Abstract
In the United States, the blacklegged tick, Ixodes scapularis, is a vector of seven human pathogens, including those causing Lyme disease, anaplasmosis, babesiosis, Borrelia miyamotoi disease, Powassan virus disease, and ehrlichiosis associated with Ehrlichia muris eauclarensis. In addition to an accelerated rate of discovery of I. scapularis-borne pathogens over the past two decades, the geographic range of the tick, and incidence and range of I. scapularis-borne disease cases, have increased. Despite knowledge of when and where humans are most at risk of exposure to infected ticks, control of I. scapularis-borne diseases remains a challenge. Human vaccines are not available, and we lack solid evidence for other prevention and control methods to reduce human disease. The way forward is discussed.
Ixodes scapularis-Borne Disease Agents Are an Increasing Public Health Concern
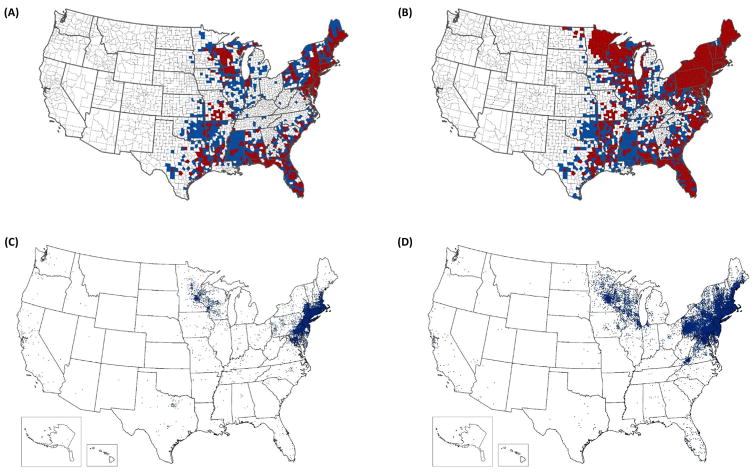
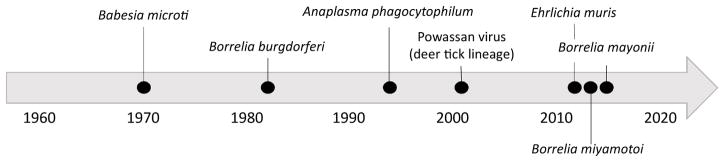
Among the approximately 50 000 locally acquired vector-borne disease cases reported annually from the contiguous United States, roughly 95% are caused by tick-borne pathogens and >70% are Lyme disease [1]. Lyme disease is caused by the spirochetes Borrelia burgdorferi sensu stricto (herein referred to as B. burgdorferi) [2], or much less commonly by Borrelia mayonii [3]; both are transmitted by the blacklegged tick, Ixodes scapularis (including the junior synonym, Ixodes dammini) in the eastern United States where the vast majority of cases occur [4,5]. Over the past two decades, we have seen expansions in both the geographic range of I. scapularis [6] (Figure 1A,B) and the incidence and geographic range of Lyme disease and other I. scapularis-borne diseases [7,8] (Figure 1C,D). In addition, new I. scapularis-borne human pathogens continue to be discovered. As of 2017, seven microorganisms transmitted by I. scapularis – including five bacteria (Anaplasma phagocytophilum, Bo. burgdorferi, Bo. mayonii, Bo. miyamotoi, and E. muris eauclarensis), one protozoan parasite (Babesia microti), and one virus (Powassan virus) – are known to cause illness in humans [7,9]. The recognition of this diverse guild of I. scapularis-borne pathogens over the last five decades marks a significant shift in the perceived medical importance of the tick; prior to 1970, I. scapularis was not considered an important vector of human pathogens (Figure 2).
Figure 1.
Reported Distribution of Ixodes scapularis in 1996 (A) and 2016 (B); and Reported Cases of Lyme Disease in 2001 (C) and 2015 (D).
Counties classified as established, based on <6 individual ticks of a single life stage or >1 life stage reported per county in a year are shown in red. Counties classified as reported based on <6 individual ticks reported in a year are shown in blue. Data from A and B are derived from Dennis et al. [30] and Eisen et al. [6], respectively. In panels C and D, one dot was placed randomly within the county of residence for each reported case. In the far-western United States, Ixodes pacificus serves as a vector of Borrelia burgdorferi.
Figure 2.
Timeline Showing Discovery of the Seven Human Pathogens Transmitted by Ixodes scapularis.
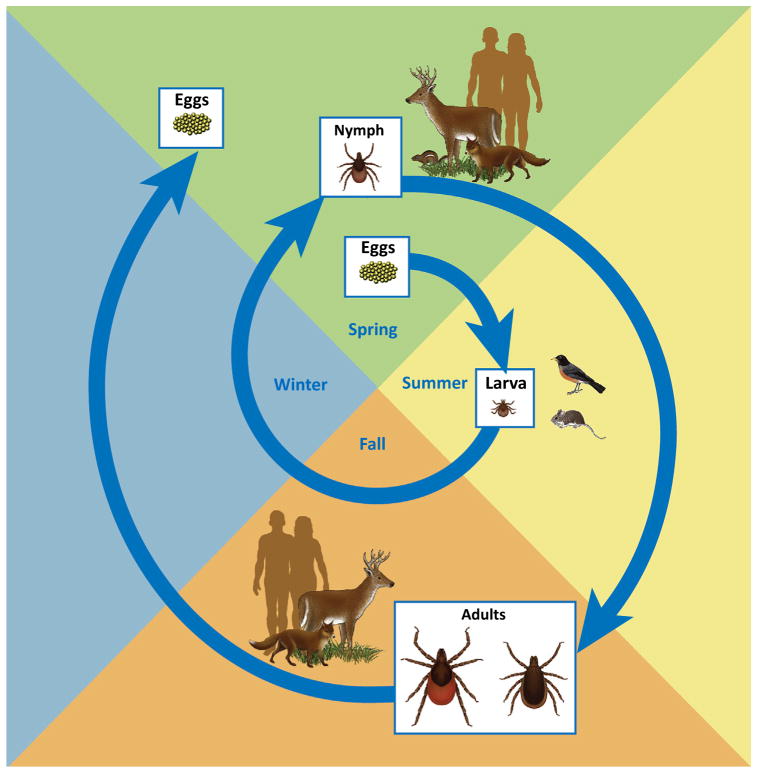
Humans are incidental hosts (see Glossary) of I. scapularis and its associated pathogens; although humans may be bitten [10,11], they are not essential for either the survival of tick populations or pathogen perpetuation (Figure 3). I. scapularis is a woodland-associated, three-host tick with a life cycle of 2–4 years [12,13]. Immature ticks (larvae and nymphs) have a broad host range, including rodents, insectivores, birds, lagomorphs, and ungulates [14,15]; whereas adults are restricted to medium- and large-sized mammals, primarily white-tailed deer (Odocoileus virginianus) [16]. With the exception of Powassan virus and Bo. miyamotoi relapsing fever spirochetes, which can be passed transovarially as well as acquired through blood-feeding [17,18], the other five human pathogens transmitted by I. scapularis are not known to be maintained transovarially, and acquisition by ticks therefore occurs during blood feeding [4,17,19–23].
Figure 3.
Life Cycle of Ixodes scapularis.
Many different personal protective measures to prevent tick bites, and control strategies to reduce tick abundance or disrupt pathogen transmission cycles, have been evaluated and demonstrated to be effective at preventing tick bites or reducing the abundance of host-seeking ticks or infection rates in ticks or reservoir hosts [24–26]. These approaches include: tick repellents and permethrin-treated clothing to prevent human–tick contact; synthetic chemicals, natural products, and biological agents to suppress host-seeking ticks; deer reduction to suppress tick populations; topical application of pesticides to reduce tick burdens on rodents and deer; and antibiotic treatment or vaccination of rodent reservoirs against Lyme borreliosis spirochetes [27]. However, very few approaches have been evaluated with tick-borne diseases as an outcome measure, and we lack evidence for any currently available personal protective measure or environmentally-based tick/pathogen-control method to consistently reduce I. scapularis-borne infections [28,29]. Herein, we describe the rise of I. scapularis and its associated diseases, and discuss control opportunities and challenges.
Ixodes scapularis Is Reclaiming Its Historical Range
Tick surveillance is not standardized or routine, thus hampering our ability to monitor changes in the distribution and abundance of I. scapularis [6,30]. Retrospective review of I. scapularis records reveals remarkable range expansion over the past century, particularly in the northern portion of the eastern United States. The earliest record of the tick in the northeast dates back to the 1920s near Cape Cod, Massachusetts [31]. By 1945, I. scapularis was recorded sporadically from states along the northern Atlantic coast, but its core distribution was primarily in the Gulf Coast states and the southeast [10]. In the early 1960s, focal populations were reported along the New England coast and in Rhode Island, and later in that decade records emerged from Long Island, New York, and northwestern Wisconsin. During the 1970s, the reported distribution of the tick expanded, and its abundance increased along the Atlantic coast from New England to the mid-Atlantic states; expansion inland continued through the 1980s and 1990s [30,31].
Moreover, compilations of I. scapularis county collection records revealed that over the past two decades the tick’s range has expanded substantially in the upper Midwest, northeast and mid-Atlantic states, but remained stable in the southeast (Figure 1A,B). As of 2016, I. scapularis had been documented in nearly half (1420 of 3110 counties) of the counties in the contiguous United States; in total, 842 counties across 35 eastern and central states are believed to have established populations [6]. Overall, during the past two decades, the number of counties in which I. scapularis is considered to be established has more than doubled. Recent habitat suitability models for I. scapularis identified many eastern counties as environmentally suitable for the tick to become established but from which it has not yet been reported, implying that the tick is still under-reported [32,33].
These geographical trends appear to represent a species reclaiming its historical range. Phylogeographic studies suggest that the tick’s historical range likely extended across much of the eastern United States. It is likely that the species originated in the southern United States a half a million years ago, with later expansion into the mid-Atlantic and northeastern United States roughly 50 000 years ago, followed by colonization of the upper Midwest in the last 20 000 years following the retreat of the Laurentide Ice Sheet [34].
Environmental changes over the past 200 years drastically altered the distribution of I. scapularis, particularly in the northeast. Rapid deforestation to accommodate agriculture and to provide fuel, coupled with near elimination of white-tailed deer through hunting and habitat loss during the 1800s and early 1900s, likely restricted the range of this woodland tick that strongly depends on deer for blood meals in the adult stage. Refugia sites were restricted to focal areas in the northeast and upper Midwest where forests remained intact [31,35]. By the second half of the 20th century, large portions of the northeast were converted from agricultural to suburban land, leading to reforestation with a spatial mosaic of woods of various ages and patch sizes intermingled with ornamental plants and maintained lawns [36]. During roughly the same time period, the increase in suitable habitat for deer resulted in dramatically increasing abundance of white-tailed deer [35].
Although compilation of presence records provides a reasonably accurate representation of the tick’s geographic range, lack of systematic vector surveillance limits accuracy in the estimation of geographic variation in the density of host-seeking I. scapularis nymphs, a variable that is more closely associated with Lyme disease incidence than measures of tick presence [37–40]. Roughly a decade ago, a systematic collection effort was undertaken to assess variation in the density of host-seeking nymphal I. scapularis ticks throughout the eastern United States [41]. The study revealed that, although I. scapularis was widely distributed, the density of host-seeking nymphs was generally higher in the north compared with the south, mirroring the reported distribution of Lyme disease cases in the eastern United States (Figure 1C,D). The findings were consistent with previous reports that, although the tick is present in southern states, host-seeking I. scapularis nymphs are rarely collected by drag sampling [42–44] and seldom bite people [11,45]. Later studies revealed distinct differences in host-seeking behavior between northern and southern clades of I. scapularis. Specifically, southern ticks are less likely than their northern counterparts to ascend vegetation when seeking hosts [46,47], thus reducing the likelihood of tick–human encounters when compared with their northern counterparts. Since the last systematic effort to document geographic variation in the density of host-seeking I. scapularis throughout its range in the United States [41], the tick’s range has expanded [6], and northern clade ticks appear to be spreading south [34]. Not surprisingly, the geographic range of counties classified as having high Lyme disease incidence has expanded following similar patterns [48]. A renewed effort to assess spatial variation in the density of host-seeking I. scapularis appears justified.
The Number of Recognized Human Disease Agents Transmitted by I. scapularis Is Growing
From 1970 through 2017, seven I. scapularis-borne human pathogens were described (Figure 2). In 1970, Ba. microti, an intraerythrocytic parasite, was first described in an otherwise healthy woman [49], (Figure 2). Shortly thereafter, Ba. microti was identified in white-footed mice (Peromysus leucopus) and in I. scapularis; experimental studies later confirmed that I. scapularis nymphs are capable of transmitting Ba. microti [50,51].
Lyme disease was first recognized in the United States as a new form of inflammatory arthritis in 1975 [52]. In 1982, a spirochete, later named Bo. burgdorferi [53], was identified as the etiological agent and shown to be transmissible by I. scapularis [2,5]. Although numerous small mammals and birds have been implicated as reservoirs of Bo. burgdorferi, the white-footed mouse is among the most important reservoirs in the eastern United States [15,36,54,55].
Human granulocytic anaplasmosis, originally described as human granulocytic ehrlichiosis (Ehrlichia phagocytophila), was first identified in six patients from northern Minnesota and Wisconsin presenting with acute febrile illnesses between 1990 and 1993. The timing of onset of cases was consistent with host-seeking activity of I. scapularis and Dermacentor variabilis and the former was implicated as a vector based on evidence that the closely related Ixodes ricinus transmits E. phagocytophila in Europe [56]. In 1996, I. scapularis was experimentally confirmed as a vector of E. phagocytophila, and P. leucopus was shown to be a competent reservoir [57]. In 2001, this intraleukocytic bacterium was renamed A. phagocytophilum [58].
Powassan virus, a flavivirus, was first recognized as a human pathogen in 1958 when it was isolated from a child who died of encephalitis [59]. Ixodes marxi, Ixodescookei, and Ixodes spinipalpis were implicated as enzootic vectors of Powassan virus in the 1960s [60–62], more than 30 years before experimental vector competence was demonstrated for I. scapularis [17]. Owing to its greater propensity to bite humans, I. scapularis is considered the primary bridging vector of Powassan virus (also referred to as ‘Deer Tick virus’ or ‘lineage II Powassan virus’) to humans [17,19,63].
In 2011, a novel obligate intracellular Gram-negative bacterium, found in I. scapularis from Minnesota and Wisconsin and later described as E. muris eauclarensis [64], was recognized to cause ehrlichiosis in humans [65]. I. scapularis was demonstrated experimentally to be a vector of E. muris eauclarensis [20,66], supporting earlier reports of natural infection in I. scapularis from Minnesota and Wisconsin [65,67,68]. E. muris eauclarensis has been detected in naturally infected white-footed mice collected in these two states [69], and reservoir competence was demonstrated in the laboratory [66].
Bo. miyamotoi, a relapsing fever spirochete, was first described in Ixodes persulcatus in Japan [70]. In 2001, the ability of I. scapularis to transmit Bo. miyamotoi while feeding, and to pass spirochetes transovarially, was demonstrated under laboratory conditions [71]. A decade later, Bo. miyamotoi was recognized as a human pathogen in a report of 46 cases from Russia [72]. Shortly thereafter, the first recognized case of Bo. miyamotoi disease in North America was described in an 80-year-old woman from New Jersey [73]. The first large case series from the northeastern United States revealed that the peak onset of illness occurs from July through August – 1 month later than for Lyme disease, anaplasmosis, and babesiosis – and thus corresponds with the peak host-seeking activity of larval rather than nymphal I. scapularis ticks [74,75]. Although white-footed mice support short-lived infections of Bo. miyamotoi transmissible to feeding ticks and likely play a role in amplification of infections [71,76], transovarial transmission may be the primary route of enzootic maintenance [76–79].
Until 2016, when Bo. mayonii was described and recognized as a causative agent of Lyme disease in Minnesota and Wisconsin [3,80], Bo. burgdorferi had been considered the sole agent of Lyme disease in the United States. Bo. mayonii has been detected in field-collected I. scapularis from Minnesota and Wisconsin [3], and vector competence has been demonstrated under laboratory conditions [4]. Bo. mayonii also was isolated from white-footed mice and an American red squirrel (Tamiasciurus hudsonicus) in Minnesota, but reservoir competence has not yet been demonstrated experimentally [81].
Coinfections Are Common in I. scapularis and May Increase Severity of Illness in Humans
Coinfections are commonly reported in I. scapularis, most often dual infections of Bo. burgdorferi with either A. phagocytophilum or Ba. microti [82–91]. Because of small sample sizes and lack of systematic efforts to assess trends over the geographic range of I. scapularis, the true prevalence of coinfections remains unknown. Based on limited data, prevalence of dual infections varies over time and by geographic region and has been reported in 1–28% of ticks tested, but commonly less than 5–10% of ticks are coinfected [89–91].
Bo. burgdorferi and Ba. microti share a common reservoir, the white-footed mouse, explaining the increased likelihood of finding coinfections more often than expected by chance [85,86,91]. Recent evidence suggests that Bo. burgdorferi promotes transmission of Ba. microti, and the former typically becomes established in new foci before the latter [85,86,91,92]. By contrast, coinfection with Bo. burgdorferi and A. phagocytophilum are typically observed at rates expected based on prevalence of each infection individually, suggesting independent enzootic transmission maintenance cycles [83,84,87,93]. Although, the efficiency of I. scapularis to transmit Bo. burgdorferi or A. phagocytophilum is not affected by coinfection [84], coinfection in mice has been shown to increase pathogen acquisition by feeding larvae, compared with rates observed when feeding on singly infected mice [94]. The relative abundance of various hosts in a community likely influences the probability of coinfections occurring. Although reported less commonly as a coinfection with Bo. burgdorferi compared with A. phagocytophilum or Ba. microti, coinfection with Bo. miyamotoi appears to occur at rates expected by chance, or lower, again suggesting independent mechanisms of persistence [76,82].
Coinfections with I. scapularis-borne pathogens in humans can arise from the bite of a single coinfected tick, or from concurrent bites by multiple singly-infected ticks. Although differences in methods of detecting infections differ across studies of persons diagnosed with tickborne diseases, and across studies from the northeastern and upper Midwestern United States, coinfection rates ranged from 0 to 67% for Lyme disease and babesiosis, 0 to 26% for Lyme disease and anaplasmosis, and 0 to 7% for anaplasmosis and babesiosis [90]. As reviewed previously, concurrent infections with Bo. burgdorferi and Ba. microti or A. phagocytophilum appear to increase severity of illness [90,91].
Incidence and Ranges of Diseases Caused by I. scapularis-Borne Pathogens Are Increasing
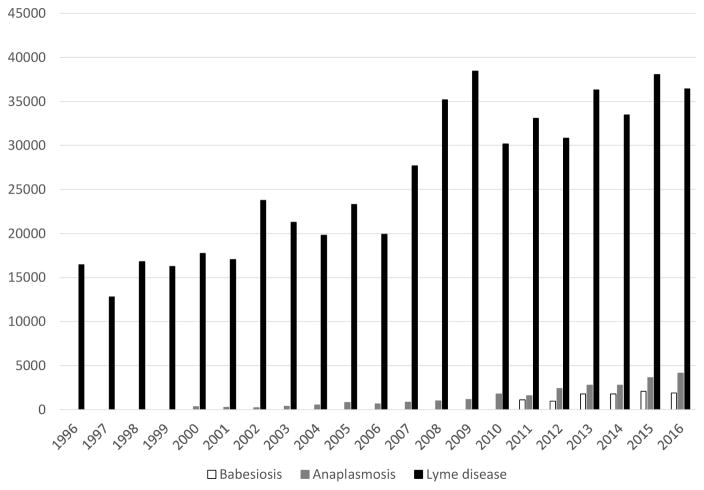
I. scapularis-borne pathogens are associated with four nationally notifiable diseases. Lyme disease was added to the list of notifiable conditions in 1991; anaplasmosis, Powassan virus disease, and babesiosis were included in 2000, 2002, and 2011, respectively. Case counts have generally increased for each of these conditions since they became notifiable (Figure 4). From 2002 to 2016, a total of 102 Powassan virus disease cases have been reported, with annual case counts ranging from 0 to 22 casesi. By contrast, since 2008, annual reported cases of Lyme disease have exceeded 30 000, marking a near tripling of reported cases since it was first notifiable in 1991 [8,95]. Notably, the number of cases reported is estimated to be approximately tenfold lower than the number of Lyme disease cases that are diagnosed annually [96,97]. Reported cases of anaplasmosis increased from 351 in 2000 to 4151 in 2016, and reported cases of babesiosis have increased from 1128 in 2011 to 1910 in i.
Figure 4.
Reported Cases of Babesiosis, Anaplasmosis, and Lyme Diseases in the United States, 1996–2016. Source: https://wwwn.cdc.gov/nndss/data-and-statistics.html (last referenced November 13, 2017).
Compared with Lyme disease, the incidence of reported anaplasmosis, Powassan virus disease, and babesiosis is several orders of magnitude lower, and their geographic distribution appears to be similar but more restricted; like the distribution of Lyme disease cases, the geographic range of anaplasmosis and babesiosis has similarly spread over time (Figure 4) [7,19,95,98,99]. Over 96% of Lyme disease cases are reported from just 14 states in the northeast, mid-Atlantic and the upper Midwest [95]. Since the mid-1990s, the number of counties with a high incidence of Lyme disease has increased by approximately 300% [48]. As it is not a notifiable condition, trends in incidence and geographic range of Bo. miyamotoi disease cases are not well characterized, but the geographic range is likely similar to that of Lyme disease [100]. By contrast, ehrlichiosis, caused by E. muris eauclarensis, thus far has been reported only from the upper Midwest [65].
Controlling I. scapularis and Reducing Tick-Borne Diseases Is Challenging
The Evidence Base for Existing Interventions to Reduce Human Tick-Borne Disease Is Weak
Perhaps the most vexing aspect of control of I. scapularis-borne diseases, as exemplified by Lyme disease, is that we already know (i) the geographic areas in which the majority of cases will occur each year, and (ii) the months of the year during which most of the infections will be acquired [95]. In the Lyme disease focus in the northeast, we also know that humans most often encounter I. scapularis ticks in peridomestic settings, including on their own residential properties [26,37]. Despite this detailed knowledge of when and where humans are most at risk for exposure to infected ticks, we remain unable to control I. scapularis-borne diseases.
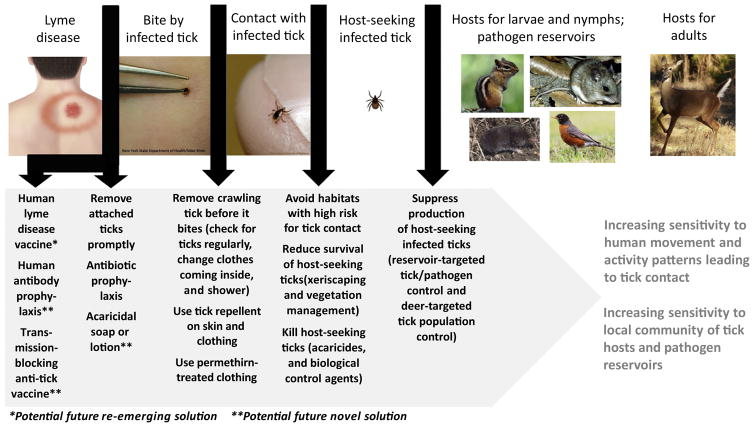
Previous reviews have addressed (i) personal protective measures to reduce human contact with I. scapularis ticks and environmentally based control methods to suppress host-seeking ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs [25–27,101]; (ii) the evidence base for such measures, and methods to reduce Lyme disease [28,29,102,103]; and (iii) the prospect for a human Lyme disease vaccine to re-emerge in the wake of the rise and fall of Lymerix, an effective licensed vaccine that was removed from the US market in 2003 [104,105]. Despite the emergence of a wide array of approaches to avoid contact with ticks through personal protective measures, suppress host-seeking I. scapularis, or disrupt enzootic B. burgdorferi transmission, we unfortunately still lack robust evidence for any method other than a human Lyme disease vaccine to reduce disease cases. When thinking about strengths and weaknesses of methods to prevent I. scapularis-borne infections, we find it useful to illustrate the chain of events leading to a case of I. scapularis-borne infection (using Lyme disease as an example) and, working backwards from the human infection, define and discuss the points where we can potentially intervene (Figure 5).
Figure 5.
Chain of Events Leading to a Lyme Disease Infection, with Possible Intervention Points for Different Control Approaches.
Disease Resulting from Bites by Infected Ticks Can Be Prevented by Early Tick Detection and Removal, Antibiotic Prophylaxis, and, in the Future, Hopefully Also by Vaccines
The most proximate intervention to prevent a human infection caused by an I. scapularis-borne pathogen is to ensure that the bite of an infected tick does not result in illness (Figure 5). Although this intuitively is the most impactful intervention point, all currently available intervention methods suffer from the shortcoming of being reliant on detection of attached ticks. The fact that I. scapularis nymphs are notoriously difficult for people to detect while biting [106] limits the usefulness of both removal of an attached infected nymph before it can transmit a pathogen [107] and antibiotic prophylaxis following a recognized tick bite [108]. This could potentially be overcome with a new type of consumer product to kill attached ticks without first having to detect their presence, such as an acaricidal skin lotion or shower soap. However, even this solution has practical limitations because it will require daily use and would be effective only if the soap or lotion is applied directly onto an unrecognized biting tick. Moreover, the tick would be attached for some period of time before being impacted, thus increasing the risk for pathogen transmission.
Potential future magic bullet solutions, capable of both ensuring that the bite of an infected tick does not result in illness and having the potential to rapidly and dramatically reduce I. scapularis-borne human infections at the population level, include (i) human vaccines or prophylactic antibody treatments against Lyme disease spirochetes or other I. scapularis-borne pathogens [104,105,109,110], and (ii) transmission-blocking anti-tick vaccines for human use with potential for simultaneous protection against multiple I. scapularis-borne pathogens [111–115]. These approaches would not require daily action and vigilance, and they would be effective regardless of whether or not bites by infected ticks are noticed. If proven to be safe, effective, and acceptable for widespread use, there is no question that the re-emergence of a human vaccine against Bo. burgdorferi, or the emergence of a prophylactic antibody treatment, would be the most effective ways to rapidly reduce Lyme disease cases. However, neither approach would address the remaining I. scapularis-borne pathogens, several of which are on the rise (Figure 4). A transmission-blocking anti-tick vaccine could potentially address that shortcoming, but only if it proves to act quickly and effectively enough on an infected tick to prevent or substantially reduce the likelihood of pathogen transmission occurring before the tick is incapacitated. These urgently needed approaches merit greater resources to expeditiously move them forward in a pipeline from prevention concept to proven solution and become, should they be successful, cornerstones in public health programs to reduce I. scapularis-borne infections.
Use of Repellents and Permethrin-Treated Clothing Can Reduce the Risk of Tick Contact Resulting in Bites
The next point of intervention to prevent a human infection is to ensure that ticks making contact with human skin or clothing do not get an opportunity to bite (Figure 5). This can be achieved by the use of tick repellents on skin and clothing, or the use of permethrin-treated clothing [27,116], combined with regular checks for crawling ticks, changing clothes when coming inside (and drying removed clothing articles at high heat), and taking a shower (ensuring removal of clothes worn outside, increasing the likelihood of detecting crawling ticks, and perhaps also dislodging crawling ticks while showering) [25,29,95]. The main problem is that all of these actions require a level of daily vigilance and effort that is hard to keep up for the period of 2–3 months during which I. scapularis nymphs are most active. Another problem is that we lack knowledge of how specific use patterns for repellents and permethrin-treated clothing, including frequency of use and extent of the body protected, may impact their protective effect against tick bites.
Risk of Contact with Host-Seeking Ticks Can Be Minimized by Behavioral Change, Environmental Modification, and Killing of Host-Seeking Ticks
Even further distant from the human infection, we can intervene by minimizing contact with host-seeking ticks (Figure 5) through (i) avoidance of habitats with a high risk for tick contact (easier said than done if it includes your backyard), (ii) reduction in longevity or survival of desiccation-sensitive host-seeking I. scapularis ticks in the peridomestic environment (e.g., xeriscaping, hardscaping, and vegetation management, including keeping grass short, clearing brush and removing leaf litter), or (iii) direct killing of host-seeking ticks with acaricides or biological control agents [24,25,27,29,117]. Here we introduce increased complexity by relying on solutions that are highly sensitive to human movement patterns. For example, controlled experimental spring applications of pyrethroids typically reduce host-seeking I. scapularis nymphs by >85% [27]. Nevertheless, a recent effort to reduce tick bites and human I. scapularis-borne infections by spraying pyrethroids along the lawn–wood interface on residential properties, rather than treating all the wooded and brushy high-risk habitat on the properties, achieved 50–70% suppression of host-seeking I. scapularis nymphs within the areas sprayed but failed to reduce either tick bites or human infection [118]. Suppression of host-seeking I. scapularis nymphs across all wooded or brushy habitats on a property, thereby getting closer to a desired goal of complete absence of ticks in this high-risk environment, intuitively should be more impactful but it is also more expensive and may come at higher environmental costs. It remains to be evaluated to what extent such an intervention can reduce tick-borne disease.
Production of Infected Ticks Can Be Suppressed by Targeting Important Tick Hosts and Pathogen Reservoirs
Finally, we can intervene by suppressing production of infected I. scapularis nymphs by disrupting enzootic pathogen transmission among tick immatures and vertebrate hosts acting as pathogen reservoirs (particularly rodents) and targeting key hosts for the adult stage (particularly white-tailed deer) to reduce overall tick populations (Figure 5). There is little doubt that the white-tailed deer is the engine that drove the remarkable surge in populations of I. scapularis seen across the northeast and upper Midwest over the last 50 years [31,35]. Intuitively, adult I. scapularis ticks feeding on white-tailed deer is the weakest point in the chain leading to tick population build-up and, ultimately, intensified enzootic transmission among tick immatures and pathogen reservoirs, and production of pathogen-infected I. scapularis nymphs. It therefore has been reasonably argued that addressing deer, either by population reduction to very low levels or topical/oral application of acaricides to a large proportion of the deer, should be viewed as a cornerstone of area-wide environmentally based integrated management programs for I. scapularis [25,26,101,103]. Although numerous methods targeting rodent reservoirs and white-tailed deer have emerged in the last 30 years [27], questions remain about the extent of available animals within a given area that need to be removed or treated to achieve reduction of human tick bites and human disease [26,28,102,103]. These methods also can be sensitive to local vertebrate community structure (if alternative pathogen reservoirs are readily available for tick immatures or alternative hosts for adult ticks are abundant) or, when relying on food baits in the implementation, to natural variation in food sources for rodents or deer over time.
Integrated Intervention Approaches Need to Be Evaluated with Human Disease Outcome Measures
Barring the emergence of a magic bullet solution (human vaccine, prophylactic antibody treatment, or transmission-blocking anti-tick vaccine), no single personal protective measure or environmentally based tick/pathogen control method is likely to substantially reduce I. scapularis-borne infections when used in isolation [28]. A few integrated intervention approaches that combine two or three environmentally based control methods have been shown to effectively reduce abundance of host-seeking I. scapularis nymphs [26,27,119–121], but none of these integrated approaches have yet been evaluated with the gold standard of human infection with an I. scapularis-borne pathogen as an outcome measure [28]. As suggested by the chain of events outlined in Figure 5, we also need to think outside the box and consider integrated intervention approaches that – rather than just combining two or more environmentally-based control methods – also include changes in human behavior and the use of existing personal protective measures.
We Need to Better Understand the Issues Relating to Cost, Acceptability, and Feasibility of Different Intervention Approaches
Another major challenge arises because control of I. scapularis and prevention of infection with its associated pathogens remains the responsibility of individual homeowners. Families therefore must make decisions regarding personal protective measures and environmentally based tick control on their properties, taking into consideration how much money they are willing to spend, under which circumstances (when and where) they wish to be protected, the level of daily effort required to achieve protection, and whether a given measure or method is acceptable to use. As illustrated in Figure 6, an ideal tick-borne disease prevention method should, from the perspective of a family, incur low cost, require minimal effort, and be globally effective (i.e., protective everywhere, all the time, and regardless of type of activity). It also must be acceptable for use. The scope of this challenge is illustrated by the most recently published survey of willingness to pay for tick control [122], which found that most residents in a Lyme disease endemic setting in Connecticut were unwilling to spend more than $100 per year and that acceptability was limited for some methods, including the use of acaricides to kill host-seeking ticks.
Figure 6.
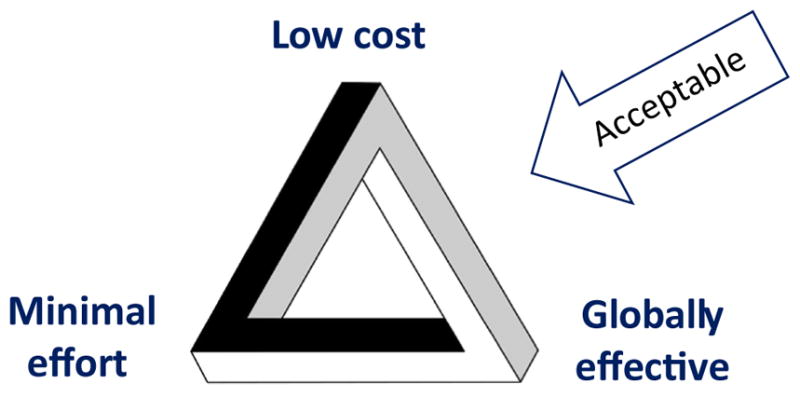
Desired Characteristics of an Ideal Tick-Borne Disease Prevention Method.
The magic bullet approaches discussed above (human vaccine, prophylactic antibody treatment, or transmission-blocking anti-tick vaccine) come closest to solving the ‘impossible tribar’ of low cost, minimal effort, and global effectiveness, should they emerge and prove to be safe, effective, and widely acceptable for use (Figure 6). All currently available personal protective measures or environmentally based control methods fall short for at least one of the three desired characteristics, and some likely also will have limited acceptability. This, in turn, raises the intriguing question of which characteristic a majority of families is willing to give up: low cost, minimal effort, or global effectiveness? We therefore need to consider not only whether solutions to reduce I. scapularis-borne infections can be applied by individual families/on individual properties or may require implementation at a neighborhood/community scale, but also which solutions can achieve specific combinations of at least two desired characteristics: low cost–minimal effort, low cost–global effectiveness, or minimal effort–global effectiveness. Finally, we note that the process of moving promising solutions to reduce I. scapularis-borne infections forward in a pipeline from prevention concept to proven solution and successful public health program currently is impeded by an order of magnitude shortcoming in the financial resources available to achieve this effectively and expeditiously.
Concluding Remarks
In recent decades, I. scapularis has become more widespread, and an increasing number of microorganisms transmitted by this tick have proven to be pathogenic in humans. In parallel, both the incidence and geographic range of reported cases of I. scapularis-borne diseases have increased, and coinfections are increasingly being recognized to contribute to severity of illness. Moreover, habitat suitability models suggest that the tick’s potential range exceeds the current reported distribution, suggesting either under-reporting of the tick’s current range or the potential for range expansion (see Outstanding Questions). Because the presence of the vector tick is a prerequisite for human tick-borne infections, we recognize a need to monitor changes in the distribution of I. scapularis. Recognizing that the density of host-seeking infected nymphs provides a better estimate of human risk for bites by infected ticks than measures of tick presence, we emphasize the need to assess spatial variation in the density of infected host-seeking nymphs in order to educate the public of changing risk patterns. Such studies should (i) use standardized sampling methodology, (ii) be conducted during the expected peak in nymphal host-seeking, (iii) use sensitive and specific pathogen-detection assays that are capable of detecting coinfections, (iv) report the density of pathogen-infected host-seeking nymphs (the life stage most often associated with human infections) per sampling site, and (v) use appropriate statistical methods to extrapolate predictions about the measured outcomes to areas that were not sampled. Moreover, there is a critical need for intervention approaches with proven capacity to reverse the growing public health problem imposed by I. scapularis (see Outstanding Questions). We need intensified and sustained efforts to develop safe and effective human vaccines, prophylactic antibody treatments, and transmission-blocking anti-tick vaccines, as well as a stronger evidence base for the capability of other already available personal protective measures and environmental control methods to reduce tick-borne disease, especially for integrated intervention approaches. Although proof-of-concept studies will logically focus on acarological or zoonotic outcomes (e.g., tick or host abundance, infection rates in ticks or hosts), ultimately evaluations of prevention strategies with human disease outcomes are needed.
Outstanding Questions.
How widespread is I. scapularis?
How does the density of infected host-seeking nymphs change across the species’ range?
Are E. muris eauclarensis and Bo. mayonii restricted to the upper Midwest and, if so, why?
As previously distinct northern I. scapularis foci in the upper Midwest and northeast are merging, how will this affect the distribution of I. scapularis-borne pathogens?
Why is the prevalence of Bo. miyamotoi in ticks so low compared with Bo. burgdorferi, given that the former is transmitted transtadially and transovarially and the latter is transmitted only transtadially?
Among the potential control strategies, which has the greatest potential to reduce the incidence of I. scapularis-borne disease cases? Given that none of the current options can combine low cost, minimal effort, and global effectiveness, which characteristic is a majority of families willing to give up: low cost, minimal effort, or global effectiveness?
Highlights.
The blacklegged tick, Ixodes scapularis, is becoming more widespread in the eastern United States.
The number of I. scapularis-borne microorganisms recognized to be pathogenic in humans is increasing.
The incidence of I. scapularis-borne disease cases continues to increase.
The geographic distribution of human cases of I. scapularis-borne diseases is expanding.
There is a critical need for control approaches with proven capacity to reverse the growing public health problem imposed by I. scapularis.
Acknowledgments
We thank Anna Perea for assistance with the figures, and Alison Hinckley and Paul Mead for helpful discussions.
Glossary
- Bridging tick vectors
ticks that acquire pathogens from zoonotic hosts involved in enzootic transmission cycles and later transmit pathogens to incidental hosts, which, in the case of tick-borne pathogens, include humans.
- Coinfection
simultaneous infection with two or more pathogens within the same vector or host.
- Drag sampling
a method of collecting host-seeking ticks in which a blanket is dragged across vegetation, typically over fixed distances or amounts of time, usually in an effort to quantify the abundance or density of host-seeking ticks. It is generally considered a better measure of the risk for human encounters with ticks than measures of tick abundance on hosts.
- Enzootic tick vectors
ticks that transmit the pathogen of interest among zoonotic hosts.
- Host-seeking
behavior displayed by a tick in an attempt to find a bloodmeal host (e.g., ascending vegetation and waiting for a host to pass by).
- Incidental hosts
hosts that are not essential to the tick’s life cycle or perpetuation of tick-associated pathogens.
- Magic bullet
something providing an effective solution to a difficult or previously unsolvable problem.
- Relapsing fever spirochetes
phylogenetically related to Lyme disease spirochetes, but relapsing fever spirochetes are typically transmitted by soft (argasid) ticks (with the notable exception of a few hard-tick-borne species, including Borrelia miyamotoi), and transovarial transmission is common. In contrast, Lyme disease spirochetes are transmitted by hard (ixodid) ticks and are not maintained transovarially.
- Reservoirs
organisms in which a pathogen can survive and reproduce, for some period of time, and that contribute to enzootic maintenance.
- Transovarial transmission
passage of infection from an infected adult female tick to her eggs.
- Vector ticks
ticks capable of acquiring infection during blood-feeding or transovarially, remaining infected through transition to subsequent life stages, and infecting a susceptible host while feeding.
Footnotes
Resources
Disclaimer Statement
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Adams DA, et al. Summary of notifiable infectious disease conditions – United States, 2014. Morb Mortal Wkly Rep. 2016;63:1–52. doi: 10.15585/mmwr.mm6354a1. [DOI] [PubMed] [Google Scholar]
- 2.Burgdorfer W, et al. Lyme disease-a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 3.Pritt BS, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16:556–564. doi: 10.1016/S1473-3099(15)00464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolan MC, et al. Vector competence of the blacklegged tick, Ixodes scapularis, for the recently recognized Lyme borreliosis spirochete Candidatus Borrelia mayonii. Ticks Tick Borne Dis. 2016;7:665–669. doi: 10.1016/j.ttbdis.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Piesman J, et al. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557–558. doi: 10.1128/jcm.25.3.557-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisen RJ, et al. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J Med Entomol. 2016;53:349–386. doi: 10.1093/jme/tjv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisen RJ, et al. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017:1–17. doi: 10.1093/ilar/ilx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz AM, et al. Surveillance for Lyme disease –United States, 2008–2015. MMWR Surveill Summ. 2017;66(SS-22):1–12. doi: 10.15585/mmw.ss6622a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paddock CD, et al. Changing paradigms for tick-borne diseases in the Americas. In: Mack A, editor. Global Health Impacts of Vector-Borne Diseases: Workshop Summary. National Academies Press; 2016. pp. 221–257. [PubMed] [Google Scholar]
- 10.Bishopp FC, Trembley HL. Distribution and hosts of certain North American ticks. J Parasitol. 1945;31:1–54. [Google Scholar]
- 11.Merten HA, Durden LA. A state-by-state survey of ticks recorded from humans in the United States. J Vector Ecol. 2000;25:102–113. [PubMed] [Google Scholar]
- 12.Hamer SA, et al. Synchronous phenology of juvenile Ixodes scapularis, vertebrate host relationships, and associated patterns of Borrelia burgdorferi ribotypes in the midwestern United States. Ticks Tick Borne Dis. 2012;3:65–74. doi: 10.1016/j.ttbdis.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Yuval B, Spielman A. Duration and regulation of the developmental cycle of Ixodes dammini (Acari: Ixodidae) J Med Entomol. 1990;27:196–201. doi: 10.1093/jmedent/27.2.196. [DOI] [PubMed] [Google Scholar]
- 14.Giardina AR, et al. Modeling the role of songbirds and rodents in the ecology of Lyme disease. Can J Zool. 2000;78:2184–2197. [Google Scholar]
- 15.LoGiudice K, et al. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci U S A. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piesman J, et al. Role of deer in the epizootiology of Babesia microti in Massachusetts, USA. J Med Entomol. 1979;15:537–540. doi: 10.1093/jmedent/15.5-6.537. [DOI] [PubMed] [Google Scholar]
- 17.Costero A, Grayson MA. Experimental transmission of Powassan virus (Flaviviridae) by Ixodes scapularis ticks (Acari: Ixodidae) Am J Trop Med Hyg. 1996;55:536–546. doi: 10.4269/ajtmh.1996.55.536. [DOI] [PubMed] [Google Scholar]
- 18.Rollend L, et al. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick Borne Dis. 2013;4:46–51. doi: 10.1016/j.ttbdis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Ebel G. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol. 2010;55:95–110. doi: 10.1146/annurev-ento-112408-085446. [DOI] [PubMed] [Google Scholar]
- 20.Karpathy SE, et al. Cofeeding transmission of the Ehrlichia muris-like agent to mice (Mus musculus) Vector Borne Zoonotic Dis. 2016;16:145–150. doi: 10.1089/vbz.2015.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mather TN, Mather ME. Intrinsic competence of three ixodid ticks (Acari) as vectors of the Lyme disease spirochete. J Med Entomol. 1990;27:646–650. doi: 10.1093/jmedent/27.4.646. [DOI] [PubMed] [Google Scholar]
- 22.Piesman J, et al. Simultaneous transmission of Borrelia burgdorferi and Babesia microti by individual nymphal Ixodes dammini ticks. J Clin Microbiol. 1987;25:2012–2013. doi: 10.1128/jcm.25.10.2012-2013.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teglas MB, Foley E. Differences in the transmissibility of two Anaplasma phagocytophilum strains by the North American ticks vector species, Ixodes pacificus and Ixodes scapularis (Acari: Ixodidae) Exp Appl Acarol. 2006;38:47–58. doi: 10.1007/s10493-005-5293-5. [DOI] [PubMed] [Google Scholar]
- 24.Piesman J, Eisen L. Prevention of tick-borne diseases. Annu Rev Entomol. 2008:323–343. doi: 10.1146/annurev.ento.53.103106.093429. [DOI] [PubMed] [Google Scholar]
- 25.Stafford KC., III . Tick Management Handbook. An Integrated Guide for Homeowners, Pest Control Operators, and Public Health Officials for the Prevention of Tick-Associated Disease. The Connecticut Agricultural Experiment Station; 2007. [Google Scholar]
- 26.Stafford KC, III, et al. Integrated pest management in controlling ticks and tick-associated diseases. J Integr Pest Manage. 2017 Published online October 17, 2017. http://dx.doi.org/10.1093/jipm/pmx018.
- 27.Eisen L, Dolan MC. Evidence for personal protective measures to reduce human contact with blacklegged ticks and for environmentally based control methods to suppress host-seeking blacklegged ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs. J Med Entomol. 2016;53:1063–1092. doi: 10.1093/jme/tjw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisen L, Gray JS. Lyme borreliosis prevention strategies: United States versus Europe. In: Braks MAH, et al., editors. Ecology and Prevention of Lyme Borreliosis. Wageningen Academic Publishers; 2016. pp. 429–450. [Google Scholar]
- 29.Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348:2424–2430. doi: 10.1056/NEJMra021397. [DOI] [PubMed] [Google Scholar]
- 30.Dennis DT, et al. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J Med Entomol. 1998;35:629–638. doi: 10.1093/jmedent/35.5.629. [DOI] [PubMed] [Google Scholar]
- 31.Spielman A, et al. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annu Rev Entomol. 1985;30:439–460. doi: 10.1146/annurev.en.30.010185.002255. [DOI] [PubMed] [Google Scholar]
- 32.Hahn MB, et al. Modeling the geographic distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the contiguous United States. J Med Entomol. 2016;53:1176–1191. doi: 10.1093/jme/tjw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hahn MB, et al. Response: The geographic distribution of Ixodes scapularis (Acari: Ixodidae) revisited: the importance of assumptions about error balance. J Med Entomol. 2017;54:1104–1106. doi: 10.1093/jme/tjx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Zee J, et al. Nuclear markers reveal predominantly north to south gene flow in Ixodes scapularis, the tick vector of the Lyme disease spirochete. PLoS One. 2015;10:e0139630. doi: 10.1371/journal.pone.0139630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spielman A. The emergence of Lyme disease and human babesiosis in a changing environment. Ann N Y Acad Sci. 1994;740:146–156. doi: 10.1111/j.1749-6632.1994.tb19865.x. [DOI] [PubMed] [Google Scholar]
- 36.Lane RS, et al. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- 37.Falco RC, Fish D. A comparison of methods for sampling the deer tick, Ixodes dammini, in a Lyme disease endemic area. Exp Appl Acarol. 1992;14:165–173. doi: 10.1007/BF01219108. [DOI] [PubMed] [Google Scholar]
- 38.Mather TN, et al. Entomologic index for human risk of Lyme disease. Am J Epidemiol. 1996;144:1066–1069. doi: 10.1093/oxfordjournals.aje.a008879. [DOI] [PubMed] [Google Scholar]
- 39.Stafford KC, III, et al. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J Clin Microbiol. 1998;36:1240–1244. doi: 10.1128/jcm.36.5.1240-1244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pepin KM, et al. Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the Eastern United States. Am J Trop Med Hyg. 2012;86:1062–1071. doi: 10.4269/ajtmh.2012.11-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diuk-Wasser MA, et al. Field and climate-based model for predicting the density of host-seeking nymphal Ixodes scapularis, an important vector of tick-borne disease agents in the eastern United States. Global Ecol Biogeogr. 2010;19:504–514. [Google Scholar]
- 42.Cilek JE, Olson MA. Seasonal distribution and abundance of ticks (Acari: Ixodidae) in northwestern Florida. J Med Entomol. 2000;37:439–444. doi: 10.1093/jmedent/37.3.439. [DOI] [PubMed] [Google Scholar]
- 43.Goddard J, Piesman J. New records of immature Ixodes scapularis from Mississippi. J Vector Ecol. 2006;31:421–422. doi: 10.3376/1081-1710(2006)31[421:nroiis]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 44.Mackay A, Foil L. Seasonal and geographical distribution of adult Ixodes scapularis say (Acari: Ixodidae) in Louisiana. J Vector Ecol. 2005;30:168–170. [PubMed] [Google Scholar]
- 45.Stromdahl EY, Hickling GJ. Beyond Lyme: aetiology of tick-borne human diseases with emphasis on the south-eastern United States. Zoonoses Public Health. 2012;59(Suppl 2):48–64. doi: 10.1111/j.1863-2378.2012.01475.x. [DOI] [PubMed] [Google Scholar]
- 46.Arsnoe IM, et al. Different populations of blacklegged tick nymphs exhibit differences in questing behavior that have implications for human Lyme disease risk. PLoS One. 2015;10:e0127450. doi: 10.1371/journal.pone.0127450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ginsberg HS, et al. Environmental factors affecting survival of immature Ixodes scapularis and implications for geographical distribution of Lyme disease: the climate/behavior hypothesis. PLoS One. 2017;12:e0168723. doi: 10.1371/journal.pone.0168723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kugeler KJ, et al. Geographic distribution and expansion of human Lyme disease, United States. Emerg Infect Dis. 2015;21:1455–1457. doi: 10.3201/eid2108.141878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Western KA, et al. Babesiosis in a Massachusetts resident. N Engl J Med. 1970;283:854–856. doi: 10.1056/NEJM197010152831607. [DOI] [PubMed] [Google Scholar]
- 50.Spielman A. Human babesiosis on Nantucket Island: transmission by nymphal Ixodes ticks. Am J Trop Med Hyg. 1976;25:784–787. doi: 10.4269/ajtmh.1976.25.784. [DOI] [PubMed] [Google Scholar]
- 51.Piesman J, Spielman A. Human babesiosis on Nantucket Island: prevalence of Babesia microti in ticks. Am J Trop Med Hyg. 1980;29:742–746. doi: 10.4269/ajtmh.1980.29.742. [DOI] [PubMed] [Google Scholar]
- 52.Steere AC, et al. Erythema chronicum migrans and Lyme arthritis: cryoimmunoglobulins and clinical activity of skin and joints. Science. 1977;196:1121–1122. doi: 10.1126/science.870973. [DOI] [PubMed] [Google Scholar]
- 53.Johnson RC, et al. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int J Syst Bacteriol. 1984;34:496–497. [Google Scholar]
- 54.Donahue JG, et al. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am J Trop Med Hyg. 1987;36:92–96. doi: 10.4269/ajtmh.1987.36.92. [DOI] [PubMed] [Google Scholar]
- 55.Mather TN, et al. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi) Am J Epidemiol. 1989;130:143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- 56.Chen SM, et al. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Telford SR, 3rd, et al. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci U S A. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dumler JS, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 59.McLean DM, Donohue WL. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J. 1959;80:708–711. [PMC free article] [PubMed] [Google Scholar]
- 60.McLean DM, et al. Powassan virus: summer infection cycle, 1964. Can Med Assoc J. 1964;91:1360–1362. [PMC free article] [PubMed] [Google Scholar]
- 61.McLean DM, et al. Powassan virus: field investigations during the summer of 1963. Am J Trop Med Hyg. 1964;13:747–753. doi: 10.4269/ajtmh.1964.13.747. [DOI] [PubMed] [Google Scholar]
- 62.McLean DM, Larke RP. Powassan and Silverwater viruses: ecology of two Ontario arboviruses. Can Med Assoc J. 1963;88:182–185. [PMC free article] [PubMed] [Google Scholar]
- 63.Telford SR, 3rd, et al. A new tick-borne encephalitis-like virus infecting New England deer ticks. Ixodes dammini Emerg Infect Dis. 1997;3:165–170. doi: 10.3201/eid0302.970209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pritt BS, et al. Proposal to reclassify Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. nov., a newly recognized tick-borne pathogen of humans. Int J Syst Evol Microbiol. 2017;67:2121–2126. doi: 10.1099/ijsem.0.001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pritt BS, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med. 2011;365:422–429. doi: 10.1056/NEJMoa1010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lynn GE, et al. Experimental evaluation of Peromyscus leucopus as a reservoir host of the Ehrlichia muris-like agent. Parasites Vectors. 2017;10:48. doi: 10.1186/s13071-017-1980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stromdahl E, et al. Comparison of phenology and pathogen prevalence, including infection with the Ehrlichia muris-like (EML) agent, of Ixodes scapularis removed from soldiers in the midwestern and the northeastern United States over a 15 year period (1997–2012) Parasites Vectors. 2014;7:553. doi: 10.1186/s13071-014-0553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Telford SR, et al. Prevalence of Ehrlichia muris in Wisconsin deer ticks collected during the mid 1990. Open Microbiol J. 2011;5:18–20. doi: 10.2174/1874285801105010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castillo CG, et al. Detection of human pathogenic Ehrlichia muris-like agent in Peromyscus leucopus. Ticks Tick Borne Dis. 2015;6:155–157. doi: 10.1016/j.ttbdis.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 70.Fukunaga M, et al. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int J Syst Bacteriol. 1995;45:804–810. doi: 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- 71.Scoles GA, et al. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- 72.Platonov AE, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gugliotta JL, et al. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med. 2013;368:240–245. doi: 10.1056/NEJMoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krause PJ, Barbour AG. Borrelia miyamotoi: the newest infection brought to us by deer ticks. Ann Intern Med. 2015;163:141–142. doi: 10.7326/M15-1219. [DOI] [PubMed] [Google Scholar]
- 75.Molloy PJ, et al. Borrelia miyamotoi disease in the Northeastern United States: a case series. Ann Intern Med. 2015;163:91–98. doi: 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- 76.Barbour AG, et al. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg. 2009;81:1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crowder CD, et al. Prevalence of Borrelia miyamotoi in Ixodes ticks in Europe and the United States. Emerg Infect Dis. 2014;20:1678–1682. doi: 10.3201/eid2010.131583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wagemakers A, et al. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol. 2015;31:260–269. doi: 10.1016/j.pt.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 79.Bunikis J, Barbour AG. Third Borrelia species in white-footed mice. Emerg Infect Dis. 2005;11:1150–1151. doi: 10.3201/eid1107.041355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pritt BS, et al. Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. Int J Syst Evol Microbiol. 2016;66:4878–4880. doi: 10.1099/ijsem.0.001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson TL, et al. Isolation of the Lyme disease spirochete Borrelia mayonii from naturally infected rodents in Minnesota. J Med Entomol. 2017;54:1088–1092. doi: 10.1093/jme/tjx062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hamer SA, et al. Increased diversity of zoonotic pathogens and Borrelia burgdorferi strains in established versus incipient Ixodes scapularis populations across the Midwestern United States. Infect Genet Evol. 2014;27:531–542. doi: 10.1016/j.meegid.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 83.Hoen AG, et al. Effects of tick control by acaricide self-treatment of white-tailed deer on host-seeking tick infection prevalence and entomologic risk for Ixodes scapularis-borne pathogens. Vector Borne Zoonotic Dis. 2009;9:431–438. doi: 10.1089/vbz.2008.0155. [DOI] [PubMed] [Google Scholar]
- 84.Levin ML, Fish D. Acquisition of coinfection and simultaneous transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis ticks. Infect Immun. 2000;68:2183–2186. doi: 10.1128/iai.68.4.2183-2186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Piesman J, et al. Concurrent Borrelia burgdorferi and Babesia microti infection in nymphal Ixodes dammini. J Clin Microbiol. 1986;24:446–447. doi: 10.1128/jcm.24.3.446-447.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prusinski MA, et al. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis (Acari: Ixodidae) collected from recreational lands in the Hudson Valley Region, New York State. J Med Entomol. 2014;51:226–236. doi: 10.1603/me13101. [DOI] [PubMed] [Google Scholar]
- 87.Schauber EM, et al. Coinfection of blacklegged ticks (Acari: Ixodidae) in Dutchess County, New York, with the agents of Lyme disease and human granulocytic ehrlichiosis. J Med Entomol. 1998;35:901–903. doi: 10.1093/jmedent/35.5.901. [DOI] [PubMed] [Google Scholar]
- 88.Schulze TL, et al. Relative encounter frequencies and prevalence of selected Borrelia, Ehrlichia, and Anaplasma infections in Amblyomma americanum and Ixodes scapularis (Acari: Ixodidae) ticks from central New Jersey. J Med Entomol. 2005;42:450–456. doi: 10.1093/jmedent/42.3.450. [DOI] [PubMed] [Google Scholar]
- 89.Nieto NC, Foley JE. Meta-analysis of coinfection and coexposure with Borrelia burgdorferi and Anaplasma phagocytophilum in humans, domestic animals, wildlife, and Ixodes ricinus-complex ticks. Vector Borne Zoonotic Dis. 2009;9:93–102. doi: 10.1089/vbz.2008.0072. [DOI] [PubMed] [Google Scholar]
- 90.Swanson SJ, et al. Coinfections acquired from ixodes ticks. Clin Microbiol Rev. 2006;19:708–727. doi: 10.1128/CMR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Diuk-Wasser MA, et al. Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends Parasitol. 2016;32:30–42. doi: 10.1016/j.pt.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dunn JM, et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS One. 2014;9:e115494. doi: 10.1371/journal.pone.0115494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levin ML, et al. Disparity in the natural cycles of Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis. Emerg Infect Dis. 1999;5:204–208. doi: 10.3201/eid0502.990203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thomas V, et al. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis alters murine immune responses, pathogen burden, and severity of Lyme arthritis. Infect Immun. 2001;69:3359–3371. doi: 10.1128/IAI.69.5.3359-3371.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mead PS. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2015;29:187–210. doi: 10.1016/j.idc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 96.Hinckley AF, et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis. 2014;59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nelson CA, et al. Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg Infect Dis. 2015;21:1625–1631. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dahlgren FS, et al. Human granulocytic anaplasmosis in the United States from 2008 to 2012: a summary of national surveillance data. Am J Trop Med Hyg. 2015;93:66–72. doi: 10.4269/ajtmh.15-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Westblade LF, et al. Babesia microti: from mice to ticks to an increasing number of highly susceptible humans. J Clin Microbiol. 2017;55:2903–2912. doi: 10.1128/JCM.00504-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krause PJ, et al. Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Infect. 2015;21:631–639. doi: 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stafford KC, III, Williams SC. Deer-targeted methods: a review of the use of topical acaricides for the control of ticks on white-tailed deer. J Integr Pest Manage. 2017 Published online July 19, 2017. http://dx.doi.org/10.1093/jipm/pmx014.
- 102.Kugeler KJ, et al. Will culling white-tailed deer prevent Lyme disease? Zoonoses Public Health. 2016;63:337–345. doi: 10.1111/zph.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Telford SR., III Deer reduction is a cornerstone of integrated deer tick management. J Integr Pest Manage. 2017 Published online September 27, 2017. http://dx.doi.org/10.1093/jipm/pmx024.
- 104.Embers ME, Narasimhan S. Vaccination against Lyme disease: past, present, and future. Front Cell Infect Microbiol. 2013;3:6. doi: 10.3389/fcimb.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Steere AC, Livey I, et al. Lyme disease vaccines. In: Plotkin SA, editor. Vaccines. 6. Elsevier; 2013. pp. 1122–1132. [Google Scholar]
- 106.Eisen L, Eisen RJ. Critical evaluation of the linkage between tick-based risk measures and the occurrence of Lyme disease cases. J Med Entomol. 2016;53:1050–1062. doi: 10.1093/jme/tjw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Piesman J, Dolan MC. Protection against Lyme disease spirochete transmission provided by prompt removal of nymphal Ixodes scapularis (Acari: Ixodidae) J Med Entomol. 2002;39:509–512. doi: 10.1603/0022-2585-39.3.509. [DOI] [PubMed] [Google Scholar]
- 108.Warshafsky S, et al. Efficacy of antibiotic prophylaxis for the prevention of Lyme disease: an updated systematic review and meta-analysis. J Antimicrob Chemother. 2010;65:1137–1144. doi: 10.1093/jac/dkq097. [DOI] [PubMed] [Google Scholar]
- 109.Shen AK, et al. The Lyme disease vaccine – a public health perspective. Clin Infect Dis. 2011;52(Suppl 3):s247–s252. doi: 10.1093/cid/ciq115. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y, et al. Pre-exposure prophylaxis with ospA-specific human monoclonal antibodies protects mice against tick transmission of Lyme disease spirochetes. J Infect Dis. 2016;214:205–211. doi: 10.1093/infdis/jiw151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de la Fuente J, et al. Targeting a global health problem: vaccine design and challenges for the control of tick-borne diseases. Vaccine. 2017;35:5089–5094. doi: 10.1016/j.vaccine.2017.07.097. [DOI] [PubMed] [Google Scholar]
- 112.de la Fuente J, et al. Strategies for new and improved vaccines against ticks and tick-borne diseases. Parasite Immunol. 2016;38:754–769. doi: 10.1111/pim.12339. [DOI] [PubMed] [Google Scholar]
- 113.Sprong H, et al. ANTIDotE: anti-tick vaccines to prevent tick-borne diseases in Europe. Parasites Vectors. 2014;7:77. doi: 10.1186/1756-3305-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Willadsen P. Anti-tick vaccines. Parasitology. 2004;129:S367–S387. doi: 10.1017/s0031182003004657. [DOI] [PubMed] [Google Scholar]
- 115.Neelakanta G, Sultana H. Transmission-blocking vaccines: focus on anti-vector vaccines against tick-borne diseases. Arch Immunol Ther Exp. 2015;63:169–179. doi: 10.1007/s00005-014-0324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miller NJ, et al. Tick bite protection with permethrin-treated summer-weight clothing. J Med Entomol. 2011;48:327–333. doi: 10.1603/me10158. [DOI] [PubMed] [Google Scholar]
- 117.Ostfeld RS, et al. Controlling ticks and tick-borne zoonoses with biological and chemical agents. Bioscience. 2006;56:383–394. [Google Scholar]
- 118.Hinckley AF, et al. Effectiveness of residential acaricides to prevent Lyme and other tick-borne diseases in humans. J Infect Dis. 2016;214:182–188. doi: 10.1093/infdis/jiv775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schulze L, et al. Ability of 4-poster passive topical treatment devices for deer to sustain low population levels of Ixodes scapularis (Acari: Ixodidae) after integrated tick management in a residential landscape. J Med Entomol. 2008;45:899–904. doi: 10.1603/0022-2585(2008)45[899:aopptt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 120.Schulze TL, et al. Integrated use of 4-poster passive topical treatment devices for deer, targeted acaricide applications, and maxforce TMS bait boxes to rapidly suppress populations of Ixodes scapularis (Acari: Ixodidae) in a residential landscape. J Med Entomol. 2007;44:830–839. doi: 10.1603/0022-2585(2007)44[830:iuoppt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 121.Williams SC, et al. Integrated control of nymphal Ixodes scapularis: effectiveness of white-tailed deer reduction, the entomopathogenic fungus Metarhizium anisopliae, and fipronil-based rodent bait boxes. Vector Borne Zoonotic Dis. 2017 doi: 10.1089/vbz.2017.2146. Published online November 27, 2017. http://dx.doi.org/10.1089/vbz.2017.2146. [DOI] [PubMed]
- 122.Gould LH, et al. Knowledge, attitudes, and behaviors regarding Lyme disease prevention among Connecticut residents, 1999–2004. Vector Borne Zoonotic Dis. 2008;8:769–776. doi: 10.1089/vbz.2007.0221. [DOI] [PubMed] [Google Scholar]