Significance
The Amazonian tropical forests have been disappearing at a fast rate in the last 50 y due to deforestation to open areas for agriculture, posing high risks of irreversible changes to biodiversity and ecosystems. Climate change poses additional risks to the stability of the forests. Studies suggest “tipping points” not to be transgressed: 4° C of global warming or 40% of total deforested area. The regional development debate has focused on attempting to reconcile maximizing conservation with intensification of traditional agriculture. Large reductions of deforestation in the last decade open up opportunities for an alternative model based on seeing the Amazon as a global public good of biological assets for the creation of high-value products and ecosystem services.
Keywords: Amazon tropical forests, Amazon sustainability, Amazon land use, Amazon savannization, climate change impacts
Abstract
For half a century, the process of economic integration of the Amazon has been based on intensive use of renewable and nonrenewable natural resources, which has brought significant basin-wide environmental alterations. The rural development in the Amazonia pushed the agricultural frontier swiftly, resulting in widespread land-cover change, but agriculture in the Amazon has been of low productivity and unsustainable. The loss of biodiversity and continued deforestation will lead to high risks of irreversible change of its tropical forests. It has been established by modeling studies that the Amazon may have two “tipping points,” namely, temperature increase of 4 °C or deforestation exceeding 40% of the forest area. If transgressed, large-scale “savannization” of mostly southern and eastern Amazon may take place. The region has warmed about 1 °C over the last 60 y, and total deforestation is reaching 20% of the forested area. The recent significant reductions in deforestation—80% reduction in the Brazilian Amazon in the last decade—opens up opportunities for a novel sustainable development paradigm for the future of the Amazon. We argue for a new development paradigm—away from only attempting to reconcile maximizing conservation versus intensification of traditional agriculture and expansion of hydropower capacity—in which we research, develop, and scale a high-tech innovation approach that sees the Amazon as a global public good of biological assets that can enable the creation of innovative high-value products, services, and platforms through combining advanced digital, biological, and material technologies of the Fourth Industrial Revolution in progress.
A number of complex problems threaten our geopolitical, environmental, social, and economic stability: the links between global food and energy markets; the unsustainable depletion of natural resources and biodiversity stocks; the increasing water insecurity around the world; and, above all, the urgent need both to decarbonize the energy systems of the world to avoid catastrophic climate change and to adapt to unavoidable climate change underway. The scale and reach of the risks associated with climate change, together with their potentially irreversible nature, make this probably the greatest market failure and the starkest example of a “tragedy of the commons” the world has ever seen. To put this comparison in perspective, the net benefit to the world economy of a 50% reduction of tropical forest deforestation and degradation has been estimated at US $3.7 trillion (1).
Of particular importance is the continued deforestation in the Amazon, which could lead to the irreversible change of its tropical forests (2, 3) and the major loss of its biodiversity (4). The Amazon ecosystems harbor about 10 to 15% of land biodiversity (5, 6); its abundant rainfall of about 2.2 m⋅y−1 makes the region an important heat source for the atmosphere (7), generating an estimated 210,000 m3⋅s−1 to 220,000 m3⋅s−1 of river discharge (8, 9), which is ∼15% of the freshwater input into the oceans (10); it stores an estimated 150 billion to 200 billion tons of carbon (11–15); and it presents a mosaic of ethnological and linguistic diversity (16, 17).
A number of large-scale drivers of environmental change are operating simultaneously and interacting nonlinearly in the Amazon, namely, land-use change and climate changes due to global warming and to deforestation, which may, in turn, induce higher frequency of extreme climate events and of vegetation fires, adding to increased tropical forests’ exposure and vulnerability. Our scientific understanding has increased about the risks associated with these drivers of change acting synergistically (18, 19). By and large, environmental change in the region is a response to the global economy. Global market demand growth for animal and vegetable protein (20–22), new transportation and energy infrastructure projects (23), and weak institutions (24) can be cited as some of key drivers in this process.
The prevailing model for rural development in the Amazon over the last half century—replacing forests with agriculture, cattle ranching, and large-scale hydropower generation—has long been outdated for a number of environmental, economic, and social reasons (25–27). For instance, for Brazil, the gross agricultural product of the Amazon represents 14.5% of Brazil's agriculture sector gross domestic product (GDP), using a deforested area of about 750,000 km2. In contrast, São Paulo state accounts for 11.3% of the agriculture sector GDP, using an area of approximately 193,000 km2 (see Tables S1–S3 in Datasets Used to Derive Agricultural Sector GVA for the Brazilian Amazon). The conclusion is inescapable: Over 50 y of a deforestation-based development model have not resulted in wealth creation or better quality of life for those living in the Amazon—the Amazônidas (28, 29). Moreover, in terms of development policy pathways for the Amazon, two modes have historically dominated: (i) a valuable nature conservation approach with large swathes of territory legally protected from any economic and human activity outside indigenous peoples and (ii) an approach that has focused on conversion or degradation of forests for the production of either protein commodities or tropical timber at the forest frontier and the build-out of massive hydropower generation capacity—which, together, have been historically responsible for massive deforestation of the Amazon (30, 31) and generated other significant negative externalities. We argue therefore that there is a “Third Way” within reach that sees the Amazon as a global public good of biological assets and biomimetic designs that can enable the creation of innovative high-value products, services, and platforms for current and for entirely new markets.
Table S1.
Brazilian states GDP description
| State | GVA at basic prices—agriculture, × R$ 1,000 | GVA at basic prices—industry, × R$ 1,000 | GVA at basic prices, for services, including administration, public health, education and social security, × R$ 1,000 | Taxes and subsides on the products at basic prices, × R$ 1,000 | GDP at current prices | |
| × R$ 1,000 | % | |||||
| Acre | 1,462,779.23 | 1,105,049.26 | 5,175,323.09 | 733,363.14 | 8,476,514.71 | 0.22 |
| Alagoas | 1,476,184.50 | 4,648,419.31 | 15,807,304.63 | 2,642,899.57 | 24,574,808.01 | 0.65 |
| Amapá | 246,394.79 | 764,615.91 | 6,666,350.06 | 588,603.90 | 8,265,964.66 | 0.22 |
| Amazonas | 3,103,963.87 | 21,925,042.78 | 24,956,435.85 | 9,793,849.34 | 59,779,291.83 | 1.59 |
| Bahia | 9,796,432.82 | 41,089,574.41 | 84,806,575.81 | 18,647,874.53 | 154,340,457.56 | 4.09 |
| Ceará | 2,837,126.10 | 16,186,732.05 | 49,239,763.43 | 9,601,793.14 | 77,865,414.73 | 2.07 |
| Distrito Federal | 334,930.28 | 8,720,979.86 | 124,179,104.52 | 16,671,304.21 | 149,906,318.88 | 3.98 |
| Espírito Santo | 4,265,131.36 | 24,272,560.85 | 38,969,444.96 | 14,614,696.98 | 82,121,834.15 | 2.18 |
| Goiás | 11,950,496.79 | 22,536,480.58 | 50,280,701.84 | 12,808,251.13 | 97,575,930.34 | 2.59 |
| Maranhão | 6,969,106.69 | 6,350,799.13 | 27,133,723.38 | 4,802,313.26 | 45,255,942.46 | 1.20 |
| Mato Grosso | 11,728,285.96 | 10,921,321.25 | 30,375,342.80 | 6,575,040.11 | 59,599,990.12 | 1.58 |
| Mato Grosso do Sul | 5,843,826.26 | 8,376,499.59 | 23,600,979.73 | 5,692,901.15 | 43,514,206.73 | 1.15 |
| Minas Gerais | 26,101,804.73 | 103,376,322.18 | 178,386,736.94 | 43,516,041.26 | 351,380,905.10 | 9.32 |
| Pará | 4,676,289.58 | 29,408,404.00 | 36,958,763.84 | 6,804,139.08 | 77,847,596.51 | 2.06 |
| Paraíba | 1,212,255.06 | 6,433,429.06 | 20,915,452.55 | 3,385,921.85 | 31,947,058.52 | 0.85 |
| Paraná | 15,871,270.44 | 51,410,752.45 | 119,980,960.17 | 30,026,693.54 | 217,289,676.60 | 5.76 |
| Pernambuco | 3,662,008.56 | 18,076,555.00 | 59,890,750.17 | 13,557,400.35 | 95,186,714.09 | 2.52 |
| Piauí | 1,215,522.19 | 3,621,609.88 | 14,773,520.88 | 2,449,508.27 | 22,060,161.22 | 0.59 |
| Rio de Janeiro | 1,449,017.83 | 96,617,935.99 | 246,338,471.02 | 62,717,368.93 | 407,122,793.76 | 10.80 |
| Rio Grande do Norte | 1,205,095.44 | 6,128,140.88 | 21,209,532.22 | 3,796,126.18 | 32,338,894.72 | 0.86 |
| Rio Grande do Sul | 19,026,837.44 | 63,989,288.97 | 136,031,908.56 | 33,434,561.85 | 252,482,596.82 | 6.70 |
| Rondônia | 4,472,190.16 | 3,046,436.44 | 13,297,907.82 | 2,744,109.31 | 23,560,643.72 | 0.62 |
| Roraima | 275,775.59 | 757,790.44 | 4,784,709.17 | 522,326.06 | 6,340,601.26 | 0.17 |
| Santa Catarina | 8,753,836.11 | 44,527,902.03 | 77,336,159.69 | 21,864,440.28 | 152,482,338.11 | 4.04 |
| São Paulo | 19,398,384.49 | 301,453,338.92 | 715,846,256.06 | 210,897,947.14 | 1,247,595,926.61 | 33.09 |
| Sergipe | 982,489.43 | 6,103,937.99 | 14,290,664.53 | 2,555,063.39 | 23,932,155.33 | 0.63 |
| Tocantins | 2,859,956.30 | 4,002,271.78 | 8,918,240.30 | 1,459,666.66 | 17,240,135.04 | 0.46 |
| Total | 171,177,392.00 | 905,852,191.00 | 2,150,151,084.00 | 542,904,204.58 | 3,770,084,871.58 | |
| Percent | 4.54 | 24.03 | 57.03 | 14.40 | ||
Data extracted from IBGE/CENSUS 2010. Available at www.ibge.gov.br/home/estatistica/economia/pibmunicipios/2010/default_base.shtm. R$, Reais (GDP in Brazilian currency).
Table S3.
GDP and GVA ratios
| Index | Values | |
| R$ 1,000 | % | |
| GDP Amazon Region | 241,772,586.29 | |
| GDP Brazil | 3,770,084,871.58 | |
| GDP Amazon Region/GDP Brazil | 6.41 | |
| GVA Amazon Region - only Agriculture | 24,224,303.17 | |
| GDP Amazon Region | 241,772,586.29 | |
| GDP Amazon Region/GVA Amazon Region - only Agriculture | 10.02 | |
| GVA Amazon Region - only Agriculture | 24,224,303.17 | |
| GVA Brazil - only Agriculture | 171,177,392.00 | |
| GVA Brazil - only Agriculture/GVA Amazon Region - only Agriculture | 14.15 | |
| GVA Amazon Region/GDP Brazil | 0.64 | |
Source is IBGE/CENSUS 2010. For these analyzes, the Amazon region was considered to be the municipalities described in Ordinance 96, published in Brazilian Federal Register in March 27, 2008, issued by the Ministry of Environment. Available at www.fundoamazonia.gov.br/FundoAmazonia/export/sites/default/site_pt/Galerias/Arquivos/Downloads/Portaria_MMA_96_08_DEFINIxO_DO_BIOMA_AMAZxNIA.pdf. The economic data—GVA, Taxes, Subsides and GDP—were extracted from the IBGE publication of CENSUS 2010. Available at www.ibge.gov.br/home/estatistica/economia/pibmunicipios/2010/default_base.shtm. The data on Agriculture CENSUS 2006 is available at IBGE webpage: www.ibge.gov.br/home/estatistica/economia/agropecuaria/censoagro/2006/default.shtm). R$, Reais (GDP in Brazilian currency).
It is urgent to halt deforestation, keeping in mind that almost 1 million square kilometers of the Amazon tropical forests have already been deforested and another equal portion finds itself in the process of degradation (27). The rate of deforestation has declined in the last several years; this decline is conspicuous in the Brazilian Amazon, where deforestation rates have been cut down by almost 80% since 2005 (32–34) at the same time that the agricultural output in the region has been increasing significantly (35). It is therefore becoming clear that economic growth is decoupled from deforestation as demonstrated by ample facts, such as the case of the reduction of deforestation rates observed between 2005 and 2014, which are opposed to the growth of the values of agricultural gross value added (GVA) in North Brazil, which almost tripled during this period (32). Since 2005, deforestation rates in Brazilian Amazon have decreased from almost 30,000 km2⋅y−1 to a rate of around 6,000 km2⋅y−1, on average, from 2011 to 2015 (33), indicating the difficulties of zero deforestation targets (36). This sharp decline of deforestation was enabled by several factors, including purpose-built satellite monitoring capabilities, effective law enforcement and compliance, industry value chain initiatives like the soy moratorium, restrictions on access to credit for farms located in deforested areas, and expansion of protected areas and indigenous territory encompassing 47% of the entire Brazilian Amazon region (37). Long-term-demand growth for agricultural commodities in the emerging markets, weak institutions, and large energy infrastructure projects may potentially contribute as underlying and proximate drivers to the return of high deforestation rates in the absence of alternative development pathways (27, 30, 38–40).
The present economic scenario continues to conspire against the Amazon by placing a higher premium on agricultural commodities such as soybeans, meat, and tropical timber than on standing forests. The long-term success of antideforestation policies must rest on firmer ground besides command and control measures to curb illegal deforestation.
The challenge, therefore, is to reconcile the current development model with a new paradigm for sustainable development of the Amazon. A corollary to this greater challenge is the urgent need to deploy a high-tech innovation ecosystem approach to serve as the basis for this new model of sustainable development for the Amazon.
In this review, we assess scientific knowledge on climate variability and extremes, on anthropogenic drivers of environmental change in the region, and on the impacts and risks for the future of the tropical forests, and we propose a paradigm for the sustainable development of the Amazon, a model that intrinsically depends on the existence of the forests.
Datasets Used to Derive Agricultural Sector GVA for the Brazilian Amazon
The data presented of fraction of agriculture sector GVA for the Brazilian Amazon region, and, for comparison purposes, of a region with higher agriculture productivity, of São Paulo state are derived from the tables presented below. Table S1 shows sectorial GVA for all Brazilian states and for all sectors. The agriculture sector GVA is seen in the second column. Table S2 shows agriculture sector GVA for the Amazon region, taking notice of the legal definition of the Brazilian Amazon region. Table S3 indicates the ratios of GVA and GDP of interest for the discussion. The area used for agriculture for São Paulo state is 192,420 km2 according to the 2006 agriculture census of Brazil.
Table S2.
Amazon region GDP description
| State | GVA at basic prices – Agriculture, × R$ 1,000 | GVA at basic prices – Industry, × R$ 1,000 | GVA at basic prices, for services, including administration, public health, education and social security, × R$ 1,000 | Taxes and subsides on the products at basic prices, × R$ 1,000 | Gross domestic product (GDP) at current prices, × R$ 1,000 |
| States entirety included | |||||
| Acre | 1,462,779.23 | 1,105,049.26 | 5,175,323.09 | 733,363.14 | 8,476,514.71 |
| Amapá | 246,394.79 | 764,615.91 | 6,666,350.06 | 588,603.90 | 8,265,964.66 |
| Amazonas | 3,103,963.87 | 21,925,042.78 | 24,956,435.85 | 9,793,849.34 | 59,779,291.83 |
| Pará | 4,676,289.58 | 29,408,404.00 | 36,958,763.84 | 6,804,139.08 | 77,847,596.51 |
| Rondônia | 4,472,190.16 | 3,046,436.44 | 13,297,907.82 | 2,744,109.31 | 23,560,643.72 |
| Roraima | 275,775.59 | 757,790.44 | 4,784,709.17 | 522,326.06 | 6,340,601.26 |
| States partially included | |||||
| Maranhão | 3,138,811.30 | 4,823,761.20 | 19,697,115.14 | 4,130,179.25 | 31,789,866.90 |
| Mato Grosso | 6,280,628.99 | 3,359,014.14 | 9,991,347.43 | 1,890,255.38 | 21,521,245.94 |
| Tocantins | 567,469.65 | 834,658.10 | 2,426,669.66 | 362,063.34 | 4,190,860.75 |
| Total | |||||
| Amazon Region | 24,224,303.17 | 66,024,772.27 | 123,954,622.05 | 27,568,888.81 | 241,772,586.29 |
Data extracted from IBGE/CENSUS 2010. Available at www.ibge.gov.br/home/estatistica/economia/pibmunicipios/2010/default_base.shtm. R$, Reais (GDP in Brazilian currency).
Climatic Variability and Extremes, and the Lengthening of the Dry Season
Precipitation Variability and Extremes.
A suite of geographical, geomorphological, and climatic factors makes the Amazon basin an area with high precipitation. The average basin-wide annual precipitation is on the order of 2,200 mm, ranging from 3,000 mm in the west, due to the influence of the Andes, to values around 1,700 mm over the southeast of the basin, areas of intense land-use and land-cover change, known as “deforestation arc” (41). High precipitation rates are maintained both by moisture flows from evaporation in the tropical Atlantic Ocean and by forest evapotranspiration (ET) recycling (7, 42). Precipitation seasonality varies markedly across the basin: minimum monthly precipitation of >150 mm⋅mo−1 and short or absent dry season in the west and northwest, in contrast to a very seasonal regime in the south and southeast with longer dry seasons (>4 mo with monthly values of <100 mm) (43, 44). Given average forest ET of 3.5 mm⋅d−1 to 4 mm⋅d−1 (45), below about 100 mm⋅mo−1 of precipitation, ET is assumed to exceed incoming precipitation, and the forest is in water deficit (46).
Precipitation pattern in the Amazon basin has a strong interannual and interdecadal variability, largely influenced by fluctuations in sea surface temperature (SST) of the tropical Pacific (related mostly to El Niño−Southern Oscillation) and tropical Atlantic (Atlantic “Dipole” Mode) Oceans. Severe droughts are associated, in general, with the occurrence of strong El Niño events affecting mostly the central and eastern portions of the Amazon, as was the case in 1906, 1912, 1926, 1983, 1992, 1998 (47), and 2015 (33). On the other hand, the warmer tropical North Atlantic and cooler tropical South Atlantic affect also the west and northwest of the basin and were responsible for the severe droughts that occurred in 1964, 2005 (48), and 2010 (49), which was recognized as one of the strongest and extensive droughts of recent decades: The 2005 drought affected about 1.9 million square kilometers, whereas the 2010 drought affected around 3 million square kilometers (50).
Although droughts and floods are part of the Amazon natural climate variability, the extreme drought and flood events that took place in the past decade (2005, 2010, and 2015 droughts; 2009 and 2012 floods) have been unusual and may have long-term implications. Global warming is projected to increase the frequency and even the intensity of extreme events (51). Drought extreme events have the potential to increase the mortality of forests, and the synergistic association between severe droughts, deforestation, and fire can be highly deleterious to the Amazon forest (see CO2 Fertilization and Forest Mortality).
The Lengthening of the Dry Season.
Another important aspect of the functioning and maintenance of the Amazon forest is the dry season length being shorter than about 4 mo. There is growing evidence of lengthening of the region’s dry season, primarily over southern and southeastern Amazon (49, 52, 53). The reasons for this lengthening are still not very clear. It has been suggested that large-scale influence of SST gradients of the North and South Atlantic (49, 54, 55), or a strong influence of dry season ET (56, 57), in response to a seasonal increase of solar radiation (58), may play a role.
In particular, this apparent lengthening of the dry season has been quantified: The dry-season length has been observed to have increased [(6.5 ± 2.5) days per decade] over southern Amazonia since 1979, primarily owing to a later onset of the wet season, and is accompanied by a prolonged fire season (53). These changes cannot be simply linked to the interannual variability of the tropical Pacific and Atlantic Oceans and may indicate that, in addition to the moisture transports from the oceans, soil moisture from continental areas could act as an important precondition for the onset of the wet season (59–61). Therefore, understanding the forests’ ability to maintain high ET rates during low-precipitation periods is an important element to better understand not only the drought-forest response, but also aspects that influence the transition from dry to wet season.
Seasonal Variability in ET.
Considering a wide range of climate variability patterns in the Amazon, it could be expected that other hydroclimatic variables also exhibited a large spatial and temporal variability. In fact, it is known that seasonal and interannual variations of the ET in tropical forests are mainly controlled by variations in the light (radiation) and soil moisture (62). Data from flux towers installed in the region as a result of the Anglo-Brazilian Amazonian Climate Observation Study (ABRACOS) and the Large-Scale Biosphere-Atmosphere Experiment in Amazonia (LBA) indicated the occurrence of ET rates as high in the dry season as in the wet season (45). However, in regions such as eastern and southern Amazon, this was not the case (63). From data obtained from the LBA flux towers networks, it was observed that, in experimental sites where the average annual rainfall exceeds 1,900 mm and the average dry season length is less than 4 mo (e.g., Manaus, Santarém, and Rondônia), ET rates tend to increase in the dry season in response to an increase in solar radiation, reaching values of around 4 mm, similar to wet season ET values (64). On the other hand, regions with average annual rainfall below 1,700 mm and longer dry seasons (>4 mo), such as in southern and southeastern Amazon, showed clear evidence of decreasing ET during the dry season, with maximum values of around 2.5 mm⋅d−1. A similar threshold of close to 2,000 mm of annual rainfall was identified in the photosynthesis and ET patterns along the Amazon forest and for tropical forests in Africa and Asia (65). In regions where the annual rainfall is above this value, water stored in the soil during the wet season seems to be able to supply ET and photosynthesis for the subsequent dry season. However, this normally is not the case for regions where the average annual rainfall is below this value, highlighting a clear spatial and seasonal pattern of ET and photosynthesis variability (66). The ET's controlling mechanisms also varied over this rainfall gradient, with climate demands (particularly radiation and vapor pressure deficit) controlling ET rates in wetter areas and soil moisture deficit controlling ET in the driest areas (64).
Due to the Amazon’s huge dimension and diversity, some Amazon regions could present high values of ET during the dry season even when annual precipitation, on average, is smaller than 2,000 mm. In Paragominas, for example, at the northeast flank of the Amazon, the mean annual precipitation of 1,800 mm can sustain high ET rates even during a dry season lasting for about 5 mo (67). Storage of water in deep, clay-rich soil layers may presumably be the reason for maintenance of ET in periods of absence of rain (67–70).
Despite some exceptions, in general, the driest regions in the Amazon are found in the southern and southeastern portions, presenting a climate pattern similar to a savannah, where the vegetation responds with loss of leaves and dormant state in response to water stress of the prolonged dry season (64). This region, known as the “Arc of Deforestation,” is experiencing heavy anthropogenic change and could be presenting signals of change in the equilibrium state, in the sense of the prevalence of a dry vegetation type (71).
Climate Change: Global and Regional Perspectives
Global Climate Change.
Climate change resulting from increased emissions of greenhouse gases, and from other anthropogenic forcings, has the potential to increase air temperature and cause complex changes in precipitation patterns (51). Despite the importance of understanding how climate affects the structure and functioning of the tropical forest as we know it today, it has not been an easy task to identify current and plausible future changes in climate variables—especially those related to the hydrological cycle—in these regions. For the Amazonian forest, this difficulty is due, in part, to the scarcity of historical data and, in part, to the natural variability of precipitation (72).
The Amazon—similarly to all continental areas of the world—has warmed about 1 °C during the last century (51, 73, 74). The temperature increase was more pronounced since the 1970s and was attributed to the global temperature increase caused primarily by greenhouse gas emissions (51, 75, 76). Temperature increases are more pronounced in deforested areas during the dry season and can reach between 1 °C and 1.5 °C, on average, solely due to the effect of deforestation, because the surface energy budget is altered and sensible heating is higher for areas covered with pastures in the Amazon (77).
It has been more difficult to identify long-term changes in rainfall patterns in the Amazon. This is due to the large natural variability, relatively small number of weather stations, and important gaps in time series (72, 78). To complement these studies, some assessments have been based on river discharge data, rather than precipitation, due to their better integrative nature and dataset consistency (79, 80). Although some studies indicated a decreasing trend in rainfall (drying) in the Amazon, in recent decades (80–83), others pointed to a wetting trend for the region, as a result of an intensification of the hydrological cycle (79, 84, 85). These wetting trends (up to 80 mm⋅mo−1 in the period 2000–2009 compared with period 1981–1990) are not homogeneously distributed around the basin, and they tend to occur during the wet season over the northwestern part of the basin. On the other hand, the decreasing wet season rainfall trend (−20 mm⋅mo−1 for the same period) was observed in the southern and southeastern parts of the basin (79).
Precipitation reductions observed in the last two decades over southern and southeastern Amazon cannot, as yet, be fully explained, but one possible explanation could be the effects of extensive land-use change in this region and relative changes in the albedo (79, 86). The increasing precipitation trend observed during the rainy season was attributed to a gradual warming of the tropical North Atlantic SST and the resulting increase of water vapor supply from the ocean toward the continent (79). The influence of the tropical North Atlantic SST anomalies, however, is more gradual and has a different pattern from that observed during 2005 and 2010 droughts, when North Atlantic SST anomalies resulted in changes in the Intertropical Convergence Zone (ITCZ) position (79). The important point here is that, even for wetter conditions, the southern and southeastern Amazon forest presented drying trends in the last two decades. Observed interdecadal trends in precipitation (87) have been associated with natural climate variability—somewhat different from the apparent lengthening of the dry season in Amazonia—and sharp shifts in precipitation patterns in the mid 1940s, 1970s, and 2000s are likely linked to phase shifts of the Pacific Decadal Oscillation (72, 80, 88, 89).
In summary, the observed absence of a significant long-term trend in Amazon river discharge—a proxy of basin-wide precipitation variations—highlights the difficulty of detecting clear long-term, anthropogenic climate change impacts in precipitation. In contrast, the observed increase in the frequency of extremes over the past decade (see Precipitation Variability and Extremes) could be an early manifestation of global climate change (90), because there have been both positive and negative precipitation extremes, which, however, did not alter significantly the long-term basin-wide averages.
Tropical Deforestation and Regional Climate Change.
Deforestation can affect vegetation through changes in the regional climate. Modeling studies suggest that a decrease in the ET rates and an increase in atmospheric temperature were caused by the large-scale replacement of the Amazon forests by pasture (7, 91, 92). It is estimated that these changes will lead to changes in local precipitation patterns, but how large these changes will be is still uncertain. Small-scale deforestation can lead to an increase in local precipitation by the so-called “wind effect” (93, 94). On the other hand, large-scale deforestation may act to decrease the precipitation rate. Increases in nonprecipitation clouds and a decrease in the dry season precipitation have been observed over deforested areas in Amazonia (95). Modeling studies simulating the rainforest replacement by pasture also showed a decrease in rainfall with an increase in deforested areas (7, 91, 92, 96–98). The extent of total deforested area also matters, with greater effects occurring for deforested areas exceeding 40% of the total area covered by forests (91). Complete deforestation could cause eastern Amazonia to warm by more than 3 °C, and precipitation from July to November could decrease by up to 40%. Crucially, these changes would be in addition to any change resulting from global warming.
Regional climate models with higher spatial resolution (25-km grid) project a smaller decrease of regional precipitation (−62 mm⋅y−1) for a complete deforestation scenario (96). Some regional models also show that low levels of deforestation (up to around 20%) increase calculated precipitation over the deforested area as a result of a heterogeneous heating of the land surfaces, which increases mesoscale convection (“vegetation breeze”) and cloud formation (96, 99). However, the importance of this effect varies according to the size and pattern of the deforestation patches (100) as well as the model considered. Most regional models agree that the effect of the vegetation breeze vanishes beyond a certain area of deforestation (around 30%) for which a decrease of regional precipitation is expected, especially during the dry season (99, 101). It is important to highlight that examples of land-use change that has been sufficiently widespread to detect its effect on river discharge over and above the effects of interannual variation or trends in precipitation patterns are currently limited to watersheds that were dominated by neighboring Cerrado vegetation, with only small fractions of Amazon forest vegetation (102, 103). It may be necessary to have a similar magnitude of land-use change in forest-only subbasins of the Amazon in order for its effect to be unequivocally established.
A key to risk analysis for the maintenance of the Amazon ecosystems is that the impacts of deforestation are greater under drought conditions, as fires used for forest clearance frequently get out of control and burn larger areas (see Extreme Droughts, Deforestation, and Fire). The impact of land-use change on simulated precipitation and temperature occurs primarily during the dry season and in regions of relatively low annual mean precipitation, projecting precipitation reductions and temperature increases (104). Reducing deforestation may help to boost forest resilience under a changing climate. Forest fires, drought, and logging increase susceptibility to further burning, and deforestation and smoke can inhibit precipitation, exacerbating fire risk (see Extreme Droughts, Deforestation, and Fire). The likely continuation of strong El Niño episodes (e.g., 1997−1998 and 2015–2016 episodes) into the near future and the possibility that the pattern of intense Amazon droughts seen in the last decade continues into the future, keeping business as usual policies, means that a large fraction of the forest will be cleared, logged, damaged by drought, or burned in the next few decades (105).
In sum, we can say that reducing deforestation could minimize these impacts as well as mitigate emissions of greenhouse gases. It has been suggested that there may be thresholds or “tipping points” that should not be transgressed for the maintenance of the Amazonian tropical forests: 40% of area deforested, beyond which forest loss causes climate impacts that cause further forest loss (91); global warming of 3 °C to 4 °C may also lead to a similar tipping point (106, 107). Although the existence of these tipping points still requires further research, interaction between climate change due to global warming and due to large-scale deforestation may make them more likely.
In sum, the observations of changes in hydroclimatic factors (e.g., the lengthening of the dry season and the enhanced occurrence of precipitation extremes)—added to global warming scenarios of increased temperatures and continued deforestation and forest fires—may wreak havoc on the stability of tropical ecosystems (see Third Way as Paradigm of Sustainable Development for the Amazon).
Impacts of Anthropogenic Drivers of Change in the Amazon
Extreme Droughts, Deforestation, and Fire.
Despite coming from different anthropogenic drivers, which act upon different scales, deforestation and extreme drought events may damage tropical forest ecosystems in an analogous way: Both have the potential to enhance mortality selectively, creating degraded areas in which the equilibrium state of the humid forest can be disrupted.
A great number of studies and advances were made in recent decades, to better understand the impacts of drought extreme events on tropical forests. However, important questions still remain. In fact, the ability of some areas of the Amazon rainforest to maintain high ET rates and, eventually, keep growing or begin leaf flushing (108) during the dry season does not guarantee that humid forest could be resilient to extreme and prolonged droughts. In situ observations of the impact of “natural” extreme droughts (109) and artificially induced droughts for several years (110–112) showed that forest responds with interruption of growth and mortality of some species during a prolonged drought period. The results of artificially induced and natural droughts have shown that the larger trees [diameter at breast height (dbh) > 30 cm], together with lianas, are the most vulnerable ones (112). This behavior is contrary to the hypothesis, previously assumed, that the larger trees would be more resilient to droughts as a result of a deeper root system, allowing them to capture water from the deeper soil layers as a drought survival strategy. It was observed, however, that these large trees could be under water stress due to a significant exposure to solar radiation, eventually dying by cavitation and embolism (113) during extreme droughts.
The vulnerability of the larger trees is a critical aspect of forest functioning and maintenance. It implies that droughts can act selectively, changing species composition and endangering local biodiversity (109). In addition, mortality of the highest species reduces the shading over lower canopy, litterfall, and soil. The increasing incident radiation in these areas enhances temperature and dryness, increasing vulnerability to subsequent droughts as well as to ignition sources and fire. Although the drought effects on stem growth could cease as soon as the drought finishes (3), this is not the case for tree mortality. In addition, the increase of dead biomass can result in a number of negative aspects: loss of habitat of endemic species, changes in the composition and biome structure, and changes in carbon budget and energy fluxes between the land surface and the atmosphere, besides potentially acting as a positive feedback to climate change.
Extreme events and deforestation can act synergistically in a two-way mode. Deforested areas can affect regional climate, and the regional climate, in its turn, can amplify the impact of deforestation, by increasing tree mortality far beyond the limits of the deforestation edges. In both situations, fire occurrence and spreading is greatly amplified. It has been observed that the forest fire scars in the Amazon increased substantially during extreme droughts years, particularly in the edges between intact forest and deforested areas. The association between a dry forest environment (soil and litterfall) and ignition sources from the anthropic activities (mainly the agricultural practices of slash and burn) promotes the leakage of fires toward the intact forest areas. The absence of rainfall, typical of the drought years, facilitates its propagation (46, 114–120).
Under normal conditions of high precipitation amounts and high atmospheric moisture, spontaneous occurrence of fire in the Amazon rainforest is quite rare (121). As a result, most of the local species are not adapted to fire, which hampers their recovery after recurrent burns. Forest areas submitted to successive fires over the years experience a change in the prevalence of secondary vegetation (119). Huge and successive fires have substantially increased tree mortality and favored the occurrence of short-life-cycle pioneer species. Invasive grasses observed in the burned areas act as a potential source of ignition during subsequent events of droughts, potentially indicating a change in the biome composition (71). This transition is more likely to occur in fragmented forest areas where disturbances are frequent and the dry season is longer (>4 mo to 5 mo) (71), or, in other words, at the southern region of the Amazon forest.
CO2 Fertilization and Forest Mortality.
In a CO2-enriched atmosphere, one of the few potentially resilience-increasing aspects for forests seems to be the effect known as “CO2 fertilization” (122). The increasing concentration of this gas in the atmosphere has been recognized as being responsible for faster growth of trees, a fact that has been proven in large-scale experiments (123–126). However, there are large uncertainties about the long-term response of the tropical forests to this effect (127), although modeling of this effect for the Amazon indicates that the impact of high degrees of climate change are attenuated, but not to the extent of avoiding forest loss (128). In a CO2-enriched environment, the plants can respond with stomatal closure to capture the same amount of CO2 required for photosynthesis (129). As a consequence, transpiration rates could decrease, potentially returning a lower amount of vapor to the atmosphere, or, in other words, altering ET rates (130). Ultimately, this behavior could impact precipitation in the Amazon, which, as shown above, plays an important role in generating dry season precipitation. Another unexpected unfavorable effect of CO2 fertilization could be the increase of mortality rates of adult trees in the tropics (131).
Terrestrial ecosystems and, in particular, tropical forests have been recognized as strong carbon sinks, comparable to the oceans (132–134). However, the potential connections between sinks of carbon and the spatial patterns of soil and tree characteristics across the region are also noteworthy. In the west, younger fertile soils (135) associated with enhanced plant growth coexist with higher tree mortality, complicating the interpretation of carbon sink (13, 136). In the eastern and central portions of the region, weathered and relatively infertile soils (135) host trees that live longer and grow taller (13, 136). Although these locations may potentially stock larger carbon pools in the long term, the responses to CO2 fertilization may be limited due to relative low nutrient availability (137). Despite uncertainties, it has been recognized that the mature forests of the Amazon store around 150 PgC to 200 PgC in their biomass (15), and the forest seems to be acting as an overall carbon sink, with assimilation rates estimated at 0.4 PgC⋅y−1 (109) over the recent past. Carbon stored in forests each year occurs in the form of growth of branches and trunks, new leaves and roots, and increase of soil organic matter (138). However, from field inventories carried out in 321 plots from 1983 to 2011, it was observed that the Amazon forest could be moving from a carbon sink to a carbon source ecosystem (131). Although the tree growth data confirmed the Earth's surface as a strong carbon sink, the same data show a decline in the growth rate of carbon accumulation and a one-third decline in net increase in aboveground biomass in the 2000s compared with the 1990s. Factors explaining this behavior are still not clear, but the main hypothesis is that the increase in forest productivity in recent years due to CO2 fertilization effect could have accelerated the life cycle of trees, anticipating their death when still young (131, 139–142). To reduce the uncertainties about the effect of increasing atmospheric CO2 concentrations on tropical ecosystems, a group of international scientists are proposing to carry out a Forest Free-Air CO2 Enrichment-type experiment in the Amazon (143).
Impacts of Hydropower Dams in the Amazon.
Major anthropogenic land-use change in the Amazon historically has been a consequence of either growing international demand for agricultural commodities or of growing energy demand domestically. In this context, the Amazon has been historically identified as a source of massive growth in hydropower capacity, and all Amazonian countries have made plans for its use in the present and in the foreseeable future. For instance, the Brazilian government has planned the expansion of hydroelectric power generation in the Brazilian Amazon to make it a large net exporter of electricity to the rest of the country. The plan calls for 30 new large dams in the next 30 y (23), with the associated flooding of ∼12,000 km2 (144). The flooding of these areas will cause a series of environmental impacts: significant increases of greenhouse gas emissions (145), disorders to local wildlife by blocking mass fish migration patterns and changes in ichthyological diversity (146, 147), loss in connectivity between upstream and downstream (148), decreased productivity in lowland Amazon floodplains due to the retention of nutrients by reservoirs (149), hydrological alterations through the water level change (150), deforestation and degradation of forests near the reservoir (27), transmission of parasitic diseases to humans such as malaria and leishmaniasis (151, 152), social impacts with the displacement of traditional populations and indigenous peoples (153, 154), and reduction of fish catch potential (155).
Climate Change, Forest−Climate Equilibrium States, and the Future of the Amazon Forests.
Taken alone, the drought impacts could be harmful enough for the ecosystem maintenance and integrity. Their joint action and the synergic effects with other anthropogenic drivers such as deforestation and fire have the potential to strongly amplify these impacts, so that the collapse of tropical rainforest (156, 157) and its transformation into a drier and impoverished savanna-like biome (7, 158) have been anticipated by computational models, and have been continuously validated by field observations. Studies in forest sites, in fact, support the assumption of a positive feedback between relatively frequent (1 to 3 y) fires and degradation (71, 115), which favor the presence of grasses and shrubs (115), secondary vegetation (119), and pioneer species that do not take long to become flammable (71). Controlled fire experiments carried out in the transition forests of northern Mato Grosso showed great incidence of lianas and tree mortality, supporting, according to the authors (71), the likelihood of “savannization” of parts of the Amazon. Depending on the scale, these changes could ultimately drive changes in the local climate, pushing the ecosystem toward a different forest−climate equilibrium state (159), that is, the one where most of the tropical forests in southern, southwestern, and southeastern Amazon are replaced by degraded savannas as predicted by models (91, 106, 158, 160). Evidence that the relationship between degraded forest, regional climate change, and a change in the equilibrium would lead to a tipping point in the Amazon region is described as a medium-confidence likelihood (161), but is already being observed in the Xingu basin (71).
At this point, it is important to assess the trends of the drivers of change conducive to savannization or forest dieback risk. In recent years, deforestation rates fell by about 80% in the Brazilian Amazon since 2005 (33). As mentioned above, the Amazon has experienced a succession of extreme climatic events since 2005 (see Precipitation Variability and Extremes). However, increased floods do not compensate fully for an increase of droughts in terms of forest vulnerability, especially due to increased fire frequency during droughts, which wreak havoc to forest resilience (18). This is supported by a trend of increasing observed forest mortality (131), together with the fact that forest fires have not decreased as expected (162). Another important driver is, of course, global warming. The Amazon region has experienced a temperature increase of close to 1 °C (51, 73), mostly due to global warming. It is estimated that 3.5 °C of global warming (that corresponds to about 4 °C temperature increase in the Amazon) could disrupt the forest−climate equilibrium, leading to substantial loss of tropical forests (106, 160, 161). In addition, as mentioned above, there is some observational evidence of a lengthening of the dry season in southern−southeastern Amazon, and this is the most important driver of forest transitioning to savanna as claimed by the hypothesis of Amazon savannization (7) because the climate envelope for tropical forests requires a dry season (less than 100 mm⋅mo−1) no longer than 4 mo.
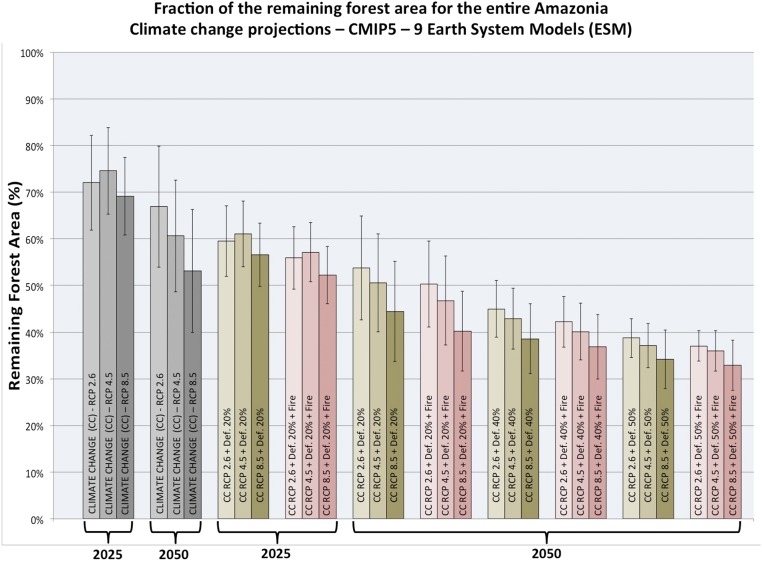
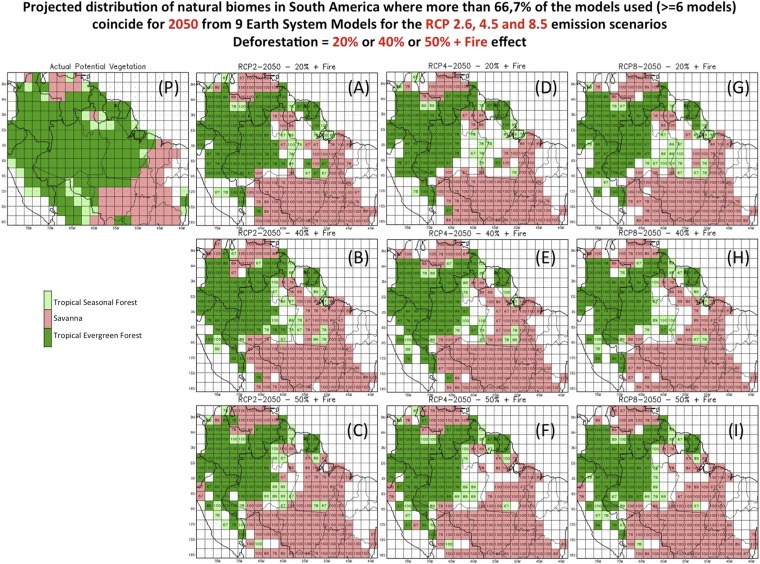
There have been attempts to use climate and vegetation models to quantify the impact of changes in climate due to global warming and due to deforestation on the distribution of major biomes in the Amazon as summarized in Modeling Efforts and Advances, including analysis (Figs. S1 and S2 and Table S4). The results show that the calculated area of tropical forest remaining, for simulations of biome distribution when only climate change forcing is considered, is about 15% smaller by 2050 for Intergovernmental Panel on Climate Change (IPCC) scenario Representative Concentration Pathway 8.5 Wm−2 (RCP8.5) in comparison with the change for scenario RCP2.6, given that the amplitude of climate change is vastly larger for the former scenario, and the effect increases in time (Fig. S1). For more drastic changes, taking into account the combined effect of climate change scenarios, regional climate change due to large-scale deforestation, and the effect of forest fire making tropical forests more vulnerable, the calculations project a substantial reduction of over 60% in the forest area in the Amazon by 2050. Most of the biome type changes occur over eastern and southern Amazon, with replacement of tropical forest by seasonal forest and tropical savanna (Fig. S2). The effect of fire in this region is important in all scenarios in further decreasing the area of tropical forest. Northwest Amazon presents the smallest changes in reduction of tropical forest, indicating that, even for substantial land-use and global climate changes, the resulting climatic conditions would still support tropical forest in that region (Fig. S2). All of the calculations assume the so-called CO2 fertilization effect (see CO2 Fertilization and Forest Mortality). In the absence of this moderating effect, the forest replacement would be considerably higher for all scenarios.
Fig. S1.
Fraction of area with remaining forest for the entire Amazon as a function of scenarios of climate change only (gray bars) and combined climate change + deforestation (moss green bars) and climate change + deforestation + fire effect (pink bars) for time slices 2020–2030 (labeled “2025”) and 2040–2060 (labeled “2050”) under 20, 40, and 50% deforestation scenarios and for IPCC AR5 scenarios RCP2.6, RCP4.5, and RCP8.5, and with or without including forest fire effects. The bars and error bars are averages and SD, respectively, for all models results. The CO2 fertilization effect is considered in the calculations by choosing photosynthetic assimilation rate as 25% of the maximum photosynthetic assimilation rate.
Fig. S2.
Grid point where more than 2/3 of the nine ESMs’ climate projections assessed (“67% consensus”) coincide as projecting the same future Amazon biome type in relation to current potential vegetation (P) (which is consistent with present climate conditions), for the time slice 2040–2060 (labeled “2050”): (A) climate change for RCP2.6 scenario + 20% deforestation + fire effect, (B) climate change for RCP2.6 scenario + 40% deforestation + fire effect, and (C) climate change for RCP2.6 scenario + 50% deforestation + fire effect; (D) climate change for RCP4.5 scenario + 20% deforestation + fire effect, (E), climate change for RCP4.5 scenario + 40% deforestation + fire effect, and (F) climate change for RCP4.5 scenario + 50% deforestation + fire effect; and (G) climate change for RCP8.5 scenario + 20% deforestation + fire effect, (H), climate change for RCP8.5 scenario + 40% deforestation + fire effect, and (I) climate change for RCP8.5 scenario + 50% deforestation + fire effect. White grid points (“Not consensus”) refer to the situation when fewer than six models project the same biome type. Biome types: dark green, tropical forest; light green, seasonal tropical forest; pink, tropical savanna.
Table S4.
CMIP5 ESMs used in this study
| Model | Model full name | Institution | Horizontal resolution |
| CCSM4 | Community Climate System Model, version 4 | National Center for Atmospheric Research (NCAR), United States | 288 × 192 |
| CSIRO Mk3.6.0 | Commonwealth Scientific and Industrial Research Organization Mark, version 3.6.0 | CSIRO and Queensland Climate Change Centre of Excellence, Australia | 192 × 96 |
| GFDL-ESM2M | Geophysical Fluid Dynamics Laboratory Earth System Model with Modular Ocean Model 4 (MOM4) component (ESM2M) | NOAA/GFDL, United States | 144 × 90 |
| GISS-E2-R | Goddard Institute for Space Studies Model E2, coupled with the Russell ocean model | NASA GISS, United States | 144 × 90 |
| HadGEM2-ES | Hadley Centre Global Environment Model, version 2–Earth System | Met Office, United Kingdom | 192 × 145 |
| IPSL-CM5A-LR | L’Institut Pierre-Simon Laplace Coupled Model, version 5A, coupled with NEMO, low resolution | L’Institut Pierre-Simon Laplace, France | 96 × 96 |
| MIROC5 | Model for Interdisciplinary Research on Climate, version 5 | Atmosphere and Ocean Research Institute (AORI) (The University of Tokyo), National Institute for Environmental Studies (NIES), and Japan Agency for Marine-Earth Science and Technology (JAMSTEC), Japan | 256 × 224 |
| MRI-CGCM3 | Meteorological Research Institute Coupled Atmosphere–Ocean General Circulation Model, version 3 | Meteorological Research Institute, Japan | 320 × 160 |
| NorESM1-M | Norwegian Earth System Model, version 1(intermediate resolution) | Norwegian Climate Centre, Norway | 144 × 96 |
Summing up, it has been established by modeling studies that the stability of Amazon forests may have at least two tipping points that, once one or both are transgressed, would entail irreversible large-scale forest dieback and a tendency for drier seasonal forests or impoverished tropical savanna to prevail over 30 to 50% of the basin, especially in the southern and eastern portions.
That is, we have identified the risks of land-use and climate change. Are there solutions to eliminate or mitigate those risks? Or, alternatively: Is the fate of the Amazon predetermined, given the likely risks to the Amazon ecosystems spelled out in Impacts of Anthropogenic Drivers of Change in the Amazon, or can we nudge a better outcome leveraging humanity’s limitless ingenuity in scientific discovery and solving seemingly intractable problems with an innovation model that has, as its foundations, a number of scientific and technological revolutions quietly transforming the world economy?
The Amazon development debate has been torn between attempting to reconcile maximizing conservation versus intensification of traditional agriculture and expansion of hydropower capacity. In Third Way as Paradigm, we argue for a Third Way based on aggressively researching, developing, and scaling up a new high-tech innovation approach that sees the Amazon as a global public good of biological and biomimetic assets that can enable the creation of innovative high-value products, services, and platforms for current and for entirely new markets through combining advanced digital, biological, and material technologies of the Fourth Industrial Revolution in progress.
Modeling Efforts and Advances
The Coupled Model Intercomparison Project phase 5 (CMIP5) models (193) simulate reasonably well some aspects of the present-day tropical South America climate. The timing of the transition in the seasonal cycle (e.g., onset of wet season) and the mean temperature in the region are well simulated, but, as a whole, the CMIP5 ensemble simulates conditions that are too dry in the Amazon basin throughout the year and, in many models, substantially so (194). That difficulty to simulate rainfall suggests that the processes controlling rainfall and, in particular, the feedbacks between land−surface latent heat flux and precipitation processes are still poorly represented (194, 195). These known model biases—especially dryness—should be taken into account in the interpretation of the projections of climate change, in the development of Amazon ecosystem-relevant climate indicators, and in using model output to run offline impacts models (196).
Projections of climate change in Amazonia from the CMIP5 ensembles show that the largest warming from 2.5 °C to 6 °C or higher for different emission scenarios by 2100 in South America occurs over the interior of the continent, including much of Amazonia and even larger temperature increases during the dry season. That range of temperature increases is worrisome because the Amazonian tropical ecosystems evolved over millions of years with relatively stable climatic conditions and adapted to fluctuations (e.g., Ice Ages), but it is uncertain how species and ecosystems will respond to such rapid warming.
Projections of precipitation present a more complex picture over the Amazon basin, particularly if annual mean changes are considered. Some models project reduction of precipitation in the Amazon, whereas other models project increases (58). By breaking the changes down by season and by subregion, more consistent signals emerge, although uncertainty remains high. The CMIP ensemble projections show a reduction of precipitation at the end of the dry season (−0.54 ± 0.64 mm⋅d−1 in September–November) and a slight increase in the months between December and May, although model agreement is low.
In general, the projections using a suite of climate change simulations indicate wetter conditions over the western basin and drier conditions in the eastern and northeastern basin (87, 128, 160, 197, 198). Model agreement is higher for the period between June and November; that is, there is much stronger model agreement for drier conditions, particularly in the northeast portion of the basin (196). This is important in connection to the projected lengthening of the dry season. Longer dry season—coupled to higher temperatures—is a key driver for savannization over eastern and southeastern Amazon (7).
To quantify how the combined impact of changes in climate due to global warming and due to deforestation and the effect of forest fires may alter the distribution of major biomes in the Amazon, we present an analysis (Figs. S1 and S2) by using a suite of climate and vegetation models.
We present additional details of our modeling scheme [nine CMIP5 models, driving the Brazilian Center for Weather Forecasting and Climate Studies Potential Vegetation Model (CPTEC-PVM2.0) and CPTEC-National Institute for Space Research (INPE) Atmospheric General Circulation Model (AGCM) driven by land-cover maps scenarios] to provide extra supporting information beyond those in the main body of the paper. We perform analyses to quantify how the impact of combined changes in climate, deforestation, and forest fire may combine to affect the distribution of major biomes in Amazonia.
To assess the effects of Amazonian deforestation on the regional climate, we used results from numerical simulations described in ref. 91 with the CPTEC-INPE AGCM (199) where the changes in land use considered deforestation scenarios of 0, 20, 40, and 50% (91, 200). Also, to assess the effects of climate change, we used results from nine Earth System Models, presented in Table S4 from CMIP5 under three representative concentration pathways (RCPs): 2.6, 4.5, and 8.5, for time slices 2015–2034 and 2040–2059 (termed “2025” and “2050” time slices). For the combination between climate change, deforestation, and fire, the CPTEC-PVM2.0 was used by combining the supposed deforestation of 20% in the “2025” and “2050” time slices, deforestation of 40% in time slice “2050” and deforestation of 50% in time slice “2050.” The climate anomalies from deforestation simulation (91) were combined with the anomalies of the CMIP5 scenarios, for each time slice to drive the CPTEC-PVM2.0. This combination between deforestation scenarios and climate projections was evaluated only for the first part of the 21st century because of the large uncertainties associated with both scenarios beyond this period. All of the calculations assume the so-called CO2 fertilization effect (see CO2 Fertilization and Forest Mortality).
Deforestation Scenarios.
The land-cover change scenarios we used are from refs. 91 and 200. These scenarios were built by assuming that deforestation trends recently observed will continue into the future, highways currently scheduled for paving will be paved, compliance with legislation requiring forest reserves on private land will remain low, and protected areas will not be enforced. Deforested areas were projected to be converted to degraded grasses (pastures), in scenarios where they expand to 20, 40, and 50% of the original extent of the Amazon forest.
Climate Change Scenarios.
We used results from nine ESMs (Table S4) from CMIP5 under three RCPs—2.6, 4.5, and 8.5—for time slices 2015–2034 and 2040–2059 (time slices “2025” and “2050”). In RCP 2.6, the atmospheric CO2 concentration in the year 2050 (2025) reaches 443 ppm (422 ppm). In RCP 4.5, the corresponding value is 486 ppm (423 ppm), and, in RCP 8.5, the corresponding value is 540 ppm (431 ppm) (201). Climate simulation for the years 1961–1990 of each model is used to evaluate the models’ anomalies. Reference precipitation and surface temperature climatologies (1961–1990) are provided at monthly/0.5° resolution from ref. 202. These data and the climate scenarios were then aggregated to T62 spectral resolution (∼2°) to drive the CPTEC-PVM2 including the land vegetation carbon cycle (128) used to evaluate biomes redistributions.
CPTEC AGCM.
CPTEC AGCM (199) results from improvements of the previous CPTEC/COLA GCM developed in cooperation with the Center for Ocean-Land-Atmosphere Studies. It has been shown that the model simulates reasonably well the main features of global climate, as well as the seasonal variability of the main atmospheric variables in the study region. CPTEC AGCM uses the Simplified Simple Biosphere Model (203) land surface scheme, where each land grid point is assigned to a vegetation type following the biomes classification of ref. 204 with a corresponding set of biophysical parameters. This study used results from numerical simulations described in ref. 91.
Potential Vegetation Model CPTEC-PVM2.0.
We used the potential vegetation model CPTEC‐ PVM2.0 (127) to quantify how these combined changes may affect the distribution of major biomes in Amazonia. CPTEC-PVM2.0 (127) preserves the particularly good performance of its predecessor CPTEC-PVM (205) for biomes in South America. The model considers seasonality in precipitation as a determinant for the delimitation of forests and savannas, and is able to account for varying atmospheric CO2 concentration on plants’ primary productivity. CPTEC-PVM2.0 assigns a biome−atmosphere equilibrium solution to each grid cell using monthly climate information (surface temperature and precipitation), incident photosynthetically active radiation, and atmospheric CO2 concentration as inputs.
The water balance routine is nearly the same as in CPTEC-PVM (205), based on ref. 206, although canopy resistance rc (1/canopy conductance gc) is calculated from net primary productivity (NPP) and atmospheric CO2 concentration, based on the formulation by ref. 207, which is used in other dynamic global vegetation models (208) and global circulation model surface schemes (e.g., ref. 209). The canopy resistance is used to calculate ET according to Penman−Monteith’s equation. This formulation enables a two-way interaction of water cycle and plant physiology. CPTEC-PVM2.0 also considers the potential for lightning-induced fires to refine the spatial distribution of savannas (210), which are sustained if thresholds of availability of natural ignition sources and fuel moisture are achieved, or, otherwise, are replaced by dry forests. All of the calculations assume the so-called CO2 fertilization effect (see CO2 Fertilization and Forest Mortality). Global and regional NPP simulated by CPTEC-PVM2.0 are similar to those from observations and other NPP models (128, 211).
Fire Effect.
Fires in the study region are normally associated with deforestation and other current land-use practices; they also contribute to the replacement of forests by secondary or degraded vegetation, and to the establishment of savannas in places that could be covered by forests in their absence (210). To account for the combined effects of deforestation and fires on the long-term distribution of tropical and seasonal forests and savannas in the Brazilian Amazon, we used relations between fire activity and climate factors that drive the spatial distribution of these major biomes.
Our relations were derived by combining climate and soil hydrology variables with fire occurrence in the region. The method, fully described in ref. 212, was applied to the results of the CPTEC-PVM2.0 to evaluate the long-term potential for tropical and seasonal forest degradation by assuming that tropical land-use dynamics and policy currently observed in the Brazilian Amazon will continue in the future. Second, we considered that fire effects are expressive only if hydrological conditions support medium-to-high fire activity.
We also assumed that edge effects will only extend to tropical and seasonal forests that are bordering savannas. This assumption is based on the fact that the access to the forests is facilitated by savannas, where fires are also a natural feature (210, 213). In addition, previous analyses also showed that the influences of deforestation on fire activity decrease substantially for distances greater than 175 km (214). Grid cells in this study are ∼180 km wide; thus it is reasonable to restrain deforestation/fires edge effect to one grid cell. Based on our method, if a grid cell—projected to be covered by tropical forests—presents environmental characteristics that support long-term medium-to-high fire activity and is adjacent to a grid cell projected to be covered by savannas, it will be adjusted to seasonal forest. If that grid cell was projected to be seasonal forest, then it would be adjusted to savanna. Taking all our assumptions together, we believe that our method is conservative in considering only medium-to-high fire potentials, and in not considering direct transitions from tropical forests to savannas that may happen in the long term in this region.
Numerical Experiments.
To evaluate the biome redistribution over Amazon forest for different forcing in the climate system, we elaborated two experiments: climate change only and climate change, deforestation, and fire.
Climate-change-only experiments.
The CPTEC-PVM2.0 was forced by monthly precipitation, surface temperature, and zonal wind inputs derived from nine CMIP5 ESMs, for the 1961–1990 period (actual climate) and two time slices in the 21st century (2015−2034 and 2040–2059), for the RCPs 2.6, 4.5, and 8.5 (Modeling Efforts and Advances, Climate Change Scenarios). The 1961–1990 model climatology for each ESM is used to derive the model’s anomalies. To filter out the effect of an ESM’s systematic errors in calculating the input fields of precipitation, surface temperature, and zonal winds, these anomalies are added to the 1961–1990 observed climatology, and this sum is used as input to CPTEC-PVM2.0, following an anomaly coupling procedure (215).
Climate change, deforestation, and fire experiments.
For the combination of climate change, deforestation, and fire, the CPTEC-PVM2.0 was used by combining the results from numerical simulations described in ref. 91 with the CPTEC-INPE AGCM (199), where the changes in land use considered deforestation scenarios of 0, 20, 40, and 50% (91, 200), and the results from nine CMIP5 models for the RCPs 2.6, 4.5, and 8.5. We supposed deforestation of 20% in time slices “2025” and “2050” time slices, deforestation of 40% in time slice “2050,” and deforestation of 50% in time slice “2050.” For each time slice, the climate anomalies from deforestation simulations were combined with the anomalies of the CMIP5 scenarios. This combination of deforestation scenarios and climate projections was evaluated only for the first part of the 21st century because of the large uncertainties associated with both scenarios beyond this period. Also, we consider the potential for occurrence of land-use fires according to the method described in Modeling Efforts and Advances, Fire Effect. The correct interpretation of the resulting equilibrium biomes in the deforestation scenarios is that those biomes would be in equilibrium with the postdeforestation climate, as though the natural biomes were left to regrow naturally after deforestation for agriculture.
Third Way as Paradigm of Sustainable Development for the Amazon
In terms of development policy pathways for the Amazon, two modes have historically dominated: (i) a valuable nature conservation approach with large swathes of territory legally protected from any economic and human activity outside indigenous peoples—which comprise 2.1 million km2, or about 43% of Brazilian Amazon (153, 163, 164); and (ii) an approach that has focused on conversion or degradation of forests for the production of either protein commodities (e.g., meat and soya) or tropical timber at the forest frontier and the build-out of massive hydropower generation capacity—which together have been historically responsible for massive deforestation of the Amazon (30, 31) and generated other significant negative externalities.
Recently, there have been suggestions of promoting grain agriculture intensification (165) and higher intensification of cattle ranching to substantially increase meat production (22) in areas already deforested through recovery of degraded pastures. However, even though these approaches can potentially provide a “transition bridge” toward a safer sustainable development model, they are inconsistent with the rigorous zero-deforestation target necessary, among other things, for climate change stabilization, because the exponential demand growth of these commodities by the emerging middle class in the emerging markets, in the end, will likely depend to a greater or lesser extent on continuously expanding the agricultural frontier.
Given Brazil’s huge underexploited central and distributed renewable energy generation and energy efficiency potential in regions much closer to the consumption centers (166, 167) versus energy demand in the Amazon that can be met by local renewable sources (168–170), we argue that it should be feasible to plan for energy generation capacity increases that do not rely on new hydropower capacity from the Amazon.
On the other hand, the Amazon biological (e.g., biomimetic) assets may hold promise for advanced energy production innovations. In nature, photosynthesis generates energy for plants, and microorganisms generate their own energy from other sources (e.g., sulfur-fixing bacteria). These processes have inspired innovations in advanced microbial fuel cells (171). In addition, the Tungara frog species that creates long-lived foams has inspired new energy generation and carbon dioxide sequestration technologies (172). Finally, plants have also directly inspired solar cell design, potentially generating much cheaper alternatives to silicon-based photovoltaics (173).
We argue therefore that there is a Third Way within reach in which we aggressively research, develop, and scale a new high-tech innovation approach that sees the Amazon as a global public good of biological assets and biomimetic designs that can enable the creation of innovative high-value products, services, and platforms for current and for entirely new markets by applying a combination of advanced digital, material, and biological technology breakthroughs to their privileged biological and biomimetic assets (please refer to Implications of the Fourth Industrial Revolution for the Sustainable Development of the Amazon for more details on the Fourth Industrial Revolution).
Biological systems in the Amazon are the result of millions of years of evolution. Humanity, as a whole, has long relied on observing and learning from nature, just as the famous Icarus tried mimicking bird flight from his prison island in ancient Greek mythology. Fast forward, and the search for biomimetic applications has developed into a scientific discipline leading inevitably to a large number of biomimicry-based innovations.
Biomimicry innovations in the Amazon should focus on learning from and then emulating Amazonian natural forms, processes, and ecosystems to create more sustainable designs and innovations (174). We are rapidly gaining understanding on how things are created in nature (materials), how organisms sense their surroundings (sensors), how they move in their environment (biomechanics and kinetics), and how they behave and function (processes) (175). In addition, there is significant innovation potential to focus on learning from the Amazon in biomimicry-enabled nanoscience, reproducing complex biological systems to solve problems on a nanomolecular scale (176, 177), create environmentally friendly process and pollution prevention/remediation technologies, design bioinspired textile structures (178), aid in energy production, and provide insight in behavioral and cognition—artificial intelligence robotic—applications (179), which are in the early phase of the innovation cycle.
In the short term and with a low-tech approach, we estimate that it is quite feasible to develop a number of biodiversity-based product value chains capable of reaching global markets with unique differentiation (180, 181). This new economy has the potential to become much larger than the present one that is based on the unsustainable use of natural resources in the Amazon. A number of biodiversity products from the Amazon, such as babassu (Attalea speciosa), cupuaçu (Theobroma grandiflorum), and the Brazil nut have already impacted the local economies, and there are plenty more to be discovered and commercialized (182). Pioneering illustrations of this new biodiversity-based economy are the recent emergence of assai (Euterpe oleracea) production that has reached the multibillion-dollar scale (183–186). The spilanthol alkaloid found in the leaves, branches, and flowers of jambu (Spilanthes oleracea) is described in patents as appropriate for anesthetic, antiseptic, antiwrinkle, toothpaste, gynecological, and antiinflammatory uses (187). Other products of the Amazon biodiversity are essential oils of species such as rosewood (Aniba rosaeodora), nhandiroba (Carapa guianensis), and copaiba (Copaifera langsdorffii) that are amenable for end-to-end processing in the Amazon and can be alternatives in the formation of a fluorine−xylo−chemical hub for cosmetic and pharmaceutical products in the Amazon (188). The bacuri (Platonia insignis) is an Amazonian fruit in increasing demand for ice cream, candy, and juice products. The oil extracted from its seeds is used as an antiinflammatory substance in traditional folk medicine and in the cosmetics industry (189). New uses of biodiversity-based products are being developed and patented more often [e.g., ucuuba (Virola Surinamensis) (188) and murumuru (Astrocaryum murumuru) butter (190)] in the cosmetics industry.
Beyond these new developments in the right direction, however, the Fourth Industrial Revolution opens a new paradigm of seeing tropical regions not only as potential sources of natural resources and biodiversity but also as reserves of precious biological biomimetic knowledge that can fuel a new development model that can benefit both local/indigenous populations and the world at large.
Our view is that transitioning to this sustainable development model will require an Amazon-specific Fourth Industrial Revolution innovation “ecosystem” that is able to rapidly prototype and scale innovations that apply a combination of advanced digital, biological, and material technologies to the Amazon’s renewable natural resources, biomimetic assets, environmental services, and biodiverse molecules and materials. This sustainable development model would then provide a basic foundation to nurture a biomimetic innovation ecosystem model for the region that would be capable of capturing synergies between private and public R&D laboratories, public−private partnerships, private and social entrepreneurs, venture capital, and innovative corporations—much like in Silicon Valley. For this innovation ecosystem to be developed over time, a new Amazon-specific innovation public−private partnership needs to be in place, together with an enabling regulatory framework that deals with good practice in dealing with biomimetic knowledge and assets. In particular, the Amazon requires an aspirational type “man on the Moon” mission that draws on frontier knowledge across digital, biological, and advanced materials to attain the goal of big science deployed to meet big problems. The role of the government and high-tech start-ups will be particularly important in the first-stage capital-intensive high-risk domains that the corporate sector tends to shy away from. Just as the development of the Internet was funded initially by the US government that, in time, created a platform for innovation for social, private, and public entrepreneurs around the world, so should the path be set for the Amazon innovation ecosystem that we aspire to develop.
The Amazon region presents limited potential for knowledge generation and capacity building partly due to a limited number of research institutions and researchers (191, 192). In this context, a critical missing element for transforming local development toward sustainability is human capital in the region. Brazilian Amazon universities produce only 2% of Ph.D.s trained in Brazil every year (330 out of 16,745 in 2014)—in contrast, the Brazilian Amazon has about 11% of the population (see Tables S5 and S6 in Datasets Used to Derive the Fraction of Ph.D.s Trained in Brazilian Amazon Universities)—and very few are trained in innovative research areas of advanced digital, biological, and material sciences that should serve as pillars of the Fourth Industrial Revolution in the region. Developing a locally innovative research capacity and attracting human capital are essential elements for long-term sustainability. This should include creating new research institutions across the region, taking notice of subregional potential of renewable natural resources and also enhancing connectivity through broadband satellite-based* and fluvial fiber optics Internet (191) [Connected Amazon Project (Amazônia Conectada) was established by Ministerial Ordinance 596, published in Brazilian Federal Register on July 23, 2015, issued by the Ministry of Science, Technology and Innovation] and through provisioning a common high-performance computing infrastructure for the region.
Table S5.
Number of PhD Student’s and CAPES fellowships
| Region | Ph.D. students | Total Ph.D. CAPES fellowships | ||
| Enrolled | Granted | Total | ||
| 2014 | ||||
| Brazil | 94,850 | 16,745 | 111,595 | 39,954 |
| Amazon region | 2,721 | 330 | 3,051 | 1,765 |
| Ratio, % | 2.9 | 2.0 | 2.7 | 4.4 |
| 2013 | ||||
| Brazil | 88,575 | 15,544 | 104,119 | 32,111 |
| Amazon region | 2,343 | 298 | 2,641 | 1,188 |
| Ratio, % | 2.6 | 1.9 | 2.5 | 3.7 |
| 2012 | ||||
| Brazil | 79,478 | 13,912 | 93,390 | 27,589 |
| Amazon region | 1,923 | 267 | 2,190 | 1,055 |
| Ratio, % | 2.4 | 1.9 | 2.3 | 3.8 |
The Amazon Region is the same as described in the legend of Table S3. The data on Ph.D. students and CAPES Fellowships are collected and published by CAPES systems. The information is public and is available at geocapes.capes.gov.br/geocapes2/.
Table S6.
Brazilian and Amazon region populations
| State | Total | Ratio, % |
| States entirely included | ||
| Acre | 733,559 | 0.38 |
| Amapá | 669,526 | 0.35 |
| Amazonas | 3,483,985 | 1.83 |
| Pará | 7,581,051 | 3.97 |
| Rondônia | 1,562,409 | 0.82 |
| Roraima | 450,479 | 0.24 |
| States partially included | ||
| Maranhão | 3,910,288 | 2.05 |
| Mato Grosso | 1,282,442 | 0.67 |
| Tocantins | 381,027 | 0.20 |
| Total | 20,054,766 | 10.51 |
The Amazon Region is the same as described in the legend of Table S3. Source is IBGE/CENSUS 2010. Brazilian population is 190,755,799. The Brazilian and Amazon Region population is described, by municipalities, in the IBGE microdata of CENSUS 2010. Available at www.censo2010.ibge.gov.br/sinopse/index.php?dados=8.
Transforming the current regional developmental model presents multidimensional challenges, which cannot be achieved through science, technology, and innovation alone. It has become vital and indeed urgent to instigate a real scientific, high-tech, and innovation revolution in the Amazon. The enormous institutional and governance challenge is to find the pathways to transform this vast and mostly unknown natural capital into a global common public good that provides a foundation for public, private, and social entrepreneurs to develop and scale innovations as a basis for a novel high-tech regional sustainable development model for the Amazon.
Implications of the Fourth Industrial Revolution for the Sustainable Development of the Amazon
Silently, over the last few years, the world economy has been undergoing a massive transformation at high speed driven by the fusion of advanced digital, material, and biological innovations, leading to the concept of the Fourth Industrial Revolution (216). The accelerating confluence of emerging technology breakthroughs, covering wide-ranging fields such as artificial intelligence, robotics, the internet of things, autonomous vehicles, 3D printing, nanotechnology, synthetic biology, DNA editing, biomimicry, advanced materials science, energy storage, and quantum computing, to name but a few, will create massive opportunities and risks (217, 218).
Many of the Fourth Industrial Revolution innovations are already reaching an inflection point in their development as they build on and amplify each other across the physical, digital, and biological worlds. For example, to complete the Human Genome Project, it took more than 10 y, at a cost of $3 billion, whereas, today, a genome can be sequenced in a few hours and cost less than a thousand dollars (219). Three-dimensional printing (220) will be combined with gene editing technologies to produce living tissues to generate skin, bone, heart, and vascular tissue. Blockchain immutable distributed ledgers in combination with artificial intelligence and Internet of Things technologies will soon revolutionize the way all biophysical assets are registered and traced along end-to-end supply chains from source to use to reuse in a global system of record (221). Disruptive system-wide applications of the Circular Economy paradigm will be scaled economy-wide, whereby businesses and consumers will shift away from the linear take−make−dispose model of natural resource use, which relies on large quantities of easily accessible resources, and toward a new industrial model where effective flows of materials, energy, labor, and now information interact with each other and promote by design a restorative, regenerative, and more productive economic system (222).
In this context and given the accelerating “reshoring” of high value-added manufacturing to advanced economies as a direct consequence of the artificial intelligence-driven automation tsunami in progress, we believe that new drivers of competitiveness for tropical developing countries will be needed urgently, given that we can no longer rely on the traditional industrialization pathway that allowed developing countries in the recent past to accumulate capital, transfer technology, and raise incomes per capita over time as productivity increased. If this traditional development pathway is effectively not available to the broader developing world given asymmetric development within the Fourth Industrial Revolution, the prospects of the grand convergence of GDP per capita across the world is indeed at risk, with the huge implications that this entails in the areas of poverty alleviation, economic development, and social stability.
Embracing the Fourth Industrial Revolution requires the development of a new model, based on the forest and its terrestrial and aquatic resources, which recognizes the economic value of the forest’s biodiversity, its biomimetic assets, and the ecosystem services it provides. Nowadays, very few value chains based on the Amazon’s natural products either involve global markets or bring benefits across the social strata (183). Actually, quite the opposite has been the case, with more and more imported products being used within the Amazon to replace traditional Amazonian goods (223).
Datasets Used to Derive the Fraction of Ph.D.s Trained in Brazilian Amazon Universities
The data on Ph.D. students enrolled or granted (Table S5) in one of the programs evaluated by the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES) were obtained by consultation of data registered on Sucupira Platform—the official data collection and archive of the National Graduate Education System in Brazil. Table S6 depicts data on the geographic distribution of the Brazilian population.
Concluding Remarks
Overcoming the risks to the integrity and functionality of Amazon ecosystems does not depend exclusively on a new local, standing forests sustainable development paradigm such as the one put forth in Third Way as Paradigm of Sustainable Development for the Amazon. Reducing tropical deforestation to nearly zero is necessary for biodiversity conservation, provision of ecosystems services, and, to some extent, climate mitigation by reducing land-cover change emissions, but it is not sufficient at all to avert the risk of global climate change. Unchecked climate change poses a great danger of exceeding tipping points for the forests. Therefore, a gargantuan global effort of decarbonizing the world economy is called for to avoid transgressing these boundaries and to meet the safeguards of maximum 2 °C global warming as set by the recent Paris Agreement during the 21st Conference of the Parties of the United Nations Framework Convention on Climate Change.
Acknowledgments
We thank Elisangela Sousa and Roberta Silva for support in the preparation of this article. This work was supported by the National Institute of Science and Technology for Climate Change under the Brazilian National Council for Scientific and Technological Development (CNPq) Grant 573797/2008-0 and the São Paulo Research Support Foundation (FAPESP) Grants 2008/57719-9 and 2009/50528-6.
Footnotes
The authors declare no conflict of interest.
*The geostationary satellite will be the first satellite fully controlled by the Brazilian government, and the project involves the Ministries of Defense, Communications, and Science, Technology and Innovation. The Telebras and Visiona Space Technology companies were responsible for the construction if equipment, which started in 2014; equipment should be ready for release in the first half 2016. The project was funded through the National High Speed Internet Program (Programa Nacional de Banda Larga-PNBL), which was established by Ministerial Decree 7.175, by the Ministry of Communications.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605516113/-/DCSupplemental.
References
- 1.Hope C, Castilla-Rubio JC. 2008. A first cost benefit analysis of action to reduce deforestation. Working paper (Cambridge Univ, Cambridge, UK). Available at digital.library.unt.edu/ark:/67531/metadc13708/
- 2.Lapola DM, et al. Pervasive transition of the Brazilian land-use system. Nat Clim Chang. 2013;4:27–35. [Google Scholar]
- 3.Davidson EA, et al. The Amazon basin in transition. Nature. 2012;481(7381):321–328. doi: 10.1038/nature10717. [DOI] [PubMed] [Google Scholar]
- 4.Barlow J, et al. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc Natl Acad Sci USA. 2007;104(47):18555–18560. doi: 10.1073/pnas.0703333104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewinshohn TM, Prado PI. Biodiversidade Brasileira. Síntese do estado atual do conhecimento. Contexto; São Paulo, Brazil: 2002. [Google Scholar]
- 6.Hubbell SP, et al. Colloquium paper: How many tree species are there in the Amazon and how many of them will go extinct? Proc Natl Acad Sci USA. 2008;105(Suppl 1):11498–11504. doi: 10.1073/pnas.0801915105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nobre CA, et al. Amazonian deforestation and regional climate. J Clim. 1991;4(10):957–988. [Google Scholar]
- 8.Callède J. L’Amazone à Óbidos (Brésil): Étude statistique des débits et bilan hydrologique. Hydrol Sci J. 2002;47(2):321–334. [Google Scholar]
- 9.Molinier M, et al. Les regimes hydroiogiques de l’Amazone et de ses affluents. IAHS Publ. 1996;238:209–222. [Google Scholar]
- 10.Barthem RB, et al. 2004. Amazon Basin – GIWA Regional Assessment 40b. (Univ Kalmar, Kalmar, Sweden)
- 11.Gibbs HK, et al. Monitoring and estimating tropical forest carbon stocks: Making REDD a reality. Environ Res Lett. 2007;2:045023. [Google Scholar]
- 12.Saatchi SS, et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc Natl Acad Sci USA. 2011;108(24):9899–9904. doi: 10.1073/pnas.1019576108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malhi Y, et al. The regional variation of aboveground live biomass in old-growth Amazonian forests. Glob Change Biol. 2006;12(7):1107–1138. [Google Scholar]
- 14.Cerri CEP, et al. Predicted soil organic carbon stocks and changes in the Brazilian Amazon between 2000 and 2030. Agric Ecosyst Environ. 2007;122(1):58–72. [Google Scholar]
- 15.Feldpausch TR, et al. Tree height integrated into pantropical forest biomass estimates. Biogeosciences. 2012;9(8):3381–3403. [Google Scholar]
- 16.Gorenflo LJ, Romaine S, Mittermeier RA, Walker-Painemilla K. Co-occurrence of linguistic and biological diversity in biodiversity hotspots and high biodiversity wilderness areas. Proc Natl Acad Sci USA. 2012;109(21):8032–8037. doi: 10.1073/pnas.1117511109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maffi L. Linguistic, cultural, and biological diversity. Annu Rev Anthropol. 2005;29:599–617. [Google Scholar]
- 18.Nobre CA, Borma LS. ‘Tipping points’ for the Amazon Forest. Curr Opin Environ Sustain. 2009;1(1):28–36. [Google Scholar]
- 19.Borma LS, et al. 2013. Response of the Amazon tropical forests to deforestation, climate, and extremes, and the occurrence of drought and fire. Vulnerability of Food Resources to Climate. Climate Vulnerability, ed Pielke R (Elsevier, New York), Vol 2, pp 153–163.
- 20.Foley JA, et al. Solutions for a cultivated planet. Nature. 2011;478(7369):337–342. doi: 10.1038/nature10452. [DOI] [PubMed] [Google Scholar]
- 21.Lambin EF, Meyfroidt P. Global land use change, economic globalization, and the looming land scarcity. Proc Natl Acad Sci USA. 2011;108(9):3465–3472. doi: 10.1073/pnas.1100480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lapola DM, et al. Indirect land-use changes can overcome carbon savings from biofuels in Brazil. Proc Natl Acad Sci USA. 2010;107(8):3388–3393. doi: 10.1073/pnas.0907318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Empresa de Pesquisa Energética (2013) Plano Decenal de Expansão de Energia 2013–2022 (Empresa Pesquisa Energética, Rio de Janeiro)
- 24.Vieira ICG, Toledo PM, Silva JM, Higuchi H. Deforestation and threats to the biodiversity of Amazonia. Braz J Biol. 2008;68(4) Suppl:949–956. doi: 10.1590/s1519-69842008000500004. [DOI] [PubMed] [Google Scholar]
- 25.Cavalcante MMA, Santos LJC. Hidrelétricas no Rio Madeira-RO: Tensões sobre o uso do território e dos recursos naturais na Amazônia. Confins. 2012;15:7758. [Google Scholar]
- 26.Schmitz C, et al. Agricultural trade and tropical deforestation: Interactions and related policy options. Reg Environ Change. 2015;15(8):1757–1772. [Google Scholar]
- 27.Chen G, et al. Spatiotemporal patterns of tropical deforestation and forest degradation in response to the operation of the Tucuruí hydroelectric dam in the Amazon basin. Appl Geogr. 2015;63:1–8. [Google Scholar]
- 28.Kahn J. False shades of green: The case of Brazilian Amazonian hydropower. Energies. 2014;7(9):6063–6082. [Google Scholar]
- 29.Fillion M, et al. Quality of life and health perceptions among fish-eating communities of the Brazilian Amazon: An ecosystem approach to well-being. EcoHealth. 2009;6(1):121–134. doi: 10.1007/s10393-009-0235-z. [DOI] [PubMed] [Google Scholar]
- 30.Walker NF, et al. From Amazon pasture to the high street: Deforestation and the Brazilian cattle product supply chain. Trop Conserv Sci. 2013;6(3):446–467. [Google Scholar]
- 31.Diniz FH, et al. Mapping future changes in livelihood security and environmental sustainability based on perceptions of small farmers in the Brazilian Amazon. Ecol Soc. 2015;20(2):26. [Google Scholar]
- 32.Assunção J, et al. Deforestation slowdown in the Brazilian Amazon: Prices or policies? J Environ Dev Econ. 2012;20(6):697–722. [Google Scholar]
- 33.Diniz CG, et al. DETER-B: The New Amazon Near Real-Time Deforestation Detection System. IEEE J Sel Top Appl Earth Obs Remote Sens. 2015;8(7):3619–3628. [Google Scholar]
- 34.Martini DZ, et al. Potential land availability for agricultural expansion in the Brazilian Amazon. Land Use Policy. 2015;49:35–42. [Google Scholar]
- 35.Verburg R, et al. The impact of commodity price and conservation policy scenarios on deforestation and agricultural land use in a frontier area within the Amazon. Land Use Policy. 2014;37:14–26. [Google Scholar]
- 36.Aguiar APD, et al. Land use change emission scenarios: Anticipating a forest transition process in the Brazilian Amazon. Glob Change Biol. 2016;22(5):1821–1840. doi: 10.1111/gcb.13134. [DOI] [PubMed] [Google Scholar]
- 37.Nepstad D, et al. Slowing Amazon deforestation through public policy and interventions in beef and soy supply chains. Science. 2014;344(6188):1118–1123. doi: 10.1126/science.1248525. [DOI] [PubMed] [Google Scholar]
- 38.Fearnside PM. Tropical hydropower in the clean development mechanism: Brazil’s Santo Antônio Dam as an example of the need for change. Clim Change. 2015;131(4):575–589. [Google Scholar]
- 39.Aguiar APD, et al. Modeling the spatial and temporal heterogeneity of deforestation-driven carbon emissions: The INPE-EM framework applied to the Brazilian Amazon. Glob Change Biol. 2012;18(11):3346–3366. [Google Scholar]
- 40.Schoenenberg R, et al. What comes after deforestation control? Learning from three attempts of land-use planning in southern Amazonia. Gaia Ecol Perspect Sci Soc. 2015;24(2):119–127. [Google Scholar]
- 41.Salati E, Vose PB. Amazon basin: A system in equilibrium. Science. 1984;225(4658):129–138. doi: 10.1126/science.225.4658.129. [DOI] [PubMed] [Google Scholar]
- 42.Salati E, et al. Recycling of water in the Amazon Basin: An isotopic study. Water Resour Res. 1979;15(5):1250. [Google Scholar]
- 43.Sombroek W. Spatial and temporal patterns of Amazon rainfall. Consequences for the planning of agricultural occupation and the protection of primary forests. Ambio. 2001;30(7):388–396. doi: 10.1579/0044-7447-30.7.388. [DOI] [PubMed] [Google Scholar]
- 44.Alves LM, et al. 2013. Classificação de anos secos na estação chuvosa e seca na Amazônia. Secas na Amazônia: Causas e Consequências, ed Borma LS, Nobre CA (Oficina Textos, Sao Paulo) Chap 4, pp 49–77.
- 45.Shuttleworth WJ. Evaporation from Amazonian rainforest. Proc Biol Sci. 1988;233(1272):321–346. [Google Scholar]
- 46.Aragão LEOC, et al. Spatial patterns and fire response of recent Amazonian droughts. Geophys Res Lett. 2007;34(7):L07701. [Google Scholar]
- 47.Marengo JA. Characteristics and spatio-temporal variability of the Amazon river basin water budget. Clim Dyn. 2005;24(1):11–22. [Google Scholar]
- 48.Marengo JA, Nobre CA, Tomasella J, Cardoso MF, Oyama MD. Hydro-climate and ecological behaviour of the drought of Amazonia in 2005. Philos Trans R Soc Lond B Biol Sci. 2008;363(1498):1773–1778. doi: 10.1098/rstb.2007.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marengo JA, et al. Extreme climatic events in the Amazon basin Climatological and hydrological context of recent floods. Theor Appl Climatol. 2012;107(1-2):73–85. [Google Scholar]
- 50.Lewis SL, et al. The 2010 Amazon drought. Science. 2011;331(6017):554. doi: 10.1126/science.1200807. [DOI] [PubMed] [Google Scholar]
- 51.Intergovernmental Panel on Climate Change . In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Stocker TF, et al., editors. Cambridge Univ Press; Cambridge, UK: 2013. [Google Scholar]
- 52.Dubreuil V, Debortoli N, Funatsu B, Nédélec V, Durieux L. Impact of land-cover change in the southern Amazonia climate: A case study for the region of Alta Floresta, Mato Grosso, Brazil. Environ Monit Assess. 2012;184(2):877–891. doi: 10.1007/s10661-011-2006-x. [DOI] [PubMed] [Google Scholar]
- 53.Fu R, et al. Increased dry-season length over southern Amazonia in recent decades and its implication for future climate projection. Proc Natl Acad Sci USA. 2013;110(45):18110–18115. doi: 10.1073/pnas.1302584110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marengo JA, et al. Onset and end of the rainy season in the Brazilian Amazon Basin. L Clim. 2001;14(5):833–852. [Google Scholar]
- 55.Rao VB, et al. Annual variation of rainfall over Brazil and water vapor characteristics over South America. J Geophys Res. 1996;101(D21):26539–26551. [Google Scholar]
- 56.Fu R, Li W. The influence of the land surface on the transition from dry to wet season in Amazonia. Theor Appl Climatol. 2004;78(1):97–110. [Google Scholar]
- 57.Li W, et al. Rainfall and its seasonality over the Amazon in the 21st century as assessed by the coupled models for the IPCC AR4. J Geophys Res Atmos. 2006;111(2):D02111. [Google Scholar]
- 58.Myneni RB, et al. Large seasonal swings in leaf area of Amazon rainforests. Proc Natl Acad Sci USA. 2007;104(12):4820–4823. doi: 10.1073/pnas.0611338104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lintner BR, Neelin JD. A prototype for convective margin shifts. Geophys Res Lett. 2007;34(5):L05812. [Google Scholar]
- 60.Lintner BR, Neelin JD. Soil moisture impacts on convective margins. J Hydrometeorol. 2009;10(4):1026–1039. [Google Scholar]
- 61.Lintner BR, et al. An idealized prototype for large-scale land-atmosphere coupling. J Clim. 2013;26(7):2379–2389. [Google Scholar]
- 62.Clark D, Clark D. Climate-induced annual variation in canopy tree growth in a Costa Rican tropical rain forest. J Ecol. 1994;82(4):865–872. [Google Scholar]
- 63.Harris PP, et al. Effect of soil moisture on canopy conductance of Amazonian rainforest. Agric Meteorol. 2004;122(3-4):215–227. [Google Scholar]
- 64.da Rocha HR, et al. Patterns of water and heat flux across a biome gradient from tropical forest to savanna in Brazil. J Geophys Res. 2009;114(G1):G00B12. [Google Scholar]
- 65.Guan K, et al. Photosynthetic seasonality of global tropical forests constrained by hydroclimate. Nat Geosci. 2015;8(4):284–289. [Google Scholar]
- 66.Restrepo-Coupe N, et al. What drives the seasonality of photosynthesis across the Amazon basin? A cross-site analysis of eddy flux tower measurements from the Brasil flux network. Agric Meteorol. 2013;182-183:128–144. [Google Scholar]
- 67.Nepstad DC, et al. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature. 1994;372(6507):666–669. [Google Scholar]
- 68.Bruno RD, et al. Soil moisture dynamics in an eastern Amazonian tropical forest. Hydrol Processes. 2006;20(12):2477–2489. [Google Scholar]
- 69.Jipp PH, et al. Deep soil moisture storage and transpiration in forests and pastures of seasonally-dry Amazonia. Clim Change. 1998;39(2):395–412. [Google Scholar]
- 70.Nepstad DC, et al. Amazon drought and its implications for forest flammability and tree growth: A basin-wide analysis. Glob Change Biol. 2004;1(5):704–717. [Google Scholar]
- 71.Brando PM, et al. Abrupt increases in Amazonian tree mortality due to drought-fire interactions. Proc Natl Acad Sci USA. 2014;111(17):6347–6352. doi: 10.1073/pnas.1305499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marengo JA. Interdecadal variability and trends of rainfall across the Amazon basin. Theor Appl Climatol. 2004;78(1):79–96. [Google Scholar]
- 73.Victoria RL, et al. Surface air temperature variations in the Amazon region and its borders during this century. J Clim. 1998;11(5):1105–1110. [Google Scholar]
- 74.Jones PD. Hemispheric surface air temperature variations: A reanalysis and an update to 1993. J Clim. 1994;7(11):1794–1802. [Google Scholar]
- 75.New M, et al. Representing twentieth-century space-time climate variability. Part I: Development of a 1961-90 mean monthly terrestrial climatology. J Clim. 1999;12(2-3):829–856. [Google Scholar]
- 76.Malhi Y, Wright J. Spatial patterns and recent trends in the climate of tropical rainforest regions. Philos Trans R Soc Lond B Biol Sci. 2004;359(1443):311–329. doi: 10.1098/rstb.2003.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gash J, et al. Amazonian Deforestation and Climate. Wiley; New York: 1996. [Google Scholar]
- 78.Costa MH, et al. 2009. Effects of climatic variability and deforestation on surface water regimes. Amazonia and Global Change, Geophysical Monograph Series, eds Keller M, et al. (AGU, Washington, DC), Vol 186, pp 543–554.
- 79.Gloor M, et al. Intensification of the Amazon hydrological cycle over the last two decades. Geophys Res Lett. 2013;40(9):1729–1733. [Google Scholar]
- 80.Marengo JA, et al. Extreme climatic events in the Amazon basin. Theor Appl Climatol. 2011;107(1-2):73–85. [Google Scholar]
- 81.Costa MH, Foley JA. Trends in the hydrologic cycle of the Amazon Basin. J Geophys Res. 1999;104(D12):14189–14198. [Google Scholar]
- 82.Dai A, et al. Changes in continental freshwater discharge from 1948 to 2004. J Clim. 2009;22(10):2773–2792. [Google Scholar]
- 83.Trenberth KE. Changes in precipitation with climate change. Clim Res. 2011;47(1-2):123–138. [Google Scholar]
- 84.Callède J, et al. Evolution du debit de l’Amazone a Obidos de 1903 a 1999. Hydrol Sci J. 2004;49(1):1–97. [Google Scholar]
- 85.Villar JCE, et al. Contrasting regional discharge evolutions in the Amazon basin (1974-2004) J Hydrol (Amst) 2009;375(3-4):297–311. [Google Scholar]
- 86.Doughty CE, et al. Theoretical impact of changing albedo on precipitation at the southernmost boundary of the ITCZ in South America. Earth Interact. 2012;16(8):1–14. [Google Scholar]
- 87.Li W, Fu R, Juárez RI, Fernandes K. Observed change of the standardized precipitation index, its potential cause and implications to future climate change in the Amazon region. Philos Trans R Soc Lond B Biol Sci. 2008;363(1498):1767–1772. doi: 10.1098/rstb.2007.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marengo JA. Long-term trends and cycles in the hydrometeorology of the Amazon basin since the late 1920s. Hydrol Processes. 2009;23(22):3236–3244. [Google Scholar]
- 89.Marengo JA, et al. The drought of 2010 in the context of historical droughts in the Amazon region. Geophys Res Lett. 2011;38(12):L12703. [Google Scholar]
- 90.Intergovernmental Panel on Climate Change . In: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Barros VR, et al., editors. Cambridge Univ Press; Cambridge, UK: 2014. [Google Scholar]
- 91.Sampaio G, et al. Regional climate change over eastern Amazonia caused by pasture and soybean cropland expansion. Geophys Res Lett. 2007;34(17):L17709. [Google Scholar]
- 92.Costa MH, et al. Climate change in Amazonia caused by soybean cropland expansion, as compared to caused by pastureland expansion. Geophys Res Lett. 2007;34(7):L07706. [Google Scholar]
- 93.Roy SB, Avissar R. Impact of land use/land cover change on regional hydrometeorology in Amazonia. J Geophys Res. 2002;107(D20):1–12. [Google Scholar]
- 94.da Silva RR, et al. Regional impacts of future land-cover changes on the Amazon Basin wet-season climate. J Clim. 2008;21(6):1153–1170. [Google Scholar]
- 95.Wang J, et al. Impact of deforestation in the Amazon basin on cloud climatology. Proc Natl Acad Sci USA. 2009;106(10):3670–3674. doi: 10.1073/pnas.0810156106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Medvigy D, et al. Effects of deforestation on spatiotemporal distributions of precipitation in South America. J Clim. 2011;24(8):2147–2163. [Google Scholar]
- 97.Lejeune Q, et al. Influence of Amazonian deforestation on the future evolution of regional surface fluxes, circulation, surface temperature and precipitation. Clim Dyn. 2015;44(9-10):2769–2786. [Google Scholar]
- 98.Silva MES, et al. Local and remote climatic impacts due to land use degradation in the Amazon “Arc of Deforestation.”. Theor Appl Climatol. 2016;125(3):609–623. [Google Scholar]
- 99.Walker R, et al. Protecting the Amazon with protected areas. Proc Natl Acad Sci USA. 2009;106(26):10582–10586. doi: 10.1073/pnas.0806059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lawrence D, Vandecar K. Effects of tropical deforestation on climate and agriculture. Nat Clim Chang. 2015;5(2):27–34. [Google Scholar]
- 101.Bagley JE, et al. Drought and deforestation: Has land cover change influenced recent precipitation extremes in the Amazon? J Clim. 2014;27(1):345–361. [Google Scholar]
- 102.Costa MH, et al. Effects of large-scale changes in land cover on the discharge of the Tocantins River, Southeastern Amazonia. J Hydrol (Amst) 2003;283(1-4):206–217. [Google Scholar]
- 103.Coe MT, et al. The effects of deforestation and climate variability on the streamflow of the Araguaia River, Brazil. Biogeochemistry. 2011;105(1):119–131. [Google Scholar]
- 104.Lee JE, et al. Land use change exacerbates tropical South American drought by sea surface temperature variability. Geophys Res Lett. 2011;38(19):L19706. [Google Scholar]
- 105.Nepstad DC. The Amazon’ s Vicious Cycles. World Wide Fund Nat; Gland, Switzerland: 2008. [Google Scholar]
- 106.Salazar LF, Nobre CA. Climate change and thresholds of biome shifts in Amazonia. Geophys Res Lett. 2010;37(17):L17706. [Google Scholar]
- 107.Lenton TM, et al. Tipping elements in the Earth’s climate system. Proc Natl Acad Sci USA. 2008;105(6):1786–1793. doi: 10.1073/pnas.0705414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saleska SR, Didan K, Huete AR, da Rocha HR. Amazon forests green-up during 2005 drought. Science. 2007;318(5850):612. doi: 10.1126/science.1146663. [DOI] [PubMed] [Google Scholar]
- 109.Phillips OL, et al. Drought sensitivity of the Amazon rainforest. Science. 2009;323(5919):1344–1347. doi: 10.1126/science.1164033. [DOI] [PubMed] [Google Scholar]
- 110.Brando PM, et al. Drought effects on litterfall, wood production and belowground carbon cycling in an Amazon forest: Results of a throughfall reduction experiment. Philos Trans R Soc Lond B Biol Sci. 2008;363(1498):1839–1848. doi: 10.1098/rstb.2007.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fisher RA, et al. The response of an Eastern Amazonian rain forest to drought stress: Results and modelling analyses from a throughfall exclusion experiment. Glob Change Biol. 2007;13(11):2361–2378. [Google Scholar]
- 112.Nepstad DC, Tohver IM, Ray D, Moutinho P, Cardinot G. Mortality of large trees and lianas following experimental drought in an Amazon forest. Ecology. 2007;88(9):2259–2269. doi: 10.1890/06-1046.1. [DOI] [PubMed] [Google Scholar]
- 113.Rowland L, et al. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature. 2015;528(7580):119–122. doi: 10.1038/nature15539. [DOI] [PubMed] [Google Scholar]
- 114.Laurance WF, et al. Rain forest fragmentation and the dynamics of Amazonian tree communities. Ecology. 1998;79(6):2032–2040. [Google Scholar]
- 115.Cochrane MA, Schulze MD. Fire as a recurrent event in tropical forests of the eastern Amazon: Effects on forest structure, biomass, and species composition. Biotropica. 1999;31(1):2–16. [Google Scholar]
- 116.Nepstad DC, et al. Large-scale impoverishment of Amazonian forests by logging and fire. Nature. 1999;398(6727):505–508. [Google Scholar]
- 117.Alencar AAC, et al. Modeling forest understory fires in an eastern Amazonian landscape. Ecol Appl. 2004;14(4):S139–S149. [Google Scholar]
- 118.Barlow J, Peres CA. Ecological responses to el Niño-induced surface fires in central Brazilian Amazonia: Management implications for flammable tropical forests. Philos Trans R Soc Lond B Biol Sci. 2004;359(1443):367–380. doi: 10.1098/rstb.2003.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barlow J, Peres CA. Fire-mediated dieback and compositional cascade in an Amazonian forest. Philos Trans R Soc Lond B Biol Sci. 2008;363(1498):1787–1794. doi: 10.1098/rstb.2007.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aragão LEOC, et al. Interactions between rainfall, deforestation and fires during recent years in the Brazilian Amazonia. Philos Trans R Soc Lond B Biol Sci. 2008;363(1498):1779–1785. doi: 10.1098/rstb.2007.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ray D, et al. Micrometeorological and canopy controls of fire susceptibility in a forested Amazon landscape. Ecol Appl. 2005;15(5):1664–1678. [Google Scholar]
- 122.Huntingford C, et al. Simulated resilience of tropical rainforests to CO2-induced climate change. Nat Geosci. 2013;6(4):268–273. [Google Scholar]
- 123.Norby RJ, et al. Rising CO2 − Future ecosystems. New Phytol. 2001;150(2):215–221. [Google Scholar]
- 124.Norby RJ, et al. Forest response to elevated CO2 is conserved across a broad range of productivity. Proc Natl Acad Sci USA. 2005;102(50):18052–18056. doi: 10.1073/pnas.0509478102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McCarthy HR, et al. Re-assessment of plant carbon dynamics at the Duke free-air CO(2) enrichment site: Interactions of atmospheric [CO(2)] with nitrogen and water availability over stand development. New Phytol. 2010;185(2):514–528. doi: 10.1111/j.1469-8137.2009.03078.x. [DOI] [PubMed] [Google Scholar]
- 126.Walker AP, et al. Comprehensive ecosystem model-data synthesis using multiple data sets at two temperate forest free-air CO2 enrichment experiments: Model performance at ambient CO2 concentration. J Geophys Res Biogeosci. 2014;119(5):937–964. [Google Scholar]
- 127.Hickler T, et al. CO2 fertilization in temperate FACE experiments not representative of boreal and tropical forests. Glob Change Biol. 2008;14(7):1531–1542. [Google Scholar]
- 128.Lapola DM, et al. Exploring the range of climate biome projections for tropical South America: The role of CO2 fertilization and seasonality. Global Biogeochem Cycles. 2009;23(3):GB3003. [Google Scholar]
- 129.de Boer HJ, et al. Climate forcing due to optimization of maximal leaf conductance in subtropical vegetation under rising CO2. Proc Natl Acad Sci USA. 2011;108(10):4041–4046. doi: 10.1073/pnas.1100555108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lammertsma EI, et al. Global CO2 rise leads to reduced maximum stomatal conductance in Florida vegetation. Proc Natl Acad Sci USA. 2011;108(10):4035–4040. doi: 10.1073/pnas.1100371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brienen RJW, et al. Long-term decline of the Amazon carbon sink. Nature. 2015;519(7543):344–348. doi: 10.1038/nature14283. [DOI] [PubMed] [Google Scholar]
- 132.Gloor M, et al. Does the disturbance hypothesis explain the biomass increase in basin-wide Amazon forest plot data? Glob Change Biol. 2009;15(10):2418–2430. [Google Scholar]
- 133.Lewis SL, et al. Increasing carbon storage in intact African tropical forests. Nature. 2009;457(7232):1003–1006. doi: 10.1038/nature07771. [DOI] [PubMed] [Google Scholar]
- 134.Pan Y, et al. A large and persistent carbon sink in the world’s forests. Science. 2011;333(6045):988–993. doi: 10.1126/science.1201609. [DOI] [PubMed] [Google Scholar]
- 135.Quesada CA, et al. Variations in chemical and physical properties of Amazon forest soils in relation to their genesis. Biogeosciences. 2010;7(5):1515–1541. [Google Scholar]
- 136.Castanho ADA, et al. Improving simulated Amazon forest biomass and productivity by including spatial variation in biophysical parameters. Biogeosciences. 2013;10(4):2255–2272. [Google Scholar]
- 137.Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE. CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc Natl Acad Sci USA. 2010;107(45):19368–19373. doi: 10.1073/pnas.1006463107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chapin FS, et al. The ecology and economics of storage in plants. Annu Rev Ecol Syst. 1990;21(1):423–447. [Google Scholar]
- 139.Bigler C, Veblen TT. Increased early growth rates decrease longevities of conifers in subalpine forests. Oikos. 2009;118(8):1130–1138. [Google Scholar]
- 140.di Filippo A, et al. Bioclimate and growth history affect beech lifespan in the Italian Alps and Apennines. Glob Change Biol. 2012;18(3):960–972. [Google Scholar]
- 141.Friend AD, et al. Carbon residence time dominates uncertainty in terrestrial vegetation responses to future climate and atmospheric CO2. Proc Natl Acad Sci USA. 2014;111(9):3280–3285. doi: 10.1073/pnas.1222477110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.LaDeau SL, Clark JS. Rising CO2 levels and the fecundity of forest trees. Science. 2001;292(5514):95–98. doi: 10.1126/science.1057547. [DOI] [PubMed] [Google Scholar]
- 143.Norby RJ, et al. Model-data synthesis for the next generation of Forest Free-Air CO2 Enrichment (FACE) experiments. New Phytol. 2016;209(1):17–28. doi: 10.1111/nph.13593. [DOI] [PubMed] [Google Scholar]
- 144.Fearnside PM. Greenhouse Gas emissions from land-use changes in Brazil’s Amazon. In: Lal R, et al., editors. Global Climate Change and Tropical Ecosystems: Advances in Soil Science. CRC Press; Boca Raton, FL: 2000. pp. 231–249. [Google Scholar]
- 145.Kemenes A, et al. Methane release below a tropical hydroelectric dam. Geophys Res Lett. 2007;34(12):12805–12809. [Google Scholar]
- 146.Barthem RB, Goulding M. The Catfish Connection: Ecology, Migration, and Conservation of Amazon Predators. Columbia Univ Press; New York: 1997. [Google Scholar]
- 147.Mérona B, et al. Fishes and Fisheries in the Lower Tocantins River: Twenty Years After the Tucuruí Hydroelectric Dam. Eletrobrás-Eletronorte; Brasilia, Brazil: 2010. [Google Scholar]
- 148.Finer M, Jenkins CN. Proliferation of hydroelectric dams in the Andean Amazon and implications for Andes-Amazon connectivity. PLoS One. 2012;7(4):e35126. doi: 10.1371/journal.pone.0035126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vörösmarty CJ, et al. Anthropogenic sediment retention: Major global impact from registered river impoundments. Global Planet Change. 2003;39(1-2):169–190. [Google Scholar]
- 150.Fearnside PM. Impacts of Brazil’s Madeira River dams: Unlearned lessons for hydroelectric development in Amazonia. Environ Sci Policy. 2014;38:164–172. doi: 10.1007/s002670010156. [DOI] [PubMed] [Google Scholar]
- 151.Hiwat H, Bretas G. Ecology of Anopheles darlingi Root with respect to vector importance: A review. Parasit Vectors. 2011;4:177. doi: 10.1186/1756-3305-4-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gunkel G, et al. The environmental and operational impacts of Curuá-Una, a reservoir in the Amazon region of Pará, Brazil. Lakes Reservoirs Res Manage. 2003;8(3-4):201–216. [Google Scholar]
- 153.Crisostomo AC, et al. Terras Indígenas na Amazônia Brasileira: Reservas de Carbono e Barreiras ao Desmatamento. Inst Pesquisa Ambiental Amazônia; Brasilia, Brazil: 2015. [Google Scholar]
- 154.Nuti MR. Integração, Usinas Hidrelétricas e Impactos Socioambientais. Inst Estudos Socioecon; Brasilia, Brazil: 2007. Análise das estimativas de população atingida por projetos hidrelétricos; pp. 57–88. [Google Scholar]
- 155.Alho CJ, Reis RE, Aquino PP. Amazonian freshwater habitats experiencing environmental and socioeconomic threats affecting subsistence fisheries. Ambio. 2015;44(5):412–425. doi: 10.1007/s13280-014-0610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature. 2000;408(6809):184–187. doi: 10.1038/35041539. [DOI] [PubMed] [Google Scholar]
- 157.Cox PM, et al. Amazonian forest dieback under climate-carbon cycle projections for the 21st century. Theor Appl Climatol. 2004;78(1-3):137–156. [Google Scholar]
- 158.Oyama MD, Nobre CA. A new climate-vegetation equilibrium state for Tropical South America. Geophys Res Lett. 2003;30(23):2199. [Google Scholar]
- 159.Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413(6856):591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- 160.Salazar LF, et al. Climate change consequences on the biome distribution in tropical South America. Geophys Res Lett. 2007;34(9):L09708. [Google Scholar]
- 161.Lenton TM. Early warning of climate tipping points. Nat Clim Change. 2011;1(4):201–209. [Google Scholar]
- 162.Alencar AAC, Brando PM, Asner GP, Putz FE. Landscape fragmentation, severe drought, and the new Amazon forest fire regime. Ecol Appl. 2015;25(6):1493–1505. doi: 10.1890/14-1528.1. [DOI] [PubMed] [Google Scholar]
- 163.Rolla A, Ricardo F. Amazônia Brasileira 2009. Instituto Socioambiental. Inst Socioambiental; Brasilia, Brazil: 2009. [Google Scholar]
- 164.Adeney JM, Christensen NL, Pimm SL. Reserves protect against deforestation fires in the Amazon. PLoS One. 2009;4(4):e5014. doi: 10.1371/journal.pone.0005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Verburg R, et al. Towards a low carbon economy in the Amazon: The role of land-use policies. Sustentabilidade Debate. 2011;2(2):83–96. [Google Scholar]
- 166.Miranda RFC, et al. Technical-economic potential of PV systems on Brazilian rooftops. Renewable Energy. 2015;75:694–713. [Google Scholar]
- 167.Nogueira LAHC, et al. Evaluation of the energy impacts of the Energy Efficiency Law in Brazil. Energy Sustainable Dev. 2015;24:58–69. [Google Scholar]
- 168.Mohammed YS, et al. Hybrid renewable energy systems for off-grid electric power: Review of substantial issues. Renewable Sustainable Energy Rev. 2014;35:527–539. [Google Scholar]
- 169.Alves JJA. Análise regional da energia eólica no Brasil. Rev Brasileira Gestão Desenvolvimento Reg. 2010;6(1):165–188. [Google Scholar]
- 170.Caneppele FL, Gabriel Filho LRA. Current overview of the use of solar energy in Brazil. UNOPAR Cient Cienc Exatas Tecnol. 2015;13(1):69–74. [Google Scholar]
- 171.Rabaey K, Verstraete W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005;23(6):291–298. doi: 10.1016/j.tibtech.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 172.Wendell D, Todd J, Montemagno C. Artificial photosynthesis in ranaspumin-2 based foam. Nano Lett. 2010;10(9):3231–3236. doi: 10.1021/nl100550k. [DOI] [PubMed] [Google Scholar]
- 173.Service RF. Artificial leaf turns sunlight into a cheap energy source. Science. 2011;332(6025):25. doi: 10.1126/science.332.6025.25. [DOI] [PubMed] [Google Scholar]
- 174.Benyus J. Biomimicry: Innovation Inspired by Nature. 1st Ed Perennial; New York: 2002. [Google Scholar]
- 175.Lurie-Luke E. Product and technology innovation: What can biomimicry inspire? Biotechnol Adv. 2014;32(8):1494–1505. doi: 10.1016/j.biotechadv.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 176.Murphy WL, Mooney DJ. Molecular-scale biomimicry. Nat Biotechnol. 2002;20(1):30–31. doi: 10.1038/nbt0102-30. [DOI] [PubMed] [Google Scholar]
- 177.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294(5547):1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 178.Eadie L, Ghosh TK. Biomimicry in textiles: Past, present and potential. An overview. J R Soc Interface. 2011;8(59):761–775. doi: 10.1098/rsif.2010.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Edwards C. Brain science helps computers separate speakers in a crowded room. Commun ACM. 2015;58(11):18–20. [Google Scholar]
- 180.Nogueira RC, de Cerqueira HF, Soares MB. Patenting bioactive molecules from biodiversity: The Brazilian experience. Expert Opin Ther Pat. 2010;20(2):145–157. doi: 10.1517/13543770903555221. [DOI] [PubMed] [Google Scholar]
- 181.Pandey RC. Prospecting for potentially new pharmaceuticals from natural sources. Med Res Rev. 1998;18(5):333–346. doi: 10.1002/(sici)1098-1128(199809)18:5<333::aid-med4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 182.Barata LES. A economia verde – Amazônia. Cienc Cult. 2012;64(3):31–35. [Google Scholar]
- 183.Nobre CA. Brazil: Boost pro-forest economics. Nature. 2014;510(7504):210. [Google Scholar]
- 184.Brondizio ES. The Amazonian Caboclo and the Açaí Palm: Forest Farmers in the Global Market. New York Botanical Garden Press; New York: 2008. p. 402. [Google Scholar]
- 185.Brondizio ES, et al. The urban market of Açaí fruit (Euterpe oleracea Mart.) and rural land use change: Ethnographic insights into the role of price and land tenure constraining agricultural choices in the Amazon estuary. Urban Ecosyst. 2002;6(1/2):67–98. [Google Scholar]
- 186. de Marajo and Paragominas I (May 10, 2001) Managing the rainforests. Economist. Available at www.economist.com/node/616834. Accessed February 2, 2016.
- 187.Homma AKO. Em favor de uma revolução tecnológica na Amazônia. Trop Plant Pathol. 2012;37:1–17. [Google Scholar]
- 188.Homma AKO. O Extrativismo do óleo Essencial de Pau-Rosa na Amazônia. Embrapa Amazônia Oriental; Belem, Brazil: 2003. [Google Scholar]
- 189.Homma AKO, Carvalho JEU, Menezes AJEA. Bacuri: Fruta amazônica em ascensão. Cienc Hoje. 2010;46:40–45. [Google Scholar]
- 190.Oliveira Dias AF, et al. 2010. FR Patent 2934495-A1, 2934495-B1; WO Patent 2011011840-A1; CA Patent 2732600-A1, 2794623-C; US Patent 2011256075-A1; and EP Patent 2459165-A1.
- 191.Brazilian Academy of Science . Amazon: The Challenge of Brazil for the 21st Century - The Need of a Scientific and Technological Revolution. Brazilian Acad Sci; Brasilia, Brazil: 2008. [Google Scholar]
- 192.Marcovitch J. A gestão da Amazônia: Ações empresariais, políticas públicas, estudos e propostas. Dir Open Access J. 2012;9(1):380–383. [Google Scholar]
- 193.Taylor KE, et al. An overview of CMIP5 and the experiment design. Bull Am Meteorol Soc. 2012;93(4):485–498. [Google Scholar]
- 194.Yin L, et al. How well can CMIP5 simulate precipitation and its controlling processes over tropical South America? Clim Dyn. 2013;41(11-12):3127–3143. [Google Scholar]
- 195.Joetzjer E, et al. Predicting the response of the Amazon rainforest to persistent drought conditions under current and future climates: A major challenge for global land surface models. Geosci Model Dev Discuss. 2014;7(4):5295–5340. [Google Scholar]
- 196.Boisier JP, et al. Projected strengthening of Amazonian dry season by constrained climate model simulations. Nat Clim Chang. 2015;5(7):656–660. [Google Scholar]
- 197.Saatchi SS, et al. Distribution of aboveground live biomass in the Amazon basin. Glob Change Biol. 2007;13(4):816–837. [Google Scholar]
- 198.Malhi Y, et al. Climate change, deforestation, and the fate of the Amazon. Science. 2008;319(5860):169–172. doi: 10.1126/science.1146961. [DOI] [PubMed] [Google Scholar]
- 199.Cavalcanti IFA, et al. Global climatological features in a simulation using the CPTEC-COLA AGCM. J Clim. 2002;15(21):2965–2988. [Google Scholar]
- 200.Soares-Filho BS, et al. Modelling conservation in the Amazon basin. Nature. 2006;440(7083):520–523. doi: 10.1038/nature04389. [DOI] [PubMed] [Google Scholar]
- 201.Moss RH, et al. The next generation of scenarios for climate change research and assessment. Nature. 2010;463(7282):747–756. doi: 10.1038/nature08823. [DOI] [PubMed] [Google Scholar]
- 202.Willmott CJ, Matsuura K. Terrestrial Air Temperature and Precipitation: Monthly and Annual Climatologies. Univ Delaware; Newark, DE: 1998. [Google Scholar]
- 203.Xue Y, et al. Simplified biosphere model for global climate studies. J Clim. 1991;4(3):345–364. [Google Scholar]
- 204.Dorman JL, Sellers PJ. A global climatology of albedo, roughness length and stomatal resistance for atmospheric general circulation models as represented by the Simple Biosphere Model (SiB) Am Meteorol Soc. 1989;28(9):833–855. [Google Scholar]
- 205.Oyama MD, Nobre CA. A simple potential vegetation model for coupling with the Simple Biosphere Model (SIB) Rev Brasileira Meteorol. 2004;19(2):203–216. [Google Scholar]
- 206.Willmott CJ, et al. Climatology of the terrestrial seasonal water cycle. Int J Climatol. 1985;5(6):589–606. [Google Scholar]
- 207.Collatz GJ, et al. Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration: A model that includes a laminar boundary layer. Agric For Meteorol. 1991;54(2-4):107–136. [Google Scholar]
- 208.Sitch S, et al. Evaluation of the terrestrial carbon cycle, future plant geography and climate-carbon feedbacks using 5 Dynamic Global Vegetation Models (DGVMs) Glob Change Biol. 2008;14(9):2015–2039. [Google Scholar]
- 209.Sellers PJ, et al. A Simple Biosphere model (SiB) for use within General Circulation Models. J Atmos Sci. 1986;43(6):505–531. [Google Scholar]
- 210.Cardoso MF, et al. Long-term potential for fires in estimates of the occurrence of savannas in the tropics. Glob Ecol Biogeogr. 2008;17(2):222–235. [Google Scholar]
- 211.Lapola DM, Oyama MD, Nobre CA, Sampaio G. A new world natural vegetation map for global change studies. An Acad Bras Cienc. 2008;80(2):397–408. doi: 10.1590/s0001-37652008000200017. [DOI] [PubMed] [Google Scholar]
- 212.Cardoso M, et al. Long-term potential for tropical-forest degradation due to deforestation and fires in the Brazilian Amazon. Biologia. 2009;64(3):433–437. [Google Scholar]
- 213.Ramos-Neto MB, Pivello VR. Lightning fires in a Brazilian savanna national park: Rethinking management strategies. Environ Manage. 2000;26(6):675–684. doi: 10.1007/s002670010124. [DOI] [PubMed] [Google Scholar]
- 214.Cardoso MF, et al. Projecting future fire activity in Amazonia. Glob Change Biol. 2003;9(5):656–669. [Google Scholar]
- 215.Kutzbach JR, et al. Climate and the biome simulations for the past 21,000 years. Quat Sci Rev. 1998;17:473–506. [Google Scholar]
- 216.World Economic Forum 2015. Deep Shift – Technology Tipping Points and Societal Impact (World Economic Forum, Geneva)
- 217.Schwab K. 2016. The Fourth Industrial Revolution (World Economic Forum, Geneva)
- 218.Brynjolfsson E, McAfee A. The Second Machine Age: Work, Progress, and Prosperity in a Time of Brilliant Technologies. W.W. Norton; New York: 2014. [Google Scholar]
- 219.National Human Genome Research Institute . DNA Sequencing Costs: Data from the NHGRI Genome Sequencing Program. Nat Human Genome Res Inst; Bethesda, MD: 2015. [Google Scholar]
- 220.World Economic Forum . Top 10 Technologies of 2015 Meta-Council on Emerging Technologies. World Econ Forum; Geneva: 2015. [Google Scholar]
- 221.Castilla-Rubio JC. UNEP Enquiry into Fintech for Sustainable Development. UN Environ Programme; Nairobi: 2016. [Google Scholar]
- 222.Ellen MacArthur Foundation . The New Plastics Economy: Rethinking the Future of Plastics. Ellen MacArthur Found; Cowes, UK: 2016. [Google Scholar]
- 223. Ciência Hoje Na cozinha com os índios. Available at chc.cienciahoje.uol.com.br/na-cozinha-com-os-indios. Accessed: March 22, 2016.