Abstract
Background
With the improvement of ultrasound technology, the likelihood of detection of major fetal structural anomalies in mid‐pregnancy has increased considerably. Upon the detection of serious anomalies, women typically are offered the option of pregnancy termination. Additionally, there are still many reasons other than fetal anomalies why women seek abortion in the mid‐trimester.
Objectives
To compare different methods of second trimester medical termination of pregnancy for their efficacy and side‐effects.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), MEDLINE, Popline and reference lists of retrieved papers and other sources.
Selection criteria
All randomised controlled trials (RCTs) examining medical regimens for termination of pregnancy of a singleton living fetus between 12‐28 weeks' gestation were analysed. The outcome measures were the induction to abortion interval, abortion rate within 24 hours, need for surgical evacuation, blood loss, uterine rupture, pain, and side‐effects.Trials including >20% fetal death, multiple pregnancies, previous uterine scars and regimens which involved cervical preparation were excluded.
Data collection and analysis
Two authors selected the trials and three authors extracted data.
Main results
Fourty RCTs were included, addressing various agents for pregnancy termination and methods of administration. When used alone, misoprostol was an effective inductive agent, though it appeared to be more effective in combination with mifepristone. However, the evidence from RCTs is limited.
Misoprostol was preferably administered vaginally, although among multiparous women sublingual administration appeared equally effective. A range of doses of vaginally administered misoprostol has been used. No randomised trials comparing doses of misoprostol were identified; however low doses of misoprostol appear to be associated with fewer side‐effects while moderate doses appear to be more efficient in completing abortion. Four RCTs showed that the induction to abortion interval with 3‐hourly vaginal administration of prostaglandins is shorter than 6‐hourly administration without an increase in side‐effects.
Many studies reported the need for surgical evacuation. Indications for surgical evacuation include retained products of the placenta and heavy vaginal bleeding. Fewer women required surgical evacuation when misoprostol was administrated vaginally compared with women receiving intra‐amniotical PGF2a. Mild, self‐limiting diarrhoea was more common among women who received misoprostol compared to other agents.
Authors' conclusions
Medical abortion in the second trimester using the combination of mifepristone and misoprostol appeared to have the highest efficacy and shortest abortion time interval. Where mifepristone is not available, misoprostol alone is a reasonable alternative. The optimal route for administering misoprostol is vaginally, preferably using tablets at 3‐hourly intervals. Apart from pain, the side‐effects of vaginal misoprostol are usually mild and self limiting. Conclusions from this review are limited by the gestational age ranges and variable medical regimens, including dosing, administrative routes and intervals of medication, of the included trials.
Plain language summary
Planned abortion after three months of pregnancy can be done using several medicines. This review looked at which medical procedure is the best.
There are many medical methods for planned termination of pregnancy in the second trimester of pregnancy (abortion after three months). We did a search of the scientific literature to find out which is the best method. We identified 38 studies and came to the conclusion that misoprostol is the drug of choice for medical pregnancy termination, preferably in combination with mifepristone which facilitates the effectiveness of misoprostol. Misoprostol works best when it is administered into the vagina. Women who had previously given birth could take misoprostol by mouth (under the tongue). Irrespective of the medication used for second trimester termination there is a considerable risk of surgical intervention because of vaginal bleeding or incomplete abortion.
Background
With the wide‐scale introduction of prenatal screening programmes the issue of second trimester abortion has become increasingly relevant, in particular for women whose pregnancies are complicated by a serious fetal anomaly (Asch 1999; Ballantyne 2009; Boyd 2008). Additionally, there are many reasons other than fetal anomalies for which women seek abortion in the midtrimester (Drey 2006; Grimes 1998; Ingham 2008). Second trimester abortion for fetal structural anomalies may have advantages over surgical abortion as it is operator independent and the intact fetus may be preferable for feto‐pathological examination (Akgun 2007; Isaksen 1998; Isaksen 1999; Kaasen 2006). Medical abortion, however, also has several limitations including the need for the hospitalisation, the need for surgical removal of (the retained products of) the placenta when indicated, and the emotional impact of the process of labor and delivery on women who choose to end a pregnancy.
Medical abortion in the first trimester of pregnancy is considered successful if complete expulsion of the conceptus occurs without the need for surgical intervention (Christin‐Maitre 2000). Beyond the first trimester, definitions differ but generally consider expulsion of the fetus separate from management of the placenta.
Several regimens for second trimester abortion have been published. Most of these are based on misoprostol or gemeprost, which are synthetic prostaglandin E1 analogues (PGE1), and used alone or misoprostol combined with mifepristone. Comparison of medical methods with surgical evacuation for mid‐trimester termination of pregnancy is the subject of another review (Lohr 2008).
Agents used for medical abortion
Prostaglandins
Prostaglandins and their analogues are widely used for medical termination of pregnancy. Prostaglandins are produced by almost every tissue in the body and play a major role in human reproduction and in many other vital processes. To date, nine groups (A, B, C, D, E, F, G, H, I) and three types (PG1, PG2, PG3) of prostaglandins have been identified. Prostaglandins of the F and E series are the most important prostaglandins involved in pregnancy, labor, delivery and puerperium. PG receptors are always present in myometrial tissue.
Misoprostol (PGE1) is increasingly used for second trimester termination of pregnancy (Friedman 2001; Goldberg 2001; Wagner 2005; Weeks 2005). Misoprostol is marketed for use in the prevention and treatment of peptic ulcer disease, and it is registered for obstetric indications, including abortion, in a few countries. It is inexpensive, stable at room temperature and it is rapidly absorbed by vaginal, sublingual, buccal and oral routes (Tang 2002; Zieman 1997). Moreover, misoprostol is reportedly associated with few, relatively minor side‐effects. Serious complications such as uterine rupture are rare.
Gemeprost (PGE1) is formulated as a vaginal suppository which requires refrigeration, and is not as widely available as misoprostol. Like other prostaglandins, it induces uterine contractions and cervical softening.
Dinoprost (PGF2α) and dinoprostone (PGE2) are natural prostaglandins which induce uterine activity and are available for intravenous, intra‐amniotic and extra‐amniotic use.
Carboprost (15 methyl PGF2α) can be given by intramuscular or intra‐amniotic injection and its methyl ester can be given as vaginal suppository. Carboprost and its methyl ester are both effective in inducing uterine contractions.
Sulprostone (PGE2) is used intravenously. The intramuscular preparation of sulprostone is no longer available because it was associated with cardiovascular complications, such as acute myocardial infarction and hypotension (Ulmann 1992).
Uterotonic agents other than prostaglandins
Mifepristone, also known as RU 486 or RU 38486, is a 19‐norsteroid that specifically blocks the receptors for progesterone and glucosteroids. It is used as pretreatment 24 to 48 hours prior to the induction of first trimester abortion with a prostaglandin analogue. It sensitizes the myometrium of the uterus to prostaglandin (Belanger 1981; Bygdeman 1985; Norman 1992; Swahn 1988).
Oxytocin is released physiologically by the posterior pituitary and stimulates uterine contractions. The sensitivity of the uterus to oxytocin increases with gestational age.
Injection techniques
Intra‐amniotic instillation
Several chemical solutions for intra‐amniotic injection techniques have been used, including formalin, glucose, hypertonic saline, urea and PGF2α. When using hypertonic saline, a spinal needle is passed through the abdominal wall into the amniotic cavity. A variable amount of the amniotic fluid surrounding the fetus is removed and replaced by 150 to 250 ml of 20% saline chloride solution that will induce abortion (Bygdeman and Gemzell‐Danielsson 2008).
Extra‐amniotic instillation
Instead of passing a spinal needle directly into the amniotic sac, effective irritants, such as ethacridine lactate or PGF2α, can be introduced through the cervix into the extra‐amniotic space, that is, the space between the uterine wall and the fetal membranes. Ethacridine lactate is an organic compound based on acridine. Its primary use is as an antiseptic in solutions of 0.1%. When used as an agent for second trimester abortion, it is thought to stimulate endogenous prostaglandin production and subsequent uterine contractions. Up to 150 ml of 0.1% ethacridine is instilled into the extra‐amniotic space using a Foley catheter. Oxytocin intravenous administration is often used concomitantly to expedite fetal expulsion (Bygdeman and Gemzell‐Danielsson 2008).
Description of the problem or issue
Second trimester abortions constitute 10% to 15% of all induced abortions worldwide but are responsible for two‐thirds of major abortion‐related complications (Drey 2006; Grimes 1998). Medical methods for second trimester induced abortion have improved considerably during the last decades in terms of efficacy and safety; however, a variety of regimens remain in use.
Why it is important to do this review
Because of improved ultrasound technology, the prenatal detection of fetal structural anomalies during the second trimester of pregnancy has improved substantially. For this reason, the demand for medical methods to terminate pregnancy during the second trimester has also increased (Grimes 1998). Additionally, there are a number of other reasons why women seek abortion in the mid‐trimester. There are many medical regimens for mid‐trimester termination of pregnancy. This review aims to identify the most effective medical regimens for mid‐trimester abortion with the fewest side‐effects.
Objectives
To evaluate the medical regimens for second trimester medical abortion in terms of efficacy and side‐effects.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials were considered for inclusion if different medical methods, routes of application or doses used for second trimester medical abortion were compared.
Types of participants
Studies which included healthy women undergoing a second trimester abortion were eligible if carrying a singleton, living fetus between 12 to 28 weeks gestation. Studies including women with multiple pregnancies, or those who had cervical preparation prior to the abortion procedure were excluded.
Types of interventions
Different medical methods, administration routes or doses of medication used for second trimester medical abortion.
Types of outcome measures
The main outcome measures were the induction to abortion interval and the number of complete abortions within 24 hours. The primary endpoint for the abortion interval is expulsion of the fetus. The secondary endpoint for the abortion interval is the expulsion of the placenta. In addition, other secondary outcome measures included the need for surgical evacuation (non‐emergency procedure, emergency procedure, or not specified, including manual removal of the placenta), blood loss (measured, need for blood transfusion or clinically relevant drop in haemoglobin), uterine rupture, pain resulting from the procedure (reported by the women or measured by use of analgesics), nausea, vomiting and diarrhoea. The table Characteristics of included studies includes whether a surgical intervention was performed routinely in the study.
Potential confounding
The sensitivity of the myometrium for uterotonic drugs increases with gestational age. Hence, the longer the gestation, the less uterotonic drugs are needed. Apart from gestational age, parity could also be viewed as a potential confounder as multiparous women appear to have a shorter time interval to abortion. Differential treatment effects could theoretically be ascribed by differences in gestational age or parity. Parity and gestational age of study participants are listed under Characteristics of included studies.
Search methods for identification of studies
See:Collaborative Review Group search strategy.
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), MEDLINE and Popline were systematically searched. Reference lists of retrieved papers were searched. Electronic literature search was conducted using the following key words: (induced abortion) AND (second trimester) AND (mifepristone OR misoprostol OR methotrexate OR dinoprost OR dinoprostone OR carboprost OR sulprostone OR gemeprost OR meteneprost OR epostane OR oxytocin OR RU 486 OR mifegyne) OR ethacridine lactate AND ((randomised controlled trial[pt] OR controlled clinical trial[pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw] ) OR ((singl* [tw] OR doubl* [tw] OR tripl* [tw] ) AND (mask* [tw] OR blind* [tw] )) OR ("latin square" [tw] ) OR placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR cross‐over studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw] ) NOT (animal [mh] NOT human [mh]) )
Data collection and analysis
Selection of studies
The selection of trials for inclusion was performed independently by two review authors after employing the research strategy described previously. Trials under consideration were evaluated for inclusion and methodological quality without consideration of the results. This review is limited to randomised controlled trials, thereby focusing on four types of medical interventions for second trimester termination of pregnancy, that is (1) mifepristone and prostaglandin, (2) misoprostol, (3) other prostaglandins and (4) hyperosmolar agents (hypertonic saline, ethacridine lactate).
A form was designed to facilitate the process of data extraction which was performed by two of the reviewers independently. There were no discrepancies between the reviewers in either decision of inclusion/exclusion of studies or in data extraction.
Trials were not excluded based on an arbitrary cut‐off limit regarding losses to follow up. Subgroup analyses were planned for early and late second trimester abortions as the performance of methods may differ with gestational age.
Trials describing the use of quinine were excluded. Trials including more than 20% fetal death at the onset of treatment (unless separate analysis was available), multiple pregnancies, women with uterine scars (if reportedly included in the trial) and regimens which included cervical preparation prior to the abortion procedure were excluded.
Data extraction and management
Data were extracted by two authors from eligible studies. Study characteristics (type of study, allocation, blinding), participants characteristics (number, gestational age), interventions, main outcome measures and results were recorded. An attempt was made to obtain additional information from authors if required.
Assessment of risk of bias in included studies
The quality of studies was assessed without blinding to authorship or journal. Bias was assessed using the following.
1. Allocation concealment. The quality score for concealment of allocation was assigned to each trial using the criteria in the Cochrane Handbook:
A adequate concealment of allocation;
B unclear whether adequate concealment of allocation;
C inadequate concealment of allocation (includes quasi‐randomised studies.
Only trials scoring A or B were included in the review.
2. Blinding of participants, clinicians and investigators.
3. Protection against exclusion bias.
4. Appropriate analysis of data.
Assessment of heterogeneity
Heterogeneity was analysed using the I² statistic (Higgins 2003). I² values of 25%, 50%, and 75% correspond to low, medium and high levels of heterogeneity.
Data synthesis
Data analyses was performed by using Revman 5 software.
Trials that were conducted within the subject of this review included the comparison of different medical methods, application methods and dose regimens. For this reason, the trials were considered by medical regimen comparing the outcome measures for each regimen as described earlier. The different comparisons are as follows.
Comparison 1: mifepristone + misoprostol versus mifepristone + gemeprost.
Comparison 2: mifepristone + misoprostol versus misoprostol alone.
Comparisons 3 to 5: routes of administration of misoprostol combined with mifepristone:
Comparison 3: vaginal use versus the oral use of misoprostol;
Comparison 4: vaginal use versus the sublingual use of misoprostol;
Comparison 5: oral use versus the sublingual use of misoprostol.
Comparison 6: dosing interval of misoprostol following mifepristone.
Comparison 7: dosing of mifepristone previous to misoprostol.
Comparison 8: combined regimen of mifepristone + gemeprost.
Comparisons 9 to 11: misoprostol versus another prostaglandin:
Comparison 9: misoprostol versus intra‐amniotic PGF2α;
Comparison 10: misoprostol versus gemeprost;
Comparison 11: misoprostol versus dinoprostone.
Comparisons 12 to 13: routes of administration of misoprostol:
Comparison 12: vaginal use versus the oral use of misoprostol;
Comparison 13: vaginal use versus the sublingual use of misoprostol.
Comparison 14: misoprostol tablet insertion versus gel insertion.
Comparisons 15 to 16: time interval for repeat dosing of misoprostol or gemeprost:
Comparison 15: Time interval of misoprostol;
Comparison 16: Time interval of gemeprost.
Comparison 17: low dose versus a higher dose of misoprostol.
Comparisons 18 to 19: prostaglandin E2 versus prostaglandin F2α:
Comparison 18: prostaglandin E2 versus prostaglandin F2α;
Comparison 19: prostaglandin E2 versus prostaglandin F2α + oxytocin.
Comparison 20: intra‐amniotic instillation of prostaglandin F2α versus intra‐amniotic instillation of hypertonic saline (20%).
Comparison 21: combined regimen intra‐amniotic prostaglandin F2α + hypertonic saline.
Comparison 22: prostaglandin E1 vaginally versus the intra‐amniotic instillation of prostaglandin F2α + hypertonic saline.
Comparison 23: prostaglandins versus ethacridine lactate.
Comparison 24: ethacridine lactate versus normal saline.
Results
Description of studies
See: Characteristics of included studies;Characteristics of excluded studies
Results of the search
Eighty‐eight studies underwent full review. Full text review excluded 52 studies as they were not randomised; used inadequate concealment (score C); included over 20% fetal demise, multiple pregnancies, patients with uterine scarring; a pre‐treatment trial, including medical or mechanical dilatation of the cervix. See Characteristics of excluded studies.
Forty studies were included in this review. Due to the diversity of the interventions, the review concerns the comparison of 24 regimens. Three trials compared more than two different groups and the interventions are therefore listed as different comparisons (Mehta 1975 a; Mehta 1975 b; Muzsnai 1979 a; Muzsnai 1979 b; Muzsnai 1979 c; Muzsnai 1979 d; Nuutila 1997 a; Nuutila 1997 b; Nuutila 1997 c). The main outcomes considered were induction to abortion interval or completed abortion within 24 hours.
Included studies
Four studies (Borgida 1995; Ho 1996; Nielsen 1975; Steyn 1993) each enrolled 50 patients or less.
Four separate interventions were used.
1. Mifepristone and prostaglandin
Three studies (210 patients) compared a regimen of mifepristone and misoprostol to mifepristone and gemeprost (Bartley 2002; el‐Refaey 1993; Ho 1996).
One study (64 patients) compared mifepristone to a placebo prior to misoprostol induced abortion (Kapp 2007).
-
Five studies (500 patients) compared different routes of administration of misoprostol combined with mifepristone:
vaginal use compared to oral use (306 patients) (El‐Refaey 1995; Ho 1997; Ngai 2000);
vaginal use compared to sublingual use (76 patients) (Hamoda 2005);
oral use compared to sublingual use (118 patients) (Tang 2005).
One study (141 patients) compared the dosing interval of misoprostol following mifepristone administration (Chai 2009).
One study (70 patients) compared the dosage of mifepristone before misoprostol was administered (Webster 1996).
One study (100 patients) compared 0.5mg and 1.0 mg gemeprost combined with mifepristone (Thong 1996).
2. Misoprostol
-
Five studies (693 patients) compared misoprostol to another prostaglandin:
one studies (125 patients) compared the vaginal use of misoprostol to PGF2α (Su 2005);
two studies (221 patients) compared the vaginal use of misoprostol to gemeprost (Nuutila 1997 a; Nuutila 1997 b; Wong 1998);
one study (130 patients) compared the vaginal use of misoprostol to dinoprostone (Makhlouf 2003).
-
Five studies (812 patients) compared different routes of administration of misoprostol:
vaginal use compared to oral use (310 patients) (Akoury 2004; Bebbington 2002; Behrashi 2008);
vaginal use compared to sublingual use (502 patients) (Bhattacharjee 2008; Tang 2004; von Hertzen 2009).
One study (148 patients) compared misoprostol tablets to gel insertion (Pongsatha 2008).
Three studies (427 patients) compared different time intervals of misoprostol or gemeprost (Armatage 1996; Herabutya 2005; Wong 2000).
Two studies (133 patients) compared different doses of misoprostol (Nuutila 1997 c; Ozerkan 2009).
2. Other prostaglandins
Three studies (143 patients) compared prostaglandin E2 to prostaglandin F2α (Borgida 1995; Sorensen 1984; Steyn 1993).
3. Hyperosmolar agents
Four studies (1670 patients) compared hypertonic saline and prostaglandin F2α (Faktor 1988; Mehta 1975 a; Mehta 1975 b; Nielsen 1975; WHO 1976).
One study (385 patients) compared different regimens of prostaglandin F2α and hypertonic saline (Muzsnai 1979 a; Muzsnai 1979 b; Muzsnai 1979 c; Muzsnai 1979 d).
One study (58 patients) compared gemeprost to prostaglandin F2α and hypertonic saline (Waldron 1990).
Three studies (302 patients) compared prostaglandins to ethacridine lactate (Inan 1997; Kelekci 2006; Olund 1978).
One study (37 patients) compared ethacridine lactate to normal saline (Zauva 1989).
Risk of bias in included studies
Only randomised controlled trials were included in this review. Thirty‐two studies reported the method of randomisation (Akoury 2004; Armatage 1996; Bartley 2002; Bebbington 2002; Bhattacharjee 2008; Borgida 1995; Chai 2009; el‐Refaey 1993; El‐refaey 1995; Hamoda 2005; Herabutya 2005; Ho 1996; Ho 1997; Kapp 2007; Kelekci 2006; Makhlouf 2003; Mehta 1975 a; Mehta 1975 b; Ngai 2000; Nuutila 1997 a; Nuutila 1997 b; Nuutila 1997 c; Ozerkan 2009; Pongsatha 2008; Sorensen 1984; Steyn 1993; Su 2005; Tang 2004; Tang 2005; Thong 1996; von Hertzen 2009; Webster 1996; WHO 1976; Wong 1998; Wong 2000). For more detailed information, see the section Included studies. Eleven studies did not state the inclusion or exclusion criteria (Bartley 2002; el‐Refaey 1993; El‐refaey 1995; Faktor 1988; Inan 1997; Mehta 1975 a; Mehta 1975 b; Nielsen 1975; Nuutila 1997 a; Nuutila 1997 b; Nuutila 1997 c; Olund 1978; Ozerkan 2009; Webster 1996).
Allocation
Allocation concealment was adequately reported in 28 studies (Akoury 2004; Armatage 1996; Bartley 2002; Bebbington 2002; Bhattacharjee 2008; Borgida 1995; Chai 2009; el‐Refaey 1993; El‐refaey 1995; Hamoda 2005; Herabutya 2005; Ho 1996; Ho 1997; Kapp 2007; Makhlouf 2003; Mehta 1975 a; Mehta 1975 b; Ngai 2000; Nuutila 1997 a; Nuutila 1997 b; Nuutila 1997 c; Steyn 1993; Su 2005; Tang 2004; Tang 2005; Thong 1996; von Hertzen 2009; Webster 1996; WHO 1976; Wong 1998; Wong 2000).
Blinding
Blinding (no further explanation was given) was reported in one study (von Hertzen 2009). Blinding of participants was reported in one study (Ngai 2000). Blinding of participants and clinicians was reported in two studies (Ho 1997; Tang 2005). Blinding of participants, clinicians and researchers was reported in one study (Kapp 2007).
Effects of interventions
For outcomes using a continuous scale, the mean difference (MD) with 95% confidence intervals (95% CI) were used to asses the effects of the intervention. For dichotomous outcomes the results were expressed using odds ratio (OR) with 95% confidence intervals (95% CI).
Comparison 1: combined regimen mifepristone + misoprostol versus mifepristone + gemeprost
Three trials (Bartley 2002; el‐Refaey 1993; Ho 1996) were included in this comparison. In total, 210 women were eligible for analysis. In regards to the induction to abortion interval, el‐Refaey 1993 did not provide standard deviations, and thus precluded inclusion of these data in the meta‐analysis. The median induction to abortion interval was 8 hrs (range 1 to 60) and 9.1 hrs (range 3 to 22) for the oral use of 400 μg misoprostol and the 1 mg gemeprost pessaries group respectively (not significant). There were no significant differences in the induction‐to‐abortion interval (Analysis 1.1), abortion rate within 24 hours (Analysis 1.2), need for surgical evacuation (Analysis 1.3) or side‐effects (pain, Analysis 1.4; nausea, Analysis 1.5; vomiting, Analysis 1.6; diarrhoea, Analysis 1.7) between regimens using mifepristone with either misoprostol or gemeprost.
1.1. Analysis.
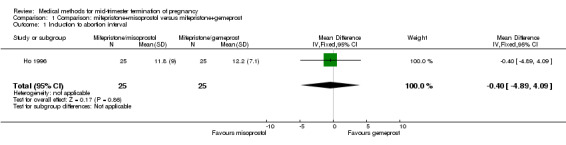
Comparison 1 Comparison: mifepristone+misoprostol versus mifepristone+gemeprost, Outcome 1 Induction to abortion interval.
1.2. Analysis.
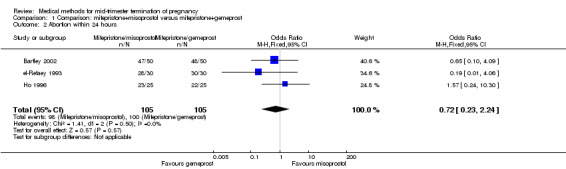
Comparison 1 Comparison: mifepristone+misoprostol versus mifepristone+gemeprost, Outcome 2 Abortion within 24 hours.
1.3. Analysis.
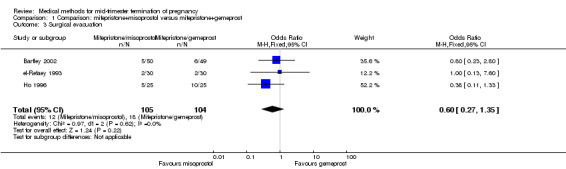
Comparison 1 Comparison: mifepristone+misoprostol versus mifepristone+gemeprost, Outcome 3 Surgical evacuation.
1.4. Analysis.
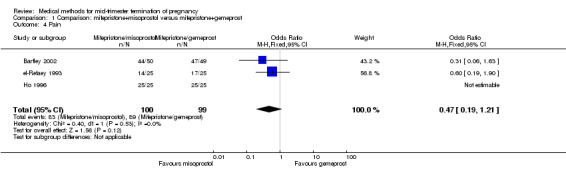
Comparison 1 Comparison: mifepristone+misoprostol versus mifepristone+gemeprost, Outcome 4 Pain.
1.5. Analysis.
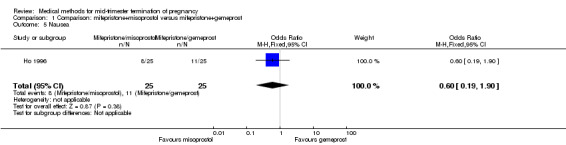
Comparison 1 Comparison: mifepristone+misoprostol versus mifepristone+gemeprost, Outcome 5 Nausea.
1.6. Analysis.
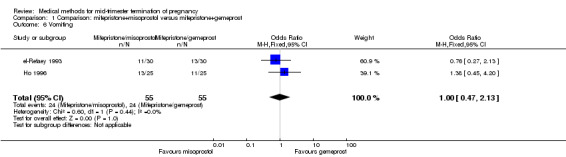
Comparison 1 Comparison: mifepristone+misoprostol versus mifepristone+gemeprost, Outcome 6 Vomiting.
1.7. Analysis.
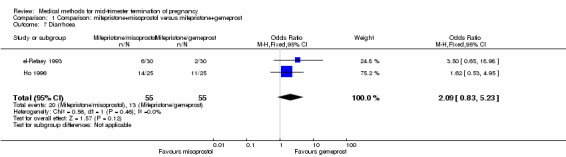
Comparison 1 Comparison: mifepristone+misoprostol versus mifepristone+gemeprost, Outcome 7 Diarrhoea.
Comparison 2: misoprostol versus mifepristone + misoprostol
One trial (Kapp 2007) was included in this comparison. In this regimen, 64 women were eligible for analysis. Women who received mifepristone + misoprostol aborted more rapidly than women who had misoprostol alone (Analysis 2.1) (MD 12.13, 95% CI 1.43 to 102.61). No difference was found in terms of the need for surgical evacuation (Analysis 2.2), pain (Analysis 2.3), vomiting (Analysis 2.5) or nausea (Analysis 2.4).
2.1. Analysis.
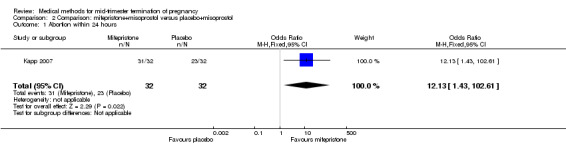
Comparison 2 Comparison: mifepristone+misoprostol versus placebo+misoprostol, Outcome 1 Abortion within 24 hours.
2.2. Analysis.
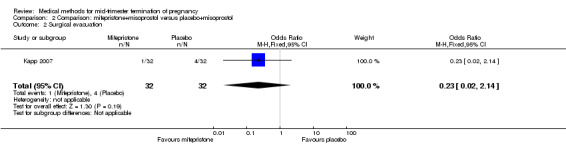
Comparison 2 Comparison: mifepristone+misoprostol versus placebo+misoprostol, Outcome 2 Surgical evacuation.
2.3. Analysis.
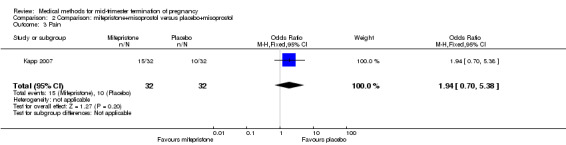
Comparison 2 Comparison: mifepristone+misoprostol versus placebo+misoprostol, Outcome 3 Pain.
2.5. Analysis.
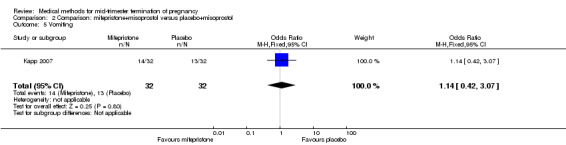
Comparison 2 Comparison: mifepristone+misoprostol versus placebo+misoprostol, Outcome 5 Vomiting.
2.4. Analysis.
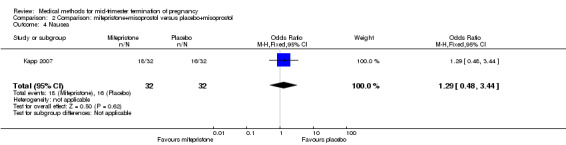
Comparison 2 Comparison: mifepristone+misoprostol versus placebo+misoprostol, Outcome 4 Nausea.
Comparisons 3 to 5: routes of administration of misoprostol combined with mifepristone
In this comparison, five trials with three comparisons (El‐refaey 1995; Hamoda 2005; Ho 1997; Ngai 2000; Tang 2005) were included.
Comparison 3: vaginal use compared to oral use of misoprostol
In this comparison, three trials (El‐refaey 1995; Ho 1997; Ngai 2000) were included. In total, 306 women were eligible for analysis. The largest trial in this analysis was conducted by Ngai, comparing the oral use of 400 μg and the vaginal use of 200 μg of misoprostol, both combined with 200 mg mifepristone administered orally 36 to 48 hours in advance. In addition to this comparison, both groups received either vaginal or oral placebo tablets. Ho 1997 conducted a randomised controlled trial comparing the use of 200 μg of misoprostol orally combined with a vaginal placebo to the vaginal use of 200 μg misoprostol combined with an oral placebo, each arm combined with 200 mg mifepristone. The vaginal use of 200 μg misoprostol was superior (MD 13.00, 95% CI 2.77 to 23.23) to the oral use of 200 μg misoprostol (Analysis 3.1). When both oral and vaginal misoprostol was administered in a low dose (200 μg), the vaginal use was superior to the oral use regarding the abortion rate within 24 hours (Analysis 3.2) (OR 0.26, 95% CI 0.09 to 0.78). In regards to the induction‐to‐abortion interval, El Refaey 1995 did not provide a standard deviation in addition to estimates which precluded the inclusion of these data in the meta‐analysis. The mean induction to abortion interval was 6.0 hrs (95% CI 5.0 to 7.2) and 6.7 hrs (95% CI 5.8 to 7.6) for the oral and the vaginal groups respectively (not significant). No significant difference was found between the oral and vaginal use of misoprostol in terms of induction‐to‐abortion interval. In addition, no difference was found between the need for surgical evacuation (Analysis 3.3), amount of pain (Analysis 3.4), nausea (Analysis 3.5) or vomiting (Analysis 3.6). However, fewer episodes of diarrhoea occurred (Analysis 3.7) (OR 2.21, 95% CI 1.06 to 4.61) when 200 μg misoprostol was administered vaginally compared to 400 μg misoprostol, orally.
3.1. Analysis.
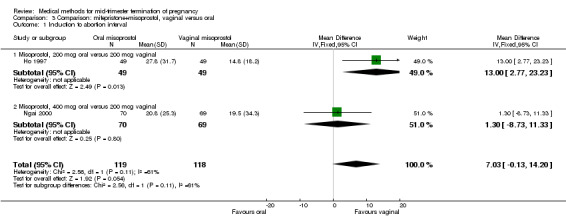
Comparison 3 Comparison: mifepristone+misoprostol, vaginal versus oral, Outcome 1 Induction to abortion interval.
3.2. Analysis.
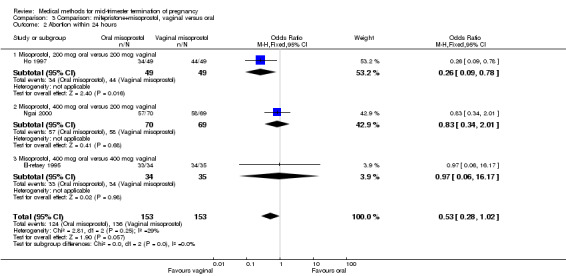
Comparison 3 Comparison: mifepristone+misoprostol, vaginal versus oral, Outcome 2 Abortion within 24 hours.
3.3. Analysis.
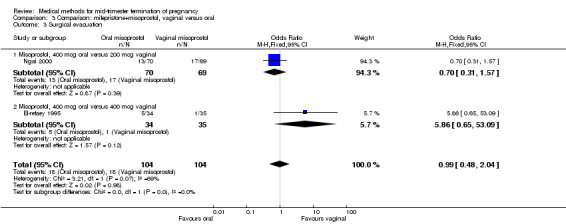
Comparison 3 Comparison: mifepristone+misoprostol, vaginal versus oral, Outcome 3 Surgical evacuation.
3.4. Analysis.
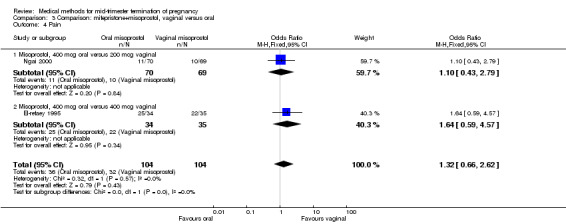
Comparison 3 Comparison: mifepristone+misoprostol, vaginal versus oral, Outcome 4 Pain.
3.5. Analysis.
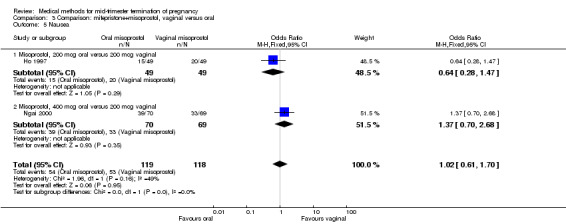
Comparison 3 Comparison: mifepristone+misoprostol, vaginal versus oral, Outcome 5 Nausea.
3.6. Analysis.
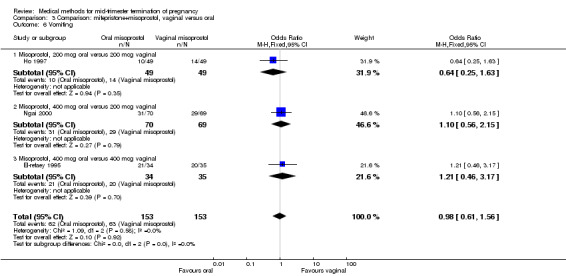
Comparison 3 Comparison: mifepristone+misoprostol, vaginal versus oral, Outcome 6 Vomiting.
3.7. Analysis.
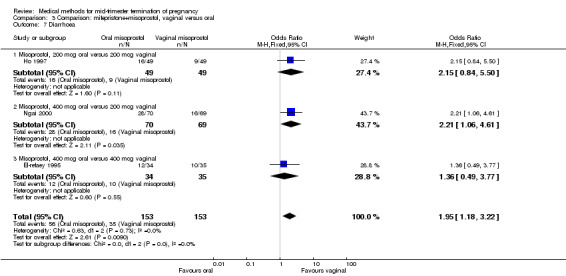
Comparison 3 Comparison: mifepristone+misoprostol, vaginal versus oral, Outcome 7 Diarrhoea.
Comparison 4: vaginal use compared to sublingual use of misoprostol
In this comparison, one trial (Hamoda 2005) was included. In total, 69 women were eligible for analyses. The comparison was made between the sublingual use of 600 μg of misoprostol and the vaginal use of 800 μg, both combined with 200 mg mifepristone. In regards to the induction‐to‐the abortion interval, no mean or SD was provided and thus precluded inclusion of these data in a meta‐analysis. The median and range of the sublingual group (median 5.27, range 0.55 to 29.35) and of the vaginal group (median 5.40, range 2.10 to 13.00) showed no significant difference (P = 0.95) for the abortion interval. In addition, no difference between the sublingual and vaginal use was found in terms of the need for surgical evacuation (Analysis 4.1) or side‐effects such as pain (Analysis 4.2), nausea (Analysis 4.3), vomiting (Analysis 4.4) or diarrhoea (Analysis 4.5).
4.1. Analysis.
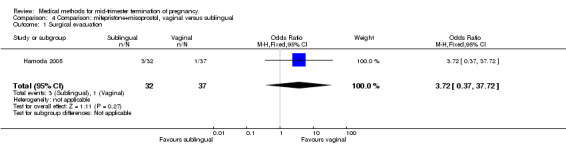
Comparison 4 Comparison: mifepristone+misoprostol, vaginal versus sublingual, Outcome 1 Surgical evacuation.
4.2. Analysis.
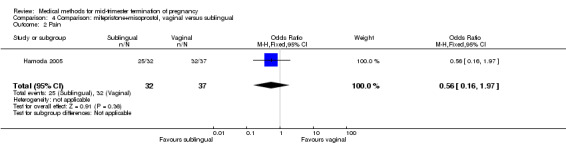
Comparison 4 Comparison: mifepristone+misoprostol, vaginal versus sublingual, Outcome 2 Pain.
4.3. Analysis.
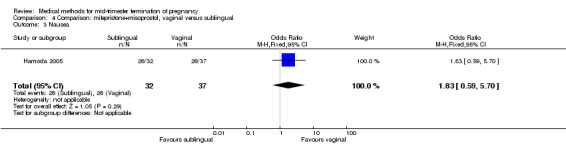
Comparison 4 Comparison: mifepristone+misoprostol, vaginal versus sublingual, Outcome 3 Nausea.
4.4. Analysis.
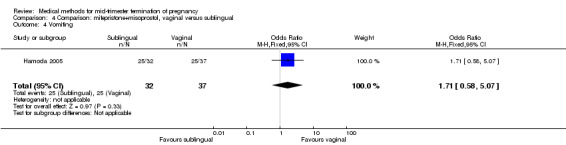
Comparison 4 Comparison: mifepristone+misoprostol, vaginal versus sublingual, Outcome 4 Vomiting.
4.5. Analysis.
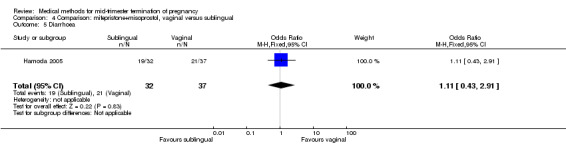
Comparison 4 Comparison: mifepristone+misoprostol, vaginal versus sublingual, Outcome 5 Diarrhoea.
Comparison 5: oral use compared to sublingual use of misoprostol
In this comparison, one trial (Tang 2005) was included. In total, 118 women were eligible for analysis. Tang 2005 compared the oral and sublingual use of 400 μg of misoprostol in combination with 200 mg of mifepristone. Both groups received oral or sublingual placebo tablets. In regards to the induction‐to‐the abortion interval, no mean or SD was provided and thus precluded inclusion of these data in a meta‐analysis. The sublingual group (median 5.5, range 1.4 to 43.2) aborted in a shorter time interval when compared to the oral group (median 7.5, range 2.4 to 38.8) (P = 0.009). No difference was found in regards to the abortion rate within 24 hours (Analysis 5.1) or side‐effects such as pain (Analysis 5.2), nausea (Analysis 5.3) or diarrhoea (Analysis 5.4).
5.1. Analysis.
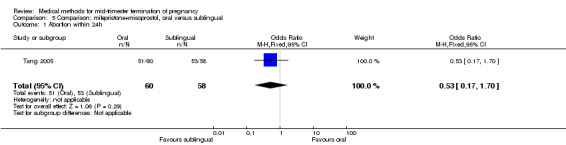
Comparison 5 Comparison: mifepristone+misoprostol, oral versus sublingual, Outcome 1 Abortion within 24h.
5.2. Analysis.
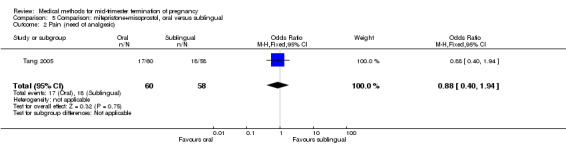
Comparison 5 Comparison: mifepristone+misoprostol, oral versus sublingual, Outcome 2 Pain (need of analgesic).
5.3. Analysis.
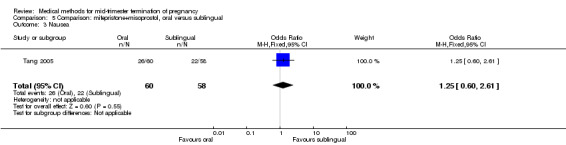
Comparison 5 Comparison: mifepristone+misoprostol, oral versus sublingual, Outcome 3 Nausea.
5.4. Analysis.
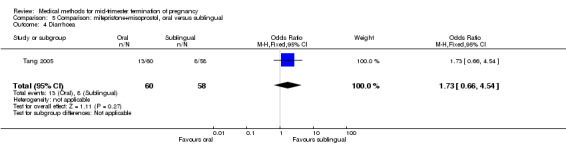
Comparison 5 Comparison: mifepristone+misoprostol, oral versus sublingual, Outcome 4 Diarrhoea.
Comparison 6: dosing interval of misoprostol following mifepristone administration
One trial (Chai 2009) was included in this comparison. There were 141 women eligible for analyses. No difference was found in the abortion rate within 24 hours (Analysis 6.1), need of surgical evacuations (Analysis 6.2), or side‐effects of pain (Analysis 6.3), nausea (Analysis 6.4) and diarrhoea (Analysis 6.5). All patients from one centre had dilatation and curettage the day following abortion, as it was the routine practice in that hospital. These patients were not included in Analysis 6.2.
6.1. Analysis.
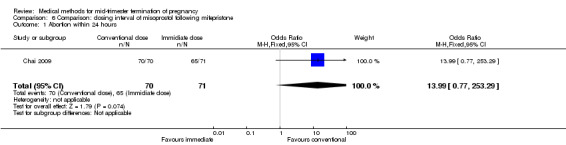
Comparison 6 Comparison: dosing interval of misoprostol following mifepristone, Outcome 1 Abortion within 24 hours.
6.2. Analysis.
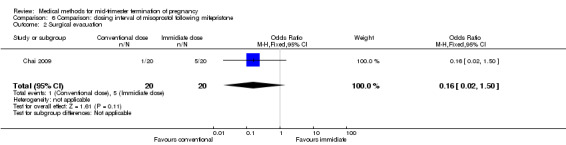
Comparison 6 Comparison: dosing interval of misoprostol following mifepristone, Outcome 2 Surgical evacuation.
6.3. Analysis.
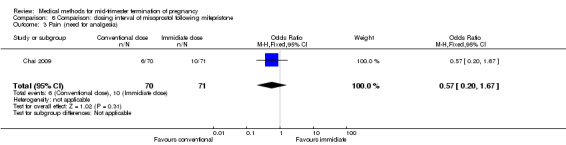
Comparison 6 Comparison: dosing interval of misoprostol following mifepristone, Outcome 3 Pain (need for analgesia).
6.4. Analysis.
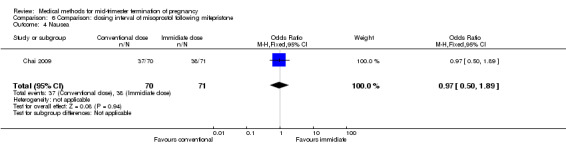
Comparison 6 Comparison: dosing interval of misoprostol following mifepristone, Outcome 4 Nausea.
6.5. Analysis.
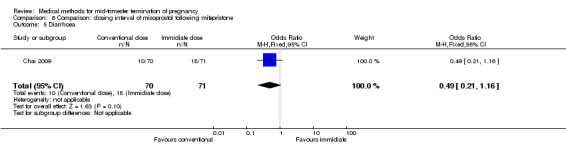
Comparison 6 Comparison: dosing interval of misoprostol following mifepristone, Outcome 5 Diarrhoea.
Comparison 7: dose of mifepristone before the administration of misoprostol
One trial (Webster 1996) was included in this comparison. In total, 70 women were eligible for analyses. No difference was found in the induction of the abortion interval (Analysis 7.1), abortion rate within 24 hours (Analysis 7.2), need of surgical evacuation (Analysis 7.3) or side‐effects of pain (Analysis 7.4), vomiting (Analysis 7.5) or diarrhoea (Analysis 7.6).
7.1. Analysis.
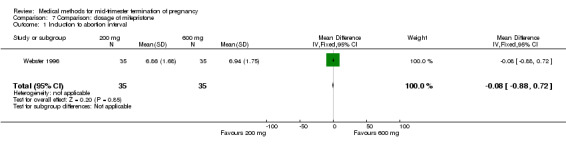
Comparison 7 Comparison: dosage of mifepristone, Outcome 1 Induction to abortion interval.
7.2. Analysis.
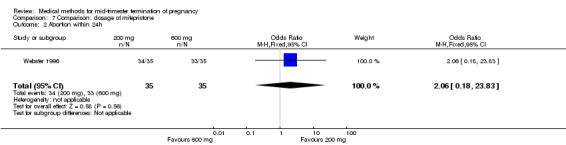
Comparison 7 Comparison: dosage of mifepristone, Outcome 2 Abortion within 24h.
7.3. Analysis.
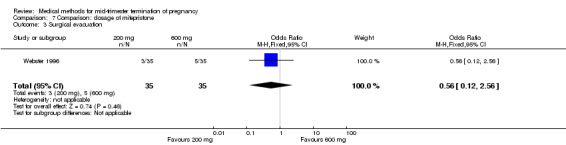
Comparison 7 Comparison: dosage of mifepristone, Outcome 3 Surgical evacuation.
7.4. Analysis.
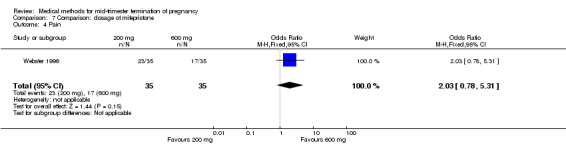
Comparison 7 Comparison: dosage of mifepristone, Outcome 4 Pain.
7.5. Analysis.
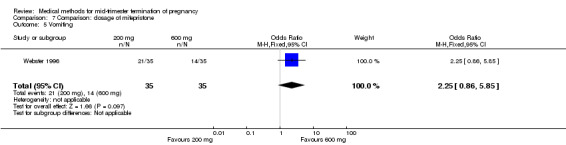
Comparison 7 Comparison: dosage of mifepristone, Outcome 5 Vomiting.
7.6. Analysis.
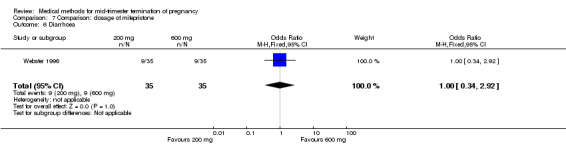
Comparison 7 Comparison: dosage of mifepristone, Outcome 6 Diarrhoea.
Comparison 8: regimen mifepristone + gemeprost 1.0 mg versus mifepristone + gemeprost 0.5 mg
One trial (Thong 1996) was included in this comparison. There were 100 women eligible for analysis. No difference was found in the abortion rate within 24 hours (Analysis 8.1), excessive blood loss (Analysis 8.2), need for surgical evacuation (Analysis 8.3) or episodes of diarrhoea (Analysis 8.5). When patients were given 0.5 mg gemeprost rather than 1 mg, they experienced fewer episodes of vomiting (Analysis 8.4) (OR 2.83, 95% CI 1.04 to 7.66).
8.1. Analysis.
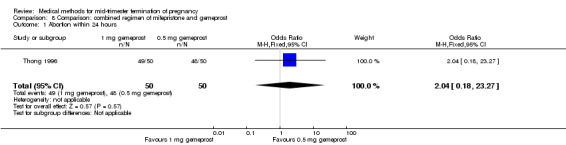
Comparison 8 Comparison: combined regimen of mifepristone and gemeprost, Outcome 1 Abortion within 24 hours.
8.2. Analysis.
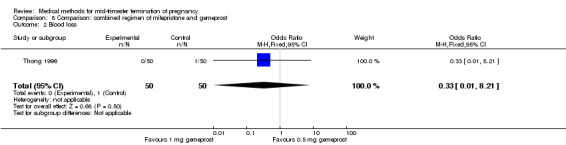
Comparison 8 Comparison: combined regimen of mifepristone and gemeprost, Outcome 2 Blood loss.
8.3. Analysis.
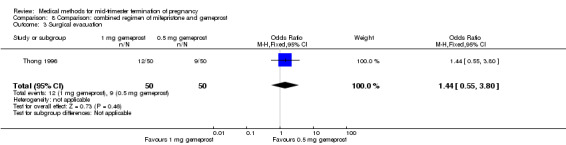
Comparison 8 Comparison: combined regimen of mifepristone and gemeprost, Outcome 3 Surgical evacuation.
8.5. Analysis.
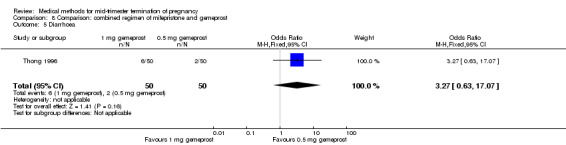
Comparison 8 Comparison: combined regimen of mifepristone and gemeprost, Outcome 5 Diarrhoea.
8.4. Analysis.
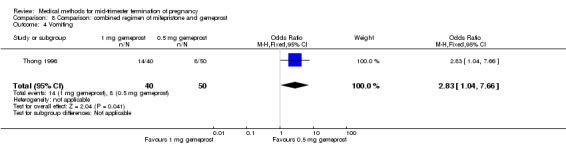
Comparison 8 Comparison: combined regimen of mifepristone and gemeprost, Outcome 4 Vomiting.
Comparisons 9 to 11: misoprostol versus another prostaglandin
Four trials with seven comparisons (Makhlouf 2003; Nuutila 1997 a; Nuutila 1997 b; Su 2005; Wong 1998) were included. Nuutila compared three interventions, that is, two doses of vaginal misoprostol and vaginal gemeprost. For this reason, this trial was considered for each of the different comparisons (see Characteristics of included studies).
Comparison 9: misoprostol versus intra‐amniotic PGF2α
In this comparison, one trial was included (Su 2005). In total, 125 women were eligible for analysis. Su 2005 compared the use of vaginal misoprostol with the use of intra‐amniotic PGF2α. Vaginal misoprostol was superior to intra‐amniotic PGF2α (MD ‐4.60, 95% CI ‐7.74 to ‐1.46) (Analysis 9.1) regarding the induction to the abortion interval. No difference was found between the groups in relation to the abortion completion rate (Analysis 9.2), need for surgical evacuations (Analysis 9.3), nausea (Analysis 9.4), vomiting (Analysis 9.5) or diarrhoea (Analysis 9.6).
9.1. Analysis.
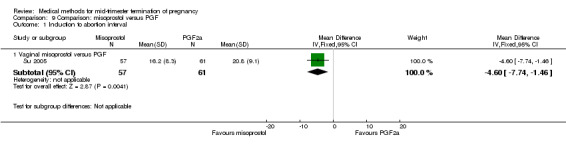
Comparison 9 Comparison: misoprostol versus PGF, Outcome 1 Induction to abortion interval.
9.2. Analysis.
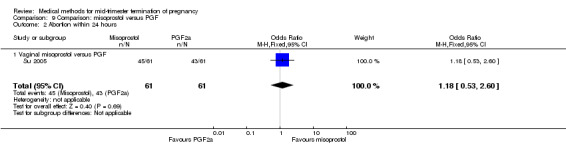
Comparison 9 Comparison: misoprostol versus PGF, Outcome 2 Abortion within 24 hours.
9.3. Analysis.
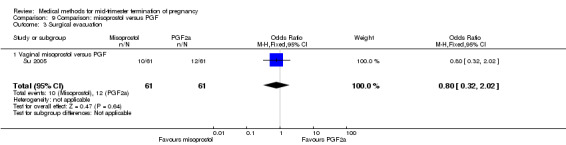
Comparison 9 Comparison: misoprostol versus PGF, Outcome 3 Surgical evacuation.
9.4. Analysis.
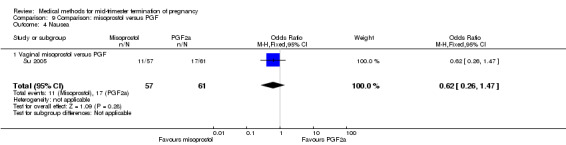
Comparison 9 Comparison: misoprostol versus PGF, Outcome 4 Nausea.
9.5. Analysis.
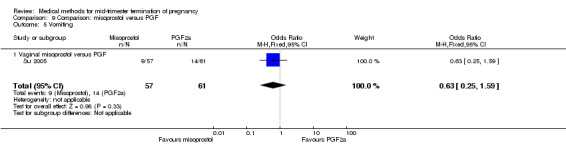
Comparison 9 Comparison: misoprostol versus PGF, Outcome 5 Vomiting.
9.6. Analysis.
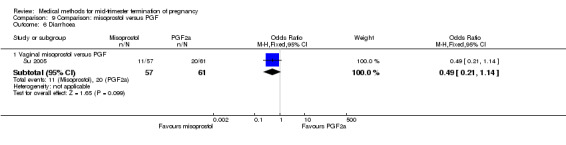
Comparison 9 Comparison: misoprostol versus PGF, Outcome 6 Diarrhoea.
Comparison 10: misoprostol versus PGE1 (gemeprost)
In this comparison, two trials with three comparisons were included (Nuutila 1997 a; Nuutila 1997 b; Wong 1998). In total, 249 women were eligible for analysis. The largest trial in this analysis was conducted by Wong 1998, comparing the vaginal use of misoprostol, 400 μg every 4 hours, to the vaginal use of gemeprost. When misoprostol was used at very low doses (100 μg) (MD 8.60, 95% CI 3.11 to 14.09) or 200 μg (MD 13.30, 95% CI 7.90 to 18.70) every 6 or 12 hours, respectively, gemeprost was superior (Analysis 10.1). In contrast, when misoprostol was used at a higher dose (400 μg) (OR 2.83, 95% CI 1.33 to 6.02) every 3 or 6 hours, more women aborted within 24 hours in comparison to gemeprost (Analysis 10.2). In regards to the side‐effects, women who received misoprostol experienced less pain (100 μg) (OR 0.22, 95% CI 0.07 to 0.71) or 200 μg (OR 0.23, 95% CI 0.07 to 0.77), vomiting (misoprostol 200 μg, OR 0.15, 95% CI 0.04 to 0.62) and diarrhoea (100 μg, OR 0.04, 95% CI 0.00 to 0.68) or 200 μg (OR 0.18, 95% CI 0.03 to 0.91) (Analysis 10.5; Analysis 10.7; Analysis 10.8). Moreover, the amount of blood loss was decreased when women received 200 μg misoprostol when compared to gemeprost (Analysis 10.3) (OR ‐146.00, 95% CI ‐219.02 to ‐72.98). No difference was found between the groups in relation to the need for surgical evacuation (Analysis 10.4) or nausea (Analysis 10.6).
10.1. Analysis.
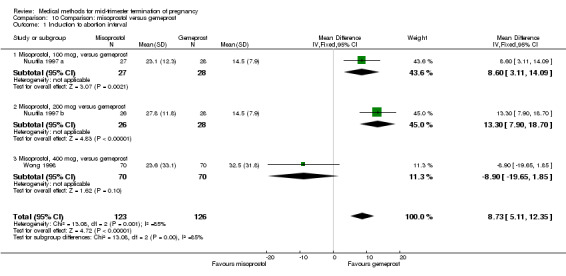
Comparison 10 Comparison: misoprostol versus gemeprost, Outcome 1 Induction to abortion interval.
10.2. Analysis.
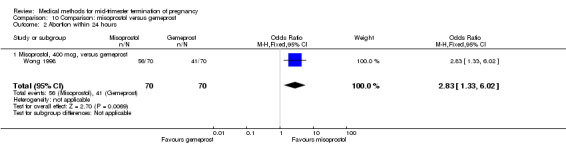
Comparison 10 Comparison: misoprostol versus gemeprost, Outcome 2 Abortion within 24 hours.
10.5. Analysis.
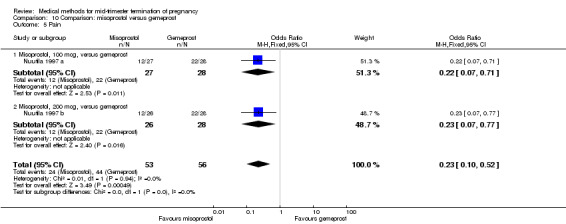
Comparison 10 Comparison: misoprostol versus gemeprost, Outcome 5 Pain.
10.7. Analysis.
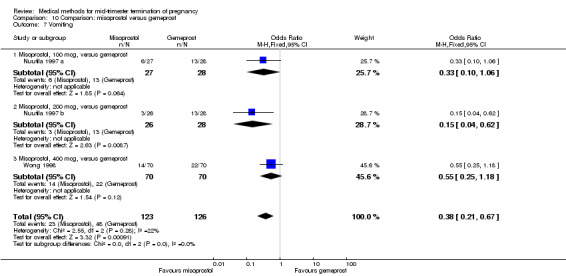
Comparison 10 Comparison: misoprostol versus gemeprost, Outcome 7 Vomiting.
10.8. Analysis.
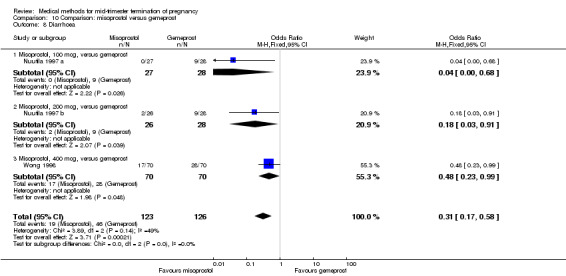
Comparison 10 Comparison: misoprostol versus gemeprost, Outcome 8 Diarrhoea.
10.3. Analysis.
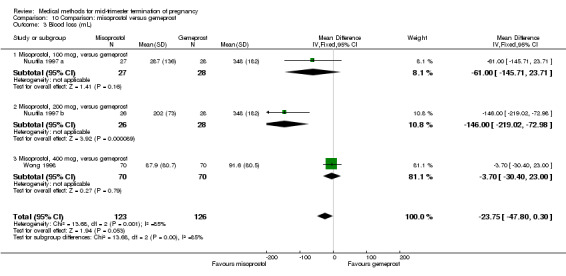
Comparison 10 Comparison: misoprostol versus gemeprost, Outcome 3 Blood loss (mL).
10.4. Analysis.
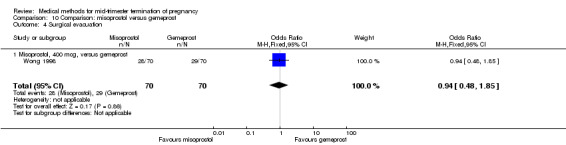
Comparison 10 Comparison: misoprostol versus gemeprost, Outcome 4 Surgical evacuation.
10.6. Analysis.
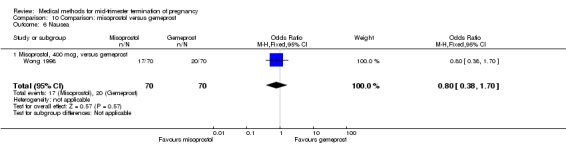
Comparison 10 Comparison: misoprostol versus gemeprost, Outcome 6 Nausea.
Comparison 11: misoprostol versus PGE2 (dinoprostone)
In this comparison, one trial was included (Makhlouf 2003). In total, 80 women were eligible for analysis. More women who were given misoprostol, 100 μg every four hours, aborted within 24 hours when compared to PGE2, 6 mg every six hours (Analysis 11.1) (OR 51.73, 95% CI 2.89 to 924.42). There was no difference regarding blood loss (Analysis 11.2), need for surgical evacuation (Analysis 11.3) or side‐effects of pain (Analysis 11.4), vomiting (Analysis 11.5) or diarrhoea (Analysis 11.6) between the groups.
11.1. Analysis.
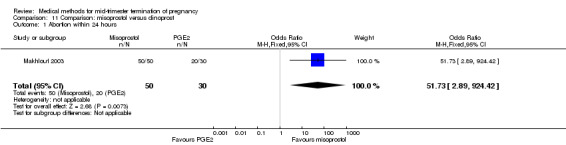
Comparison 11 Comparison: misoprostol versus dinoprost, Outcome 1 Abortion within 24 hours.
11.2. Analysis.
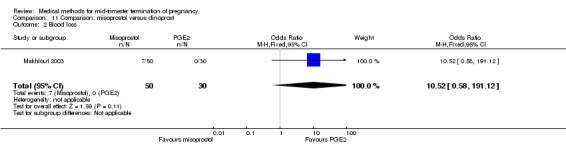
Comparison 11 Comparison: misoprostol versus dinoprost, Outcome 2 Blood loss.
11.3. Analysis.
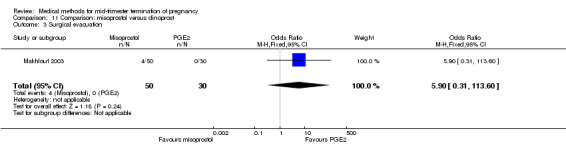
Comparison 11 Comparison: misoprostol versus dinoprost, Outcome 3 Surgical evacuation.
11.4. Analysis.
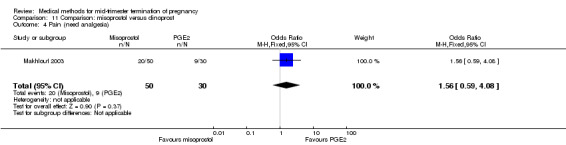
Comparison 11 Comparison: misoprostol versus dinoprost, Outcome 4 Pain (need analgesia).
11.5. Analysis.
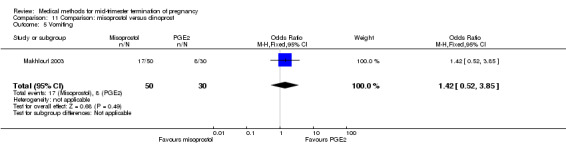
Comparison 11 Comparison: misoprostol versus dinoprost, Outcome 5 Vomiting.
11.6. Analysis.
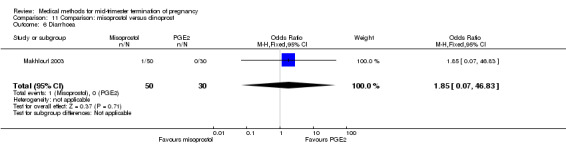
Comparison 11 Comparison: misoprostol versus dinoprost, Outcome 6 Diarrhoea.
Comparisons 12 to 13: routes of misoprostol for misoprostol used alone
In this comparison, six trials with two comparisons (Akoury 2004; Bebbington 2002; Behrashi 2008; Bhattacharjee 2008; Tang 2004; von Hertzen 2009) were included.
Comparison 12: vaginal use of misoprostol versus the oral use of misoprostol
In this comparison, three trials were included (Akoury 2004; Bebbington 2002; Behrashi 2008). In total, 310 women were eligible for analysis. The largest trial was conducted by Akoury, comparing the oral and vaginal use of misoprostol and the intra‐amniotic use of PGF2α. The vaginal administration of misoprostol was superior to the oral route (Analysis 12.1), at both a lower dose (200 μg) (mean difference (MD) ‐14.90, 95% CI ‐23.33 to ‐6.47) and a higher dose (400 μg) (MD ‐6.04, 95% CI ‐8.51 to ‐3.58). The abortion rate after 24 hours with vaginal use was also superior to the oral use of 200 μg of misoprostol (OR 9.60, 95% CI 3.74 to 24.66) (Analysis 12.2). Fewer women experienced nausea when the misoprostol was given vaginally when compared to the oral use of 400 μg of misoprostol (Analysis 12.6) (OR 0.41, 95% CI 0.18 to 0.93). No difference was found between the groups in regard to the amount of blood loss (Analysis 12.3), pain (Analysis 12.4), need for surgical evacuation (Analysis 12.5), vomiting (Analysis 12.7) or diarrhoea (Analysis 12.8).
12.1. Analysis.
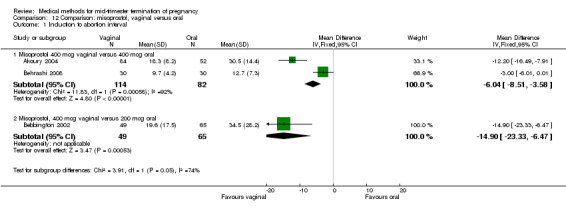
Comparison 12 Comparison: misoprostol, vaginal versus oral, Outcome 1 Induction to abortion interval.
12.2. Analysis.
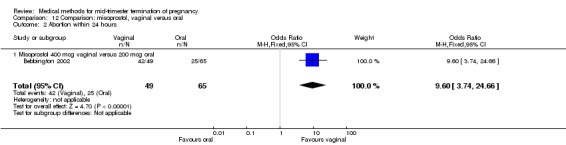
Comparison 12 Comparison: misoprostol, vaginal versus oral, Outcome 2 Abortion within 24 hours.
12.6. Analysis.
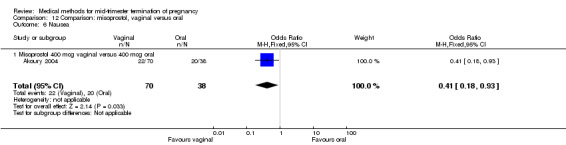
Comparison 12 Comparison: misoprostol, vaginal versus oral, Outcome 6 Nausea.
12.3. Analysis.
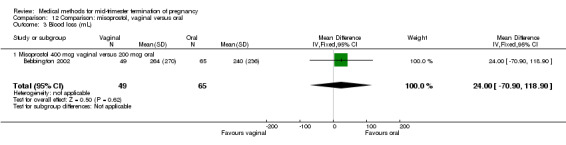
Comparison 12 Comparison: misoprostol, vaginal versus oral, Outcome 3 Blood loss (mL).
12.4. Analysis.
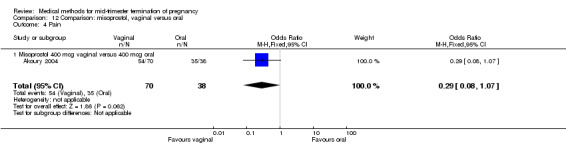
Comparison 12 Comparison: misoprostol, vaginal versus oral, Outcome 4 Pain.
12.5. Analysis.
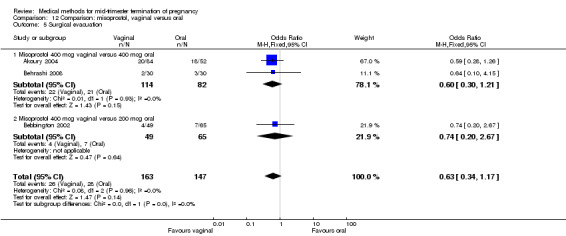
Comparison 12 Comparison: misoprostol, vaginal versus oral, Outcome 5 Surgical evacuation.
12.7. Analysis.
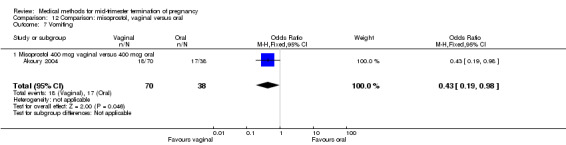
Comparison 12 Comparison: misoprostol, vaginal versus oral, Outcome 7 Vomiting.
12.8. Analysis.
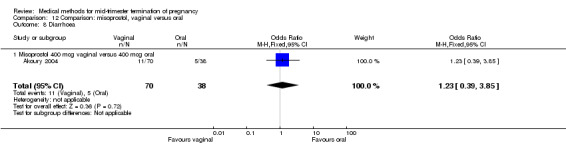
Comparison 12 Comparison: misoprostol, vaginal versus oral, Outcome 8 Diarrhoea.
Comparison 13: vaginal use of misoprostol versus the sublingual use of misoprostol
In this comparison, three trials were included (Bhattacharjee 2008; Tang 2004; von Hertzen 2009). In total, 1178 women were eligible for analysis. The largest trial was conducted by von Hertzen, comparing the vaginal use of 400 μg misoprostol to the sublingual use of 400 μg misoprostol with the use of placebo tablets. No difference was found between the vaginal or the sublingual use of misoprostol for the induction to abortion interval in one smaller study (Analysis 13.1). Von Hertzen was not included in this analyses, because no mean (SD) was provided. However, authors did provide median (range). In the vaginal group, the induction to abortion interval was longer (median 12.3, range 3.2 to 48.0) than the sublingual group (median 12.0, range 4.1 to 61.8). However, this difference was not significant. The abortion rate after 24 hours with vaginal use was superior to the sublingual use (Analysis 13.2) (OR 1.39, 95% CI 1.05 to 1.83). However, the significant heterogeneity for the analysis (Analysis 13.2) (I2 = 63%) must be noted despite use of identical regimens precludes confidence in these combined estimates. The heterogeneity between these data could potentially be explained by the difference in included numbers of multigravidas between both trials; Tang included 36% to 39%, while Bhattacharjee included 80% of women studied. Because of the possibility that among nulliparous women, vaginal misoprostol is associated with higher rates of complete abortion within 24 hours, the analysis were separated. Vaginal misoprostol is associated with significantly higher rates of complete abortion within 24 hours among nulliparous women (OR 2.31, 95% CI 1.17 to 4.54), while among multiparous women, there is no difference between vaginal or sublingual administration (OR 0.90, 95% CI 0.55 to 1.47). Von Hertzen conducted a stratified analysis by parity because there was a highly significant interaction of treatment by parity. When success rates at 24 h were analysed according to parity, vaginal administration was clearly superior to sublingual administration in nulliparous women (87.3% versus 68.5%) but the difference between treatments was not present among parous women: 84.7% (vaginal) versus 88.5% (sublingual).
13.1. Analysis.
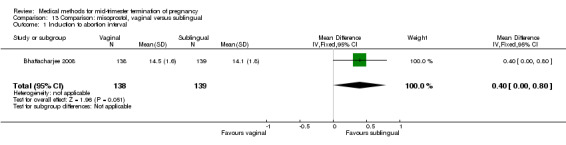
Comparison 13 Comparison: misoprostol, vaginal versus sublingual, Outcome 1 Induction to abortion interval.
13.2. Analysis.
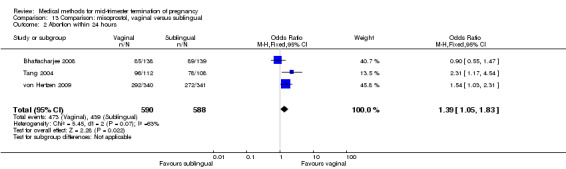
Comparison 13 Comparison: misoprostol, vaginal versus sublingual, Outcome 2 Abortion within 24 hours.
No differences were noted in the meta‐analysis for the occurrence of complications of blood loss (Analysis 13.3) or surgical evacuations (Analysis 13.5) or side‐effects of pain (Analysis 13.4), nausea (Analysis 13.6), vomiting (Analysis 13.7) or diarrhoea (Analysis 13.8).
13.3. Analysis.
Comparison 13 Comparison: misoprostol, vaginal versus sublingual, Outcome 3 Blood loss.
13.5. Analysis.
Comparison 13 Comparison: misoprostol, vaginal versus sublingual, Outcome 5 Surgical evacuation.
13.4. Analysis.
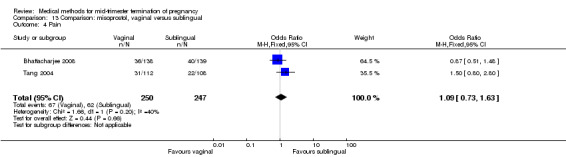
Comparison 13 Comparison: misoprostol, vaginal versus sublingual, Outcome 4 Pain.
13.6. Analysis.
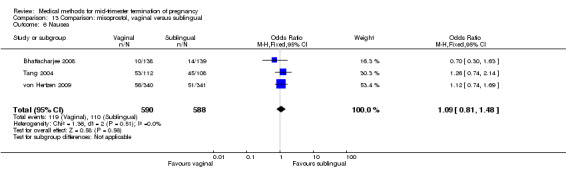
Comparison 13 Comparison: misoprostol, vaginal versus sublingual, Outcome 6 Nausea.
13.7. Analysis.
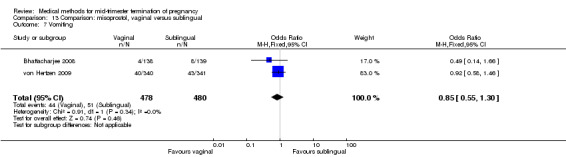
Comparison 13 Comparison: misoprostol, vaginal versus sublingual, Outcome 7 Vomiting.
13.8. Analysis.
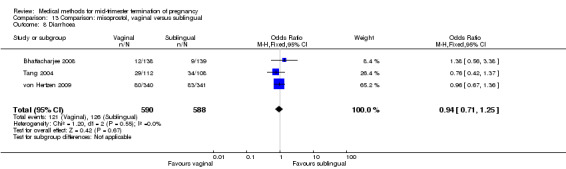
Comparison 13 Comparison: misoprostol, vaginal versus sublingual, Outcome 8 Diarrhoea.
Comparison 14: misoprostol tablet insertion versus gel insertion
One trial (Pongsatha 2008) was included in this comparison. For analysis, 148 women were eligible for analysis. In terms of adverse outcomes, women who had misoprostol inserted with gel experienced more diarrhoea (Analysis 14.7) (OR 0.15, 95% CI 0.03 to 0.71). No significant difference was observed between the two groups in terms of abortion within 24 hours (Analysis 14.1), excessive blood loss (Analysis 14.2), need for surgical evacuation (Analysis 14.3), pain (Analysis 14.4), nausea (Analysis 14.5) or vomiting (Analysis 14.6).
14.7. Analysis.
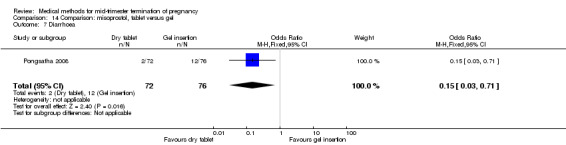
Comparison 14 Comparison: misoprostol, tablet versus gel, Outcome 7 Diarrhoea.
14.1. Analysis.
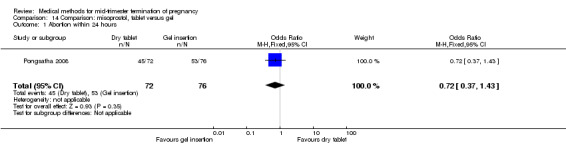
Comparison 14 Comparison: misoprostol, tablet versus gel, Outcome 1 Abortion within 24 hours.
14.2. Analysis.
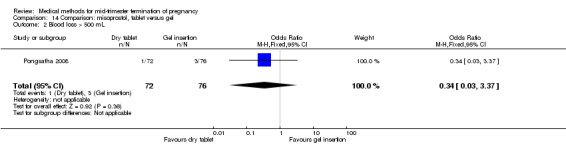
Comparison 14 Comparison: misoprostol, tablet versus gel, Outcome 2 Blood loss > 500 mL.
14.3. Analysis.
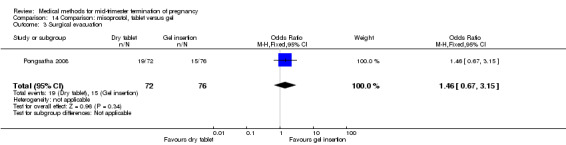
Comparison 14 Comparison: misoprostol, tablet versus gel, Outcome 3 Surgical evacuation.
14.4. Analysis.
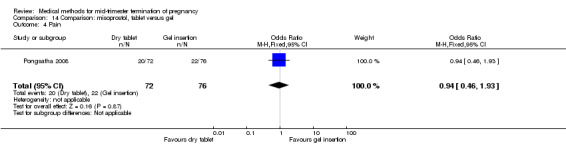
Comparison 14 Comparison: misoprostol, tablet versus gel, Outcome 4 Pain.
14.5. Analysis.
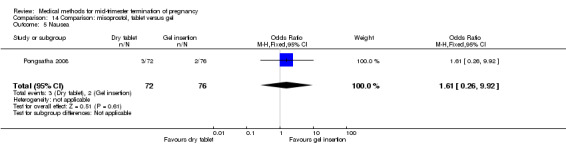
Comparison 14 Comparison: misoprostol, tablet versus gel, Outcome 5 Nausea.
14.6. Analysis.
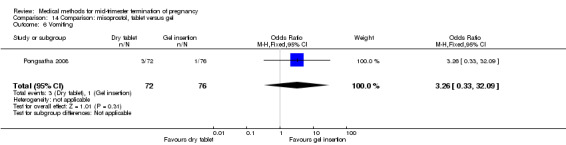
Comparison 14 Comparison: misoprostol, tablet versus gel, Outcome 6 Vomiting.
Comparisons 15 to 16: time interval and dose of misoprostol or gemeprost
Four trials with two comparisons (Armatage 1996; Herabutya 2005; Wong 2000) were included.
Comparison 15: time interval of misoprostol
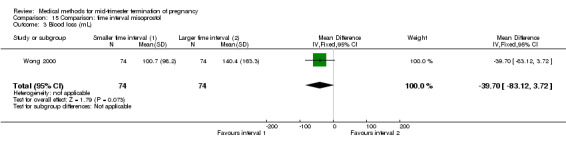
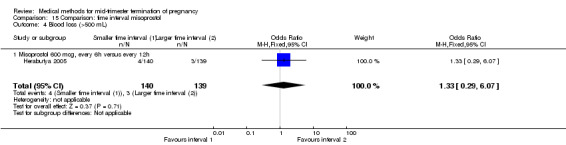
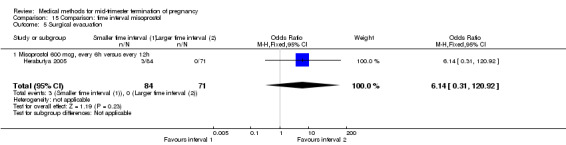
Two trials (Herabutya 2005; Wong 2000) were included in this comparison. In total, 427 women were eligible for analysis. The largest trial in this analysis was conducted by Wong 2000 who examined the optimal time interval for the vaginal use of 400 μg misoprostol, either administered every three or every six hours. When the time interval was shorter, the interval to abortion was shorter (three hours) (Analysis 15.1) (MD ‐19.20, 95% CI ‐36.02 to ‐2.38) compared to the longer time interval (six hours). In regards to the induction to abortion interval, no mean or SD was provided by Herabutya 2005 and thus precluded inclusion of these data in the meta‐analysis. The median induction‐to‐abortion interval was 15.8 hrs (25, 75 centiles: 12, 26) and 16.0 hrs (25, 75 centiles: 12, 30) for the group given misoprostol with a shorter time interval and the group with a longer time interval respectively (P =0.80). No effect was found for the time interval in relation to the abortion rate within 24 hours (Analysis 15.2), excessive blood loss (Analysis 15.3; Analysis 15.4), need for surgical evacuation (Analysis 15.5), or side‐effects of pain (Analysis 15.6), nausea (Analysis 15.7), vomiting (Analysis 15.8) or diarrhoea (Analysis 15.9).
15.1. Analysis.
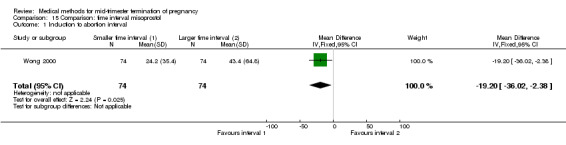
Comparison 15 Comparison: time interval misoprostol, Outcome 1 Induction to abortion interval.
15.2. Analysis.
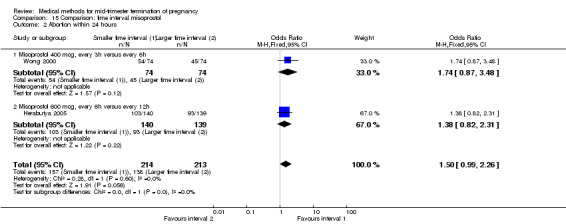
Comparison 15 Comparison: time interval misoprostol, Outcome 2 Abortion within 24 hours.
15.3. Analysis.
Comparison 15 Comparison: time interval misoprostol, Outcome 3 Blood loss (mL).
15.4. Analysis.
Comparison 15 Comparison: time interval misoprostol, Outcome 4 Blood loss (>500 mL).
15.5. Analysis.
Comparison 15 Comparison: time interval misoprostol, Outcome 5 Surgical evacuation.
15.6. Analysis.
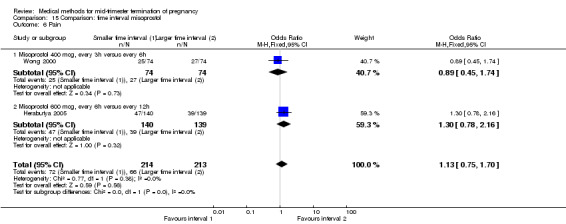
Comparison 15 Comparison: time interval misoprostol, Outcome 6 Pain.
15.7. Analysis.
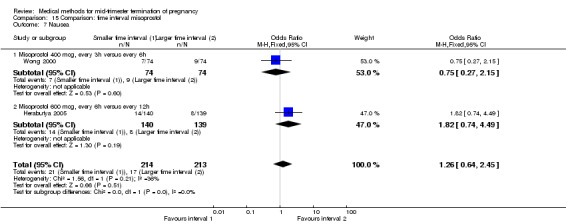
Comparison 15 Comparison: time interval misoprostol, Outcome 7 Nausea.
15.8. Analysis.
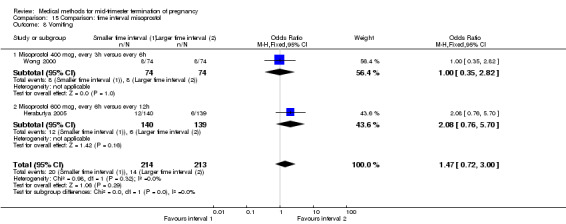
Comparison 15 Comparison: time interval misoprostol, Outcome 8 Vomiting.
15.9. Analysis.
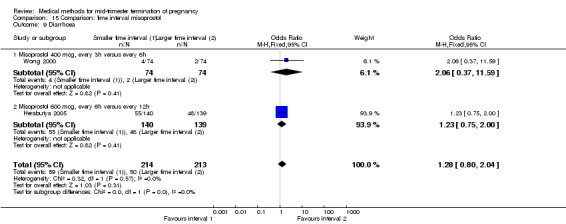
Comparison 15 Comparison: time interval misoprostol, Outcome 9 Diarrhoea.
Comparison 16: time interval of gemeprost
One trial (Armatage 1996) was included in this comparison. In total, 99 women were eligible for analysis. In regards to the induction to abortion interval, no mean or SD was given, and thus precluded inclusion of these data in a meta‐analysis. The median induction‐to‐abortion interval was 16 hrs (25, 75 centiles: 12, 26) and 15 hrs (25, 75 centiles: 11.4, 28.5) for the group given gemeprost with a shorter time interval compared with the longer time interval, respectively (not significant). In addition, no significant difference was found between both groups in the abortion rate after 24 hours (Analysis 16.1), need for surgical evacuation (Analysis 16.2) or pain (Analysis 16.3).
16.1. Analysis.
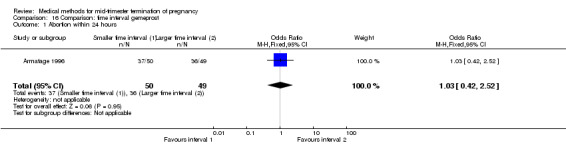
Comparison 16 Comparison: time interval gemeprost, Outcome 1 Abortion within 24 hours.
16.2. Analysis.
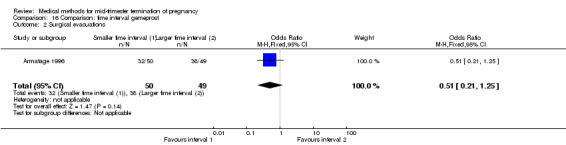
Comparison 16 Comparison: time interval gemeprost, Outcome 2 Surgical evacuations.
16.3. Analysis.
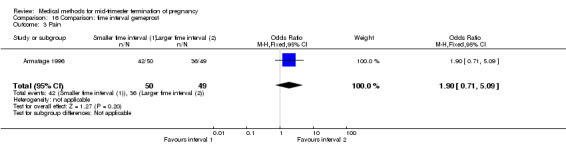
Comparison 16 Comparison: time interval gemeprost, Outcome 3 Pain.
Comparison 17: low dose versus a higher dose of misoprostol
Two trials (Nuutila 1997 c; Ozerkan 2009) were included in this comparison. In total, 133 women were eligible for analyses. Ozerkan 2009 compared the use of 600 µg with the use of 400 µg of misoprostol. An initial first dose of 600 µg of misoprostol was found to be more effective than 400 µg (Analysis 17.1) (MD 6.40, 95% CI 0.40 to 12.40). While gestational age or parity were not found to be related to the duration of the termination procedure, a higher parity was shown to be correlated with a shorter induction to fetal‐expulsion period in the low dose, but not in the high dose group. Nuutila 1997 c compared the use of 100 µg of misoprostol to the use of 200 µg of misoprostol. The study found no difference between these groups in terms of the induction of the abortion interval (Analysis 17.1). The significant heterogeneity for the analysis (Analysis 17.1) (I2 = 84%) must be noted. This is most likely do to the different misoprostol doses the trials used. Both studies found no difference in the amount of pain (Analysis 17.2), and side effects such as vomiting (Analysis 17.4) and diarrhoea (Analysis 17.5).
17.1. Analysis.
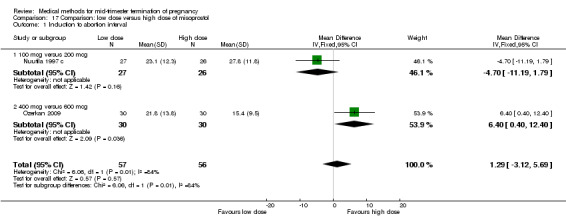
Comparison 17 Comparison: low dose versus high dose of misoprostol, Outcome 1 Induction to abortion interval.
17.2. Analysis.
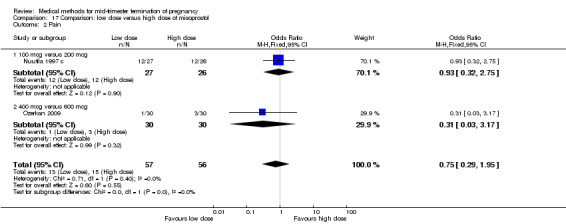
Comparison 17 Comparison: low dose versus high dose of misoprostol, Outcome 2 Pain.
17.4. Analysis.
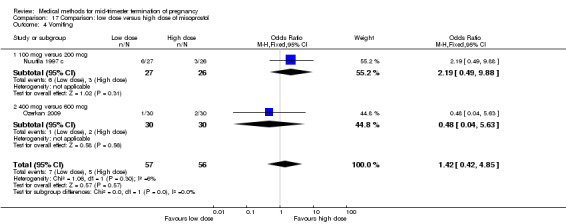
Comparison 17 Comparison: low dose versus high dose of misoprostol, Outcome 4 Vomiting.
17.5. Analysis.
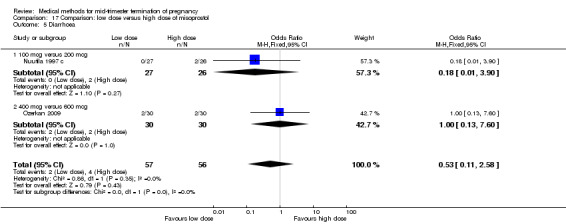
Comparison 17 Comparison: low dose versus high dose of misoprostol, Outcome 5 Diarrhoea.
Comparisons 18 to 19: prostaglandin E2 versus prostaglandin F2α
Three trials with two comparisons (Borgida 1995; Sorensen 1984; Steyn 1993) were included.
Comparison 18: prostaglandin E2 versus prostaglandin F2α
One trial (Borgida 1995) was included in this comparison. In total, 50 women were eligible for analysis. When women were given prostaglandin E2intravaginally, the interval to abortion was shorter (Analysis 18.1) (MD ‐9.10, 95% CI ‐13.68 to ‐4.52) when compared to the women receiving prostaglandin F2α intramuscularly. In addition, more women given prostaglandin E2 aborted within 24 hours (Analysis 18.2) (OR 11.29, 95% CI 1.29 to 98.89). No difference was found between the occurrence of side‐effects of pain (Analysis 18.3), nausea (Analysis 18.4), vomiting (Analysis 18.5) or diarrhoea (Analysis 18.6).
18.1. Analysis.
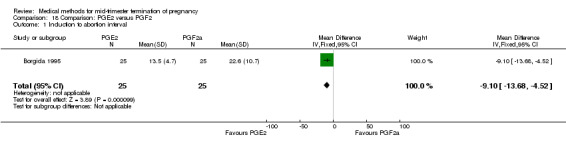
Comparison 18 Comparison: PGE2 versus PGF2, Outcome 1 Induction to abortion interval.
18.2. Analysis.
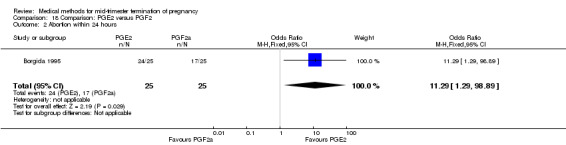
Comparison 18 Comparison: PGE2 versus PGF2, Outcome 2 Abortion within 24 hours.
18.3. Analysis.
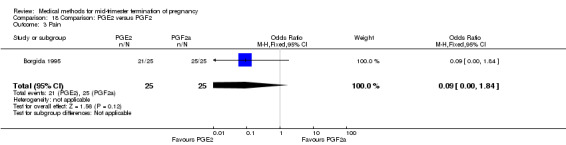
Comparison 18 Comparison: PGE2 versus PGF2, Outcome 3 Pain.
18.4. Analysis.
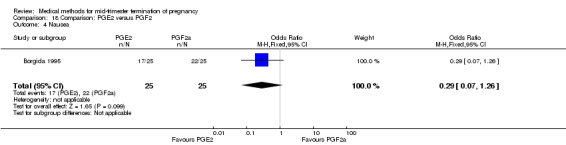
Comparison 18 Comparison: PGE2 versus PGF2, Outcome 4 Nausea.
18.5. Analysis.
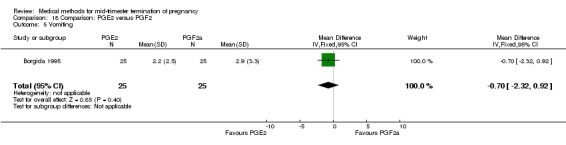
Comparison 18 Comparison: PGE2 versus PGF2, Outcome 5 Vomiting.
18.6. Analysis.
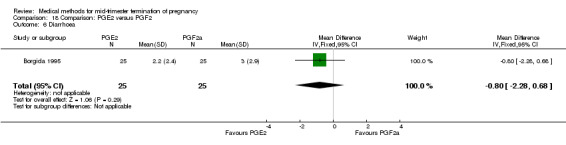
Comparison 18 Comparison: PGE2 versus PGF2, Outcome 6 Diarrhoea.
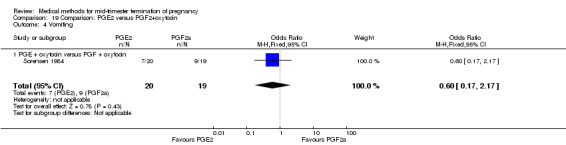
Comparison 19: prostaglandin E2 versus prostaglandin F2α + oxytocin
Two trials (Sorensen 1984; Steyn 1993) were included in this comparison. In total, 59 women were eligible for analysis. In regards to the induction‐to‐abortion interval, Steyn 1993 did not provide SDs, and thus precluded inclusion of these data in a meta‐analysis. The median induction to abortion interval was 38 hrs (range 19 to 61) and 23 hrs (range 11 to 54.5) for the intra‐amniotic prostaglandin F2α and the extra‐amniotic prostaglandin E2 group, respectively (not significant). When women were given prostaglandin E2 + oxytocin, the interval to abortion was longer (Analysis 19.1) (MD 2.00, 95% CI 0.90 to 3.10) compared to the women receiving prostaglandin F2α + oxytocin. Fewer surgical evacuations were performed in the prostaglandin E2 + oxytocin group (Analysis 19.2) (OR 0.25, 95% CI 0.07 to 0.90) and fewer women experienced pain (Analysis 19.3) (OR 0.03, 95% CI 0.00 to 0.72) when compared to the use of prostaglandin F2α + oxytocin. No difference was found regarding the episodes of vomiting or diarrhoea (Analysis 19.4; Analysis 19.5).
19.1. Analysis.
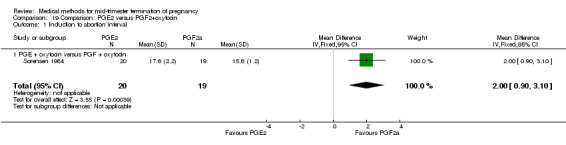
Comparison 19 Comparison: PGE2 versus PGF2+oxytocin, Outcome 1 Induction to abortion interval.
19.2. Analysis.
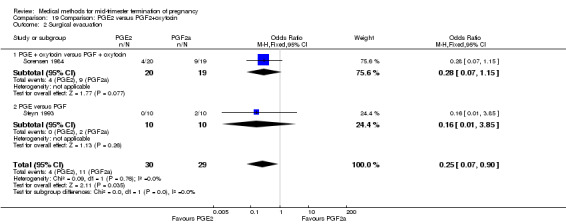
Comparison 19 Comparison: PGE2 versus PGF2+oxytocin, Outcome 2 Surgical evacuation.
19.3. Analysis.
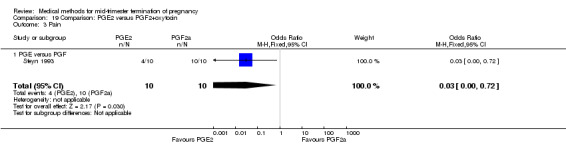
Comparison 19 Comparison: PGE2 versus PGF2+oxytocin, Outcome 3 Pain.
19.4. Analysis.
Comparison 19 Comparison: PGE2 versus PGF2+oxytocin, Outcome 4 Vomiting.
19.5. Analysis.
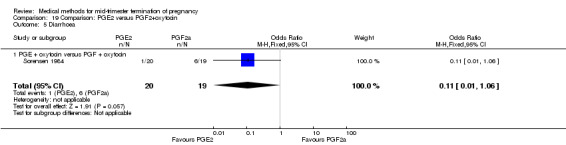
Comparison 19 Comparison: PGE2 versus PGF2+oxytocin, Outcome 5 Diarrhoea.
Comparison 20: intra‐amniotic instillation of prostaglandin F2α versus intra‐amniotic instillation of hypertonic saline (20%)
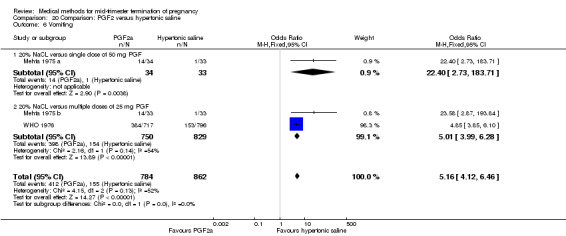
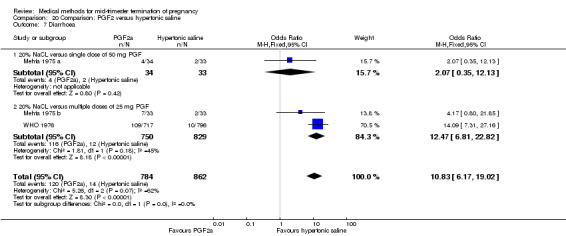
Four trials (Faktor 1988;Mehta 1975 a; Mehta 1975 b;Nielsen 1975;WHO 1976) were included for analysis. In total, 1703 women were eligible for analysis. The largest trial in this analysis was conducted by the WHO 1976, comparing the intra‐amniotic use of 25 mg of prostaglandin F2α to the intra‐amniotic instillation of 20% saline. In regards to the induction to abortion interval, Nielsen 1975. and the WHO trials did not provide standard deviations, and thus precluded inclusion of these data in a meta‐analysis. The median induction to abortion interval in the study by Nielsen 1975 was 21.5 hrs (ranges not given) and 14.2 hrs (ranges not given) for the hypertonic saline and the PGF2α group, respectively (P < 0.01). The median induction‐to‐abortion interval in the study by the WHO was 30.4 hrs (ranges not given) and 19.7 hrs (ranges not given) for the hypertonic saline and the PGF2α group respectively (P <0.001). Based on analysis of only 25 women, a single dose of 40 mg prostaglandin F2α proved to be more effective than 20% hypertonic saline in terms of induction‐ to‐ abortion interval (Analysis 20.1) (MD ‐5.30, 95% CI ‐6.67 to ‐3.93). The analysis of the abortion rate within 24 hours included 1678 women. Multiple doses of PGF2α proved to be more effective than 20% hypertonic saline (Analysis 20.2) (OR 6.14, 95% CI 4.91 to 7.68). On the other hand, women who received hypertonic saline experienced fewer complications, such as the need for surgical evacuation (Analysis 20.8) (single dose of 50 mg PGF2α OR 7.89, 95% CI 2.01 to 30.95; multiple doses of 25 mg PGF2α OR 1.52, 95% CI 1.24 to 1.87) episodes of nausea (Analysis 20.5) (OR 3.01, 95% CI 1.17 to 7.72), vomiting (Analysis 20.6) (single dose of 50 mg PGF2α OR 22.40, 95% CI 2.73 to 183.71; and multiple doses of 25 mg PGF OR 5.01, 95% CI 3.99 to 6.28) and diarrhoea (Analysis 20.7) (multiple doses of 25mg PGF2α OR 12.47, 95% CI 6.81 to 22.82). The WHO trial reported more episodes of excessive blood loss in women receiving PGF2α (Analysis 20.4) (OR 3.05, 95% CI 1.56 to 5.97).
20.1. Analysis.
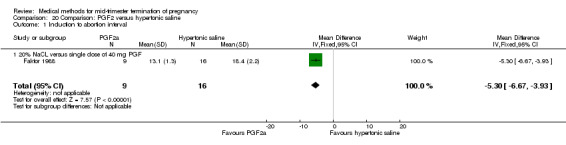
Comparison 20 Comparison: PGF2 versus hypertonic saline, Outcome 1 Induction to abortion interval.
20.2. Analysis.
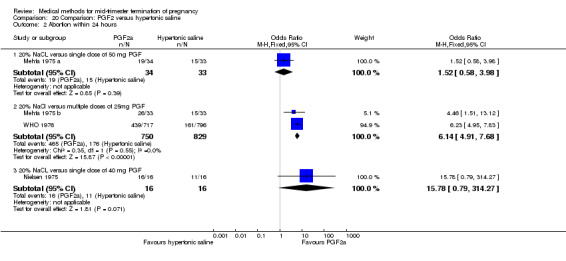
Comparison 20 Comparison: PGF2 versus hypertonic saline, Outcome 2 Abortion within 24 hours.
20.8. Analysis.
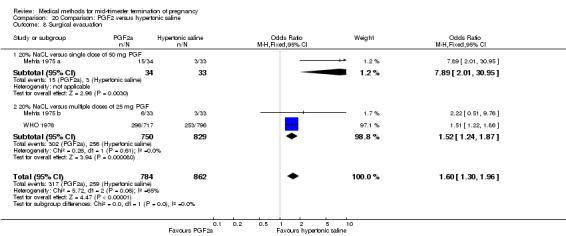
Comparison 20 Comparison: PGF2 versus hypertonic saline, Outcome 8 Surgical evacuation.
20.5. Analysis.
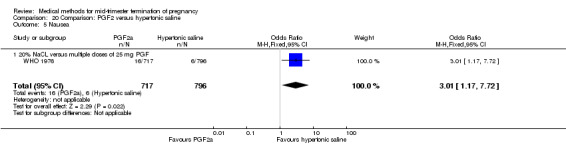
Comparison 20 Comparison: PGF2 versus hypertonic saline, Outcome 5 Nausea.
20.6. Analysis.
Comparison 20 Comparison: PGF2 versus hypertonic saline, Outcome 6 Vomiting.
20.7. Analysis.
Comparison 20 Comparison: PGF2 versus hypertonic saline, Outcome 7 Diarrhoea.
20.4. Analysis.
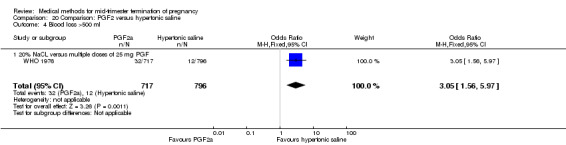
Comparison 20 Comparison: PGF2 versus hypertonic saline, Outcome 4 Blood loss >500 ml.
Comparison 21: combined regimen prostaglandin F2α and hypertonic saline
One trial (Muzsnai 1979 a; Muzsnai 1979 b; Muzsnai 1979 c; Muzsnai 1979 d) was included for analysis. In total, 770 women were eligible for analysis. The instillation of 25 ml 20% NaCl (5 g) + PGF2α (20 mg) was superior to the instillation of 100 ml 10% NaCl (10 g) + PGF2α (20 mg) (Analysis 21.1) in terms of the induction to the abortion interval (MD ‐2.96, 95% CI ‐5.29 to ‐0.64), but also in terms of the 24 hour abortion rate (OR 2.30, 95% CI 1.38 to 3.86) (Analysis 21.2). No significant difference was found between those who received 5 g of hypertonic saline versus 10 g in terms of excessive blood loss (Analysis 21.3), need for surgical evacuation (Analysis 21.4), vomiting (Analysis 21.6) and diarrhoea (Analysis 21.7). When given 25 ml of 20% hypertonic saline, women experienced less nausea (Analysis 21.5) (OR 0.33, 95% CI 0.17 to 0.62) than those who received 100 ml of 10% hypertonic saline.
21.1. Analysis.
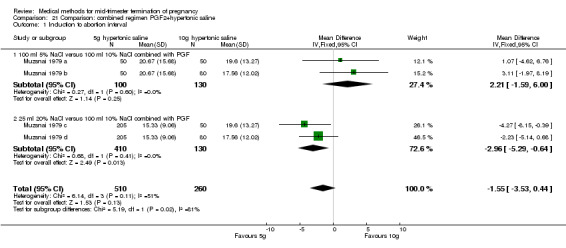
Comparison 21 Comparison: combined regimen PGF2+hypertonic saline, Outcome 1 Induction to abortion interval.
21.2. Analysis.
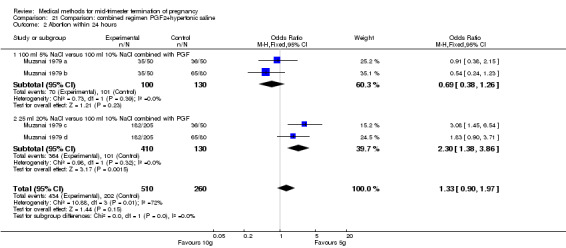
Comparison 21 Comparison: combined regimen PGF2+hypertonic saline, Outcome 2 Abortion within 24 hours.
21.3. Analysis.
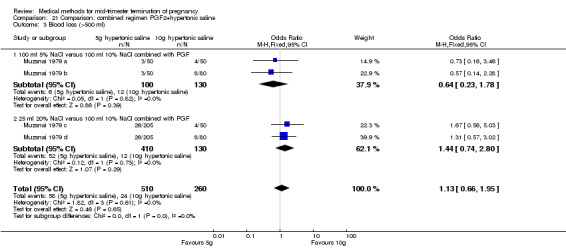
Comparison 21 Comparison: combined regimen PGF2+hypertonic saline, Outcome 3 Blood loss (>500 ml).
21.4. Analysis.
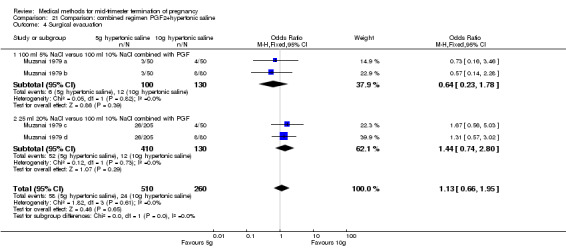
Comparison 21 Comparison: combined regimen PGF2+hypertonic saline, Outcome 4 Surgical evacuation.
21.6. Analysis.
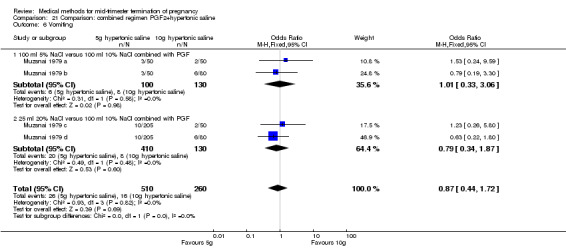
Comparison 21 Comparison: combined regimen PGF2+hypertonic saline, Outcome 6 Vomiting.
21.7. Analysis.
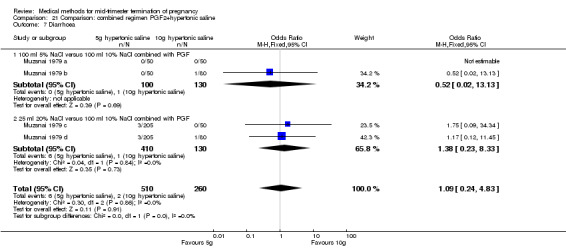
Comparison 21 Comparison: combined regimen PGF2+hypertonic saline, Outcome 7 Diarrhoea.
21.5. Analysis.
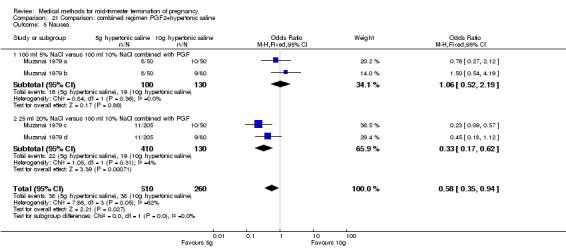
Comparison 21 Comparison: combined regimen PGF2+hypertonic saline, Outcome 5 Nausea.
Comparison 22: prostaglandin E1 (gemeprost) vaginally versus intra‐amniotic instillation of prostaglandin F2α + hypertonic saline
One trial (Waldron 1990) was included in this comparison. In total, 58 women were eligible for analysis. Women who had intra‐amniotic instillation of prostaglandin F2α + hypertonic saline aborted more rapidly than women who received vaginally administered gemeprost (Analysis 22.1) (MD 0.90, 95% CI 0.10 to 0.70) and the 24 hour abortion rate was significantly higher (Analysis 22.2) (OR 0.16, 95% CI 0.04 to 0.67). In addition, women who received gemeprost experienced more episodes of vomiting (Analysis 22.6) (OR 3.11, 95% CI 1.06 to 9.08) and diarrhoea (Analysis 22.7) (OR 19.13, 95% CI 3.80 to 96.18). No significant difference was found in terms of excessive blood loss (Analysis 22.3), need for surgical evacuation (Analysis 22.4) or pain (Analysis 22.5).
22.1. Analysis.
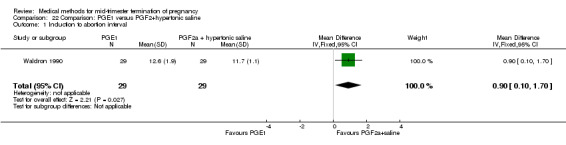
Comparison 22 Comparison: PGE1 versus PGF2+hypertonic saline, Outcome 1 Induction to abortion interval.
22.2. Analysis.
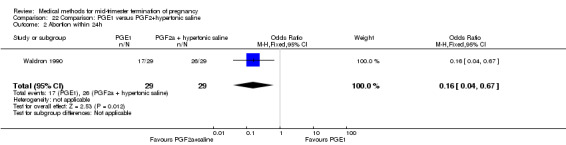
Comparison 22 Comparison: PGE1 versus PGF2+hypertonic saline, Outcome 2 Abortion within 24h.
22.6. Analysis.
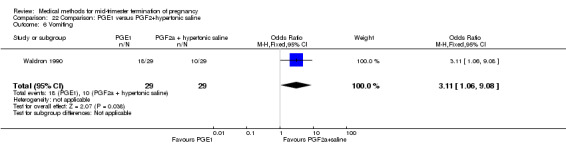
Comparison 22 Comparison: PGE1 versus PGF2+hypertonic saline, Outcome 6 Vomiting.
22.7. Analysis.
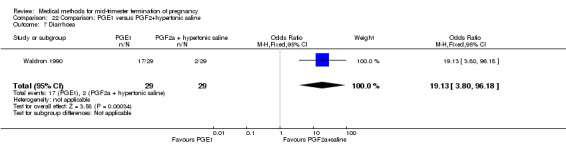
Comparison 22 Comparison: PGE1 versus PGF2+hypertonic saline, Outcome 7 Diarrhoea.
22.3. Analysis.
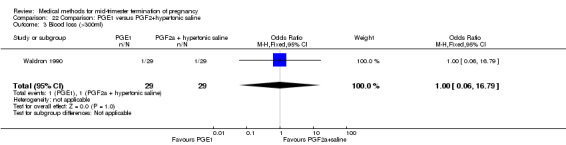
Comparison 22 Comparison: PGE1 versus PGF2+hypertonic saline, Outcome 3 Blood loss (>300ml).
22.4. Analysis.
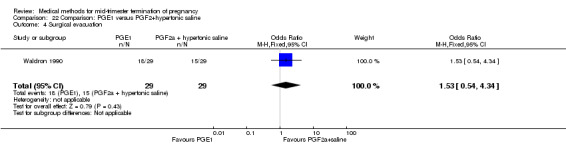
Comparison 22 Comparison: PGE1 versus PGF2+hypertonic saline, Outcome 4 Surgical evacuation.
22.5. Analysis.
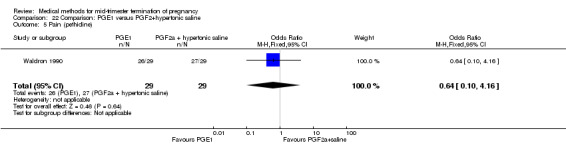
Comparison 22 Comparison: PGE1 versus PGF2+hypertonic saline, Outcome 5 Pain (pethidine).
Comparison 23: prostaglandins versus ethacridine lactate
Three trials (Inan 1997; Kelekci 2006; Olund 1978) were included in this comparison. For analyses, 302 women were eligible. No significant difference in induction to abortion interval was found (Analysis 23.1). Olund 1978 provided no standard deviation and could therefore not enter our analysis. The mean and range of the abortion interval of the ethacridine lactate group (29.9, 23.9 to 47.2) and of the prostaglandin F2α group (26.7, 8.9 to 63.0) showed no significant difference. More women in the ethacridine lactate group aborted within 24 hours (Analysis 23.2) (OR 0.18, 95% CI 0.06 to 0.48) in comparison to prostaglandin E2, but not in comparison to misoprostol. No differences were found in regard to the amount of blood loss (Analysis 23.3) or side‐effects, such as nausea (Analysis 23.4), vomiting (Analysis 23.5) or diarrhoea (Analysis 23.6). Kelekci 2006 provided no information about the side‐effects of each group, but found similar occurrences in both groups. Other side‐effects described by Inan 1997 included endometritis (ethacridine lactate group 4.1%, PGE2 group 3.3%; difference not significant).
23.1. Analysis.
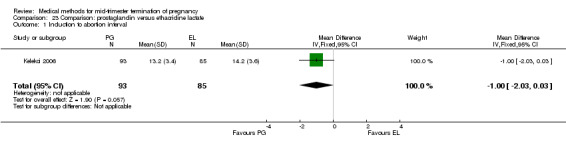
Comparison 23 Comparison: prostaglandin versus ethacridine lactate, Outcome 1 Induction to abortion interval.
23.2. Analysis.
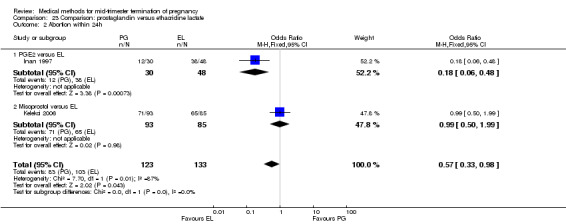
Comparison 23 Comparison: prostaglandin versus ethacridine lactate, Outcome 2 Abortion within 24h.
23.3. Analysis.
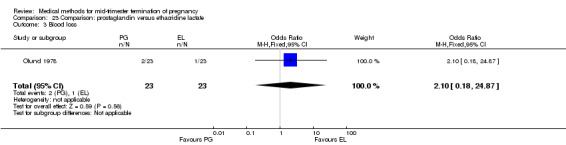
Comparison 23 Comparison: prostaglandin versus ethacridine lactate, Outcome 3 Blood loss.
23.4. Analysis.
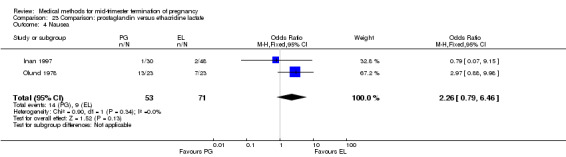
Comparison 23 Comparison: prostaglandin versus ethacridine lactate, Outcome 4 Nausea.
23.5. Analysis.
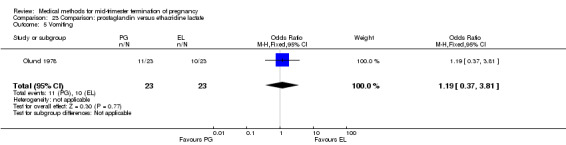
Comparison 23 Comparison: prostaglandin versus ethacridine lactate, Outcome 5 Vomiting.
23.6. Analysis.
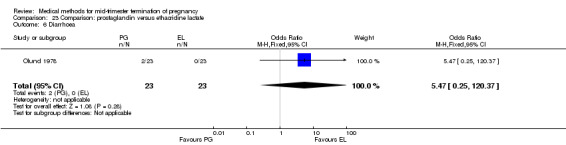
Comparison 23 Comparison: prostaglandin versus ethacridine lactate, Outcome 6 Diarrhoea.
Comparison 24: ethacridine lactate versus normal saline
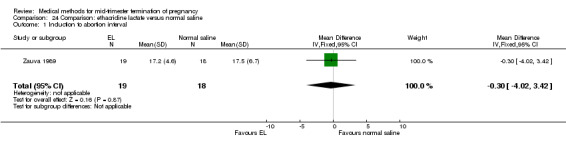
One trial (Zauva 1989) was included in this comparison. In total, 37 women were eligible for analysis. No differential effect was found between extra‐amniotic ethacridine lactate and extra‐amniotic normal saline regarding the induction‐to‐abortion interval (Analysis 24.1), excessive blood loss (Analysis 24.2), pain (Analysis 24.3), vomiting (Analysis 24.4) or rate of uterine rupture (Analysis 24.5).
24.1. Analysis.
Comparison 24 Comparison: ethacridine lactate versus normal saline, Outcome 1 Induction to abortion interval.
24.2. Analysis.
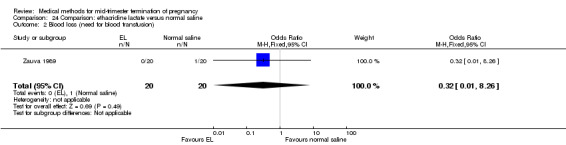
Comparison 24 Comparison: ethacridine lactate versus normal saline, Outcome 2 Blood loss (need for blood transfusion).
24.3. Analysis.
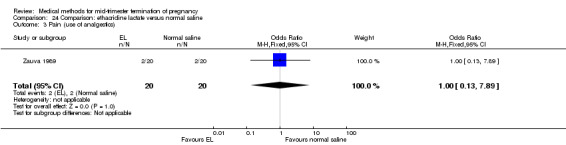
Comparison 24 Comparison: ethacridine lactate versus normal saline, Outcome 3 Pain (use of analgestics).
24.4. Analysis.
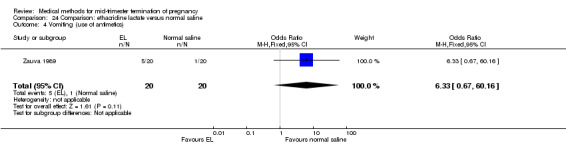
Comparison 24 Comparison: ethacridine lactate versus normal saline, Outcome 4 Vomiting (use of antimetics).
24.5. Analysis.
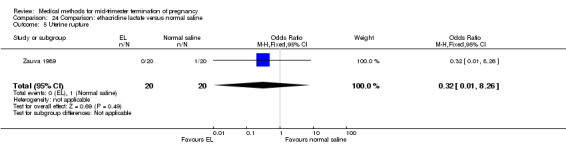
Comparison 24 Comparison: ethacridine lactate versus normal saline, Outcome 5 Uterine rupture.
Discussion
Second trimester medical abortion regimens have evolved greatly over the past 20 years with increasing availability of prostaglandin analogues and anti‐progesterone agents such as mifepristone. Older regimens such as instillation of hypertonic saline or prostaglandin F2α although effective in provoking abortion, were associated with higher rates of serious adverse events than are modern methods (Bygdeman and Gemzell‐Danielsson 2008).
Randomised comparisons included in this review demonstrate that misoprostol is the prostaglandin analogue of choice: it is as effective or more effective than other studied prostaglandins and has the preferable characteristics of heat stability and multiple administrative routes. However, in settings where prostaglandins are not available for second trimester medical abortion, extra‐amniotic instillation of ethacridine lactate may be an alternative (Comparisons 23, 24) (Hou 2010). However, limited information is available percentage of women needing a surgical intervention for incomplete abortion and the safety outcomes of ethacridine lactate, given the small number of subjects studied. When using extra‐amniotic instillation of drugs, the catheter tends to be expelled as the cervix dilates, before the abortion process is self‐sustaining. For this reason, supplementary infusions of oxytocin are commonly used (Kelekci 2006; WHO technical report series) which also increases the associated costs. Furthermore, intra‐amniotic injection of drugs is potentially dangerous as accidental injection into maternal tissue or placenta can result in local tissue damage or harmful absorption into the maternal circulation (WHO technical report series). For this reason, the drugs should only be given by skilled operators. Intra‐amniotic injection of drugs may also induce infection into the amniotic cavity (WHO technical report series).
Misoprostol when used alone is an effective inductive agent; however, it appears more efficient when combined with mifepristone, although the evidence from randomised trials is limited. In fact, there is only one relatively small randomised study (Kapp 2007) comparing the effect of misoprostol + mifepristone with misoprostol only (Comparison 2). This study demonstrated that the addition of mifepristone in second trimester abortion reduces the induction to abortion interval from 18 hours (95% CI 1 to 22) to 10 hours (95% CI 8 to12), while the occurrence of side‐effects in both groups was similar. Indirect evidence, however, suggests a beneficial effect of adding mifepristone to prostaglandin tablets or gel since the induction‐to‐abortion interval is generally shorter in regimens using mifepristone + prostaglandins (Comparisons 1, 3, 4, 5 and 7) than those using prostaglandins alone (Comparisons 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 and 19). Additionally, mifepristone is known to potentiate the uterine effect of misoprostol and is superior to misoprostol alone in first trimester abortion.
Misoprostol may be administered by different routes, the oral route being the least effective (Comparisons 3, 4 and 5). For regimens using misoprostol, vaginal dosing appears to be the most efficient when compared to both oral and sublingual regimens. Among multiparous women undergoing medical abortion with misoprostol alone, sublingual administration appears equally effective as vaginal administration. No study of second trimester medical abortion has compared vaginal with buccal administration of misoprostol.
The optimal dose of vaginally administered misoprostol is difficult to ascertain since there are no randomised studies comparing various dosing schemes for vaginal administration. Four randomised clinical trials showed that the induction to abortion interval with 3‐hourly vaginal administration of prostaglandins was significantly shorter than 6‐hourly administration without significant increase in side‐effects (Comparisons 15 and 16).
There is insufficient data to make any gestational, age‐specific recommendations on the dosage and regimen for abortion. Since the uterus becomes more sensitive to prostaglandins with increasing gestational age, reducing the dosage or frequency of administration should be considered at later gestational ages (Ho 2007). The age range considered in this review includes 12 through 28 weeks of gestation. Overall, from the design of the included studies, there is no indication for confounding by gestational age.
Other considerations for second trimester medical abortion regimens which could not be addressed in this review include the effect on the abortion process of the use of pre‐procedure feticide to avoid the occurrence of a fetus with signs of life at abortion, and therapeutic strategies for women who have not aborted after 24 hours of treatment.
There are considerable differences in practices regarding the management of the placenta following the expulsion of the fetus. We considered surgical evacuation any procedure where an instrument was introduced into the uterine cavity. Indications for surgical evacuation include the removal of retained products of the placenta and heavy vaginal bleeding, where reported. Fewer women required surgical evacuation when misoprostol was administrated vaginally when compared to women having mid‐trimester abortion by intra‐amniotic instillation of PGF2α (OR 0.52, 95% CI 0.31 to 0.87) (Comparison 9). Apart from the latter finding, there were no statistically significant differences in reported frequencies of surgical removal of the placenta among women undergoing misoprostol‐induced abortions when compared to other regimens.
Diarrhoea is the most common adverse reaction that has been reported consistently with misoprostol, but it is usually mild and self limiting. Nausea and vomiting may also occur and generally resolves in two to six hours (Tang 2007). Uterine rupture is a rare but serious complication of abortion in the second trimester of pregnancy, especially in women with a previous uterine scar (Berghella 2009). Uterine rupture is uncommon and did not occur during any of the included trials; thus, its relative risk with differing medical regimens are not informed by this review.
Summary of main results
Thirty‐six randomised controlled trials were included in the review. The included studies addressed the various agents for pregnancy termination and methods of administration which were grouped into 28 comparisons. When used alone, misoprostol is an effective inductive agent, though it appears to be more effective in combination with mifepristone.
Misoprostol is preferably administered vaginally, although among multiparous women sublingual administration appears equally effective. The optimal dose of vaginally administered misoprostol could not be determined, as no randomised studies could be identified. Low doses of misoprostol are associated with fewer side‐effects, while moderate doses are more efficient in completing abortion. Four randomised controlled trials showed that the induction to abortion interval with 3‐hourly vaginal administration of prostaglandins is significantly shorter than 6‐hourly administration without a significant increase in side‐effects.
Many studies reported the need for surgical evacuation in a considerable number of women undergoing mid‐trimester termination. Indications for surgical evacuation include the removal of retained products of the placenta and heavy vaginal bleeding. Fewer women required surgical evacuation when misoprostol was administrated vaginally when compared with those having intra‐amniotic instillation of PGF2a. Apart from the latter finding, there were no statistically significant differences in reported frequencies of surgical removal of the placenta among women undergoing misoprostol‐induced abortions when compared to other regimens. Diarrhoea was more common among women having misoprostol when compared to other agents. However, diarrhoea is reportedly mild and self limiting.
Overall completeness and applicability of evidence
The results of this review fit well into the current practices of mid‐trimester termination of pregnancy.
Quality of the evidence
All randomised controlled trials, most of these being unblinded. Given the heterogeneity of the some studies included in the review, the internal validity of the findings is limited.
Potential biases in the review process
None.
Agreements and disagreements with other studies or reviews
Agree with recent Society for Family Planning Guidelines, in press.
Authors' conclusions
Implications for practice.
The results of this review suggest that the most efficient regimen for medical abortion in the second trimester is the combination of mifepristone and misoprostol. If mifepristone is not available, misoprostol alone is a reasonable alternative. The available data suggest that vaginal administration is the most efficient route of administration, and 3‐hourly intervals of administration are more effective than 6‐hourly intervals. Meta‐analysis of the various randomised controlled trials on misoprostol was hampered by the heterogeneity in medical regimens used among the included trials. Included studies indicate that adverse effects of misoprostol are usually mild and dose dependant. Apart from pain resulting from uterine contractions, diarrhoea is the most common side‐effect that has been reported consistently with misoprostol. There are considerable differences in practices regarding the management of the placenta following the expulsion of the fetus.
Implications for research.
This review highlights the importance of developing a standardised medical method for women requesting mid‐trimester abortion. Further research is needed to evaluate the gestational‐age‐specific dosage of misoprostol for mid‐trimester abortion. In addition, more data are needed to guide medical and/or surgical strategies for women with a uterine scar resulting from prior hysterotomy (see Berghella 2009) and for those who failed to abort within 24 hours or five doses of misoprostol. Finally, more research is needed to evaluate the additional value, optimal dose and timing of mifepristone when used in combination with misoprostol.
Notes
None
Acknowledgements
None
Data and analyses
Comparison 1. Comparison: mifepristone+misoprostol versus mifepristone+gemeprost.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐4.89, 4.09] |
| 2 Abortion within 24 hours | 3 | 210 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.23, 2.24] |
| 3 Surgical evacuation | 3 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.27, 1.35] |
| 4 Pain | 3 | 199 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.19, 1.21] |
| 5 Nausea | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.19, 1.90] |
| 6 Vomiting | 2 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.47, 2.13] |
| 7 Diarrhoea | 2 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.83, 5.23] |
Comparison 2. Comparison: mifepristone+misoprostol versus placebo+misoprostol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abortion within 24 hours | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.13 [1.43, 102.61] |
| 2 Surgical evacuation | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.02, 2.14] |
| 3 Pain | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.70, 5.38] |
| 4 Nausea | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.48, 3.44] |
| 5 Vomiting | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.42, 3.07] |
Comparison 3. Comparison: mifepristone+misoprostol, vaginal versus oral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 2 | 237 | Mean Difference (IV, Fixed, 95% CI) | 7.03 [‐0.13, 14.20] |
| 1.1 Misoprostol, 200 mcg oral versus 200 mcg vaginal | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [2.77, 23.23] |
| 1.2 Misoprostol, 400 mcg oral versus 200 mcg vaginal | 1 | 139 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐8.73, 11.33] |
| 2 Abortion within 24 hours | 3 | 306 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.28, 1.02] |
| 2.1 Misoprostol, 200 mcg oral versus 200 mcg vaginal | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.09, 0.78] |
| 2.2 Misoprostol, 400 mcg oral versus 200 mcg vaginal | 1 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.34, 2.01] |
| 2.3 Misoprostol, 400 mcg oral versus 400 mcg vaginal | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 16.17] |
| 3 Surgical evacuation | 2 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.48, 2.04] |
| 3.1 Misoprostol, 400 mcg oral versus 200 mcg vaginal | 1 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.31, 1.57] |
| 3.2 Misoprostol, 400 mcg oral versus 400 mcg vaginal | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.86 [0.65, 53.09] |
| 4 Pain | 2 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.66, 2.62] |
| 4.1 Misoprostol, 400 mcg oral versus 200 mcg vaginal | 1 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.43, 2.79] |
| 4.2 Misoprostol, 400 mcg oral versus 400 mcg vaginal | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.59, 4.57] |
| 5 Nausea | 2 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.61, 1.70] |
| 5.1 Misoprostol, 200 mcg oral versus 200 mcg vaginal | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.28, 1.47] |
| 5.2 Misoprostol, 400 mcg oral versus 200 mcg vaginal | 1 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.70, 2.68] |
| 6 Vomiting | 3 | 306 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.61, 1.56] |
| 6.1 Misoprostol, 200 mcg oral versus 200 mcg vaginal | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.25, 1.63] |
| 6.2 Misoprostol, 400 mcg oral versus 200 mcg vaginal | 1 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.56, 2.15] |
| 6.3 Misoprostol, 400 mcg oral versus 400 mcg vaginal | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.46, 3.17] |
| 7 Diarrhoea | 3 | 306 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.18, 3.22] |
| 7.1 Misoprostol, 200 mcg oral versus 200 mcg vaginal | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.84, 5.50] |
| 7.2 Misoprostol, 400 mcg oral versus 200 mcg vaginal | 1 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.06, 4.61] |
| 7.3 Misoprostol, 400 mcg oral versus 400 mcg vaginal | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.49, 3.77] |
Comparison 4. Comparison: mifepristone+misoprostol, vaginal versus sublingual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Surgical evacuation | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.72 [0.37, 37.72] |
| 2 Pain | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.16, 1.97] |
| 3 Nausea | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.59, 5.70] |
| 4 Vomiting | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.58, 5.07] |
| 5 Diarrhoea | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.43, 2.91] |
Comparison 5. Comparison: mifepristone+misoprostol, oral versus sublingual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abortion within 24h | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.17, 1.70] |
| 2 Pain (need of analgesic) | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.40, 1.94] |
| 3 Nausea | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.60, 2.61] |
| 4 Diarrhoea | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.66, 4.54] |
Comparison 6. Comparison: dosing interval of misoprostol following mifepristone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abortion within 24 hours | 1 | 141 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.99 [0.77, 253.29] |
| 2 Surgical evacuation | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.50] |
| 3 Pain (need for analgesia) | 1 | 141 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.20, 1.67] |
| 4 Nausea | 1 | 141 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.50, 1.89] |
| 5 Diarrhoea | 1 | 141 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.21, 1.16] |
Comparison 7. Comparison: dosage of mifepristone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.88, 0.72] |
| 2 Abortion within 24h | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.18, 23.83] |
| 3 Surgical evacuation | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.12, 2.56] |
| 4 Pain | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.78, 5.31] |
| 5 Vomiting | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.86, 5.85] |
| 6 Diarrhoea | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.34, 2.92] |
Comparison 8. Comparison: combined regimen of mifepristone and gemeprost.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abortion within 24 hours | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.18, 23.27] |
| 2 Blood loss | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.21] |
| 3 Surgical evacuation | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.55, 3.80] |
| 4 Vomiting | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [1.04, 7.66] |
| 5 Diarrhoea | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.63, 17.07] |
Comparison 9. Comparison: misoprostol versus PGF.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Vaginal misoprostol versus PGF | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐7.74, ‐1.46] |
| 2 Abortion within 24 hours | 1 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.53, 2.60] |
| 2.1 Vaginal misoprostol versus PGF | 1 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.53, 2.60] |
| 3 Surgical evacuation | 1 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.32, 2.02] |
| 3.1 Vaginal misoprostol versus PGF | 1 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.32, 2.02] |
| 4 Nausea | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.26, 1.47] |
| 4.1 Vaginal misoprostol versus PGF | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.26, 1.47] |
| 5 Vomiting | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.25, 1.59] |
| 5.1 Vaginal misoprostol versus PGF | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.25, 1.59] |
| 6 Diarrhoea | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Vaginal misoprostol versus PGF | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.21, 1.14] |
Comparison 10. Comparison: misoprostol versus gemeprost.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 3 | 249 | Mean Difference (IV, Fixed, 95% CI) | 8.73 [5.11, 12.35] |
| 1.1 Misoprostol, 100 mcg, versus gemeprost | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 8.60 [3.11, 14.09] |
| 1.2 Misoprostol, 200 mcg versus gemeprost | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 13.3 [7.90, 18.70] |
| 1.3 Misoprostol, 400 mcg, versus gemeprost | 1 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐8.90 [‐19.65, 1.85] |
| 2 Abortion within 24 hours | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [1.33, 6.02] |
| 2.1 Misoprostol, 400 mcg, versus gemeprost | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [1.33, 6.02] |
| 3 Blood loss (mL) | 3 | 249 | Mean Difference (IV, Fixed, 95% CI) | ‐23.75 [‐47.80, 0.30] |
| 3.1 Misoprostol, 100 mcg, versus gemeprost | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐61.0 [‐145.71, 23.71] |
| 3.2 Misoprostol, 200 mcg, versus gemeprost | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐146.0 [‐219.02, ‐72.98] |
| 3.3 Misoprostol, 400 mcg, versus gemeprost | 1 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐30.40, 23.00] |
| 4 Surgical evacuation | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.48, 1.85] |
| 4.1 Misoprostol, 400 mcg, versus gemeprost | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.48, 1.85] |
| 5 Pain | 2 | 109 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.10, 0.52] |
| 5.1 Misoprostol, 100 mcg, versus gemeprost | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.07, 0.71] |
| 5.2 Misoprostol, 200 mcg, versus gemeprost | 1 | 54 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.77] |
| 6 Nausea | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.38, 1.70] |
| 6.1 Misoprostol, 400 mcg, versus gemeprost | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.38, 1.70] |
| 7 Vomiting | 3 | 249 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.21, 0.67] |
| 7.1 Misoprostol, 100 mcg, versus gemeprost | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.10, 1.06] |
| 7.2 Misoprostol, 200 mcg, versus gemeprost | 1 | 54 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.62] |
| 7.3 Misoprostol, 400 mcg, versus gemeprost | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.25, 1.18] |
| 8 Diarrhoea | 3 | 249 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.17, 0.58] |
| 8.1 Misoprostol, 100 mcg, versus gemeprost | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.68] |
| 8.2 Misoprostol, 200 mcg, versus gemeprost | 1 | 54 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.03, 0.91] |
| 8.3 Misoprostol, 400 mcg, versus gemeprost | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.23, 0.99] |
Comparison 11. Comparison: misoprostol versus dinoprost.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abortion within 24 hours | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 51.73 [2.89, 924.42] |
| 2 Blood loss | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 10.52 [0.58, 191.12] |
| 3 Surgical evacuation | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.90 [0.31, 113.60] |
| 4 Pain (need analgesia) | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.59, 4.08] |
| 5 Vomiting | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.52, 3.85] |
| 6 Diarrhoea | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.07, 46.83] |
Comparison 12. Comparison: misoprostol, vaginal versus oral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Misoprostol 400 mcg vaginal versus 400 mcg oral | 2 | 196 | Mean Difference (IV, Fixed, 95% CI) | ‐6.04 [‐8.51, ‐3.58] |
| 1.2 Misoprostol, 400 mcg vaginal versus 200 mcg oral | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐14.9 [‐23.33, ‐6.47] |
| 2 Abortion within 24 hours | 1 | 114 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.6 [3.74, 24.66] |
| 2.1 Misoprostol 400 mcg vaginal versus 200 mcg oral | 1 | 114 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.6 [3.74, 24.66] |
| 3 Blood loss (mL) | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 24.00 [‐70.90, 118.90] |
| 3.1 Misoprostol 400 mcg vaginal versus 200 mcg oral | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 24.00 [‐70.90, 118.90] |
| 4 Pain | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.07] |
| 4.1 Misoprostol 400 mcg vaginal versus 400 mcg oral | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.07] |
| 5 Surgical evacuation | 3 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.34, 1.17] |
| 5.1 Misoprostol 400 mcg vaginal versus 400 mcg oral | 2 | 196 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.30, 1.21] |
| 5.2 Misoprostol 400 mcg vaginal versus 200 mcg oral | 1 | 114 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.20, 2.67] |
| 6 Nausea | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.18, 0.93] |
| 6.1 Misoprostol 400 mcg vaginal versus 400 mcg oral | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.18, 0.93] |
| 7 Vomiting | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.98] |
| 7.1 Misoprostol 400 mcg vaginal versus 400 mcg oral | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.98] |
| 8 Diarrhoea | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.39, 3.85] |
| 8.1 Misoprostol 400 mcg vaginal versus 400 mcg oral | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.39, 3.85] |
Comparison 13. Comparison: misoprostol, vaginal versus sublingual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 1 | 277 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.00, 0.80] |
| 2 Abortion within 24 hours | 3 | 1178 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.05, 1.83] |
| 3 Blood loss | 1 | 277 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.52, 6.31] |
| 4 Pain | 2 | 497 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.73, 1.63] |
| 5 Surgical evacuation | 2 | 497 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.52, 1.58] |
| 6 Nausea | 3 | 1178 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.81, 1.48] |
| 7 Vomiting | 2 | 958 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.55, 1.30] |
| 8 Diarrhoea | 3 | 1178 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.25] |
Comparison 14. Comparison: misoprostol, tablet versus gel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abortion within 24 hours | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.37, 1.43] |
| 2 Blood loss > 500 mL | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.03, 3.37] |
| 3 Surgical evacuation | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.67, 3.15] |
| 4 Pain | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.46, 1.93] |
| 5 Nausea | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.26, 9.92] |
| 6 Vomiting | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.33, 32.09] |
| 7 Diarrhoea | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.03, 0.71] |
Comparison 15. Comparison: time interval misoprostol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 1 | 148 | Mean Difference (IV, Fixed, 95% CI) | ‐19.2 [‐36.02, ‐2.38] |
| 2 Abortion within 24 hours | 2 | 427 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.99, 2.26] |
| 2.1 Misoprostol 400 mcg, every 3h versus every 6h | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.87, 3.48] |
| 2.2 Misoprostol 600 mcg, every 6h versus every 12h | 1 | 279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.82, 2.31] |
| 3 Blood loss (mL) | 1 | 148 | Mean Difference (IV, Fixed, 95% CI) | ‐39.7 [‐83.12, 3.72] |
| 4 Blood loss (>500 mL) | 1 | 279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.29, 6.07] |
| 4.1 Misoprostol 600 mcg, every 6h versus every 12h | 1 | 279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.29, 6.07] |
| 5 Surgical evacuation | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.14 [0.31, 120.92] |
| 5.1 Misoprostol 600 mcg, every 6h versus every 12h | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.14 [0.31, 120.92] |
| 6 Pain | 2 | 427 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.75, 1.70] |
| 6.1 Misoprostol 400 mcg, every 3h versus every 6h | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.45, 1.74] |
| 6.2 Misoprostol 600 mcg, every 6h versus every 12h | 1 | 279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.78, 2.16] |
| 7 Nausea | 2 | 427 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.64, 2.45] |
| 7.1 Misoprostol 400 mcg, every 3h versus every 6h | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.27, 2.15] |
| 7.2 Misoprostol 600 mcg, every 6h versus every 12h | 1 | 279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.74, 4.49] |
| 8 Vomiting | 2 | 427 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.72, 3.00] |
| 8.1 Misoprostol 400 mcg, every 3h versus every 6h | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.35, 2.82] |
| 8.2 Misoprostol 600 mcg, every 6h versus every 12h | 1 | 279 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.76, 5.70] |
| 9 Diarrhoea | 2 | 427 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.80, 2.04] |
| 9.1 Misoprostol 400 mcg, every 3h versus every 6h | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.37, 11.59] |
| 9.2 Misoprostol 600 mcg, every 6h versus every 12h | 1 | 279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.75, 2.00] |
Comparison 16. Comparison: time interval gemeprost.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abortion within 24 hours | 1 | 99 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.42, 2.52] |
| 2 Surgical evacuations | 1 | 99 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.21, 1.25] |
| 3 Pain | 1 | 99 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.71, 5.09] |
Comparison 17. Comparison: low dose versus high dose of misoprostol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | 1.29 [‐3.12, 5.69] |
| 1.1 100 mcg versus 200 mcg | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐4.70 [‐11.19, 1.79] |
| 1.2 400 mcg versus 600 mcg | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 6.4 [0.40, 12.40] |
| 2 Pain | 2 | 113 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.29, 1.95] |
| 2.1 100 mcg versus 200 mcg | 1 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.32, 2.75] |
| 2.2 400 mcg versus 600 mcg | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 3.17] |
| 3 Nausea | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.10, 4.15] |
| 3.1 400 mcg versus 600 mcg | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.10, 4.15] |
| 4 Vomiting | 2 | 113 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.42, 4.85] |
| 4.1 100 mcg versus 200 mcg | 1 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.19 [0.49, 9.88] |
| 4.2 400 mcg versus 600 mcg | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.63] |
| 5 Diarrhoea | 2 | 113 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.11, 2.58] |
| 5.1 100 mcg versus 200 mcg | 1 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.90] |
| 5.2 400 mcg versus 600 mcg | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.60] |
17.3. Analysis.
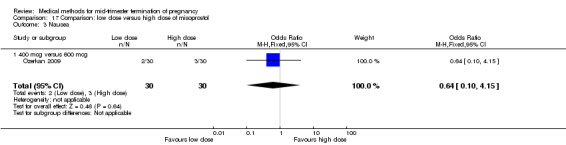
Comparison 17 Comparison: low dose versus high dose of misoprostol, Outcome 3 Nausea.
Comparison 18. Comparison: PGE2 versus PGF2.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐9.10 [‐13.68, ‐4.52] |
| 2 Abortion within 24 hours | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.29 [1.29, 98.89] |
| 3 Pain | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.84] |
| 4 Nausea | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.07, 1.26] |
| 5 Vomiting | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐2.32, 0.92] |
| 6 Diarrhoea | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐2.28, 0.68] |
Comparison 19. Comparison: PGE2 versus PGF2+oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [0.90, 3.10] |
| 1.1 PGE + oxytocin versus PGF + oxytocin | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [0.90, 3.10] |
| 2 Surgical evacuation | 2 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.07, 0.90] |
| 2.1 PGE + oxytocin versus PGF + oxytocin | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.07, 1.15] |
| 2.2 PGE versus PGF | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.85] |
| 3 Pain | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.72] |
| 3.1 PGE versus PGF | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.72] |
| 4 Vomiting | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.17, 2.17] |
| 4.1 PGE + oxytocin versus PGF + oxytocin | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.17, 2.17] |
| 5 Diarrhoea | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.06] |
| 5.1 PGE + oxytocin versus PGF + oxytocin | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.06] |
Comparison 20. Comparison: PGF2 versus hypertonic saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐6.67, ‐3.93] |
| 1.1 20% NaCL versus single dose of 40 mg PGF | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐6.67, ‐3.93] |
| 2 Abortion within 24 hours | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 20% NaCL versus single dose of 50 mg PGF | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.58, 3.98] |
| 2.2 20% NaCl versus multiple doses of 25mg PGF | 2 | 1579 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.14 [4.91, 7.68] |
| 2.3 20% NaCL versus single dose of 40 mg PGF | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 15.78 [0.79, 314.27] |
| 3 Blood loss >100 ml | 3 | 165 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.50 [0.79, 7.91] |
| 3.1 20% NaCL versus single dose of 50 mg PGF | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.30 [0.96, 71.72] |
| 3.2 20% NaCL versus multuple doses of 25 mg PGF | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.23] |
| 3.3 20% NaCL versus single dose of 40 mg PGF | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.23, 11.26] |
| 4 Blood loss >500 ml | 1 | 1513 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.05 [1.56, 5.97] |
| 4.1 20% NaCL versus multiple doses of 25 mg PGF | 1 | 1513 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.05 [1.56, 5.97] |
| 5 Nausea | 1 | 1513 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.01 [1.17, 7.72] |
| 5.1 20% NaCL versus multiple doses of 25 mg PGF | 1 | 1513 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.01 [1.17, 7.72] |
| 6 Vomiting | 3 | 1646 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.16 [4.12, 6.46] |
| 6.1 20% NaCL versus single dose of 50 mg PGF | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 22.4 [2.73, 183.71] |
| 6.2 20% NaCL versus multiple doses of 25 mg PGF | 2 | 1579 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.01 [3.99, 6.28] |
| 7 Diarrhoea | 3 | 1646 | Odds Ratio (M‐H, Fixed, 95% CI) | 10.83 [6.17, 19.02] |
| 7.1 20% NaCL versus single dose of 50 mg PGF | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.35, 12.13] |
| 7.2 20% NaCL versus multiple doses of 25 mg PGF | 2 | 1579 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.47 [6.81, 22.82] |
| 8 Surgical evacuation | 3 | 1646 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.30, 1.96] |
| 8.1 20% NaCL versus single dose of 50 mg PGF | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.89 [2.01, 30.95] |
| 8.2 20% NaCL versus multiple doses of 25 mg PGF | 2 | 1579 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.24, 1.87] |
20.3. Analysis.
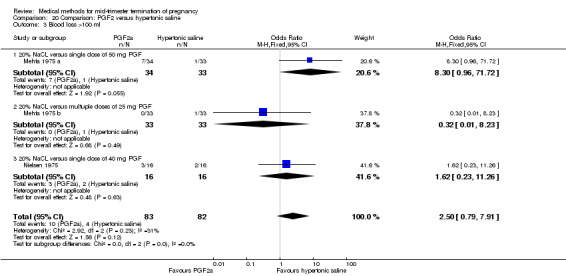
Comparison 20 Comparison: PGF2 versus hypertonic saline, Outcome 3 Blood loss >100 ml.
Comparison 21. Comparison: combined regimen PGF2+hypertonic saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 4 | 770 | Mean Difference (IV, Fixed, 95% CI) | ‐1.55 [‐3.53, 0.44] |
| 1.1 100 ml 5% NaCl versus 100 ml 10% NaCl combined with PGF | 2 | 230 | Mean Difference (IV, Fixed, 95% CI) | 2.21 [‐1.59, 6.00] |
| 1.2 25 ml 20% NaCl versus 100 ml 10% NaCl combined with PGF | 2 | 540 | Mean Difference (IV, Fixed, 95% CI) | ‐2.96 [‐5.29, ‐0.64] |
| 2 Abortion within 24 hours | 4 | 770 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.90, 1.97] |
| 2.1 100 ml 5% NaCl versus 100 ml 10% NaCl combined with PGF | 2 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.38, 1.26] |
| 2.2 25 ml 20% NaCl versus 100 ml 10% NaCl combined with PGF | 2 | 540 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.30 [1.38, 3.86] |
| 3 Blood loss (>500 ml) | 4 | 770 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.66, 1.95] |
| 3.1 100 ml 5% NaCl versus 100 ml 10% NaCl combined with PGF | 2 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.23, 1.78] |
| 3.2 25 ml 20% NaCl versus 100 ml 10% NaCl combined with PGF | 2 | 540 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.74, 2.80] |
| 4 Surgical evacuation | 4 | 770 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.66, 1.95] |
| 4.1 100 ml 5% NaCl versus 100 ml 10% NaCl combined with PGF | 2 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.23, 1.78] |
| 4.2 25 ml 20% NaCl versus 100 ml 10% NaCl combined with PGF | 2 | 540 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.74, 2.80] |
| 5 Nausea | 4 | 770 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.35, 0.94] |
| 5.1 100 ml 5% NaCl versus 100 ml 10% NaCl combined with PGF | 2 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.52, 2.19] |
| 5.2 25 ml 20% NaCl versus 100 ml 10% NaCl combined with PGF | 2 | 540 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.17, 0.62] |
| 6 Vomiting | 4 | 770 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.44, 1.72] |
| 6.1 100 ml 5% NaCl versus 100 ml 10% NaCl combined with PGF | 2 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.33, 3.06] |
| 6.2 25 ml 20% NaCl versus 100 ml 10% NaCl combined with PGF | 2 | 540 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.34, 1.87] |
| 7 Diarrhoea | 4 | 770 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.24, 4.83] |
| 7.1 100 ml 5% NaCl versus 100 ml 10% NaCl combined with PGF | 2 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.02, 13.13] |
| 7.2 25 ml 20% NaCl versus 100 ml 10% NaCl combined with PGF | 2 | 540 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.23, 8.33] |
Comparison 22. Comparison: PGE1 versus PGF2+hypertonic saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.10, 1.70] |
| 2 Abortion within 24h | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.67] |
| 3 Blood loss (>300ml) | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.79] |
| 4 Surgical evacuation | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.54, 4.34] |
| 5 Pain (pethidine) | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.10, 4.16] |
| 6 Vomiting | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.11 [1.06, 9.08] |
| 7 Diarrhoea | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 19.13 [3.80, 96.18] |
Comparison 23. Comparison: prostaglandin versus ethacridine lactate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 1 | 178 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.03, 0.03] |
| 2 Abortion within 24h | 2 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.33, 0.98] |
| 2.1 PGE2 versus EL | 1 | 78 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.06, 0.48] |
| 2.2 Misoprostol versus EL | 1 | 178 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.50, 1.99] |
| 3 Blood loss | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.18, 24.87] |
| 4 Nausea | 2 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.79, 6.46] |
| 5 Vomiting | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.37, 3.81] |
| 6 Diarrhoea | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.47 [0.25, 120.37] |
Comparison 24. Comparison: ethacridine lactate versus normal saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction to abortion interval | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐4.02, 3.42] |
| 2 Blood loss (need for blood transfusion) | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.26] |
| 3 Pain (use of analgestics) | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.89] |
| 4 Vomiting (use of antimetics) | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.33 [0.67, 60.16] |
| 5 Uterine rupture | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akoury 2004.
| Methods | Computer‐generated randomisation sequence with stratification for participating centre and gestational age (≥20 weeks versus < 20 weeks) using blocks of 6. A central office allocated study patients to groups using sealed opaque envelopes. Women were randomly assigned. | |
| Participants | 136 pregnant women (group I: 84, group II: 52) Inclusion criteria: singleton, live fetus at 15 to 24 weeks’ gestation with a complex fetal anomaly and/or abnormal fetal karyotype were included. Exclusion criteria: allergy to prostaglandins, a previous classic cesarean section or hysterotomy, active bleeding, severe asthma, severe oligohydramnios, pre‐labor rupture of membranes. |
|
| Interventions | Group I: 400 mg of misoprostol in the posterior fornix of the vagina every 4 hours for a total of 6 doses or until delivery occurred. If after 24 hours no labor commenced, an intravenous solution of oxytocin, 100 U/of L Ringer’s lactate at 100 mL per hour, was commenced. Group II: 400 μg misoprostol orally every 4 hours for a total of 6 doses or until delivery occurred. If after 24 hours no labor commenced, an intravenous solution of oxytocin, 100 U/of L Ringer’s lactate at 100 mL per hour, was commenced. |
|
| Outcomes | Primary outcome: time from the start of the procedure to placental delivery. Secondary outcomes: incidence of major and minor maternal complications, women’s views of the method and the success rate for culture of fetal umbilical cord. |
|
| Notes | In this study, women were randomly assigned to 1 of 3 groups: intra‐amniotic PGF2a, vaginal misoprostol, or oral misoprostol. (n=217). The women receiving PGF2a were excluded from our analyses, because of the use of laminaria. Definition of abortion: expulsion of the fetus and placenta. No clear information was provided regarding the policy of evacuation of the uterus. No major complications occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Armatage 1996.
| Methods | Patients were randomised into 2 groups using sealed sequentially numbered envelopes. | |
| Participants | 99 pregnant women (group I: 50, group II: 49) Inclusion criteria: uncomplicated pregnancies, between 12‐20 weeks gestation. Exclusion criteria: multiple pregnancy, known fetal abnormality, significant maternal illness. |
|
| Interventions | Group I: gemeprost pessaries at 3‐hourly intervals up to a maximum of 5 in 24 hours, until fetal expulsion. Group II: gemeprost pessaries at 6‐hourly intervals until fetal expulsion. Where abortion did not occur within 48 hours, an intravenous oxytocin infusion was commenced unless delivery was deemed imminent. |
|
| Outcomes | Primary outcome: abortion interval, abortion rates. Secondary outcomes: analgesia, side‐effects, surgical evacuations. |
|
| Notes | Definition of abortion: expulsion of the fetus. Following delivery of the fetus, intramuscular Syntometrine (ergometrine maleate 500 μg and oxytocin 5 iu, Sandez Products Limited) was given. One women received a blood transfusion (group 1). Women underwent surgical evacuation if the placenta was retained or did not appear intact. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Bartley 2002.
| Methods | Randomisation was carried out using opaque envelopes. These envelopes were sealed, then shuffled and numbered consecutively in two batches of 50. | |
| Participants | 100 pregnant women (group I: 50, group II: 50). Inclusion criteria: gestation 12 to 20 weeks. No exclusion criteria were reported. A history of previous caesarean section was not considered a reason for exclusion. A history of previous caesarean section was not considered a reason for exclusion. |
|
| Interventions | All: 200 mg mifepristone and admission followed approximately 36 hours later: Group I: 800 μg misoprostol tablets inserted in the posterior vaginal fornix followed by 400 μg misoprostol tablets orally every 3 hours for a maximum of four doses over the first 24 hours; Group II: 1 mg gemeprost inserted in the posterior vaginal fornix every 6 hours for a maximum of four doses over the first 24 hours. If abortion did not occur within 24 hours, 1 mg vaginal gemeprost was administered every 3 hours to a maximum of five doses over the next 12 hours. If abortion did not occur after this course of gemeprost, the abortion was completed by intravenous oxytocin, repeated course of gemeprost or dilatation and evacuation. |
|
| Outcomes | Primary outcome: prostaglandin to abortion interval. Secondary outcomes: differences in percentage of women delivered by 24 hours, incidence in side effects and adverse events. |
|
| Notes | No clear definition of abortion. One woman required a blood transfusion and an emergency evacuation of the uterus due to severe haemorrhage. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Bebbington 2002.
| Methods | Randomization was performed with a series of sequentially numbered opaque envelopes that contained allocations determined through the use of a random number table. | |
| Participants | 114 pregnant women (group I: 49, group II: 65) Inclusion criteria: midtrimester abortion. Exclusion criteria: hypersensitivity to prostaglandins, inability to understand English to ensure informed consent. |
|
| Interventions | Group I: misoprostol 400 μg in the posterior vaginal fornix every 4 hours. Group II: misoprostol 200 μg orally every hour for 3 hours and then 400 μg orally every 4 hours. If the patient was undelivered after 24 hours, the attending physician determined further management. The options available were to increase the dosage of misoprostol using the same route of administration, to change the route of administration of the misoprostol, to proceed with a high‐dose oxytocin infusion, or to proceed with surgical evacuation of the uterus. |
|
| Outcomes | Primairy outcomes: induction to abortion interval. Secondary outcomes: maternal fever >38°C; maternal infection defined as maternal fever, elevated white blood cell count, and the need for antibiotics in the postabortion period; maternal side effects from the medication including nausea or diarrhoea, blood loss, the need for additional operative intervention; and the failure to achieve a medical termination of pregnancy. |
|
| Notes | Definition of abortion: expulsion of the fetus. If the placenta remained undelivered after 2h, an attempt was made at manual extraction under general anaesthesia. No major complications reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Behrashi 2008.
| Methods | Random assignment, not specified. | |
| Participants | 60 pregnant women (group I: 30, group II: 30) Inclusion criteria: 14‐28 weeks gestation. Exclusion criteria: contraindications to prostaglandin therapy, placenta previa, cervical changes, uncontrolled convulsion, glaucoma, inflammatory bowel disease. |
|
| Interventions | Group I: 400 μg misoprostol, vaginally Group II: 400 μg misoprostol, orally These regimens was followed by 400 μg of misoprostol up to 3 doses, if needed. After delivery: 30 unit oxytocin (in 1000 ml Ringer's solution). |
|
| Outcomes | Complete expulsion, induction to abortion interval, side‐effects, surgical evacuation. | |
| Notes | Definition of abortion: expulsion of fetus and placenta. No major complications occurred. No time interval was given by the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | B ‐ unclear |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Bhattacharjee 2008.
| Methods | The patients were randomly allocated into two groups using a computer‐generated randomisation protocol. A computer‐generated randomisation sequence was used to assign participants into two treatment groups. The allocation was concealed in sealed, sequentially numbered, brown envelopes, which had been prepared by the statistician of each centre and handed over to the respective pharmacy department. | |
| Participants | 277 pregnant women (group I: 139, group II: 138) Inclusion criteria: 13 ‐ 20 weeks singleton pregnancy, young healthy women. Exclusion criteria: gestation < 13 or > 20 weeks, contraindication for misoprostol use. |
|
| Interventions | Group I: vaginal administration 400 μg misoprostol at the interval of three hours, up to a maximum
five doses over 24h. Group II: sublingual administration 400 μg misoprostol, at the interval of three hours, up to a maximum five doses over 24h. The patients were instructed to keep the tablets under the tongue until these were dissolved and not to spit out or swallow the content for at least one hour post‐administration. Those women, who failed to abort within 24 h of initiation of the treatment,received a second course of misoprostol, with the same allocated regimen, over a period of another 24 h. If a woman failed to abort after 48 h, the regimen was declared unsuccessful and she was offered a regimen of extra amniotic 0.1% ethacridine lactate infusion (single instillation) or repeated doses of dinoprostone gel (0.5 mg) in the cervical canal six‐hourly up to a maximum of three doses. |
|
| Outcomes | Primary outcomes: induction to abortion interval, abortion within 24 and 48 hours. Secondary outcomes: blood loss, surgical evacuations, side‐effects. |
|
| Notes | Definition of abortion: expulsion of fetus and placenta without operative intervention. Exploration of the uterus was performed under deep sedation or short general anaesthesia if the placenta was found to be incompletely expelled. No major complications reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Borgida 1995.
| Methods | Sequentially numbered, sealed, opaque envelopes containing indicator cards and were opened at enrolment. The randomisation sequence was determined by a random‐number table and a block size of 6. | |
| Participants | 50 pregnant women (group I: 27, group II: 23) Inclusion criteria: abnormal 14‐24 weeks pregnancy, age 18‐45 years. Exclusion criteria: allergies to medications, cardiac/pulmonary/renal disease. |
|
| Interventions | All: pre‐med (25 mg diphenhydramine hydrochloride, 10 mg metoclopramide hydrochloride, 5 mg diphenoxylate hydrochloride, 650 mg acetaminophen) every 4‐6 hours + 30 minutes after first dose: Group I: 250 μg IM 15M PGF2α injections every 3 hours; Group II: 20 mg intravaginal PGE2 every 3 hours. After delivery, all patients received oxytocin 40 U/L, and if the placenta was not delivered within approximally 2 hours or excessive bleeding occurred, a curettage was performed. |
|
| Outcomes | Primary outcomes: induction to abortion interval, abortion within 24 hours. Secondary outcomes: surgical evacuation, side‐effects. |
|
| Notes | Definition of abortion: expulsion of fetus. No major complications reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Chai 2009.
| Methods | The randomisation was done by computer‐generated random numbers and the group assignments were put into sealed, opaque envelopes. The randomisation envelope was opened by the research nurse after recruitment. The investigating team members and the research nurse responsible for recruitment were not aware of the randomisation. | |
| Participants | 141 pregnant women (group I: 70, group II: 71) were recruited from the Hong Kong centre and Shanghai centre. Inclusion criteria: healthy women aged 18 or older who requested termination of second trimester pregnancy at 12–20 weeks of gestation and were willing to comply with the schedule of follow‐up visits. Exclusion criteria: any contraindications to mifepristone, including adrenal disease or steroid‐dependent cancer; any contraindications to misoprostol, including mitral stenosis, glaucoma, sickle cell anaemia, diastolic pressure over 100 mmHg, severe asthma or known allergy to prostaglandin; history or evidence of thrombo‐embolism, severe or recurrent liver disease or pruritus of pregnancy; a known history of or active medical disease; a history of regular use of prescription drugs; an intrauterine contraceptive device in utero; a haemoglobin level ,100 g/l or abnormal liver or renal function tests; breastfeeding or heavy smoker of more than 20 cigarettes per day. |
|
| Interventions | Group I: 200 mg mifepristone and 36–38 h later: 600 μg misoprostol vaginally every 3 h for a maximum of four doses. Group II: 200 mg mifepristone was given orally and 600 μg misoprostol was given vaginally simultaneously, followed by 400 μg vaginal misoprostol every 3 h for a maximum of four doses. The patient was reassessed if abortion had not occurred after 24 h. If there were no signs and symptoms suggestive of imminent abortion, a second course of vaginal misoprostol was given for a maximum of five doses (600 μg for the first dose followed by 400 μg every 3 h for a maximum of four doses). If abortion still did not occur, gemeprost was given to terminate the pregnancy. |
|
| Outcomes | Primary outcomes: success rate at 24 h. Secondary outcomes: difference in the induction‐to‐abortion interval and the frequency of side effects between two groups. |
|
| Notes | Definition of abortion: expulsion of fetus. No major complications reported. Six patients from Hong Kong centre (five from the immediate dosing group and one from the conventional dosing group) required suction evacuation of the uterus for retained placenta before discharge from the hospital. All patients from Shanghai centre had dilatation and curettage the day following abortion, as it was the routine practice in that hospital. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
el‐Refaey 1993.
| Methods | Randomisation by sealed envelope selection. | |
| Participants | 60 pregnant women (group I: 30, group II: 30) Inclusion criteria: 13‐20 weeks gestation. Exclusion criteria: none reported. |
|
| Interventions | 600mg mifepristone (36‐48 hours later followed by): Group I: misoprostol 400 μg orally, every 3 hrs, max 3 doses. If abortion did not occur: two further doses of vaginal gemeprost 1 mg, every 3 hrs; Group II: gemeprost 1 mg pessaries vaginally, every 3 hrs, max 5 doses. |
|
| Outcomes | Primary outcome: abortion within 24 hours. Secondary outcomes: surgical evacuation, side‐effects. |
|
| Notes | Definition of abortion: expulsion of fetus and placenta. No information was provided regarding the policy of evacuation of the uterus. No major complications reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
El‐refaey 1995.
| Methods | Randomisation using computer‐generated random number tables. A series of numbered, sealed, opaque envelopes was prepared containing allocation. | |
| Participants | 69 pregnant women (group I: 34, group II: 35) Inclusion criteria: 13‐20 weeks gestation. Exclusion criteria: none reported. |
|
| Interventions | All: mifepristone 600 μg orally + vaginal misoprostol 600 μg (first dose) Group I: oral misoprostol 400 μg every 3 hours, max 5d; Group II: vaginal misoprostol 400 μg every 3 hours, max 5d. If after the fifth dose, abortion had not occurred, 1 mg gemeprost was administered the next morning. |
|
| Outcomes | Primary outcomes: induction to abortion interval, abortion within 24 hours. Secondary outcomes: side‐effects. |
|
| Notes | Definition of abortion: abortion occurring after the fifth dose. One patient suffered from rigours, vomiting and eruption of a maculopapular rash following the administration of 600 μg misoprostol. If the placenta was retained, the uterus was surgically evacuated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Faktor 1988.
| Methods | Randomisation. Authors report that there was 'no selection bias in the choice of the patients'. | |
| Participants | 77 pregnant women (group Ia: 35, group Ib: 17, group II: 16, group III: 9) Inclusion criteria: mid‐trimester abortion (15‐26 weeks gestation). Exclusion criteria: none given. |
|
| Interventions | Group Ia: 1.0g oxytetracycline hydrochloride, dissolved in 16‐20 ml of normal physiological saline, intra‐amniotic. Patients received oxytocin i.v. in increasing dosage after the appearance of uterine contractions un till time of abortion. Group Ib: 1.0g oxytetracycline hydrochloride, dissolved in 16‐20 ml of normal physiological saline, intra‐amniotic. No oxytocin was given. Group II: 200 cm3 amniotic fluid was exchanged for 200 cm3 of 20% of hypertonic saline. Group III: 40 mg of PGF2α, intra‐amniotic. Group I is considered as the intervention group and group II and III are considered control groups. |
|
| Outcomes | Abortion interval, side‐effects. | |
| Notes | Definition of abortion: expulsion of the fetus. After expulsion of the fetus, all patients underwent revision of the uterine cavity under general anaesthesia. No major complications described. For our analysis, we did not include oxytetracycline hydrochloride. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | B ‐ unclear |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Unclear risk | No baseline characteristics of the separate groups were provided. |
Hamoda 2005.
| Methods | Randomisatin by opening consecutive sealed opaque envelopes generated using random number tables. | |
| Participants | 69 pregnant women (group I: 32, group II: 37) Inclusion criteria: singleton intrauterine pregnancy, 13‐20 weeks gestation. Exclusion criteria: < 16 years, severe asthma, haemorrhagic disorders, treatment with anticoagulants, known allergy to prostaglandins, history of cardiac disease, smoking over the age of 35 years with ECG abnormalities, breast feeding. |
|
| Interventions | All: mifepristone 200mg followed 36‐48 hours later by: Group I: misoprostol 600 μg sublingually and misoprostol 400 μg sublingually every 3h; Group II: misoprostol 800 μg vaginally and misoprostol 400mg μg vaginally every 3h. |
|
| Outcomes | Primary outcome: induction to abortion interval. Secondary outcomes: acceptability of the route of misoprostol administration to the women and staff, side‐effects. |
|
| Notes | Definition of abortion: not specified. Surgical evacuation was offered to women if the placenta was not delivered within 1h of delivery of the fetus. Two women suffered from heavy bleeding during the abortion and needed a surgical evacuation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Herabutya 2005.
| Methods | Random allocation by computer generated numbers. The assignments were put into sealed envelopes, which were opened when the women were recruited. | |
| Participants | 279 pregnant women (group I: 140, group II: 139) Inclusion criteria: 14‐26 weeks gestation (abortion was not offered > 22 weeks apart from lethal fetal conditions). Exclusion criteria: unstable cardiac disease, recent severe asthmatic attack, severe hepatic or renal impairment, ruptured membranes. |
|
| Interventions | All: 600 μg misoprostol vaginally Group I: every 6 hrs, max 9 d; Group II: every 12 hrs, max 5 d. |
|
| Outcomes | Primary outcome: induction to abortion interval, abortion within 24 hours. Secondary outcomes: side‐effects. |
|
| Notes | Definition of abortion: expulsion of fetus. If the placenta was incomplete or failed to be expelled after 1h, an evacuation of the uterus was carried out under general anaesthesia. No major complications reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Ho 1996.
| Methods | Randomisation schedule was prepared as described by Meinert. Sealed envelopes with serial numbers on the front and containing the group to which the woman was randomised were opened at recruitment. | |
| Participants | 50 pregnant women (group I: 25, group II: 25) Inclusion criteria: 14‐20 weeks gestation. Exclusion criteria: regular use of prescription drugs, IUD in utero, nursing mothers, multiple pregnancies, heavy smokers. |
|
| Interventions | All: 200mg mifepristone orally (36‐48 hours later): Group I: 400 μg misoprostol orally, every 3 h, max 5 doses; Group II: 1mg gemeprost vaginally, every 6 hours, max 4 doses. The patient was reassessed after 24h. If there were no signs or symptoms suggestive of imminent abortion, the pregnancy was terminated with 1 mg gemeprost every 3 hours. |
|
| Outcomes | Primary outcome: induction of abortion interval, abortion within 24 hours. Secondary outcomes: side‐effects, uterine contractions, blood pressure, pulse rate. |
|
| Notes | Definition of abortion: not specified. If the placenta was incomplete, an evacuation of the uterus was carried out. No major complications reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and gravidity. |
Ho 1997.
| Methods | Randomisation schedule as described by Meinert. Schedules were unknown to both patient and clinicans. | |
| Participants | 98 pregnant women (group I: 49, group II: 49) Inclusion criteria: good general health, age 16‐35 years, singleton pregnancy, 14‐20 weeks gestation. Exclusion criteria: past or present ill health, nursing mothers, IUD, smoking >10 cigarettes/day. |
|
| Interventions | All: mifepristone 200 mg 36‐48 hours later: Group I: misoprostol 200 μg orally, and a placebo vaginally every 3 hours, max 5 doses; Group II: misoprostol 200 μg vaginally, and a placebo orally, every 3 hours, max 5 doses. |
|
| Outcomes | Primary outcome: induction of abortion, abortion within 24 hours. Secondary outcomes: side‐effects, uterine contractions, blood pressure, pulse rate. |
|
| Notes | Definition of abortion: expulsion of fetus. If the placenta was incomplete or failed to be expelled after 1/2h, an evacuation of the uterus was carried out. No major complications reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | Low risk | Blinding of participants and clinicians. |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Inan 1997.
| Methods | Randomly assigned. | |
| Participants | 78 pregnant women (group I: 48, group II: 30) Inclusion criteria: 13‐24 weeks gestation, Bishop score <4. Exclusion criteria: none described. |
|
| Interventions | Group I: extra‐amniotic ethacridine lactate (Rivanol). A No 16 Foley catheter was placed into the uterus. Following inflammation of the balloon of the catheter to 20‐30 ml, an average of 10 ml of 0.1 % sterile ethacridine lactate solution per gestational week was instilled extra‐amniotically. The catheter was left in place for 24 hours, if not expelled earlier. Group II: 2.5 ml gel containing 0.5 mg PGE2, intracervical (Cerviprost 0.5 mg gel Organon). Group III: extra‐amniotic ethacridine lactate combined with oxytocin infusion. 10‐20 units/5% DW IV oxytocin induction was started within 2‐4 hours following the ethacridine lactate instillation. For analyses, we did not include group III. |
|
| Outcomes | Successful abortion rates, side‐effects. | |
| Notes | Definition of abortion: Complete evacuation of fetus and placental tissues from the uterus within 24 hours. No major complications described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | B ‐ unclear |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Kapp 2007.
| Methods | Sequentially distributed study number in an allocation ratio of 1:1. The randomisation scheme used permuted blocks of eight, selected by a random number generator created using SAS V.9.3. The pharmacy dispensed the study medication. | |
| Participants | 64 pregnant women (group I: 32, group II: 32) Inclusion criteria: 18‐23 weeks of gestation. Exclusion criteria: known allergy to mifepristone/misoprostol/prostaglandins, preexisting intrauterine fetal demise, premature preterm rupture of membranes, IUD in place, history of chronic adrenal failure, porphyrias, concurrent long term corticosteroid treatment. |
|
| Interventions | All: intra‐amniotic injection of 1.5 mg digoxin, then: Group I: 200 mg mifepristone, 20–24 hours after study capsule: misoprostol induction using 400 μg misoprostol, followed by 200 μg every 6h (buccally); Group II: 2 placebo tablets (vitamin C). 20–24 hours after study capsule: misoprostol induction using 400 μg misoprostol, followed by 200 μg every 6h (buccally). |
|
| Outcomes | Primary outcome: median interval from first misoprostol dose to fetal expulsion. Secondary outcomes: women delivering within 24 hours, proportion of women with a complete delivery requiring additional treatment for retained placenta, the amount of required pain medication, length of hospital stay, side‐effects. |
|
| Notes | Definition of abortion: expulsion of fetus. If the placenta was incomplete or failed to be expelled after 4h, an evacuation of the uterus was carried out under general anaesthesia. Heavy bleeding occurred in two women. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | Low risk | Blinding for participants, clinicians, and researchers. |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Kelekci 2006.
| Methods | Patients were randomised to one of four treatment groups by a series of computer‐generated random numbers. | |
| Participants | 178 pregnant women (group I: 93, group II: 85) Inclusion criteria: genetic indications, 13‐24 weeks gestation. Exclusion criteria: previous uterine scar, pulmonary, hepatic, renal or cardiovascular disease, intrauterine death, vaginal bleeding, uterine contractions, any signs of cervical dilatation, a Bishop score of 4, vaginal infection, a discrepancy of 2 weeks between the gestational age determined by last menstrual period and ultrasonographic gestational age |
|
| Interventions | Group I: 200 μg misoprostol, vaginally, followed by 100 μg of oral misoprostol every 4 hour for 24 hrs. Group II: extra‐amniotic ethacridine lactate, 10 ml instilled per gestational week, to a maximum of 200 ml. Group III: combination of misoprostol and oxytocin. 200 μg misoprostol, vaginally, followed by 100 μg of oral misoprostol every 4 hour for 24 hrs. An initial dose of 6 mU/min oxytocin was given, followed by additional 6 mU/min doses every 20 min. Group IV: combination of ethacridine lactate and oxytocin. Ethacridine lactate was given extra‐amniotic, 10 ml instilled per gestational week, to a maximum of 200 ml. Oxytocin was administered in a similar way as in group III. For analyses, we did not include group III and IV. |
|
| Outcomes | Time to induce abortion, success/failure rates, side‐effects and complications. | |
| Notes | Definition of abortion: complete evacuation of fetal and placental tissues within 24 h of the initiation of medical abortion. 14 cases of endometritis and 20 cases of incomplete abortions were described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | B ‐unclear |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Makhlouf 2003.
| Methods | First 90 patient were randomly assigned into three groups (see notes). Randomisation of the remainig 40 patients involved the group using misoprostol and glyceryl trinitrate only, because of shortage of finance to buy more prostaglandin tablets. Randomisation involved computer‐generated random tables. | |
| Participants | 80 pregnant women (group I: 50, group II: 30) Inclusion criteria: 13‐28 weeks gestation, Bishop score ≤ 4. Exclusion criteria: contra‐indication to induction of abortion by medical methods, e.g. placenta previa, preterm rupture of membranes (PROM) and transverse lie, grand‐multiparous women (parity ≥ 5), previous scarred uterus or contra‐indications to the drugs. |
|
| Interventions | Group I: 100 μg misoprostol, vaginally every 4 hours, with a maximum dose of 500 μg (five doses). Group II: 6 mg prostaglandin E2 , vaginally every 6 hours, with a maximum of 24 mg (four doses). Women with a method failure and a Bishop score ≤ 4 or absence of uterine activity continued abortion by using a Foley's catheter. If uterine contractions started or the Bishop score was > 4, but expulsion did not occur after 24 hours of after expulsion of the Foley catheter, intravenous 5mIU/min of oxytocin infusion was used. We excluded the outcome 'induction to abortion interval' because of this method after 24 hour. |
|
| Outcomes | Induction to abortion interval, abortion within 24 hours, side‐effects. | |
| Notes | Definition of method failure: absence of fetal expulsion of absence of signs of impending expulsion (regular uterine contractions and cervical dilatation) at the end of 24 hours. No clear information was provided regarding the policy of evacuation of the uterus. No major complications described. Study included 130 pregnant women into three groups. The third group (n = 50) were randomised for the use of nitric oxide donor (glyceryl trinitrate) tablets. Women received 500 μg of glyceryl trimitrate evert 6h, with a maximum of 5 doses. For this review, this group was excluded. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Mehta 1975 a.
| Methods | Prepared envelopes indicating one of the methods were picked up serially; the investigators being blind to what the envelopes contained till they opened them. | |
| Participants | 67 pregnant women (group I: 33, group II: 34) Inclusion criteria: 15‐20 weeks gestation. Exclusion criteria: none given. |
|
| Interventions | Group I: 20% hypertonic saline, 200 ml. Group II: single dose, 50mg PGF2α. |
|
| Outcomes | Abortion rates, side‐effects. | |
| Notes | Definition of abortion: when complete (spontaneous evacuation of all products of conception) or incomplete abortion (total or partial retainment of placenta or membranes) occurred within 72 hours. No information was provided regarding the policy of evacuation of the uterus. One women in the single dose PGF2α group received a blood transfusion. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Mehta 1975 b.
| Methods | Prepared envelopes indicating one of the methods were picked up serially; the investigators being blind to what the envelopes contained till they opened them. | |
| Participants | 66 pregnant women (group I: 33, group II: 33) Inclusion criteria: 15‐20 weeks gestation. Exclusion criteria: none given. |
|
| Interventions | Group I: 20% hypertonic saline, 200 ml. Group II: multiple doses of 25mg PGF2α, given at 0 hours and 6 hours. Similar doses were instilled at 24 hours and 30 hours when necessary. |
|
| Outcomes | Abortion rates, side‐effects. | |
| Notes | Definition of abortion: when complete (spontaneous evacuation of all products of conception) or incomplete abortion (total or partial retainment of placenta or membranes) occurred within 72 hours. No information was provided regarding the policy of evacuation of the uterus. One women in the single dose PGF2α group received a blood transfusion. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Muzsnai 1979 a.
| Methods | Random assignment. | |
| Participants | 100 pregnant women (group I: 50, group II: 50) Inclusion criteria: 16‐24 weeks of gestation. Exclusion criteria: history of uterine surgery. |
|
| Interventions | Group I: PGF2α (20mg) + 100 mL 5% NaCl (5g), intra‐amniotic. No amniotic fluid removed. Group II: PGF2α (20mg) + 100 mL 10% NaCl (10g), intra‐amniotic. No amniotic fluid removed. All patients received i.v. oxytocin stimulation 40 mU/min. |
|
| Outcomes | Instillation to abortion time, abortion interval, complications, side‐effects. | |
| Notes | Definition of abortion: none given. Incomplete abortion: if placenta was not expelled within 2h after delivery of the fetus, of if haemorrhage occurred. Failure of abortion: if fetus was not expelled within 48h. The procedure was then repeated. Instillation abortion interval: time from amniocentesis to expulsion of fetus. No major complications described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | B ‐ unclear |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Muzsnai 1979 b.
| Methods | Random assignment. After completion of 50 patients in group I and II, the remaining patients were assigned to group II, because of favourable outcome. | |
| Participants | 130 pregnant women (group I: 50, group II:80) Inclusion criteria: 16‐24 weeks of gestation. Exclusion criteria: history of uterine surgery. |
|
| Interventions | Group I: PGF2α (20mg) + 100 mL 5% NaCl (5g), intra‐amniotic. No amniotic fluid removed. Group II: PGF2α (20mg) + 100 mL 10% NaCl (10g), intra‐amniotic. 100mL amniotic fluid removed. All patients received i.v. oxytocin stimulation 40 mU/min. |
|
| Outcomes | Instillation to abortion time, abortion interval, complications, side‐effects. | |
| Notes | Definition of abortion: none given. Incomplete abortion: if placenta was not expelled within 2h after delivery of the fetus, of if haemorrhage occurred. Failure of abortion: if fetus was not expelled within 48h. The procedure was then repeated. Instillation abortion interval: time from amniocentesis to expulsion of fetus. No major complications described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | B ‐ unclear |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Muzsnai 1979 c.
| Methods | Random assignment. After completion of 50 patients in group I and II, the remaining patients were assigned to group I, because of favourable outcome. | |
| Participants | 255 pregnant women (group I: 205, group II: 50) Inclusion criteria: 16‐24 weeks of gestation. Exclusion criteria: history of uterine surgery. |
|
| Interventions | Group I: PGF2α (20mg) + 25 mL 20% NaCl (5g), intra‐amniotic. All amniotic fluid removed. Group II: PGF2α (20mg) + 100 mL 10% NaCl (10g), intra‐amniotic. No amniotic fluid removed. All patients received i.v. oxytocin stimulation 40 mU/min. |
|
| Outcomes | Instillation to abortion time, abortion interval, complications, side‐effects. | |
| Notes | Definition of abortion: none given. Incomplete abortion: if placenta was not expelled within 2h after delivery of the fetus, of if haemorrhage occurred. Failure of abortion: if fetus was not expelled within 48h. The procedure was then repeated. Instillation abortion interval: time from amniocentesis to expulsion of fetus. No major complications described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | B ‐ unclear |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Muzsnai 1979 d.
| Methods | Random assignment. After completion of 50 patients in group I and II, the remaining patients were assigned to both groups, because of favourable outcome. | |
| Participants | 285 pregnant women (group I: 205, group II: 80) Inclusion criteria: 16‐24 weeks of gestation. Exclusion criteria: history of uterine surgery. |
|
| Interventions | Group I: PGF2α (20mg) + 25 mL 20% NaCl (5g), intra‐amniotic. All amniotic fluid removed. Group II: PGF2α (20mg) + 100 mL 10% NaCl (10g), intra‐amniotic. 100mL amniotic fluid removed. All patients received i.v. oxytocin stimulation 40 mU/min. |
|
| Outcomes | Instillation to abortion time, abortion interval, complications, side‐effects. | |
| Notes | Definition of abortion: none given. Incomplete abortion: if placenta was not expelled within 2h after delivery of the fetus, of if haemorrhage occurred. Failure of abortion: if fetus was not expelled within 48h. The procedure was then repeated. Instillation abortion interval: time from amniocentesis to expulsion of fetus. No major complications described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | B ‐ unclear |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Ngai 2000.
| Methods | Randomisation schedule as described by Meinert. Sealed envelopes with serial numbers were prepared. At enrolment, a serial number was given according to the sequence of entry. | |
| Participants | 139 pregnant women (group I: 70, group II: 69) Inclusion criteria: healthy women between 16‐35 years, 14‐20 weeks gestation. Exclusion criteria: regular use of prescription drugs, IUD in utero, nursing mothers, multiple pregnancies, heavy smokers. |
|
| Interventions | All: 200mg mifepristone + (36 ‐ 48 h later): Group I: 400 μg misoprostol oral and a vaginal placebo (vitamin B6) every 3 hours; Group II: 200 μg misoprostol vaginally and an oral placebo (vitamin B6) every 3 hours. |
|
| Outcomes | Primary outcome: abortion within 24 hours. Secondary outcomes: induction to abortion interval, side‐effects. |
|
| Notes | Definition of abortion: not specified. If the placenta was incomplete, an evacuation of the uterus was carried out under general anaesthesia. No major complications occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | Low risk | Blinding for participants. |
| Free of other bias? | Unclear risk | No statistically significant differences between the groups in terms of maternal age and gestational age. |
Nielsen 1975.
| Methods | Randomisation. | |
| Participants | 32 pregnant women (group I: 16, group II: 16) Inclusion criteria: gestation more than 14 weeks. Exclusion criteria: none reported. |
|
| Interventions | Group I: suprapublical/transvaginal injection of 20% saline, preceded by removal of 50 mL of amniotic fluid. Amount of saline depending on gestation; 14th week 75 mL, 15th week 100 mL, >16th week 150 mL. Group II: suprapublical/transvaginal injection of 40 mg of PGF2α, preceded by removal of 50 mL of amniotic fluid. Both groups received an 10 IU/h oxytocin drip (100IU in one litre of 5% glucose) within half an hour. If abortion did not occur before 200IU oxytocin was given, the infusion was stopped for 6‐8h and then restarted. PG/saline injection was not repeated. |
|
| Outcomes | Abortion interval, complications, side‐effects. | |
| Notes | Definition of abortion: none given. Curettage was performed in cases which were considered incomplete. No major complications described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | B ‐ unclear |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No apparant differences between the groups in terms of maternal age, gestational age and parity. |
Nuutila 1997 a.
| Methods | Randomisation was done using random numbers tables into three groups. A series of numbered, sealed envelopes were prepared containing the allocation. | |
| Participants | 55 pregnant women (group I: 27, group II: 28) Inclusion criteria: 12‐24 weeks gestation, singleton pregnancies. Exclusion criteria: none reported. |
|
| Interventions | Group I: 100 μg misoprostol vaginally, every 6 hours, max max 6 doses. Group II: 1 mg gemeprost vaginally, every 3 hours, max 8 doses. |
|
| Outcomes | Primary outcome: induction to abortion interval. Secondary outcomes: side‐effects. |
|
| Notes | Definition of abortion: expulsion of fetus and placenta. Within 1h after the passage of the fetus, whether or not the placenta was passed, an evacuation of the uterus was carried out under general anaesthesia. No major complications occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Nuutila 1997 b.
| Methods | Randomisation was done using random numbers tables into three groups. A series of numbered, sealed envelopes were prepared containing the allocation. | |
| Participants | 54 pregnant women (group I: 26, group II: 28) Inclusion criteria: 12‐24 weeks gestation, singleton pregnancies. Exclusion criteria: none reported. |
|
| Interventions | Group I: 200 μg misoprostol vaginally, every 12 hours, max 3 doses. Group II: 1 mg gemeprost vaginally, every 3 hours, max 8 doses. |
|
| Outcomes | Primary outcome: induction to abortion interval. Secondary outcomes: side‐effects. |
|
| Notes | Definition of abortion: expulsion of fetus and placenta. Within 1h after the passage of the fetus, whether or not the placenta was passed, an evacuation of the uterus was carried out under general anaesthesia. No major complications occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Nuutila 1997 c.
| Methods | Randomisation was done using random numbers tables into three groups. A series of numbered, sealed envelopes were prepared containing the allocation. | |
| Participants | 53 pregnant women (group I: 27, group II: 26) Inclusion criteria: 12‐24 weeks gestation, singletone pregnancies. Exclusion criteria: none reported. |
|
| Interventions | Group I: 100 μg misoprostol vaginally, every 6 hours, max 6 doses. Group II: 200 μg misoprostol vaginally, every 12 hours, max 3 doses. |
|
| Outcomes | Primary outcome: induction to abortion interval. Secondary outcomes: side‐effects. |
|
| Notes | Definition of abortion: expulsion of fetus and placenta. Within 1h after the passage of the fetus, whether or not the placenta was passed, an evacuation of the uterus was carried out under general anaesthesia. No major complications occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Olund 1978.
| Methods | Randomly assigned. | |
| Participants | 92 pregnant women (group I: 23, group II: 23, group III: 23, group IV: 23) Inclusion criteria: 13‐25 weeks gestation. Exclusion criteria: none described. |
|
| Interventions | Group I: extra‐amniotic instillation of 0.1% solution of rivanol, 10 ml per gestational week. Maximum of 150 ml. Group II: extra‐amniotic instillation of 1 ml of saline containing 0.25 mg PGF2α per ml, 3 ml every 2 hours for up to 24 hours. At a gestational age >16 weeks, the dose was doubled. Group III: Rivanol + PGF2α, as in group I and II. Group IV: Rivanol + a half dose of PGF2α, as in group III, except for a half dose of PGF2α. NB: For our analysis, we included group I and II. |
|
| Outcomes | Abortion time, abortion interval, side‐effects. | |
| Notes | No definition of abortion was given. No major complications described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | B ‐ unclear |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Ozerkan 2009.
| Methods | Randomisation by computer‐generated number lists to two groups of 30. | |
| Participants | 60 pregnant women (group I: 30, group II: 30) Inclusion criteria: 13‐24 weeks gestation. Exclusion criteria: none reported. |
|
| Interventions | Group I: 400 μg of misoprostol, vaginally, with an additional 200 μg at two‐hour intervals up to five doses. Group II: 600 μg of misoprostol, vaginally, with an additional 400μg at four‐hour intervals up to two doses. Patients in either group received a maximum total dose of 1400g of misoprostol. The next dose was skipped whenever there were effective uterine contractions. If the procedure failed on the first day, it was undertaken the next day using the same protocol. Another method of termination was called in case the procedure failed on two consecutive days. |
|
| Outcomes | Primary outcome: success rates, time to termination, blood loss, complications, side‐effects and cervical features defined ultrasonographically. | |
| Notes | Definition of abortion: expulsion of fetus. Post‐abortion curettage of the uterine cavity. No major complications reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | B ‐ unclear |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age and gestational age. |
Pongsatha 2008.
| Methods | Randomisation through block randomisation by the authors. | |
| Participants | 148 pregnant women (group I: 72, group II: 76) Inclusion criteria: second trimester abortion, live fetuses, closed/uneffaced cervix without labor. Exclusion criteria: previous uterine scar, hypersensitivity to prostaglandins. |
|
| Interventions | Group I: 400 mg misoprostol tablet insertion, every 3h. Group II: 400 mg misoprostol gel insertion, every 3h. |
|
| Outcomes | Primary outcome: abortion within 24 hours. Secondary outcomes: side‐effects. |
|
| Notes | Misoprostol in gel form: mixing misoprostol with 3 mL 1% carboxy methyl cellulose. Definition of abortion: expulsion of fetus. No information was provided regarding the policy of evacuation of the uterus. No major complications reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | B ‐ unclear |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Sorensen 1984.
| Methods | Use of random numbers. | |
| Participants | 39 pregnant women (group I: 20, group II: 19) Inclusion criteria:13‐24 weeks of gestation. Exclusion criteria: intra‐uterine fetal death, cardiopulmonary disease, nephropathy, liver diseases, previous operation on the uterus |
|
| Interventions | Group I: 2x PGE2 0.75 mg within 5 hours intracervical/extra amniotic, 5 hours, later followed by oxytocin infusion 0.15 IU/min if no contractions. Group II: PGF2α 40 mg intra‐amniotically 5 hours later followed by oxytocin drip 0.15 IU/min if no contractions. |
|
| Outcomes | Primary outcomes: abortion success rate, induction‐abortion interval. Secondary outcomes: side‐effects. |
|
| Notes | Definition of abortion: expulsion of fetus. If the placenta was not expelled within 2 hours, an evacuation of the uterus was performed. No major complications reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | B ‐ unclear |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Steyn 1993.
| Methods | Randomisation using the balanced block method. Instructions were placed in sealed envelopes. | |
| Participants | 20 pregnant women (group I: 10, group II: 10) Inclusion criteria: 14‐26 weeks of gestation. Exclusion criteria: fetal death on admission, previous uterine scars, history of asthma, active vaginal or intra‐uterine infection, anhydramnios. |
|
| Interventions | Group I: 1.5 mg prostaglandin E2 (PGE2) gel extra‐amniotically. Group II: 25 mg prostaglandin F2α (PGF2α) intra‐amniotically. Patients in both groups received oxytocin to a maximum dosage of 120 mU per minute if they had not aborted 18 hours after the original administration of either prostaglandin regimen. If the patient had not aborted within 36h, the method was regarded unsuccessful and the managing physician was free to change the management of choice. |
|
| Outcomes | Primary outcome: induction to abortion interval. Secondary outcomes: complications, side effects. Proportion of successful inductions and complications. |
|
| Notes | Definition of abortion: none given. No information was provided regarding the policy of evacuation of the uterus. No major complications reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Su 2005.
| Methods | Random allocation; computer‐generated schedule randomisation; numbered, sealed, opaque envelope; envelopes were drawn in consecutive order. | |
| Participants | 125 pregnant women (group I: 61, group II: 64) Inclusion criteria: 12‐24 weeks gestation. Exclusion criteria: multiple pregnancies, ≥ 2 previous cesarean sections, missed abortion, oligohydramnios, severe asthma, allergy to prostaglandins. |
|
| Interventions | Group I: vaginal misoprostol 400 μg /3h, max 5d in 24 hrs. Group II: intra amniotic PGF2α 1,5mg, max 5d in 24 hrs. |
|
| Outcomes | Primary outcome: induction to abortion interval. Secondary outcomes: abortion within 24 and 48 hours, the need for repeat course of medications, evacuation of uterus, adverse effects. |
|
| Notes | Definition of abortion: expulsion of the fetus. Evacuation of the uterus was not performed routinely. No major complications reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Tang 2004.
| Methods | Computer‐generated random numbers; sealed envelopes, opened at recruitment. | |
| Participants | 220 pregnant women (group I: 112, group II: 108) Inclusion criteria: 12‐20 weeks gestation. Exclusion criteria: regular use of prescription drugs, IUD in utero, nursing mothers, multiple pregnancies, heavy smokers. |
|
| Interventions | Group I: Vaginal administration 400 μg misoprostol every 3h, max 5 doses in 24 hours. Group II: Sublingual administration 400 μg misoprostol every 3h, max 5 doses in 24 hours. |
|
| Outcomes | Primary outcome: success rate at 48 hours. Secondary outcomes: success rate at 24 hours, side‐effects. |
|
| Notes | Definition of abortion: expulsion of the fetus and placenta. If the placenta was incomplete, evacuation of the uterus was performed. No major complications occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Tang 2005.
| Methods | Computer‐generated randomisation sequence; sealed, sequentially numbered treatment packs, which were filled and labelled in accordance with the list of randomisation. | |
| Participants | 118 women (group I: 58, group II: 60) Inclusion criteria: >18 years, 12‐20 weeks gestation. Exclusion criteria: regular use of prescription drugs, IUD in utero, nursing mothers, multiple pregnancies, heavy smokers. |
|
| Interventions | all: mifepristone 200mg orally, 36‐48h later: Group 1: misoprostol 400 μg sublingual and 2 placebo tablets orally every 3 hrs, max 5 d; Group 2: misoprostol 400 μg orally and 2 placebo tablets sublingually every 3 hrs, max 5 d. |
|
| Outcomes | Primary outcome: success rate at 24 h. Secondary outcomes: induction‐to‐abortion interval, side‐effects. |
|
| Notes | Definition of abortion: not specified. If the placenta was incomplete, evacuation of the uterus was performed. No major complications occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | Low risk | Blinding for participants and clinicians. |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Thong 1996.
| Methods | Sealed, opaque envelopes containing either of the two treatment groups. These envelopes were shuffled and numbered consecutively. | |
| Participants | 100 pregnant women (group I: 50, group II: 50) Inclusion criteria: 12‐19 weeks gestation. Exclusion criteria: <16 years. |
|
| Interventions | All: 200mg mifepristone, 36 hours later: Group I: 1mg pessary gemeprost vaginally in 6 hour intervals, max 4 doses in 24 hour. After 24 hours without abortion 1 mg gemeprost in 3 hour intervals, max 24 hours; Group II: 0.5mg pessary gemeprost vaginally in 6 hour intervals, max 4 doses in 24 h. After 24 hours 1 mg gemeprost in 3 hour intervals. |
|
| Outcomes | Primary outcome: abortion within 24h. Secondary outcomes: side‐effects. |
|
| Notes | Definition of abortion: expulsion of fetus and placenta. Evacuation of the uterus was carried out if the placenta was not expelled spontaneously or if it was judged to be incomplete. One woman in group II required a blood transfusion of two units because of heavy bleeding in association with a retained placenta after expulsion of the fetus. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No apparent differences between the groups in terms of maternal age, gestational age and parity. |
von Hertzen 2009.
| Methods | A computer‐generated randomisation sequence was produced by WHO staff in Geneva to assign participants within each centre to sublingual or vaginal treatment group by randomly permuted blocks with a fixed block size of six. Allocation was concealed by using sealed, opaque, sequentially numbered envelopes, which were filled and labelled in accordance with the list of randomization for each centre by Magistra, Geneva, Switzerland. | |
| Participants | 681 pregnant women (group I: 340, group II: 341) Inclusion criteria: healthy, older than the age of legal consent, had a single intrauterine pregnancy of 13–20 weeks (91–140 days) duration as verified by ultrasound and had haemoglobin 100 g/l or higher. Exclusion criteria: any indication of serious past or present illness; an allergy to misoprostol; a habit of heavy smoking (.20 cigarettes/day); a scar in the uterus or cervix or any gynaecological anomaly detected with ultrasound; mitral stenosis, glaucoma or sickle cell anaemia; diastolic blood pressure .90 mmHg; uncontrolled bronchial asthma; systolic blood pressure ,90 mmHg; history or evidence of thromboembolism or liver disease; presence of an intrauterine device; or haemolytic disorders. |
|
| Interventions | Group I: 400 μg misoprostol vaginally, 2 placebo tablets sublingually, every 3 hours up to five doses until abortion took place. Group II: 400 μg misoprostol sublingually, 2 placebo tablets vaginally, every 3 hours up to five doses until abortion took place. Placebo tablets were manufactured by Labatec, Geneva, Switzerland; similar shape and colour as misoprostol tablets. The blisters were labelled indicating which tablets were to be taken sublingually and which tablets vaginally. Additional misoprostol tablets were provided to the centres to be used sublingually for those women who did not abort within 24 h. After expulsion of the fetus, one additional dose of the tablets was administered. |
|
| Outcomes | Primary outcome: successful abortion (including complete and incomplete abortion) within 24 h. Secondary outcome: successful abortion within 48 h induction‐to‐abortion interval (the start of treatment to expulsion of fetus, side effects and women’s perceptions of the method. |
|
| Notes | Definition of abortion: complete or incomplete abortion, while treatment failures included missed abortion, continuing pregnancy and undetermined outcomes. Ten women received a blood transfusion and three women required hospitalization after discharge, two of them for surgical evacuation of the uterus and one for reasons unrelated to the study. After abortion, the products of gestation were examined to see whether the abortion was complete. If necessary, or if it was a local routine practice (three centres), exploration and evacuation of the uterus was performed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | Low risk | |
| Free of other bias? | Low risk | A stratified analysis was conducted by parity because there was a highly significant interaction of treatment by parity. No apparent differences between the groups in terms of maternal age and gestational age. |
Waldron 1990.
| Methods | Randomisation. | |
| Participants | 58 pregnant women (group I: 29, group II: 29) Inclusion criteria: 14‐20 weeks of gestation. Exclusion criteria: signs or symptoms of spontaneous abortion, known or suspected hypersensitivity to prostaglandins, cardio‐pulmonary disease, hypertension, urticaria, eczema, ulcerative colitis, diabetes, epilepsy, renal disease, liver disease. |
|
| Interventions | Group I: 1 mg gemeprost in vaginal pessaries, 3h interval, maximum of 5 doses. Group II: 20 mg of PGF2α in 40 ml of 20% NaCl, intra‐amniotic. If abortion had not occurred within 24 hours, an alternative treatment was commenced at the discretion of the clinician. Following delivery of the fetus, oxytocin or ergometrine in routine dosages were used at the clinician's discretion, and surgical evacuation of the uterus was performed if the placenta was not delivered within two hours. |
|
| Outcomes | Abortion interval, side‐effects, complications. | |
| Notes | Definition of abortion: abortion within 24 hours. If the placenta was not expelled within 2 hours, an evacuation of the uterus was performed. No major complications described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | B ‐ unclear |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No apparent differences between the groups in terms of maternal age, gestational age and parity. |
Webster 1996.
| Methods | Women were randomly allocated using a series op opaque envelopes that had been prepared by using random number tables. | |
| Participants | 70 pregnant women (group I: 35, group II: 35) Inclusion criteria: 13‐20 weeks of gestation. Exclusion criteria: none given. |
|
| Interventions | Group I: 200 mg mifepristone prior to admission for prostaglandin. A first dose of 800 μg of misoprostol (8.00 am) was given vaginally, followed by 400 μg doses administered orally on a 3h basis, to a maximum of 4 doses. Group II: 600 mg mifepristone prior to admission for prostaglandin. A first dose of 800 μg of misoprostol (8.00 am) was given vaginally, followed by 400 μg doses administered orally on a 3h basis, to a maximum of 4 doses. If abortion had not occured following the final dose of misoprostol, the treatment was considered to be a failure and mifepristone 600 mg was given at midnight, followed by a course of 1 mg gemeprost pessaries at 3h intervals commenced the following morning. |
|
| Outcomes | Induction to abortion interval, side effects. | |
| Notes | Complete abortion: on clinical grounds, and then no further interventions were undertaken. Surgical evacuation of the uterus was performed if women did not pass the complete placenta. One woman from each group required a blood transfusion as a result of blood loss at this time. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | Women in the 600 mg group were significantly older than their counterparts in the 200 mg group. No apparent differences between the groups in terms of gestational age and parity. |
WHO 1976.
| Methods | Computer‐generated randomisation table. Identity of the compound was kept in a sealed envelope until the patient was accepted for the study. | |
| Participants | 1513 pregnant women (group I: 717, group II: 796) Inclusion criteria: 13‐22 weeks of gestation. Exclusion criteria: previous heart disease, hypertension, respiratory disease, ulcerative colitis, diabetes mellitus, disorders of blood coagulation, kidney disease, liver disease, sickle‐cell anaemia, severe hypersensitivity, serious systemic disease, contraindication to transperitoneal uterine puncture (previous abdominal surgery on the body of the uterus, large uterine myomata/pelvic tumors, major congenital abnormalities of the uterus, rupture of membranes, earlier failed saline induction). |
|
| Interventions | Both groups were punctured with a fine‐bore needle and a small amount of amniotic fluid withdrawn to confirm the intra‐amniotic position. Group I: 200 mL 20% saline was slowly injected, intra‐amniotic; Group II: 5 mL tromethamine salt of PGF2α (= 25mg PGF2α) was injected, intra‐amniotic. A catheter was left in position and 6h later a second injection op 25mg PGF2α was given. |
|
| Outcomes | Abortion interval, complications, side‐effects. | |
| Notes | Definition of abortion: spontaneous expulsion of placenta through the cervix into the vagina. If the placenta was incomplete, evacuation of the uterus was performed. Re‐admission to hospital was necessary for 17 patients given PGF2α and 13 patients given saline (excessive blood loss, retained products of conception, signs of genital tract infection). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No apparant differences between the groups in terms of maternal age, gestational age and parity. |
Wong 1998.
| Methods | Randomisation schedule, (sealed) envelopes bearing the subject number and allocation were prepared as described by Meinert. The envelopes were opened only when recruited. | |
| Participants | 140 pregnant women (group I: 70, group II: 70) Inclusion criteria: healthy women, age 16‐40 years, 14‐20 weeks gestation. Exclusion criteria: regular use of prescription drugs, cardiac disorders, IUD in situ, missed abortion, multiple pregnancy, nursing mothers. |
|
| Interventions | Group I: misoprostol 400 μg vaginally every 3 hours, max 5d. Group II: gemeprost 1 mg vaginally every 3 hours, max 5d. |
|
| Outcomes | Primary outcomes: induction‐abortion interval, rates of successful abortion (within 24 h), complete abortion. Secondary outcomes: side‐effects. |
|
| Notes | Definition of abortion: expulsion of fetus. If the placenta was incomplete, evacuation of the uterus was performed under general anaesthesia. No major complications reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Wong 2000.
| Methods | Randomisation schedule, envelopes bearing the subject number and allocation were prepared as described by Meinert. The envelopes were opened only when recruited. | |
| Participants | 148 pregnant women (group I: 74, group II: 74) Inclusion criteria: healthy women, 14‐20 weeks' gestation. Exclusion criteria: regular use of prescription drugs, IUD in situ, missed abortion, multiple pregnancy, nursing mothers. |
|
| Interventions | All: vaginal misoprostol 400 μg Group I: every 3 hours; Group II: every 6 hours. |
|
| Outcomes | Primary outcomes: induction to abortion interval, abortion within 24 hours, complete abortion. Secondary outcomes: side‐effects. |
|
| Notes | Definition of abortion: expulsion of fetus and placenta. If the placenta was incomplete, evacuation of the uterus was performed under general anaesthesia. No major complications reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ adequate |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Zauva 1989.
| Methods | Random allocation. | |
| Participants | 37 pregnant women (group I: 19, group II: 18) Inclusion criteria: normal physical investigations, 12‐20 weeks gestation, regular menstrual cycles, certain last menstrual period. Exclusion criteria: previous Caesarian section, hysterotomy, myomectomy, any other surgery on the uterus. |
|
| Interventions | Group I: 150 ml (0.1%) emcredil by extra‐amniotic instillation. Group II: 150 ml normal saline. |
|
| Outcomes | Induction‐abortion interval, side‐effects. | |
| Notes | Definition of abortion: expulsion of the fetus. No major complications described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | B ‐ unclear |
| Blinding? All outcomes | High risk | |
| Free of other bias? | Low risk | No statistically significant differences between the groups in terms of maternal age, gestational age and parity. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allahbadia 1992 | No randomisation |
| Ballard 1981 | No randomisation |
| Ben‐Meir 2009 | Fetal demise > 20% |
| Caliskan 2005 | Fetal demise > 20% |
| Caliskan 2009 | Fetal demise > 20% |
| Carbonell 2008 | Different primary outcomes |
| Dickinson 1998 | Multiple pregnancies, previous uterine scar included |
| Dickinson 2002 | Multiple pregnancies, previous uterine scar, fetal death included |
| Dickinson 2003 | Multiple pregnancies included, previous uterine scar included |
| Feldman 2003 | 27% of the women did not meet the inclusion criteria |
| Frydman 1988 | Pre‐treatment trial |
| Ghorab 1998 | Fetal demise > 20%, cervix dilatation before treatment |
| Ghosh 1980 | Authors excluded women with incomplete abortion |
| Gilbert 2001 | Simple randomisation |
| Goswami 1982 | No randomisation |
| Guix 2005 | Concealment score: C |
| Herabutya 2001 | Odd and even number randomisation, biased and concealment score C |
| Hidar 2001 | Inclusion of fetal death, premature rupture of membranes |
| Hill 1991 | No randomisation |
| Ho 1993 | Mifepristone pre‐treatment trial |
| Jain 1994 | Fetal demise > 20%, live fetuses received a lethal cardiac injection |
| Jain 1999 | Fetal demise included |
| Jansen 2008 | Cervical dilatation |
| Jarnbert 1999 | Cervical ripening |
| Kamali 1998 | No randomisation |
| Kapp 2007 (2) | Fetal demise > 20% |
| Klinte 1983 | No randomisation |
| le Roux 2002 | Trilostane and danazol as pre‐treatments to misoprostol |
| Manabe 1981 | Inclusion of quinine |
| Munthali 2001 | Fetal demise > 20% |
| Nigam 2006 | Simple randomisation |
| Niromanesh 2005 | Concealment allocation: C |
| Nor Azlin 2006 | Cervical dilatation |
| Nuthalapaty 2005 | Use of catheter which was left in place during treatment to promote cervical ripening |
| Olund 1979 | No randomisation |
| Owen 1996 | First dilatation with hygroscopic dilatators and oxytocin infusion |
| Owen 1999 | Women received laminaria for cervical ripening |
| Perry 1999 | Complex and unconventional regime, use of laminaria and cardiac injection |
| Pulkkinen 1980 | No randomisation |
| Ragab 1976 | No primary outcomes could be read off the tables |
| Ramsey 2004 | Cervical dilatation |
| Shukla 1984 | No randomisation |
| Sørensen 1986 | Open list of random numbers |
| Thong 1993 | No randomisation |
| Thong KJ, Baird 1992 | Dilapan laminaria tents, cervical dilatation |
| Wong 1996 | Pre‐treatment trial |
| Yapar 1996 | Fetal demise > 20% |
| Yilmaz 2007 | Fetal demise > 20% |
Differences between protocol and review
None
Contributions of authors
HIJ Wildschut: study design, writing, editing and overall supervision
MI Both: literature search, data analyses and writing
S Medema: literature search and data management
E Thomee: data management
MF Wildhagen: statistics
N Kapp: review design, writing, editing and critical evaluation
Declarations of interest
None declared
Edited (no change to conclusions)
References
References to studies included in this review
Akoury 2004 {published data only}
- Akoury HA, Hannah ME, Chitayat D, Thomas M, Winsor E, Ferris LE, et al. Randomized controlled trial of misoprostol for second‐trimester pregnancy termination associated with fetal malformation. American Journal of Obstetrics and Gynecology 2004;190(3):755‐62. [DOI] [PubMed] [Google Scholar]
Armatage 1996 {published data only}
- Armatage RJ, Luckas MJ. A randomized trial of 2 regimens for the administration of vaginal prostaglandins (gemeprost) for the induction of midtrimester abortion. The Australian & New Zealand Journal of Obstetrics & Gynaecology 1996;36(3):296‐9. [DOI] [PubMed] [Google Scholar]
Bartley 2002 {published data only}
- Bartley J, Baird DT. A randomised study of misoprostol and gemeprost in combination with mifepristone for induction of abortion in the second trimester of pregnancy. BJOG 2002;109(11):1290‐4. [DOI] [PubMed] [Google Scholar]
Bebbington 2002 {published data only}
- Bebbington MW, Kent N, Lim K, Gagnon A, Delisle MF, Tessier F, et al. A randomized controlled trial comparing two protocols for the use of misoprostol in midtrimester pregnancy termination. American Journal of Obstetrics and Gynecology 2002;187(4):853‐7. [DOI] [PubMed] [Google Scholar]
Behrashi 2008 {published data only}
- Behrashi M, Mahdian M. Vaginal versus oral misoprostol for second trimester pregnancy termination: a randomised trial. Pakistan Journal of Biological Sciences 2008;11(21):2505‐8. [DOI] [PubMed] [Google Scholar]
Bhattacharjee 2008 {published data only}
- Bhattacharjee N, Saha SP, Ghosroy SC, Bhowmik S, Barui G. A randomised comparative study on sublingual versus vaginal administration of misoprostol for termination of pregnancy between 13 to 20 weeks. The Australian & New Zealand Journal of Obstetrics & Gynaecology 2008;48:165‐71. [DOI] [PubMed] [Google Scholar]
Borgida 1995 {published data only}
- Borgida AF, Rodis JF, Hanlon W, Craffey A, Ciarleglio L, Campbell WA. Second‐trimester abortion by intramuscular 15‐methyl‐prostaglandin F2 alpha or intravaginal prostaglandin E2 suppositories: a randomized trial. Obstetrics and Gynecology 1995;85(5):697‐700. [DOI] [PubMed] [Google Scholar]
Chai 2009 {published data only}
- Chai J, Tang OS, Hong QQ, Chen QF, Cheng LN, Ng E, Ho PC. A randomized trial to compare two dosing intervals of misoprostol following mifepristone administration in second trimester medical abortion. Human Reproduction 2009;24(2):320‐4. [DOI] [PubMed] [Google Scholar]
el‐Refaey 1993 {published data only}
- El‐Refaey H, Hinshaw K, Templeton A. The abortifacient effect of misoprostol in the second trimester. A randomized comparison with gemeprost in patients pre‐treated with mifepristone (RU486). Human Reproduction 1993;8(10):1744‐6. [DOI] [PubMed] [Google Scholar]
El‐refaey 1995 {published data only}
- El‐Refaey H, Templeton A. Induction of abortion in the second trimester by a combination of misoprostol and mifepristone: a randomized comparison between two misoprostol regimens. Human Reproduction 1995;10(2):475‐8. [DOI] [PubMed] [Google Scholar]
Faktor 1988 {published data only}
- Faktor JH, Frenkel Y, Mashiach S, Serr DM. Intra‐amniotic injection of oxytetracycline hydrochloride for termination of mid trimester abortion. Gynecologic and Obstetric Investvestigation 1988;26:177‐80. [DOI] [PubMed] [Google Scholar]
Hamoda 2005 {published data only}
- Hamoda H, Ashok PW, Flett GMM, Templeton A. A randomized trial of mifepristone in combination with misoprostol administered sublingually or vaginally for medical abortion at 13‐20 weeks gestation. Human Reproduction 2005;20(8):2348‐54. [DOI] [PubMed] [Google Scholar]
Herabutya 2005 {published data only}
- Herabutya Y, Chanrachakul B, Punyavachira P. A randomised controlled trial of 6 and 12 hourly administration of vaginal misoprostol for second trimester pregnancy termination. BJOG 2005;112(9):1297‐301. [DOI] [PubMed] [Google Scholar]
Ho 1996 {published data only}
- Ho PC, Chan JF, Lau W. Misoprostol is as effective as gemeprost in termination of second trimester pregnancy when combined with mifepristone: a randomised comparative trial. Contraception 1996;53(5):281‐3. [PubMed] [Google Scholar]
Ho 1997 {published data only}
- Ho PC, Ngai SW, Liu KL, Wong GC, Lee SW. Vaginal misoprostol compared with oral misoprostol in termination of second‐trimester pregnancy. Obstetrics and Gynecology 1997;90(5):735‐8. [DOI] [PubMed] [Google Scholar]
Inan 1997 {published data only}
- Inan I, Kelekci S, Yazar D. Comparison of ethacridine lactate and prostaglandin E2 in second trimester medical abortion. Acta Obstetricia et Gynecologica Scandinavica 1996;76:680‐3. [DOI] [PubMed] [Google Scholar]
Kapp 2007 {published data only}
- Kapp N, Borgatta L, Stubblefield P, Vragovic O, Moreno N. Mifepristone in second‐trimester medical abortion: A randomized controlled trial. Obstetrics and Gynecology 2007;110(6):1304‐10. [DOI] [PubMed] [Google Scholar]
Kelekci 2006 {published data only}
- Kelekci S, Erdemoglu E, Inan I. Randomized study on the effect of adding oxytocin to ethacridine lactate or misoprostol for second‐trimester termination of pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2006;85:825‐9. [DOI] [PubMed] [Google Scholar]
Makhlouf 2003 {published data only}
- Makhlouf AM, Al‐Hussaini TK, Habib DM, Makarem MH. Second‐trimester pregnancy termination: comparison of three different methods. Journal of Obstetrics and Gynaecology 2003;23(4):407‐11. [DOI] [PubMed] [Google Scholar]
Mehta 1975 a {published data only}
- Mehta A, Ghatge V, Dave S, Chaina M, Shah P. Termination of second trimester pregnancies: a blind study using hypertonic saline and prostaglandins F2a ‐ a preliminary report. Journal of Obstetrics and Gynaecology India 1975;25(2):165‐75. [PubMed] [Google Scholar]
Mehta 1975 b {published data only}
- Mehta A, Ghatge V, Dave S, CHaina M, Shah P. Termination of second trimester pregnancies: a blind study using hypertonic saline and prostaglandins F2a ‐ a preliminary report. Journal of Obstetrics and Gynaecology India 1975;25(2):165‐75. [PubMed] [Google Scholar]
Muzsnai 1979 a {published data only}
- Muzsnai D, Kerenyi T. Use of prostaglandin, hypertonic saline and oxytocin for second‐trimester abortion. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1979;9/6:385‐9. [DOI] [PubMed] [Google Scholar]
Muzsnai 1979 b {published data only}
- Muzsnai D, Kerenyi T. Use of prostaglandin, hypertonic saline and oxytocin for second‐trimester abortion. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1979;9/6:385‐9. [DOI] [PubMed] [Google Scholar]
Muzsnai 1979 c {published data only}
- Muzsnai D, Kerenyi T. Use of prostaglandin, hypertonic saline and oxytocin for second‐trimester abortion. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1979;9/6:385‐9. [DOI] [PubMed] [Google Scholar]
Muzsnai 1979 d {published data only}
- Muzsnai D, Kerenyi T. Use of prostaglandin, hypertonic saline and oxytocin for second‐trimester abortion. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1979;9/6:385‐9. [DOI] [PubMed] [Google Scholar]
Ngai 2000 {published data only}
- Ngai SW, Tang OS, Ho PC. Randomized comparison of vaginal (200 µg every 3 hrs) and oral (400 µg every 3 hrs) misoprostol when combined with mifepristone in termination of second trimester pregnancy. Human Reproduction 2000;15(10):2205‐8. [DOI] [PubMed] [Google Scholar]
Nielsen 1975 {published data only}
- Nielsen KR, Gregersen E, Larsen JF, Olsen CE. Prostaglandin F2alpha and oxytocin compared with hypertonic saline and oxytocin for the induction of second trimester abortion.. Acta Obstetricia et Gynecologica Scandinavica. Supplement 1975;37:57‐60. [DOI] [PubMed] [Google Scholar]
Nuutila 1997 a {published data only}
- Nuutila M, Toivonen J, Ylikorkala O, Halmesmäki E. A comparison between two doses of intravaginal misoprostol and gemeprost for induction of second‐trimester abortion. Obstetrics and Gynecology 1997;90(6):896‐900. [DOI] [PubMed] [Google Scholar]
Nuutila 1997 b {published data only}
- Nuutila M, Toivonen J, Ylikorkala O, Halmesmäki E. A comparison between two doses of intravaginal misoprostol and gemeprost for induction of second‐trimester abortion. Obstetrics and Gynecology 1997;90(6):896‐900. [DOI] [PubMed] [Google Scholar]
Nuutila 1997 c {published data only}
- Nuutila M, Toivonen J, Ylikorkala O, Halmesmäki E. A comparison between two doses of intravaginal misoprostol and gemeprost for induction of second‐trimester abortion. Obstetrics and Gynecology 1997;90(6):896‐900. [DOI] [PubMed] [Google Scholar]
Olund 1978 {published data only}
- Olund A, Larsson B. Comparison of extra‐amniotic instillation of rivanol and PGF2a either separately or in combination followed by oxytocin for second trimester abortion. Acta Obstetricia et Gynecologica Scandinavica 1978;57:333‐6. [DOI] [PubMed] [Google Scholar]
Ozerkan 2009 {published data only}
- Ozerkan K, Ocakoglu G, Rehimli S, Uncu G, Develioglu O. A comparison of low‐dose and high‐dose protocols of vaginal misoprostol for second trimester termination of pregnancy. Clinical and Experimental Obstetrics & Gynecology 2009;36(4):245‐7. [PubMed] [Google Scholar]
Pongsatha 2008 {published data only}
- Pongsatha S, Tongsong T. Randomized comparison of dry tablet insertion versus gel form of vaginal misoprostol for second trimester pregnancy termination. The Journal of Obstetrics and Gynaecology Research 2008;34(2):199‐203. [DOI] [PubMed] [Google Scholar]
Sorensen 1984 {published data only}
- Sorensen SS, Wolf P. Randomized trial of intracervical prostaglandin E2 gel and intra‐amniotic prostaglandin F2alfa for induction of second trimester abortion. Contraception 1984;29(2):171‐9. [DOI] [PubMed] [Google Scholar]
Steyn 1993 {published data only}
- Steyn DW, Pienaar MP. Mid‐trimester termination of pregnancy‐‐a randomised controlled trial of two prostaglandin regimens. South African Medical Journal 1993;83(10):737‐8. [PubMed] [Google Scholar]
Su 2005 {published data only}
- Su LL, Biswas A, Choolani M, Kalaichelvan V, Singh K. A prospective, randomized comparison of vaginal misoprostol versus intra‐amniotic prostaglandins for midtrimester termination of pregnancy. American Journal of Obstetrics and Gynecology 2005;193(4):1410‐4. [DOI] [PubMed] [Google Scholar]
Tang 2004 {published data only}
- Tang OS, Chan CC, Kan AS, Ho PC. A prospective randomized comparison of sublingual and vaginal misoprostol in second trimester termination of pregnancy. BJOG 2004;111:1001‐5. [DOI] [PubMed] [Google Scholar]
Tang 2005 {published data only}
- Tang OS, Chan CC, Kan AS, Ho PC. A prospective randomized comparison of sublingual and oral misoprostol when combined with mifepristone for medical abortion at 12‐20 weeks gestation. Human Reproduction 2005;20(11):3062‐6. [DOI] [PubMed] [Google Scholar]
Thong 1996 {published data only}
- Thong KJ, Lynch P, Baird DT. A randomised study of two doses or gemeprost in combination with mifepristone for induction of abortion in the second trimester of pregnancy. Contraception 1996;54(2):97‐100. [DOI] [PubMed] [Google Scholar]
von Hertzen 2009 {published data only}
- Hertzen H, Piaggio G, Wojdyla D, Nguyen TM, Marions L, Okoev G, et al. WHO Research Group on Post‐ovulatory Methods of Fertility Regulation. Comparison of vaginal and sublingual misoprostol for second trimester abortion: randomised controlled equivalence trial. Human Reproduction 2009;24(1):106‐12. [DOI] [PubMed] [Google Scholar]
Waldron 1990 {published data only}
- Waldron KW, Renou PM, Lolatgis N, Morris ND, Mamers PM, Oldham J, Healy DL. Second‐trimester termination with 16,16 dimethyl‐PGE1‐methyl ester (gemeprost) compared with a regimen that included intra‐amniotic PGF2a and hypertonic saline. Reproduction, Fertility, and Development 1990;2:495‐8. [DOI] [PubMed] [Google Scholar]
Webster 1996 {published data only}
- Webster D, Penney GC, Templeton A. A comparison of 600 and 200 mg mifepristone prior to second trimester abortion with the prostaglandin misoprostol. British Journal of Obstetrics and Gynaecology 1996;103(7):706‐9. [DOI] [PubMed] [Google Scholar]
WHO 1976 {published data only}
- No authors listed. Comparison of intra‐amniotic prostaglandin F2alpha and hypertonic saline for induction of second‐trimester abortion. British Medical Journal 1976;1:1373‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 1998 {published data only}
- Wong KS, Ngai CS, Wong AY, Tang LC, Ho PC. Vaginal misoprostol compared with vaginal gemeprost in termination of second trimester pregnancy. A randomized trial. Contraception 1998;58(4):207‐10. [DOI] [PubMed] [Google Scholar]
Wong 2000 {published data only}
- Wong KS, Ngai CS, Yeo EL, Tang LC, Ho PC. A comparison of two regimens of intravaginal misoprostol for termination of second trimester pregnancy: a randomized comparative trial. Human Reproduction 2000;15(3):709‐12. [DOI] [PubMed] [Google Scholar]
Zauva 1989 {published data only}
- Zauva BL, Gupta I, Dhall GI. Mid‐trimester abortion by extra‐amniotic emcredil versus normal saline. The Australian & New Zealand Journal of Obstetrics & Gynaecology 1989;29(3(2)):299‐302. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allahbadia 1992 {published data only}
- Allahbadia G. Comparative study of midtrimester termination of pregnancy using hypertonic saline, ethacridine lactate, prostaglandin analogue and iodine‐saline. Journal of the Indian Medical Association 1992;90(9):237‐9. [PubMed] [Google Scholar]
Ballard 1981 {published data only}
- Ballard CA. The vaginal administration of 9‐deoxo‐16,16‐dimethyl‐9‐methylene PGE2 for second trimester abortion. Contraception 1981;24(2):151‐7. [DOI] [PubMed] [Google Scholar]
Ben‐Meir 2009 {published data only}
- Ben‐Meir A, Erez Y, Feigenberg T, Hamani Y, Laufer N, Rojansky N. Mifepristone followed by high‐dose oxytocin drip for second‐trimester abortion. The Journal of Reproductive Medicine 2009;54(8):511‐6. [PubMed] [Google Scholar]
Caliskan 2005 {published data only}
- Caliskan E, Dilbaz S, Doger E, Ozeren S, Dilbaz B. Randomized comparison of 3 misoprostol protocols for abortion induction at 13‐20 weeks of gestation. The Journal of Reproductive Medicine 2005;50(3):173‐80. [PubMed] [Google Scholar]
Caliskan 2009 {published data only}
- Caliskan E, Doger E, Cakiroglu Y, Corakci A, Yucesoy I. Sublingual misoprostol 100 microgram versus 200 microgram for second trimester abortion: a randomised trial. The European Journal of Contraception & Reproductive Health Care 2009;14(1):55‐60. [DOI] [PubMed] [Google Scholar]
Carbonell 2008 {published data only}
- Carbonell JL, Torres MA, Reyes R, Ortega L, García‐Gallego F, Sánchez C. Second‐trimester pregnancy termination with 600‐μg vs. 400‐μg vaginal misoprostol and systematic curettage post expulsion: a randomized trial. Contraception 2007;77(1):50‐5. [DOI] [PubMed] [Google Scholar]
Dickinson 1998 {published data only}
- Dickinson JE, Godfrey M, Evans SF. Efficacy of intravaginal misoprostol in second‐trimester pregnancy termination: a randomized controlled trial. The Journal of Maternal‐Fetal Medicine 1998;7(3):115‐9. [DOI] [PubMed] [Google Scholar]
Dickinson 2002 {published data only}
- Dickinson JE, Evans SF. The optimization of intravaginal misoprostol dosing schedules in second trimester pregnancy termination. American Journal of Obstetrics and Gynecology 2002;186(3):470‐4. [DOI] [PubMed] [Google Scholar]
Dickinson 2003 {published data only}
- Dickinson JE, Evans SF. A comparison of oral misoprostol with vaginal misoprostol administration in second‐trimester pregnancy termination for fetal abnormality. Obstetrics and Gynecology 2003;101(6):1294‐9. [DOI] [PubMed] [Google Scholar]
Feldman 2003 {published data only}
- Feldman DM, Borgida AF, Rodis JF, Leo MV, Campbell WA. A randomized comparison of two regimens of misoprostol for second‐trimester pregnancy termination. American Journal of Obstetrics and Gynecology 2003;189(3):710‐3. [DOI] [PubMed] [Google Scholar]
Frydman 1988 {published data only}
- Frydman R, Fernandez H, Pons JC, Ulmann A. Mifepristone (RU486) and therapeutic late pregnancy termination: a double‐blind study of two different doses. Human Reproduction 1988;3(6):803‐6. [DOI] [PubMed] [Google Scholar]
Ghorab 1998 {published data only}
- Ghorab MN, Helw BA. Second‐trimester termination of pregnancy by extra‐amniotic prostaglandin F2alpha or endocervical misoprostol. A comparative study. Acta Obstetricia et Gynecologica Scandinavica 1998;77(4):429‐32. [PubMed] [Google Scholar]
Ghosh 1980 {published data only}
- Ghodh AK, Konar JR. The relative value of two concentrations of hypertonic saline for midtrimester abortion. International Journal of Gynaecology and Obstetrics 1980;17:368‐71. [DOI] [PubMed] [Google Scholar]
Gilbert 2001 {published data only}
- Gilbert A, Reid R. A randomised trial of oral versus vaginal administration of misoprostol for the purpose of mid‐trimester termination of pregnancy. The Australian & New Zealand Journal of Obstetrics & Gynaecology 2001;41(4):407‐10. [DOI] [PubMed] [Google Scholar]
Goswami 1982 {published data only}
- Goswami BK, Raha A, Gupta A, Mukherjee K. Midtrimester abortion by ethacridine lactate. Journal of the Indian Medical Association 1982;79(1‐2):7‐9. [PubMed] [Google Scholar]
Guix 2005 {published data only}
- Guix C, Palacio M, Figueras F, Bennasar M, Zamora L, Coll O, et al. Efficacy of two regimens of misoprostol for early second‐trimester pregnancy termination. Fetal Diagnosis and Therapy 2005;20(6):544‐8. [DOI] [PubMed] [Google Scholar]
Herabutya 2001 {published data only}
- Herabutya Y, Chanrachakul B, Punyavachira P. Second trimester pregnancy termination: a comparison of 600 and 800 micrograms of intravaginal misoprostol. The Journal of Obstetrics and Gynaecology Research 2001;27(3):125‐8. [DOI] [PubMed] [Google Scholar]
Hidar 2001 {published data only}
- Hidar S, Fekih M, Chaieb, Bibi M, Mellouli R, Khairi H. Oxytocin and misoprostol administered intravaginally for termination of pregnancy at 13 to 29 weeks of amenorrhea. A prospective randomized trial [Apport de l'association d'oxytocine au misoprostol administre en intravaginal au cours des interruptions de grossesses entre 13 et 29 semaines d'amenorrhee]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2001;30(5):439‐43. [PubMed] [Google Scholar]
Hill 1991 {published data only}
- Hill NC, Selinger M, Ferguson J, MacKenzie IZ Hill NC, Selinger M, Ferguson J, et al. Mid‐trimester termination of pregnancy with 16,16‐dimethyl‐trans‐delta 2 PGE1 vaginal pessaries: a comparison with intra‐ and extra‐amniotic prostaglandin E2 administration. International Journal of Gynaecology and Obstetrics 1991;35(4):337‐40. [DOI] [PubMed] [Google Scholar]
Ho 1993 {published data only}
- Ho PC, Ma HK. Termination of second trimester pregnancy with sulprostone and mifepristone: a randomized double‐blind placebo‐controlled trial. Contraception 1993;47:123‐9. [DOI] [PubMed] [Google Scholar]
Jain 1994 {published data only}
- Jain JK, Mishell DR Jr. A comparison of intravaginal misoprostol with prostaglandin E2 for termination of second‐trimester pregnancy. The New England Journal of Medicine 1994;331(5):290‐3. [DOI] [PubMed] [Google Scholar]
Jain 1999 {published data only}
- Jain JK, Kuo J, Mishell DR Jr. A comparison of two dosing regimens of intravaginal misoprostol for second‐trimester pregnancy termination. Obstetrics and Gynecology 1999;93(4):571‐5. [DOI] [PubMed] [Google Scholar]
Jansen 2008 {published data only}
- Jansen NE, Pasker‐De Jong PC, Zondervan HA. Mifepristone and misoprostol versus dilapan and sulprostone for second trimester termination of pregnancy. The Journal of Maternal‐Fetal & Neonatal Medicine 2008;21(11):847‐51. [DOI] [PubMed] [Google Scholar]
Jarnbert 1999 {published data only}
- Järnbert A, Klang B, Thi Vinh N, Ngoc Hamb N. Comparative study of cervical laminar tents prior to extra‐amniotic injection of ethacridine lactate (rivanol) and a condom‐nelathon catheter method for second‐trimester pregnancy interruption in Vietnam. Gynecologic and Obstetric Investigation 1999;48:113‐8. [DOI] [PubMed] [Google Scholar]
Kamali 1998 {published data only}
- Kamali P, Hohmann M, Herrero J, Künzel W. Gemeprost, sulprostone and dinoprostone for induced abortion in the 15th‐24th week of pregnancy. Zentralblatt fur Gynakologie 1998;120(6):293‐300. [PubMed] [Google Scholar]
Kapp 2007 (2) {published data only}
- Kapp N, Todd CS, Yadgarova KT, Alibayeva G, Nazarova D, Loza O, Babadjanova GS. A randomized comparison of misoprostol to intrauterine instillation of hypertonic saline plus a prostaglandin F2α analogue for second‐trimester induction termination in Uzbekistan. Contraception 2007;76(6):461‐6. [DOI] [PubMed] [Google Scholar]
Klinte 1983 {published data only}
- Klinte I, Hamberger L, Wiqvist N. Second‐trimester abortion by extra‐amniotic instillation of Rivanol combined with intravenous administration of oxytocin or prostaglandin F2 alpha. Acta Obstetricia et Gynecologica Scandinavica 1983;62(4):303‐6. [DOI] [PubMed] [Google Scholar]
le Roux 2002 {published data only}
- Roux PA, Tregoning SK, Zinn PM, Spuy ZM. Inhibition of progesterone secretion with trilostane for midtrimester termination of pregnancy: randomized controlled trials. Human Reproduction 2002;17(6):1483‐9. [DOI] [PubMed] [Google Scholar]
Manabe 1981 {published data only}
- Manabe Y, Manabe A. Abortion during mid‐pregnancy by rivanol‐catheter supplemented with PGF2a drip‐infusion or quinine hydrochloride. Contraception 1981;23(6):621‐8. [DOI] [PubMed] [Google Scholar]
Munthali 2001 {published data only}
- Munthali J, Moodley J. The use of misoprostol for mid‐trimester therapeutic termination of pregnancy. Tropical Doctor 2001;31(3):157‐61. [DOI] [PubMed] [Google Scholar]
Nigam 2006 {published data only}
- Nigam A, Singh VK, Prakash A. Vaginal vs. oral misoprostol for mid‐trimester abortion. International Journal of Gynaecology and Obstetrics 2006;92(3):270‐1. [DOI] [PubMed] [Google Scholar]
Niromanesh 2005 {published data only}
- Niromanesh S, Hashemi‐Fesharaki M, Mosavi‐Jarrahi A. Second trimester abortion using intravaginal misoprostol [brief communication]. International Journal of Gynaecology and Obstetrics 2005;89(3):276‐7. [DOI] [PubMed] [Google Scholar]
Nor Azlin 2006 {published data only}
- Nor Azlin MI, Abdullah HS, Zainul Rashid MR, Jamil MA. Misoprostol (alone) in second trimester terminations of pregnancy: as effective as gemeprost?. Journal of Obstetrics and Gynaecology 2006;26(6):546‐9. [DOI] [PubMed] [Google Scholar]
Nuthalapaty 2005 {published data only}
- Nuthalapaty FS, Ramsey PS, Biggio JR, Owen J. High‐dose vaginal misoprostol versus concentrated oxytocin plus low‐dose vaginal misoprostol for midtrimester labor induction: a randomized trial. American Journal of Obstetrics and Gynecology 2005;193(3 Pt 2):1065‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olund 1979 {published data only}
- Olund A. The effect of indomethacin on the instillation‐abortion interval in rivanol‐induced mid‐trimester abortion. Acta Obstetricia et Gynecologica Scandinavica 1979;58(1):121‐2. [DOI] [PubMed] [Google Scholar]
Owen 1996 {published data only}
- Owen J, Hauth JC. Concentrated oxytocin plus low‐dose prostaglandin E2 compared with prostaglandin E2 vaginal suppositories for second‐trimester pregnancy termination. Obstetrics and Gynecology 1996;88(1):110‐3. [DOI] [PubMed] [Google Scholar]
Owen 1999 {published data only}
- Owen J, Hauth JC. Vaginal misoprostol vs. concentrated oxytocin plus low‐dose prostaglandin E2 for second trimester pregnancy termination. J Matern Fetal Med 1999;8(2):48‐50. [DOI] [PubMed] [Google Scholar]
Perry 1999 {published data only}
- Perry KG Jr, Rinehart BK, Terrone DA, Martin RW, May WL, Roberts WE. Second‐trimester uterine evacuation: A comparison of intra‐amniotic (15S)‐15‐methyl‐prostaglandin F2alpha and intravaginal misoprostol. American Journal of Obstetrics and Gynecology 1999;181(5):1057‐61. [DOI] [PubMed] [Google Scholar]
Pulkkinen 1980 {published data only}
- Pulkkinen MO, Kajanoja P, Kivikoski A, Saastamoinen J, Selander K, Tuimala R. Abortion with sulprostone, a prostaglandin E2 derivative. International Journal of Gynaecology and Obstetrics 1980;18(1):40‐3. [DOI] [PubMed] [Google Scholar]
Ragab 1976 {published data only}
- Ragab MI, Edelman DA. Midtrimester abortion: a comparison of intra‐amniotic Prostaglandin F2a and hypertonic saline. International Journal of Gynaecology and Obstetrics 1976;14:393‐6. [DOI] [PubMed] [Google Scholar]
Ramsey 2004 {published data only}
- Ramsey PS, Savage K, Lincoln T, Owen J. Vaginal misoprostol versus concentrated oxytocin and vaginal PGE2 for second trimester labor induction. Obstetrics and Gynecology 2004;104(1):138‐45. [DOI] [PubMed] [Google Scholar]
Shukla 1984 {published data only}
- Shukla S, Sapre S, Olyai P. Mid‐trimester pregnancy termination with ethacridine lactate. Journal of the Indian Medical Association 1984;82(12):432‐4. [PubMed] [Google Scholar]
Sørensen 1986 {published data only}
- Stampe Sørensen S, Heisterberg L, Wolf P. Midtrimester abortion by intracervical prostaglandin E2. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1986;21(3):165‐71. [DOI] [PubMed] [Google Scholar]
Thong 1993 {published data only}
- Thong KJ, Baird DT. Induction of second trimester abortion with mifepristone and gemeprost. British Journal of Obstetrics and Gynaecology 1993;100(8):758‐61. [DOI] [PubMed] [Google Scholar]
Thong KJ, Baird 1992 {published data only}
- Thong KJ, Baird DT. A study of gemeprost alone, dilapan of mifepristone in combination with gemeprost for the termination of second trimester pregnancy. Contraception 1992;46:11‐7. [DOI] [PubMed] [Google Scholar]
Wong 1996 {published data only}
- Wong KS, Ngai CS, Chan KS, Tang LC, Ho PC. Wong KS, Ngai CS, et al. Termination of second trimester pregnancy with gemeprost and misoprostol: a randomized double‐blind placebo‐controlled trial. Contraception 1996;54(1):23‐5. [DOI] [PubMed] [Google Scholar]
Yapar 1996 {published data only}
- Yapar EG, Senoz S, Orkiittir M, Batioglu S, Gokmen O. Second trimester pregnancy termination including fetal death:comparison of five different methods. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1996;69:97‐102. [DOI] [PubMed] [Google Scholar]
Yilmaz 2007 {published data only}
- Yilmaz B, Kelekci S, Ertas IE, Ozel M, Sut N, Mollamahmutoglu L, Danisman N. Randomized comparison of second trimester pregnancy termination utilizing saline moistened or dry misoprostol. Archives of Gynecology and Obstetrics 2007;276(5):511‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Akgun 2007
- Akgun H, Basbug M, Ozgun MT, Canoz O, Tokat F, Murat N, Ozturk F. Correlation between prenatal ultrasound and fetal autopsy findings in fetal anomalies terminated in the second trimester. Prenatal Diagnosis 2007;27(5):457‐62. [DOI] [PubMed] [Google Scholar]
Asch 1999
- Asch A. Prenatal diagnosis and selective abortion: a challenge to practice and policy. American Journal of Public Health 1999;89(11):1649‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballantyne 2009
- Ballantyne A, Newson A, Luna F, Ashcroft R. Prenatal diagnosis and abortion for congenital abnormalities: is it ethical to provide one without the other?. The American Journal of Bioethics 2009;9(8):48‐56. [DOI] [PubMed] [Google Scholar]
Belanger 1981
- Bélanger A, Philibert D, Teutsch G. Regio and stereospecific synthesis of 11beta‐substituted 19‐norsteroids. Steroids 1981;37(4):361‐82. [DOI] [PubMed] [Google Scholar]
Berghella 2009
- Berghella V, Airoldi J, O'Neill AM, Einhorn K, Hoffman M. Misoprostol for second trimester pregnancy termination in women with prior caesarean: a systematic review. BJOG 2009;116(9):1151‐7. [DOI] [PubMed] [Google Scholar]
Boyd 2008
- Boyd PA, Devigan C, Khoshnood B, Loane M, Garne E, Dolk H, EUROCAT WorkingGroup. Survey of prenatal screening policies in Europe for structural malformations and chromosome anomalies, and their impact on detection and termination rates for neural tube defects and Down's syndrome. BJOG 2008;115(6):689‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bygdeman 1985
- Bygdeman M, Swahn ML. Progesterone receptor blockage. Effect on uterinecontractility and early pregnancy. Contraception 1985;32(1):45‐51. [DOI] [PubMed] [Google Scholar]
Bygdeman and Gemzell‐Danielsson 2008
- Bygdeman M, Gemzell‐Danielsson K. An historical overview of second trimester abortion methods. Reproductive Health Matters 2008;16 Suppl(31):196‐204. [DOI] [PubMed] [Google Scholar]
Christin‐Maitre 2000
- Christin‐Maitre S, Bouchard P, Spitz IM. Medical termination of pregnancy. The New England Journal of Medicine 2000;342(13):946‐56. [DOI] [PubMed] [Google Scholar]
Drey 2006
- Drey EA, Foster DG, Jackson RA, Lee SJ, Cardenas LH, Darney PD. Risk factors associated with presenting for abortion in the second trimester. Obstetrics and Gynecology 2006;107(1):128‐35. [DOI] [PubMed] [Google Scholar]
Friedman 2001
- Friedman MA. Manufacturer's warning regarding unapproved uses of misoprostol. The New England Journal of Medicine 2001;344(1):61. [DOI] [PubMed] [Google Scholar]
Goldberg 2001
- Goldberg AB, Greenberg MB, Darney PD. Misoprostol and pregnancy. The New England Journal of Medicine 2001;344(1):38‐47. [DOI] [PubMed] [Google Scholar]
Grimes 1998
- Grimes DA. The continuing need for late abortions. JAMA 1998;280(8):747‐50. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 2007
- The continuing need for late abortions. Misoprostol for the termination of pregnancy with a live fetus at 13 to 26 weeks. International Journal of Gynaecology and Obstetrics 2007;99 Suppl 2:178‐81. [DOI] [PubMed] [Google Scholar]
Hou 2010
- Hou S, Chen Q, Zhang L, Fang A, Cheng L. Mifepristone combined with misoprostol versus intra‐amniotic injection of ethacridine lactate for the termination of second trimester pregnancy: a prospective, open‐label, randomized clinical trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2010;151(2):149‐53. [DOI] [PubMed] [Google Scholar]
Ingham 2008
- Ingham R, Lee E, Clements SJ, Stone N. Reasons for second trimester abortions in England and Wales. Reproductive Health Matters 2008;1631 Suppl:18‐29. [DOI] [PubMed] [Google Scholar]
Isaksen 1998
- Isaksen CV, Eik‐Nes SH, Blaas HG, Torp SH. Comparison of prenatal ultrasound and postmortem findings in fetuses and infants with central nervous system anomalies. Ultrasound in Obstetrics & Gynecology 1998;11(4):246‐53. [DOI] [PubMed] [Google Scholar]
Isaksen 1999
- Isaksen CV, Eik‐Nes SH, Blaas HG, Tegnander E, Torp SH. Comparison of prenatal ultrasound and postmortem findings in fetuses and infants with congenital heart defects. Ultrasound in Obstetrics & Gynecology 1999;13(2):117‐26. [DOI] [PubMed] [Google Scholar]
Kaasen 2006
- Kaasen A, Tuveng J, Heiberg A, Scott H, Haugen G. Correlation between prenatal ultrasound and autopsy findings: A study of second‐trimester abortions. Ultrasound in Obstetrics & Gynecology 2006;28(7):925‐33. [DOI] [PubMed] [Google Scholar]
Lohr 2008
- Lohr PA, Hayes JL, Gemzell‐Danielsson K. Surgical versus medical methods for second trimester induced abortion. Cochrane Database of Systematic Reviews 2008;23(1):CD006714. [DOI] [PubMed] [Google Scholar]
Norman 1992
- Norman JE, Thong KJ, Rodger MW, Baird DT. Medical abortion in women of less than or equal to 56 days amenorrhoea: a comparison between gemeprost (a PGE1 analogue) alone and mifepristone and gemeprost. British Journal of Obstetrics and Gynaecology 1992;99(7):601‐6. [DOI] [PubMed] [Google Scholar]
Swahn 1988
- Swahn ML, Johannisson E, Daniore V, Torre B, Bygdeman M. The effect of RU486 administered during the proliferative and secretory phase of the cycle on the bleeding pattern, hormonal parameters and the endometrium. Human Reproduction 1988;3(7):915‐21. [DOI] [PubMed] [Google Scholar]
Tang 2002
- Tang OS, Schweer H, Seyberth HW, Lee SWH, Ho PC. Pharmacokinetics of different routes of administration of misoprostol. Human Reproduction 2002;17:332‐6. [DOI] [PubMed] [Google Scholar]
Tang 2007
- Tang OS, Gemzell‐Danielsson K, Ho PC. Misoprostol: pharmacokinetic profiles,effects on the uterus and side‐effects. Int J Gynaecol Obstet. 2007;99 Suppl 2:S160‐7. [DOI] [PubMed] [Google Scholar]
Ulmann 1992
- Ulmann A, Silvestre L, Chemama L, Rezvani Y, Renault M, Aguillaume CJ, Baulieu EE. Medical termination of early pregnancy with mifepristone (RU 486) followed by a prostaglandin analogue. Study in 16,369 women. Acta Obstetricia et Gynecologica Scandinavica 1992;71(4):278‐83. [DOI] [PubMed] [Google Scholar]
Wagner 2005
- Wagner M. Off‐label use of misoprostol in obstetrics: a cautionary tale. BJOG 2005;112(3):266‐8. [DOI] [PubMed] [Google Scholar]
Weeks 2005
- Weeks AD, Fiala C, Safar P. Misoprostol and the debate over off‐label drug use. BJOG 2005;112(3):269‐72. [DOI] [PubMed] [Google Scholar]
Zieman 1997
- Zieman M, Fong SK, Benowitz NL, Banskter D, Darney PD. Absorption kinetics of misoprostol with oral or vaginal administration. Obstetrics and Gynecology 1997;90(1):88‐92. [DOI] [PubMed] [Google Scholar]



