Abstract
The Cambrian Explosion was a key event in the evolution of life on Earth. This event took place at a time when sea surface temperatures have been proposed to reach about 60 °C. Such high temperatures are clearly above the upper thermal limit of 38 °C for modern marine invertebrates and preclude a major biological revolution. To address this dichotomy, we performed in situ δ18O analyses of Cambrian phosphatic brachiopods via secondary ion mass spectrometry (SIMS). The δ18Ophosphate data, which are considered to represent the most primary δ18Oseawater signature, were identified by evaluating the diagenetic alteration of the analyzed shells. Assuming ice-free conditions for the Cambrian ocean and no change in δ18Oseawater (-1.4‰ to -1‰; V-SMOW) through time, our temperatures vary between 35 °C ± 12 °C and 41 °C ± 12 °C. They are thus clearly above (1) recent subequatorial sea surface temperatures of 27 °C–35 °C and (2) the upper lethal limit of 38 °C of marine organisms. Our new data can therefore be used to infer a minimal depletion in early Cambrian δ18Oseawater relative to today of about -3‰. With this presumption, our most pristine δ18Ophosphate values translate into sea surface temperatures of about 30 °C indicating habitable temperatures for subequatorial oceans during the Cambrian Explosion.
Subject terms: Palaeoceanography, Environmental impact, Marine chemistry
Introduction
The Cambrian Explosion was one of the most important events in the history of life on Earth. Within a few million years, the simple life of the Precambrian evolved to highly organized organisms with modern anatomical characteristics such as a chorda dorsalis, complex eyes and legs, and biomineralized skeletal parts1–5. During this fundamental biotic event, the metazoans invaded all kinds of marine habitats and evolved complex ecosystems and food webs for the first time6,7. Insights into this often odd-looking Cambrian world are provided by a variety of global fossil Lagerstätten such as the Burgess Shale (Canada)8, Chengjiang (South China)9, or Sirius Passet (Greenland)10 (Fig. 1). The reasons for this radical faunal turnover are still poorly understood but changes in ocean chemistry11,12, the rise in atmospheric and oceanic oxygen concentration to modern levels13,14, and the presumed evolutionary arms race between predators and prey15,16 have been discussed as major driving mechanisms. Additionally, the establishment of habitable seawater temperatures seems to be most essential for the evolution of diverse marine metazoan life. Information on marine paleotemperatures is inferred from studies of oxygen isotopes (δ18O) from bulk carbonate samples, marine cements, cherts, phosphorites, or fossils of calcitic or phosphatic composition. However, independent of the material analyzed, these oxygen isotope ratios become progressively depleted in the heavier 18O towards older stratigraphic units17–20, resulting in a rising trend of reconstructed seawater temperatures with increasing age. For the Cambrian, temperatures of about 60 °C or even higher have been proposed17–23, which is intolerable for most animals in modern oceans. Whether this trend reflects (1) an increased diagenetic alteration of older rocks, (2) secular changes in the oxygen isotopic composition of ancient ocean seawater, or (3) higher original seawater temperatures in the past, is still controversial17–23. If seawater oxygen isotope composition is considered as constant throughout Earth history, a δ18O value of ~ -1‰ is generally assumed to characterize an ice-free planet24. In contrast, assuming that seawater δ18O changed substantially over time, a global average of Cambrian seawater δ18O of up to -6.5‰ or even -8‰ has been proposed25. These presumptions would shift the reconstructed temperature of ancient seawater towards higher or lower values, respectively.
Figure 1.
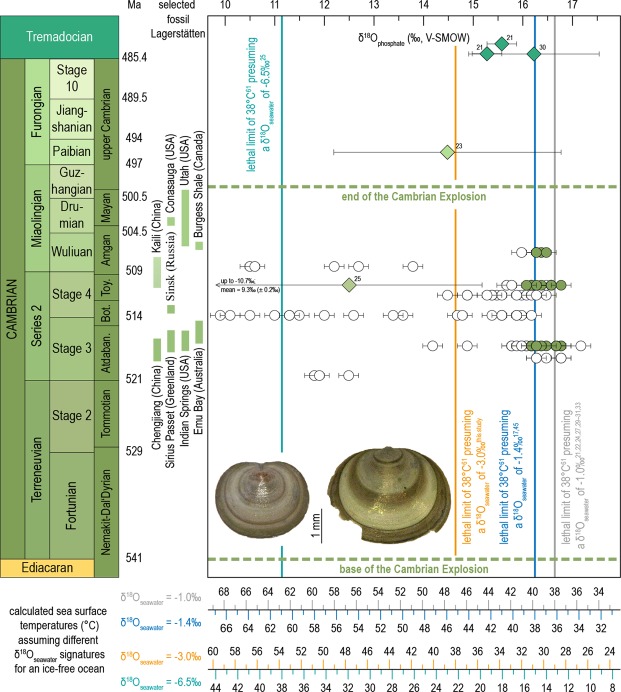
Oxygen isotope compositions of Cambrian and Tremadocian brachiopods and conodonts. Our δ18Ophosphate data range from 9.9‰ to 17.2‰ (V-SMOW; 1σ ± 0.2‰). Calculated seawater surface temperatures assuming δ18Oseawater signatures of -1.0‰21,22,24,27,29–31,33 and -1.4‰17,45 vary between 34 °C ± 12 °C and 68 °C ± 11 °C. Excluding altered and probably altered δ18Ophosphate data, our calculated sea surface temperatures range from 35 °C ± 12 °C to 41 °C ± 12 °C. In contrast, assuming a Cambrian δ18Oseawater signature of -6.5‰25, reconstructed sea surface temperatures are incredible low, ranging from 12 °C ± 14 °C to 16 °C ± 14 °C. Our new data therefore clearly support the hypothesis of a secular change in the ocean δ18O during Earth history. A minimal depletion in early Cambrian δ18Oseawater relative to today of about -3‰ is assumed. With this presumption, our most pristine δ18Ophosphate values translate into sea surface temperatures of 28 °C ± 13 °C to 32 °C ± 13 °C. Our most pristine δ18Ophosphate values are illustrated by green circles, (probably) altered data by white circles. Additional data points of the Cambrian Series 2–Tremadocian were generated from bulk sample analyses of conodonts and brachiopods23,30 and in situ measurements of conodonts21 and brachiopods25. SIMS data of ref.25 vary between -10.6‰ (±0.1‰) and 14.8‰ (±0.4‰); mean = 9.3‰ (±0.2‰). Global Cambrian time scale and selected fossil Lagerstätten are from ref.38,43. Siberian nomenclature from ref.41. Atdaban. = Atdabanian Stage, Bot. = Botoman Stage, Toy. = Toyonian Stage.
Oxygen isotopes of marine carbonates (δ18Ocarbonate) have been used for studies of seawater temperatures since the beginning of geochemical research. One drawback of carbonate mineralogy is its susceptibility to diagenetic alteration, making the application of δ18Ocarbonate more doubtful for older stratigraphic successions which are typically characterized by intense alteration and recrystallization. The analysis of phosphate minerals (i.e. apatite), which are considered to be more resistant to diagenetic processes than carbonate, are therefore an attractive alternative, or at least a complementary tool, for assessing paleoclimate conditions18,26,27. In Paleozoic successions, conodonts and phosphatic brachiopods have been used in addition to calcite-shelled brachiopods to provide information about the seawater temperatures via their oxygen isotope composition with high stratigraphic resolution27–29. Seawater temperatures calculated from δ18Ophosphate vary between 36 and 53 °C (or even 62 °C) for the late Cambrian (brachiopods)23 and between 33 and 41 °C for the Early Ordovician (Tremadocian; brachiopods and conodonts)30, in both cases assuming an ice-free ocean and no significant change in δ18Oseawater over time. However, the traditional δ18O method of analysis of phosphates requires bulk samples of about 0.5–1 mg29,31 which equates to several hundred conodont elements or several complete brachiopod shells depending on their size and weight. The pooling of various specimens may result in significant problems. Even if carefully evaluated, sampling of altered material cannot be avoided with certainty as these areas are not observable macroscopically. Incorporation of only a fraction of altered sample material will result in δ18Ophosphate data that do not represent the primary oxygen isotopic composition of ambient seawater. A possible alternative may be the analysis of clumped isotopes, which could allow the discrimination of the diagenetic isotopic signal from the primary one32. A critical drawback of this method, however, is the enormous sample size of up to 12 mg and 200 mg required for calcite and phosphate samples, respectively32. Against this background, in situ measurements of oxygen isotopes have been applied using SIMS and similar high precision in situ analyses21,25,33–37, which minimize sample sizes and therefore the risk of contamination of the primary oxygen isotope signal. Again, assuming ice-free conditions and no significant variation in seawater δ18O over time, the corresponding values of Ordovician δ18Oconodont in such analyses range from 15.3‰ to 19.6‰ (V-SMOW)21. Temperatures calculated from these values vary from 25 °C to 44 °C21. Even higher calculated temperatures are reported from middle Cambrian brachiopods with a mean value of 71 °C ± 11 °C and a minimum temperature of 46 °C ± 10 °C25. However, assuming a Cambrian δ18O seawater composition of -6.5‰ these Cambrian temperatures were re-calculated to a minimum seawater temperature of 22 °C ± 10 °C (mean value of 47 °C ± 12 °C) and interpreted to represent the original seawater temperature of early–middle Cambrian southern latitudes (65°S to 70°S)25.
Here we present a study of in situ oxygen isotope analyses of Cambrian phosphatic brachiopods using the SIMS technique (CAMECA ims1280 at the Nordsim laboratory, Swedish Museum of Natural History, Stockholm), in order to shed new light on the unexpected high seawater temperatures estimated for the Cambrian Period and their aforementioned implications.
Stratigraphic Setting and Material
Thirteen exceptionally conserved Cambrian lingulid and acrotetid brachiopod shells (Fig. 1; Supplementary Material) were analyzed for their oxygen isotopic composition. The shells are composed of fluorapatite. They come from the Siberian Platform which is characterized by an almost horizontally bedded Cambrian sedimentary succession showing no or only minor metamorphic or tectonic overprint. Within the sedimentary rocks covering the Terreneuvian–Miaolingian38 interval, three distinct facies realms are developed, characterizing an eastward deepening of the depositional environment39,40. From west to east these are the restricted–lagoonal Turukhansk-Irkutsk-Olekma, the open marine Yudoma-Olenek, and the transitional Anabar-Sinsk facies realms (Fig. 2). The thirteen brachiopods analyzed herein belong to the open marine Yudoma-Olenek facies realm. Sections investigated are located along the Malaya Kuonamka (sections 96-1 and 96B-1) and Bol’shaya Kuonamka (sections 96-7, 96B-7, and 96-8) rivers on the eastern flank of the Anabar Uplift of the Siberian Platform (Fig. 2). The fauna from the sections includes trilobites, brachiopods, echinoderms, and other shelly fossils41 typical for shallow marine Cambrian carbonate environments with normal salinity.
Figure 2.
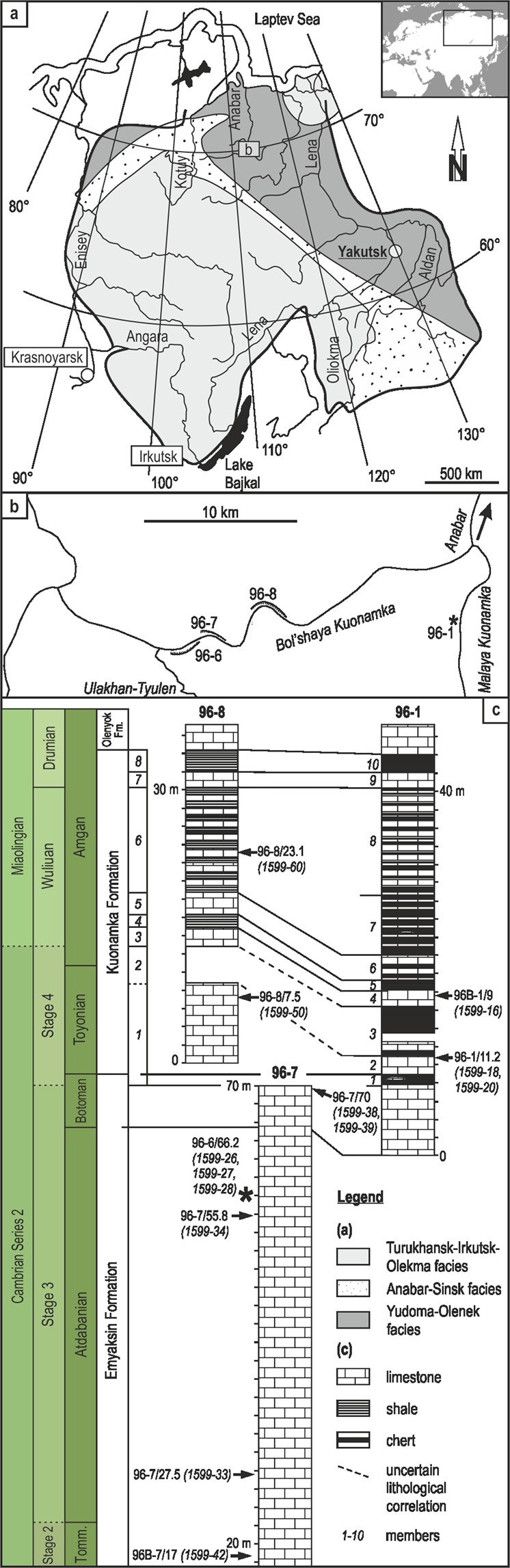
Geology of the sample area and stratigraphy of the samples. (a) Geological map of the northern Siberian Platform. (b) Geographic positions of the sections investigated. Section 96-1 is located on the Malaya Kuonamka River, sections 96-6, 96-7, and 96-8 are located on the Bol’shaya Kuonamka River. (c) Stratigraphic columns of the sections investigated. Derivation of brachiopod samples analyzed herein are marked by arrows with numbers. Tomm. = Tommotian Stage, Fm. = Formation.
Samples cover the Tommotian–Amgan interval (according to the Siberian nomenclature41,42) respectively the Terreneuvian (Cambrian Stage 2)–Miaolingian (Wuliuan Stage) interval (according to the international nomenclature38,43; Figs 1 and 2), and thus offer a unique window into the time of the Cambrian Explosion.
Results
Calculation of seawater temperatures
Ninety-six spots on 13 brachiopod shells were analyzed for their oxygen isotopic composition. The δ18Ophosphate values obtained range from 9.9‰ to 17.2‰ (V-SMOW; 1σ ± 0.2‰). Oxygen isotope data vary between specimens, but also show intra-sample variations of about 3‰ up to 6‰ (samples 1599-16, 1599-38, 1599-39; see Supplementary Table 1). For calculation of paleotemperature we applied the equation of Lécuyer and co-authors44. The major part of the Terreneuvian–Miaolingian interval is considered as an ice-free period. Assuming (1) an ice-free Cambrian ocean and therefore a δ18Oseawater value of -1.0‰21,22,24,27,29–31,33 or -1.4‰17,45, and (2) considering error propagation, our reconstructed sea surface temperatures vary between 34 °C ± 12 °C and 68 °C ± 11 °C, (Fig. 1, and see Supplementary Material for additional information). In contrast, presuming an increasing δ18Oseawater over time and a global Cambrian ocean average δ18O of -6.5‰25, calculated temperatures become incredible cooler, ranging from 11 °C ± 14 °C to 44 °C ± 12 °C (Fig. 1).
Diagenetic constraints
It is generally considered, that phosphate minerals are less susceptible to diagenetic alteration than carbonates. However, several studies indicate that skeletal phosphate minerals are metastable and were typically recrystallized during early diagenesis17,46,47. Whereas no widespread diagenetic processes are known to result in an enrichment of 18O in phosphates17, more negative δ18O values are generally considered as a sign of diagenetic alteration27.
In our samples, reflected light optical images already indicate recrystallization of individual shells and shell portions, which are characterized by considerably darker color and inhomogeneous appearance (Supplementary Material). SEM photographs confirm this first order identification by revealing the brachiopod shell ultrastructure in detail (Fig. 3; Supplementary Material). Dense crystalline shell material without any indication for recrystallization and alteration is characterized by δ18Ophosphate values generally more positive than 16.0‰, which thus appear to represent the most primary δ18Oseawater signature. Pores, laminae, and secondary fissures in other shell portions potentially act as pathways for fluid migration and therewith for diagenetic alteration. The visually identified recrystallized shells or portions of shells often, if not ubiquitously, yield lower δ18Ophosphate values, and are thus interpreted as most probably diagenetically altered (see Fig. 1 and Supplementary Material). Even for spots showing only initial recrystallization or partially pores, diagenetic alteration cannot be excluded with certainty. All these (probably) altered data are not included in our final discussion. The co-occurrence of both, dense crystalline and recrystallized areas in one and the same shell also explains large intra-shell variations as identified in samples 1599-16, 1599-38, or 1599-39.
Figure 3.
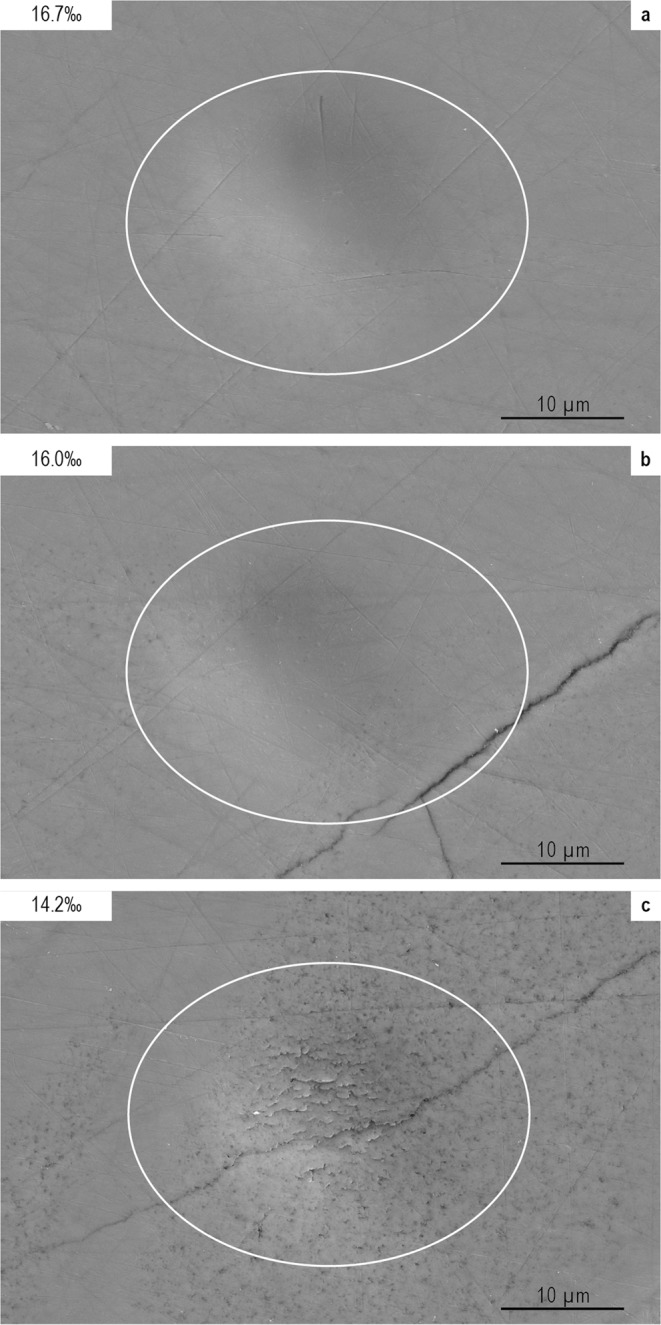
SEM images of selected spots from sample 1599-28 showing different stages of alteration. (a) Dense material (e.g., spot 1; 16.7‰) without any indication for recrystallization processes is generally characterized by more positive δ18Ophosphate values. We interpret such parts as representing the most primary δ18Oseawater signature. (b) Slightly more negative δ18Ophosphate values (e.g., spot 3; 16.0‰) most probably mirror diagenetic alteration caused by pores, fractures, or laminae acting as pathways for fluid migration. (c) Fractures and recrystallized shell material correspond to a considerably lower δ18Ophosphate values (spot 6; 14.2%), thus appear to represent diagenetically altered shell portions.
Analytical constrains
Despite the obvious advantages of δ18O analyses on biogenic apatite using SIMS (e.g., analyses of very small samples or distinct shell portions), a number of sources for probable errors have to be addressed, not all of which are presently fully understood. The general chemical formula of apatite Ca5(PO4, CO3)3(CO3, F, OH) contains three common oxygen bearing molecular groups. Whereas δ18Ophosphate analyses performed by the conventional IRMS method only isolate the PO43− group as trisilverphosphate (Ag3PO4) and subsequently analyze the oxygen isotopes by high-temperature reduction27,29,31,37,48, the SIMS technique indiscriminately samples oxygen from PO43−, CO32−, OH−, as well as residual organics36,49. The integration of oxygen from these different sources will likely influence the SIMS-determined δ18Ophosphate values. This problem is relevant when analyzing e.g. organo-phosphatic brachiopods characterized by an alternation of carbonate-fluorapatite and organic-rich laminae. Studies on recent and fossil lingulid brachiopods have documented intra-shell δ18Ophosphate variations often exceeding 4‰ that probably represent vital fractionation effects50. Similar intra-element δ18Ophosphate variations were detected from conodonts36. Therefore, care should be taken when interpreting δ18Ophosphate values generated by SIMS. However, variations in δ18Ophosphate can be minimized by high spatial resolution SIMS analyses on the most pristine areas36.
Multitude of paired SIMS and IRMS δ18Ophosphate analyses on conodonts have shown, that SIMS normally yields values 0.5–1.0‰ higher21,33,35,36. There is still uncertainty about the systematic offset between both methods in measuring the δ18Ophosphate values in phosphate brachiopods. This unknown offset would of course bias the calculation of paleotemperatures to higher values. In this context it should be also mentioned, that coefficients in the available thermometer equations44,51,52 were determined by IRMS (isotope ratio mass spectrometer) analyses. Whether these equations have to be adjusted for treatment of SIMS data, needs further investigation.
A probable further weakness arises from the Durango apatite standard which is commonly used to calibrate the δ18Ophosphate measurements generated by SIMS. Crystals of Durango apatite are generally assumed to be homogeneous in δ18O53,54. A recent study, however, questions this presumption and identifies conspicuous intra-crystalline heterogeneity in δ18O in all apatite standards available53. However, δ18O analyses of our samples and in house Durango apatite were performed on restricted crystal portions and δ18ODurango values show no significant variation and a reproducibility generally better than ±0.2‰ (1σ).
Discussion
Biological and paleogeographical considerations
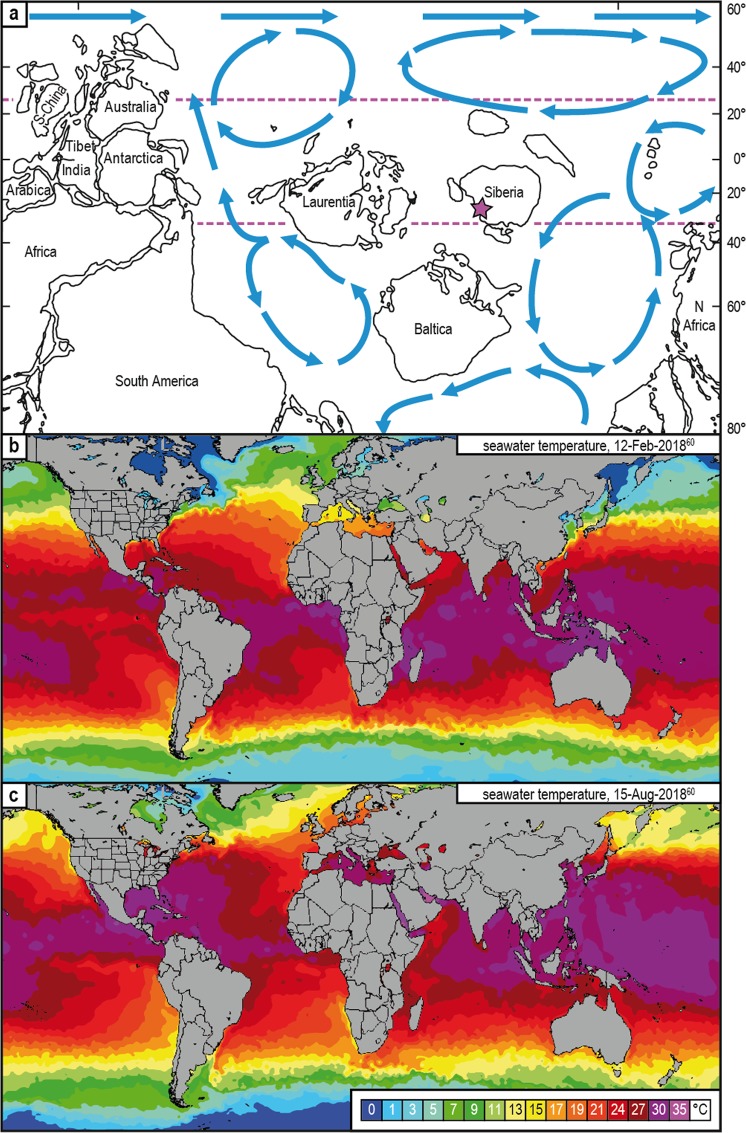
During the early and middle Cambrian, Siberia was located in a subtropical position south of the equator55 (Fig. 4a). Most recent simulations indicate mean sea surface temperatures of more than 35 °C for this paleogeographic region with minimum and maximum values of about 33 °C (austral winter) and 40 °C (austral summer), respectively25. Similar data were modeled for Furongian to the middle Silurian equatorial to subequatorial oceans, with values varying between 30 and 35 °C56. Comparable warm temperate ocean conditions of about 30 °C with maxima up to 37 °C (or even approaching 40 °C) are not unusual in earth history. They have also been estimated for the Triassic, Middle Jurassic, and Cretaceous times57–59.
Figure 4.
Reconstruction of the Terreneuvian earth and illustration of the recent seawater temperature. (a) Reconstruction of lower Cambrian paleogeography and ocean currents (blue arrows). Dotted purple lines correspond with the 35 °C isotherms of ref.25. The location of the sections investigated has been identified (purple star). (b,c) Illustration of the current seawater temperatures during austral summer (b)/winter (c) is based on the open source ref.60. Recent subequatorial seawater temperatures vary between 24 °C and 27 °C (austral winter) and between 27 °C and 35 °C (austral summer). Comparable mean annual temperatures could be also assumed for the Siberian carbonate shelf, located in a similar paleogeographic position during the Terreneuvian–Cambrian Series 2 interval.
Average temperatures of present subequatorial ocean areas of comparable latitude to the position of Siberia in the early Cambrian vary between 24 °C (austral winter) and 35 °C (austral summer), respectively60 (e.g., eastern Indian ocean, Java-, Banda-, Timor-, and Arafura seas; Fig. 4b,c). Assuming an ice-free Cambrian ocean and a δ18Oseawater value of -1.0‰21,22,24,27,29–31,33 or -1.4‰17,45, our temperatures calculated from the most primary δ18Ophosphate values vary between 35 °C ± 12 °C and 41 °C ± 12 °C. They are thus clearly higher than modern annual subequatorial temperatures. They also often exceed the upper lethal temperature for modern marine species which is typically considered to be 38 °C with habitat-dependent fluctuations towards higher and lower values61–64. The biological tolerance limit of 38 °C can be probably applied to Cambrian life forms, even if modern restrictions may not be strictly assumed for deep geological time.
Implications for the interpretation of the Paleozoic seawater temperature
Considering the thermal limit of 38 °C as a critical threshold, secular changes in the oxygen isotopic composition of ocean seawater are necessary to explain the presence of marine life in the Paleozoic. Based on our interpretation of the Siberian data a minimal depletion in oxygen isotopic composition of early–middle Cambrian seawater relative to today would be about -3‰. With this assumption and excluding altered and probably altered δ18Ophosphate values, calculated subtropical sea surface temperatures vary between 28 °C ± 13 °C and 32 °C ± 13 °C (Fig. 1). The minimum temperature becomes even lower (26 °C ± 13 °C) if we involve the most positive δ18Ophosphate value (17.2‰) into our calculation. These sea surface temperatures would be (1) clearly below the upper lethal limit of 38 °C of marine organisms (Fig. 1) and (2) comparable to temperatures of present subequatorial ocean areas (Fig. 4). Unexpected high temperatures calculated from the in situ measurements and bulk sample analyses of brachiopods23,25 for the Cambrian are thus most probably an artifact of diagenetic alteration. However, the possibility of larger 18O depletions up to -6.5‰ or even -8‰25 cannot be excluded completely if (1) the observed spread of data in our samples represent more than just an artifact of alteration and/or (2) other thermometer equations (e.g., Pucéat and co-authors52) are applied for temperature calculation.
Methods
Oxygen isotope analyses
For oxygen isotope (δ18O) analyses, brachiopod shells were mounted centrally on two-side tape fixed on a 4.5 × 4.5 cm acrylic glass (Supplementary Material). Six grains of an in house Durango apatite standard were added and both (samples and standard) were embedded in an epoxy pellet of 2.5 cm in diameter. After hardening of about 24 hours, pellets were removed from the tape and polished to a low relief in order to minimize analytical artifacts65,66. To facilitate navigation during SIMS analyses, a photograph image of the stub was generated using the Olympus cellSens Standard software (Supplementary Material). Prior to measurements, samples were coated with 30 nm of gold.
δ18O analyses were performed on a CAMECA ims1280 large-geometry ion microprobe at the Department of Geosciences of the Swedish Museum of Natural History (Nordsim facility). For analyses, a 133Cs+ ion beam with an intensity of ~3 nA was critically focused and a small raster applied to homogenize the beam profile on the sample, resulting in an analyzed volume of about 10 µm width and about 2 µm depth. For calibration, two–four analyses of the Durango apatite were performed before and after six analyses of brachiopod shells. Results are reported in ‰ relative to the V-SMOW (Vienna Standard Mean Ocean Water) standard with reproducibility generally better than ± 0.2‰ (1σ). A total of 134 measurements (96 samples and 38 standard) was performed (Supplementary Material & Supplementary Table 1). Parallel to oxygen isotope measurements each analyzing spot and surrounding shell material was imaged.
Scanning electron microscopy and energy-dispersive X-ray spectroscopy
Scanning electron microscopy (SEM) was performed on the Zeiss Sigma 300 VP at the Department of Paleontology of the University of Cologne. We used the gold-coated stubs already utilized for δ18O analyses. In addition to the SEM images, energy-dispersive X-ray spectroscopy (EDS or EDXS) was applied using a X-MaxN 80 Silicon Drift Detector (Oxford Instruments) connected to the SEM. No identification of inconsistencies in element concentration could be shown by the SEM-EDS analyses (see Supplementary Material for selected spots).
All data generated or analyzed during this study are included in this published article (and its Supplementary Material).
Supplementary information
Acknowledgements
We acknowledge financial support from SYNTHESYS (SE-TAF 6454 to T.W.) which was financed by the European Community – Research Infrastructure Action under the Seventh Framework Program. We thank K. Lindén and L. Ilyinsky for sample preparation prior to ion microprobe analyses. SEM and EDS analyses were aided by H. Cieszynski and S. Gilbricht.
Author Contributions
T.W. conceived the study. A.K. collected samples used in this study. T.W., M.W. and C.B.S. did the analyses. T.W. wrote the manuscript, with contributions from C.B.S., M.W. and A.K. All authors contributed to the discussions and interpretations of the data.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-42719-4.
References
- 1.Shu D-G, et al. Lower Cambrian vertebrates from South China. Nature. 1999;402:42–46. doi: 10.1038/46965. [DOI] [Google Scholar]
- 2.Lee MSY, et al. Modern optics in exceptionally preserved eyes of Early Cambrian arthropods from Australia. Nature. 2011;474:631–634. doi: 10.1038/nature10097. [DOI] [PubMed] [Google Scholar]
- 3.Ma X, Hou X, Edgecombe GD, Strausfeld NJ. Complex brain and optic lobes in an early Cambrian arthropod. Nature. 2012;490:258–262. doi: 10.1038/nature11495. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Zhang X, Yun H, Li G. Complex hierarchical microstructures of Cambrian mollusk Pelagiella: insight into early biomineralization and evolution. Sci. Rep. 2017;7:1935. doi: 10.1038/s41598-017-02235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao F, Bottjer DJ, Hu S, Yin Z, Zhu M. Complexity and diversity of eyes in Early Cambrian ecosystems. Sci. Rep. 2013;3:2751. doi: 10.1038/srep02751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conway-Morris S. The Cambrian “explosion”: Slow-fuse or megatonnage? PNAS. 2000;97:4426–4429. doi: 10.1073/pnas.97.9.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conway-Morris S. The Cambrian “explosion” of metazoans and molecular biology: Would Darwin be satisfied? Int. J. Dev. Biol. 2003;47:505–515. [PubMed] [Google Scholar]
- 8.Briggs, D. E. G., Erwin, D. H. & Collier, F. J. The Fossils of the Burgess Shale. Smithsonian Institution Press, 238 pp. (1994).
- 9.Hou, X.-G., Aldridge, R. J., Bergström, J., Siveter, D. J. & Feng, X.-H. The Cambrian Fossils of Chengjang, China: The Flowering of Early Animal Life. Blackwell Science Ltd., 233 pp. (2004).
- 10.Peel JS, Ineson JR. The extent of the Sirius Passet Lagerstätte (early Cambrian) of North Greenland. Bull. Geosci. 2011;86:535–543. doi: 10.3140/bull.geosci.1269. [DOI] [Google Scholar]
- 11.Brennan ST, Lowenstein TK, Horita J. Seawater chemistry and the advent of biocalcification. Geology. 2004;32:473–476. doi: 10.1130/G20251.1. [DOI] [Google Scholar]
- 12.Tatzel M, von Blanckenburg F, Oelze M, Bouchez J, Hippler D. Late Neoproterozoic seawater oxygenation by siliceous sponges. Nat. Commun. 2017;8:621. doi: 10.1038/s41467-017-00586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen Y, Zhang T, Hoffman PF. On the coevolution of Ediacaran oceans and animals. Proc. Natl. Acad. Sci. U.S.A. 2008;105:7376–7381. doi: 10.1073/pnas.0802168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, X. et al. Rise to modern levels of ocean oxygenation coincided with the Cambrian radiation of animals. Nat. Commun. 6, 7142 (2015). [DOI] [PMC free article] [PubMed]
- 15.Dzik J. Behavioral and anatomical unity of the earliest burrowing animals and the cause of the “Cambrian explosion”. Paleobiol. 2005;31:503–521. doi: 10.1666/0094-8373(2005)031[0503:BAAUOT]2.0.CO;2. [DOI] [Google Scholar]
- 16.Zhang H, et al. Armored kinorhynch-like scalidophoran animals from the early Cambrian. Sci. Rep. 2015;5:16521. doi: 10.1038/srep16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaffrés JBD, Shields GA, Wallmann K. The oxygen isotope evolution of seawater: A critical review of a long-standing controversy and an improved geological water cycle model for the past 3.4 billion years. Earth-Sci. Rev. 2007;83:83–112. doi: 10.1016/j.earscirev.2007.04.002. [DOI] [Google Scholar]
- 18.Prokoph A, Shields GA, Veizer J. Compilation and time-series analysis of a marine carbonate δ18O, δ13C, 87Sr/86Sr and δ34S database through Earth history. Earth-Sci. Rev. 2008;87:113–133. doi: 10.1016/j.earscirev.2007.12.003. [DOI] [Google Scholar]
- 19.Veizer J, Prokoph A. Temperatures and oxygen isotopic composition of Phanerozoic oceans. Earth-Sci. Rev. 2015;146:92–104. doi: 10.1016/j.earscirev.2015.03.008. [DOI] [Google Scholar]
- 20.Veizer J, et al. 87Sr/86Sr, δ13C and δ18O evolution of Phanerozoic seawater. Chem. Geol. 1999;161:59–88. doi: 10.1016/S0009-2541(99)00081-9. [DOI] [Google Scholar]
- 21.Trotter JA, Williams IS, Barnes CR, Lécuyer C, Nicoll RS. Did cooling oceans trigger Ordovician biodiversification? Evidence from conodont thermometry. Science. 2008;321:550–554. doi: 10.1126/science.1155814. [DOI] [PubMed] [Google Scholar]
- 22.Wenzel B, Joachimski MM. Carbon and oxygen isotopic composition of Silurian brachiopods (Gotland/Sweden): palaeoceanographic implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996;122:143–166. doi: 10.1016/0031-0182(95)00094-1. [DOI] [Google Scholar]
- 23.Elrick M, Rieboldt S, Saltzman M, McKay RM. Oxygen-isotope trends and seawater temperature changes across the Late Cambrian Steptoean positive carbon-isotope excursion (SPICE event) Geology. 2011;39:987–990. doi: 10.1130/G32109.1. [DOI] [Google Scholar]
- 24.Savin SM. The history of the earth’s surface temperature during the past 100 million years. Ann. Rev. Earth Planet. Sci. 1977;5:319–355. doi: 10.1146/annurev.ea.05.050177.001535. [DOI] [Google Scholar]
- 25.Hearing TW, et al. An early Cambrian greenhouse climate. Sci. Adv. 2018;4:eaar5690. doi: 10.1126/sciadv.aar5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mine AH, et al. Microprecipitation and δ18O analysis of phosphate for paleoclimate and biogeochemistry research. Chem. Geol. 2017;460:1–14. doi: 10.1016/j.chemgeo.2017.03.032. [DOI] [Google Scholar]
- 27.Wenzel B, Lécuyer C, Joachimski MM. Comparing oxygen isotope records of Silurian calcite and phosphate—δ18O compositions of brachiopods and conodonts. Geochim. Cosmochim. Acta. 2000;64:1859–1872. doi: 10.1016/S0016-7037(00)00337-9. [DOI] [Google Scholar]
- 28.Luz B, Kolodny Y, Novach J. Oxygen isotope variations in phosphate of biogenic apatites, III. Conodonts. Earth Planet. Sci. Lett. 1984;69:255–262. doi: 10.1016/0012-821X(84)90185-7. [DOI] [Google Scholar]
- 29.Joachimski MM, van Geldern R, Breisig S, Buggisch W, Day J. Oxygen isotope evolution of biogenic calcite and apatite during the Middle and Late Devonian. Int. J. Earth Sci. 2004;93:542–553. doi: 10.1007/s00531-004-0405-8. [DOI] [Google Scholar]
- 30.Bassett D, MacLeod K, Miller JF, Ethington RL. Oxygen isotopic composition of biogenic phosphate and the temperature of Early Ordovician seawater. Palaios. 2007;22:98–103. doi: 10.2110/palo.2005.p05-089r. [DOI] [Google Scholar]
- 31.Rigo M, Joachimski MM. Palaeoecology of Late Triassic conodonts: Constraints from oxygen isotopes in biogenic apatite. Acta Pal. Pol. 2010;55:471–478. doi: 10.4202/app.2009.0100. [DOI] [Google Scholar]
- 32.Bergmann KD, et al. A paired apatite and calcite clumped isotope thermometry approach to estimating Cambro-Ordovician seawater temperatures and isotopic composition. Geochim. Cosmochim. Acta. 2018;224:18–41. doi: 10.1016/j.gca.2017.11.015. [DOI] [Google Scholar]
- 33.Trotter JA, Williams IS, Barnes CR, Männik P, Simpson A. New conodont δ18O records of Silurian climate change: Implications for environmental and biological events. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016;443:34–48. doi: 10.1016/j.palaeo.2015.11.011. [DOI] [Google Scholar]
- 34.Roelofs B, et al. Assessing the fidelity of marine vertebrate microfossil δ18O signatures and their potential for palaeo-ecological and –climatic reconstructions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017;465:79–92. doi: 10.1016/j.palaeo.2016.10.018. [DOI] [Google Scholar]
- 35.Chen J, et al. High-resolution SIMS oxygen isotope analysis on conodont apatite from South China and implications for the end-Permian mass extinction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016;448:26–38. doi: 10.1016/j.palaeo.2015.11.025. [DOI] [Google Scholar]
- 36.Wheeley JR, Smith MP, Boomer I. Oxygen isotope variability in conodonts: implications for reconstructing Palaeozoic palaeoclimates and palaeooceanography. J. Geol. Soc. 2012;169:239–250. doi: 10.1144/0016-76492011-048. [DOI] [Google Scholar]
- 37.Rigo M, Trotter JA, Preto N, Williams IS. Oxygen isotopic evidence for Late Triassic monsoonal upwelling in the northwestern Tethys. Geology. 2012;40:515–518. doi: 10.1130/G32792.1. [DOI] [Google Scholar]
- 38.Zhao, Y. et al. Proposed Global Standard Stratotype-Section and Point for the base of the Mialongian series and Wuliuan stage (provisional Cambrian Series 3 and Stage 5). Proposal, prepared for the International Subcommission on Cambrian Stratigraphy, 46 pp. (2016).
- 39.Brasier MD, Sukhov SS. The falling amplitude of carbon isotope oscillations through the Lower to Middle Cambrian: Northern Siberia data. Can. J. Earth Sci. 1998;35:353–373. doi: 10.1139/e97-122. [DOI] [Google Scholar]
- 40.Kouchinsky AS, et al. The SPICE carbon excursion in Siberia: a combined study of the upper Middle Cambrian–lowermost Ordovician Kulyumbe River section, northwestern Siberian Platform. Geol. Mag. 2008;145:609–622. doi: 10.1017/S0016756808004913. [DOI] [Google Scholar]
- 41.Kouchinsky A, Bengtson S, Clausen S, Vendrasco MJ. An early Cambrian fauna of skeletal fossils from the Emyaksin Formation, northern Siberia. Acta Pal. Pol. 2015;60:421–512. [Google Scholar]
- 42.Kouchinsky A, et al. A middle Cambrian fauna of skeletal fossils from the Kuonamka Formation, northern Siberia. Alcheringa. 2011;35:123–189. doi: 10.1080/03115518.2010.496529. [DOI] [Google Scholar]
- 43.Gradstein, F. M., Ogg, J. G., Schmitz, M. D. & Ogg, G. M. The Geologic Time Scale 2012, Volume 2. Elsevier BV, 1144 pp. (2012).
- 44.Lécuyer C, Amiot R, Touzeau A, Trotter J. Calibration of the phosphate δ18O thermometer with carbonate-water oxygen fraction equations. Chem. Geol. 2013;347:217–226. doi: 10.1016/j.chemgeo.2013.03.008. [DOI] [Google Scholar]
- 45.Lhomme N, Clarke GKC. Global budget of water isotopes inferred from polar ice sheets. Geophys. Res. Lett. 2005;32:L20502. doi: 10.1029/2005GL023774. [DOI] [Google Scholar]
- 46.Kolodny Y, Luz B, Sander M, Clemens WA. Dinosaur bones: fossils or pseudomorphs? The pitfalls of physiology reconstruction from apatitic fossils. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996;126:161–171. doi: 10.1016/S0031-0182(96)00112-5. [DOI] [Google Scholar]
- 47.Sharp ZD, Atudorei V, Furrer H. The effect of diagenesis on oxygen isotope ratios of biogenic phosphates. Am. J. Sci. 2000;300:222–237. doi: 10.2475/ajs.300.3.222. [DOI] [Google Scholar]
- 48.O’Neil JR, Roe JL, Reinhardt E, Blake RE. A rapid and precise method of oxygen isotope analysis of biogenic phosphate. Isr. J. Earth Sci. 1994;43:203–212. [Google Scholar]
- 49.Kocsis L, Dulai A, Bitner MA, Vennemann T, Cooper M. Geochemical compositions of Neogene phosphatic brachiopods: Implications for ancient environmental and marine conditions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012;326–328:66–77. doi: 10.1016/j.palaeo.2012.02.004. [DOI] [Google Scholar]
- 50.Rodland DL, Kowalewski M, Dettman DL, Flessa KW, Atudorei V, Sharp ZD. High-resolution analysis of δ18O in the biogenic phosphate of modern and fossil lingulid brachiopods. J. Geol. 2003;111:441–453. doi: 10.1086/375283. [DOI] [Google Scholar]
- 51.Kolodny Y, Luz B, Navon O. Oxygen isotope variations in phosphate of biogenic apatites, I. Fish bone apatite – rechecking the rules of the game. Earth Planet. Sci. Lett. 1983;64:398–404. doi: 10.1016/0012-821X(83)90100-0. [DOI] [Google Scholar]
- 52.Pucéat E, et al. Revised phosphate-water fractionation equation reassessing paleotemperatures derived from biogenic apatite. Earth Planet. Sci. Lett. 2010;298:135–142. doi: 10.1016/j.epsl.2010.07.034. [DOI] [Google Scholar]
- 53.Sun Y, et al. Chemical and oxygen isotope composition of gem-quality apatites: Implications for oxygen isotope reference materials for secondary ion mass spectrometry (SIMS) Chem. Geol. 2016;440:164–178. doi: 10.1016/j.chemgeo.2016.07.013. [DOI] [Google Scholar]
- 54.Žigaitė Ž, Whitehouse M. Stable oxygen isotopes of dental biomineral: differentiation at the intra- and inter-tissue level of modern shark teeth. GFF. 2014;136:337–340. doi: 10.1080/11035897.2013.878747. [DOI] [Google Scholar]
- 55.Brock GA, et al. Palaeobiogeographic affinities of Australian Cambrian Faunas. Mem. Ass. Austral. Pal. 2000;23:1–61. [Google Scholar]
- 56.Nardin E, et al. Modeling the early Paleozoic long-term climatic trend. GSA Bull. 2011;123:1181–1192. doi: 10.1130/B30364.1. [DOI] [Google Scholar]
- 57.Sun Y, et al. Lethally hot temperatures during the Early Triassic greenhouse. Science. 2012;338:366–370. doi: 10.1126/science.1224126. [DOI] [PubMed] [Google Scholar]
- 58.Littler K, Robinson SA, Bown PR, Nederbragt AJ, Pancost RD. High sea-surface temperatures during the Early Cretaceous Epoch. Nat. Geosci. 2011;4:169–172. doi: 10.1038/ngeo1081. [DOI] [Google Scholar]
- 59.Alsenz H, et al. Sea surface temperature record of a Late Cretaceous tropical Southern Tethys upwelling system. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013;392:350–358. doi: 10.1016/j.palaeo.2013.09.013. [DOI] [Google Scholar]
- 60.World Sea Temperatures, https://www.seatemperature.org/ (12-Feb-2018 & 15-Aug-2018).
- 61.Brock TD. Life at high temperatures. Science. 1985;230:132–138. doi: 10.1126/science.230.4722.132. [DOI] [PubMed] [Google Scholar]
- 62.Compton TJ, Rijkenverg MJA, Drent J, Piersma T. Thermal tolerance ranges and climate variability: A comparison between bivalves from differing climates. J. Exp. Mar. Biol. Ecol. 2007;352:200–211. doi: 10.1016/j.jembe.2007.07.010. [DOI] [Google Scholar]
- 63.Rothschild LJ, Mancinelli RL. Life in extreme environments. Nature. 2001;409:1092–1101. doi: 10.1038/35059215. [DOI] [PubMed] [Google Scholar]
- 64.Kinne, O. Marine Ecology: A Comprehensive, Integrated Treatise on Life in Oceans and Coastal Waters. Volume I: Environmental Factors, Part 1. Wiley-Interscience, 681 pp. (1970).
- 65.Kita NT, Ushikubo T, Fu B, Valley JW. High precision SIMS oxygen isotope analysis and the effect of sample topography. Chem. Geol. 2009;264:43–57. doi: 10.1016/j.chemgeo.2009.02.012. [DOI] [Google Scholar]
- 66.Whitehouse MJ, Nemchin AN. High precision, high accuracy measurement of oxygen isotopes in a large lunar zircon by SIMS. Chem. Geol. 2009;261:32–42. doi: 10.1016/j.chemgeo.2008.09.009. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.