Abstract
Background
Childhood asthma has become a critical public health problem because of its high morbidity and increasing prevalence. The impact of nutrition and other exposures during pregnancy on long-term health and development of children has been of increasing interest.
Objective
We performed a systematic review and meta-analysis of the association of folate and folic acid intake during pregnancy and risk of asthma and other allergic outcomes in children.
Design
We performed a systematic search of 8 electronic databases for articles that examined the association between prenatal folate or folic acid exposure and risk of asthma and other allergic outcomes (eg, allergy, eczema, and atopic dermatitis) in childhood. We performed a meta-analysis by using a random-effects model to derive a summary risk estimate of studies with similar exposure timing, exposure assessment, and outcomes.
Results
Our meta-analysis provided no evidence of an association between maternal folic acid supplement use (compared with no use) in the prepregnancy period through the first trimester and asthma in childhood (summary risk estimate: 1.01; 95% CI: 0.78, 1.30). Because of substantial heterogeneity in exposures and outcomes, it was not possible to generate summary measures for other folate indicators (eg, blood folate concentrations) and asthma or allergy-related outcomes; however, the preponderance of primary risk estimates was not elevated.
Conclusions
Our findings do not support an association between periconceptional folic acid supplementation and increased risk of asthma in children. However, because of the limited number and types of studies in the literature, additional research is needed.
INTRODUCTION
Asthma is a chronic condition of inflammation and airway constriction that results in clinical symptoms, including wheezing and shortness of breath. The diagnosis of asthma has been increasing, and in 2010, it has been estimated that asthma affected ~26 million persons in the United States, including 9.3% of children (7 million) <18 y of age (1). The diagnosis of asthma is based on the presence of signs and symptoms and tests of pulmonary function through spirometry (not recommend before 5 y of age) (2, 3). Therefore, a formal asthma diagnosis is generally not given to infants and toddlers (3). Children who have had wheezing and comorbid conditions such as eczema, atopic dermatitis, and allergic reactions are at higher risk to have a later diagnosis of asthma than those without these comorbidities; however, 60% of children with wheezing before age 3 y will no longer wheeze at age 6 y (4–7). It is unclear whether asthma is 1 disorder with many triggers or causes or a group of different disorders with similar clinical manifestations.
Causes of asthma are considered to be an interaction of both genetic and environmental risk factors. Risk factors for asthma include but are not limited to the following: family history, infections in infancy (eg, respiratory syncytial virus), prematurity, fetal growth pattern, exposure to pollution and irritants, prenatal and postnatal exposure to cigarette smoke, and exposure to allergens (8, 9). Both the immune system and lungs begin development in early pregnancy and continue well into childhood (5, 8, 9). This long window of development of critical systems allows for a multitude of exposures that could alter asthma risk.
Folate (both natural food folate and synthetic folic acid) is a 1-carbon source critical for the replication of DNA and RNA during the cell division and methylation of DNA, histones, and other proteins. Clinical folate deficiency is associated with weight loss, slow growth in children, and, in severe cases, megaloblastic anemia. In pregnancy, low folate intake (but above clinical deficiency) is associated with increased risk of neural tube defects (10), and these birth defects are prevented by folic acid supplementation in the periconceptional period (before neural tube closure at day 28 of pregnancy) (11). It is recommended that women capable of becoming pregnant consume 400 μg folic acid/d (12), and in the United States, enriched cereal grain products are fortified (~140 μg folic acid/d) (13). Questions have emerged about the role of in utero exposure to folate through maternal intake and the development of asthma and atopic disease. In transgenic mice, Hollingsworth et al (14) observed that, compared with a lower–methyldonor diet, a diet supplemented with methyl donors, including folic acid, choline, L-methionine, and betaine, produced progeny with airway hyperactivity, inflammatory response, changes in DNA methylation patterns, and reduced gene expression. Although animal models have provided valuable insight, the genomic regulation of inflammatory diseases differs substantially between humans and mice (15). In humans, wheezing in 4-y-olds has been associated with DNA hypomethylation at ALOX12 in their cord blood (16), but it is unknown if maternal folate intake or folic acid supplementation during pregnancy changes fetal DNA methylation (17) or risk of asthma. The objective of this study was to perform a systematic review and meta-analysis of the association between prenatal folate and folic acid exposure and risk of asthma and other allergic outcomes in childhood.
METHODS
Search strategy
The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (18). See “supplemental data – protocol” under “Supplemental data” in the online issue for our protocol. With the assistance of a research librarian, we searched PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Medline (http://www.ncbi.nlm.nih.gov/), Embase (http://www.embase.com), Cumulative Index to Nursing and Allied Health Literature (http://www.ebscohost.com/academic/cinahl-plus-with-full-text), Cochrane (http://www.thecochranelibrary.com/view/0/index.html), Web of Science (http://thomsonreuters.com/web-of-science/), Population Information Online (http://www.popline.org/), and Education Resources Information Center (http://eric.ed.gov/) for the period from the inception of the database through February 2013 by using the term folic acid and its variants (folate, folacin, and vitamin B-9) combined with terms specific to allergic and respiratory outcomes (eg, asthma, respiratory, wheeze, reactive airway, atopy, and allergy) and their associated Medical Subject Headings. To ensure maximum sensitivity, no language or other limits were set. See “supplemental data – search strategies” under “Supplemental data” in the online issue for full search strategies for each database. We scanned reference lists of all included studies as well as those of relevant systematic review articles returned in the search, and articles with potentially relevant titles were pulled for screening against inclusion criteria.
Inclusion criteria
To be included in the systematic review, studies had to meet the following criteria: 1): be a randomized controlled trial, cohort, case-control, or cross-sectional study; 2) report the exposure of natural food folate intake, folic acid intake from fortified foods, total folate intake from foods (eg, dietary folate equivalents), folic acid intake from supplements, or maternal or cord blood serum, plasma, or red blood cell folate concentrations; 3) have an exposure timing during the periconceptional period or during pregnancy; 4) provide results on at least one allergic or respiratory outcome; and 5) include an evaluation of the direct association between folic acid exposure and one of the outcomes of interest.
Two independent reviewers (KSC and AMC) reviewed titles and, when available, the abstracts of search results and identified potentially relevant articles (Figure 1). The same authors independently reviewed the full text of each potentially relevant article against inclusion criteria. There were no discrepancies between authors on articles meriting inclusion.
FIGURE 1.
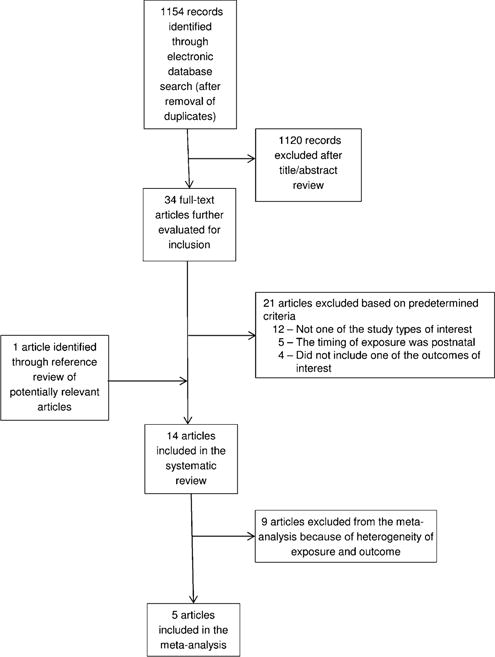
Study selection process flowchart.
Data extraction
One reviewer (AMC) extracted data into a piloted form, and the extracted data were independently cross-checked for accuracy with the original study by 2 additional reviewers (KSC and JM). The following elements were extracted: citation, study design (study type, study time period, study location, and study name), population (the number of study participants), exposure (type, measure, range, cutoffs, and methods of assessment), outcomes [type (eg, asthma, wheezing, allergy, eczema, and atopic dermatitis), how defined or measured, and timing], and results [the adjusted RR (aRR)5, adjusted OR (aOR), or adjusted prevalence ratio that reflected the greatest degree of control for confounders, CIs, and covariates adjusted for in the analysis].
Risk of bias and meta-analysis
We performed meta-analyses on articles with similar exposures and outcomes with Comprehensive Meta-Analysis Software (version 2; Biostat). Only the subset of studies of periconceptional folic acid exposure (month before and through the first trimester) and risk of asthma or wheezing was large enough to perform a meta-analysis of risk (criteria defined as ≥3 studies of sufficient quality). We measured the summary effect size for asthma alone and performed a sensitivity analysis with asthma combined with wheezing, which is sometimes used as a proxy for asthma. We combined the data from cohort studies and nested-case control studies by using ORs to approximate RRs. We used a random-effects model in our analyses. The risk estimate with the greatest control for confounders from each study was used in all analyses; see Supplemental Table 1 under “Supplemental data” in the online issue for a list of confounders adjusted for in each study. When risk estimates were provided for multiple exposure time points within the periconceptional period, exposure timing was prioritized in the following order for inclusion in the quantitative analysis (or meta-analysis), to prioritize exposure time periods closest to the DNA methylation programing in the early embryo: periconceptional, prepregnancy, and first trimester.
Statistical heterogeneity was tested with the Q statistic and evaluated by using the I2 statistic (19), which provides an estimate of the proportion of the variance that is not attributable to random error. Heterogeneity was considered to be substantial if I2 was greater than the conventional value of 50%. Publication bias was assessed by a visual inspection of funnel plots. The trim-and-fill analysis of Duval and Tweedie (20) was used to investigate the potential influence that unpublished studies could have on the summary estimates. P < 0.05 was considered statistically significant in all analyses.
Two authors (KSC and YPQ) independently graded each study included in the meta-analysis by using the Newcastle Ottawa Scale (NOS) as recommended by the Cochrane Non-Randomized Studies Methods Working Group (21). Studies assessed by using the NOS can receive a maximum score of 9. For our analyses, studies with a score ≥6 were considered to be of sufficient quality to include in the calculation of pooled risk estimates.
RESULTS
The database search produced 1154 articles after deduplication. As shown in Figure 1, after a title and abstract review, 34 articles were identified as being potentially relevant for inclusion and were reviewed in full. After a full-text review, 12 articles were excluded because they were not of appropriate study design (4 commentaries and editorials, 6 conference highlights, posters, and abstracts, 1 review, and 1 correspondence). Five articles were excluded because the assessment of folic acid was of a nonpregnant population. Four articles did not evaluate the association between folic acid exposure and one of the outcomes of interest (eg, asthma, wheeze, or atopy in childhood). Thirteen articles from the database search and one article identified through a review of reference lists of potentially relevant articles and relevant review articles met inclusion criteria for the systematic review (Figure 1). Ten of these studies were cohort studies, 3 studies were nested case-control studies, and 1 study was a case-control study. No randomized controlled trials of folate and folic acid in pregnancy and asthma, wheezing, or atopy in childhood were identified. All of the studies that met inclusion criteria were published in the English language. The studies reported results from 8 different countries.
See Supplemental Table 1 under “Supplemental data” in the online issue for details of the characteristics of each individual study. There was substantial heterogeneity in the type of folate exposure assessed [ie, folic acid supplements, dietary folate, total folate (supplement and diet combined), blood folate concentrations (maternal red blood cell, maternal plasma, and cord blood)], the timing of exposure assessment (eg, week or trimester of pregnancy), and the measurement method of folate used for analysis [ie, dichotomous (use compared with no use), categorical (quantiles of exposure), or continuous (per 100 or 1000 μg/d)]. In all studies, dietary folate intake was self-reported via food-frequency questionnaires and supplement use was assessed via questionnaires. Outcomes were generally assessed by self-report of parents in response to a questionnaire (Table 1). All studies included in the review that assessed asthma or wheezing did so on the basis of parental response to a questionnaire or a structured interview, with the outcome definition varying slightly in studies. Definitions for asthma included a parental report of physician-diagnosed asthma, parental report of asthma symptoms (at least one attack of wheezing or dyspnea or shortness of breath), child ever having taken inhalation medication, or a combination of these items. Studies defined wheezing as a parental report of wheezing, whistling, chest tightness, or dyspnea in a specified time period (generally the previous 12 mo) (Table 1). Of the 12 studies that examined the association between folic acid and asthma or wheeze, only 2 studies also looked at clinical test results in addition to a parental report. Both Bekkers et al (27) and Magdelijins et al (25) tested for allergic sensitization by measuring IgE in blood samples of children and tested their lung function by measuring the forced expiratory volume. The timing of the outcome assessment in children ranged from birth to 8 y of age.
TABLE 1.
Dose, timing of exposure and outcome, and outcome definitions of studies included in the meta-analysis
| Reference; study type; sample size; country; time period | Folic acid dose | Folic acid–exposure timing | Outcomes reported (timing of assessment) | Definition of outcomes |
|---|---|---|---|---|
| Kiefte-de Jong et al (22); cohort; 8742; Netherlands; 2002–2006 | ~400–500 μg/d | First trimester | Wheezing (up to age 4 y) | Parental report of child having “had problems with a wheezing chest during the last year” or “problems with tightness of the chest or shortness of breath during the past year” |
| Håberg et al (23); cohort; 32,077; Norway; 2000–2005 | No mean or median reported; authors mentioned 400 μg as a recommendation | First trimester | Wheezing (at 6–18 mo) | Maternal report of child having “chest congestion/tightness or whistling/wheezing in the chest between 6 and 18 months of age” |
| Whitrow et al (24); cohort; 490; Australia; 1998–2000 | No mean or median reported for prepregnancy; for pregnancy <16 wk, medians (ranges) were 700 μg/d (42.9–5500 μg/d) at 3.5 y and 666.6 μg/d (42.9–5500 μg/d) at 5.5 y of age | Prepregnancy | Asthma (3.5 y or 5.5 y) | Maternal report of physician-diagnosed asthma at 3.5 or 5.5 y |
| Magdelijns et al (25); nested case-control; 2834; Netherlands; 2000 | 400 μg | Periconceptional (−4 to 8 wk) | Asthma (at 6–7 y) | “Ever physician diagnosed asthma with clinical symptoms [having had at least 1 attack of wheeze or dyspnea in the past 12 months] and/or the use of asthma medication [everyday use during at least 2 months or use associated with physical activity of short-acting inhalation bronchodilators or the use of inhaled corticosteroids and medication use according to the Dutch guidelines of treatment of bronchial asthma in children] in the last 12 months” |
| Wheezing (until 6–7 y) | Maternal report of child having “suffer[ed] from wheezing in the past 12 months” or since last follow-up | |||
| Martinussen et al (26); nested case-control; 1499; United States; 2003–2007 | Mean ± SD: 497 ± 301 μg | First trimester | Asthma (at 6 y) | Positive maternal response to both of the following questions: “Has the child ever been diagnosed by a doctor or health professional as having asthma?” and “Has your child had wheezing or whistling in the chest in the past 12 months?” |
Studies were grouped by the timing of folate exposure [early (preconceptional, periconceptional, and first trimester) compared with late (second and third trimesters)] because widespread demethylation and subsequent remethylation occur in the early embryo, and exposures around this time could have different and more widespread effects than in later pregnancy (17). In addition, studies were further stratified by association with asthma or wheezing (Table 2) or other allergic outcomes (eg, atopy or eczema) (Table 3) and then by folate measure (supplementation, dietary, or blood folate) to allow for grouping of related measures.
TABLE 2.
Summary of studies of folate exposure and asthma or wheezing by pregnancy stage, outcome, and exposure1
| Exposure | Periconceptional or first trimester
|
Second and third trimesters
|
Any/throughout pregnancy
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome (timing) | Change in risk | Ref | Outcome (timing) | Change in risk | Ref | Outcome (timing) | Change in risk | Ref | |
| Folic acid–containing supplement use (yes or no) | |||||||||
| W (6–18 mo) | ▲ | 24 | W (6–18 mo) | ▬ | 24 | W (6–18 mo) | ▲ | 24 | |
| PC: A (3.5 y, 5.5 y) | ▬ ▬ | 25 | A (3, 4, 5, 6, 7, 8 y) | ▬ ▬ ▬ ▬ ▬ ▬ | 22 | A, W (6–7 y) | ▬ ▬ | 232 | |
| A, W (6–7 y) | ▬ ▬ | 232 | W (1, 2, 3, 4, 5, 6, 7, 8 y) | ▲ ▬ ▬ ▬ ▬ ▬ ▬ ▬ | 22 | ||||
| Peri, 10 wk: W (to 4 y) (to 4 y) | ▬ ▬ | 26 | |||||||
| PC, 1, 2, 3 mo: A (6 y) | ▬ ▬ ▬ ▬ | 272 | |||||||
| Folic acid intake (other than supplement (yes or no) | |||||||||
| W (2 y) | ▬ | 28 | A (3.5 y, 5.5 y) | ▲ ▬ | 25 | ||||
| A (3.5 y, 5.5 y) | ▬ ▬ | 25 | W (1 y) | ▬ | 31 | ||||
| A (6 y), W (1 y) | ▼▼ | 29 | |||||||
| PC, 1, 2, 3 mo: A (6 y) | ▬ ▬ ▬ ▬ | 272 | |||||||
| Dietary folate intake | |||||||||
| A (3.5 y, 5.5 y) | ▬ ▬ | 25 | A (3.5 y, 5.5 y) | ▬ ▬ | 25 | W (16–24 mo) | ▬ | 35 | |
| A, W (5 y) | ▬ ▬ | 32 | |||||||
| Blood folate concentration | |||||||||
| PF Qr4:Qr1: W (to 4 y) | ▬ | 26 | PF Qn5:Qn1: A (3 y) | ▲ | 332 | ||||
| CBF T3:T2, T1:T2: W (1 y) | ▬ ▬ | 31 | |||||||
| RBC Qn5:Qn1: A, W (6–7 y) | ▬ ▬ | 232 | |||||||
Studies that reported multiple associations within a time period for different subsets of the time period (eg, periconceptional and at 10 wk of gestation) or variations of the same outcomes (eg, asthma and wheeze at 1 y or asthma and wheeze at 5 y) have multiple findings denoted to avoid bias of only reporting positive findings. However, if there are findings that group individual outcomes (eg, asthma at 3.5, 5.5, and both 3.5 and 5.5 y), only individual findings are shown. We included only findings that fell within the denoted exposure time frames. See Supplemental Table 1 under “Supplemental data” in the online issue for all reported findings. Symbols are defined as follows: ▲, significant increased risk; ▬, no association; ▼, significant decreased risk. A, asthma; CBF, cord blood folate; PC, preconception; Peri, periconceptional; PF, plasma folate; Qn, quintile; Qr, quartile; RBC, red blood cell folate; Ref, reference; T, tertile; W, wheeze.
Nested case-control studies.
TABLE 3.
Summary of studies of folate exposure and allergic outcomes by pregnancy stage, outcome, and exposure1
| Exposure | Periconceptional or first trimester
|
Second and third trimesters
|
Any/throughout pregnancy
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome (timing) | Change in risk | Ref | Outcome (timing) | Change in risk | Ref | Outcome (timing) | Change in risk | Ref | |
| Folic acid–containing supplement use (yes or no) | |||||||||
| LRTIs, Hosp (18 mo) | ▲▲ | 24 | 18 wk, 32 wk: AT (7–8 y) | ▬ ▬ | 34 | LRTIs, Hosp (18 mo) | ▬ ▬ | 24 | |
| AD; Specific IgE; Tot IgE (2 y) | ▬ ▬ ▬ | 232 | LRTIs, Hosp (18 mo) | ▬ ▬ | 24 | AD, Specific IgE, Tot IgE (2 y) | ▬ ▬ ▬ | 232 | |
| E, FEV, FVC (6–7 y) | ▬ ▬ ▬ | 232 | URTIs (1–8 y) | ▬ ▬ ▬ ▬ ▬ ▬ ▬ ▬ | 22 | E, FEV, FVC (6–7 y) | ▬ ▬ ▬ | 232 | |
| PC, 10 wk: AD (to 4 y) | ▬ ▬ | 26 | LRTIs (1–8 y) | ▬ ▬ ▬ ▬ ▬ ▬ ▬ ▬ | 22 | Peanut allergy | ▬ | 303 | |
| PC, 10 wk: SB (to 4 y) | ▬ ▬ | 26 | |||||||
| Peanut allergy | ▬ | 303 | Freq. RTI (1–8 y) | ▬ ▬ ▬ ▬ ▬ ▬ ▬ ▬ | 22 | ||||
| Eczema (1–8 y) | ▬ ▬ ▬ ▬ ▬ ▬ ▬ ▬ | 22 | |||||||
| S, BHR (8 y) | ▬ ▬ | 22 | |||||||
| Folic acid intake other than supplement (yes or no) | |||||||||
| E (2 y) | ▬ | 28 | Allergy, S, E (1 y) | ▬ ▬ ▬ | 31 | ||||
| Food rxn, IgE, S (1 y) | ▬ ▬ ▬ | 31 | |||||||
| Dietary folate intake | |||||||||
| PF Qr4:Qr1: AD, SB (to 4y) | ▲ ▬ | 26 | Allergic rhinitis, E (5 y) | ▬ ▬ | 32 | E (16–24 mo) | ▬ | 35 | |
| AT (7–8 y) | ▬ | 34 | |||||||
| RBC Qn5:Qn1: AD, AS, Tot IgE (2 y) | ▬ ▬ ▬ | 232 | |||||||
| RBC: Qn5:Qn1: E, FEV, FVC (6–7 y) | ▬ ▬ ▬ | 232 | |||||||
| CBF T3:T.2: Allergy, S, E (1 y) CBF |
▬ ▲ ▬ | 31 | |||||||
| CBF T1:T.2: Allergy, S, E (1 y) | ▬ ▲ ▬ | 31 | |||||||
| CBF T3:T.2- Food rxn, IgE, S (1 y) | ▬ ▬ ▬ | 31 | |||||||
| CBF T1:T.2: Food rxn, IgE, S (1 y) | ▬ ▬ ▬ | 31 | |||||||
Studies that reported multiple associations periconceptional and at 10 wk of gestation) or variations of the same outcomes (eg, asthma and wheeze at 1 y or asthma and wheeze at 5 positive findings. However, if there were findings that group individual outcomes (eg, asthma at 3.5, 5.5, and both 3.5 and 5.5 y), only individual denoted exposure time frames. See Supplemental Table 1 under “Supplemental data” in the online issue for all reported findings. Symbols are defined as follows ▲, significant increased risk; ▬, no association. AD, atopic dermatitis; AS, allergic sensitization; AT, atopy; BHR, bronchial hyper-responsiveness; CBF, cord blood folate; E, eczema; FEV, forced expiratory volume in 1 s; FVC, forced vital capacity; Hosp, hospitalized for lower respiratory infections; LRTI, lower respiratory tract infection; PC, preconception; PF, plasma folate; Qn, quintile; Qr, quartile; RBC, red blood cell folate; Ref, reference; RTI, respiratory tract infection; rxn, reaction; S, sensitization; SB, shortness of breath; T, tertile; Tot, total; URTI, upper respiratory tract infection.
Nested case-control study.
Case-control study.
Outcomes: asthma or wheezing in childhood
Exposure: periconceptional or first-trimester use of a supplement containing folic acid (yes or no)
Three cohort studies (22–24) and 2 nested case-control studies (25, 26) met the inclusion criteria and examined the effect of maternal folic acid supplement use compared with no use (yes or no) in the periconceptional period or first trimester only on risk of asthma or wheezing in childhood (Table 2; see Supplemental Table 1 under “Supplemental data” in the online issue).
Our meta-analysis of studies (24–26) that analyzed folic acid supplement use as a dichotomous variable and reported its association with asthma in childhood showed a pooled risk of RR of 1.01 (95% CI: 0.78, 1.30; P = 0.95; I2 = 0.00, P = 0.73) (Figure 2). However, the combination of reports of wheezing in infants and toddlers (22, 23) with reports of asthma (23, 25, 27) resulted in a significantly elevated summary risk estimate (RR: 1.05; 95% CI: 1.02, 1.09; P < 0.01; I2 = 0.00, P = 0.68) (Figure 3). The study by Håberg et al (23) assessed children at a younger age than did other studies, and its large study population size contributed more heavily to the data (Figure 3). The removal of Håberg et al (23) from the analysis reduced the summary risk estimate to a nonsignificant level (RR: 1.04; 95% CI: 0.92, 1.17). A visual inspection of the funnel plot (data not shown) revealed no evidence of a publication bias in the literature on folic acid and asthma, although the small number of studies was a limitation. When we combined asthma and wheezing in a single meta-analysis, the funnel plot suggested some asymmetry (see Supplemental Figure 1 under “Supplemental data” in the online issue). According to the trim-and-fill analysis, 1 small study to the right of the mean might have been missing. However, the inclusion of this potential study in the meta-analysis did not change the summary effect (see Supplemental Figure 1 under “Supplemental data” in the online issue). All studies received a score of ≥6 (of a possible score of 9) on the NOS in risk of bias assessment (see Supplemental Table 2 under “Supplemental data” in the online issue), and thus, sensitivity analyses were not conducted. This meta-analysis suggested that the collective literature to date does not support an association between periconceptional and first-trimester folic acid supplementation and risk of asthma in early childhood.
FIGURE 2.
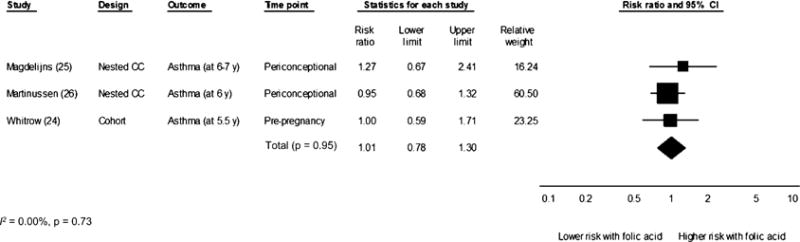
Random-effects meta-analysis of the association between maternal folic acid supplement use (compared with no use) in the periconceptional period through the first trimester of pregnancy and asthma in childhood. Black squares represent point estimates, and the size of each square is proportional to the weight of the study in the analysis. Horizontal lines represent CIs. The black diamond represents the summary effect. CC, case-control.
FIGURE 3.
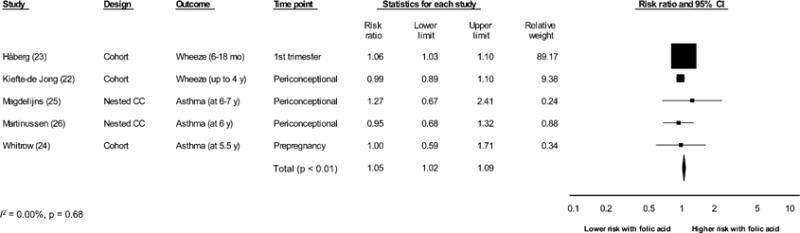
Random-effects meta-analysis of the association between maternal folic acid supplement use (compared with no use) in the periconceptional period through the first trimester of pregnancy and asthma or wheezing in childhood. Black squares represent point estimates, and the size of each square is proportional to the weight of the study in the analysis. Horizontal lines represent CIs. The black diamond represents the summary effect. CC, case-control.
Exposures: periconceptional and first-trimester folic acid intake other than supplement (yes or no), dietary folate intake, and blood folate concentrations
Five studies (22, 24, 26, 28, 29) examined the association of folate (folic acid use not dichotomized as yes or no, dietary folate, or blood folate measurements) in the first trimester with childhood asthma or wheezing (12 comparisons); no significantly increased risks were reported.
Outcomes: other allergic (eg, atopy, eczema, and atopic dermatitis)
Exposure: periconceptional and first-trimester use of a supplement containing folic acid (yes or no)
Many other outcomes are associated with asthma risk, such as atopy, eczema, dermatitis and respiratory tract infections. Two cohort studies (22, 23), a nested case-control study (25), and a case-control study (34) examined exposure to maternal folic acid supplement use compared with no use (yes or no) in the periconceptional period or first trimester only and risk of other allergic outcomes (Table 3). Of the 13 reported associations across 4 studies, 2 associations in one study reported increased risk in 2 outcomes. Håberg et al (23) reported that folic acid supplement use (compared with no use) in the first trimester of pregnancy was associated with increased risks for lower respiratory tract infections as reported by mothers (aRR: 1.09; 95% CI: 1.02, 1.15) and hospitalizations because of lower respiratory tract infections (aRR: 1.24; 95% CI: 1.09, 1.41) at 0–18 mo of age.
Exposures: periconceptional and first-trimester folic acid intake other than supplement (yes or no), dietary folate intake, and blood folate concentrations
Two cohort studies (22, 28) examined the association of folate (folic acid use not dichotomized as yes or no, dietary folate, or blood folate measurements) periconceptionally or in the first trimester with other allergic outcomes (Table 3; see Supplemental Table 1 under “Supplemental data” in the online issue). Of the 3 reported associations across 2 studies, only 1 association in 1 study reported increased risk. Kiefte de-Jong et al (22) showed first-trimester maternal plasma folate concentrations (highest compared with lowest quartiles) were associated with increased risk of atopic dermatitis (≤4 y of age) (aOR: 1.18; 95% CI: 1.05, 1.33).
Outcomes: asthma or wheezing in childhood
Exposures: second- and third-trimester use of a supplement containing folic acid (yes or no)
Two cohort studies (23, 27) examined the association between prenatal use of a supplement containing folic acid (compared with no use) in the second or third trimester and asthma or wheezing in childhood (Table 2; see Supplemental Table 1 under “Supplemental data” in the online issue). Of the 15 associations across 2 studies, only one association was significantly elevated. Bekkers et al (27) showed that maternal use of folic acid in the third trimester was associated with increased risk of maternal report of wheezing at 1 y (adjusted prevalence ratio: 1.20; 95% CI: 1.04, 1.39) but not at any time after 1 y of age.
Exposures: second- and third-trimester folic acid intake other than supplement (yes or no), dietary folate intake, and blood folate concentrations
Three cohort studies (24, 30, 31) and 2 nested case-control studies (25, 32) examined the association between folate (folic acid use not dichotomized as yes or no, dietary folate, or blood folate measurements) after the first trimester and childhood asthma or wheezing (Table 2). Across the 5 studies and 12 reported associations, 3 associations were significant. Håberg et al (32) reported maternal plasma folate concentrations (highest compared with lowest quintiles) in the second trimester were associated with increased risk of asthma at age 3 y (aOR: 1.66; 95% CI: 1.16, 2.37). Whitrow et al (24) showed high-dose folic acid supplement use (1000 μg/d) plus dietary folate intake at 30–34 wk of gestation was associated with both asthma at 3.5 y (aRR: 1.26; 95% CI: 1.09, 1.47) and persistent asthma (defined as asthma at both 3.5 and 5.5 y; aRR: 1.32; 95% CI: 1.03, 1.69) (data not reported in Table 2). No significant associations were shown for asthma at 5.5 y (aRR: 1.16; 95% CI: 0.94, 1.43).
Outcomes: other allergic (eg, atopy, eczema, and atopic dermatitis)
Exposures: second- and third-trimester use of a supplement containing folic acid (yes or no)
Three cohort studies (23, 27, 35) examined the use of supplements containing folic acid and risk of other allergic outcomes (eg, atopy, eczema, and atopic dermatitis; Table 3). No significant findings were reported across the 38 reported associations.
Exposures: second- and third-trimester folic acid intake other than supplement (yes or no), dietary folate intake, and blood folate concentrations
Three cohort studies (30, 31, 35) and 1 nested case-control study (25) examined the association of folate (folic acid use not dichotomized as yes or no, dietary folate, or blood folate measurements) after the first trimester with other allergic outcomes (Table 3). Two of 27 reported associations across the 4 studies showed a significant increased risk. Dunstan et al (30) showed no associations between cord blood folate and any outcome until the values were categorized in tertiles (eg, for logistic regression models). A U-shaped association was then shown for cord blood folate and increased risk of sensitization in the third compared with second tertiles (aOR: 3.3; 95% CI: 1.3, 8.0) and for the first compared with second tertiles (aOR: 2.7; 95% CI: 1.1, 7.0).
Any time or throughout pregnancy
Studies that examined folate at any time in pregnancy (eg, not stratified by the trimester of use) or throughout pregnancy are detailed in Tables 2 and 3 (see Supplemental Table 1 under “Supplemental data” in the online issue for additional details). Three of the studies showed no association (25, 33, 34) between folic acid and any of the outcomes assessed, whereas one study (24) showed slight increased risk between folic acid supplement use (compared with no use) and wheezing at 6–18 mo of age (aRR: 1.07; 95% CI: 1.02, 1.12) (24).
DISCUSSION
Our systematic review did not support a causal link between the use of folic acid supplements during pregnancy and increased risk of asthma in children. Our meta-analysis of folic acid supplement use in the periconceptional period through the first trimester and asthma in childhood showed no increased risk (summary risk estimate: 1.01; 95% CI: 0.78, 1.30), although this finding was limited by the small number of studies. Because of the substantial heterogeneity in exposures and outcomes, it was not possible to generate summary statistics for other folate measures and asthma or allergy-related outcomes; however, the preponderance of risk estimates was not elevated (Tables 2 and 3).
Limitations of current literature
One of the significant limitations of the current literature is the lack of randomized clinical trials because of ethical issues that would be involved in conducting such a study. Randomization of exposure is critical for the assessment of the effect of exposure to multivitamin supplements and folic acid supplements because the women who use folic acid supplements have different health behaviors and characteristics than do women who do not take supplements. Håberg et al (23) observed that supplement users were more likely to have atopic conditions, more education, lower smoking rates, and other health-behavior differences. Wald and Morris (36) published a reply to the study of Håberg et al (23) and argued that the weak association shown in the study was unlikely to be causal and more likely a result of confounding because women who are more health conscious are both more likely to take folic acid supplements and to report respiratory problems in their children. Other studies also showed that maternal folic acid supplement use was associated with maternal self-report of asthma or allergy (22, 26, 35). Several studies reported that children with asthma, wheezing, or atopy were significantly more likely to have allergic mothers (29, 30, 32, 33), and 2 studies reported oversampling of allergic mothers by study design (26, 30). In addition, Kiefte-de Jong et al (22) reported that mothers who used supplements were significantly different from mothers who did not use supplements for 12 of 16 maternal and child characteristics including the following: birth weight, parental history of atopy, age, ethnicity, education, and smoking and alcohol use during pregnancy. This degree of difference was unlikely to be amenable to statistical correction and likely indicative of residual confounding because of another unmeasured factor.
Of all of the studies in our review that examined folic acid supplementation, dietary folate intake, or blood folate concentrations in the periconceptional period or first trimester and risk of asthma or wheezing at any age, the only study with a significant positive association was the marginally elevated finding by Håberg et al (23) (aRR: 1.06; CI: 1.03, 1.10). Results from the other studies in our review did not confirm this finding (22, 24–26, 28, 29); however, none of these studies were of a size that would allow them to detect an estimate so modestly elevated.
Limitations of exposure assessment and definition
The determination of how much natural food folate and its synthetic form, folic acid (used in multivitamins, prenatal supplements, and food fortification) was consumed is difficult. In addition, concerns about recall and self-report can lead to a misclassification of exposure. It would be ideal to use a biomarker such as red blood cell or serum folate in mothers or cord blood folate to determine folate status. However, not only does this require sampling at the critical time of exposure, which precludes its use in retrospective studies and makes very large cohort studies difficult, it can also be very costly; hence, there were a limited number of studies that used this methodology. In addition, there were a number of different folate exposure measures reported (Tables 2 and 3), and it clearly is not possible to combine and interpret different exposures measured on different scales to generate a single summary estimate.
Limitations of outcome assessment and definition
There is no single diagnostic biomarker of asthma. Symptoms of asthma, such as wheezing, are common in infants and young children and, although correlated with increased risk of asthma later in life, are often transient (4–7). The single elevated increased risk association by Håberg et al (23) was limited to the maternal report of wheezing at the young ages of 6–18 mo; a substantial portion of these infants and children are unlikely to be diagnosed with asthma. This was evident in the studies reviewed in this article, in which an elevated risk of asthma or wheezing was reported at some ages but not others (24, 27). In the meta-analysis we performed in which reports of wheezing with those of asthma were combined, we showed a moderately elevated risk estimate (RR: 1.05; 95% CI: 1.02, 1.09). However, this result was driven by the very large cohort assessed in Håberg et al (23) (89% of the summary RR weight), and the removal of it from the analysis resulted in a nonsignificant finding (RR: 1.04; 95% CI: 0.92, 1.17) (Figure 3). Because of known limitations of the association of wheezing in infancy and early toddlerhood with later asthma, the meta-analysis that was limited to asthma in children >5 y of age should be considered the more-robust estimate of the association of periconceptional folic acid use and asthma risk in childhood.
Limitations of current review
Cohort studies included in the review reported a variety of exposures (eg, folate, folic acid, serum folate, red blood cell folate, and cord blood folate), exposure time periods (eg, early compared with late pregnancy), and outcomes (eg, wheezing at 6 mo to 6 y of age, asthma diagnosis at a variety of ages, allergies, IgE concentrations, and atopy), which rendered the question of the association between folic acid exposure in pregnancy and risk of childhood asthma difficult to answer. When possible, we attempted to group studies (first-trimester supplement use and asthma or wheezing at ages 1–8 y) but fully acknowledge the limitations of the summary estimate. To date, the studies could not rule out that higher dosages consumed in the third trimester are associated with increased risk in some groups. Note that Dunstan et al (30) examined cord blood samples from an Australian cohort (n = 484) and reported a U-shaped association with increased risk of sensitization for lower and higher tertiles compared with the middle tertile, and no associations were shown when the data were not analyzed in this manner. Observed increased risk of both higher and lower cord blood could be indicative of an optimal blood folate concentration, although it was impossible to determine whether the association was because of other characteristics of these groups or a product of multiple testing in a single study.
Research agenda
To fully address the question of a possible association of folic acid supplement use in pregnancy and increased risk of asthma in children suggested by the Hollingsworth et al (14) animal model, a number of additional studies are warranted. There are at least 3 main questions that remain to be answered:
Are there epigenetic changes in utero that are associated with risk of asthma?
Are these epigenetic changes associated with folate or folic acid supplementation at specific critical developmental windows during pregnancy?
Is there a dose effect of folate or folic acid (ie, a minimum, maximum, and optimal intake) on risk of asthma, wheezing, or allergy-related outcome?
The undertaking of a randomized control trial of the effects of folic acid supplementation in pregnancy on allergic outcomes in children would be ideal, although clearly ethically problematic. Women would have to be exposed to folic acid supplementation in the periconceptional period because of the known benefits for the prevention of neural tube defects. An alternative way to address the research questions proposed in this review is to use previous trials and follow up the children whose mothers participated in those randomized controlled trials in which exposure was randomly assigned. However, the original trials were not designed to address many of the questions surrounding the timing of supplement use during gestation and may not easily address those hypotheses.
To circumvent the need for a new randomized controlled tiral, existing cohorts and Mendelian random assignment could be used to look for interactions and effects with genetic variants involved in one-carbon metabolism and folic acid intake. Some studies have looked for associations of risk with 5,10-methylenetetrahydrofolate reductase (MTHFR) (35, 37), but none to date have looked for the interaction of intake and MTHFR. It is known that individuals with the MTHFR677 CC genotype have higher blood folate concentrations for a given folic acid intake (17, 38). Because genotype is assigned at birth, it can act as a surrogate of randomization. These types of studies require substantial sample sizes to achieve a reasonable statistical power. In addition, if the causal pathway is independent of the downstream metabolites of folic acid or dietary folate, this approach would be ineffective.
In conclusion, the analysis of the published literature to date is difficult to summarize because of heterogeneity in folate- and folic acid–exposure windows during pregnancy, a variety of asthma and allergy-associated outcomes, and the limited numbers of studies. Our meta-analysis does not support the association of folic acid supplement use in the first trimester with risk of asthma in childhood.
Supplementary Material
Footnotes
Findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Supported by the CDC and in part by an appointment to the Research Participation Program at the CDC administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the CDC.
Abbreviations used: aOR, adjusted OR; aRR, adjusted RR; NOS, Newcastle Ottawa Scale
The authors’ responsibilities were as follows—KSC, AMC, NFD, and RJB: designed the research; KSC, AMC, YPQ, and JM: conducted the research; KSC and AMC: analyzed data and wrote the manuscript; and KSC: had primary responsibility for the final content of the manuscript. None of the authors had a conflict of interest.
References
- 1.Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, Liu X. National surveillance of asthma: United States, 2001–2010. National Center for Health Statistics Vital Health Statistics. 2012;3(35) [PubMed] [Google Scholar]
- 2.Koterba AP, Saltoun CA. Chapter 9: asthma classification. Allergy Asthma Proc. 2012;33(suppl 1):S28–31. doi: 10.2500/aap.2012.33.3539. [DOI] [PubMed] [Google Scholar]
- 3.National Heart Lung and Blood Institute, National Asthma Education and Prevention Program. Expert panel report 3: guidelines for the diagnosis and management of asthma: full report 2007. Washington, DC: US Department of Health and Human Services; 2007. [Google Scholar]
- 4.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ, The Group Health Medical Associates Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 5.Hedlin G, Konradsen J, Bush A. An update on paediatric asthma. Eur Respir Rev. 2012;21:175–85. doi: 10.1183/09059180.00003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robison RG, Singh AM. Chapter 11: the infant and toddler with wheezing. Allergy Asthma Proc. 2012;33(suppl 1):S36–8. doi: 10.2500/aap.2012.33.3543. [DOI] [PubMed] [Google Scholar]
- 7.Guilbert TW, Morgan WJ, Zeiger RS, Bacharier LB, Boehmer SJ, Krawiec M, Larsen G, Lemanske RF, Liu A, Mauger DT, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004;114:1282–7. doi: 10.1016/j.jaci.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Turner S, Prabhu N, Danielan P, McNeill G, Craig L, Allan K, Cutts R, Helms P, Seaton A, Devereux G. First- and second-trimester fetal size and asthma outcomes at age 10 years. Am J Respir Crit Care Med. 2011;184:407–13. doi: 10.1164/rccm.201012-2075OC. [DOI] [PubMed] [Google Scholar]
- 9.Henderson AJ, Warner JO. Fetal origins of asthma. Semin Fetal Neonatal Med. 2012;17:82–91. doi: 10.1016/j.siny.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Daly LE, Kirke PN, Molloy A, Weir DG, Scott JM. Folate levels and neural tube defects. Implications for prevention. JAMA. 1995;274:1698–702. doi: 10.1001/jama.1995.03530210052030. [DOI] [PubMed] [Google Scholar]
- 11.Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, Mulinare J, Zhao P, Wong LY, Gindler J, et al. Prevention of neural-tube defects with folic acid in China. China-US Collaborative Project for Neural Tube Defect Prevention. N Engl J Med. 1999;341:1485–90. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- 12.Food and Nutrtion Board, Institute of Medicine. Dietary references intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 13.Yeung L, Yang Q, Berry RJ. Contributions of total daily intake of folic acid to serum folate concentrations. JAMA. 2008;300:2486–7. doi: 10.1001/jama.2008.742. [DOI] [PubMed] [Google Scholar]
- 14.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, Bailey N, Potts EN, Whitehead G, Brass DM, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118:3462–9. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110:3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morales E, Bustamante M, Vilahur N, Escaramis G, Montfort M, de Cid R, Garcia-Esteban R, Torrent M, Estivill X, Grimalt JO, et al. DNA hypomethylation at ALOX12 is associated with persistent wheezing in childhood. Am J Respir Crit Care Med Am J Respir Crit Care Med. 2012;185:937–43. doi: 10.1164/rccm.201105-0870OC. [DOI] [PubMed] [Google Scholar]
- 17.Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr. 2012;3:21–38. doi: 10.3945/an.111.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 21.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (cited 7 January 2013)
- 22.Kiefte-de Jong JC, Timmermans S, Jaddoe VW, Hofman A, Tiemeier H, Steegers EA, de Jongste JC, Moll HA. High circulating folate and vitamin B-12 concentrations in women during pregnancy are associated with increased prevalence of atopic dermatitis in their offspring. J Nutr. 2012;142:731–8. doi: 10.3945/jn.111.154948. [DOI] [PubMed] [Google Scholar]
- 23.Håberg SE, London SJ, Stigum H, Nafstad P, Nystad W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child. 2009;94:180–4. doi: 10.1136/adc.2008.142448. (Published erratum appears in Arch Dis Child 2009;94:485.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitrow MJ, Moore VM, Rumbold AR, Davies MJ. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol. 2009;170:1486–93. doi: 10.1093/aje/kwp315. [DOI] [PubMed] [Google Scholar]
- 25.Magdelijns FJH, Mommers M, Penders J, Smits L, Thijs C. Folic acid use in pregnancy and the development of atopy, asthma, and lung function in childhood. Pediatrics. 2011;128:e135–44. doi: 10.1542/peds.2010-1690. [DOI] [PubMed] [Google Scholar]
- 26.Martinussen MP, Risnes KR, Jacobsen GW, Bracken MB. Folic acid supplementation in early pregnancy and asthma in children aged 6 years. Am J Obstet Gynecol. 2012;206:72 e1–7. doi: 10.1016/j.ajog.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bekkers MBM, Elstgeest LEM, Soholtens S, Haveman-Nies A, de Jongste JC, Kerkhof M, Koppelman GH, Gehring U, Smit HA, Wijga AH. Maternal use of folic acid supplements during pregnancy, and childhood respiratory health and atopy. Eur Respir J. 2012;39:1468–74. doi: 10.1183/09031936.00094511. [DOI] [PubMed] [Google Scholar]
- 28.Litonjua AA, Rifas-Shiman SL, Ly NP, Tantisira KG, Rich-Edwards JW, Camargo CA, Jr, Weiss ST, Gillman MW, Gold DR. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am J Clin Nutr. 2006;84:903–11. doi: 10.1093/ajcn/84.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Triche EW, Lundsberg LS, Wickner PG, Belanger K, Leaderer BP, Bracken MB. Association of maternal anemia with increased wheeze and asthma in children. Ann Allergy Asthma Immunol. 2011;106:131–9. doi: 10.1016/j.anai.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunstan JA, West C, McCarthy S, Metcalfe J, Meldrum S, Oddy WH, Tulic MK, D’Vaz N, Prescott SL. The relationship between maternal folate status in pregnancy, cord blood folate levels, and allergic outcomes in early childhood. Allergy. 2012;67:50–7. doi: 10.1111/j.1398-9995.2011.02714.x. [DOI] [PubMed] [Google Scholar]
- 31.Nwaru BI, Erkkola M, Ahonen S, Kaila M, Kronberg-Kippila C, Ilonen J, Simell O, Knip M, Veijola R, Virtanen SM. Intake of antioxidants during pregnancy and the risk of allergies and asthma in the offspring. Eur J Clin Nutr. 2011;65:937–43. doi: 10.1038/ejcn.2011.67. [DOI] [PubMed] [Google Scholar]
- 32.Håberg SE, London SJ, Nafstad P, Nilsen RM, Ueland PM, Vollset SE, Nystad W. Maternal folate levels in pregnancy and asthma in children at age 3 years. J Allergy Clin Immunol. 2011;127:262–4. 264.e1. doi: 10.1016/j.jaci.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyake Y, Sasaki S, Tanaka K, Hirota Y. Maternal B vitamin intake during pregnancy and wheeze and eczema in Japanese infants aged 16–24 months: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2011;22:69–74. doi: 10.1111/j.1399-3038.2010.01081.x. [DOI] [PubMed] [Google Scholar]
- 34.Binkley KE, Leaver C, Ray JG. Antenatal risk factors for peanut allergy in children. Allergy Asthma Clin Immunol. 2011;7:17. doi: 10.1186/1710-1492-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Granell R, Heron J, Lewis S, Davey Smith G, Sterne JA, Henderson J. The association between mother and child MTHFR C677T polymorphisms, dietary folate intake and childhood atopy in a population-based, longitudinal birth cohort. Clin Exp Allergy. 2008;38:320–8. doi: 10.1111/j.1365-2222.2007.02902.x. (Published erratum appears in Clin Exp Allergy 2008;38:699.) [DOI] [PubMed] [Google Scholar]
- 36.Wald N, Morris J. Folic acid supplementation and childhood respiratory health? Available from: http://www.wolfson.qmul.ac.uk/epm/tlkscmmnts/docs/Haberg%20comments.26.0.pdf (cited 14 October 2012)
- 37.Thuesen BH, Husemoen LL, Ovesen L, Jorgensen T, Fenger M, Gilderson G, Linneberg A. Atopy, asthma, and lung function in relation to folate and vitamin B-12 in adults. Allergy. 2010;65:1446–54. doi: 10.1111/j.1398-9995.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 38.Crider KS, Zhu JH, Hao L, Yang QH, Yang TP, Gindler J, Maneval DR, Quinlivan EP, Li Z, Bailey LB, et al. MTHFR 677C->T genotype is associated with folate and homocysteine concentrations in a large, population-based, double-blind trial of folic acid supplementation. Am J Clin Nutr. 2011;93:1365–72. doi: 10.3945/ajcn.110.004671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



