Abstract
The Genetic Epidemiology of COPD (COPDGene) study is a noninterventional, multicenter, longitudinal analysis of > 10,000 subjects, including smokers with a ≥ 10 pack-year history with and without COPD and healthy never smokers. The goal was to characterize disease-related phenotypes and explore associations with susceptibility genes. The subjects were extensively phenotyped with the use of comprehensive symptom and comorbidity questionnaires, spirometry, CT scans of the chest, and genetic and biomarker profiling. The objective of this review was to summarize the major advances in the clinical epidemiology of COPD from the first 10 years of the COPDGene study. We highlight the influence of age, sex, and race on the natural history of COPD, and the impact of comorbid conditions, chronic bronchitis, exacerbations, and asthma/COPD overlap.
Key Words: chronic bronchitis, comorbidities, COPD, COPDGene, epidemiology, sex
Abbreviations: AA, non-Hispanic African-American; ACO, asthma/COPD overlap; CB, chronic bronchitis; CHF, congestive heart failure; CVD, cardiovascular disease; DM, diabetes mellitus; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GWAS, genome-wide association studies; NHLBI, National Heart, Lung, and Blood Institute; NHW, non-Hispanic white; PA:A, pulmonary artery to aorta ratio; SGRQ, St. George’s Respiratory Questionnaire
COPD is one of the most prevalent chronic pulmonary diseases worldwide. A better understanding of the natural history, epidemiology, and the multiple clinical features that affect this heterogeneous disease is required to employ personalized management and improve outcomes. Several subject characteristics, such as frequent exacerbations and the presence of various comorbidities, have been associated with worse outcomes, although the underlying mechanisms have not been clearly elucidated. Only a few longitudinal studies of adequate size have attempted to address these important issues, and many aspects of the clinical epidemiology of COPD are incompletely understood.1, 2, 3
The Genetic Epidemiology of COPD (COPDGene) study is an observational, multicenter, longitudinal analysis of > 10,000 subjects with a ≥ 10 pack-year cigarette smoking history with and without COPD and healthy never smokers to characterize disease-related phenotypes and explore associations with susceptibility genes (Table 1).1 The subjects were extensively phenotyped with comprehensive symptom and comorbidity questionnaires, pre- and post-bronchodilator spirometry, CT scans of the chest, and genetic and biomarker profiling. Five-year follow-up data are available on > 9,000 subjects.
Table 1.
Baseline Characteristics of Subjects Enrolled in the Genetic Epidemiology of COPD Study
| Parameter | Value |
|---|---|
| No. of subjects enrolled | 10,198 |
| Age, y | 59.5 ± 9.0 |
| Female sex | 4,746 (46.5) |
| African-American race | 3,412 (33.5) |
| BMI, kg/m2 | 28.8 ± 6.3 |
| Current smoker | 5,416 (53.1) |
| Oxygen supplementation | 1,174 (11.5) |
| Lung function | |
| FEV1, L | 2.24 ± 0.9 |
| FEV1, % predicted | 76.3 ± 26 |
| FVC, L | 3.30 ± 1.0 |
| FVC, % predicted | 86.9 ± 18 |
| FEV1/FVC | 0.67 ± 0.2 |
| GOLD severity, % | |
| Stage 0 | 4,387 (43.0) |
| Stage I | 788 (7.7) |
| Stage II | 1,925 (18.9) |
| Stage III | 1,164 (11.4) |
| Stage IV | 606 (5.9) |
| PRISm | 1,323 (12.4) |
| SGRQ score | 27.4 ± 23 |
| 6-Min walk distance, feet | 1,350 ± 399 |
| mMRC score (IQR) | 1.37 (3) |
| % Emphysema (–950 Hu), total lung | 6.3 ± 9.8 |
| % Gas trapping (–856 Hu), total lung | 21.4 ± 19.7 |
Data are presented as mean ± SD or No. (%) unless otherwise indicated. Hu = Hounsfield units; IQR = interquartile range; mMRC = Modified Medical Research Council; PRISm = preserved ratio impaired spirometry; SGRQ = St. George’s Respiratory Questionnaire.
COPDGene has published multiple novel findings that have furthered the understanding of the natural history of COPD, including the impact of exacerbations and comorbidities. COPDGene has also offered new insights in special subgroups of patients; for example, those with chronic bronchitis (CB), asthma/COPD overlap (ACO), comorbidities, and a history of exacerbations, and in women, those of advanced age, and of non-Hispanic African-American (AA) race. The current review highlights some of the most important epidemiologic findings of the COPDGene study described over the past decade.
Genetic Findings in COPDGene
Identifying genetic determinants of COPD has been a major goal of the COPDGene study since its inception. Most genetic studies in COPDGene thus far have been based on genome-wide panels of hundreds of thousands of common genetic variants (known as single nucleotide polymorphisms), which have been tested in genome-wide association studies (GWAS) with the presence/absence of COPD and with multiple COPD-related phenotypes, including imaging features, clinical characteristics, COPD subtypes, and protein biomarkers.4
COPDGene has participated in progressively larger collaborative GWAS analyses of COPD through the International COPD Genetics Consortium; the most recent collaborative GWAS identified > 80 genomic regions associated with COPD.4 Further studies within these regions are needed to identify the functional genetic variants driving those associations and to determine the genes that those functional variants influence.
In addition to the identification of novel COPD genetic determinants, COPDGene has provided evidence supporting the increased risk for COPD among carriers for the alpha1-antitrypsin Z allele (genotype PiMZ).5 Whole genome sequencing of the entire COPDGene cohort is being obtained in conjunction with the National Heart, Lung, and Blood Institute (NHLBI) Trans-Omics for Precision Medicine (TOPMed) program, which will enable the identification of rare genetic determinants of COPD and COPD-related phenotypes.
Chronic Bronchitis
The importance of CB is frequently overlooked, particularly among patients with milder disease and those without airflow obstruction. CB is present in about one-quarter of the COPDGene participants with COPD and is associated with heightened exacerbation risk, worse health status, more dyspnea, and reduced 6-min walk distance compared with those without CB.6, 7, 8 In COPDGene, historical exacerbation rates were nearly twice as high in those with CB as in those without CB. St. George’s Respiratory Questionnaire (SGRQ) values (minimal clinical important difference of four units) were also dramatically higher (48.0 ± 21.3 vs 30.6 ± 21.8; P < .001), indicating lower health-related quality of life.6
Because of the inconsistent definition of CB in the literature and the complicated series of questions to support the classic definition of chronic cough and phlegm for ≥ 3 months per year for the last 2 years, a clinician-friendly definition was developed from COPDGene data, using two questions from the SGRQ.9, 10 Subjects were characterized as having SGRQ CB if they responded “almost every day” or “most days a week” to the following two questions: “Over the last 4 weeks, I have coughed:” and “Over the last 4 weeks, I have brought up phlegm (sputum).” The SGRQ definition identified 53% more subjects with CB than the classic definition, and those subjects had a clinical profile (sex and racial distributions, more current smoking, more exacerbations, more dyspnea, and worse SGRQ scores) nearly identical to those with the classic CB definition.7 The SGRQ CB definition is user friendly, has been shown to identify more subjects at risk for poor outcomes, and is able to assess changes over time.7, 8
CB is also common in smokers without airflow obstruction. In 4,880 current or ex-smokers without airflow obstruction in COPDGene, 12.2% reported CB.10 Individuals with nonobstructed CB had more respiratory exacerbations, lower 6-min walk distances, more dyspnea, and worse health-related quality of life compared with subjects without airflow limitations.11 Interestingly, CB in patients without airflow obstruction has also been associated with worse quality of life compared with those with airflow obstruction without CB. Using a multivariate logistic regression model, Kim et al8 found that current smoking was associated with an OR of 3.53 (95% CI, 2.72-4.58; P < .0001) for CB.7 CB is more likely to persist with continued smoking (OR, 5.77), improve with smoking cessation (OR, 4.29), and to newly develop if smoking is resumed (OR, 4.59).
Asthma/COPD Overlap
The heterogeneity of COPD and new therapies for asthma have resulted in a renewed interest in ACO. This patient population has been difficult to study because such patients are often excluded from both COPD and asthma studies. In COPDGene, the major inclusion criterion was a history of cigarette smoking, and subjects with a history of asthma were not excluded nor were they targeted for inclusion.1
Hardin et al12 identified 915 subjects with a COPD spirometric Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage II to IV and a concurrent diagnosis of asthma prior to 40 years of age. Compared with subjects with COPD alone, subjects with coexisting asthma were younger, had less tobacco exposure, and were more frequently AA. Subjects with ACO had more exacerbations in the year prior to enrollment and had worse health-related quality of life.12, 13 COPDGene provided the first descriptions of a large group (n = 167) of AA subjects with ACO.13 AA subjects were more likely to have coexisting COPD and asthma than non-Hispanic white (NHW) subjects, supporting the theory that these individuals have a different COPD susceptibility and clinical presentation than other populations.12, 13
There is controversy regarding the definition of ACO. Beyond a physician diagnosis of asthma, other characteristics have been explored for their ability to further refine the definition. Data from COPDGene were used to evaluate the significance of adding bronchodilator responsiveness and the degree of emphysema to the definition of ACO.14 Subjects with ACO and bronchodilator responsiveness had less airflow limitation, emphysema, and gas trapping on CT imaging. Despite these characteristics, subjects with ACO were equally likely to have frequent exacerbations compared with those with COPD without a history of asthma. Based on the findings of these studies, subjects with ACO represent a distinct high-risk population with a high propensity for exacerbations. Identifying these subjects early in their clinical course may aid in the prevention of future exacerbations. In fact, subjects from COPDGene who had asthma early in life (ie, diagnosed by a health professional at < 16 years of age) have a higher risk of developing COPD and more respiratory symptoms compared with those without childhood asthma.15
Impact of Sex, Race, and Age
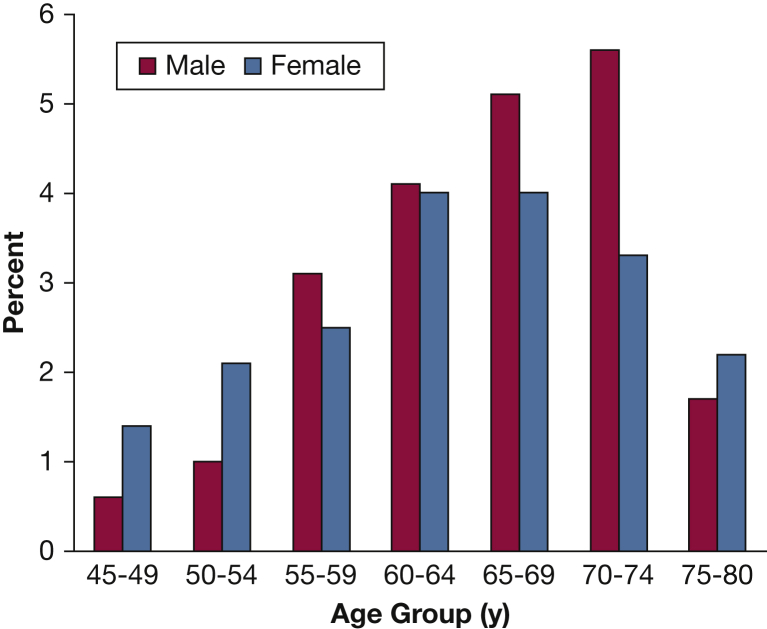
Data from COPDGene describe the important sex and racial differences in the phenotypic expression of COPD. These differences translate into higher risk of disease severity, particularly in women. For example, patients with severe COPD (FEV1 < 50% predicted) diagnosed at an early age (< 55 years) revealed female predominance (66%) and association with maternal smoking and maternal COPD (Fig 1).16 It was also noted that the airways of female subjects who smoke exhibit higher wall area percentage but a lower luminal area, internal diameter, and airway thickness compared with male smokers.17 Younger women with COPD have more severe dyspnea and airflow limitation and 1.53 higher OR for exacerbations than younger men.18 Despite less cigarette smoking, older women are more likely to have more severe dyspnea and more severe COPD than older men.19 In three distinct female phenotypic subgroups (early-onset COPD, subjects with severe emphysema, and GOLD stage IV COPD), female subjects have radiographic emphysema comparable to male subjects despite significantly fewer pack-years of smoking.20
Figure 1.
Sex distribution according to age groups of subjects with an FEV1 < 50% predicted (n = 532). A female predominance is noted at both ends of the spectrum: ages 45 to 54 years and 75 to 80 years. (Adapted with permission from Foreman et al.16)
Moreover, longitudinal data in COPDGene revealed that female sex is independently associated with risk of acute episodes of respiratory disease independent of other relevant covariates.21 Therefore, women, compared with men, seem to be at greater risk for development of COPD and may present with more severe disease. Conversely, following adjustment for steroid use, age, pack-years of smoking, current smoking, and exacerbations, COPD and radiographic emphysema were associated with low volumetric bone mineral density and vertebral fractures, with male smokers having a small but significantly greater risk than female smokers.22 The reasons for these observations are incompletely understood but highlight the importance of evaluating sex differences in COPD.
Assessment of racial differences in COPD suggests that clinical expression of disease may vary along racial lines. Although health-related quality of life scores were similar for AA and NHW subjects with COPD without exacerbations, scores are worse for AA subjects who experience exacerbations, particularly exacerbations requiring hospitalization.23 According to quantitative CT scan parameters, however, AA subjects had less emphysema but the same degree of airway disease compared with NHW subjects.24 Common comorbidities, particularly gastroesophageal reflux disease and osteoarthritis, were associated with worse outcomes in AA subjects compared with NHW subjects.25
With the growing population of patients aged > 65 years, there is an increased interest in the effects of age in COPD. Parulekar et al26 explored the effects of age using data from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) and COPDGene cohorts. In this cross-sectional study, patients with COPD who were aged > 65 years had worse lung function and exercise tolerance, more frequent use of long-term oxygen therapy, and increased likelihood of having comorbidities compared with younger patients. Interestingly, older age was associated with decreased exacerbation rates and improved quality of life. It is possible that these findings may be the result of changes in patient expectations and an ability of older patients to adapt to the burden of the disease, but longitudinal studies are still required to better understand the impact of age on the progression of the disease.
Thus, sex, race, and age influence differences in how COPD is expressed. It is anticipated that ongoing analysis of the COPDGene longitudinal data will provide clarity to COPD expression and inform development of future studies targeting therapeutic interventions.
COPD Exacerbations
COPDGene used subject self-report to identify exacerbations in the 12 months prior to enrollment.1 Subsequent exacerbations during longitudinal follow-up were assessed by using automated telephone calls every 3 to 6 months.27 COPDGene evaluated exacerbations in relation to demographic characteristics, radiographic abnormalities, lung function, and biomarkers.
Although a previous study reported that exacerbations are associated with accelerated lung function decline, this study included subjects with moderate to severe disease, and the impact of exacerbations in mild disease was not clear.2 COPDGene explored the effect of COPD exacerbations on lung function decline over 5 years.28 Exacerbations in subjects with COPD were associated with decline in FEV1, and this effect was most pronounced in those with less severe airflow obstruction. For subjects with GOLD stage I, each moderate exacerbation was associated with an additional 23 mL per year decline (95% CI, 2-44; P = .03) in FEV1, whereas each severe event was associated with an additional 87 mL per year decline (95% CI, 23-151; P = .008). In subjects without COPD, acute respiratory events were not associated with decline in lung function. These findings suggest that special attention should be paid to patients with mild airflow obstruction and exacerbations, as these patients have the highest risk of lung function loss over time.
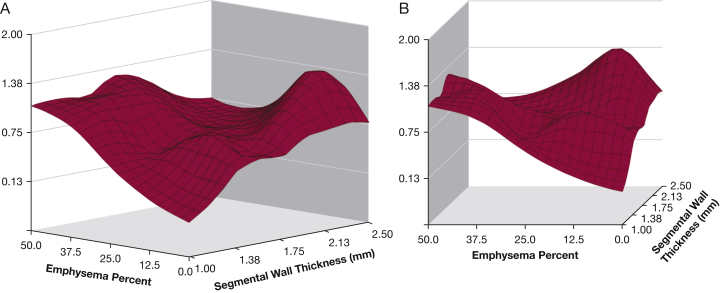
There is significant interest in identifying patients at risk of future exacerbations by using measures beyond lung function and exacerbation history. A study by Han et al29 evaluated different radiologic phenotypes and their associations with COPD exacerbations. Following adjustments for lung function, increasing bronchial wall thickness and total lung emphysema were associated with the frequency of COPD exacerbations (Fig 2). Subjects with ≥ 35% total emphysema had a 1.18-fold increase in the exacerbation rate for every 5% increase in emphysema (P = .047). Increases in bronchial thickness of 1 mm was linked to a 1.84-fold increase in the annualized COPD exacerbation rate (P = .004).
Figure 2.
Three-dimensional surface plots showing the relations between emphysema percentage, wall thickness, and COPD exacerbation frequency (vertical axis). A, Surface view of entire relation shows the largely independent effects of emphysema percentage and segmental wall thickness. B, Horizontal view emphasizes the increased exacerbation frequency at greater levels of emphysema. The very strong effect of the bronchial wall thickness at low levels of emphysema is also noted. (Adapted with permission from Han et al.29)
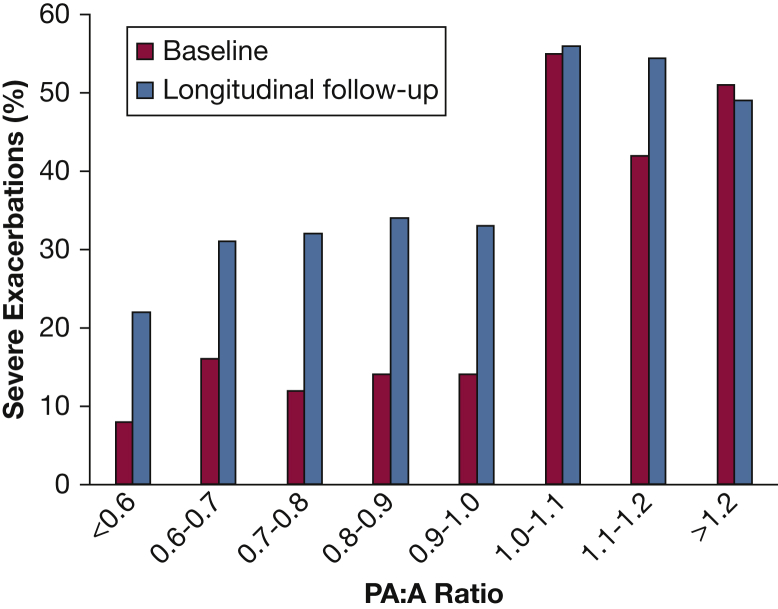
COPDGene has provided insight on novel methods of evaluating patients with COPD. For example, a study evaluated the impact of the pulmonary artery (PA) enlargement using the PA to aorta ratio (PA:A) ratio, a CT surrogate for pulmonary hypertension.30, 31 Subjects were classified on the basis of a PA:A ratio ≤ 1 or > 1. Importantly, PA enlargement was associated with poor lung function, the need for supplemental oxygen, comorbidities including congestive heart failure (CHF) and OSA, worse symptoms, and more exacerbations in the 12 months prior to enrollment (Fig 3). These results suggest that a simple CT measurement of PA enlargement can identify subgroups of patients with increased risk of exacerbation and more severe disease independent of lung function.
Figure 3.
Relation between the PA:A ratio and the occurrence of severe exacerbations at baseline and during follow-up in the Genetic Epidemiology of COPD (COPDGene) study. The rates of exacerbations are shown by increments of 0.1 unit. The risk increased for exacerbations at the threshold of PA:A ratio of 1. PA:A = pulmonary artery:aorta. (Adapted with permission from Wells et al.30)
Smokers not meeting criteria for COPD are usually excluded from lung disease studies. Nevertheless, these patients experience acute respiratory episodes. During follow-up of the COPDGene cohort, predictors of acute respiratory episodes in current and former smokers without COPD were evaluated.21 Although current and former smokers without COPD had lower rates of acute respiratory episodes compared with patients with COPD, they had similar risk factors, and there were more episodes in the former group. The strongest predictors for future acute respiratory episodes were baseline pulmonary symptoms, FEV1 percent predicted, and the number of episodes 12 months prior to enrollment. AA race was independently associated with severe episodes. These findings have important implications and suggest that other characteristics beyond lung function should be considered in smokers or former smokers without COPD to identify high-risk populations.
Utilizing data from the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) and the COPDGene cohorts, the value of 90 serum or plasma candidate proteins as predictors of exacerbations was investigated.32 Although clinical features, such as the degree of airflow limitation and previous exacerbations, were useful predictors of future exacerbations, no COPDGene biomarker proven to significantly add to the predictive value of clinical variables in predicting future exacerbations was validated in a similar independent cohort (SPIROMICS). Future studies are needed to determine the role of biomarkers as predictors of COPD exacerbations; these results, however, highlight the importance of clinical characteristics in assessing the risk for future exacerbations and the utility of replicating findings in large cohorts.
Comorbidities
The extensive collection of data regarding comorbidities and medication exposure during the recruitment phase of COPDGene and the longitudinal design served as an ideal platform for evaluating the impact of coexisting diseases on patients with COPD and different degrees of severity. A self-reported list of current medications was obtained and comorbid conditions were ascertained based on subject recall of a physician diagnosis except for obesity, which was based on BMI and imaging.1
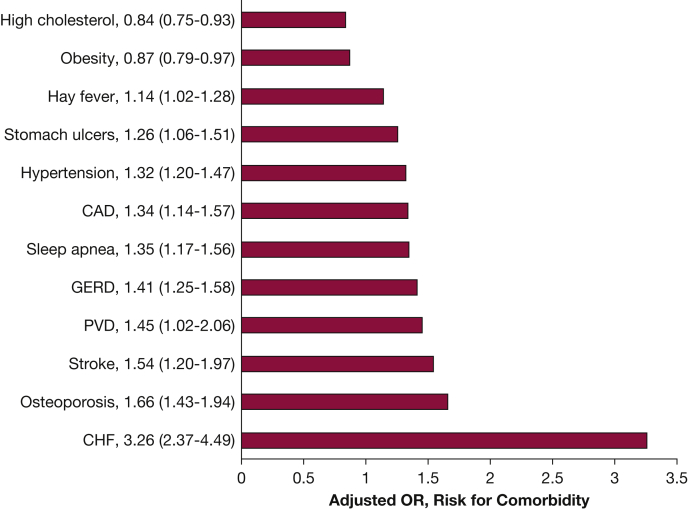
A study evaluating the burden and impact of various comorbidities among 8,078 individuals enrolled in COPDGene noted that compared with control subjects without COPD, comorbid conditions among COPD cases were significantly more prevalent (3.3 vs 2.4 conditions).25 Following adjustments for confounders, COPD case status was associated with increased risk of (in descending order of magnitude) CHF (OR, 3.36; 95% CI, 2.37-4.49), osteoporosis, stroke, peripheral vascular disease, gastroesophageal reflux disease, coronary artery disease, hypertension, OSA, stomach ulcers, and hay fever (Fig 4). Several comorbidities were independently associated with reduced 6-min walk distance (CHF, obesity, OSA, peripheral vascular disease, coronary artery disease, osteoporosis, diabetes mellitus [DM], and osteoarthritis) and worse dyspnea (OSA, CHF, stroke, obesity, stomach ulcers, gastroesophageal reflux disease, osteoporosis, and osteoarthritis). Furthermore, this report was one of the first detailing the disparate impact of comorbid conditions in AA subjects with COPD. Although comorbidities were less prevalent in AA subjects, when present, these diseases were associated with a higher risk of poor outcomes compared with NHW subjects. A subsequent study showed that the number of comorbidities added prediction ability for future clinical outcomes in COPD, a finding that was robust to internal and external validation.33 A simple count of comorbidities was shown to be as useful as more complex calculated comorbidity scores in assessing their impact on COPD. An increased number of comorbidities was associated with more respiratory symptoms and exacerbations, and lower exercise capacity.
Figure 4.
ORs with 95% CIs (in parentheses) for the risk of specific comorbidities based on COPD case status. Adjusted for age at enrollment, sex, race, pack-years smoked, and education level. For all conditions shown, COPD case status was significantly associated with increased risk for the condition with the exception of high cholesterol and obesity. CAD = coronary artery disease; CHF = congestive heart failure; GERD = gastroesophageal reflux disease; PVD = peripheral vascular disease. (Adapted with permission from Putcha et al.25)
Bronchiectasis is often seen in patients with COPD, and the bronchiectasis-COPD overlap is associated with worse prognosis than COPD alone. A COPDGene analysis has provided insight into the pulmonary circulation in smokers with mild bronchiectasis.34 Among 486 subjects, 155 (31.9%) had bronchiectasis on CT imaging, and those subjects with bronchiectasis had a loss of distal pulmonary vessels as measured by using a noninvasive imaging technique compared with those without this airway abnormality. Furthermore, subjects with bronchiectasis and loss of distal vessels had lower FEV1 and exercise capacity than those with bronchiectasis without loss of distal vessels, suggesting that abnormalities of the pulmonary vasculature might be of clinical relevance.
Low-body weight has been linked to poor outcomes in COPD. For instance, a study of the ECLIPSE and COPDGene cohorts evaluated the low fat-free mass index as an independent risk factor for mortality. The fat-free mass index was determined by using the pectoralis muscle area according to CT imaging, and it was shown that subjects with COPD and a low fat-free mass index had an increased risk of death (hazard ratio, 1.6; P < .001).35 It is incompletely understood if individuals with obesity have similarly increased risk. Using data from the chest CT scans, Diaz et al36 evaluated the abdominal visceral and subcutaneous adipose tissue of 1,267 individuals with COPD. Those with increased abdominal visceral adipose tissue had increased odds of myocardial infarction history after adjusting for multiple variables. These findings were supported by a study of 3,631 subjects from COPDGene that explored outcomes in individuals with COPD based on BMI values.37 Obesity (BMI ≥ 30 kg/m2) occurred in 35% of individuals, and the prevalence of comorbid conditions increased with increasing obesity class. Obesity was independently associated with worse respiratory-specific and general quality of life, reduced 6-min walk distance, increased dyspnea, and increased risk of severe exacerbations in a dose-dependent manner based on obesity class and independent of the presence of other comorbidities.
Exploring the radiographic phenotypes of COPD, a study using the COPDGene cohort found that DM was more prevalent in airway-predominant COPD, defined as a 1.75-mm bronchial wall thickness, compared with an emphysema-predominant group (9.3% vs 19.5%; P = .03).29, 38 In the COPDGene cohort, DM was not only common, but it was also found to be associated with a modest decrease in lung function, reduced exercise tolerance, and poor quality of life.39 A longitudinal evaluation of 7,080 individuals from COPDGene explored factors related to incident DM in 392 individuals who did not report the disease at enrollment but who reported the development of DM at the 5-year follow-up visit.40 These individuals had higher BMI, higher rates of hypertension and hyperlipidemia, decreased 6-min walk distances, and increased COPD exacerbations. These findings have important implications regarding screening for DM and selection of therapy, particularly in patients with COPD and non-CB. For example, the phosphodiesterase-4 inhibitor roflumilast has been linked to improved glucose control.41 Although COPDGene provided evidence that DM has a deleterious effect on COPD, future studies should explore if adequate glucose control is associated with improved outcomes in COPD.
Black-Shinn et al42 evaluated the first 2,500 COPDGene subjects and reported that cardiovascular disease (CVD) was independently related to the presence of COPD (OR, 1.61; 95% CI, 1.2-2.2; P = .01) and increased prevalence of reported hospitalization due to exacerbation in the past year. Subjects with CVD also had more respiratory symptoms and shorter 6-min walk distances. The data suggest a strong influence of CVD on various outcomes and COPD, and subjects with coexisting disease should be monitored closely. Moreover, a study exploring the use of beta-blockers in 3,464 COPDGene individuals revealed that exposure to this class of medications was in fact associated with a significant reduction in COPD exacerbations after adjusting for underlying CVD.43 Importantly, other antihypertensive agents were not associated with this reduction. These data add to the growing evidence that beta-blockers are not only safe in COPD but may also have potential benefits in patients with coexisting COPD and CVD. Shared risk factors, such as smoking and age, do not fully explain the associations of these conditions, and it is possible that activated inflammatory pathways may affect the cardiovascular system in a subgroup of patients.44
Martinez et al45 found that gastroesophageal reflux disease, which occurred in approximately one-third of the COPDGene subjects, was associated with poor quality of life and more frequent COPD exacerbations. These observations persisted after adjusting for medication use, such as proton pump inhibitors, and other covariates.
The results from COPDGene exploring the associations of comorbidities with COPD prevalence and morbidity highlight the importance of these diseases in a wide range of outcomes. From the clinical perspective, understanding the influence of various comorbidities should be an integral part of the care of patients with COPD.
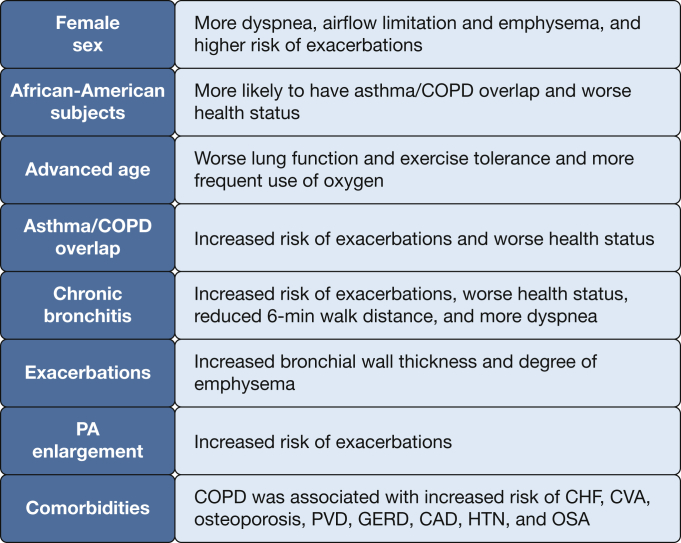
Implications for Disease Classification and Treatment
The GOLD statement currently categorizes COPD based on lung function, the history of exacerbations, and respiratory symptoms.46 Although this classification has been widely accepted, evidence suggests that there are many different phenotypes of COPD beyond these three patient characteristics. COPDGene has provided further insights regarding the risk of specific outcomes (Fig 5). Some predictors are nonmodifiable, such as age, race, and sex, and the presence of CB and ACO. Others may be modifiable, such as tobacco exposure and comorbidities that can be treated. In addition, in all patients newly diagnosed with COPD, taking into consideration the patient’s history, exposures, physical examination, and lung function, other studies (eg, chest radiograph, chest CT scan, echocardiogram) may provide additional information relevant to the diagnosis and individualized treatment approach.
Figure 5.
Epidemiologic associations and findings of COPDGene. CVA = cerebrovascular accident; HTN = hypertension. See Figure 3 and 4 legends for expansion of other abbreviations.
Limitations
COPDGene has several limitations that deserve mention. First, several of the variables, such as previous exacerbations and comorbid conditions, were based on self-report and may be subject to recall bias or physician misdiagnosis; particularly in the setting of asthma, the diagnosis can be subject to misclassification. Second, the study is limited by the loss of some subjects during the follow-up time. This loss is common in longitudinal observational studies but may bias the estimations caused by missing data points. Furthermore, missing subjects potentially may represent a more advanced disease subgroup. Third, some biomarkers (ie, IgE), were tested in only a subgroup of patients based on ancillary protocols specific to one or several study sites. It is unclear if these findings are applicable to all study sites. Finally, COPDGene is limited by its observational design, and the findings described here may not be generalizable to other patient populations.
Future Directions and Research Needs
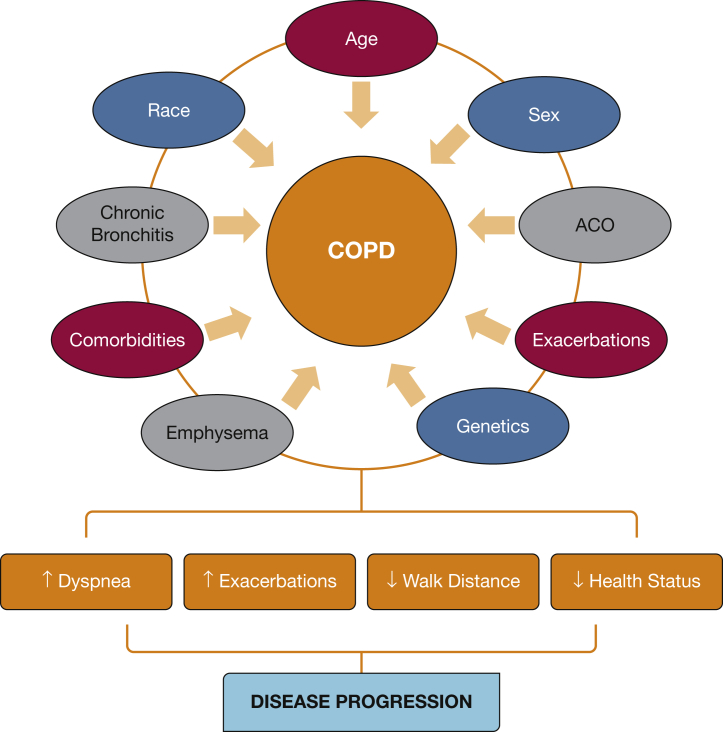
COPDGene is ongoing, and phase III, recently funded by the NHLBI, will invite subjects to return for a third visit 10 years following their initial enrollment. The longitudinal evaluation of this richly characterized cohort will continue to provide additional information regarding the natural history of the disease and implications of subject characteristics on various outcomes. Because the subjects were assessed in a multidimensional fashion, demographic characteristics, symptom scores, comorbidities, pulmonary function, imaging, biomarker, and genetic profiling data will be further evaluated to enhance our understanding of the complex structural and functional changes underpinning the evolution of this chronic disease (Fig 6). The long-term follow-up will enable a better understanding of the natural history of the symptomatic smokers and disease progression in those with airflow obstruction. With increasing use of powerful statistical and deep learning tools, we anticipate that the COPDGene study will uncover additional risk factors for disease progression and contribute to the precision medicine of subjects with COPD.
Figure 6.
Complex and multifactorial influence of demographic characteristics, comorbidities, emphysema, chronic bronchitis, and genetics on the disease progression of COPD. ACO = asthma/COPD overlap.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: D. J. M. reports consulting fees for GlaxoSmithKline, Sunovion, Sanofi/Regeneron, AstraZeneca, and Novartis. A. A. reports consultancy for Boehringer Ingelheim, AstraZeneca, Novartis, and Sunovion. S. P. B. is supported by the National Institutes of Health (NIH) [K23HL133438] and has received research grants from AstraZeneca and ProterixBio. R. P. B. reports consulting fees for Boehringer Ingelheim, AstraZeneca, and GlaxoSmithKline. D. L. D. reports consulting fees from Novartis and grants from the NIH. A. A. D. reports research grants from the NIH and Brigham and Women’s Hospital; and personal fees from Novartis. M. T. D. has received grant support from the NIH and Department of Defense; consulting fees from AstraZeneca, Boehringer Ingelheim, Genentech, and GlaxoSmithKline; and contracted clinical trial support from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, PneumRx/BTG, Pulmonx, and Yungjin. N. A. H. reports consulting fees for Roche, Teva, Sanofi, Boehringer Ingelheim, and Novartis; and research support from Chiesi, Boehringer Ingelheim, GlaxoSmithKline, and Roche. C. P. H. reports consulting fees from AstraZeneca, Concert Pharmaceuticals, Mylan, and 23andMe; and research grants from Boehringer Ingelheim and Novartis. V. K. reports grants from the NHLBI; and consulting fees from Medscape, CSA Medical, AstraZeneca, Concert Pharmaceuticals, the American Board of Internal Medicine, and Gala Therapeutics. N. P. is supported by the NIH/NHLBI [K23HL123594]. J. M. W. reports grants from the NIH/NHLBI [K08HL123940], the Cystic Fibrosis Foundation [SORCH15RO], Bayer AG, and UAB Health Services Foundation; and consulting fees from Quintiles, GlaxoSmithKline, Mereo BioPharma, and Mylan. E. K. S. reports grants from the NIH; personal fees from Novartis; and grant and travel support from GlaxoSmithKline. M. K. H. reports support from the NIH, and the COPD Foundation; consulting fees from GlaxoSmithKline, Boehringer Ingelheim, Novartis, AstraZeneca, and Sunovion; and research support by the NIH/NHLBI, Novartis, and Sunovion. B. J. M. reports grants and personal fees from the NHLBI, AstraZeneca, Spiration, Sunovion, Novartis, CSL Behring, Verona, Boehringer Ingelheim, Theravance, and Circassia; and support for Continuing Medical Education activities from Consensus Medical Education, Integrity Medical Education, Mt. Sinai Medical Center, WebMD, UpToDate, National Jewish Health, SPIRE Learning, the American College of Chest Physicians, Projects in Knowledge, Hybrid Communications, Peer Review Institute, Cleveland Clinic, Medscape, and Ult Medical Academy. Nothing declared (A. F., M. G. F., G. L. K., E. S. W., G. E. W., K. A. Y.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors would like to acknowledge the generous support to the COPDGene study (NCT00608764) from the National Heart, Lung, and Blood Institute (Grant U01 HL089897 and U01 HL089856) and the support of the COPD Foundation through contributions made to an Industry Advisory Committee composed of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Sunovion.
Footnotes
FUNDING/SUPPORT: This research was supported by the National Heart, Lung, and Blood Institute [Grants U01 HL089897 and U01 HL089856]. The COPDGene study (NCT00608764) is also supported by the COPD Foundation through contributions made to an Industry Advisory Committee composed of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Sunovion.
References
- 1.Regan E.A., Hokanson J.E., Murphy J.R. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vestbo J., Anderson W., Coxson H.O. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) Eur Respir J. 2008;31(4):869–873. doi: 10.1183/09031936.00111707. [DOI] [PubMed] [Google Scholar]
- 3.Couper D., LaVange L.M., Han M. Design of the subpopulations and intermediate outcomes in COPD study (SPIROMICS) Thorax. 2014;69(5):491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverman E.K., Vestbo J., Agusti A. Opportunities and challenges in the genetics of COPD 2010: an International COPD Genetics Conference report. COPD. 2011;8(2):121–135. doi: 10.3109/15412555.2011.558864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foreman M.G., Wilson C., DeMeo D.L. Alpha-1 antitrypsin PiMZ genotype is associated with chronic obstructive pulmonary disease in two racial groups. Ann Am Thorac Soc. 2017;14(8):1280–1287. doi: 10.1513/AnnalsATS.201611-838OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim V., Han M.K., Vance G.B. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene study. Chest. 2011;140(3):626–633. doi: 10.1378/chest.10-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim V., Davey A., Comellas A.P. Clinical and computed tomographic predictors of chronic bronchitis in COPD: a cross sectional analysis of the COPDGene study. Respir Res. 2014;15:52. doi: 10.1186/1465-9921-15-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim V., Zhao H., Boriek A.M. Persistent and newly developed chronic bronchitis are associated with worse outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(7):1016–1025. doi: 10.1513/AnnalsATS.201512-800OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferris B.G. Epidemiology standardization project (American Thoracic Society) Am Rev Respir Dis. 1978;118(6 pt 2):1–120. [PubMed] [Google Scholar]
- 10.Kim V., Crapo J., Zhao H. Comparison between an alternative and the classic definition of chronic bronchitis in COPDGene. Ann Am Thorac Soc. 2015;12(3):332–339. doi: 10.1513/AnnalsATS.201411-518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez C.H., Kim V., Chen Y. The clinical impact of non-obstructive chronic bronchitis in current and former smokers. Respir Med. 2014;108:491–499. doi: 10.1016/j.rmed.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardin M., Silverman E.K., Barr R.G. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardin M., Cho M., McDonald M.L. The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J. 2014;44(2):341–350. doi: 10.1183/09031936.00216013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosentino J., Zhao H., Hardin M. Analysis of asthma-chronic obstructive pulmonary disease overlap syndrome defined on the basis of bronchodilator response and degree of emphysema. Ann Am Thorac Soc. 2016;13(9):1483–1489. doi: 10.1513/AnnalsATS.201511-761OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayden L.P., Hardin M.E., Qiu W. Asthma is a risk factor for respiratory exacerbations without increased rate of lung function decline: five-year follow-up in adult smokers from the COPDGene study. Chest. 2018;153(2):368–377. doi: 10.1016/j.chest.2017.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foreman M.G., Zhang L., Murphy J. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene study. Am J Respir Crit Care Med. 2011;184(4):414–420. doi: 10.1164/rccm.201011-1928OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y.I., Schroeder J., Lynch D. Gender differences of airway dimensions in anatomically matched sites on CT in smokers. COPD. 2011;8(4):285–292. doi: 10.3109/15412555.2011.586658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Busch R., Han M.K., Bowler R.P. Risk factors for COPD exacerbations in inhaled medication users: the COPDGene study biannual longitudinal follow-up prospective cohort. BMC Pulm Med. 2016;16:28. doi: 10.1186/s12890-016-0191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sørheim I.C., Johannessen A., Gulsvik A., Bakke P.S., Silverman E.K., DeMeo D.L. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax. 2010;65(6):480–485. doi: 10.1136/thx.2009.122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardin M., Foreman M., Dransfield M.T. Sex-specific features of emphysema among current and former smokers with COPD. Eur Respir J. 2016;47(1):104–112. doi: 10.1183/13993003.00996-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowler R.P., Kim V., Regan E. Prediction of acute respiratory disease in current and former smokers with and without COPD. Chest. 2014;146(4):941–950. doi: 10.1378/chest.13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaramillo J.D., Wilson C., Stinson D.S. Reduced bone density and vertebral fractures in smokers. Men and COPD patients at increased risk. Ann Am Thorac Soc. 2015;12(5):648–656. doi: 10.1513/AnnalsATS.201412-591OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han M.K., Curran-Everett D., Dransfield M.T. Racial differences in quality of life in patients with COPD. Chest. 2011;140(5):1169–1176. doi: 10.1378/chest.10-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansel N.N., Washko G.R., Foreman M.G. Racial differences in CT phenotypes in COPD. COPD. 2013;10(1):20–27. doi: 10.3109/15412555.2012.727921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putcha N., Han M.K., Martinez C.H. Comorbidities of COPD have a major impact on clinical outcomes, particularly in African Americans. Chronic Obstr Pulm Dis. 2014;1(1):105–114. doi: 10.15326/jcopdf.1.1.2014.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parulekar A.D., Martinez C., Tsai C.L. examining the effects of age on health outcomes of chronic obstructive pulmonary disease: results from the Genetic Epidemiology of Chronic Obstructive Pulmonary Disease Study and evaluation of chronic obstructive pulmonary disease longitudinally to identify predictive surrogate endpoints cohorts. J Am Med Dir Assoc. 2017;18(12):1063–1068. doi: 10.1016/j.jamda.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart J.I., Moyle S., Criner G.J. Automated telecommunication to obtain longitudinal follow-up in a multicenter cross-sectional COPD study. COPD. 2012;9(5):466–472. doi: 10.3109/15412555.2012.690010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dransfield M.T., Kunisaki K.M., Strand M.J. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(3):324–330. doi: 10.1164/rccm.201605-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han M.K., Kazerooni E.A., Lynch D.A. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells J.M., Washko G.R., Han M.K. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–921. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung K.S., Kim Y.S., Kim S.K. Functional and prognostic implications of the main pulmonary artery diameter to aorta diameter ratio from chest computed tomography in Korean COPD patients. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keene J.D., Jacobson S., Kechris K. Biomarkers predictive of exacerbations in the SPIROMICS and COPDGene cohorts. Am J Respir Crit Care Med. 2017;195(4):473–481. doi: 10.1164/rccm.201607-1330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Putcha N., Puhan M.A., Drummond M.B. A simplified score to quantify comorbidity in COPD. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz A.A., Maselli D.J., Rahaghi F. Pulmonary vascular pruning in smokers with bronchiectasis. ERJ Open Res. 2018;4(4) doi: 10.1183/23120541.00044-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald M.N., Diaz A.A., Rutten E. Chest computed tomography-derived low fat-free mass index and mortality in COPD. Eur Respir J. 2017;50(6) doi: 10.1183/13993003.01134-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diaz A.A., Young T.P., Kurugol S. Abdominal visceral adipose tissue is associated with myocardial infarction in patients with COPD. Chronic Obstr Pulm Dis. 2015;2(1):8–16. doi: 10.15326/jcopdf.2.1.2015.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert A.A., Putcha N., Drummond M.B. Obesity is associated with increased morbidity in moderate to severe COPD. Chest. 2017;151(1):68–77. doi: 10.1016/j.chest.2016.08.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hersh C.P., Make B.J., Lynch D.A. Non-emphysematous chronic obstructive pulmonary disease is associated with diabetes mellitus. BMC Pulm Med. 2014;14:164. doi: 10.1186/1471-2466-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinney G.L., Black-Shinn J.L., Wan E.S. Pulmonary function reduction in diabetes with and without chronic obstructive pulmonary disease. Diabetes Care. 2014;37(2):389–395. doi: 10.2337/dc13-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinney G.L., Baker E.H., Klein O.L. Pulmonary predictors of incident diabetes in smokers. Chronic Obstr Pulm Dis. 2016;3(4):739–747. doi: 10.15326/jcopdf.3.4.2016.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wouters E.F., Bredenbröker D., Teichmann P. Effect of the phosphodiesterase 4 inhibitor roflumilast on glucose metabolism in patients with treatment-naive, newly diagnosed type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97(9):E1720–E1725. doi: 10.1210/jc.2011-2886. [DOI] [PubMed] [Google Scholar]
- 42.Black-Shinn J.L., Kinney G.L., Wise A.L. Cardiovascular disease is associated with COPD severity and reduced functional status and quality of life. COPD. 2014;11(5):546–651. doi: 10.3109/15412555.2014.898029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhatt S.P., Wells J.M., Kinney G.L. β-Blockers are associated with a reduction in COPD exacerbations. Thorax. 2016;71(1):8–14. doi: 10.1136/thoraxjnl-2015-207251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Aymerich J., Gómez F.P., Benet M. Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypes. Thorax. 2011;66(5):430–437. doi: 10.1136/thx.2010.154484. [DOI] [PubMed] [Google Scholar]
- 45.Martinez C.H., Okajima Y., Murray S. Impact of self-reported gastroesophageal reflux disease in subjects from COPDGene cohort. Respir Res. 2014;15:62. doi: 10.1186/1465-9921-15-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Global Strategy for Asthma Management and the Prevention and the Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. 2019 update. https://goldcopd.org/gold-reports/