Abstract
Ciliary opsins were classically thought to function only in vertebrates for vision, but they have also been identified recently in invertebrates for non-visual photoreception. Larvae of the annelid Platynereis dumerilii are used as a zooplankton model, and this zooplankton species possesses a “vertebrate-type” ciliary opsin (named c-opsin) in the brain. Platynereis c-opsin is suggested to relay light signals for melatonin production and circadian behaviors. Thus, the spectral and biochemical characteristics of this c-opsin would be directly related to non-visual photoreception in this zooplankton model. Here we demonstrate that the c-opsin can sense UV to activate intracellular signaling cascades and that it can directly bind exogenous all-trans-retinal. These results suggest that this c-opsin regulates circadian signaling in a UV-dependent manner and that it does not require a supply of 11-cis-retinal for photoreception. Avoidance of damaging UV irradiation is a major cause of large-scale daily zooplankton movement, and the observed capability of the c-opsin to transmit UV signals and bind all-trans-retinal is ideally suited for sensing UV radiation in the brain, which presumably lacks enzymes producing 11-cis-retinal. Mutagenesis analyses indicated that a unique amino acid residue (Lys-94) is responsible for c-opsin–mediated UV sensing in the Platynereis brain. We therefore propose that acquisition of the lysine residue in the c-opsin would be a critical event in the evolution of Platynereis to enable detection of ambient UV light. In summary, our findings indicate that the c-opsin possesses spectral and biochemical properties suitable for UV sensing by the zooplankton model.
Keywords: brain, G protein-coupled receptor (GPCR), molecular evolution, photobiology, rhodopsin, ultraviolet-visible spectroscopy (UV-Vis spectroscopy)
Introduction
Most animals possess visual and non-visual photoreceptive functions. Vision is primarily mediated by visual photoreceptor cells in the eyes. The eyes of vertebrates (deuterostomes) and invertebrates (protostomes) typically possess ciliary and rhabdomeric photoreceptor cells, respectively (1, 2), with some exceptions (3). Thus, vertebrate and invertebrate visual pigments, which function as photoreceptive proteins in visual photoreceptor cells, are called ciliary and rhabdomeric opsins, respectively. Ciliary and rhabdomeric opsins are classified into different groups in the opsin family (Fig. 1A) and coupled with different G proteins (2, 4). However, recent studies have revealed that vertebrates possess rhabdomeric opsins, and invertebrates have ciliary opsins for non-visual photoreception. For example, mammals, including humans, possess a rhabdomeric opsin named melanopsin (Opn4), a ciliary opsin, Opn3, as well as visual pigments and another non-visual opsin, Opn5 (neuropsin) (4). Melanopsin is expressed in some portions of retinal ganglion cells in mammals, and light input via melanopsin plays important roles in circadian clock photoentrainment and pupil constriction (5, 6). We reported recently that mammalian melanopsins possess molecular properties relevant to their non-visual functions (7). On the other hand, many invertebrates possess ciliary opsins, some of which are expressed in the brain (8, 9). This fact suggests that they are involved in some non-visual photoreception and have characteristics suitable for their physiological functions. These ciliary opsins in invertebrates, along with vertebrate Opn3s and its homologs, can be classified into the group “Opn3 homolog” in the opsin family (Fig. 1A) (10).
Figure 1.
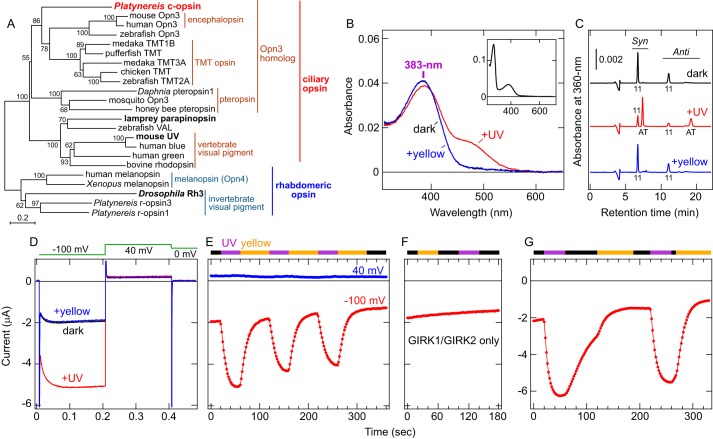
Characterization of Platynereis c-opsin. A, molecular phylogenetic relationship between Platynereis c-opsin and other ciliary and rhabdomeric opsins. The phylogenetic tree was constructed by the neighbor-joining method using MEGA6 software (76). The groups and subgroups in the opsin family are indicated. UV-sensitive opsins are highlighted in boldface. The bootstrap probabilities are indicated at each branch node, and a scale bar (0.2 substitutions per site) is also shown. B, absorption spectra of purified Platynereis c-opsin. Spectra in the dark (black), after UV illumination (red), and after subsequent yellow light (>480 nm) illumination (blue) are shown. The black and blue spectra are almost superimposed. In the dark, the λmax value is 383 nm in the UV-A region. Inset, absorption spectrum of Platynereis c-opsin in the dark in a wider wavelength range. C, HPLC analyses of the retinal configurations in purified Platynereis c-opsin. Chromatographs of the opsin in the dark (black), after UV light illumination (red), and after subsequent yellow light (>480 nm) illumination (blue) are shown. Peaks labeled 11 and AT indicate the 11-cis and all-trans isomers, respectively. Syn and Anti indicate respective isomers of the retinal oxime. D, photoresponses of a Xenopus oocyte expressing Platynereis c-opsin WT as well as GIRK1/GIRK2. Representative voltage clamp current recording data of an oocyte expressing Platynereis c-opsin with GIRK1/GIRK2 in the dark (black), after UV light illumination (red), and after subsequent yellow light (>480 nm) illumination (blue) are shown. Clamped voltage values are schematically indicated (see “Experimental Procedures”). E, Changes in the inward current upon light illumination. Current amplitudes recorded from the oocyte at −100 mV (red) and 40 mV (blue) as a function of time are shown. E–G, UV (violet bar) and yellow (>480 nm, orange bar) light illumination are indicated, and the black bar indicates that the oocyte was kept in the dark. The UV-dependent increase in inward current at −100 mV and little change at 40 mV indicate that the inward current through an inwardly rectifying potassium channel, GIRK1/GIRK2, was successfully measured. F, photoresponses of an oocyte injected with only GIRK1/GIRK2 (without c-opsin). Current amplitude at −100 mV recorded from the oocyte as a function of time is shown. The oocyte was incubated with 11-cis-retinal before measurement and illuminated with yellow (>480 nm) or UV light. G, decay of UV-induced current in light-dependent and light-independent manners. Current amplitude at −100 mV from a Xenopus oocyte expressing Platynereis c-opsin is plotted. Note that, after termination of UV illumination, the current was gradually reduced, and illumination with yellow light accelerated the current decay.
Opn3 was originally found as encephalopsin (or panopsin) in the mammalian brain (11, 12). Subsequent studies have found Opn3 homologs in other vertebrates (deuterostomes) (13–15) and invertebrates (protostomes) (8–10, 16). These Opn3 homologs are divided into various subgroups in the ciliary opsin group (Fig. 1A). For example, TMT opsin was found in a wide variety of tissues of non-mammalian vertebrates (13–15). Pteropsin was identified in the brain of the honeybee and subsequently found in other ecdysozoans (9, 10, 16, 17). In particular, a ciliary opsin (hereafter called c-opsin) in the marine ragworm Platynereis dumerilii (Polychaeta, Annelida, Lophotrochozoa) has been extensively studied, and its expression pattern (8) and physiological roles (18) are well understood.
The larvae of Platynereis possess ciliary photoreceptor cells in the brain, and c-opsin expression is localized to the cells (8). Further, the brain photoreceptor cells are involved in melatonin production controlling circadian swimming behavior (18). Platynereis larvae live as zooplankton and have been studied as a zooplankton model (18, 19). Many zooplankton species, including polychaetes, show synchronized circadian movement, named diel vertical migration (DVM)2, moving downward in water during daytime and upward at night (20–22). In this context, a relationship between the ciliary brain photoreceptor cells and DVM is suggested (18, 23).
Because DVM is important to avoid UV damage from sunlight and predation by fish (24, 25), detection of ambient UV signals is essential for the physiology and ecology of Platynereis larvae and other zooplankton. Nevertheless, it is unclear how zooplankton receive ambient UV signals. For example, Platynereis larvae possess several types of eyes responsible for phototaxis, but the UV sensitivity of these eyes is low (19, 26). Thus, molecule(s) capable of sensing UV signals should exist outside of the eyes. As mentioned above, Platynereis c-opsin in the brain is a suitable candidate molecule for receiving and transmitting UV signals for circadian behavior. However, Opn3 homologs in chickens, fish, and mosquitos have been reported to sense visible light (10, 15, 27, 28), not UV light. In this study, we aimed to elucidate the spectral and biochemical properties of Platynereis c-opsin and found that the opsin can transmit UV signals and directly bind exogenous all-trans-retinal. Mutational analyses revealed that a single amino acid residue, Lys-94, underlies the two molecular properties. We discuss the relationship between molecular properties of the ciliary opsin and non-visual photoreception in Platynereis.
Results
UV sensitivity and bistability of Platynereis c-opsin
Full-length Platynereis c-opsin with the 1D4 tag on its C terminus was expressed in cultured COS-1 cells, mixed with 11-cis-retinal, and purified by immunoaffinity chromatography (see supplemental Fig. S1 and “Experimental Procedures”). The absorption spectrum of the purified Platynereis c-opsin showed an absorption maximum (λmax) at 383 nm in the UV-A region (Fig. 1B). We also confirmed that the covalent bond with retinal (the Schiff base linkage) is formed in the opsin using acid denaturing of the pigment (supplemental Fig. S2A). After irradiation with UV light, the spectrum showed two peaks in the UV-A (∼390 nm) and visible (∼490 nm) regions (Fig. 1B). HPLC analyses of retinal isomers extracted from Platynereis c-opsin in the dark or after UV irradiation showed that the retinal chromophore was isomerized from the 11-cis to the all-trans form (Fig. 1C). Illumination of the photoproduct with yellow (visible) light (>480 nm) completely brought the retinal isomer back to its original 11-cis-retinal chromophore, with its spectrum indicating the 383 nm peak as the λmax (Fig. 1, B and C). Thus, the photoproduct is in equilibrium between the UV-absorbing and visible light-absorbing forms. These results clearly indicate that the invertebrate ciliary opsin is a UV-sensitive and bistable pigment.
Ability of Platynereis c-opsin to transmit UV signals to intracellular signaling cascades
We next assessed the ability of Platynereis c-opsin to transmit UV signals into cells. The c-opsin and G protein-coupled inwardly rectifying potassium channel GIRK1/GIRK2 (Kir3.1/Kir3.2) were heterologously co-expressed in Xenopus oocytes because ciliary opsins have been reported to be Gi/o-coupled (2, 10, 27), and GIRK1/GIRK2 is activated by Gβγ subunits released from Gi/o (29). Thus, coupling of receptors with Gi/o proteins has been investigated using this method (30–32). UV light irradiation of an oocyte expressing Platynereis c-opsin and GIRK1/GIRK2 caused an increase in inward current, and the UV-induced current was shut off by subsequent yellow light (>480 nm) illumination (Fig. 1, D and E). Such photoresponses were not observed in an oocyte expressing GIRK1/GIRK2 only (Fig. 1F), clearly indicating that the photocurrent is due to c-opsin. These results also indicate that the photoproduct with all-trans-retinal (Fig. 1, B and C) is the active form to activate endogenous Gi/o in the oocyte and that the inactive and active forms are photo-interconvertible. It should be noted that, even without yellow light illumination, termination of UV irradiation caused gradual decay of the photocurrent, but the decay was accelerated by yellow light illumination (Fig. 1G). Because the photoproduct spectrum showed little changes at least for 1 h at 25 °C (supplemental Fig. S2B), either thermal decay of the active photoproduct or thermal reversion to the original (dark) form would not occur during the electrophysiological measurement (for ∼6 min at room temperature, Fig. 1G). It is unknown how the opsin is deactivated light-independently, but visible light illumination, causing all-trans to 11-cis isomerization (Fig. 1C), more rapidly and efficiently shuts off the response. Taken together, Platynereis c-opsin can transmit external UV signals to intracellular signaling cascade(s) via UV-dependent Gi/o activation, and the signal transmission is shut off by visible light that converts the activated form back to the inactive (dark) form.
Ability to directly bind exogenous all-trans-retinal
Vertebrate visual pigments, classified as ciliary opsins (Fig. 1A), are also activated via cis-trans isomerization of the retinal chromophore as Opn3 homologs, but they cannot directly bind all-trans-retinal (33). In contrast, some (but not all) bistable pigments (opsins) can bind exogenous all-trans-retinal (34–37). Direct binding of all-trans-retinal would be particularly important for opsins expressed outside of the eyes because sophisticated multienzyme systems (“visual cycle”) producing a thermally unstable 11-cis-retinal isomer probably exist specifically in eyes of vertebrates and invertebrates (2, 38). Because Opn3 homologs are expressed in the brain of various animals, including Platynereis (8, 9, 11–15, 39), direct binding of all-trans-retinal would be important for the Opn3s, too. Thus, we tested the retinal isomers that can directly bind Platynereis c-opsin protein, although it is unknown how 11-cis-retinal is synthesized and supplied in the animal.
COS-1 membranes expressing the c-opsin were incubated with a 9-cis, 11-cis, 13-cis, or all-trans retinal isomer, and the retinal-bound proteins were purified (Fig. 2A). After incubation with 9-cis-retinal, the absorption spectrum was slightly blue-shifted from that of the 11-cis-retinal–bound form (Fig. 2A). Such a blueshift, upon binding of the 9-cis isomer compared with the 11-cis-retinal–bound form, has been observed in various opsins (40–43). The absorption spectra of c-opsin after incubation with 13-cis and all-trans retinals (Fig. 2, B and C) were very similar to the spectrum of the photoproduct having photo-isomerized all-trans-retinal (Fig. 1B). HPLC analyses of retinal isomers in these samples clearly show that both products possess all-trans-retinal as a chromophore (Fig. 2B). Because all-trans- and 13-cis-retinals are interconvertible via thermal isomerization (10, 44), the c-opsin prefers to bind all-trans-retinal rather than 13-cis-retinal. These results clearly indicate that Platynereis c-opsin can act as a photoreceptive protein even without the supply of 11-cis-retinal (see “Discussion”). Mosquito Opn3 (pteropsin) prefers to bind 13-cis-retinal over the all-trans isomer (10). Thus, the preference of Opn3 homologs for retinal isomers varies between species.
Figure 2.
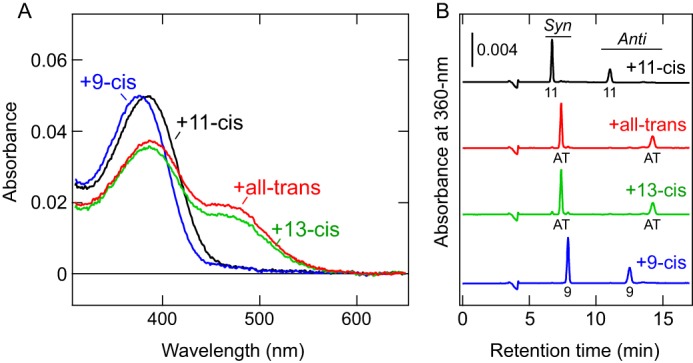
Ability of Platynereis c-opsin to bind exogenous all-trans-retinal directly. A, absorption spectra of purified WT Platynereis c-opsin that were incubated with 11-cis (black), all-trans (red), 13-cis (green), or 9-cis (blue) retinal. B, HPLC analyses determining retinal isomers actually bound to the opsin proteins. Chromatographs of samples after incubation with 11-cis (black), all-trans (red), 13-cis (green), or 9-cis (blue) retinal are shown. Peaks labeled 11, AT, and 9 indicate the 11-cis, all-trans, and 9-cis isomers, respectively. Syn and Anti indicate respective isomers of the retinal oxime. Note that incubation with 13-cis-retinal resulted in all-trans-retinal binding to the opsin proteins (green).
A single lysine residue is responsible for UV-sensing ability
The spectral and biochemical data described above revealed that Platynereis c-opsin is UV-sensitive and able to bind exogenous all-trans-retinal directly. In contrast, previous studies using Opn3 homologs showed that they possess λmax values in the visible (blue to green) region (∼460–500 nm) (10, 15, 27, 28), and mosquito Opn3 prefers to bind 13-cis-retinal rather than the all-trans isomer (10). These facts suggest that Platynereis c-opsin possesses specific amino acid residues responsible for UV reception and/or the binding of exogenous all-trans-retinal.
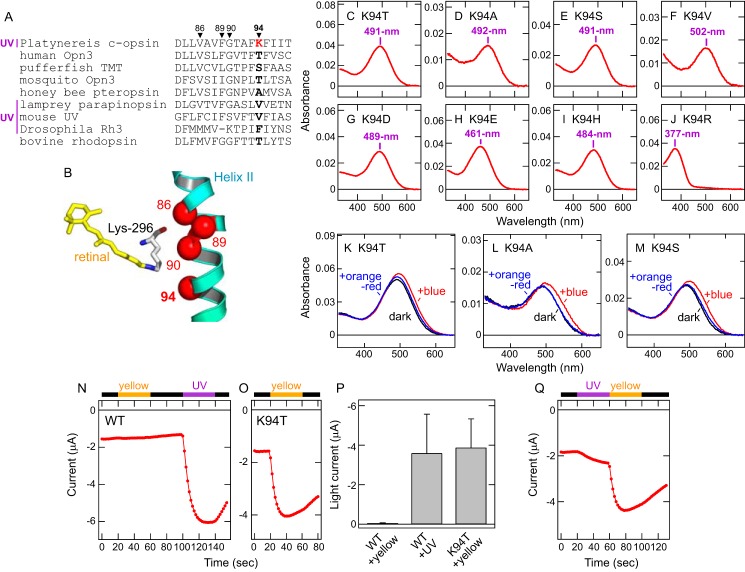
In UV-sensitive visual pigments of vertebrates and invertebrates, amino acid residues near the retinal Schiff base are responsible for UV sensitivity (45, 46). Based on the sequence alignment of ciliary opsins and the crystal structures of bovine rhodopsin (47–49), we compared amino acid residues near the retinal Schiff base in Platynereis c-opsin and noticed a unique lysine residue at position 94 in the second transmembrane helix (Fig. 3, A and B). Because in other ciliary opsins, this position is mainly occupied by Thr, Ser, Ala, or Val (Fig. 3A), we substituted Lys-94 in Platynereis c-opsin with these amino acids. We also tested substitutions of Lys-94 with Asp, Glu, His, or Arg, which possess a negative or positive charge in the side chain. The mutants K94T, K94S, K94A, K94V, K94D, K94E, and K94H showed λmax values in the visible region (461–502 nm) (Fig. 3, C–I), whereas the K94R mutant retained UV sensitivity (λmax = 377 nm) (Fig. 3J). These results clearly indicate that, in Platynereis c-opsin, the lysine residue at position 94 is essential for UV reception. Also, the retained UV sensitivity in the K94R mutant suggests that a positive charge in the side chain of the amino acid residue at position 94 converts the spectrum of Platynereis c-opsin to the UV-A region (see “Discussion”). In addition, the K94T, K94S, and K94A mutants, all of which were converted to “visible pigments”, formed stable (visible) photoproducts that are partially converted back to the original state by subsequent orange-red light illumination (Fig. 3, K–M), indicating that Lys-94 is not essential for photo-interconvertibility between the original (dark) state and the photoproduct. This result is consistent with the fact that mosquito Opn3, having Thr-94, and medaka and pufferfish TMT opsins, having Ser-94, show photo-interconvertibility between the dark state and the activated state by illumination with different colors of light (10, 27).
Figure 3.
Lys-94 is essential for UV (UV-A) reception and transmission by Platynereis c-opsin. A, amino acid sequence alignment of opsins around position 94 in the second transmembrane helix. Position 94 is highlighted in boldface, and positions 86, 89, and 90 are also indicated. Lys-94 in Platynereis c-opsin is highlighted in red. UV-sensitive opsins are labeled UV. Lys-90 in Drosophila Rh3 can be regarded as Lys-89 because of a gap at the site. B, arrangement of 11-cis-retinal and position 94 in the second transmembrane helix. 11-cis-retinal (yellow) and positions 94 as well as 86, 89, and 90 (red spheres) in the crystal structure of bovine rhodopsin (Protein Data Bank code 1U19) (48) are shown. Lys-296, which forms the retinal Schiff base linkage, is also shown. C–J, absorption spectra of the Platynereis c-opsin mutants K94T (C), K94A (D), K94S (E), K94V (F), K94D (G), K94E (H), K94H (I), and K94R (J). The λmax values of the mutants are indicated. K–M, spectral changes of Platynereis c-opsin mutants K94T, K94A, and K94S upon light illumination. The absorption spectra of the Platynereis c-opsin mutants K94T (K), K94A (L), and K94S (M) in the dark (black), after illumination with blue (440 nm) light (red), and after subsequent illumination with orange-red (>580 nm) light (blue). N and O, photo-induced changes of current amplitudes at −100 mV from Xenopus oocytes expressing Platynereis c-opsin WT (N) or the K94T mutant (O). Illumination with UV (violet bar) and yellow light (>480 nm, orange bar) is indicated, and the black bar indicates that the oocytes were kept in the dark. P, comparison of photo-induced currents by Platynereis c-opsin WT and the K94T mutant. Differences in current amplitudes at −100 mV before and after light illumination are plotted. The error bars represent S.D. values (n = 9 for WT and n = 5 for K94T). Q, photo-induced changes of current amplitude at −100 mV from a Xenopus oocyte expressing the Platynereis c-opsin K94T mutant. The oocyte is illuminated by yellow (>480 nm) light followed by UV light.
To clarify the role of Lys-94 in cellular responses, we compared light-induced inward currents in Xenopus oocytes expressing Platynereis c-opsin WT or the K94T mutant with GIRK1/GIRK2. In particular, we compared photoresponses upon UV or visible light illumination. Oocytes expressing Platynereis c-opsin WT showed little response following visible light illumination, but subsequent UV light illumination caused an increase in the inward current (Fig. 3, N and P). In contrast, oocytes expressing the K94T mutant showed a significant increase in inward current with the initial visible light irradiation (Fig. 3, O and P). In addition, oocytes expressing the K94T mutant showed a small increase in inward current with initial UV light illumination, with the response further increased by subsequent visible light illumination (Fig. 3Q). These results clearly indicate that visible light inactivates WT opsin (Figs. 1E and 3N) but activates the K94T mutant (Fig. 3, O and P). This difference is consistent with the dramatic redshift (from 383 to 491 nm for λmax) induced by K94T substitution (Fig. 3C). Taken together, the K94T mutation converted the ability to transmit UV signals to the ability to transmit visible light signals. In other words, Lys-94 is responsible for the ability of Platynereis c-opsin to receive and transmit UV signals.
Dual roles of Lys-94 in Platynereis c-opsin
Next we tested the K94T mutant for its ability to bind retinal isomers and found that, in contrast to the WT, the mutant cannot directly bind exogenous all-trans-retinal. COS-1 membranes expressing the K94T mutant were incubated with all-trans-retinal or 13-cis-retinal as well as 11-cis or 9-cis isomers, and the pigments were purified. As is the case for the WT (Fig. 2A), the purified K94T samples, after incubation with 9-cis-retinal, showed a blue-shifted λmax (478 nm) (Fig. 4D) compared with the 11-cis-retinal–bound form (491 nm) (Fig. 4A). In contrast, after incubation with all-trans-retinal or 13-cis-retinal, the mutant showed no significant absorption in the visible or UV regions, except for protein absorbance at ∼280 nm (Fig. 4, B and C). These results clearly show that, unlike the WT, the K94T mutant does not bind exogenous all-trans- or 13-cis-retinal. This indicates that Lys-94 has dual functions of enabling the c-opsin to sense UV signals and directly binding exogenous all-trans-retinal. Both properties are probably important for the ciliary opsin to function as a photoreceptive protein in the brain, and acquisition of the Lys residue in molecular evolution is a critical event for non-visual photoreception in Platynereis (see “Discussion”).
Figure 4.
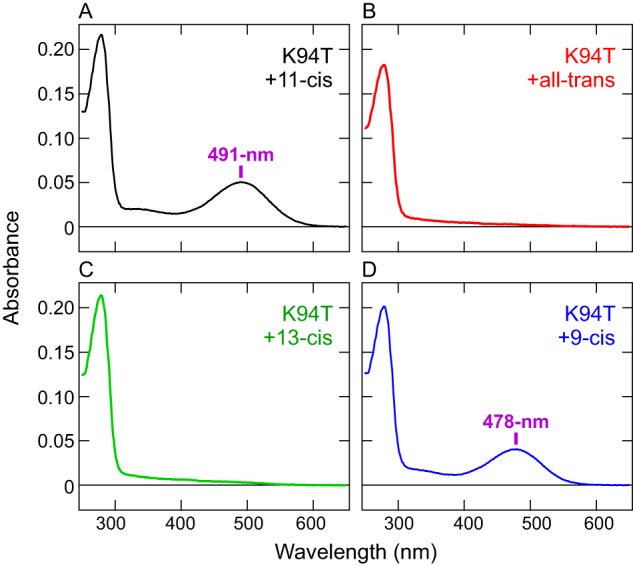
Loss of binding ability for exogenous all-trans-retinal in the Platynereis c-opsin K94T mutant. A–D, absorption spectra of Platynereis c-opsin K94T mutant samples that were purified after incubation with 11-cis (A), all-trans (B), 13-cis (C), or 9-cis (D) retinal. The λmax values after incubation with 11-cis or 9-cis retinal are indicated. Note that, after incubation with all-trans or 13-cis retinal, no absorbance except protein absorbance at ∼280 nm was observed.
Discussion
Molecular characteristics of Platynereis c-opsin as a UV receptor in the brain
As mentioned under “Introduction,” ciliary opsins have been identified in the brains of various vertebrates and invertebrates (8, 9, 11, 14, 15). Thus, expression of ciliary opsin in the brain may be widely spread among animals. In particular, Platynereis c-opsin is localized ciliary photoreceptor cells in the brain (8), and the c-opsin-expressing photoreceptor cells control circadian swimming behavior via melatonin production (18). Thus, the spectral and biochemical properties of Platynereis c-opsin are directly linked to light regulation of circadian signaling. This study revealed that Platynereis c-opsin is a UV-sensitive and bistable pigment (Fig. 1). To the best of our knowledge, this is the first study showing that an opsin expressed in an invertebrate brain has UV sensitivity, whereas UV-sensing opsins, such as parapinopsin (50, 51) or Opn5 (35, 52, 53), function in the brains of some vertebrates. (After submission of this paper, a study reported that Rh7 functions in the brain of Drosophila for photoentrainment of the circadian clock (75). Rh7 may be a UV-sensitive pigment, but violet (or white) light was used to activate the pigment in the study.) It should be noted that many invertebrates, in particular insects, possess UV-sensitive visual (rhabdomeric) photoreceptor cells in their eyes (54, 55), and UV-sensing (rhabdomeric, Gq-coupled) visual pigments have been identified (56–61).
Our electrophysiological analyses using Xenopus oocytes showed that Platynereis c-opsin can activate the Gi/o signaling pathway upon UV reception (Fig. 1, D and E), and a previous study reported that Gi is co-expressed with the opsin in native tissues (18). Taken together, in ciliary photoreceptor cells, ambient UV light would affect melatonin production via UV-dependent Gi activation by the ciliary opsin, leading to regulation of circadian behaviors.
Besides UV sensitivity, Platynereis c-opsin can directly bind exogenous all-trans-retinal as well as 11-cis-retinal (Fig. 2). We propose that this binding ability is important for the opsin to function in the brain, which probably lacks enzyme systems (visual cycle) producing and supplying 11-cis-retinal (2, 38). Thus, the two abilities of Platynereis c-opsin, UV sensitivity and direct binding of all-trans-retinal, would be necessary to enable brain photoreceptor cells to detect UV signals. In other words, Platynereis c-opsin has acquired and kept two properties to introduce UV-sensing ability in brain photoreceptor cells.
Acquisition of Lys-94 in molecular evolution
Previous spectroscopic studies of Opn3 homologs in various animals showed that these pigments possess λmax values in the blue to green range (∼460–500-nm) upon binding of 11-cis-retinal (10, 15, 27, 28). In contrast, Platynereis c-opsin possesses λmax in the UV-A region (383 nm, Fig. 1B), indicating that specific amino acid residues make the opsin UV-sensitive. Our mutagenesis analyses revealed that Lys-94 enables Platynereis c-opsin to not only detect UV signals (Fig. 3) but also to directly bind exogenous all-trans-retinal (Fig. 4), indicating that the UV-sensing ability and binding ability for exogenous all-trans-retinal are related to each other in this opsin and are conferred by the single Lys-94 residue. A previous study using Opn5 in vertebrates showed that amino acid substitution at position 168 alters the direct binding of all-trans-retinal, but does not affect UV sensitivity (37), indicating that the UV-sensing ability and the direct binding of exogenous all-trans-retinal are independent in vertebrate Opn5s, unlike what is the case for Platynereis c-opsin.
UV-sensing visual and non-visual opsins have been identified in vertebrates and invertebrates, and the spectral tuning mechanisms have been extensively studied through mutagenesis (45, 46, 51, 55, 62, 63). These studies and our data indicate that not identical but spatially close amino acid residues are responsible for UV sensitivity in different types of opsin. For example, amino acid residues at positions 86 and 90 are primary residues for spectral tuning in vertebrate UV-sensitive visual pigments (46, 62, 63), a residue at position 90 (or 89) is critical for UV sensitivity in insect visual pigments (45, 56), and residues at positions 86 and 89 are suggested to be important for UV sensing in parapinopsin, a pineal photoreceptor in lower vertebrates (51) (Fig. 3A). Positions 86, 89, 90, and 94 are located on the same face of the second transmembrane helix and close to the retinal Schiff base (Fig. 3B). Taken together, UV-sensing ability has been independently accomplished in different subgroups of the opsin family, but the UV-sensing opsins nonetheless utilize adjacent amino acid residues (55). In particular, Platynereis c-opsin and Drosophila Rh3 (45) (and other UV-sensing rhabdomeric opsins) achieve UV sensitivity with the help of a single lysine residue at position 94 and 90, respectively (Fig. 3A). In both opsins, the positive charge in the side chain of each Lys residue would block protonation of the Schiff base, resulting in the spectral shift to the UV region. Thus, both UV-sensing opsins have established UV sensitivity by very similar mechanisms, although they are classified into different groups in the opsin family (the ciliary and rhabdomeric opsin groups, respectively; Fig. 1A).
Lys-94 also confers the ability to directly bind all-trans-retinal (Fig. 4). It is well known that vertebrate visual pigments do not bind all-trans-retinal directly (33), but T94I substitution in bovine rhodopsin, a night blindness mutant, enables it to directly bind all-trans-retinal and causes a blueshift of its λmax value (64). These data suggest that, in ciliary opsins, interaction between the amino acid residue at position 94 and the Schiff base is important not only for spectral tuning but also for retinal isomers to bind. Also, another study using bovine rhodopsin showed that direct binding of all-trans-retinal depends on the conformational dynamics of the retinal-free state (65). Thus, Lys-94 may increase the conformational flexibility of Platynereis c-opsin. As mentioned above, mosquito Opn3 having Thr-94 preferably binds 13-cis-retinal rather than the all-trans form (10). Thus, the preference for retinal isomers has varied in the molecular evolution of ciliary opsins. Future structural and computational studies of ciliary opsins will determine how retinal binding selectivity is regulated in detail.
Potential physiological roles of ciliary opsin in Platynereis and other zooplankton
Positive and negative phototactic behaviors in Platynereis larvae have been reported (19, 26, 66). Phototaxis is primarily based on eyespots (larval eyes) and adult eyes having rhabdomeric photoreceptor cells and is efficiently driven by blue (∼420 nm) and/or green (∼480 nm) light. These photoreceptive properties are governed by rhabdomeric opsins named r-opsins (8, 67) and a Go-coupled opsin (26) in the eyes. Because avoiding UV damage from sunlight is considered a major cause of DVM (see “Introduction”) (24, 25), zooplankton species need to somehow detect UV signals. At least in the case of Platynereis larvae, the eyes are apparently not suitable for UV detection, suggesting that other organs should receive UV signals for DVM.
Our study revealed that Platynereis c-opsin can transmit UV signals to intracellular signaling cascades via Gi/o activation. Furthermore, because the opsin is activated by UV light and inactivated by visible light (Fig. 1, B, C, and E), the opsin should efficiently detect UV light intensity in ambient light. c-opsin and Gi are co-expressed in ciliary photoreceptor cells in the brain, and the cells produce melatonin to regulate swimming behavior (18) that correlates with a vertical position in the water column (68). Taken together, the UV-sensitive c-opsin in ciliary photoreceptor cells is ideally suitable as a UV sensor for DVM because characteristics of the opsin enable integration of internal circadian melatonin signaling with external UV signals in the same cells. Furthermore, because the c-opsin is activated by UV-A light and inactivated by visible light (Fig. 1), the opsin would be able to detect the ratio of UV light relative to visible light. Detection of the UV/visible light ratio at different depths through the one photopigment might be useful to recognize the vertical position. Of course, we cannot exclude the possibility that other opsins also participate in UV detection. For example, neuropsin (Opn5) is expressed in the Platynereis brain (18), and neuropsins in some vertebrates are UV-sensitive (35, 52, 53). In addition, one of the most studied zooplankton, Daphnia magna, possesses UV-sensitive visual (rhabdomeric) photoreceptor cells in its compound eye (69), implying that, in some zooplankton species, UV reception via eyes is involved in the regulation of DVM (24).
Zooplankton consist of aquatic animals classified into various animal phyla. Ciliary opsins (Opn3 homologs) have been identified in the brains of various animals, including protostomes and deuterostomes. Thus, ciliary opsin-dependent UV reception in the brain could be a major mechanism regulating DVM in some zooplankton species, such as annelids. Elucidation of the expression pattern and the spectral properties of ciliary opsins in various zooplankton species would help us to understand the molecular mechanisms underlying DVM, possibly the largest daily biomass movement on earth (19, 21).
Experimental procedures
Ethics statement
All animal experiments in this study were compliant with the guidelines of the Animal Care Committee of the National Institute for Physiological Sciences (Okazaki, Japan) and were performed with approval of the committee.
Construction of Platynereis c-opsin WT and mutants for heterologous expression in mammalian cultured cells
The cDNA of Platynereis c-opsin (AY692353.1) (8) was synthesized by GenScript (Piscataway, NJ). The cDNAs of the WT and mutants of Platynereis c-opsin were inserted into the EcoRI/NotI site in a mammalian expression vector pMT using in-Fusion HD (Takara, Kusatsu, Japan). On the C terminus, the coding sequence of the 1D4 tag (ETSQVAPA), which is a recognition sequence of the antibody 1D4, was added for purification using 1D4 antibody columns. Mutations at position 94 were introduced by conventional PCR reactions on the pMT expression vector containing cDNA of Platynereis c-opsin WT with the 1D4 tag as a template. PCR primers for the reactions are listed in supplemental Table S1. A PCR product using “c-opsin_forward” and “K94X_reverse” primers and another PCR product using “K94X_forward” and “c-opsin_1D4_reverse” primers were inserted into the EcoRI/NotI site in pMT using in-Fusion HD. DNA sequences were confirmed with a sequencer (ABI Prism 3130xl, Applied Biosystems, Waltham, MA). The location of position 94 in the amino acid sequence of Platynereis c-opsin is indicated in supplemental Fig. S1. In this paper, residue numbering is based on the amino acid sequence of bovine rhodopsin (supplemental Fig. S1).
Protein expression and purification
Platynereis c-opsin WT and mutants were transiently expressed in COS-1 cells (typically 10–20 plates), and the cells were harvested 48 h after transfection as described previously (7, 70). The collected cells were incubated with 11-cis-retinal overnight, and membrane proteins were solubilized with 1.25% DDM (Dojindo, Kumamoto, Japan), 20 mm HEPES, 140 mm NaCl, 0.25% cholesterol hemisuccinate (Sigma-Aldrich, St. Louis, MO) 25 mm Tris, and 10% glycerol (pH 7.0). The solubilized materials were mixed with 1D4-agarose overnight, and the mixture was transferred into Bio-Spin columns (Bio-Rad). The columns were washed with 0.05% DDM, 2 mm ATP, 1 m NaCl, 3 mm MgCl2, 0.01% cholesterol hemisuccinate, 1 mm Tris, and 10% glycerol in PBS and subsequently washed with 0.05% DDM, 140 mm NaCl, 0.01% cholesterol hemisuccinate, 1 mm Tris, 10% glycerol, and 20 mm HEPES (pH 7) (buffer A). The 1D4-tagged pigments were eluted with buffer A containing 0.45 mg/ml 1D4 peptide (TETSQVAPA) (Toyobo, Osaka, Japan).
UV-visible spectroscopy and photoreaction of Platynereis c-opsin
The absorption spectra of purified photopigments were recorded with a Shimadzu UV-2450 spectrophotometer (Shimadzu, Kyoto, Japan). The samples were kept at 10 °C (except in supplemental Fig. S2B). An optical interference filter (YIF-BP340-390S, Sigma-Koki, Tokyo, Japan), which transmits light between 340 and 390 nm, was used for illumination of Platynereis c-opsin with UV light (duration, 2 min; light intensity, ∼9 mW/cm2; light source, 250-W halogen lamp). To convert the photoproduct to the original state, a longpass filter (SCF-50S-48Y, Sigma-Koki) was used (duration, 30 s; light intensity, ∼460 mW/cm2; light source, 250-W halogen lamp). For illumination of Platynereis c-opsin K94T, K94S, and K94A mutants (Fig. 3, K–M), an interference filter (VPF-50S-10-45-440000, Sigma-Koki) that transmits light at 440 nm was used (duration, 1 min; light intensity, ∼48 mW/cm2; light source, 250-W halogen lamp), and the photoproduct was converted back to the original state by illumination with a longpass filter (SCF-50S-58Y, Sigma-Koki) (duration, 30 s; light intensity, ∼220 mW/cm2; light source, 250-W halogen lamp).
HPLC analysis to determine retinal isomer content
Retinal configurations of Platynereis c-opsin samples (Figs. 1C and 2B) were analyzed as described previously (7, 10). Briefly, this analysis involved mixing 0.2 ml of each purified c-opsin sample after incubation at 37 °C with 50 μl of 1 m hydroxylamine and 400 μl of cold 90% methanol to convert retinal chromophore into retinal oxime, followed by extraction of the retinal oxime with 0.7 ml of n-hexane. The extract (150 μl) was then injected into a YMC-Pack SIL column (particle size, 3 μm; diameter, 150 × 6.0 mm; YMC Co. Ltd., Kyoto, Japan) and eluted with n-hexane containing 15% ethyl acetate and 0.15% ethanol at a flow rate of 1 ml/min. The absorbance at 360 nm was monitored using an SPD-20A detector (Shimadzu).
Preparation of Xenopus oocytes
Xenopus oocytes were isolated from frogs as described previously (71, 72). Briefly, Xenopus oocytes were surgically collected from frogs anesthetized in water containing 0.15% tricaine and treated with 2 mg/ml collagenase (Sigma-Aldrich) for 3–4 h to remove the follicular membrane. 5′-capped cRNA was prepared from the pGEMHE vector containing cDNA of Platynereis c-opsin WT and the K94T mutant (inserted in the EcoRI/HindIII site) using an in vitro transcription kit (mMESSAGE mMACHINE Kit, Life Technologies). Typically, we injected the c-opsin cRNA (∼250 pg/oocyte) with rat GIRK1 and mouse GIRK2 cRNAs (∼25 ng/oocyte and ∼12.5 ng/oocyte, respectively) in 50 nl of water/oocyte into ∼40 oocytes. After the injection of cRNA, the oocytes were transferred to another dish to separate them from other (uninjected) oocytes. The oocytes injected with cRNA were incubated in the standard frog Ringer solution (MBSH), a standard frog Ringer solution (88 mm NaCl, 1 mm KCl, 0.3 mm Ca(NO3)2, 0.41 mm CaCl2, 0.82 mm MgSO4, 2.4 mm NaHCO3, and 15 mm HEPES (pH 7.6) with 0.1% penicillin-streptomycin solution), in the dark at 17 °C for 2 days to make sufficient proteins expressed on the plasma membrane for electrophysiological measurements. We performed the cRNA injection and the electrophysiological measurements (see below) six times in 3 months and obtained similar results.
Electrophysiology
We used a conventional two-electrode voltage clamp technique (73, 74) to measure photoresponses caused by Platynereis c-opsin. Before electrophysiological recording, oocytes injected with cRNAs were incubated in the standard frog Ringer solution (MBSH) containing 5 μm 11-cis-retinal for ∼30 min at 17 °C to form photosensitive pigments (Fig. 1B). Light-induced electrophysiological responses were recorded in a bath solution (96 mm KCl, 3 mm MgCl2, and 5 mm HEPES (pH 7.4)). The tip resistance of the glass electrodes was 0.2–0.5 megohm when filled with the pipette solution (3 m potassium acetate and 10 mm KCl). The increase in inward K+ current as a result of Gi/o activation was monitored by two-electrode voltage clamp technique using an OC-725C amplifier (Warner Instruments, Hamden, CT) at room temperature with continuous hyperpolarizing pulses of 0.2 s to −100 mV every 2 s from the holding potential of 0 mV (32) and subsequent 0.2-s pluses of 40 mV (Fig. 1D). Typically, after 10 repetitions of the hyperpolarizing pulses (∼20 s), Platynereis c-opsin molecules were activated by illumination with UV light (light intensity, ∼0.6 mW/cm2; light source, 100-W halogen lamp), and subsequently the opsin was deactivated by yellow (>480-nm) light (light intensity, ∼3 mW/cm2; light source, 3-W LED light). For the K94T mutant of Platynereis c-opsin, efficient activation was induced by the yellow light (>480 nm) (Fig. 3, O and Q), in contrast with the WT. Data acquisition was performed by a digital converter (Digidata 1440, Molecular Devices, Sunnyvale, CA) and pCLAMP 10 software (Molecular Devices).
Author contributions
H. T. designed the research, conducted experiments, and analyzed data. I. S. C. and Y. K. designed and supervised the electrophysiological experiments. Y. F. supervised the overall research. All authors wrote and edited the paper.
Supplementary Material
Acknowledgments
We thank Drs. Shozo Yokoyama (Emory University), Akihisa Terakita (Osaka City University), and Mitsumasa Koyanagi (Osaka City University) for valuable discussions and insightful suggestions; Dr. Robert Molday (University of British Columbia) for providing hybridoma cells producing 1D4 antibody; Dr. David Farrens (Oregon Health and Science University) for providing COS-1 cells and pMT vectors; Drs. Yoshinori Shichida, Takahiro Yamashita, and Kazumi Sakai (Kyoto University) for instructions on how to prepare the 1D4 antibody; and Dr. Kristin Tessmar-Raible (University of Vienna) for providing unpublished materials and helpful discussions. We also thank Hiroe Motomura and the Functional Genomics Facility, NIBB Core Research Facilities (Okazaki, Japan) for technical support.
This work was supported by JSPS KAKENHI Grants 25840122 and 17K15109 (to H. T.) and 26708002 (to Y. F.), the Uehara Memorial Foundation, the Center for the Promotion of Integrated Sciences of SOKENDAI, and the Cooperative Study Program of the National Institute for Physiological Sciences. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains supplemental Figs. S1 and S2 and Table S1.
- DVM
- diel vertical migration
- DDM
- dodecyl maltoside
- mW
- milliwatt
- GIRK
- G protein–coupled inwardly rectifying potassium channel.
References
- 1. Eakin R. M. (1979) Evolutionary significance of photoreceptors: in retrospect. Am. Zool. 19, 647–653 [Google Scholar]
- 2. Yau K. W., and Hardie R. C. (2009) Phototransduction motifs and variations. Cell 139, 246–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Randel N., and Jékely G. (2016) Phototaxis and the origin of visual eyes. Philos. Trans. R Soc. Lond. B Biol. Sci. 371, 20150042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Terakita A. (2005) The opsins. Genome Biol. 6, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Do M. T., and Yau K. W. (2010) Intrinsically photosensitive retinal ganglion cells. Physiol. Rev. 90, 1547–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koyanagi M., and Terakita A. (2008) Gq-coupled rhodopsin subfamily composed of invertebrate visual pigment and melanopsin. Photochem. Photobiol. 84, 1024–1030 [DOI] [PubMed] [Google Scholar]
- 7. Tsukamoto H., Kubo Y., Farrens D. L., Koyanagi M., Terakita A., and Furutani Y. (2015) Retinal attachment instability is diversified among mammalian melanopsins. J. Biol. Chem. 290, 27176–27187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arendt D., Tessmar-Raible K., Snyman H., Dorresteijn A. W., and Wittbrodt J. (2004) Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science 306, 869–871 [DOI] [PubMed] [Google Scholar]
- 9. Velarde R. A., Sauer C. D., Walden K. K., Fahrbach S. E., and Robertson H. M. (2005) Pteropsin: a vertebrate-like non-visual opsin expressed in the honey bee brain. Insect. Biochem. Mol. Biol. 35, 1367–1377 [DOI] [PubMed] [Google Scholar]
- 10. Koyanagi M., Takada E., Nagata T., Tsukamoto H., and Terakita A. (2013) Homologs of vertebrate Opn3 potentially serve as a light sensor in nonphotoreceptive tissue. Proc. Natl. Acad. Sci. U.S.A. 110, 4998–5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blackshaw S., and Snyder S. H. (1999) Encephalopsin: a novel mammalian extraretinal opsin discretely localized in the brain. J. Neurosci. 19, 3681–3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Halford S., Freedman M. S., Bellingham J., Inglis S. L., Poopalasundaram S., Soni B. G., Foster R. G., and Hunt D. M. (2001) Characterization of a novel human opsin gene with wide tissue expression and identification of embedded and flanking genes on chromosome 1q43. Genomics 72, 203–208 [DOI] [PubMed] [Google Scholar]
- 13. Fischer R. M., Fontinha B. M., Kirchmaier S., Steger J., Bloch S., Inoue D., Panda S., Rumpel S., and Tessmar-Raible K. (2013) Co-expression of VAL- and TMT-opsins uncovers ancient photosensory interneurons and motorneurons in the vertebrate brain. PLoS Biol. 11, e1001585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moutsaki P., Whitmore D., Bellingham J., Sakamoto K., David-Gray Z. K., and Foster R. G. (2003) Teleost multiple tissue (tmt) opsin: a candidate photopigment regulating the peripheral clocks of zebrafish? Brain Res. Mol. Brain Res. 112, 135–145 [DOI] [PubMed] [Google Scholar]
- 15. Kato M., Sugiyama T., Sakai K., Yamashita T., Fujita H., Sato K., Tomonari S., Shichida Y., and Ohuchi H. (2016) Two opsin 3-related proteins in the chicken retina and brain: a TMT-type opsin 3 is a blue-light sensor in retinal horizontal cells, hypothalamus, and cerebellum. PLoS ONE 11, e0163925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brandon C. S., Greenwold M. J., and Dudycha J. L. (2017) Ancient and recent duplications support functional diversity of Daphnia opsins. J. Mol. Evol. 84, 12–28 [DOI] [PubMed] [Google Scholar]
- 17. Futahashi R., Kawahara-Miki R., Kinoshita M., Yoshitake K., Yajima S., Arikawa K., and Fukatsu T. (2015) Extraordinary diversity of visual opsin genes in dragonflies. Proc. Natl. Acad. Sci. U.S.A. 112, E1247–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tosches M. A., Bucher D., Vopalensky P., and Arendt D. (2014) Melatonin signaling controls circadian swimming behavior in marine zooplankton. Cell 159, 46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jékely G., Colombelli J., Hausen H., Guy K., Stelzer E., Nédélec F., and Arendt D. (2008) Mechanism of phototaxis in marine zooplankton. Nature 456, 395–399 [DOI] [PubMed] [Google Scholar]
- 20. Alldredge A. L., and King J. M. (1980) Effects of moonlight on the vertical migration patterns of demersal zooplankton. J. Exp. Mar. Biol. Ecol. 44, 133–156 [Google Scholar]
- 21. Brierley A. S. (2014) Diel vertical migration. Curr. Biol. 24, R1074–1076 [DOI] [PubMed] [Google Scholar]
- 22. Bianchi D., Galbraith E. D., Carozza D. A., Mislan K., and Stock C. A. (2013) Intensification of open-ocean oxygen depletion by vertically migrating animals. Nat. Geosci. 6, 545–548 [Google Scholar]
- 23. Schippers K. J., and Nichols S. A. (2014) Deep, dark secrets of melatonin in animal evolution. Cell 159, 9–10 [DOI] [PubMed] [Google Scholar]
- 24. Rhode S. C., Pawlowski M., and Tollrian R. (2001) The impact of ultraviolet radiation on the vertical distribution of zooplankton of the genus Daphnia. Nature 412, 69–72 [DOI] [PubMed] [Google Scholar]
- 25. Williamson C. E., Fischer J. M., Bollens S. M., Overholt E. P., and Breckenridge J. K. (2011) Toward a more comprehensive theory of zooplankton diel vertical migration: integrating ultraviolet radiation and water transparency into the biotic paradigm. Limnol. Oceanogr. 56, 1603–1623 [Google Scholar]
- 26. Gühmann M., Jia H., Randel N., Verasztó C., Bezares-Calderón L. A., Michiels N. K., Yokoyama S., and Jékely G. (2015) spectral tuning of phototaxis by a go-opsin in the rhabdomeric eyes of Platynereis. Curr. Biol. 25, 2265–2271 [DOI] [PubMed] [Google Scholar]
- 27. Sakai K., Yamashita T., Imamoto Y., and Shichida Y. (2015) Diversity of active states in TMT opsins. PLoS ONE 10, e0141238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sugihara T., Nagata T., Mason B., Koyanagi M., and Terakita A. (2016) Absorption characteristics of vertebrate non-visual opsin, Opn3. PLoS ONE 11, e0161215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wickman K., and Clapham D. E. (1995) Ion channel regulation by G proteins. Physiol. Rev. 75, 865–885 [DOI] [PubMed] [Google Scholar]
- 30. Friedmann D., Hoagland A., Berlin S., and Isacoff E. Y. (2015) A spinal opsin controls early neural activity and drives a behavioral light response. Curr. Biol. 25, 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saitoh O., Kubo Y., Miyatani Y., Asano T., and Nakata H. (1997) RGS8 accelerates G-protein-mediated modulation of K+ currents. Nature 390, 525–529 [DOI] [PubMed] [Google Scholar]
- 32. Chen I. S., Furutani K., Inanobe A., and Kurachi Y. (2014) RGS4 regulates partial agonism of the M2 muscarinic receptor-activated K+ currents. J. Physiol. 592, 1237–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jäger S., Palczewski K., and Hofmann K. P. (1996) Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin. Biochemistry 35, 2901–2908 [DOI] [PubMed] [Google Scholar]
- 34. Tsukamoto H., Terakita A., and Shichida Y. (2005) A rhodopsin exhibiting binding ability to agonist all-trans-retinal. Proc. Natl. Acad. Sci. U.S.A. 102, 6303–6308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamashita T., Ohuchi H., Tomonari S., Ikeda K., Sakai K., and Shichida Y. (2010) Opn5 is a UV-sensitive bistable pigment that couples with Gi subtype of G protein. Proc. Natl. Acad. Sci. U.S.A. 107, 22084–22089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsukamoto H. (2014) in Evolution of Visual and Non-visual Pigments (Hunt D. M., Hankins M. W., Collin S. P., and Marshall N. J., eds) pp. 219–239, Springer, New York [Google Scholar]
- 37. Yamashita T., Ono K., Ohuchi H., Yumoto A., Gotoh H., Tomonari S., Sakai K., Fujita H., Imamoto Y., Noji S., Nakamura K., and Shichida Y. (2014) Evolution of mammalian Opn5 as a specialized UV-absorbing pigment by a single amino acid mutation. J. Biol. Chem. 289, 3991–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang X., Wang T., Ni J. D., von Lintig J., and Montell C. (2012) The Drosophila visual cycle and de novo chromophore synthesis depends on rdhB. J. Neurosci. 32, 3485–3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nissilä J., Mänttäri S., Särkioja T., Tuominen H., Takala T., Timonen M., and Saarela S. (2012) Encephalopsin (OPN3) protein abundance in the adult mouse brain. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 198, 833–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hubbard R., Gregerman R. I., and Wald G. (1953) Geometrical isomers of retinene. J. Gen. Physiol. 36, 415–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kefalov V. J., Estevez M. E., Kono M., Goletz P. W., Crouch R. K., Cornwall M. C., and Yau K. W. (2005) Breaking the covalent bond: a pigment property that contributes to desensitization in cones. Neuron 46, 879–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hubbard R., and St. George R. C. (1958) The rhodopsin system of the squid. J. Gen. Physiol. 41, 501–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fukada Y., Okano T., Shichida Y., Yoshizawa T., Trehan A., Mead D., Denny M., Asato A. E., and Liu R. S. (1990) Comparative study on the chromophore binding sites of rod and red-sensitive cone visual pigments by use of synthetic retinal isomers and analogues. Biochemistry 29, 3133–3140 [DOI] [PubMed] [Google Scholar]
- 44. Groenendijk G. W., Jacobs C. W., Bonting S. L., and Daemen F. J. (1980) Dark isomerization of retinals in the presence of phosphatidylethanolamine. Eur. J. Biochem. 106, 119–128 [DOI] [PubMed] [Google Scholar]
- 45. Salcedo E., Zheng L., Phistry M., Bagg E. E., and Britt S. G. (2003) Molecular basis for ultraviolet vision in invertebrates. J. Neurosci. 23, 10873–10878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yokoyama S. (2008) Evolution of dim-light and color vision pigments. Annu. Rev. Genomics Hum. Genet. 9, 259–282 [DOI] [PubMed] [Google Scholar]
- 47. Li J., Edwards P. C., Burghammer M., Villa C., and Schertler G. F. (2004) Structure of bovine rhodopsin in a trigonal crystal form. J. Mol. Biol. 343, 1409–1438 [DOI] [PubMed] [Google Scholar]
- 48. Okada T., Sugihara M., Bondar A. N., Elstner M., Entel P., and Buss V. (2004) The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J. Mol. Biol. 342, 571–583 [DOI] [PubMed] [Google Scholar]
- 49. Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., Yamamoto M., and Miyano M. (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739–745 [DOI] [PubMed] [Google Scholar]
- 50. Koyanagi M., Kawano E., Kinugawa Y., Oishi T., Shichida Y., Tamotsu S., and Terakita A. (2004) Bistable UV pigment in the lamprey pineal. Proc. Natl. Acad. Sci. U.S.A. 101, 6687–6691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koyanagi M., Wada S., Kawano-Yamashita E., Hara Y., Kuraku S., Kosaka S., Kawakami K., Tamotsu S., Tsukamoto H., Shichida Y., and Terakita A. (2015) Diversification of non-visual photopigment parapinopsin in spectral sensitivity for diverse pineal functions. BMC Biol. 13, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kojima D., Mori S., Torii M., Wada A., Morishita R., and Fukada Y. (2011) UV-sensitive photoreceptor protein OPN5 in humans and mice. PLoS ONE 6, e26388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sato K., Yamashita T., Haruki Y., Ohuchi H., Kinoshita M., and Shichida Y. (2016) Two UV-sensitive photoreceptor proteins, Opn5m and Opn5m2 in Ray-finned fish with distinct molecular properties and broad distribution in the retina and brain. PLoS ONE 11, e0155339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Menzel R. (1979) in Handbook of Sensory Biology Vol. VIII6A: Invertebrate Photoreceptors (Autrum H., ed.), pp 503–580, Springer Verlag, Berlin [Google Scholar]
- 55. Cronin T. W., and Bok M. J. (2016) Photoreception and vision in the ultraviolet. J. Exp. Biol. 219, 2790–2801 [DOI] [PubMed] [Google Scholar]
- 56. Gärtner W. (2000) Invertebrate visual pigments. Handbook of Biological Physics 3, 297–388 [Google Scholar]
- 57. Kitamoto J., Ozaki K., and Arikawa K. (2000) Ultraviolet and violet receptors express identical mRNA encoding an ultraviolet-absorbing opsin: identification and histological localization of two mRNAs encoding short-wavelength-absorbing opsins in the retina of the butterfly Papilio xuthus. J. Exp. Biol. 203, 2887–2894 [DOI] [PubMed] [Google Scholar]
- 58. Fryxell K. J., and Meyerowitz E. M. (1987) An opsin gene that is expressed only in the R7 photoreceptor cell of Drosophila. EMBO J. 6, 443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Montell C., Jones K., Zuker C., and Rubin G. (1987) A second opsin gene expressed in the ultraviolet-sensitive R7 photoreceptor cells of Drosophila melanogaster. J. Neurosci. 7, 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Townson S. M., Chang B. S., Salcedo E., Chadwell L. V., Pierce N. E., and Britt S. G. (1998) Honeybee blue- and ultraviolet-sensitive opsins: cloning, heterologous expression in Drosophila, and physiological characterization. J. Neurosci. 18, 2412–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zuker C. S., Montell C., Jones K., Laverty T., and Rubin G. M. (1987) A rhodopsin gene expressed in photoreceptor cell R7 of the Drosophila eye: homologies with other signal-transducing molecules. J. Neurosci. 7, 1550–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fasick J. I., Applebury M. L., and Oprian D. D. (2002) Spectral tuning in the mammalian short-wavelength sensitive cone pigments. Biochemistry 41, 6860–6865 [DOI] [PubMed] [Google Scholar]
- 63. Tada T., Altun A., and Yokoyama S. (2009) Evolutionary replacement of UV vision by violet vision in fish. Proc. Natl. Acad. Sci. U.S.A. 106, 17457–17462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ramon E., del Valle L. J., and Garriga P. (2003) Unusual thermal and conformational properties of the rhodopsin congenital night blindness mutant Thr-94 → Ile. J. Biol. Chem. 278, 6427–6432 [DOI] [PubMed] [Google Scholar]
- 65. Schafer C. T., Fay J. F., Janz J. M., and Farrens D. L. (2016) Decay of an active GPCR: conformational dynamics govern agonist rebinding and persistence of an active, yet empty, receptor state. Proc. Natl. Acad. Sci. U.S.A. 113, 11961–11966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Randel N., Asadulina A., Bezares-Calderon L. A., Veraszto C., Williams E. A., Conzelmann M., Shahidi R., and Jekely G. (2014) Neuronal connectome of a sensory-motor circuit for visual navigation. eLife 3, e02730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Randel N., Bezares-Calderón L. A., Gühmann M., Shahidi R., and Jékely G. (2013) Expression dynamics and protein localization of rhabdomeric opsins in Platynereis larvae. Integr. Comp. Biol. 53, 7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Conzelmann M., Offenburger S. L., Asadulina A., Keller T., Münch T. A., and Jékely G. (2011) Neuropeptides regulate swimming depth of Platynereis larvae. Proc. Natl. Acad. Sci. U.S.A. 108, E1174–E1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smith K. C., and Macagno E. R. (1990) UV photoreceptors in the compound eye of Daphnia magna (Crustacea, Branchiopoda): a fourth spectral class in single ommatidia. J. Comp. Physiol. A 166, 597–606 [DOI] [PubMed] [Google Scholar]
- 70. Tsukamoto H., and Farrens D. L. (2013) A constitutively activating mutation alters the dynamics and energetics of a key conformational change in a ligand-free G protein-coupled receptor. J. Biol. Chem. 288, 28207–28216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fujiwara Y., and Kubo Y. (2002) Ser165 in the second transmembrane region of the Kir2.1 channel determines its susceptibility to blockade by intracellular Mg2+. J. Gen. Physiol. 120, 677–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kubo Y., Miyashita T., and Murata Y. (1998) Structural basis for a Ca2+-sensing function of the metabotropic glutamate receptors. Science 279, 1722–1725 [DOI] [PubMed] [Google Scholar]
- 73. Baumgartner W., Islas L., and Sigworth F. J. (1999) Two-microelectrode voltage clamp of Xenopus oocytes: voltage errors and compensation for local current flow. Biophys. J. 77, 1980–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., and Sakmann B. (1986) Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 407, 577–588 [DOI] [PubMed] [Google Scholar]
- 75. Ni J. D., Baik L. S., Holmes T. C., and Montell C. (2017) A rhodopsin in the brain functions in circadian photoentrainment in Drosophila. Nature 545, 340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tamura K., Stecher G., Peterson D., Filipski A., and Kumar S. (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.