Abstract
Rationale:
The initial symptoms and signs of Takayasu arteritis vary due to the heterogeneity of affected vessels. Moreover, the vascular lesions are difficult to detect at initial presentation, making diagnosis even more challenging. Although cases of aortic dissection with arteritis history have been reported, Takayasu arteritis in men with aortic dissection as initial presentation is very rare.
Patient concerns:
A 37-year-old man presenting with persistent chest and back pain for 6 days was transferred to our hospital for further treatment. Left hand pulse was absent and right lower limb pulse was feeble. Blood pressure was 144/83 mmHg in the right arm but only 114/62 mmHg in the left arm.
Diagnoses:
Computed tomography angiography revealed aortic dissection (DeBakey type III b) from the descending aorta to the distal abdominal aorta.
Interventions:
High-dose glucocorticoid therapy and immunosuppressive therapy have been used to control inflammatory reaction during acute period of Takayasu arteritis. Endovascular graft exclusion (EVGE) surgery was performed to cover the primary entry tear and re-expand true lumen during inactive stage.
Outcomes:
His pain symptoms improved progressively and he was followed in our outpatient clinic after discharged from hospital, without recurrence.
Lessons:
Timely therapy (glucocorticoid and immunosuppressive) and corrective surgery (endovascular graft exclusion) for Takayasu arteritis with aortic dissection at the inactive stage is essential and beneficial.
Keywords: aortic dissection, endovascular graft exclusion, stenosis, Takayasu arteritis
1. Introduction
Takayasu arteritis (TA), known as “pulseless disease” or nonspecific aortoarteritis, is a form of large vessel vascularitis primarily afflicting young women. It is characterized by ischemic symptoms stemming from inflammatory damage to the aorta and its main branches, while small or medium-sized vessels are rarely involved.[1] Some researchers have proposed that cellular immunity against vascular endothelial cells causes inflammatory damage to large vessels.[2] Takayasu arteritis has a worldwide distribution, but there is a predisposition in Asian ethnicities such as Japanese, Chinese, Korean, and Middle-Eastern.[3] Clinical manifestations of TA differ markedly depending on the sites of inflammation within involved vessels, and may include upper body pain, headache, dizziness, syncope, visual deterioration, intermittent claudication, diminished pulse, vascular bruits, and a gap in systolic blood pressure between the 2 arms (>10 mmHg). Aortic dissection, characterized by a tear in the intima of the aortic wall, is considered a rare complication of TA. Our study describes a rare case of TA initially presenting with aortic dissection. Written informed patient consent was obtained in the present study.
2. Case report
A 37-year-old man without history of hypertension, hyperlipidemia, or coronary heart disease reported persistent chest and back pain for 6 days, and was transferred to our hospital for further examination after unsuccessful symptomatic treatment at a community hospital. Computed tomography angiography (CTA) of the chest and abdomen revealed aortic dissection (DeBakey type IIIb) from the descending aorta (T7–T8 vertebral level) to the distal abdominal aorta (Figs. 1 and 2) as well as stenosis in many sites including the left subclavian artery, abdominal aorta, and iliac artery. Laboratory tests showed liver dysfunction as evidenced by elevated aspartate aminotransferase (AST 64U/L) and alanine aminotransferase (ALT 156U/L), in addition to increased erythrocyte sedimentation rate (ESR, 96 mm/h), C-reactive protein (CRP, 117 mg/L), and interleukin-6 (IL-6, 39 pg/mL). The dynamic changes of ESR and CRP during hospitalization are shown in Fig. 3. On clinical examination, left hand pulse was absent and the right lower limb pulse was weak. Blood pressure was 144/83 mmHg in the right arm but only 114/62 mmHg in the left arm. According to these observations, the diagnosis of Takayasu arteritis was established.
Figure 1.
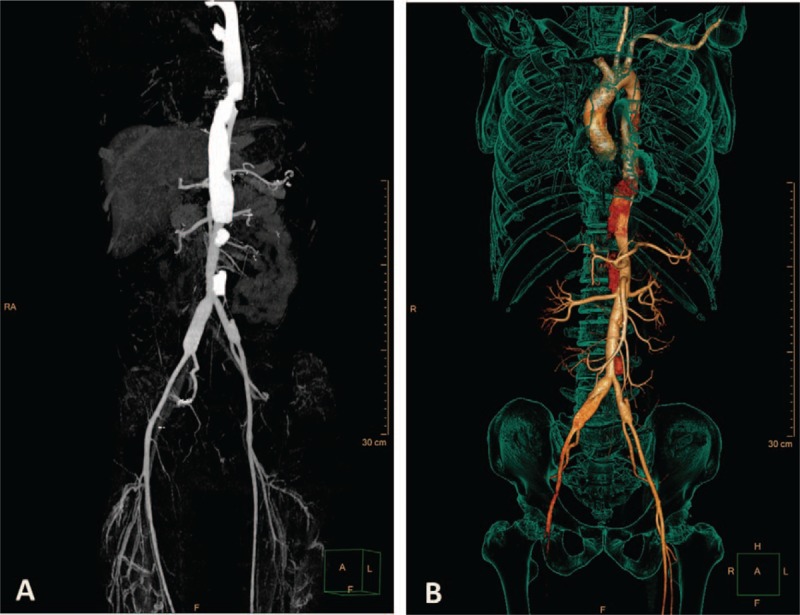
Preoperative CTA findings. Aortic dissections were localized from descending aorta to distal abdominal aorta. CTA = computed tomography angiography.
Figure 2.
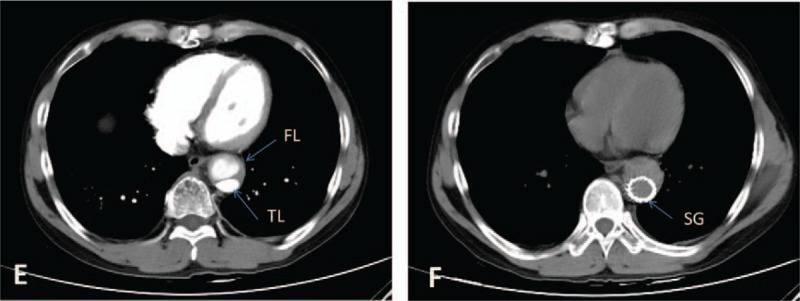
Picture E, preoperative CT image. Picture F, postoperative CT image. CT = computed tomography, FL = false lumen, TL = true lumen,SG = stent graft.
Figure 3.
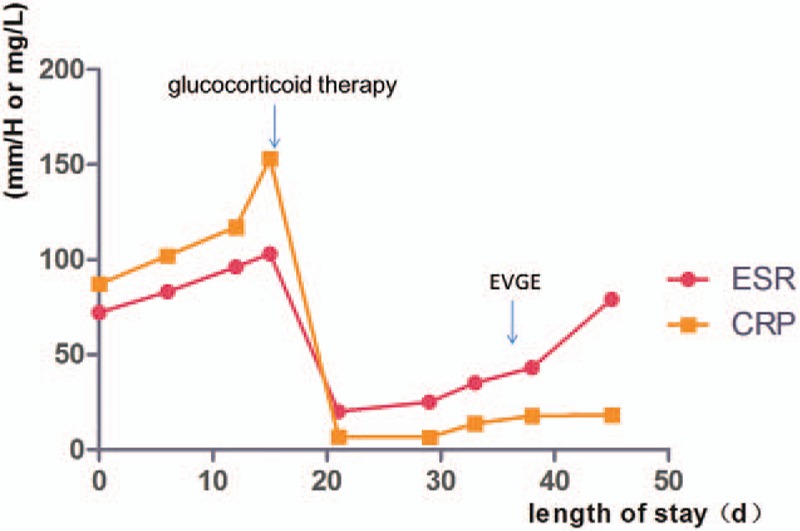
Dynamic changes in ESR and CRP during hospitalization. The 2 arrows indicate the time (day) of glucocorticoid therapy initiation and EVGE surgery, respectively. CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, EVGE = endovascular graft exclusion.
The patient was treated with sufentanil and tramadol for pain relief, metoprolol for heart rate control and reduction in left ventricular ejection force, and candesartan for blood pressure control. We began glucocorticoid therapy at day 20 and immunosuppressive therapy at day 34 with the following regimen: methylprednisolone sodium succinate (7.15 mg/kg from day 20 to day 22, qd, then 1 mg/kg from day 23 to day 33, qd), prednisone (1 mg/kg from day 34 to hospital discharge, qd), and cyclophosphamide (2.85 mg/kg from day 34 to hospital discharge, biw). On day 36, the patient received endovascular graft exclusion (EVGE) under general anesthesia and guidance by digital subtraction angiography. Six hours after the operation, the patient was conscious and vital signs were stable. Reexamination by CTA revealed that the stent graft was in good shape and little contrast agent flowed into the false lumen of the descending aorta. Intramural hematoma formation was also found in the false lumen of the descending aorta dissection (Figs. 2 and 4). After discharged from our hospital, the patient gradually reduced the dose of prednisone and cyclophosphamide every month according to ESR and CRP levels under the supervision of a physician. On follow-up at 6 months, the patient was doing well physically and enjoying full function without complaints of chest or back pain.
Figure 4.
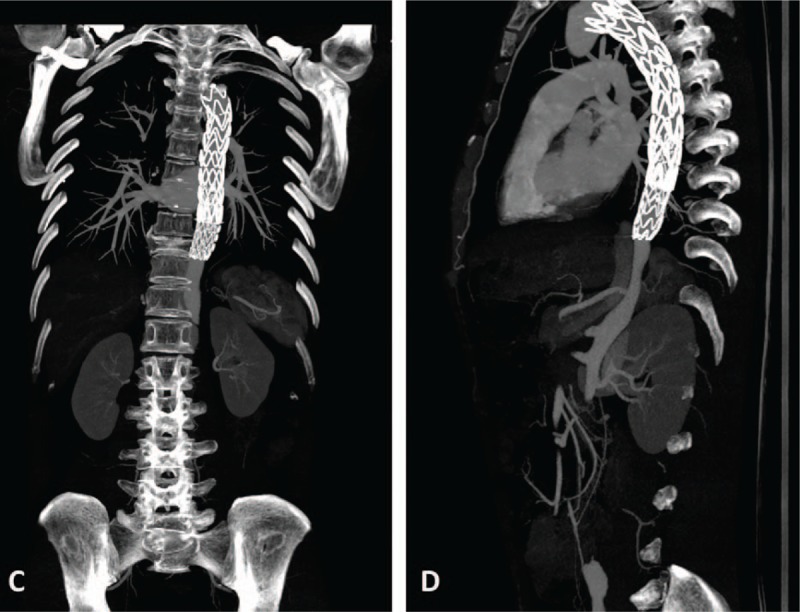
Postoperative CTA findings. Stent graft was in good shape and little contrast agent flowed into the false lumen of the descending aorta. CTA = computed tomography angiography.
3. Discussion
Takayasu arteritis was originally reported by the ophthalmologist Mikito Takayasu in 1908. During progression, TA may cause segmental stenosis, occlusion, dilatation, or aneurysm formation in the vessel wall.[4] Diagnosis and classification criteria for TA were established by the American College of Rheumatology,[5] but assessing the inflammatory status and pattern of arterial damage is still a major challenge.[6] In the clinic, the commonly adopted approach for judging TA activity includes acute-phase reactants (such as ESR or CRP), new bruits, and new angiographic features. Wang et al[7] found that high resolution sonography was able to discern tiny dissected intimae and provide precise images for evaluation of TA. Many factors, such as arteriosclerosis, long-term hypertension, dyslipidemia, and connective tissue disorders, have been identified that can damage the aortic wall and lead to dissection.[8] Autoimmune diseases, including TA, can cause persistent inflammation of the aorta, but rarely dissection because of the dense adventitial fibrosis and intimal scarring.[9] Some researchers have speculated that decreased wall elasticity and aortic mobility due to fibrous adhesion between fibrous adventitia and surrounding tissue may cause dissection in TA cases.[10]
In the present study, our patient was a young man admitted to hospital with aortic dissection as initial presentation and diagnosed with TA after further examination. Though the scope of dissection tear was extensive, thoracic endovascular aortic repair carries great risks during the active (progressive) stage of TA. According to the results of CT (day 1 and day 12) and MRI (day 18), the aortic dissection was stable, so we began glucocorticoid therapy and immunosuppressive therapy. The most effective drugs for TA are still corticosteroids, but early diagnosis and implementation of immunosuppressive agents are essential to prevent vascular complications.[11] Many biological agents have been used effectively for refractory cases, such as tocilizumab, rituximab, and anti-tumor necrosis factor agents.[12] After disease activity was controlled (ESR: 43 mm/h, CRP: 27.9 mg/L), the patient received successful EVGE surgery. In general, TA patients with severe ischemic symptoms (limb claudication, heart failure, etc.) require endovascular intervention or vascular surgery in addition to medical therapy.[13] Though restenosis and occlusion risks are still high with vascular interventions, a previous study found lower restenosis rate when vascular surgery was performed during the stable stage.[14] Therefore, we conclude that corrective surgery at the inactive stage is essential and beneficial for treatment of TA with aortic dissection.
Footnotes
Abbreviations: AD = aortic dissection, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BP = blood pressure, CRP = C-reactive protein, CTA = computed tomography angiography, ESR = erythrocyte sedimentation rate, EVGE = endovascular graft exclusion, TA = Takayasu arteritis.
The authors have no conflicts of interest to disclose.
References
- [1].Isobe M. Takayasu arteritis revisited: current diagnosis and treatment. Int J Cardiol 2013;168:3–10. [DOI] [PubMed] [Google Scholar]
- [2].Seko Y, Minota S, Kawasaki A, et al. Perforin-secreting killer cell infiltration and expression of a 65-kD heat-shock protein in aortic tissue of patients with Takayasu's arteritis. J Clin Invest 1994;93:750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aydin SZ, Merkel PA, Direskeneli H. Outcome measures for Takayasu's arteritis. Curr Opin Rheumatol 2015;27:32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Direskeneli H. Clinical assessment in Takayasu's arteritis: major challenges and controversies. Clin Exp Rheumatol 2017;35(suppl):189–93. [PubMed] [Google Scholar]
- [5].Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990;33:1129–34. [DOI] [PubMed] [Google Scholar]
- [6].Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014;53:793–801. [DOI] [PubMed] [Google Scholar]
- [7].Wang J, Lee YZ, Cheng Y, et al. Sonographic characterization of arterial dissections in Takayasu arteritis. J Ultrasound Med 2016;35:1177–91. [DOI] [PubMed] [Google Scholar]
- [8].Elsayed RS, Cohen RG, Fleischman F, et al. Acute Type A aortic dissection. Cardiol Clin 2017;35:331–45. [DOI] [PubMed] [Google Scholar]
- [9].Tyagi S, Nigam A. Aortic dissection: a rare presenting manifestation of Takayasu's aortitis. Indian Heart J 2008;60:58–60. [PubMed] [Google Scholar]
- [10].Kameyama K, Kuramochi S, Ueda T, et al. Takayasu's aortitis with dissection in systemic lupus erythematosus. Scand J Rheumatol 1999;28:187–8. [DOI] [PubMed] [Google Scholar]
- [11].Mason JC. Takayasu arteritis—advances in diagnosis and management. Nat Rev Rheumatol 2010;6:406–15. [DOI] [PubMed] [Google Scholar]
- [12].Mekinian A, Comarmond C, Resche-Rigon M, et al. Efficacy of biological-targeted treatments in takayasu arteritis: multicenter, retrospective study of 49 patients. Circulation 2015;132:1693–700. [DOI] [PubMed] [Google Scholar]
- [13].Seyahi E. Takayasu arteritis: an update. Curr Opin Rheumatol 2017;29:51–6. [DOI] [PubMed] [Google Scholar]
- [14].Fields CE, Bower TC, Cooper LT, et al. Takayasu's arteritis: operative results and influence of disease activity. J Vasc Surg 2006;43:64–71. [DOI] [PubMed] [Google Scholar]



