Abstract
There is general agreement that both motivation and cognitive control play critical roles in shaping goal-directed behavior, but only recently has scientific interest focused around the question of motivation-control interactions. Here we briefly survey this literature, organizing contemporary findings around three issues: 1) whether motivation preferentially impacts cognitive control processes, 2) the neural mechanisms that underlie motivation-cognition interactions, and 3) why motivation might be relevant for overcoming the costs of control. Dopamine (DA) is discussed as a key neuromodulator in these motivation-cognition interactions. We conclude by highlighting open issues, specifically Pavlovian versus instrumental control distinctions and effects of motivational valence and conflict, which could benefit from future research attention.
Introduction
There has been a longstanding interest in investigating motivation and cognitive control as modulators of goal-directed behavior. However, only recently have researchers begun to examine these two processes in terms of their integrated influence on behavior and the brain [1,2]. Here we review recent studies on motivation-cognition interactions, while highlighting key unresolved issues in this burgeoning domain.
The Challenge of Operationalizing Motivation
René Descartes once contended that the ‘passions’ in human nature “dispose our soul to want the things that nature decides are useful for us, and to persist in this volition; and [to bring about] the agitation of the spirits which customarily causes them to dispose the body to those movements that help bring about those useful things [3].” While to modern ears this quotation initially seems overly baroque, upon deeper inspection it reveals a surprisingly apt description of what could be considered the four central dimension of motivation: value/utility (things of use), anticipatory affect (passion, desire, and persistence), activation/energization (agitation of the spirits), and directed action (disposing the body to movement).
In the scientific literature, motivation has been characterized as the energization and direction of behavior, response vigor, arousal and intensity of motor output, or as a biologically-driven impulse that compels an organism to act [4–7]. Since the concept of motivation was created to provide a theoretical framework to describe a diverse range of behaviors (e.g., approach, avoidance), a precise operationalization of this multifaceted construct has proved to be challenging. Despite valiant efforts, researchers have yet to agree upon a unified definition and comprehensive framework for this elusive construct [7,8].
Nevertheless, the term ‘motivation’ is consistently used to describe when an external or internal incentive alters the biological system (i.e., generates a ‘motivated state’) to stimulate an observable change in behavior. It is generally assumed that providing incentives (e.g., offering rewards or threats/penalties) can induce such motivational states, which then lead to dynamic adjustments in cognitive processing, and consequently, influence behavior. However, open questions remain regarding the mechanisms that underlie such motivation-cognition interactions. A further point to acknowledge is that motivation has been found to influence a broad range of cognitive processes, i.e., attention [9,10], learning [11,12], memory [13,14], and perception [15,16]. Here, we emphasize studies that have examined motivation as it relates to cognitive control, a key interaction underlying goal-directed behavior.
The interactions of motivation and cognitive control
The recent literature in this domain can be organized around three central questions. First, is there evidence that motivation selectively enhances cognitive control? Second, what are the neural mechanisms that give rise to these interactions? Third, why is motivation relevant for overcoming costs of cognitive control?
Selective motivation – cognitive control enhancements?
The claim that motivation preferentially impacts tasks with higher cognitive control demands is compelling and provocative [2]. Demonstrating it would require two steps: 1) isolation of a selective measure of cognitive control and 2) showing that this measure is significantly enhanced under high motivational value conditions (e.g., when incentives are offered). Some tasks include selective behavioral measures that isolate control processing, such as task switching, conflict paradigms (e.g., Stroop, flanker), response inhibition tasks (e.g., go/no-go, stop signal) and context processing paradigms (e.g., AX-CPT). In task-switching paradigms the control measure is the mixing or switching cost (i.e., switch–no switch), while in the Stroop and flanker tasks it is the interference effect (i.e., incongruent–congruent), and in the stop-signal task it is the stop-signal reaction time (i.e., time required to inhibit an initiated response). Finally, in AX-CPT tasks, cognitive control is indexed by performance on AY and BX lure trials (Figure 1).
Figure 1.
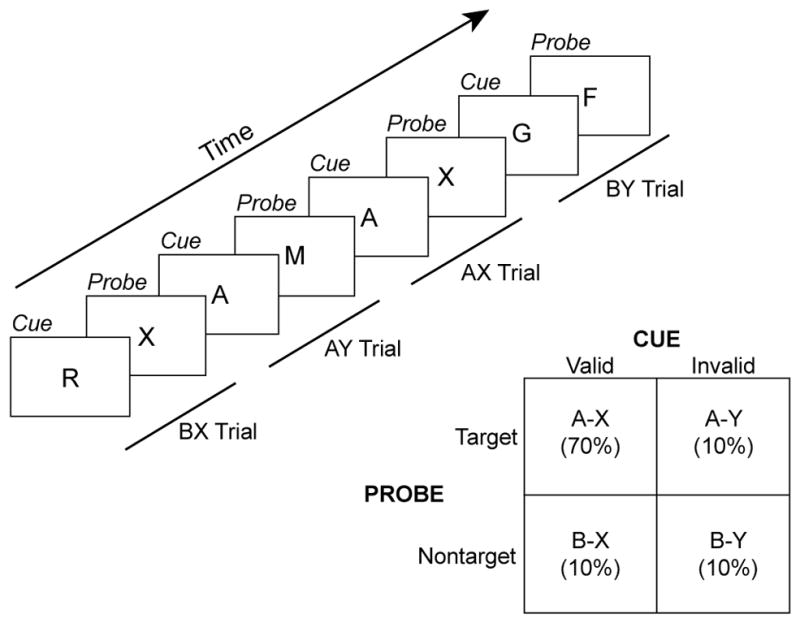
Schematic of classic version of AX-CPT paradigm. Single letters are visually displayed as a series of cue-probe pairs. Here, the target pair is the occurrence of an X probe followed immediately an A cue. Of the three nontarget trial types, BY trials (where B refers to any non-A cue and Y refers to any non-X probe) provide a low-demand baseline general performance index, while BX and AY serve as low-frequency lures that selectively index cognitive control (each lure type typically occurs with 10% frequency). A range of studies with this paradigm have found that optimal utilization of contextual cues can eliminate typical interference effects observed in BX trials, because in these trials the contextual cue allows for fully accurate preparation of a non-target response [74–76]. In contrast, enhanced proactive control increases interference on AY trials, since contextually based preparation of a target response is invalid in these trials.
Monetary incentives appear to enhance cognitive control performance via increased proactive control – the utilization of preparatory tasks and/or contextual cues to increase accuracy and reduce response times [17–19]. Recent studies have observed that anticipation of reward incentives selectively reduces switch costs in task-switching paradigms [20,21]. Likewise, Chiew and Braver (2016) found that task-informative cues in the flanker task (indicating whether an upcoming trial was incongruent) could reduce interference effects, but only when cues were combined with rewards and sufficient preparation time [17]. Thus, reward incentives seem most effective in modulating cognitive control in a proactive and preparatory manner [22].
However, motivational enhancements of proactive control can both benefit and impair task performance. This phenomenon has been most directly examined within the AX-CPT paradigm [23–26]. Hefer & Dreisbach (2017) observed that reward motivation manipulations led to persistent increased use of contextual cue information, even under conditions which result in sub-optimally high AY errors (i.e., reduced BX interference but increased AY interference) [26], providing evidence for both costs and benefits of reward motivation on proactive control.
Others have found that motivational incentives enhance reactive control (i.e., rapid adjustment of control in response to performance monitoring). Boehler et al. (2014) observed that rewards speeded up response inhibition in a stop-signal task without preparatory cues, revealing that rewards also facilitate inhibitory responding, even when proactive mechanisms are likely not engaged [27]. More research is needed to determine the relevant boundary conditions for when motivational manipulations will result in proactive versus reactive control enhancements.
What are the neural mechanisms by which motivation impacts cognitive control?
Dopamine (DA) is hypothesized to play a key role in motivation-cognition interface [28], with tonic DA activity postulated to mediate the relationship between average reward and movement vigor [29,30]. Movement vigor appears to reflect a general arousal process, which leads to quicker responding in a high reward context, and has been considered a characteristic behavioral measure of motivation [31,32]. Rigoli and colleagues (2016) examined this hypothesis in a visual search task in which participants received monetary rewards which varied block-wise ($1, $6, $11), independent of a $3 reward earned for accurate performance within each trial [33]. Higher average reward (manipulated across blocks) was associated with increased motor vigor (measured by button pressing force). These effects were mediated by activation in subcortical brain regions with high DA neuronal concentration, bolstering the role of DA as critical in regulating movement vigor. However, increased vigor in higher reward contexts may not directly entail selective enhancement of cognitive control mechanisms (e.g., vigor may alternatively reflect priming of the motor systems).
Another hypothesis is that motivated cognitive control arises from DA modulation of both striatum and PFC via parallel neural pathways (Figure 2). Cools (2016) hypothesized that PFC DA facilitates stabilization of current goal representations (via tonic DA release), whereas striatal DA disrupts these representations via attention shifting and/or task-set updating in response to unexpected relevant stimuli (via phasic DA release) [34]. This dynamic tradeoff between cognitive stability and flexibility [22] may explain the sometimes paradoxical detrimental effects of monetary rewards on cognitive control, as both excessive and insufficient PFC DA may impair the ability to maintain task representations in working memory over time (i.e., U-shaped DA effects) [35–37].
Figure 2.
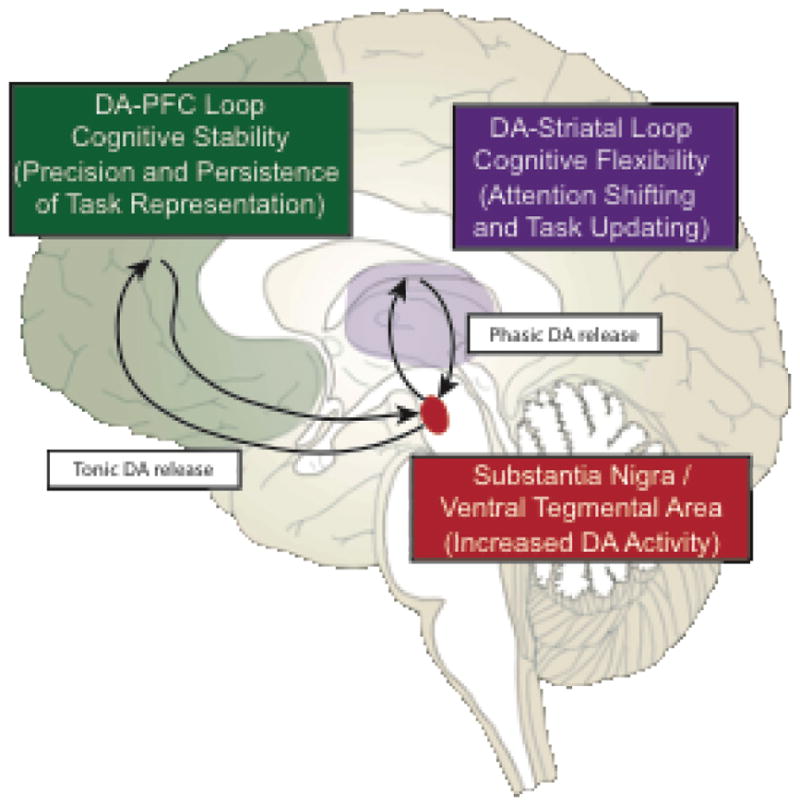
Dopamine (DA) may have differential effects on motivated behavior. It has been hypothesized that tonic release of DA in the prefrontal cortex (PFC) may facilitate the precision and persistence of current task goal representations (i.e., cognitive stability). In contrast, phasic release of DA in the striatum may facilitate attention shifting and updating of task goal representations based upon unexpected, behaviorally important stimuli (i.e., cognitive flexibility). Tonic DA is also hypothesized to mediate the relationship between reward rate and response vigor in tasks, although the neural pathway of such effects has not been well investigated (not shown in figure).
Interactions between motivational signals from dopaminergic midbrain and PFC consistently enhance cognitive control [38,39], and may even selectively target specific levels of cognitive control hierarchy in lateral PFC (e.g., posterior-to-anterior gradient corresponding to task rule abstraction). Bahlmann and colleagues (2015) found evidence consistent with this idea, and observed that the strongest motivational effects in lateral PFC corresponded to mid-level task representations, which were accompanied by increased functional coupling between the DA midbrain and lateral PFC [40].
However, the exact mechanism by which motivation enhances control remains unknown. One hypothesis is that motivation improves the signal-to-noise ratio in the neural coding of task rules within PFC, thus increasing the efficacy and precision of cognitive control performance. Such an account would be consistent with classical experimental and computational studies of DA effects on PFC activation [41–45]. Etzel et al. (2015) reported data consistent with this account, as they used multivariate pattern analyses to decode task representations on a trial-by-trial basis under reward motivation manipulations. There, incentives not only sharpened task representations in frontoparietal cortex (i.e., more discriminable voxel patterns in each task), but also increased task decoding accuracy, with the latter statistically mediating improvements in task performance [46]. Together, these studies suggest that motivational incentives impact cognitive control via dopaminergic signaling to frontoparietal control network, thus facilitating more effective, stable, and precise task representations.
Why is motivation relevant for overcoming the costs of cognitive control?
Cognitive control, specifically utilizing cognitive resources in the service of decision-making, is intrinsically costly [47,48]. The term “cognitive effort” typically refers to the subjective experience of up-regulating the cognitive control system during goal-pursuit, and is often considered to be a canonical metric for the cost of cognitive control engagement [47]. Individuals are less willing to engage in cognitively demanding tasks if the allocated effort costs outweigh the expected benefit [49–51].
Motivation may act as a modulatory factor that offsets these effort costs. Manohar and colleagues (2015) proposed a computational framework which argued that motivation improves task performance beyond normal bounds (e.g., faster and more accurate/precise choices in motor and decision tasks) [52]. Thus, task performance can be improved without contravening the speed-accuracy tradeoff. However, missing from this framework is an explanation of why cognitive control is costly to begin with. One speculation is that recruiting cognitive control detracts from available cognitive resources in a limited capacity system, thus representing an opportunity cost [53]. Importantly, what makes such costs ‘expensive’ or ‘cheap’ depends on whether using that resource involves forgoing another beneficial use or not, respectively.
These ideas are consistent with the Value-Based Cognitive Control (VBCC) framework, which posits that engaging cognitive control can be construed as an economic decision between the estimated subjective/computational costs of control weighed against the expected benefits of enhanced control [54]. Broadly, VBCC opens up a novel domain of quantifying effort, and argues that motivation and cognitive effort are juxtaposed: higher motivational value can offset higher effort costs in shaping control policy selection and behavior. In other words, appetitive motivation should increase the subjective value of the current option, thus decreasing the opportunity cost of exerting cognitive control to obtain it. Conversely, aversive motivation should offset potential benefits of reward, such that a negative option should decrease cognitive control engagement.
In this framework, DA is theorized to modulate the efficacy of control through titration of the precision and persistence of task representations [55]. Precision refers to the clarity of the task goal representation in the brain (i.e., sharpness, signal-to-noise ratio), whereas persistence refers to the duration over which these task goal representations are actively maintained (i.e., sustained elevations in neuronal activity). Because of the well-established limited capacity of active goal maintenance, the degree to which an individual commits to representing a task goal clearly and persistently incurs an opportunity cost, i.e., they forgo the chance to use those cognitive resources for alternative tasks. Thus, motivationally triggered DA release in the PFC should facilitate goal-directed task performance and reduce control costs.
Although these ideas regarding the role of DA in motivating cognitive control have been laid out conceptually [56], there is still a lack of convincing experimental support. The most direct evidence would be to demonstrate a multi-way link between increased motivational value, increased DA release and neural changes within PFC, which together mediate improvements in behavioral cognitive control measures. Such evidence may be hard to obtain with current neuroscience methods, but a potentially promising route is to utilize simultaneous PET-fMRI to co-localize changes in DA release (via radioligand binding) with changes in PFC BOLD activity.
Open Issues
In our opinion, two important factors require further investigation to make progress in this domain: 1) Pavlovian versus instrumental influences of motivational incentives; and 2) effects of motivational valence and conflict.
Pavlovian vs. Instrumental Effects of Motivational Incentives
The dichotomy between Pavlovian versus instrumental control of behavior has long played an influential role in the study of motivation [57], but researchers have only recently started to examine this distinction in terms of effects on human decision-making [58,59]. Pavlovian control refers to a behavioral reflex elicited by predictive stimuli associated with appetitive or aversive outcome (e.g., approach, withdrawal), while instrumental control refers to learning of the stimulus-dependent contingency between responses and outcomes (e.g., a rat must press a lever to earn a food pellet reward). Thus, in instrumental paradigms, motivational incentives are typically used to reinforce or punish behavioral responding (e.g., presenting food pellets will increase lever pressing, whereas presenting shocks will decrease lever pressing).
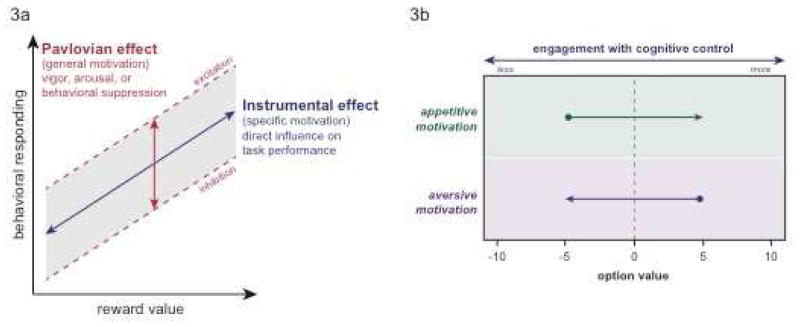
Researchers have attempted to disentangle these dissociable influences in simple decision tasks (e.g., stimulus-response associations, go-no-go tasks) [60]. Some have proposed that instrumental responses are unsigned and therefore not sensitive to the valence of the motivational incentive driving the behavior, whereas Pavlovian conditioned responses are evolutionarily hard wired and thus explicitly linked to incentive valence [61]. These distinct mechanisms appear to have orthogonal effects in modulating simple decisions, with instrumental influences giving rise to more specific enhancement of behavioral responding, whereas Pavlovian influences may lead to a general excitatory or inhibitory bias on instrumental responding (Figure 3a).
Figure 3.
3a) Reward incentives may have parallel effects on motivation and behavioral responding via both Pavlovian and instrumental control influences. The Pavlovian effect may reflect a general motivation mechanism, such that the valence of an incentive biases responding in an overall excitatory (e.g., arousal, vigor) or inhibitory manner (e.g., behavioral suppression). Instrumental influences may implement a more directed motivation mechanism (e.g., specific enhancement of task performance). These two effects may occur simultaneously and alter behavioral responding via parallel mechanisms, but this distinction has not yet been fully explored within the context of cognitive control. 3b). The engagement of cognitive control might be construed as an economic decision. In the case of motivational conflict – consideration of an option that has both associated costs and benefits (e.g., money and saltwater) – appetitive motivation can be used to increase the subjective value of an option, thus offsetting the cost of engaging cognitive control (which would otherwise have negative subjective value; top panel). In contrast, aversive motivation has the opposite influence, decreasing the value of an otherwise attractive option (i.e., with positive appetitive value), and reducing cognitive control engagement via motivational conflict (bottom panel).
However, it remains ambiguous whether motivational enhancements of cognitively controlled behavior might reflect Pavlovian as well as instrumental mechanisms. One approach for investigating this question would be to use well-established Pavlovian Instrumental Transfer (PIT) paradigms [62], although to date these have not been examined within the context of motivation-cognition interactions.
Valence and Motivational Conflict
Although motivational incentives are inherently valenced (e.g., appetitive, aversive), surprisingly few studies have examined this dimension in motivation-cognition interaction studies. Moreover, a broader unanswered question is how control processes are modulated by motivational conflict (i.e., integration of both appetitive and aversive incentives) [63,64].
A recent novel design developed by Yee et al. (2016) highlights these issues within the context of a cued task-switching paradigm [65], in which individuals must exert cognitive control to earn money, but are provided with liquid incentives of differing valences (e.g., appetitive, neutral, aversive) as task performance feedback [66]. Motivational conflict occurs in the aversive liquid block, as subjects must integrate the prospect of saltwater delivery (as performance feedback) with potential monetary earnings in deciding whether to enhance cognitive control and maximize reward rate. The parametric effect of these bundled incentives suggests that humans indeed integrate different incentives into a net motivational value that modulates cognitively controlled behavior (i.e., better performance on juice+money trials, poorer performance on saltwater+money trials compared with tasteless liquid+money; Figure 3b). As the foregoing suggests, motivational valence is an important dimension that should be more systematically explored in future investigations of motivation-cognition interactions, as it may lead to deeper insights regarding more complex issues, such as incentive integration (i.e., bundling) and motivational conflict [67–69].
Conclusions
Recent studies of motivation-cognitions have primarily focused on understanding whether, how, and why motivation interacts with cognitive control. The recent VBCC framework conceptualizes the motivation-cognition interaction as a decision-making process that juxtaposes motivation and cognitive effort costs. We suggest promising future directions regarding how to incorporate Pavlovian vs. instrumental influences and motivational valence / conflict into this research domain. It is our hope that this review spurs future innovative investigations, which could also extend into broader relevant issues such as aging and developmental trajectories [70–72], and the neural mechanisms of psychopathology [73].
Highlights.
Motivation may have a preferential impact on proactive cognitive control mechanisms
Dopamine signals may enhance control by modulating prefrontal task representations
Cognitive control can be construed as a form of economic decision-making
Motivational value can offset subjective/computational costs of effortful control
Future research targets: Pavlovian/instrumental and appetitive/aversive distinctions
Acknowledgments
DMY was supported by funding from the National Institute on Drug Abuse (F31-DA042574). TSB was supported by the National Institute of Mental Health (R21-MH105800; R37-MH066078, R21-AG058206-01). Additionally, the authors gratefully acknowledge the members of the CCP lab for helpful productive discussion and assistance in the investigation of these topics.
Footnotes
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended Readings
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Braver TS, Krug MK, Chiew KS, Kool W, Westbrook JA, Clement NJ, et al. Mechanisms of motivation-cognition interaction: challenges and opportunities. Cogn Affect Behav Neurosci. 2014;14:443–72. doi: 10.3758/s13415-014-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2•.Botvinick MM, Braver TS. Motivation and Cognitive Control: From Behavior to Neural Mechanism. Annu Rev Psychol. 2015 doi: 10.1146/annurev-psych-010814-015044. A comprehrensive review of motivation and cognitive control that addresses contemporary issues and theoretical perspectives, and proposes several behavioral and neural mechanisms underlying this interaction. [DOI] [PubMed] [Google Scholar]
- 3.The Passions of the Soul: an English Translation of Les Passions De l’Âme. Indianapolis: Hackett Pub. Co; 1989. Descartes 1596-1650 R. ©1989; n.d. [Google Scholar]
- 4.Niv Y, Joel D, Dayan P. A normative perspective on motivation. Trends Cogn Sci. 2006;10:375–81. doi: 10.1016/j.tics.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Stellar JR, Stellar E. The Neurobiology of Motivation and Reward. New York: Springer-Verlag New York, Inc; 1985. [Google Scholar]
- 6.Stellar E. The Physiology of Motivation. Psychol Rev. 1954:61. doi: 10.1037/h0060347. [DOI] [PubMed] [Google Scholar]
- 7.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Dickinson A, Balleine B. Motivational Control of Instrumental Action. Curr Dir Psychol Sci. 1995;4:162–7. [Google Scholar]
- 9.Pessoa L, Engelmann JB. Embedding reward signals into perception and cognition. Front Neurosci. 2010;4:1–8. doi: 10.3389/fnins.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothkirch M, Schmack K, Deserno L, Darmohray D, Sterzer P. Attentional Modulation of Reward Processing in the Human Brain. Hum Brain Mapp. 2014;35:3036–51. doi: 10.1002/hbm.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daw ND, Shohamy D. The Cognitive Neuroscience of Motivation and Learning. Soc Cogn. 2008;26:593–620. doi: 10.1521/soco.2008.26.5.593. [DOI] [Google Scholar]
- 12.Dayan P, Balleine B. Reward, motivation, and reinforcement learning. Neuron. 2002;36:285–98. doi: 10.1016/S0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 13.Miendlarzewska EA, Bavelier D, Schwartz S. Neuroscience and Biobehavioral Reviews Influence of reward motivation on human declarative memory. Neurosci Biobehav Rev. 2016;61:156–76. doi: 10.1016/j.neubiorev.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-Motivated Learning: Mesolimbic Activation Precedes Memory Formation. Neuron. 2006:507–17. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 15.Spaniol J, Voss A, Bowen HJ, Grady CL. Motivational incentives modulate age differences in visual perception. Psychol Aging. 2011;26:932–9. doi: 10.1037/a0023297. [DOI] [PubMed] [Google Scholar]
- 16.Rothkirch M, Sterzer . Motiv Cogn Control. New York: Routledge; 2015. The Role of Motivation in Visual Information Processing; pp. 23–49. [Google Scholar]
- 17••.Chiew KS, Braver TS. Reward Favors the Prepared: Incentive and Task-Informative Cues Interact to Enhance Attentional Control. J Exp Psychol Hum Percept Perform. 2016;42:52–66. doi: 10.1037/xhp0000129. A behavioral study which observed that task-informative cues and reward effects in a flanker task, suggesting that reward incentives may modulate proactive control mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreisbach G, Fischer R. The role of affect and reward in the conflict-triggered adjustment of cognitive control. Front Hum Neurosci. 2012;6:342. doi: 10.3389/fnhum.2012.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braem S, Hickey C, Duthoo W, Notebaert W. Reward Determines the Context-Sensitivity of Cognitive Control Reward Determines the Context-Sensitivity of Cognitive Control. J Exp Psychol Hum Percept Perform. 2014;40:1769–78. doi: 10.1037/a0037554. [DOI] [PubMed] [Google Scholar]
- 20.Umemoto A, Holroyd CB. Task-specific effects of reward on task switching. Psychol Res. 2015:698–707. doi: 10.1007/s00426-014-0595-z. [DOI] [PubMed] [Google Scholar]
- 21.Kleinsorge T, Rinkenauer G. Effects of Monetary Incentives on Task Switching. Exp Psychol. 2012;59:216–26. doi: 10.1027/1618-3169/a000146. [DOI] [PubMed] [Google Scholar]
- 22•.Goschke T, Bolte A. Emotional modulation of control dilemmas: The role of positive affect, reward, and dopamine in cognitive stability and flexibility. Neuropsychologia. 2014;62:403–23. doi: 10.1016/j.neuropsychologia.2014.07.015. An extensive review which discusses the role of reward motivation and dopamine release in modulating cognitive stability and flexilibity of task representations during goal-directed behavior. [DOI] [PubMed] [Google Scholar]
- 23.Chiew KS, Braver TS. Temporal dynamics of motivation-cognitive control interactions revealed by high-resolution pupillometry. Front Psychol. 2013:4. doi: 10.3389/fpsyg.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiew KS, Braver TS. Dissociable influences of reward motivation and positive emotion on cognitive control. Cogn Affect Behav Neurosci. 2014;14:509–29. doi: 10.3758/s13415-014-0280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fröber K, Dreisbach G. The differential influences of positive affect, random reward, and performance-contingent reward on cognitive control. Cogn Affect Behav Neurosci. 2014;14:530–47. doi: 10.3758/s13415-014-0259-x. [DOI] [PubMed] [Google Scholar]
- 26••.Hefer C, Dreisbach G. How Performance-Contingent Reward Prospect Modulates Cognitive Control: Increased Cue Maintenance at the Cost of Decreased Flexibility. J Exp Psychol Learn Mem Cogn. 2017 doi: 10.1037/xlm0000397. A behavioral study using the AX-CPT paradigm illustrating that reward-related enhancement of proactive control can both benefit and impair task performance; specifcally, improving the ability to perform tasks that utilize contextual information and impairing the ability performan tasks where contextually-based preparation is invalid. [DOI] [PubMed]
- 27••.Boehler CN, Schevernels H, Hopf J-M, Stoppel CM, Krebs RM. Reward prospect rapidly speeds up response inhibition via reactive control. Cogn Affect Behav Neurosci. 2014 doi: 10.3758/s13415-014-0251-5. A behavioral study which demonstrates that anticipation of reward incentives can enhance reactive control mechanisms. [DOI] [PubMed] [Google Scholar]
- 28.Aarts E, van Holstein M, Cools R. Striatal dopamine and the interface between motivation and cognition. Front Psychol. 2011;2:1–11. doi: 10.3389/fpsyg.2011.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niv Y. Cost, benefit, tonic, phasic: what do response rates tell us about dopamine and motivation? Ann N Y Acad Sci. 2007;1104:357–76. doi: 10.1196/annals.1390.018. [DOI] [PubMed] [Google Scholar]
- 30.Beierholm U, Guitart-Masip M, Economides M, Chowdhury R, Düzel E, Dolan R, et al. Dopamine modulates reward-related vigor. Neuropsychopharmacology. 2013;38:1495–503. doi: 10.1038/npp.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dudman JT, Krakauer JW. The basal ganglia: from motor commands to the control of vigor. Curr Opin Neurobiol. 2016;37:158–66. doi: 10.1016/j.conb.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Manohar SG, Finzi RD, Drew D, Husain M. Distinct Motivational Effects of Contingent and Noncontingent Rewards. Psycholological Sci. 2017:1–11. doi: 10.1177/0956797617693326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Rigoli F, Chew B, Dayan P, Dolan RJ. The Dopaminergic Midbrain Mediates an Effect of Average Reward on Pavlovian Vigor. J Cogn Neurosci. 2016;26:1–15. doi: 10.1162/jocn_a_00972. An fMRI study which examined the relationship between average reward rate (manipulated by monetary incentives) and motor vigor, and observed that BOLD signal from dopaminergic brain regions mediated this relationship. [DOI] [PubMed] [Google Scholar]
- 34•.Cools R. The costs and benefits of brain dopamine for cognitive control. WIREs Cogn Sci. 2016;7:317–29. doi: 10.1002/wcs.1401. A recent review which argues that dopamine plays central role in cost-benefit decision making, specifically that differential dopaminergic release to the PFC and striatum may influence cognitive stability vs. flexibility, respectively. [DOI] [PubMed] [Google Scholar]
- 35.Aarts E, Wallace DL, Dang LC, Jagust WJ, Cools R, D’Esposito M. Dopamine and the cognitive downside of a promised bonus. Psychol Sci. 2014;25:1003–9. doi: 10.1177/0956797613517240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloemendaal M, Van Schouwenburg MR, Miyakawa A, Aarts E, D’Esposito M, Cools R. Dopaminergic modulation of distracter-resistance and prefrontal delay period signal. Psychopharmacology (Berl) 2015;232:1061–70. doi: 10.1007/s00213-014-3741-9. [DOI] [PubMed] [Google Scholar]
- 37.Fallon SJ, Williams-Gray CH, Barker RA, Owen AM, Hampshire A. Prefrontal dopamine levels determine the balance between cognitive stability and flexibility. Cereb Cortex. 2013;23:361–9. doi: 10.1093/cercor/bhs025. [DOI] [PubMed] [Google Scholar]
- 38.Ardenne KD, Eshel N, Luka J, Lenartowicz A, Leigh E, December N, et al. Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proc Natl Acad Sci U S A. 2012;109:19900–9. doi: 10.1073/pnas.1116727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parro C, Dixon ML, Christoff K. The Neural Basis of Motivational Influences on Cognitive Control: An ALE. 2017:1–26. doi: 10.1002/hbm.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Bahlmann J, Aarts E, D’Esposito M. Influence of Motivation on Control Hierarchy in the Human Frontal Cortex. J Neurosci. 2015;35:3207–17. doi: 10.1523/JNEUROSCI.2389-14.2015. An fMRI study that examined the motivational effects of monetary reward incentives on different levels of the cognitive control hierarchy in lateral prefrontal cortex (PFC), as well as functional coupling between dopamiergic midbrain and PFC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol. 2002:223–9. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- 42.Durstewitz D, Seamans JK. The computational role of dopamine D1 receptors in working memory. Neural Networks. 2002;15:561–72. doi: 10.1016/s0893-6080(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 43.Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol. 1994;71:515–28. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- 44.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–84. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 45.Thurley K, Senn W, Lu H, Thurley K, Senn W. Dopamine Increases the Gain of the Input-Output Response of Rat Prefrontal Pyramidal Neurons. J Neurophysiol. 2008;99:2985–97. doi: 10.1152/jn.01098.2007. [DOI] [PubMed] [Google Scholar]
- 46.Etzel J, Cole MW, Zacks JM, Kay KN, Braver TS. Reward motivation enhances task representation coding in frontoparietal cortex. Cereb Cortex. 2015:1–13. doi: 10.1093/cercor/bhu327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westbrook A, Kester D, Braver TS. What Is the Subjective Cost of Cognitive Effort? Load, Trait, and Aging Effects Revealed by Economic Preference. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0068210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kool W, Botvinick M. The intrinsic cost of cognitive control. Behav Brain Sci. 2013;36:697–8. doi: 10.1017/S0140525X1300109X. [DOI] [PubMed] [Google Scholar]
- 49.Kool W, McGuire JT, Rosen ZB, Botvinick MM. Decision making and the avoidance of cognitive demand. J Exp Psychol Gen. 2010;139:665–82. doi: 10.1037/a0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dixon ML, Christoff K. The Decision to Engage Cognitive Control Is Driven by Expected Reward-Value: Neural and Behavioral Evidence. PLoS One. 2012:7. doi: 10.1371/journal.pone.0051637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shenhav A, Musslick S, Lieder F, Kool W, Griffiths TL, Cohen JD, et al. Toward a Rational and Mechanistic Account of Mental Effort. Annu Rev Neurosci. 2017;40:99–124. doi: 10.1146/annurev-neuro-072116-031526. [DOI] [PubMed] [Google Scholar]
- 52••.Manohar SG, Chong TT, Apps MAJ, Jarman PR, Bhatia KP, Husain M, et al. Reward Pays the Cost of Noise Reduction in Motor and Cognitive Control. Curr Biol. 2015;25:1–10. doi: 10.1016/j.cub.2015.05.038. A computational and behavioral study which proposes that motivational may offset the comptuational costs of exerting cognitive control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Boureau Y, Sokol-hessner P, Daw ND. Deciding How To Decide: Self-Control and Meta-Decision Making. Trends Cogn Sci. 2015;19:700–10. doi: 10.1016/j.tics.2015.08.013. A recent review which present the idea that humans have limited available cognitive resources, and that exerting cognitive control may be ‘expensive’ because it incurs an opportunity cost. [DOI] [PubMed] [Google Scholar]
- 54.Westbrook A, Braver T. Cognitive effort: A neuroeconomic approach. Cogn Affect Behav Neurosci. 2015:395–415. doi: 10.3758/s13415-015-0334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durstewitz D, Seamans JK. The Dual-State Theory of Prefrontal Cortex Dopamine Function with Relevance to Catechol-O-Methyltransferase Genotypes and Schizophrenia. Biol Psychiatry. 2008;64:739–49. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 56•.Westbrook A, Braver TS. Dopamine Does Double Duty in Motivating Cognitive Effort. Neuron. 2016;89:695–710. doi: 10.1016/j.neuron.2015.12.029. A proposed theoretical framework for the dual role of dopamine in functional modulation of motivated cognition and cognitive effort, with prefrontal DA modulating working memory circuits and striatal DA modulating value-based decision making. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rescorla RA, Solomon RL. Two-Process Learning Theory: Relationships Between Pavlovian Conditioning and Instrumental Learning. 1967:74. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- 58.Geurts DEM, Huys QJM, den Ouden HEM, Cools R. Aversive Pavlovian Control of Instrumental Behavior in Humans. J Cogn Neurosci. 2013;25:1428–41. doi: 10.1162/jocn_a_00425. [DOI] [PubMed] [Google Scholar]
- 59•.Guitart-Masip M, Huys QJM, Fuentemilla L, Dayan P, Duzel E, Dolan RJ. Go and no-go learning in reward and punishment: Interactions between affect and effect. Neuroimage. 2012;62:154–66. doi: 10.1016/j.neuroimage.2012.04.024. A recent framework outlining Pavlovian vs. instrumental influences in terms of their disocciable effects on decision-making and behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huys QJM, Cools R, Glözer M, Friedel E, Heinz A, Dolan RJ, et al. Disentangling the roles of approach, activation and valence in instrumental and pavlovian responding. PLoS Comput Biol. 2011:7. doi: 10.1371/journal.pcbi.1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guitart-Masip M, Duzel E, Dolan R, Dayan P. Action versus valence in decision making. Trends Cogn Sci. 2014;18:194–202. doi: 10.1016/j.tics.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cartoni E, Balleine B, Baldassarre G. Appetitive Pavlovian-instrumental transfer: a review. Neurosci Biobehav Rev. 2016;71:829–48. doi: 10.1016/j.neubiorev.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 63.Aupperle RL, Melrose AJ, Francisco A, Paulus MP, Stein MB. Neural substrates of approach-avoidance conflict decision-making. Hum Brain Mapp. 2015;36:449–62. doi: 10.1002/hbm.22639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pessiglione M, Delgado MR. The good, the bad and the brain: Neural correlates of appetitive and aversive values underlying decision making. Curr Opin Behav Sci. 2015;5:78–84. doi: 10.1016/j.cobeha.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Minear M, Shah P. Training and transfer effects in task switching. Mem Cognit. 2008;36:1470–83. doi: 10.3758/MC.336.8.1470. [DOI] [PubMed] [Google Scholar]
- 66••.Yee D, Krug MK, Allen A, Braver TS. Humans Integrate Monetary and Liquid Incentives to Motivate Cognitive Task Performance. Front Psychol. 2016;6:1–17. doi: 10.3389/fpsyg.2015.02037. A behavioral study which examined valence effects (i.e., appetive vs. aversive motivation) on cognitively controlled behavior, especially in the case of motivational conflict. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Camille N, Griffiths CA, Vo K, Fellows LK, Kable JW. Ventromedial Frontal Lobe Damage Disrupts Value Maximization in Humans. J Neurosci. 2011;31:7527–32. doi: 10.1523/JNEUROSCI.6527-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.FitzGerald THB, Seymour B, Dolan RJ. The role of human orbitofrontal cortex in value comparison for incommensurable objects. J Neurosci. 2009;29:8388–95. doi: 10.1523/JNEUROSCI.0717-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barron HC, Dolan RJ, Behrens TEJ. Online evaluation of novel choices by simultaneous representation of multiple memories. Nat Neurosci. 2013;16:1492–8. doi: 10.1038/nn.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Samanez-Larkin GR, Knutson B. Decision making in the ageing brain: changes in affective and motivational circuits. Nat Rev Neurosci. 2015:16. doi: 10.1038/nrn3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luna B, Paulsen DJ, Padmanabhan A, Geier C. The Teenage Brain: Cognitive Control and Motivation. Curr Dir Psychol Sci. 2013;22:94–100. doi: 10.1177/0963721413486971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luciana M, Collins PF. Incentive Motivation, Cognitive Control, and the Adolescent Brain: Is It Time for a Paradigm Shift? Child Dev Perspect. 2012;6:392–9. doi: 10.1111/j.1750-8606.2012.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Foussias G, Siddiqui I, Fervaha G, Mann S, McDonald K, Agid O, et al. Motivated to do well: An examination of the relationships between motivation, effort, and cognitive performance in schizophrenia. Schizophr Res. 2015;166:276–82. doi: 10.1016/j.schres.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 74.Braver TS, Cohen JD, Barch DM. The role of prefrontal cortex in normal and disordered cognitive control: A cognitive neuroscience perspective. In: Stuss DT, Knight RT, editors. Princ Front Lobe Funct. Oxford, England: Oxford University Press; 2002. pp. 428–48. [Google Scholar]
- 75.Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:7351–6. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonthier C, Macnamara BN, Chow M, Conway ARA, Braver TS. Inducing proactive control shifts in the AX-CPT. Front Psychol. 2016;7:1–14. doi: 10.3389/fpsyg.2016.01822. [DOI] [PMC free article] [PubMed] [Google Scholar]