Supplemental Digital Content is available in the text.
Abstract
Background:
Carcinogenic risks of internal exposures to alpha-emitters (except radon) are poorly understood. Since exposure to alpha particles—particularly through inhalation—occurs in a range of settings, understanding consequent risks is a public health priority. We aimed to quantify dose–response relationships between lung dose from alpha-emitters and lung cancer in nuclear workers.
Methods:
We conducted a case–control study, nested within Belgian, French, and UK cohorts of uranium and plutonium workers. Cases were workers who died from lung cancer; one to three controls were matched to each. Lung doses from alpha-emitters were assessed using bioassay data. We estimated excess odds ratio (OR) of lung cancer per gray (Gy) of lung dose.
Results:
The study comprised 553 cases and 1,333 controls. Median positive total alpha lung dose was 2.42 mGy (mean: 8.13 mGy; maximum: 316 mGy); for plutonium the median was 1.27 mGy and for uranium 2.17 mGy. Excess OR/Gy (90% confidence interval)—adjusted for external radiation, socioeconomic status, and smoking—was 11 (2.6, 24) for total alpha dose, 50 (17, 106) for plutonium, and 5.3 (−1.9, 18) for uranium.
Conclusions:
We found strong evidence for associations between low doses from alpha-emitters and lung cancer risk. The excess OR/Gy was greater for plutonium than uranium, though confidence intervals overlap. Risk estimates were similar to those estimated previously in plutonium workers, and in uranium miners exposed to radon and its progeny. Expressed as risk/equivalent dose in sieverts (Sv), our estimates are somewhat larger than but consistent with those for atomic bomb survivors.
See video abstract at, http://links.lww.com/EDE/B232.
Knowledge of long-term health effects of ionizing radiation is chiefly derived from studies of populations exposed to photons (X and γ rays), particularly the Japanese atomic bomb survivors1 and populations receiving external radiation doses through occupational, medical, and environmental exposures.2 Less is known about long-term impacts of internal exposure to emitters of alpha particles. Because internal exposures to alpha-emitters occur in environmental, malicious, accidental, or occupational settings, understanding attendant long-term risks is a priority in radiation protection.
The nuclear industry encompasses a range of activities, for example, energy and isotope production, fuel manufacturing and reprocessing, production of nuclear weapons, and research. Monitoring of external photon exposures is relatively straightforward and many cohorts have complete records from the industry’s inception. Previous epidemiologic studies of nuclear workers have chiefly examined associations between cancers and photon radiation exposure.3–5 However, at some facilities—particularly those involved in the fuel cycle and weapons production—workers may receive internal doses from incorporation of radionuclides, in particular uranium and plutonium, by inhalation, ingestion, or contamination of wounds. After inhalation/incorporation, doses to organs or tissues depend on the radionuclide/element, its physicochemical form, and intake route.6 Densely ionizing radiations such as alpha particles have a greater relative biologic effectiveness than sparsely ionizing radiation such as X and γ rays for many cell toxicity endpoints. In radiological protection—where the prevention of cancer is generally the limiting factor—the increased relative biologic effectiveness of alpha particles is taken into account through the use of a radiation weighting factor (wR) of 20.7 While sufficient evidence exists to classify internalized alpha-emitting radionuclides as carcinogenic, and there is sufficient evidence to declare some radionuclides as human carcinogens (e.g., plutonium and radon progeny), data on risks at low doses are lacking for plutonium, and direct evidence of carcinogenicity in humans is limited for uranium.8
Relatively large lung doses can result from inhaling uranium/plutonium, depending on lung solubilities of materials inhaled. Lung cancer risk from internal exposure to these radionuclides is therefore of interest. It is well established that prolonged exposure to radon progeny increases lung cancer risk.9,10 Studies of cancer risks associated with plutonium have been conducted in highly-exposed individuals in the Mayak worker cohort (MWC)11 and less highly-exposed workers at Sellafield,12 with uranium and plutonium in nuclear workers in the United States,13–16 and with thorium and radium in medical patients.17 Dose–response analyses have been conducted on individually-assigned plutonium doses in Mayak and Sellafield cohorts. The MWC currently provides most of the information in humans on plutonium dose–response for several outcomes18–22 though it is limited by scant bioassay data and no monitoring in a substantial portion of the population. Dosimetry in the few other studies in which individual-level lung doses from alpha-emitters have been reconstructed has largely been based on limited bioassay data, resulting in uncertain doses, particularly at low doses.
An increased risk of solid cancer in relation to external radiation dose was found in the International Collaborative Study (ICS), a 15-country cohort study of nuclear workers23,24 and in the International Nuclear Workers Study (INWORKS), a continuation of the ICS restricted to United States, United Kingdom, and France.5 The ICS excluded workers with potential for internal exposure to alpha-emitters as dose reconstruction was impracticable at the cohort level; smoking information was unavailable for the ICS and INWORKS. To complement these studies, and to address some limitations of MWC data, the current study was conducted to improve lung cancer risks associated with internal exposure to alpha-emitters, particularly at low doses, and to account for potential confounders, for example, smoking. Specifically, this study aimed to quantify any dose–response relationship between cumulated lung dose from alpha-emitters and lung cancer mortality among nuclear workers monitored for uranium and/or plutonium exposure in five European cohorts.
METHODS
Study Design
We conducted a case–control study of lung cancer mortality, nested in a set of five cohorts identified in the much larger ICS, comprising workers from Belgium, France, and the United Kingdom (Table 1). This design reduced data collection (internal monitoring and smoking) and individual dose reconstruction compared with a cohort study. Activities covered by these facilities include: nuclear research and development, waste treatment, fuel production and reprocessing, construction and operation of experimental reactors, development of fast breeder reactors, and nuclear weapons research and production.
TABLE 1.
Study Populations, Study Periods, and t1 Date
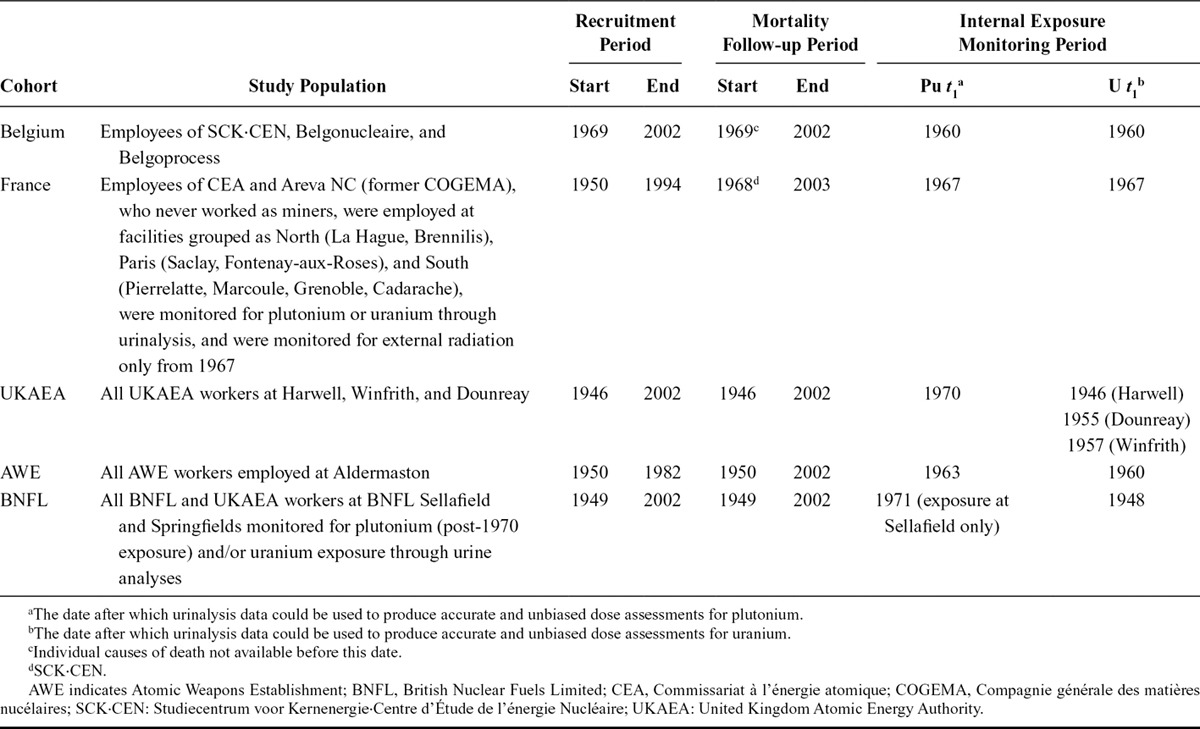
Study Population
The study population included all workers monitored for internal exposure to plutonium and/or uranium through urinalysis on or after a date, t1, and employed for ≥1 year at a facility in a study cohort. t1 was defined as the date after which bioassay (urine) data could be used to produce accurate and unbiased dose assessments (Table 1).25 For uranium, t1 often corresponded to the start of operation of a facility; for plutonium, early bioassay measurements were less reliable. Workers with only pre-t1 data were excluded. Workers employed for <1 year were excluded, as they may not be comparable to longer-term workers in terms of cancer risk. Contract workers were excluded as work histories and exposures could not be comprehensively reconstructed, and mortality could not be comprehensively followed up.
We defined cases as all deaths within the study populations and mortality follow-up periods (Table 1) for which lung cancer was either the underlying cause of death or an associated cause where no other cancer was listed as an underlying cause. Controls were alive and at risk in the year of death of the case (“reference date”); one to three controls were matched to each case by age at reference date (within 5 years), sex and facility (for cohorts with more than one geographically distinct facility, the facility was used as the matching variable). Controls born closest to the date of birth of the case were preferentially selected. Controls were eligible for reselection as a control for another case and for reselection as a case.
Dosimetry
We obtained individual annual external dose estimates based on personal dosimeters, compiled for each worker for the purposes of other epidemiologic studies.24,26 We estimated individual lung doses from internally incorporated alpha-emitters—chiefly plutonium and uranium and some others (radium, actinium, thorium, curium, polonium, protactinium, and americium)—for each subject annually from start of potential exposure up to and including the reference date of the relevant case. Doses were reconstructed from bioassay data (primarily urinalysis but also fecal analysis and in vivo monitoring if available) using a common methodology developed and tested by the study dosimetry subcommittee as detailed elsewhere.25 Start of potential exposure to plutonium and uranium was determined from work history records or set to just before the start of bioassay monitoring. Where study subjects had both pre- and post-t1 monitoring data during their work history, pre-t1 data were included and doses calculated for pre-t1 years. Long retention of plutonium and uranium in the body means that later more reliable bioassay data can provide information on earlier (pre-t1) exposures.
For workers employed at more than one facility or several times at the same facility, we reconstructed dosimetric and occupational histories, with final dose assessments conducted at the facility of last employment.
Calculated doses depended on assumptions regarding the physicochemical properties of the radioactive materials to which a worker was exposed, lung absorption characteristics, and intake (exposure) regimes (acute or chronic). We generated four indices of internal dose: total alpha dose (all radionuclides); alpha dose from plutonium; alpha dose from uranium; and dose from other alpha-emitters.
We estimated doses using the Human Respiratory Tract Model (HRTM),27 in which the lung is partitioned into three regions: bronchial (BB), bronchiolar (bb), and alveolar interstitial (AI). Doses were estimated to each region, and to the whole lung, because doses to these regions from radionuclide-bearing aerosols vary and the radiosensitivities of cells in these regions are assumed to differ. We estimated doses for four cell types: basal (BBbas) and secretory (BBsec) cells (BB region), Clara cells (bb region), and endothelial cells (AI region). We summed regional doses using weights of 1/3 for AI and bb regions and 1/6 for BBsec and BBbas subregions.27 This weighted lung dose has been used in epidemiologic studies of radon and its progeny28 and is extensively used in radiation protection.9 Studies of MWC have used a different approach, wherein dose is calculated as total energy deposited in the lung divided by lung tissue mass. We estimated mass-averaged doses for sensitivity analysis (weights: BB = 0.0006; bb = 0.0017; AI = 0.9977).
Potential Confounding
Information was available on the matching variables—age, sex, and facility—and socioeconomic status (SES). Facility is important in controlling for potential occupational coexposures and geographically determined factors (e.g., natural background radiation). SES is a surrogate for lifestyle factors associated with cancer including smoking and diet. SES was assigned to each subject based on job title—either at time of hiring (France), last job (UKAEA, AWE), or job of longest duration (other cohorts). For AWE, Belgium, France, and UKAEA, we assigned SES according to a regrouping of the British Registrar General’s classification, from 1 (unskilled) to 4 (intermediate and professional).29 For BNFL, detailed classification was not possible so workers were assigned categories 5 and 6, indicating “industrial” (paid weekly) and “nonindustrial” (paid monthly) work, respectively (eTable 1; http://links.lww.com/EDE/B205); “nonindustrial” included managerial, scientific, and clerical staff; “industrial” comprised the remainder. For sensitivity analysis, we recoded SES: categories 1, 2, and 5 were combined (lower SES), and categories 3, 4, and 6 were combined (higher SES).
Smoking is the main risk factor for lung cancer and an important potential confounder in this study. The record-based nature of this study precluded collection of comprehensive information on lifetime smoking status. Smoking information was extracted from occupational medical records from routine medical examinations. For Belgium and AWE, smoking information was well documented for entry examinations but not in subsequent ones. For UKAEA and BNFL, information was available for ~40% of workers but completeness of records was heterogeneous. In France, smoking information was not recorded in early time periods. From the mid-1970s, such information was collected more systematically in all facilities. Given the heterogeneous data available for each worker’s employment history, and no definitive retrospectively-collected data regarding lifetime smoking habits, “never smoker” was assigned if any data after the age of 40 indicated the worker never having smoked. “Ever smoker” was assigned when any record of smoking appeared in medical records. Where neither condition was met (or information was unavailable) “unknown” status was assigned.
Statistical Analysis
The aim of the study was to derive estimates of the excess relative risk (in terms of the excess odds ratio) of lung cancer per gray (Gy) of absorbed dose to the lung from alpha-emitters as a group and for plutonium and uranium separately. All information was truncated at the reference date. We fitted conditional logistic regression models based on matched sets using EPICURE software.30 The excess OR/Gy was estimated using a mixture model comprising a linear function of radiation dose (both internal and external) and a loglinear function of covariates, as is typical in radiation epidemiology, defined:
where 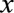 is a vector of covariates,
is a vector of covariates, 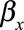 are the covariate parameters to be estimated,
are the covariate parameters to be estimated, 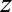 is a vector of cumulated doses (both internal and external) minus lag,
is a vector of cumulated doses (both internal and external) minus lag, 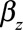 is a vector of dose–response slopes, that is, the excess OR/Gy to be estimated, and
is a vector of dose–response slopes, that is, the excess OR/Gy to be estimated, and 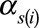 is a set of stratum parameters indicating numbers of cases and controls in each matched set. In common with most modern radiation epidemiology studies, we investigated the magnitude of increased cancer risk associated with radiation exposure, and therefore followed the convention of reporting 90% confidence intervals (CIs) and one-sided P values. Profile likelihood-based CIs were estimated.
is a set of stratum parameters indicating numbers of cases and controls in each matched set. In common with most modern radiation epidemiology studies, we investigated the magnitude of increased cancer risk associated with radiation exposure, and therefore followed the convention of reporting 90% confidence intervals (CIs) and one-sided P values. Profile likelihood-based CIs were estimated.
Main analyses were adjusted systematically for external dose, SES, and smoking through inclusion in the model—external dose in the linear subterm, all other covariates in the loglinear subterm—and for age, sex, and facility through matching. Age at start of employment and duration of employment were investigated as potential confounders of associations between lung cancer and alpha dose, by including them in the model and evaluating whether estimates of radiation-induced risk changed by ≥10%. We investigated effect modification of the association of lung cancer with alpha dose by attained age, age at start of employment, and duration of exposure, and by sex, cohort, smoking, and SES. For each model an interaction term was introduced as an exponent of alpha dose. Heterogeneity of risk was evaluated using a likelihood ratio test between models including and excluding the interaction. Departures from linear dose–response for alpha doses—including quadratic and logarithmic transformations in the linear term (logarithm of dose + 1 to prevent exclusion of zero-dose subjects)—were investigated. We assessed fit using likelihood ratio test (dose + dose2) and Bayesian information criterion (log(dose + 1)).
We lagged cumulated doses—both internal and external—by 10 years as is typical in studies of nuclear workers and lung cancer.23 Doses lagged by 5 and 15 years were estimated for sensitivity analysis. Other sensitivity analyses included: no adjustment for SES or smoking, restricting to men, and exploring heterogeneity among cohorts by running models that omitted cohorts one by one. The highly skewed dose data (a large proportion of workers had zero or near-zero doses) precluded extensive categorical analysis: four categories were defined based on inspection of histograms of dose.
RESULTS
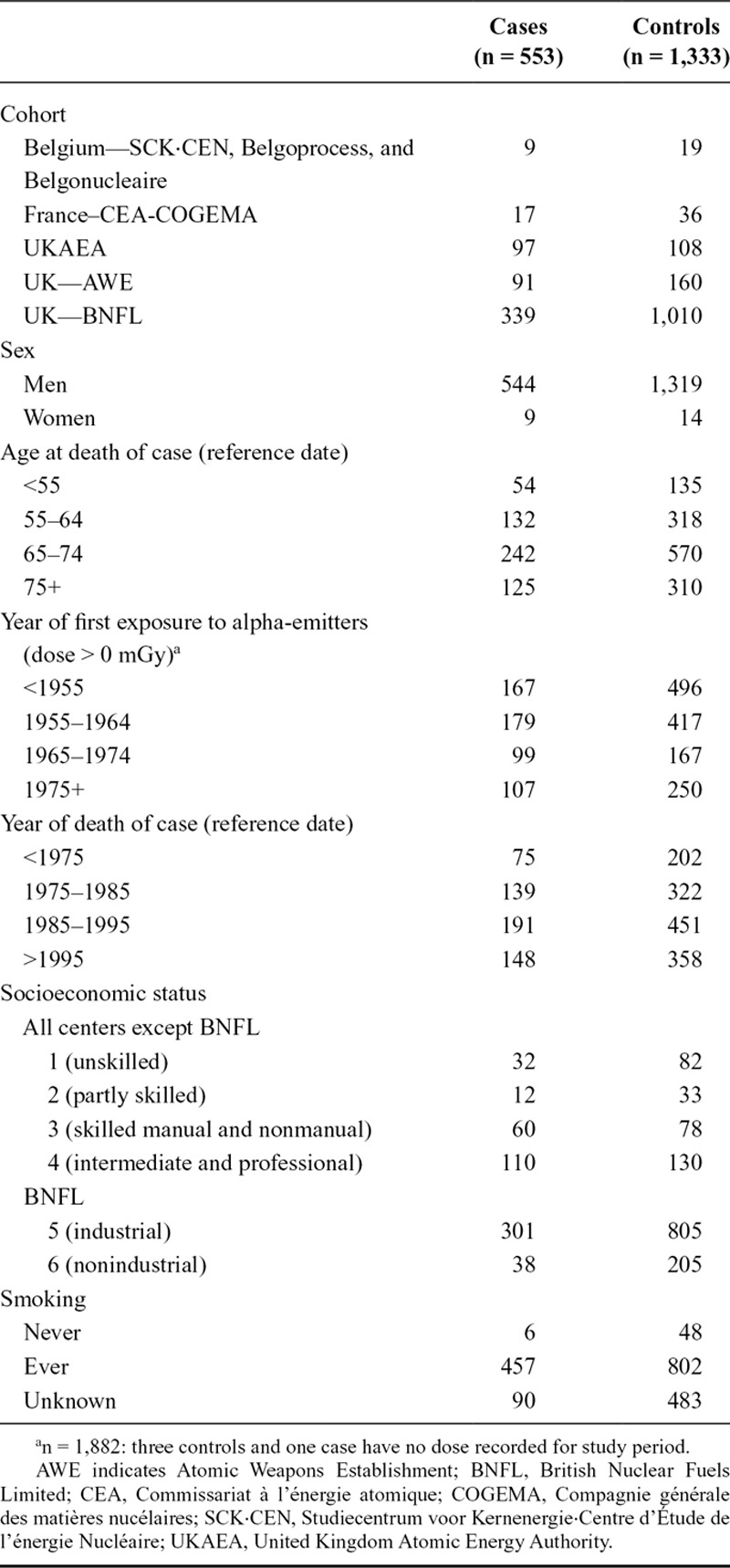
In total, 553 cases of lung cancer and 1,333 controls were included (Table 2); nine controls subsequently became cases. A total of 67% of subjects were ≥65 years old at reference date. Most began employment before 1965, ~35% before 1955. Mean date of death was 1989 (median: 1988, SD: 10.5). BNFL contributed the largest number of subjects (339 cases; 1,010 controls).
TABLE 2.
Distribution of Key Characteristics of Subjects
Distributions of internal alpha doses and photon doses were highly positively skewed (eTable 2; http://links.lww.com/EDE/B205). Median positive alpha dose to the lung from all radionuclides was 2.43 mGy (mean: 8.13 mGy; maximum: 316 mGy; interquartile range width [IQRw]: 7.76 mGy). Median positive alpha doses were 1.27 mGy for plutonium (mean: 5.09 mGy; maximum: 110 mGy; IQRw: 4.27 mGy) and 2.17 mGy for uranium (mean: 6.45 mGy; maximum: 302 mGy; IQRw: 5.93 mGy). The number of subjects with positive doses was 711 for plutonium, 1,409 for uranium, and 56 for other alpha-emitters.
Median total alpha doses were highest to BBsec and lowest to BBbas (eFigure 1; http://links.lww.com/EDE/B205). Regional doses were highly correlated (Spearman’s rho ≥0.93 for uranium and plutonium). Ranges and medians of average lung doses generated using the alternative weighting scheme were similar (eFigure 2; http://links.lww.com/EDE/B205).
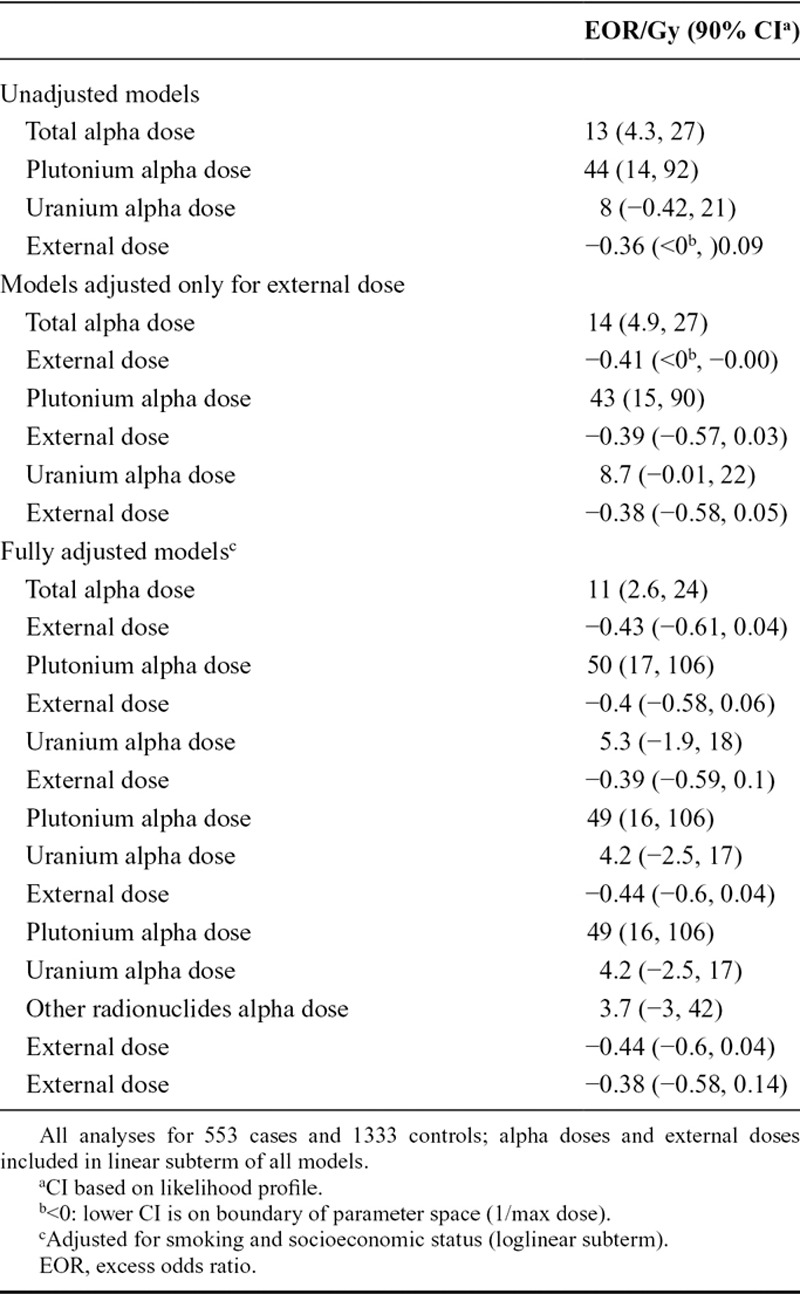
We found a dose–response relationship for total alpha dose: the excess OR/Gy adjusted for external dose, smoking, and SES was 11 (90% CI: 2.6, 24; Table 3). The excess OR/Gy for plutonium and uranium adjusted for external dose, smoking, and SES were 50 (90% CI: 17, 106) and 5.3 (90% CI: −1.9, 18), respectively. Although CIs were wide and overlapped, we demonstrated a difference between deviances of models (adjusted for SES, smoking status, and photon dose) of combined uranium and plutonium doses and of the two doses separately (likelihood ratio test P value = 0.03). Repeating the analysis for plutonium omitting the seven subjects with plutonium dose ≥50 mGy gave an excess OR/Gy of 35 (90% CI: 3.5, 88), which was lower but statistically compatible with the main plutonium result. No dose–response relationship was found for external radiation dose: excess OR/Gy adjusted for smoking and SES was −0.38 (90% CI: −0.58; 0.14; Table 3).
TABLE 3.
EOR per Gy for Lung Cancer—Matched on Sex, Age, and Cohort, Lag of 10 Years
Most subjects smoked during their lifetime (84% of cases; 62% of controls). Few workers never smoked (1% of cases; 4% of controls). Smoking status was unknown for 17% of cases and 38% of controls. Odds ratios of lung cancer due to smoking were 9.2 (90% CI: 4, 21) for ever smokers and 2.5 (90% CI: 1.1, 5.9) for those of unknown smoking status (eTable 3; http://links.lww.com/EDE/B205).
Removing SES or smoking from the model (eTable 4; http://links.lww.com/EDE/B205) led to ≥10% change in excess OR/Gy for total alpha, uranium, and plutonium doses (with the exception of SES for plutonium) suggesting that both variables confound associations between lung cancer and alpha dose. Reclassification of SES into two groups resulted in little change to the risk estimates (eTable 5; http://links.lww.com/EDE/B205).
We found no evidence for effect modification of total alpha dose by smoking (P value: 0.35), attained age (P value: >0.50), age at start of employment (P value: >0.50), SES (P value: 0.08), cohort (maximum likelihood estimate for some parameters could not be calculated), or duration of employment (P: >0.50). Similarly, no effect modification by any covariate was identified for dose from plutonium or uranium individually.
We detected no evidence of departures from linearity in the associations for total alpha, plutonium, or uranium. Likelihood ratio tests indicated that the fits of models of dose + dose2 were no improvement over linear models for total alpha (P: 0.12), plutonium (P: 0.07), and uranium (P: 0.14). Information criteria also indicated that fits of models of log(dose + 1) were no improvement over linear models of dose for total alpha (Bayesian information criterion 1,203.72 vs. 1,203.57 for linear dose model), plutonium (Bayesian information criterion 1,199.54 vs. 1,199.44), and uranium (Bayesian information criterion 1,208.31 vs. 1,208.25).
Results of categorical analyses are plotted in the Figure. For plutonium, the excess OR for the highest category was very high, but based on small numbers of subjects (Table 4), with very wide CIs that just include the estimate from the model using the continuous form of the exposure variable. Sensitivity analyses were carried out with reference categories of zero dose: patterns of risk estimates for all internal dose variables were similar to those observed in the main analyses (eTable 6; http://links.lww.com/EDE/B205).
TABLE 4.
Distribution of Subjects by Dose Category and Results of Categorical Analysis, Adjusted for External Dose, Socioeconomic Status and Smoking, 10-year Lag
FIGURE.
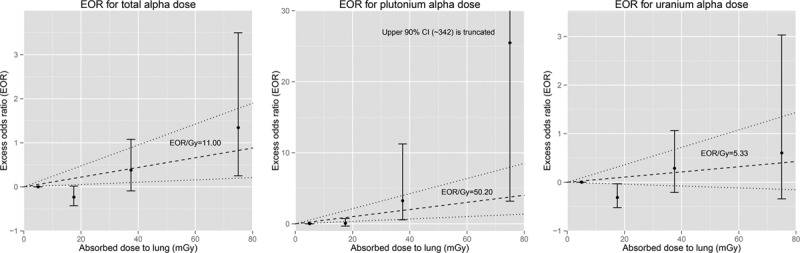
Excess odds ratio (90% confidence intervals) for categorical analysis of alpha dose, adjusted for smoking, socioeconomic status, and external dose with trend from continuous analysis of alpha dose (90% confidence interval).
Sensitivity analyses of 5- and 15-year lags produced excess OR/Gy similar to those for 10-year lags (eTable 7; http://links.lww.com/EDE/B205). Restricting to males (n = 1,863) gave an excess OR/Gy for total alpha dose of 11 (90% CI: 2.6, 24), essentially identical the main analysis result. Numbers were too low to restrict to females.
Estimates of excess OR/Gy for individual lung regions were heterogeneous, particularly for total alpha dose (eTable 8; http://links.lww.com/EDE/B205). Generally, however, CIs on the dose–response estimates were wide and overlapping. Deviances of models of excess OR/Gy of regional alpha doses were consistently lowest for the AI region for all nuclides. Although median average lung doses were similar to International Commission on Radiological Protection (ICRP)- weighted lung doses (analyses restricted to UK cohorts as doses to individual lung regions were not available elsewhere), the risk estimates for the two sets of doses differed although CIs were wide and overlapped (eTable 9; http://links.lww.com/EDE/B205). Differences between the ratios of the risk estimates for plutonium versus uranium for individual regions of the lung were small (eTable 8; http://links.lww.com/EDE/B205). Risks for plutonium were greater than those for uranium by factors of ~7 (BBbas) to ~15 (bb). Risk estimates for plutonium using average lung dose were ~80% of those estimated using the ICRP weighted dose, compared with ~200% for uranium. Risks for total alpha dose were higher for average lung dose than ICRP weighted dose. For all dose indices, however, CIs were wide and the results obtained using either weighting system are similar.
DISCUSSION
This multinational case–control study, combining data from those European nuclear industry cohorts with substantial numbers of workers exposed to uranium and/or plutonium, is the first large-scale study in which lung doses from both nuclides have been estimated using common dosimetric models.
We found strong evidence that internal exposure to alpha-emitters in the lung increases lung cancer risk even at the low doses experienced by nuclear industry workers. A linear model proved adequate to describe the shape of dose–response for total alpha, plutonium, and uranium doses. External radiation dose did not confound these associations. Smoking and SES confounded these associations; the degree to which confounding by smoking was controlled was potentially limited by a low proportion of lifetime nonsmokers and relatively high proportion of subjects for which definitive smoking data were not available (eTable 3; http://links.lww.com/EDE/B205).
In radionuclide-specific analyses, excess OR/Gy were higher for plutonium than for uranium. Although CIs associated with estimates for plutonium and uranium were wide and overlapped, the difference in the estimates was statistically significant. Given uncertainties in doses (not presented here), we cannot draw clear conclusions regarding this difference. Also, since the dosimetry protocol was agreed, modifications to the HRTM have been recommended32 and proposals submitted for updated lung solubility parameters for plutonium and uranium compounds which might differentially affect doses. Although impacts of these modifications were considered in an uncertainty analysis (not presented here), it would be useful to estimate risk in this population using dosimetry including the updated model, and to explore the influence on risk estimates of individual-level dosimetric information, such as numbers of bioassays, bioassay data below detection limits, solubility assumptions, and calculated intakes.
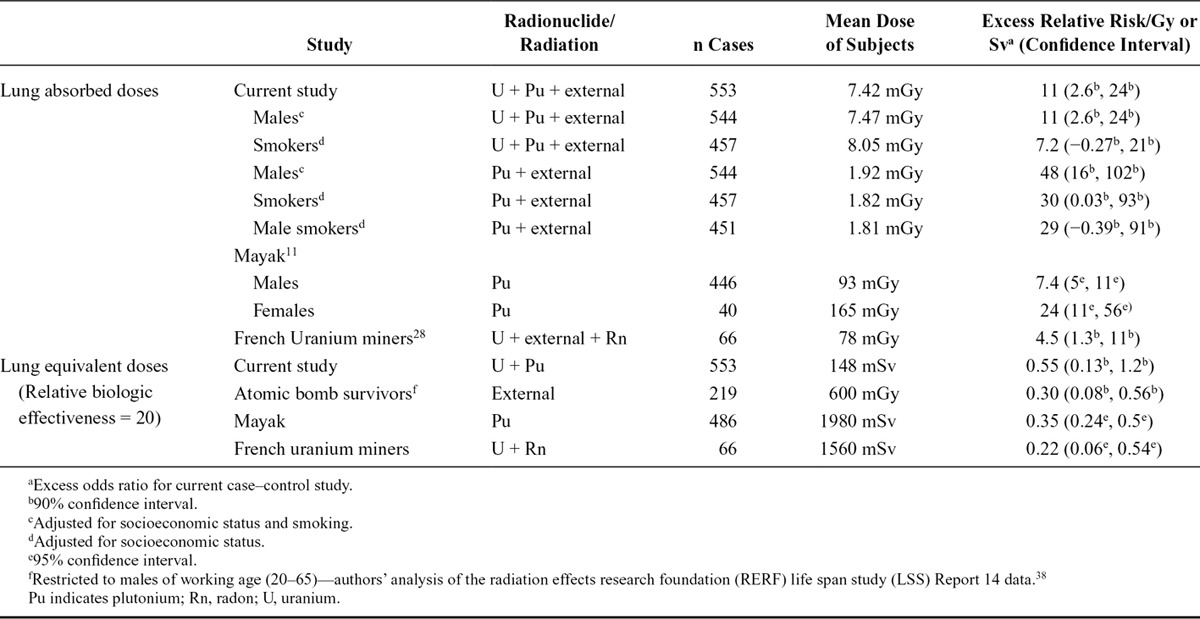
Our estimate of excess OR/Gy for total alpha dose is higher than, but compatible with, that of previous studies of prolonged exposure to alpha-emitters (Table 5), namely for radon exposure in the French uranium miners cohort (FUMC)28 and for plutonium in the MWC,11 where doses were higher than in our study. The risk estimates in the present study for plutonium dose—and specifically for male smokers, for comparison with the MWC—are higher than those reported for the MWC.11,22,33 The reasons for this are unclear, although differences between the current study cohorts and MWC in terms of monitoring regimes, dosimetry and associated uncertainties, distributions of dose, and potential confounding by occupational carcinogens may play a role. The dosimetry used in our study was based on more extensive individual biomonitoring data than MWC which led to more accurate (though still uncertain) dose estimates, particularly at low doses.25 Our findings are particularly important as other studies of populations internally-exposed to plutonium at doses lower than those in the MWC12,14,34,35 have not provided clear evidence of increased risk of lung cancer with increasing plutonium dose.11 Using a wR of 20 to express risk in terms of equivalent dose to the lung in Sv, our estimates are compatible with those of the atomic bomb survivors.1 We were unable to compare our results with many underground miner studies that present risk in terms of working level months. The FUMC study, however, reported ERR/Gy for lung cancer mortality and lung alpha doses of 4.5 (95% CI: 1.3, 11),28 which is compatible with our results for total alpha dose.
TABLE 5.
Comparison of Current Study Results with Other Studies
We found no clear evidence of effect modification by age at start of employment, or duration of employment, although the generally low doses meant we had little statistical power to test for this. The similarity of results for different lags reflects much of the exposure having occurred many years before reference dates (mainly before the 1960s).
Although we found no evidence of effect modification by smoking, an excess OR/Gy of 7.2 (90% CI: −0.3, 21) was estimated for total alpha dose (adjusted for SES) in an analysis restricted to smokers, which indirectly suggests a similar pattern to that observed in the MWC, cohorts of uranium miners, residents exposed to environmental radon, and atomic bomb survivors,36 where the modifying effect of smoking on radiation is submultiplicative.
We found no clear evidence of effect modification by cohort. We could not estimate excess OR/Gy for some cohorts individually due to small numbers: models for Belgium, France, and UKAEA failed to converge; effect measures for AWE and BNFL individually were imprecise, prohibiting interpretation (results for BNFL in eTable 10; http://links.lww.com/EDE/B205). However, heterogeneities in risk estimates observed when omitting cohorts one-by-one (eTable 11; http://links.lww.com/EDE/B205) suggest differences in risks among them. Omitting Belgium, France, or UKAEA had a limited impact on excess OR/Gy for total alpha, plutonium, or uranium. Omitting both Belgium and France (see ICRP weighted lung dose estimates in eTable 9; http://links.lww.com/EDE/B205) also had limited impact. Omitting AWE decreased excess OR/Gy for total alpha, plutonium, and uranium doses. It appears that AWE subjects with relatively higher doses may be influential in the analyses. Omitting BNFL had little impact on excess OR/Gy for plutonium dose, but increased excess OR/Gy for uranium dose (and subsequently for total alpha dose). Some of the heterogeneity may result from subtle differences in matching: although controls were matched on facility in all cohorts, in BNFL this resulted in a tendency to match on radionuclide (Sellafield workers were typically exposed only to plutonium, workers at Springfields only to uranium). This would potentially reduce contrasts in dose between cases and their controls and lead to attenuation of dose–response. Relatively low plutonium doses and relatively high uranium doses at BNFL compared with AWE would explain why such attenuation would only affect the uranium excess OR/Gy. Also, the plutonium and uranium materials to which each cohort was predominantly exposed depended upon the facilities that the cohort covered. For example, exposures in the BNFL cohort were predominantly to soluble plutonium materials, whereas AWE workers were mainly exposed to insoluble plutonium materials. While the effect that different material types have on lung dose was taken into account in the dose assessment process, it is possible that some systematic bias in dose due to material type remained that would manifest itself on a cohort basis.6,25 Information on nonradiological carcinogenic coexposures such as organic solvents and cutting fluids, metals such as beryllium and lithium, and asbestos, was sparse and hence could not be included in risk models.
Differences in excess OR/Gy estimated for lung regions reflect heterogeneity of dose across the lung: risk estimates are highest for regions receiving lower doses and vice versa. It is unclear which region, if any, is the most appropriate for assessing risk as our analysis considered mortality and lacked information regarding tumor location, type, or histology. Relatively low deviances of models of excess OR/Gy for total alpha dose to the AI region suggest that dose estimation to that region may provide better predictions of lung cancer risk. However, given uncertainties in doses, this finding cannot necessarily be considered to provide etiologically meaningful information on risk.
A few other cohorts exist in which substantial numbers of workers have been exposed to uranium, plutonium, and other alpha-emitters, in the former USSR and North America. Although studies of uranium and plutonium workers in the United States have been conducted, apart from one study of uranium workers,37 individual dose reconstruction of the type performed here has not been conducted. Applying the present methodology to other cohorts would potentially provide more precise, comprehensive dose estimates on which to base multinational risk analyses. Results could then be compared with those derived from the different exposure situation of the MWC, which currently provides the most information on risk associated with internal exposures to plutonium.
CONCLUSIONS
This study is the first in which individual estimates of dose from multiple alpha-emitters have been used to estimate the risk of lung cancer mortality in large European cohorts of nuclear industry workers. Most subjects in the current study had low lung doses from uranium and/or plutonium. We found an increased risk of lung cancer associated with doses from these alpha-emitters, and although the risk appears greater for plutonium than for uranium, CIs are wide and overlapping. Our estimates of the excess OR per Sv for alpha dose are higher than—but compatible with—those reported in the FUMC, the MWC, and atomic bomb survivors. The results lend further support to existing accepted risk estimates associated with internal alpha-emitters and the radiation protection systems based on them, although uncertainties remain and these findings cannot be considered definitive.
ACKNOWLEDGMENTS
The authors thank Trident Medical Services, the Approved Dosimetry Service and Radiation Protection Group at AWE (for work on the AWE cohort); CEA-AREVA staff and the Groupe de Travail dosimetrists (France); Dallas Law, Gill Froud, Jennifer Clark, Helen Beddow, Phil Adsley, and Tom Horrocks (for work on the UKAEA cohort); the workforce and their representatives at former BNFL sites for permission to use their data; Keith Binks (formerly Westlakes) for his work on the BNFL cohort and Michael Gillies (PHE) for information on UK cohorts.
This report makes use of data obtained from the Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan. RERF is a private, non-profit foundation funded by the Japanese Ministry of Health, Labour and Welfare and the U.S. Department of Energy, the latter through the National Academy of Sciences. The conclusions in this report are those of the authors and do not necessarily reflect the scientific judgment of RERF or its funding agencies.
Supplementary Material
Footnotes
Supported by the European Commission under the 6th Framework Programme of the European Atomic Energy Community for nuclear research and training activities as a Specific Targeted Research Programme entitled “Alpha-risk: quantification of cancer and non-cancer risks associated with multiple chronic radiation exposures: Epidemiological studies, organ dose calculation and risk assessment” (Grant Number 516483). Work on the worker cohort at former BNFL sites was supported by the Nuclear Decommissioning Authority.
The authors report no conflicts of interest.
J.G. is currently affiliated with European Centre for Environment & Human Health, University of Exeter Medical School, Truro, United Kingdom. I.G.C. is currently affiliated with Institute for Work and Health (IST), University of Lausanne, Lausanne, Switzerland. M.T. is currently affiliated with Autorité de Sûreté Nucléaire, Paris, France. W.A., D.B., R.B., R.C., A.F., and A.E.R. are employed currently or were employed in the nuclear industry. C.H. is now retired.
Deceased.
This report makes use of data obtained from the Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan. RERF is a private, nonprofit foundation funded by the Japanese Ministry of Health, Labour and Welfare and the US Department of Energy, the latter through the National Academy of Sciences. The conclusions in this report are those of the authors and do not necessarily reflect the scientific judgment of RERF or its funding agencies.
Availability of data and code for replication: Due to the confidential nature of the human data used in the research that this article is based on, it is not possible to make these data available for replication. The scripts written to analyze the data would not themselves be informative without these data and are therefore also not supplied.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
REFERENCES
- 1.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. [DOI] [PubMed] [Google Scholar]
- 2.UNSCEAR. UNSCEAR 2006 Report: Volume 1. Annex A: Epidemiological Studies of Radiation and Cancer. 2006Vienna, Austria; [Google Scholar]
- 3.Cardis E, Gilbert ES, Carpenter L, et al. Effects of low doses and low dose rates of external ionizing radiation: cancer mortality among nuclear industry workers in three countries. Radiat Res. 1995;142:117–132. [PubMed] [Google Scholar]
- 4.Leuraud K, Richardson DB, Cardis E, et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol. 2015;2:e276–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson DB, Cardis E, Daniels RD, et al. Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ. 2015;351:h5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhivin S, Laurier D, Guseva Canu I.Health effects of occupational exposure to uranium: do physicochemical properties matter? Int J Radiat Biol. 2014;90:1104–1113. [DOI] [PubMed] [Google Scholar]
- 7.ICRP. 1990 Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. Ann ICRP. 1991;21:1–3. [PubMed] [Google Scholar]
- 8.IARC. A Review of Human Carcinogens. Part D: Radiation. Vol 2009100D Lyon, France: IARC Press; [Google Scholar]
- 9.ICRP. Lung cancer risk from radon and progeny and statement on radon - ICRP Publication 115. Ann ICRP. 2010;40:1-64. [DOI] [PubMed] [Google Scholar]
- 10.Kreuzer M, Schnelzer M, Tschense A, Walsh L, Grosche B.Cohort profile: The German uranium miners cohort study (WISMUT cohort), 1946–2003. Int J Epidemiol. 2010;39:980–987. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert ES, Sokolnikov ME, Preston DL, et al. Lung cancer risks from plutonium: an updated analysis of data from the Mayak worker cohort. Radiat Res. 2013;179:332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omar RZ, Barber JA, Smith PG.Cancer mortality and morbidity among plutonium workers at the Sellafield plant of British Nuclear Fuels. Br J Cancer. 1999;79:1288–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boice JD, Cohen SS, Mumma MT, et al. Updated mortality analysis of radiation workers at Rocketdyne (Atomics International), 1948–2008. Radiat Res. 2011;176:244–258. [DOI] [PubMed] [Google Scholar]
- 14.Brown SC, Schonbeck MF, McClure D, et al. Lung cancer and internal lung doses among plutonium workers at the Rocky Flats Plant: a case-control study. Am J Epidemiol. 2004;160:163–172. [DOI] [PubMed] [Google Scholar]
- 15.Richardson DB, Wing S.Lung cancer mortality among workers at a nuclear materials fabrication plant. Am J Ind Med. 2006;49:102–111. [DOI] [PubMed] [Google Scholar]
- 16.Wing S, Richardson D, Wolf S, Mihlan G.Plutonium-related work and cause-specific mortality at the United States Department of Energy Hanford Site. Am J Ind Med. 2004;45:153–164. [DOI] [PubMed] [Google Scholar]
- 17.Harrison JD, Muirhead CR.Quantitative comparisons of cancer induction in humans by internally deposited radionuclides and external radiation. Int J Radiat Biol. 2003;79:1–13. [PubMed] [Google Scholar]
- 18.Kreisheimer M, Koshurnikova NA, Nekolla E, et al. Lung cancer mortality among male nuclear workers of the Mayak facilities in the former Soviet Union. Radiat Res. 2000;154:3–11. [DOI] [PubMed] [Google Scholar]
- 19.Koshurnikova NA, Gilbert ES, Sokolnikov M, et al. Bone cancers in Mayak workers. Radiat Res. 2000;154:237–245. [DOI] [PubMed] [Google Scholar]
- 20.Hunter N, Kuznetsova IS, Labutina E V, Harrison JD.Solid cancer incidence other than lung, liver and bone in Mayak workers: 1948–2004. Brit J Canc. 2013;109:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sokolnikov ME, Gilbert ES, Preston DL, et al. Lung, liver and bone cancer mortality in Mayak workers. Int J Cancer. 2008;123:905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbert ES, Koshurnikova NA, Sokolnikov ME, et al. Lung cancer in Mayak workers. Radiat Res. 2004;162:505–516. [DOI] [PubMed] [Google Scholar]
- 23.Cardis E, Vrijheid M, Blettner M, et al. Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. BMJ. 2005;331:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardis E, Vrijheid M, Blettner M, et al. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: estimates of radiation-related cancer risks. Radiat Res. 2007;167:396–416. [DOI] [PubMed] [Google Scholar]
- 25.Bingham D, Bérard P, Birchall A, et al. Reconstruction of internal doses for the alpha-risk case–control study of lung cancer and leukaemia among European Nuclear Workers. Radiat Prot Dosim. 2017;174 (4):485–494. [DOI] [PubMed] [Google Scholar]
- 26.Thierry-Chef I, Marshall M, Fix JJ, et al. The 15-country collaborative study of cancer risk among radiation workers in the nuclear Industry: study of errors in dosimetry. Radiat Res. 2007;167:380–395. [DOI] [PubMed] [Google Scholar]
- 27.ICRP. Human respiratory tract model for radiological protection. ICRP Publication 66. Ann ICRP. 1994;24:482. [PubMed] [Google Scholar]
- 28.Rage E, Vacquier B, Blanchardon E, et al. Risk of lung cancer mortality in relation to lung doses among French uranium miners: follow-up 1956–1999. Radiat Res. 2012;177:288–297. [DOI] [PubMed] [Google Scholar]
- 29.OPCS. Classification of Occupations 1980. 1980London: HMSO; [Google Scholar]
- 30.Preston DL, Lubin JH, Pierce DAHiroSoft International Corporation, EPICURE (EpiWin) Version 1.81. 2008.
- 31.Puncher M, Birchall A, Bull RK.Uncertainties on lung doses from inhaled plutonium. Radiat Res. 2011;176:494–507. [DOI] [PubMed] [Google Scholar]
- 32.ICRP. Occupational intakes of radionuclides, part 1. ICRP Publication 130. Ann ICRP. 2015;44:1-188. [DOI] [PubMed] [Google Scholar]
- 33.Kreisheimer M, Sokolnikov ME, Koshurnikova NA, et al. Lung cancer mortality among nuclear workers of the Mayak facilities in the former Soviet Union. An updated analysis considering smoking as the main confounding factor. Radiat Env Biophys. 2003;42:129–135. [DOI] [PubMed] [Google Scholar]
- 34.Voelz GL, Lawrence JN, Johnson ER.Fifty years of plutonium exposure to the Manhattan Project plutonium workers: an update. Heal Phys. 1997;73:611–619. [DOI] [PubMed] [Google Scholar]
- 35.Wiggs LD, Johnson ER, Cox-DeVore CA, Voelz GL.Mortality through 1990 among white male workers at the Los Alamos National Laboratory: considering exposures to plutonium and external ionizing radiation. Heal Phys. 1994;67:577–588. [DOI] [PubMed] [Google Scholar]
- 36.Furukawa K, Preston DL, Lönn S, et al. Radiation and smoking effects on lung cancer incidence among atomic bomb survivors. Radiat Res. 2010;174:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silver SR, Bertke SJ, Hein MJ, et al. Mortality and ionising radiation exposures among workers employed at the Fernald Feed Materials Production Center (1951–1985). Occup Env Med. 2013;70:453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozasa K, Shimizu Y, Suyama A, et al. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;243:229–243. [DOI] [PubMed] [Google Scholar]



