The majority of women with gestational trophoblastic disease can be successfully managed with preservation of their reproductive capacity.
Abstract
This review summarizes the current evaluation and management of gestational trophoblastic disease, including evacuation of hydatidiform moles, surveillance after evacuation of hydatidiform mole and the diagnosis and management of gestational trophoblastic neoplasia. Most women with gestational trophoblastic disease can be successfully managed with preservation of reproductive function. It is important to manage molar pregnancies properly to minimize acute complications and to identify gestational trophoblastic neoplasia promptly. Current International Federation of Gynecology and Obstetrics guidelines for making the diagnosis and staging of gestational trophoblastic neoplasia allow uniformity for reporting results of treatment. It is important to individualize treatment based on their risk factors, using less toxic therapy for patients with low-risk disease and aggressive multiagent therapy for patients with high-risk disease. Patients with gestational trophoblastic neoplasia should be managed in consultation with an individual experienced in the complex, multimodality treatment of these patients.
Gestational trophoblastic disease is a spectrum of interrelated disease processes originating from the placenta (Box 1). Gestational trophoblastic neoplasia refers to lesions that have the potential for local invasion and metastasis. Before the development of sensitive assays for human chorionic gonadotropin (hCG) and effective chemotherapy, mortality from all forms of malignant gestational trophoblastic neoplasia was substantial.
Box 1.
Gestational Trophoblastic Disease Terminology
-
I. Hydatidiform moles—benign placental tumors with malignant potential
a. Complete mole
b. Partial mole
-
II. Gestational trophoblastic neoplasia—placental tumors with malignant behavior
a. Postmolar gestational trophoblastic neoplasia
b. Invasive mole
c. Gestational choriocarcinoma
d. Placental site trophoblastic tumor
e. Epithelioid trophoblastic tumor
Currently, most women can be cured, and their reproductive function can be preserved, but it is important that the initial management and follow-up of patients be timely and appropriate. Practicing obstetrician–gynecologists are most likely to be involved in the care of women with hydatidiform moles. They should be able to manage this disease, including making the diagnosis of postmolar gestational trophoblastic neoplasia and evaluating the patient's risk status to allow appropriate referral for treatment. Obstetrician–gynecologists may be consulted for patients who have an unclear diagnosis associated with an elevated hCG level and should be able to direct evaluation for “phantom hCG” and malignant gestational trophoblastic neoplasia.
Estimates for the incidence of various types of gestational trophoblastic disease vary. In the United States, hydatidiform moles are observed in approximately 1 in 600 therapeutic abortions and 1 in 1,000–1,200 pregnancies.1,2 Women at the extremes of reproductive life are at increased risk, especially those older than age 45 years. Approximately 15–20% of patients will be treated for gestational trophoblastic neoplasia after evacuation of complete hydatidiform mole.3 Gestational choriocarcinoma occurs in approximately 1 in 20,000–40,000 pregnancies.1,2 Approximately 50% of choriocarcinomas present after term pregnancies, 25% after molar pregnancies, and the remainder after other gestational events.1–3 Although much rarer than hydatidiform mole or gestational choriocarcinoma, placental site trophoblastic tumors and epithelioid trophoblastic tumors can develop after any type of pregnancy.3
HYDATIDIFORM MOLE
Hydatidiform moles are usually diagnosed during the first half of pregnancy. The most common presenting symptom is abnormal bleeding, occasionally with passage of hydropic villi. Other classic signs and symptoms include uterine enlargement greater than expected for gestational dates, absent fetal heart tones, cystic enlargement of the ovaries (theca lutein cysts), hyperemesis, pregnancy-induced hypertension in the first trimester, and an abnormally high level of hCG for gestational dates.3 As discussed later, the incidence of these presenting symptoms has decreased significantly because of the more frequent use of ultrasonography in early pregnancy, with an early diagnosis of “abnormal” pregnancy.
Classification of Hydatidiform Moles
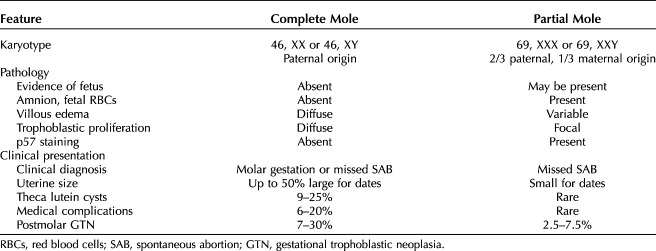
Partial and complete hydatidiform moles are distinct diseases (Table 1).4,5 In both conditions, through a defect in gametogenesis or fertilization, the placental villi become edematous, forming small grape-like (hydatidiform) structures. Despite the cytogenetic, pathologic, and clinical differences, the management of patients with complete and partial moles is similar.
Table 1.
Features of Complete and Partial Moles
Complete moles almost always have a chromosomal complement totally derived from the paternal genome. The 46, XX karyotype is most common4,5 representing reduplication of the haploid genome of the sperm and exclusion of the maternal chromosomal complement. Approximately 5–10% of complete moles have a Y chromosome consistent with dispermic fertilization causing about 10–20% of complete moles. Fetal development, vessels, or red blood cells are not observed in complete moles because the fetus resorbs before the development of the circulatory system.5,6
Partial moles usually have complete trisomy derived from two paternal and one maternal haploid sets of chromosomes.4,5 Most have a 69, XXX or 69, XXY karyotype derived from a haploid ovum with either reduplication of the paternal haploid set from a single sperm, or less frequently, from dispermic fertilization. Trisomy with XYY karyotype is rarely seen,3 and YYY karyotype is not observed.5,6 Gross or histologic evidence of fetal development, such as amnion or vessels with fetal red blood cells, is a prominent pathologic feature.4,5
Germline mutations in NLRP7 and KHDC3L are observed in 48–80% and 10–14% of patients with repetitive moles, respectively.6–8 These are maternal effect genes, and mutations cause defects in maternal imprinting potentially resulting in defective oocytes and possible creation of a maternal environment hostile to embryonic implantation. In contrast to complete and partial moles, these are biparental moles with a normal chromosomal compliment.6–8
The p57 cyclin-dependent kinase inhibitor is a paternally imprinted but maternally expressed gene.9 In complete moles that lack maternal genome, p57 is not expressed. In contrast, partial moles and nonmolar gestations have maternal genome and do express the p57 gene. Therefore, p57 immunohistochemical staining cannot differentiate partial moles from hydropic spontaneous abortions, but can be helpful in distinguishing complete from partial moles.9
Among complete moles the extent of hydropic villi and trophoblastic proliferation generally exceeds that observed in partial moles or hydropic abortions (Figs. 1 and 2).4,5 Cytologic atypia and frequent mitoses are often observed in the trophoblast component of complete moles. Serum hCG levels are usually higher in patients with complete moles than in partial moles. Many patients with complete moles have a clinical or ultrasonographic diagnosis of hydatidiform mole, but complete moles early in the first trimester and partial moles often have minimal ultrasonographic evidence of abnormal villi.
Fig. 1. Low-power photomicrograph of complete hydatidiform mole demonstrates markedly edematous avascular hydropic villi with sheets of trophoblastic cells at the periphery (arrows). Hematoxylin-eosin stain, ×40 magnification. Image courtesy of Dr. Siobhan M. O’Connor. Used with permission.
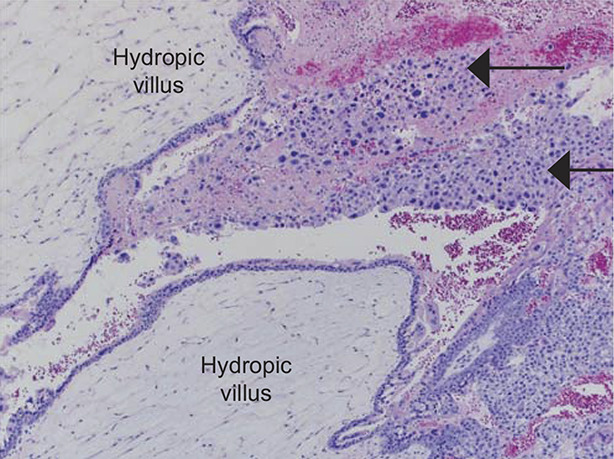
Soper. Gestational Trophoblastic Disease. Obstet Gynecol 2021.
Fig. 2. Low-power photomicrograph of partial hydatidiform mole: decidualized endometrium is in the upper field (*), with characteristic villi centrally (†). In contrast to complete moles, there is variable edema of the villi, with scalloping at the edges and trophoblastic inclusions (arrows) within villi. Trophoblastic proliferation is less pronounced and focal compared with complete moles. Image courtesy of Dr. Rex Bentley. Used with permission.
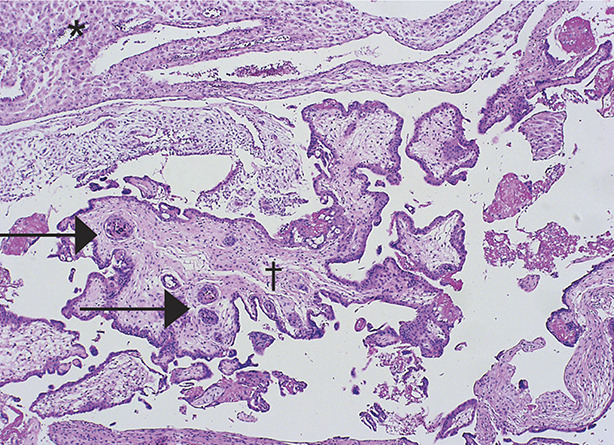
Soper. Gestational Trophoblastic Disease. Obstet Gynecol 2021.
Uterine enlargement beyond the expected gestational age, theca lutein cysts, and medical complications of molar pregnancy such as pregnancy-induced hypertension, hyperthyroidism, anemia, respiratory distress, and hyperemesis more frequently affect patients with complete moles diagnosed later in pregnancy.3 Postmolar gestational trophoblastic neoplasia is diagnosed more frequently after complete than partial moles (Table 1).3
Diagnosis of Hydatidiform Mole
Ultrasound examination has replaced all other noninvasive means of establishing the diagnosis. Molar tissue typically is identified as a mixed echogenic pattern replacing the placenta (Fig. 3), produced by villi and intrauterine blood clots, but these findings may be subtle or lacking in cases of early complete moles and partial moles. The combination of ultrasound findings with elevation of hCG above expected for gestational age is highly suggestive of molar pregnancy.
Fig. 3. Ultrasonogram of an unevacuated complete mole demonstrates intrauterine tissue with a mixed echogenic pattern.
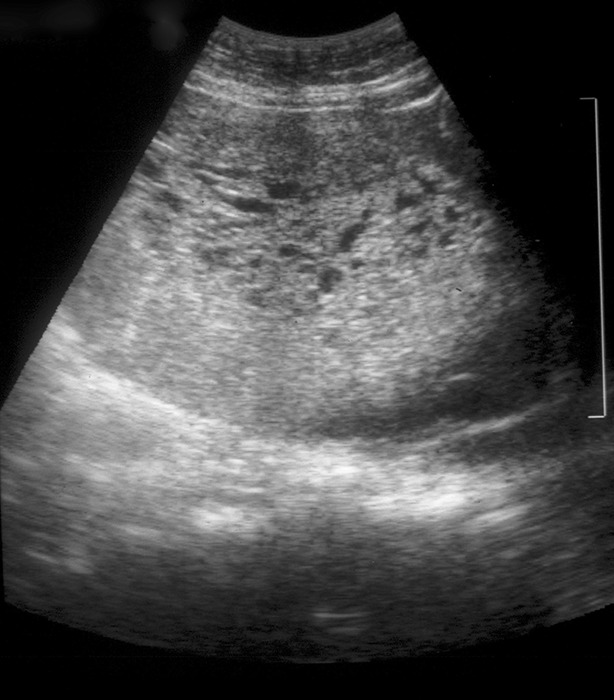
Soper. Gestational Trophoblastic Disease. Obstet Gynecol 2021.
With the increased availability of sensitive hCG assays and increased use of routine early ultrasound evaluations in pregnancy leading to more frequent diagnosis in early pregnancy, the classical features of hydatidiform moles are observed less often than previously.10–13 Medical complications occur in approximately 25% of patients with uterine enlargement greater than 14–16-weeks gestational size, but less frequently among patients with smaller uteri.3 Several studies have compared the clinical presentation of women with hydatidiform moles in different time intervals and have documented that patients in the more contemporary cohorts are evacuated more often in the first trimester and have significantly decreased medical complications, compared with the earlier cohorts.10–12 Sun and associates report that even the classical presentation of hydatidiform mole with symptoms of abnormal bleeding significantly decreased during the duration of their study from 84% in the early cohort of patients to 46% in the later cohort.12 Although the frequency of medical complications of moles was significantly decreased and first-trimester evacuation was more frequent in later patient populations, the incidence of postmolar gestational trophoblastic neoplasia appears to be unchanged.
Management of Hydatidiform Mole
Complete or partial mole will sometimes be diagnosed only by pathology after dilation and evacuation (D&E) is performed for a suspected incomplete abortion. In these cases, patients should be monitored afterward with serum quantitative hCG values. When suspected beforehand, the mole should be evacuated as soon as possible after a brief workup (Box 2) and stabilization of any medical complications.
Box 2.
Management of Clinically Diagnosed Hydatidiform Mole
-
I. Pre-evacuation evaluation
a. Serum quantitative β-hCG
b. Complete blood count, clotting studies (PT and PTT), renal and liver functions, blood type and screen
c. Pelvic ultrasound examination
d. Chest X-ray
e. Thyroid function tests if hyperthyroidism is suspected
-
II. Evacuation
-
a. Suction D&E
i. Serial dilation of cervix without sounding the uterus
ii. Begin Pitocin 20 units/L infusion after cervical dilation
iii. 12–14 mm suction cannula—initially allow the uterus to involute around the cannula
iv. Complete evacuation with suction curettage
v. Role of sharp curettage unclear
b. Hysterectomy should be considered in women older than age 40 y
-
-
III. Management after evacuation
a. Rhogam if Rh-negative
b. Serial SaO2 monitoring in patients with uterine enlargement greater than 14 weeks in size
c. Postevacuation hCG
hCG, human chorionic gonadotropin; PT, prothrombin time; PTT, partial thromboplastin time; D&E, dilation and evacuation; SaO2, oxygen saturation.
Suction D&E is the preferred method of evacuation for most patients who desire fertility preservation,3 usually performed under ultrasound guidance.
The procedure is usually performed under general anesthesia, but local or regional anesthesia may be used for a cooperative patient with a small uterus. After suction curettage has emptied the uterus, sharp curettage can be performed; however, the utility of sharp curettage has not been prospectively evaluated. This may increase the risk of uterine perforation and risk of uterine synechiae. It is our practice to begin intravenous oxytocin after the cervix is dilated. This is maintained for several hours after evacuation if there is significant uterine bleeding after D&E. Other oxytocics such as methergine or Cytotec can be used. Rhogam should be given to Rh-negative women after molar evacuation because the RhD factor is expressed by trophoblast.3
Pulmonary complications are observed around the time of molar evacuation in less than 1% of patients overall,12 but more than 20% among patients with uterine enlargement greater than 14–16 weeks in size.14 Baseline arterial blood gases and serial oxygen saturation determinations may be valuable in managing moles with this degree of uterine enlargement. Respiratory distress syndrome may be caused by trophoblastic embolization, high-output congestive heart failure caused by anemia, hyperthyroidism, preeclampsia, or iatrogenic fluid overload.14 In general, these complications should be treated aggressively with therapy directed by central hemodynamic monitoring and ventilator support.
Medical complications usually regress promptly after evacuation of the mole and may not require specific therapy. Theca lutein cysts are associated with hCG hyperstimulation of the ovaries. The resolution of theca lutein cysts lags behind the drop in hCG values but rarely require surgical intervention for rupture or torsion.15
Hysterectomy is an alternative to suction D&E for molar evacuation in selected patients who do not wish to preserve childbearing (Box 2), especially in women older than the age of 40 years, because these patients have a higher risk of postmolar gestational trophoblastic neoplasia.3,13,16 Usually the adnexa may be preserved, even if theca lutein cysts are present. Hysterectomy reduces the risk of malignant postmolar sequelae to approximately 3–5% compared with approximately 15–20% after evacuation by D&E.3,13,16 Because hysterectomy does not eliminate the possibility of postmolar gestational trophoblastic neoplasia these patients should also be monitored postoperatively with serial hCG levels.3,13,16
Surveillance After Molar Evacuation
Serial quantitative serum hCG determinations should be performed after molar evacuation using one of several commercially available assays capable of detecting β-hCG to baseline values (less than 5 milli-international units/mL). For monitoring patients with gestational trophoblastic disease, an hCG assay that can detect all forms of hCG is needed because these neoplasms often secrete abnormal forms of hCG. Free beta, nicked-free beta, C-terminal hCG, beta core and hyperglycosylated hCG should be detectable by the assay.3 Ideally, serum hCG levels should be obtained within 48 hours of molar evacuation and followed every 1–2 weeks while elevated.
The rationale for recommending an interval of monitoring after normalization of hCG value is to identify patients who develop postmolar malignant gestational trophoblastic neoplasia after achieving normal hCG values. Although rare instances of long latent periods preceding postmolar gestational trophoblastic neoplasia have been reported, almost all episodes of malignant sequelae occur within 6–12 months of evacuation.3 After normalization of hCG has been confirmed with a second hCG value, the risk of developing postmolar gestational trophoblastic neoplasia is extremely small.
In the largest study reported from the United Kingdom, Coyle and associates followed more than 20,000 women with hydatidiform moles after evacuation.17 For partial moles, the risk of postmolar gestational trophoblastic neoplasia was 1:3,195 after normalization of hCG; it was 1:406 for complete moles.17 The risk of postmolar gestational trophoblastic neoplasia dropped rapidly during the first 6 months of monitoring. Women with complete mole where hCG normalized more than 56 days after evacuation had a 3.8-fold increased higher risk of gestational trophoblastic neoplasia after normalization of hCG.17 They recommended that hCG monitoring could be stopped for partial moles after a confirmatory hCG, but recommended continuing to monitor complete moles for 6 months. The International Federation of Gynecology and Obstetrics (FIGO) also recommends confirming hCG normalization with a second value and discontinuing monitoring for partial moles, but to continue monthly monitoring for 6 months after complete mole.3 Others have suggested shortening the surveillance period for complete moles after confirming a normal hCG value with two hCG values.18 The National Comprehensive Cancer Network practice guidelines for gestational trophoblastic neoplasia recommends monitoring with two hCG values at 3-month intervals after three consecutive normal hCG values.19
Pregnancies that occur within 12 months after molar evacuation are usually normal gestations.20,21 However, an early pregnancy obscures the interpretation of hCG values during monitoring. For this reason, reliable contraception is recommended during hCG surveillance. Several studies, including a randomized study performed by the Gynecologic Oncology Group, established that moderate-low dose oral contraceptives do not increase the incidence of postmolar gestational trophoblastic neoplasia and significantly decrease intercurrent pregnancies during postmolar surveillance, compared with barrier contraception.22,23
After completion of surveillance documenting remission, pregnancy can be encouraged. Patients with prior partial or complete mole have a 1–2% incidence of a second mole in subsequent pregnancies.24,25 All future pregnancies should be evaluated by a first-trimester obstetric ultrasound examination. The risk of repetitive molar pregnancies increases substantially if a woman has had two or more prior moles. Women with consecutive molar pregnancies should undergo germline genetic testing for mutations in NLRP7 and KHDC3L, because these mutations are identified in more than half of such women. Assisted reproduction techniques with the use of donor eggs are recommended for future pregnancies if mutations are identified.6,7
Patients are at heightened risk for postmolar gestational trophoblastic neoplasia if they have any of the following: age older than 40 years, pre-evacuation hCG greater than 100,000 milli-international units/mL, excessive uterine enlargement, or theca lutein cysts greater than 6 cm.19 In a Cochrane meta-analysis of three randomized studies, prophylactic chemotherapy with brief methotrexate or dactinomycin regimens reduced the incidence of postmolar gestational trophoblastic neoplasia in women after molar evacuation compared with control patients who did not receive chemotherapy (relative risk [RR] 0.37, 95% CI 0.24–0.57, P<.001).26 There are anecdotal cases of fatalities caused by prophylactic chemotherapy. Because of low morbidity and mortality achieved by monitoring patients with serial hCG determinations and instituting chemotherapy only in patients with postmolar gestational trophoblastic neoplasia, prophylactic chemotherapy is usually not recommended unless a patient has high-risk features and barriers for reliable hCG surveillance.19,26
Coexistent Mole and Fetus
Coexistence of a fetus with molar change of the placenta is rare, occurring in 1 of 22,000–100,000 pregnancies.3,27–30 The majority of the relevant literature consists of individual case reports, small series, and limited collective reviews. Both complete and partial moles with a coexistent normal fetus have been reported. Most of these twin pregnancies are diagnosed antepartum by ultrasound findings of a complex, cystic placental component distinct from the fetoplacental unit, but in a few cases the diagnosis is not suspected until examination of the placenta after delivery. Medical complications such as early-onset, pregnancy-induced hypertension or bleeding requiring termination of pregnancies are frequent, yet many patients can deliver viable live births.
In a combined case series from Brazil and the United States, 60% of pregnancies managed expectantly resulted in viable live births.30 The overall risk of gestational trophoblastic neoplasia was 46%, increased in patients with higher hCG levels and among patients with termination of pregnancy for medical complications.30 Among 72 patients with coexistent mole twin pregnancies reported from Japan, 45.2% of 31 patients who required evacuation during the second trimester for medical indications subsequently developed postmolar gestational trophoblastic neoplasia, significantly more than the 20.8% and 17.6% who delivered in the first and third trimesters, respectively.28 Compared with singleton hydatidiform moles, twin pregnancies with fetus and mole have increased risk for postmolar gestational trophoblastic neoplasia, with a higher proportion of patients having metastatic disease or requiring multiagent chemotherapy.27–30 Major congenital abnormalities have not been reported in surviving neonates.
Postmolar Gestational Trophoblastic Neoplasia
Postmolar gestational trophoblastic neoplasia is a clinical diagnosis occurring after evacuation of hydatidiform mole when hCG levels have a sustained rise or plateau, indicating the need for evaluation and treatment. In 2000, FIGO standardized the hCG criteria used for the diagnosis of postmolar gestational trophoblastic neoplasia31 to include (Box 3).
Box 3.
Diagnostic Criteria for Gestational Trophoblastic Neoplasia
-
I. hCG criteria after molar evacuation
a. Sustained hCG level plateau ±10% of 4 values over a 3-wk duration
b. Sustained hCG level rise greater than 10% of 3 values over a 2-wk duration
c. Persistence of detectable hCG more than 6 mo after molar evacuation
II. Presence of metastatic disease
-
III. Histologic diagnosis of GTN
a. Invasive mole
b. Gestational choriocarcinoma
c. Placental site trophoblastic disease
d. Epithelioid trophoblastic disease
hCG, human chorionic gonadotropin; GTN, gestational trophoblastic neoplasia.
Data from Ngan HY, Bender H, Benedet JL, Jones H, Montruccoli GC, Pecorelli S; FIGO Committee on Gynecologic Oncology. Gestational trophoblastic neoplasia, FIGO 2000 staging and classification Int J Gynecol Obstet 2003;83:175-7.
The role of repeat D&E in the setting of an hCG rise or plateau is controversial. Some investigators have reported that repeat curettage induces remission or influences treatment in less than 20% of patients, with uterine perforation occurring in 4.8–8% of patients.32,33 In contrast, Pezeshki and associates report that 368 (68%) of 544 patients entered spontaneous remission after repeat D&E, with no patients requiring hysterectomy for perforation.34 Patients with persistent histologic evidence of gestational trophoblastic disease at second curettage and those with hCG levels greater than 1,500 international units/L were significantly less likely to respond to the second curettage.34 These retrospective series included patients who received curettage performed for a variety of indications, even when hCG values were falling after initial D&E.32–34
In a phase II trial conducted by the Gynecologic Oncology Group, 64 low-risk nonmetastatic gestational trophoblastic neoplasia patients underwent a second curettage as initial management of gestational trophoblastic neoplasia.35 In this study, 40% of the patients had a surgical cure and did not need additional treatment. They observed one uterine perforation and one grade 3 hemorrhage. Three patients were diagnosed with placental site trophoblastic tumors, which would have not been detected before treatment without the second D&E.35 Patients treated with a second curettage should be followed with weekly hCG values, similar to surveillance after initial D&E. In a randomized trial, Hemida and associates report that the amount of chemotherapy required to treat postmolar gestational trophoblastic neoplasia was not affected by a second D&E.36 Long-term effects from a second curettage, such as uterine synechiae and effects on future fertility, are unknown.
GESTATIONAL TROPHOBLASTIC NEOPLASIA
Apart from patients with placental site trophoblastic tumors and epithelioid trophoblastic tumors, the clinical presentation of the patient with gestational trophoblastic neoplasia is more important for determining treatment and outcome than the precise histologic diagnosis.
Histology of Gestational Trophoblastic Neoplasia
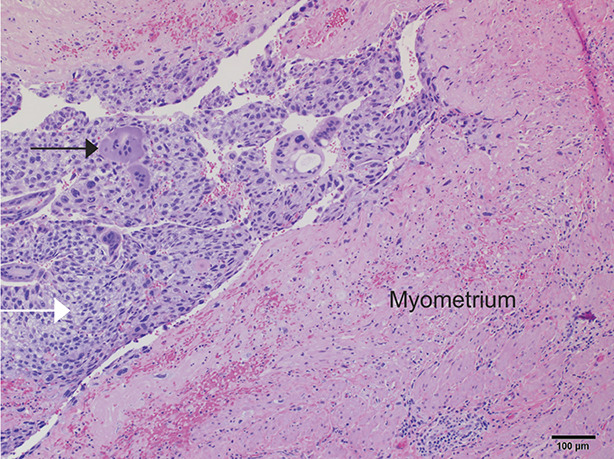
Postmolar gestational trophoblastic neoplasia refers to a clinical diagnosis after molar evacuation on the basis of hCG values, most often without histologic verification. Proliferative moles without myometrial invasion are treated on the basis of hCG values. These cases lack radiologic or histologic evidence of myometrial invasion. Invasive moles are diagnosed histologically by the identification of direct myometrial invasion by hydropic villi with trophoblastic proliferation by uterine curettings or hysterectomy (Fig. 4). They rarely metastasize and are usually self-limited, but are treated with chemotherapy to prevent morbidity and mortality caused by uterine perforation, hemorrhage, or infection.3,19
Fig. 4. In this gross photograph of an invasive complete mole treated with hysterectomy, the hydropic villi make up the majority of the mole (black arrow). A focus of myometrial invasion is present in the myometrium to the right of the large intrauterine tumor (blue arrow).
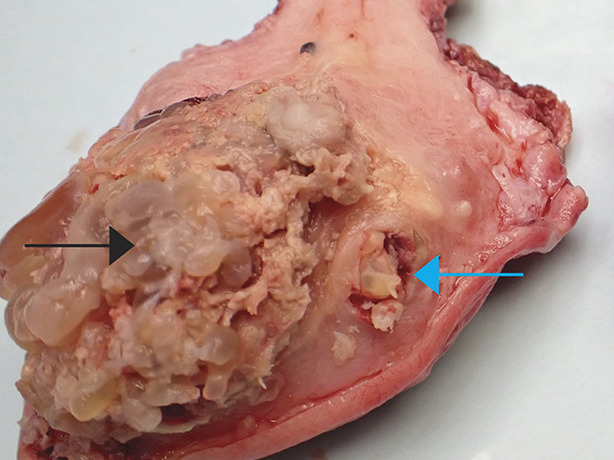
Soper. Gestational Trophoblastic Disease. Obstet Gynecol 2021.
Gestational choriocarcinoma is a pure epithelial malignancy, comprising neoplastic intermediate trophoblast, cytotrophoblast, and syncytiotrophoblast elements without chorionic villi (Fig. 5).3 Cytologic atypia is common and most cases have high mitotic counts. Aneuploidy is often identified.2 Central necrosis and hemorrhage are frequently observed in tumor nodules of choriocarcinoma. Patients with gestational choriocarcinomas tend to develop early systemic metastasis, and chemotherapy is almost always indicated when histologically diagnosed, even in the absence of metastases.3,19
Fig. 5. This high-power photomicrograph of gestational choriocarcinoma demonstrates the multinucleated syncytiotrophoblast (black arrow) and polygonal cytotrophoblast cell populations (white arrow). Hematoxylin-eosin stain, ×40 magnification. Image courtesy of Dr. Siobhan M. O’Connor. Used with permission.
Soper. Gestational Trophoblastic Disease. Obstet Gynecol 2021.
Placental site trophoblastic tumor is usually diagnosed by D&E or hysterectomy. Histologically, it is characterized by absence of villi, proliferation of intermediate trophoblast cells without syncytiotrophoblast cells (Appendix 1, available online at http://links.lww.com/AOG/C176), and low mitotic counts.2,3 Because syncytiotrophoblast cells are lacking, relatively lower levels of hCG are secreted by these tumors. Although the cells stain positively for human placental lactogen, serum human placental lactogen is not a reliable tumor marker. Immunohistochemical staining is also diffusely positive for Mel-CAM and cytokeratin. Serum free β-hCG subunit may be a marker for this disease.37
Epithelioid trophoblastic tumors are very rare and most often histologically diagnosed by curettage or cervical biopsy.3 They are also derived from intermediate trophoblastic cells. They often arise in the cervix or lower uterine segment, invading deeply into surrounding tissues. Microscopically this tumor comprises nests of relatively bland intermediate trophoblastic cells with frequent eosinophilic to clear cytoplasm (Appendix 2, available online at http://links.lww.com/AOG/C176) and strong expression of p63.2,3 A hyaline-like matrix (Appendix 2, http://links.lww.com/AOG/C176) and extensive necrosis are usually observed. Epithelioid trophoblastic tumors can be misdiagnosed as a squamous carcinoma.
In general, placental site trophoblastic tumors and epithelioid trophoblastic tumors can occur after any type of antecedent pregnancy. They are not as sensitive to chemotherapy as other forms of gestational trophoblastic neoplasia. The majority of patients who present with these lesions confined to the uterus are treated by hysterectomy.3,21
Diagnosis and Pretherapy Evaluation of Gestational Trophoblastic Neoplasia
Postmolar gestational trophoblastic neoplasia is most frequently diagnosed on the basis of hCG values as discussed above, without a histologic diagnosis. After nonmolar pregnancies, women with gestational trophoblastic neoplasia often present with subtle signs and symptoms, making the diagnosis difficult.38 However, abnormal uterine bleeding after any pregnancy should be evaluated with hCG testing to exclude the diagnosis. Metastases of gestational choriocarcinoma have been reported in virtually every body site and often produce abnormal bleeding from that site. Central nervous system lesions may produce subtle neurologic symptoms or dramatic symptoms of intracranial hemorrhage. Pulmonary lesions may present clinically with signs and symptoms of pulmonary embolism, hemoptysis, or as asymptomatic pulmonary nodules. Gestational choriocarcinoma should be considered in any premenopausal woman presenting with metastatic disease from an unknown primary site. A serum hCG determination and exclusion of normal pregnancy are all that are required to diagnose metastatic gestational trophoblastic neoplasia in these circumstances, which may spare the patient an unnecessary surgical procedure to establish a diagnosis.3,38
When gestational trophoblastic neoplasia is diagnosed, immediate evaluation for metastases is part of the anatomical staging system. Along with the history and physical examinations, the following evaluation should be performed: complete blood count, clotting function studies, renal and liver function studies, blood type and screen, and determination of pretherapy hCG level.3,19 It is emphasized that the hCG level used to assess risk scoring is the hCG level obtained at diagnosis of gestational trophoblastic neoplasia, not at initial evacuation of a hydatidiform mole.3,19,31 A pelvic examination should be performed to exclude vaginal or pelvic metastases; if a lesion is detected, it should not be biopsied owing to risk of hemorrhage.19 Radiographic evaluation should include chest computerized tomography (CT) scan,39 and contrasted abdominopelvic CT.3,19 Pelvic ultrasonography or a magnetic resonance imaging scan may be helpful if better visualization is needed of the uterine tumor or due to enlarged ovaries. Disseminated metastasis usually occurs only after pulmonary metastases are established.3,31,39,40 Brain magnetic resonance imaging or CT scan with contrast should be performed in any patient with pulmonary metastases or neurologic symptoms.19,41 In patients with atypical presentations, phantom hCG should be excluded (Appendix 3, available online at http://links.lww.com/AOG/C176).
Classification and Staging of Gestational Trophoblastic Neoplasia
Two systems are used to categorize patients with gestational trophoblastic neoplasia. Both systems correlate with clinical outcomes and identify patients at risk for failure of treatment.3,19,31,42–44
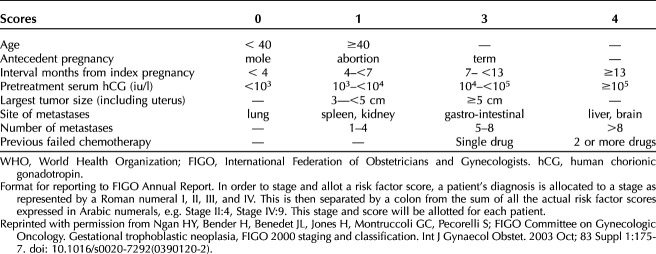
Currently, the 2000 FIGO staging system (Table 2)31 is the standard classification, but patients are also assigned a modified World Health Organization (WHO) prognostic index score (Table 3). In one retrospective study, the 2000 FIGO stage correlated better with outcome than the modified WHO prognostic index score used at the investigator’s institution.45 A WHO risk score of 6 or lower is classified as low-risk, and scores higher than 6 are classified as high-risk.31
Table 2.
FIGO 2000 Staging and Classification of GTN
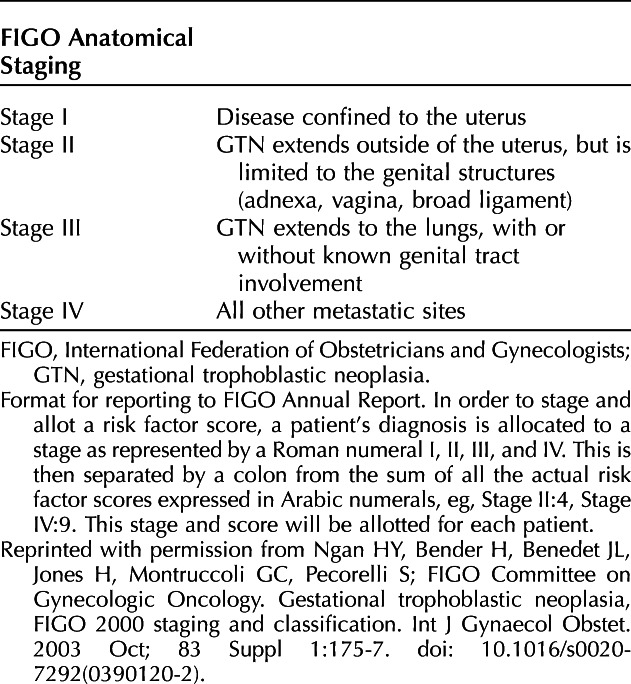
Table 3.
Modified WHO Risk Scoring System as Adapted by FIGO
The primary treatment for most forms of gestational trophoblastic neoplasia is chemotherapy, based on the individual patient’s risk. Because of the relative rarity of this diagnosis, very few randomized trials of therapy have been completed. Instead, the majority of studies are retrospective analyses of single-institution experiences, yet these confirm high activity for a variety of agents in the treatment of gestational trophoblastic neoplasia.3,19
Management of Low-Risk Gestational Trophoblastic Neoplasia
Primary remission rates of patients treated for low-risk gestational trophoblastic neoplasia are high using a variety of chemotherapy agents.3,19,46 Essentially all patients with low-risk gestational trophoblastic neoplasia can be cured, usually without the need for hysterectomy if fertility preservation is desired.
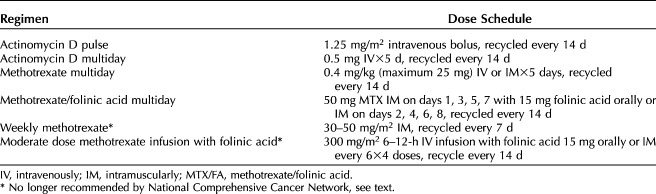
The most frequently used single-agent regimens with methotrexate and actinomycin D are listed in Table 4.46 The Gynecologic Oncology Group trial comparing weekly intramuscular methotrexate 30 mg/m2 to bolus intravenous actinomycin D 1.25 mg/m2 is the largest randomized study completed in patients with gestational trophoblastic neoplasia.47 Among 216 women treated in this trial, bolus actinomycin D demonstrated a superior primary remission rate compared with weekly methotrexate (70% vs 53%, P=.01).47 Primary remission rates with actinomycin D regimens range from 69% to 94%.45 Similar primary remission rates have been reported for multiday methotrexate regimens, ranging from 87 to 94% for 5 days methotrexate and 74–93% for the 8-day methotrexate-folinic acid regimens.45 The most recent Cochrane Review of randomized trials of treatment regimens for low-risk gestational trophoblastic neoplasia concluded that actinomycin D regimens were more likely to produce primary remission with a higher risk of serious side effects.46 The current National Comprehensive Cancer Network guidelines recommend initial multiday methotrexate or methotrexate-folinic acid regimens, with actinomycin D regimens for patients with contraindications for methotrexate therapy.19 Weekly methotrexate is not recommended by National Comprehensive Cancer Network guidelines based on the GOG trial.19,47 Moderate high-dose methotrexate infusion is not recommended because it had an inferior sustained remission rate of 62% when retrospectively compared with the 84% sustained remission rate for patients treated with 8-day methotrexate–folinic acid regimen (P<.001).19,48
Table 4.
Actinomycin D and Methotrexate Regimens for Low-Risk Gestational Trophoblastic Neoplasia
Serum hCG levels are monitored during chemotherapy and when hCG values normalize, additional cycles of consolidation therapy are usually administered. Surwit and Hammond49 report that treating low-risk patients with at least one cycle of chemotherapy after the first normal hCG value reduced the relapse rate to 3%. Multiple studies of patients with low-risk disease who received between one and three cycles of consolidation therapy report relapse rates ranging from 0% to 8.4%, with the majority having relapse rates less than 5%.3,19,47–50 The FIGO and National Comprehensive Cancer Network recommend two to three cycles of consolidation chemotherapy after hCG normalization.4,21
Early hysterectomy will shorten the duration and amount of chemotherapy required to produce remission in low-risk disease.51–53 Therefore, a patient’s desire for fertility should be evaluated at the onset of treatment. But postoperative chemotherapy will still be needed as well as hCG monitoring similar to patients managed exclusively with chemotherapy.
If hCG values have not fallen by at least 10% over three cycles of therapy or have risen more than 10% over two cycles, or if significant toxicity has developed, alternative therapy should be used.3,19 With an hCG rise, the patient should be re-evaluated with examination and imaging for evidence of new metastases and a new WHO risk score assigned. Treatment should be changed to an alternative single-agent regimen unless there is evolution to a high-risk score. In patients previously treated with multiday methotrexate regimens, 5-day actinomycin D is preferred over the pulsed regimen and will produce response rates of greater than 70%.54–56 If there is failure of alternative single-agent chemotherapy, the patient should be treated with multiagent chemotherapy. Hysterectomy should be considered for refractory disease that is confined to the uterus.51 Patients with nonmetastatic disease, FIGO risk scores lower than 4, and patients who do not have choriocarcinoma are less likely to require second-line therapy than patients with metastatic disease, FIGO scores of 5 or 6, or histologic diagnosis of choriocarcinoma. Overall, 85–95% of patients in these categories can be cured without multiagent chemotherapy or hysterectomy. The overall cure rate for patients with low-risk disease approaches 100%3,19,45–48,50–56 with recurrence rates less than 5%.
Management of High-Risk Gestational Trophoblastic Neoplasia
Patients with a FIGO risk score of 7 or higher should initially be treated with aggressive multiagent chemotherapy; surgery or radiation or both are often incorporated into treatment. Survival reported by trophoblastic disease centers exceeds 86%.3 In contrast to patients with low-risk disease, early hysterectomy does not appear to improve the outcome in women with high-risk metastatic disease.51 It is recommended that all patients with high-risk gestational trophoblastic neoplasia be referred to specialists with experience in the management of this disease.3,21
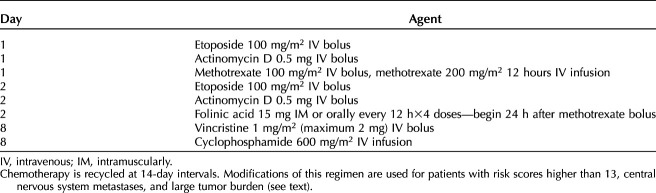
Multiagent regimens incorporate etoposide, with or without cisplatin, into cyclical combination chemotherapy with high rates of success and lower acute toxicity. Unfortunately, the cumulative dose of etoposide has been associated with increased risk for leukemia. Currently, EMA–CO (etoposide, methotrexate, and dactinomycin alternating with cyclophosphamide, and vincristine) is used most frequently (Table 5).3,19,57,58 Randomized studies, however, have not established the superiority of any single regimen.
Table 5.
Fourteen-Day Alternating Etoposide-Methotrexate-Actinomycin D/Cyclophosphamide-Vincristine Chemotherapy for High-Risk Gestational Trophoblastic Neoplasia
High-risk metastatic sites include brain, liver, and kidney lesions. Many patients with high-risk metastatic sites of disease will require coordinated multimodality therapy for optimal treatment. Even with intense chemotherapy, additional surgery may be necessary in high-risk patients to control hemorrhage from metastases, remove chemoresistant disease, or treat complications to stabilize high-risk patients during therapy.3,19
Patients with FIGO risk scores of 13 or higher have a higher risk of mortality when treated initially with intense multiagent therapy, especially if their increased risk score is due to large tumor burden or metastases to the brain or liver.3,19,58,59 Patients with these characteristics may develop an initial massive tumor response, with tumor lysis syndrome, catastrophic hemorrhage from metastatic sites, multiple organ failure, myelosuppression, sepsis, and are at risk for death early in the course of treatment. Relatively low-dose induction chemotherapy with a 2-day regimen of cisplatin 20 mg/m2 and etoposide 100 mg/m2 repeated weekly for one to three cycles appeared to reduce the risk of early deaths, in the experience of investigators in the United Kingdom and others.58,59
Management of High-Risk Metastatic Sites of Disease
Management of cerebral metastases is controversial. Radiation therapy has been used concurrently with chemotherapy in an attempt to limit acute hemorrhagic complications from brain metastases. Whole brain irradiation combined with systemic chemotherapy results in cure rates up to 75% among patients who initially present with brain metastases.60 However, a similar primary remission rate has also been reported among patients treated with modification of the EMA–CO regimen that incorporated high-dose systemic methotrexate infusion (1 g/m2) combined with intrathecal methotrexate infusions.61 This approach relies on early neurosurgical intervention for complications rather than brain irradiation.61 Others report similar outcomes using intense multiagent chemotherapy without intrathecal methotrexate or radiation.62
The best treatment for liver or other high-risk sites of metastases has not been established; these patients are most often managed with highly individualized multimodality therapy, incorporating chemotherapy, with potential surgical resection of isolated metastases, embolization of liver metastases, or localized radiation therapy.3,19,63
Resistant High-Risk Gestational Trophoblastic Neoplasia
Treatment of high-risk gestational trophoblastic neoplasia that is resistant to initial chemotherapy is challenging. Sites of persistence, exposure to prior chemotherapeutic agents and tolerance of prior therapy must be taken into consideration.3,19 Patients with relatively low-level hCG plateaus during EMA–CO can often be salvaged with a regimen substituting etoposide-cisplatin (EMA/EP) for the CO.3,19,64 Other frequently used salvage regimens include alternating paclitaxel-etoposide with cisplatin-etoposide, multiday etoposide-cisplatin regimens, or high-dose chemotherapy with stem cell support.3,19,64
Recently, programmed death ligand 1 has been identified in almost all gestational trophoblastic disease lesions and there are reports of patients with drug resistant gestational trophoblastic neoplasia salvaged with check-point inhibition using immunotherapy.65–67 Agents such as pembrolizumab and avelumab neutralize programmed death ligand 1, enhancing immune native immunologic response to malignancies. In the initial report, three of four patients with multidrug resistance had sustained remissions after treatment with pembrolizumab.65 A subsequent case report documented a patient with complete response to 6 months treatment with pembrolizumab, resulting in a relatively short remission of only 6 months before hCG levels started rising after completion of treatment.66 More recently, a phase II trial of avelumab in low-risk gestational trophoblastic neoplasia who had failed prior methotrexate or actinomycin D was reported by You and associates.67 Eight (53.3%) entered sustained remission and one patient subsequently had a normal pregnancy.67 The role of immunotherapy for treatment of gestational trophoblastic neoplasia remains to be established. Current trials are investigating anti–programmed death ligand 1 therapy as single-agent therapy or combined with chemotherapy for gestational trophoblastic neoplasia.
Chemotherapy is continued until hCG values have normalized, and this is followed by at least two to three courses of consolidation chemotherapy for the purpose of eradicating all viable tumor.3,19 Despite using sensitive hCG assays and maintenance chemotherapy, up to 12.5% of patients with high-risk disease will recur after achieving an initial remission.68–70 Risk factors for recurrence in high-risk patients include large-volume disseminated disease and inappropriate initial therapy.
Surveillance After Treatment for Gestational Trophoblastic Neoplasia
After hCG remission has been achieved, patients with gestational trophoblastic neoplasia should be managed with serial determinations of hCG levels at 2-week intervals during the first 3 months of remission and then at monthly intervals for at least 12 months. The risk of recurrence after 1 year of remission is less than 1% and is higher for patients with high-risk gestational trophoblastic neoplasia.68–70 Sustained remissions can be achieved in more than 50% of patients treated for an episode of recurrent gestational trophoblastic neoplasia.68,69 Therefore, high-risk patients should receive hCG monitoring at 6- to 12-month intervals beyond the first year of remission.
Contraception, preferably with oral contraceptives, should be used during chemotherapy and the first year of remission after treatment of gestational trophoblastic neoplasia. Because of the 1–2% risk for a second mole in subsequent pregnancy, early ultrasound examination is recommended during all future pregnancies in addition to histologic evaluation of the placenta, and postdelivery hCG but there are no studies detailing the utility of these measures. There does not appear to be an increase in the risk of congenital malformations or other complications related to pregnancy.71–73
Savage et al74 conducted a survey in the United Kingdom comparing the incidence of secondary malignancies and early menopause after successful treatment of gestational trophoblastic neoplasia to rates observed in the normal population. They report no increased risk for patients treated with methotrexate/folinic acid or EMA–CO, but an increased risk for leukemia in patients treated with prolonged exposure to etoposide and alkylating agents. Oral and pharyngeal cancers, melanoma, and meningioma had an apparent increased risk, but the study was limited in small numbers of malignancies and relatively short-term follow up of patients treated with EMA–CO.74 Patients treated with single-agent chemotherapy had minimal effect on early or premature menopause, whereas patients treated with EMA–CO had a 13% risk of menopause at age 40 years and 36% by age 45 years. The risk of early menopause in this group was increased in women treated after age 30 years.74
PLACENTAL SITE TROPHOBLASTIC TUMORS AND EPITHELIOID TROPHOBLASTIC TUMORS
These neoplasms are comprised of neoplastic intermediate trophoblastic cells (Appendices 1 and 2, http://links.lww.com/AOG/C176). They are similar in behavior and treatment.3,19,75–77 The majority of patients with placental site trophoblastic tumors and epithelioid trophoblastic tumors will be diagnosed at the time of D&E for uterine bleeding and disease is limited to the uterus or cervix. Because these lesions are not responsive to methotrexate and actinomycin D, surgery is a main component of therapy.3,19,75–77 Radiologic staging similar to other forms of gestational trophoblastic neoplasia should be performed before treatment. Rare patients with localized lesions have been cured with uterine curettage or myometrial resection and chemotherapy, but most have diffuse uterine disease and hysterectomy is usually incorporated into treatment.3,19,75–77 The most important factors influencing survival are FIGO stage and, in some series, prolonged interval since the antecedent pregnancy. Chemotherapy with EMA/EP, paclitaxel-cisplatin/etoposide-cisplatin combination, or multiday etoposide-platin regimens are usually used to treat high-risk localized disease or advanced stage lesions.3,19,75–77
SUMMARY
Most women with all forms of gestational trophoblastic disease can be successfully diagnosed and treated with preservation of their reproductive function. It is important to manage molar pregnancies properly to minimize acute complications and identify postmolar gestational trophoblastic neoplasia promptly. It is important to individualize treatment for women with all forms of gestational trophoblastic neoplasia based on known risk factors, using less-toxic therapy for patients with low-risk disease and aggressive multiagent therapy for those with high-risk disease. Patients with high-risk gestational trophoblastic neoplasia should be managed at centers or with consultants experienced in the complex multimodality treatment of these patients.
CME FOR THE CLINICAL EXPERT SERIES
Learning Objectives for “Gestational Trophoblastic Disease: Current Evaluation and Management”
After completing this learning experience, the involved learner should be able to:
List the current guidelines from the International Federation of Gynecology and Obstetrics (FIGO) for making the diagnosis
Outline the steps in management for patients with gestational trophoblastic disease
Discuss the treatment and surveillance of patients with hydatidiform mole
Institute a plan of care for those who develop trophoblastic neoplasia
Instructions for Obtaining AMA PRA Category 1 Credits™
Continuing Medical Education credit is provided through joint providership with the American College of Obstetricians and Gynecologists.
Obstetrics & Gynecology includes CME-certified content that is designed to meet the educational needs of its readers. This article is certified for 2 AMA PRA Category 1 Credits.™ This activity is available for credit through February 29, 2024.
Accreditation Statement
ACCME Accreditation
The American College of Obstetricians and Gynecologists is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
AMA PRA Category 1 Credit(s)™
The American College of Obstetricians and Gynecologists designates this journal-based CME activity for a maximum of 2 AMA PRA Category 1 Credits.™ Physicians should claim only the credit commensurate with the extent of their participation in the activity.
College Cognate Credit(s)
The American College of Obstetricians and Gynecologists designates this journal-based CME activity for a maximum of 2 Category 1 College Cognate Credits. The College has a reciprocity agreement with the AMA that allows AMA PRA Category 1 Credits™ to be equivalent to College Cognate Credits.
Disclosure of Faculty and Planning Committee Industry Relationships
In accordance with the College policy, all faculty and planning committee members have signed a conflict of interest statement in which they have disclosed any financial interests or other relationships with industry relative to article topics. Such disclosures allow the participant to evaluate better the objectivity of the information presented in the articles.
How to Earn CME Credit
To earn CME credit, you must read the article in Obstetrics & Gynecology and complete the quiz, answering at least 70 percent of the questions correctly. For more information on this CME educational offering, visit the Lippincott CMEConnection portal at https://cme.lww.com/browse/sources/196 to register and to complete the CME activity online. ACOG Fellows will receive 50% off by using coupon code, ONG50.
Hardware/software requirements are a desktop or laptop computer (Mac or PC) and an Internet browser. This activity is available for credit through February 29, 2024. To receive proper credits for this activity, each participant will need to make sure that the information on their profile for the CME platform (where this activity is located) is updated with 1) their date of birth (month and day only) and 2) their ACOG ID. In addition, participants should select that they are board-certified in obstetrics and gynecology.
The privacy policies for the Obstetrics & Gynecology website and the Lippincott CMEConnection portal are available at http://www.greenjournal.org and https://cme.lww.com/browse/sources/196, respectively.
Contact Information
Questions related to transcripts may be directed to educationcme@acog.org. For other queries, please contact the Obstetrics & Gynecology Editorial Office, 202-314-2317 or obgyn@greenjournal.org. For queries related to the CME test online, please contact ceconnection@wolterskluwer.com or 1-800-787-8985.
Footnotes
Financial Disclosure The author did not report any potential conflicts of interest.
The author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C177.
Figure.
No available caption
REFERENCES
- 1.Smith HO. Gestational trophoblastic disease epidemiology and trends. Clin Obstet Gynecol 2003;46:541–56. Doi: 10.1097/00003081-2000309000-00006 [DOI] [PubMed] [Google Scholar]
- 2.Altieri A, Franceschi S, Ferlay J, La Vecchia C. Epidemiology and aetiology of gestational trophoblastic diseases. Lancet Oncol 2003;4:670–78. Doi: 10.1016/s1470-2045(03)01245-2 [DOI] [PubMed] [Google Scholar]
- 3.Ngan HYS, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, et al. FIGO cancer report 2018. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynecol Obstet 2018;143:79–85. Doi: 10.1002/ijgo.12615 [DOI] [PubMed] [Google Scholar]
- 4.Szulman AE, Surti U. The syndrome of hydatidiform mole. I. Cytogenetics and morphologic correlation. Am J Obstet Gynecol 1978;131:665–71. Doi: 10.1016/0002-9378(78)90829-3 [DOI] [PubMed] [Google Scholar]
- 5.Szulman AE, Surti U. The syndrome of hydatidiform mole. II. Morphologic evaluation of the complete and partial mole. Am J Obstet Gynecol 1978;132:20–7. doi: 10.1016/0002-9378(78)90792-5 [DOI] [PubMed] [Google Scholar]
- 6.Fisher RA, Khatoon R, Paradinas FJ, Roberts AP, Newlands ES. Repetitive complete hydatidiform mole can be biparental in origin and either male or female. Hum Reprod 2000;15:594–8. doi: 10.1093/humanrep/15.3.594 [DOI] [PubMed] [Google Scholar]
- 7.Andreasen L, Christiansen OB, Niemann I, Bolund L, Sunde L. NLRP7 or KhDC3L genes and the etiology of molar pregnancies and recurrent miscarriage. Mol Hum Reprod 2013;19:773–81. doi: 10.1093/molehr/gat056 [DOI] [PubMed] [Google Scholar]
- 8.Kalogiannidis I, Kalinderi K, Kalindris M, Miliaras D, Tariatzis B, Athanasiadis A. Recurrent complete hydatidiform mole: where are we, is there a safe gestational horizon? Opinion and mini-review. J Assist Reprod Genet 2018;35:967–73. doi: 10.1007/s10815-018-1202-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mondal SK, Mandal S, Bhattacharya S, Panda UK, Ray A, Alsi SM. Expression of P57 immunomarker in the classification and differential diagnosis of partial and complete hydatidiform moles. J Lab Physicians 2019;11:270–74. doi: 10.4103/JLP.JLP_130_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soto-Wright V, Bernstein M, Goldstein DP, Berkowitz RS. The changing clinical presentation of complete molar pregnancy. Obstet Gynecol 1995;86:775–9. doi: 10.1016/0029-7844(95)00268-V [DOI] [PubMed] [Google Scholar]
- 11.Coukos G, Makrigiannakis A, Chung J, Randall TC, Rubin SC, Benjamin I. Complete hydatidiform mole: a disease with a changing profile. J Reprod Med 1999;44:698–704. [PubMed] [Google Scholar]
- 12.Sun SY, Melamed A, Goldstein DP, Bernstien MR, Horowitz NS, Moron AF, et al. Changing presentation of complete hydatidiform mole at the New England Trophoblastic Disease Center over the past three decades: does early diagnosis alter risk for gestational trophoblastic neoplasia? Gynecol Oncol 2015;138:46–9. doi: 10.1016/j.ygyno.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 13.Curry SL, Hammond CB, Tyrey L, Creasman WT, Parker RT. Hydatidiform mole: diagnosis, management and long-term follow-up of 347 patients. Obstet Gynecol 1975;45:1–8. [PubMed] [Google Scholar]
- 14.Twiggs LB, Morrow CP, Schlaerth JB. Acute pulmonary complications of molar pregnancy. Am J Obstet Gynecol 1979;135:189–94. doi: 10.1016/002-9378(79)90341-7 [DOI] [PubMed] [Google Scholar]
- 15.Montz FJ, Schlaerth JB, Morrow CP. The natural history of theca lutein cysts. Obstet Gynecol 1988; 72;247–51. [PubMed] [Google Scholar]
- 16.Zhao P, Lu Y, Tong B, Lu VV. Total hysterectomy versus uterine evacuation for preventing post-molar gestational trophoblastic neoplasia in patients who are at least 40 years old: a systematic review and meta-analysis. BMC Cancer 2019;19:13. doi: 10.1186/s12885-018-5168-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coyle C, Short D, Jackson L, Sebire NJ, Kaur B, Harvey R, et al. What is the optimal duration of human chorionic gonadotropin surveillance following evacuation of a molar pregnancy? A retrospective analysis on over 20,000 consecutive patients. Gynecol Oncol 2018;148:254–57. doi: 10.1016/j.ygyno.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 18.Albright BB, Shorter JM, Mastroyannis SA, Ko EM, Schreiber CA, Sonalkar S. Gestational trophoblastic neoplasia after human chorionic gonadotropin normalization following molar pregnancy. A systematic review and meta-analysis. Obstet Gynecol 2020;135:12–23. doi: 10.1097/AOG.000000000003566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Rustum NR, Yashar CM, Bean S, Bradley K, Campos SM, Chon HS, et al. Gestational trophoblastic neoplasia, version 2.2019. J Natl Compr Canc Netw 2019;17:1374–91. doi: 10.6004/jnccn.2019.0053 [DOI] [PubMed] [Google Scholar]
- 20.Kohorn EI. How soon is it safe to undertake pregnancy after trophoblastic tumor?. Gynecol Oncol 1999;94:343–44. doi: 10.1006/gyno.1999.5468 [DOI] [PubMed] [Google Scholar]
- 21.Tuncer ZS, Berstein MR, Goldstein DP, Lu KH, Berkowitz RS. Outcome of pregnancies occurring within 1 year of hydatidiform mole. Obstet Gynecol 1999;94:588–90. doi: 10.1016/s0029-7844(99)00395-6 [DOI] [PubMed] [Google Scholar]
- 22.Curry SL, Schlaerth JB, Kohorn EI, Boyce JB, Gore H, Twiggs LB, et al. Hormonal contraception and trophoblastic sequelae after hydatidiform mole. Am J Obstet Gynecol 1989;160:805–11. doi: 10.1016/0002-9378(89)90295-0 [DOI] [PubMed] [Google Scholar]
- 23.Costa HLFF, Doyle P. Influence of oral contraceptives in the development of post-molar trophoblastic neoplasia – a systematic review. Gynecol Oncol 2006;100:579–85. doi: 10.1016/j.ygyno.2005.09.031 [DOI] [PubMed] [Google Scholar]
- 24.Sebire NJ, Fisher RA, Foskett M, Rees H, Seckl MJ, Newlands ES. Risk of recurrent hydatidiform mole and subsequent pregnancy outcome following complete or partial hydatidiform molar pregnancy. BJOB 2003;110:22–6. [PubMed] [Google Scholar]
- 25.Vargas R, Barroilhet LM, Esselen K, Diver E, Bernstein M, Goldstein DP, et al. Subsequent pregnancy outcomes after complete and partial molar pregnancy, recurrent molar pregnancy and gestational trophoblastic neoplasia: an update from the New England Trophoblastic Disease Center. J Reprod Med 2014;59:188–94. [PubMed] [Google Scholar]
- 26.Wang Q, Fu J, Fang F, Xie L, Chen H, He F, et al. Prophylactic chemotherapy for hydatidiform mole to prevent gestational trophoblastic neoplasia. The Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No.:CD007289. doi: 10.1002/14651858.CD007289.pub3 [DOI] [PMC free article] [PubMed]
- 27.Fishman DA, Padilla LA, Keh P, Cohen D, Frederiksen M, Lurain JR. Management of twin pregnancies consisting of a complete hydatidiform mole and normal fetus. Obstet Gynecol 1998;91:546–50. doi: 10.1016/s0029-7844(97)00720-5 [DOI] [PubMed] [Google Scholar]
- 28.Matsui H, Sekiya S, Hando T, Wake N, Tomoda Y. Hydatidiform mole coexistent with a twin fetus: a national collaborative study in Japan. Hum Reprod 2000;15:608–11. doi: 10.1093/humrep/15.3.608 [DOI] [PubMed] [Google Scholar]
- 29.Sebire NJ, Foskett M, Paradinas FJ, Fisher RA, Francis RJ, Short D, et al. Outcome of twin pregnancies with complete hydatidiform mole and healthy co-twin. Lancet 2002;359:2165–66. doi:10.1016/S0140-6736(02)09085-2 [DOI] [PubMed] [Google Scholar]
- 30.Lin LH, Maestrá I, Braga A, Sun SY, Fushida K, Francisco RPV, et al. Multiple pregnancies with complete mole and coexisting normal fetus in North and South America: a retrospective multicenter cohort and literature review. Gynecol Oncol 2017;145:88–95. doi: 10.1016/j.ygyno.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 31.Ngan HY Bender H , Benedet JL Jones H Montruccoli GC Pecorelli S. FIGO Committee on Gynecologic Oncology. Gestational trophoblastic neoplasia, FIGO 2000 staging and classification Int J Gynecol Obstet 2003;83:175–7. [DOI] [PubMed] [Google Scholar]
- 32.Schlaerth JB, Morrow CP, Rodriguez M. Diagnostic and therapeutic curettage in gestational trophoblastic disease. Am J Obstet Gynecol 1990;162:1465–71. [DOI] [PubMed] [Google Scholar]
- 33.Van Trommel NE, Massuger LF, Verheijen RH, Sweep FC, Thomas CM. The curative effect of a second curettage in persistent trophoblastic disease: a retrospective cohort survey. Gynecol Oncol 2005;99:6–13. doi: 10.1016/0002-9378(90)90907-o [DOI] [PubMed] [Google Scholar]
- 34.Pezeshki M, Hancock BW, Silcocks P, Everard JE, Coleman J, Gillespi AM, et al. The role of repeat uterine evacuation in the management of persistent gestational trophoblastic disease. Gynecol Oncol 2004;95:423–9. doi: 10.1016/j.ygyno.2004.08.045 [DOI] [PubMed] [Google Scholar]
- 35.Osborne RJ, Filliaci VL, Schink JC, Mannel RS, Behbekht K, Hoffman JS, et al. Second curettage for low-risk gestational trophoblastic neoplasia. Obstet Gynecol 2016;128:535–42. doi: 10.1097/AOG.0000000000001554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hemida R, Vos EL, Arafa M, Toson E, Burger CW, et al. Second curettage and number of chemotherapy courses in postmolar gestational trophoblastic neoplasia: a randomized controlled trial. Obstet Gynecol 2019;133:1024–31. doi: 10.1097/AOG.0000000000003232 [DOI] [PubMed] [Google Scholar]
- 37.Cole LA, Khanlian SA, Muller CY, Giddings A, Kohorn E, Berkowitz R. Gestational trophoblastic diseases: 3. Human chorionic gonadotropin-free B-subunit, a reliable marker of placental site trophoblastic tumors. Gynecol Oncol 2006;102:160–4. doi: 10.1016/j.ygymo.2005.12.046 [DOI] [PubMed] [Google Scholar]
- 38.Tidy JA, Rustin GJ, Newlands ES, Foskett M, Fuller S, Short D, et al. Presentation and management of choriocarcinoma after nonmolar pregnancy. Br J Obstet Gynaecol 1995;102:715–19. doi: 10.1111/j.1471-0528.1995.tb11429.x [DOI] [PubMed] [Google Scholar]
- 39.Hunter V, Raymond E, Christensen C, Olt G, Soper J, Hammond C. Efficacy of the metastatic screening in the staging of gestational trophoblastic disease. Cancer 1990;65:1647–50. doi: 10.1002/1097-0142(19900401)65:7<1647::aid-cncr282065072>3.0.co;2-9 [DOI] [PubMed] [Google Scholar]
- 40.Soper JT, Clarke-Pearson DL, Hammond CB. Metastatic gestational trophoblastic disease: prognostic factors in previously untreated patients. Obstet Gynecol 1988:71:338–43. [PubMed] [Google Scholar]
- 41.Mutch DG, Soper JT, Baker ME, Bandy LC, Cox EB, Clarke-Pearson DL, et al. Role of computed axial tomography of the chest in staging patients with non-metastatic gestational trophoblastic disease. Obstet Gynecol 1986;68:348–52. doi: 10.1097/00006250-198609000-00011 [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization Scientific Group. Gestational trophoblastic disease. WHO Tech Rep Ser 1983;692:1–80. [PubMed] [Google Scholar]
- 43.Soper JT, Evans AC, Conaway MR, Clarke-Pearson DL, Berchuck A, Hammond CB, et al. Evaluation of prognostic factors and staging in gestational trophoblastic tumor. Obstet Gynecol 1994;84:969–73. [PubMed] [Google Scholar]
- 44.Hancock BW, Welch EM, Gillespie AM, Newlands ES. A retrospective comparison of current and proposed staging and scoring systems for persistent gestational trophoblastic disease. Int J Gynecol Cancer 2000;10:318–22. doi: 10.1046/j.1525-1438.2000.010004318.x [DOI] [PubMed] [Google Scholar]
- 45.Goldstein DP, Berkowitz RS, Horowitz NS. Optimal management of low-risk gestational trophoblastic neoplasia. Expert Rev Anti Cancer Ther 2015;15:1293–304. [DOI] [PubMed] [Google Scholar]
- 46.Lawrie TA, Alazzam M, Tidy J, Hancock BW, Osborne R. First-line chemotherapy in low-risk gestational trophoblastic neoplasia. The Cochrane Database of Systematic Reviews 2016, Issue 6. Art. No.:CD007102. doi: 10.1002/14651858.CD007102.pub4 [DOI] [PMC free article] [PubMed]
- 47.Osborne RJ, Filiaci V, Schink JC, Mannel RS, Alvarez Secord A, Kelley JL, et al. Phase III trial of weekly methotrexate or pulsed dactinomycin for low-risk gestational trophoblastic neoplasia: a gynecologic oncology group study. J Clin Oncol 2011;29:825–31. doi: 10.1200/JCO/2010.30.4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maestá I, Nitecki R, Horowitz NS, Goldstein DP, de Freitas Segalla Moreira M. Effectiveness and toxicity of first-line methotrexate chemotherapy in low-risk postmolar gestational trophoblastic neoplasia: the New England Trophoblastic Disease Center experience. Gynecol Oncol 2018;148:161–7. doi: 10.1016/j.ygyno.2017.10.028 [DOI] [PubMed] [Google Scholar]
- 49.Surwit EA, Hammond CB. Recurrent gestational trophoblastic disease. Gynecol Oncol 1981;12:177–85. doi: 10.1016/0090-8258(81)90147-5 [DOI] [PubMed] [Google Scholar]
- 50.Lybol C, Sweep FCGJ, Harvey R, Mitchell H, Short D, Thomas CMG, et al. Relapse rates after two versus three consolidation courses of methotrexate in the treatment of low-risk gestational trophoblastic neoplasia. Gynecol Oncol 2012;125:576–9. doi 10.1016/j.ygyno2012.03.003 [DOI] [PubMed] [Google Scholar]
- 51.Hammond CB, Weed JC, Jr, Currie JL. The role of operation in the current therapy of gestational trophoblastic disease. Am J Obstet Gynecol 1980;136:844–58. doi: 10.1016/0002-9378(80)91041-8 [DOI] [PubMed] [Google Scholar]
- 52.Suzuka K, Matsui H, Iitsuka Y, Yamazawa K, Seki K, Sekiya S. Adjuvant hysterectomy in low-risk gestational trophoblastic disease. Obstet Gynecol 2001;97:431–34. doi 10.1016/s0029-7844(00)01169-8 [DOI] [PubMed] [Google Scholar]
- 53.Bolze PA, Mathe M, Hajri T, You B, Dabi Y, Schott AM, et al. First-line hysterectomy for women with low-risk non-metastatic gestational trophoblastic neoplasia no longer wishing to conceive. Gynecol Oncol 2018;150:202–87. doi: 10.1016/j/ygyno.2018.05.030 [DOI] [PubMed] [Google Scholar]
- 54.Lurain JR, Chapman-Davis E, Hoekstra AV, Schink JC. Actinomycin D for methotrexate-failed low-risk gestational trophoblastic neoplasia. J Reprod Med 2012;56:283–7. [PubMed] [Google Scholar]
- 55.Winter MC, Tidy JA, Hills A, Ireson J, Gillett S, Singh K, et al. Risk adapted single-agent dactinomycin or carboplatin for second-line treatment of methotrexate resistant low-risk gestational trophoblastic neoplasia. Gynecol Oncol 2016;143:565–70. doi: 10.1016/j.ygyno.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 56.Prouvot C, Golfier F, Massardier J, You B, Lotz JP, Patrier S, et al. Efficacy and safety of second-line 5-day dactinomycin in case of methotrexate failure for gestational trophoblastic neoplasia. Int J Gynecol Cancer 2018;28:1038–44doi. 10.1097/IGC.0000000000001248 [DOI] [PubMed] [Google Scholar]
- 57.Bower M, Newlands ES, Holden J, Short D, Brock C, Rustin GJ, et al. EMA/CO for high-risk gestational trophoblastic tumors: results from a cohort of 272 patients. J Clin Oncol 1997;15:2636–43. doi: 10.1200/JCO.1997.15.7.2636 [DOI] [PubMed] [Google Scholar]
- 58.Alifragis C, Agarwal R, Short D, Fisher RA, Sebire NJ, Harvey R, et al. EMA/CO for high-risk gestational trophoblastic neoplasia: god outcomes with induction low-dose etoposide-cisplatin and genetic analysis. J Clin Oncol 2013;31:280–86. doi: 10.1200/JCO.2921.43.1817 [DOI] [PubMed] [Google Scholar]
- 59.Bolze PA, Riedl C, Massardier J, Lotz JP, You B, Schott AM, et al. Mortality rate of gestational trophoblastic neoplasia with a FIGO score of > 13. Am J Obstet Gynecol 2016;214:390–99. doi: 10.1016/j.ajog.2015.09.083 [DOI] [PubMed] [Google Scholar]
- 60.Evans AC, Soper JT, Clarke-Pearson DL, Berchuck A, Rodriguez GC, Hammond CB, et al. Gestational trophoblastic disease metastatic to the central nervous system. Gynecol Oncol 1995;59:226–30. doi: 10.1006/gyno.1995.0013 [DOI] [PubMed] [Google Scholar]
- 61.Savage P, Kelpanides I, Tuthill M, Seckl M. Brain metastases in gestational trophoblast neoplasia: an update on incidence, management and outcome. Gynecol Oncol 2015;137:73–6. doi: 10.1016/j.ygyno.2015.01.530 [DOI] [PubMed] [Google Scholar]
- 62.Gavanier D, Leport H, Massardier J, Abbas F, Schott AM, Golfier F, et al. Gestational trophoblastic neoplasia with brain metastases at initial presentation: a retrospective study. Int J Clin Oncol 2019;24:153–60. doi: 10.1007/s10147-018-1337-9 [DOI] [PubMed] [Google Scholar]
- 63.Soper JT. Role of surgery and radiation therapy in the management of gestational trophoblastic disease. Best Prac Res Clin Obstet Gynaecol 2003;17:943–57. [DOI] [PubMed] [Google Scholar]
- 64.Alazzam M, Tidy J, Osborne R, Coleman R, Hancock BW, Lawrie TA. Chemotherapy for resistant or recurrent gestational trophoblastic neoplasia. The Cochrane Database of Systematic Reviews 2016, Issue 1. Art. No.:CD00891. doi: 10.1002/14651858.CD008891 [DOI] [PMC free article] [PubMed]
- 65.Ghorani E, Kaur B, Fisher RA, Short D, Joneborg U, Carlson JW, et al. Pembrolizumab is effective for drug-resistant gestational trophoblastic neoplasia. Lancet 2017;390:2343–45. doi: 10.1016/S0140-6736(17)32894-5 [DOI] [PubMed] [Google Scholar]
- 66.Goldfarb JA, Dinoi G, Mariani A, Langstraat CL. A case of multi-agent drug resistant choriocarcinoma treated with pembrolizumab. Gynecol Oncol Rep 2020;32:100574. doi: 10.1016/j.gore.2020.100574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.You B, Bolze PA, Lotz JP, Massardier J, Gladief K. Avelumab in patients with gestational trophoblastic tumors with resistance to single-agent chemotherapy: cohort A of the TROPHIMMUN phase II trial. J Clin Oncol 2020;38:3129–37. doi: 10.1200/JCO.20.00803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mutch DG, Soper JT, Babcock CJ, Clarke-Pearson DL, Hammond CB. Recurrent gestational trophoblastic disease: experience of the southeastern regional trophoblastic disease center. Cancer 1990;66:978–82. doi: 10.1002/1097-0142(19900901)66:5<978::aid-cncr2820660529>3.0.co;2-3 [DOI] [PubMed] [Google Scholar]
- 69.Ngan HYS, Tam K, Lam KW, Chan KKL: Relapsed gestational trophoblastic neoplasia: a 20 year experience. J Reprod Med 2006;51:829–34. [PubMed] [Google Scholar]
- 70.Balachandran K, Salawu A, Ghorani E, Kaur B, Sebire NJ, Short D, et al. When to stop human chorionic gonadotropin (hCG) surveillance after treatment with chemotherapy for gestational trophoblastic neoplasia (GTN): a national analysis on over 4,000 patients. Gynecol Oncol 2019;155:8–12. doi: 10.106/j.ygyno.2019.07.024 [DOI] [PubMed] [Google Scholar]
- 71.Loret de Mola JR, Goldfarb JM. Reproductive performance of patients after gestational trophoblastic disease. Semin Oncol 1995;22:193–97. [PubMed] [Google Scholar]
- 72.Kim JH, Park DC, Bae SN, Namkoong SE, Kim SJ. Subsequent reproductive experience after treatment for gestational trophoblastic disease. Gynecol Oncol 1998;71:108–12. doi: 10.1006/gyno.1998.5167 [DOI] [PubMed] [Google Scholar]
- 73.Woolas RP, Brower M, Newlands ES, Seckl M, Short D, Holden I. Influence of chemotherapy for gestational trophoblastic disease on subsequent pregnancy outcome. Br J Obstet Gynaecol 1998;103:1032–35. doi: 10.1111/j.1471-0528.1998.tb10271.x [DOI] [PubMed] [Google Scholar]
- 74.Savage P, Cooke R, O’Nions J, Krell J, Kwan A, Camarta M, et al. Effects of single-agent and combination chemotherapy for gestational trophoblastic tumors on risks of second malignancy and early menopause. J Clin Oncol 2015;33:472–28. doi: 10.1200/JCO.2014.57.5332 [DOI] [PubMed] [Google Scholar]
- 75.Zhao J, Lv WG, Feng FZ, Wan XR, Liu JH, Yi XF, et al. Placental site trophoblastic tumor: a review of 108 cases and their implications for prognosis and treatment. Gynecol Oncol 2016;142:102–8. doi: 10.1016/j.ygyno.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 76.Moutte A, Doret M, Hajri T, Peyron N, Chateau F, Massardier J, et al. Placental site and epithelioid trophoblastic tumours: diagnostic pitfalls. Gynecol Oncol 2013;128:568–72. doi: 10.1016/j.ygyno.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 77.Frijstein MM, Lok CAR, van Trommel NE, ten Kate-Booij MJ, Massuger LFAG, van Werkhoven E, et al. Management and prognostic factors of epithelioid trophoblastic tumors: results from the International Society for the Study of Trophoblastic Diseases database. Gynecol Oncol 2019;152:361–67. doi: 10.1016/j.ygyno.2018.11.015 [DOI] [PubMed] [Google Scholar]