Abstract
Bupropion has been used as an antidepressant for over 20 years, though its licence for such use varies and it is typically a third- or fourth-line agent. It has a unique pharmacology, inhibiting the reuptake of noradrenaline and dopamine, potentially providing pharmacological augmentation to more common antidepressants such as selective serotonergic reuptake inhibitors (SSRIs). This systematic review and meta-analysis identified 51 studies, dividing into four categories: bupropion as a sole antidepressant, bupropion coprescribed with another antidepressant, bupropion in ‘other’ populations (e.g. bipolar depression, elderly populations) and primary evaluation of side effects.
Methodologically more robust trials support the superiority of bupropion over placebo, and most head-to-head antidepressant trials showed an equivalent effectiveness, though some of these are hindered by a lack of a placebo arm. Most work on the coprescribing of bupropion with another antidepressant supports an additional effect, though many are open-label trials. Several large multi-medication trials, most notably STAR*D, also support a therapeutic role for bupropion; in general, it demonstrated similar effectiveness to other medications, though this literature highlights the generally low response rates in refractory cohorts. Effectiveness has been shown in ‘other’ populations, though there is an overall dearth of research. Bupropion is generally well tolerated, it has very low rates of sexual dysfunction, and is more likely to cause weight loss than gain. Our findings support the use of bupropion as a sole or coprescribed antidepressant, particularly if weight gain or sexual dysfunction are, or are likely to be, significant problems. However there are notable gaps in the literature, including less information on treatment naïve and first presentation depression, particularly when one considers the ever-reducing rates of response in more refractory illness. There are some data to support bupropion targeting specific symptoms, but insufficient information to reliably inform such prescribing, and it remains uncertain whether bupropion pharmacodynamically truly augments other drugs.
Keywords: antidepressant, bupropion, efficacy
Introduction
Major depressive disorders (MDD) are a leading global cause of morbidity [Ferrari et al. 2013; Kessler et al. 2003; Murray et al. 2013] affecting up to a fifth of individuals [Hirschfeld, 2012], four fifths of whom will have multiple illness episodes [Bulloch et al. 2014].
Guidelines generally advocate selective serotonergic reuptake inhibitors (SSRIs) as the first-line pharmacological intervention, primarily due to their more benign side-effect profile rather than any superiority in efficacy [NICE, 2009]. A so-called ‘therapeutic trial’ of a minimum recognized dose over about 6 weeks is ordinarily recommended before changing medication, and treatment resistance is usually defined as failure to respond to two such trials. Disappointing data indicate that about half of patients discontinue their treatment during such an initial time frame [Melfi et al. 1998].
Response, usually defined as a ⩾50% symptom reduction, is typically seen in about half to three quarters on a first trial of an antidepressant, with symptom remission occurring in about a third [Nemeroff et al. 2008; Trivedi et al. 2006b]. Unfortunately many individuals prove resistant to multiple first- and second-line pharmacological interventions [Coplan et al. 2014; Rush et al. 2004]. Treatment options thereafter include changing drug class or adding a second agent, though there are not convincing data to clearly support one strategy over the other [Rush, 2007]; the overall literature on pharmacological ‘next steps’ has numerous options, but most without strong evidence bases [Taylor et al. 2015].
Most antidepressants act through increasing the synaptic levels of serotonin or noradrenaline (norepinephrine) through various pharmacological mechanisms. Bupropion is an aminoketone, and has a unique pharmacology, inhibiting the reuptake of both noradrenaline and dopamine. It has no effects on serotonin, histamine, acetylcholine or adrenaline (epinephrine) receptors; it is thus not associated with significant sedation, cognitive or anticholinergic gastrointestinal or hypotensive side effects [Stahl et al. 2004].
Bupropion has been licensed for depression in the United States since the late 1990s [Fava et al. 2005]; an extended formulation version became available in 2007. In the United Kingdom it is only licensed for the treatment of nicotine addiction, though it is prescribed off-licence for depression. There is literature supporting its effectiveness as an antidepressant in various populations, and data that it can cause weight loss and help sexual dysfunction. However to date there have been no systematic reviews or meta-analyses of its effectiveness and side-effect profile in affective disorders.
Aims
The aim of this study was to provide a systematic evaluation and meta-analysis of the effectiveness of bupropion as an antidepressant, both when prescribed alone and in combination with other antidepressants, and describe its side-effect profile, particularly the effects on weight and sexual dysfunction.
Methods
Search strategy
An electronic search was conducted between 19 and 27 March 2015. Potentially relevant studies were identified by searching the following databases: PsycInfo (1806–27 March 2015), Medline (1946–27 March 2015), Embase (1980–27 March 2015) via OvidSP, PubMed, Web of Science (Core Collection) and The Cochrane Library. The search criteria were as follows: ‘bupropion’ OR ‘Wellbutrin’ OR ‘Aplenzin’ OR ‘Forfivo’ OR ‘Zyban’ OR ‘Amfebutamone’ combined with AND ‘major depress*’ OR ‘(MDD)’ OR ‘depress*’ OR ‘mood disorder’ OR ‘depressive-disorder’ OR ‘bipolar’ OR ‘unipolar’ OR ‘bipolar affective disorder’ OR ‘seasonal affective disorder’. The review was limited to articles published in English, and a thorough search of grey literature was not undertaken. The reference list of each included study and relevant reviews were examined for potential studies.
Participants
Studies that looked at adult (18–65) and elderly populations (⩾65) with a diagnosis of MDD, bipolar affective disorder, seasonal affective disorder (SAD), dysthymia, or postnatal (postpartum) depression (as defined by DSM-V or ICD-10, or previous versions of these diagnostic manuals) were included.
Intervention
Randomized controlled trials (RCTs) and open-label trials evaluating the effect of bupropion (any dose or formulation) on depressive symptoms were included.
Comparator intervention
RCTs with a placebo-arm, head-to-head trials with no placebo-arm and augmentation trials were deemed eligible for review.
Outcomes
Only studies evaluating effectiveness through validated measures were included, namely the following: Hamilton Depression Rating Scale (HDRS/HAM-D) [Hamilton, 1960]; Montgomery Asberg Depression Rating Scale (MADRS) [Montgomery and Asberg, 1979]; Inventory for Depressive Symptomology (IDS) [Rush et al. 1986]; Structured Interview Guide for the Hamilton Depression Rating Scale–Seasonal Affective Disorder Version (SIGH-SAD) [Williams et al. 1988].
Study design
Only journal articles post 1990 were included for review, with case studies (⩽3 participants), expert opinions and poster presentations excluded.
Study selection
We initially identified 14,372 reports (see Figure 1); after adjusting for duplicates (9817) 4555 articles remained. We excluded 4417 articles as unsuitable based on title and abstract. In total 138 full-text articles were evaluated, 87 of which were excluded for being conference abstracts or presentations, pre-1990 trials, case studies, a non-included patient population, failure to separate results according to treatment or disorder and for not containing original data. Ultimately there were 51 studies that met the criteria for qualitative synthesis, which we divided into four major groups: those evaluating bupropion as a sole pharmacological intervention in MDD (n = 27); those evaluating its coprescribing with a second antidepressant in MDD (n = 13); the treatment of ‘other’ populations such as bipolar depression and the elderly (n = 11); and primary evaluation of side effects (n = 13).
Figure 1.
A summary of the search strategy, following PRISMA guidelines.
Data extraction
Data was extracted by two authors (KP and SA). Extracted data concerning patient characteristics and study results are available in Tables 1 and 2. For quantitative analysis, effect sizes were converted to Hedge’s g, to decrease the risk of bias associated with standardized mean differences.
Table 1.
Table of demographic and clinical characteristics.
| Study | Age (years) | Gender | Length of current episode | Primary outcome measure(s) | Secondary measures | Response criteria | Remission criteria | Washout period | Concurrent medication | Diagnosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Monotherapy | Jefferson et al. [2006] | Bupropion: 40.0 (±12) Placebo: 39.8 (±12.5) |
Bupropion: 66% Female Placebo: 69% Female |
⩾12 weeks and ⩽2 years Bupropion: 40.4 weeks Placebo: 43.2 weeks |
IDS-IVR-30 | IDS-C-30 CGI |
⩾50% reduction in IDS-IVR-30 & IDS-C-30 | IDS-IVR-30 ⩽15 IDS-C-30 ⩽13 |
Yes | Zolpidem or zaleplon was allowed during the study through to treatment day 10. | MDD |
| Koshino et al. [2013] | Bupropion 150 mg: 36.0 (±10.42) Bupropion 300 mg: 37.5 (±10.96) Placebo: 37.9 (±11.09) |
Bupropion 150 mg: 52% Female Bupropion 300 mg: 56% Female Placebo: 54% Female |
⩾8 weeks and ⩽2 years Bupropion 150mg: 26.5 weeks Bupropion 300 mg: 28.7 weeks Placebo: 26.8 weeks |
MADRS | ⩾50% reduction in MADRS | MADRS ⩽11 | Yes | Nil | MDD | ||
| Lineberry et al. [1990] | Bupropion: 41.9 Placebo: 40.8 |
Bupropion: 64.6% Female Placebo: 65.1% Female |
⩾4 weeks and ⩽2 years | HAM-D-21 | MADRS CGI-I CGI-S |
⩾50% reduction in HAMD-D | Not stated | Yes | Nil | MDD | |
| Reimherr et al. [1998] | Bupropion 150 mg: 38.3 (±11.0) Bupropion 300 mg: 38.6 (±10.7) Placebo: 40.2 (±12.2) |
Bupropion 150 mg: 72% Female Bupropion 300 mg: 79% Female Placebo: 59% Female |
⩾4 weeks and ⩽2 years | HAM-D-17 | CGI-I CGI-S |
Not stated | Not stated | Yes | Nil | MDD | |
| Brown et al. [2007] | Not stated | Bupropion: 88.9% Female |
Not stated | HAM-D-17 HAM-A IDS-SR |
ACQ | ⩾50% reduction in HAMD-D | HAM-D ⩽7 | Not stated | Not stated | MDD | |
| Gross et al. [2007] | Bupropion: 33.54 (±9.32) |
Bupropion: 76.9% Female |
Not stated | HAM-D-17 CGI-S |
MEI | Not stated | Not stated | Yes | Not stated | MDD | |
| Weihs et al. [2002] | Bupropion: 39.9 (±13.25) Placebo: 39.4 (±13.75) |
Bupropion: 64% Female Placebo: 66% Female |
⩾8 weeks and ⩽2 years | Time to depression relapse (time of randomisation to first prescription of new pharmacotherapy or ECT) | HAM-D-21 HAM-A CGI-S |
Not stated | Not stated | Not stated | Nil | MDD | |
| Fava et al. [2003] | Bupropion: 37.8 (±9.8) |
Bupropion: 46.2% Female |
Not stated | HAM-D | CGI-S CGI-I |
⩾50% reduction in HAMD-D | HAM-D ⩽7 | Nil | Not stated | MDD | |
| Ferguson et al. [1994] | Bupropion: 42.1 (±10.3) |
Bupropion: 70% Female |
Not stated | HAM-D-28 | CGI-S CGI-I HAM-A |
⩾50% reduction in HAMD-D | Not stated | Yes | Choral hydrate permitted for first 14 days of the treatment phase | MDD | |
| Walker et al. [1993] | Bupropion: 45 |
Bupropion: 56% Female |
Not stated | HAM-D-28 | CGI-S CGI-I |
Not stated | Not stated | Yes | Not stated | MDD | |
| Tomarken et al. [2004] | Bupropion: 39.4 (±9.8) Placebo: 37.5 (±7.8) |
Bupropion: 60% Female Placebo: 66.6% Female |
Not stated | MASQ HAM-D-17 HAM-A |
Not stated | Not stated | Yes | Not stated | MDD | ||
| Bupropion versus SSRIs | Clayton et al. [2006] | Bupropion: 37 (±12) Escitalopram: 35 (±11) Placebo: 36 (±11) |
Bupropion: 58% Female Escitalopram: 57% Female Placebo: 60% Female |
⩾12 weeks and ⩽2 years Bupropion: 38 weeks Escitalopram: 40 weeks Placebo: 44 weeks |
HAM-D-17 | CGI-S CGI-I HAD |
⩾50% reduction in HAMD-D | HAM-D ⩽7 | Yes | Zolpidem, zaleplon or nonprescription sleep aids were allowed during the study through to treatment day 10. | MDD |
| Coleman et al. [1999] | Bupropion: 38.1 (±11.5) Sertraline: 38.3 (±13.75) Placebo: 38.5 (±11.75) |
Bupropion: 56% Female Sertraline: 54% Female Placebo: 59% Female |
⩾8 weeks and ⩽2 years | HAM-D-31 | HAM-A CGI-S CGI-I |
⩾50% reduction in HAMD-D | Not stated | Yes | Choral hydrate permitted for first 14 days of the treatment phase | Moderate to severe, recurrent major depression | |
| Coleman et al. [2001] | Bupropion: 36.6 (±12.25) Fluoxetine: 37.1 (±14.5) Placebo: 36.7 (±10.75) |
Bupropion: 63% Female Fluoxetine: 66% Female Placebo: 61% Female |
⩾8 weeks and ⩽2 years | HAM-D-21 | ⩾50% reduction in HAMD-D | HAM-D ⩽8 | Yes | Nil | Moderate to severe, recurrent major depression | ||
| Croft et al. [1999] | Bupropion: 35.9 (±12.75) Sertraline: 36.0 (±10.5) Placebo: 37.4 (±12.75) |
Bupropion: 51% Female Sertraline: 50% Female Placebo: 50% Female |
⩾8 weeks and ⩽2 years | HAM-D-31 | HAM-A CGI-S CGI-I |
⩾50% reduction in HAMD-D | Not stated | Yes | Nil | Moderate to severe, recurrent major depression | |
| Feighner et al. [1991] | Bupropion: 40.9 Fluoxetine: 42.9 |
Bupropion: 64.4% Female Fluoxetine: 63.3% Female |
⩾4 weeks and ⩽2 years | HAM-D-21 | HAM-A CGI-S CGI-I |
⩾50% reduction in HAMD-D | Not stated | Yes | Not stated | MDD | |
| Grunebaum et al. [2012] | Bupropion: 37.9 (±11.9) Paroxetine: 35.2 (±12.8) |
Bupropion: 53.9% Female Paroxetine: 58.3% Female |
Not stated | mHDRS-171 (subtracting suicide items) | Not stated | Not stated | Nil | Not stated | MDD | ||
| Kavoussi et al. [1997] | Bupropion: 39 (±14.25) Sertraline: 40 (±14) |
Bupropion: 48% Female Sertraline: 48% Female |
⩾4 weeks and ⩽2 years | HAM-D | HAM-A CGI-S CGI-I |
Not stated | Not stated | Yes | Chloral hydrate permitted for first 2 weeks | MDD | |
| Kennedy et al. [2006] | 37.8 (±10.5) | 48% Female | ⩾4 weeks | HDRS | ⩾50% reduction in HAMD-D | HAM-D ⩽7 | Yes | Zopiclone for sedation for first 2 weeks | MDD | ||
| Rush et al. [2001] | Bupropion: 39 (±14.25) Sertraline: 40 (±14) |
Bupropion: 48% Female Sertraline: 48% Female |
Not stated | HAM-D | HAM-A CGI-S CGI-I |
⩾50% reduction in HAMD-D | HAM-D ⩽8 | Not stated | Not stated | MDD | |
| Rush et al. [2006] | Bupropion: 41.9 (±12.9) Sertraline: 42.6 (±12.7) Venlafaxine: 41.1 (±12.6) |
Bupropion: 56.9% Female Sertraline: 55.0% Female Venlafaxine: 64.0% Female |
Not stated | HAM-D ⩽7 remission rate | QIDS-SR-16 | ⩾50% reduction in QIDS-SR | QIDS-SR ⩽5 | Nil | Concomitant medication for general medical conditions, anxiolytic and hypnotic medication permitted | MDD | |
| Bupropion versus SNRIs | Hewett et al. [2009] | Bupropion: 41.8 (±11.68) Venlafaxine: 42.7 (±11.48) Placebo: 41.8 (±11.56) |
Bupropion: 74% Female Venlafaxine: 68% Female Placebo: 72% Female |
⩾8 weeks | MADRS | HAM-A CGI-S CGI-I |
⩾50% reduction in MADRS | MADRS ⩽11 | Yes | Nil | MDD |
| Hewett et al. [2010b] | Bupropion: 45.6 (±11.76) Venlafaxine: 44.1 (±11.54) Placebo: 44.5 (±10.79) |
Bupropion: 63% Female Venlafaxine: 68% Female Placebo: 67% Female |
⩾8 weeks | MADRS | CGI-S HAM-A |
⩾50% reduction in MADRS | MADRS ⩽11 | Yes | Nil | MDD | |
| Rosso et al. [2012] | Bupropion: 46.6 (±13.1) Duloxetine: 47.6 (±12.6) |
Bupropion: 71.4% Female Duloxetine: 64% Female |
Not stated | HAM-D-17 | CGI-S CGI-I GAF |
⩾50% reduction in HAMD-D | HAM-D ⩽8 | Yes | Benzodiazepine’s allowed in the first two weeks | MDE-TR | |
| Thase et al. [2006] | Bupropion: 37.1 (±12.3) Venlafaxine: 37.4 (±11.6) |
Bupropion: 56% Female Venlafaxine: 64% Female |
⩾8 weeks and ⩽2 years | HAM-D-17 | CGI-S CGI-I |
⩾50% reduction in HAMD-D | HAM-D ⩽7 | Yes | Nil | MDD | |
| Bupropion versus SARIs | Weisler et al. [1994] | Bupropion: 40.2 Trazadone: 40.8 |
Bupropion: 52.4% Female Trazadone: 65% Female |
Not stated | HAM-D-21 | CGI-S CGI-I HAM-A |
⩾50% reduction in HAMD-D | HAM-D ⩽10 | Yes | Not stated | MDD |
| Bupropion versus TCAs | Masco et al. [1994] | Bupropion: 46.3 Nortriptyline: 42.5 |
Bupropion: 50% Female Nortriptyline: 60% Female |
⩾4 weeks and ⩽2 years | HAM-D-21 | CGI-S CGI-I HAM-A |
⩾50% reduction in HAMD-D | Not stated | Yes | Nil | MDD |
| Augmentation | Bares et al. [2013] |
ADM= (bupropion, mirtazapine, milnacipran, escitalopram imipramine): 46.7 (±12.3) CAD= (Mirtazapine and Milnacipran Escitalopram and Bupropion Imipramine and (drug not stated): 45.6 (±10.2) |
Bupropion: 72% Female |
ADM= (bupropion, mirtazapine, milnacipran, escitalopram imipramine): 80.3 months CAD= (Mirtazapine and Milnacipran: Escitalopram and Bupropion Imipramine and drug not stated): 94.7 months |
MADRS | BDI-SF, CGI FIBSER |
⩾50% reduction of MADRS | MADRS ⩽12 | Yes | Benzodiazepines throughout trial only for severe anxiety symptoms or insomnia. | MDD |
| Bech et al. [2012] | Bupropion: 40.8 (±12.9) Buspirone: 41.5 (±12.6) |
Bupropion: 61.6% Female Buspirone: 55.9% Female |
Not stated | HAM-D-6 IDS-C-6 |
Not stated | Not stated | Not stated | Nil | MDD | ||
| Blier et al. [2010] | Fluoxetine and placebo: 43.8 (±1.8) Mirtazapine and Fluoxetine: 45.8 (±2.4) Mirtazapine and Venlafaxine: 41.9 (±2.2) Mirtazapine and Bupropion: 43.8 (±2.6) |
Not stated | Not stated | HAM-D | MADRS CGI-S CGI-I |
⩾50% reduction in HAM-D | HAM-D ⩽7 | Not stated | Clonazepam (1 mg/day), zopiclone (7.5 mg/day), zolpidem (10 mg/day) |
MDD | |
| Clayton et al. [2014] | Bupropion and Aripiprazole: 45.0 (±12.9) SSRI/SNRI and Aripiprazole: 46.6 (±12.6) |
SSRI Sertraline and Aripiprazole: 13.4% Female Escitalopram and Aripiprazole: 22.7% Female Paroxetine and Aripiprazole: 9.6% Female Paroxetine CR and Aripiprazole: 3.4% Female Fluoxetine and Aripiprazole: 14.4% Female SNRI Duloxetine and Aripiprazole: 2.4% Female Venlafaxine XR and Aripiprazole: 17.2% Female |
Not stated | MGH-SFI CGI-S |
MADRS | ⩾50% improvement on MGH-SFI | Not stated | Yes | Nil | MDD | |
| DeBattista et al. [2003] | SSRI and Bupropion: 44 (±10.25) |
SSRI and Bupropion: 57% Female |
Not stated | HDRS | BDI CGI |
⩾50% reduction in HDRS | Not stated | Not stated | Nil | MDD | |
| Fornaro et al. [2014] | Duloxetine and Placebo: 35.48 (±10.08) Duloxetine and Bupropion: 42.61 (±12.05) |
Duloxetine and Placebo: 50% Female Duloxetine and Bupropion: 50% Female |
Not stated | HAM-D-21 | MADRS | ⩾50% reduction in HAM-D-21 | HAM-D ⩽7 | Yes | Lorazepam on days 1-8 | MDD | |
| Gulrez et al. [2012] | Sertraline and Placebo: 43.23 (±2.67) Sertraline and Bupropion: 39.23 (±2.21) |
Sertraline and Placebo: 50% Female Sertraline and Bupropion: 54% Female |
Not stated | HDRS | MADRS | Not stated | HDRS ⩽7 MDRS ⩽7 |
Not stated | Nil | MDD | |
| Lam et al. [2004] | Citalopram and Bupropion: 37.5 (±10.0) Bupropion or Citalopram monotherapy: 36.4 (±9.3) |
Citalopram and Bupropion SR: 68% Female Bupropion or Citalopram monotherapy: 69% Female |
Not stated | SIGH-SAD | ⩾50% improvement on SIGH-SAD | ⩾50% improvement on SIGH-SAD | Nil | Not stated | MDD | ||
| Leuchter et al. [2008] | Escitalopram and Bupropion: 45 (± 14) |
Escitalopram and Bupropion: 72% Female |
⩾5 months | QIDS-SR-16 | QIDS-C-16 | QIDS-SR-16 ⩾50% | QIDS-C16 ⩽5 | Nil | Not stated | MDD | |
| Mohan et al. [2009] | Bupropion and Escitalopram combination and monotherapy: 31.1 (± 11.6) |
Bupropion and Escitalopram combination and monotherapy: 66.1% Female |
Not stated | MADRS CGI-I |
HAM-D-17 BDI |
⩾50% reduction in MADRS and HAMD-17 | MADRS ⩽12 HAM-D ⩽8 |
Nil | Zolpidem Zopiclone |
MDD | |
| Spier [1998] | SSRI and Bupropion: 49.4 (±17.6) |
Not stated | Not stated | CGI | Not stated | Not stated | Not stated | Nil | MDD: Unipolar and Bipolar depression | ||
| Trivedi et al. [2006a] | Bupropion: 40.8 (±12.9) Buspirone: 41.5 (±12.6) |
Bupropion: 61.6% Female Buspirone: 55.9% Female |
Not stated | HRSD-17 | QIDS-SR-16 | ⩾50% reduction in QIDS-C-16. | HRSD-17 ⩽5 | Not stated | Nil | MDD | |
| Weissman et al. [2015] | Escitalopram: 38.2 (±5.7) Bupropion: 39.9 (±5.6) Bupropion and Escitalopram: 43.7 (±6.4) |
100% Female | Not stated | HAM-D-17 | QIDS-SR MADRS SAS-SR |
Not stated | HAMD-17 ⩽7 | Not stated | Nil | MDD | |
| Older Adults | Hewett et al. [2010a] | Bupropion: 70.9 (± 5.6) Placebo: 71.3 (± 5.9) |
Bupropion: 74% Female Placebo: 70% Female |
Not stated | MADRS | CGI-S CGI-I |
⩾50% reduction in MADRS | MADRS ⩽11 | Not stated | Nil | MDD |
| Weihs et al. [2000] | Bupropion: 69.2 Paroxetine: 71.0 |
Bupropion: 54% Female Paroxetine: 60% Female |
2–6 months: Bupropion 33% Paroxetine 33% 7–12 months: bupropion 31% Paroxetine 37% 13–24 months: Bupropion 35% Paroxetine 31% |
HAM-D | HAM-A CGI-S CGI-I |
⩾50% reduction in HAM-D | Not stated | Yes | Nil | MDD | |
| Steffens et al. [2001] | Bupropion: 68.5 (± 7.2) |
Bupropion: 65% Female |
Not stated | MADRS CGI |
MADRS ⩽15 | CGI severity score of 1 or 2 | Not stated | Nil | MDD | ||
| Bipolar Affective Disorder | Grossman et al. [1999] | Idazoxan: Male: 45.7 (±1.5) Female: 39.5 (± 11.2) Bupropion: Male: 38.5 (± 13.4) Female: 36.6 (± 5.9) |
Idazoxan: 57% Female Bupropion: 71% Female |
Not stated | HDRS | Not stated | Not stated | Yes | Nil | Bipolar Disorder Type I | |
| McIntyre et al. [2002] | Topiramate: 39 (±4.75) Bupropion SR: 43 (±6) |
Topiramate: 39% Female Bupropion SR: 44% Female |
Topiramate: 6.5 months Bupropion: 7.5 months |
HDRS-17 | MADRS YMRS CGI-S CGI-I |
⩾50% reduction in HDRS-17 | HDRS-17 ⩽7 | Not stated | Continued with current medication including atypical antipsychotics, lithium, divalproex sodium. |
Bipolar Disorder Type I/II | |
| Post et al. [2006] | Bupropion: 41 (± 11.8) Sertraline: 43.4 (± 14.2) Venlafaxine: 40.6 (± 12.1) |
Bupropion: 54.9% Female Sertraline: 44.8% Female Venlafaxine: 50.8% Female |
Not stated | IDS CGI-BP |
⩾50% improvement in IDS score or a reduction in the CGI-BP depression score of ⩾ 2 points | IDS score ⩽12 and/or a CGI-BP depression severity score of 1 | Not stated | Continued with current medication including lithium, valproate, carbamazepine, lamotrigine, typical antipsychotics, atypical antipsychotics |
Bipolar Type I: 73% Bipolar Type II: 26% Bipolar NOS: 1% Rapid cycling: 27% |
||
| Seasonal Affective Disorder | Modell et al. [2005] | Bupropion and Placebo: Study A: 42 (±11) Study B: 42 (±11) Study C: 41 (±12) |
Bupropion: Study A: 74% Female Study B: 68% Female Study C: 69% Female |
Not stated | SIGH-SAD | N/A | N/A | Not stated | Nil | SAD | |
| Seo et al. [2013] | Bupropion: 40.3 (±15.4) |
Bupropion: 74.5% Female |
Not stated | SIGH-SAD | SIGH-SAD (atypical symptoms subscale) CGI-S SDS ESQ |
⩾ 50 % reduction in SIGH-SAD | SIGH-SAD ⩽7 | Yes | Concomitant medications for insomnia and anxiety including triazolam, zolpidem, buspirone, alprazolam, lorazepam | MDD with atypical features | |
| Dilsaver et al. [1992] | Bupropion: 33.9 (± 8.9) |
Bupropion: 80% Female |
Not stated | mHAM-D2
HAM-D |
= 6–10 score on mHAM-D | mHAM-D ⩽5 | Not stated | Nil | MDD with a seasonal pattern |
||
| Dysthymic disorder | Hellerstein et al. [2001] | Bupropion: 46.71 (± 10.17) |
Bupropion: 42.9% Female |
Not stated | HAM-D-24 Cornell Dysthymia Rating Scale |
CGI GAF BDI SCL-90-R |
⩾50% reduction in HAM-D-24 CGI improvement score of 1 or 2 |
HAM-D-17 ⩽4 HAM-D item 1 score of 0 |
Not stated | Nil | Dysthymic Disorder |
| Postpartum depression | Nonacs et al. [2005] | Bupropion: 31.5 |
Bupropion: 100% Female |
2–10 weeks | HAM-D | CGI KSQ |
⩾50% reduction in HAM-D | HAM-D ⩽7 | Not stated | Concomitant treatment with zolpidem for insomnia and lorazepam for anxiety and sleep disturbance | PPD |
ACQ, Asthma Control Questionnaire; BDI, Beck Depression Inventory; BDI-SF, Beck Depression Inventory – Short Form; CGI, Clinical Global Impression; CGI-BP, Clinical Global Impression-Bipolar Disorder; CGI-S, Clinical Global Impression—Severity of Illness Scale; CGI-I, Clinical Global Impressions—Improvement Scale; ESQ, Epworth Sleepiness Questionnaire; FIBSER, Frequency, Intensity, and Burden of Side Effects; GAF, Global Assessment of Functioning; HAD, Hospital Anxiety Depression Scale; HDRS, Hamilton Depression Rating Scale; HAM-A, Hamilton Anxiety Rating Scale; HAM-D, Hamilton Rating Scale for Depression; HAM-D6, Six Item Clinician-Administrated Hamilton Depression Scale; HAM-D-17, 17-item Hamilton Rating Scale for Depression; HAM-D-21, 21-item Hamilton Rating Scale for Depression; HRSD-17, 17-item Hamilton Rating Scale for Depression; IDS-C, Inventory of Depressive Symptomology Clinician Reported; IDS-C6, Six point Inventory of Depressive Symptomology Clinician Reported; IDS-IVR, Inventory of Depressive Symptomology Self-Reported; KSQ, Kellner Symptom Questionnaire Depression subscale; MADRS, Montgomery–Asberg Depression Rating Scale; MEI, Motivation and Energy Inventory; MDD, major depressive disorder; MDE-TR, major depressive episode—treatment resistant; MGH-SFI, Massachusetts General Hospital Sexual Functioning Inventory; MASQ, Mood and Anxiety Symptoms Questionnaire; mHDRS1, modified Hamilton Depression Rating Scale (i.e. subtracted suicide item); mHAM-D2, a modified version of the Hamilton Rating Scale for Depression including ratings of hypersomnia, increased appetite and carbohydrate craving; PPD, postpartum depression; QIDS-C, Quick Inventory of Depressive Symptomatology-Clinician Rating; QID-SR, Quick Inventory of Depressive Symptomology – Self-Reported; QIDS-SR16, 16-item Quick Inventory of Depressive Symptomology – Self-Reported; SAS-SR, Social Adjustment Scale-Self-Report; SAD, seasonal affective disorder; SARI, serotonin antagonists and reuptake inhibitor; SCL-90-R, Symptom Checklist-90-Revised; SDS, Sheehan Disability Scale; SIGH-SAD, Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders; SNRI, selective norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; YMRS, Young Mania Rating Scale.
Table 2.
Table of results of studies.
| Study | Subjects (ITT) | Intervention (range, mg/day) | Mean dosage (mg) | Control | Blinded/randomized | Treatment phase (weeks) | Response (%) | Remission (%) | Compliance rates (capsule, %) | Main findings | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Monotherapy Trials | Jefferson et al. [2006] | Bupropion: 133 Placebo: 137 |
Bupropion: 150–450 |
59%: 450 38%: 300 3%: 150 |
Placebo | Double blinded Randomised |
8 | IDS-IVR-30: Bupropion: 53% Placebo: 45% IDS-C-30: Bupropion: 50% Placebo: 35% |
IDS-IVR-30: Bupropion: 41% Placebo: 27% IDS-C-30: Bupropion: 32% Placebo: 18% |
Not stated | Bupropion XL group showed greater mean improvement compared with placebo group on the IDS-IVR-30 (p = 0.018) and IDS-C-30 (p < 0.001) total score at study endpoint. IDS-IVR-30 response rate was higher in bupropion group but not significantly so at end of study. IDS-C-30 response rates were significantly higher than placebo group at study end. IDS-IVR-30 and IDS-C-30 remission rates were both significantly higher in bupropion group compared to placebo group. |
| Koshino et al. [2013] | Bupropion 150 mg: 190 Bupropion 300 mg: 188 Placebo: 186 |
Bupropion: 150 Bupropion: 300 |
Not stated | Placebo | Double blinded Randomised |
8 | Bupropion 150: 51.6% Bupropion 300: 43.6% Placebo: 46.2% |
Bupropion 150: 31.6% Bupropion 300: 29.8% Placebo: 28.5% |
Not stated | Bupropion SR (150 mg/day and 300 mg/day) groups were not statistically different to placebo on the mean change in MADRS total score at study endpoint. No statistical difference between bupropion groups and placebo for MADRS response or remission rates. |
|
| Lineberry et al. [1990] | Bupropion: 110 Placebo: 106 |
Bupropion: 200–300 |
286 | Placebo | Double blinded Randomised | 6 | Bupropion: 51% Placebo: 34% |
Not stated | 95% | Bupropion was statistically significant superior to placebo in the combined centre analysis (p = <0.05) and 3/5 individual centres at study endpoint (p ⩽ 0.05). Bupropion group were statistically superior to placebo group on response rates (p = 0.01) in the combined centre analysis but not individual centre analysis. |
|
| Reimherr et al. [1998] | Bupropion 150 mg: 120 Bupropion 300 mg: 116 Placebo: 117 |
Bupropion: 150 Bupropion: 300 |
Bupropion 150 mg: 147 Bupropion 300 mg: 290 |
Placebo | Double blinded randomized | 8 | Not stated | Not stated | 97% | Bupropion group had significantly greater improvement on the HAM-D at study endpoint compared to placebo (p ⩽ 0.05). Significant difference also seen across CGI-S and CGI-I scores (p ⩽ 0.05 and p ⩽ 0.01, respectively). |
|
| Weihs et al. [2002] | Bupropion: 207 Placebo: 210 |
Bupropion: 150–300 | 290 | Placebo | Double blinded randomized | 44 | Not stated | Not stated | Bupropion: 97% Placebo: 98% |
There was a statistically significant difference in favour of bupropion over placebo when comparing survival curves for the two treatment groups (p = 0.004). In placebo patients the median time to relapse was 24 weeks compared with bupropion patients (44 weeks). Of those patients who relapsed, mean HAM-D scores was 21 (SD 4.4). No additional data on HAM-D or CGI-S scores was reported. |
|
| Brown et al. [2007] | Bupropion: 14 |
Bupropion: 150 | Not stated | Nil | Open Label | 12 | Bupropion: 27.8% |
Bupropion: 16.7% |
Not stated | Statistically significant improvement in HAM-D scores(p = 0.02). No significant difference in IDS-SR scores(p = 0.09). |
|
| Gross et al. [2007] | Bupropion: 26 |
Bupropion: 150–450 |
265.38 | Nil | Open Label | 8 | Not stated | Not stated | Not stated | Statistically significant improvement in HAM-D and CGI-S scores at study endpoint (p < 0.01, for both). | |
| Fava et al. [2003] | Bupropion: 26 |
Bupropion: 150–400 |
353.3 | Nil | Open Label | 8 | Bupropion: 34.6% |
Bupropion: 23.1% |
Not stated | A statistically significant difference in HAM-D-17 scores was observed at study endpoint from week 1 (p < 0.001). A statistically significant difference in CGI-S scores was observed at study endpoint from week 3 (p < 0.001). |
|
| Ferguson et al. [1994] | Bupropion: 37 |
Bupropion: 300–450 |
390 | Nil | Single blind | 8 | Bupropion: 49% |
Not stated | Not stated | Statistically significant reduction in HAM-D and HAM-A scores were recorded at study endpoint. HAM-D (p < 0.0001, for both). | |
| Walker et al. [1993] | Bupropion: 36 |
Bupropion: 150–450 |
329 | Nil | Open label | 8 | Not stated | Not stated | Not stated | Both HAM-D and CGI-S scores decreased significantly from baseline to study endpoint (p ⩽ 0.01). 58% (21/36) were rated as ‘much’ or ‘very much’ improved on the CGI-I scale. |
|
| Tomarken et al. [2004] | Bupropion: 7 Placebo: 9 |
Phase I:
Bupropion: 100–300 Phase II: Bupropion: 300–400 |
Not stated | Placebo |
Phase I:
Double blinded, randomized Phase II: Open label |
12 | Not stated | Not stated | Not stated | Linear declines in HAM-D scores during phase I for both bupropion and placebo(p < 0.001). Rate of change was greater for the bupropion group than placebo (p = 0.04). Declines were seen across phase II (p = 0.005) with no between group differences (p > 0.30). |
|
| Bupropion versus SSRI | Clayton et al. [2006] | Bupropion: 263 Escitalopram: 266 Placebo: 256 |
Bupropion: 150–450 Escitalopram: 10–20 |
Trial 1:
Bupropion: 323 Escitalopram: 13 Trial 2: Bupropion: 309 Escitalopram: 13 |
Placebo | Double blinded, double dummy randomized |
8 |
*Bupropion: 62% Escitalopram: 65% Placebo: 52% |
*Bupropion: 43% Escitalopram: 45% Placebo: 34% |
Not stated | Bupropion XL did not differ significantly from placebo on the HAM-D-17 scores. Bupropion group did not meet criteria for response in study 1 or study 2. Met criteria in pooled data set. Bupropion group met criteria for remission in study 1 (not study 2) and pooled data set. Bupropion did not statistically differ from escitalopram on mean change in HAM-D total scores, response rate, or remission rate. |
| Coleman et al. [1999] | Bupropion: 118 Sertraline: 109 Placebo: 117 |
Bupropion: 150–400 Sertraline: 50–200 |
Bupropion: 290 Sertraline: 106 |
Placebo | Double blinded, double dummy randomized |
8 | Bupropion: 66% Sertraline: 61% Placebo: 56% |
Not stated | Bupropion: 97.9 Sertraline: 98.3 Placebo: 98.4 |
Bupropion was statistically superior to placebo on mean HAM-D-31 scores at end of study (p < 0.05). Patients in the placebo group did not demonstrate ⩾50% reduction in scores until study endpoint. More patients treated with bupropion met the criteria for HAM-D response than placebo, but not significantly so. Sertraline was not statistically superior to placebo at any time during the study. |
|
| Coleman et al. [2001] | Bupropion: 136 Fluoxetine: 146 Placebo: 145 |
Bupropion: 150–400 Fluoxetine: 20 −60 |
Bupropion: 319 Fluoxetine: 26 |
Placebo | Double blinded, double dummy randomized |
8 | Bupropion: 56% Fluoxetine: 57% Placebo: 50% |
Bupropion: 47% Fluoxetine: 40% Placebo: 32% |
Bupropion: 98.0 Fluoxetine: 98.5 Placebo: 98.8 |
Bupropion was superior to placebo at week 8 for HAM-D-21 total scores (p < 0.05). Statistically significantly more bupropion patients met criteria for remission compared with placebo group (p < 0.05) at week 8, however there was no difference in response rates. Bupropion and fluoxetine were associated with comparable improvement in antidepressant efficacy. |
|
| Croft et al. [1999] | Bupropion: 116 Sertraline: 116 Placebo: 116 |
Bupropion: 150–400 Sertraline: 50–200 |
Bupropion: 293 Sertraline: 121 |
Placebo | Double blinded, double dummy randomized |
8 | Bupropion: 66% Sertraline: 68% Placebo: 47% |
Not stated | Bupropion: 98.0 Sertraline: 97.2 Placebo: 97.9 |
Bupropion group statistically superior to placebo group on mean HAM-D scores (p < 0.05) by week 6, maintained to week 8. Significantly more patients treated with bupropion (p < 0.004) had a positive clinical response based on HAM-D scores compared with patients who received placebo. Bupropion and sertraline were associated with comparable improvement in antidepressant efficacy. |
|
| Feighner et al. [1991] | Bupropion: 59 Fluoxetine: 60 |
Bupropion: 225–450 Fluoxetine: 20–80 |
Bupropion: 345 Fluoxetine: 26 |
Nil | Double blinded, doubledummy randomized |
6 | Bupropion: 62.7% Fluoxetine: 58.3% |
Not stated | Bupropion: 97.3 Fluoxetine: 99.0 |
Bupropion did not statistically differ from fluoxetine on mean change in HAM-D total scores or response rate. | |
| Grunebaum et al. [2012] | Bupropion: 38 Paroxetine: 36 |
Bupropion: 150–450 Paroxetine: 25–50 |
Bupropion: 275.3 Paroxetine: 33.7 |
Nil | Double blinded randomized |
24 | Not stated | Not stated | Not stated | A numerical reduction in mHDRS1 scores was observed in both bupropion and paroxetine patients, but p values were not reported. Treatment × mHDRS scores were statistically significant in favour of paroxetine (p = 0.002). For each point more severe in the mHDRS baseline these symptoms were 0.46 points lower with paroxetine compared with bupropion in weeks 1–8. |
|
| Kavoussi et al. [1997] | Bupropion: 119 Sertraline: 122 |
Bupropion: 100–300 Sertraline: 50–200 |
Bupropion: 238 Sertraline: 114 |
Nil | Double blinded, double dummy randomized |
16 | Not stated | Not stated | Bupropion: 98 Sertraline: 99 |
Both bupropion and sertraline groups demonstrated a greater than 50% improvement in HAM-D scores from day 42 to study endpoint. There was no statistically significant difference between groups at any treatment week. |
|
| Kennedy et al. [2006] | Bupropion: 65 Paroxetine: 66 |
Bupropion: 150–300 Paroxetine: 20–40 |
Bupropion: 178.5 Paroxetine: 23.3 |
Nil | Double blinded randomized |
8 | Bupropion Male: 64.9% Bupropion Female: 53.6% Paroxetine Male: 59.4% Paroxetine Female: 52.9% |
Bupropion Male: 43.2% Bupropion Female: 32.1% Paroxetine Male: 37.5% Paroxetine Female: 35.3% |
Not stated | Both bupropion and paroxetine groups demonstrated a significant reduction in HDRS scores by study endpoint (p < 0.001). No significant difference between bupropion and paroxetine was identified on HDRS scores, response or remission rate. |
|
| Rush et al. [2001] | Bupropion: 122 Sertraline: 126 |
Bupropion: 100–300 Sertraline: 50–200 |
Bupropion: 259 Sertraline: 123 |
Nil | Double blinded randomized |
16 | Bupropion: 66% Sertraline: 74% |
Bupropion: 55% Sertraline: 63% |
Not stated | There was a numerical reduction in HAM-D scores for both treatment groups, however p values were not provided. There was no difference between active treatment groups on HAM-D scores, response or remission rate. |
|
| Rush et al. [2006] | Bupropion: 189 Sertraline: 194 Venlafaxine: 200 |
Bupropion: 150–400 Sertraline: 50–200 Venlafaxine: 37.5–375 |
Bupropion: 282.7 Sertraline: 135.5 Venlafaxine: 193.6 |
Nil | Open label randomized |
14 | Not stated | Bupropion: 21.3% Sertraline: 17.6% Venlafaxine: 24.8% |
Not stated | On the basis of HRDS-17 scores no differential treatment effect in regard to remission was found between treatment groups. Treatment did not differ significantly with respect to QIDS-16 total scores, response or remission rate. |
|
| Bupropion versus SNRI | Hewett et al. [2009] | Bupropion: 187 Venlafaxine: 185 Placebo: 197 |
Bupropion: 150–300 Venlafaxine: 75–150 |
Bupropion: 170 Venlafaxine: 86.3 |
Placebo | Double blinded, double dummy randomized |
8 | Bupropion: 57% Venlafaxine: 65% Placebo: 46% |
Bupropion: 47% Venlafaxine: 51% Placebo: 32% |
Not stated | Efficacy of bupropion and venlafaxine was significantly superior to placebo on MADRS total score (p = 0.006 and p < 0.001, respectively). Statistically significant proportion of bupropion and venlafaxine group met the criteria for MADRS responders (p = 0.033 and p < 0.001, respectively) and remitters (p = 0.004 and p < 0.001, respectively) compared with placebo group. Bupropion and venlafaxine were not statistically different on depression scores and showed comparable antidepressant activity. |
| Hewett et al. [2010b] | Bupropion: 203 Venlafaxine: 198 Placebo: 187 |
Bupropion: 150–300 Venlafaxine: 75–150 |
Bupropion: 180 Venlafaxine: 85 |
Placebo | Double blinded, double dummy randomized |
8 | Bupropion: 57% Venlafaxine: 66% Placebo: 49% |
Bupropion: 45% Venlafaxine: 56% Placebo: 38% |
Not stated | Bupropion and placebo groups were not significantly different on MADRS total scores, response or remission rate. The 95% confidence interval for the mean difference between the bupropion XR and venlafaxine XR groups (0.3–4.3) failed to include zero indicating a difference in favour of venlafaxine XR. |
|
| Descriptive statistics yielded significant differences in mean MDRS scores in venlafaxine over placebo (p < 0.001) and response and remission rate (p < 0.05). | |||||||||||
| Rosso et al. [2012] | Bupropion: 21 Duloxetine: 25 |
Bupropion: 150–300 Duloxetine: 60–120 |
Not stated | Nil | Single blind randomized |
6 | Bupropion: 71.4% Duloxetine: 64% |
Bupropion: 38.1% Duloxetine: 32% |
Not stated | Statistically significant difference in HAM-D scores observed for bupropion (p < 0.001) and duloxetine (p < 0.001). No differences were observed between the treatment groups at any time point. Observed superior response and remission rates in both treatment groups, but not significantly so. |
|
| Thase et al. [2006] | Bupropion: 160 Venlafaxine: 164 |
Bupropion: 150–450 Venlafaxine: 75–225 |
Bupropion: 299.6 Venlafaxine: 149.8 |
Nil | Double blind, double dummy randomized |
12 | Bupropion: ~55% Venlafaxine: ~48% |
Bupropion: ~47% Venlafaxine: ~29% |
Bupropion: 95% Venlafaxine: 98% |
Mean changes in HAM-D scores were comparable between bupropion and venlafaxine. A significant difference in remission rate was observed for bupropion patients. |
|
| Bupropion versus SARI | Weisler et al. [1994] | Bupropion: 59 Trazadone: 52 |
Bupropion: 225–450 Trazadone: 150–400 |
Bupropion: 279 Trazadone: 168 |
Nil | Double blinded, double dummy randomized |
6 |
*Bupropion: 55.9% *Trazadone: 40.4% |
*Bupropion: 46% *Trazadone: 31% |
Bupropion: 84.7% Trazadone: 90.1% |
*No statistically significant differences were observed between bupropion or trazadone treatment groups at study endpoint, with the exception of a statistically significant reduction in mean HAM-D scores in favour of the trazadone treatment group at week 1 (p < 0.01). No statistically significant difference in response or remission rate was observed between treatment groups. |
| Bupropion versus TCA | Masco et al. [1994] | Bupropion: 55 Nortriptyline: 50 |
Bupropion: 225–450 Nortriptyline: 75–150 |
Bupropion: 333 Nortriptyline: 111 |
Nil | Double blinded, double dummy randomised |
6 |
*Bupropion: 40% *Nortriptyline: 48% |
Not stated |
*Bupropion: 97% *Nortriptyline: 96% |
*Overall improvement in HAM-D scores and response rate was comparable across bupropion and nortriptyline groups. The mean observed HAM-D score decreased by 47% in the bupropion treatment group and by 50% in the nortriptyline treatment group between baseline and day 42. Statistically significant differences were demonstrated at individual assessment times for the nortriptyline group (p < 0.05) but not for bupropion group. |
| Augmentation | Bares et al. [2013] |
ADM = Bupropion, Mirtazapine, Milnacipran, Escitalopram: 30 CAD = Mirtazapine and Milnacipran, Escitalopram and Bupropion: 30 |
ADM = Bupropion, Mirtazapine, Milnacipran, Escitalopram 10.7–11.3 CAD = Mirtazapine and Milnacipran, Escitalopram and Bupropion 10–10.8 |
Nil | Nil | Open label, randomized | 6 |
ADM = Bupropion, Mirtazapine, Milnacipran, Escitalopram: 48% CAD = Mirtazapine and Milnacipran. Escitalopram and Bupropion: 58% |
ADM = Bupropion, Mirtazapine, Milnacipran, Escitalopram: 41% CAD = Mirtazapine and Milnacipran. Escitalopram and Bupropion: 45% |
Not stated. | There were no differences from baseline between ADM and CAD groups on MADRS scores or response rates. N.B. Whilst bupropion was administered to participants in this study, the total number who received bupropion in the ADM or CAD groups was not reported limiting the interpretation of results. |
| Bech et al. [2012] | Bupropion SR and Citalopram: 189 Buspirone and Citalopram: 198 |
Bupropion: 200–400 Buspirone: 15–50 |
Not stated | Nil | Double blinded, Randomized (STAR*D) | 6 | Not stated | Not stated | Not stated | IDS-C-6 and HAM-D-6 response rates indicated a statistically significant change at study endpoint in favour of bupropion and citalopram (p = 0.05 and p = 0.02, respectively). | |
| Blier et al. [2010] | Mirtazapine and Bupropion: 26 Fluoxetine and Placebo: 28 Mirtazapine and Fluoxetine: 25 Mirtazapine and Venlafaxine: 26 |
Bupropion: 150–300 Mirtazapine: 30 Fluoxetine: 20 Venlafaxine: 75–225 |
Not stated | Nil | Double blinded, randomized | 6 | Mirtazapine and Bupropion: 65% Fluoxetine monotherapy: 54% Mirtazapine and Fluoxetine: 68% Mirtazapine and Venlafaxine: 73% |
Mirtazapine and Bupropion: 46% Fluoxetine monotherapy: 25% Mirtazapine and Fluoxetine: 52% Mirtazapine and Venlafaxine: 58% |
Within the range of 80–120% | HAM-D scores demonstrated statistical difference amongst the four treatment groups in favour of fluoxetine monotherapy (p = 0.011). There was no statistical difference in response rates amongst the four treatments. There were also no significant differences between treatment groups on CGI-S (p = 0.22) or CGI-I scores (p = 0.08). |
|
| Clayton et al. [2014] | Bupropion and aripiprazole: 47 SSRI/SNRI and aripiprazole 245 |
Not stated | Aripiprazole: 9.3 and 9.6 Escitalopram: 16.8 Fluoxetine: 41.2 Sertraline: 125 Paroxetine CR: 29.3 Paroxetine: 34.4 Venlafaxine: 167 Duloxetine: 60 Bupropion: 340 Mirtazapine: Not stated |
Nil | Open label | 52 weeks | Not stated | Not stated | Not stated | No significant differences were observed on CGI-S scores between bupropion and SSRI/SNRI and bupropion and aripiprazole treatment groups by study endpoint. SSRI/SNRI and aripiprazole showed a reduction in symptom severity on CGI-I scores compared with bupropion and aripiprazole, although not significantly so. |
|
| DeBattista et al. [2003] | Bupropion/SSRIs: 28 |
Bupropion: 150–300 Fluoxetine: 20 Paroxetine 20 Sertraline 50 Venlafaxine: Not stated |
Bupropion: Not stated Fluoxetine: 40 Paroxetine: 34 Sertraline: 113 Venlafaxine: 75 |
Nil | Open label | 6 | Bupropion/SSRIs: 54% | Not stated | Not stated | Bupropion augmentation demonstrated statistically significant mean change in HDRS scores (p < 0.0001) and BDI scores (p < 0.001) in patients not responding to their existing antidepressant monotherapies by study endpoint. | |
| Fornaro et al. [2014] | Duloxetine and placebo: 22 Duloxetine and bupropion: 23 |
Bupropion: 60–120 Duloxetine: 60–120 |
Duloxetine and Placebo: 91.3 Duloxetine (and Bupropion): Duloxetine: 86.09 Bupropion: 215.22 |
Nil | Double blinded, double dummy randomized |
6 | Duloxetine and Bupropion: 29.6% Duloxetine and Placebo: 31.8% |
Not stated | Not stated | A reduction in HAM-D-21 scores was demonstrated in both duloxetine and bupropion and duloxetine monotherapy groups (no p values provided). No statistical difference between groups was demonstrated. |
|
| Gulrez et al. [2012] |
SSRI and placebo:
Sertraline 10 Citalopram 3 Escitalopram 15 Paroxetine 2 SSRI and bupropion: Bupropion and sertraline: 14 Bupropion and citalopram: 5 Bupropion and escitalopram: 7 Bupropion and paroxetine: 4 |
Bupropion: 150–300 Escitalopram: 10-30 Paroxetine: 25–75 Sertraline: 50–200 Citalopram: 20–60 |
SSRI and placebo:
Sertraline: 160 mg Citalopram: 37 mg Escitalopram: 25 mg Paroxetine: 38 mg SSRI and bupropion: Sertraline: 68 mg Citalopram: 22 mg Escitalopram: 13 mg Paroxetine: 31 mg Bupropion: Not stated |
Nil | Single blind, double dummy randomized |
4 | Not stated | SSRI and placebo: MADRS 27% HDRS 23% SSRI and Bupropion: MADRS 63% HDRS 60% |
Not stated | A statistically significant reduction in mean HDRS scores was demonstrated in the SSRI and bupropion group compared with SSRI monotherapy group by study endpoint (p < 0.05). A statistically significant difference was also demonstrated in MADRS and HDRS remission rates in the SSRI and bupropion group compared with SSRI monotherapy (p < 0.05). |
|
| Lam et al. [2004] | Citalopram and bupropion: 32 Citalopram or bupropion monotherapy: 29 |
Not stated |
Citalopram and bupropion
Bupropion 248.4 Citalopram 33.1 Monotherapy Bupropion 283.3 Citalopram 38.8 |
Nil | Open label naturalistic | 6 | Citalopram and Bupropion: 56% Citalopram or Bupropion monotherapy: 38% |
Citalopram and Bupropion: 28% Citalopram or Bupropion monotherapy: 7% |
Not stated | Statistically significant improvement with citalopram and bupropion combination (p < 0.04) compared with switching to bupropion or citalopram monotherapy was demonstrated on the SIGH-SAD. The response rates were numerically higher in the combination group compared with the monotherapy switch groups, however this difference did not reach significance (p > 0.15). There was statistically higher remission rates in the combinations group compared with the monotherapy group (p < 0.05). |
|
| Leuchter et al. [2008] | Bupropion and escitalopram: 51 |
Bupropion: 150–400 Escitalopram: 10–20 |
Bupropion: 329 Escitalopram: 18 |
Nil | Open label | 12 | Bupropion and escitalopram: 62% |
Bupropion and escitalopram: 50% |
Not stated | A numerical reduction in QIDS-C and QIDS-SR is demonstrated by study endpoint, however p values are not provided. 62% (28/45) of patients had responded and 50% (23/46) had remitted at study exit. |
|
| Mohan et al. [2009] | Bupropion and escitalopram: 41 Escitalopram: 135 |
Bupropion: 150–300 Escitalopram: 10–20 |
Not stated | Nil | Open label naturalistic | 12 weeksNon-responders (augmenta-tion):6 weeks | Escitalopram and bupropion: 61.7% Escitalopram monotherapy: 60.7% |
Escitalopram and bupropion: 53.7% Escitalopram monotherapy: 58.5% |
Not stated | At study endpoint (week 6 of augmentation) 61% (25/41) of patients were defined as responders, and 53.7% (22/41) achieved remission. 31.7 (13/41) did not respond to bupropion augmentation. Interestingly, the melancholic features of depression were associated with insufficient or partial response to escitalopram and were reduced by bupropion augmentation. |
|
| Spier [1998] | Inadequate response to SRI or venlafaxine (augmented with bupropion): 11 Inadequate response to bupropion (augmented with SRI): 4 Intolerance to SRI side effects (augmented with bupropion): 10 |
Bupropion: 75–450 Fluoxetine: 5–40 Sertraline: 25–125 Paroxetine: 10–40 Venlafaxine: 37.5–225 |
Bupropion: 230 Fluoxetine: 19.5 Sertraline: 55 Paroxetine: 25 Venlafaxine: 131 |
Nil | Open label | Not stated | Inadequate response to SRI or venlafaxine (augmented with bupropion): 9/11 (82%) Inadequate response to Bupropion (augmented with SRI): 3/4 (75%) Intolerance to SRI side effects (augmented with bupropion): 2/10 (20%) |
Not stated | Not stated | Of those augmented with bupropion after showing an inadequate response to SRI or venlafaxine, 82% showed a response. Of those augmented with an SRI after showing an inadequate response to bupropion, 75% showed a response. |
|
| In patients who were intolerable to SRI 20% showed a response when medication was augmented with bupropion. A numerical reduction in CGI scores was observed after response to second agent had stabilized, although this did not reach significance. |
|||||||||||
| Trivedi et al. [2006a] | Citalopram and bupropion: 279 Citalopram and buspirone: 286 |
Bupropion: 200–400 Buspirone: 15–60 Citalopram: Not stated |
Bupropion: 267.5 Buspirone: 40.9 Citalopram (with bupropion adjunct): 54.2 Citalopram (with Buspirone adjunct) 54.9 |
Nil | Open label, randomized (STAR*D) |
12 | Bupropion: 31.8% Buspirone: 26.9 % |
Bupropion: 39% Buspirone: 32.9% |
Not stated | QIDS-SR-16 scores significantly decreased with citalopram and bupropion compared to citalopram and buspirone (p < 0.02) by study endpoint. There were no significant differences in remission rates in citalopram and bupropion and citalopram and buspirone groups. |
|
| Weissman et al. [2015] | Bupropion: Mothers: 20 Children: 35 Escitalopram: Mothers: 29 Children: 46 Bupropion and escitalopram: Mothers: 27 Children: 54 |
Bupropion: 150–450 Escitalopram: 10-40 |
Bupropion: 244.8 Escitalopram: 23.8 Bupropion and citalopram: 314.3 |
Nil | Double blinded randomized | 12 | Not stated | 67% | Not stated | A significant decrease in HAM-D scores in all treatment groups was reported (p = 0.001), no significant differences in scores were observed between groups. Mean CDI scores declined significantly among children whose mothers received escitalopram monotherapy compared with both bupropion monotherapy (p = 0.04) and combination treatment of bupropion and escitalopram (p = 0.001). Significant effect of mothers’ HAM-D scores on children’s CDI score was also demonstrated (p = 0.001). |
|
| Older Adults | Hewett et al. [2010a] | Bupropion: 211 Placebo: 207 |
Bupropion: 150–300 |
Bupropion: 179 | Placebo | Double blinded randomized |
10 | Bupropion: 53% Placebo: 43% |
Bupropion: 38% Placebo: 33% |
Bupropion: 77% Placebo: 78% |
Bupropion showed statistically significant improvement on MADRS scores compared with placebo at study endpoint (p = 0.033). Bupropion group had significantly greater improvement on MADRS scores at study endpoint compared with placebo (p = 0.021). |
| Weihs et al. [2000] | Bupropion: 48 Paroxetine: 52 |
Bupropion: 100–300 Paroxetine: 10–40 |
Bupropion: 197 Paroxetine: 22 |
Nil | Double blinded, double dummy randomized |
6 | Bupropion: 71 % Paroxetine: 77 % |
Not stated | Bupropion: 95% Paroxetine: 98% |
No statistically significant differences in mean HAM-D score were found between bupropion and paroxetine. No statistically significant differences in mean CGI-S scores were found between bupropion and paroxetine. |
|
| Steffens et al. [2001] | Bupropion: 15 Bupropion and SSRI: 16 |
Bupropion: 150–450 SSRI: Not stated |
Bupropion SR: 240 Bupropion IR: 258 |
Nil | Naturalistic | 12 | Bupropion: 67.7% Bupropion and SSRI: 81.2% |
Bupropion: 50% Bupropion and SSRI: 56.2% |
100% | In subjects on bupropion monotherapy, 67% were responders and 50% achieved full or partial remission. Out of the total sample, 74% were responders and 53% achieved a partial or complete remission. |
|
| Bipolar Affective Disorder | Grossman et al. [1999] | Bupropion: 7 Idazoxan: 7 |
Not stated | Bupropion: 450 Idazoxan: 240 | Nil | Double blinded randomized |
6 | Not stated | Not stated | Not stated | Both idazoxan and bupropion demonstrated reductions in HDRS total score by the end of the 6 week trial (p = 0.01, for both treatments). No significant between group differences were observed. |
| McIntyre et al. [2002] | Bupropion: 18 Topiramate: 18 |
Bupropion: 100–400 Topiramate: 50–300 |
Bupropion: 250 Topiramate:176 |
Nil | Single blind Randomized |
8 | Bupropion: 58.7% Topiramate: 56.2% |
Bupropion: 27.5% Topiramate: 24.8% |
Not stated | Statistically significant improvements in mean HDRS-17 scores were observed at study endpoint in both bupropion and topiramate groups (p = 0.001) No significant differences were found between the bupropion and topiramate groups (p = 0.09). |
|
| Post et al. [2006] | Bupropion: 51 Sertraline: 58 Venlafaxine: 65 |
Bupropion: 75–450 Sertraline: 50–200 Venlafaxine: 37.5–375 |
Bupropion: 286 Sertraline: 192 Venlafaxine: 195 |
Nil | Open label, (n = 27) double blinded, double dummy randomized (n = 147) |
10 | Bupropion: 49% Sertraline: 53% Venlafaxine: 51% |
Bupropion: 41% Sertraline: 36% Venlafaxine: 34% |
Not stated | No significant differences were found between bupropion, sertraline and venlafaxine in IDS or CGI response and remission rates. | |
| Seasonal Affective Disorder | Modell et al. [2005] | Study A Bupropion: 140 Placebo: 132 Study B: Bupropion: 156 Placebo: 150 Study C: Bupropion: 238 Placebo: 226 |
Bupropion: 150–300 | Not stated | Placebo | Double blinded randomized |
Not stated | N/A | N/A | Not stated | Recurrence rates were significantly lower in bupropion groups than placebo groups across study A, study B and study C (p = 0.026, p = 0.049, p < 0.001, respectively). Survival analyses for depression onset favoured bupropion over placebo across study A, study B and study C (p = 0.081, p = 0.057, and p = 0.001, respectively). |
| Seo et al. [2013] | Bupropion: 51 |
Bupropion: 150–300 | Not stated | Nil | Open label | 8 | Bupropion: 51.2% |
Bupropion: 24.4% |
Not stated | Statistically significant reduction in SIGH-SAD scores by study endpoint (p < 0.001). | |
| Dilsaver et al. [1992] | Bupropion: 15 |
Bupropion: 200–400 | Not stated | Nil | Open label | 5 | Bupropion: 33.3% |
Bupropion: 66.7% |
Not stated | Statistically significant reduction in HAM-D scores by study endpoint (p < 0.0001). Statistically significant reduction in mHAM-D2 scores by study endpoint (p < 0.0001). |
|
| Dysthymic Disorder | Hellerstein et al. [2001] | Bupropion: 21 |
Bupropion: 150–400 |
Bupropion: 364 | Nil | Open label | 8 | Bupropion: 71.4% |
Bupropion: 42.9% |
Not stated | Statistically significant reduction in HAM-D scores by study endpoint (p < 0.001). |
| Postpartum depression | Nonacs et al. [2005] | Bupropion: 8 |
Bupropion: 37.5–300 |
Bupropion: 189.1 | Nil | Open label | 8 | Bupropion: 75% |
Bupropion: 37.5% |
Not stated | Statistically significant reduction in HAM-D scores by study endpoint (p < 0.05). |
ADM, antidepressant monotherapy; BDI, Beck’s Depression Inventory; BID, twice a day; CAD, combination antidepressants; CDI, Children’s Depression Inventory; CGI-I, Clinical Global Impressions—Improvement Scale; CGI-S, Clinical Global Impression—Severity of Illness Scale; CR, controlled; HAM-D, Hamilton Rating Scale for Depression; HDRS, Hamilton Depression Rating Scale; HDRS-17, Seventeen Point Hamilton Depression Rating Scale; HAM-D6, Six Item Clinician-Administrated Hamilton Depression Scale; IDS-C6, Six Point Inventory of Depressive Symptomology Clinician Reported; IDS-C30, Thirty Point Inventory of Depressive Symptomology Clinician Reported; IR, immediate release; ITT, intention to treat; LOCF, last observation carried forward; MADRS, Montgomery–Ashberg Depression Rating Scale; mHDRS1, modified Hamilton Depression Rating Scale (i.e. subtracted suicide item); mHAM-D2, a modified version of the Hamilton Rating Scale for Depression including ratings of hypersomnia, increased appetite and carbohydrate craving; QIDS-SR16, 16-item Quick Inventory of Depressive Symptomology – Self-Reported; SARI, serotonin antagonist and reuptake inhibitor; SNRI, serotonin norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; SR, slow release; TCA, tricyclic antidepressant; XL/XR, extended release.
Results reported are for the pooled data set.
Data analysis
In light of the limited amount of data for quantitative analysis as well as the hypothesis that true effect sizes would differ depending on sample and treatment characteristics, random-effects models were chosen as most appropriate for the meta-analysis. Statistical procedures were carried out using Stata [StataCorp, 2009], using the metan package for the meta-analysis, and the metafunnel and metabias packages for assessment of publication bias. p values below 0.05 were accepted as being statistically significant.
Results
Bupropion monotherapy
There were 27 trials evaluating bupropion in the treatment of MDD, running from 6 to 44 weeks in treatment duration. Of these, 21 trials were double-blinded RCTs, of which one had a two-phase design (double-blinded RCT, followed by an open-label phase), one was single blinded, and five were open-label. Eleven studies compared bupropion with placebo; ten compared with an SSRI (of which five additionally had a placebo arm); four compared with an serotonin–norepinephrine reuptake inhibitor (SNRI) (of which two had a placebo arm); and one trial compared bupropion with an serotonin antagonist and reuptake inhibitor (SARI) and one to a tricyclic antidepressant (TCA) of which neither had a placebo control. Bupropion was efficacious in reducing depression scores in 24 of the 27 trials, and, where evaluated, showed comparable levels of efficacy to the other classes of antidepressant.
Placebo trials
Five of the six open-label placebo trials reported a statistically significant clinical improvement in favour of bupropion [Brown et al. 2007; Fava et al. 2003; Ferguson et al. 1994; Gross et al. 2007; Walker et al. 1993] and one [Tomarken et al. 2004] did not demonstrate between group differences: they are described in Table 2. Of the six double-blinded, placebo-controlled RCTs, two evaluated flexible dosing of bupropion. In the earlier study [Lineberry et al. 1990] (n = 216) bupropion was titrated to a maximum of 100 mg/TID. Intention-to-treat (ITT) analysis demonstrated significantly greater HAM-D, MADRS, and CGI changes for the bupropion group over 6 weeks in both observed case analysis (p < 0.05 for all) and in last-observation-carried-forward (LOCF) analysis (p < 0.01 for all). A total of 54% attained response (⩾50% reduction in HAM-D total scores) on bupropion, significantly more (p = 0.01) than the 34% in the placebo group. In the work by Jefferson and colleagues (n = 270) the majority of patients (59%) received maximum dosage (450 mg/day) bupropion (versus 38% on 300 mg/day) [Jefferson et al. 2006]. Clinician reported response rates (IDS-C-30) were statistically significant for the bupropion group (50% versus 35%, p = 0.009) but self-reported response rates were not (53% versus 45%, p = 0.084) at the 8-week endpoint; remission rates were significantly greater in both clinician (41% versus 27%, respectively, p = 0.01) and self-reports (32% versus 18%, respectively, p = 0.005).
Two RCTs evaluated fixed dosing regimens over 8-week timeframes, with contrasting results. In the most recent study [Koshino et al. 2013] ITT analysis of 564 Japanese and Korean participants (randomized to bupropion 150 or 300 mg/day or placebo in a 1:1:1 ratio) reported no statistical differences between bupropion SR and placebo on MADRS total scores (p = 0.853) response (⩾50% reduction in MADRS) or remission (MADRS ⩽ 11) rates, though the authors note that significant changes in their placebo group by the study end could have reduced between-group differences. Post hoc analysis showed a trend towards response in those diagnosed with severe MDD. The study had notably stringent inclusion criteria to reduce the potential enrolment of patients with mild depression, a factor reported to impact placebo response rates in clinical trials [Posternak et al. 2002]. However, mean MADRS baseline scores (31.8–32.1) did not seem to differ widely with those reported by other authors in this review who used the same measure. Conversely, an earlier study of similar design (n = 362) [Reimherr et al. 1998] reported that bupropion dosed at 150 and 300 mg/day significantly reduced HAM-D and CGI-I total scores (p < 0.05 and p < 0.01, respectively) compared with placebo. Notably, this study had a lower study completion rate (54%) and, interestingly, of the 46% of patients that had prematurely discontinued, the majority were from the placebo group, with withdrawal due to inadequate response or because their condition had deteriorated.
A two-phase trial evaluated the long-term efficacy and weight change (described later) of fixed dose (300 mg/day) bupropion. Weihs and colleagues administered bupropion dosed at 150-300mg/day in an open-label design for 8 weeks (phase I) [Weihs et al. 2002]; those who responded were then recruited for a 44-week double-blind, placebo-controlled randomized fixed-dose (300 mg/day) study (phase II). In this study the primary outcome measure was time to relapse, defined as the prescription of a new antidepressant or the use of electroconvulsive therapy (ECT) and the study did not report additional data on changes in HAM-D scores. In phase II (n = 417) of those patients who relapsed, mean HAM-D scores were 21 (SD 4.4, range 11–30) and a statistically significant difference in favour of bupropion over placebo was demonstrated when comparing the survival curves for the two treatment groups (p = 0.004). In the placebo treatment group median time to relapse was 24 weeks after randomization compared with 44 weeks for the bupropion treatment group. Furthermore, survival estimates demonstrated that 52% of the placebo group would become depressed by the end of the study compared with 37% of the bupropion treatment group (p = 0.004) and that by end of year 1 the odds of placebo group requiring treatment were 1.83 times greater than for those in the bupropion treatment group.
In an inverse two-phase design with a much smaller sample (n = 16) Tomarken and colleagues administered bupropion dosed at 100–300 mg/day in a double-blinded, placebo-controlled randomized design for 6 weeks (phase I); at study endpoint participants from the bupropion group were titrated up to 400 mg/day and participants from the placebo group to 300 mg/day of medication, in an open-label design (phase II) for a further 6 weeks [Tomarken et al. 2004]. The primary aim of this study was evaluating efficacy of bupropion on specific symptom dimensions of depression (which are discussed later); however, the authors also administered the HAM-D. Both bupropion and placebo demonstrated linear declines on the HAM-D during phase I (p < 0.001) though the rate of change was greater for bupropion than placebo (p = 0.04). Although declines in mean scores were replicated in phase II (p = 0.005) no significant differences were observed between groups (p > 0.3).
In summary, bupropion demonstrated efficacy compared with placebo in five out of the six RCTs, and all but one of the open-label trials, with one of the two fixed dosing studies showing no difference. Study sizes in the RCTs were generally reasonable, with the exception of the Tomarken and colleagues cohort; follow-up periods were generally relatively brief, but consistent with typical antidepressant trials.
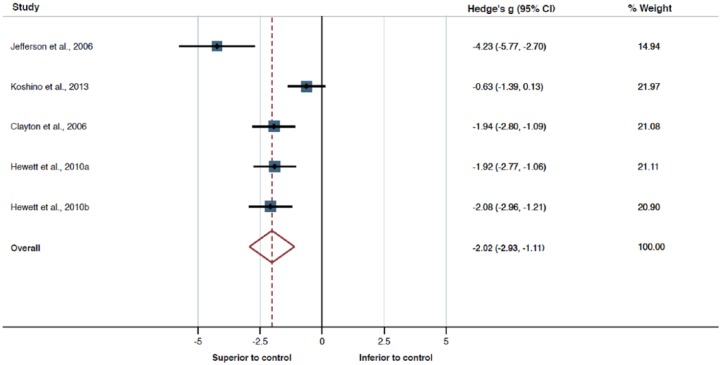
A subset of five studies reported sufficient data to allow for quantitative analysis using meta-analysis. Meta-analysis for the main effect of bupropion on depression scores as compared with placebo control showed a consistent large effect favouring bupropion (Hedge’s g = 2.02, df = 4, p < 0.001, 95% confidence interval [CI] 2.93–1.11; Figure 2). However, this analysis also revealed high heterogeneity of study findings (p = 0.001, I2 = 79.4%, τ2 = 0.832), which is reflected in a large prediction interval (PI 5.28 to −1.24). Moreover, both Egger’s (p = 0.043) and Begg’s (p = 0.027) tests for publication bias produced significant results. Thus, the strength of evidence produced by the present meta-analysis needs to be considered with extreme caution.
Figure 2.
A forest plot of the efficacy of bupropion compared with placebo.
Bupropion versus SSRI
Ten RCTs identified evaluated the efficacy of bupropion against an SSRI: one compared with escitalopram [Clayton et al. 2006], five with sertraline [Coleman et al. 1999; Croft et al. 1999; Kavoussi et al. 1997; Rush et al. 2001, 2006], two with fluoxetine [Coleman et al. 2001; Feighner et al. 1991] and two with paroxetine [Grunebaum et al. 2012; Kennedy et al. 2006]. Nine trials employed a double-blinded, randomized design: six of which utilized a double dummy approach, which is a technique for retaining blinding when the two drugs cannot be made to appear identical; all participants thus take two treatments, one of which (depending upon the arm they are in) would be a placebo; and five also had a placebo arm. Trials varied in length from 8 to 24 weeks, and number of participants from 74 to 785.
Bupropion versus escitalopram
A large (n = 785) RCT by Clayton and colleagues randomized participants to receive bupropion XL (300–450 mg/day, n = 276), escitalopram (10–20 mg/day, n = 281) or placebo (n = 273) [Clayton et al. 2006]. A primary measure, reported later, was sexual functioning, but compared with placebo both drugs had statistically superior response (p = 0.015 and p < 0.001, respectively) and remission rates (p = 0.018 and p < 0.005, respectively), as measured by the HAM-D, with no difference between the treatment groups. However, separation from placebo was not achieved at the statistical level of 0.05 for bupropion for mean HAM-D total scores in the individual or pooled analysis. This is surprising given a statistically significant response rate was achieved in the pooled analysis for bupropion. The authors attribute this discrepancy to their sample size and large placebo response (53%).
Bupropion versus sertraline
Five studies compared bupropion and sertraline, two with placebo arms [Coleman et al. 1999; Croft et al. 1999]. Three employed a double-blinded, double dummy design [Coleman et al. 1999; Croft et al. 1999; Kavoussi et al. 1997], one a double-blinded design [Rush et al. 2001] and one was not blinded [Rush et al. 2006]. Trials ranged from 8 to 16 weeks and from 248 to 583 patients. The most recent RCT [Rush et al. 2001], which is 14 years old, employed a double-blind, randomized parallel design (n = 248) over a 16-week treatment phase. Mean HAM-D scores reduced for both treatment groups, with high response (bupropion, 66% and sertraline, 74%) and remission (bupropion, 55% and sertraline, 63%) rates reported, with no between-group differences. Interestingly a larger (n = 583) open-label trial by the same group [Rush et al. 2006] recorded significantly lower response rates despite administering bupropion at a similar mean dose; however, there was a broader inclusion of patients with other comorbid somatic or psychiatric disorders, and it is not clear to what degree these factors might have altered the results. Findings from the RCT are in accord with the earlier, similarly designed (n = 241) trial by Kavoussi and colleagues [Kavoussi et al. 1997]. Both groups demonstrated a ⩾50% improvement in HAM-D scores from week 6 to study endpoint (week 16) with no statistical between-group difference on the HAM-D, CGI-S or CGI-I. Both groups also demonstrated ⩾50% study-end improvement in anxiety scores, as measured by the HAM-A. Neither trial had a placebo arm, nor were p values reported.
Two early studies employed more rigorous, and similar, methodological designs. Coleman and colleagues reported mean HAM-D scores in the bupropion, sertraline and placebo groups improved by ⩾50% by week 8, but only bupropion was statistically significantly superior to placebo by this point (p < 0.05) [Coleman et al. 1999]. Croft and colleagues reported the same reduction in depression scores by week 8 (from week 6) (p < 0.05) LOCF analysis for both active treatment groups, with no statistically significant between them [Croft et al. 1999]. A statistically significant HAM-D clinical response was observed in bupropion (66%, p < 0.004) and sertraline groups (68%, p = 0.002) compared with placebo (47%). These two trials, plus work by Reimherr and colleagues [Reimherr et al. 1998] reported that individuals on bupropion showed significantly greater improvement on CGI-S and CGI-I scores. In the Coleman and colleagues study no difference between sertraline and placebo was established at any time for the CGI-S or CGI-I [Coleman et al. 1999]. Croft and colleagues reported that although no statistical difference between the groups was observed both treatments were statistically superior to placebo in CGI-S (bupropion, p = 0.005 and sertraline, p = 0.05) and CGI-I scores (p < 0.01) [Croft et al. 1999].
In summary, all trials that included a placebo arm reported bupropion as being significantly superior to placebo. In the majority of the studies, bupropion and sertraline demonstrated comparable efficacy, with the exception of the Coleman and colleagues [Coleman et al. 1999] cohort where bupropion but not sertraline was superior to placebo.
Bupropion versus fluoxetine
Two RCTs evaluated bupropion and fluoxetine, with similar study designs including placebo arms. The most recent trial was in 2001 [Coleman et al. 2001]; over the 8-week intervention mean HAM-D scores decreased across all groups (total n = 427). No statistical difference in response rates was observed between bupropion (56%), fluoxetine (57%) and placebo (50%); however, for remission (47%, 40%, 32%, respectively), a statistically significant greater rate was seen for bupropion, but not fluoxetine, over placebo (p < 0.05). The earlier trial [Feighner et al. 1991] had a smaller sample (n = 119) and shorter treatment phase (6 weeks); data similarly demonstrated no statistical difference in HAM-D, CGI-S or CGI-I scores, or response rates (bupropion, 62.5% and fluoxetine, 58.3%), with no statistical difference demonstrated between treatment groups.
Bupropion versus paroxetine
Two RCTs compared the efficacy of bupropion with paroxetine, neither utilizing a placebo arm. Grunebaum utilized an initial 8-week treatment phase (bupropion n = 38, paroxetine n = 36) in individuals with MDD and elevated suicidal risk factors, followed by a 16-week continuation phase design for those patients who had initially responded to treatment [Grunebaum et al. 2012]. The primary outcome was suicidal behaviour and ideation, measured on a modified HDRS scores (mHDRS; subtracting the suicide item). A reduction in mHDRS scores was reported for both active treatment groups; however, for each point more severe at mHDRS baseline, symptoms were 0.46 points lower with paroxetine by week 8. These data suggests that patients with more severe global depression symptoms (minus suicidality) improved modestly more with paroxetine when controlling for the suicidal ideation index at baseline. The earlier trial by Kennedy and colleagues had a larger sample (n = 131) and lower mean dosage of medication for both drugs, and reported a statistically significant reduction in HDRS scores for both treatment groups (p < 0.01), with no significant differences between bupropion and paroxetine on the HDRS scores, response or remission rates [Kennedy et al. 2006]. Overall the lack of a placebo arm hinders the ability to determine the absolute efficacy of the drug, though they produced comparable effects in terms of reduction in mean HDRS scores, with the latest study showing a particular advantage for paroxetine in suicidal patients.
Bupropion versus SNRIs
Four trials evaluated bupropion and a SNRI, three of which looked at venlafaxine [Hewett et al. 2009, 2010b; Thase et al. 2006] and one duloxetine [Rosso et al. 2012]. The venlafaxine trials were all double-blinded, double dummy designs, and two contained a placebo arm; sample size varied from 324 to 569 subjects and treatment phase from 8 to 12 weeks. The single trial evaluating bupropion and duloxetine had a small sample (n = 46) and short treatment duration (6 weeks) [Rosso et al. 2012].
Bupropion versus venlafaxine
The trial by Hewett and colleagues (n = 384) (Hewett et al. 2009) reported a statistically significant difference in mean MADRS total scores for bupropion and venlafaxine compared with placebo in the LOCF (p = 0.006 and p = 0.001, respectively) and observed case analyses (p = 0.003 and p < 0.001, respectively), as well as on CGI-I (p < 0.001 and p = 0.009), CGI-S (p = 0.003 and p < 0.001) and HAM-A (p = 0.019 and p < 0.001). A statistically significantly greater proportion of bupropion and venlafaxine patients, compared with placebo, met the criteria for response (p = 0.033 and p < 0.001, respectively) and remission (p = 0.004 and p < 0.001, respectively) at week 8, with no significant differences between the two active treatment groups.
However a larger (n = 390) subsequent trial by the same group [Hewett et al. 2010b] reported no statistical significance in the least squares mean change from baseline MADRS scores, at study endpoint (week 8) for bupropion (180 mg/day) compared with placebo. Given the limitations of the studies analysis protocol, no further comparisons at the 0.05 level between active treatment groups and placebo were deemed appropriate, and further results are therefore purely descriptive. Comparison of patients classified as responders or remitters (according to MADRS and CGI-I criteria) were significant for venlafaxine (p < 0.05) but not bupropion, and this was also the case for CGI-S and HAM-A scores (p < 0.01, venlafaxine). In this latter study, Hewett and colleagues argued that the study enrolled a population that was inherently less responsive to bupropion, and more so to venlafaxine, although they recognized that such an argument was speculative.
Thase and colleagues conducted a randomized, double-blind, non-placebo-controlled study comparing bupropion and venlafaxine in 324 outpatients with MDD, and reported similar response and remission rates for the bupropion and venlafaxine treatment groups [Thase et al. 2006]. Although the study reported that a significant difference in favour of bupropion was observed in the LOCF analysis for percentage of patients categorized under remission (for both HAM-D and CGI-I criteria) no p values were provided to further interpret this, and antidepressant efficacy was a secondary aim of the study.
In summary bupropion up to doses of 450 mg and venlafaxine up to 225 mg showed comparable levels of antidepressant activity in two trials. One trial indicated superiority of venlafaxine over bupropion and placebo, however results should be interpreted as descriptive. In some studies mean severity scores were trending downward at the end of treatment, suggesting there was potential for patients to achieve remission if treatment phases were extended.
Bupropion versus duloxetine
One study evaluated the efficacy of bupropion (300 mg/day) versus duloxetine (120 mg/day) in a small-scale (n = 46) 6-week single-blinded, randomized trial in individuals with MDD resistant to SSRIs [Rosso et al. 2012]. Rosso and colleagues reported that both groups demonstrated a statistically significant reduction in mean HAM-D and CGI-S scores at study endpoint (p < 0.001), however no differences between treatment groups were found at any time point. Although a similar percentage of patients were categorized as responders (60–70%) and remitters (30–40%), there were no statistical differences between the treatment groups or from baseline to study endpoint (week 6). Whilst the results are positive, given the sample’s refractory cohort, and support class change in MDD refractory to SSRIs, interpretation is hindered by the lack of a placebo arm and the small sample size.
Bupropion versus trazadone
One study, a RCT, evaluated the relative efficacy of bupropion (225–450 mg/day) and trazadone (150–400 mg/day), a SARI [Weisler et al. 1994]. After a 1-week placebo lead in, 124 outpatients with mild–moderate depression were randomized to one of the two drugs. At the 6-week follow up there were no significant differences between the compounds, though trazadone showed earlier gains that might have been due to improved sleep. A total of 58% of bupropion and 46% of trazadone patients were rated as much or very much improved by the end, though the study lacked a placebo arm, and only numerical values of improvement were provided.
Bupropion versus TCAs
A double-blind, double dummy RCT [Masco et al. 1994] evaluated bupropion (n = 55) against nortriptyline (n = 50) over a 6-week treatment phase. Improvements on HAM-D scores and response rate (bupropion, 40% versus nortriptyline, 48%) were comparable across the two treatment groups. As with the trazadone study, there were some early benefits for nortriptyline that the authors considered might be due to a disproportionate weighting of the sleep index on the HAM-D.
Bupropion combined with other medication
Thirteen reports evaluated bupropion coprescribing, with sample sizes from 25 to 565 participants, and trial durations of 4–52 weeks: five were RCTs, of which four were double-blinded; seven were open-label studies; and one was a longitudinal study. Two were part of the STAR*D trial, of which one was a double-blinded, randomized trial [Bech et al. 2012] and one an open-label, randomized trial [Trivedi et al. 2006a].
Bupropion coprescribed with SSRIs
Five reports evaluated the efficacy of adding bupropion to SSRIs. A methodologically unique work by Weissman and colleagues followed the progress of mothers with depression (n=76) randomized to receive bupropion, escitalopram, or their combination over 12 weeks [Weissman et al. 2015]; their children’s (n = 135) wellbeing was independently assessed. There were no between-group differences for the women, all groups showing statistically significant improvements as measured on the HAM-D (p < 0.001); there was an overall remission rate of 67%. However, the effect upon their children varied, and depended upon the mothers’ baseline symptom profiles; mean Children’s Depression Inventory (CDI) scores declined significantly among children whose mothers received escitalopram monotherapy compared with both bupropion monotherapy (p < 0.04) and combination treatment of bupropion and escitalopram (p < 0.001). Subanalysis of this interesting finding showed that those children with mothers with a so-called high ‘negative affectivity’ (which includes the symptoms of guilt, irritability and fear/anxiety) only improved when their mothers were on escitalopram; mothers in this group reported improvements in their abilities to listen to their children, and the children described the mothers as becoming more caring during treatment. Negative affectivity has been linked with serotonergic dysfunction, which may explain bupropion’s lack of impact on this domain.
A single-blind RCT [Gulrez et al. 2012] evaluated 60 outpatients showing a partial response on SSRIs (escitalopram 10–30 mg/day, citalopram 20–60 mg/day, paroxetine 25–75 mg/day and sertraline 50–200 mg/day; all on treatment for 4 weeks). Participants were randomly assigned to have either placebo or bupropion SR (150–300 mg) added to their antidepressant. By the end of week 4, both groups had significantly improved, but the decrease in depression scores (measured on the HDRS, MADRS and ADI) were significantly greater in those coprescribed bupropion.
Three open-label studies evaluated the addition of bupropion to citalopram or escitalopram, a SSRI that is highly selective for the serotonin reuptake transporter. A novel design naturalistic study by Lam and colleagues explored treatment strategies, whether to augment or switch medication, in 61 individuals taking either citalopram or bupropion SR (at a therapeutic dose but showing minimal improvement) for at least 6 weeks [Lam et al. 2004]. Eligible participants, all of whom had failed to respond to at least one previous antidepressant, had, in alternate months, the other medication added to their treatment, or were switched to the other drug. An advantage of such a methodology is that it attempts to address the issue of whether it is the combination of medications (which could be additive or synergistic) or the novel compound that accounts for any improvement. At 6 weeks treatment, the combination condition (n = 32) was superior to a monotherapy switch (n = 29) in terms of clinical change on the SIGH-SAD score (p < 0.04) and the proportion of participants in remission (28% versus 7%, p < 0.05). The combination remission rate was low, but with the caveat that it was measured at 6 weeks treatment. Leuchter and colleagues examined the coprescribing of citalopram (mean dose 18 mg/day) and bupropion SR (mean dose 329 mg/day) over 12 weeks in 51 medication-free outpatients with chronic or recurrent MDD [Leuchter et al. 2008]. Participants were commenced on escitalopram 10 mg/day, with bupropion SR 150 mg/day added at week 1; they were eligible for an increase in either medication by week 4 (and for bupropion also at weeks 6–10) if they failed to show any, or suboptimal, improvement; mean doses by the trial end were 18 and 329 mg/day, respectively. A total of 62% showed a response to treatment, 50% attained remission (QIDS-C16 ⩽ 5). Rates of discontinuation due to side effects were low, at 6%. Similarly, Mohan and colleagues prescribed 10-20 mg/day of escitalopram to 135 participants with MDD in a 12-week trial (those showing ⩽50% decrease in MADRS scores at week 4 being put on the higher dose) [Mohan et al. 2009]; nonresponders at week 12 were then coprescribed 150–300 mg/day (depending upon response) bupropion for an additional 6 weeks. By the end of 12 weeks 60.7% were responders, 58.5% remitters on citalopram monotherapy; 41 participants defined as nonresponders entered the coprescribing phase, and 61.0% attained response, 53.7% remission in the 6-week follow on. Interestingly, subanalysis of symptom changes showed that melancholic features of depression were less responsive to escitalopram monotherapy, but that these appeared to respond well to bupropion augmentation.
Overall, with regards to adding bupropion to SSRIs, there was only one double-blinded RCT, though this did not have a placebo group, and was in a specific cohort of depressed mothers. In this study bupropion and bupropion coprescribing showed results comparable, though not superior to, SSRI prescribing; the data are interesting in terms of the differential outcomes in the children. The data from the single-blinded RCT and open-label trials support superior efficacy of bupropion, though the sample sizes are modest, and methodologically, open-label trials, with their lack of placebo, are open to challenge.
Bupropion coprescribed with non-SSRIs
Two studies compared the efficacy of bupropion in adjunction with a non-SSRI antidepressant, in both instances a SNRI.
A 6-week double-blind placebo-controlled RCT [Fornaro et al. 2014] evaluated bupropion SR (150 or 300 mg/day) or placebo added to duloxetine (60–120 mg/day) in 48 outpatients with MDD (all of whom had failed at least one drug trial) with DSM-IV criteria atypical features (which include increased appetite and food consumption, weight gain, hypersomnia and atypical diurnal variation in mood). No statistically significant differences were seen between groups, with measurements including the Structured Interview Guide for the HAM-D with Atypical Depression Supplement (SIGH-ADS) in addition to the GAF and HAM-D; 26.1% (duloxetine) and 21.7% (placebo) response rates were attained. The authors noted that the presence of a higher number of atypical features, which generally occur in up to a fifth of those with MDD, significantly predicted nonresponse, as has been demonstrated in other studies, and may have affected their outcomes.
Spier evaluated the use of adding bupropion to patients with MDD in a private practice clinic already on venlafaxine or an SSRI in an open-label trial assessing symptom relief and management of drug-induced side effects in monotherapy nonresponders (n = 15) and responders (n = 10) with side effects, respectively [Spier, 1998]. A numerical reduction in average CGI scores was observed after response to second agent had stabilized, scores reducing from 5.2 (4–7) to 2.2 (1–4). A total of 12 of the 15 monotherapy nonresponders showed a response after the addition of bupropion, but only two of the ten prescribed this to manage medication side effects showed any amelioration of their problems. The small sample size, and a very heterogeneous population, inevitably means these results should be treated with caution.
Bupropion as part of multiple medication trials
Six studies compared the efficacy of bupropion in multiple medication trials.
As part of the highly influential STAR*D project, Trivedi and colleagues randomized (but in an unblinded fashion) 851 individuals with MDD not remitting on citalopram (mean dose 55 mg/day, mean duration 11.9 weeks) to additionally receive either bupropion XL (n = 565, dose of up to 400 mg/day) or buspirone (n = 286, dose of up to 60 mg/day); there was no placebo arm [Trivedi et al. 2006a]. Both treatments had similar results on the HRSD-17 remission, QIDS-SR-16 remission, and QIDS-SR-16 response rates; however bupropion had a greater reduction (p < 0.04) and overall score (p < 0.02) in QIDS-SR-16 total scores, and a lower side-effect drop-out rate (p < 0.009) by the 12-week study end. Subsequent analysis by the same group [Bech et al. 2012] focused on what the authors termed the ‘pharmacopsychometric triangle’ in comparing bupropion with buspirone augmentation. Under this model, which composites the domains of antidepressive activity, side effects and quality of life, bupropion was superior in all domains (though not to statistical significance for ‘quality of life’).
In a double-blind RCT Blier and colleagues randomized 105 medication-free participants with MDD to receive either fluoxetine monotherapy (n = 28, dose 20 mg/day) or one of three mirtazapine (30 mg/day) combination groups: with fluoxetine (20 mg/day, n = 25); with venlafaxine (225 mg/day, n = 26); or with bupropion (150 mg/day, n = 26) over a 6-week period [Blier et al. 2010]. Although, similarly to the STAR*D work, there was no placebo group, the monotherapy group allowed for evaluation of the hypothesis that any response or remission might be solely due to having more time on an initial compound. There were no significant differences in inter-group drop-out rates, suggesting that polytherapy was tolerated well; and all of the combination groups showed significantly greater improvement on HAM-D scores compared to fluoxetine monotherapy, producing a number needed to treat (NNT) of 3–5 over monotherapy. However, the combination protocols did not result in any greater rapidity of response and there were no significant between-combination-group differences on the MADRS or CGI. Furthermore, discontinuation of any drug in those who had shown a marked response led to relapse in approximately 40% of patients.
A post-hoc analysis of data from a subgroup of patients enrolled in a large (n = 296) 52-week open-label multicentre study [Clayton et al. 2014] evaluated the addition of aripiprazole to either bupropion (n = 47) or SSRIs/SNRIs (n = 245, data pooled) in individuals with MDD. The primary outcome measurements were the safety and tolerability of these combinations, which are reported elsewhere in this paper. For both groups, LOCF improvements occurred over the course of the 1-year trial; mean changes of −1.4 points in CGI-S scores occurred in the bupropion group and −1.5 points in the SSRI/SNRI group (observed case n = 76). In a 6-week prospective open-label study, DeBattista and colleagues examined the efficacy of bupropion XL (150 or 300 mg/day) added to 25 participants’ existing antidepressants (all with MDD and an inadequate response on their current treatment of ⩾4 weeks), which included SSRIs (fluoxetine, paroxetine and sertraline) and the SNRI venlafaxine [DeBattista et al. 2003]. A statistically significant reduction in HDRS symptoms was demonstrated by the trial end (p < 0.001), with 54% demonstrating a clinical response of ⩾50% symptom reduction. No between drug differences were noted, though the sample size was very small. Bares and colleagues compared the efficacy of antidepressant monotherapy (ADM) with combinational antidepressant (CAD) treatment in 60 inpatients with treatment-resistant depression (TRD) in a randomized 6-week open-label study [Bares et al. 2013]. After a short initial washout period of a day or two, eligible participants were randomly allocated to either 6-week ADM or CAD groups, with responders from both entered into a further 8-week follow-up protocol. The new treatments were chosen by clinicians based upon professional judgement of the patients mental state and past psychiatric histories, excluding medications that had previously failed, or drugs of the same class (with the exception of SSRIs). There were no differences from baseline between ADM and CAD groups as measured by change on MADRS or response rates; whilst bupropion was administered to participants in this study, the total number on this in both groups (and the combinations in the CAD group) are not recorded, nor are the other drugs reported.
Interpreting these findings, the methodologically strongest work, by Trivedi and colleagues and Blier and coworkers support the addition of bupropion to a range of existing first-line antidepressants [Trivedi et al. 2006a; Blier et al. 2010]. However, there is little to delineate treatment options further, and the lack of placebo arms is notable.
Other population groups
Older adults
Three studies evaluated the use of bupropion in older adults (⩾65 years of age) with MDD. Two were RCTs, evaluating bupropion in comparison with placebo [Hewett et al. 2010a] and with paroxetine [Weihs et al. 2000]. One naturalistic study investigated the use of both bupropion monotherapy and its combination with SSRIs (Steffens et al. 2001). Trials ranged from 6-12 weeks with bupropion doses ranging from 100-400 mg/day. All three studies showed bupropion to be significantly efficacious.
The most recent double-blind RCT, by Hewett and colleagues, demonstrated efficacy for bupropion XR in comparison with placebo (p < 0.05) over 10 weeks in a large sample (n = 418) [Hewett et al. 2010a]; from baseline the median changes in MADRS total score were −15.0 and −11.0 for bupropion XR and placebo, respectively. Weihs and colleagues (n = 100) found significant efficacy for both bupropion SR (n = 48) and paroxetine (n = 52) for the treatment of depression in a double-blinded, double dummy RCT [Weihs et al. 2000]. Mean HAM-D (LOCF) scores were similar at baseline for both treatment groups with a 59% reduction in total score for bupropion SR and a 63% reduction in total score for paroxetine by week 6. LOCF analyses found no significant difference in mean HAM-D scores between the two drugs. Secondary analyses of CGI-S, CGI-I and HAM-A scores showed improvement in both treatment groups at week 6. No significant differences were found between treatment groups in mean CGI-S, CGI-I or HAM-A scores.
In the naturalistic trial, Steffens and colleagues (n = 31) investigated the use of bupropion SR or IR monotherapy (n = 15) and its combination with an SSRI (n = 16):with prescribing based upon clinicians’ choices [Steffens et al. 2001]. For bupropion monotherapy, 61% were classified as responders (MADRS < 15), with 50% achieving partial (CGI = 2) or complete (CGI = 1) remission. Of those receiving bupropion and SSRI combination therapy 81.2% were classified as responders (MADRS < 15) with 56.2% showing partial (CGI = 2) or complete (CGI = 1) remission. Across both types of intervention, 74.2% were classed as responders (defined as a MADRS < 15), with 53.3% achieving a partial (CGI = 2) or complete (CGI = 1) remission.
Of the three studies identified that evaluated the use of bupropion in the treatment of MDD in the elderly all demonstrated significant improvement on depression scores. However, given the general dearth of research in this population and that the current studies were not methodologically strong results should be interpreted with caution.
Bipolar affective disorder
Although defined by pathological mood elevation, depression (so-called bipolar depression) constitutes the majority of illness burden [Lloyd et al. 2011]. Three studies investigated the use of bupropion in this group: one double-blind RCT compared it to the selective α2 adrenergic antagonist idazoxan [Grossman et al. 1999], a single-blinded RCT compared with the anticonvulsant and mood stabilizer topiramate [McIntyre et al. 2002], and a mixed-design study compared bupropion with sertraline and venlafaxine in combination with mood stabilizers [Post et al. 2006]. Trial length varied from 6 to 8 weeks with bupropion doses ranging from 75 to 450 mg/day.
In a small study of 14 individuals with bipolar depression, Grossman and colleagues found a significant reduction in HDRS total score by the end of the 6-week trial for both bupropion and idazoxan [Grossman et al. 1999]. In a slightly larger (n = 36) and longer trial, McIntyre and colleagues reported a significant improvement in HDRS-17 total score from baseline to endpoint for both bupropion SR and topiramate (p < 0.001) [McIntyre et al. 2002]. Both treatment groups demonstrated significant response rates (≥ 50% reduction in HDRS-17); bupropion SR (59%) and topiramate (56%) (p = 0.03 and p = 0.04, respectively), with no significant between-group differences being observed (p = 0.097). This was also reflected in CGI-I total scores, where a significant reduction was observed for both groups at week 8 (p < 0.005), with no significant differences between bupropion and topiramate (p = 0.092).
Post et al. (2006) evaluated the effects of bupropion, sertraline and venlafaxine as adjuncts to mood stabilizers. This 10-week study consisted of a cohort of 174 outpatients with bipolar disorder; 28 of which were treated with open medication and 156 studied in a randomized, double-blind double dummy fashion. Continued medication included lithium (n=68), valproate (n=93), carbamazepine (n=16), lamotrigine (n=8), typical antipsychotics (n=8) and atypical antipsychotics (n=30). Similar response (≥50% improvement in IDS score) and remission rates (IDS score <12) were recorded across all three treatment groups at study end, with no statistically significant differences demonstrated (p = 0.68 and p = 0.29, respectively).
Collectively these studies suggest efficacy of bupropion in the treatment of bipolar depression, although no significant differences were observed between the active comparators. However, interpretation is once again restricted due to the dearth in research within this population and notable methodological issues including a small sample size and absence of a placebo group.
Seasonal affective disorder
Three studies evaluated the use of bupropion in SAD. One prospective study utilized bupropion prophylactically in a double-blinded, randomized design [Modell et al. 2005], and two adopted open-label designs [Dilsaver et al. 1992; Seo et al. 2013]. Studies ranged from 5 to 8 weeks in length, with doses of bupropion ranging from 150 to 400 mg/day.
In their large (n = 1042) multisite RCT of individuals with a diagnosis of SAD but currently asymptomatic, Modell and colleagues randomized participants to receive either bupropion or placebo, and found a significant reduction in recurrence rates by the following Spring in the active group [Modell et al. 2005]. Of the total sample, 16% of patients experienced a recurrence of major depression whilst on bupropion XL, compared with 28% of patients in the placebo group, with an overall relative risk of 0.56. Bupropion XL was favoured over placebo across all three study sites p = 0.081, p = 0.057 and p = 0.001, respectively). Suggesting there is potentially a role for bupropion in preventing recurrence of seasonal major depressive episodes, although further methodologically sound research is required to support this initial data.
Both open-label trials demonstrated a significant reduction in depressive symptoms. The more recent work by Seo and colleagues (n = 52) found a significant reduction in SIGH-SAD total score by 53% from baseline (27.6 ± 6.5) to week 8 (12.2 ± 6.3) (p < 0.001) [Seo et al. 2013]. The earlier, and considerably smaller study (n = 15) by Dilsaver and colleagues found a significant improvement on HAM-D total score from baseline (18.3 ± 6.9) to study end (week 5; 3.2 ± 2.2) with an average reduction of 15.2 (p < 0.0001) [Dilsaver et al. 1992]; there were also significant reductions of modified HAM-D (mHAM-D; modified to include scores on hyperphagia and weight gain) total score from baseline (25.5 ± 6.4) to treatment end (4.1 ± 3.1) with an average reduction of 21.4 (p < 0.0001).
In summary, Modell and colleagues demonstrated the potential prophylactic potential of bupropion in patients with SAD [Modell et al. 2005] although this finding is yet to be replicated. Open-label trials have also demonstrated the efficacy of bupropion in reducing depressive symptomology. However, given the limited number of trials these findings should be considered preliminary.
Dysthymic disorder
A single open-label study evaluated bupropion SR in the treatment of dysthymic disorder, in an 8-week trial consisting of 21 patients [Hellerstein et al. 2001]. A significant improvement in depression scores was demonstrated at study end; HAM-D scores decreased by 73% from 21.71 (SD 5.57) at baseline to 5.90 (SD 3.60) at week 8 (p < 0.001) as did the Cornell Dysthymia Rating Scale (CDRS) total score from baseline (36.33, SD 9.85) to week 8 (12.43, SD 7.90) (p < 0.001). Although in support of bupropion in the treatment of dysthymia, methodologically sound replication is required to validate these preliminary positive findings.
Postpartum depression
One small (n = 8) study evaluated bupropion SR in the treatment of postpartum depression in an 8-week open-label trial [Nonacs et al. 2005]. Overall, patients showed a significant response to bupropion treatment, with a significant reduction in HAM-D by week 4 (p < 0.05), CGI and Kellner depression scores at study end (p < 0.005). Given the small sample and the study design, further validation of findings is required in support of this initial data.
Side effects
Weight change
Fourteen studies (total n = 8137 participants with MDD), ranging from 6-52 weeks study duration reported bupropion-related weight changes (see Table 3). Overall, the majority concurred that there was significant weight loss in bupropion-treated patients [Coleman et al. 1999; 2001; Croft et al. 1999; 2002; Jefferson et al. 2006; Reimherr et al. 1998; Settle et al. 1999; Weihs et al. 2002], though some studies reported a small weight increase [Blier et al. 2010; Clayton et al. 2014] or no significant change [Hewett et al. 2009, 2010b; Koshino et al. 2013; Thase et al. 2006].
Table 3.
Table of side effects.
| Study | Subjects (ITT) | Treatment phase (weeks) | Results, mean (SD) |
Discontinuation rates due to side effects (%) | |
|---|---|---|---|---|---|
| Weight Change | Blier et al. [2010] | Fluoxetine and placebo: 15 Fluoxetine and mirtazapine: 16 Mirtazapine and venlafaxine: 18 Mirtazapine and bupropion: 17 |
6 | There was no significant weight change in the fluoxetine monotherapy group (n = 25) compared with baseline, +0.1 kg (1.5). Patients in the combination groups had statistically significant increase in weight compared with fluoxetine monotherapy (p < 0.001). |
Not stated. |
| Clayton et al. [2014] | Bupropion and aripiprazole: 47 SSRI/SNRI and aripiprazole: 245 |
52 | 8.7% of the group taking bupropion with aripiprazole reported weight gain as a side effect compared with 19.0% of the SSRI/SNRI and aripiprazole group. | Not stated. | |
| Coleman et al. [1999] | Bupropion: 122 Sertraline: 118 Placebo: 124 |
8 | Patients in the active treatment groups experienced small and similar decreases in mean body weight (−0.9 kg with bupropion and −0.5 kg with sertraline); patients in the placebo group experienced an increase in mean body weight of 0.1 kg. | Placebo 2% Bupropion 6% Sertraline 8% |
|
| Coleman et al. [2001] | Placebo: 152 Bupropion: 150 Fluoxetine: 154 |
8 | Patients in the active-treatment groups experienced small, similar decreases in mean body weight (–1.49 kg, bupropion; −1.41 kg, fluoxetine); patients in the placebo group experienced an increase in mean body weight of 0.20 kg. | Placebo: 3% Bupropion: 9% Fluoxetine: 4% |
|
| Croft et al. [1999] | Placebo: 119 Sertraline: 118 Bupropion: 118 |
8 | Weight loss was associated with active treatment (1.06 kg with bupropion and 0.79 kg with sertraline); patients in the placebo group experienced a minor increase (0.2 1 kg) (p > 0.05). | Placebo: 0% Bupropion: 7% Sertraline: 3% |
|
| Croft et al. [2002] | Bupropion open label: 816 Bupropion double blind: 210 Placebo: 213 |
8 open label + 44 double-blind |
At the end of the open-label phase, patients reported losing between 0.5 and 1.8 kg. At the end of double-blind treatment, patients reported losing between 0.1 and 2.4 kg. The rate of change in body weight during the double-blind phase was statistically significant compared with baseline BMI (p < 0.001). |
Not stated. | |
| Hewett et al. [2009] | Placebo: 197 Bupropion: 187 Venlafaxine: 187 |
8 | Bupropion: 9% of patients gained more than 2.5 kg, and 5% lost more than 2.5 kg. Venlafaxine: 10% of patients gained more than 2.5 kg and 8% lost more than 2.5 kg. Placebo: 10% of patients gained and 8% of patients lost more than 2.5 kg. |
Not stated. | |
| Hewett et al. [2010b] | Placebo: 187 Bupropion: 203 Venlafaxine: 198 |
8 | Bupropion: 7% of subjects gained more than 2.5 kg, and 12% lost more than 2.5 kg. Venlafaxine: 8% of subjects gained and 13% lost more than 2.5kg. Placebo: 13% of subjects gained and 7% of subjects lost more than 2.5 kg. |
Not stated. | |
| Jefferson et al. [2006] | Bupropion: 135 Placebo: 139 |
8 | The bupropion group had a mean weight decrease of 1.1 kg, while the placebo group showed a mean increase of 0.2 kg (p < 0.05). | Placebo: 2% Bupropion 9% |
|
| Reimherr et al. [1998] | Placebo: 121 Bup150: 121 Bup300: 120 |
9 | The largest decrease in mean weight was seen in the Bup300 group (1.0 kg), followed by the Bup150 (0.5 kg) group, and placebo (0.2kg). There was a statistically significant difference in mean weight change between the Bup300 and placebo groups throughout the study (p < 0.001). | Placebo: 3% Bup150 8% Bup300 11% |
|
| Settle et al. [1999] |
Study 1
Placebo: 121 Bup150: 121 Bup300: 120 Study 2 Placebo: 124 Bup100: 119 Bup200: 120 Bup300: 120 Bup400: 119 Study 3 Placebo: 154 Bup50-150: 152 Bup100-300: 150 |
9 | Weight loss occurred in a dose-dependent manner; 11% of bupropion-treated patients and 6% of placebo recipients had a weight loss of >2.3 kg (p = 0.002). | Bupropion: 7%* Placebo: 4% |
|
| Thase et al. [2006] | Bupropion: 168 Venlafaxine: 174 |
12 | Mean weight change at week 12 was minimal for both groups: bupropion group −0.1 kg and venlafaxine group + 0.1 kg. For bupropion, 10% of subjects gained more than 2.5 kg, and 20% lost more than 2.5 kg. The corresponding percentages of subjects with weight changes for venlafaxine were 15% and 12%, respectively. | Bupropion: 11% Venlafaxine: 6% |
|
| Weihs et al. [2002] | Open-label bupropion: 816 Double-blind placebo: 213 Double-blind bupropion: 210 |
8 + 44 | Statistically significant difference in weight loss (p < 0.05) between the placebo (no change) and bupropion treatment groups beginning at week 9 and continuing through week 28. | Open-label bupropion: 9% Double-blind bupropion: 4% Double-blind placebo: <1% |
|
| Sexual Function | Koshino et al. [2013] | Placebo: 187 Bup150: 190 Bup300: 192 |
8 | There were no significant findings in subjects treated with bupropion in regard to sexual dysfunction. | Placebo: 2% Bup150 6% Bup300 5% |
| Clayton et al. [2001] | 11 | 8 | Global CSFQ scores revealed significant improvement in global CSFQ scores from week 0 to week 2 and from week 2 to 4. Each of the mean scores at weeks 2, 4 and 8 were significantly greater than the mean score at week 0, indicating improvement after starting bupropion. | Not reported. | |
| Clayton et al. [2004] | 42 | 4 | The difference in global sexual functioning (total CSFQ score) was not significant between the 2 groups at week 4. Self-reported arousal, desire and frequency showed a significantly greater improvement among those patients receiving bupropion compared with placebo (p = 0.024). |
Bupropion: 6% Placebo: 5% |
|
| Clayton et al. [2006] | Bupropion: 276 Escitalopram: 281 Placebo: 273 |
8 | In both the individual studies and the pooled dataset, the incidence of orgasm dysfunction and worsened sexual functioning at week 8 were statistically significantly lower with bupropion than with escitalopram (p < 0.05) but not statistically different between bupropion and placebo (p = 0.067). | Bupropion: 9% Placebo: 3% Fluoxetine: 4% |
|
| Coleman et al. [2001] | Bupropion: 150 Placebo: 152 Fluoxetine: 154 |
8 | Orgasm dysfunction occurred in significantly more fluoxetine patients than either bupropion or placebo patients (p < 0.001). There were no statistically significant differences in the occurrence of orgasm dysfunction between the bupropion and placebo group at any treatment week. | Bupropion: 6% Placebo: 2% Sertraline: 8% |
|
| Coleman et al. [1999] | Bupropion: 122 Placebo: 124 Sertraline: 118 |
8 | More sertraline treated patients experienced orgasm dysfunction and sexual desire disorder (p < 0.05). The onset of orgasm dysfunction occurred in significantly more sertraline-treated patients (15%; p < 0.05) compared with either placebo (5%) or bupropion treated patients (4%). Bupropion treated patients were more satisfied with their sexual functioning (p < 0.05). | Bupropion: 7% Placebo: 0% Sertraline: 3% |
|
| Croft et al. [1999] | Bupropion: 118 Placebo: 119 Sertraline: 118 |
8 | Significantly more patients treated with sertraline experienced orgasmic dysfunction throughout the study than did subjects treated with bupropion or placebo (p < 0.001). By day 42, significantly fewer bupropion-treated patients (19%; p < 0.05) had sexual desire disorder than did sertraline-treated patients (30%) and placebo patients (31%) | Not stated. | |
| Dobkin et al. [2006] | 18 | 10 | There were significant improvements in desire (p < 0.001), arousal (p < 0.001), and orgasm (p < 0.001) after 8 weeks on bupropion. | Venlafaxine: 11% Bupropion: 6% |
|
| Hewett et al. [2010b] | Placebo: 187 Bupropion: 203 Venlafaxine: 198 |
8 | Bupropion did not differ significantly from placebo on measures of sexual functioning. | Not stated. | |
| Kennedy et al. [2006] | Bupropion: 65 Paroxetine: 66 |
8 | Bupropion was superior to paroxetine on global sexual function in males (p < 0.01); and subcategories: desire, p < 0.01; arousal, p < 0.01; overall satisfaction, p < 0.01. | Not stated. | |
| Safarinejad et al. [2011] | Bupropion + SSRI: 109 Bupropion + Placebo: 109 |
12 | Female Sexual Function Index total score was higher in the bupropion group than in the placebo group (p = 0.001). | Bupropion + SSRI: 2.8% Bupropion + placebo: 0.9% |
|
| Segraves et al. [2000] | Bupropion: 124 Sertraline: 124 |
16 | More patients in the sertraline group experienced sexual desire disorder (63% of men and 41% of women) and sexual arousal disorder (sertraline: 16% (p = 0.005); 19% in men (p = 0.02) and 12% in women (p = 0.05) compared with bupropion: (4%), 7% in men, 2% in women. | Not stated. | |
| Settle et al. [1999] |
Study 1 Placebo: 121 Bup150: 121 Bup300: 120 Study 2 Placebo: 124 Bup100: 119 Bup200: 120 Bup300: 120 Bup400: 119 Study 3 Placebo: 154 Bup50-150: 152 Bup100-300: 150 |
9 | 1% of patients on bupropion reported sexual dysfunction; this did not differ significantly from the placebo group. | Bupropion: 7% Placebo: 4% |
|
| Thase et al. [2006] | Bupropion: 168 Venlafaxine: 174 |
12 | For men, there was a statistically significant difference in sexual functioning in favour of bupropion (p = 0.048). For women, this was also true for comparisons at week 5, week 6, and the average across weeks 5–12 (p = 0.043). | Bupropion: 11% Venlafaxine: 6% |
BMI, body mass index; Bup, bupropion; CSFQ, Changes in Sexual Functioning Questionnaire; ITT, intention-to-treat population; SD, standard deviation; SE, side effects.
Six double-blinded RCTs reported significant weight loss during bupropion treatment, including two long-term (52-week) follow-up studies. In the work by Weihs and colleagues (the antidepressant efficacy data are reported earlier in this review), there was an initial 8-week open-label phase of 518 participants with recurrent major depression treated with bupropion SR 300 mg/day [Weihs et al. 2002]; following this the responders (n = 471) entered a 44-week double-blind, placebo-controlled phase where they were randomized to continue bupropion or to switch to placebo. At week 52, no fluctuations in weight were found in the placebo group compared with baseline (+0.18 kg; SD 11.0), whereas the bupropion SR group had a mean weight loss of 1.13 kg (SD 15.8); as noted earlier the bupropion cohort had significant improvements in mood over placebo, and one might otherwise expect more illness-driven weight loss in the more depressed placebo group. Interestingly, one bupropion-treated patient discontinued from the study due to weight loss of approximately 7.8 kg or 15.2% of her baseline body weight. In a similarly designed open-label study, Croft et al. (2002) reported a mean weight loss of 1.4 kg by week 8 in the open-label phase; following double-blind randomization at that point, mean weight changes of −1.15 kg were reported for the bupropion group (N = 2101) and +0.02 kg for the placebo arm (n = 213) by week 52 [Croft et al. 2002].
Five briefer 8-week, multicentre, randomized, double-blind, double dummy placebo-controlled studies (totalling 2957 subjects with MDD), also reported weight decreases with bupropion [Coleman et al. 1999, 2001; Croft et al. 1999; Reimherr et al. 1998; Settle et al. 1999]. Across these trials median weight loss ranged from 0.9 to 1.49 kg, compared with losses of 0.5 kg on sertraline and 1.49 kg on fluoxetine, and weight gain of 0.1–0.2 kg in placebo groups. In the studies by Reimherr and colleagues and Settle and coworkers, weight loss was dose dependent, with larger mean decreases on higher dosing regimens [Reimherr et al. 1998; Settle et al. 1999].
Four double-blind RCTs reported minimal weight changes over 8 to 12 weeks of bupropion treatment (n = 2070), though only the work by Koshino and colleagues reported statistical comparisons [Koshino et al. 2013]; they found no significant differences in weight change in a cohort of 569 depressed Asian subjects treated with bupropion 150 mg/day, 300 mg/day or placebo. Thase and colleagues found a mean weight decrease in the bupropion XL group of 0.1 kg compared with a gain of 0.1 kg in the venlafaxine XR group [Thase et al. 2006]. Hewett and colleagues described minimal 8-week weight changes for bupropion, venlafaxine and placebo groups [Hewett et al. 2009, 2010b].
Two studies (n = 358) reported mean weight gains associated with bupropion treatment, though these were studies where it was co-administered with another antidepressant. Blier and colleagues (n = 66) reported significant mean weight increases in the bupropion plus mirtazapine (M = 2.7 kg, SD 2.4), venlafaxine plus mirtazapine (M = 2.2 kg, SD 2.5) and fluoxetine plus mirtazapine groups (M = 3.1 kg, SD 2.5) as compared with fluoxetine monotherapy (p < 0.001) [Blier et al. 2010]. In a 52-week open-label study (n = 292) by Clayton and colleagues mean weight gains of +3.1 kg for bupropion plus aripiprazole, and +2.4 kg for an SSRI/SNRI plus aripiprazole were reported [Clayton et al. 2014].
Sexual functioning
Eleven studies (total participant n = 5582) measured effect of bupropion on sexual functioning in patients with MDD (see Table 3). The majority divided sexual dysfunction into sexual desire disorder, sexual arousal disorder and orgasm dysfunction, while one study reported sexual functioning using a single measure. The consensus across the literature was that bupropion did not differ significantly from placebo in causing sexual dysfunction; whilst it improved sexual functioning in patients experiencing SSRI-induced sexual dysfunction.
Regarding causing sexual dysfunction, Croft and colleagues and Coleman and coworkers both reported that significantly more patients treated with sertraline experienced orgasmic dysfunction than subjects treated with bupropion SR or placebo, with an onset of orgasm dysfunction occurring as early as day 7 in up to a sixth of those on sertraline [Croft et al. 1999; Coleman et al. 1999]. Following a 16-week randomized clinical trial (N = 248), Segraves and colleagues reported that compared with bupropion, more patients in the sertraline group experienced sexual desire disorder (63% of men and 41% of women) and sexual arousal disorder (19% in men, p = 0.02; 12% in women, p = 0.05) [Segraves et al. 2000]; bupropion treatment was related with an increase in sexual satisfaction (from 57% to 79%). Clayton and colleagues combined data from two double-blind, placebo-controlled RCTs comparing bupropion (n = 276) to escitalopram (n = 281) on measures of sexual functioning [Clayton et al. 2006]. They found, in both the individual studies and the pooled dataset, that the incidence of orgasm dysfunction and the incidence of worsened sexual functioning at the end of the treatment (week 8) were statistically significantly lower with bupropion and placebo than with escitalopram (p < 0.05), and not statistically different between bupropion and placebo. Similarly, Hewett and colleagues reported no significant difference between bupropion patients and placebo on the Changes in Sexual Functioning Questionnaire (CSFQ), while venlafaxine treated subjects experienced significant decreases on arousal and orgasm subscales (p < 0.05) in an 8-week double-blind, placebo-controlled RCT (n = 591) [Hewett et al. 2010b]. A double-blind, double dummy RCT [Coleman et al. 2001] compared bupropion with fluoxetine. The overall incidence of sexual desire disorder significantly decreased only in the bupropion group by the week 8 endpoint (p < 0.05). Sexual arousal disorder occurred in significantly more fluoxetine- than bupropion- or placebo-treated patients (p < 0.05), with no difference between bupropion and placebo at any point. The incidence of orgasm dysfunction in those on fluoxetine 20 mg/day was more than twice as high than in those on bupropion 300 mg/day and, interestingly, three times higher than that of those on bupropion 400 mg/day. Subjectively, more patients in the bupropion group were satisfied with their sexual functioning than in the placebo or fluoxetine groups.
The important issue of potential gender differences in sexual functioning was taken into account by two randomized, double-blind trials [Kennedy et al. 2006; Thase et al. 2006] (total n = 473 subjects with MDD but without sexual dysfunction). Thase and colleagues reported that for men, there was a statistically significant difference in sexual functioning in favour of bupropion XL for all comparisons with venlafaxine (p ⩽ 0.048); for women, this was also true at week 5, week 6, and for the average across weeks 5–12 (p ⩽ 0.043). Notably, bupropion XL was also superior to venlafaxine XR on the CSFQ subscales for pleasure, desire/frequency, desire/interest, arousal and orgasm throughout the study period. Following an 8-week treatment period with either bupropion or paroxetine, Kennedy and colleagues found that women (n = 68) in the bupropion group experienced significantly higher levels of Sexual Functioning (Sex FX; total, p < 0.01; desire, p < 0.01; arousal, p < 0.01; overall satisfaction, p < 0.01) [Kennedy et al. 2006]. In men (n = 73), the analysis revealed a significant treatment effect by visit for Sex FX total (p < 0.001), desire (p < 0.001) and overall satisfaction (p < 0.01). The effect was due to a significant decrease in sexual functioning during paroxetine treatment (Sex FX total, p < 0.002; desire, p < 0.005; arousal, p < 0.005; and overall satisfaction, p < 0.057); at week 8 the paroxetine groups displayed a significant deterioration from baseline, on Sex FX total (p < 0.01), desire (p < 0.01), arousal (p < 0.05), orgasm (p < 0.01) and overall satisfaction (p < 0.01) scores, whereas no significant change was observed in any of these measures across visits in men randomised to the bupropion SR group.
Several studies evaluated bupropion’s effectiveness at reducing existing sexual dysfunction: four studies measured changes in sexual functioning when subjects were either switched from their previous SSRI/SNRI to bupropion monotherapy or bupropion was added to their current SSRI treatment plan. Clayton and colleagues reported significant improvements in global CSFQ scores by week 2 in the coprescribing group (SSRI/SNRI+bupropion) and from week 2 to 4 in the monotherapy group (discontinuation from SSRI/SNRI to bupropion monotherapy), with gains over baseline maintained by the 8-week endpoint (p < 0.05) [Clayton et al. 2001]. A similar pattern was observed on the subscales of sexual desire and orgasm, mean scores differing significantly (p < 0.05) from baseline to weeks 4 and 8, indicating improvement following SSRI discontinuation and the start of bupropion monotherapy, but not for coprescribing. In a later, placebo-controlled, double-blinded study (n = 42) by the same group, Clayton and colleagues reported that neither the addition of bupropion nor placebo produced a change in CSFQ total scores after 4 weeks [Clayton et al. 2004]; however, those coprescribed bupropion self-reported improved desire and frequency of sexual activity (p = 0.024). Dobkin and colleagues conducted a 10-week open-label study of 18 ethnic minority depressed women with poor response to current SSRI treatment and hypoactive sexual desire [Dobkin et al. 2006]. Compared with baseline measurement, after 8 weeks of bupropion monotherapy (i.e. week 10), there were significant improvements in desire (p < 0.001), arousal (p < 0.001) and orgasm (p < 0.001). Post-hoc analyses indicated that improvements were observed as early as week 2 (end of cross-taper) for desire scores (p = 0.001) and at week 4 for arousal (p < 0.001) and orgasm scores (p < 0.001). More recently, a 12-week double-blind RCT of 218 women with SSRI-induced sexual dysfunction reported the Female Sexual Function Index total score was higher in those coprescribed bupropion SR than in those randomized to placebo (p = 0.001). In addition, at the end of the trial the mean scores for desire, arousal, lubrication, orgasm, and satisfaction were significantly higher in the bupropion group (p < 0.01) [Safarinejad, 2011].
Other side effects
The majority of studies identified for review described side effects as frequently reported (generally defined as occurring in at least 5% of patients). Overall, following bupropion treatment, side effects experienced were generally of mild to moderate intensity, and the most commonly reported were: dry mouth (5–34.5%), insomnia (1–27.8%), headache (3–34%) and nausea (7–21%). Other rarely reported side effects included restlessness, anxiety, constipation, dizziness, nasopharyngitis and fatigue. Discontinuation rates due to adverse events ranged from 0 to 55%, however, bupropion was not significantly different from other antidepressants and in some cases placebo treatments.
Discussion
This review set out to provide a systematic evaluation and meta-analysis of the existing evidence for the use of bupropion as an antidepressant. A total of 51 studies were identified, clustering into four groups: the sole use of bupropion; bupropion coprescribing; ‘other’ populations; and side effects. Regarding the methodologically more robust data, bupropion showed superiority to placebo in most, but not all RCTs; study sizes were generally reasonable, encompassing several hundred participants, though follow-up times were typically relatively brief, lasting in the range of 8 weeks or so. Meta-analysis of trials where sufficient data were provided to allow sufficient extraction produced a result further supporting bupropion in this group; however the small number of trials included means that caution should be exerted in interpreting this. Comparator trials generally showed bupropion as having equivalent effectiveness to other antidepressants, but a considerable number of studies did not have placebo arms, most drugs had few trials (sertraline 5; fluoxetine 2; paroxetine 2; venlafaxine 3; and one each for escitalopram, duloxetine, trazadone and nortriptyline); furthermore, many of the studies are now of considerable age. The RCT by Clayton and colleagues is notable both for its large size (n = 785) and its inclusion of a placebo arm in addition to bupropion and escitalopram [Clayton et al. 2006]; however, whilst the pooled active drug data were superior to placebo, analysis of bupropion alone failed to show separation from placebo. The most evaluated head-to-head drug was sertraline, and all trials with placebo arms showed bupropion’s superiority to placebo, and equivalence with the comparator drug.
Bupropion, arguably, has had a greater role in clinical practice as a coprescribed antidepressant; this may be due to several factors including (in some jurisdictions) limited or lack of licensing, a lack of clear evidence or recommendations as a first-line drug, and the fact that its pharmacodynamics would suggest it might augment existing first-line medications. This latter factor would appear to be borne out in the studies adding bupropion to the highly serotonergic drug (es)citalopram. Data from these trials are supportive of bupropion coprescribing, with the caveat that most were open-label. Results from coprescribing with non-SSRIs are sparse, with a single trial of duloxetine and one of venlafaxine: the former had disappointing results, whilst the latter is again marked by an open-label methodology. It is perhaps the multi-medication trials that have most raised clinicians’ awareness of bupropion, notably the STAR*D work that has been widely reported. STAR*D supports the addition of bupropion as an intervention of value, but with the caveats that overall outcomes in TRD are disappointing, and numbers going into remission are low in all secondary treatment arms. The study emphasises the variation and difficulty in predicting individuals’ responses. The work by Blier and colleagues is consistent with this, albeit in a trial with far fewer participants [Blier et al. 2010]. Both of these support the principle of coprescribing, but highlight that bupropion, whilst a reasonable option, is not superior to other drug choices.
Data from the ‘other’ section are interesting, but generally marked by a dearth of research. Positive results were seen in bipolar depression, older adults, SAD and dysthymia, but considerable caution is required in interpretation due to the general lack of studies in this area. Of this section the work by Modell and colleagues on SAD is noteworthy due to its large size (n = 1042), randomized nature and the fact it was undertaken prophylactically in asymptomatic individuals [Modell et al. 2005]. Their results support the prophylactic prescribing of bupropion in SAD, though the absolute numbers of those relapsing were low in both groups, which raises the issue of benefit:risk ratios in prescribing medications.
With regards to side effects, bupropion is generally a well-tolerated drug, the type, severity and frequency of problems fitting with that of most SSRIs. There are two clear notable differences however: weight change and sexual functioning. The evidence is in favour of bupropion producing no weight gain, and typically weight loss, and that it can improve sexual functioning in depressed individuals. This is potentially very important, as these are considerable drug- and illness-induced difficulties for many with MDD.
Interpreting this broad literature, several challenges emerge. As has been mentioned, the methodology of many studies can be questioned, there were many open-label trials, and many of the head-to-head and multi-drug studies did not have placebo arms. Inevitably cross-comparison between different head-to-head drugs is difficult, not least as some drugs have single trials, some of which are almost 20 years old. Follow-up periods were typically brief, in the order of a couple of months or so. However, in general where response to an antidepressant occurs, it tends to be within such a timeframe [Fornaro et al. 2014].
Regarding participants, there was a wide range of inclusion and exclusion criteria, not least in defining (or not defining) if individuals were treatment refractory, and indeed no clear consensus on what that meant. This factor is critically important if one considers that one of the major outcomes of STAR*D was showing how poor responses are in general as one moves through sequential stages of depression management. As such, much bupropion prescribing is thus potentially in individuals fundamentally more refractory to pharmacological intervention, but the variation in study criteria makes this very difficult to elucidate as a factor; work on first-episode and treatment-naïve individuals would be welcomed.
Zimmerman and colleagues reported that the targeting of specific symptoms and the desire to avoid certain side effects were the most frequently given reasons expressed by a small US sample (n = 10) of psychiatrists for choosing bupropion, namely hypersomnia, hyperphagia and fatigue [Zimmerman et al. 2004]; high levels of anxiety, irritability, poor sleep and appetite were significantly less often cited as a reason for prescribing. For approximately half of the patients, the desire to avoid weight gain or sexual dysfunction was reported as a reason for choosing bupropion. The issue of bupropion’s novel pharmacology is also one of the primary reasons behind many of coprescribing studies, with the consideration that its differing mechanism of action might compliment, or enhance, another drug. This is a complex and incompletely understood area, and there are several possibilities, none yet clearly proven. The first is a potential pharmacokinetic effect wherein coprescribing will change the plasma protein binding and availability of the drugs; thus, additional effects might arise solely through an effective increase in the unbound and active level of one or other, or both, drugs. A second possibility is that there is a pharmacodynamic additive effect through the use of two compounds, each producing different pharmacological effects. Finally it is further possible that there is a synergistic effect, with the combined compounds pharmacokinetically or pharmacodynamically producing an effect greater than the sum of their individual actions. Problematically, there are few RCTs, and fewer of sufficient size or duration, that might allow elucidation of these factors; an issue compounded by the earlier mentioned variable population responses. It is just as possible in open-label work that the effective factor is time; a second drug is added, and a response is seen, but this is, in fact, just a delayed response to the first compound.
Following on from this, depressive disorders are becoming ever more to be seen as fundamentally heterogeneous conditions with as yet incompletely understood but highly complex pathway disorders involving numerous gene and environmental interactions [Koutsouleris et al. 2015]. In psychosis studies there has been an argument to reconceptualize ‘the schizophrenias’ [Arnedo et al. 2015] and we would argue that ‘the depressions’ are equally likely. As such, the bupropion data face a similar problem to that of the wider pharmacological literature; the critical question, that does not have an answer at this time, is in whom and when might bupropion work, rather than the typical cruder class-effect query of ‘is it effective in depression?’. This is of particular interest with bupropion given its differing mechanism of action, that is primarily noradrenergic and dopaminergic (but no serotonergic) effects, raising the interesting question about whether it might target specific symptoms in depression, or certain types of depression.
With specific consideration of the bupropion literature, there are some interesting findings in this regard, though the field is nascent. The study by Weissman and colleagues, in depressed mothers, is a thought-provoking example [Weissman et al. 2015]. Whilst bupropion was as effective as escitalopram, it was less effective with regards to their children’s mental health; subanalyses showed that escitalopram was more effective with regards to treating ‘negative affectivity’ (guilt, irritability, fear/anxiety), which the authors posit has a more serotonergic underpinning. Another domain-specific negative finding was the work by Grunebaum and colleagues which found bupropion less effective at managing suicidal feelings than paroxetine [Grunebaum et al. 2012]; this may fit with a literature suggesting that serotonergic dysfunctioning is associated with suicidality. Fornaro and colleagues intentionally coprescribed bupropion with duloxetine in a cohort with atypical depression (carbohydrate craving, hypersomnia, etc.) on the basis that bupropion’s additional dopaminergic and noradrenergic functioning might provide additional therapeutic gains, though this was not borne out in the (relatively small) sample [Fornaro et al. 2014]. Conversely, Mohan and colleagues, in their study of bupropion coprescribed with escitalopram, showed that so-called ‘melancholic features’ of depression were more effectively treated by bupropion [Mohan et al. 2009].
Of the studies that have explored the possibility of bupropion producing a more distinct pattern of changes across specific symptom dimensions, differential response to bupropion has been identified with regards to negative affect. One study reported in this review in the context of HAM-D scores, also used a dimensional assessment of mood based on the tripartite model of mood disorders [Clark and Watson, 1991] administering the mood and anxiety symptom questionnaire (MASQ AD Scale) [Tomarken et al. 2004]. The authors reported that bupropion exerted a more robust effect on the positive affect dimension (energy, motivation, enjoyment) but not negative affect (general distress and somatic anxiety). Interestingly, during the earlier phase when bupropion was dosed at a lower range (100–300 mg/day) a stronger effect was observed on the affectively negative dimensions, but later within the open-label phase where bupropion was dosed at a higher range (300–400 mg/day) a notably higher effect was observed on the affectively positive dimensions, perhaps suggesting a dose-dependent relationship or due to a longer time in treatment. In a closer examination of the effects of bupropion on negative affect, through a post hoc analysis of monotherapy treatment (bupropion versus escitalopram) in patients with MDD (n = 163), Gerra and colleagues reported that whilst response to escitalopram did not differ significantly between low- and high-negative-affect patients, bupropion was significantly more effective for patients with low negative affect than high negative affect (p < 0.03) [Gerra et al. 2014]. Conversely, escitalopram was significantly more effective than bupropion for high-negative-affect patients (p = 0.017). This suggests that bupropion may be more suitable in the treatment of the core components of depression rather than for the commonly encountered illness-associated anxiety.
The issue of bupropion’s lack of a licence is problematic in some jurisdictions, including the UK. The existing evidence would support bupropion’s addition to the pharmacological armamentarium; however, the burdens of obtaining a European licence are bureaucratically and financially considerable. For a pharmaceutical company, this may present an unappealing prospect; having gone through this process, it is likely to be some time before guidelines or local policies promote bupropion, at such point probably recommending it as a third, fourth, fifth (or worse) choice of drug. The head-to-head data do not suggest it should have a higher role than this, but it means that financial returns are likely to be scant in an already bloated market place, acting as a further disincentive to obtain a licence to market this medication. However, this presents clinicians with a problem; whilst most research shows rough equivalence between various antidepressants, undoubtedly individual responses vary considerably, and there are, and will be, patients who will respond well to bupropion, perhaps better than to other compounds. Of course a current scientific frustration, by no means limited to bupropion, is prospectively identifying such individuals, but the field of pharmacogenomics remains in its infancy, with disappointing results thus far (Penn and Tracy, 2012). However, the lack of a licence means that many clinicians (and potentially patients) will be hesitant to try the drug, and may face further problems such as having difficulties having such prescriptions continued in primary care.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors have no conflicts of interest to declare.
Contributor Information
Krisna Patel, Cognition, Schizophrenia and Imaging Laboratory, Department of Psychosis Studies, the Institute of Psychiatry, Psychology and Neuroscience, King’s College, London, UK.
Sophie Allen, Cognition, Schizophrenia and Imaging Laboratory, Department of Psychosis Studies, the Institute of Psychiatry, Psychology and Neuroscience, King’s College, London, UK.
Mariam N. Haque, Cognition, Schizophrenia and Imaging Laboratory, Department of Psychosis Studies, the Institute of Psychiatry, Psychology and Neuroscience, King’s College, London, UK
Ilinca Angelescu, Cognition, Schizophrenia and Imaging Laboratory, Department of Psychosis Studies, the Institute of Psychiatry, Psychology and Neuroscience, King’s College, London, UK.
David Baumeister, Department of Psychology, Institute of Psychiatry, Psychology and Neuroscience, King’s College, London, UK.
Derek K. Tracy, Consultant Psychiatrist, Green Parks House, Princess Royal University Hospital, Oxleas NHS Foundation Trust, London BR6 8NY, UK.
References
- Arnedo J., Svrakic D., Del Val C., Romero-Zaliz R., Hernandez-Cuervo H. Molecular Genetics of Schizophrenia Consortium and Zwir I. (2015) Uncovering the hidden risk architecture of the schizophrenias: confirmation in three independent genome-wide association studies. Am J Psychiat 172: 139–153. [DOI] [PubMed] [Google Scholar]
- Bares M., Novak T., Kopecek M., Stopkova P., Cermak J., Kozeny J., et al. (2013) Antidepressant monotherapy compared with combinations of antidepressants in the treatment of resistant depressive patients: A randomized, open-label study. Int J Psychiat Clin Pract 17: 35–43. [DOI] [PubMed] [Google Scholar]
- Bech P., Fava M., Trivedi M., Wisniewski S., Rush A. (2012) Outcomes on the pharmacopsychometric triangle in bupropion-SR versus buspirone augmentation of citalopram in the STAR*D trial. Acta Psychiatrica Scandinavica 125:342–348. [DOI] [PubMed] [Google Scholar]
- Blier P., Ward H., Tremblay P., Laberge L., Hebert C., Bergeron R. (2010) Combination of antidepressant medications from treatment initiation for major depressive disorder: A double-blind randomized study. Am J Psychiat 167: 281–288. [DOI] [PubMed] [Google Scholar]
- Brown E., Vornik L., Khan D., Rush A. (2007) Bupropion in the treatment of outpatients with asthma and major depressive disorder. Int J Psychiat Med 37: 23–28. [DOI] [PubMed] [Google Scholar]
- Bulloch A., Williams J., Lavorato D., Patten S. (2014) Recurrence of major depressive episodes is strongly dependent on the number of previous episodes. Depress Anxiety 31: 72–76. [DOI] [PubMed] [Google Scholar]
- Clark L., Watson D. (1991) Tripartite model of anxiety and depression – psychometric evidence and taxonomic implications. J Abnorm Psychol 100: 316–336. [DOI] [PubMed] [Google Scholar]
- Clayton A., Baker R., Sheehan J., Cain Z., Forbes R., Marler S., et al. (2014) Comparison of adjunctive use of aripiprazole with bupropion or selective serotonin reuptake inhibitors/serotonin-norepinephrine reuptake inhibitors: analysis of patients beginning adjunctive treatment in a 52-week, open-label study. BMC Res Note 7: 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A., Croft H., Horrigan J., Wightman D., Krishen A., Richard N., et al. (2006) Bupropion extended release compared with escitalopram: Effects on sexual functioning and antidepressant efficacy in 2 randomized, double-blind, placebo-controlled studies. J Clin Psychiat 67: 736–746. [DOI] [PubMed] [Google Scholar]
- Clayton A., McGarvey E., Abouesh A., Pinkerton R. (2001) Substitution of an SSRI with bupropion sustained release following SSRI-induced sexual dysfunction. J Clin Psychiat 62: 185–190. [DOI] [PubMed] [Google Scholar]
- Clayton A., Warnock J., Kornstein S., Pinkerton R., Sheldon-Keller A., McGarvey E. (2004) A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiat 65: 62–67. [DOI] [PubMed] [Google Scholar]
- Coleman C., Cunningham L., Foster V., Batey S., Donahue R., Houser T., et al. (1999) Sexual dysfunction associated with the treatment of depression: a placebo-controlled comparison of bupropion sustained release and sertraline treatment. Ann Clin Psychiat 11: 205–215. [DOI] [PubMed] [Google Scholar]
- Coleman C., King B., Bolden-Watson C., Book M., Taylor Segraves R., Richard N., et al. (2001) A placebo-controlled comparison of the effects on sexual functioning of bupropion sustained release and fluoxetine. Clin Ther 23: 1040–1058. [DOI] [PubMed] [Google Scholar]
- Coplan J., Gopinath S., Abdallah C., Berry B. (2014) A neurobiological hypothesis of treatment-resistant depression-mechanisms for selective serotonin reuptake inhibitor non-efficacy. Front Behav Neurosci 8: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft H., Houser T., Jamerson B., Leadbetter R., Bolden-Watson C., Donahue R., et al. (2002) Effect on body weight of bupropion sustained-release in patients with major depression treated for 52 weeks. Clin Ther 24: 662–672. [DOI] [PubMed] [Google Scholar]
- Croft H., Settle E., Houser T., Batey S., Donahue R., Ascher J. (1999) A placebo-controlled comparison of the antidepressant efficacy and effects on sexual functioning of sustained-release bupropion and sertraline. Clin Ther 21: 643–658. [DOI] [PubMed] [Google Scholar]
- DeBattista C., Solvason H., Poirier J., Kendrick E., Schatzberg A. (2003) A prospective trial of bupropion SR augmentation of partial and non-responders to serotonergic antidepressants. J Clin Psychopharmacol 23: 27–30. [DOI] [PubMed] [Google Scholar]
- Dilsaver S., Qamar A., Del Medico V. (1992) The efficacy of bupropion in winter depression: Results of an open trial. J Clin Psychiat 53: 252–255. [PubMed] [Google Scholar]
- Dobkin R., Menza M., Marin H., Allen L., Rousso R., Leiblum S. (2006) Bupropion improves sexual functioning in depressed minority women. An open-label switch study. J Clin Psychopharmacol 26: 21–26. [DOI] [PubMed] [Google Scholar]
- Fava M., Papakostas G., Petersen T., Mahal Y., Quitkin F., Stewart J., et al. (2003) Switching to bupropion in fluoxetine-resistant major depressive disorder. Ann Clin Psychiat 15: 17–22. [DOI] [PubMed] [Google Scholar]
- Fava M., Rush A., Thase M., Clayton A., Stahl S., Pradko J., et al. (2005) 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Primary Care Comp J Clin Psychiat 7: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighner J., Gardner E., Johnston J., Batey S., Khayrallah M., Ascher J., et al. (1991) Double-blind comparison of bupropion and fluoxetine in depressed outpatients. J Clin Psychiat 52: 329–335. [PubMed] [Google Scholar]
- Ferguson J., Cunningham L., Merideth C., Apter J., Feighner J., Ionescu-Pioggia M., et al. (1994) Bupropion in tricyclic antidepressant nonresponders with unipolar major depressive disorder. Ann Clin Psychiat 6: 153–160. [DOI] [PubMed] [Google Scholar]
- Ferrari A., Charlson F., Norman R., Patten S., Freedman G., Murray C., et al. (2013) Burden of depressive disorders by country, sex, age and year: findings from the global burden of disease study 2010. PLoS Med 10: e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M., Martino M., Mattei C., Prestia D., Vinciguerra V., De Berardis D., et al. (2014) Duloxetine-bupropion combination for treatment-resistant atypical depression: A double-blind, randomized, placebo-controlled trial. Eur Neuropsychopharmacol 24: 1269–1278. [DOI] [PubMed] [Google Scholar]
- Gerra M., Marchesi C., Amat J., Blier P., Hellerstein D., Stewart J. (2014) Does negative affectivity predict differential response to an SSRI versus a non-SSRI antidepressant? J Clin Psychiat 75: e939–e944. [DOI] [PubMed] [Google Scholar]
- Gross P., Nourse R., Wasser T., Bukenya D. (2007) Safety and efficacy of bupropion extended release in treating a community sample of Hispanic and African American adults with major depressive disorder: An open-label study. Primary Care Comp J Clin Psychiat 9: 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman F., Potter W., Brown E., Maislin G. (1999) A double-blind study comparing idazoxan and bupropion in bipolar depressed patients. J Affect Dis 56:237–243. [DOI] [PubMed] [Google Scholar]
- Grunebaum M., Ellis S., Duan N., Burke A., Oquendo M., John Mann J. (2012) Pilot randomized clinical trial of an SSRI vs bupropion: Effects on suicidal behavior, ideation and mood in major depression. Neuropsychopharmacology 37: 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulrez G., Badyal D., Deswal R., Sharma A. (2012) Bupropion as an augmenting agent in patients of depression with partial response. Basic Clin Pharmacol Toxicol 110:227–230. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. J Neurol Neurosurg Psychiat 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstein D., Batchelder S., Kreditor D., Fedak M. (2001) Bupropion sustained-release for the treatment of dysthymic disorder: An open-label study. J Clin Psychopharmacol 21: 325–329. [DOI] [PubMed] [Google Scholar]
- Hewett K., Chrzanowski W., Jokinen R., Felgentreff R., Shrivastava R., Gee M., et al. (2010a) Double-blind, placebo-controlled evaluation of extended-release bupropion in elderly patients with major depressive disorder. J Psychopharmacol 24: 521–529. [DOI] [PubMed] [Google Scholar]
- Hewett K., Chrzanowski W., Schmitz M., Savela A., Milanova V., Gee M., et al. (2009) Eight-week, placebo-controlled, double-blind comparison of the antidepressant efficacy and tolerability of bupropion XR and venlafaxine XR. J Psychopharmacol 23: 531–538. [DOI] [PubMed] [Google Scholar]
- Hewett K., Gee M., Krishen A., Wunderlich H., Le Clus A., Evoniuk G., et al. (2010b) Double-blind, placebo-controlled comparison of the antidepressant efficacy and tolerability of bupropion XR and venlafaxine XR. J Psychopharmacol 24: 1209–1216. [DOI] [PubMed] [Google Scholar]
- Hirschfeld R. (2012) Depression epidemiology and its treatment evolution. J Clin Psychiat 73: e29. [DOI] [PubMed] [Google Scholar]
- Jefferson J., Rush A., Nelson J., VanMeter S., Krishen A., Hampton K., et al. (2006) Extended-release bupropion for patients with major depressive disorder presenting with symptoms of reduced energy, pleasure and interest: Findings from a randomized, double-blind, placebo-controlled study. J Clin Psychiat 67: 865–873. [DOI] [PubMed] [Google Scholar]
- Kavoussi R., Segraves R., Hughes A., Ascher J., Johnston J. (1997) Double-blind comparison of bupropion sustained release and sertraline in depressed outpatients. J Clin Psychiat 58: 532–537. [DOI] [PubMed] [Google Scholar]
- Kennedy S., Fulton K., Bagby R., Greene A., Cohen N., Rafi-Tari S. (2006) Sexual function during bupropion or paroxetine treatment of major depressive disorder. Can J Psychiat 51: 234–242. [DOI] [PubMed] [Google Scholar]
- Kessler R., Berglund P., Demler O., Jin R., Koretz D., Merikangas K. National Comorbidity Survey Replication (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). J Am Med Assoc 289: 3095–3105. [DOI] [PubMed] [Google Scholar]
- Koshino Y., Bahk W., Sakai H., Kobayashi T. (2013) The efficacy and safety of bupropion sustained-release formulation for the treatment of major depressive disorder: A multi-center, randomized, double-blind, placebo-controlled study in Asian patients. Neuropsychiat Dis Treat 9: 1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N., Meisenzahl E., Borgwardt S., Riecher-Rossler A., Frodl T., Kambeitz J., et al. (2015) Individualized differential diagnosis of schizophrenia and mood disorders using neuroanatomical biomarkers. Brain 138: 2059–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam R., Hossie H., Solomons K., Yatham L. (2004) Citalopram and bupropion-SR: combining versus switching in patients with treatment-resistant depression. J Clin Psychiat 65:337–340. [PubMed] [Google Scholar]
- Leuchter A., Lesser I., Trivedi M., Rush A., Morris D., Warden D., et al. (2008) An open pilot study of the combination of escitalopram and bupropion-SR for outpatients with major depressive disorder. J Psychiat Pract 14: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lineberry C., Johnston J., Raymond R., Samara B., Feighner J., Harto N., et al. (1990) A fixed-dose (300 mg) efficacy study of bupropion and placebo in depressed outpatients. J Clin Psychiat 51: 194–199. [PubMed] [Google Scholar]
- Lloyd L., Giaroli G., Taylor D., Tracy D. (2011) Bipolar depression: clinically missed, pharmacologically mismanaged. Ther Adv Psychopharmacol 1: 153–162. DOI: 10.1177/2045125311420752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan E., Eller T., Vasar V., Nutt D. (2009) Effects of bupropion augmentation in escitalopram-resistant patients with major depressive disorder: An open-label, naturalistic study. J Clin Psychiat 70: 1054–1056. [DOI] [PubMed] [Google Scholar]
- Masco H., Kiev A., Holloman L., Batey S., Johnston J., Lineberry C. (1994) Safety and efficacy of bupropion and nortriptyline in outpatients with depression. Curr Ther Res Clin Exp 55: 851–863. [Google Scholar]
- McIntyre R., Mancini D., McCann S., Srinivasan J., Sagman D., Kennedy S. (2002) Topiramate versus Bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: A preliminary single-blind study. Bipolar Dis 4: 207–213. [DOI] [PubMed] [Google Scholar]
- Melfi C., Chawla A., Croghan T., Hanna M., Kennedy S., Sredl K. (1998) The effects of adherence to antidepressant treatment guidelines on relapse and recurrence of depression. Arch Gen Psychiat 55: 1128–1132. [DOI] [PubMed] [Google Scholar]
- Modell J., Rosenthal N., Harriett A., Krishen A., Asgharian A., Foster V., et al. (2005) Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biol Psychiat 58: 658–667. [DOI] [PubMed] [Google Scholar]
- Montgomery S., Asberg M. (1979) A new depression scale designed to be sensitive to change. Br J Psychiat 134: 382–389. [DOI] [PubMed] [Google Scholar]
- Murray C., Atkinson C., Bhalla K., Birbeck G., Burstein R., Chou D. and US Burden of Disease Collaborators (2013) The state of US health, 1990–2010: burden of diseases, injuries and risk factors. J Am Med Assoc 310: 591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff C., Entsuah R., Benattia I., Demitrack M., Sloan D., Thase M. (2008) Comprehensive analysis of remission (COMPARE) with venlafaxine versus SSRIs. Biol Psychiat 63: 424–434. [DOI] [PubMed] [Google Scholar]
- NICE (2009) Depression in adults: The treatment and management of depression in adults. http://www.nice.org.uk/CG90.
- Nonacs R., Soares C., Viguera A., Pearson K., Poitras J., Cohen L. (2005) Bupropion SR for the treatment of postpartum depression: A pilot study. Int J Neuropsychopharmacol 8: 445–449. [DOI] [PubMed] [Google Scholar]
- Penn E., Tracy D. (2012) The drugs don’t work? Antidepressants and the current and future pharmacological management of depression. Ther Adv Psychopharmacol 2: 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post R., Altshuler L., Leverich G., Frye M., Nolen W., Kupka R., et al. (2006) Mood switch in bipolar depression: Comparison of adjunctive venlafaxine, bupropion and sertraline. Br J Psychiat 189: 124–131. [DOI] [PubMed] [Google Scholar]
- Posternak M., Zimmerman M., Keitner G., Miller I. (2002) A reevaluation of the exclusion criteria used in antidepressant efficacy trials. Am J Psychiat 159: 191–200. [DOI] [PubMed] [Google Scholar]
- Reimherr F., Cunningham L., Batey S., Johnston J., Ascher J. (1998) A multicenter evaluation of the efficacy and safety of 150 and 300 mg/d sustained-release bupropion tablets versus placebo in depressed outpatients. Clin Ther 20: 505–516. [DOI] [PubMed] [Google Scholar]
- Rosso G., Rigardetto S., Bogetto F., Maina G. (2012) A randomized, single-blind, comparison of duloxetine with bupropion in the treatment of SSRI-resistant major depression. J Affect Dis 136172–176. [DOI] [PubMed] [Google Scholar]
- Rush A. (2007) STAR*D: what have we learned? Am J Psychiat 164: 201–204. [DOI] [PubMed] [Google Scholar]
- Rush A., Fava M., Wisniewski S., Lavori P., Trivedi M., Sackeim H., et al. (2004) Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials 25:119–142. [DOI] [PubMed] [Google Scholar]
- Rush A., Giles D., Schlesser M., Fulton C., Weissenburger J., Burns C. (1986) The inventory for depressive symptomatology (IDS): Preliminary findings. Psychiat Res 18: 65–87. [DOI] [PubMed] [Google Scholar]
- Rush A., Trivedi M., Carmody T., Donahue R., Houser T., Bolden-Watson C., et al. (2001) Response in relation to baseline anxiety levels in major depressive disorder treated with bupropion sustained release or sertraline. Neuropsychopharmacology 25: 131–138. [DOI] [PubMed] [Google Scholar]
- Rush A., Trivedi M., Wisniewski S., Stewart J., Nierenberg A., Thase M., et al. (2006) Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. New Engl J Med 354: 1231–1242. [DOI] [PubMed] [Google Scholar]
- Safarinejad M. (2011) Reversal of SSRI-induced female sexual dysfunction by adjunctive bupropion in menstruating women: a double-blind, placebo-controlled and randomized study. J Psychopharmacol 25: 370–378. [DOI] [PubMed] [Google Scholar]
- Segraves R., Kavoussi R., Hughes A., Batey S., Johnston J., Donahue R., et al. (2000) Evaluation of sexual functioning depressed outpatients: A double-blind comparison of sustained-release bupropion and sertraline treatment. J Clin Psychopharmacol 20: 122–128. [DOI] [PubMed] [Google Scholar]
- Seo H., Lee B., Seok J., Jeon H., Paik J., Kim W., et al. (2013) An open-label, rater-blinded, 8-week trial of bupropion hydrochloride extended-release in patients with major depressive disorder with atypical features. Pharmacopsychiatry 46: 221–226. [DOI] [PubMed] [Google Scholar]
- Settle E., Stahl S., Batey S., Johnston J., Ascher J. (1999) Safety profile of sustained-release bupropion in depression: Results of three clinical trials. Clin Ther 21: 454–463. [DOI] [PubMed] [Google Scholar]
- Spier S. (1998) Use of bupropion with SRIs and venlafaxine. Depress Anxiety 7(2:73–75. [PubMed] [Google Scholar]
- Stahl S., Pradko J., Haight B., Modell J., Rockett C., Learned-Coughlin S. (2004) A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Primary Care Comp J Clin Psychiat 6: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens D., Doraiswamy P., McQuoid D. (2001) Bupropion SR in the naturalistic treatment of elderly patients with major depression. Int J Geriatr Psychiat 16: 862–865. [DOI] [PubMed] [Google Scholar]
- Taylor D., Paton C., Kapur S. (2015) The Maudsley Prescribing Guidelines in Psychiatry (12 ed). London, UK: Wiley Blackwell. [Google Scholar]
- Thase M., Clayton A., Haight B., Thompson A., Modell J., Johnston J. (2006) A double-blind comparison between bupropion XL and venlafaxine XR: Sexual functioning, antidepressant efficacy and tolerability. J Clin Psychopharmacol 26: 482–488. [DOI] [PubMed] [Google Scholar]
- Tomarken A., Dichter G., Freid C., Addington S., Shelton R. (2004) Assessing the effects of bupropion SR on mood dimensions of depression.J Affect Dis 78: 235–241. [DOI] [PubMed] [Google Scholar]
- Trivedi M., Fava M., Wisniewski S., Thase M., Quitkin F., Warden D., et al. (2006a) Medication augmentation after the failure of SSRIs for depression. N Engl J Med 354: 1243–1252. [DOI] [PubMed] [Google Scholar]
- Trivedi M., Rush A., Wisniewski S., Nierenberg A., Warden D., Ritz L., et al. (2006b) Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiat 163: 28–40. [DOI] [PubMed] [Google Scholar]
- Walker P., Cole J., Gardner E., Hughes A., Johnston J., Batey S., et al. (1993) Improvement in fluoxetine-associated sexual dysfunction in patients switched to bupropion. J Clin Psychiat 54: 459–465. [PubMed] [Google Scholar]
- Weihs K., Houser T., Batey S., Ascher J., Bolden-Watson C., Donahue R., et al. (2002) Continuation phase treatment with bupropion SR effectively decreases the risk for relapse of depression. Biol Psychiat 51: 753–761. [DOI] [PubMed] [Google Scholar]
- Weihs K., Settle E., Jr, Batey S., Houser T., Donahue R., Ascher J. (2000) Bupropion sustained release versus paroxetine for the treatment of depression in the elderly. J Clin Psychiat 61: 196–202. [DOI] [PubMed] [Google Scholar]
- Weisler R., Johnston J., Lineberry C., Samara B., Branconnier R., Billow A. (1994) Comparison of bupropion and trazodone for the treatment of major depression. J Clin Psychopharmacol 14: 170–179. [PubMed] [Google Scholar]
- Weissman M., Wickramaratne P., Pilowsky D., Poh E., Batten L., Hernandez M., et al. (2015) Treatment of maternal depression in a medication clinical trial and its effect on children. Am J Psychiat 172: 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J., Link M., Rosenthal N., Terman M. (1988) Structured Interview Guide for the Hamilton Depression Rating Scale — Seasonal Affective Disorders Version (SIGH-SAD). New York: New York State Psychiatric Institute. [Google Scholar]
- Zimmerman M., Posternak M., Friedman M., Attiullah N., Baymiller S., Boland R., et al. (2004) Which factors influence psychiatrists’ selection of antidepressants? Am J Psychiat 161: 1285–1289. [DOI] [PubMed] [Google Scholar]