Abstract
Concept
Fatigue is a major concern for patients with multiple sclerosis (MS). A clear definition of MS-related fatigue is a prerequisite for appropriate instruments for fatigue assessment. In turn, accurate assessment of fatigue in MS will enhance exploration of plausible mechanisms underlying this common and distressing symptom.
Content/Objectives
To provide an integrative review of the current literature on theoretical models used to study fatigue in MS, instruments used to assess fatigue and other factors that impact fatigue during the various phases of MS.
Methods
Data sources: PUBMED, OVID, Ovid Health Star, Ovid MEDINE, CINAHL, Health and Psychosocial Instruments (HaPI), and PsycINFO. Seventeen articles fit the inclusion criteria and were included in the review.
Results
Definitions of MS-related fatigue are reviewed. Several studies found a link with neurotransmitter dysfunction, circadian rhythm, and the timing of fatigue. Central fatigue in MS is associated with neurotransmitters disruptions as well as circadian rhythm disorders, but the evidence is not strong. Perceptions of fatigue or fatigability may arise as either a primary or secondary manifestation of disease. Based on findings from the literature review, a theoretical model of fatigue in MS is proposed.
Conclusion
Future research on MS-related fatigue may consider a longitudinal design with a carefully selected self-report instrument to advance understanding of the underlying pathological mechanisms.
Keywords: Multiple sclerosis, Central fatigue, Electroencephalogram, Demyelination
Introduction
Multiple sclerosis (MS) is a progressive and disabling neurologic disease resulting from immune-mediated damage in the central nervous system (CNS). It differs among people in its clinical manifestations (e.g. motor, sensory visual and autonomic systems).1 Furthermore, depending on the location of lesions (e.g. in brain or spinal cord) fatigue may be more pronounced. Fatigue is associated with decreased quality of life (QOL) and disability even when controlling for other symptoms, such as depression.2 The prevalence and severity of fatigue in people with MS has been described in several studies, and is reported as the most disabling symptom by as many as 60% of patients.3–6 In addition, compared with other neurological symptoms that characterize MS, fatigue is common across diagnostic subtypes (relapsing vs. progressive forms), with estimates of lifetime prevalence in MS as high as 80%.7 Whereas historically it has been difficult to discern the impact of MS-related fatigue compared with fatigue related to other chronic conditions, several recent studies suggest that the annual prevalence of fatigue in MS is higher in severity and frequency compared with that in both healthy people and those with chronic fatigue syndrome (CFS),8 systemic lupus erythematosus (SLE),9 and cancer.10
As early as 1989, Krupp11 reported a greater impact of fatigue on daily living in patients with MS and SLE compared with healthy controls, and proposed that the pathological mechanisms of MS-related fatigue are different compared to those of SLE. Although the pathological (disease-related) mechanisms may be different, the experience of fatigue in both conditions can entail primary (e.g. lassitude), secondary (e.g. medications or sleep problems), and tertiary (e.g. inactivity/stress) factors.7 Specific to pathogenesis of MS-related fatigue, there is evidence of peripheral and central mechanisms, with peripheral fatigue originating in the muscles and related tissues, whereas central fatigue develops in the central nervous system.12 Central fatigue generally refers to performance decrements on a cognitive task, changes in motivation, effects of fatigue on CNS function, or CNS causes of fatigability.13 Recently, considerable interest has been generated about the possible role of neurotransmitter disruptions and circadian rhythm in precipitating fatigue in MS. Although the cause of MS-related fatigue has not been elucidated, it remains a significant problem.14
Magnetic resonance imaging (MRI) studies have contributed to the identification of factors related to fatigue in MS.15 Although studies to date have yielded conflicting results,16 recent reports have described an association between fatigue and higher lesion load as well as demyelination.17,18 Moreover, altered brain activity in certain subcortical areas, such as the thalamus and basal ganglia, may be reflected in oscillatory cortical electrical wave activity that can be measured by electroencephalogram (EEG). For example, central fatigue assessed during task performance (odd ball test) has been related to increased brain activity in certain bandwidths.19
While these studies have provided new information regarding the brain regions associated with fatigue, a major challenge in translation to patient care has been variation across studies in how the concept of fatigue is quantified. In order to accelerate translation to the clinical setting, an operational definition of MS-related fatigue with clear case/non-case criteria and linked with objectives measures will facilitate identification and treatment. Similar challenges have been confronted with the concept of cancer-related fatigue (CRF), a condition which still lacks a clear etiology.20 Dysregulation of several systems, both biochemical and physiological, are likely involved in CRF, and include both peripheral and central mechanisms (Table 1). Proposed mechanisms of CRF include cytokine dysregulation, hypothalamic-pituitary-adrenal (HPA) axis dysfunction,5 hydroxy tryptophan (5-HT) neurotransmitter dysregulation, circadian rhythm disruption, alterations in adenosine triphosphate (ATP) and muscle metabolism, and vagal afferent activation.21,22 As the body of evidence continues to grow regarding how these mechanisms contribute to CRF, there has been less attention given to fatigue in MS despite the documented impact on patient QOL.
Table 1.
Differing characteristics between central and peripheral fatigue
| Fatigue type | Key Features | Physiological | Psychological | Neurochemicals Released |
|---|---|---|---|---|
| Central | Presence of both physical and mental fatigue, failure to sustain sustained mental tasks (e.g. mental arithmetic, remembering). | Impaired brain function Sleep problems Altered thought processes Brain atrophy Autonomous response (altered heart rate) during cognitive challenge. |
Lassitude Inability to concentrate |
↑ Cytokines (e.g. IL-6; IFN-alpha) ↑ hypocretin-1 ↓melatonin ↓HPA axis function |
| Peripheral | Failure to achieve motor and muscle activation and voluntary strength for maximum muscle force | Motor Weakness Reduced strength and endurance |
↑ATP ↑TNF a ↑Interleukin 6 ↓acetylcholine |
IL-6 = interleukin; IFN = interferon's
Therefore, we performed an integrative review as a first step toward the development of an operational definition of MS-related fatigue. The purpose of this review was to thoughtfully integrate biological studies focused on the mechanisms of MS-related fatigue in order to examine the current state of knowledge and propose future directions for research and clinical practice. The review of the literature was guided by the following questions:
How has fatigue been defined and measured in patients with MS?
What factors have been found to influence fatigue in MS?
What theoretical models have been used to study fatigue in MS?
Methods
Search method
A detailed literature review addressing fatigue and MS was conducted for this article. The literature search was performed through the databases Ovid HealthStar, Ovid MEDINE, CINAHL, Health and Psychosocial Instruments (HaPI), and PsycINFO. The time frame of the search was from 2009 to 2014, resulting in a total of 164 articles. An initial generic search in PubMed published at any date using the following key words as titles, “Fatigue AND multiple sclerosis,” yielded 101 articles. These common key words/phrases include: (“fatigue” [MeSH Terms] OR “central fatigue” [All Fields]) AND (“circadian rhythm” [MeSH Terms] OR “neurotransmitters” [All Fields] OR “serotonin” [All Fields] OR “suprachiasmatic nucleus (SCN)” [All Fields]). The refined search yielded 30 articles by eliminating inclusion and exclusion criteria. We then incorporated “brain activity” AND “disability.” The search included articles written in English published between the years 2000–2014 and focused on fatigue in patients with MS. Twenty-three publications were included in this review. One article was found by incidental review of the literature.23 The earliest article was published in 199424 and the remainder (n = 16) were published from 2004 to the present (Table 2).
Table 2.
Summary of articles focusing on MS-related fatigue
| References | Participants | Purpose | Significant Findings | Conclusions |
|---|---|---|---|---|
| Bailey et al.25 | 14 with chronic progressive MS. | Examine cognitive and subjective fatigue. First phase: A continuous n-back task, involving attention; Second phase: a task involving working memory (1-back). Subjective fatigue was rated at regular intervals during each session. | The MS group reported a greater increase in the level of subjective fatigue during the 1-back testing session compared to the control group. Fatigue measure: FSS |
Given the small sample size, there was limited evidence of objective cognitive fatigue in the MS group, as assessed by the change in n-back performance during the sessions. The use of the FSS does allow for consistency of fatigue measurement. |
| Chang et al. (2011) | 9 MS. | Evaluate effects of a surface functional electrical stimulation (FES) on muscle strength and fatigability in MS subjects and to determine whether the surface FES training relieved central or peripheral fatigue. | Central fatigue was weighted over 5 times higher than peripheral fatigue. Eight weeks of surface FES training led to increases in resistance to general fatigue and resistance to central fatigue. Fatigue measure: General Fatigue Index (FI), Central Fatigue Index (CFI), Peripheral Fatigue Index, and MFIS |
Since EDSS were 1 and 4, outcomes on subjects with more severe motor deficits cannot be determined. Given the small sample, seven out of nine participants completed the study; central fatigue could not be evaluated. The use of the MFIS does allow for consistency of measurement. |
| Engstrom et al.26 | 15 MS; self-reported fatigue; matched to controls. | Determine if disruptions of the thalamo-striato-cortical network correctly predicted fatigue using functional MRI (fMRI) during the performance of a complex working memory task. | In MS group, parietal cortex was activated in both hemispheres and elicited less activation in the thalamus and several regions of the basal ganglia. Brain activation in the left posterior parietal cortex and the right substantia nigra was positively correlated with perceived fatigue ratings. Fatigue measure: Swedish FIS |
In a small sample study, thalamo-striato-cortical network was involved in the pathophysiology of fatigue in MS and possibly central fatigue in general. |
| Fisk24 | 85 MS; 20 hypertensive subjects. | Evaluate the impact of fatigue in relation to other symptoms. | MS group reported fatigue as either the worst (14%), or one of the worst (55%) symptoms. Fatigue measure: FIS |
Perceived fatigue is a significant factor for the MS group; Fatigue significantly impacts outcomes of subjects with MS over the course of the disease. The use of the FIS allows for consistent use of a measurement tool. |
| Heesen et al.27 | 23 MS; 25 controls. | Examine if the immune response to a cognitive task is a variant of psychological stress in MS subjects; distinct from healthy controls. | MS group scored high on a disease-specific fatigue measure, whereas baseline cytokine patterns did not differ between the groups. MS subjects displayed a blunted response of IFNγ (P = 0.03) whereas TNFα and IL-10 responses did not change. Additionally MS subjects showed less of an increase in heart rate exercise (P < 0.001). Fatigue measure: MFIS and VAS |
Small sample size; and a reduced cardiac response might indicate an autonomic dysfunction in this group of subjects. The use of consistent tools strengthens this study. |
| Iriarte et al.28 | 155 (105 women, 50 men) with MS. | Demonstrate if there was a mechanism for each of the different varieties of fatigue. | Immunoactivation parameters were associated with asthenia (P < 0.001), and pyramidal tract involvement was associated with fatigability (P < 0.001). Sleep disorders, anxiety and depression were linked with fatigue in a few subjects. Fatigue measure: Fatigue Descriptive Scale (FDS) and FSS |
Relative small sample size; fatigue in MS seemed to be a heterogeneous with asthenia and fatigability remaining different clinical entities. This finding gives strength to the use of FSS as a subjective measure of fatigue. |
| Johansson et al.27 | 200 MS. | Describe longitudinal variations in MS-related fatigue while simultaneously exploring predictors for variations in fatigue. | 54% of MS group reported fatigue, 27% were persistently fatigued and 19% were persistently non-fatigued (P = 0.02); with predictors of increased fatigue being depression, not working, and disease severity ( > than 10 years since diagnosis or a progressive course). Fatigue measure: FSS |
Since disease severity was a predictor of disease, classification of subtypes is needed in future studies. The use of the FSS is a strength of this study. |
| Kim et al.30 | 49 MS; FSS score > 4. | Develop and evaluate a real-time measurement for fatigue. | Mean RDFS significantly correlated with FSS (P < 0.001) and MFIS (P < 0.001); mean RDFS did not correlate with BDI or EDSS. RDFS captured significant variability in fatigue throughout the day with an incremental increase in circadian fatigue as the day progressed (all values P < 0.05 with the majority of values P < 0.001) Fatigue measure: FSS and MFIS |
Small sample size; compliance for the RDFS all four times each day for three weeks was 64%. The use of the FSS and MFIS are strengths of this study. No mention of MS subtypes; thus classification of MS subtypes and distinct diagnostic parameters need to be considered. |
| Krupp & Elkins31 | 45 MS and 14 healthy controls. | Complete a baseline neuropsychological battery, a continuous effortful cognitive task, and a repeat neuropsychological battery. | MS group declines in measures of verbal memory and conceptual planning. | Due to the disparate sample size between groups, these findings may not be useful for showing a relationship in mental and physical fatigue. |
| Melamud et al.32 | 13 females with RRMS and 12 matched healthy controls. | Evaluate fatigue, sleep and day/night levels of 6-sulphatoxy-melatonin were performed before, during, and after starting IFN-beta treatment. | Mean urine melatonin metabolite levels in the MS group pre-treatment were significantly lower than in the control group (P < 0.001). During treatment with IFN-beta patient's levels of 6SMT gradually increased and the 4-month levels were significantly higher compared to pre-treatment levels (P = 0.001). Fatigue measure: FSS |
Small sample size; however, melatonin levels may be related to fatigue in subjects with RRMS. The use of a reliable fatigue measure strengthens this study. |
| Mills et al (2013)33 | 208 MS. | Determine the minimum clinically important difference (MCID) of the NFIMS. The instrument was administered before and after expected change or stability in fatigue. | The largest MCID increased 2.49 points on the Summary scale, 2.36 points on the Physical scale, 0.84 points on the Cognitive scale, 0.97 on the Diurnal Sleep scale and 1.95 on the Nocturnal Sleep scale. | Conclusion was that the NFI-MS responded as expected to changes in fatigue and had desirably small MCID scores. The NFI-MS may be a useful tool for measuring fatigue |
| Mills & Young34 | 1223 with MS. | Examine relationships between fatigue, disability, sleep, and depression in MS subjects using a large sample and a newly developed patient reported outcome for fatigue (NFI-MS). | Fatigue higher in those with an ambulatory disability (P < 0.001) with regards to the EDSS and strong linear correlation to the MFIS physical scale. Those with progressive disease had more fatigue than those with RRMS (P < 0.001). Those who slept during the day had greater fatigue (P < 0.002) a borderline linear correlation of fatigue and hours of day sleep. Fatigue was much greater with broken nocturnal sleep (P < 0.001) with no linear relationship between fatigue and duration of nocturnal sleep. Anxiety, depression, and non-working groups had greater fatigue (P < 0.001). Fatigue measure: NFI-MS |
Due to fair response rate at 52%, it is difficult to determine if NFI-MS is better in comparing fatigue than the MFIS-29 |
| Mills et al.35 | 40 MS. | Analyze a coherent definition of fatigue, and a potential 52-item measure (FIS). | Four potential subscales were identified by factor analysis: ‘physical’, ‘cognitive’, ‘relief by diurnal sleep or rest’ and ‘abnormal nocturnal sleep and sleepiness’. Summary scale comprising items from the Physical and Cognitive subscales. | Qualitative analysis alone is not a reliable method to define fatigue given the small sample size. |
| Najafi et al.36 | 120 MS; 60 healthy controls (age and sex matched). | Compare sleep quality, circadian rhythm and fatigue severity. | CRSD was more frequent in MS group (P = 0.002). Sleep quality, using the Pittsburgh Sleep Quality Index and fatigue were worse in MS subjects (P = 0.0001) Fatigue measure: FSS |
The use of sleep quality as an indicator of fatigue may be useful with additional subtypes with age- matched controls. The use of the FSS expands the reliability of this study. |
| Papuć et al.37 | 38 MS; 15 healthy controls. | Investigate the hypocretin-1 levels in CSF of MS subjects in relation to different neurological deficit measures. | Positive correlation between hypocretin-1 and fatigue in a total group of MS subjects (P = 0.016); association was stronger in a subgroup of MS subjects who experienced more severe fatigue (P = 0.006). Fatigue measure: FSS |
Small sample size. Clinical significance should be tested on larger MS population especially with different clinical subgroups and well-defined groups (e.g. relapsing-remitting versus progressive subtype). The use of the FSS expands the reliability of this study. |
| Pokryszko-Dragan et al.23 | 86 MS; 42 healthy controls age- and sex-matched. | Evaluate visual and brainstem auditory EP with regard to fatigue and disease-related variables (duration, subtype). P100 component of VEP and the I–V components of BAEP were analyzed. The results of EP were compared between non-fatigued, moderately and severely fatigued MS subjects and controls. |
The amplitude of the EP was lower in fatigued subjects and correlated with FSS/FSS-5. Significant VEP and BAEP abnormalities were found in fatigued MS group, with no relationships to disease related variables. Fatigue measure: FSS |
EP may be considered an electrophysiological marker of fatigue severity. The use of the FSS expands the reliability of this study. |
| Steens et al.38 | 20 RRMS (EDSS < 5.5); 20 healthy controls. | Investigate associations between perceived fatigue and measures of fatigability after correction for differences in maximal voluntary contraction (MVC). | MS and controls developed similar amounts of muscle fatigue during the sustained contraction but muscle fatigue and MVC was positively associated with perceived fatigue in MS (P = 0.01). Voluntary activation during the sustained contraction was negatively associated with perceived fatigue (P = 0.02). Fatigue measure: FSS |
Given the small sample of RRMS subjects, the isolation of muscle fatigue to determine perceived fatigue requires more study. No reliable or valid self-report measure of fatigue was used. |
| Streckis et al.39 | 9 men with MS and 9 women, age, BMI, EDSS matched as 3.5–5.5 (moderately disabled). | Evaluate the effect of time of day on central and peripheral fatigue during a continuous 2-min maximal voluntary contraction of the quadriceps muscle in women and men with MS. | Central fatigue increased and peripheral fatigue decreased in women during the 2-min MVC in the evening. Fatigue measure: FSS |
In this small sample, the FSS was not used to determine fatigue changes for time of day. No specific MS subtype mentioned. |
| Tartaglia et al.19 | 10 RRMS; 7 controls. | Assess the impact of mental fatigue on motor task-related cerebral activation. | Challenging mental tasks altered the pattern and increased the volume of cerebral activation on an unrelated motor task in fatigued MS subjects. Fatigue measure: FSS |
In a small sample, mental task could be sufficient to increase fatigue as objective measure in subjects with RRMS. |
| Tedeschi et al.40 | 222 RRMS with low disability scores. | Evaluate the correlation between fatigue and lesion load, white matter (WM) and gray matter (GM) in MRI. | Fatigue group had higher WM and GM atrophy and lesion load. In MS, independent of disability, WM and GM atrophy is a risk factor for fatigue. Fatigue measure: FSS |
The use of WM and GM in MRI, with FSS, is essential to determine neural disruption and may prove useful for defining fatigue. Yet to be determined if this method would be useful in other subtypes of MS (e.g. progressive). The use of the FSS expands the reliability of this study. |
| Tomasevic et al.41 | 20 mildly disabled RRMS subjects :EDSS ≤ 2). | Test structural and functional (cortico-muscular coherence [CMC]) measures were recruited in two fatigue-dependent groups. | Both groups were similar in terms of demographic, clinical and imaging features, as well as task execution accuracy and weariness. In the absence of any fatigue-dependent brain and muscular oscillatory activity alterations, CMC higher fatigue, explaining 67% of fatigue variance (P = 0.002). Fatigue measure: MFIS |
Given the small sample size, CMC may not be a reliable method for measuring central fatigue. |
| Vetrugno et al.42 | 6 MS; 10 age- and sex-matched controls. | Measure sleep-wake cycles with body core temperature (BCT) measurement using polysomnography. | Both groups displayed a normal BCT 24-h rhythm. Mesor, amplitude and acrophase of BCT rhythm did not show significant differences between MS and controls. Fatigue measure: FSS |
Small sample size; abnormal sleep or abnormal BCT were unlikely mechanisms of fatigue. The use of sleep circadian rhythm and BCT may not be an advantage for measuring fatigue. |
BDI = Beck Depression Inventory; CRSD = Circadian Rhythm Sleep Disorder; EP = Evoked Potential; ESS = Epworth Sleepiness Scale; EDSS = Expanded Disability Status Scale; FIS = Fatigue Impact Scale; FSS = Fatigue Severity Scale; HADS = Hospital Anxiety and Depression Scale; MFIS = Modified Fatigue Impact Scale; MSIS-29 = MS Impact Scale; NFI-MS = Neurological Fatigue Index for MS; RDFS = Real-time Digital Fatigue Scores; RRMS = Relapsing Remitting Multiple Sclerosis; VAS = Visual Analogue Scale
Inclusion criteria
All RCTs, quasi-randomized and quasi-experimental designs with comparative controls, and controlled before-and-after studies were included. Whenever RCTs were lacking, a search for relevant observational studies was conducted. Studies involving other medical conditions where data were specifically provided for MS-related fatigue were also included. Descriptive studies and narrative reviews were explored to identify policies, protocols, and gaps in service provision. Systematic reviews and meta-analysis were excluded. No restrictions were set for type of MS, disease severity, level of disability, or demographic characteristics (gender/race/ethnicity) of study participants. Studies were excluded if they targeted non-adults (aged younger than 18 years old), or included populations other than MS. Results and inclusion were discussed until consensus was reached among all three authors. A summary of the publications included in the review is provided in Table 2.
Results
How has fatigue been defined and measured in patients with MS?
A host of assessments have been used to measure MS-related fatigue, many of which have been used in clinical trials (Table 3). Several recent reviews of published MS assessments highlight the strengths and weaknesses of each available assessment.25,27,43,44 These reviews confirm the Fatigue Task Force by the American Association of Neuroscience Nurses (AANN)27 recommendations that researchers should select the most appropriate instrument to achieve the study goals.
Table 3.
Reliability and validity of fatigue measures
| Measure | Forum | Subscales | Test-Retest Reliability | Internal Consistency | Construct Validity | Criteron Validity | Time (minutes) | #Items |
|---|---|---|---|---|---|---|---|---|
| FSS | IP/OP Self-report |
NA | 0.91 | 0.94 | 0.80 with Fatigue scale11 | 5 | 9 | |
| MFIS43 | IP/OP | Physical | 0.70–0.86 | NA | 5 to 10 | 9 | ||
| Self-report | Cognitive | 0.77–0.90 | 10 | |||||
| Psychosocial | 0.81 | 2 | ||||||
| Total | 0.94–0.96 | 21 | ||||||
| NFI-MS | IP/OPSelf-report | Physical | 0.85 | NA | 0.96 | 5 to 10 | 16 | |
| Cognitive | 0.82 | 0.85 | 8 | |||||
| Nocturnal Sleep | 0.83 | 0.62 | 7 | |||||
| Diurnal Sleep | 0.79 | 0.65 | 8 | |||||
| Summary | 0.86 | 10 |
FSS = Fatigue Severity Scale; MFIS = Modified Fatigue Inventory Scale; NFI-MS = Neurological Fatigue Index Scale; IP = inpatient; OP = outpatient forum.
Self-report measures of MS-related fatigue
As a subjective symptom, MS-related fatigue is measured via self-report. Most self-report scales identified in the literature review address both the sensation and impact of fatigue,25 and some scales include additional domains such as reduced motivation, energy or vitality, or diurnal variation.7 A brief description of the major self-report fatigue instruments is provided to illustrate variation in measurement.
The Fatigue Severity Scale (FSS) instructs patients to assign a score of between 1 (completely disagree) and 7 (completely agree) for each of the 9 FSS items. The items are designed to rate the extent of fatigue symptoms and their impact on patient functioning (including motivation, exercise, physical functioning, carrying out duties, and interfering with work, family, or social life). Examples of the items include: “exercise brings on my fatigue” and “my fatigue is very debilitating.” A higher score indicates a higher degree of fatigue for all items.11 Scoring of the FSS is performed by dividing the sum of the 9 items to produce an FSS total score that ranges from 1 (no fatigue) to 7 (very severe fatigue).
The MFIS-21 is a modified version of the 40-item Fatigue Impact Scale (FIS), which was originally developed to assess the effects of fatigue on quality of life in patients with chronic diseases, specifically MS.23 The FIS asks patients to rate the extent to which fatigue has affected their life in the past 4 weeks on a questionnaire consisting of 10 “physical” items, 10 “cognitive” items, and 20 “social” items, with 0 indicating “no problem” and 4 indicating “extreme problem.” The maximum possible score is 160. The MFIS evolved from the FIS during the development of a clinical inventory assessing overall QOL in people with MS (the Multiple Sclerosis Quality of Life Inventory (MSQLI). During the phase 2 field testing of the MSQLI the 40-item FIS was abbreviated into the 21-item MFIS by “eliminating items which appeared both content-redundant and had high inter-item correlations.”43,44 The maximum possible score is 84, with higher scores indicating a greater impact on QOL.
The Neurological Fatigue Index for MS (NFI-MS) (28) consists of 23 items in four subscales of Physical (8 items), Cognitive (4 items), Relief by diurnal sleep or rest (6 items), and Abnormal nocturnal sleep and sleepiness (5 items). A 10-item Summary Scale derived from physical and cognitive items is also available. Wording of the scales is both simple and concise; the use of the word ‘fatigue’ was deliberately avoided because of its associated semantic ambiguities. All items are worded in such a way as to be scored in the same direction. Each item has a four point Likert response option with headings of ‘strongly disagree,’ ‘disagree,’ ‘agree,’ and ‘strongly agree,’ which progress in the natural reading direction (i.e. left to right), and are scored 0, 1, 2, 3. There is a single sentence instruction at the start of the scale asking respondents to consider their experience over the previous four weeks. The scale has good construct validity across subscales in persons with MS.35
In summary, the publications included in this review used the FSS, MFIS, and NFI-MS to measure fatigue in people with MS. Of the publications identified, almost all used the FSS,11,19,28–30,32,37,38,40,41 whereas five 20,21,33,35,41,45 used the MFIS and three33–35 used the NFI-MS. In comparing these commonly used instruments, it is noted that the FSS does not include questions regarding cognitive fatigue whereas the MFIS does include questions of cognitive fatigue as well as the impact of fatigue on social functioning. In contrast, the NFI-MS focuses on measurement of physical and cognitive fatigue as well as the impact of sleep quality, quantity, and sleepiness of levels of fatigue. Because different constructs of fatigue are measured in each instrument, the results cannot be compared across studies. Also, there are no adequate measures for the evaluation of fatigue in control populations to allow for true comparison, as a majority of fatigue scales are disease specific. It is, therefore, reasonable to assume that more research on the various measures described here is needed to identify change in the physical and cognitive domains.45
Physiological factors associated with fatigue: neurotransmitter dysfunction
Several studies reviewed investigated the relationships among fatigue and levels of neurochemical modulators. Melamud et al.32 assessed the influence of IFN-β treatment on melatonin secretion, fatigue, and sleep characteristics in a sample of 15 patients with MS compared to healthy controls (HC). Using the Modified Fatigue Impact Scale (MFIS), sleep was assessed by actigraphy, and day/night levels of 6-sulphatoxy-melatonin (6-SMT) in urine were determined using a highly specific ELISA assay. They found that patients with MS had significantly decreased levels of 6-SMT and disrupted circadian regulation of its secretion, which was increased with IFN-β treatment. Furthermore the IFN-β improved fatigue, suggesting dysregulation of melatonin secretion in MS. The results call for further characterization of the role of neuro-hormones, such as melatonin, in MS and their cross-regulation with immune-mediators.
Using an experimental design, Papuć et al.37 found hypocretin system functions may be involved in fatigue levels. The hypocretin system is linked to wakefulness and arousal in the brain. The sample size was small with 25 people diagnosed with relapsing-remitting MS and 13 diagnosed with secondary progressive MS, along with healthy control participants (HCs). All participants completed the Fatigue Severity Scale (FSS), and Epworth Sleepiness Scale (ESS). CSF hypocretin-1 levels did not differ between patients with MS and HCs (P > 0.05), however, a positive correlation between hypocretin-1 level and fatigue level was found in patients with MS who were in the relapse phase (P < 0.05), an effect even stronger in the MS subgroup suffering from fatigue (P = 0.01). The hypocretin system seems to be generally unchanged in MS, but a relationship between hypocretin-1 level and fatigue may indicate involvement of some compensatory mechanisms stimulating the production of the neuropeptide in patients with MS.
In a study by Heesen et al.21 there was an altered cytokine response to cognitive stress during MS-related fatigue. Patients (n = 23) and controls (n = /25) participated in a cognitive task lasting 40 minutes in which the heart rate was continuously monitored. Patients scored high on a disease-specific fatigue score compared to controls, whereas baseline cytokine patterns did not differ between the groups. Patients with MS displayed a blunted response of IFN γ (P = / 0.03) whereas TNF α and IL-10 responses did not change. Additionally patients with MS showed a significantly lower heart rate increase after the task (P < / 0.001). Cognitive impairment was associated with a decreased heart rate reactivity (P = /0.02) while depressive symptoms correlated with stronger IL-10 responses (P < /0.05), supporting autonomic dysfunction in the MS group.
In Iriarte et al.28 a sample of 155 patients (105 women, 50 men) with clinically definite MS were studied. Fatigue was measured using the Fatigue Descriptive Scale (FDS) and the Fatigue Severity Scale (FSS). Patients were to report on three clinically different entities (asthenia, fatigability, and worsening of symptoms with effort). Fatigue was a symptom in 118 patients (76.13%.); 26 patients (22.03%) described it as asthenia (fatigue at rest); 85 patients (72.03%) as fatigability (fatigue with exercise), and seven patients (5.9%) as worsening of symptoms. The authors concluded that asthenia (fatigue at rest) and fatigability (fatigue with exercise) may be different clinical entities.
The publications reviewed suggest that there are differences in levels of neurochemical mediators, such as melatonin and hypocretin in people with MS who report fatigue compared to non-fatigued patients with MS and healthy controls. Although inflammatory activation is often cited as a potential mediator of fatigue in people with MS, there is not enough evidence currently available to support this assumption.
Circadian rhythm and timing of fatigue
Several publications reported findings on the relevance of time of day to fatigue severity. In a recent study, Streckis and colleagues39 evaluated the effect of time of day on central and peripheral fatigue during quadriceps exercise in men and women with MS. The sample consisted of age-matched patients with MS (age range 40–50) who completed the FSS to ascertain any difference in men and women with MS in both morning and evening. They found a significant gender difference in central activation ratio (CAR) in the evening. At the end of the 2-min maximal voluntary contraction (MVC), the voluntary torque decreased by about 65% in men and women with MS in both the morning and evening. The most interesting finding of that study was that central fatigue increased, whereas peripheral fatigue decreased markedly in the evening only in women. It remains unclear why women's central fatigue is greater in the evening than in the morning.
In a similar study, Kim et al.30 used the FSS and MFIS in a sample of 49 patients with MS. The authors used a cut-off score of FSS > 4 and had patients wear a wrist-worn device four times a day over 3 weeks. FSS and MFIS were evaluated and compared with real time digital fatigue scores (RDFS). Mean RDFS significantly correlated with FSS (r = 0.55, P < 0.001) and MFIS (r = 0.55, P < 0.001). RDFS captured circadian variations in fatigue, with scores increasing from mean 3.4 at 9 am, to 4.0 at 1 pm, 4.5 at 5 pm, and 5.0 at 9 pm.
Likewise, in a large cross sectional study (n = 635) in the UK, Mills and Young34 reported fatigue was worse in those with progressive disease and clearly worsened once ambulation was started. Fatigue was not related to disease duration or patient age. Fatigue levels were minimum at nocturnal sleep duration of 7.5 hours.
Conversely in a longitudinal study, Johansson and colleagues29 sought to describe variations in fatigue over the course of 2 years in a sample of persons with MS. These authors found that FSS scores varied significantly (P = 0.02); 54% changed FSS category one or several times, 27% were persistently fatigued, and 19% persistently non-fatigued. Furthermore, moderate disease severity predicted increase in fatigue when combined with 10 years since diagnosis, or a progressive course.
In Iran, Najafi et al.36 used a case-control study performed with 120 patients with MS and 60 healthy subjects, who were age-sex matched. Sleep quality, rhythm, and fatigue severity were assessed using PSQI (Pittsburgh Sleep Quality Index) and FSS questionnaires, respectively. They observed that circadian rhythm sleep disorder (CRSD) was more frequent in patients with MS relative to healthy subjects (P = 0.002). Also, CRSD was higher in patients with MS with severe fatigue relative to patients with MS with mild fatigue (P < 0.05). The patients were divided by fatigue severity of 49.9 ± 8.2 and 22.5 ± 7.4 in the first and second group, respectively. The PSQI index was 7.9 ± 4.5 in patients with severe fatigue and 5.9 ± 4.5 in patients with mild fatigue, and 4.5 ± 2.4 in the control group (P = 0.0001). They concluded that distinguishing fatigue and sleep among this population is highly important, as fatigue is difficult to define. Some of the reasons for these findings could be attributed to a shared mechanism between fatigue and sleep.34
In contrast, Vetrugno et al.42 reported on sleep-wake and body core temperature (BCT) circadian rhythms in patients with MS associated with chronic fatigue in Italy. They used 48 consecutive hours of polysomnography (PSG) with BCT measurement, followed by a Multiple Sleep Latency Test (MSLT) in six patients with MS and chronic fatigue. Fatigue was also assessed by standardized, self-administered questionnaires that included the FSS and the MFIS. They found normal sleep–wake rhythmicity, sleep structure, and BCT rhythm in six drug-free patients with MS and chronic fatigue, and without sleep-related breathing disorders. However, due to the small sample, the authors concluded that other factors, such as co-occurring sleep symptoms, should be addressed.
It has been documented previously that CRSD may increase fatigue levels.45 The possible common mechanisms shared by MS and sleep disturbances may be connected with CRSD combined with compromised melatonin secretion, reducing input to the suprachiasmatic nucleus (SCN) due to increased levels of proinflammatory cytokines.46 Najafi et al.36 reported that sleep phase syndrome and irregular sleep wake patterns in patients with MS with mild and severe fatigue were compared with healthy subjects. CRSDs were significantly higher in patients with MS in relation to healthy subjects.47,48 Furthermore, it is correlated with increased symptoms of tiredness, fatigue, and lack of energy in patients with MS.40,49
In summary, the literature concerning the impact of circadian rhythm and timing on fatigue suggests that fatigue may worsen over the course of the day,28,42 with disease severity,39,50 levels of activity39 and with diagnosis of CRSD.30,34 A few studies found that the later in the day, the more severe the fatigue for patients with MS.28,42,44,51 Fatigue has been shown to show noticeable daily cycles, with greater fatigue at the end of the day in some people.40 Explanations are unclear, but it is postulated that this may be caused by early neuronal damage in the brain, or influenced by psychological factors, muscle exhaustion,42 or an overlap of these factors.28 Particularly in patients with more progressive forms of MS, fatigue may start or intensify with excessive physical activity, emotional stress or cognitive changes.22,51
Factors related to central fatigue
A recent study23 found that evoked potential (EP) in EEG may be useful as indicator of neural disruption and not just the subjective nature of fatigue. For example, abnormalities of EP in fatigued patients, independent from MS-related variables, may support the hypothesis of disturbed bioelectrical activity due to CNS damage as the background of fatigue, which contradicts the idea of its purely subjective origin.
While there are differing etiologic theories underlying the pathogenesis of MS-related fatigue, it is clear that damage to the central nervous system is an inciting event. Neuroimaging procedures have demonstrated that select areas of CNS disruption are tied to symptoms of fatigue. Such techniques have shown reduced cerebral blood flow and overall cerebral atrophy in both people with MS and chronic fatigue syndrome,15 even though the clinical patterns of fatigue differ between these disease states. Other work using fMRI demonstrate impaired functionality of the cortical and subcortical areas in association with increased levels of fatigue as measured by the FSS.16 In that study, people without fatigue displayed significantly higher activation of the primary somatomotor cortex and several other areas of the brain associated with motor movement and activation. Similarly, those with more severe MS-related fatigue demonstrate increased lesion load, and atrophy of both white and gray matter.36 Other authors conclude that damage to CNS connectivity results in a need for increased activation of other brain areas in order to maintain function, and such increases are tied to resultant fatigue.19
In summary, it appears that MS-related fatigue is associated with white matter changes in areas associated with motivation and reward processing, in a manner beyond that traditionally associated with the central serotonergic model.52 Disease associated damage of the CNS is associated with the manifestation of fatigue in those with MS.
What theoretical models have been used to study fatigue in MS?
Conceptual framework for the definition of MS-related fatigue
A conceptual definition of fatigue commonly used in people with MS is the “subjective lack of physical and/or mental energy that is perceived by the individual to interfere with usual and desired activities.”48 However, a clear conceptual framework for MS-related fatigue is still lacking. Building on the subjective experience of fatigue, explanatory theories have been offered explaining the pathogenesis of MS. A major theory of central fatigue is the thalamo-striato-cortical theory. This theory implicates central fatigue results from disruptions of cortical networks. Work, by Alexander and Crutcher54 examining this perspective has found the thalamo-striato-cortical network as specific hypothesis of fatigue in MS. The authors state that communication between the striatum and prefrontal cortex is clearly related to neurotransmitters. To further explain this theory, Chaudhuri and Behan54 suggest that central fatigue might arise due to the “failure of the non-motor functions of the basal ganglia.”37 One paper currently reviewed by Engstrom et al.26 reported a physiological model (e.g. dysfunction of the thalamo-striato-cortical network) explanation of fatigue in MS. Subjects in their study experienced an altered brain response in the thalamo-striato-cortical network during performance of a complex working memory task. In essence, MRI reveals that fatigue results from the disruption of communication between these regions.
The model proposed here (Fig. 1) is our unique contribution. In our model assessing neurotransmitter dysfunction, thalamo-striato-cortical network, such as in EEG findings, as well as other distressing symptoms could help to further unravel the pathophysiological mechanism of MS-related fatigue.55 Inflammation is a biological response of the immune system to a number of different stimuli. If serum levels of cytokines reflect general systemic inflammation and CSF levels of cytokines are more closely related to disease-specific processes in the central nervous system of patients with MS, simultaneous testing of multiple cytokines in serum and CSF may shed new light on the possible mechanism of inflammation in MS-related fatigue.56 Based on similar findings, symptom overlap of cognition, depression, and sleep problems may occur due to thalamo-striato-cortical network dysfunction.58 Although possible, this theory remains inconclusive in our experience, and thus, we did not include this exact brain response in our model due to the potential for symptom confounders.
Figure 1.
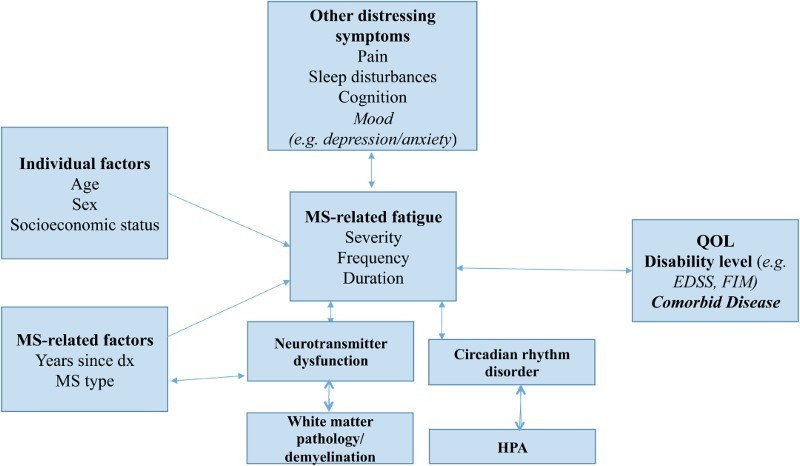
Organizational framework for MS-related fatigue. Concepts from the literature search related to physiology, clinical components, symptoms, and individual demographic factors. Dx = diagnosis; EDSS = Expanded Disability Status Scale; FIM = Functional Independence Measure; HPA = hypothalamus-pituitary-adrenal; QOL = quality of life.
Discussion
The literature review was guided by three questions regarding the concept of fatigue in MS with the goal of providing a synthesis of the measures used, factors that influence fatigue, and theoretical frameworks used to study fatigue across MS populations. Based on the literature review, we identified several seminal instruments that have been used to quantify the symptom of fatigue. Each instrument offers measurement of different fatigue constructs such as: physical and/or cognitive; the impact of fatigue on different functional domains; and the influence of sleep quality, quantity, and sleepiness.
Conceptual clarity of MS-related fatigue may help to distinguish patients with definite MS-related fatigue versus fatigue from other causes. MS-related fatigue has remarkable phenomenological and neuroimmune overlaps with CRF44; however, a defining feature of MS-related fatigue is the anatomical hallmark of a progressive destruction of the myelin coating of axons. Autonomic dysfunction, diminished cardiac responses, and a relapsing-remitting or progressive course with infections and psychosocial stress, play a part in driving MS fatigue symptoms. However, both MS and CRF exhibit evidence of autoimmunity and activated immunoinflammatory pathways.33 As seen with CRF, patients with MS-related fatigue may experience behavioral variables such as anxiety, depressed mood, and reduced activity, as well as biobehavioral signs of cognitive slowing, loss of executive function during rest, and memory loss as a confounder to fatigue51 (Table 3).
The concept that MS fatigue as central in origin is related to a disruption of cortical-subcortical involvement in motor planning and execution during simple motor tasks. For example, fatigue in MS is related to a functional impairment of the motor system at the cortical level, up from the corticospinal tracts. This has shown to be evident even during the performance of a simple reaction time task (e.g. odd ball test) during electroencephalography (EEG). Furthermore, there are differences between central and peripheral fatigue along both physiologic and psychological pathways (Table 1). Central fatigue is a phenomenon prevalent in many pathological conditions including multiple sclerosis, Parkinson's disease, cancer, and human immunovirus/autoimmune deficiency syndrome (HIV/AIDS). Fatigue in these conditions is multidimensional, comprising both primary pathophysiological mechanisms and secondary contributing factors. Central fatigue in MS is recognized as multifactorial in origin, and may be associated with immunomodulatory drugs used for treatment, depression, and sleep disorders. Impairment of volitional drive to the descending motor pathways54 has been proposed as a mechanism determining central fatigue by functional neuroimaging and electrophysiological studies.59
MS-related fatigue comprises self-reported lack of energy, and physical and mental tiredness which may be present irrespective of physical effort or intense exercise, and which may not be alleviated with rest. It is reported by people to be amongst their worst symptoms, contributes to disability, and has a highly detrimental impact upon perceived QOL.51,57,60 A combined approach using neurophysiology and neuroimaging allowed evaluation of both functional and structural abnormalities in cortical areas involved in motor programming and execution. One such study61 found that brain-muscle circuits play a role in fatigue during EEG and handgrip tasks. Thus, fatigue resulting from CNS disturbances is clearly different in nature than that resulting from metabolic disruption of energy utilization in peripheral muscle.62
Other factors related to fatigue derived from this review include the time of day,39,42 activity level,39 and CRSD.30,34 However, many gaps still remain as to the usefulness of such factors. The authors used small samples and various fatigue measures. For example, in one case-control study, Najafi et al.36 discuss limitations of their study of small sample size, lack of MS subtypes, and fatigue as multifaceted symptom. The evidence suggests that these are important factors to consider in studies evaluating fatigue in people with MS.
A well-defined theoretical framework or model of MS-related fatigue is still lacking. Research has shown that fatigue does not occur in isolation and is associated other symptoms, such as depression and disability.3,4 Due to the common presence of co-occurring symptoms in MS, the Theory of Unpleasant Symptoms63 may provide a theoretical model in which to study the patient experience. The Theory of Unpleasant Symptoms addresses the influences of physiological (e.g. existence of any pathological condition [sleep]), psychological (e.g. affective reaction or mood), and situational (e.g. social support or physical environment) factors that impact each symptom to one another (Table 1). Incorporating the Theory of Unpleasant Symptoms in the design of studies focused on MS-related fatigue would enable a more holistic64 investigation that is based upon well-established constructs and conceptual relationships. Based on a growing body of evidence, the model incorporates potential contributions of genetic and epigenetic factors on stress responses and vulnerability to symptom phenotypes.
Evoked potentials (EP) parameters seem promising as possible electrophysiological markers of fatigue with the asymmetry of their abnormalities deserving special attention. A limitation of our study is the fact that the assessment of fatigue and EP parameters was performed only once, without re-testing to check for reliability of the results. Considering the common fluctuations of MS, large longitudinal studies are needed to determine mechanism of MS-fatigue (Table 1).
Strengths and limitations
The strength of this integrative review is that it provides a current evaluation of the instruments used to measure MS-related fatigue, factors that influence fatigue, and theoretical models65 used to understand the phenomenon in patients with MS. Several studies found a link with neurotransmitter dysfunction, CSRD, and the timing of fatigue.66 The findings can be useful in the development of intervention programs, and while providing care to patients with MS who experience fatigue. The limitations present in the review include that there were too few randomized control trials (RCT) studies available using large samples to provide a more comprehensive review of central fatigue mechanisms. Likewise, careful classification of well-defined MS subtypes and disability levels is needed for future studies.
Conclusions
The review of literature identified three instruments that are frequently used to quantify fatigue in MS populations; each instrument offers a unique strength. Factors identified in the literature that influence fatigue include other symptoms, time of day, and CRDS.66 Although there may be clinical overlap and interactions between symptoms and fatigue, they are distinct experiences. Further, MS-related fatigue may result from impairment in cortico-subcortical [thalamus and basal ganglia] interactions utilized in motor planning and execution.16 Along with the organizational framework, this review is unique in the integration of biobehavioral measures to develop explanations of MS-related fatigue.
Acknowledgments
The authors acknowledge Ms. Margaret O'Connor, Office of Nursing Research Writer/Editor and Mary Bordner, Graduate Nursing Student, for their thoughtful review of this manuscript.
Disclaimer statements
Contributors None.
Funding None.
Conflicts of interest None.
Ethics approval None.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet 2008;372(9648):1502–17. doi: 10.1016/S0140-6736(08)61620-7 [DOI] [PubMed] [Google Scholar]
- 2.Stroud NM, Minahan CL. The impact of regular physical activity on fatigue, depression and quality of life in persons with multiple sclerosis. Health Qual Life Outcomes 2009;7:68. doi: 10.1186/1477-7525-7-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merkelbach S, Schulz H, Kölmel HW, Gora G, Klingelhöfer J, Dachsel R, et al. . Fatigue, sleepiness, and physical activity in patients with multiple sclerosis. J Neurol 2011;258(1):74–9. doi: 10.1007/s00415-010-5684-3 [DOI] [PubMed] [Google Scholar]
- 4.Jougleux-Vie C, Duhin E, Deken V, Outteryck O, Vermersch P, Zephir H. Does fatigue complaint reflect memory impairment in multiple sclerosis? Mult Scler Int 2014;2014:692468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron MH, Peterson V, Boudreau EA, Downs A, Lovera J, Kim E, et al. . Fatigue is associated with poor sleep in people with multiple sclerosis and cognitive impairment. Mult Scler Int 2014;2014:872732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hildebrandt H, Eling P. A longitudinal study on fatigue, depression, and their relation to neurocognition in multiple sclerosis. J Clin Exp Neuropsychol 2014;36(4):410–7. doi: 10.1080/13803395.2014.903900 [DOI] [PubMed] [Google Scholar]
- 7.Krupp LB, Serafin DJ, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother 2010;10(9):1437–47. doi: 10.1586/ern.10.99 [DOI] [PubMed] [Google Scholar]
- 8.Ocon AJ. Caught in the thickness of brain fog: exploring the cognitive symptoms of chronic fatigue syndrome. Front Physiol 2013;4:63. doi: 10.3389/fphys.2013.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonseca R, Bernardes M, Terroso G, de Sousa M, Figueiredo-Braga M. Silent burdens in disease: fatigue and depression in SLE. Autoimmune Dis 2014;2014:790724 Epub 2014 Jan 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tell D, Mathews HL, Janusek LW. Day-to-day dynamics of associations between sleep, napping, fatigue, and the cortisol diurnal rhythm in women diagnosed as having breast cancer. Psychosom Med 2014;76(7):519–28. doi: 10.1097/PSY.0000000000000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46(10):1121–3. doi: 10.1001/archneur.1989.00520460115022 [DOI] [PubMed] [Google Scholar]
- 12.Silverman MN, Heim CM, Nater UM, Marques AH, Sternberg EM. Neuroendocrine and immune contributors to fatigue. PM R 2010;2(5):338–46. doi: 10.1016/j.pmrj.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363(9413):978–88. doi: 10.1016/S0140-6736(04)15794-2 [DOI] [PubMed] [Google Scholar]
- 14.Bol Y, Duits AA, Hupperts RM, Verlinden I, Verhey FR. The impact of fatigue on cognitive functioning in patients with multiple sclerosis. Clin Rehabil 2010;24(9):854–62. doi: 10.1177/0269215510367540 [DOI] [PubMed] [Google Scholar]
- 15.Morris G, Maes M. Myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics. BMC Med 2013;11:205. doi: 10.1186/1741-7015-11-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filippi M, Rocca MA, Colombo B, Falini A, Codella M, Scotti G, et al. . Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. NeuroImage 2002;15(3):559–67. doi: 10.1006/nimg.2001.1011 [DOI] [PubMed] [Google Scholar]
- 17.Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, Lassmann H, et al. . Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 2011;365(23):2188–97. doi: 10.1056/NEJMoa1100648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vercellino M, Masera S, Lorenzatti M, Condello C, Merola A, Mattioda A, et al. . Demyelination, inflammation, and neurodegeneration in multiple sclerosis deep gray matter. J Neuropathol Exp Neurol 2009;68(5):489–502. doi: 10.1097/NEN.0b013e3181a19a5a [DOI] [PubMed] [Google Scholar]
- 19.Tartaglia MC, Narayanan S, Arnold DL. Mental fatigue alters the pattern and increases the volume of cerebral activation required for a motor task in multiple sclerosis patients with fatigue. Eur J Neurol 2008;15(4):413–9. doi: 10.1111/j.1468-1331.2008.02090.x [DOI] [PubMed] [Google Scholar]
- 20.Barsevick AM, Newhall T & Brown S.. Management of cancer-related fatigue. Clin J Oncol Nurs 2008;12(5 Suppl):21–5. doi: 10.1188/08.CJON.S2.21-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heesen C, Koehler G, Gross R, Tessmer W, Schulz KH, Gold SM. Altered cytokine responses to cognitive stress in multiple sclerosis patients with fatigue. Mult Scler 2005;11(1):51–7. doi: 10.1191/1352458505ms1129oa [DOI] [PubMed] [Google Scholar]
- 22.Heesen C, Nawrath L, Reich C, Bauer N, Schulz KH, Gold SM. Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry 2006;77(1):34–9. doi: 10.1136/jnnp.2005.065805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pokryszko-Dragan A, Bilinska M, Gruszka E, Kusinska E, Podemski R. Assessment of visual and auditory evoked potentials in multiple sclerosis patients with and without fatigue. Neurol Sci 2015;36(2):235–42. doi: 10.1007/s10072-014-1953-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci 1994;21(1):9–14. doi: 10.1017/S0317167100048691 [DOI] [PubMed] [Google Scholar]
- 25.Bailey A, Channon S, Beaumont JG. The relationship between subjective fatigue and cognitive fatigue in advanced multiple sclerosis. Mult Scler 2007;(1):73–80. doi: 10.1177/1352458506071162 [DOI] [PubMed] [Google Scholar]
- 26.Engstrom M, Flensner G, Landtblom AM, Ek AC, Karlsson T. Thalamo-striato-cortical determinants to fatigue in multiple sclerosis. Brain Behav 2013;3(6):715–28. doi: 10.1002/brb3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Association of Neuroscience Nurses (AANN), Association of Rehabilitation Nurses (ARN), International Organization of Multiple Sclerosis Nurses (IOMSN) Nursing management of the patient with multiple sclerosis. Glenview (IL): American Association of Neuroscience Nurses (AANN); 2011. [Google Scholar]
- 28.Iriarte J, Subirá ML, Castro P. Modalities of fatigue in multiple sclerosis: correlation with clinical and biological factors. Mult Scler 2000;6(2):124–30. doi: 10.1191/135245800678827572 [DOI] [PubMed] [Google Scholar]
- 29.Johansson S, Ytterberg C, Gottberg K, Widén Holmqvist L, von Koch L. Use of health services in people with multiple sclerosis with and without fatigue. Mult Scler 2008;15(1):88–95. doi: 10.1177/1352458508095730 [DOI] [PubMed] [Google Scholar]
- 30.Kim E, Lovera J, Schaben L, Melara J, Bourdette D, Whitham R. Novel method for measurement of fatigue in multiple sclerosis: real-time digital fatigue score. J Rehabil Res Dev 2010;47(5):477–84. doi: 10.1682/JRRD.2009.09.0151 [DOI] [PubMed] [Google Scholar]
- 31.Debouverie M, Pittion-Vouyovitch S, Brissart H, Guillemin F. Physical dimension of fatigue correlated with disability change over time in patients with multiple sclerosis. J Neurol 2008; 255(5):633–6. [Google Scholar]
- 32.Melamud L, Golan D, Luboshitzky R, Lavi I, Miller A. Melatonin dysregulation, sleep disturbances and fatigue in multiple sclerosis. J Neurol Sci 2012;314(1–2):37–40. doi: 10.1016/j.jns.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 33.Mills RJ, Calabresi M, Tennant A, Young CA. Perceived changes and minimum clinically important difference of the Neurological Fatigue Index for multiple sclerosis (NFI-MS). Mult Scler 2013;19(4):502–5. doi: 10.1177/1352458512457840 [DOI] [PubMed] [Google Scholar]
- 34.Mills RJ, Young CA. The relationship between fatigue and other clinical features of multiple sclerosis. Mult Scler 2011;17(5):604–12. doi: 10.1177/1352458510392262 [DOI] [PubMed] [Google Scholar]
- 35.Mills RJ, Young CA, Pallant J, Tennant A. Development of a patient reported outcome scale for fatigue in multiple sclerosis: the neurological fatigue index (NFI). Health Qual Life Outcomes 2010;8:22. doi: 10.1186/1477-7525-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Najafi MR, Toghianifar N, Etemadifar M, Haghighi S, Maghzi AH, Akbari M. Circadian rhythm sleep disorders in patients with multiple sclerosis and its association with fatigue: a case-control study. J Res Med Sci 2013;18(Suppl 1):S71–3. [PMC free article] [PubMed] [Google Scholar]
- 37.Papuć E, Stelmasiak Z, Grieb P, Pawel G, Rejdak K. CSF hypocretin-1 concentrations correlate with the level of fatigue in multiple sclerosis patients. Neurosci Lett 2010;479(3):9–12. doi: 10.1016/j.neulet.2010.02.062 [DOI] [PubMed] [Google Scholar]
- 38.Steens A, de Vries A, Hemmen J, Heersema T, Heerings M, Maurits N, et al. . Fatigue perceived by multiple sclerosis patients is associated with muscle fatigue. Neurorehabil Neural Repair 2012;26(1):48–57. doi: 10.1177/1545968311416991 [DOI] [PubMed] [Google Scholar]
- 39.Streckis V, Skurvydas A, Mamkus G. Effect of the time of day on central and peripheral fatigue during 2-min maximal voluntary contractions in persons with multiple sclerosis: gender differences. J Electromyogr Kinesiol 2014;24(5):601–6. doi: 10.1016/j.jelekin.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 40.Tedeschi G, Dinacci D, Lavorgna L, Prinster A, Savettieri G, Quattrone A, et al. . Correlation between fatigue and brain atrophy and lesion load in multiple sclerosis patients independent of disability. J Neurol Sci 2007;263(1–2):15–9. doi: 10.1016/j.jns.2007.07.004 [DOI] [PubMed] [Google Scholar]
- 41.Tomasevic L, Zito G, Pasqualetti P, Filippi M, Landi D, Ghazaryan A, et al. . Cortico-muscular coherence as an index of fatigue in multiple sclerosis. Mult Scler 2013;19(3):334–43. doi: 10.1177/1352458512452921 [DOI] [PubMed] [Google Scholar]
- 42.Vetrugno R, Stecchi S, Scandellari C, Pierangeli G, D'Angelo R, Provini F, et al. . Sleep-wake and body core temperature rhythms in multiple sclerosis with fatigue. Clin Neurophysiol 2007;118(1):228–34. doi: 10.1016/j.clinph.2006.09.021 [DOI] [PubMed] [Google Scholar]
- 43.Ritvo PG, Fischer JS, Miller DM, Andrews H, Paty DW, LaRocca NG. MSQLI: multiple sclerosis quality of life inventory: a user's manual. New York, NY: National Multiple Sclerosis Society; 1997. [Google Scholar]
- 44.Larson RD. Psychometric properties of the modified fatigue impact scale. Int J MS Care 2013;15(1):15–20. doi: 10.7224/1537-2073.2012-019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhat S, Chokroverty S. Fatigue in neurologic disorders. Sleep Med Clin 2013;8(2):191–212. doi: 10.1016/j.jsmc.2013.02.006 [DOI] [Google Scholar]
- 46.Barwick F, Arnett P, Slobounov S. EEG correlates of fatigue during administration of a neuropsychological test battery. Clin Neurophysiol 2012;123(2):278–84. doi: 10.1016/j.clinph.2011.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adamczyk-Sowa M, Pierzchala K, Sowa P, Mucha S, Sadowska-Bartosz I, Adamczyk J, et al. . Melatonin acts as antioxidant and improves sleep in MS patients. Neurochem Res 2014;39(8):1585–93. doi: 10.1007/s11064-014-1347-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andreasen AK, Spliid PE, Anderson H, Jakobsen J. Fatigue and processing speed are related in multiple sclerosis. Eur J Neurol 2010;17(2):212–18. doi: 10.1111/j.1468-1331.2009.02776.x [DOI] [PubMed] [Google Scholar]
- 49.Côté I, Trojan DA, Kaminska M, Cardoso M, Benedetti A, Weiss D, et al. . Impact of sleep disorder treatment on fatigue in multiple sclerosis. Mult Scler 2013;19(4):480–9. doi: 10.1177/1352458512455958 [DOI] [PubMed] [Google Scholar]
- 50.Hanken K, Eling P, Hildebrandt H. The representation of inflammatory signals in the brain - a model for subjective fatigue in multiple sclerosis. Front Neurol 2014;5:264. doi: 10.3389/fneur.2014.00264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider S, Stone AA, Schwartz JE, Broderick JE. Peak and end effects in patients’ daily recall of pain and fatigue: a within-subjects analysis. J Pain 2011;12(2):228–35. doi: 10.1016/j.jpain.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris G, Berk M, Walder K, Maes M. Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med 2015;13(1):28. doi: 10.1186/s12916-014-0259-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-L [DOI] [PubMed] [Google Scholar]
- 54.Chaudhuri A, Behan PO. Fatigue and basal ganglia. J Neurol Sci 2000;179(S1–2):34–42. doi: 10.1016/S0022-510X(00)00411-1 [DOI] [PubMed] [Google Scholar]
- 55.Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology 2013;80(4):409–16. doi: 10.1212/WNL.0b013e31827f07be [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malekzadeh A, Van de Geer-Peeters W, De Groot V, Teunissen C, Beckerman H, Trefams-Ace Study Group . Fatigue in patients with multiple sclerosis: is it related to pro- and anti-inflammatory cytokines? Dis Markers 2015;2015:758314. doi: 10.1155/2015/758314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weier K, Penner IK, Magon S, Amann M, Naegelin Y, Andelova M, et al. . Cerebellar abnormalities contribute to disability including cognitive impairment in multiple sclerosis. PLoS One 2014;9(1):e86916. doi: 10.1371/journal.pone.0086916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melief J, de Wit SJ, van Eden CG, Teunissen C, Hamann J, Uitdehaag BM, et al. . HPA axis activity in multiple sclerosis correlates with disease severity, lesion type and gene expression in normal-appearing white matter. Acta Neuropathol 2013;126(2):237–49. doi: 10.1007/s00401-013-1140-7 [DOI] [PubMed] [Google Scholar]
- 59.Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer related fatigue. Oncologist 2007;12 Suppl 1:22–34. doi: 10.1634/theoncologist.12-S1-22 [DOI] [PubMed] [Google Scholar]
- 60.Alvarenga-Filho H, Papais-Alvarenga RM, Carvalho SR, Clemente HN, Vasconcelos CC, Dias RM. Does fatigue occur in MS patients without disability? Int J Neurosci 2015;125(2):107–15. doi: 10.3109/00207454.2014.909415 [DOI] [PubMed] [Google Scholar]
- 61.Leocani L, Columbo B, Comi G. Physiopathology of fatigue in multiple sclerosis. Neurol Sci 2008;Suppl 2:S241–3. doi: 10.1007/s10072-008-0950-1 [DOI] [PubMed] [Google Scholar]
- 62.Edeschi G, Dinacci D, Lavorgna L, Prinster A, Savettieri G, Quattrone A, et al. . Correlation between fatigue and brain atrophy and lesion load in multiple sclerosis patients independent of disability. J Neurol Sci 2007;263(1–2):15–9. doi: 10.1016/j.jns.2007.07.004 [DOI] [PubMed] [Google Scholar]
- 63.Lenz ER, Suppe F, Gift AG, Pugh LC, Milligan RA. Collaborative development of middle-range nursing theories: toward a theory of unpleasant symptoms. ANS Adv Nurs Sci 1995;17(3):1–13 doi: 10.1097/00012272-199503000-00003 [DOI] [PubMed] [Google Scholar]
- 64.Multiple Sclerosis Council for Clinical Practice Guidelines Fatigue and multiple sclerosis: evidence-based management strategies for fatigue in multiple sclerosis. Washington, DC: Paralyzed Veterans of America; 1998. [Google Scholar]
- 65.Marrie RA, Cutter G, Tyry T, Hadjimichael O, Campagnolo D, Vollmer T. Validation of the NARCOMS registry: fatigue assessment. Mult Scler 2005;11(5):583–4. doi: 10.1191/1352458505ms1216oa [DOI] [PubMed] [Google Scholar]
- 66.Finke C, Schlichting J, Papazoglou S, Scheel M, Freing A, Soemmer C, et al. . Altered basal ganglia functional connectivity in multiple sclerosis patients with fatigue. Mult Scler 2015;21(7):925–34. doi: 10.1177/1352458514555784 [DOI] [PubMed] [Google Scholar]



