recently, a further role of LcrV has been described in the deployment of YopB, which is in turn essential for the vectorial translocation of Yops into eukaryotic cells (246a). In agreement with these observations, LcrV interacts with both YopB and YopD (294), as well as with LcrG (247, 294). On the basis of these results, it was suggested that LcrV constitutes a third component of an organized delivery apparatus (246a, 294).
Conclusion.
In conclusion, data available today suggest that the translocation apparatus could act like the perforin from the cytotoxic T lymphocytes, forming a pore through which the effectors are translocated. This putative pore is probably composed of YopB and YopD, which are secreted and possibly assembled with the assistance of LcrV. Inside the bacterium, YopB and YopD are capped with their chaperone SycD/LcrH. LcrG, the first protein encoded by the lcrGV sycD yopBD operon, is also involved in translocation, but its exact status needs to be clarified (see below).
Control of Yop Release
The Ca2+ paradox and the role of eukaryotic cell contact.
We have seen that in vitro, Yersinia spp. secrete Yops only in the absence of Ca2+. Since the Ca2+ concentrations in the mammalian intracellular compartment are low (micromolar range) but those in the extracellular medium are high (about 2.5 mM), this would suggest that Yops are essentially produced in the intracellular environment. However, this hypothesis contradicts the evidence that Yersinia spreads and multiplies extracellularly (140, 141, 209, 210, 309). There is thus a paradox: in vivo, Yersinia proliferates under conditions that are supposed to be nonpermissive for Yop production, but it does produce Yops, as evidenced by the specific anti-Yop immune response that develops during an infection (150, 220, 325). The discovery of Yop translocation into eukaryotic cells (285, 321) led to a better understanding of the system and a solution to the Ca2+ paradox. Translocation of YopE into target cells occurs in cell culture media containing about 1 mM Ca2+, a medium not permissive for Yop secretion. Since translocation is achieved only by extracellular bacteria adhering at the cell surface (see above), one must assume that eukaryotic cells can somehow replace Ca2+ chelation to trigger Yop secretion. In vivo, contact is thus the real signal inducing Yop secretion.
Proteins involved in control of Yop release by Ca2+ chelation: YopN, TyeA, and LcrG.
The isolation of Ca2+-blind mutants (383) allowed the identification of three genes involved in the control of Yop release: yopN (99), tyeA (172), and lcrG (295, 311). These mutants are deregulated for Yop secretion in the sense that they secrete Yops even in the presence of Ca2+. LcrG was also described in the previous section. YopN and TyeA are described here and in Table 5.
TABLE 5.
Control of Yop release
| Protein | Size (kDa) | Structural featuresa | Role(s) | Localization
|
Relevant similarities | References | |
|---|---|---|---|---|---|---|---|
| Minus Ca2+ | Plus Ca2+ | ||||||
| YopN | 32.6 | Coiled-coil structures (aa 62–108 and aa 248–272) | Element of the putative plug closing the secretion channel; associated with TyeA | Secreted, cytosolic | Cytosolic, surface associated | MxiC (Shigella spp.); InvE (Salmonella spp.); PopN (P. aeruginosa); HrpI (E. amylovora); CopN (C. psittaci) | 6, 38, 99, 117, 165, 172, 379 |
| TyeA | 10.8 | Element of the putative plug closing the secretion channel; binds to the second coiled-coil domain of YopN; binds to YopD; required for translocation of YopE and YopH but not YopM, YopO, YopP, YopT | Surface associated, cytosolic | Surface associated, cytosolic | Pcr1 (P. aeruginosa); SsaL (Salmonella spp.) | 99, 154, 170, 172, 360, 379 | |
| LcrG | 11.0 | Forms dimers; heparin-binding domain | Involved in control of secretion; required for efficient delivery of all Yop effectors; binds to heparan proteoglycans | Mainly cytosolic | Mainly cytosolic | PcrG (P. aeruginosa) | 48, 246a, 247, 294, 295, 311, 312, 379 |
aa, amino acids.
YopN, also known as LcrE, is a 32.6-kDa protein encoded by the first gene of a locus that also contains tyeA and three other ORFs that have been sequenced and characterized (99, 107a, 172, 360). Sequence analysis shows that it is devoid of hydrophobic domains (99) and that the regions spanning amino acids 62 to 108 and 248 to 272 could form coiled-coil structures (172). YopN is massively secreted at 37°C in the absence of Ca2+ ions. However, in contrast to the other Yop proteins, YopN can be detected in bacteria grown at 37°C in the presence of Ca2+. In the presence of Ca2+, YopN is accessible to proteases exogenously added to intact bacteria, can be extracted from the bacterial surface with xylene (99, 172), and fractionates with the Triton X-100-insoluble membrane fraction (172). Thus, under low-Ca2+ conditions, most of the YopN produced is released in the culture supernatant, while in the presence of Ca2+, the protein is not released but is exposed at the bacterial surface.
TyeA is a 92-amino-acid protein (10.8 kDa) encoded immediately downstream of yopN (99, 360). This protein, previously called ORF1 (99, 360), has been named TyeA by Iriarte et al. (172) because it plays a role in translocation of some Yop effectors (see below). TyeA is detected in the bacterial cytosolic fraction and in the Triton X-100-insoluble membrane fraction but not in the culture supernatant irrespective of the presence of Ca2+ in the culture medium. Like YopN, TyeA is accessible to proteases exogenously added to intact bacteria and can be removed from the bacterial surface with xylene, indicating that it is loosely associated with the membrane. TyeA has the capacity to bind to the second coiled coil of YopN (172).
LcrG is a 96-amino-acid protein (11.0 kDa) (311) that controls the release of Yops in vitro (295, 311) but is also required for efficient translocation of the Yop effectors (295). LcrG has been shown to be primarily cytosolic, but it has also been detected in the membrane and in the extracellular media (247, 311). Localization of the LcrG protein upon infection of cells by bacteria has not been investigated. LcrG has been shown to bind to LcrV (247, 294). Nilles et al. (246a, 247) presented a model in which LcrG blocks Yop secretion and LcrV is required to remove the LcrG-imposed block. The experimental arguments that support this model are that (i) some lcrV mutants are blocked for total Yop secretion (312) and (ii) strains carrying defects in both LcrG and LcrV are calcium blind like single lcrG mutants (246a, 312). At this stage, no definitive proof is available. LcrG has also been shown to bind heparan sulfate proteoglycans on the surface of HeLa cells (see below), but the role of this interaction in translocation remains to be investigated (48).
Contact control.
We have seen that secretion and subsequent injection of Yops is an oriented phenomenon in the sense that Yops are essentially directed into the eukaryotic cell and not into the culture medium. Thus, the three proteins that are involved in the in vitro control of Yop release by Ca2+ (YopN, LcrG, and TyeA) could also be involved in the contact-induced control of Yop release (41, 98, 172, 259, 285, 295). As expected, yopN45 mutants producing a 45-amino-acid truncated YopN secrete more Yops into the eukaryotic cell medium than do wild-type Yersinia strains. It has been suggested that YopN could function as a sensor and a stop valve controlling Yop secretion. After contact with the eukaryotic cell, the YopN sensor would interact with a ligand on the target cell surface, be removed, and allow Yop secretion and delivery to the target cell (285). However, YopN has never been shown to interact either with Ca2+ or with a cell receptor. The fact that lcrG and tyeA mutants are also deregulated for Yop secretion in the presence of Ca2+ or depolarized in the presence of eukaryotic cells (172, 295, 311) suggests that control of the delivery of the effectors requires not simply YopN but, rather, a complex system comprising at least these three proteins.
Interpenetration of the control and translocation systems.
Yersinia producing YopN45 can still deliver Yops into the cytosol of the target cell. However, since secretion is deregulated, the ratio between the amount of Yops delivered into cells and the amount secreted into the culture medium is very low (1:100). This suggests that translocation is independent of the control of Yop release. However, the situation is more complex and there appears to be interpenetration of the two systems. Indeed, a tyeA mutant is deregulated for Yop secretion but impaired in translocation of YopE and YopH but, surprisingly, not of YopM, YopO/YpkA, YopP/YopJ, and YopT (170, 172). Moreover, a yopNΔ248–272 mutant, producing a truncated YopN protein with the binding domain for TyeA deleted, has the same phenotype as a tyeA mutant. This suggests that when YopN is inserted in the control complex, its interaction with TyeA is required for translocation of YopE and YopH but not the other effectors (170, 172). tyeA has a dominant phenotype over yopN since a double mutant, the yopN45 tyeA mutant, does not translocate YopE (172), which indicates that TyeA is not required simply for removal of the YopN stop plug. Finally, as discussed above, LcrG is required for efficient translocation of all the known Yop effectors into macrophages (295), but it is also involved in the control of Yop release, since the lcrG mutants are Ca2+ blind (295, 311).
It is still difficult to establish a model that integrates the dual function of YopN, TyeA, and LcrG. The fact that TyeA is required for translocation of YopE and YopH but not YopM, YopO/YpkA, YopP/YopJ, or YopT raises the hypothesis that some Yops could be specifically delivered to particular cell types. TyeA could be a bacterial ligand with some specificity for given cell types. Although appealing, this hypothesis is purely speculative. One could also speculate that TyeA is required for translocation of Yops having a Syc chaperone and not for the others, but this is contradicted by the fact that YopT has a chaperone and does not require TyeA for its translocation (170).
Heparin interferes with translocation of YopE into HeLa cells.
If the control of Yop release is induced by contact with eukaryotic cells, one can speculate that YopN, LcrG, and TyeA form a recognition complex at the bacterial surface that interacts with a receptor on the surface of eukaryotic cells. Information about this hypothetical receptor is still scarce, but the first element appeared recently.
Proteoglycans, i.e., surface proteins to which glycosaminoglycans are attached, are found on practically all types of eukaryotic cells, and they have been shown to be receptors for a variety of microorganisms via their glycosaminoglycans. Boyd et al. (48) tested the possibility that proteoglycans are responsible for the binding of a Yersinia sensor to the eukaryotic cell. They observed that LcrG binds HeLa cells by interacting with heparan sulfate proteoglycans (48). LcrG, which has heparin-binding motifs, also binds directly to heparin-agarose beads. Addition of exogenous heparin decreased the level of YopE translocation into HeLa cells. Translocation of YopE was also decreased by treatment of HeLa cells with heparitinase. Thus, heparan sulfate proteoglycans play a role in the delivery of Yops into HeLa cells. However, the addition of heparin was unable to completely abolish translocation. This suggests that perhaps the requirement for heparan sulfate-LcrG interaction can be partially compensated for by another bacterium-eukaryotic cell interaction. Thus, the heparan sulfate-LcrG interaction can be viewed as maximizing the efficiency of LcrG in the translocation process but may not be absolutely essential.
Homologs of YopN, TyeA, and LcrG in other bacteria.
Homologs to YopN, LcrG, and TyeA have been identified in other type III secretion systems. YopN is similar to InvE from Salmonella typhimurium (117), MxiC from Shigella flexneri (6), HrpI of Erwinia amylovora (38), PopN of Pseudomonas aeruginosa (379), and CopN of Chlamydia (165). Homologs to TyeA and LcrG have been identified in the type III secretion system of P. aeruginosa (379). TyeA also shows some similarity (25% identity over 97 amino acids) to the C terminus of SsaL, a component of the type III secretion apparatus of S. typhimurium pathogenicity island 2 (154). The existence of homologs for all three proteins reinforces the view that they are important pieces of the type III secretion-translocation system.
Yop Effectors and Their Targets
YopE.
YopE, originally described as Yop25 in Y. enterocolitica (229) and as YOP5 in Y. pseudotuberculosis (97), is a 23-kDa protein, containing an N-terminal secretion domain of 15 amino acids and a translocation domain of 50 amino acids (320). Bacterial mutants defective in yopE are less virulent in mice after oral infection, intraperitoneal infection, and intravenous injection than are wild-type strains (282, 330). As seen previously, YopE contributes to the ability of Yersinia to resist phagocytosis (282). Infection of epithelial cells with Yersinia leads to disruption of the microfilament structure of the host cell, due to the action of YopE (283). Within minutes after infection, the host cell rounds up and detaches from the extracellular matrix, a phenomenon referred to as cytotoxicity (282). In HeLa cells infected with Y. pseudotuberculosis, the YopE protein is enriched in the perinuclear region (285). As the infection of HeLa cells progresses, the microfilament structure of the cells changes from ordered filaments to a disordered granular appearance, leading to a complete disruption of the actin microfilaments (283). However, the actual enzyme activity and the target of YopE remain to be identified, since YopE does not act directly on actin (283). Since GTPases regulate the polymerization of actin in eukaryotic cells (216), it can be hypothesized that YopE acts on the cytoskeleton through interaction with GTPases.
YopE is homologous to the N-terminal noncatalytic region of the P. aeruginosa ADP-ribosyltransferase ExoS (195) (see below), but YopE is not known to act as an ADP-ribosyltransferase.
YopH.
YopH, originally described as Yop51 (226) and Yop2b (46), is probably the best characterized Yop. It is a PTPase of 51.0 kDa, composed of several domains, which include two N-terminal domains, of 17 and 71 amino acids, which are important for secretion and translocation, respectively (259, 320); a sequence of 39 amino acids that shows unexpected homology to the regulatory protein YscM/LcrQ (see below) (275, 327); a central proline-rich sequence that binds host cell Src homology 3 (SH3) domains (31); and finally a C-terminal 262-amino-acid domain that is homologous to the catalytic domains of eukaryotic PTPases (32, 132) (Fig. 8). Phosphorylation is one of the mechanisms through which both bacteria and eukaryotic cells modulate protein activity in response to environmental stimuli (216). Phosphorylation of eukaryotic proteins commonly occurs on serine or threonine; only 0.01% of the total phosphoamino acids within a eukaryotic cell exist as phosphotyrosines. The protein tyrosine phosphorylation process forms part of the signal transduction pathways that control many cellular functions, including fundamental processes such as phagocytosis, mitogenesis, and cell division (216).
The YopH tyrosine phosphatase activity is optimal around pH 5.0, and the protein is active in vitro against synthetic proteins and eukaryotic proteins, such as the insulin receptor, that are phosphorylated on tyrosine (132). The catalytic region of PTPases contains a highly conserved cysteine residue (132). Conversion of this conserved cysteine at position 403 of YopH to alanine (C403A) abolishes the PTPase activity of the recombinant enzyme expressed in E. coli (132). A study of the kinetic properties of the purified recombinant Yersinia PTPase has shown that this enzyme is by far the most active PTPase known, with a kcat value 25-fold higher than that of the mammalian PTP1 enzyme (385, 386). The PTPase reaction proceeds in two steps. First, the phosphate is transferred from tyrosine to a functional group on the enzyme, and then the phosphoenzyme intermediate is hydrolyzed (133). For YopH, the invariant cysteine 403 residue is directly involved in the formation of a covalent phosphoenzyme intermediate (132).
The three-dimensional structure of the catalytic domain of YopH has been resolved by Su et al. (340) and Stuckey et al. (339). The YopH PTPase domain consists of an eight-stranded β-sheet surrounded by seven α-helices (Fig. 9). The most prominent characteristic of the structure is a strand-loop-helix motif, representing the phosphate recognition loop (P-loop), that is composed of amino acids 403 to 410 (CGRAGVGRT) in which cysteine 403 is positioned at the center of the protein. The P-loop provides the framework of hydrogen bonds that initially stabilize the negatively charged thiolate of the catalytic cysteine 403 residue. During the catalysis reaction, a substrate-induced conformational change takes place. By analyzing crystals of YopH complexed with the phosphate analog tungsten, a competitive inhibitor of YopH, it was shown that an adjacent loop of amino acids (residues 350 to 360) moves 7 Å into the active site, thereby placing an invariant aspartic acid into the active site, where it participates in phosphotyrosine hydrolysis (339, 387) (Fig. 9). It is remarkable that the structure of the P-loop and the invariant cysteine (position 403) and arginine (position 356) residues is conserved among species from bacteria to mammals. Moreover, the mechanism of dephosphorylation is also conserved from bacteria to mammals (387).
FIG. 9.
Catalytic domain of YopH. A ribbon diagram of the (C403S) Yersinia PTPase is shown. The sulfate anion has been left out of the active site for clarity. A few critical conserved residues are depicted with stick bonds in yellow and are labeled. The P-loop is depicted by a long arrow, and the flexible loop is indicated by a short arrow. The general acid on the flexible loop, aspartic acid at position 356, can be seen in the “closed” conformation over the active site. Serine 403 is seen in place of the catalytic cysteine, although the two residues have very similar conformations. Diagram courtesy of J. Dixon (University of Michigan Medical School).
Several studies have demonstrated that YopH is an important virulence determinant of Yersinia (42, 330). Insertional inactivation of yopH has no measurable effect on bacterial gene expression, growth, or cell viability outside the host. However, yopH mutants derived from Y. pestis or Y. pseudotuberculosis are significantly reduced in virulence. As explained above, Y. pseudotuberculosis yopH mutants are less able to resist phagocytosis in vitro by macrophages than are their parental strains (281).
Immunofluorescence studies have demonstrated that after infection of HeLa cells, YopH is localized largely in the cytoplasm of the cells, although a small fraction is colocalized with the plasma membrane (259). During infection of cultured human epithelial cells and macrophages, two host proteins of 55 and 120 kDa are dephosphorylated by wild-type Y. pseudotuberculosis within 15 min (34, 36), whereas these proteins are not dephosphorylated in cells infected with the C403A mutant. For identification of the YopH substrates and their localization within the host cell, mutant Yersinia strains producing YopH C403A (258) or C403S (35) were used, because these catalytically inactive PTPases form stable complexes with their substrate. After infection of HeLa cells with Yersinia, YopH (C403A) interacts with tyrosine-phosphorylated forms of FAK and p130CAS and colocalizes with these proteins in focal adhesions. In contrast, the active YopH leads to inhibition of bacterial uptake, dephosphorylation of p130CAS and FAK, and disruption of peripheral focal complexes (35, 258, 281). Focal adhesions are sites where integrin receptors serve as a transmembrane bridge between extracellular matrix proteins and intracellular signaling proteins. FAK is involved in the early steps of the integrin-mediated signaling cascade and is therefore believed to function as a transmitter and amplifier (216). The kinase substrate p130CAS interacts with FAK via an interaction between its proline-rich sequence and a C-terminal SH3 domain of p130CAS (35, 258). In macrophages, the inactive YopH specifically recognizes and interacts with tyrosine-phosphorylated p130CAS (88).
As described above, YopH inhibits phagocytosis by PMNs and macrophages, mediated by complement receptors (291) or Fc receptors (87), respectively. Interestingly, these receptors also mediate phosphorylation of FAK or p130CAS upon stimulation (135, 260). Dephosphorylation of p130CAS by YopH could prevent the early steps required for the formation of focal adhesions. Integrin-mediated adhesion of phagocytes to endothelia or extracellular matrix proteins plays an important role during inflammation. Interference with this process due to YopH-mediated dephosphorylation of p130CAS might therefore have an important implication for leukocyte function during Yersinia infection.
YopM.
YopM, originally described as Yop48 in Y. enterocolitica (74, 239) and as Yop2a in Y. pseudotuberculosis (97), was first sequenced in Y. pestis (208). It is a strongly acidic protein with an isoelectric point of 4.06 and a mass of 41.6 kDa (41, 208). It is hydrophobic at both the N- and C-terminal ends and contains 12 leucine-rich repeated motifs (LRRs) (193) (Fig. 8). There is some interstrain variability in the size of YopM (40). In particular, Boland et al. (40) noted that YopM from a Y. enterocolitica O:8 strain isolated from a patient with a severe infection is 56.9 kDa instead of 41.6 kDa. This increased size results from the duplication of part of the gene that probably occurred because of the repetitive nature of this gene (40). Therefore, unlike the other Yop effector proteins that are well conserved among different Yersinia species, YopM is somewhat heterogeneous.
According to the 50% lethal dose test, both Y. pestis and Y. enterocolitica yopM mutants have a strongly reduced virulence in mice (207, 239). Furthermore, bacterial counts in the liver and spleen of Y. enterocolitica-infected animals showed that the yopM mutant had a reduced ability to multiply in the host (239).
YopM is significantly homologous to IpaH of Shigella (148) and y4fR of Rhizobium (104); however, no function is known for either of these proteins. Due to the presence of LRRs, YopM shows a moderate similarity to a great number of proteins containing LRRs, including the α-chain of the platelet membrane glycoprotein 1b (GP1bα). GP1bα binds thrombin and von Willebrand factor (208), and therefore the ability of YopM to bind thrombin was studied. In vitro studies showed that purified YopM has thrombin-binding activity and competitively inhibits thrombin-induced platelet activation in vitro, suggesting that YopM is an extracellular effector (207, 274). However, this role remains to be confirmed, since no thrombin-binding site has been identified so far in YopM and since the domains of GP1bα that are known to be involved in the interaction with thrombin are located outside the region with homology to YopM (77, 125). Furthermore, the similarity to GP1bα is less than 24% and is essentially due to the LRR repeats. Today, the data bases contain many proteins, in particular proteoglycans, containing LRR motifs that are more similar to YopM than is GP1bα.
Recently, by using the Yop-Cya approach, Boland et al. (41) demonstrated that YopM is delivered inside eukaryotic cells. The secretion signal of YopM is contained within the first 40 N-terminal residues, and the translocation domain is contained within the first 100 residues. However, the directionality of intracellular Yop delivery is not as high as that of the other delivered Yops. Therefore, although YopM is likely to react with an as yet unknown intracellular target, an extracellular role cannot be excluded.
YpkA/YopO.
YpkA/YopO had been described first in Y. enterocolitica as Yop84 (74), and later, when a letter code was adopted, it was renamed YopO (239). When the gene encoding this Yop in Y. pseudotuberculosis was sequenced and found to be homologous to the eukaryotic serine/threonine kinases, it was named YpkA, for “Yersinia protein kinase A.” The protein is thus named YopO in Y. enterocolitica and YpkA in Y. pseudotuberculosis and in Y. pestis (257a, 330). YopO/YpkA shows some similarity to the COT (cancer Osaka thyroid) oncogene product, a cytosolic serine/threonine protein kinase expressed in hematopoietic cells and implicated in signal transduction by growth factors (155).
Galyov et al. (110) described the ability of the 81.7-kDa YpkA protein to catalyze autophosphorylation of a serine residue in vitro. Removal of a major part of the catalytic domain (from amino acids 207 to 388) does not totally abolish phosphorylation. However, a disruption of the ORF downstream from the catalytic domain results in a kinase null mutant, indicating that at least part of the kinase function is located outside the catalytic domain. The effect of this mutation on virulence was studied by challenging mice orally. No lethal infection could be observed with the mutant strain, in contrast to a challenge with the wild-type strain. Nevertheless, the mutant strain was able to colonize the Peyer’s patches to an extent similar to that shown by the wild type at the initial stage of the infection. Furthermore, no colonization of the spleen was observed and the colonization of the Peyer’s patches decreased in the later stages of the infection (111).
Infection of HeLa cells with a multiple yop mutant overproducing YpkA leads to a morphological alteration of the cells, different from those mediated by YopE and YopH. The cells round up but do not detach from the extracellular matrix. Inside the cells, the YpkA protein is targeted to the inner surface of the plasma membrane (137). No target protein of YpkA/YopO has been identified yet.
YopJ/YopP.
The 32.5-kDa YopJ/YopP was first detected as YopJ in Y. pestis (330) and as Yop30 (74) and later YopP (69a) in Y. enterocolitica. YopP contains 288 residues (232), and for a while it was thought that YopJ contains 264 residues (111); however, recent data suggest that YopJ also contains 288 residues (251). YopJ/YopP, together with YopO/YpkA, is encoded by a single operon (74, 111). It is considered a “minor” Yop in the sense that in vitro, it is secreted in smaller amounts than most of the other Yops, e.g., YopE. A yopJ mutant strain appeared to be fully virulent in an intravenous mouse model (111, 330, 332). However, in vitro, both YopP and YopJ induce apoptosis in murine macrophages (232, 237). Induction of apoptosis requires type III secretion and a translocation step (Fig. 4). The apoptotic process is cell type dependent, since Yersinia is not able to induce apoptosis in epithelial cells (232, 237, 290) or in fibroblasts (237). By using the mechanism of apoptosis during the infection process, Yersinia might eliminate macrophages without inducing an inflammatory response and thereby might favor extracellular proliferation in lymphoid tissues (see above).
Interestingly, YopP and YopJ have a high level of similarity to AvrRxv from Xanthomonas campestris (372), AvrA from Salmonella (144), and y410 from Rhizobium (104). No function is known for AvrA and y410. However, AvrRxv is one of many plant pathogen avirulence proteins that mediate the hypersensitive response, a process that is likely to result from the activation of a programmed cell death pathway (234, 372). However, no cytotoxic effect has been described for AvrA so far. Animal and plant pathogens therefore share a type III secretion-dependent effector to elicit programmed cell death in their respective hosts.
YopT.
YopT is a 35.5-kDa Yop effector that has been described and characterized recently (170). It induces a cytotoxic effect in HeLa cells and macrophages. The effect on HeLa cells consists of disruption of the actin filaments and alteration of the cell cytoskeleton. YopT shows some similarity to the C-terminal end of p76, an immunoglobulin-binding protein present in the serum-resistant strains of Haemophilus somnus (67), but the relevance of this similarity is not known.
Conclusion.
In conclusion, the presence of at least six Yop effectors (Table 6) could reflect the interaction of Yersinia with one or more cell types at different stages of activation or development, thereby inducing different cell responses. The outcome of this multifactorial process is the ability of Yersinia to obstruct a cellular immune response. Remarkably, all the effector Yops have homologs in a taxonomically diverse group of pathogens including animal and plant pathogens. Interestingly, Salmonella secretes the SptP/StpA protein, whose C terminus exhibits homology to YopH while the N terminus exhibits sequence similarity to YopE and ExoS (14, 184). This implies that these shared genes were already present before the divergence of these pathogens into different species, that they result from a successful coevolution, or that they were recruited by horizontal transfer. The last hypothesis is favored by the fact that the mechanism of type III secretion is also highly conserved and widespread among bacterial species that are taxonomically distant.
TABLE 6.
Yop effectors
| Protein | Size (kDa) | Action on the cell | Enzymatic activity | Relevant similarities | Localization in the target cell | Sufficient signals fora:
|
Reference(s) | |
|---|---|---|---|---|---|---|---|---|
| Secretion (aa or codons) | Translocation (aa) | |||||||
| YopE | 22.9 | Disruption of the cytoskeleton; rounding up of cells; detachment from extracellular matrix | Cytotoxicity | ExoS (P. aeruginosa); SptP/StpA (Salmonella) | Perinuclear region | 11 | 50 | 10, 14, 184, 282, 283, 300, 321, 378 |
| YopH | 51.0 | Disruption of peripheral focal complexes | PTPase | Eukaryotic phosphatases; SptP/StpA (Salmonella) | Cytoplasm | 17 | 71 | 14, 31, 32, 46, 132, 184, 258, 321, 340 |
| YopM | 41.6 | Unknown | Unknown | y4fR (Rhizobium); IpaH (Shigella); numerous proteoglycans; GP1bα | Unknown | 40 | 100 | 41, 104, 148, 207, 208 |
| YpkA/YopO | 81.7 | Rounding up of cells | Ser/Thr kinase | Ser/Thr kinases; COT oncogene | Inner surface of plasma membrane | 77 | 77 | 74, 110, 137, 324 |
| YopP/YopJ | 32.5 | Apoptosis of macrophages; inhibition of TNF-α release | Cytotoxicity | AvRxv (X. campestris); AvrA (Salmonella) | Cytoplasm | 99 | 99 | 41, 144, 232, 237, 251, 324 |
| YopT | 35.5 | Disruption of actin filaments | Cytotoxicity | p76 (H. somnus) | Unknown | 124 | 124 | 170 |
aa, amino acids.
Functional Conservation among Different Bacterial Species
We have seen that several other bacterial species possess a type III virulence system. Are these systems functionally interchangeable in the sense that effectors from one system could be secreted or even delivered intracellularly by another system? The N-terminal domain (217 residues) of the ADP-ribosyltransferase ExoS from P. aeruginosa (453 residues total) has 54% similarity to the entire YopE (see above), and the protein encoded by the gene next to exoS (ORF1) is very similar to SycE/YerA (368). These observations prompted Frithz-Lindsten et al. (105) to introduce the two genes from P. aeruginosa, transcribed from the plac promoter, into Y. pseudotuberculosis. Since they observed that the recombinant Y. pseudotuberculosis could secrete ExoS, they next investigated whether ExoS would be delivered by a recombinant Y. pseudotuberculosis into HeLa cells, just like YopE. They introduced the exoS gene and ORF1 into a noncytotoxic double yopE yopH mutant of Y. pseudotuberculosis and used the mutant to infect HeLa cells. The result was clear cytotoxicity, indicating that ExoS is translocated across the HeLa cell plasma membrane and also that ExoS has a cytotoxic activity. Repeating the experiment with a mutated form of ExoS that has a 2,000-fold-reduced ADP-ribosyltransferase activity, they still observed cytotoxicity, which indicated that ExoS is a bifunctional protein endowed with a YopE-like cytotoxic activity. These experiments demonstrated that the closely related Yersinia and Pseudomonas type III systems are functionally interchangeable. Given the taxonomic distance between these two species, the observation is important because it strengthens the idea of horizontal spread of these type III systems.
The group of H. Wolf-Watz also observed that Y. pseudotuberculosis can secrete IpaB from Shigella flexneri and that Salmonella typhimurium can secrete YopE (284). The latter recombinant Salmonella strain is also cytotoxic for HeLa cells, suggesting that YopE could even be translocated across the cell plasma membrane.
YADA ADHESIN AND YLPA LIPOPROTEIN
Discovery and Description
Y. enterocolitica and Y. pseudotuberculosis synthesize a pYV-encoded outer membrane protein called YadA (formerly called Yop1 or P1) (20, 43, 45). YadA is named for “Yersinia adhesin A,” a name that was given during the 1990 Keystone meeting on Yersinia, in Frisco, Colo. The expression of the gene encoding this protein (yadA, formerly yopA) is under the control of VirF, and it is expressed only at 37°C, like the yop and ysc genes (43, 187). YadA is thus a Yop regulon protein (see “Regulation of transcription of the virulon genes,” below), but unlike the Yops, it is produced in the presence and absence of Ca2+ (43, 228, 315). YadA is not expressed by Y. pestis even though its pYV plasmid contains the yadA gene. The yadA gene of Y. pestis has a single-base-pair deletion that results in a shift in the reading frame of the gene and an mRNA with a reduced half-life, and so the YadA protein is not produced (268, 286, 316).
yadA encodes a 44.1- to 47.1-kDa protein (the exact size depends on the strain) that is seen on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) when prepared in the presence of high concentrations of urea or upon prolonged boiling in the presence of excess reducing agent (313, 384). Sample preparation by boiling for shorter periods in the presence of SDS results in the appearance on SDS-PAGE of an approximately 200-kDa band that is a polymer of YadA. By electron microscopy, YadA has been seen as a fibrillar extracellular matrix around the bacteria (188). Each individual fibrilla had a diameter of 1.5 to 2.0 Å and a length of 50 to 125 Å. In another study, YadA was seen as tack-like projections protruding from the bacterial surface (384). The protein causes autoagglutination of Yersinia and mannose-resistant haemagglutination of guinea pig erythrocytes (20, 188, 198, 221, 313).
YadA can bind a variety of eukaryotic extracellular and cell surface molecules including collagens, fibronectin, and laminins (85, 96, 304, 305, 345). It has been shown to bind to rabbit intestinal tissue (both the brush border membranes and mucus), eye lens capsule basement membranes, and human intestinal submucosa (96, 249, 250, 314). YadA also mediates the binding of Yersinia to cultured cells, such as HeLa and HEp-2 (152, 282). Thus, YadA is a major adhesin of Yersinia for attachment to eukaryotic cells. Although invasin, an outer membrane protein encoded by the chromosome of Yersinia, is the major determinant for internalization of the bacteria into eukaryotic cells via interaction with β1-integrins (176, 177), YadA too can mediate internalization of the bacteria into eukaryotic cells, and this is due, at least in part, to interaction with β1-integrins (35, 380).
Role of YadA in Virulence
YadA plays a protective role for Yersinia. As discussed above, it contributes to the protection of Y. enterocolitica against killing by PMN extracts and against killing by human serum (20, 221, 362). This latter ability is due to the binding of factor H by YadA, which leads to the inactivation of C3b and a subsequent decrease in the deposition of membrane attack complexes on the bacterial surface (66, 262). YadA also mediates the inhibition of the anti-invasive effect of interferon (52).
The role played by YadA in the resistance of Y. enterocolitica to phagocytosis and killing by eukaryotic cells is disputed (65, 152, 291, 361), but it has been suggested that YadA would act by binding to eukaryotic cells and, in doing so, allow delivery of the Yops (see above) (291, 321).
There is a major difference between Y. enterocolitica and Y. pseudotuberculosis in the influence of a yadA mutation on virulence in mice. A Y. enterocolitica yadA mutant is attenuated for virulence (254, 277, 278, 342). Due to impaired colonisation of the Peyer’s patches, Y. enterocolitica bacteria are eliminated and smaller numbers of bacteria are found in the mesenteric lymph nodes, spleen, and liver (188, 277). In addition, there is less inflammation and necrosis in the liver (254). The virulence attenuation is not a result of YadA being required for movement of Yersinia from the intestinal lumen to the Peyer’s patches—the yadA mutant strain does this very well (277). It is due to the requirement of YadA for persistence, i.e., survival and multiplication, in the Peyer’s patches, and perhaps for dissemination of the bacteria from the Peyer’s patches to other sites in the body (254, 277). In contrast, a Y. pseudotuberculosis yadA mutant is just as virulent as the wild type (45, 139, 286) and can colonize the Peyer’s patches just as efficiently. It was previously reported that an inv yadA double mutant was hypervirulent compared to the wild-type strain or the yadA or inv single mutants (286). However, more recent results suggest that this inv yadA double mutant was not isogenic to the parental wild-type strain. A newly constructed inv yadA double-mutant strain maintained the same virulence as the parental strain, demonstrating that neither invasin nor YadA plays an important role during Y. pseudotuberculosis infection (139). The severe virulence of Y. pestis is thus probably not due to the inv yadA mutant phenotype, as originally suggested, but could be due to additional virulence factors, which are lacking in Y. enterocolitica and Y. pseudotuberculosis (139). The differences between the Yersinia species with regard to the role of YadA in virulence probably result from the importance of the interplay of additional adhesion factors.
Structure-Function Analysis of YadA from Y. enterocolitica
The YadA proteins from various strains of Y. pseudotuberculosis and Y. enterocolitica differ as a result of substitutions and deletions/insertions within the coding DNA (316). Studies on the structure-function relationships of YadA have focused primarily on Y. enterocolitica serotype O:8 (Fig. 10). The N terminus of each YadA comprises a typical signal sequence (316). YadA is thus not exported by the Ysc secretion machinery but is instead probably exported by the Sec system. A Y. enterocolitica mutant lacking amino acids 29 to 81, a region that varies among the Yersinia species, does not adhere to PMNs and so does not cause inhibition of the oxidative burst (278). This mutant is attenuated in virulence and is comparable to a complete yadA mutant strain. This suggests that the effect on PMNs mediated by YadA is important for the virulence of Y. enterocolitica. Residues 83 to 101 compose one of the conserved hydrophobic domains of YadA. Deletion of these amino acids results in a YadA protein that cannot promote autoagglutination, does not bind collagen and has impaired ability to bind to basement membranes (342). This domain is also involved in the binding of YadA to human intestinal submucosa via collagen and laminin (314). Most importantly, this mutant is avirulent, reinforcing the importance of YadA binding to eukaryotic cells for virulence of Y. enterocolitica. The conserved histidines 156 and 159 are necessary for collagen binding, are important for binding to fibronectin and laminin, and are needed for binding to HEp-2 cells (277). A Y. enterocolitica strain carrying a yadA gene with mutations in these two histidine residues colonizes the Peyer’s patches but does not disseminate to the mesenteric lymph nodes, spleen, or liver. These results indicate that YadA plays a role in the dissemination of Y. enterocolitica from the Peyer’s patches to other sites in the body. Finally, the conserved hydrophobic C-terminal amino acids of YadA have been shown to be involved in surface exposure of the protein and polymer formation (342).
FIG. 10.
Representation of the YadA protein of Y. enterocolitica O:8 showing the mapped domains and their functions (277, 278, 314, 316, 342).
Conclusion for YadA
To summarize, YadA is a Yersinia adhesin that binds eukaryotic cells by a number of molecules. In Y. enterocolitica, the adhesive ability of YadA is essential for virulence and could be required for the translocation of the Yop proteins into eukaryotic cells and so for the effects of these Yop proteins on the target cells. A number of domains of YadA have been mapped as being important for the various functions of the protein and for virulence.
YlpA
YlpA is a pYV-encoded lipoprotein that is produced at 37°C in the absence of Ca2+ (64). Its expression is dependent on VirF, making it a member of the Yop regulon. Like YadA, YlpA has a typical signal sequence and is localized to the outer membrane independent of the Ysc secretion machinery. YlpA is highly homologous to the TraT proteins of a number of bacterial plasmids (up to 88% identity at the amino acid level). TraT proteins are involved in serum resistance (235), surface exclusion during conjugation (1), and inhibition of phagocytosis by macrophages (2), but the involvement of YlpA in these processes is unknown. A mutation in YlpA does not affect virulence in the mouse model (64).
GENETICS
Regulation of Transcription of the Virulon Genes
Effect of temperature and Ca2+ on in vitro transcription.
As discussed above, Yop secretion in vitro occurs only at 37°C in the absence of Ca2+. This secretion correlates with growth arrest, a phenomenon known for a long time as Ca2+ dependency (50). By contrast, the production of YadA is independent of the Ca2+ concentration but is still thermoregulated (43, 187). This was the first indication that temperature and Ca2+ influence two different regulatory networks. The first permits full expression of all the pYV-encoded virulence functions when the environment is ideal and the temperature reaches 37°C, while the second prevents the production of the Yops and YlpA only in the presence of 2.5 mM Ca2+ ions (for reviews, see references 70 and 335). Neither of these two regulatory networks is perfectly understood so far, but it is quite clear that they are independent of each other. They are summarized in Fig. 11.
FIG. 11.
Schematic representation of the two circuits regulating yop gene transcription. IM, inner membrane; OM, outer membrane.
The transcriptional activator VirF/LcrF.
In 1986, Yother et al. mutagenized the virulence plasmid pCD1 of Y. pestis with Tn5 and identified a locus which positively controls the transcription of two loci (called trtA and trtB at that time) in response to temperature. They named this regulatory locus lcrF (382). In 1987, Cornelis et al. (74) described virF, the Y. enterocolitica homolog of lcrF, and showed that a mutation in this locus, which is located between virB and virC (Fig. 3), drastically reduces the transcription of a yopH-lacZ operon fusion. The virF gene was isolated and sequenced by the same group 2 years later (72).
VirF/LcrF is a 30.9-kDa protein that belongs to the AraC family of regulators (72). This family includes regulators of degradative pathways in E. coli and Pseudomonas putida as well as regulators involved in the control of virulence of Shigella spp., enterotoxigenic E. coli, P. aeruginosa, and the phytopathogen Ralstonia solanacearum (80, 114, 164, 180; for a review, see reference 109). Transcription of many pYV genes, including all the yop genes, sycE, ylpA, yadA, and the virC operon, is dependent on VirF/LcrF (64, 72, 228, 315, 366). These genes and operons constitute the virF/lcrF regulon. By contrast, VirF/LcrF seems to be dispensable or less important for transcription of the virA and virB operons, which encode the Ysc secretion apparatus (199), and of some other genes such as sycH (365). All these genes, dependent on or independent of VirF/LcrF, are silent at low temperature but strongly expressed at 37°C. They constitute the yop stimulon. Note that the yop stimulon is larger than the virF/lcrF regulon.
DNase I footprinting experiments carried out by Wattiau and Cornelis (367) on four promoters (yopE, yopH, virC, and lcrGVsycDyopBD) showed that VirF binds to a 40-bp region localized immediately upstream from the RNA polymerase binding site. These VirF-binding sequences are located in an AT-rich region and appear either isolated or repeated in opposite orientation. This site contains the 13-bp consensus sequence TTTTaGYcTtTat (in which nucleotides conserved in ≥60% of the sequences are in capital letters and Y indicates C or T) (367).
In Y. enterocolitica, the virF gene itself is strongly thermoregulated. This thermoinduction still occurs in E. coli containing an isolated virF gene transcribed from its own promoter (72). Expression of the virF gene must thus be thermoregulated by a chromosomal gene rather than by a pYV gene. The fact that virF is itself thermoregulated can explain why the Yops are produced only at 37°C. However, it does not prove that temperature control occurs only through the regulation of virF. In fact, when virF is transcribed at low temperature from a tac promoter, the yop and yadA genes are only poorly transcribed. In contrast, at 37°C, the response to isopropyl-β-d-thiogalactopyranoside (IPTG) mimics the normal response to thermal induction (199). In conclusion, expression of the yop stimulon is first controlled by temperature but expression of some of its genes is reinforced by the action of VirF, whose synthesis is also temperature controlled.
In Y. pestis, transcription of lcrF::lacZ transcriptional fusions is independent of temperature (160). However, comparison of the amount of LcrF protein produced per unit of message at low and high temperature indicates that the efficiency of translation of the lcrF mRNA increases with temperature (159). To account for this observation, Hoe and Goguen (159) presented a model in which a secondary structure of mRNA could sequester the lcrF Shine-Dalgarno sequence and so regulate LcrF synthesis.
With regard to these two models, one might think that there are two separate modes of regulation in Y. enterocolitica and in Y. pestis. However, VirF and LcrF are so similar in their sequence and in their function that it is difficult to imagine that they could be regulated differently. Data from the two groups could be reconciled in a model in which both transcription and translation of virF/lcrF are temperature dependent.
Role of the histone-like protein YmoA and chromatin structure.
To identify the chromosomal regulator of the yop stimulon, Cornelis et al. (71) carried out transposon mutagenesis of a Y. enterocolitica strain which carried lacZ fused to yopH. Two chromosomal mutants strongly transcribed yopH, yopE, and yadA at 28°C but did not secrete the Yops at this temperature (71). Transcription of the regulatory gene virF was itself increased at 28°C, which could account for the increased transcription of the genes of the regulon. Although expression of these genes was deregulated at low temperature in the mutants, there was still an increase of transcription upon transfer to 37°C. Hence, the thermal response was not abolished but, rather, “modulated.” The phenotype is thus not that of a classical repressor-minus mutant, but the mutations nevertheless specifically affected a component of the temperature response. In both mutants, the transposon was inserted in the same small gene that Cornelis et al. (71) called ymoA for “Yersinia modulator.” The ymoA gene encodes an 8.1-kDa protein extremely rich in positively and negatively charged residues. Although there is no sequence similarity between YmoA and the histone-like proteins HU, IHF, and H-NS (H1), it is very likely that YmoA is a histone-like protein (71). This idea has been reinforced by the fact that the level of supercoiling was higher in ymoA mutants than in the wild-type strain, as shown by chloroquine agarose gel electrophoresis of plasmid DNA (71). To determine whether the chromatin structure influences expression of yop genes, Lambert de Rouvroit et al. (199) measured the expression of a yopH-cat operon fusion in the ymoA mutant. They showed that the yopH promoter becomes independent of VirF in the ymoA mutant but still remains thermoinducible. This result suggested that chromatin structure and temperature strongly affect the yopH promoter itself. The most likely hypothesis is that temperature could somehow modify the structure of the chromatin, making the promoters more accessible to VirF. Rohde et al. (279) confirmed that temperature alters DNA supercoiling and DNA bending by VirF in Y. enterocolitica and hypothesized that temperature dislodges a repressor, perhaps YmoA, bound on promoter regions of VirF-sensitive genes and of some other thermoregulated genes.
The role of chromatin structure in thermoregulation of virulence gene transcription is now well established. It is reviewed and discussed in detail by Dorman and Ni Bhriain (81). All the properties of the ymoA mutants are strikingly reminiscent of the virR mutants of Shigella flexneri lacking H-NS (123). YmoA is, however not the counterpart of H-NS from E. coli or Shigella. A homolog of YmoA was discovered in E. coli as a regulator of hemolysin production (246). The gene encoding this regulator, called hha, can complement the ymoA mutations of Y. enterocolitica, provided that it is expressed at an adequate level (230). YmoA and Hha are thus the first representatives of a new class of histone-like proteins regulating the expression of topologically sensitive promoters. YmoA can thus be listed along with the histone-like proteins H-NS, IHF, FIS, HU, and LRP. It is striking that the searches for thermoregulators in Yersinia spp., in Shigella spp., and in uropathogenic E. coli converged on histone-like proteins (156).
Feedback control of Yop synthesis by the secretion apparatus.
The first molecular studies on the transcription of pYV genes in Y. pestis and Y. enterocolitica were done in the mid-1980s by using mini-Mu dlac insertion mutants. This strategy permits researchers to monitor the expression of various genes under different conditions simply by measuring the β-galactosidase activity of promoters fused to lacZ. In 1984, Goguen et al. showed that Ca2+ ions do not affect the transcription of lcrA-lacZ, lcrB-lacZ, and lcrC-lacZ gene fusions in Y. pestis (120). This was confirmed for their homologs in Y. enterocolitica (virA, virB, and virC) by Cornelis et al. (73). While Ca2+ had little effect on transcription of these loci, which are now known to encode the Ysc secretion apparatus, it had a very clear effect on yop expression: expression of yop-lacZ operon fusions was dramatically decreased by the presence of 2.5 mM Ca2+ in the medium (73, 74, 239, 330). Later, Northern blotting experiments confirmed that Ca2+ abolishes the transcription of yopE and yopH (46, 100). Thus, the presence of Ca2+ ions blocks not only the secretion of Yops but also their synthesis. It is difficult to imagine how an ion like Ca2+ could penetrate into the bacterial cytosol to block transcription. It is more plausible that the abundance of Ca2+ could influence Yop secretion from the bacterial surface. Cornelis et al. (74) thus suggested that Ca2+ would essentially stop secretion and that a feedback inhibition mechanism would block transcription of yop genes when secretion is compromised. This hypothesis is reinforced by the fact that mutations in the virA, virB, and virC loci, which encode the Ysc secretion apparatus, severely downregulate expression of the yop genes (9, 72, 264, 265, 375). It is also consistent with the fact that transcription of the ysc genes themselves is not strongly influenced by Ca2+ (see above).
As described above, yopN/lcrE, lcrG, and tyeA mutants have a Ca2+-blind phenotype: they express and secrete Yops in the presence, as well as in the absence, of Ca2+ in the media (99, 172, 311). The fact that these mutants were derepressed for Yop expression even under repressive conditions (presence of Ca2+ ions) leads to the idea that feedback regulation is of the negative type.
As we have seen previously, this regulation by Ca2+ permits control of Yop secretion in vitro while contact with eukaryotic cells is probably the signal triggering Yop secretion in vivo. In 1996, Pettersson et al. (261) studied the transcriptional activity of a Yersinia yopE-luxAB fusion in the presence of HeLa cells. They observed that transcription of yopE was induced in the bacteria associated with HeLa cells whereas no signal was observed in the bacteria attached to the glass coverslip. These observations clearly demonstrate that the bacteria initiated yop transcription only after contact with the target cell had been established.
LcrQ/YscM.
By analogy with the secreted anti-sigma factor involved in the regulation of flagellum synthesis (49, 169, 197), Rimpiläinen et al. (275) suggested that feedback inhibition could be mediated by a negative regulator that is normally expelled via the Yop secretion machinery. They suggested that in Y. pseudotuberculosis, LcrQ, a 12.4-kDa secreted protein encoded by the last gene of the virC locus, could be this hypothetical regulator because overproduction of this protein abolishes Yop production. In the absence of secretion (presence of Ca2+ or mutation in the genes coding for the secretion machinery), an lcrQ mutant indeed synthesizes more Yops than the wild type does. In the presence of Ca2+, this mutant secretes YopD and LcrV. Recently, the same group showed that LcrQ is rapidly secreted when bacteria are shifted from a medium containing 2.5 mM Ca2+ (nonpermissive conditions for Yop secretion) to a medium containing a Ca2+ chelator (permissive for Yop secretion), which fits quite well with the “secreted negative regulator” hypothesis (261). In Y. enterocolitica, the situation appeared to be slightly different: YscM, the counterpart of LcrQ, is also a secreted protein (327), but a yscM mutant does not show any sign of derepression of Yop synthesis in the absence of Yop secretion, although overproduction of YscM blocks Yop synthesis (8). The reason for this discrepancy was recently elucidated by the discovery of a gene related to yscM (now called yscM1) on the pYV virulence plasmid of Y. enterocolitica, which was called yscM2. A yscM1 yscM2 double mutant of Y. enterocolitica shows the same phenotype as the lcrQ mutant of Y. pseudotuberculosis. Thus, two different YscM proteins in Y. enterocolitica behave like LcrQ in Y. pseudotuberculosis (327).
The hypothesis that LcrQ and YscM are secreted negative regulators is essentially based on two observations: (i) an lcrQ single mutant and a yscM1 yscM2 double mutant display a reduction of feedback inhibition when Yop secretion is prevented, and (ii) overproduction of any one of these proteins shuts off Yop synthesis. However, some observations must be made. First, lcrQ and yscM1 yscM2 mutants secrete YopD and LcrV in the presence of Ca2+. How would the lack of a negative regulator remove the external stop-valve YopN to open the secretion channel, and, if the secretion channel is open, why would the other Yops not be secreted? Second, Stainier et al. (327) observed that overexpression of these proteins in a simplified system consisting only of a yopH-cat reporter gene and virF had no effect on yopH transcription, while a negative effect was observed in the presence of a pYV plasmid. Taken together, these various observations suggest that LcrQ/YscM is not a transcriptional repressor and that one or more pYV-encoded proteins are required to act with this protein in the feedback inhibition mechanism.
Recently, Williams and Straley (373) suggested that in Y. pestis, YopD acts with LcrQ in the feedback inhibition mechanism. This idea is supported by the fact that a yopD mutant secretes some YopM and LcrV in the presence of Ca2+, despite the presence of LcrQ in the bacterial cell. Overexpression of LcrQ in this mutant does not have the same strong repressive effect as it does in the wild-type strain. These results suggests that LcrQ requires YopD to function as a negative regulator.
Lack of interference with flagellum assembly.
It has been known for a long time that Yersinia is motile (369). The genes coding for the three flagellins and for proteins implicated in the building of the flagellum in Y. enterocolitica were described recently (89, 185). Production of flagella in Y. enterocolitica occurs below 30°C and requires sigma factor 28 of the RNA polymerase (185, 186). The similarity between some Ysc proteins and proteins involved in flagellar assembly suggests that the Yop secretion system might have evolved from the secretion system operating in flagellum biogenesis. Therefore, one could imagine that these two systems are regulated in the same way. However, it has been shown that yop genes are expressed only at 37°C and expression is independent of sigma factor 28 (174). By making use of two different sigma factors, Yersinia could thus avoid the simultaneous expression of the two systems, which could functionally interfere with each other.
Role of RpoS on pYV-encoded virulence factor expression.
Virulence genes of enteropathogenic bacteria are often regulated by growth phase and environmental signals. Iriarte et al. (173) wondered whether the alternative sigma factor RpoS is required for Yersinia virulence. They constructed a nonpolar rpoS mutant of Y. enterocolitica and analyzed the secretion of Yop proteins and the colonization of mouse tissues. The rpoS mutant secreted all the Yops as well as the wild-type strain did. In contrast to what has been observed with Salmonella (245, 374), there was no difference between the abilities of an rpoS mutant strain and its wild-type parent strain to colonize the Peyer’s patches and spleen of mice after intragastric inoculation (173). Badger and Miller (18) similarly showed that a Y. enterocolitica rpoS mutant is not affected in virulence for mice.
Organization of the pYV Plasmid
Ancillary functions: replication and stabilization.
The pYV plasmid is nonconjugative and incompatible with the sex factor F as a result of the presence of an incompatibility incD determinant which is part of the partition system (19, 28). This partition system, like that of F, consists of two proteins, called SpyA and SpyB, and a site called spyC (171, 355). The replicon is of the RepFIIA type (archetype R100), like that of many large antibiotic resistance and virulence plasmids (354). The replication machinery consists of an origin of replication (oriR) and two genes, repA and repB, encoding proteins of 33.5 and 9.5 kDa, respectively (354). RepA is the replicase, while RepB acts as a regulator (354). In the pYV plasmid of Y. enterocolitica O:9, the replication and partition regions are separated by about 20 kb, which contains the yadA gene and vestigial transposons, one of which is Tn2502 (see below).
The genetic maps of the pYV plasmids from the various Yersinia species are quite similar except for the presence of the ars transposon and some reshuffling that occurred during evolution. The most striking differences between Y. enterocolitica and Y. pseudotuberculosis are a large inversion of almost half the plasmid (28) and, within the inverted region, the inversion of the region containing the partition system spy and the yopE and sycE genes (Fig. 3).
Operon encoding arsenic resistance in Y. enterocolitica.
The pYV plasmid of the low-virulence strains of Y. enterocolitica (O:1,2,3, O:1,2, O:3, O:9, and O:5,27) contains a class II transposon, Tn2502, which confers arsenite and arsenate resistance (244). This resistance involves four genes: three are the homologs to the arsRBC genes present on the E. coli chromosome, but the fourth, arsH, has no known homolog. ArsR is an arsenite-inducible transcriptional repressor, ArsB forms a transmembrane channel, and ArsC catalyzes the reduction of arsenate to arsenite, which is in turn expelled by the ArsB transport system. One can only speculate on the role of the 26.4-kDa ArsH protein. The most likely hypothesis is that it acts as a regulator. The ars operon is not present on the pYV plasmid of the more virulent “American” strains (serotypes O:4, O:8, O:13a,13b, O:18, O:20, and O:21) of Y. enterocolitica or on the pYV plasmid of Y. pseudotuberculosis or Y. pestis. The presence of this operon thus constitutes the first significant difference between the virulence plasmids from different Yersinia species (244). It is also the first example of a virulence plasmid carrying resistance genes (244). The fact that the ars genes are present in all the serotypes of low-virulence Y. enterocolitica suggests that the low-virulence strains, which are distributed worldwide, constitute a single clone that probably emerged quite recently. At present, pigs represent the major reservoir of pathogenic strains of Y. enterocolitica, and pork meat is recognized as the major source of human contamination (204, 343). Neyt et al. (244) speculated that the ars transposon might have favored the establishment of a strain of Yersinia in pigs. Arsenic compounds were largely used before World War II as therapeutic agents to protect pigs from diarrhea caused by Serpulina hyodysenteriae.
CONCLUSIONS AND FUTURE PERSPECTIVES
The Yop story can be compared to the fairy tale “the beauty and the beast,” in the sense that it started as a nightmare with the somehow repulsive “Ca2+ dependency” phenomenon to end up as the innovative concept that we describe in this review. However, unlike a fairy tale, the Yop story is far from being complete. Many intriguing questions remain in every aspect of the system. Let us first consider the control of Yop synthesis and release. In spite of enormous efforts invested, it is fair to say that we still do not understand the feedback inhibition control. We have a candidate for a secreted repressor (LcrQ/YscM), but its mode of action is not understood. Contact control is one of the most appealing aspects of the system, but two major questions remain: (i) what is the degree of “directionality,” and (ii) how does contact lead to the opening of the channel? Generally, “contact” means an interaction between a receptor and a ligand. However, in this case, no specific receptor at the cell surface has been identified. Recognition of heparan sulfate proteoglycans by LcrG is an initial clue but certainly not the definite answer. Although several pieces of the control system have been identified and characterized at the bacterial surface, the actual bacterial ligand has not yet been identified. Is it YopN, the YopN-associated TyeA, or LcrG whose exact localization remains elusive? Another question concerning control is whether de novo protein synthesis is required for Yop translocation or is every bacterium equipped with a lethal dose of Yops before contact? Now, let us consider the translocation apparatus that is deployed at the interface between the bacterium and the eukaryotic cell. Although this is not firmly established, the existence of a pore is very likely, as suggested by the contact hemolysis shown by Håkansson et al. (138), but this pore remains to be characterized. Does it consist of YopB alone or of YopB associated with YopD? What is the role of LcrV in the assembly of this hypothetical pore? Could it form some kind of a pilus underneath YopB and YopD? What is the function of LcrG in translocation? Electron microscopy techniques such as those that provided wonderful images of the assembling flagellum (248) and of the type III secretion apparatus of S. typhimurium (194a) and structural biology will probably provide the conclusive evidence. Understanding the translocation process itself will probably still require years of effort by molecular biologists. The unravelling of the Ysc secretion pathway will also require long collaborative efforts. There are still more than 25 components to localize in the bacterium and to assemble. However, in this area, the hottest question is that of the secretion signal. Does the Ysc apparatus recognize mRNA, and how? What is the exact role of the Syc chaperones? Are they bodyguards, pilots, or both? Finally, cell biology has a lot of answers to give. Why does Yersinia deliver six effectors to the same target cell? Are the six effectors needed to neutralize the same phagocyte? Would the action of some effectors be very rapid (YopH?) but reversible and the action of others slower but irreversible (YopP/YopJ)? Alternatively, would some effectors be specifically designed for some cell types and others for other cell types?
Besides answering all these fascinating basic questions, one could also envision developing possible medical applications of the new concepts that came into sight. One can envision that such a sophisticated virulence apparatus could be an appropriate target for “antipathogenicity drugs.” Would the spectrum of such drugs be broad enough? One may also consider engineered Yersinia cells as vectors to deliver antigens when a cytotoxic T-lymphocyte response is desirable. This second application is probably close to realization, at least in the laboratory.
In conclusion, research on Yersinia has been extremely fruitful in terms of concepts. Since the Yop virulon is at the leading edge of “type III secretion,” it probably represents the most suitable system to investigate its most basic aspects such as control, secretion, and translocation. Once again, fundamental research on a system with very little if any commercial or health interest led to new ideas, which we believe will pay off sooner or later.
ACKNOWLEDGMENTS
We thank M. Monteforte and D. Desnoeck for providing artwork and for expert handling of the references, tables, and text. We thank James Bliska and Jürgen Heesemann for communicating results prior to their publication.
C.G. is financed by a postdoctoral fellowship from the European Union (contract ERBFMBICT972047). A.P.B. is the recipient of an H. and A. Brenninkmeijer ICP fellowship. A.B. and C.N. are research assistants funded by the Belgian “Fonds National de la Recherche Scientifique.” The Yersinia project is supported by the Belgian “Fonds National de la Recherche Scientifique Médicale” (Convention 3.4595.97), the “Direction générale de la Recherche Scientifique-Communauté Française de Belgique” (Action de Recherche Concertée” 94/99-172), and the “Interuniversity Poles of Attraction Program—Belgian State, Prime Minister’s Office, Federal Office for Scientific, Technical and Cultural affairs” (PAI 4/03).
REFERENCES
- 1.Achtman M, Kennedy N, Skurray R. Cell-cell interactions in conjugating Escherichia coli: role of traT protein in surface exclusion. Proc Natl Acad Sci USA. 1977;74:5104–5108. doi: 10.1073/pnas.74.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguero M E, Aron L, DeLuca A G, Timmis K N, Cabello F C. A plasmid-encoded outer membrane protein, TraT, enhances resistance of Escherichia coli to phagocytosis. Infect Immun. 1984;46:740–746. doi: 10.1128/iai.46.3.740-746.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albertini A M, Caramori T, Crabb W D, Scoffone F, Galizzi A. The flaA locus of Bacillus subtilis is part of a large operon coding for flagellar structures, motility functions, and an ATPase-like polypeptide. J Bacteriol. 1991;173:3573–3579. doi: 10.1128/jb.173.11.3573-3579.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfano J R, Collmer A. The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. J Bacteriol. 1997;179:5655–5662. doi: 10.1128/jb.179.18.5655-5662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Alfano J R, Kim H S, Delaney T P, Collmer A. Evidence that the Pseudomonas syringae pv. syringae hrp-linked hrmA gene encodes an Avr-like protein that acts in an hrp-dependent manner within tobacco cells. Mol Plant-Microbe Interact. 1997;10:580–588. doi: 10.1094/MPMI.1997.10.5.580. [DOI] [PubMed] [Google Scholar]
- 5.Allaoui A, Sansonetti P J, Parsot C. MxiJ, a lipoprotein involved in secretion of Shigella Ipa invasins, is homologous to YscJ, a secretion factor of the Yersinia Yop proteins. J Bacteriol. 1992;174:7661–7669. doi: 10.1128/jb.174.23.7661-7669.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allaoui A, Sansonetti P J, Parsot C. MxiD, an outer membrane protein necessary for the secretion of the Shigella flexneri Ipa invasins. Mol Microbiol. 1993;7:59–68. doi: 10.1111/j.1365-2958.1993.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 7.Allaoui A, Scheen R, Lambert de Rouvroit C L, Cornelis G R. VirG, a Yersinia enterocolitica lipoprotein involved in Ca2+ dependency, is related to ExsB of Pseudomonas aeruginosa. J Bacteriol. 1995;177:4230–4237. doi: 10.1128/jb.177.15.4230-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allaoui A, Schulte R, Cornelis G R. Mutational analysis of the Yersinia enterocoliticia virC operon: characterization of yscE, F, G, I, J, K required for Yop secretion and yscH encoding YopR. Mol Microbiol. 1995;18:343–355. doi: 10.1111/j.1365-2958.1995.mmi_18020343.x. [DOI] [PubMed] [Google Scholar]
- 9.Allaoui A, Woestyn S, Sluiters C, Cornelis G R. YscU, a Yersinia enterocolitica inner membrane protein involved in Yop secretion. J Bacteriol. 1994;176:4534–4542. doi: 10.1128/jb.176.15.4534-4542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson D M, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- 11.Andersson K, Carballeira N, Magnusson K E, Persson C, Stendahl O, Wolf-Watz H, Fällman M. YopH of Yersinia pseudotuberculosis interrupts early phosphotyrosine signalling associated with phagocytosis. Mol Microbiol. 1996;20:1057–1069. doi: 10.1111/j.1365-2958.1996.tb02546.x. [DOI] [PubMed] [Google Scholar]
- 12.Andrews G P, Maurelli A T. mxiA of Shigella flexneri 2a, which facilitates export of invasion plasmid antigens, encodes a homolog of the low-calcium-response protein, LcrD, of Yersinia pestis. Infect Immun. 1992;60:3287–3295. doi: 10.1128/iai.60.8.3287-3295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold R, Scheffer J, Konig B, Konig W. Effects of Listeria monocytogenes and Yersinia enterocolitica on cytokine gene expression and release from human polymorphonuclear granulocytes and epithelial (HEp-2) cells. Infect Immun. 1993;61:2545–2552. doi: 10.1128/iai.61.6.2545-2552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arricau N, Hermant D, Waxin H, Popoff M Y. Molecular characterization of the Salmonella typhi StpA protein that is related to both Yersinia YopE cytotoxin and YopH tyrosine phosphatase. Res Microbiol. 1997;148:21–26. doi: 10.1016/S0923-2508(97)81896-7. [DOI] [PubMed] [Google Scholar]
- 15.Autenrieth I B, Firsching R. Penetration of M cells and destruction of Peyer’s patches by Yersinia enterocolitica: an ultrastructural and histological study. J Med Microbiol. 1996;44:285–294. doi: 10.1099/00222615-44-4-285. [DOI] [PubMed] [Google Scholar]
- 16.Autenrieth I B, Heesemann J. In vivo neutralization of tumor necrosis factor-alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica infection in mice. Med Microbiol Immunol. 1992;181:333–338. doi: 10.1007/BF00191545. [DOI] [PubMed] [Google Scholar]
- 17.Autenrieth I B, Kempf V, Sprinz T, Preger S, Schnell A. Defense mechanisms in Peyer’s patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect Immun. 1996;64:1357–1368. doi: 10.1128/iai.64.4.1357-1368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badger J L, Miller V L. Role of RpoS in survival of Yersinia enterocolitica to a variety of environmental stresses. J Bacteriol. 1995;177:5370–5373. doi: 10.1128/jb.177.18.5370-5373.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakour R, Laroche Y, Cornelis G. Study of the incompatibility and replication of the 70-kb virulence plasmid of Yersinia. Plasmid. 1983;10:279–289. doi: 10.1016/0147-619x(83)90042-2. [DOI] [PubMed] [Google Scholar]
- 20.Balligand G, Laroche Y, Cornelis G. Genetic analysis of virulence plasmid from a serogroup 9 Yersinia enterocolitica strain: role of outer membrane protein P1 in resistance to human serum and autoagglutination. Infect Immun. 1985;48:782–786. doi: 10.1128/iai.48.3.782-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barinaga M. Life-death balance within the cell. Science. 1996;274:724. doi: 10.1126/science.274.5288.724. [DOI] [PubMed] [Google Scholar]
- 22.Barve S S, Straley S C. lcrR, a low-Ca2+-response locus with dual Ca2+-dependent functions in Yersinia pestis. J Bacteriol. 1990;172:4661–4671. doi: 10.1128/jb.172.8.4661-4671.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beg A A, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 24.Bergman T, Erickson K, Galyov E E, Persson C, Wolf-Watz H. The lcrB (yscN/U) gene cluster of Yersinia pseudotuberculosis is involved in Yop secretion and shows high homology to the spa gene clusters of Shigella flexneri and Salmonella typhimurium. J Bacteriol. 1994;176:2619–2626. doi: 10.1128/jb.176.9.2619-2626.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergman T, Håkansson S, Forsberg A, Norlander L, Macellaro A, Backman A, Bolin I, Wolf-Watz H. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J Bacteriol. 1991;173:1607–1616. doi: 10.1128/jb.173.5.1607-1616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beuscher H U, Rodel F, Forsberg A, Rollinghoff M. Bacterial evasion of host immune defense: Yersinia enterocolitica encodes a suppressor for tumor necrosis factor alpha expression. Infect Immun. 1995;63:1270–1277. doi: 10.1128/iai.63.4.1270-1277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhakdi S, Mackman N, Nicaud J M, Holland I B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986;52:63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biot T, Cornelis G R. The replication, partition and yop regulation of the pYV plasmids are highly conserved in Yersinia enterocolitica and Y. pseudotuberculosis. J Gen Microbiol. 1988;134:1525–1534. doi: 10.1099/00221287-134-6-1525. [DOI] [PubMed] [Google Scholar]
- 29.Bischoff D S, Ordal G W. Identification and characterization of FliY, a novel component of the Bacillus subtilis flagellar switch complex. Mol Microbiol. 1992;6:2715–2723. doi: 10.1111/j.1365-2958.1992.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 30.Bischoff D S, Weinreich M D, Ordal G W. Nucleotide sequences of Bacillus subtilis flagellar biosynthetic genes fliP and fliQ and Identification of a novel flagellar gene, fliZ. J Bacteriol. 1992;174:4017–4025. doi: 10.1128/jb.174.12.4017-4025.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Black D S, Bliska J B. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 1997;16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bliska J B. Crystal structure of the Yersinia tyrosine phosphatase. Trends Microbiol. 1995;3:125–127. doi: 10.1016/S0966-842X(00)88898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bliska J B, Black D S. Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect Immun. 1995;63:681–685. doi: 10.1128/iai.63.2.681-685.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bliska J B, Clemens J C, Dixon J E, Falkow S. The Yersinia tyrosine phosphatase: specificity of a bacterial virulence determinant for phosphoproteins in the J774A.1 macrophage. J Exp Med. 1992;176:1625–1630. doi: 10.1084/jem.176.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bliska J B, Copass M C, Falkow S. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect Immun. 1993;61:3914–3921. doi: 10.1128/iai.61.9.3914-3921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bliska J B, Guan K L, Dixon J E, Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci USA. 1991;88:1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogdanove A J, Beer S V, Bonas U, Boucher C A, Collmer A, Coplin D L, Cornelis G R, Huang H C, Hutcheson S W, Panopoulos N J, Van Gijsegem F. Unified nomenclature for broadly conserved hrp genes of phytopathogenic bacteria. Mol Microbiol. 1996;20:681–683. doi: 10.1046/j.1365-2958.1996.5731077.x. [DOI] [PubMed] [Google Scholar]
- 38.Bogdanove A J, Wei Z M, Zhao L, Beer S V. Erwinia amylovora secretes harpin via a type III pathway and contains a homolog of yopN of Yersinia spp. J Bacteriol. 1996;178:1720–1730. doi: 10.1128/jb.178.6.1720-1730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boland A, Cornelis G R. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect Immun. 1998;66:1878–1884. doi: 10.1128/iai.66.5.1878-1884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boland, A., S. Havaux, and G. R. Cornelis. Heterogeneity of the Yersinia YopM protein. Microb. Pathog., in press. [DOI] [PubMed]
- 41.Boland A, Sory M P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 42.Bölin I, Forsberg A, Norlander L, Skurnik M, Wolf-Watz H. Identification and mapping of the temperature-inducible, plasmid-encoded proteins of Yersinia spp. Infect Immun. 1988;56:343–348. doi: 10.1128/iai.56.2.343-348.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bölin I, Norlander L, Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982;37:506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bölin I, Portnoy D A, Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985;48:234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bölin I, Wolf-Watz H. Molecular cloning of the temperature-inducible outer membrane protein 1 of Yersinia pseudotuberculosis. Infect Immun. 1984;43:72–78. doi: 10.1128/iai.43.1.72-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bölin I, Wolf-Watz H. The plasmid encoded Yop2b protein of Yersinia pseudotuberculosis is a virulence determinant regulated by calcium and temperature at the level of transcription. Mol Microbiol. 1988;2:237–245. doi: 10.1111/j.1365-2958.1988.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 47.Boyd, A. P. 1998. Unpublished data.
- 48.Boyd A P, Sory M-P, Iriarte M, Cornelis G R. Heparin interferes with translocation of Yop proteins into HeLa cells and binds to LcrG, a regulatory component of the Yersinia Yop apparatus. Mol Microbiol. 1998;27:425–436. doi: 10.1046/j.1365-2958.1998.00691.x. [DOI] [PubMed] [Google Scholar]
- 49.Brown K L, Hughes K T. The role of anti-sigma factors in gene regulation. Mol Microbiol. 1995;16:397–404. doi: 10.1111/j.1365-2958.1995.tb02405.x. [DOI] [PubMed] [Google Scholar]
- 50.Brubaker, R. R. 1983. The Vwa+ virulence factor of yersiniae: the molecular basis of the attendant nutritional requirement for Ca++. Rev. Infect. Dis. 5(Suppl. 4):S748–S758. [DOI] [PubMed]
- 51.Brubaker R R. Factors promoting acute and chronic diseases caused by yersiniae. Clin Microbiol Rev. 1991;4:309–324. doi: 10.1128/cmr.4.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bukholm G, Kapperud G, Skurnik M. Genetic evidence that the yopA gene-encoded Yersinia outer membrane protein Yop1 mediates inhibition of the anti-invasive effect of interferon. Infect Immun. 1990;58:2245–2251. doi: 10.1128/iai.58.7.2245-2251.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52a.Burdack S, Schmidt A, Knieschies E, Rollinghoff M, Beuscher H U. Tumor necrosis factor-alpha expression induced by anti-YopB antibodies coincides with protection against Yersinia enterocolitica infection in mice. Med Microbiol Immunol. 1997;185:223–229. doi: 10.1007/s004300050034. [DOI] [PubMed] [Google Scholar]
- 53.Burrows T W, Bacon G A. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br J Exp Pathol. 1956;37:481–493. [PMC free article] [PubMed] [Google Scholar]
- 54.Caron E, Gross A, Liautard J P, Dornand J. Brucella species release a specific, protease-sensitive, inhibitor of TNF-α expression, active on human macrophage-like cells. J Immunol. 1996;156:2885–2893. [PubMed] [Google Scholar]
- 55.Caron E, Peyrard T, Kohler S, Cabane S, Liautard J P, Dornand J. Live Brucella spp. fail to induce tumor necrosis factor alpha excretion upon infection of U937-derived phagocytes. Infect Immun. 1994;62:5267–5274. doi: 10.1128/iai.62.12.5267-5274.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carpenter P B, Ordal G W. Bacillus subtilis FlhA: a flagellar protein related to a new family of signal-transducing receptors. Mol Microbiol. 1993;7:735–743. doi: 10.1111/j.1365-2958.1993.tb01164.x. [DOI] [PubMed] [Google Scholar]
- 57.Carpenter P B, Zuberi A R, Ordal G W. Bacillus subtilis flagellar proteins FliP, FliQ, FliR and FlhB are related to Shigella flexneri virulence factors. Gene. 1993;137:243–245. doi: 10.1016/0378-1119(93)90014-t. [DOI] [PubMed] [Google Scholar]
- 58.Cavanaugh D C, Randall R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J Immunol. 1959;83:348–363. [PubMed] [Google Scholar]
- 59.Charnetzky W T, Shuford W W. Survival and growth of Yersinia pestis within macrophages and an effect of the loss of the 47-megadalton plasmid on growth in macrophages. Infect Immun. 1985;47:234–241. doi: 10.1128/iai.47.1.234-241.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen L M, Kaniga K, Galan J E. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 61.Chen L Y, Chen D Y, Miaw J, Hu N T. XpsD, an outer membrane protein required for protein secretion by Xanthomonas campestris pv. campestris, forms a multimer. J Biol Chem. 1996;271:2703–2708. doi: 10.1074/jbc.271.5.2703. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y, Smith M R, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng L W, Anderson D M, Schneewind O. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol Microbiol. 1997;24:757–765. doi: 10.1046/j.1365-2958.1997.3831750.x. [DOI] [PubMed] [Google Scholar]
- 64.China B, Michiels T, Cornelis G R. The pYV plasmid of Yersinia encodes a lipoprotein, YlpA, related to TraT. Mol Microbiol. 1990;4:1585–1593. doi: 10.1111/j.1365-2958.1990.tb02070.x. [DOI] [PubMed] [Google Scholar]
- 65.China B, N’Guyen B T, de Bruyere M, Cornelis G R. Role of YadA in resistance of Yersinia enterocolitica to phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1994;62:1275–1281. doi: 10.1128/iai.62.4.1275-1281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.China B, Sory M P, N’Guyen B T, de Bruyere M, Cornelis G R. Role of the YadA protein in prevention of opsonization of Yersinia enterocolitica by C3b molecules. Infect Immun. 1993;61:3129–3136. doi: 10.1128/iai.61.8.3129-3136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cole S P, Guiney D G, Corbeil L B. Molecular analysis of a gene encoding a serum-resistance-associated 76 kDa surface antigen of Haemophilus somnus. J Gen Microbiol. 1993;139:2135–2143. doi: 10.1099/00221287-139-9-2135. [DOI] [PubMed] [Google Scholar]
- 68.Collazo C M, Galan J E. Requirement for exported proteins in secretion through the invasion-associated type III system of Salmonella typhimurium. Infect Immun. 1996;64:3524–3531. doi: 10.1128/iai.64.9.3524-3531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collazo C M, Zierler M K, Galan J E. Functional analysis of the Salmonella typhimurium invasion genes invI and invJ and identification of a target of the protein secretion apparatus encoded in the inv locus. Mol Microbiol. 1995;15:25–38. doi: 10.1111/j.1365-2958.1995.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 69a.Cornelis G R. Yersiniae, finely tuned pathogens. In: Hormaeche C E, Penn C W, Smyth C J, editors. Molecular biology of bacterial infection: current status and future perspectives. Cambridge, England: Cambridge University Press and Society for General Microbiology Ltd; 1992. pp. 231–265. [Google Scholar]
- 70.Cornelis G R, Iriarte M, Sory M P. Environmental control of virulence functions and signal transduction in Yersinia enterocolitica. In: Rappuoli R, Scarlato V, Arico B, editors. Signal transduction and bacterial virulence. R. G. Austin, Tex: Landes Co.; 1995. pp. 95–110. [Google Scholar]
- 71.Cornelis G R, Sluiters C, Delor I, Geib D, Kaniga K, Lambert de Rouvroit C L, Sory M P, Vanooteghem J C, Michiels T. ymoA, a Yersinia enterocolitica chromosomal gene modulating the expression of virulence functions. Mol Microbiol. 1991;5:1023–1034. doi: 10.1111/j.1365-2958.1991.tb01875.x. [DOI] [PubMed] [Google Scholar]
- 72.Cornelis G R, Sluiters C, Lambert de Rouvroit C L, Michiels T. Homology between virF, the transcriptional activator of the Yersinia virulence regulon, and AraC, the Escherichia coli arabinose operon regulator. J Bacteriol. 1989;171:254–262. doi: 10.1128/jb.171.1.254-262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cornelis G R, Sory M P, Laroche Y, Derclaye I. Genetic analysis of the plasmid region controlling virulence in Yersinia enterocolitica O:9 by Mini-Mu insertions and lac gene fusions. Microb Pathog. 1986;1:349–359. doi: 10.1016/0882-4010(86)90067-7. [DOI] [PubMed] [Google Scholar]
- 74.Cornelis G R, Vanooteghem J C, Sluiters C. Transcription of the yop regulon from Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb Pathog. 1987;2:367–379. doi: 10.1016/0882-4010(87)90078-7. [DOI] [PubMed] [Google Scholar]
- 75.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 76.Cover T L, Aber R C. Yersinia enterocolitica. N Engl J Med. 1989;321:16–24. doi: 10.1056/NEJM198907063210104. [DOI] [PubMed] [Google Scholar]
- 77.De Marco L, Mazzucato M, Masotti A, Ruggeri Z M. Localization and characterization of an α-thrombin-binding site on platelet glycoprotein Ibα. J Biol Chem. 1994;269:6478–6484. [PubMed] [Google Scholar]
- 78.Demuth A, Goebel W, Beuscher H U, Kuhn M. Differential regulation of cytokine and cytokine receptor mRNA expression upon infection of bone marrow-derived macrophages with Listeria monocytogenes. Infect Immun. 1996;64:3475–3483. doi: 10.1128/iai.64.9.3475-3483.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Descoteaux A, Matlashewski G. c-fos and tumor necrosis factor gene expression in Leishmania donovani-infected macrophages. Mol Cell Biol. 1989;9:5223–5227. doi: 10.1128/mcb.9.11.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dorman C J. The VirF protein from Shigella flexneri is a member of the AraC transcription factor superfamily and is highly homologous to Rns, a positive regulator of virulence genes in enterotoxigenic Escherichia coli. Mol Microbiol. 1992;6:1575. doi: 10.1111/j.1365-2958.1992.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 81.Dorman C J, Ni Bhriain N. DNA topology and bacterial virulence gene regulation. Trends Microbiol. 1993;1:92–99. doi: 10.1016/0966-842x(93)90114-7. [DOI] [PubMed] [Google Scholar]
- 82.Easmon C S, Cole P J, Williams A J, Hastings M. The measurement of opsonic and phagocytic function by luminol-dependent chemiluminescence. Immunology. 1980;41:67–74. [PMC free article] [PubMed] [Google Scholar]
- 83.Eichelberg K, Ginocchio C C, Galan J E. Molecular and functional characterization of the Salmonella typhimurium invasion genes invB and invC: homology of InvC to the F0F1 ATPase family of proteins. J Bacteriol. 1994;176:4501–4510. doi: 10.1128/jb.176.15.4501-4510.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- 85.Emödy L, Heesemann J, Wolf-Watz H, Skurnik M, Kapperud G, O’Toole P, Wadstrom T. Binding to collagen by Yersinia enterocolitica and Yersinia pseudotuberculosis: evidence for yopA-mediated and chromosomally encoded mechanisms. J Bacteriol. 1989;171:6674–6679. doi: 10.1128/jb.171.12.6674-6679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ewald J H, Heesemann J, Rudiger H, Autenrieth I B. Interaction of polymorphonuclear leukocytes with Yersinia enterocolitica: role of the Yersinia virulence plasmid and modulation by the iron-chelator desferrioxamine B. J Infect Dis. 1994;170:140–150. doi: 10.1093/infdis/170.1.140. [DOI] [PubMed] [Google Scholar]
- 87.Fällman M, Andersson K, Håkansson S, Magnusson K E, Stendahl O, Wolf-Watz H. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect Immun. 1995;63:3117–3124. doi: 10.1128/iai.63.8.3117-3124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fallman M, Persson C, Wolf-Watz H. Yersinia proteins that target host cell signaling pathways. J Clin Invest. 1997;99:1153–1157. doi: 10.1172/JCI119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fauconnier A, Allaoui A, Campos A, Van Elsen A, Cornelis G R, Bollen A. Flagellar flhA, flhB and flhE genes, organized in an operon, cluster upstream from the inv locus in Yersinia enterocolitica. Microbiology. 1997;143:3461–3471. doi: 10.1099/00221287-143-11-3461. [DOI] [PubMed] [Google Scholar]
- 90.Felmlee T, Welch R A. Alterations of amino acid repeats in the Escherichia coli hemolysin affect cytolytic activity and secretion. Proc Natl Acad Sci USA. 1988;85:5269–5273. doi: 10.1073/pnas.85.14.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fenselau S, Balbo I, Bonas U. Determinants of pathogenicity in Xanthomonas campestris pv. vesicatoria are related to proteins involved in secretion in bacterial pathogens of animals. Mol Plant-Microbe Interact. 1992;5:390–396. doi: 10.1094/mpmi-5-390. [DOI] [PubMed] [Google Scholar]
- 92.Fenselau S, Bonas U. Sequence and expression analysis of the hrpB pathogenicity operon of Xanthomonas campestris pv. vesicatoria which encodes eight proteins with similarity to components of the Hrp, Ysc, Spa, and Fli secretion systems. Mol Plant-Microbe Interact. 1995;8:845–854. doi: 10.1094/mpmi-8-0845. [DOI] [PubMed] [Google Scholar]
- 93.Fields K A, Plano G V, Straley S C. A low-Ca2+ response (LCR) secretion (ysc) locus lies within the lcrB region of the LCR plasmid in Yersinia pestis. J Bacteriol. 1994;176:569–579. doi: 10.1128/jb.176.3.569-579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 95.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Flugel A, Schulze-Koops H, Heesemann J, Kühn K, Sorokin L, Burkhardt H, von der Mark K, Emmrich F. Interaction of enteropathogenic Yersinia enterocolitica with complex basement membranes and the extracellular matrix proteins collagen type IV, laminin-1 and -2, and nidogen/entactin. J Biol Chem. 1994;269:29732–29738. [PubMed] [Google Scholar]
- 97.Forsberg A, Bolin I, Norlander L, Wolf-Watz H. Molecular cloning and expression of calcium-regulated, plasmid-coded proteins of Y. pseudotuberculosis. Microb Pathog. 1987;2:123–137. doi: 10.1016/0882-4010(87)90104-5. [DOI] [PubMed] [Google Scholar]
- 98.Forsberg A, Rosqvist R, Wolf-Watz H. Regulation and polarized transfer of the Yersinia outer proteins (Yops) involved in antiphagocytosis. Trends Microbiol. 1994;2:14–19. doi: 10.1016/0966-842x(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 99.Forsberg A, Viitanen A M, Skurnik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 100.Forsberg A, Wolf-Watz H. The virulence protein Yop5 of Yersinia pseudotuberculosis is regulated at transcriptional level by plasmid-plB1-encoded trans-acting elements controlled by temperature and calcium. Mol Microbiol. 1988;2:121–133. doi: 10.1111/j.1365-2958.1988.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 101.Forsberg A, Wolf-Watz H. Genetic analysis of the yopE region of Yersinia spp.: identification of a novel conserved locus, yerA, regulating yopE expression. J Bacteriol. 1990;172:1547–1555. doi: 10.1128/jb.172.3.1547-1555.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frank D W. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol Microbiol. 1997;26:621–629. doi: 10.1046/j.1365-2958.1997.6251991.x. [DOI] [PubMed] [Google Scholar]
- 103.Frank D W, Iglewski B H. Cloning and sequence analysis of a trans-regulatory locus required for exoenzyme S synthesis in Pseudomonas aeruginosa. J Bacteriol. 1991;173:6460–6468. doi: 10.1128/jb.173.20.6460-6468.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Freiberg C, Fellay R, Bairoch A, Broughton W J, Rosenthal A, Perret X. Molecular basis of symbiosis between Rhizobium and legumes. Nature. 1997;387:394–401. doi: 10.1038/387394a0. [DOI] [PubMed] [Google Scholar]
- 105.Frithz-Lindsten E, Du Y, Rosqvist R, Forsberg A. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol Microbiol. 1997;25:1125–1139. doi: 10.1046/j.1365-2958.1997.5411905.x. [DOI] [PubMed] [Google Scholar]
- 106.Frithz-Lindsten E, Rosqvist R, Johansson L, Forsberg A. The chaperone-like protein YerA of Yersinia pseudotuberculosis stabilizes YopE in the cytoplasm but is dispensible for targeting to the secretion loci. Mol Microbiol. 1995;16:635–647. doi: 10.1111/j.1365-2958.1995.tb02426.x. [DOI] [PubMed] [Google Scholar]
- 107.Galan J E, Bliska J B. Cross-talk between bacterial pathogens and their host cells. Annu Rev Cell Biol. 1996;12:221–255. doi: 10.1146/annurev.cellbio.12.1.221. [DOI] [PubMed] [Google Scholar]
- 108.Galan J E, Ginocchio C C, Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol. 1992;174:4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gallegos M T, Schleif R, Bairoch A, Hofmann K, Ramos J L. AraC/XylS family of transcriptional regulators. Microbiol Mol Biol Rev. 1997;61:393–410. doi: 10.1128/mmbr.61.4.393-410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Galyov E E, Håkansson S, Forsberg A, Wolf-Watz H. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature. 1993;361:730–732. doi: 10.1038/361730a0. [DOI] [PubMed] [Google Scholar]
- 111.Galyov E E, Håkansson S, Wolf-Watz H. Characterization of the operon encoding the YpkA Ser/Thr protein kinase and the YopJ protein of Yersinia pseudotuberculosis. J Bacteriol. 1994;176:4543–4548. doi: 10.1128/jb.176.15.4543-4548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gemski P, Lazere J R, Casey T, Wohlhieter J A. Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect Immun. 1980;28:1044–1047. doi: 10.1128/iai.28.3.1044-1047.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Genin S, Boucher C A. A superfamily of proteins involved in different secretion pathways in gram-negative bacteria: modular structure and specificity of the N-terminal domain. Mol Gen Genet. 1994;243:112–118. doi: 10.1007/BF00283883. [DOI] [PubMed] [Google Scholar]
- 114.Genin S, Gough C L, Zischek C, Boucher C A. Evidence that the hrpB gene encodes a positive regulator of pathogenicity genes from Pseudomonas solanacearum. Mol Microbiol. 1992;6:3065–3076. doi: 10.1111/j.1365-2958.1992.tb01764.x. [DOI] [PubMed] [Google Scholar]
- 115.Ginocchio C C, Galan J E. Functional conservation among members of the Salmonella typhimurium InvA family of proteins. Infect Immun. 1995;63:729–732. doi: 10.1128/iai.63.2.729-732.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ginocchio C C, Olmsted S B, Wells C L, Galan J E. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell. 1994;76:717–724. doi: 10.1016/0092-8674(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 117.Ginocchio C C, Pace J, Galan J E. Identification and molecular characterization of a Salmonella typhimurium gene involved in triggering the internalization of salmonellae into cultured epithelial cells. Proc Natl Acad Sci USA. 1992;89:5976–5980. doi: 10.1073/pnas.89.13.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Glaser P, Sakamoto H, Bellalou J, Ullmann A, Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988;7:3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goguen J D, Walker W S, Hatch T P, Yother J. Plasmid-determined cytotoxicity in Yersinia pestis and Yersinia pseudotuberculosis. Infect Immun. 1986;51:788–794. doi: 10.1128/iai.51.3.788-794.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goguen J D, Yother J, Straley S C. Genetic analysis of the low calcium response in Yersinia pestis mu d1(Ap lac) insertion mutants. J Bacteriol. 1984;160:842–848. doi: 10.1128/jb.160.3.842-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gomez-Duarte O G, Kaper J B. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect Immun. 1995;63:1767–1776. doi: 10.1128/iai.63.5.1767-1776.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gooding L R. Virus proteins that counteract host immune defenses. Cell. 1992;71:5–7. doi: 10.1016/0092-8674(92)90259-f. [DOI] [PubMed] [Google Scholar]
- 122a.Goranson J, Hovey A K, Frank D W. Functional analysis of exsC and exsB in regulation of exoenzyme S production by Pseudomonas aeruginosa. J Bacteriol. 1997;179:1646–1654. doi: 10.1128/jb.179.5.1646-1654.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goransson M, Sonden B, Nilsson P, Dagberg B, Forsman K, Emanuelsson K, Uhlin B E. Transcriptional silencing and thermoregulation of gene expression in Escherichia coli. Nature. 1990;344:682–685. doi: 10.1038/344682a0. [DOI] [PubMed] [Google Scholar]
- 124.Gough C L, Genin S, Lopes V, Boucher C A. Homology between the HrpO protein of Pseudomonas solanacearum and bacterial proteins implicated in a signal peptide-independent secretion mechanism. Mol Gen Genet. 1993;239:378–392. doi: 10.1007/BF00276936. [DOI] [PubMed] [Google Scholar]
- 125.Gralnick H R, Williams S, McKeown L P, Hansmann K, Fenton II J W, Krutzsch H. High-affinity α-thrombin binding to platelet glycoprotein Ibα: identification of two binding domains. Proc Natl Acad Sci USA. 1994;91:6334–6338. doi: 10.1073/pnas.91.14.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grebner J V, Mills E L, Gray G H, Quie P G. Comparison of phagocytic and chemiluminescence response of human polymorphonuclear neutrophils. J Lab Clin Med. 1977;89:153–159. [PubMed] [Google Scholar]
- 127.Green S P, Hamilton J A, Phillips W A. Zymosan-triggered tyrosine phosphorylation in mouse bone-marrow-derived macrophages is enhanced by respiratory-burst priming agents. Biochem J. 1992;288:427–432. doi: 10.1042/bj2880427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Green S P, Hartland E L, Robins Browne R M, Phillips W A. Role of YopH in the suppression of tyrosine phosphorylation and respiratory burst activity in murine macrophages infected with Yersinia enterocolitica. J Leukoc Biol. 1995;57:972–977. doi: 10.1002/jlb.57.6.972. [DOI] [PubMed] [Google Scholar]
- 129.Greenberg S, Chang P, Silverstein S C. Tyrosine phosphorylation of the γ subunit of Fcγ receptors, p72syk, and paxillin during Fc receptor-mediated phagocytosis in macrophages. J Biol Chem. 1994;269:3897–3902. [PubMed] [Google Scholar]
- 130.Groisman E A, Ochman H. Cognate gene clusters govern invasion of host epithelial cells by Salmonella typhimurium and Shigella flexneri. EMBO J. 1993;12:3779–3787. doi: 10.1002/j.1460-2075.1993.tb06056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grutzkau A, Hanski C, Hahn H, Riecken E O. Involvement of M cells in the bacterial invasion of Peyer’s patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut. 1990;31:1011–1015. doi: 10.1136/gut.31.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guan K L, Dixon J E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990;249:553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- 133.Guan K L, Dixon J E. Evidence for protein-tyrosine-phosphatase catalysis proceeding via a cysteine-phosphate intermediate. J Biol Chem. 1991;266:17026–17030. [PubMed] [Google Scholar]
- 134.Haddix P L, Straley S C. Structure and regulation of the Yersinia pestis yscBCDEF operon. J Bacteriol. 1992;174:4820–4828. doi: 10.1128/jb.174.14.4820-4828.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Haimovich B, Regan C, DiFazio L, Ginalis E, Ji P, Purohit U, Rowley R B, Bolen J, Greco R. The FcγRII receptor triggers pp125FAK phosphorylation in platelets. J Biol Chem. 1996;271:16332–16337. doi: 10.1074/jbc.271.27.16332. [DOI] [PubMed] [Google Scholar]
- 136.Håkansson S, Bergman T, Vanooteghem J C, Cornelis G, Wolf-Watz H. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect Immun. 1993;61:71–80. doi: 10.1128/iai.61.1.71-80.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Håkansson S, Galyov E E, Rosqvist R, Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 138.Håkansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homblé F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 139.Han Y W, Miller V L. Reevaluation of the virulence phenotype of the inv yadA double mutants of Yersinia pseudotuberculosis. Infect Immun. 1997;65:327–330. doi: 10.1128/iai.65.1.327-330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hanski C, Kutschka U, Schmoranzer H P, Naumann M, Stallmach A, Hahn H, Menge H, Riecken E O. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O:8 with intestinal mucosa during experimental enteritis. Infect Immun. 1989;57:673–678. doi: 10.1128/iai.57.3.673-678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hanski C, Naumann M, Grutzkau A, Pluschke G, Friedrich B, Hahn H, Riecken E O. Humoral and cellular defense against intestinal murine infection with Yersinia enterocolitica. Infect Immun. 1991;59:1106–1111. doi: 10.1128/iai.59.3.1106-1111.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hardie K R, Lory S, Pugsley A P. Insertion of an outer membrane protein in Escherichia coli requires a chaperone-like protein. EMBO J. 1996;15:978–988. [PMC free article] [PubMed] [Google Scholar]
- 143.Hardie K R, Seydel A, Guilvout I, Pugsley A P. The secretin-specific, chaperone-like protein of the general secretory pathway: separation of proteolytic protection and piloting functions. Mol Microbiol. 1996;22:967–976. doi: 10.1046/j.1365-2958.1996.01539.x. [DOI] [PubMed] [Google Scholar]
- 144.Hardt W-D, Galan J E. A secreted Salmonella protein with homology to an avirulence determinant of plant pathogenic bacteria. Proc Natl Acad Sci USA. 1997;94:9887–9892. doi: 10.1073/pnas.94.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reference deleted.
- 146.Hartland E L, Bordun A M, Robins Browne R M. Contribution of YopB to virulence of Yersinia enterocolitica. Infect Immun. 1996;64:2308–2314. doi: 10.1128/iai.64.6.2308-2314.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hartland E L, Green S P, Phillips W A, Robins Browne R M. Essential role of YopD in inhibition of the respiratory burst of macrophages by Yersinia enterocolitica. Infect Immun. 1994;62:4445–4453. doi: 10.1128/iai.62.10.4445-4453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hartman A B, Venkatesan M, Oaks E V, Buysse J M. Sequence and molecular characterization of a multicopy invasion plasmid antigen gene, ipaH, of Shigella flexneri. J Bacteriol. 1990;172:1905–1915. doi: 10.1128/jb.172.4.1905-1915.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Heesemann J, Algermissen B, Laufs R. Genetically manipulated virulence of Yersinia enterocolitica. Infect Immun. 1984;46:105–110. doi: 10.1128/iai.46.1.105-110.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Heesemann J, Eggers C, Schröder J. Serological diagnosis of yersiniosis by immunoblot technique using virulence-associated antigen of enteropathogenic yersiniae. Contrib Microbiol Immunol. 1987;9:285–289. [PubMed] [Google Scholar]
- 151.Heesemann J, Gross U, Schmidt N, Laufs R. Immunochemical analysis of plasmid-encoded proteins released by enteropathogenic Yersinia sp. grown in calcium-deficient media. Infect Immun. 1986;54:561–567. doi: 10.1128/iai.54.2.561-567.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heesemann J, Grüter L. Genetic evidence that the outer membrane protein Yop1 of Yersinia enterocolitica mediates adherence and phagocytosis resistance to human epithelial cells. FEMS Microbiol Lett. 1987;40:37–41. [Google Scholar]
- 153.Heesemann J, Laufs R. Double immunofluorescence microscopic technique for accurate differentiation of extracellularly and intracellularly located bacteria in cell culture. J Clin Microbiol. 1985;22:168–175. doi: 10.1128/jcm.22.2.168-175.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hensel M, Shea J E, Raupach B, Monack D M, Falkow S, Gleeson C, Kubo T, Holden D W. Functional analysis of ssaJ and the ssaK/U operon, 13 genes encoding components of the type III secretion apparatus of Salmonella Pathogenicity Island 2. Mol Microbiol. 1997;24:155–167. doi: 10.1046/j.1365-2958.1997.3271699.x. [DOI] [PubMed] [Google Scholar]
- 155.Higashi T, Sasai H, Suzuki F, Miyoshi J, Ohuchi T, Taikai S, Mori T, Kakunaga T. Hamster cell line suitable for transfection assay of transforming genes. Proc Natl Acad Sci USA. 1990;87:2409–2412. doi: 10.1073/pnas.87.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Higgins C F, Dorman C J, Stirling D A, Waddell L, Booth I R, May G, Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988;52:569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- 157.Higuchi K, Smith J L. Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J Bacteriol. 1961;81:605–608. doi: 10.1128/jb.81.4.605-608.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hilbi H, Chen Y, Thirumalai K, Zychlinsky A. The interleukin 1β-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect Immun. 1997;65:5165–5170. doi: 10.1128/iai.65.12.5165-5170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hoe N P, Goguen J D. Temperature sensing in Yersinia pestis: translation of the LcrF activator protein is thermally regulated. J Bacteriol. 1993;175:7901–7909. doi: 10.1128/jb.175.24.7901-7909.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hoe N P, Minion F C, Goguen J D. Temperature sensing in Yersinia pestis: regulation of yopE transcription by lcrF. J Bacteriol. 1992;174:4275–4286. doi: 10.1128/jb.174.13.4275-4286.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Holmström A, Pettersson J, Rosqvist R, Håkansson S, Tafazoli F, Fallman M, Magnusson K E, Wolf-Watz H, Forsberg A. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 162.Holmström A, Rosqvist R, Wolf-Watz H, Forsberg A. Virulence plasmid-encoded YopK is essential for Yersinia pseudotuberculosis to cause systemic infection in mice. Infect Immun. 1995;63:2269–2276. doi: 10.1128/iai.63.6.2269-2276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hoover D L, Friedlander A M, Rogers L C, Yoon I K, Warren R L, Cross A S. Anthrax edema toxin differentially regulates lipopolysaccharide-induced monocyte production of tumor necrosis factor alpha and interleukin-6 by increasing intracellular cyclic AMP. Infect Immun. 1994;62:4432–4439. doi: 10.1128/iai.62.10.4432-4439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hovey A K, Frank D W. Analyses of the DNA-binding and transcriptional activation properties of ExsA, the transcriptional activator of the Pseudomonas aeruginosa exoenzyme S regulon. J Bacteriol. 1995;177:4427–4436. doi: 10.1128/jb.177.15.4427-4436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hsia R, Pannekoek Y, Ingerowski E, Bavoil P M. Type III secretion genes identify a putative virulence locus of Chlamydia. Mol Microbiol. 1997;25:351–359. doi: 10.1046/j.1365-2958.1997.4701834.x. [DOI] [PubMed] [Google Scholar]
- 165a.Hu P, Elliott J, McCready P, Skowronski E, Garnes J, Kobayashi A, Carrano A V, Brubaker R, Garcia E. Yersinia pestis plasmid pCD1, complete plasmid sequence. 1998. GenBank accession no. AF053946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Huang H C, He S Y, Bauer D W, Collmer A. The Pseudomonas syringae pv. syringae 61 hrpH product, an envelope protein required for elicitation of the hypersensitive response in plants. J Bacteriol. 1992;174:6878–6885. doi: 10.1128/jb.174.21.6878-6885.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Huang H C, Lin R H, Chang C J, Collmer A, Deng W L. The complete hrp gene cluster of Pseudomonas syringae pv. syringae 61 includes two blocks of genes required for harpinPss secretion that are arranged colinearly with Yersinia ysc homologs. Mol Plant-Microbe Interact. 1995;8:733–746. doi: 10.1094/mpmi-8-0733. [DOI] [PubMed] [Google Scholar]
- 168.Huang H C, Xiao Y, Lin R H, Lu Y, Hutcheson S W, Collmer A. Characterization of the Pseudomonas syringae pv. syringae 61 hrpJ and hrpI genes: homology of HrpI to a superfamily of proteins associated with protein translocation. Mol Plant-Microbe Interact. 1993;6:515–520. doi: 10.1094/mpmi-6-515. [DOI] [PubMed] [Google Scholar]
- 169.Hughes K T, Gillen K L, Semon M J, Karlinsey J E. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262:1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- 170.Iriarte M, Cornelis G R. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol Microbiol. 1998;29:915–929. doi: 10.1046/j.1365-2958.1998.00992.x. [DOI] [PubMed] [Google Scholar]
- 170a.Iriarte, M., and G. R. Cornelis. Assignment of SycN, YscX, and YscY, three new elements of the Yersinia Yop virulon. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 171.Iriarte, M., I. Lambermont, C. Kerbourch, and G. R. Cornelis. The complete sequence of the Yersinia enterocolitica virulence plasmid. A global view of a type III virulence apparatus archetype. Submitted for publication.
- 172.Iriarte M, Sory M P, Boland A, Boyd A P, Mills S D, Lambermont I, Cornelis G R. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 1998;17:1907–1918. doi: 10.1093/emboj/17.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Iriarte M, Stainier I, Cornelis G R. The rpoS gene from Yersinia enterocolitica and its influence on expression of virulence factors. Infect Immun. 1995;63:1840–1847. doi: 10.1128/iai.63.5.1840-1847.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Iriarte M, Stainier I, Mikulskis A V, Cornelis G R. The fliA gene encoding ς28 in Yersinia enterocolitica. J Bacteriol. 1995;177:2299–2304. doi: 10.1128/jb.177.9.2299-2304.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Iriarte M, Vanooteghem J C, Delor I, Diaz R, Knutton S, Cornelis G R. The Myf fibrillae of Yersinia enterocolitica. Mol Microbiol. 1993;9:507–520. doi: 10.1111/j.1365-2958.1993.tb01712.x. [DOI] [PubMed] [Google Scholar]
- 176.Isberg R R, Leong J M. Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 177.Isberg R R, Voorhis D L, Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987;50:769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- 178.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jerse A E, Yu J, Tall B D, Kaper J B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jordi B J, Dagberg B, de Haan L A, Hamers A M, van der Zeijst B A, Gaastra W, Uhlin B E. The positive regulator CfaD overcomes the repression mediated by histone-like protein H-NS (H1) in the CFA/I fimbrial operon of Escherichia coli. EMBO J. 1992;11:2627–2632. doi: 10.1002/j.1460-2075.1992.tb05328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jung H C, Eckmann L, Yang S K, Panja A, Fierer J, Morzycka Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kaniga K, Bossio J C, Galan J E. The Salmonella typhimurium invasion genes invF and invG encode homologues of the AraC and PulD family of proteins. Mol Microbiol. 1994;13:555–568. doi: 10.1111/j.1365-2958.1994.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 183.Kaniga K, Tucker S, Trollinger D, Galan J E. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella entry into host cells. J Bacteriol. 1995;177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kaniga K, Uralil J, Bliska J B, Galan J E. A secreted protein tyrosine phosphatase with modular effector domains in the bacterial pathogen Salmonella typhimurium. Mol Microbiol. 1996;21:633–641. doi: 10.1111/j.1365-2958.1996.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 185.Kapatral V, Minnich S A. Co-ordinate, temperature-sensitive regulation of the three Yersinia enterocolitica flagellin genes. Mol Microbiol. 1995;17:49–56. doi: 10.1111/j.1365-2958.1995.mmi_17010049.x. [DOI] [PubMed] [Google Scholar]
- 186.Kapatral V, Olson J W, Pepe J C, Miller V L, Minnich S A. Temperature-dependent regulation of Yersinia enterocolitica class III flagellar genes. Mol Microbiol. 1996;19:1061–1071. doi: 10.1046/j.1365-2958.1996.452978.x. [DOI] [PubMed] [Google Scholar]
- 187.Kapperud G, Namork E, Skarpeid H J. Temperature-inducible surface fibrillae associated with the virulence plasmid of Yersinia enterocolitica and Yersinia pseudotuberculosis. Infect Immun. 1985;47:561–566. doi: 10.1128/iai.47.2.561-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kapperud G, Namork E, Skurnik M, Nesbakken T. Plasmid-mediated surface fibrillae of Yersinia pseudotuberculosis and Yersinia enterocolitica: relationship to the outer membrane protein YOP1 and possible importance for pathogenesis. Infect Immun. 1987;55:2247–2254. doi: 10.1128/iai.55.9.2247-2254.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 190.Kenny B, Finlay B B. Secretion of proteins by enteropathogenic E. coli which mediate signaling in host epithelial cells. Proc Natl Acad Sci USA. 1995;97:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Khisty V J, Munske G R, Randall L L. Mapping of the binding frame for the chaperone SecB within a natural ligand, galactose-binding protein. J Biol Chem. 1995;270:25920–25927. doi: 10.1074/jbc.270.43.25920. [DOI] [PubMed] [Google Scholar]
- 192.Kihara M, Homma M, Kutsukake K, Macnab R M. Flagellar switch of Salmonella typhimurium: gene sequences and deduced protein sequences. J Bacteriol. 1989;171:3247–3257. doi: 10.1128/jb.171.6.3247-3257.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kobe B, Deisenhofer J. The leucine-rich repeat: a versatile binding motif. Trends Biochem Sci. 1994;19:415–420. doi: 10.1016/0968-0004(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 194.Koster M, Bitter W, de Cock H, Allaoui A, Cornelis G R, Tommassen J. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol Microbiol. 1997;26:789–798. doi: 10.1046/j.1365-2958.1997.6141981.x. [DOI] [PubMed] [Google Scholar]
- 194a.Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, Sukhan A, Galan J E, Aizawa S-I. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 195.Kulich S M, Yahr T L, Mende-Mueller L M, Barbieri J T, Frank D W. Cloning the structural gene for the 49-kDa form of exoenzyme S (exoS) from Pseudomonas aeruginosa strain 388. J Biol Chem. 1994;269:10431–10437. [PubMed] [Google Scholar]
- 196.Kumamoto C A, Beckwith J. Evidence for specificity at an early step in protein export in Escherichia coli. J Bacteriol. 1985;163:267–274. doi: 10.1128/jb.163.1.267-274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kutsukake K. Excretion of the anti-ς factor through a flagellar substructure couples flagellar gene expression with flagellar assembly in Salmonella typhimurium. Mol Gen Genet. 1994;243:605–612. doi: 10.1007/BF00279569. [DOI] [PubMed] [Google Scholar]
- 198.Lachica R V, Zink D L. Determination of plasmid-associated hydrophobicity of Yersinia enterocolitica by a latex particle agglutination test. J Clin Microbiol. 1984;19:660–663. doi: 10.1128/jcm.19.5.660-663.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lambert de Rouvroit C L, Sluiters C, Cornelis G R. Role of the transcriptional activator, VirF, and temperature in the expression of the pYV plasmid genes of Yersinia enterocolitica. Mol Microbiol. 1992;6:395–409. [PubMed] [Google Scholar]
- 200.Laroche Y, Van Bouchaute M, Cornelis G. A restriction map of virulence plasmid pVYE439-80 from a serogroup 9 Yersinia enterocolitica strain. Plasmid. 1984;12:67–70. doi: 10.1016/0147-619x(84)90069-6. [DOI] [PubMed] [Google Scholar]
- 201.Lee C A. Type III secretion systems: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol. 1997;5:148–156. doi: 10.1016/S0966-842X(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 202.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 203.Lee J C, Young P R. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukoc Biol. 1996;59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- 204.Lee L A, Taylor J, Carter G P, Quinn B, Farmer III J J, Tauxe R V. Yersinia enterocolitica O:3: an emerging cause of pediatric gastroenteritis in the United States. J Infect Dis. 1991;163:660–663. doi: 10.1093/infdis/163.3.660. [DOI] [PubMed] [Google Scholar]
- 204a.Lee V T, Anderson D M, Schneewind O. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol Microbiol. 1998;28:593–601. doi: 10.1046/j.1365-2958.1998.00822.x. [DOI] [PubMed] [Google Scholar]
- 205.Lehrer R I, Rosenman M, Harwig S S, Jackson R, Eisenhauer P. Ultrasensitive assays for endogenous antimicrobial polypeptides. J Immunol Methods. 1991;137:167–173. doi: 10.1016/0022-1759(91)90021-7. [DOI] [PubMed] [Google Scholar]
- 206.Leong J M, Morrissey P E, Marra A, Isberg R R. An aspartate residue of the Yersinia pseudotuberculosis invasin protein that is critical for integrin binding. EMBO J. 1995;14:422–431. doi: 10.1002/j.1460-2075.1995.tb07018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Leung K Y, Reisner B S, Straley S C. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect Immun. 1990;58:3262–3271. doi: 10.1128/iai.58.10.3262-3271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Leung K Y, Straley S C. The yopM gene of Yersinia pestis encodes a released protein having homology with the human platelet surface protein GPIb α. J Bacteriol. 1989;171:4623–4632. doi: 10.1128/jb.171.9.4623-4632.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lian C J, Hwang W S, Kelly J K, Pai C H. Invasiveness of Yersinia enterocolitica lacking the virulence plasmid: an in-vivo study. J Med Microbiol. 1987;24:219–226. doi: 10.1099/00222615-24-3-219. [DOI] [PubMed] [Google Scholar]
- 210.Lian C J, Hwang W S, Pai C H. Plasmid-mediated resistance to phagocytosis in Yersinia enterocolitica. Infect Immun. 1987;55:1176–1183. doi: 10.1128/iai.55.5.1176-1183.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lian C J, Pai C H. Inhibition of human neutrophil chemiluminescence by plasmid-mediated outer membrane proteins of Yersinia enterocolitica. Infect Immun. 1985;49:145–151. doi: 10.1128/iai.49.1.145-151.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lidell M C, Hutcheson S W. Characterization of the hrpJ and hrpU operons of Pseudomonas syringae pv. syringae Pss61: similarity with components of enteric bacteria involved in flagellar biogenesis and demonstration of their role in HarpinPss secretion. Mol Plant-Microbe Interact. 1994;7:488–497. doi: 10.1094/mpmi-7-0488. [DOI] [PubMed] [Google Scholar]
- 212a.Linderoth N A, Model P, Russel M. Essential role of a sodium dodecyl sulfate-resistant protein IV multimer in assembly-export of filamentous phage. J Bacteriol. 1996;178:1962–1970. doi: 10.1128/jb.178.7.1962-1970.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212b.Linderoth N A, Simon M N, Russel M. The filamentous phage pIV multimer visualized by scanning transmission electron microscopy. Science. 1997;278:1635–1638. doi: 10.1126/science.278.5343.1635. [DOI] [PubMed] [Google Scholar]
- 213.Lindler L E, Tall B D. Yersinia pestis pH6 antigen forms fimbriae and is induced by intracellular association with macrophages. Mol Microbiol. 1993;8:311–324. doi: 10.1111/j.1365-2958.1993.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 214.Lindler L E, Klempner M S, Straley S C. Yersinia pestis pH6 antigen: genetic, biochemical, and virulence characterization of a protein involved in pathogenesis in bubonic plague. Infect Immun. 1990;58:2569–2577. doi: 10.1128/iai.58.8.2569-2577.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Liu Z G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 216.Lodish H, Baltimore D, Berk A, Zipursky S L, Matsudaira P, Darnel J E. In integrative and specialized cellular activities. In: Darnel J E, editor. Molecular cell biology. New York, N.Y: Scientific American Books Inc.; 1995. pp. 850–1342. [Google Scholar]
- 217.Lupas A, van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 218.Malakooti J, Ely B, Matsumura P. Molecular characterization, nucleotide sequence, and expression of the fliO, fliP, fliQ, and fliR genes of Escherichia coli. J Bacteriol. 1994;176:189–197. doi: 10.1128/jb.176.1.189-197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Malakooti J, Komeda Y, Matsumura P. DNA sequence analysis, gene product identification, and localization of flagellar motor components of Escherichia coli. J Bacteriol. 1989;171:2728–2734. doi: 10.1128/jb.171.5.2728-2734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Martinez R J. Plasmid-mediated and temperature-regulated surface properties of Yersinia enterocolitica. Infect Immun. 1983;41:921–930. doi: 10.1128/iai.41.3.921-930.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Martinez R J. Thermoregulation-dependent expression of Yersinia enterocolitica protein 1 imparts serum resistance to Escherichia coli K-12. J Bacteriol. 1989;171:3732–3739. doi: 10.1128/jb.171.7.3732-3739.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Meinhardt L W, Krishnan H B, Balatti P A, Pueppke S G. Molecular cloning and characterization of a sym plasmid locus that regulates cultivar-specific nodulation of soybean by Rhizobium fredii USDA257. Mol Microbiol. 1993;9:17–29. doi: 10.1111/j.1365-2958.1993.tb01665.x. [DOI] [PubMed] [Google Scholar]
- 223.Menard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Menard R, Sansonetti P J, Parsot C. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 1994;13:5293–5302. doi: 10.1002/j.1460-2075.1994.tb06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Menard R, Sansonetti P J, Parsot C, Vasselon T. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell. 1994;79:515–525. doi: 10.1016/0092-8674(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 226.Michiels T, Cornelis G. Nucleotide sequence and transcription analysis of yop51 from Yersinia enterocolitica W22703. Microb Pathog. 1988;5:449–459. doi: 10.1016/0882-4010(88)90006-x. [DOI] [PubMed] [Google Scholar]
- 227.Michiels T, Cornelis G R. Secretion of hybrid proteins by the Yersinia Yop export system. J Bacteriol. 1991;173:1677–1685. doi: 10.1128/jb.173.5.1677-1685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Michiels T, Vanooteghem J C, Lambert de Rouvroit C L, China B, Gustin A, Boudry P, Cornelis G R. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J Bacteriol. 1991;173:4994–5009. doi: 10.1128/jb.173.16.4994-5009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Michiels T, Wattiau P, Brasseur R, Ruysschaert J M, Cornelis G. Secretion of Yop proteins by yersiniae. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mikulskis A V, Cornelis G R. A new class of proteins regulating gene expression in enterobacteria. Mol Microbiol. 1994;11:77–86. doi: 10.1111/j.1365-2958.1994.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 231.Miller S I, Pesci E C, Pickett C L. A Campylobacter jejuni homolog of the LcrD/FlbF family of proteins is necessary for flagellar biogenesis. Infect Immun. 1993;61:2930–2936. doi: 10.1128/iai.61.7.2930-2936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mills S D, Boland A, Sory M P, Van der Smissen P, Kerbourch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Minamino T, Iino T, Kutuskake K. Molecular characterization of the Salmonella typhimurium flhB operon and its protein products. J Bacteriol. 1994;176:7630–7637. doi: 10.1128/jb.176.24.7630-7637.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mittler R, Lam E. Sacrifice in the face of foes: pathogen-induced programmed cell death in plants. Trends Microbiol. 1996;4:10–15. doi: 10.1016/0966-842x(96)81499-5. [DOI] [PubMed] [Google Scholar]
- 235.Moll A, Manning P A, Timmis K N. Plasmid-determined resistance to serum bacterial activity: a major outer membrane protein, the traT gene product, is responsible for plasmid-specified serum resistance in Escherichia coli. Infect Immun. 1980;28:359–367. doi: 10.1128/iai.28.2.359-367.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Monack, D. M. Unpublished results.
- 237.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mulder B, Michiels T, Simonet M, Sory M P, Cornelis G. Identification of additional virulence determinants on the pYV plasmid of Yersinia enterocolitica W227. Infect Immun. 1989;57:2534–2541. doi: 10.1128/iai.57.8.2534-2541.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nakajima R, Brubaker R R. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993;61:23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Nakajima R, Motin V L, Brubaker R R. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect Immun. 1995;63:3021–3029. doi: 10.1128/iai.63.8.3021-3029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Newhall W J, Wilde C E, Sawyer W D, Haak R A. High-molecular-weight antigenic protein complex in the outer membrane of Neisseria gonorrhoeae. Infect Immun. 1980;27:475–482. doi: 10.1128/iai.27.2.475-482.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Neyt, C., and G. R. Cornelis. Role of SycD, the chaperone of the Yersinia Yop translocators YopB and YopD. Mol. Microbiol., in press. [DOI] [PubMed]
- 244.Neyt C, Iriarte M, Ha Thi V, Cornelis G R. Virulence and arsenic resistance in yersiniae. J Bacteriol. 1997;179:612–619. doi: 10.1128/jb.179.3.612-619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Nickerson C A, Curtiss R., III Role of sigma factor RpoS in initial stages of Salmonella typhimurium infection. Infect Immun. 1997;65:1814–1823. doi: 10.1128/iai.65.5.1814-1823.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Nieto J M, Carmona M, Bolland S, Jubete Y, de la Cruz F, Juarez A. The hha gene modulates haemolysin expression in Escherichia coli. Mol Microbiol. 1991;5:1285–1293. doi: 10.1111/j.1365-2958.1991.tb01902.x. [DOI] [PubMed] [Google Scholar]
- 246a.Nilles M L, Fields K A, Straley S C. The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG. J Bacteriol. 1998;180:3410–3420. doi: 10.1128/jb.180.13.3410-3420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Nilles M L, Williams A W, Skrzypek E, Straley S C. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J Bacteriol. 1997;179:1307–1316. doi: 10.1128/jb.179.4.1307-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ohnishi K, Ohto Y, Aizawa S I, Macnab R M, Iino T. FlgD is a scaffolding protein needed for flagellar hook assembly in Salmonella typhimurium. J Bacteriol. 1994;176:2272–2281. doi: 10.1128/jb.176.8.2272-2281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pærregaard A, Espersen F, Jensen O M, Skurnik M. Interactions between Yersinia enterocolitica and rabbit ileal mucus: growth, adhesion, penetration, and subsequent changes in surface hydrophobicity and ability to adhere to ileal brush border membrane vesicles. Infect Immun. 1991;59:253–260. doi: 10.1128/iai.59.1.253-260.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Pærregaard A, Espersen F, Skurnik M. Role of the Yersinia outer membrane protein YadA in adhesion to rabbit intestinal tissue and rabbit intestinal brush border membrane vesicles. APMIS. 1991;99:226–232. doi: 10.1111/j.1699-0463.1991.tb05143.x. [DOI] [PubMed] [Google Scholar]
- 251.Palmer L E, Hobbie S, Galan J E, Bliska J B. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNFα production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 252.Payne P L, Straley S C. YscO of Yersinia pestis is a mobile core component of the Yop secretion system. J Bacteriol. 1998;180:3882–3890. doi: 10.1128/jb.180.15.3882-3890.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252a.Payne, P. L., and S. C. Straley. YscP of Yersinia pestis is a secreted component of the Yop secretion system. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 253.Pegues D A, Hantman M J, Behlau I, Miller S I. PhoP/PhoQ transcriptional repression of Salmonella typhimurium invasion genes: evidence for a role in protein secretion. Mol Microbiol. 1995;17:169–181. doi: 10.1111/j.1365-2958.1995.mmi_17010169.x. [DOI] [PubMed] [Google Scholar]
- 254.Pepe J C, Wachtel M R, Wagar E, Miller V L. Pathogenesis of defined invasion mutants of Yersinia enterocolitica in a BALB/c mouse model of infection. Infect Immun. 1995;63:4837–4848. doi: 10.1128/iai.63.12.4837-4848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Perry R D, Fetherston J D. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Perry R D, Haddix P L, Atkins E B, Soughers T K, Straley S C. Regulation of expression of V antigen and outer membrane proteins in Yersinia pestis. Contrib Microbiol Immunol. 1987;9:173–178. [PubMed] [Google Scholar]
- 257.Perry R D, Harmon P A, Bowmer W S, Straley S C. A low-Ca2+ response operon encodes the V antigen of Yersinia pestis. Infect Immun. 1986;54:428–434. doi: 10.1128/iai.54.2.428-434.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257a.Perry R D, Straley S C, Fetherston J D, Rose D J, Gregor J, Blattner F R. DNA sequencing and analysis of the low-Ca2+ response plasmid pCD1 of Yersinia pestis KIM5. Infect Immun. 1998;66:4611–4623. doi: 10.1128/iai.66.10.4611-4623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Persson C, Carballeira N, Wolf-Watz H, Fällman M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Persson C, Nordfelth R, Holmström A, Håkansson S, Rosqvist R, Wolf-Watz H. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol Microbiol. 1995;18:135–150. doi: 10.1111/j.1365-2958.1995.mmi_18010135.x. [DOI] [PubMed] [Google Scholar]
- 260.Petruzzelli L, Takami M, Herrera R. Adhesion through the interaction of lymphocyte function-associated antigen-1 with intracellular adhesion molecule-1 induces tyrosine phosphorylation of p130cas and its association with c-CrkII. J Biol Chem. 1996;271:7796–7801. doi: 10.1074/jbc.271.13.7796. [DOI] [PubMed] [Google Scholar]
- 261.Pettersson J, Nordfelth R, Dubinina E, Bergman T, Gustafsson M, Magnusson K E, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 262.Pilz D, Vocke T, Heesemann J, Brade V. Mechanism of YadA-mediated serum resistance of Yersinia enterocolitica serotype O3. Infect Immun. 1992;60:189–195. doi: 10.1128/iai.60.1.189-195.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Plano G V, Barve S S, Straley S C. LcrD, a membrane-bound regulator of the Yersinia pestis low-calcium response. J Bacteriol. 1991;173:7293–7303. doi: 10.1128/jb.173.22.7293-7303.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Plano G V, Straley S C. Multiple effects of lcrD mutations in Yersinia pestis. J Bacteriol. 1993;175:3536–3545. doi: 10.1128/jb.175.11.3536-3545.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Plano G V, Straley S C. Mutations in yscC, yscD, and yscG prevent high-level expression and secretion of V antigen and Yops in Yersinia pestis. J Bacteriol. 1995;177:3843–3854. doi: 10.1128/jb.177.13.3843-3854.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Portnoy D A, Falkow S. Virulence-associated plasmids from Yersinia enterocolitica and Yersinia pestis. J Bacteriol. 1981;148:877–883. doi: 10.1128/jb.148.3.877-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Portnoy D A, Moseley S L, Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981;31:775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Portnoy D A, Wolf-Watz H, Bolin I, Beeder A B, Falkow S. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984;43:108–114. doi: 10.1128/iai.43.1.108-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Price S B, Cowan C, Perry R D, Straley S C. The Yersinia pestis V antigen is a regulatory protein necessary for Ca2+-dependent growth and maximal expression of low-Ca2+ response virulence genes. J Bacteriol. 1991;173:2649–2657. doi: 10.1128/jb.173.8.2649-2657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Price S B, Leung K Y, Barve S S, Straley S C. Molecular analysis of lcrGVH, the V antigen operon of Yersinia pestis. J Bacteriol. 1989;171:5646–5653. doi: 10.1128/jb.171.10.5646-5653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Price S B, Straley S C. lcrH, a gene necessary for virulence of Yersinia pestis and for the normal response of Y. pestis to ATP and calcium. Infect Immun. 1989;57:1491–1498. doi: 10.1128/iai.57.5.1491-1498.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ramakrishnan G, Zhao J L, Newton A. The cell cycle-regulated flagellar gene flbF of Caulobacter crescentus is homologous to a virulence locus (lcrD) of Yersinia pestis. J Bacteriol. 1991;173:7283–7292. doi: 10.1128/jb.173.22.7283-7292.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Reimann T, Buscher D, Hipskind R A, Krautwald S, Lohmann Matthes M L, Baccarini M. Lipopolysaccharide induces activation of the Raf-1/MAP kinase pathway. A putative role for Raf-1 in the induction of the IL-1β and the TNF-α genes. J Immunol. 1994;153:5740–5749. [PubMed] [Google Scholar]
- 274.Reisner B S, Straley S C. Yersinia pestis YopM: thrombin binding and overexpression. Infect Immun. 1992;60:5242–5252. doi: 10.1128/iai.60.12.5242-5252.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Rimpiläinen M, Forsberg A, Wolf-Watz H. A novel protein, LcrQ, involved in the low-calcium response of Yersinia pseudotuberculosis shows extensive homology to YopH. J Bacteriol. 1992;174:3355–3363. doi: 10.1128/jb.174.10.3355-3363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Roggenkamp A, Geiger A M, Leitritz L, Kessler A, Heesemann J. Passive immunity to infection with Yersinia spp. mediated by anti-recombinant V antigen is dependent on polymorphism of V antigen. Infect Immun. 1997;65:446–451. doi: 10.1128/iai.65.2.446-451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Roggenkamp A, Neuberger H-R, Flügel A, Schmoll T, Heesemann J. Substitution of two histidine residues in YadA protein of Yersinia enterocolitica abrogates collagen binding, cell adherence and mouse virulence. Mol Microbiol. 1995;16:1207–1219. doi: 10.1111/j.1365-2958.1995.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 278.Roggenkamp A, Ruckdeschel K, Leitritz L, Schmitt R, Heesemann J. Deletion of amino acids 29 to 81 in adhesion protein YadA of Yersinia enterocolitica serotype O:8 results in selective abrogation of adherence to neutrophils. Infect Immun. 1996;64:2506–2514. doi: 10.1128/iai.64.7.2506-2514.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Rohde J R, Fox J M, Minnich S A. Thermoregulation in Yersinia enterocolitica is coincident with changes in DNA supercoiling. Mol Microbiol. 1994;12:187–199. doi: 10.1111/j.1365-2958.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 280.Rose D M, Winston B W, Chan E D, Riches D W, Gerwins P, Johnson G L, Henson P M. Fc γ receptor cross-linking activates p42, p38, and JNK/SAPK mitogen-activated protein kinases in murine macrophages: role for p42MAPK in Fc γ receptor-stimulated TNF-α synthesis. J Immunol. 1997;158:3433–3438. [PubMed] [Google Scholar]
- 281.Rosqvist R, Bölin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rosqvist R, Forsberg A, Rimpiläinen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 283.Rosqvist R, Forsberg A, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Rosqvist R, Håkansson S, Forsberg A, Wolf-Watz H. Functional conservation of the secretion and translocation machinery for virulence proteins of yersiniae, salmonellae and shigellae. EMBO J. 1995;14:4187–4195. doi: 10.1002/j.1460-2075.1995.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Rosqvist R, Skurnik M, Wolf-Watz H. Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature. 1988;334:522–525. doi: 10.1038/334522a0. [DOI] [PubMed] [Google Scholar]
- 287.Rosqvist R, Wolf-Watz H. Virulence plasmid-associated HeLa cell induced cytotoxicity of Yersinia pseudotuberculosis. Microb Pathog. 1986;1:229–240. doi: 10.1016/0882-4010(86)90047-1. [DOI] [PubMed] [Google Scholar]
- 288.Ruckdeschel K, Harb S, Roggenkamp A, Hornef M, Zumbihl R, Kohler S, Heesemann J, Rouot B. Yersinia enterocolitica impairs activation of transcription factor NF-κB: involvement in the induction of programmed cell death and in the suppression of the macrophage TNF-α production. J Exp Med. 1998;187:1069–1079. doi: 10.1084/jem.187.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ruckdeschel K, Machold J, Roggenkamps A, Schubert S, Pierre J, Zumbihl R, Liautard J P, Heesemann J, Rouot B. Yersinia enterocolitica promotes deactivation of macrophage mitogen-activated protein kinases extracellular signal-regulated kinase-1/2, p38, and c-Jun NH2-terminal kinase. J Biol Chem. 1997;272:15920–15927. doi: 10.1074/jbc.272.25.15920. [DOI] [PubMed] [Google Scholar]
- 290.Ruckdeschel K, Roggenkamp A, Lafont V, Mangeat P, Heesemann J, Rouot B. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect Immun. 1997;65:4813–4821. doi: 10.1128/iai.65.11.4813-4821.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ruckdeschel K, Roggenkamp A, Schubert S, Heesemann J. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect Immun. 1996;64:724–733. doi: 10.1128/iai.64.3.724-733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Russel M. Phage assembly: a paradigm for bacterial virulence factor export? Science. 1994;265:612–614. doi: 10.1126/science.8036510. [DOI] [PubMed] [Google Scholar]
- 293.Sanders L A, Van Way S, Mullin D A. Characterization of the Caulobacter crescentus flbF promoter and identification of the inferred FlbF product as a homolog of the LcrD protein from a Yersinia enterocolitica virulence plasmid. J Bacteriol. 1992;174:857–866. doi: 10.1128/jb.174.3.857-866.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sarker M R, Neyt C, Stainier I, Cornelis G R. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J Bacteriol. 1998;180:1207–1214. doi: 10.1128/jb.180.5.1207-1214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sarker M R, Sory M-P, Boyd A P, Iriarte M, Cornelis G R. LcrG is required for efficient translocation of Yersinia Yop effector proteins into eukaryotic cells. Infect Immun. 1998;66:2976–2979. doi: 10.1128/iai.66.6.2976-2979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Sarker, M. R., and G. R. Cornelis. Unpublished data.
- 297.Sarmento A M, Appelberg R. Relationship between virulence of Mycobacterium avium strains and induction of tumor necrosis factor alpha production in infected mice and in in vitro-cultured mouse macrophages. Infect Immun. 1995;63:3759–3764. doi: 10.1128/iai.63.10.3759-3764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Sasakawa C, Adler B, Tobe T, Okada N, Nagai S, Yoshikawa M. Functional organization and nucleotide sequence of virulence region-2 on the large virulence plasmid in Shigella flexneri 2a. Mol Microbiol. 1989;3:1191–1201. doi: 10.1111/j.1365-2958.1989.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 299.Sasakawa C, Komatsu K, Tobe T, Suzuki T, Yoshikawa M. Eight genes in region 5 that form an operon are essential for invasion of epithelial cells by Shigella flexneri 2a. J Bacteriol. 1993;175:2334–2346. doi: 10.1128/jb.175.8.2334-2346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Schesser K, Frithz-Lindsten E, Wolf-Watz H. Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J Bacteriol. 1996;178:7227–7233. doi: 10.1128/jb.178.24.7227-7233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Reference deleted.
- 302.Schmitz A, Josenhans C, Suerbaum S. Cloning and characterization of the Helicobacter pylori flbA gene, which codes for a membrane protein involved in coordinated expression of flagellar genes. J Bacteriol. 1997;179:987–997. doi: 10.1128/jb.179.4.987-997.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Schulte R, Wattiau P, Hartland E L, Robins Browne R M, Cornelis G R. Differential secretion of interleukin-8 by human epithelial cell lines upon entry of virulent or nonvirulent Yersinia enterocolitica. Infect Immun. 1996;64:2106–2113. doi: 10.1128/iai.64.6.2106-2113.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Schulze-Koops H, Burkhardt H, Heesemann J, Kirsch T, Swoboda B, Bull C, Goodman S, Emmrich F. Outer membrane protein YadA of enteropathogenic yersiniae mediates specific binding to cellular but not plasma fibronectin. Infect Immun. 1993;61:2513–2519. doi: 10.1128/iai.61.6.2513-2519.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Schulze-Koops H, Burkhardt H, Heesemann J, von der Mark K, Emmrich F. Plasmid-encoded outer membrane protein YadA mediates specific binding of enteropathogenic yersiniae to various types of collagen. Infect Immun. 1992;60:2153–2159. doi: 10.1128/iai.60.6.2153-2159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Shea J E, Hensel M, Gleeson C, Holden D W. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Shevchik V, Robert-Baudouy J, Condemine G. Specific interaction between OutD, an Erwinia chrysanthemi outer membrane protein of the general secretory pathway, and secreted proteins. EMBO J. 1997;16:3007–3016. doi: 10.1093/emboj/16.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Shi L, Mai S, Israels S, Browne K, Trapani J A, Greenberg A H. Granzyme B (GraB) autonomously crosses the cell membrane and perforin initiates apoptosis and GraB nuclear localization. J Exp Med. 1997;185:855–866. doi: 10.1084/jem.185.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Simonet M, Richard S, Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990;58:841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Simonet M, Riot B, Fortineau N, Berche P. Invasin production by Yersinia pestis is abolished by insertion of an IS200-like element within the inv gene. Infect Immun. 1996;64:375–379. doi: 10.1128/iai.64.1.375-379.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Skrzypek E, Straley S C. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J Bacteriol. 1993;175:3520–3528. doi: 10.1128/jb.175.11.3520-3528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Skrzypek E, Straley S C. Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J Bacteriol. 1995;177:2530–2542. doi: 10.1128/jb.177.9.2530-2542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Skurnik M, Bolin I, Heikkinen H, Piha S, Wolf-Watz H. Virulence plasmid-associated autoagglutination in Yersinia spp. J Bacteriol. 1984;158:1033–1036. doi: 10.1128/jb.158.3.1033-1036.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Skurnik M, el Tahir Y, Saarinen M, Jalkanen S, Toivanen P. YadA mediates specific binding of enteropathogenic Yersinia enterocolitica to human intestinal submucosa. Infect Immun. 1994;62:1252–1261. doi: 10.1128/iai.62.4.1252-1261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Skurnik M, Toivanen P. LcrF is the temperature-regulated activator of the yadA gene of Yersinia enterocolitica and Yersinia pseudotuberculosis. J Bacteriol. 1992;174:2047–2051. doi: 10.1128/jb.174.6.2047-2051.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Skurnik M, Wolf-Watz H. Analysis of the yopA gene encoding the Yop1 virulence determinants of Yersinia spp. Mol Microbiol. 1989;3:517–529. doi: 10.1111/j.1365-2958.1989.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 317.Smith G L. Virus strategies for evasion of the host response to infection. Trends Microbiol. 1994;2:81–88. doi: 10.1016/0966-842x(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 318.Sodeinde O A, Sample A K, Brubaker R R, Goguen J D. Plasminogen activator/coagulase gene of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect Immun. 1988;56:2749–2752. doi: 10.1128/iai.56.10.2749-2752.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Sodeinde O A, Goguen J D. Genetic analysis of the 9.5-kilobase virulence plasmid of Yersinia pestis. Infect Immun. 1988;56:2743–2748. doi: 10.1128/iai.56.10.2743-2748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Sory M P, Boland A, Lambermont I, Cornelis G R. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci USA. 1995;92:11998–12002. doi: 10.1073/pnas.92.26.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Sory M P, Cornelis G R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 322.Sory M P, Hermand P, Vaerman J P, Cornelis G R. Oral immunization of mice with a live recombinant Yersinia enterocolitica O:9 strain that produces the cholera toxin B subunit. Infect Immun. 1990;58:2420–2428. doi: 10.1128/iai.58.8.2420-2428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Sory M P, Kaniga K, Goldenberg S, Cornelis G R. Expression of the eukaryotic Trypanosoma cruzi CRA gene in Yersinia enterocolitica and induction of an immune response against CRA in mice. Infect Immun. 1992;60:3830–3836. doi: 10.1128/iai.60.9.3830-3836.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Sory, M. P., C. Kerbourch, and G. R. Cornelis. Unpublished data.
- 325.Stahlberg T H, Granfors K, Toivanen A. Immunoblot analysis of human IgM, IgG, and IgA responses to plasmid-encoded antigens of Yersinia enterocolitica serovar O:3. J Med Microbiol. 1987;24:157–163. doi: 10.1099/00222615-24-2-157. [DOI] [PubMed] [Google Scholar]
- 326.Stainier, I., and G. R. Cornelis. Unpublished data.
- 326a.Stainier, I., L. Karmani, and G. R. Cornelis. Unpublished data.
- 327.Stainier I, Iriarte M, Cornelis G R. YscM1 and YscM2, two Yersinia enterocolitica proteins causing down regulation of yop transcription. Mol Microbiol. 1997;26:833–843. doi: 10.1046/j.1365-2958.1997.6281995.x. [DOI] [PubMed] [Google Scholar]
- 328.Starnbach M N, Bevan M J. Cells infected with Yersinia present an epitope to class I MHC-restricted CTL. J Immunol. 1994;153:1603–1612. [PMC free article] [PubMed] [Google Scholar]
- 329.Straley, S. C. 1988. The plasmid-encoded outer-membrane proteins of Yersinia pestis. Rev. Infect. Dis. 10(Suppl. 2):S323–S326. [DOI] [PubMed]
- 330.Straley S C, Bowmer W S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986;51:445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Straley S C, Brubaker R R. Cytoplasmic and membrane proteins of yersiniae cultivated under conditions simulating mammalian intracellular environment. Proc Natl Acad Sci USA. 1981;78:1224–1228. doi: 10.1073/pnas.78.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Straley S C, Cibull M L. Differential clearance and host-pathogen interactions of YopE− and YopK− YopL−Yersinia pestis in BALB/c mice. Infect Immun. 1989;57:1200–1210. doi: 10.1128/iai.57.4.1200-1210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Straley S C, Harmon P A. Yersinia pestis grows within phagolysosomes in mouse peritoneal macrophages. Infect Immun. 1984;45:655–659. doi: 10.1128/iai.45.3.655-659.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Straley S C, Harmon P A. Growth in mouse peritoneal macrophages of Yersinia pestis lacking established virulence determinants. Infect Immun. 1984;45:649–654. doi: 10.1128/iai.45.3.649-654.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Straley S C, Perry R D. Environmental modulation of gene expression and pathogenesis in Yersinia. Trends Microbiol. 1995;3:310–317. doi: 10.1016/s0966-842x(00)88960-x. [DOI] [PubMed] [Google Scholar]
- 336.Straley S C, Plano G V, Skrzypek E, Haddix P L, Fields K A. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol Microbiol. 1993;8:1005–1010. doi: 10.1111/j.1365-2958.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 337.Straley S C, Skrzypek E, Plano G V, Bliska J B. Yops of Yersinia spp. pathogenic for humans. Infect Immun. 1993;61:3105–3110. doi: 10.1128/iai.61.8.3105-3110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Strathdee C A, Lo R Y. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989;171:916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Stuckey J A, Schubert H L, Fauman E B, Zhang Z Y, Dixon J E, Saper M A. Crystal structure of Yersinia protein tyrosine phosphatase at 2.5 A and the complex with tungstate. Nature. 1994;370:571–575. doi: 10.1038/370571a0. [DOI] [PubMed] [Google Scholar]
- 340.Su X D, Taddei N, Stefani M, Ramponi G, Nordlund P. The crystal structure of a low-molecular-weight phosphotyrosine protein phosphatase. Nature. 1994;370:575–578. doi: 10.1038/370575a0. [DOI] [PubMed] [Google Scholar]
- 341.Taglialatela G, Robinson R, Perez Polo J R. Inhibition of nuclear factor kappa B (NFkappaB) activity induces nerve growth factor-resistant apoptosis in PC12 cells. J Neurosci Res. 1997;47:155–162. [PubMed] [Google Scholar]
- 342.Tamm A, Tarkkanen A M, Korhonen T K, Kuusela P, Toivanen P, Skurnik M. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol Microbiol. 1993;10:995–1011. doi: 10.1111/j.1365-2958.1993.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 343.Tauxe R V, Vandepitte J, Wauters G, Martin S M, Goossens V, De Mol P, Van Noyen R, Thiers G. Yersinia enterocolitica infections and pork: the missing link. Lancet. 1987;ii:1129–1132. doi: 10.1016/s0140-6736(87)91683-7. [DOI] [PubMed] [Google Scholar]
- 344.Tertti R, Eerola E, Lehtonen O P, Stahlberg T H, Viander M, Toivanen A. Virulence-plasmid is associated with the inhibition of opsonization in Yersinia enterocolitica and Yersinia pseudotuberculosis. Clin Exp Immunol. 1987;68:266–274. [PMC free article] [PubMed] [Google Scholar]
- 345.Tertti R, Skurnik M, Vartio T, Kuusela P. Adhesion protein YadA of Yersinia species mediates binding of bacteria to fibronectin. Infect Immun. 1992;60:3021–3024. doi: 10.1128/iai.60.7.3021-3024.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Thirumalai K, Kim K S, Zychlinsky A. IpaB, a Shigella flexneri invasin, colocalizes with interleukin-1β-converting enzyme in the cytoplasm of macrophages. Infect Immun. 1997;65:787–793. doi: 10.1128/iai.65.2.787-793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Trotta R, Kanakaraj P, Perussia B. Fc γ R-dependent mitogen-activated protein kinase activation in leukocytes: a common signal transduction event necessary for expression of TNF-α and early activation genes. J Exp Med. 1996;184:1027–1035. doi: 10.1084/jem.184.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Valdivia R H, Falkow S. Fluorescence-based isolation of bacterial genes expressed within host cells. Science. 1997;277:2007–2011. doi: 10.1126/science.277.5334.2007. [DOI] [PubMed] [Google Scholar]
- 349.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 350.Van Antwerp D J, Martin S J, Verma I M, Green D R. Inhibition of TNF-induced apoptosis by NF-κB. Trends Cell Biol. 1998;8:107–111. doi: 10.1016/s0962-8924(97)01215-4. [DOI] [PubMed] [Google Scholar]
- 351.Van den Ackerveken G, Bonas U. Bacterial avirulence proteins as triggers of plant disease resistance. Trends Microbiol. 1997;5:394–398. doi: 10.1016/S0966-842X(97)01124-4. [DOI] [PubMed] [Google Scholar]
- 352.Van Gijsegem F, Genin S, Boucher C. Conservation of secretion pathways for pathogenicity determinants of plant and animal bacteria. Trends Microbiol. 1993;1:175–180. doi: 10.1016/0966-842x(93)90087-8. [DOI] [PubMed] [Google Scholar]
- 353.Van Gijsegem F, Gough C L, Zischek C, Niqueux E, Arlat M, Genin S, Barberis P, German S, Castello P, Boucher C. The hrp gene locus of Pseudomonas solanacearum, which controls the production of a type III secretion system, encodes eight proteins related to components of the bacterial flagellar biogenesis complex. Mol Microbiol. 1995;15:1095–1114. doi: 10.1111/j.1365-2958.1995.tb02284.x. [DOI] [PubMed] [Google Scholar]
- 354.Vanooteghem J-C, Cornelis G R. Structural and functional similarities between the replication region of the Yersinia virulence plasmid and the RepFIIA replicons. J Bacteriol. 1990;172:3600–3608. doi: 10.1128/jb.172.7.3600-3608.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Vanooteghem, J.-C., and G. R. Cornelis. Unpublished observation.
- 356.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 357.Venkatesan M M, Buysse J M, Oaks E V. Surface presentation of Shigella flexneri invasion plasmid antigens requires the products of the spa locus. J Bacteriol. 1992;174:1990–2001. doi: 10.1128/jb.174.6.1990-2001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Vesikari T, Nurmi T, Maki M, Skurnik M, Sundqvist C, Granfors K, Gronroos P. Plasmids in Yersinia enterocolitica serotypes O:3 and O:9: correlation with epithelial cell adherence in vitro. Infect Immun. 1981;33:870–876. doi: 10.1128/iai.33.3.870-876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Vesikari T, Sundqvist C, Maki M. Adherence and toxicity of Yersinia enterocolitica O:3 and O:9 containing virulence-associated plasmids for various cultured cells. Acta Pathol Microbiol Immunol Scand Sect B. 1983;91:121–127. doi: 10.1111/j.1699-0463.1983.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 360.Viitanen A M, Toivanen P, Skurnik M. The lcrE gene is part of an operon in the lcr region of Yersinia enterocolitica O:3. J Bacteriol. 1990;172:3152–3162. doi: 10.1128/jb.172.6.3152-3162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Visser L G, Annema A, van Furth R. Role of Yops in inhibition of phagocytosis and killing of opsonized Yersinia enterocolitica by human granulocytes. Infect Immun. 1995;63:2570–2575. doi: 10.1128/iai.63.7.2570-2575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Visser L G, Hiemstra P S, van den Barselaar M T, Ballieux P A, van Furth R. Role of YadA in resistance to killing of Yersinia enterocolitica by antimicrobial polypeptides of human granulocytes. Infect Immun. 1996;64:1653–1658. doi: 10.1128/iai.64.5.1653-1658.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Vogler A P, Homma M, Irikura V M, Macnab R M. Salmonella typhimurium mutants defective in flagellar filament regrowth and sequence similarity of FliI to F0F1, vacuolar, and archaebacterial ATPase subunits. J Bacteriol. 1991;173:3564–3572. doi: 10.1128/jb.173.11.3564-3572.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363a.Wainwright L A, Kaper J B. EspB and EspD require a specific chaperone for proper secretion from enteropathogenic Escherichia coli. Mol Microbiol. 1998;27:1247–1260. doi: 10.1046/j.1365-2958.1998.00771.x. [DOI] [PubMed] [Google Scholar]
- 364.Wang C Y, Mayo M W, Baldwin A S J. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 365.Wattiau P, Bernier B, Deslee P, Michiels T, Cornelis G R. Individual chaperones required for Yop secretion by Yersinia. Proc Natl Acad Sci USA. 1994;91:10493–10497. doi: 10.1073/pnas.91.22.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Wattiau P, Cornelis G R. SycE, a chaperone-like protein of Yersinia enterocolitica involved in the secretion of YopE. Mol Microbiol. 1993;8:123–131. doi: 10.1111/j.1365-2958.1993.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 367.Wattiau P, Cornelis G R. Identification of DNA sequences recognized by VirF, the transcriptional activator of the Yersinia yop regulon. J Bacteriol. 1994;176:3878–3884. doi: 10.1128/jb.176.13.3878-3884.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Wattiau P, Woestyn S, Cornelis G R. Customized secretion chaperones in pathogenic bacteria. Mol Microbiol. 1996;20:255–262. doi: 10.1111/j.1365-2958.1996.tb02614.x. [DOI] [PubMed] [Google Scholar]
- 369.Wauters G. Antigens of Yersinia enterocolitica. In: Bottone E J, editor. Yersinia enterocolitica. Boca Raton, Fla: CRC Press, Inc.; 1981. pp. 41–53. [Google Scholar]
- 370.Wei Z M, Beer S V. HrpI of Erwinia amylovora functions in secretion of harpin and is a member of a new protein family. J Bacteriol. 1993;175:7958–7967. doi: 10.1128/jb.175.24.7958-7967.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Welch R A. Pore-forming cytolysins of gram-negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 372.Whalen M C, Wang J F, Carland F M, Heiskell M E, Dahlbeck D, Minsavage G V, Jones J B, Scott J W, Stall R E, Staskawicz B J. Avirulence gene avrRxv from Xanthomonas campestris pv. vesicatoria specifies resistance on tomato line Hawaii 7998. Mol Plant-Microbe Interact. 1993;6:616–627. doi: 10.1094/mpmi-6-616. [DOI] [PubMed] [Google Scholar]
- 373.Williams A W, Straley S C. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J Bacteriol. 1998;180:350–358. doi: 10.1128/jb.180.2.350-358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Wilmes-Riesenberg M R, Foster J W, Curtiss R., III An altered rpoS allele contributes to the avirulence of Salmonella typhimurium LT2. Infect Immun. 1997;65:203–210. doi: 10.1128/iai.65.1.203-210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Woestyn S, Allaoui A, Wattiau P, Cornelis G R. YscN, the putative energizer of the Yersinia Yop secretion machinery. J Bacteriol. 1994;176:1561–1569. doi: 10.1128/jb.176.6.1561-1569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Woestyn S, Sory M P, Boland A, Lequenne O, Cornelis G R. The cytosolic SycE and SycH chaperones of Yersinia protect the region of YopE and YopH involved in translocation across eukaryotic cell membranes. Mol Microbiol. 1996;20:1261–1271. doi: 10.1111/j.1365-2958.1996.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 377.Wolf-Watz H, Portnoy D A, Bolin I, Falkow S. Transfer of the virulence plasmid of Yersinia pestis to Yersinia pseudotuberculosis. Infect Immun. 1985;48:241–243. doi: 10.1128/iai.48.1.241-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Yahr T L, Goranson J, Frank D W. Exoenzyme S of Pseudomonas aeruginosa secreted by a type III secretion pathway. Mol Microbiol. 1996;22:991–1003. doi: 10.1046/j.1365-2958.1996.01554.x. [DOI] [PubMed] [Google Scholar]
- 379.Yahr T L, Mende-Mueller L M, Friese M B, Frank D W. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J Bacteriol. 1997;179:7165–7168. doi: 10.1128/jb.179.22.7165-7168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Yang Y, Isberg R R. Cellular internalization in the absence of invasin expression is promoted by the Yersinia pseudotuberculosis yadA product. Infect Immun. 1993;61:3907–3913. doi: 10.1128/iai.61.9.3907-3913.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Yang Y, Merriam J J, Mueller J P, Isberg R R. The psa locus is responsible for thermoinducible binding of Yersinia pseudotuberculosis to cultured cells. Infect Immun. 1996;64:2483–2489. doi: 10.1128/iai.64.7.2483-2489.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Yother J, Chamness T W, Goguen J D. Temperature-controlled plasmid regulon associated with low calcium response in Yersinia pestis. J Bacteriol. 1986;165:443–447. doi: 10.1128/jb.165.2.443-447.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Yother J, Goguen J D. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J Bacteriol. 1985;164:704–711. doi: 10.1128/jb.164.2.704-711.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383a.Yuk M H, Harvill E T, Miller J F. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol Microbiol. 1998;28:945–959. doi: 10.1046/j.1365-2958.1998.00850.x. [DOI] [PubMed] [Google Scholar]
- 384.Zaleska M, Lounatmaa K, Nurminen M, Wahlström E, Mäkelä P H. A novel virulence-associated cell surface structure composed of 47-kd protein subunits in Yersinia enterocolitica. EMBO J. 1985;4:1013–1018. doi: 10.1002/j.1460-2075.1985.tb03732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Zhang Z Y. Kinetic and mechanistic characterization of a mammalian protein-tyrosine phosphatase, PTP1. J Biol Chem. 1995;270:11199–11204. doi: 10.1074/jbc.270.19.11199. [DOI] [PubMed] [Google Scholar]
- 386.Zhang Z Y, Clemens J C, Schubert H L, Stuckey J A, Fischer M W F, Hume D M, Saper M A, Dixon J E. Expression, purification, and physicochemical characterization of a recombinant Yersinia protein tyrosine phosphatase. J Biol Chem. 1992;267:23759–23766. [PubMed] [Google Scholar]
- 387.Zhang Z Y, Wang Y, Wu L, Fauman E B, Stuckey J A, Schubert H L, Saper M A, Dixon J E. The Cys(X)5Arg catalytic motif in phosphoester hydrolysis. Biochemistry. 1994;33:15266–15270. doi: 10.1021/bi00255a007. [DOI] [PubMed] [Google Scholar]
- 388.Zheng Z M, Specter S. Dynamic production of tumour necrosis factor-α (TNF-α) messenger RNA, intracellular and extracellular TNF-α by murine macrophages and possible association with protein tyrosine phosphorylation of STAT1α and ERK2 as an early signal. Immunology. 1996;87:544–550. doi: 10.1046/j.1365-2567.1996.513591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Zierler M K, Galan J E. Contact with cultured epithelial cells stimulates secretion of Salmonella typhimurium invasion protein InvJ. Infect Immun. 1995;63:4024–4028. doi: 10.1128/iai.63.10.4024-4028.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Zink D L, Feeley J C, Wells J G, Vanderzant C, Vickery J C, Roof W D, O’Donovan G A. Plasmid-mediated tissue invasiveness in Yersinia enterocolitica. Nature. 1980;283:224–226. doi: 10.1038/283224a0. [DOI] [PubMed] [Google Scholar]
- 391.Zychlinsky, A. Personnal communication.
- 392.Zychlinsky A, Kenny B, Menard R, Prevost M C, Holland I B, Sansonetti P J. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994;11:619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 393.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 394.Zychlinsky A, Sansonetti P J. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 1997;5:201–204. doi: 10.1016/S0966-842X(97)01044-5. [DOI] [PubMed] [Google Scholar]
- 395.Zychlinsky A, Sansonetti P J. Perspectives series: host/pathogen interactions. Apoptosis in bacterial pathogenesis. J Clin Invest. 1997;100:493–495. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]