Abstract
The Burgersdorp Formation of South Africa is a richly fossiliferous rock sequence at the top of the Permian–Triassic Beaufort Group and is known for its abundance of Early–Middle Triassic vertebrate remains, particularly cynodonts. Fossils from the Burgersdorp Formation are referred biostratigraphically to the Cynognathus Assemblage Zone (CAZ), which is further divided into three subzones: Langbergia-Garjainia, Trirachodon-Kannemeyeria, and Cricodon-Ufudocyclops. Each subzone is characterised by the presence of a distinct species of trirachodontid, a group of gomphodont cynodonts found relatively abundantly throughout the CAZ, with the lower two subzones characterised by the medium-sized trirachodontids Langbergia and Trirachodon. The uppermost part of the formation, the Cricodon-Ufudocyclops subzone, yields trirachodontids of larger size. The majority of these trirachodontid specimens have previously been referred to Cricodon metabolus, a taxon also known from the Manda Beds of Tanzania and the Ntawere Formation of Zambia. Here we identify one of the specimens (BP/1/5538) previously referred to Cricodon as a new taxon, Guttigomphus avilionis. Guttigomphus can be distinguished from other gomphodont cynodonts by features of the upper postcanine teeth, such as an asymmetric crown in occlusal view (crown narrower along the lingual margin than the labial). Our phylogenetic analysis recovers Guttigomphus as a basal member of Trirachodontidae, outside of the clade including Cricodon, Langbergia and Trirachodon.
Keywords: Middle Triassic, South Africa, Phylogenetics, Taxonomy, Anatomy, Anisian, Cynodont, Synapsid, Burgersdorp Formation, Trirachodontid
Introduction
The Karoo Supergroup is a highly fossiliferous sequence of rocks that crops out across two thirds of the land area of present-day South Africa and preserves a time span stretching from the Carboniferous to the Early Jurassic (Schluter, 2008). In this span, it encompasses two mass extinctions, providing the best terrestrial record of the end-Permian mass extinction and the subsequent Triassic recovery period. The Permian–Triassic boundary occurs within the Beaufort Group, the most extensive subdivision of the Karoo Supergroup in terms of outcrop area and second most extensive in terms of geological time (since revision of the chronostratigraphy of the Stormberg Group; Bordy et al., 2020). Seven temporally distinct faunal assemblages are now recognized within the Beaufort Group (Smith et al., 2020), the youngest of which is the Cynognathus Assemblage Zone (CAZ). The CAZ has in turn been divided into three subunits. These were initially informally labeled subzones A–C and characterised largely by temnospondyl amphibian and cynodont index taxa (Hancox et al., 1995; Hancox, 1998, 2000). These subunits have now been formalized as the Langbergia-Garjainia (formerly subzone A), Trirachodon-Kannemeyeria (B), and Cricodon-Ufudocyclops (C) subzones (Hancox, Neveling & Rubidge, 2020), with trirachodontid cynodonts as the primary index taxa.
The Karoo Basin provides an excellent record of the early members of Cynodontia, the therapsid subclade that includes mammals. The earliest cynodonts currently known, Abdalodon diastematicus and Charassognathus gracilis, are known from rare remains in the Lopingian-aged (Wuchiapingian) Teekloof Formation (Endothiodon Assemblage Zone) (Botha, Abdala & Smith, 2007; Day et al., 2015; Kammerer, 2016; Day & Smith, 2020). Additional taxa, such as Procynosuchus delaharpeae, Cynosaurus suppostus, and the recently described Vetusodon elikhulu, are known from slightly younger (Wuchiapingian–Changhsingian) strata in the latest Permian Cistecephalus–Daptocephalus assemblage zones (Viglietti et al., 2016; Van den Brandt & Abdala, 2018; Abdala et al., 2019). In the earliest Triassic (Induan), additional cynodont taxa are found, including well-known species such as Galesaurus planiceps (Owen, 1859; Parrington, 1934; Pusch, Kammerer & Fröbisch, 2019) and Thrinaxodon liorhinus (Seeley, 1894; Estes, 1961; Jasinoski, Abdala & Fernandez, 2015).
The CAZ is currently thought to encompass the boundary between the late Lower (Olenekian) and early Middle (Anisian) Triassic (Hancox et al., 1995; although see Ottone et al. (2014) for an alternate proposal) and is characterised by abundant remains of the large-bodied cynognathian cynodonts Cynognathus and Diademodon. General concordance between the biostratigraphical CAZ (originally just “Cynognathus Zone”) and the lithostratigraphical Burgersdorp Formation has long been recognized (e.g., Watson, 1914), but the precise extent of the latter has been subject to some debate. Earlier works (e.g., Keyser & Smith, 1978; Groenewald, Kitching & Rubridge, 1995) considered the lowest parts of the Burgersdorp Formation to fall within the Lystrosaurus Assemblage Zone, with the CAZ corresponding to the rest of the formation. These studies recognised an ‘impoverished zone’ typified by fossil paucity at the boundary between the two assemblage zones (Fig. 1), corresponding to the “Procolophon Zone” of Broom (1906). This area of fossil paucity has also been proposed as an extinction event (Lucas, 1998), reflected in the abrupt faunal turnover existing between the two assemblages (Anderson & Cruickshank, 1978). However, more recent studies (Neveling, 2002, 2004) have revealed typical fossils for the CAZ in the ‘impoverished zone’ (e.g., the temnospondyl Kestrosaurus and trirachodontid cynodonts). Neveling, Hancox & Rubidge (2006) provided updated range data for tetrapod taxa in the ‘impoverished zone’, demonstrating that the entire Burgersdorp Formation correlates with the CAZ.
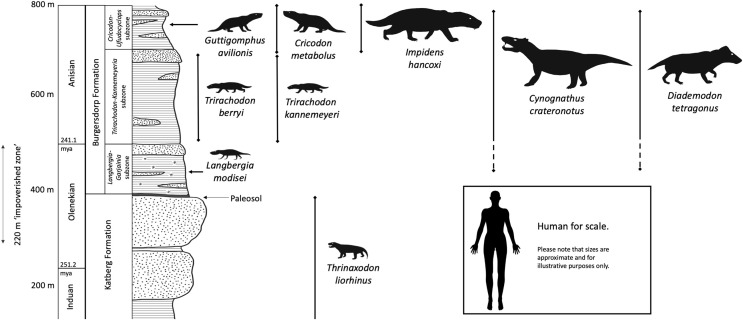
Figure 1. Lithology and ranges of key cynodont taxa from the Burgersdorp Formation and underlying Katberg Formation.
This is a generalized interpretation based on the area around Sterkstroom and modified from the work of Hancox (1998), then updated based on information in Hancox, Neveling & Rubidge (2020). The palaeosol marking the boundary between the upper Katberg and lower Burgersdorp formations is only found across some of the outcrop. Taxa are shown approximately to scale in relation to each other. Silhouettes modified from life reconstructions by Mojca Janezic, Nobu Tamura, and Gabriel Ugueto.
Hancox et al. (1995) initially proposed division of the CAZ into three subzones (A, B, and C), based primarily on the recognition of differences in the temnospondyl fauna. The lowest subzone (A) was characterised by the presence of the temnospondyl Kestrosaurus and also fossils of gomphodont cynodonts and an erythrosuchid archosauriform (later described as Garjainia madiba Gower et al., 2014). The middle subzone (B) is the most extensive (in thickness and area of exposure) and fossiliferous of the three. In addition to the temnospondyl index fossil Xenotosuchus africanus (see Morales & Shishkin, 2002), subzone B yields numerous cynodonts, bauriid therocephalians, the erythrosuchid Erythrosuchus africanus, and abundant remains of the kannemeyeriid dicynodont Kannemeyeria. Finally, the uppermost subzone (C) was originally considered to be characterised by “advanced capitosauroid amphibians” (Hancox et al., 1995; later described as Paracyclotosaurus morganorum Damiani & Hancox, 2003), archosaurs (although this has yet to be verified), and a “new tuskless kannemeyeriid dicynodont” (later referred to the Tanzanian taxon Angonisaurus (see Hancox & Rubidge, 1996), but more recently confirmed as a new taxon, Ufudocyclops mukanelai Kammerer et al., 2019).
Subsequently, additional support for the threefold subdivision of the CAZ has come from taxonomic revisions of the trirachodontid cynodont fauna (previously considered to represent a single genus, Trirachodon, throughout the CAZ; Hancox et al., 1995; Neveling, Hancox & Rubidge, 2006). Abdala, Hancox & Neveling (2005), Abdala, Neveling & Welman (2006) considered Trirachodon proper to be restricted to subzone B, recognising subzone A specimens as a new taxon (Langbergia modisei) and referring subzone C specimens to Cricodon metabolus, a species originally known from the Manda Beds of Tanzania (Crompton, 1955). Referral of the subzone C specimens to Cricodon was based primarily on their greater size compared to other CAZ trirachodontids and similarities in postcanine morphology (Abdala, Hancox & Neveling, 2005). This taxonomic scheme for trirachodontids underlies the recent formal nomenclature for the CAZ subunits (see above), which are based on co-occurrence of a particular trirachodontid genus with archosauromorph or dicynodont secondary index taxa (Hancox, Neveling & Rubidge, 2020). However, there is substantial variation in morphology within the subzone C specimens previously referred to Cricodon metabolus, which suggests that more than one taxon may be present (Hendrickx, Abdala & Choiniere, 2019).
Here, we describe one of the specimens (BP/1/5538) previously assigned to Cricodon metabolus as a new species, Guttigomphus avilionis. This specimen was recovered from the Cricodon-Ufudocyclops Subzone of the CAZ in the southern exposures of the Burgersdorp Formation, near Sterkstroom (Fig. 2, location 1). We test and discuss its phylogenetic relationships with other gomphodont cynodonts and reconsider the record of Cricodon metabolus from South Africa. This new taxonomic record for the Cricodon-Ufudocyclops Subzone complements the recent description (Tolchard et al., 2021) of another novel trirachodontid (Impidens hancoxi) from this subzone, indicating high diversity in the group even in the youngest known strata where they occur.
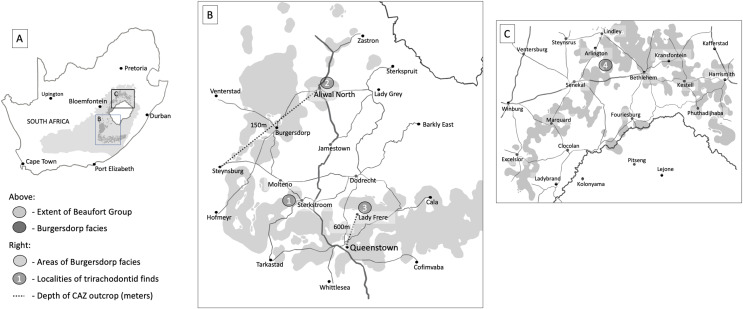
Figure 2. Areas of significant outcrops of the Burgersdorp Formation.
(A) Map of South Africa showing the extent of the Burgersdorp facies and wider Beaufort Group along with the positioning of (B), the southern Burgersdorp facies and (C), the northern Burgersdorp facies. Site 1, Avilion and Thala farms west of the town of Sterkstroom, localities for specimen BP/1/5538 (holotype of Guttigomphus avilionis gen. et sp. nov.), along with BP/1/5540, a complete skull in occlusion referred to Cricodon cf. C. metabolus, and the recently described Impidens hancoxi. Site 2, Aliwal North, locality of the holotype specimen of Trirachodon kannemeyeri (Seeley, 1895a). Site 3, Lady Frere, locality of the holotype specimen of Trirachodon berryi (Seeley, 1895a). Site 4, Langberg, Paul Roux district, locality of the holotype of Langbergia modisei (Abdala, Hancox & Neveling, 2005). The Burgersdorp outcrop is at its thickest in the area between Lady Frere (Cacadu) and Queenstown (Komani) where it reaches over 600 m. The unit then thins gradually extending from this area to a thickness of just 150–200 m in the area between Aliwal North and Burgersdorp.
Materials and Methods
Material
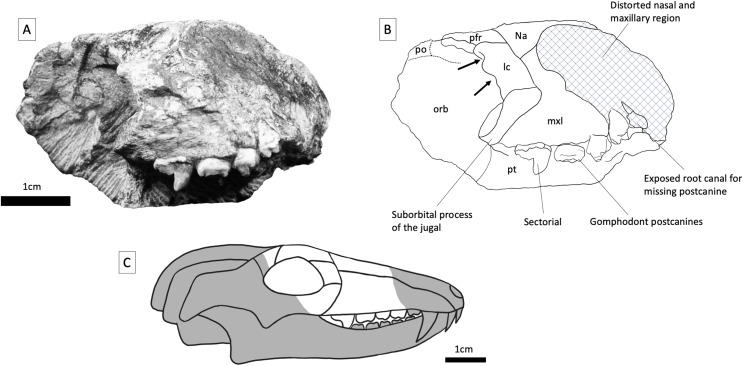
Specimen BP/1/5538 was recovered from Avilion Farm, west of the town of Sterkstroom (Fig. 2, location 1), by John Hancox in May 1993. BP/1/5538 consists of a partial skull preserving the posterior portion of the snout and part of the interorbital region (Figs. 3–5). It has undergone transverse and oblique compression such that the right maxillary region has been compressed inwards towards the left orbit. It preserves a large portion of the anterior and dorsal margins of the right orbit, parts of the anterior skull roofing bones, and three reasonably preserved posterior postcanines on each side plus the right sectorial postcanine. BP/1/5538 was previously referred to Cricodon metabolus by Abdala, Hancox & Neveling (2005) on the basis of comparative size and tooth morphology, but later excluded from the genus by Hendrickx, Abdala & Choiniere (2019) and recovered outside of Trirachodontidae and inside Traversodontidae in a subsequent phylogenetic analysis (Hendrickx et al., 2020). Hendrickx, Abdala & Choiniere (2019) argued that BP/1/5538 differed from C. metabolus (and might therefore represent a new taxon) based on the strong apical curvature of the main cusp and two distal accessory cusps on the upper sectorial postcanine (only a single accessory cusp is present in C. metabolus) and the longer and more ovoid upper gomphodont postcanines with a large labial-mesial accessory cusp and no distal cingulum or ‘step-like’ contact between crowns.
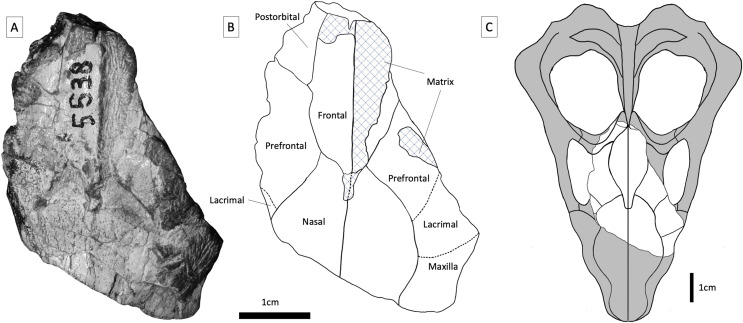
Figure 3. BP/1/5538, holotype of Guttigomphus avilionis gen. et sp. nov.
(A) Dorsal view. (B) Interpretative drawing. (C) Skull reconstruction showing the part of the skull represented in the specimen in white. The taxa Langbergia modisei and Cricodon metabolus were used to make informed reconstruction of missing regions of the skull.
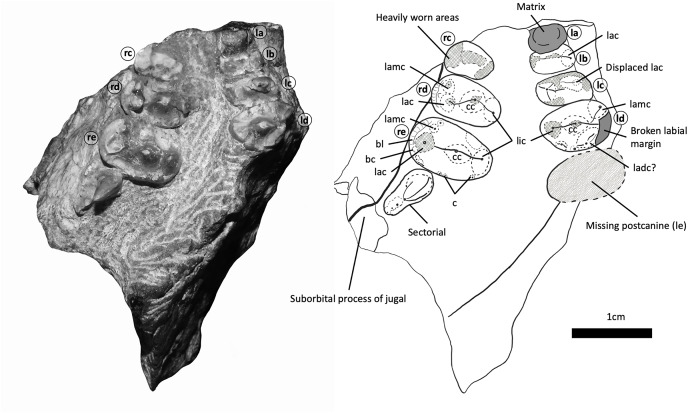
Figure 5. BP/1/5538, holotype of Guttigomphus avilionis gen. et sp. nov., in palatal view with interpretive drawing.
Teeth have been assigned as Left A (la), Left B (lb), Left C (lc) and Left D (ld), with the alveolus of (missing) Left E (le) and Right C (rc), Right D (rd) and Right E (re). Numerical positional values have not been provided since the number of postcanines in the row cannot be confidently assessed with the material available. Letters have been assigned with the posterior-most gomphodont postcanine being designated as ‘E’ and working forwards across the tooth row. For the purposes of comparative anatomy, the dentitions of other closely related taxa were also characterized in this way. Since the total number of postcanines are subject to intraspecific variation among trirachodontids, the letters assigned in this study will correspond to different numerical loci in different specimens. Abbreviations: bc, buccal cingulum; bl, buccal ledge; c, cuspule; cc, central cusp; lac, labial cusp; ladc, labial distal cusp; lamc, labial mesial cusp; lic, lingual cusp.
Methods
For the phylogenetic analysis, a data matrix containing a total of 81 craniodental characters across 24 taxa was assembled, the majority of which were taken from previous studies conducted by Abdala, Neveling & Welman (2006), Kammerer et al. (2008), Gao et al. (2010), and Sidor & Hopson, 2018. To these we added six new characters. Additional characters were: Character 11—absence, presence, or enlargement of the diastema between the upper canines and postcanines, similar to character 3 of Gao et al. (2010) which scored the presence or absence of a diastema between the incisors and canine; Character 12—anterior postcanines are conical, sectorial, less transversely expanded postcanines or the same as more posterior teeth in the row; Character 13—whether the labial or lingual cusp on the upper postcanines has greater sagittal depth; Character 14—whether the wider cusp on the upper postcanines is on the labial or lingual side, or if they are occlusally symmetrical; Character 16—the number of cuspules around the mesial and distal borders of the postcanine crown and Character 17—the percentage difference in the transverse width of upper postcanines in proportion to their length. Scoring of additional characters was conducted based on primary literature as well as photographs of some taxa. The primary objective of our analysis was to determine the relationships of BP/1/5538, so we focused on ingroup relationships within Gomphodontia, the large cynodont clade including Gomphognathidae, Trirachodontidae, and Traversodontidae.
Multiple previous cladistic analyses have recovered Cynognathus as the sister taxon of Gomphodontia in the larger clade Cynognathia. Therefore, we used Cynognathus as the outgroup for our analysis of gomphodont relationships, as in previous studies (e.g., Hopson, 1991; Hopson & Kitching, 2001; Abdala, Neveling & Welman, 2006; Kammerer et al., 2008; Gao et al., 2010; Sidor & Hopson, 2018). Analyses were conducted in TNT version 1.5 for Windows, made freely available with the sponsorship of the Willi Hennig Society (Goloboff & Catalano, 2016), with all characters weighted equally. Fourteen characters were treated as ordered (characters 1, 2, 7, 8, 10, 11, 16, 21, 36, 50, 53, 66, 67, 81). Analyses initially used the default New Technology settings with a driven search, which aims to reduce the number of trees obtained during independent replication (Goloboff & Pol, 2007). The recovered trees were saved to RAM and used as the basis for a traditional search using the tree bisection and reconnection (TBR) swapping algorithm. Eight characters were parsimony uninformative but were retained primarily for the benefit of future studies, as their inclusion in previous matrices across different taxa has been informative. Support values for the results were calculated using bootstrap analysis (1,000 replicates). Synapomorphies were mapped onto the resultant strict consensus of the recovered most parsimonious trees.
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved, and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: 57E697ED-DEC1-4E2D-A2E9-A6EC2E9A0FCA. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
Results
Systematic palaeontology
THERAPSIDA Broom, 1905
CYNODONTIA Owen, 1861
GOMPHODONTIA Seeley, 1895a
TRIRACHODONTIDAE Crompton, 1955
GUTTIGOMPHUS AVILIONIS gen. et sp. nov.
Holotype—BP/1/5538. A partial skull preserving the posterior snout and right interorbital region.
Horizon and locality—BP/1/5538 was recovered from Avilion Farm, west of the town of Sterkstroom in the Karoo Basin of South Africa from the Cricodon-Ufudocyclops subzone of the Cynognathus Assemblage Zone, Burgersdorp Formation and as such is most likely dated to the early Middle Triassic (Anisian, Hancox et al., 1995).
Etymology—From the Latin gutta, meaning a fluid drop, and gomphus, meaning a peg, referring to the somewhat droplet-like shape (narrower at one end) of the gomphodont postcanines in occlusal view. The species name avilionis refers to Avilion Farm, where BP/1/5538 was collected.
Diagnosis—A combination of features distinguishes Guttigomphus avilionis from other early gomphodonts, including trirachodontids and basal traversodontids such as Pascualgnathus and Etjoia. The new taxon can be distinguished from Langbergia modisei by the absence of distinct, well-developed cingular cuspules on the upper gomphodont postcanines, location of the central cusp closer to the lingual cusp (rather than equidistant from lingual and labial cusps as in Langbergia) on the upper gomphodont postcanines, and the absence of a mesial accessory cusp on the sectorial postcanine(s). The new taxon can be distinguished from Trirachodon (both species) and Cricodon metabolus by the shape of the upper gomphodont postcanines (more ovate, with the lingual margin narrower than the labial margin; the opposite is the case in Trirachodon and Cricodon), presence of a well-developed labial-mesial accessory cusp on the upper gomphodont postcanines, weaker development of the transverse crest connecting the three main cusps of the upper gomphodont postcanines, and presence of two distal accessory cusps on the upper sectorial postcanine. The new taxon can be distinguished from the newly described Impidens hancoxi by the possession of fewer sectorials in the postcanine tooth row (and shorter contribution of the sectorials to the length of the tooth row), upper gomphodont postcanines that are wider labially than those of Impidens, in which these teeth are wider lingually (it should be noted that the postcanines are not preserved in Impidens; this inference is therefore based on the different outline of the alveolus in occlusal view), more pronounced transverse expansion of the upper postcanines than in Impidens, less oblique inclination in the posterior-most postcanines in relation to the axis of the skull than in Impidens, and much smaller size than in Impidens (which is unlikely to be attributable to immaturity in BP/1/5538, given the observed tooth wear).
Distinction from the traversodontid gomphodonts Pascualgnathus and Etjoia is predominately on the basis of a wider labial cusp on the upper postcanines (lingual cusp is wider in Etjoia, Cricodon, and Andescynodon; these cusps are generally symmetrical in other cynognathians) and the number and prominence of cuspules around the crown borders, with Guttigomphus avilionis presenting only faint cuspules similar to those found on more derived gomphodonts such as Scalenodon angustifrons, whereas Etjoia presents a small number of larger cuspules closer to the morphological condition found more commonly in Trirachodontidae.
Description
Maxilla—Parts of the maxilla are preserved on both sides of the skull (Figs. 3 and 4). The right maxilla has been compressed inwards towards the left orbit, causing distortion to the palate (Fig. 5). The lateral surfaces of both maxillae are badly eroded. The best-preserved portion of the lateral maxillary surface is immediately above the tooth row of the right maxilla, but even here there are visible trabeculae, indicating that this is not intact bone surface. On the left side, the fragmentary section of the left maxilla contains three gomphodont posterior postcanines as well as a fragment of the base of the crown of a preceding postcanine that is heavily obscured by matrix. Also preserved is the anterior part of the alveolus of the missing most posterior gomphodont postcanine (Fig. 5). On the right side, the anterior portion of the right maxilla has been eroded off, but the preserved portion includes the last three gomphodont postcanines and one sectorial postcanine. At the anterior edge of the right maxilla there is part of an additional postcanine, nearly all of which has been sheared off to expose the internal cavity of the root of the tooth. On the right side of the specimen, the suborbital process of the jugal contacts the maxilla above the sectorial tooth, with this suture extending anterodorsally at approximately 45 degrees to the horizontal. The maxillary postcanines are only weakly inset from the lateral maxilla surface, and there is not a distinct labial shelf, but given the amount of distortion and erosion of the maxilla this should not be taken as a definite indication of this structure’s absence in life. The lacrimal contacts the maxilla posterodorsally, also above the sectorial postcanine. Cross sections through the maxillae are exposed anteriorly, where the specimen has been damaged, but provide little useful information. The maxillae are transversely thick in the postcanine region, comparable to the dimensions of other gomphodonts (e.g., Massetognathus; Crompton et al., 2017).
Figure 4. BP/1/5538, holotype of Guttigomphus avilionis gen. et sp. nov.
(A) Right lateral view. (B) Interpretative drawing. (C) Skull reconstruction showing the part of the skull represented in the specimen in white. Abbreviations: lc, lacrimal; mxl, maxilla; na, nasal; orb, orbital region; pfr, postfrontal; pft, prefrontal; po, postorbital; pt, palatine. Arrows denote location of lacrimal canals.
Nasal—Large portions of the posterior parts of the nasals are present (Fig. 3), but their anterior parts have broken away. The dorsal surface of the paired nasals is flat to gently convex transversely. The contact between each nasal and the prefrontal extends from anterolateral to posteromedial at an acute angle, making the posterior processes of the nasals almost equilaterally triangular, which is not typical among gomphodonts. At their posterior ends, the nasals are separated from one another by a triangular anterior projection of the paired frontals.
Frontal—The right frontal is almost entirely intact, with only the posterior tip missing, but the left frontal is either missing or almost entirely obscured by overlying matrix. The sutural contact between the frontal and prefrontal runs anteromedial to posterolateral at a weak angle. The frontal margin appears to be convex at its contact with the prefrontal on the right side; by contrast, on the left side this suture is largely straight. This variation may reflect taphonomic distortion or simply natural asymmetry. Although damaged and partially obscured by matrix, a pointed process of each frontal is apparent at its anterior end: together, these processes form a triangular projection between the nasals in dorsal view. Narrow anterior processes of the frontals are frequently present in traversodontids (e.g., Dadadon; Ranivoharimanana et al., 2011). By contrast, anterior processes of the frontals are usually present but relatively short and broad in trirachodontids (e.g., Sidor & Hopson, 2018). The posterior portion of the frontal is missing on each side, but based on the right side the frontal appears to taper towards the midline, flanked by the postorbital, as seen in other trirachodontid specimens (e.g., the Langbergia modisei skulls NMQR 3255 and NMQR 3280).
Prefrontal and lacrimal—The right lacrimal is clearly identifiable due to the presence of paired lacrimal canals on the internal surface of the anterior orbital margin. Lacrimal canals cannot be identified on the left side, as the specimen is broken just before the anterior border of the orbit. The lacrimal contacts the nasal and maxilla anteriorly and the suborbital process of the jugal ventrally. The contact between the lacrimal and the prefrontal is not readily identifiable due to the preservation of the specimen but would have been around the anterodorsal corner of the orbit. Part of the prefrontal is preserved on both sides of the specimen (although the left prefrontal is largely covered in matrix), and this element is trapezoidal in shape, with weakly-angled contacts with the nasal and frontal dorsally. Posteriorly, the prefrontal contacts the postorbital, excluding the frontal from the orbital margin.
Postorbital—Only a small fragment of the postorbital is present on the right side. It formed part of the posterodorsal margin of the orbit. A triangular anteromedial postorbital process extends between the frontal and prefrontal in this region.
Orbit—The anterior and dorsal margins of the right orbit are preserved, and are formed by the postorbital, prefrontal, lacrimal, and suborbital process of the jugal. There is a large amount of matrix within the inner surface of the orbit, obscuring some of the internal boundaries. On the left side, portions of the lacrimal and prefrontal are present but broken just before the orbital boundary, so that only parts of the inner orbit can be identified.
Dentition—Three upper gomphodont postcanine crowns are preserved on each side of the specimen (Fig. 5). Since it is not possible to confidently assess the anterior end of the tooth row, individual teeth have been designated as letters rather than numerical identifiers. In both our description of the new taxon and our comparative anatomy the letter ‘E’ has been assigned to the posteriormost gomphodont postcanine, immediately before the first sectorial, or in taxa where no sectorials are present, to the last tooth in the row. The letters then work sequentially forwards from this point, with teeth A–E representing the five posteriormost gomphodont postcanines. The anteriormost preserved postcanine on the right side (Fig. 5, rc) is heavily worn and only the occlusal outline and a portion of occlusal basin surface area can be seen. The lingual cusp is partially covered by matrix, making accurate measurement difficult, but the transverse width is approximately 5.0 mm and mesiodistal length of the labial cusp is 3.2 mm. The transverse crest was positioned roughly on the midline of the crown but has broken away. The second tooth on the right side (Fig. 5, rd) is better preserved and has two distinct cusps on the labial margin, a primary labial cusp and a smaller but distinct labial-mesial cusp, both of which are missing the apex. The mesiolingual portion of the tooth is slightly obscured by matrix and the main lingual cusp is present but damaged and has undergone slight displacement towards the posterior of the tooth due to taphonomic distortion. The central cusp is missing the apex and is positioned at a slightly oblique angle so that the labial part of the transverse crest is angled towards the distal margin of the tooth and the lingual part is angled towards the mesial margin. The distal border of the crown, where a distal cingulum is usually present in gomphodonts, is heavily worn. The transverse width of tooth rd is 7.2 mm and the wider labial margin is well exposed, measuring 4 mm in mesiodistal length at the widest point compared to the 3.3 mm mesiodistal length of the widest point of the lingual margin.
The posteriormost gomphodont postcanine on the right side (Fig. 5, re; Fig. 6) is the best-preserved member of the series and clearly displays the unique occlusal outline of this taxon, with a transverse width of 8.5 mm, the labial cusp measuring 4.8 mm in mesiodistal length at the widest point, and the lingual base measuring 4.1 mm at the widest point. The main labial cusp has been broken near the base. Lateral to the base of this cusp, a shelf-like expanse of crown separates the cusp from the tooth margin and is interpreted as a cingulum. This contrasts with the condition in Cricodon metabolus, where the labial cusp is part of the labial tooth margin and the entire border of the crown slopes gently upwards to an apex that displays slight curvature towards the lingual side (Figs. 6 and 7). A well-developed labial-mesial accessory cusp is present in postcanine re, situated anterior to the transverse midpoint of the main cusp. The labial-mesial cusp is mesiodistally longer than wide. Distal to the main labial cusp, two small cuspules are present at the labial corner of the distal cingulum. These we interpret as members of the series of cuspules usually present along the cingulum in trirachodontids, with the rest lost to wear and/or damage. We agree with the observations of Hendrickx, Abdala & Choiniere (2019), who considered the distal cingulum absent in this specimen, contrary to the original description of Abdala, Hancox & Neveling (2005) that it is present but worn. The relatively small size of the preserved cuspules is similar to that of most trirachodontids and some traversodontids, but differs strongly from that of Langbergia, where the cingular cuspules are unusually well-developed. The central and lingual cusps in this tooth are well preserved, the transverse crest is straight, running between the lingual and labial cusps and bisecting the central cusp; without the oblique angle observed in the preceding tooth (tooth rd). The central cusp is positioned closer to the lingual than the labial margin, and there are deep occlusal basins to either side forming a concave surface between the cingula and the main cusps. The labial borders of teeth rd and re have a very gently sloping, almost flat ledge, 1–2 mm across, positioned labial to the main labial cusp. On the lingual side, the base of the main cusp merges into the border of the crown with no separating ledge.
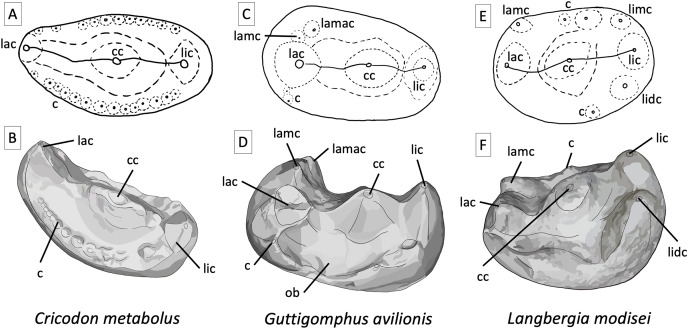
Figure 6. Comparative tooth morphology for trirachodontids, showing the uniquely droplet shaped occlusal outline found in Guttigomphus avilionis gen. et sp. nov. and the clear ring of cuspules and more pronounced labial cusp present in Cricodon metabolus.
(A) Cricodon metabolus upper postcanine, interpretive drawing of occlusal outline from UMZC T905; (B) Cricodon metabolus, artistic rendition of upper postcanine from UMZC T905; (C) Guttigomphus avilionis gen. et sp. nov., interpretative drawing of occlusal outline from BP/1/5538 (re Fig. 5); (D) Guttigomphus avilionis gen. et sp. nov., artistic rendition of upper postcanine from BP/1/5538 (tooth re, Fig. 5); (E) Langbergia modisei upper postcanine, interpretive drawing of occlusal outline from NMQR 3255; (F) Langbergia modisei, artistic rendition of upper postcanine from NMQR 3255. Abbreviations: c, cuspule; cc, central cusp; lac, labial cusp; lamac, labial mesial accessory cusp; lamc, labial mesial cusp; lic, lingual cusp; lidc, lingual distal cusp; limc, lingual mesial cusp; ob, occlusal basin.
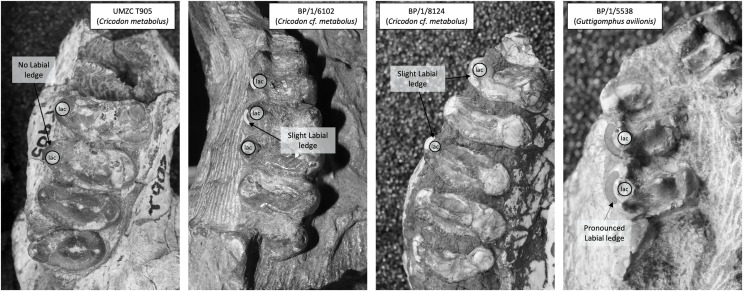
Figure 7. Comparison of the mesial border of the upper postcanines in Cricodon metabolus and Guttigomphus avilionis.
Comparison of the mesial border of the upper postcanines in Cricodon metabolus (UMZC T905), two additional specimens of Cricodon cf. metabolus from the Cricodon-Ufudocyclops subzone (BP/1/6102 and BP/1/8124) and Guttigomphus avilionis (BP/1/5538), highlighting the difference in position of the labial cusp. lac, labial cusp.
The sectorial postcanine on the right side is well preserved but incompletely erupted, and measures 2.2 mm wide transversely and 6 mm long mesiodistally. The main cusp displays a posterior curvature towards the apex (comparative to other gomphodonts) and is very faintly serrated (difficult to observe with the naked eye). At least two distal accessory cusps (similar to those of Langbergia) are present on the sectorial tooth, but their apices are broken. Consistent with the rest of the post-canine series, the sectorial also possesses a sectorial labial ledge, a feature commented on by Hendrickx et al. (2020) who suggest it might result from ‘lingomesial rotation of the broken crown’. Given the presence of a ledge on the gomphodont postcanines (Fig. 7) it is possible this is a genuine anatomical feature.
On the left side, the anterior-most tooth position (Fig. 5, la) is largely obscured by matrix and only a tiny portion of the labial margin of a tooth can be seen in lateral view. Assuming that its dimensions are accurately reflected by the overlying chunk of sediment, the crown is approximately 3.5 mm in transverse width. The second most anterior tooth on the left side (Fig. 5, lb) is heavily eroded and partially overlaid by matrix, preventing accurate measurement of the lingual cusp; however, the difference between the mesiodistal lengths of the lingual cusp and labial cusp appears less pronounced on tooth lb than the other postcanines in the series. The labial cusp is mostly preserved, missing the apex and cracked at the base, indicating that minor taphonomic displacement may have occurred. The central ridge sits at a slightly oblique angle as seen in tooth rd, so that the lingual side of the ridge is positioned towards the mesial border of the crown and the labial side of the ridge meets the midline. Tooth lb measures 4.6 mm in transverse width. The subsequent tooth (Fig. 5, tooth lc) shows substantial damage to the mesial and distal borders of the crown. The lingual cusp is only partially preserved, and the labial cusp has been displaced and sits over the central cusp with a small portion of the labial border of the crown missing. Teeth lb and lc both have the transverse ridge positioned fractionally more towards the anterior border of the crown. Tooth lc measures 5.6 mm in transverse width and 2.4 mm in mesiodistal length at the base of the lingual cusp. The labial cusp cannot be accurately measured due to damage.
The posteriormost tooth on the left side (Fig. 5, ld) has sustained breakage of the labial side so that a large portion of the tooth root, including some of the internal cavity, has been exposed laterally on the specimen. Only the base of the lingual cusp is preserved, along with an intact smaller distal lingual accessory cusp (which also appears to bound the edge of the distal cingulum) and faint distal cuspules. The central cusp is well preserved, missing only the apex. It exhibits the slight distortion of the angle of the transverse ridge with the lingual side being angled towards the mesial margin and the labial side being angled towards the missing labial cusp. This angling of the transverse ridge is also observed in Trirachodon berryi (BP/1/4658). There is a labial-mesial cusp and a large feature which is possibly a labial distal cusp, but since the distolabial portion of the tooth is missing it is not possibly to accurately gauge this feature in relation to the labial cusp or crown borders. Tooth ld measures 6.2 mm in transverse width and 2.9 mm in mesiodistal length across the base of the lingual cusp. Measurement of the labial cusp is not possible due to damage, but the widening trajectory of the mesial and distal crown borders clearly display the hallmark widening of the tooth on the labial side forming the droplet shaped occlusal outline.
The morphology of the upper postcanine teeth provides the primary evidence for recognition of Guttigomphus avilionis as a new species. The overall morphology appears somewhat intermediate between Langbergia modisei and Cricodon metabolus (Fig. 6). The shared features which typify trirachodontids, as first outlined by Crompton (1955), are that they all display transverse expansion of the postcanines, three cusps in a transverse row across the upper postcanines, and cuspules around the crown borders. Guttigomphus shares with Langbergia a distinct labial-mesial accessory cusp, which is absent in Cricodon and Trirachodon. Transverse expansion of the crowns in Cricodon, Langbergia and Guttigomphus is less than in Trirachodon berryi and T. kannemeyeri, with the labiolingual width being typically 75–95% greater than the mesiodistal length, compared to a labiolingual width in excess of twice the mesiodistal length in Trirachodon (Table 1). Details of the occlusal surfaces further differ between Guttigomphus and Cricodon/Trirachodon, with the latter having a broader lingual margin and the former having a broader labial margin. There are also often distinct minor accessory cusps on the labial and lingual sides that are more prominent than the crown border cuspules in Cricodon and Trirachodon, which appear as small spherical ‘bumps’. In Guttigomphus, these smaller accessory cusps rise with the sagittal (dorsal:ventral) plane in line with the border of the crown and form individual pointed apices, rather than appearing as rounded relief structures on the occlusal surface of the crown (Fig. 6).
Table 1. Comparative measurements of transverse width and mesiodistal length in upper postcanines across all sampled trirachodontid species.
| Cricodon metabolus (NHCC LB28) | Cricodon metabolus (UMZC T905) | Trirachodon kannemeyeri (SAM-PK-K12168) | Trirachodon kannemeyeri (SAM-PK-K171) | Trirachodon berryi (NHMUK PV R3579) | Langbergia modesei (NMQR 3255) | Guttigomphus avilionis (BP/1/5538) | |
|---|---|---|---|---|---|---|---|
| Approximate skull length | 180 mm | 170 mm | 115 mm | 100 mm | 120 mm | 160 mm | 170 mm |
| Approximate length postcanine row | 50 mm | 55 mm | 30 mm | 30 mm | 30 mm | 40 mm | 47 mm |
| Upper PCb width | 5 mm | 12.5 mm | 6.2 mm | 7.6 mm | 5.5 mm | 7.5 mm | 8.4 mm |
| Upper PCb length | 3 mm | 6.5 mm | 3 mm | 3.5 mm | 2.4 mm | 3.7 mm | 5.1 mm |
| % difference | 66.7 | 92.3 | 106.6 | 117.7 | 131 | 102.7 | 64.7 |
| Upper PCc width | 6.75 mm | 13 mm | 6.4 mm | 7.8 mm | 4.7 mm | 6.5 mm | 6.5 mm |
| Upper PCc length | 3.4 mm | 6 mm | 2.4 mm | 3.7 mm | 3.2 mm | 3.5 mm | 3.9 mm |
| % difference | 98.52 | 116.6 | 166.7 | 166.7 | 46.8 | 85.7 | 66.7 |
| Upper PCd width | 7.4 mm | 11 mm | 4.7 mm | 7 mm | 4.7 mm | 6 mm | 6 mm |
| Upper PCd length | 3.75 mm | 6 mm | 2.5 mm | 2.8 mm | 2.4 mm | 3.2 mm | 3.5 mm |
| % difference | 97.30 | 83.3 | 88 | 150 | 95 | 87.5 | 71.4 |
| Upper PCe width | 4.2 mm | 10 mm | 3.75 mm | 6.3 mm | 6.2 mm | 5.2 mm | 5 mm |
| Upper PCe length | 3.2 mm | 5.5 mm | 2 mm | 2.7 mm | 2.4 mm | 3 mm | 2.5 mm |
| % difference | 31.25 | 81.81 | 87.5 | 133.3 | 158 | 73.3 | 100 |
| Upper PC average width | 5.8 mm | 11.6 mm | 5.3 mm | 7.2 mm | 5.5 mm | 6.3 mm | 6.5 mm |
| Upper PC average length | 3.3 mm | 6 mm | 2.5 mm | 3.2 mm | 2.6 mm | 3.3 mm | 3.7 mm |
| Average % difference | 80.1 | 93.6 | 112.9 | 126.1 | 120 | 46.8 | 75.6 |
Note:
Tooth ‘E’ represents the posterior most gomphodont postcanine, immediately prior to the sectorials. This sequence then runs forwards across the tooth row, teeth A–E are the five most posterior gomphodont postcanines (see Fig. 5). Comparison has been made in this way due to the entire tooth row not being present in all taxa examined, including Guttigomphus avilionis gen. et sp. nov. It should be noted that this means ‘tooth E’ will have a different numerical locus identifier across different taxa, as the total number of postcanines is subject to intraspecific variation.
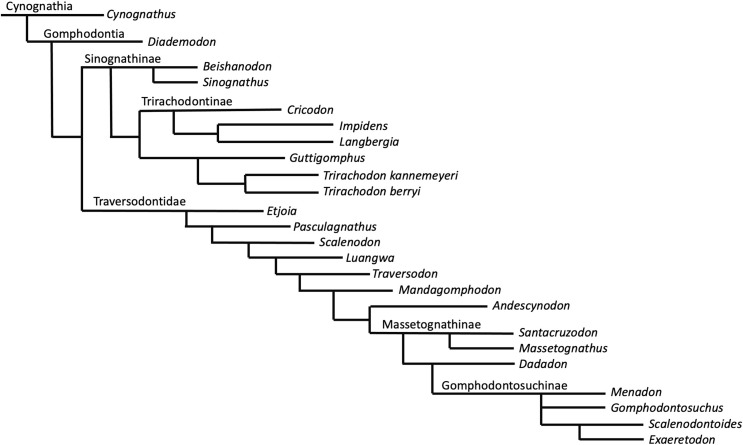
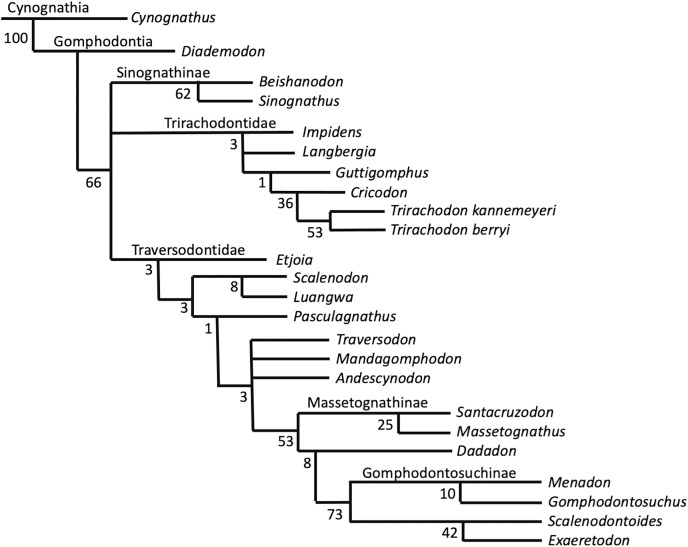
Phylogenetic results—The New Technology search in TNT recovered two most parsimonious trees (MPTs) of 233 steps. Subsequently, a traditional search with TBR did not recover any further MPTs. The strict consensus of these two MPTs is displayed in Fig. 8. Bootstrap support is generally low, with a bootstrap value of only 1% for the placement of Guttigomphus as the sister taxon to Cricodon + Trirachodon (Fig. 9), and 62% support for Sinognathinae. The relationship between Cricodon and Trirachodon has a support value of 36% and the support value for T. kannemeyeri and T. berryi as sister taxa is 53% (Fig. 9).
Figure 8. Strict consensus of two equally most parsimonious trees recovered by the phylogenetic analysis showing relationships among gomophodont cynodonts.
Figure 9. Bootstrap support values for phylogenetic results shown in Fig. 8.
Guttigomphus avilionis is characterised by the following local autapomorphies in this analysis:
Character 14; character state 2, upper postcanine widest on labial cusp.
Character 17; character state 1, cuspules around mesial and distal border of upper postcanine crowns are very faint.
Character 25; character state 1, distal cingulum on the upper postcanine absent (Abdala, Neveling & Welman, 2006)
Character 26; character state 1, upper postcanine labial cingulum absent (Sidor & Hopson, 2018)
Character 28; character state 1, deep occlusal basins present (Abdala, Neveling & Welman, 2006)
Our phylogenetic results largely support the results of previous phylogenetic analyses (Gao et al., 2010; Abdala, Neveling & Welman, 2006; Kammerer et al., 2008; Sidor & Hopson, 2018). The only notable differences are that our results do not resolve Trirachodon kannemeyeri within the genus Cricodon, as suggested by Sidor & Hopson (2018). It has been suggested by other researchers that Trirachodon kannemeyeri does not constitute a valid taxon and is instead an ontogenetic variation of Trirachodon berryi (Hendrickx, Abdala & Choiniere, 2019). As such, we do not here use the generic recombination of Cricodon kannemeyeri proposed by Sidor & Hopson (2018). We retain T. kannemeyeri in Trirachodon based on historical usage and recognize that additional work is required to address trirachodontid alpha taxonomy. In addition, we do not resolve Guttigomphus avilionis within Traversodontidae, unlike the results of Hendrickx et al. (2020). However, we note that these phylogenetic positions are unstable and that morphological similarities, such as the presence of cuspules around the borders of the postcanines, exist between the basal members of both Trirachodontidae and Traversodontidae.
Trirachodontidae can be considered as a monophyletic group of gomphodont cynodonts that now numbers six species and is currently found exclusively in the Cynognathus AZ of South Africa and correlated faunal assemblages in Tanzania and Zambia (if the disputed Chinese taxa Sinognathus and Beishanodon are not included within the clade, see discussion).
Synapomorphy mapping in TNT reconstructs the following character state transformations as unambiguous synapomorphies of Trirachodontidae:
Character 12: character state 2, the anterior most postcanines on the maxillary tooth row are less transversely expanded post canines (rather than conical or sectorial).
Character 17: character state 2, a multitude of tiny cuspules present on the mesial and distal upper postcanine crown border.
Character 18: character state 2, the upper postcanine row consists of gomphodont and sectorial postcanines (conical postcanines absent).
Character 40: character state 2, three cusps on the transverse row of the lower postcanines.
Discussion
The discovery of Guttigomphus avilionis adds to our expanding knowledge of cynodont diversity in the Cynognathus Assemblage Zone. There are now at least nine cynodont taxa reported from probable Middle Triassic beds in the Karoo Basin of South Africa: Diademodon tetragonus (Kitching, 1958; Brink, 1963), Cynognathus crateronotus (Brink, 1963), Lumkuia fuzzi (Hopson & Kitching, 2001), Bolotridon frerensis (Seeley, 1895b; Pusch, Kammerer & Fröbisch, 2021), Trirachodon berryi (Seeley, 1895a; Huene, 1911), Trirachodon kannemeyeri (Seeley, 1895a; Colbert & Kitching, 1977), Cricodon metabolus (Crompton, 1955; Abdala, Hancox & Neveling, 2005), Impidens hancoxi (Tolchard et al., 2021) and now Guttigomphus avilionis (the poorly known Cistecynodon parvus may represent a tenth, but requires restudy to confirm its validity). Of these, the specimen that we identify here as Guttigomphus avilionis was one of a number of specimens from CAZ Subzone C previously referred to Cricodon metabolus.
Guttigomphus falls out as the earliest branching member of Trirachodontidae. It shares some features in common with the recently described early traversodontid Etjoia, such as the extent of transverse expansion on the upper post-canines, the presence of a mesial cingulum and the presence of deep occlusal basins. These features are absent in more derived trirachodontids but commonplace within Traversodontidae. These two taxa reflect the symplesiomorphies of the common ancestor between trirachodontids and traversodontids.
Discussion of trirachodontid material from the Cricodon-Ufudocyclops Subzone
Specimen BP/1/5538 (Guttigomphus avilionis) was recovered in May 1993 by John Hancox, from the same general location as specimens BP/1/5540, BP/1/5835, and BP/1/6102. All of these were previously referred to Cricodon metabolus by Abdala, Hancox & Neveling (2005) and then ‘tentatively referred to Cricodon’ by Hendrickx, Abdala & Choiniere (2019). In addition to these specimens, collaborative fieldwork in 2014 between the University of Birmingham and the University of the Witwatersrand recovered an additional trirachodontid specimen: BP/1/8124. A full evaluation of the taxonomic status of all these specimens is the subject of ongoing work, and we provide only some brief comments here.
BP/1/5540 is a complete but poorly preserved skull with the lower jaws in occlusion, measuring 180 mm from the anterior of the snout to the posterior of cranium (Fig. 10A), and is comparable in size to the Cricodon metabolus holotype. It is not possible to confidently identify BP/1/5540 as Cricodon without examination of postcanine morphology. Due to the iron-heavy composition of the matrix, CT data have proved uninformative to date. Based upon external morphology, nothing was identified to exclude BP/1/5540 from Cricodon metabolus. If BP/1/5540 does prove to be an additional example of Cricodon metabolus, it provides additional morphological information on phylogenetic characters that were scored as unknown on the holotype, given that the preservation of the dentary, palate, and some other skull elements are good. BP/1/5540 provides information on the greatest width of the zygomatic arch, the length of the snout in proportion to the temporal region, and the widest part of the temporal fenestra, which are uncertain in the type material. At present, however, we support previous researchers in only tentative assignment to C. metabolus.
Figure 10. Cynodont specimens from the Cricodon-Ufudocyclops subzone.
(A) BP/1/5540, Cricodon cf. C. metabolus. Full skull in occlusion, right lateral view. (B) BP/1/5835, large cynognathian, probably Cynognathus, right lateral view of anterior part of skull.
BP/1/5835 is an anterior portion of snout broken just posterior to the canines (Fig. 10B). In addition to this there are two fragments of upper and lower jaw in occlusion (Abdala, Hancox & Neveling, 2005), although this additional material was not available for examination for the purposes of this article. Estimating the skull length with such fragmentary material is more challenging, but it appears to represent a larger individual with a total skull length of up to 230 mm. The canine appears proportionately smaller than in BP/1/5540. BP/1/5835 and BP/1/5540 differ in the presence of procumbent incisors in the latter. BP/1/5540 also possesses canines and incisors with proportionately greater sagittal (dorsal:ventral) depth relative to the basal width than BP/1/5835 where the incisors and canines appear less slender (Fig. 10). The additional material indicates the sequence of the postcanine row does not provide information on distinct gomphodont postcanine morphology without CT data. Based on its size and the morphological differences observed in the incisors, it seems unlikely that BP/1/5835 is a trirachodontid. Firm conclusions cannot be drawn from such fragmentary material, but based on the larger size, lack of incisor procumbency, and proportionately stouter mesial:distal depth to basal width in the canines and incisors, it seems more consistent with basal members of Cynognathia, such as Cynognathus or possibly Diademodon. Further examination of this specimen, particularly through obtaining informative CT data of the postcanine morphology, is required in the future.
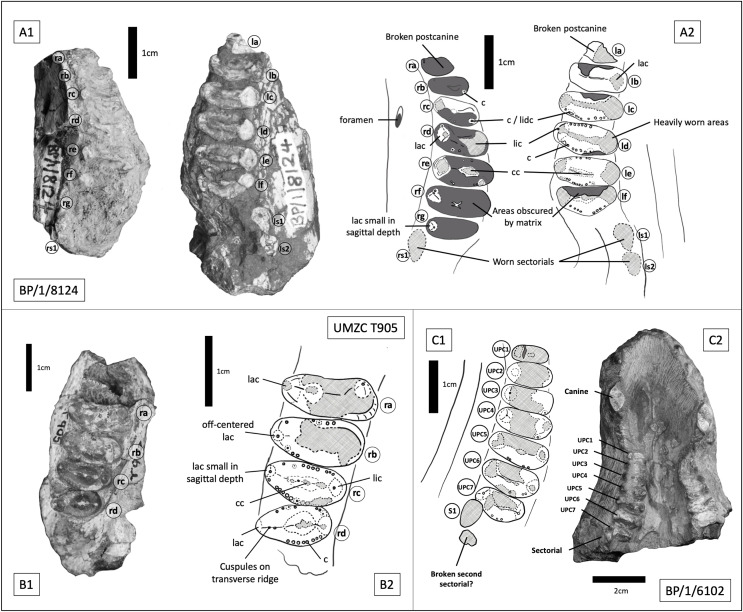
BP/1/8124 consists of two maxillary tooth row fragments, from opposite sides of what is inferred to be a single skull, with the dentition of the right side being heavily obscured by matrix. On the right side there are seven gomphodont postcanines and a single sectorial preserved, whereas on the left there are six gomphodont postcanines and two sectorials (Figs. 11 A, 11B). The estimated skull length for BP/1/8124 is approximately 180 mm. BP/1/6102 consists of a large portion of snout, with maxilla, premaxilla and nasals present (Fig. 11 C). Its estimated skull length is identical to that for BP/1/8124. Measurements for BP/1/8124 and BP/1/6102 use specimen BP/1/5540 as a proxy. Both BP/1/6102 and BP/1/5540 measure 42 mm from front of premaxilla to the back of the caniniform. In BP/1/8124 the extend of the postcanine row is comparable to the other two specimens. Four incisors on each side remain well preserved, as are the basal portions of both canines, seven gomphodont postcanines on the right side plus one clear sectorial and an additional fragment which may represent a second sectorial. The left side is less well preserved and contains six heavily worn gomphodont postcanines and the alveolus for the seventh, posteriormost postcanine. Based on their similarity in size, near identical occlusal outline of gomphodont postcanines and in-stepping arrangement of the tooth row (teeth at the anterior most end are smaller and less transversely expanded, there is a curvature of the tooth row towards the lingual side in the middle of the row and becomes more labially offset towards the posterior end of the row) it can be determined that BP/1/8124 and BP/1/6102 represent two specimens of the same taxon. Based on the seven postcanines of BP/1/6102 and the smaller size of the anterior most postcanine on BP/1/8124, it seems probable that the right side of BP/1/8124 represents the entire maxillary tooth row and that both specimens present a total of seven gomphodont postcanines and two sectorials.
Figure 11. Trirachodontid specimens from the Cricodon-Ufudocyclops subzone of South Africa and the Manda Beds of Tanzania.
(A1) BP/1/8124, Cricodon cf. C. metabolus, Cricodon-Ufudocyclops subzone, South Africa. Left and right fragments of maxillary cheek teeth row, occlusal view. (A2) Interpretive drawing of BP/1/8124, occlusal view. (B1) UMZC T905, Cricodon metabolus holotype, Manda Beds, Tanzania. (B2) Interpretive drawing of UMZC T905. (C1) BP/1/6102, Cricodon cf. C. metabolus, Cricodon-Ufudocyclops subzone, South Africa. Interpretative drawing in occlusal view. (C2) BP/1/6102, photograph in occlusal view. Abbreviations: c, cuspule; cc, central cusp; lac, labial cusp; lic, lingual cusp; lidc, lingual distal cusp; 1s1, left sectorial one; ls2, left sectorial two; rs1, right sectorial one. Teeth have been assigned as Left A (la), Left B (lb), Left C (lc) and Left D (ld) and Right C (rc), Right D (rd) and Right E (re) to distinguish left from right (see caption to Fig. 5). Numerical values have not been provided since the number of postcanines in the row cannot be confidently assessed with the material available.
Sidor & Hopson (2018) identified paired foramina positioned above the 4th to 5th and 5th to 6th upper postcanines as a diagnostic feature of Cricodon metabolus. A single foramen can be identified on the left side of BP/1/8124 above the 3rd to 4th postcanine and on the right side of BP/1/6102 above the 3rd to 4th postcanine. Other features of the postcanines also suggest BP/1/8124 is referable to Cricodon metabolus, including the ring of distinctly spherical cuspules found across mesial and distal borders of the crown and a distinct transverse crest which runs from labial to lingual cusps. Labial and lingual borders of the crown present a singular main cusp with no prominent accessory cusps (although teeth rd and rf on BP/1/8124 and tooth rb on UMZC T905 all present a ‘labiomesial cuspule’ which sits more prominently than the other border cuspules immediately to the anterior of the main labial cusp) and a comparable level of gomphodont transverse expansion (Figs. 11A2, 11B2). The Cricodon metabolus holotype (UMZC T905) and specimen BP/1/8124 both display a labial cusp small in sagittal depth, appearing more like an accessory cusp or large cuspule than the prominent labial cusp on other teeth (Fig. 11A2, tooth rg; Fig. 11B2, tooth rc).
It is worth noting that Cricodon metabolus presents labial cusps that are much narrower at the base than the lingual cusp and the labial cusp itself rises with the labial margin of the tooth, presenting no ledge or cingulum to the labial side of the labial cusp (Figs. 11B1, 11B2). Both BP/1/8124 and BP/1/6102 lack the distinction of the narrower labial cusp and have labial and lingual cusps that are approximately symmetrical in basal width. BP/1/8124, BP/1/6102 and Cricodon all have central cusps which sit closer to the lingual border, but in BP/1/8124 and BP/1/6102 they are slightly closer to the centre. The poor state of preservation of these specimens makes confident assessment of the labial margin challenging, but BP/1/8124 and BP/1/6102 also appear to have the labial cusp sitting fractionally in from the labial margin as seen in other trirachodontids (Fig. 7), unlike in the holotype and referred specimens of Cricodon metabolus. The anterior upper postcanines in the referred specimen of Cricodon metabolus from Zambia (NHCC LB28, Sidor & Hopson (2018) are tricuspid teeth with the cusps arranged mesiodistally, and they resemble sectorials in lateral view. In BP/1/6102, the anteriormost teeth in the row are transversely expanded gomphodont postcanines. In addition, NHCC LB28 presents a total of 10–11 gomphodont upper postcanines rather than the 7 found in BP/1/8124 and BP/1/6102. These differences could be ontogenetic, although it is worth noting that BP/1/8124 and BP/1/6102 are the larger specimens and are unlikely to be juveniles. It is not unheard of for this sort of ontogenetic variation to occur in gomphodonts; in Exaeretodon the juveniles have a greater number of postcanines (Abdala, Barberena & Dornelles, 2002). However, juveniles with a greater number of postcanines would be unusual compared to the growth series observed in Trirachodon (Hopson, 2005), therefore we must consider the possibility that this difference presents evidence that BP/1/8124 and BP/1/6102 might represent a novel species of Cricodon. As such, we currently consider BP/1/5540, BP/1/8124 and BP/1/6102 to represent Cricodon cf. C. metabolus and exclude BP/1/5835, instead proposing that the latter specimen is more likely Cynognathus.
Discussion of disputed trirachodontid taxa
The Chinese taxa Sinognathus gracilis (Young, 1959) and Beishanodon youngi (Gao et al., 2010) have been difficult to place phylogenetically, and their classification as trirachodontids has been disputed in different cladistic analyses. In their original description of Beishanodon youngi, Gao et al. (2010) conducted an analysis of 43 craniodental characters across 18 taxa and found some support in their majority rule consensus tree for the grouping of Sinognathinae and Trirachodontidae. Liu & Abdala (2014) conducted a large-scale phylogenetic analysis across 30 taxa to resolve the interrelationships of Transversodontidae using 18 cranial characters, 32 dental morphology characters, 16 characters pertaining to dental positioning, and 10 postcranial characters, and resolved Sinognathus and Beishanodon as closest to Langbergia. An identical taxonomic resolution was also found by Gaetano & Abdala (2015). However, Sidor & Hopson (2018) argued that Sinognathinae do not belong within trirachodontids, recovering them as traversodontids in their phylogenetic analysis. They speculated that they may instead be probainognathians that have convergently evolved transverse expansion of the postcanines. They identified several morphological features in the Chinese taxa that they considered to support a placement as traversodontids, such as the absence of the parietal foramen and jugal process and the significantly bowed zygomatic arch (Sidor & Hopson, 2018). Another analysis by Hendrickx et al. (2020) recovered Cricodon alongside Sinognathinae and suggested that Trirachodontidae is a paraphyletic grouping at the base of Traversodontidae.
Our analysis attempted to include all potentially parsimony informative traits among Cynognathia and the only characters that we excluded that were included in the matrix of Sidor & Hopson (2018) were those that identified differences between more basal taxa (Procynosuchus, Galesaurus, Thrinaxodon) and Probainognathia; for example the absence of the prefrontal (found in Tritylodontidae, Pachygenelus, and Morganucodon) and postorbital, shape of the internarial vomer, the secondary palatal plate not reaching the midline, length of secondary palate relative to the tooth row (shorter in all of Cynognathia) and the dorsal extent of the zygomatic arch. We also excluded characters for postcranial material due to insufficient availability of material for scoring in either the Chinese taxa or our newly described species.
We do not clearly resolve these Chinese taxa as being either trirachodontids or traversodontids. Although recovered as the sister taxon of the African trirachodontids in our consensus tree, the placement of Sinognathinae is extremely poorly supported, and taxon sampling in our analysis does not permit testing of the probainognathian hypothesis. Further research is required into the phylogenetic positions of the sinognathines, and indeed all the relationships within Gomphodontia. It is possible that convergent trait evolution is obscuring the correct placement of sinognathines. Until the recent description of Etjoia, sinognathines were the only taxa within Cynognathia, other than Diademodon, to possess conical anterior-most postcanines and they have an unusually slender snout in comparison to the rest of Cynognathia.
Conclusions
Guttigomphus avilionis is the sixth known trirachodontid species, and along with the recently described Impidens hancoxi it presents a rapidly expanding understanding of the Cricodon-Ufudocyclops subzone of the Karoo Basin’s Burgersdorp Formation. All currently undisputed trirachodontids are known from South Africa, Tanzania, and Zambia. A broader geographic range for the Trirachodontidae is possible, but confirmation requires additional study of various poorly known extra-African taxa, most notably the Chinese Sinognathinae. The temporal range of trirachodontids is also currently questionable; although an Anisian age is usually cited for the main CAZ fauna, the possibility that the upper Cricodon-Ufudocyclops Subzone is later (Ladinian or even Carnian) cannot be excluded at present. Radioisotopic data for these strata are needed to test whether they are coeval with “Cynognathus Zone-like” faunas globally.
Supplemental Information
Acknowledgments
Thanks to paleoartist Gabriel Ugueto for use of his artwork in Fig. 1. The silhouettes for Guttigomphus avilionis, Trirachodon kannemeyeri, Langbergia modisei and Impidens hancoxi are adaptations of life restorations from his work presented here with his permission. We thank Andrew Jones (Lapworth Museum, University of Birmingham) for providing 3D scanned images of the specimen. Thanks to John Hancox and Christophe Hendrickx for discussion and assistance for fieldwork, and to all those who have participated in our Cynognathus Zone fieldwork, including Emese Bordy, Daniel Cashmore, Michael Day, Kathleen Dollman, Emma Dunne, Martin Ezcurra, Pedro Godoy, John Hancox, Andrew Jones, Blair McPhee, James Neenan, Roland Sookias and Pia Viglietti. We thank Fernando Abdala, Leandro Gaetano and an anonymous reviewer and the editor for comments that helped improve the final version of the manuscript.
Abbreviations
- BP
Evolutionary Studies Institute (formerly the Bernard Price Institute for Palaeontological Research), University of the Witwatersrand, South Africa
- BSPG
Bayerische Staatssammlung für Paläontologie und Geologie, Munich, Germany
- NMQR
National Museum, Bloemfontein, South Africa
- UMZC
University Museum of Zoology, Cambridge, UK
Funding Statement
Funding for field work was provided by a Marie Curie Career Integration Grant (630123 to RJB), the NRF African Origins Platform (98800 to JNC), and by Palaeontological Scientific Trust (JNC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Romy R. Rayner conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Richard J. Butler conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Christian F. Kammerer conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Jonah N. Choiniere conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
The University of the Witwatersrand, Johannesburg, South Africa.
Data Availability
The following information was supplied regarding data availability:
The raw data used for phylogenetic analysis in TNT and characters and gradings are available in the Supplemental Files.
New Species Registration
The following information was supplied regarding the registration of a newly described species:
Guttigomphus avilionis LSID: urn:lsid:zoobank.org:act:57E697ED-DEC1-4E2D-A2E9-A6EC2E9A0FCA
Publication LSID: urn:lsid:zoobank.org:pub:61EC40CA-DA44-4E1A-8316-84BCE847BE20
References
- Abdala, Barberena & Dornelles (2002).Abdala F, Barberena MC, Dornelles J. A new species of the traversodontid cynodont Exaeretodon from the Santa Maria Formation (Middle/Late Triassic) of southern Brazil. Journal of Vertebrate Paleontology. 2002;22:313–325. doi: 10.1671/0272-4634(2002)022[0313:ANSOTT]2.0.CO;2. [DOI] [Google Scholar]
- Abdala et al. (2019).Abdala F, Gaetano LC, Smith RMH, Rubidge BS. A new large cynodont from the Late Permian (Lopingian) of the South African Karoo Basin and its phylogenetic significance. Zoological Journal of the Linnean Society. 2019;186:983–1005. doi: 10.1093/zoolinnean/zlz004. [DOI] [Google Scholar]
- Abdala, Hancox & Neveling (2005).Abdala F, Hancox PJ, Neveling J. Cynodonts from the uppermost Burgersdorp Formation, South Africa, and their bearing on the biostratigraphy and correlation of the Triassic Cynognathus Assemblage Zone. Journal of Vertebrate Paleontology. 2005;25:192–199. doi: 10.1671/0272-4634(2005)025[0192:CFTUBF]2.0.CO;2. [DOI] [Google Scholar]
- Abdala, Neveling & Welman (2006).Abdala F, Neveling J, Welman J. A new trirachodontid cynodont from the lower levels of the Burgersdorp Formation (Lower Triassic) of the Beaufort Group, South Africa and the cladistic relationships of Gondwanan gomphodonts. Zoological Journal of the Linnean Society. 2006;147:383–413. doi: 10.1111/j.1096-3642.2006.00224.x. [DOI] [Google Scholar]
- Anderson & Cruickshank (1978).Anderson JM, Cruickshank ARI. The biostratigraphy of the Permian and the Triassic. Part 5. A review of the classification and distribution of the Permo-Triassic tetrapods. Paleontologia Africana. 1978;21:15–44. [Google Scholar]
- Bordy et al. (2020).Bordy EM, Rampersadh A, Abrahams M, Lockley MG, Head HV. Tracking the Pliensbachian—Toarcian Karoo firewalkers. Trackways of quadruped and biped dinosaurs and mammaliaforms. PLOS ONE. 2020;15:e0226847. doi: 10.1371/journal.pone.0226847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha, Abdala & Smith (2007).Botha J, Abdala F, Smith R. The oldest cynodont: new clues on the origin and early diversification of the Cynodontia. Zoological Journal of the Linnean Society. 2007;149:477–492. doi: 10.1111/j.1096-3642.2007.00268.x. [DOI] [Google Scholar]
- Brink (1963).Brink AS. On Bauria cynops Broom. Palaeontologia Africana. 1963;8:39–56. [Google Scholar]
- Broom (1905).Broom R. On the use of the term Anomodontia. Records of the Albany Museum. 1905;1:266–269. [Google Scholar]
- Broom (1906).Broom R. V.—On the Permian and Triassic faunas of South Africa. Geological Magazine. 1906;3(1):29–30. doi: 10.1017/S001675680012271X. [DOI] [Google Scholar]
- Colbert & Kitching (1977).Colbert E, Kitching JW. Triassic cynodont reptiles from Antarctica. American Museum Novitates. 1977;2611:1–30. [Google Scholar]
- Crompton (1955).Crompton AW. On some Triassic cynodonts from Tanganyika. Proceedings of the Zoological Society of London. 1955;125(3–4):617–669. doi: 10.1111/j.1096-3642.1955.tb00620.x. [DOI] [Google Scholar]
- Crompton et al. (2017).Crompton AW, Owerkowicz T, Bhullar BS, Musinksy C. Structure of the nasal region of non-mammalian cynodonts and mammaliaforms: speculations on the evolution of mammalian endothermy. Journal of Vertebrate Paleontology. 2017;37:e1269116. doi: 10.1080/02724634.2017.1269116. [DOI] [Google Scholar]
- Damiani & Hancox (2003).Damiani RJ, Hancox PJ. New mastodonsaurid temnospondyls from the Cynognathus Assemblage Zone (Upper Beaufort Group: Karoo Basin) of South Africa. Journal of Vertebrate Paleontology. 2003;23:54–66. doi: 10.1671/0272-4634(2003)23[54:NMTFTC]2.0.CO;2. [DOI] [Google Scholar]
- Day et al. (2015).Day MO, Ramezani J, Bowring SA, Sadler PM, Erwin DH, Abdala F, Rubidge BS. When and how did the terrestrial mid-Permian mass extinction occur? Evidence from the tetrapod record of the Karoo Basin, South Africa. Proceedings of the Royal Society B. 2015;282:20150834. doi: 10.1098/rspb.2015.0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day & Smith (2020).Day MO, Smith RMH. Biostratigraphy of the Endothiodon Assemblage Zone (Beaufort Group, Karoo Supergroup), South Africa. South African Journal of Geology. 2020;123:165–180. doi: 10.25131/sajg.123.0011. [DOI] [Google Scholar]
- Estes (1961).Estes R. Cranial anatomy of the cynodont reptile Thrinaxodon liorhinus. Bulletin, Museum of Comparative Zoology, Harvard University. 1961;125:165–180. [Google Scholar]
- Gaetano & Abdala (2015).Gaetano LC, Abdala F. The stapes of gomphodont cynodonts: insights into the middle ear structure of non-mammaliaform cynodonts. PLOS ONE. 2015;10:e0131174. doi: 10.1371/journal.pone.0131174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al. (2010).Gao K-Q, Fox RC, Zhou C-F, Li D-Q. A new nonmammalian eucynodont (Synapsida: Therapsida) from the Triassic of northern Gansu Province, China, and its biostratigraphic and biogeographic implications. American Museum Novitates. 2010;3685:1–25. [Google Scholar]
- Goloboff & Catalano (2016).Goloboff PA, Catalano SA. TNT version 1. 5, including a full implementation of phylogenetic morphometrics. Cladistics. 2016;32:221–238. doi: 10.1111/cla.12160. [DOI] [PubMed] [Google Scholar]
- Goloboff & Pol (2007).Goloboff PA, Pol D. On divide-and-conquer strategies for parsimony analysis of large data sets: Rec-I-DCM3 versus TNT. Systematic Biology. 2007;56(3):485–495. doi: 10.1080/10635150701431905. [DOI] [PubMed] [Google Scholar]
- Gower et al. (2014).Gower DJ, Hancox PJ, Botha-Brink J, Sennikov AG, Butler RJ. A new species of Garjainia Ochev, 1958 (Diapsida, Archosauriformes: Erythrosuchidae) from the Early Triassic of South Africa. PLOS ONE. 2014;9:e111154. doi: 10.1371/journal.pone.0111154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewald, Kitching & Rubridge (1995).Groenewald GH, Kitching JW, Rubridge BS. Biostratigraphy of the Beaufort Group (Karoo Supergroup) Vol. 1. Pretoria: Council for Geosciences; 1995. Biostratigraphy of the Lystrosaurus Assemblage Zone; pp. 35–39. [Google Scholar]
- Hancox (1998).Hancox PJ. A stratigraphic, sedimentological and palaeoenvironmental synthesis of the Beaufort-Molteno contact in the Karoo Basin. PhD thesis (unpublished) 1998. University of the Witwatersrand, Johannesburg, South Africa.
- Hancox (2000).Hancox PJ. The continental Triassic of South Africa. Zentralblatt für Geologie und Paläontologie, Teil 1. 2000;11–12:1285–1324. [Google Scholar]
- Hancox, Neveling & Rubidge (2020).Hancox PJ, Neveling J, Rubidge BS. Biostratigraphy of the Cynognathus Assemblage Zone (Beaufort Group, Karoo Supergroup), South Africa. South African Journal of Geology. 2020;123:217–238. doi: 10.25131/sajg.123.0016. [DOI] [Google Scholar]
- Hancox & Rubidge (1996).Hancox PJ, Rubidge BS. The first specimen of the Mid-Triassic dicynodont Angonisaurus from the Karoo of South Africa: implications for the dating and biostratigraphy of the Cynognathus Assemblage Zone, Upper Beaufort Group. South African Journal of Science. 1996;92:391–392. [Google Scholar]
- Hancox et al. (1995).Hancox PJ, Shishkin MA, Rubidge BS, Kitching JW. A threefold subdivision of the Cynognathus Assemblage Zone (Beaufort Group, South Africa) and its palaeogeographical implications. South African Journal of Science. 1995;91:143–144. [Google Scholar]
- Hendrickx, Abdala & Choiniere (2019).Hendrickx C, Abdala F, Choiniere JN. A proposed terminology for the dentition of gomphodont cynodonts and dental morphology in Diademodontidae and Trirachodontidae. PeerJ. 2019;7:e6752. doi: 10.7717/peerj.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx et al. (2020).Hendrickx C, Gaetano LC, Choiniere JN, Mocke H, Abdala F. A new traversodontid cynodont with a peculiar postcanine dentition from the middle/late Triassic of Namibia and dental evolution in basal gomphodonts. Journal of Systematic Palaeontology. 2020;18:1669–1706. doi: 10.1080/14772019.2020.1804470. [DOI] [Google Scholar]
- Hopson (1991).Hopson JA. Systematics of the nonmammalian Synapsida and implications for patterns of evolution in synapsids. In: Schultze HD, Trueb L, editors. Origin of the Higher Groups of Tetrapods; Controversy and Consensus. Ithaca, NY: Comstock Publishing Associates, Cornell University Press; 1991. pp. 635–693. [Google Scholar]
- Hopson (2005).Hopson JA. A juvenile gomphodont cynodont specimen from the Cynognathus Assemblage Zone of South Africa: implications for the origin of gomphodont postcanine morphology. Palaeontologia Africana. 2005;41:53–66. [Google Scholar]
- Hopson & Kitching (2001).Hopson JA, Kitching JW. A probainognathian cynodont from South Africa and the phylogeny of non-mammalian cynodonts. Bulletin of the Museum of Comparative Zoology. 2001;155:445–475. [Google Scholar]
- Huene (1911).Huene FV. Über Erythrosuchus, Vertreter der neuen Reptil-Ordnung Pelycosimia. Geologische und Palaontologische Abhandlungen, Neue Folge. 1911;10:1–60. [Google Scholar]
- Jasinoski, Abdala & Fernandez (2015).Jasinoski S, Abdala F, Fernandez V. Ontogeny of the Early Triassic cynodont Thrinaxodon liorhinus (Therapsida): cranial morphology. The Anatomical Record. 2015;298:1440–1464. doi: 10.1002/ar.23116. [DOI] [PubMed] [Google Scholar]
- Kammerer (2016).Kammerer CF. A new taxon of cynodont from the Tropidostoma Assemblage Zone (Upper Permian) of South Africa, and the early evolution of Cynodontia. Papers in Palaeontology. 2016;2:387–397. doi: 10.5061/dryad.52064. [DOI] [Google Scholar]
- Kammerer et al. (2008).Kammerer CF, Flynn JJ, Ranivoharimanana L, Wyss AR. New material of Menadon besairiei (Cynodontia: Traversodontidae) from the Triassic of Madagascar. Journal of Vertebrate Paleontology. 2008;28:445–462. doi: 10.1671/0272-4634(2008)28[445:NMOMBC]2.0.CO;2. [DOI] [Google Scholar]
- Kammerer et al. (2019).Kammerer CF, Viglietti PA, Hancox PJ, Butler RJ, Choiniere JN. A new kannemeyeriiform dicynodont (Ufudocyclops mukanelai, gen. et sp. nov.) from Subzone C of the Cynognathus Assemblage Zone, Triassic of South Africa, with implications for biostratigraphic correlation with other African Triassic faunas. Journal of Vertebrate Paleontology. 2019;39:e1596921. doi: 10.1080/02724634.2019.1596921. [DOI] [Google Scholar]
- Keyser & Smith (1978).Keyser AW, Smith RMH. Vertebrate biozonation of the Beaufort Group with special reference to the Western Karoo Basin. Republic of South Africa: Geological Survey, Department of Mineral and Energy Affairs; 1978. [Google Scholar]
- Kitching (1958).Kitching JW. A new small stereospondylus labyrinthodont from the Triassic beds of South Africa. Palaeontologia Africana. 1958;5:67–82. [Google Scholar]
- Liu & Abdala (2014).Liu J, Abdala F. Chapter 15: phylogeny and taxonomy of the Traversodontidae. In: Kammerer CF, Angielczyk KD, Fröbisch J, editors. Early Evolutionary History of the Synapsida. New York: Springer; 2014. pp. 255–279. [Google Scholar]
- Lucas (1998).Lucas SG. Global Triassic tetrapod biostratigraphy and biochronology. Palaeogeography, Palaeoclimatology, Palaeoecology. 1998;143:347–384. doi: 10.1016/S0031-0182(98)00117-5. [DOI] [Google Scholar]
- Morales & Shishkin (2002).Morales M, Shishkin MA. A re-assessment of Parotosuchus africanus (Broom), a capitosauroid temnospondyl amphibian from the Triassic of South Africa. Journal of Vertebrate Paleontology. 2002;21:1–11. doi: 10.1671/0272-4634(2002)022[0001:ARAOPA]2.0.CO;2. [DOI] [Google Scholar]
- Neveling (2002).Neveling J. Biostratigraphic and sedimentological investigations of the contact between the Lystrosaurus and Cynognathus Assemblage Zones (Beaufort Group: Karoo Supergroup) 2002. PhD thesis (unpublished), University of the Witwatersrand, Johannesburg, South Africa.
- Neveling (2004).Neveling J. Council for Geoscience Bulletin. (South Africa) 2004. Stratigraphic and sedimentological investigation of the contact between the Lystrosaurus and Cynognathus Assemblage Zones (Beaufort Group: Karoo Supergroup) p. 137. [Google Scholar]
- Neveling, Hancox & Rubidge (2006).Neveling J, Hancox PJ, Rubidge BS. Biostratigraphy of the lower Burgersdorp Formation (Beaufort Group; Karoo Supergroup) of South Africa—implications for the stratigraphic ranges of Early Triassic tetrapods. Paleontologia Africana. 2006;41:81–87. [Google Scholar]
- Ottone et al. (2014).Ottone EG, Monti M, Marsicano CA, de la Fuente MS, Naipauer M, Armstrong R, Mancuso AC. A new late Triassic age for the Puesto Viejo Group (San Rafael depocenter, Argentina): SHRIMP U-Pb zircon dating and biostratigraphic correlations across southern Gondwana. Journal of South American Earth Sciences. 2014;56:186–199. doi: 10.1016/j.jsames.2014.08.008. [DOI] [Google Scholar]
- Owen (1859).Owen R. On some reptilian remains from South Africa. New Philosophical Journal. 1859;10:1–289. [Google Scholar]
- Owen (1861).Owen R. Palaeontology, or, a systematic summary of extinct animals and their geological relations. Edinburgh, U.K: Adam and Charles Black; 1861. [Google Scholar]
- Parrington (1934).Parrington FR. On the cynodont genus Galesaurus, with a note on the functional significance of the changes in the evolution of the theriodont skull. Annals and Magazine of Natural History. 1934;10:38–67. [Google Scholar]
- Pusch, Kammerer & Fröbisch (2019).Pusch LC, Kammerer CF, Fröbisch J. Cranial anatomy of the early cynodont Galesaurus planiceps and the origin of mammalian endocranial characters. Journal of Anatomy. 2019;234:592–621. doi: 10.1111/joa.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch, Kammerer & Fröbisch (2021).Pusch LC, Kammerer CF, Fröbisch J. Cranial anatomy of Bolotridon frerensis, an enigmatic cynodont from the Middle Triassic of South Africa, and its phylogenetic significance. PeerJ. 2021;9:e11542. doi: 10.7717/peerj.11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranivoharimanana et al. (2011).Ranivoharimanana L, Kammerer CF, Flynn JJ, Wyss AR. New material of Dadadon isaloi (Cynodontia, Traversodontidae) from the Triassic of Madagascar. Journal of Vertebrate Paleontology. 2011;31:1292–1302. doi: 10.1080/02724634.2011.618154. [DOI] [Google Scholar]
- Schluter (2008).Schluter T. Geological atlas of Africa. Berlin, Heidelberg: Springer; 2008. [Google Scholar]
- Seeley (1894).Seeley HG. Researches on the Structure, Organisation, and Classification of the Fossil Reptilia.—Part IX., Section 1. On the Therosuchia. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1894;185:987–1018. doi: 10.1098/rstb.1894.0021. [DOI] [Google Scholar]
- Seeley (1895a).Seeley HG. Researches on the structure, organisation, and classification of the fossil Reptilia.—Part IX., Section 4. On the Gomphodontia. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1895a;186:1–57. doi: 10.1098/rstb.1895.0001. [DOI] [Google Scholar]
- Seeley (1895b).Seeley HG. Researches on the structure, organization, and classification of the fossil Reptilia. Part IX, Section 5. On the skeleton in new Cynodontia from the Karroo Rocks. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1895b;186:59–148. doi: 10.1098/rstb.1895.0002. [DOI] [Google Scholar]
- Sidor & Hopson (2018).Sidor CA, Hopson JA. Cricodon metabolus (Cynodontia: Gomphodontia) from the Triassic Ntawere Formation of northeastern Zambia: patterns of tooth replacement and a systematic review of the Trirachodontidae. Journal of Vertebrate Paleontology. 2018;37:39–64. doi: 10.1080/02724634.2017.1410485. [DOI] [Google Scholar]
- Smith et al. (2020).Smith RMH, Rubidge BS, Day MO, Botha J. Introduction to the tetrapod biozonation of the Karoo Supergroup. South African Journal of Geology. 2020;123:131–140. doi: 10.25131/sajg.123.0009. [DOI] [Google Scholar]
- Tolchard et al. (2021).Tolchard F, Kammerer CF, Butler RJ, Hendrickx C, Benoit J, Abdala F, Choiniere J. A new large gomphodont from the Triassic of South Africa and its implications for Gondwanan biostratigraphy. Journal of Vertebrate Paleontology. 2021;41:e1929265. doi: 10.1080/02724634.2021.1929265. [DOI] [Google Scholar]
- Van den Brandt & Abdala (2018).Van den Brandt MJ, Abdala F. Cranial morphology and phylogenetic analysis of Cynosaurus suppostus (Therapsida, Cynodontia) from the upper Permian of the Karoo Basin, South Africa. Palaeontologia Africana. 2018;52:201–221. [Google Scholar]
- Viglietti et al. (2016).Viglietti PA, Smith RMH, Angielczyk KD, Kammerer CF, Fröbisch J, Rubidge BS. The Daptocephalus Assemblage Zone (Lopingian), South Africa: a proposed biostratigraphy based on a new compilation of stratigraphic ranges. Journal of African Earth Sciences. 2016;113:153–164. doi: 10.1016/j.jafrearsci.2015.10.011. [DOI] [Google Scholar]
- Watson (1914).Watson DMS. The zones of the Beaufort Beds of the Karroo System in South Africa. Geological Magazine. 1914;1:203–208. doi: 10.1017/S001675680019675X. [DOI] [Google Scholar]
- Young (1959).Young CC. Note on the first cynodont from the Sinokannemeyeria-faunas in Shansi, China. Vertebrata Palasiatica. 1959;3:124–132. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data used for phylogenetic analysis in TNT and characters and gradings are available in the Supplemental Files.