Abstract
Background
This is the second updated version of the Cochrane Review published in Issue 3, 2010 and first updated in Issue 5, 2013.
People with a primary brain tumour often experience depression, for which drug treatment may be prescribed. However, they are also at high risk of epileptic seizures, cognitive impairment, and fatigue, all of which are potential adverse side effects of antidepressants. The benefit, or harm, of pharmacological treatment of depression in people with a primary brain tumour is unclear.
Objectives
To assess the benefits and harms of pharmacological treatment of depression in people with a primary brain tumour.
Search methods
We updated the search to include CENTRAL, MEDLINE, Embase, and PsycINFO to September 2019. As in the original review, we also handsearched Neuro‐Oncology, Journal of Neuro‐Oncology, Journal of Neurology, Neurosurgery and Psychiatry, and Journal of Clinical Oncology: for the current update we handsearched the latest three years of articles from these journals (up to November 2019).
Selection criteria
We searched for all randomised controlled trials (RCTs), controlled clinical trials, cohort studies, and case‐control studies of any pharmacological treatment of depression in people with a histologically diagnosed primary brain tumour.
Data collection and analysis
No studies met the inclusion criteria.
Main results
We found no eligible studies evaluating the benefits of any pharmacological treatment of depression in people with a primary brain tumour.
Authors' conclusions
We identified no high‐quality studies that investigated the value of pharmacological treatment of depression in people with a primary brain tumour. RCTs and detailed prospective studies are required to inform the effective pharmacological treatment of this common and important complication of brain tumours. Since the last version of this review none of the related new literature has provided additional information to change these conclusions.
Plain language summary
Pharmacological treatment of depression in people with a primary brain tumour
The issue
People with brain tumours may experience epilepsy, memory problems, and fatigue. Depression is also common, and doctors might choose to treat this with antidepressants, since antidepressants are thought to be effective in other patients. However, antidepressants could be less effective, or could cause more side effects, in people with a primary brain tumour.
The aim of the review
We researched whether any drugs have been proven to be effective, and whether they cause significant side effects when prescribed to treat depression in people with primary brain tumours.
Main findings
We searched the medical journal literature to find high‐quality studies comparing the effectiveness of any one drug treatment for depression in people with a brain tumour against another treatment. Despite a thorough search, we could not find any studies and so cannot determine whether any drug is of benefit.
What are the conclusions?
We conclude that it is important to research whether drugs can treat depression safely and effectively in people with primary brain tumours.
Quality of evidence
No studies were eligible for this review.
Background
This is the second updated version of the Cochrane Review published in Issue 3, 2010 and first updated in Issue 5, 2013.
The reported prevalence of depression in cancer varies widely, depending on the study population, diagnostic method, and time at which people are assessed. In people with primary brain tumours, the cross‐sectional prevalence of depression as determined by clinician‐rated methods is approximately 20% (Huang 2017). However, depression may pass undiagnosed (Baltenberger 2014), and, even when identified, may be inadequately treated (Litofsky 2009; Sharpe 2004).
Description of the condition
Primary brain tumours
The global incidence of primary brain tumours is estimated to be 10.8 per 100,000 person‐years (de Robles 2015), and increasing (Patel 2019). As a group, primary brain tumours differ from metastatic tumours, which spread to the brain from cancer elsewhere in the body. Primary brain tumours can be categorised according to World Health Organization (WHO) criteria reflecting their cell of origin, degree of malignancy, and molecular parameters (Louis 2016). The most common type of malignant primary brain tumour is glioma (Lapointe 2018), and most gliomas (70% to 80%) are 'high‐grade' aggressive tumours. With current treatment, approximately 10% of people with the most aggressive form of glioma (glioblastoma multiforme, GBM) may survive five years after diagnosis (Stupp 2009). A minority of gliomas are slowly growing 'low‐grade' tumours associated with a median survival of approximately seven years (Claus 2015). Low‐grade gliomas are more frequently associated with epileptic seizures than high‐grade tumours (Pallud 2019). Meningiomas are another common type of primary brain tumour, which occurs more frequently in women and the elderly and may be cured by surgery (Wiemels 2010).
Depression
Major depressive disorder (depression) is a clinical syndrome of sadness or loss of interest or/enjoyment, or both; negative beliefs (e.g. feelings of guilt, hopelessness, or low self‐esteem); psychomotor abnormalities (e.g. fatigue and psychomotor retardation); and biological manifestations (e.g. sleep and appetite disturbances) (Gelder 2012). Because sadness, negative thoughts (e.g. about loss of health or death) and physical symptoms can be normal in people with cancer, the gold‐standard method of diagnosing depression is a structured clinical interview conducted by a specialist in mental health (APA 2016). Significant depressive symptoms can also be identified in people with cancer using rating scales (e.g. Zigmond 1983), which use cut‐off scores to estimate the likelihood of depression and, being easier to administer, are often used as a 'proxy' diagnosis in clinical research.
Description of the intervention
Antidepressants are recommended as a first‐line treatment for moderate to severe depression in national guidelines (APA 2016; NICE 2009). Three prominent pharmacological classes of antidepressant are: selective serotonin re‐uptake inhibitors (SSRI), tricyclic antidepressants (TCA), and monoamine oxidase inhibitors (MAOI). A fourth class of newer, 'second‐generation' compounds is chemically diverse. Other drug classes, for example psychostimulants, Weitzner 1999, or acetylcholinesterase inhibitors, Shaw 2006, have also been proposed for use in people with primary brain tumours. Although the precise mode of action is unknown (Moncrieff 2004), meta‐analyses of randomised controlled trials (RCTs) enrolling people without brain tumours report antidepressants to be effective in the depressed elderly, Mottram 2006, and in those with chronic low‐level depression, Lima 2005, or severe or psychotic depression, Wijkstra 2005.
How the intervention might work
Antidepressants may reduce depressive symptoms in people with other neurological illnesses (Hackett 2008). In the physically ill, however, a limited response to treatment and high relapse rates may often be observed (Iosifescu 2004). Non‐pharmacological treatments for depression (e.g. psychotherapy) are not covered in this review.
Why it is important to do this review
Clinical depression can affect up to 20% of people with a primary brain tumour in the first eight months after diagnosis (Rooney 2011), and can have important consequences for the affected person and their family. In glioma, depression has been associated with physical functional impairment (Rooney 2011), cognitive impairment (Sayyah 2016), reduced quality of life (Mainio 2006a), higher mortality (Gathinji 2009; Litofsky 2004; Mainio 2006b), a greater frequency of medical complications (Litofsky 2004), and reduced work productivity (Feuerstein 2007). People with glioma may have the highest risk of all people with cancer of psychiatric hospitalisation in the year following diagnosis (Dalton 2009). Depression is a strong risk factor for suicide, Rihmer 2007, and epilepsy, Hesdorffer 2000, in people without a brain tumour. If depression in people with a primary brain tumour was effectively treated, personal or family suffering could potentially be reduced. However, it is possible that the side effects of antidepressants outweigh any benefit. Antidepressants might lower seizure threshold (Hill 2015), impair memory (Sayyah 2016), or cause fatigue (Cassano 2004). People with primary brain tumours are already at high risk of these complications. Despite clinical uncertainty, the effectiveness and potential adverse impact of pharmacotherapy for depression in primary brain tumours has not been systematically reviewed.
Objectives
To assess the benefits and harms of pharmacological treatment of depression in people with a primary brain tumour.
Methods
Criteria for considering studies for this review
Types of studies
For evidence of efficacy we aimed to include:
RCTs.
For evidence of adverse effects we also aimed to include:
controlled clinical trials;
cohort studies;
case‐control studies.
We aimed to find all published and unpublished studies, in any language.
Types of participants
Inclusion criteria
Adults (aged 18 years and over)
A diagnosis of depression at baseline (diagnosed by any validated method and satisfying the original trial authors that pharmacological treatment of depression was reasonable and necessary)
A comorbid histological diagnosis of any primary brain tumour
Exclusion criteria
Studies recruiting only people with metastatic (i.e. non‐primary) brain tumours were excluded.
Types of interventions
Any drug prescribed to treat depression compared, where relevant, with a control group receiving any other treatment. We anticipated that the most common drug class would be an antidepressant, but any category of prescription drug was eligible. Studies administering only unlicensed herbal remedies (e.g. St John's wort) were excluded.
Types of outcome measures
Primary outcomes
The primary outcome was change in depression at final follow‐up. We anticipated that this could be measured in several different ways:
the mean change in depression scale score (measured by a validated scale, e.g. Center for Epidemiological Studies‐Depression Scale (CES‐D; Radloff 1977); Hospital Anxiety and Depression Scale (HADS; Zigmond 1983); Beck Depression Inventory‐II (BDI‐II; Beck 1996);
the proportion of participants meeting predefined criteria for improvement in scale score;
changes in the proportion of participants with a categorical diagnosis of depression ('present' or 'absent').
Where both categorical (present or absent) and continuous (scale score) depression data were presented, we aimed to analyse both.
Secondary outcomes
Health‐related quality of life (measured by a validated scale, e.g. using the European Organisation for Research and Treatment of Cancer QLQ‐C30 (EORTC QLQ‐C30; Aaronson 1993); Functional Assessment of Cancer Therapy (FACT; Weitzner 1995); 36‐item Short Form Health Survey (SF‐36; McHorney 1993).
General emotional distress (measured by a validated scale, e.g. Hospital Anxiety and Depression Scale (HADS; Zigmond 1983).
Length of time from diagnosis of a primary brain tumour to death.
Although antidepressants can have many side effects, we identified those we considered most likely to occur in people with a brain tumour:
seizure frequency (measured by participant or carer report);
cognitive dysfunction (e.g. poor memory, measured by validated neuropsychological testing);
fatigue (measured by a validated rating scale, e.g. Brief Fatigue Inventory (Mendoza 1999), Functional Assessment of Cancer Therapy ‐ Fatigue (Yellen 1997), Multidimensional Fatigue Symptom Inventory (Stein 2004)).
Search methods for identification of studies
We ran searches for the original review in July 2009. We ran the subsequent (update) search in October 2012, and the latest search in September 2019.
Electronic searches
For this review update, we searched the following databases up to 30 September 2019:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 9) in the Cochrane Library;
MEDLINE via Ovid (October 2012, week 3 to September 2019, week 3);
Embase via Ovid (2012, week 42 to 2019, week 39);
PsycINFO (October 2012 to September 2019).
We had previously searched the British Nursing Index, LILACS (Latin American and Caribbean Health Science Information database), PSYNDEX, the NHS National Research Register, the NHS Centre for Reviews and Dissemination's Database of Abstracts of Reviews of Effects (DARE), and Web of Knowledge (covering Science SciSearch, Social Sciences Citation Index, and Biological Abstracts) (to July 2009).
In the original review we constructed individual search strategies for each database (see Appendix 1 to Appendix 2). In the update, we amended search strategies where relevant to remove duplicate terms (Appendix 3 to Appendix 4). Because we aimed to include studies other than RCTs, we conducted topic searches only.
Searching other resources
For the original review we contacted:
pharmaceutical companies manufacturing all antidepressants listed in the British National Formulary (BNF);
International Network of Agencies for HTA (INAHTA);
British Library Document Supply Centre (BLDSC).
This yielded no relevant information and was not repeated for the update.
We handsearched the following journals (up to November 2019) for articles and conference abstracts:
Journal of Neurology, Neurosurgery and Psychiatry;
Journal of Neuro‐Oncology;
Journal of Clinical Oncology;
Neuro‐Oncology.
We aimed to handsearch:
reference lists of eligible studies;
book chapters identified in the reference lists of eligible studies.
Data collection and analysis
Selection of studies
Two review authors (ZB and SH) initially filtered studies based on the title and abstract. We then obtained full copies of potentially relevant studies, which two review authors (ZB and SH) independently assessed for eligibility, supervised by an experienced research fellow (FB).
Data extraction and management
Two review authors (ZB and SH) were to independently extract data from eligible studies using a predefined data extraction form. For the categorical outcome of depression, we planned to extract the number of depressed participants in each arm at final follow‐up in order to estimate risk ratio (RR). For continuous outcomes, we planned to extract the mean and standard deviation (SD) of the outcome of interest in each arm at final follow‐up. We planned to estimate from the means and SDs whether the data were skewed; if they were, we would write to the study authors requesting the mean and SDs of log transformed data. For time‐to‐death data, we planned to extract the hazard ratio (HR) and its variance from trial reports; if these were not presented, we would attempt to either estimate them or extract relevant data from Kaplan‐Meier survival curves. Failing this, we would estimate RR as outlined above. One review author (ZB or SH) would enter data into Review Manager 5, and the other review author would check the data entry (Review Manager 2014).
Assessment of risk of bias in included studies
We intended to assess and report on the methodological risk of bias of included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), which recommends the explicit reporting of RCTs, using the 'Risk of bias' 1 tool, including all of the domains in this tool: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and other sources of bias. We would categorise risk of bias as high, low, or unclear, and present this information in a ‘Risk of bias’ table. We would discuss the risk of bias in the included studies, resolving any persisting disagreements between the authors by consulting a third review author.
Measures of treatment effect
When summarising the categorical outcome of depression, we planned to calculate risk ratios (RR) with 95% confidence intervals (CI). When comparing continuous outcome data from studies using different rating scales, we would use the standardised mean difference (SMD); we would calculate the mean difference (MD) for studies using the same scale. Both would be presented with 95% CI for individual outcomes in individual studies. For time‐to‐death data, we planned to pool HRs using the generic inverse variance facility of Review Manager 5 (Review Manager 2014). We planned to analyse secondary outcomes as continuous variables only (MDs or SMDs). Where possible, participants were to be analysed in the groups to which they had been randomised, irrespective of the treatment they actually received.
Unit of analysis issues
We would take different levels of randomisation (e.g. at the level of participants or groups) into account. When there were long‐term follow‐up assessments available within trials, we would analyse outcomes for two different follow‐up categories: short term (i.e. 0 to 6 months); or medium to long term (i.e. 6 months and more). If we identified studies with multiple intervention groups, we would make pair‐wise comparisons between all possible pairs of intervention groups. We would ensure that double‐counting of participants in the analysis did not occur.
Dealing with missing data
We would calculate missing measures of uncertainty (e.g. CIs and SDs) where possible or contact the study authors to request the data. We did not intend to impute missing data.
We aimed to record the number of participants in each intervention arm whose outcomes were not reported at the end of the study, noting if loss to follow‐up was not reported. Controlled non‐randomised studies were to be similarly assessed, with analysis of whether:
the treatment and comparison groups were comparable at baseline;
the analysis was adjusted for potential confounding factors.
If as a result of this assessment we considered it likely that the study was biased, we would not include it in meta‐analysis.
Assessment of heterogeneity
We aimed to calculate statistical heterogeneity using the I2 statistic, considering an I2 value of > 50% as evidence of substantial heterogeneity, and visually inspect forest plots for heterogeneity. As we expected a certain degree of heterogeneity, we planned to use a random‐effects model for meta‐analysis.
Assessment of reporting biases
If at least 10 studies were included, we would draw funnel plots of treatment effect versus precision with the data from all studies (Higgins 2011). We would visually inspect the funnel plots to assess whether there had been selective reporting of outcomes.
Data synthesis
If outcomes differed amongst included studies, we would pool them if measuring the same construct, or report on each outcome systematically for those not measuring the same construct.
We would perform a meta‐analysis if we included two or more RCTs with a low risk of bias in which the study population, intervention, and outcome measures were comparable. We would present this in a ‘Summary of findings’ table, and would include the primary and secondary outcomes listed above. For each outcome, we would report the number of participants, the overall quality of the evidence according to the GRADE approach, and the effect size. If a meta‐analysis was not possible, we would synthesise the findings of the included studies in a table, employing the GRADE levels of evidence (Higgins 2011). We would report the individual effect sizes of the studies and 95% CI. We would use Review Manager 5 for analyses (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
If we included a sufficient number of studies, that is at least two for each subgroup, we would perform subgroup analysis by antidepressant class, tumour histology, and WHO grade of tumour.
Sensitivity analysis
We aimed to conduct sensitivity analyses by excluding studies that did not report adequate: (i) concealment of allocation or (ii) blinding of the outcome assessor.
Results
Description of studies
Results of the search
A PRISMA diagram displaying records identified and screened is shown in Figure 1.
1.
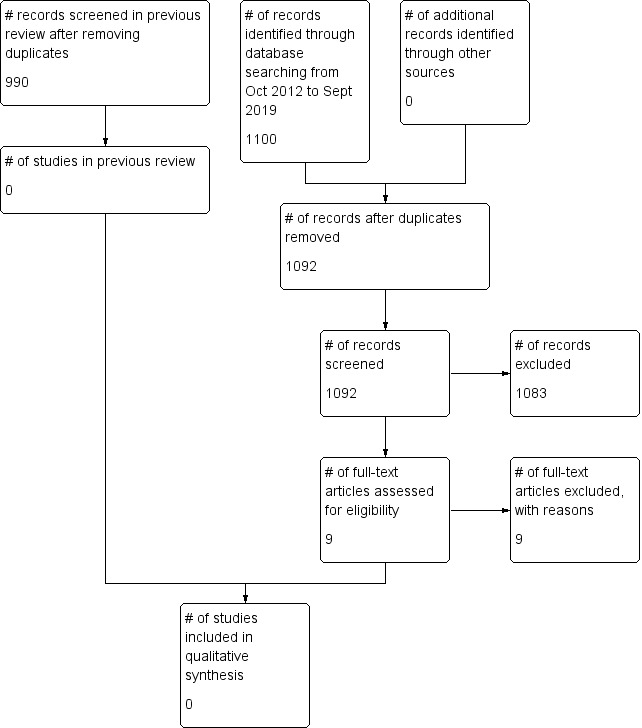
PRISMA study flow diagram.
Included studies
We found no eligible studies for any of our primary or secondary outcomes.
Excluded studies
Studies that were of interest to the research question but did not meet the inclusion criteria of the review are as follows.
Knudsen‐Baas 2018 conducted a cohort study reporting the prevalence and associations of psychotropic medication, including antidepressants, in a Danish population‐based study. The authors did not test the efficacy of any antidepressant interventions, or measure specific side effects.
Boele 2013 tested a psychostimulant (modafinil) in 37 participants with primary brain tumours. In this RCT, the researchers screened for fatigue, which was the primary outcome. Depression was included as a secondary outcome, but participation was not limited to depressed people.
Blackhall 2009 conducted a similar study looking at modafinil to treat cancer‐related fatigue in multiple cancers, and also looked at depression along with neurocognitive function as a secondary outcome. The authors reported some improvement in anxiety and depression, but participation in the study was not limited to depressed people.
Wirz 2010 also conducted an intervention study testing modafinil for reducing fatigue in people with cancer who were undergoing both treatment and pain relief, but did not select people with depression. No people with primary brain tumours were included.
Gehring 2012 compared two psychostimulants (methylphenidate and modafinil) in 24 people with primary brain tumours in an RCT. Depressive symptoms were assessed as a secondary outcome only, and participation was not limited to depressed people.
Maschio 2013 conducted an RCT to determine the effect of pregabalin on seizure control in people with brain tumour. However, depression was a secondary outcome only, and participants did not have to suffer from depression to be included.
Walker 2010 looked at the benefit of using tricyclic antidepressants for mortality reduction in people with glioma or colorectal cancer. They did not investigate management of depression, but found that tricyclic antidepressants did not improve mortality.
Attia 2012 conducted a phase II trial to investigate whether the botanical product Ginkgo biloba had any effect on improving cognitive functioning in irradiated people with brain tumours. Mood (depression) was included as a secondary outcome only, and participants were not selected based on an existing diagnosis of depression.
Gothelf 2005 conducted a pilot study to assess safety and tolerability of a selective serotonin re‐uptake inhibitor (SSRI) antidepressant (fluvoxamine) in children with cancer and comorbid depression or anxiety disorders (Gothelf 2005). The treatment was well tolerated and appeared to reduce depressive symptoms. Participants did not include adults, and only one participant was diagnosed with a brain tumour.
Wellisch 2006 conducted an RCT of a psychostimulant (methylphenidate) in people with brain tumour, with depression as a secondary outcome. However, it was not clear from the published conference abstract whether participation was limited to people with depression, and we were unable to contact the authors for clarification.
Morrow 2003 reported an RCT of an antidepressant (paroxetine) in a mixed population of people with cancer, including those with brain tumours. Although depression was measured as an outcome, the intervention was given primarily to treat fatigue, and participation was not limited to people with depression. Data for the small number of participants with brain tumours were not reported separately.
Moss 2006 conducted an open‐label phase II trial of an antidepressant (bupropion) on fatigue in a mixed population of people with cancer, including those with brain tumour, and measured levels of depression. However, participation was restricted to people with high levels of fatigue, not depression.
Shaw 2006 enrolled 35 participants with brain tumours to an open‐label phase II trial of an acetylcholinesterase inhibitor (donepezil) and measured depression using a rating scale. However, participation was not limited to people with depression.
Meyers 1998 evaluated the effects of a psychostimulant (methylphenidate) in an open‐label phase II trial of 30 participants with high‐grade glioma, measuring depressive symptom severity amongst other outcomes. However, the authors explicitly excluded individuals with major depression at study baseline.
Litofsky 2004 conducted a prospective cohort study of adults with high‐grade glioma, examining the relationship between depression and survival in glioma. However, the authors did not systematically prescribe treatment for depression and did not limit participation to people with depression.
Koval'chuk 2007 conducted a prospective cohort study of 225 participants following operation for brain tumour. The authors measured depression amongst other outcomes, and assessed the influence of different antidepressants on the postoperative normalisation of mood. However, again, participation was not limited to people with depression.
Caudill 2011 conducted a retrospective cohort study of the frequency and toxicity of SSRI prescription in 160 people with glioblastoma presenting to a tertiary neuro‐oncology service over a 10‐year period. The authors reported that being prescribed SSRIs was associated with improved survival at two years postdiagnosis, after controlling for relevant confounders. Participants being prescribed an SSRI reported similar levels of toxicities as those on no SSRI. However, antidepressant prescription was uncontrolled, and the study was not limited to people with depression.
Other studies that were ineligible on methodological grounds were a small uncontrolled case series, Rabey 1985, and a single case study, Oyewumi 1981.
Ongoing studies
We identified one ongoing study. The PASSION trial is testing the effect of ketamine on perioperative depressive symptoms in people undergoing brain tumour resection. Only adults with moderate to severe depressive symptoms will be recruited. The trial was scheduled to finish in February 2019, but to date no results have been published (Zhou 2018). We contacted the authors but have not received a response.
Risk of bias in included studies
No eligible studies have been identified.
Effects of interventions
No eligible studies have been identified.
Discussion
Summary of main results
We found no eligible studies of the pharmacological treatment of depression in adults with primary brain tumours. Several studies have examined related aspects of drug treatment of symptoms in people with a primary brain tumour. However, all trials to date recruited both depressed and non‐depressed participants, and all cohort studies reported usual clinical care rather than systematically evaluating a specific treatment. Consequently, the results do not easily generalise to the question that is the focus of this review, that is treating depressed people with primary brain tumours. The lack of evidence is an important finding because depression is a common complication of brain tumours (Huang 2017; Litofsky 2004), with serious consequences for quality of life, Gazzotti 2011, and possibly survival (Gathinji 2009; Litofsky 2004; Mainio 2006b).
Although national guidelines for the treatment of depression exist (NICE 2009), they cannot have been based on evidence specific to brain tumours because we have been unable to identify any studies that were eligible for this review. Antidepressants appear to be effective in people with other medical illnesses, but the effectiveness in people with a primary brain tumour should not be assumed. Brain tumours are rapidly progressive, biologically active, and with significant structural and functional effects upon the main organ implicated in depression. Brain tumours (and their surgical treatment) may disrupt neuronal circuits crucial to the experience of emotion (Litofsky 2009), or alter local levels of biochemical mediators (Mainio 2005). Antiepileptic drugs can affect the bioavailability of antidepressants (Hachad 2002; Spina 2016), and corticosteroids have been associated with mood changes in glioma (Litofsky 2004). There are reasons to be cautious about assuming that antidepressants are effective in people with primary brain tumours.
Brain tumours are also associated with epilepsy, cognitive dysfunction, and fatigue (Ruda 2010; Wen 2008), which are recognised side effects of antidepressants (Cassano 2004; Montgomery 2005; Peretti 2000). It is important to highlight the lack of evidence about the possible adverse effects of antidepressants in this high‐risk group. Antidepressants could also impact on these outcomes, since depression has itself been associated with epilepsy (Mula 2017), impaired cognition (Rock 2014), and fatigue (Moss 2006).
Overall completeness and applicability of evidence
Nine excluded studies used a pharmacological agent other than an antidepressant (a psychostimulant, acetylcholinesterase inhibitor, antiepileptic drug, or botanical product). These drugs could help treat a wider range of symptoms than antidepressants. However, to date no studies have primarily evaluated the benefit, or harm, of pharmacological treatment of depression in people with a primary brain tumour. Direct study of this question requires that eligibility is restricted to people with high levels of depression at baseline.
Quality of the evidence
With no eligible studies identified, we could not assess the quality of evidence according to the GRADE levels of evidence.
Potential biases in the review process
We searched three databases electronically and handsearched four journals for this update (we previously searched more databases in the original review). We searched for ongoing trials, and contacted the authors for any unpublished data (Zhou 2018). Despite this rigorous review process, the possibility remains that we did not identify an eligible trial. Regular reviews of this update are therefore needed.
Agreements and disagreements with other studies or reviews
Over 20 years ago, Weitzner and colleagues argued that people with primary brain tumours should receive psychotropic medication for depression if indicated (Weitzner 1999). However, now as then, no RCTs have studied the effectiveness of drug treatment for clinical depression in people with primary brain tumours. Several research groups recognise the potentially profound impact of depression on quality of life in people with primary brain tumours (Huang 2017; Litofsky 2009; Pangilinan 2007); yet people living with a primary brain tumour may in theory be more sensitive to adverse side effects from the drugs used to treat depression. The risk of antidepressant‐associated delirium, seizures, and drug‐drug interactions remains unknown (Price 2008). As a result, the risk‐benefit of treating depression with drugs in these patients remains unclear. Consequently, there is general agreement in the need for RCTs of pharmacological treatment of clinical depression in people with a primary brain tumour ((Huang 2017; Litofsky 2009; Pangilinan 2007; Price 2008; Weitzner 1999).
Authors' conclusions
Implications for practice.
Since the last version of this review, no new relevant studies have provided additional information to change our conclusions. There is currently no high‐quality evidence as to whether pharmacological treatments for depression in people with primary brain tumours are either effective or harmful. Best practice would suggest that doctors treating depressed people with a primary brain tumour discuss the lack of evidence with the individual, document the views of the patient, and use their clinical judgement. If drug treatment is started, close follow‐up may help detect any adverse effects.
Implications for research.
Detailed prospective studies and randomised controlled trials addressing the risks and benefits of antidepressants are vital. Important research questions include whether, amongst people with a primary brain tumour and depression, there is any evidence that treatment:
is effective in treating depression;
has clinically significant effects on seizure frequency, fatigue, and cognition;
has clinically significant pharmacokinetic interactions with antiepileptic drugs or chemotherapy;
has a clinically significant effect on survival.
What's new
| Date | Event | Description |
|---|---|---|
| 15 July 2020 | New citation required but conclusions have not changed | Nine new studies added to excluded studies (Attia 2012; Blackhall 2009; Boele 2013; Gehring 2012; Gothelf 2005; Knudsen‐Baas 2018; Maschio 2013; Walker 2010; Wirz 2010). One new study was added to ongoing studies (Zhou 2018). The update has not altered the conclusions from the last publication of this review. |
| 15 July 2020 | New search has been performed | Search updated up to September 2019 and review updated accordingly. |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 3, 2010
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
| 7 April 2013 | New citation required but conclusions have not changed | Text updated. One new study added to excluded studies (Caudill 2011). The update has not altered the conclusions from the last publication of this review. |
| 26 October 2012 | New search has been performed | Updated search of MEDLINE, EMBASE, CENTRAL, and PsycINFO. |
Acknowledgements
We thank Robin Grant for his contributions to previous versions of this review.
We thank Heather Dickinson, Steff Lewis, Dr NS Litofsky, Dr E Davies, Sandi Pniauskas, and Lesley Smith for their helpful comments on previous versions of this review. We thank Jane Hayes for help updating and running search strategies for the previous versions of this review.
We thank Joanne Platt and Clare Jess from Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers for their help with this review update.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy
Conducted 22 July 2009
Ovid MEDLINE (R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE (R) 1950 to present (July week 2 2009)
1. exp depressive disorder/~ 2. *Depressive Disorder, Major/ 3. *Dysthymic Disorder/ 4. Depression, involutional.mp. 5. Depress$.ab,ti. 6. Affective disorder$.ab,ti. 7. Mood disorder$.ab,ti. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. exp Glioma/ 10. exp Brain Neoplasms/ 11. brain tumo$.ab,ti. 12. astrocytoma$.ab,ti. 13. meningioma$.ab,ti. 14. oligodendroglioma$.ab,ti. 15. glioblastoma$.ab,ti. 16. 9 or 10 or 11 or 12 or 13 or 14 or 15 17. exp Antidepressive Agents/ 18. exp Serotonin Uptake Inhibitors/ 19. exp Monoamine Oxidase Inhibitors/ 20. exp Drug Therapy/ 21. Antidepress$.ab,ti. 22. TCA$.ab,ti. 23. Tricyclic$.ab,ti. 24. MAOI$.ab,ti. 25. SSRI$.ab,ti. 26. Antidepressive Agents, Tricyclic/ 27. Antidepressive Agents, Second Generation/ 28. 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 29. 8 and 16 and 28 30. (animals not (humans and animals)).sh. 31. 29 not 30
(n = 57)
Appendix 2. Web of Knowledge search strategy
Searched 22 July 2009
www.isiknowledge.com
Topic=(depression OR depressive disorder OR major depression OR mood disorder OR depress* OR affectiv* OR dysthym* OR mood*) AND Topic=(antidepress* OR SSRI OR MAOI OR TCA) AND Topic=(brain neoplas* OR brain tumo* OR glioma OR astrocytoma OR oligodendroglioma OR meningioma)
(n = 306)
Appendix 3. MEDLINE updated search strategy
1 exp Depressive Disorder/ 2 Depression/ 3 exp Mood Disorders/ 4 depress*.mp. 5 ((affective or mood) adj disorder*).mp. 6 1 or 2 or 3 or 4 or 5 7 exp Brain Neoplasms/ 8 exp Glioma/ 9 (brain adj5 (tumor* or tumour* or neoplas* or malignan* or cancer* or carcinoma*)).mp. 10 (glioma* or astrocytoma* or meningioma* or oligodendroglioma* or glioblastoma*).mp. 11 7 or 8 or 9 or 10 12 drug therapy.fs. 13 exp Antidepressive Agents/ 14 exp Serotonin Uptake Inhibitors/ 15 exp Monoamine Oxidase Inhibitors/ 16 (SSRI* or TCA* or MAOI* or tricyclic* or antidepress* or anti‐depress*).mp. 17 12 or 13 or 14 or 15 or 16 18 6 and 11 and 17
Appendix 4. CENTRAL updated search strategy
#1 MeSH descriptor: [Depressive Disorder] explode all trees #2 MeSH descriptor: [Depression] explode all trees #3 MeSH descriptor: [Mood Disorders] explode all trees #4 depress* #5 (affective or mood) near disorder* #6 #1 or #2 or #3 or #4 or #5 #7 MeSH descriptor: [Brain Neoplasms] explode all trees #8 MeSH descriptor: [Glioma] explode all trees #9 brain near/5 (tumor* or tumour* or neoplas* or malignan* or cancer* or carcinoma*) #10 glioma* or astrocytoma* or meningioma* or oligodendroglioma* or glioblastoma* #11 #7 or #8 or #9 or #10 #12 Any MeSH descriptor with qualifier(s): [Drug therapy ‐ DT] in all MeSH products #13 MeSH descriptor: [Antidepressive Agents] explode all trees #14 MeSH descriptor: [Serotonin Uptake Inhibitors] explode all trees #15 MeSH descriptor: [Monoamine Oxidase Inhibitors] explode all trees #16 SSRI* or TCA* or MAOI* or tricyclic* or antidepress* or anti‐depress* #17 #12 or #13 or #14 or #15 or #16 #18 #6 and #11 and #17
Appendix 5. CENTRAL search strategy
Searched 22 July 2009
EBM Reviews ‐ Cochrane Central Register of Controlled Trials 3rd Quarter 2009
1. Glioma/ 2. exp Brain Neoplasms/ 3. Astrocytoma/ 4. Meningioma/ 5. glioma.ti,kw,ab. 6. brain tumo$.ti,kw,ab. 7. astrocytoma$.ti,kw,ab. 8. meningioma$.ti,kw,ab. 9. oligodendroglioma$.ti,kw,ab. 10. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 11. exp Antidepressive Agents/ 12. exp Antidepressive Agents, Second‐Generation/ 13. Antidepressive Agents, Tricyclic/ 14. Serotonin Uptake Inhibitors/ 15. Monoamine Oxidase Inhibitors/ 16. Antidepress$.ti,kw,ab. 17. SSRI$.ti,kw,ab. 18. MAOI$.ti,kw,ab. 19. TCA$.ti,kw,ab. 20. drug therap$.ti,kw,ab. 21. exp Drug Therapy/ 22. pharmacotherapy.mp. 23. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 24. exp Mental Disorder/ 25. Depression/ 26. Affective Symptoms/ 27. depress$.ti,kw,ab. 28. mood$.ti,kw,ab. 29. affectiv$.ti,kw,ab. 30. dysthym$.ti,kw,ab. 31. 24 or 25 or 26 or 27 or 28 or 29 or 30 32. 10 and 23 and 31
(n = 8)
Appendix 6. LILACS search strategy
Searched 22 July 2009
1. Depression OR Depressive OR Mood OR Affect 2. Glioma OR Glioblastoma OR Meningioma OR Brain neoplasm 3. Antidepressant OR Antidepressive OR SSRI OR MAOI OR TCA 4. 1 and 2 and 3
(n = 0)
Appendix 7. PSYNDEX search strategy
Searched 22 July 2009
(all search terms free text)
1. Depression OR Depressive OR Mood OR Affectiv? 2. Gliom? OR gehirntumo? OR Krebs OR Astrocytom? Or Oligodendrogliom? OR Meningiom? 3. Antidepress OR Serotonin OR SSRI OR MAOI OR TCA OR Pharmacotherap? 4. 1 and 2 and 3
(n = 1)
Appendix 8. National Research Register archive search strategy
Searched 22 July 2009
(Database was only active until 2007)
1. "Glioma"
(n = 272)
Appendix 9. DARE search strategy
Searched 22 July 2009
Database of Abstracts of Reviews of Effectiveness 3rd Quarter 2009
1. depress$.ab,ti. 2. affective disorder$.ab,ti. 3. mood disorder$.ab,ti. 4. 1 or 2 or 3 5. brain tumo$.ab,ti. 6. astrocytoma$.ab,ti. 7. meningioma$.ab,ti. 8. oligodendroglioma$.ab,ti. 9. glioblastoma$.ab,ti. 10. 5 or 6 or 7 or 8 or 9 11. antidepress$.ab,ti. 12. TCA$.ab,ti. 13. Tricyclic$.ab,ti. 14. MAOI$.ab,ti. 15. SSRI$.ab,ti. 16. 11 or 12 or 13 or 14 or 15 17. 4 and 10 and 16
(n = 0)
Appendix 10. Embase search strategy
Conducted 22 July 2009
EMBASE 1980 to 2009 Week 29
1. exp Depression/ 2. exp Mood Disorder/ 3. "Depress*".ti,ab. 4. "Affect*".ti,ab. 5."Dysthym*".ti,ab. 6. 1 or 2 or 3 or 4 or 5 7. exp Glioma/ 8. exp Brain Tumor/ 9. Astrocytoma/ 10.Meningioma/ 11. (glioma* or brain tumo* or astrocytoma* or meningioma*).ti,ab. 12. 7 or 8 or 9 or 10 or 11 13. exp Antidepressant Agent/ 14. Monoamine Oxidase Inhibitor/ 15. Tricyclic Antidepressant Agent/ 16. Serotonin Uptake Inhibitor/ 17. (antidepress* or SSRI* or MAOI* or TCA* or tricyclic*).ti,ab. 18. 13 or 14 or 15 or 16 or 17 19. animal.sh 20. human.sh 21. 19 not (19 and 20) 22. 6 and 12 and 18 23. 22 not 21
(n = 287)
Appendix 11. PsycINFO search strategy
Searched 22 July 2009
PsycINFO 1806 to July Week 2 2009
1. exp Endogenous Depression/ 2. exp Reactive Depression/ 3. exp Major Depression/ 4. exp Mental Disorders/ 5. exp Affective Disorders/ 6. (depress$ or mood$ or affective$ or dysthym$).ab,ti. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp Brain Neoplasms/ 9. brain tumo?r.mp. 10. Oligodendroglioma$.mp. 11. Astrocytoma$.mp. 12. Meningioma$.mp. 13. (glioma$ or brain tumo$ or astrocytoma$ or meningioma$ or oligodendroglioma$).ab,ti. 14. 8 or 9 or 10 or 11 or 12 or 13 15. exp Drug Therapy/ 16. pharmacotherapy.mp. 17. exp Antidepressant Drugs/ 18. exp Tricyclic Antidepressant Drugs/ 19. exp Monoamine Oxidase Inhibitors/ 20. exp Serotonin Reuptake Inhibitors/ 21. (antidepress$ or SSRI$ or MAOI$ or TCA$ or tricyclic$ or drug therap$ or pharmacotherap$).ab,ti. 22.15 or 16 or 17 or 18 or 19 or 20 or 21 23. 7 and 14 and 22
(n = 39)
Appendix 12. Embase updated search strategy
1 exp mood disorder/ 2 depress*.mp. 3 ((affective or mood) adj disorder).mp. 4 1 or 2 or 3 5 exp brain tumor/ 6 exp glioma/ 7 (brain adj5 (tumor* or tumour* or neoplas* or malignan* or cancer* or carcinoma*)).mp. 8 (glioma* or astrocytoma* or meningioma* or oligodendroglioma* or glioblastoma*).mp. 9 5 or 6 or 7 or 8 10 dt.fs. 11 exp antidepressant agent/ 12 (SSRI* or TCA* or MAOI* or tricyclic* or antidepress* or anti‐depress*).mp. 13 10 or 11 or 12 14 4 and 9 and 13
Appendix 13. PsycINFO updated search strategy
1 exp affective disorders/ 2 depress*.mp. 3 ((affective or mood) adj disorder*).mp. 4 1 or 2 or 3 5 brain neoplasms/ 6 glioma/ 7 (brain adj5 (tumor* or tumour* or neoplas* or malignan* or cancer* or carcinoma*)).mp. 8 (glioma* or astrocytoma* or meningioma* or oligodendroglioma* or glioblastoma*).mp. 9 5 or 6 or 7 or 8 10 exp drug therapy/ 11 exp antidepressant drugs/ 12 exp Serotonin Reuptake Inhibitors/ 13 exp Monoamine Oxidase Inhibitors/ 14 (SSRI* or TCA* or MAOI* or tricyclic* or antidepress* or anti‐depress*).mp. 15 10 or 11 or 12 or 13 or 14 16 4 and 9 and 15
Appendix 14. British Nursing Index search strategy
Searched 22 July 2009
British Nursing Index and Archive 1985 to July 2009
1. exp depression/ 2. exp psychiatric disorders/ 3. (depress$ or mood$ or affectiv$ or dysthym$).ti,ab. 4. 1 or 2 or 3 5. exp cancer/ 6. (glioma$ or brain tumo$ or astrocytoma$ or meningioma$ or oligodendroglioma$).ti,ab. 7. 5 or 6 8. exp Drug Therapy/ 9. exp Psychiatroc disorders: Drug Therapy/ 10. SSRI$.mp. 11. MAOI$.mp. 12. Tricyclic.mp. 13. (antidepress$ or SSRI$ or MAOI$ or TCA$ or drug therap$ or pharmacotherap$).ti,ab. 14. 8 or 9 or 10 or 11 or 12 or 13 15. 4 and 7 and 14
(n = 20)
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Attia 2012 | Depression was not the primary outcome; the study did not require participants to have depression. The drug used, Gingko biloba, is a herbal supplement unlicenced for the treatment of depression. |
| Blackhall 2009 | Depression was not the primary outcome, and participants were not explicitly screened for depression. |
| Boele 2013 | Depression was not the primary outcome, and participants were not explicitly screened for depression. |
| Caudill 2011 | Treatment of depression was based on routine clinical care (not systematically controlled). |
| Gehring 2012 | Depression was not the primary outcome, and participants were not explicitly screened for depression. |
| Gothelf 2005 | No adult brain tumour patients included. |
| Knudsen‐Baas 2018 | Not an intervention study |
| Koval'chuk 2007 | Depression was not an inclusion criterion. |
| Litofsky 2004 | Treatment of depression was not systematically controlled. Depression was not an inclusion criterion. |
| Maschio 2013 | Depression was not the primary outcome, and participants were not explicitly screened for depression. |
| Meyers 1998 | Patients with severe depression were excluded, and the study was not limited to those with milder depression. |
| Morrow 2003 | Depression was not an inclusion criterion. Data for brain tumour patients were not presented separately. |
| Moss 2006 | Depression was not an inclusion criterion. Data for brain tumour patients were not presented separately. |
| Oyewumi 1981 | Single case study |
| Rabey 1985 | 3 case studies; brain tumour not diagnosed at time of antidepressant prescription |
| Shaw 2006 | Depression was not an inclusion criterion. |
| Walker 2010 | Did not investigate antidepressant as treatment for depression |
| Wellisch 2006 | Could not confirm whether depression was an inclusion criterion |
| Wirz 2010 | No patients with primary brain tumours included. |
Characteristics of ongoing studies [ordered by study ID]
Zhou 2018.
| Study name | Effect of low‐dose ketamine on PerioperAtive depreSsive Symptoms in patients undergoing Intracranial tumOr resectioN (PASSION): study protocol for a randomized controlled trial |
| Methods | Single‐centre randomised, placebo controlled, and double‐blinded; 1:1 split between treatment and placebo arms. Participants randomised and stratified by severity of perioperative depression symptoms (moderate and severe). |
| Participants | 80 |
| Interventions | Participants in the ketamine arm receive a low‐dose ketamine infusion, and participants in the placebo arm receive a saline infusion. |
| Outcomes | Primary endpoint is perioperative depressive symptoms 3 days postsurgery. |
| Starting date | 2017 |
| Contact information | Ruguan Han, Department of Anesthesiology, Beijing Tiantan Hospital, Capital Medical University, No.6, Tiantan Xili, Dongcheng District, Beijing, 100050, People's Republic of China |
| Notes |
Differences between protocol and review
We changed the title to specify that the review was of interventions for the treatment of depression (as opposed to prevention).
We searched the British Nursing Index in place of CINAHL (Cumulative Index to Nursing and Allied Health Literature) because CINAHL was not available on Ovid. We did not search CancerLit because it is covered by MEDLINE. We did not search Applied Science and Technology Plus, as it is an engineering database. General Science Plus could not be accessed from Edinburgh University. The search strategy outlined in the protocol was altered, as necessary, for individual databases; for full details see the Appendices.
We did not widen our search terms to include psychostimulants and acetylcholinesterase inhibitors as we said we would in the original protocol because publications on these drugs came up in the search, indicating that our search strategy was already adequate.
Contributions of authors
ZB and SH screened abstracts, supervised by FB and AR. All authors contributed to writing the update.
Sources of support
Internal sources
-
NHS Lothian Neuro‐oncology Endowment Fund, UK
Salary for AR
External sources
No sources of support supplied
Declarations of interest
Zachary Beevers: none known Sana Hussain: none known Florien W Boele: none known Alasdair G Rooney: none known
Joint First Author
Joint First Author
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
Attia 2012 {published data only}
- Attia A, Rapp S, Case L, D'Agostino R, Lesser G, Naughton M, et al. Phase II study of ginkgo biloba in irradiated brain tumour patients: effect on cognitive function, quality of life, and mood. Journal of Neuro-Oncology 2012;109(2):357-63. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blackhall 2009 {published data only}
- Blackhall K, Petronic G, Shu J, Baum L, Farace E. A pilot study evaluating the safety and efficacy of modafinil for cancer-related fatigue. Journal of Palliative Medicine 2009;12(5):433-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Boele 2013 {published data only}
- Boele F, Duow L, Groot M, Thuijl H, Cleijne W, Heimans J, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Journal of Neuro-Oncology 2013;15(10):1420-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caudill 2011 {published data only}
- Caudill JS, Brown PD, Cerhan JH, Rummans TA. Selective serotonin reuptake inhibitors, glioblastoma multiforme, and impact on toxicities and overall survival. The Mayo Clinic experience. American Journal of Clinical Oncology 2011;34:385-7. [DOI] [PubMed] [Google Scholar]
Gehring 2012 {published data only}
- Gehring K, Patwardhan S, Collins R, Groves M, Etzel C, Meyers A, et al. A randomized trial of the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumour. Journal of Neuro-Oncology 2012;107(1):165-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gothelf 2005 {published data only}
- Gothelf D, Rubinstein M, Shemesh E, Miller O, Farbstein I, Klein A, et al. Pilot study: fluvoxamine treatment for depression and anxiety disorders in children and adolescents with cancer. Journal of the American Academy of Child and Adolescent Psychiatry 2005;44(12):1258-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Knudsen‐Baas 2018 {published data only}
- Knudsen-Baas K, Johannesen T, Myklebust T, Aarseth J, Owe J, Gilhus E, et al. Antiepileptic and psychiatric medication in a nationwide cohort of patients with glioma WHO grade II-IV. Journal of Neuro-Oncology 2018;140(3):739-48. [PMID: ] [DOI] [PubMed] [Google Scholar]
Koval'chuk 2007 {published data only}
- Koval'chuk VV. Pharmacologic correction of psycho-emotive disorders in the rehabilitation of patients after removal of brain tumors. Voprosy Onkologii 2007;53(6):704-10. [PubMed] [Google Scholar]
Litofsky 2004 {published data only}
- Litofsky NS, Farace E, Anderson F Jr. Depression in patients with high-grade glioma: results of the Glioma Outcomes Project. Neurosurgery 2004;54(2):358-66. [DOI] [PubMed] [Google Scholar]
Maschio 2013 {published data only}
- Maschio M, Dinapoli L, Sperati F, Pace A, Fabi A, Vidiri A, et al. Effect of pregabalin add-on treatment on seizure control, quality of life, and anxiety in patients with brain tumour-related epilepsy: a pilot study. Epileptic Disorders 2013;14:388-97. [PMID: ] [DOI] [PubMed] [Google Scholar]
Meyers 1998 {published data only}
- Meyers CA, Weitzner MA, Valentine AD, Levin VA. Methylphenidate therapy improves cognition, mood, and function of brain tumor patients. Journal of Clinical Oncology 1998;16(7):2522-7. [DOI] [PubMed] [Google Scholar]
Morrow 2003 {published data only}
- Morrow GR, Hickok JT, Roscoe JA, Raubertas RF, Andrews PLR, Flynn PJ. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Journal of Clinical Oncology 2003;21(24):4635-41. [DOI] [PubMed] [Google Scholar]
Moss 2006 {published data only}
- Moss EL, Simpson JSA, Pelletier G, Forsyth P. An open-label study of the effects of bupropion SR on fatigue, depression and quality of life of mixed-site cancer patients and their partners. Psycho-Oncology 2006;15:259-67. [DOI] [PubMed] [Google Scholar]
Oyewumi 1981 {published data only}
- Oyewumi LK, Lapierre YD. Efficacy of lithium in treating mood disorder occurring after brain stem injury. American Journal of Psychiatry 1981;138(1):110-2. [DOI] [PubMed] [Google Scholar]
Rabey 1985 {published data only}
- Rabey JM, Avrahami E. Unmasking of cerebellar tumours by amitriptyline in depressive patients. Journal of Neurology, Neurosurgery and Psychiatry 1985;48(3):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaw 2006 {published data only}
- Shaw EG, Rosdhal R, D'Agostino RB Jr, Lovato J, Naughton MJ, Robbins ME, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. Journal of Clinical Oncology 2006;24(9):1415-20. [DOI] [PubMed] [Google Scholar]
Walker 2010 {published data only}
- Walker A, Grainge M, Bates T, Card T. Survival of glioma and colorectal cancer patients using tricyclic antidepressants post-diagnosis. Cancer Causes & Control 2012;23(12):1959-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wellisch 2006 {published data only}
- Wellisch DK, Steh B, Kaleita TA, Graham CA, Cloughes TF. A modafinil therapeutic trial for adult brain tumor patients: III. Depression outcomes. Psycho-Oncology 2006;15 Suppl:455. [Google Scholar]
Wirz 2010 {published data only}
- Wirz S, Nadstawek J, Kuhn J, Vater S, Junker U, Wartenberg H. Modafinil for the treatment of cancer-related fatigue: an intervention study [Modafinil zur behandlung der tumorfatigue]. Schmerz 2010;24:587-95. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Zhou 2018 {published and unpublished data}
- Zhou Y, Peng Y, Fang J, Sun W, Zhang G, Zhen L, et al. Effect of low-dose ketamine on PerioperAtive depreSsive Symptoms in patients undergoing Intracranial tumOr resectioN (PASSION): study protocol for randomized controlled trial. Trials 2018;19:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993;85(5):365-76. [DOI] [PubMed] [Google Scholar]
APA 2016
- American Psychiatric Association. User's Guide for the Structured Clinical Interview for DSM-5 Disorders - Clinical Version (SCID-5-CV). Washington, DC: American Psychiatric Association, 2016. [Google Scholar]
Baltenberger 2014
- Baltenberger E, Schmitt G, Thomas C. Treatment of depressive symptoms in patients with cancer. Mental Health Clinician 2014;4(3):114-7. [Google Scholar]
Beck 1996
- Beck AT, Brown G, Steer R. Beck Depression Inventory-II. Pearson, 1996. [Google Scholar]
Cassano 2004
- Cassano P, Fava M. Tolerability issues during long-term treatment with antidepressants. Annals of Clinical Psychiatry 2004;16(1):15-25. [DOI] [PubMed] [Google Scholar]
Claus 2015
- Claus E, Walsh K, Wiencke J, Molinaro A, Wiermels J, Schildkraut, et al. Survival and low grade glioma: the emergence of genetic information. Neurosurgery Focus 2015;38(1):E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalton 2009
- Dalton SO, Laursen TM, Ross L, Mortensen PB, Johansen C. Risk for hospitalisation with depression after a cancer diagnosis: a nationwide, population-based study of cancer patients in Denmark from 1973 to 2003. Journal of Clinical Oncology 2009;27:1440-5. [DOI] [PubMed] [Google Scholar]
de Robles 2015
- Robles P, Fiest KM, Frolkis AD, Pringsheim T, Atta C, St Germaine-Smith C. The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis. Neuro-Oncology 2015;17(6):776-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feuerstein 2007
- Feuerstein M, Hansen JA, Calvio LC, Johnson L, Ronquillo JG. Work productivity in brain tumor survivors. Journal of Occupational and Environmental Medicine 2007;49:803-11. [DOI] [PubMed] [Google Scholar]
Gathinji 2009
- Gathinji M, McGirt M, Attenello F, Chaichana K, Than K, Olivi A, et al. Association of preoperative depression and survival after resection of malignant brain astrocytoma. Surgical Neurology 2009;71(3):299-303. [DOI] [PubMed] [Google Scholar]
Gazzotti 2011
- Gazzotti M, Malheiros S, Alith M, Nascimento O, Santoro I, Jardim J, et al. Quality of life and physical limitation in primary brain tumour patients. Quality of Life Research 2011;20(10):1639-43. [DOI] [PubMed] [Google Scholar]
Gelder 2012
- Gelder M, Andreasen N, Lopez-Ibor J, Geddes J. Oxford Textbook of Psychiatry. 2nd edition. Oxford: Oxford University Press, 2012. [Google Scholar]
Hachad 2002
- Hachad H, Ragueneau-Majlessi I, Levy RH. New antiepileptic drugs: review on drug interactions. Therapeutic Drug Monitoring 2002;24(1):91-103. [DOI] [PubMed] [Google Scholar]
Hackett 2008
- Hackett ML, Anderson CS, House AO, Xia J. Interventions for treating depression after stroke. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003437.pub3] [DOI] [PubMed] [Google Scholar]
Hesdorffer 2000
- Hesdorffer DC, Hauser WA, Annegers JF, Cascino G. Major depression is a risk factor for seizures in older adults. Annals of Neurology 2000;47:246-9. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hill 2015
- Hill T, Coupland C, Morriss R, Arthur A, Moore M, Hippisley-Cox J. Antidepressant use and risk of epilepsy and seizures in people aged 20 to 64 years: cohort study using a primary care database. BMC Psychiatry 2015;15:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2017
- Huang J, Zeng C, Xiao J, Zhao D, Tang H, Wu H, et al. Association between depression and brain tumour: a systematic review and meta-analysis. Oncotarget 2017;8(55):94932-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iosifescu 2004
- Iosifescu DV, Bankier B, Fava M. Impact of medical co-morbid disease on antidepressant treatment of major depressive disorder. Current Psychiatry Reports 2004;6(3):193-201. [DOI] [PubMed] [Google Scholar]
Lapointe 2018
- Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet 2018;392(10145):432-46. [DOI] [PubMed] [Google Scholar]
Lima 2005
- Lima MS, Moncrieff J, Soares BGO. Drugs versus placebo for dysthymia. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001130] [DOI] [PubMed] [Google Scholar]
Litofsky 2009
Louis 2016
- Louis DN, Perry A, Reifenberger G, Deimling A, Figaraella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathalogica 2016;131:803-20. [DOI] [PubMed] [Google Scholar]
Mainio 2005
- Mainio A, Hakko H, Timonen M, Niemelä A, Koivukangas J, Räsänen P. Depression in relation to survival among neurosurgical patients with a primary brain tumor: a 5-year follow-up study. Neurosurgery 2005;56:1234-42. [DOI] [PubMed] [Google Scholar]
Mainio 2006a
- Mainio A, Hakko H, Niemela A. Gender difference in relation to depression and quality of life among patients with a primary brain tumour. European Psychiatry: The journal of the European Psychiatric Association 2006;21(3):194-9. [DOI] [PubMed] [Google Scholar]
Mainio 2006b
- Mainio A, Tuunanen S, Hakko H. Decreased quality of life and depression as predictors for shorter survival among patients with low-grade gliomas: a follow-up from 1990 to 2003. European Archives of Psychiatry and Clinical Neuroscience 2006;256(8):516-21. [DOI] [PubMed] [Google Scholar]
McHorney 1993
- McHorney CA, Ware JE, Raczek AE. The MOS 3-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care 1993;31(3):247-63. [DOI] [PubMed] [Google Scholar]
Mendoza 1999
- Mendoza T, Wang S, Cleeland C, Morrissey M, Johnson B, Wendt J, et al. The rapid assessment of fatigue severity in cancer patients. Cancer 1999;85(5):1186-96. [DOI] [PubMed] [Google Scholar]
Moncrieff 2004
- Moncrieff J, Wessely S, Hardy R. Active placebos versus antidepressants for depression. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003012.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 2005
- Montgomery SA. Antidepressants and seizures: emphasis on newer agents and clinical implications. International Journal of Clinical Practice 2005;59(12):1435-40. [DOI] [PubMed] [Google Scholar]
Mottram 2006
- Mottram P, Wilson K, Strobl J. Antidepressants for depressed elderly. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD003491.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mula 2017
- Mula M. Depression in epilepsy. Current Opinion in Neurology 2017;30(2):180-6. [DOI] [PubMed] [Google Scholar]
NICE 2009
- NICE. Depression in adults with a chronic physical health problem: the NICE guideline on treatment and management: National Clinical Practice Guideline 91 NICE. www.nice.org.uk/guidance/cg91 (accessed prior to 29 June 2020) 2009.
Pallud 2019
- Pallud J, McKhann G. Diffuse low-grade glioma related epilepsy. Neurosurgery Clinics of North America 2019;30(1):43-54. [DOI] [PubMed] [Google Scholar]
Pangilinan 2007
- Pangilinan PH Jr, Kelly BM, Pangilinan JM. Depression in the patient with brain cancer. Community Oncology 2007;4:533-7. [Google Scholar]
Patel 2019
- Patel AP, Fisher JL, Nichols E, Feigin VL, Murray CJL, Fitzmaurice C, et al. Global, regional, and national burden of brain and other CNS cancer, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology 2019;18(4):376-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peretti 2000
- Peretti S, Judge R, Hindmarch I. Safety and tolerability considerations: tricyclic antidepressants vs. selective serotonin re-uptake inhibitors (Antidepressant selection: Proceedings from a TCA/SSRI consensus conference). Acta Psychiatrica Scandinavia 2000;101 Suppl 403:17-25. [DOI] [PubMed] [Google Scholar]
Price 2008
- Price TRP, Goetz KL, Lovell MR. Neuropsychiatric aspects of brain tumors. In: Yudofsky SC, Hales RE, editors(s). The American Psychiatric Publishing Textbook of Neuropsychiatry and Behavioural Neurosciences. 5th edition. Arlington (VA): American Psychiatric Publishing Inc, 2008. [Google Scholar]
Radloff 1977
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement 1977;1(3):385-401. [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rihmer 2007
- Rihmer Z. Suicide risk in mood disorders. Current Opinion in Psychiatry 2007;20:17-22. [DOI] [PubMed] [Google Scholar]
Rock 2014
- Rock P, Roiser J, Riedel W, Blackwell A. Cognitive impairment in depression: a systematic review and meta-analysis. Psychological Medicine 2014;44(10):2029-40. [DOI] [PubMed] [Google Scholar]
Rooney 2011
- Rooney AG, McNamara S, MacKinnon M, Fraser M, Rampling R, Carson A, et al. Frequency, clinical associations, and longitudinal course of major depressive disorder in adults with cerebral glioma. Journal of Clinical Oncology 2011;29:4307-12. [DOI] [PubMed] [Google Scholar]
Ruda 2010
- Ruda R, Trevisan E, Soffietti R. Epilepsy and brain tumours. Current Opinion in Oncology 2010;22(6):611-20. [DOI] [PubMed] [Google Scholar]
Sayyah 2016
- Sayyah M, Eslami K, AlaiShehni S, Kouti L. Cognitive function before and during treatment with selective serotonin reuptake inhibitors in patients with depression or obsessive-compulsive disorder. Psychiatry Journal 2016 Aug 15 [Epub ahead of print]. [DOI: 10.1155/2016/5480391] [PMCID: PMC5002481] [DOI] [PMC free article] [PubMed]
Sharpe 2004
- Sharpe M, Strong V, Allen K. Major depression in outpatients attending a regional cancer centre: screening and unmet treatment needs. British Journal of Cancer 2004;90:314-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spina 2016
- Spina E, Pisani F, deLeon J. Clinically significant pharmacokinetic drug interactions of antiepileptic drugs with new antidepressants and new antipsychotics. Pharmacological Research 2016;106:72-86. [DOI] [PubMed] [Google Scholar]
Stein 2004
- Stein K, Jacobsten P, Blanchard C, Thors C. Further validation of the Multidimensional Fatigue Symptom Inventory-Short Form. Journal of Pain Symptom Management 2004;27(1):14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stupp 2009
- Stupp R, Hegi ME, Mason WP, den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncology 2009;10(5):459-66. [DOI] [PubMed] [Google Scholar]
Weitzner 1995
- Weitzner MA, Myers C, Gelke C, Byrne K, Levin C, Cella D. The functional assessment of cancer therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer 1995;75(5):1151–61. [DOI] [PubMed] [Google Scholar]
Weitzner 1999
- Weitzner MA. Psychosocial and neuropsychiatric aspects of patients with primary brain tumors. Cancer Investigation 1999;17(4):285-91. [DOI] [PubMed] [Google Scholar]
Wen 2008
- Wen PY, Kesari S. Malignant gliomas in adults. New England Journal of Medicine 2008;359:492-507. [DOI] [PubMed] [Google Scholar]
Wiemels 2010
- Wiemels J, Wrensch M, Claus E. Epidemiology and etiology of meningioma. Journal of Neuro-Oncology 2010;99(3):307-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wijkstra 2005
- Wijkstra J, Lijmer J, Balk F, Geddes J, Nolen WA. Pharmacological treatment for psychotic depression. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004044.pub2] [DOI] [PubMed] [Google Scholar]
Yellen 1997
- Yellen S, Cella D, Webster K, Blendowksi C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. Journal of Pain Symptom Management 1997;13(2):63-74. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavia 1983;67(6):361-70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Rooney 2010
- Rooney A, Grant R. Pharmacological treatment of depression in patients with a primary brain tumour. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD006932.pub2] [DOI] [PubMed] [Google Scholar]
Rooney 2013
- Rooney AG, Grant R. Pharmacological treatment of depression in patients with a primary brain tumour. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD006932.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]



