Abstract
The Brugada syndrome (BrS) is an inherited cardiac arrhythmia syndrome first described as a new clinical entity in 1992. Electrocardiographically characterized by distinct coved type ST segment elevation in the right precordial leads, the syndrome is associated with a high risk for sudden cardiac death in young adults, and less frequently in infants and children. The ECG manifestations of the BrS are often concealed and may be unmasked or aggravated by sodium channel blockers, a febrile state, vagotonic agents, as well as by tricyclic and tetracyclic antidepressants. An implantable cardioverter defibrillator (ICD) is the most widely accepted approach to therapy. Pharmacological therapy is designed to produce an inward shift in the balance of currents active during the early phases of the right ventricular action potential and can be used to abort electrical storms or as an adjunct or alternative to device therapy when use of an ICD is not possible. Isoproterenol, cilostazol and milrinone boost calcium channel current and drugs like quinidine, bepridil and the Chinese herb extract Wenxin Keli inhibit the transient outward current, acting to diminish the action potential (AP) notch and thus to suppress the substrate and trigger for VT/VF. Radiofrequency ablation of the right ventricular outflow tract epicardium of BrS patients has recently been shown to reduce arrhythmia-vulnerability and the ECG-manifestation of the disease, presumably by destroying the cells with more prominent AP notch. This review provides an overview of the clinical, genetic, molecular and cellular aspects of the BrS as well as the approach to therapy.
1. Clinical Characteristics and Diagnostic Criteria
The Brugada syndrome typically manifests in the third or fourth decade of life (average age of 41±15 years), although patients have been diagnosed with the syndrome at an age as young as 2 days and as old as 84 years. Prevalence of BrS ECG in the general population varies significantly among continents, countries and ethnic groups.1–22 The prevalence of the disease is highest in Southeast Asia where the syndrome is endemic, estimated to be greater than 5 per 10,000 inhabitants.23 In Japan, a Brugada syndrome ECG (Type 1) is observed in 12 per 10,000 inhabitants; Type 2 and 3 ECGs, which are not diagnostic of BrS, are much more prevalent, appearing in 58 per 10,000 inhabitants.24 The true prevalence of the disease in the general population is difficult to estimate because the ECG pattern is often concealed.
Sudden unexplained nocturnal death syndrome (SUNDS also known as SUDS) and Brugada syndrome have been shown to be phenotypically, genetically and functionally the same disorder.25 Most patients with a Brugada ECG are asymptomatic, usually diagnosed incidentally and they often remain asymptomatic for life. A small minority develop, nocturnal agonal breathing, syncope and/or palpitations, leading to ventricular tachycardia/fibrillation (VT/VF) or SCD. Approximately 20% of BrS patients also develop supraventricular arrhythmias, including atrial flutter, fibrillation, AV nodal reentry and pre-excitation syndromes such as WPW syndrome. Atrial fibrillation (AF) is reported in approximately 10–20% of cases. AV nodal reentrant tachycardia (AVNRT) and Wolf-Parkinson-White (WPW) syndrome have been described by Eckart et al. 26 Prolonged sinus node recovery time and sino-atrial conduction time 27 as well as slowed atrial conduction and atrial standstill have been reported in association with the syndrome. 28 Ventricular inducibility is positively correlated with a history of atrial arrhythmias 29. The incidence of atrial arrhythmias is 27% in Brugada syndrome patients with an indication for ICD vs 13% in patients without an indication for ICD, suggesting a more advanced disease process in patients with spontaneous atrial arrhythmias. 29
The Brugada syndrome is characterized by an ST segment elevation in the right precordial leads. Three types of ST segment elevation are generally recognized 30–32. Type 1 is characterized by a coved ST segment elevation ≥ 2 mm (0.2 mV) followed by a negative T wave (Figure 1). The initial guidelines also required one of the following for the definitive diagnosis of BrS: documented, polymorphic VT or VF, a family history of SCD (< 45 years old), coved type ECGs in family members, syncope or nocturnal agonal respiration. In the latest guidelines, clinical symptoms remain important in risk stratification, although they are no longer listed among the diagnostic criteria.33 The diagnosis of BrS hinges exclusively on the presence of a Type 1 ST segment elevation in one or more right-precordial lead (V1–V3), 34 spontaneously or in the presence of provocative agents, regardless of presence or absence of symptoms. This is a major departure from the original recommendation of the 2002 and 2005 Consensus Conferences.31, 32, 35 While this may serve to draw more attention to the syndrome, it disregards the fact that many subjects manifesting a Brugada pattern remain asymptomatic throughout life.
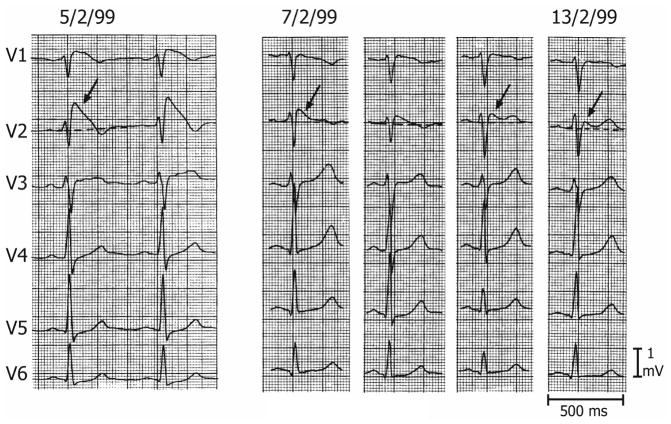
Figure 1.
Three Types of ST segment elevation generally observed in patients with the Brugada syndrome. Shown are precordial leads recorded from a patient diagnosed with the Brugada syndrome. Note the dynamic ECG changes occurring over a period of a week. The left panel shows a clear Type 1 ECG, which is diagnostic of the Brugada syndrome. A saddleback ST segment elevation (Type 2) is observed a day later. The ST segment is further normalized a week later, showing a Type 3 ECG. Modified from 31, with permission.
Patients with a Brugada Type 1 ECG have an approximate cardiac event-rate/year of 7.7% in patients with aborted SCD, 1.9% in patients with syncope, and 0.5% in asymptomatic patients.36 Sodium channel blockers, including ajmaline, procainamide, flecainide, disopyramide, propafenone and pilsicainide are useful in the differential diagnosis when ST segment elevation is not diagnostic under baseline conditions.37–41 However a negative INa-block test does not exclude a latent form of BrS (e.g. negative predictive value of flecainide-testing is 36%).40, 42 Ajmaline and pilsicainide seem to be more effective tools in unmasking BrS, compared to flecainide 43, 44 or procainamide38. The electrocardiographic manifestations of the Brugada syndrome when concealed can be also be unmasked by bradycardia, febrile state or with vagotonic agents. 37, 45–50
Placement of the right precordial leads in a superior position (one or two intercostal space above normal) can increase the sensitivity of the ECG for detecting the Brugada phenotype in some patients, both in the presence or absence of a drug challenge. 51–53,54–56
There are several conditions that produce a Brugada-like ECG-morphology, which should be distinguished from Brugada syndrome. They can be divided into two main categories: acute and persistent conditions. The most commonly observed acute conditions are acute coronary events, pericarditis, myocarditis, pulmonary embolism, metabolic disorders, impaired ion-balance, dissecting aorta aneurism, thiamine deficiency, electric shock and certain pharmacologic agents (see below)57. These manifestations usually disappear with cessation of the provoking event. Most common permanent conditions producing right precordial ST-elevation are: left ventricular hypertrophy, athlete’s heart, right bundle branch block (RBBB), pectus excavatum, septal hypertrophy, arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D), autonomic nervous system abnormalities, Duchenne-dystrophy, Friedreich’s ataxia, mediastinal tumor and Chagas disease31, 58–60. Some investigators have labelled these “phenocopies” indicating that they mimic the phenotype without a genetic predisposition. We would prefer to avoid this term because data as to a genetic predisposition is lacking in almost all cases.
Additionally, inappropriate settings of the recording device can be misleading. In some circumstances, highly-set low cut filters can lead to “false” ST-elevation or altered shape.58, 61, 62
Differentiating RBBB from BrS is not always easy, particularly because RBBB has been reported to mask BrS.63, 64 Pre-excitation of the right ventricle has been shown to be helpful in unmasking BrS under these circumstances. Criteria have been proposed to distinguish RBBB from BrS ECG pattern 58, 65, 66. Thirty-eight consecutive patients with either Type 2 or Type 3 Brugada patterns that were referred for sodium block challenge were included in the study. Before administration of the provocative agent, α and β angles alone and in combination with QRS duration were measured from ECG leads V1 and/or V2 showing incomplete RBBB:
α angle was defined as the angle between a vertical line and the down slope of the r'-wave. Patients with BrS displayed wider α angles than patients with incomplete RBBB.
β angle was defined as the angle between the upslope of the S-wave and the down slope of the r'-wave. The mean β angle was significantly smaller in the 14 patients with incomplete RBBB than the 24 patients with BrS (positive response to sodium block) 36 ± 20° vs. 62 ± 20° (p < 0.01), respectively.
When the angles were combined with QRS duration, it improved discrimination. We must await further tests of the utility and validity of these diagnostic tools.
While most cases of BrS display prominent J waves, often appearing as ST segment elevation, limited to the right precordial leads, isolated cases of inferior lead 67 or left precordial lead 68 ST segment elevation have been reported in Brugada-like syndromes, in some cases associated with SCN5A mutations 69. Coexistence of inferolateral early repolarization (ER) in BrS is not uncommon and is a recently identified risk factor for ventricular fibrillation.70,71, 72 However, in another interpretation, the presence of ER in inferior, lateral and anterior (right precordial) leads is referred to as global ER and designated as Type 3 early repolarization pattern (ERS3).73
Minor prolongation of the QT-interval may accompany ST segment elevation in the Brugada syndrome .41, 74, 75 The QT-interval is prolonged more in the right vs. left precordial leads, probably due to a preferential prolongation of action potential duration (APD) in RV epicardium secondary to accentuation of the action potential notch. 76, 77 Depolarization abnormalities including prolongation of P wave duration, PR- and QRS-intervals are frequently observed, particularly in patients linked to SCN5A mutations.78 PR prolongation likely reflects HV conduction delay. 74
Risk stratification in BrS
Because patients with BrS have a relatively low annual rate of cardiac events, risk stratification is critical in determination of appropriate therapy.
I. Commonly accepted risk factors
Spontaneous Type 1 BrS ECG
History of cardiac events or syncope likely due to VT/VF33, 36, 79, 80
Aborted sudden cardiac death
Documented VT/VF
Nocturnal agonal respiration
Late potentials on epicardial bipolar electrogram or SAECG 81–87
T wave amplitude variability 82
Short ventricular refractory period (VRP < 200 ms) 79
Prolonged QRS duration 89
Early repolarization pattern in the inferolateral leads. 71, 72
II. Controversial risk stratifiers
Family history
There is currently no consensus regarding its prognostic value. The most recent studies have denied its usefulness in the prediction of major arrhythmic events.36, 80, 90, 91
Inducible VT/VF
Inducibility of VT/VF using programmed electrical stimulation (PES) was considered to be a reliable predictor of arrhythmic events in early studies, more recent comprehensive clinical studies have discounted its value. Although the discrepancy between the different studies derives partly from the fact that there is no consensus protocol for PES in BrS, in the latest guidelines inducibility by PES is not considered to have a reliable prognostic value.36, 79, 80, 90–94 It is noteworthy, that when PES is limited to one or two extrastimuli, the results are more prognostic than with triple extrastimuli.95
III. Promising new risk stratifiers
2. Genetic Basis
Inheritance of the Brugada syndrome is via an autosomal dominant mode of transmission. Mutations in 19 genes have been identified as associated with the Brugada phenotype (Table 1). These mutations cause either a decrease in inward sodium or calcium current or an increase in outward potassium currents resulting in an outward shift in the balance of current active during the early phases of the action potential.
Table 1.
Brugada Syndrome Susceptibility Genes.
| Locus | Gene | Ion Channel | % of Probands | |
|---|---|---|---|---|
| BrS1 | 3p21 | SCN5A, Nav1.5 | ↓ INa | 11–28% |
| BrS2 | 3p24 | GPD1L | ↓ INa | Rare |
| BrS3 | 12p13.3 | CACNA1C, Cav1.2 | ↓ ICa | 6.6% |
| BrS4 | 10p12.33 | CACNB2b, Cavβ2b | ↓ ICa | 4.8% |
| BrS5 | 19q13.1 | SCN1B, Navβ1 | ↓ INa | 1.1% |
| BrS6 | 11q13–14 | KCNE3, MiRP2 | ↑ Ito | Rare |
| BrS7 | 11q23.3 | SCN3B, Navβ3 | ↓ INa | Rare |
| BrS8 | 12p11.23 | KCNJ8, Kir6.1 | ↑ IK-ATP | 2% |
| BrS9 | 7q21.11 | CACNA2D1, Cavα2δ | ↓ ICa | 1.8% |
| BrS10 | 1p13.2 | KCND3, Kv4.3 | ↑ Ito | Rare |
| BrS11 | 17p13.1 | RANGRF, MOG1 | ↓ INa | Rare |
| BrS12 | 3p21.2-p14.3 | SLMAP | ↓ INa | Rare |
| BrS13 | 12p12.1 | ABCC9, SUR2A | ↑ IK-ATP | Rare |
| BrS14 | 11q23 | SCN2B, Navβ2 | ↓ INa | Rare |
| BrS15 | 12p11 | PKP2, Plakophillin-2 | ↓ INa | Rare |
| BrS16 | 3q28 | FGF12, FHAF1 | ↓ INa | Rare |
| BrS17 | 3p22.2 | SCN10A, Nav1.8 | ↓ INa | 16.7% |
| BrS18 | 6q | HEY2 (transcriptional factor) | ↓ INa | Rare |
| BrS19 | 7p12.1 | SEMA3A, Semaphorin | ↑ Ito | Rare |
1) Mutations causing a loss of function of sodium channel current
SCN5A
The first gene to be linked to the Brugada syndrome is SCN5A, the gene encoding for the α-subunit of the voltage-gated cardiac sodium channel (Nav1.5) 106. To date more than 300 BrS-related mutations in SCN5A have been described 107–109, accounting for the vast majority of BrS genotype-positive cases but only 11 to 28% of total BrS probands.110 Several of these mutations have been functionally expressed and shown to cause a loss-of-function of INa.
GPD1-L
More than a decade ago, Weiss et al. described a new BrS-associated locus, near to SCN5A.111 The locus was later identified to be the GPD1-L gene, encoding the glycerol-3-phosphat dehydrogenase 1 -like protein112, which has been found to be in close structural and functional association with NaV1.5. 113. Impaired enzymatic activity leads eventually to decreased INa, via GPD1L-dependent phosphorylation of Nav1.5. 114
SCN1B
BrS-related mutations in SCN1B gene, encoding the auxiliary NaVβ1 subunit of the voltage-gated cardiac sodium channel, was first identified to cause a loss function of peak INa by Watanabe et al. 115 In a subsequent study, co-expression of mutant SCN1B with WT-SCN5A and WT-KCND3 (separately) induced a 55.6% decrease in peak INa and 70.6% gain of function in Ito, moreover, co-immunoprecipitation revealed structural association between NaVβ1B, NaV1.5 and KV4.3, suggesting that the elevated level of transient outward potassium current is predominantly responsible for pathogenesis in these cases of BrS.116
SCN3B
Hu et al. reproted missense mutations in SCN3B encoding the Navβ3 subunit of the cardiac sodium channel, which led to a decrease in peak sodium current density, accelerated inactivation, and slowed reactivation. The study revealed that the mutation in this subunit impaired the intracellular transport and cell surface expression of the cardiac sodium channel. 117 A further study by Ishikawa et al confirmed these findings. 118
SCN2B
The SCN2B gene encodes the β2-subunit of the cardiac sodium channel. Mutation in the gene leads to a significant reduction in sodium current density due to a decreased Nav1.5 cell surface expression119.
SCN10A
Hu et al recently identified SCN10A as a major susceptibility gene for BrS. The gene encodes Nav1.8, a neuronal sodium channel, which appears to play a role in the heart. The study showed that coexpression of SCN5A-WT with SCN10A-WT results in a gain of function in INa, whereas co-expression of SCN5A-WT with SCN10A-mutant leads to a major loss of function in INa, thus contributing to the manifestation of Brugada syndrome 110. Previous studies (including genome-wide association studies) have associated SCN10A variants with BrS, although the prinipal loci were intronic. 120 Othere GWAS studies have idenitifed SCN10A variants with altered cardiac function and arrhythmogenesis, suggesting that Nav1.8 plays an important role in the electrical function of the heart in both health and disease.121–129 With the identification of SCN10A as BrS susceptibility gene in 16.7% of probands, potentially causative gene mutations can now be detected in more than 50% of BrS patients, further enhancing the impact of genotyping in risk stratification and in screening of family members.
HEY2
The trascriptional factor HEY2 was also identified as associated with BrS in the GWAS study of Bezzina et al. 120, 130,131
FGF12 is another recently idetified BrS susceptibility gene. FGF12 encodes for a fibroblast growth factor homologous factor (FHF-1). 132 FHFs exert modulatory effects on cardiac sodium -and calcium-channels.133,134 A Q7R missense mutation in FGF12, associated with BrS, has been shown to reduce INa. 132
PKP2
Mutations in PKP2, encoding the desmosomal protein plakophillin-2, a known susceptibility gene for arrhyhtmgenic right ventriccualr cardiomypathy (ARVC), has been associated with BrS. The disruption of desmosomes has been shown to result in loss of sodium channel function.135
RANGRF
Missense mutations in RANGRF, encoding MOG1, a protein known to modulate NaV1.5, have been associated with BrS and shown to reduce cardiac INa due to impaired trafficking of the sodium-channel.136, 137
SLMAP
Mutations in the sarcolemmal membrane-associated protein (SLMAP), a component of T-tubules and sarcoplasmic reticulum with unknown functional role, have been associated with BrS and shown to impair Nav1.5 trafficking, thus causing a loss of function in INa. 138
2) Mutations causing a loss of function of calcium channel current
CACNA1C
Loss of function mutations in CACNA1C, encoding the α-subunit of the human L-type voltage-gated calcium channel, CaV1.2, have been associated with many cases of BrS.139, 140
CACNB2B, a gene that encodes the β-subunit of CaV1.2, Cavβ2, is involved in regulation and intracellular trafficking of ICaL.141, 142 Several loss of function mutations in ICaL function have been associated with BrS. 140, 143,139, 140
CACNA2D1 encodes the α2β-subunit of the voltage-dependent calcium channel and shares similar functional properties with Cavβ2144. Loss of function mutations in this gene are reported to contribute to SQTS, IVF, ERS and BrS. 139
3) Mutations causing a gain of function of potassium channel currents
KCNE3
Gain of function mutations in KCNE3 (MiRP2) have been asssocaited with BrS. The functional role of MiRP2 is modulation of several cardiac potassium currents, including Ito and IKs. Co-expression of KCNE3 mutations with WT-KCND3 leads to a gain-of-function and accelerated kinetics of Ito.145
KCND3
Gain of function mutation in KCND3 has been also associated to BrS and shown to increase Ito. KCND3 encodes Kv4.3, the α-subunit of the Ito channel. 146
SCN1B
As dicussed above, BrS-related mutations in SCN1B gene, encoding the auxiliary NaVβ1 subunit of the voltage-gated cardiac sodium channel, in addition to reducing INa can also increase Ito when co-expressed with WT-KCND3. 116
KCNJ8
Mutations in KCNJ8, encoding Kir6.1, have been reported to result in gain-of-function in IK-ATP, a channel which, under normoxic conditons, is closed. This leads to accentuation of the action potenial notch as well as depression of the plateau, leading to BrS phenotype or SQTS phenotype due to abbreivation of action potential duration. 147–149
ABCC9
Mutations in ABCC9, encoding SUR2A, the ATP-binding cassette transporter of the IK-ATP channel, have been recently identified as causative of BrS.150 Mutations in ABCC9 can reduce the ATP-sensitivity of the receptor-complex, resulting in a gain of function due to reduced tonic inhibitory effect of ATP.
4) Other candidate cenes
TRPM4
The transient receptor potential melastatin protein 4 gene (TRPM4), which encodes a calcium-activated non-selective cation channel has been proposed as a BrS-susceptibility gene. Mutations causing both a gain- and loss of function of the channel have been associated with BrS. The cellular basis as well its role in the pathophysiology of BrS requires further clarification. 151
5) Genetic variants capable of modulating but not necessarily causing BrS
KCNE5
encodes one of the regulatory β-subunits of the Ito and IKs channels. Mutations in this gene can modualte expression of BrS by increasing Ito. 152 Evidence is currently insufficient to demonstrate causation.
KCNH2
Gain of function mutation in KCNH2, encoding the α-subunit of the rapid delayed rectifier potassium channel (hERG), results in increased IKr current, giving rise to SQTS and modulating the expression of BrS.153 Evidence is currently insufficient to demsontrate causality.
HCN4
Loss of function mutations in HCN4 encoding the hyperpolarization-activated cyclic nucleotide-gated channel 4 protein, the main monomer-isoform constituting the tetramer-complex of the human cardiac pacemaker channel (If), has been associated with BrS. 154–156 Reduction in If can lead to bradycardia, which is known to accentuate or unmask the BrS phenotype 107. HCN4 (and HCN2) are mainly expressed in cells with high automaticity (SA-node > AV-node > His-Purkinje-system≫>working myocardium). 154, 157 Their very low expression level in ventricular myocardium argues against their direct invovlemet in causing BrS.
Role of Genetic Testing
Genetic testing is recommended for support of the clinical diagnosis, for early detection of relatives at potential risk, and particularly for the purpose of advancing research and consequently our understanding of genotype-phenotype relations. The role of genetic markers in risk stratification of BrS is a matter of debate. 158 According to the latest guidelines (HRS/EHRA consensus statement) 159, genetic testing is recommended (Class I) for relatives of an index case with an identified BrS-causative mutation. Genetic testing can be useful (Class II) “for any patient in whom a cardiologist has established a clinical index of suspicion for BrS based on examination of the patient’s clinical history, family history and expressed electrocardiographic (resting 12-lead ECGs and/or provocative drug challenge testing) phenotype”. On the other hand, the latest CCS/CHRS joint position paper 160 and other studies 156 suggest that a Type 1 BrS ECG alone should be enough of an indication (Class I recommendation) for genetic testing. In individuals expressing isolated Type 2 or 3 BrS pattern, genetic testing has a Class III recommendation. 159 Although identification of a genotype may not be helpful in the approach to therapy at present time, it could be argued that with additional evidence, some genotypes may offer innovative therapeutic strategies, e.g., use of IK-ATP blockers in cases involving a gain of function of IK-ATP or use of IKr blockers in cases of IKr gain of function.
3. Cellular and Ionic Basis
An outward shift in the balance of currents active during phases 1 and 2 of the epicardial action potential via either a reduction of inward current (INa or ICa ) or increase in outward current (IKr or IK-ATP), allows the already prominent Ito to accentuate phase 1 repolarization (Figs. 2 and 3). When phase-1 is repolarized beyond the voltage range at which L-type Ca+2 channels activate, the Ca+2 channels fail to activate, resulting in loss of the action potential plateau, predominantly in the right ventricular subepicardial cells where Ito is most prominent .
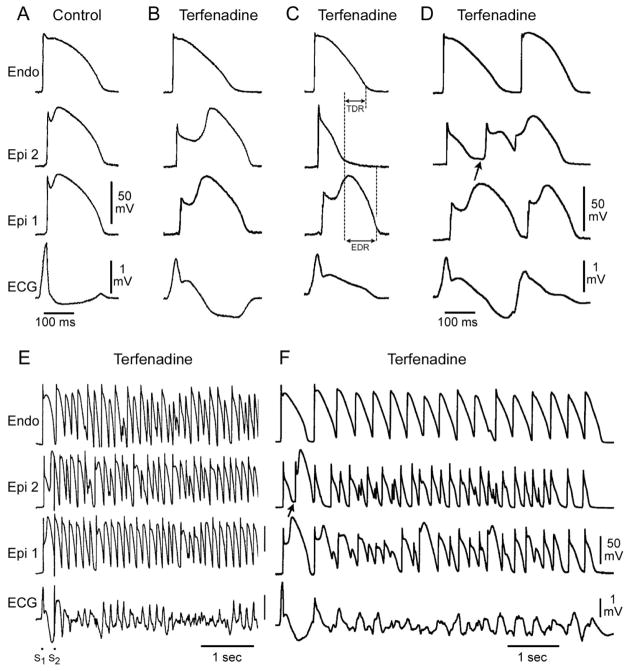
Figure 2.
Cellular basis for electrocardiographic and arrhythmic manifestation of BrS. Each panel shows transmembrane action potentials from one endocardial (top) and two epicardial sites together with a transmural ECG recorded from a canine coronary-perfused right ventricular wedge preparation. A: Control (Basic cycle length (BCL) 400 msec). B: Combined sodium and calcium channel block with terfenadine (5 μM) accentuates the epicardial action potential notch creating a transmural voltage gradient that manifests as an ST segment elevation or exaggerated J wave in the ECG. C: Continued exposure to terfenadine results in all-or-none repolarization at the end of phase 1 at some epicardial sites but not others, creating a local epicardial dispersion of repolarization (EDR) as well as a transmural dispersion of repolarization (TDR). D: Phase 2 reentry occurs when the epicardial action potential dome propagates from a site where it is maintained to regions where it has been lost giving rise to a closely coupled extrasystole. E: Extrastimulus (S1–S2 = 250 msec) applied to epicardium triggers a polymorphic VT. F: Phase 2 reentrant extrasystole triggers a brief episode of polymorphic VT. (Modified from reference169, with permission)
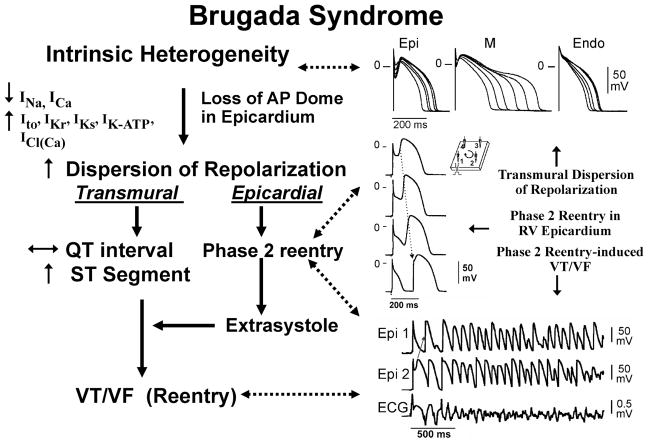
Figure 3.
Proposed mechanism for the Brugada syndrome. An outward shift in the balance of currents serves to amplify existing heterogeneities by causing loss of the action potential dome at some epicardial, but not endocardial sites. A vulnerable window develops as a result of the dispersion of repolarization and refractoriness within epicardium as well as across the wall. Epicardial dispersion leads to the development of phase 2 reentry, which provides the extrasystole that captures the vulnerable window and initiates VT/VF via a circus movement reentry mechanism. Modified from 261, with permission.
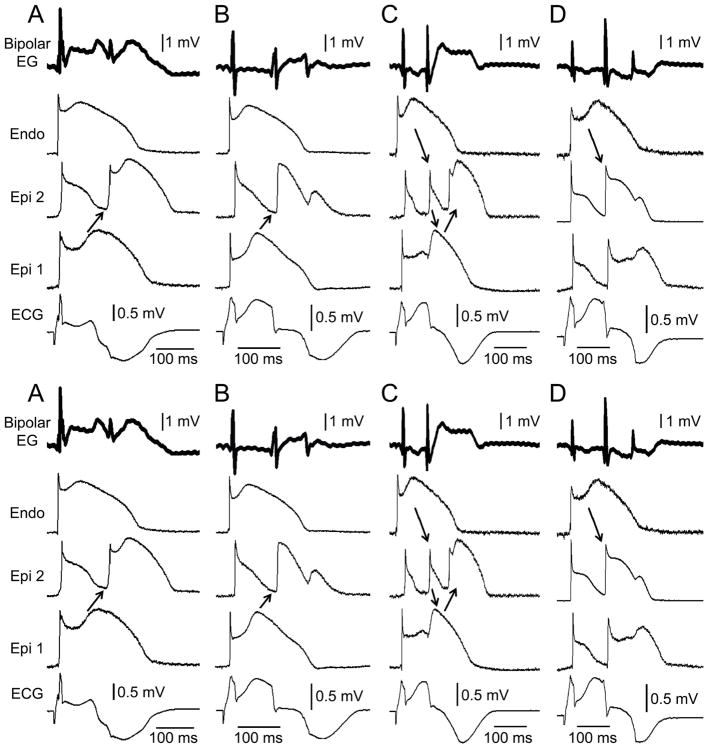
Conduction of the AP dome from epicardial sites at which it is maintained to sites at which it is lost results in the development of phase 2 reentry, giving rise to a very closely coupled extrasystole.77 Interestingly, these repolarization abnormalities give rise to low voltage fractionated electrogram activity (Figure 4) and high frequency late potentials when a bipolar electrogram is recorded in the region of the RVOT (Figure 5).161 The low voltage fractionated electrogram activity is due to dysynchrony in the appearance of the epicardial action potential dome secondary to accentuation of the action potential notch and the high frequency late potentials are due to concealed phase 2 reentry.
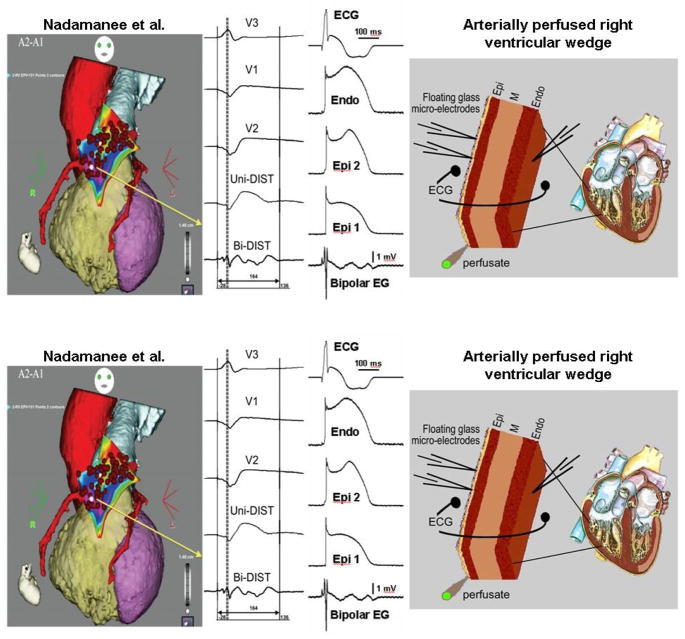
Figure 4.
Heterogeneities in the appearance of the epicardial action potential second upstroke gives rise to fractionated epicardial electrogram (EG) activity in the setting of Brugada syndrome (BrS). Left panel: Shown are right precordial lead recordings and unipolar and bipolar EGs recorded form the right ventricular outflow tract of a BrS patient (from Nademanee et al. 83). Right panel: ECG, action potentials from endocardium (Endo) and two epicardial (Epi) sites, and a bipolar epicardial EG (Bipolar EG) all simultaneously recorded from a coronary-perfused right ventricular wedge preparation treated with the Ito agonist NS5806 (5 μM) and the calcium channel blocker verapamil (2 μM) to induce the Brugada phenotype. Basic cycle length=1000 ms. Reproduced from161, with permission.
Figure 5.
Concealed phase 2 reentry as the basis for late potential and fractionated bipolar epicardial (Epi) electrogram (Bipolar EG) activity in an experimental model of Brugada syndrome. Each panel shows (from top to bottom) a Bipolar EG, action potentials recorded from endocardium (Endo) and two Epi sites and an ECG all simultaneously recorded from a coronary-perfused right ventricular wedge preparation exposed to NS5806 (5 μM) and verapamil (2 μM) to induce the Brugada phenotype. Heterogeneous loss of the dome at epicardium caused local re-excitation via a ‘concealed’ phase 2 re-entry mechanism, leading to the development of late potentials and fractionated bipolar epicardial EG activity. No major delays in conduction of the primary beat were ever observed. Each panel shows results from a different preparation. Basic cycle length=1000 ms. Reproduced from 161, with permission.
The ability of the right ventricular action potential to lose its dome, giving rise to phase 2 reentry and other characteristics of the Brugada syndrome were identified in the early 1990’s and evolved in parallel with the clinical syndrome. 162–165
The development of prominent J waves, appearing as ST segment elevation, in the right precordial leads of BrS patients is believed to be due to accentuation of the right ventricular epicardial action potential notch secondary to the rebalancing of the currents active at the end of phase 1 (see 166 for references). A spike and dome morphology due to a prominent transient outward current (Ito), or notch, in ventricular epicardium, but not endocardium, generates a transmural voltage gradient that inscribes the electrocardiographic J wave in larger mammals and in man. The ST segment is typically isoelectric because of the absence of transmural voltage gradients at the level of the action potential plateau. Under pathophysiologic conditions such as those associated with BrS, accentuation of the right ventricular action potential notch leads to exaggeration of transmural voltage gradients and thus to accentuation of the J wave, causing an apparent ST segment elevation. 166 The repolarization waves take on a saddleback or coved appearance depending on the timing of repolarization of epicardium relative to endocardium. A delay in epicardial activation and repolarization time leads to progressive inversion of the T wave.
Accentuation of the action potential notch in the right ventricular outflow tract (RVOT) can give rise to the typical Brugada ECG without creating an arrhythmogenic substrate, as illustrated in Figure 2B. The arrhythmogenic substrate could then arise with a further shift in the balance of currents causing loss of the action potential dome at some epicardial sites but not others. Disappearance of the action potential dome in epicardium but not endocardium results in marked transmural dispersion of repolarization and refractoriness, which is responsible for the development of a vulnerable window, as illustrated in Figure 2C. Loss of the epicardial action potential dome is usually heterogeneous, leading to the development of epicardial dispersion of repolarization. Propagation of the action potential dome from RVOT sites at which it is maintained to sites at which it is lost can cause local re-excitation via a phase 2 reentry mechanism (P2R). The phase 2 reentrant extrasystole is often concealed because it is surrounded by refractory tissue. These concealed P2Rs are responsible for the appearance of high frequency late potentials (Figure 5) in epicardial electrograms recorded over these epicardial sites in patients with BrS.161 When the P2R succeeds in propgating out of its protected focus and capturing the vulnerable window, it can trigger a circus movement reentry, usually manifest as polymorphic VT/VF (Figure 2F). 167–169 The phase 2 reentrant beat fuses with the negative T wave of the basic response. Support for these hypotheses derives in part from experiments involving arterially perfused right ventricular wedge preparations as well as from studies such as those of Kurita et al. who obtained monophasic action potential (MAP) recordings form the epicardial and endocardial surfaces of the right ventricular outflow tract (RVOT) of patients with the Brugada syndrome.164, 170 Figure 2E illustrates an example of programmed electrical stimulation-induced VT/VF under similar conditions.
Although the genetic mutations are equally distributed between the sexes, the clinical phenotype is 8 to 10 times more prevalent in males than in females. The basis for this sex-related distinction was shown to be due to a more prominent Ito-mediated action potential notch in the right ventricular (RV) epicardium of males vs. females.171 The more prominent Ito causes the end of phase 1 of the RV epicardial action potential to repolarize to more negative potentials in tissue and arterially perfused wedge preparations from males, facilitating loss of the action potential dome and the development of phase 2 reentry and polymorphic VT. The gender distinction is not seen in all families; some authors described families without a male predominance of the Brugada phenotype.172
Available data support the hypothesis that the Brugada syndrome is the result of amplification of heterogeneities intrinsic to the early phases of the action potential among the different transmural cell types. This is due to a rebalancing of currents active during phases 1 and 2 of the acion potential secondary to a decrease in INa or ICa or augmentation of outward currents including IKr, IKs, ICl(Ca) or Ito (Figure 3). The accentuation of the action potential notch leads to elevation of the ST segment, eventually leading to loss of the action potential dome in right ventricular epicardium, where Ito is most prominent. Loss of the dome causes a transmural as well as epicardial dispersion of repolarization. The transmural dispersion contributes to development of ST segment elevation and the generation of a vulnerable window across the ventricular wall, whereas the epicardial dispersion leads to development of phase 2 reentry, thus providing a closely coupled extrasystole that captures the vulnerable window and precipitates VT/VF. The polymorphic VT generated resembles a rapid form of Torsade de Pointes (Figures 2F and 3).
The cellular mechanisms underlying Brugada syndrome have long been a matter of debate 173, 174. Two principal hypotheses have been advanced: 1) The repolarization hypothesis maintains that an outward shift in the balance of currents in right ventricular epicardium leads to repolarization abnormalities resulting in the development of phase 2 reentry, which generates closely-coupled premature beats capable of precipitating VT/VF; 2) The depolarization hypothesis maintains that slow conduction in the right ventricular outflow tract plays a primary role in the development of the electrocardiographic and arrhythmic manifestations of the syndrome. Although these theories are not mutually exclusive and may indeed be synergistic, from the standpoint of appropriate therapy, correct assessment of the cellular pathophysiology is important.
To date, the most compelling evidence in support of the depolarization hypothesis was the demonstration by Nademanee et al.83 of late potentials and fractionated electrogram (EGs) recorded from the right ventricular outflow tract (RVOT) of BrS using bipolar electrograms. They further demonstrated that radiofrequency (RF) ablation these epicardial sites significantly reduced the arrhythmia-vulnerability and ECG-manifestation of the disease. These authors concluded that the high frequency late potential (LP) and low voltage fractionated electrogram activity are due to conduction delays within the RVOT and elimination of the sites of slow conduction is the basis for the ameliorative effect of ablation therapy83. In a direct test of this hypothesis, our group recently suggested an alternative cellular electrophysiological mechanism as the basis for late potentials and fractionated electrogram activity in the setting of BrS.161 Using the coronary-perfused wedge model of BrS, Szel et al. 161 demonstrated that the high frequency late potentials are due to concealed phase 2 reentry and that the low-voltage fractionated electrogram activity recorded from the RV epicardium is due to different timing of the second upstroke of the action potential secondary to accentuation of the epicardial action potential notch (Figures 4 and 5).
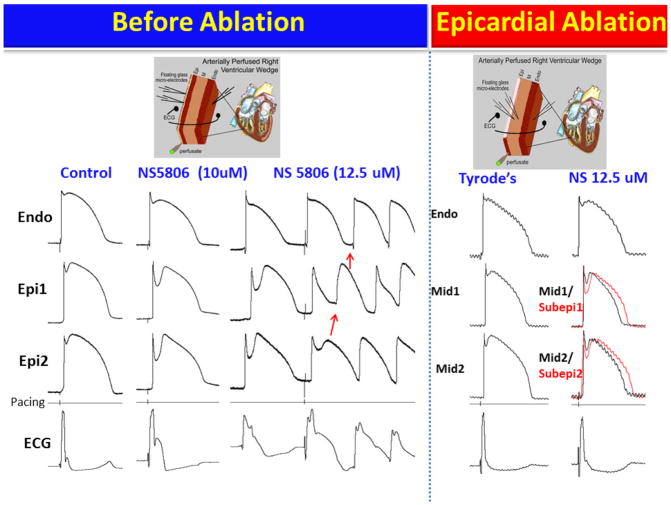
If late potentials and fractionated electrogram activity recorded from the RVOT do not reflect depolarization and conduction abnormalities, what is the basis for the ameliorative effect of RVOT ablation? As we will discuss below, our preliminary data strongly support the hypothesis that ablation destroys the cells with the most prominent action potential notch, thus eliminating the cells responsible for the repolarization abnormalities that give rise to phase 2 reentry and VT/VF (Figure 6).
Figure 6.
Epicardial radiofrequency ablation abolishes the electrographic and arrhythmic manifestations of Brugada syndrome (BrS) in coronary-perfused canine right ventricular wedge-model. Transmembrane action potentials (AP) were simultaneously recorded from one endocardial (Endo) and two epicardial (Epi) sites together with a transmural pseudo-ECG. (The model is schematically illustrated in the top panels). Stimulus marker (pacing) is indicated in the 4th row. All recordings were obtained at 1000 ms basic cycle length.
Column 1: Control. Column 2: Recorded 20 min after the addition of the Ito-agonist NS5806 (10μM) to the coronary perfusate. The much greater accentuation of the Epicardial (Epi) vs. Endocardial (Endo) AP notch was associated with accentuation of the J wave in the ECG. Column 3: Recorded 15 min after increasing the concentration of NS5806 to 12.5 μM. A stimulated premature beat induced VT (later VF) via a phase 2 reentry (P2R) mechanism. The abbreviated cycle length caused loss of the AP dome in Epi1 due to use-dependent block of INa by NS5806. In the Endo and Epi2 sites the dome is maintained and the notch is slightly reduced because slow recovery of Ito overwhelmed the use-dependent inhibition of INa. The pronounced epicardial and transmural dispersion of repolarization created the substrate for P2R and VT/VF. Column 4: Recorded after washout of NS5806 and 70 min after RF ablation of the epicardial surface. APs were recorded from the subepicardial layer (Subepi) just below the ablation border and from the midmyocardium (Mid) because the Epi layer was destroyed. Column 5: Recorded 45 min after re-introduction of NS5806 (12.5μM) to the coronary perfusate. After ablation of epicardium, NS5806 was no longer able to induce the ECG or arrhythmic manifestations of BrS.
Factors that Modulate ECG and Arrhythmic Manifestations of the Brugada Syndrome
ST segment elevation in the Brugada syndrome is often dynamic. The Brugada ECG is often concealed and can be unmasked or modulated by sodium channel blockers, a febrile state, vagotonic agents, α adrenergic agonists, β adrenergic blockers, tricyclic or tetracyclic antidepressants, a combination of glucose and insulin, hyperkalemia, hypokalemia, hypercalcemia, and by alcohol and cocaine toxicity 37, 45, 46, 175–181. A number of precipitants for ventricular arrhythmias have been reported and should be addressed acutely. These agents may also induce acquired forms of the Brugada syndrome (Table 2). All patients should be promptly treated for fevers and avoid drugs that unmask or aggravate BrS. A definitive list of agents to avoid in the Brugada syndrome can be found in the website www.brugadadrugs.org , and are briefly summarized in Table 2.
Table 2.
Drug-induced Brugada-like ECG Patterns
| I. Antiarrhythmic drugs |
| 1. Na+ channel blockers |
| Class IC drugs (Flecainide 37, 38, 262–264, Pilsicainide 265, 266, Propafenone 267) |
| Class IA drugs (Ajmaline 37, 268, Procainamide 37, 46, Disopyramide 31, 46, Cibenzoline 269) |
| 2. Ca2+ channel blockers |
| Verapamil |
| II. Antianginal drugs |
| 1. Ca2+ channel blockers |
| Nifedipine, Diltiazem. |
| 2. Nitrate |
| Isosorbide dinitrate, Nitroglycerine 270. |
| 3. K+ channel openers |
| Nicorandil. |
| III. Psychotropic drugs |
| 1. Tricyclic antidepressants |
| Amitriptyline271, 272, Nortriptyline 177, Desipramine 175, Clomipramine 176. |
| 2. Tetracyclic antidepressants |
| Maprotiline 271. |
| 3. Phenothiazine |
| Perphenazine 271, Cyamemazine., Trifluoperazine272 |
| 4. Other antipsychotics |
| Loxapin 272, |
| 5. Selective serotonin reuptake inhibitors |
| Fluoxetine 272. |
| 6. Anticonvulsives |
| Oxcarbazepine 273 |
| IV.Anesthetic and analgesics |
| 1. Bupivacaine274,275 |
| 2. Procaine 276 |
| 3. Propofol277–279 |
| V. Other drugs |
| 1. Histaminic H1 receptor antagonists |
| Dimenhydrinate 181. |
| 2. Cocaine intoxication 178, 280 |
| 3. Alcohol Intoxication |
| 4. Cannabis 281 |
| 5. Ergonovine 182 |
| 6. Acetilcholine46, 182 |
Modified from44, with permission
Acute ischemia or myocardial infarction due to vasospasm involving the RVOT mimics ST segment elevation similar to that in Brugada syndrome. This effect is secondary to the depression of ICa and the activation of IK-ATP during ischemia, and suggests that patients with congenital and possibly acquired forms of Brugada syndrome may be at a higher risk for ischemia-related sudden cardiac death. 182
VF and sudden death in the Brugada syndrome usually occur at rest and at night. Circadian variation of sympatho-vagal balance, hormones and other metabolic factors likely contribute this circadian pattern. Bradycardia, due to altered symaptho-vagal balance or other factors, may contribute to arrhythmia initiation. 183–185 Abnormal 123I-MIBG uptake in 8 (17%) of the 17 Brugada syndrome patients but none in the control group was demonstrated by Wichter et al.186 There was segmental reduction of 123I-MIBG in the inferior and the septal left ventricular wall indicating presynaptic sympathetic dysfunction. Of note, imaging of the right ventricle, particularly the RVOT, is difficult with this technique, so insufficient information is available concerning sympathetic function in the regions known to harbor the arrhythmogenic substrate. Moreover, it remains unclear what role the reduced uptake function plays in the arrhythmogenesis of the Brugada syndrome. If indeed the RVOT is similarly affected, this defect may alter the symaptho-vagal balance in favor of the development of an arrhythmogenic substrate.168, 187
Kies and coworkers 188 assessed autonomic nervous system function noninvasively in patients with the Brugada syndrome, quantifying myocardial presynaptic and postsynaptic sympathetic function by means of positron emission tomography with the norepinephrine analogue 11C-Hydroxyephedrine (11C-HED) and the nonselective beta-blocker 11C-CGP 12177 (11C-CGP). Presynaptic sympathetic norepinephrine recycling, assessed by 11C-HED, was found to be globally increased in patients with Brugada syndrome compared with a group of age-matched healthy control subjects, whereas postsynaptic β adrenoceptor density, assessed by 11C-CGP, was similar in patients and control. This study provides further evidence in support of an autonomic dysfunction in Brugada syndrome.
Hypokalemia has been implicated as a contributing cause for the high prevalence of SUDS in the Northeastern region of Thailand where potassium deficiency is endemic.189,180 Serum potassium in the Northeastern population is significantly lower than that of the population in Bangkok, which lies in the central part of Thailand, where potassium is abundant in the food. A recent case report highlights the ability of hypokalemia to induce VF in a 60 year old man who had asymptomatic Brugada syndrome, without a family history of sudden cardiac death.180 This patient was initially treated for asthma by steroids, which lowered serum potassium from 3.8 mmol/L on admission to 3.4 and 2.9 mmol/L on the 7th day and 8th day of admission, respectively. Both were associated with unconsciousness. VF was documented during the last episode, which reverted spontaneously to sinus rhythm.
Accelerated inactivation of the sodium channel in SCN5A mutations associated with the Brugada syndrome has been shown to be accentuated at higher temperatures 190, suggesting that a febrile state may unmask the Brugada syndrome by causing loss of function secondary to premature inactivation of INa. Indeed, numerous case reports have emerged since 1999 demonstrating that febrile illness could reveal the Brugada ECG and precipitate VF.47, 191–197 Anecdotal reports point to hot baths as a possible precipitating factor. Of note, the Northeastern part of Thailand, where the Brugada syndrome is most prevalent, is known for its very hot climate.
Oxidative stress has recently been added to the list of modulating factors. Tanaka et al. reported that elevated oxidative stress can modulate the development of VF in SCN5A-negative BrS patients.198 There was no evidence of histological structural changes in this cohort.
5. Approach to Therapy
Table 3 lists device and pharmacologic therapies suggested on the basis of clinical experience or experimental stduies.
Table 3.
Device and Pharmacologic Approach to Therapy of the Brugada Syndrome
| Devices and Ablation |
| ICD 199 |
| Radiofrequency Ablation 83, 208–212 |
| ? Pacemaker 204–206 |
| Pharmacologic Approach to Therapy |
| Ineffective or Proarrhythmic |
| Amiodarone 282 |
| β Blockers 282 |
| Class IC antiarrhythmics |
| Flecainide 38 |
| Propafenone 267 |
| ? Disopyramide 214 |
| Class IA antiarrhythmics |
| Procainamide37 |
| Effective for Treatment of Electrical Storms |
| β Adrenergic agonists – isoproterenol 46, 51, denopamine230, orciprenaline 226, 236 |
| Phosphodiesterase III Inhibitors-cilostazol 235 |
| Effective General Therapy |
| Quinidine 168, 218–220, 231–233 |
| Bepridil - Ito-inhibition and INa augmentation258 |
| Cilostazol combined with bepridil 251 |
| Experimental Therapy |
| Ito Blockers - cardioselective and ion channel specific |
| Quinidine 168 |
| 4-aminopyridine 168 |
| Tedisamil 283 |
| AVE0118 284 |
| PDE-3-inhibitors |
| Cilostazol – Increase in ICa and inhibition of Ito 253, 285 |
| Milrinone - ICa augmentation 253, 285 |
| Traditional Chinese Medicine |
| Dimethyl lithospermate B – Increase in INa due to slowed inactivation |
| Wenxin Keli - combined Ito-block and tyramine-like effect216 |
Device Therapy
Implantable cardioverter defibrillator (ICD)
Implantation of an ICD is first line therapy for patients presenting with aborted SCD or documented VT/VF with or without syncope. 199, 200 The HRS/EHRA/APHRS expert consensus statement 33 recommendations for ICD implantation are illustrated in Table 3 and summarized as follows:
-
Recommended (Class I):
Symptomatic patients presenting with a Type 1 Brugada ECG (spontaneously or after sodium channel blockade) and aborted sudden death or documented VTT/VF should receive an ICD as a Class I indication. Electrophysiologic study (EPS) is recommended in symptomatic patients only for the assessment of supraventricular arrhythmia.
Can be useful (Class IIa) in symptomatic patients with Type I pattern, in whom syncope is presumed to be likely caused by VT/VF.
-
May be considered (Class IIb) in asymptomatic patients inducible by PES.
The annual rate of arrhythmic events in asymptomatic patients is relatively low (0.5% vs. 7.7–10.2% in patients with VF and 0.6–1.2% in patients with syncope). This warrants careful consideration for ICDs in asymptomatic patients36, 158.
ICDs are not indicated (Class III) in asymptomatic patients33.
The validity of HRS/EHRA/APHRS (Class II) recommendation for patients with a history of syncope and spontaneous Type 1 ECG was confirmed by Takagi et al in a multicenter large-cohort study in the Japanese population.201 These authors reported that in patients with Class II indication, a history of syncope in combination with a spontaneous Type 1 ECG may be an important factor in distinguishing intermediate- from low-risk patients with BrS in Japan.
The effectiveness of ICD in preventing sudden cardiac death was 100% in a multicenter trial in which 258 patients diagnosed with Brugada syndrome. Appropriate shocks were delivered in 14%, 20%, 29%, 38% and 52% of cases at 1, 2, 3, 4, and 5 years of follow-up, respectively. In initially asymptomatic patients, appropriate ICD shocks were delivered in 4%, 6%, 9%, 17% and 37% at 1, 2, 3, 4, and 5 years of follow-up, respectively. Subsequent long-term follow-up studies have confirmed the safety and effectiveness of this approach.202, 203
Pacemaker therapy
Despite the fact that life-threatening arrhythmias generally occur during sleep or at rest and associated with slow heart rates, a potential therapeutic role for cardiac pacing remains largely unexplored204 and limited to a few case reports.205, 206
BrS patients who suffer from electrical storms leading to numerous appropriate ICD discharges highlight the need for adjunctive therapy. Before the development of ablation techniques (see below)83 , a heart transplantation was the only option for these patients.207
Ablation therapy
The idea of using ablation to suppress focal arrhythmogenic substrates in BrS stems back to the turn of the century. Several studies reported on the effect of focal endocardial radiofrequency ablation of sites generating monomorphic extrasystoles.208–211
A promising innovation in the treatment of BrS using radiofrequency (RF) ablation was recently reported by Nademanee et al.83 The authors showed that RF ablation of epicardial sites displaying late potentials and fractionated bipolar electrograms (EGs) in the RVOT of BrS patients significantly reduced arrhythmia-vulnerability and ECG-manifestation of the disease. Ablation at these sites was able to render VT/VF non-inducible and to normalize the Brugada ECG pattern in the majority of patients over a period of weeks or months. No recurrent VT/VF was observed over a 20+6 month follow-up, with only 1 patient on medical therapy with amiodarone. Subsequent case reports have been published in support of these effects.212 Ablation therapy may be life-saving in uncontrollable cases, or BrS cases in which ICD therapy is impractical (e.g. contraindications or financial hardship in developing countries). RF ablation was assigned a Class IIb indication in the recent HRS/EHRA/APHRS expert consensus guidelines for BrS-patients with frequent appropriate ICD-shocks due to recurrent electrical storms.33
The cellular basis for the ameliorative effect of epicardial ablation in BrS is a matter of debate. Szel et al.161 showed that late potentials and fractionated electrogram activity are due to concealed phase 2 reentry and dysnchrony in the emergence of the second action potetnial upstroke secondary to heterogeneous accentuation of the epicardial action potential notch (Figure 4 and 5). We hypothesized that RF ablation of the RVOT would eliminate these sites of abnormal repolarization and thus suppress the substrate for developmet of arrhyhtmias. Employing the canine right ventricular wedge model of BrS, we recently conducted studies to provide evidence in support of this hypothesis. As ilustrated in Figure 6, epicardial ablation is observed to destroy the cells with the most prominent action potential notch, thus annihilating the cells responsible for the repolarization abnormalities that give rise to phase 2 reentry and VT/VF213.
Pharmacologic approach to therapy
A pharmacologic approach to therapy has been sought because ICD implantation is not an appropriate solution for infants and young children or for patients residing in regions of the world where an ICD is out of reach because of economic factors. Pharmacologic-mediated therapy aimed at rebalancing the currents active during the early phases of the epicardial action potential in the RVOT so as to reduce the magnitude of the action potential notch and/or restore the action potential dome, has been the focus of much basic and clinical research. Table 3 lists the various pharmacologic agents investigated in recent years. Amiodarone and β blockers have been shown to be ineffective, whereas Class IC antiarrhythmic drugs (such as flecainide and propafenone) and class IA agents, such as procainamide, are contraindicated because of their effects to unmask BrS and to induce life-threatening arrhythmias. Disopyramide, a class IA antiarrhythmic, has been shown to normalize ST segment elevation in some BrS patients but to unmask the syndrome in others. 214
Because a prominent transient outward current, Ito, is central to the mechanism underlying BrS, the most rationale approach to therapy, regardless of the ionic or genetic basis for the disease, is to partially inhibit Ito. Regrettably, cardio-selective and Ito-specific blockers are not currently available. 4-aminopyridine (4-AP), although selective of Ito at low concentrations, is not cardio-selective because it inhibits Ito in the nervous system. It is very effective in suppressing arrhythmogenesis in wedge models of the Brugada syndrome, 168 but is unlikely to be of clinical benefit because of neurally-mediated and other side effects.
The only drug available in the United States and other part of the world with significant Ito blocking properties is quinidine. It is for this reason that we suggested in 1999 that this agent may be of therapeutic value in BrS. Experimental studies have shown quinidine to be effective in restoring the epicardial action potential dome, thus normalizing the ST segment and preventing the development of phase 2 reentry and polymorphic VT, regardless of which pharmacologic agents were used to mimic the BrS-phenotype. 161, 168, 215, 216 Interestingly, a recent experimental study suggests that quinidine exerts a protective effect against hypothermia-induced VT/VF in a J wave syndrome model.217
Clinical evidence of the effectiveness of quinidine in normalizing ST segment elevation and preventing arrhythmic events in patients with the BrS is abundant. 218,203, 218–230 The first prospective study to examine the effects of quinidine to prevent inducible and spontaneous VT/VF was reported by Belhassen and coworkers. All 25 patients enrolled had inducible VF at baseline. Relatively high doses of quinidine (1483±240 mg) were used and shown to prevent VF induction in 22 of the 25 patients (88%). With a follow-up period of 6 months to 22.2 years, all patients survived. Of nineteen patients treated with oral quinidine for 6 to 219 months (56±67 months), none developed any arrhythmic events. The main side effect, diarrhea, occurred in 36% of patients administered quinidine and was readily reversed after drug discontinuation. The study concluded that quinidine effectively suppresses spontaneous as well as inducible VT/VF and may be useful as an adjunct to ICD therapy or as an alternative to ICD in cases in which an ICD is refused, is unaffordable or under other circumstances in which ICD implantation undesirable or not feasible. These results are consistent with those reported the same group in prior years 219, 231 and subsequently by other investigators 232, 233. The data highlight the need for randomized clinical trials to assess the effectiveness of quinidine, preferably in patients with frequent events who have already received an ICD. Hermida et al. reported 76 % efficacy in prevention of VF induced by PES.232
Quinidine may has been proposed as a preventative meassure in asymptomatic patients, however this has not been evaluated in large double-blinded clinical trials221. In a more recent trial conducted at two French centers, 44 asymptomatic BrS patients with inducible VT/VF were enrolled (47 ± 10 years, 95% male). Of these, 34 (77%) were no longer inducible while treated with 600 mg/day hydroquinidine (HQ) for 6.2 ± 3 years. Among the 10 other patients (22%), who remained inducible and received ICD (Group PVS+), none received appropriate shocks during a mean follow-up of 7.7 ± 2 years. The overall annual rate of arrhythmic events was 1.04%, without significant difference between inducibility under HQ. One-third of patients experienced device-related complications.234
A prospective registry of empiric quinidine for asymptomatic Brugada syndrome has been established. The study appears at the National Institutes of Health website (ClinicalTrials.gov) and can be accessed at http://clinicaltrials.gov/ct2/show/NCT00789165?term_brugada&rank_2. Doses between 600 and 900 mg are recommended, if tolerated. 221
Because of the GI side effects of high dose quinidine, low-dose quinidine (<600mg) has been investigated as a therapeutic option. Marquez et al. evaluated the clinical history of symptomatic patients with recurrent arrhythmias and frequent ICD discharges and reported that relatively low dose quinidine, as adjunctive therapy, completely prevented arrhythmias in 85 % of the patients (median follow-up of 4years).223
The recent HRS/EHRA/APHRS expert consensus statement, gave quinidine a Class IIa recommendation in BrS patients who are qualified for an ICD but in whom hindering factors are present, IIa in ICD-patients with electrical storms, and IIb recommendation in asymptomatic BrS-patients displaying a spontaneous Type I ECG. 33
The development of a more cardio-selective and Ito-specific blocker would be a most welcome addition to the limited therapeutic armamentarium currently available to combat this disease.
Agents that augment the L-type calcium channel current, such as β adrenergic agents like isoproterenol, denopamine or orciprenaline have been shown to be useful 166, 168, 226, 230, 235, 236. Isoproterenol, sometimes in combination with quinidine, has been utilized successfully to suppress electrical storms and to normalize ST elevation particularly in children 51, 218, 219, 230, 233, 237–243,46, 224, 244–247. The occurrence of spontaneous VF in patients with Brugada syndrome is often related to an increase in vagal tone and correspondingly electrical storm is sometimes treatable by the increase of sympathetic tone via isoproterenol administration. In the latest HRS/EHRA/APHRS guideline, isoprotereonol has a Class IIa recommendation for BrS patients presenting with electrical storms.33
Administration of the phosphodiesterase III inhibitor cilostazol has been proposed as another promising approach to therapy of BrS.230, 235, 248 Cilostazol has been shown to normalize ST segment elevation, most likely by augmenting calcium current (ICa) as well as by reducing Ito secondary to an increase in cAMP and heart rate.249 Other effects of cilostazol may contribute as well. (e.g.: adenosine uptake inhibition 250) Its efficacy in combination with bepridil in preventing VF-episodes was recently reported by Shinohara et al.251 It is noteworthy that failure of cilostazol in the treatment of a BrS-patient has been reported in a single case report252.
Milrinone is another phosphodiesterase III inhibitor recently identified as a more potent alternative to cilostazol in suppressing ST elevation and arrhythmogenesis in an experimental model of BrS161, 253. No clinical reports have appeared as yet.
Wenxin Keli, a traditional Chinese medicine (TCM), in addition to its actions to suppress atrial fibrillation by atrial-selective inhibition of INa-dependent parameters254, has been shown to inhibit Ito and thus to suppress VT/VF in experimental models of BrS when combined with low concentrations of quinidine (5 μM). 216 A recent study has also reported the effect of Wenxin Keli to suppress ischemia-induced ventricular arrhythmias.255
Agents that augment INa active during the early phases of the action potential, including bepridil and dimethyl lithospermate B (dmLSB), have also been suggested to be of value in the pharmacologic approach to therapy of BrS. Several studies have reported an effect of bepridil to suppress VT/VF in patients with BrS.230, 256–258 The drug’s action are thought to be mediated by: 1) inhibition of Ito; 2) augmentation of INa via upregulation of the channels259; and 3) prolongation of QT interval at slow rates thus increasing the QT/RR slope.256, 258 Dimethyl lithospermate B, an extract of Danshen, a traditional Chinese herbal remedy, has been shown to slow inactivation of INa thus increasing inward current during the early phases of the action potential (AP) and to be effective in suppressing arrhythmogenesis in experimental models of BrS.260
Acknowledgments
Funding sources: This study was supported by grant HL47678 from NHLBI (CA), NYSTEM grant # C026424 (CA) and the Masons of New York, Florida, Massachusetts, Connecticut, Maryland, Delaware, New Hampshire and Wisconsin.
Footnotes
Conflicts of interest: Dr. Antzelevith is a conusltant to Gilead Scciences in Foster City, CA and has received research support forn Gilead Sciences and the Buchang Group in Xi’An China.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hermida JS, Lemoine JL, Aoun FB, Jarry G, Rey JL, Quiret JC. Prevalence of the brugada syndrome in an apparently healthy population. Am J Cardiol. 2000;86:91–94. doi: 10.1016/s0002-9149(00)00835-3. [DOI] [PubMed] [Google Scholar]
- 2.Postema PG. About Brugada syndrome and its prevalence. Europace. 2012;14:925–928. doi: 10.1093/europace/eus042. [DOI] [PubMed] [Google Scholar]
- 3.Patel SS, Anees SS, Ferrick KJ. Prevalence of a Brugada pattern electrocardiogram in an urban population in the United States. Pacing Clin Electrophysiol. 2009;32:704–708. doi: 10.1111/j.1540-8159.2009.02354.x. [DOI] [PubMed] [Google Scholar]
- 4.Pecini R, Cedergreen P, Theilade S, Haunso S, Theilade J, Jensen GB. The prevalence and relevance of the Brugada-type electrocardiogram in the Danish general population: data from the Copenhagen City Heart Study. Europace. 1907;12:982–986. doi: 10.1093/europace/euq077. [DOI] [PubMed] [Google Scholar]
- 5.Sinner MF, Pfeufer A, Perz S, Schulze-Bahr E, Monnig G, Eckardt L, Beckmann BM, Wichmann HE, Breithardt G, Steinbeck G, Fabritz L, Kaab S, Kirchhof P. Spontaneous Brugada electrocardiogram patterns are rare in the German general population: results from the KORA study. Europace. 2009;11:1338–1344. doi: 10.1093/europace/eup205. [DOI] [PubMed] [Google Scholar]
- 6.Schukro C, Berger T, Stix G, Pezawas T, Kastner J, Hintringer F, Schmidinger H. Regional prevalence and clinical benefit of implantable cardioverter defibrillators in Brugada syndrome. Int J Cardiol. 2010;144:191–194. doi: 10.1016/j.ijcard.2009.03.136. [DOI] [PubMed] [Google Scholar]
- 7.Tsuji H, Sato T, Morisaki K, Iwasaka T. Prognosis of subjects with Brugada-type electrocardiogram in a population of middle-aged Japanese diagnosed during a health examination. Am J Cardiol. 2008;102:584–587. doi: 10.1016/j.amjcard.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 8.Uhm JS, Hwang IU, Oh YS, Choi MS, Jang SW, Shin WS, Kim JH, Lee MY, Rho TH, Kim YH, Sung JH, Lee YS, Cho JG, Oh DJ, Kim DK, Namgung J, Park KM, Kim YH, Kim YN, Lim HE, Cha TJ, On YK, Shin DG, Pak HN, Kim NH. Prevalence of electrocardiographic findings suggestive of sudden cardiac death risk in 10,867 apparently healthy young Korean men. Pacing Clin Electrophysiol. 2011;34:717–723. doi: 10.1111/j.1540-8159.2010.03024.x. [DOI] [PubMed] [Google Scholar]
- 9.Juang JM, Phan WL, Chen PC, Lai LP, Tsai MH, Lin JW, Cheng PH, Chiu WY, Cheng BW, Hwang JJ, Tseng CD, Hsu KL, Tseng YZ, Lin JL, Chiang FT. Brugada-type electrocardiogram in the Taiwanese population--is it a risk factor for sudden death? J Formos Med Assoc. 2011;110:230–238. doi: 10.1016/S0929-6646(11)60035-1. [DOI] [PubMed] [Google Scholar]
- 10.Letsas KP, Gavrielatos G, Efremidis M, Kounas SP, Filippatos GS, Sideris A, Kardaras F. Prevalence of Brugada sign in a Greek tertiary hospital population. Europace. 2007;9:1077–1080. doi: 10.1093/europace/eum221. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo K, Akahoshi M, Nakashima E, Suyama A, Seto S, Hayano M, Yano K. The prevalence, incidence and prognostic value of the Brugada-type electrocardiogram: a population-based study of four decades. J Am Coll Cardiol. 2001;38:765–770. doi: 10.1016/s0735-1097(01)01421-8. [DOI] [PubMed] [Google Scholar]
- 12.Furuhashi M, Uno K, Tsuchihashi K, Nagahara D, Hyakukoku M, Ohtomo T, Satoh S, Nishimiya T, Shimamoto K. Prevalence of asymptomatic ST segment elevation in right precordial leads with right bundle branch block (Brugada-type ST shift) among the general Japanese population. Heart. 2001;86:161–166. doi: 10.1136/heart.86.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gervacio-Domingo G, Isidro J, Tirona J, Gabriel E, David G, Amarillo ML, Morales D, Dans A. The Brugada type 1 electrocardiographic pattern is common among Filipinos. J Clin Epidemiol. 2008;61:1067–1072. doi: 10.1016/j.jclinepi.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher MM, Forleo GB, Behr ER, Magliano G, De LL, Morgia V, De LF, Romeo F. Prevalence and significance of Brugada-type ECG in 12,012 apparently healthy European subjects. Int J Cardiol. 2008;130:44–48. doi: 10.1016/j.ijcard.2007.07.159. [DOI] [PubMed] [Google Scholar]
- 15.Junttila MJ, Raatikainen MJ, Karjalainen J, Kauma H, Kesaniemi YA, Huikuri HV. Prevalence and prognosis of subjects with Brugada-type ECG pattern in a young and middle-aged Finnish population. Eur Heart J. 2004;25:874–878. doi: 10.1016/j.ehj.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Ito H, Yano K, Chen R, He Q, Curb JD. The prevalence and prognosis of a Brugada-type electrocardiogram in a population of middle-aged Japanese-American men with follow-up of three decades. Am J Med Sci. 2006;331:25–29. doi: 10.1097/00000441-200601000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Donohue D, Tehrani F, Jamehdor R, Lam C, Movahed MR. The prevalence of Brugada ECG in adult patients in a large university hospital in the western United States. Am Heart Hosp J. 2008;6:48–50. doi: 10.1111/j.1751-7168.2008.06418.x. [DOI] [PubMed] [Google Scholar]
- 18.Bozkurt A, Yas D, Seydaoglu G, Acarturk E. Frequency of Brugada-type ECG pattern (Brugada sign) in Southern Turkey. Int Heart J. 2006;47:541–547. doi: 10.1536/ihj.47.541. [DOI] [PubMed] [Google Scholar]
- 19.Bigi MA, Aslani A, Shahrzad S. Prevalence of Brugada sign in patients presenting with palpitation in southern Iran. Europace. 2007;9:252–255. doi: 10.1093/europace/eum023. [DOI] [PubMed] [Google Scholar]
- 20.Greer RW, Glancy DL. Prevalence of the Brugada electrocardiographic pattern at the Medical Center of Louisiana in New Orleans. J La State Med Soc. 2003;155:242–246. [PubMed] [Google Scholar]
- 21.Sakabe M, Fujiki A, Tani M, Nishida K, Mizumaki K, Inoue H. Proportion and prognosis of healthy people with coved or saddle-back type ST segment elevation in the right precordial leads during 10 years follow-up. Eur Heart J. 2003;24:1488–1493. doi: 10.1016/s0195-668x(03)00323-3. [DOI] [PubMed] [Google Scholar]
- 22.Wajed A, Aslam Z, Abbas SF, Irfan M, Bangash K, Rehman S, Amin F. Frequency of Brugada-type ECG pattern (Brugada sign) in an apparently healthy young population. J Ayub Med Coll Abbottabad. 2008;20:121–124. [PubMed] [Google Scholar]
- 23.Nademanee K, Veerakul G, Nimmannit S, Chaowakul V, Bhuripanyo K, Likittanasombat K, Tunsanga K, Kuasirikul S, Malasit P, Tansupasawadikul S, Tatsanavivat P. Arrhythmogenic marker for the sudden unexplained death syndrome in Thai men. Circulation. 1997;96:2595–2600. doi: 10.1161/01.cir.96.8.2595. [DOI] [PubMed] [Google Scholar]
- 24.Miyasaka Y, Tsuji H, Yamada K, Tokunaga S, Saito D, Imuro Y, Matsumoto N, Iwasaka T. Prevalence and mortality of the Brugada-type electrocardiogram in one city in Japan. J Am Coll Cardiol. 2001;38:771–774. doi: 10.1016/s0735-1097(01)01419-x. [DOI] [PubMed] [Google Scholar]
- 25.Vatta M, Dumaine R, Varghese G, Richard TA, Shimizu W, Aihara N, Nademanee K, Brugada R, Brugada J, Veerakul G, Li H, Bowles NE, Brugada P, Antzelevitch C, Towbin JA. Genetic and biophysical basis of sudden unexplained nocturnal death syndrome (SUNDS), a disease allelic to Brugada syndrome. Hum Mol Genet. 2002;11:337–345. doi: 10.1093/hmg/11.3.337. [DOI] [PubMed] [Google Scholar]
- 26.Eckardt L, Kirchhof P, Johna R, Haverkamp W, Breithardt G, Borggrefe M. Wolff-Parkinson-White syndrome associated with Brugada syndrome. Pacing Clin Electrophysiol. 2001;24:1423–1424. doi: 10.1046/j.1460-9592.2001.01423.x. [DOI] [PubMed] [Google Scholar]
- 27.Morita H, Fukushima-Kusano K, Nagase S, Miyaji K, Hiramatsu S, Banba K, Nishii N, Watanabe A, Kakishita M, Takenaka-Morita S, Nakamura K, Saito H, Emori T, Ohe T. Sinus node function in patients with Brugada-type ECG. Circ J. 2004;68:473–476. doi: 10.1253/circj.68.473. [DOI] [PubMed] [Google Scholar]
- 28.Takehara N, Makita N, Kawabe J, Sato N, Kawamura Y, Kitabatake A, Kikuchi K. A cardiac sodium channel mutation identified in Brugada syndrome associated with atrial standstill. J Intern Med. 2004;255:137–142. doi: 10.1046/j.0954-6820.2003.01247.x. [DOI] [PubMed] [Google Scholar]
- 29.Bordachar P, Reuter S, Garrigue S, Cai X, Hocini M, Jais P, Haissaguerre M, Clementy J. Incidence, clinical implications and prognosis of atrial arrhythmias in Brugada syndrome. Eur Heart J. 2004;25:879–884. doi: 10.1016/j.ehj.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Wilde AA, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Brugada P, Corrado D, Hauer RN, Kass RS, Nademanee K, Priori SG, Towbin JA. Proposed diagnostic criteria for the Brugada syndrome. Eur Heart J. 2002;23:1648–1654. doi: 10.1053/euhj.2002.3382. [DOI] [PubMed] [Google Scholar]
- 31.Wilde AA, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Brugada P, Corrado D, Hauer RN, Kass RS, Nademanee K, Priori SG, Towbin JA. Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation. 2002;106:2514–2519. doi: 10.1161/01.cir.0000034169.45752.4a. [DOI] [PubMed] [Google Scholar]
- 32.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, Shimizu W, Schulze-Bahr E, Tan H, Wilde A. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 33.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, Kannankeril P, Krahn A, Leenhardt A, Moss A, Schwartz PJ, Shimizu W, Tomaselli G, Tracy C. Executive Summary: HRS/EHRA/APHRS Expert Consensus Statement on the Diagnosis and Management of Patients with Inherited Primary Arrhythmia Syndromes. Heart Rhythm. 2013;15:1389–1406. [Google Scholar]
- 34.Richter S, Sarkozy A, Paparella G, Henkens S, Boussy T, Chierchia GB, Brugada R, Brugada J, Brugada P. Number of electrocardiogram leads displaying the diagnostic coved-type pattern in Brugada syndrome: a diagnostic consensus criterion to be revised. Eur Heart J. 2010;31:1357–1364. doi: 10.1093/eurheartj/ehq049. [DOI] [PubMed] [Google Scholar]
- 35.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, Shimizu W, Schulze-Bahr E, Tan H, Wilde A. Brugada syndrome: report of the second consensus conference. Heart Rhythm. 2005;2:429–440. doi: 10.1016/j.hrthm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Probst V, Veltmann C, Eckardt L, Meregalli PG, Gaita F, Tan HL, Babuty D, Sacher F, Giustetto C, Schulze-Bahr E, Borggrefe M, Haissaguerre M, Mabo P, Le Marec H, Wolpert C, Wilde AAM. Long-term prognosis of aatients diagnosed with Brugada syndrome: results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121:635–643. doi: 10.1161/CIRCULATIONAHA.109.887026. [DOI] [PubMed] [Google Scholar]
- 37.Brugada R, Brugada J, Antzelevitch C, Kirsch GE, Potenza D, Towbin JA, Brugada P. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000;101:510–515. doi: 10.1161/01.cir.101.5.510. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu W, Antzelevitch C, Suyama K, Kurita T, Taguchi A, Aihara N, Takaki H, Sunagawa K, Kamakura S. Effect of sodium channel blockers on ST segment, QRS duration, and corrected QT interval in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2000;11:1320–1329. doi: 10.1046/j.1540-8167.2000.01320.x. [DOI] [PubMed] [Google Scholar]
- 39.Hong K, Brugada J, Oliva A, Berruezo-Sanchez A, Potenza D, Pollevick GD, Guerchicoff A, Matsuo K, Burashnikov E, Dumaine R, Towbin JA, Nesterenko VV, Brugada P, Antzelevitch C, Brugada R. Value of electrocardiographic parameters and ajmaline test in the diagnosis of Brugada syndrome caused by SCN5A mutations. Circulation. 2004;110:3023–3027. doi: 10.1161/01.CIR.0000144299.17008.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meregalli PG, Ruijter JM, Hofman N, Bezzina CR, Wilde AA, Tan HL. Diagnostic value of flecainide testing in unmasking SCN5A-related Brugada syndrome. J Cardiovasc Electrophysiol. 2006;17:857–864. doi: 10.1111/j.1540-8167.2006.00531.x. [DOI] [PubMed] [Google Scholar]
- 41.Priori SG, Napolitano C, Gasparini M, Pappone C, Della BP, Brignole M, Giordano U, Giovannini T, Menozzi C, Bloise R, Crotti L, Terreni L, Schwartz PJ. Clinical and genetic heterogeneity of right bundle branch block and ST-segment elevation syndrome: A prospective evaluation of 52 families. Circulation. 2000;102:2509–2515. doi: 10.1161/01.cir.102.20.2509. [DOI] [PubMed] [Google Scholar]
- 42.Gavrielatos G, Letsas KP, Pappas LK, Efremidis M, Sideris A, Kardaras F. Sensitivity and specificity of sodium channel blocking test in the diagnosis of Brugada syndrome. Int J Cardiol. 2010;141:e31–e33. doi: 10.1016/j.ijcard.2008.11.147. [DOI] [PubMed] [Google Scholar]
- 43.Wolpert C, Echternach C, Veltmann C, Antzelevitch C, Thomas GP, Sphel S, Streitner F, Kuschyk J, Schimpf R, Haase KK, Borggrefe M. Intravenous drug challenge using flecainide and ajmaline in patients with Brugada syndrome. Heart Rhythm. 2005;2:254–260. doi: 10.1016/j.hrthm.2004.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimizu W. Acquired forms of Brugada syndrome. In: Antzelevitch C, editor. The Brugada Syndrome: From Bench to Bedside. Oxford, UK: Blackwell Futura; 2004. pp. 166–177. [Google Scholar]
- 45.Brugada P, Brugada J, Brugada R. Arrhythmia induction by antiarrhythmic drugs. Pacing Clin Electrophysiol. 2000;23:291–292. doi: 10.1111/j.1540-8159.2000.tb06751.x. [DOI] [PubMed] [Google Scholar]
- 46.Miyazaki T, Mitamura H, Miyoshi S, Soejima K, Aizawa Y, Ogawa S. Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J Am Coll Cardiol. 1996;27:1061–1070. doi: 10.1016/0735-1097(95)00613-3. [DOI] [PubMed] [Google Scholar]
- 47.Antzelevitch C, Brugada R. Fever and the Brugada syndrome. Pacing Clin Electrophysiol. 2002;25:1537–1539. doi: 10.1046/j.1460-9592.2002.01537.x. [DOI] [PubMed] [Google Scholar]
- 48.Peng J, Cui YK, Yuan FH, Yi SD, Chen ZM, Meng SR. Fever and Brugada syndrome: report of 21 cases. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:432–434. [PubMed] [Google Scholar]
- 49.Junttila MJ, Gonzalez M, Lizotte E, Benito B, Vernooy K, Sarkozy A, Huikuri HV, Brugada P, Brugada J, Brugada R. Induced Brugada-type electrocardiogram, a sign for imminent malignant arrhythmias. Circulation. 2008;117:1890–1893. doi: 10.1161/CIRCULATIONAHA.107.746495. [DOI] [PubMed] [Google Scholar]
- 50.Adler A, Topaz G, Heller K, Zeltser D, Tzioni-Ohayon T, Rozovski U, Halkin A, Rosso R, Ben-Shachar S, Antzelevitch C, Viskin S. Fever-induced Brugada pattern: How common is it and what does it mean? Heart Rhythm. 2013;10:1375–1382. doi: 10.1016/j.hrthm.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimizu W, Matsuo K, Takagi M, Tanabe Y, Aiba T, Taguchi A, Suyama K, Kurita T, Aihara N, Kamakura S. Body surface distribution and response to drugs of ST segment elevation in Brugada syndrome: clinical implication of eighty-seven-lead body surface potential mapping and its application to twelve-lead electrocardiograms. J Cardiovasc Electrophysiol. 2000;11:396–404. doi: 10.1111/j.1540-8167.2000.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 52.Sangwatanaroj S, Prechawat S, Sunsaneewitayakul B, Sitthisook S, Tosukhowong P, Tungsanga K. New electrocardiographic leads and the procainamide test for the detection of the Brugada sign in sudden unexplained death syndrome survivors and their relatives. Eur Heart J. 2001;22:2290–2296. doi: 10.1053/euhj.2001.2691. [DOI] [PubMed] [Google Scholar]
- 53.Teijeiro R, Garro HA, Acunzo RS, Albino E, Chiale PA. Recording of high V1-V3 precordial leads may be essential to the diagnosis of Brugada syndrome during the ajmaline test. J Cardiovasc Pharmacol Ther. 2006;11:153–155. doi: 10.1177/1074248406288760. [DOI] [PubMed] [Google Scholar]
- 54.Miyamoto K, Yokokawa M, Tanaka K, Nagai T, Okamura H, Noda T, Satomi K, Suyama K, Kurita T, Aihara N, Kamakura S, Shimizu W. Diagnostic and prognostic value of a type 1 Brugada electrocardiogram at higher (third or second) V1 to V2 recording in men with Brugada syndrome. Am J Cardiol. 2007;99:53–57. doi: 10.1016/j.amjcard.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 55.Marquez MF, Allende R, Morales JL. Unmasking the Brugada syndrome with high parasternal leads. Europace. 2007 doi: 10.1093/europace/eum229. [DOI] [PubMed] [Google Scholar]
- 56.Govindan M, Batchvarov VN, Raju H, Shanmugam N, Bizrah M, Bastiaenen R, Kiotsekoglou A, Camm J, Behr ER. Utility of high and standard right precordial leads during ajmaline testing for the diagnosis of Brugada syndrome. Heart. 2010;96:1904–1908. doi: 10.1136/hrt.2010.201244. [DOI] [PubMed] [Google Scholar]
- 57.Baranchuk A, Nguyen T, Ryu MH, Femenia F, Zareba W, Wilde AA, Shimizu W, Brugada P, Perez-Riera AR. Brugada phenocopy: new terminology and proposed classification. Ann Noninvasive Electrocardiol. 2012;17:299–314. doi: 10.1111/j.1542-474X.2012.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bayes de Luna A, Brugada J, Baranchuk A, Borggrefe M, Breithardt G, Goldwasser D, Lambiase P, Riera AP, Garcia-Niebla J, Pastore C, Oreto G, McKenna W, Zareba W, Brugada R, Brugada P. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45:433–442. doi: 10.1016/j.jelectrocard.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 59.Arce M, Riera AR, Femenia F, Baranchuk A. Brugada electrocardiographic phenocopy in a patient with chronic Chagasic cardiomyopathy. Cardiol J. 2010;17:525–527. [PubMed] [Google Scholar]
- 60.Brito MR, Miranda CE, Rabelo W, Marino RL. Type 1 electrocardiographic Brugada pattern in a woman with Chagas disease: a case report. Europace. 2010;12:1345–1346. doi: 10.1093/europace/euq129. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Niebla J, Llontop-Garcia P, Valle-Racero JI, Serra-Autonell G, Batchvarov VN, De Luna AB. Technical mistakes during the acquisition of the electrocardiogram. Ann Noninvasive Electrocardiol. 2009;14:389–403. doi: 10.1111/j.1542-474X.2009.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garcia-Niebla J, Serra-Autonell G. Effects of inadequate low-pass filter application. J Electrocardiol. 2009;42:303–304. doi: 10.1016/j.jelectrocard.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Chiale PA, Garro HA, Fernandez PA, Elizari MV. High-degree right bundle branch block obscuring the diagnosis of Brugada electrocardiographic pattern. Heart Rhythm. 2012;9:974–976. doi: 10.1016/j.hrthm.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 64.Aizawa Y, Takatsuki S, Kimura T, Nishiyama N, Fukumoto K, Tanimoto Y, Tanimoto K, Miyoshi S, Suzuki M, Yokoyama Y, Chinushi M, Watanabe I, Ogawa S, Aizawa Y, Antzelevitch C, Fukuda K. Ventricular fibrillation associated with complete right bundle branch block. Heart Rhythm. 2013;10:1028–1035. doi: 10.1016/j.hrthm.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chevallier S, Forclaz A, Tenkorang J, Ahmad Y, Faouzi M, Graf D, Schlaepfer J, Pruvot E. New electrocardiographic criteria for discriminating between brugada types 2 and 3 patterns and incomplete right bundle branch block. J Am Coll Cardiol. 2011;58:2290–2298. doi: 10.1016/j.jacc.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 66.Corrado D, Pelliccia A, Heidbuchel H, Sharma S, Link M, Basso C, Biffi A, Buja G, Delise P, Gussac I, Anastasakis A, Borjesson M, Bjornstad HH, Carre F, Deligiannis A, Dugmore D, Fagard R, Hoogsteen J, Mellwig KP, Panhuyzen-Goedkoop N, Solberg E, Vanhees L, Drezner J, Estes NA, III, Iliceto S, Maron BJ, Peidro R, Schwartz PJ, Stein R, Thiene G, Zeppilli P, McKenna WJ. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur Heart J. 2010;31:243–259. doi: 10.1093/eurheartj/ehp473. [DOI] [PubMed] [Google Scholar]
- 67.Kalla H, Yan GX, Marinchak R. Ventricular fibrillation in a patient with prominent J (Osborn) waves and ST segment elevation in the inferior electrocardiographic leads: a Brugada syndrome variant? J Cardiovasc Electrophysiol. 2000;11:95–98. doi: 10.1111/j.1540-8167.2000.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 68.Horigome H, Shigeta O, Kuga K, Isobe T, Sakakibara Y, Yamaguchi I, Matsui A. Ventricular fibrillation during anesthesia in association with J waves in the left precordial leads in a child with coarctation of the aorta. J Electrocardiol. 2003;36:339–343. doi: 10.1016/s0022-0736(03)00079-7. [DOI] [PubMed] [Google Scholar]
- 69.Potet F, Mabo P, Le Coq G, Probst V, Schott JJ, Airaud F, Guihard G, Daubert JC, Escande D, Le Marec H. Novel brugada SCN5A mutation leading to ST segment elevation in the inferior or the right precordial leads. J Cardiovasc Electrophysiol. 2003;14:200–203. doi: 10.1046/j.1540-8167.2003.02382.x. [DOI] [PubMed] [Google Scholar]
- 70.Letsas KP, Sacher F, Probst V, Weber R, Knecht S, Kalusche D, Haissaguerre M, Arentz T. Prevalence of early repolarization pattern in inferolateral leads in patients with Brugada syndrome. Heart Rhythm. 2008;5:1685–1689. doi: 10.1016/j.hrthm.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 71.Kawata H, Morita H, Yamada Y, Noda T, Satomi K, Aiba T, Isobe M, Nagase S, Nakamura K, Fukushima KK, Ito H, Kamakura S, Shimizu W. Prognostic significance of early repolarization in inferolateral leads in Brugada patients with documented ventricular fibrillation: a novel risk factor for Brugada syndrome with ventricular fibrillation. Heart Rhythm. 2013;10:1161–1168. doi: 10.1016/j.hrthm.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 72.Takagi M, Aonuma K, Sekiguchi Y, Yokoyama Y, Aihara N, Hiraoka M. The prognostic value of early repolarization (J wave) and ST-segment morphology after J wave in Brugada syndrome: Multicenter study in Japan. Heart Rhythm. 2013;10:533–539. doi: 10.1016/j.hrthm.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 73.Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm. 2010;7:549–558. doi: 10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alings M, Wilde A. “Brugada” syndrome: clinical data and suggested pathophysiological mechanism. Circulation. 1999;99:666–673. doi: 10.1161/01.cir.99.5.666. [DOI] [PubMed] [Google Scholar]
- 75.Bezzina C, Veldkamp MW, van Den Berg MP, Postma AV, Rook MB, Viersma JW, Van Langen IM, Tan-Sindhunata G, Bink-Boelkens MT, Der Hout AH, Mannens MM, Wilde AA. A single Na+ channel mutation causing both long-QT and Brugada syndromes. Circ Res. 1999;85:1206–1213. doi: 10.1161/01.res.85.12.1206. [DOI] [PubMed] [Google Scholar]
- 76.Pitzalis MV, Anaclerio M, Iacoviello M, Forleo C, Guida P, Troccoli R, Massari F, Mastropasqua F, Sorrentino S, Manghisi A, Rizzon P. QT-interval prolongation in right precordial leads: an additional electrocardiographic hallmark of Brugada syndrome. J Am Coll Cardiol. 2003;42:1632–1637. doi: 10.1016/j.jacc.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 77.Antzelevitch C. Brugada syndrome. Pacing Clin Electrophysiol. 2006;29:1130–1159. doi: 10.1111/j.1540-8159.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smits JP, Eckardt L, Probst V, Bezzina CR, Schott JJ, Remme CA, Haverkamp W, Breithardt G, Escande D, Schulze-Bahr E, LeMarec H, Wilde AA. Genotype-phenotype relationship in Brugada syndrome: electrocardiographic features differentiate SCN5A-related patients from non-SCN5A-related patients. J Am Coll Cardiol. 2002;40:350–356. doi: 10.1016/s0735-1097(02)01962-9. [DOI] [PubMed] [Google Scholar]
- 79.Priori SG, Gasparini M, Napolitano C, Della BP, Ottonelli AG, Sassone B, Giordano U, Pappone C, Mascioli G, Rossetti G, De NR, Colombo M. Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J Am Coll Cardiol. 2012;59:37–45. doi: 10.1016/j.jacc.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 80.Delise P, Allocca G, Marras E, Giustetto C, Gaita F, Sciarra L, Calo L, Proclemer A, Marziali M, Rebellato L, Berton G, Coro L, Sitta N. Risk stratification in individuals with the Brugada type 1 ECG pattern without previous cardiac arrest: usefulness of a combined clinical and electrophysiologic approach. Eur Heart J. 2011;32:169–176. doi: 10.1093/eurheartj/ehq381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ikeda T, Sakurada H, Sakabe K, Sakata T, Takami M, Tezuka N, Nakae T, Noro M, Enjoji Y, Tejima T, Sugi K, Yamaguchi T. Assessment of noninvasive markers in identifying patients at risk in the Brugada syndrome: insight into risk stratification. J Am Coll Cardiol. 2001;37:1628–1634. doi: 10.1016/s0735-1097(01)01197-4. [DOI] [PubMed] [Google Scholar]
- 82.Yoshioka K, Amino M, Zareba W, Shima M, Matsuzaki A, Fujii T, Kanda S, Deguchi Y, Kobayashi Y, Ikari Y, Kodama I, Tanabe T. Identification of high-risk brugada syndrome patients by combined analysis of late potential and T-wave amplitude variability on ambulatory electrocardiograms. Circ J. 2013;77:610–618. doi: 10.1253/circj.cj-12-0932. [DOI] [PubMed] [Google Scholar]
- 83.Nademanee K, Veerakul G, Chandanamattha P, Chaothawee L, Ariyachaipanich A, Jirasirirojanakorn K, Likittanasombat K, Bhuripanyo K, Ngarmukos T. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]
- 84.Aizawa Y, Chinushi M, Tagawa M, Furushima H, Okada S, Iijima K, Izumi D, Watanabe H, Komura S. A post-QRS potential in Brugada syndrome: its relation to electrocardiographic pattern and possible genesis. J Am Coll Cardiol. 2008;51:1720–1721. doi: 10.1016/j.jacc.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 85.Furushima H, Chinushi M, Okamura K, Iijima K, Komura S, Tanabe Y, Okada S, Izumi D, Aizawa Y. Comparison of conduction delay in the right ventricular outflow tract between Brugada syndrome and right ventricular cardiomyopathy: investigation of signal average ECG in the precordial leads. Europace. 2007;9:951–956. doi: 10.1093/europace/eum128. [DOI] [PubMed] [Google Scholar]
- 86.Nagase S, Kusano KF, Morita H, Fujimoto Y, Kakishita M, Nakamura K, Emori T, Matsubara H, Ohe T. Epicardial electrogram of the right ventricular outflow tract in patients with the Brugada syndrome: using the epicardial lead. J Am Coll Cardiol. 2002;39:1992–1995. doi: 10.1016/s0735-1097(02)01888-0. [DOI] [PubMed] [Google Scholar]
- 87.Huang Z, Patel C, Li W, Xie Q, Wu R, Zhang L, Tang R, Wan X, Ma Y, Zhen W, Gao L, Yan GX. Role of signal-averaged electrocardiograms in arrhythmic risk stratification of patients with Brugada syndrome: a prospective study. Heart Rhythm. 2009;6:1156–1162. doi: 10.1016/j.hrthm.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 88.Morita H, Kusano KF, Miura D, Nagase S, Nakamura K, Morita ST, Ohe T, Zipes DP, Wu J. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118:1697–1704. doi: 10.1161/CIRCULATIONAHA.108.770917. [DOI] [PubMed] [Google Scholar]
- 89.Junttila MJ, Brugada P, Hong K, Lizotte E, de Zutter M, Sarkozy A, Brugada J, Benito B, Perkiomaki JS, Makikallio TH, Huikuri HV, Brugada R. Differences in 12-lead electrocardiogram between symptomatic and asymptomatic Brugada syndrome patients. J Cardiovasc Electrophysiol. 2008;19:380–383. doi: 10.1111/j.1540-8167.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- 90.Sarkozy A, Sorgente A, Boussy T, Casado R, Paparella G, Capulzini L, Chierchia GB, Yazaki Y, de AC, Coomans D, Brugada J, Brugada P. The value of a family history of sudden death in patients with diagnostic type I Brugada ECG pattern. Eur Heart J. 2011;32:2153–2160. doi: 10.1093/eurheartj/ehr129. [DOI] [PubMed] [Google Scholar]
- 91.Sarkozy A, Paparella G, Boussy T, Casado-Arroyo R, Yazaki Y, Chierchia GB, de AC, Bayrak F, Namdar M, Richter S, Brugada J, Brugada P. The usefulness of the consensus clinical diagnostic criteria in Brugada syndrome. Int J Cardiol. 2013;167:2700–2704. doi: 10.1016/j.ijcard.2012.06.115. [DOI] [PubMed] [Google Scholar]
- 92.Gehi AK, Duong TD, Metz LD, Gomes JA, Mehta D. Risk stratification of individuals with the brugada electrocardiogram: a meta-analysis. J Cardiovasc Electrophysiol. 2006;17:577–583. doi: 10.1111/j.1540-8167.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 93.Paul M, Gerss J, Schulze-Bahr E, Wichter T, Vahlhaus C, Wilde AA, Breithardt G, Eckardt L. Role of programmed ventricular stimulation in patients with Brugada syndrome: a meta-analysis of worldwide published data. Eur Heart J. 2007;28:2126–2133. doi: 10.1093/eurheartj/ehm116. [DOI] [PubMed] [Google Scholar]
- 94.Giustetto C, Drago S, Demarchi PG, Dalmasso P, Bianchi F, Masi AS, Carvalho P, Occhetta E, Rossetti G, Riccardi R, Bertona R, Gaita F. Risk stratification of the patients with Brugada type electrocardiogram: a community-based prospective study. Europace. 2009;11:507–513. doi: 10.1093/europace/eup006. [DOI] [PubMed] [Google Scholar]
- 95.Makimoto H, Kamakura S, Aihara N, Noda T, Nakajima I, Yokoyama T, Doi A, Kawata H, Yamada Y, Okamura H, Satomi K, Aiba T, Shimizu W. Clinical impact of the number of extrastimuli in programmed electrical stimulation in patients with Brugada type 1 electrocardiogram. Heart Rhythm. 2012;9:242–248. doi: 10.1016/j.hrthm.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 96.Castro Hevia J, Antzelevitch C, Tornés Bárzaga F, Dorantes Sánchez M, Dorticós Balea F, Zayas Molina R, Quiñones Pérez MA, Fayad RY. Tpeak-Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006;47:1828–1834. doi: 10.1016/j.jacc.2005.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Letsas KP, Weber R, Astheimer K, Kalusche D, Arentz T. Tpeak-Tend interval and Tpeak-Tend/QT ratio as markers of ventricular tachycardia inducibility in subjects with Brugada ECG phenotype. Europace. 2010;12:271–274. doi: 10.1093/europace/eup357. [DOI] [PubMed] [Google Scholar]
- 98.Karim Talib A, Sato N, Sakamoto N, Tanabe Y, Takeuchi T, Saijo Y, Kawamura Y, Hasebe N. Enhanced transmural dispersion of repolarization in patients with J wave syndromes. J Cardiovasc Electrophysiol. 2012;23:1109–14. doi: 10.1111/j.1540-8167.2012.02363.x. [DOI] [PubMed] [Google Scholar]
- 99.Lambiase PD. Tpeak-Tend interval and Tpeak-Tend/QT ratio as markers of ventricular tachycardia inducibility in subjects with Brugada ECG phenotype. Europace. 2010;12:158–9. doi: 10.1093/europace/eup424. [DOI] [PubMed] [Google Scholar]
- 100.Uzieblo-Zyczkowska B, Gielerak G, Michalkiewicz D. Usefulness of patient's history and non-invasive electrocardiographic parameters in prediction of ajmaline test results in patients with suspected Brugada syndrome. Archives of medical science : AMS. 2014;10:899–912. doi: 10.5114/aoms.2013.36928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang JF, Shan QJ, Yang B, Chen ML, Zou JG, Chen C, Xu DJ, Cao KJ. Tpeak-Tend interval and risk of cardiac events in patients with Brugada syndrome. Zhonghua xin xue guan bing za zhi. 2007;35:629–32. [PubMed] [Google Scholar]
- 102.Talib AK, Sato N, Kawabata N, Sugiyama E, Sakamoto N, Tanabe Y, Fujino T, Takeuchi T, Saijo Y, Akasaka K, Kawamura Y, Hasebe N. Repolarization characteristics in early repolarization and brugada syndromes: insight into an overlapping mechanism of lethal arrhythmias. J Cardiovasc Electrophysiol. 2014;25:1376–84. doi: 10.1111/jce.12566. [DOI] [PubMed] [Google Scholar]
- 103.Makimoto H, Nakagawa E, Takaki H, Yamada Y, Okamura H, Noda T, Satomi K, Suyama K, Aihara N, Kurita T, Kamakura S, Shimizu W. Augmented ST-segment elevation during recovery from exercise predicts cardiac events in patients with Brugada syndrome. J Am Coll Cardiol. 2010;56:1576–1584. doi: 10.1016/j.jacc.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 104.Makimoto H, Takaki H, Doi A, Yokoyama T, Yamada Y, Okamura H, Noda T, Satomi K, Suyama K, Aihara N, Kamakura S, Shimizu W. Clinical significance of early heart rate recovery after exercising testing in patients with Brugada syndrome. Circulation. 2009;120:S671. [Google Scholar]
- 105.Tatsumi H, Takagi M, Nakagawa E, Yamashita H, Yoshiyama M. Risk stratification in patients with Brugada syndrome: analysis of daily fluctuations in 12-lead electrocardiogram (ECG) and signal-averaged electrocardiogram (SAECG) J Cardiovasc Electrophysiol. 2006;17:705–711. doi: 10.1111/j.1540-8167.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- 106.Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, Ortiz-Lopez R, Wang Z, Antzelevitch C, O'Brien RE, Schultze-Bahr E, Keating MT, Towbin JA, Wang Q. Genetic basis and molecular mechanisms for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 107.Antzelevitch C. J wave syndromes: molecular and cellular mechanisms. J Electrocardiol. 2013;46:510–518. doi: 10.1016/j.jelectrocard.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Antzelevitch C. Genetic, molecular and cellular mechanisms underlying the J wave syndromes. Circ J. 2012;76:1054–1065. doi: 10.1253/circj.cj-12-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, Brugada P, Fressart V, Guerchicoff A, Harris-Kerr C, Kamakura S, Kyndt F, Koopmann TT, Miyamoto M, Pfeiffer R, Pollevick GD, Probst V, Zumhagen S, Vatta M, Towbin JA, Shimizu W, Schulze-Bahr E, Antzelevitch C, Salisbury BA, Guicheney P, Wilde AAM, Brugada R, Schott JJ, Ackerman MJ. An international compendium of mutations in the SCN5A encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. doi: 10.1016/j.hrthm.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu D, Barajas-Martinez H, Pfeiffer R, Dezi F, Pfeiffer J, Buch T, Betzenhauser MJ, Belardinelli L, Kahlig KM, Rajamani S, DeAntonio HJ, Myerburg RJ, Ito H, Deshmukh P, Marieb M, Nam GB, Bhatia A, Hasdemir C, Haissaguerre M, Veltmann C, Schimpf R, Borggrefe M, Viskin S, Antzelevitch C. Mutations in SCN10A are responsible for a large fraction of cases of Brugada syndrome. J Am Coll Cardiol. 2014;64:66–79. doi: 10.1016/j.jacc.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weiss R, Barmada MM, Nguyen T, Seibel JS, Cavlovich D, Kornblit CA, Angelilli A, Villanueva F, McNamara DM, London B. Clinical and molecular heterogeneity in the Brugada syndrome: a novel gene locus on chromosome 3. Circulation. 2002;105:707–713. doi: 10.1161/hc0602.103618. [DOI] [PubMed] [Google Scholar]
- 112.London B, Michalec M, Mehdi H, Zhu X, Kerchner L, Sanyal S, Viswanathan PC, Pfahnl AE, Shang LL, Madhusudanan M, Baty CJ, Lagana S, Aleong R, Gutmann R, Ackerman MJ, McNamara DM, Weiss R, Dudley SC., Jr Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 2007;116:2260–2268. doi: 10.1161/CIRCULATIONAHA.107.703330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shy D, Gillet L, Abriel H. Cardiac sodium channel NaV1.5 distribution in myocytes via interacting proteins: the multiple pool model. Biochim Biophys Acta. 2013;1833:886–894. doi: 10.1016/j.bbamcr.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 114.Valdivia CR, Ueda K, Ackerman MJ, Makielski JC. GPD1L links redox state to cardiac excitability by PKC-dependent phosphorylation of the sodium channel SCN5A. Am J Physiol Heart Circ Physiol. 2009;297:H1446–H1452. doi: 10.1152/ajpheart.00513.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watanabe H, Koopmann TT, SLS, Yang T, Ingram CR, Schott JJ, Demolombe S, Probst V, Anselme F, Escande D, Wiesfeld AC, Pfeufer A, Kaab S, Wichmann HE, Hasdemir C, Aizawa Y, Wilde AA, Roden DM, Bezzina CR. Sodium channel β1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest. 2008;118:2260–2268. doi: 10.1172/JCI33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hu D, Barajas-Martinez H, Medeiros-Domingo A, Crotti L, Veltmann C, Schimpf R, Urrutia J, Alday A, Casis O, Pfeiffer R, Burashnikov E, Caceres G, Tester DJ, Wolpert C, Borggrefe M, Schwartz P, Ackerman MJ, Antzelevitch C. A novel rare variant in SCN1Bb linked to Brugada syndrome and SIDS by combined modulation of Na(v)1.5 and K(v)4.3 channel currents. Heart Rhythm. 2012;9:760–769. doi: 10.1016/j.hrthm.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu D, Barajas-Martinez H, Burashnikov E, Springer M, Wu Y, Varro A, Pfeiffer R, Koopmann TT, Cordeiro JM, Guerchicoff A, Pollevick GD, Antzelevitch C. A mutation in the beta 3 subunit of the cardiac sodium channel associated with Brugada ECG phenotype. Circ Cardiovasc Genet. 2009;2:270–278. doi: 10.1161/CIRCGENETICS.108.829192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ishikawa T, Takahashi N, Ohno S, Sakurada H, Nakamura K, On YK, Park JE, Makiyama T, Horie M, Arimura T, Makita N, Kimura A. Novel SCN3B mutation associated with brugada syndrome affects intracellular trafficking and function of Nav1.5. Circ J. 2013;77:959–967. doi: 10.1253/circj.cj-12-0995. [DOI] [PubMed] [Google Scholar]
- 119.Riuro H, Beltran-Alvarez P, Tarradas A, Selga E, Campuzano O, Verges M, Pagans S, Iglesias A, Brugada J, Brugada P, Vazquez FM, Perez GJ, Scornik FS, Brugada R. A missense mutation in the sodium channel ß2 subunit reveals SCN2B as a new candidate gene for Brugada syndrome. Hum Mutat. 2013;34:961–966. doi: 10.1002/humu.22328. [DOI] [PubMed] [Google Scholar]
- 120.Bezzina CR, Barc J, Mizusawa Y, Remme CA, Gourraud JB, Simonet F, Verkerk AO, Schwartz PJ, Crotti L, Dagradi F, Guicheney P, Fressart V, Leenhardt A, Antzelevitch C, Bartkowiak S, Schulze-Bahr E, Zumhagen S, Behr ER, Bastiaenen R, Tfelt-Hansen J, Olesen MS, Kaab S, Beckmann BM, Weeke P, Watanabe H, Endo N, Minamino T, Horie M, Ohno S, Hasegawa K, Makita N, Nogami A, Shimizu W, Aiba T, Froguel P, Balkau B, Lantieri O, Torchio M, Wiese C, Weber D, Wolswinkel R, Coronel R, Boukens BJ, Bezieau S, Charpentier E, Chatel S, Despres A, Gros F, Kyndt F, Lecointe S, Lindenbaum P, Portero V, Violleau J, Gessler M, Tan HL, Roden DM, Christoffels VM, Le MH, Wilde AA, Probst V, Schott JJ, Dina C, Redon R. Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat Genet. 2013;45:1044–1049. doi: 10.1038/ng.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van den Boogaard M, Wong LY, Tessadori F, Bakker ML, Dreizehnter LK, Wakker V, Bezzina CR, 't Hoen PA, Bakkers J, Barnett P, Christoffels VM. Genetic variation in T-box binding element functionally affects SCN5A/SCN10A enhancer. J Clin Invest. 2012;122:2519–2530. doi: 10.1172/JCI62613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van den Boogaard M, Smemo S, Burnicka-Turek O, Arnolds DE, van de Werken HJ, Klous P, McKean D, Muehlschlegel JD, Moosmann J, Toka O, Yang XH, Koopmann TT, Adriaens ME, Bezzina CR, de Laat W, Seidman C, Seidman JG, Christoffels VM, Nobrega MA, Barnett P, Moskowitz IP. A common genetic variant within SCN10A modulates cardiac SCN5A expression. J Clin Invest. 2014;124:1844–1852. doi: 10.1172/JCI73140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Verkerk AO, Remme CA, Schumacher CA, Scicluna BP, Wolswinkel R, de JB, Bezzina CR, Veldkamp MW. Functional Nav1.8 channels in intracardiac neurons: the link between SCN10A and cardiac electrophysiology. Circ Res. 2012;111:333–343. doi: 10.1161/CIRCRESAHA.112.274035. [DOI] [PubMed] [Google Scholar]
- 124.Yang T, Atack TC, Stroud DM, Zhang W, Hall L, Roden DM. Blocking Scn10a channels in heart reduces late sodium current and is antiarrhythmic. Circ Res. 2012;111:322–332. doi: 10.1161/CIRCRESAHA.112.265173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ritchie MD, Denny JC, Zuvich RL, Crawford DC, Schildcrout JS, Bastarache L, Ramirez AH, Mosley JD, Pulley JM, Basford MA, Bradford Y, Rasmussen LV, Pathak J, Chute CG, Kullo IJ, McCarty CA, Chisholm RL, Kho AN, Carlson CS, Larson EB, Jarvik GP, Sotoodehnia N, Manolio TA, Li R, Masys DR, Haines JL, Roden DM. Genome- and phenome-wide analyses of cardiac conduction identifies markers of arrhythmia risk. Circulation. 2013;127:1377–1385. doi: 10.1161/CIRCULATIONAHA.112.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jeff JM, Ritchie MD, Denny JC, Kho AN, Ramirez AH, Crosslin D, Armstrong L, Basford MA, Wolf WA, Pacheco JA, Chisholm RL, Roden DM, Hayes MG, Crawford DC. Generalization of variants identified by genome-wide association studies for electrocardiographic traits in African Americans. Ann Hum Genet. 2013;77:321–332. doi: 10.1111/ahg.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chambers JC, Zhao J, Terracciano CM, Bezzina CR, Zhang W, Kaba R, Navaratnarajah M, Lotlikar A, Sehmi JS, Kooner MK, Deng G, Siedlecka U, Parasramka S, El-Hamamsy I, Wass MN, Dekker LR, De Jong JS, Sternberg MJ, McKenna W, Severs NJ, de Silva R, Wilde AA, Anand P, Yacoub M, Scott J, Elliott P, Wood JN, Kooner JS. Genetic variation in SCN10A influences cardiac conduction. Nat Genet. 2010;42:149–152. doi: 10.1038/ng.516. [DOI] [PubMed] [Google Scholar]
- 128.Hu D, Barajas-Martinez H, Kahlig K, Rajamani S, Belardinelli L, Pfeiffer R, Dezi F, Antzelevitch C. Genetic variants in SCN10A associated with Brugada sydrome, right bundle branch block and atrioventricular block. Heart Rhythm. 2012;9:S395. [Google Scholar]
- 129.Sotoodehnia N, Isaacs A, de Bakker PI, Dorr M, Newton-Cheh C, Nolte IM, van der HP, Muller M, Eijgelsheim M, Alonso A, Hicks AA, Padmanabhan S, Hayward C, Smith AV, Polasek O, Giovannone S, Fu J, Magnani JW, Marciante KD, Pfeufer A, Gharib SA, Teumer A, Li M, Bis JC, Rivadeneira F, Aspelund T, Kottgen A, Johnson T, Rice K, Sie MP, Wang YA, Klopp N, Fuchsberger C, Wild SH, Mateo LI, Estrada K, Volker U, Wright AF, Asselbergs FW, Qu J, Chakravarti A, Sinner MF, Kors JA, Petersmann A, Harris TB, Soliman EZ, Munroe PB, Psaty BM, Oostra BA, Cupples LA, Perz S, de Boer RA, Uitterlinden AG, Volzke H, Spector TD, Liu FY, Boerwinkle E, Dominiczak AF, Rotter JI, van HG, Levy D, Wichmann HE, Van Gilst WH, Witteman JC, Kroemer HK, Kao WH, Heckbert SR, Meitinger T, Hofman A, Campbell H, Folsom AR, Van Veldhuisen DJ, Schwienbacher C, O'Donnell CJ, Volpato CB, Caulfield MJ, Connell JM, Launer L, Lu X, Franke L, Fehrmann RS, Te MG, Groen HJ, Weersma RK, van den Berg LH, Wijmenga C, Ophoff RA, Navis G, Rudan I, Snieder H, Wilson JF, Pramstaller PP, Siscovick DS, Wang TJ, Gudnason V, van Duijn CM, Felix SB, Fishman GI, Jamshidi Y, Ch Stricker BH, Samani NJ, Kaab S, Arking DE. Common variants in 22 loci are associated with QRS duration and cardiac ventricular conduction. Nat Genet. 2010;42:1068–1076. doi: 10.1038/ng.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boukens BJ, Sylva M, de Gier-de VC, Remme CA, Bezzina C, Christoffels VM, Coronel R. Reduced sodium channel function unmasks residual embryonic slow conduction in the adult right ventricular outflow tract. Circ Res. 2013;113:137–141. doi: 10.1161/CIRCRESAHA.113.301565. [DOI] [PubMed] [Google Scholar]
- 131.Hartman ME, Liu Y, Zhu WZ, Chien WM, Weldy CS, Fishman GI, Laflamme MA, Chin MT. Myocardial deletion of transcription factor CHF1/Hey2 results in altered myocyte action potential and mild conduction system expansion but does not alter conduction system function or promote spontaneous arrhythmias. FASEB J. 2014;28:3007–3015. doi: 10.1096/fj.14-251728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hennessey JA, Marcou CA, Wang C, Wei EQ, Wang C, Tester DJ, Torchio M, Dagradi F, Crotti L, Schwartz PJ, Ackerman MJ, Pitt GS. FGF12 is a candidate Brugada syndrome locus. Heart Rhythm. 2013;10:1886–1894. doi: 10.1016/j.hrthm.2013.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang C, Hennessey JA, Kirkton RD, Wang C, Graham V, Puranam RS, Rosenberg PB, Bursac N, Pitt GS. Fibroblast growth factor homologous factor 13 regulates Na+ channels and conduction velocity in murine hearts. Circ Res. 2011;109:775–782. doi: 10.1161/CIRCRESAHA.111.247957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hennessey JA, Wei EQ, Pitt GS. Fibroblast growth factor homologous factors modulate cardiac calcium channels. Circ Res. 2013;113:381–388. doi: 10.1161/CIRCRESAHA.113.301215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cerrone M, Lin X, Zhang M, Agullo-Pascual E, Pfenniger A, Chkourko GH, Novelli V, Kim C, Tirasawadischai T, Judge DP, Rothenberg E, Chen HV, Napolitano C, Priori S, Delmar M. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation. 2013;129:1092–1103. doi: 10.1161/CIRCULATIONAHA.113.003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kattygnarath D, Maugenre S, Neyroud N, Balse E, Ichai C, Denjoy I, Dilanian G, Martins RP, Fressart V, Berthet M, Schott JJ, Leenhardt A, Probst V, Le MH, Hainque B, Coulombe A, Hatem SN, Guicheney P. MOG1: a new susceptibility gene for Brugada syndrome. Circ Cardiovasc Genet. 2011;4:261–268. doi: 10.1161/CIRCGENETICS.110.959130. [DOI] [PubMed] [Google Scholar]
- 137.Olesen MS, Jensen NF, Holst AG, Nielsen JB, Tfelt-Hansen J, Jespersen T, Sajadieh A, Haunso S, Lund JT, Calloe K, Schmitt N, Svendsen JH. A novel nonsense variant in Nav1.5 cofactor MOG1 eliminates its sodium current increasing effect and may increase the risk of arrhythmias. Can J Cardiol. 2011;27:523–523. doi: 10.1016/j.cjca.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 138.Ishikawa T, Sato A, Marcou CA, Tester DJ, Ackerman MJ, Crotti L, Schwarts PJ, On YK, Park JE, Nakamura K, Hiraoka M, Nakazawa K, Sakurada H, Arimura T, Makita N, Kimura A. A novel disease gene for Brugada syndrome: sarcolemmal membrane-associated protein gene mutations impair intracellular trafficking of hNav1.5. Circ Arrhythm Electrophysiol. 2012;5:1098–1107. doi: 10.1161/CIRCEP.111.969972. [DOI] [PubMed] [Google Scholar]
- 139.Burashnikov E, Pfeiffer R, Barajas-Martinez H, Delpon E, Hu D, Desai M, Borggrefe M, Haissaguerre M, Kanter R, Pollevick GD, Guerchicoff A, Laino R, Marieb M, Nademanee K, Nam GB, Robles R, Schimpf R, Stapleton DH, Viskin S, Winters S, Wolpert C, Zimmern S, Veltmann C, Antzelevitch C. Mutations in the cardiac L-type calcium channel associated J wave sydnrome and sudden cardiac death. Heart Rhythm. 2010;7:1872–1882. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cordeiro JM, Marieb M, Pfeiffer R, Calloe K, Burashnikov E, Antzelevitch C. Accelerated inactivation of the L-type calcium due to a mutation in CACNB2b due to a mutation in CACNB2b underlies Brugada syndrome. J Mol Cell Cardiol. 2009;46:695–703. doi: 10.1016/j.yjmcc.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cornet V, Bichet D, Sandoz G, Marty I, Brocard J, Bourinet E, Mori Y, Villaz M, De Waard M. Multiple determinants in voltage-dependent P/Q calcium channels control their retention in the endoplasmic reticulum. Eur J Neurosci. 2002;16:883–895. doi: 10.1046/j.1460-9568.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 142.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 143.Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawaz Y, Guerchicoff A, Pfeiffer R, Oliva A, Wollnik B, Gelber P, Bonaros EP, Jr, Burashnikov E, Wu Y, Sargent JD, Schickel S, Bhatia A, Hsu LF, Haissaguerre M, Schimpf R, Borggrefe M, Wolpert C. Loss-of-function mutations in the cardiac calcium channel underline a new clinical entity characterized by ST segment elevation, short QT intervals, and sudden cardiac death. Circ Res. 2006;99:1279. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gurnett CA, De WM, Campbell KP. Dual function of the voltage-dependent Ca2+ channel alpha 2 delta subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 145.Delpón E, Cordeiro JM, Núñez L, Thomsen PEB, Guerchicoff A, Pollevick GD, Wu Y, Kanters JK, Larsen CT, Burashnikov A, Christiansen M, Antzelevitch C. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythm Electrophysiol. 2008;1:209–218. doi: 10.1161/CIRCEP.107.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Giudicessi JR, Ye D, Tester DJ, Crotti L, Mugione A, Nesterenko VV, Albertson RM, Antzelevitch C, Schwartz PJ, Ackerman MJ. Transient outward current (Ito) gain-of-function mutations in the KCND3-encoded Kv4.3 potassium channel and Brugada syndrome. Heart Rhythm. 2011;8:1024–1032. doi: 10.1016/j.hrthm.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Barajas-Martinez H, Hu D, Ferrer T, Onetti CG, Wu Y, Burashnikov E, Boyle M, Surman T, Urrutia J, Veltmann C, Schimpf R, Borggrefe M, Wolpert C, Ibrahim BB, Sanchez-Chapula JA, Winters S, Haissaguerre M, Antzelevitch C. Molecular genetic and functional association of Bugada and early repolarization syndromes with S422L missense mutation in KCNJ8. Heart Rhythm. 2012;9:548–555. doi: 10.1016/j.hrthm.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Delaney JT, Muhammad R, Blair MA, Kor K, Fish FA, Roden DM, Darbar D. A KCNJ8 mutation associated with early repolarization and atrial fibrillation. Europace. 2012;14:1428–1432. doi: 10.1093/europace/eus150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Medeiros-Domingo A, Tan BH, Crotti L, Tester DJ, Eckhardt L, Cuoretti A, Kroboth SL, Song C, Zhou Q, Kopp D, Schwartz PJ, Makielski JC, Ackerman MJ. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010;7:1466–1471. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hu D, Barajas-Martinez H, Terzic A, Park S, Pfeiffer R, Burashnikov E, Wu Y, Borggrefe M, Veltmann C, Schimpf R, Cai JJ, Nam GB, Deshmukh P, Scheinman M, Preminger M, Steinberg J, Lopez-Izquierdo A, Ponce-Balbuena D, Wolpert C, Haissaguerre M, Sanchez-Chapula JA, Antzelevitch C. ABCC9 is a novel Brugada and early repolarization syndrome susceptibility gene. Int J Cardiol. 2014;171:431–442. doi: 10.1016/j.ijcard.2013.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu H, Chatel S, Simard C, Syam N, Salle L, Probst V, Morel J, Millat G, Lopez M, Abriel H, Schott JJ, Guinamard R, Bouvagnet P. Molecular genetics and functional anomalies in a series of 248 Brugada cases with 11 mutations in the TRPM4 channel. PLoS ONE. 2013;8:e54131. doi: 10.1371/journal.pone.0054131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ohno S, Zankov DP, Wei-Guang D, Makiyama T, Hattori T, Doi T, Shizuta S, Kimura T, Matsuura H, Horie M. Novel KCNE5 mutations are associated with Brugada syndrome and idiopathic ventricular fibrillation. Circulation. 2009;120:S696. [Google Scholar]
- 153.Itoh H, Sakaguchi T, Ashihara T, Ding WG, Nagaoka I, Oka Y, Nakazawa Y, Yao T, Jo H, Ito M, Nakamura K, Ohe T, Matsuura H, Horie M. A novel KCNH2 mutation as a modifier for short QT interval. Int J Cardiol. 2009;137:83–85. doi: 10.1016/j.ijcard.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 154.Ueda K, Hirano Y, Higashiuesato Y, Aizawa Y, Hayashi T, Inagaki N, Tana T, Ohya Y, Takishita S, Muratani H, Hiraoka M, Kimura A. Role of HCN4 channel in preventing ventricular arrhythmia. J Hum Genet. 2009;54:115–121. doi: 10.1038/jhg.2008.16. [DOI] [PubMed] [Google Scholar]
- 155.Ueda K, Nakamura K, Hayashi T, Inagaki N, Takahashi M, Arimura T, Morita H, Higashiuesato Y, Hirano Y, Yasunami M, Takishita S, Yamashina A, Ohe T, Sunamori M, Hiraoka M, Kimura A. Functional characterization of a trafficking-defective HCN4 mutation, D553N, associated with cardiac arrhythmia. J Biol Chem. 2004;279:27194–27198. doi: 10.1074/jbc.M311953200. [DOI] [PubMed] [Google Scholar]
- 156.Crotti L, Marcou CA, Tester DJ, Castelletti S, Giudicessi JR, Torchio M, Medeiros-Domingo A, Simone S, Will ML, Dagradi F, Schwartz PJ, Ackerman MJ. Spectrum and prevalence of mutations Involving BrS1- through BrS12-susceptibility genes in a cohort of unrelated patients referred for Brugada syndrome genetic testing: implications for genetic testing. J Am Coll Cardiol. 2012;60:1410–1418. doi: 10.1016/j.jacc.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem. 2001;268:1646–1652. doi: 10.1046/j.1432-1327.2001.02036.x. [DOI] [PubMed] [Google Scholar]
- 158.Mizusawa Y, Wilde AA. Brugada syndrome. Circ Arrhythm Electrophysiol. 2012;5:606–616. doi: 10.1161/CIRCEP.111.964577. [DOI] [PubMed] [Google Scholar]
- 159.Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, Hershberger RE, Judge DP, Le MH, McKenna WJ, Schulze-Bahr E, Semsarian C, Towbin JA, Watkins H, Wilde A, Wolpert C, Zipes DP. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 160.Gollob MH, Blier L, Brugada R, Champagne J, Chauhan V, Connors S, Gardner M, Green MS, Gow R, Hamilton R, Harris L, Healey JS, Hodgkinson K, Honeywell C, Kantoch M, Kirsh J, Krahn A, Mullen M, Parkash R, Redfearn D, Rutberg J, Sanatani S, Woo A. Recommendations for the use of genetic testing in the clinical evaluation of inherited cardiac arrhythmias associated with sudden cardiac death: Canadian Cardiovascular Society/Canadian Heart Rhythm Society joint position paper. Can J Cardiol. 2011;27:232–245. doi: 10.1016/j.cjca.2010.12.078. [DOI] [PubMed] [Google Scholar]
- 161.Szel T, Antzelevitch C. Abnormal repolarization as the basis for late potentials and fractionated electrograms recorded from epicardium in experimental models of brugada syndrome. J Am Coll Cardiol. 2014;63:2037–2045. doi: 10.1016/j.jacc.2014.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, Di Diego JM, Gintant GA, Liu DW. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res. 1991;69:1427–1449. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- 163.Krishnan SC, Antzelevitch C. Sodium channel block produces opposite electrophysiological effects in canine ventricular epicardium and endocardium. Circ Res. 1991;69:277–291. doi: 10.1161/01.res.69.2.277. [DOI] [PubMed] [Google Scholar]
- 164.Antzelevitch C, Brugada P, Brugada J, Brugada R, Shimizu W, Gussak I, Perez Riera AR. Brugada syndrome: a decade of progress. Circ Res. 2002;91:1114–1119. doi: 10.1161/01.res.0000046046.53721.90. [DOI] [PubMed] [Google Scholar]
- 165.Krishnan SC, Antzelevitch C. Flecainide-induced arrhythmia in canine ventricular epicardium. Phase 2 reentry? Circulation. 1993;87:562–572. doi: 10.1161/01.cir.87.2.562. [DOI] [PubMed] [Google Scholar]
- 166.Antzelevitch C. The Brugada syndrome: ionic basis and arrhythmia mechanisms. J Cardiovasc Electrophysiol. 2001;12:268–272. doi: 10.1046/j.1540-8167.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 167.Lukas A, Antzelevitch C. Phase 2 reentry as a mechanism of initiation of circus movement reentry in canine epicardium exposed to simulated ischemia. Cardiovasc Res. 1996;32:593–603. [PubMed] [Google Scholar]
- 168.Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 169.Fish JM, Antzelevitch C. Role of sodium and calcium channel block in unmasking the Brugada syndrome. Heart Rhythm. 2004;1:210–217. doi: 10.1016/j.hrthm.2004.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kurita T, Shimizu W, Inagaki M, Suyama K, Taguchi A, Satomi K, Aihara N, Kamakura S, Kobayashi J, Kosakai Y. The electrophysiologic mechanism of ST-segment elevation in Brugada syndrome. J Am Coll Cardiol. 2002;40:330–334. doi: 10.1016/s0735-1097(02)01964-2. [DOI] [PubMed] [Google Scholar]
- 171.Di Diego JM, Cordeiro JM, Goodrow RJ, Fish JM, Zygmunt AC, Peréz GJ, Scornik FS, Antzelevitch C. Ionic and cellular basis for the predominance of the Brugada syndrome phenotype in males. Circulation. 2002;106:2004–2011. doi: 10.1161/01.cir.0000032002.22105.7a. [DOI] [PubMed] [Google Scholar]
- 172.Hong K, Berruezo-Sanchez A, Poungvarin N, Oliva A, Vatta M, Brugada J, Brugada P, Towbin JA, Dumaine R, Pinero-Galvez C, Antzelevitch C, Brugada R. Phenotypic characterization of a large European family with Brugada syndrome displaying a sudden unexpected death syndrome mutation in SCN5A. J Cardiovasc Electrophysiol. 2004;15:64–69. doi: 10.1046/j.1540-8167.2004.03341.x. [DOI] [PubMed] [Google Scholar]
- 173.Wilde AA, Postema PG, Di Diego JM, Viskin S, Morita H, Fish JM, Antzelevitch C. The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J Mol Cell Cardiol. 2010;49:543–553. doi: 10.1016/j.yjmcc.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morita H, Zipes DP, Wu J. Brugada syndrome: insights of ST elevation, arrhythmogenicity, and risk stratification from experimental observations. Heart Rhythm. 2009;6:S34–S43. doi: 10.1016/j.hrthm.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 175.Babaliaros VC, Hurst JW. Tricyclic antidepressants and the Brugada syndrome: an example of Brugada waves appearing after the administration of desipramine. Clin Cardiol. 2002;25:395–398. doi: 10.1002/clc.4950250809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goldgran-Toledano D, Sideris G, Kevorkian JP. Overdose of cyclic antidepressants and the Brugada syndrome. N Engl J Med. 2002;346:1591–1592. doi: 10.1056/NEJM200205163462020. [DOI] [PubMed] [Google Scholar]
- 177.Tada H, Sticherling C, Oral H, Morady F. Brugada syndrome mimicked by tricyclic antidepressant overdose. J Cardiovasc Electrophysiol. 2001;12:275–275. doi: 10.1046/j.1540-8167.2001.00275.x. [DOI] [PubMed] [Google Scholar]
- 178.Ortega-Carnicer J, Bertos-Polo J, Gutierrez-Tirado C. Aborted sudden death, transient Brugada pattern, and wide QRS dysrrhythmias after massive cocaine ingestion. J Electrocardiol. 2001;34:345–349. doi: 10.1054/jelc.2001.26318. [DOI] [PubMed] [Google Scholar]
- 179.Nogami A, Nakao M, Kubota S, Sugiyasu A, Doi H, Yokoyama K, Yumoto K, Tamaki T, Kato K, Hosokawa N, Sagai H, Nakamura H, Nitta J, Yamauchi Y, Aonuma K. Enhancement of J-ST-segment elevation by the glucose and insulin test in Brugada syndrome. Pacing Clin Electrophysiol. 2003;26:332–337. doi: 10.1046/j.1460-9592.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 180.Araki T, Konno T, Itoh H, Ino H, Shimizu M. Brugada syndrome with ventricular tachycardia and fibrillation related to hypokalemia. Circ J. 2003;67:93–95. doi: 10.1253/circj.67.93. [DOI] [PubMed] [Google Scholar]
- 181.Pastor A, Nunez A, Cantale C, Cosio FG. Asymptomatic Brugada syndrome case unmasked during dimenhydrinate infusion. J Cardiovasc Electrophysiol. 2001;12:1192–1194. doi: 10.1046/j.1540-8167.2001.01192.x. [DOI] [PubMed] [Google Scholar]
- 182.Noda T, Shimizu W, Taguchi A, Satomi K, Suyama K, Kurita T, Aihara N, Kamakura S. ST-segment elevation and ventricular fibrillation without coronary spasm by intracoronary injection of acetylcholine and/or ergonovine maleate in patients with Brugada syndrome. J Am Coll Cardiol. 2002;40:1841–1847. doi: 10.1016/s0735-1097(02)02494-4. [DOI] [PubMed] [Google Scholar]
- 183.Kasanuki H, Ohnishi S, Ohtuka M, Matsuda N, Nirei T, Isogai R, Shoda M, Toyoshima Y, Hosoda S. Idiopathic ventricular fibrillation induced with vagal activity in patients without obvious heart disease. Circulation. 1997;95:2277–2285. doi: 10.1161/01.cir.95.9.2277. [DOI] [PubMed] [Google Scholar]
- 184.Proclemer A, Facchin D, Feruglio GA, Nucifora R. Recurrent ventricular fibrillation, right bundle-branch block and persistent ST segment elevation in V1–V3: a new arrhythmia syndrome? A clinical case report. G Ital Cardiol. 1993;23:1211–1218. [PubMed] [Google Scholar]
- 185.Mizumaki K, Fujiki A, Tsuneda T, Sakabe M, Nishida K, Sugao M, Inoue H. Vagal activity modulates spontaneous augmentation of ST elevation in daily life of patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2004;15:667–673. doi: 10.1046/j.1540-8167.2004.03601.x. [DOI] [PubMed] [Google Scholar]
- 186.Wichter T, Matheja P, Eckardt L, Kies P, Schafers K, Schulze-Bahr E, Haverkamp W, Borggrefe M, Schober O, Breithardt G, Schafers M. Cardiac autonomic dysfunction in Brugada syndrome. Circulation. 2002;105:702–706. doi: 10.1161/hc0602.103677. [DOI] [PubMed] [Google Scholar]
- 187.Litovsky SH, Antzelevitch C. Differences in the electrophysiological response of canine ventricular subendocardium and subepicardium to acetylcholine and isoproterenol. A direct effect of acetylcholine in ventricular myocardium. Circ Res. 1990;67:615–627. doi: 10.1161/01.res.67.3.615. [DOI] [PubMed] [Google Scholar]
- 188.Kies P, Wichter T, Schafers M, Paul M, Schafers KP, Eckardt L, Stegger L, Schulze-Bahr E, Rimoldi O, Breithardt G, Schober O, Camici PG. Abnormal myocardial presynaptic norepinephrine recycling in patients with Brugada syndrome 1. Circulation. 2004;110:3017–3022. doi: 10.1161/01.CIR.0000146920.35020.44. [DOI] [PubMed] [Google Scholar]
- 189.Nimmannit S, Malasit P, Chaovakul V, Susaengrat W, Vasuvattakul S, Nilwarangkur S. Pathogenesis of sudden unexplained nocturnal death (lai tai) and endemic distal renal tubular acidosis. Lancet. 1991;338:930–932. doi: 10.1016/0140-6736(91)91786-t. [DOI] [PubMed] [Google Scholar]
- 190.Dumaine R, Towbin JA, Brugada P, Vatta M, Nesterenko DV, Nesterenko VV, Brugada J, Brugada R, Antzelevitch C. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res. 1999;85:803–809. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- 191.Gonzalez Rebollo G, Madrid H, Carcia A, Garcia de Casto A, Moro AM. Reccurrent ventricular fibrillation during a febrile illness in a patient with the Brugada Syndrome. Rev Esp Cardiol. 2000;53:755–757. doi: 10.1016/s0300-8932(00)75151-7. [DOI] [PubMed] [Google Scholar]
- 192.Madle A, Kratochvil Z, Polivkova A. The Brugada syndrome. Vnitr Lek. 2002;48:255–258. [PubMed] [Google Scholar]
- 193.Saura D, Garcia-Alberola A, Carrillo P, Pascual D, Martinez-Sanchez J, Valdes M. Brugada-like electrocardiographic pattern induced by fever. Pacing Clin Electrophysiol. 2002;25:856–859. doi: 10.1046/j.1460-9592.2002.t01-1-00856.x. [DOI] [PubMed] [Google Scholar]
- 194.Porres JM, Brugada J, Urbistondo V, Garcia F, Reviejo K, Marco P. Fever unmasking the Brugada syndrome. Pacing Clin Electrophysiol. 2002;25:1646–1648. doi: 10.1046/j.1460-9592.2002.01646.x. [DOI] [PubMed] [Google Scholar]
- 195.Kum L, Fung JWH, Chan WWL, Chan GK, Chan YS, Sanderson JE. Brugada syndrome unmasked by febrile illness. Pacing Clin Electrophysiol. 2002;25:1660–1661. doi: 10.1046/j.1460-9592.2002.01660.x. [DOI] [PubMed] [Google Scholar]
- 196.Ortega-Carnicer J, Benezet J, Ceres F. Fever-induced ST-segment elevation and T-wave alternans in a patient with Brugada syndrome. Resuscitation. 2003;57:315–317. doi: 10.1016/s0300-9572(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 197.Dzielinska Z, Bilinska ZT, Szumowski L, Grzybowski J, Michalak E, Przybylski A, Lubiszewska B, Walczak F, Ruzyllo W. Recurrent ventricular fibrillation during a febrile illness as the first manifestation of Brugada syndrome - a case report 1. Kardiol Pol. 2004;61:269–273. [PubMed] [Google Scholar]
- 198.Tanaka M, Nakamura K, Kusano KF, Morita H, Ohta-Ogo K, Miura D, Miura A, Nakagawa K, Tada T, Murakami M, Nishii N, Nagase S, Hata Y, Kohno K, Ouchida M, Shimizu K, Yutani C, Ohe T, Ito H. Elevated oxidative stress is associated with ventricular fibrillation episodes in patients with Brugada-type electrocardiogram without SCN5A mutation. Cardiovasc Pathol. 2011;20:e37–e42. doi: 10.1016/j.carpath.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 199.Brugada J, Brugada R, Brugada P. Pharmacological and device approach to therapy of inherited cardiac diseases associated with cardiac arrhythmias and sudden death. J Electrocardiol. 2000;33 (Suppl):41–47. doi: 10.1054/jelc.2000.20322. [DOI] [PubMed] [Google Scholar]
- 200.Brugada P, Brugada R, Brugada J, Geelen P. Use of the prophylactic implantable cardioverter defibrillator for patients with normal hearts. Am J Cardiol. 1999;83:98D–100D. doi: 10.1016/s0002-9149(98)01009-1. [DOI] [PubMed] [Google Scholar]
- 201.Takagi M, Sekiguchi Y, Yokoyama Y, Aihara N, Hiraoka M, Aonuma K. Long-term prognosis in patients with Brugada syndrome based on Class II indication for implantable cardioverter-defibrillator in the HRS/EHRA/APHRS Expert Consensus Statement: Multicenter study in Japan. Heart Rhythm. 2014 doi: 10.1016/j.hrthm.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 202.Sarkozy A, Boussy T, Kourgiannides G, Chierchia GB, Richter S, De Potter T, Geelen P, Wellens F, Spreeuwenberg MD, Brugada P. Long-term follow-up of primary prophylactic implantable cardioverter-defibrillator therapy in Brugada syndrome. Eur Heart J. 2007;28:334–344. doi: 10.1093/eurheartj/ehl450. [DOI] [PubMed] [Google Scholar]
- 203.Rosso R, Glick A, Glikson M, Wagshal A, Swissa M, Rosenhek S, Shetboun I, Khalamizer V, Fuchs T, Boulos M, Geist M, Strasberg B, Ilan M, Belhassen B. Outcome after implantation of cardioverter defribrillator in patients with Brugada syndrome: a multicenter Israeli study (ISRABRU) Isr Med Assoc J. 2008;10:435–439. [PubMed] [Google Scholar]
- 204.van Den Berg MP, Wilde AA, Viersma TJW, Brouwer J, Haaksma J, van der Hout AH, Stolte-Dijkstra I, Bezzina TCR, Van Langen IM, Beaufort-Krol GC, Cornel JH, Crijns HJ. Possible bradycardic mode of death and successful pacemaker treatment in a large family with features of long QT syndrome type 3 and Brugada syndrome. J Cardiovasc Electrophysiol. 2001;12:630–636. doi: 10.1046/j.1540-8167.2001.00630.x. [DOI] [PubMed] [Google Scholar]
- 205.Bertomeu-Gonzalez V, Ruiz-Granell R, Garcia-Civera R, Morell-Cabedo S, Ferrero A. Syncopal monomorphic ventricular tachycardia with pleomorphism, sensitive to antitachycardia pacing in a patient with Brugada syndrome. Europace. 2006;8:1048–1050. doi: 10.1093/europace/eul117. [DOI] [PubMed] [Google Scholar]
- 206.Lee KL, Lau C, Tse H, Wan S, Fan K. Prevention of ventricular fibrillation by pacing in a man with Brugada syndrome. J Cardiovasc Electrophysiol. 2000;11:935–937. doi: 10.1111/j.1540-8167.2000.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 207.Ayerza MR, de Zutter M, Goethals M, Wellens F, Geelen P, Brugada P. Heart transplantation as last resort against Brugada syndrome. J Cardiovasc Electrophysiol. 2002;13:943–944. doi: 10.1046/j.1540-8167.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- 208.Haissaguerre M, Extramiana F, Hocini M, Cauchemez B, Jais P, Cabrera JA, Farre G, Leenhardt A, Sanders P, Scavee C, Hsu LF, Weerasooriya R, Shah DC, Frank R, Maury P, Delay M, Garrigue S, Clementy J. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation. 2003;108:925–928. doi: 10.1161/01.CIR.0000088781.99943.95. [DOI] [PubMed] [Google Scholar]
- 209.Nakagawa E, Takagi M, Tatsumi H, Yoshiyama M. Successful radiofrequency catheter ablation for electrical storm of ventricular fibrillation in a patient with Brugada syndrome. Circ J. 2008;72:1025–1029. doi: 10.1253/circj.72.1025. [DOI] [PubMed] [Google Scholar]
- 210.Shah AJ, Hocini M, Lamaison D, Sacher F, Derval N, Haissaguerre M. Regional substrate ablation abolishes Brugada syndrome. J Cardiovasc Electrophysiol. 2011;22:1290–1291. doi: 10.1111/j.1540-8167.2011.02054.x. [DOI] [PubMed] [Google Scholar]
- 211.Sunsaneewitayakul B, Yao Y, Thamaree S, Zhang S. Endocardial mapping and catheter ablation for ventricular fibrillation prevention in Brugada syndrome. J Cardiovasc Electrophysiol. 2012;23 (Suppl 1):S10–S16. doi: 10.1111/j.1540-8167.2012.02433.x. [DOI] [PubMed] [Google Scholar]
- 212.Cortez-Dias N, Placido R, Marta L, Bernardes A, Sobral S, Carpinteiro L, de SJ. Epicardial ablation for prevention of ventricular fibrillation in a patient with Brugada syndrome. Rev Port Cardiol. 2014;33:305–305. doi: 10.1016/j.repc.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 213.Patocskai B, Szel T, Yoon N, Antzelevitch C. Cellular mechanisms underlying the fractionated and late potentials on epicardial electrograms and the ameliorative effect of epicardial radiofrequency ablation in an experimental model of Brugada syndrome. Program and abstracts of the 24th Annual Upstate New York Cardiac Electrophysiology Society Meeting; November 3, 2014; Buffalo, NY. 2014. p. Abstact 006. [Google Scholar]
- 214.Chinushi M, Aizawa Y, Ogawa Y, Shiba M, Takahashi K. Discrepant drug action of disopyramide on ECG abnormalities and induction of ventricular arrhythmias in a patient with Brugada syndrome. J Electrocardiol. 1997;30:133–136. doi: 10.1016/s0022-0736(97)80021-0. [DOI] [PubMed] [Google Scholar]
- 215.Minoura Y, Di Diego JM, Barajas-Martinez H, Zygmunt AC, Hu D, Sicouri S, Antzelevitch C. Ionic and cellular mechanisms underlying the development of acquired Brugada syndrome in patients treated with antidepressants. J Cardiovasc Electrophysiol. 2012;23:423–432. doi: 10.1111/j.1540-8167.2011.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Minoura Y, Panama BK, Nesterenko VV, Betzenhauser M, Barajas-Martinez H, Hu D, Di Diego JM, Antzelevitch C. Effect of Wenxin Keli and quinidine to suppress arrhythmogenesis in an experimental model of Brugada syndrome. Heart Rhythm. 2013;10:1054–1062. doi: 10.1016/j.hrthm.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gurabi Z, Koncz I, Patocskai B, Nesterenko VV, Antzelevitch C. Cellular mechanism underlying hypothermia-induced VT/VF in the setting of early repolarization and the protective effect of quinidine, cilostazol and milrinone. Circ Arrhythm Electrophysiol. 2014;7:134–142. doi: 10.1161/CIRCEP.113.000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Alings M, Dekker L, Sadee A, Wilde A. Quinidine induced electrocardiographic normalization in two patients with Brugada syndrome. Pacing Clin Electrophysiol. 2001;24:1420–1422. doi: 10.1046/j.1460-9592.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- 219.Belhassen B, Viskin S, Antzelevitch C. The Brugada syndrome: is an implantable cardioverter defibrillator the only therapeutic option? Pacing Clin Electrophysiol. 2002;25:1634–1640. doi: 10.1046/j.1460-9592.2002.01634.x. [DOI] [PubMed] [Google Scholar]
- 220.Belhassen B, Viskin S. Pharmacologic approach to therapy of Brugada syndrome: quinidine as an alternative to ICD therapy? In: Antzelevitch C, Brugada P, Brugada J, Brugada R, editors. The Brugada Syndrome: From Bench to Bedside. Oxford: Blackwell Futura; 2004. pp. 202–211. [Google Scholar]
- 221.Viskin S, Wilde AA, Tan HL, Antzelevitch C, Shimizu W, Belhassen B. Empiric quinidine therapy for asymptomatic Brugada syndrome: time for a prospective registry. Heart Rhythm. 2009;6:401–404. doi: 10.1016/j.hrthm.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Belhassen B, Glick A, Viskin S. Excellent long-term reproducibility of the electrophysiologic efficacy of quinidine in patients with idiopathic ventricular fibrillation and Brugada syndrome. Pacing Clin Electrophysiol. 2009;32:294–301. doi: 10.1111/j.1540-8159.2008.02235.x. [DOI] [PubMed] [Google Scholar]
- 223.Marquez MF, Bonny A, Hernandez-Castillo E, De SA, Gomez-Flores J, Nava S, Hidden-Lucet F, Iturralde P, Cardenas M, Tonet J. Long-term efficacy of low doses of quinidine on malignant arrhythmias in Brugada syndrome with an implantable cardioverter-defibrillator: A case series and literature review. Heart Rhythm. 2013;9:1995–2000. doi: 10.1016/j.hrthm.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 224.Pellegrino PL, Di BM, Brunetti ND. Quinidine for the management of electrical storm in an old patient with Brugada syndrome and syncope. Acta Cardiol. 2013;68:201–203. doi: 10.1080/ac.68.2.2967280. [DOI] [PubMed] [Google Scholar]
- 225.Probst V, Evain S, Gournay V, Marie A, Schott JJ, Boisseau P, Le MH. Monomorphic ventricular tachycardia due to Brugada syndrome successfully treated by hydroquinidine therapy in a 3-year-old child. J Cardiovasc Electrophysiol. 2006;17:97–100. doi: 10.1111/j.1540-8167.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- 226.Schweizer PA, Becker R, Katus HA, Thomas D. Successful acute and long-term management of electrical storm in Brugada syndrome using orciprenaline and quinine/quinidine. Clin Res Cardiol. 2010;99:467–470. doi: 10.1007/s00392-010-0145-7. [DOI] [PubMed] [Google Scholar]
- 227.Hasegawa K, Ashihara T, Kimura H, Jo H, Itoh H, Yamamoto T, Aizawa Y, Horie M. Long-term pharmacological therapy of Brugada syndrome: is J-wave attenuation a marker of drug efficacy? Intern Med. 2014;53:1523–1526. doi: 10.2169/internalmedicine.53.1829. [DOI] [PubMed] [Google Scholar]
- 228.Marquez MF, Rivera J, Hermosillo AG, Iturralde P, Colin L, Moragrega JL, Cardenas M. Arrhythmic storm responsive to quinidine in a patient with Brugada syndrome and vasovagal syncope. Pacing Clin Electrophysiol. 2005;28:870–873. doi: 10.1111/j.1540-8159.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 229.Viskin S, Antzelevitch C, Marquez MF, Belhassen B. Quinidine: a valuable medication joins the list of 'endangered species'. Europace. 2007;12:1105–1106. doi: 10.1093/europace/eum181. [DOI] [PubMed] [Google Scholar]
- 230.Ohgo T, Okamura H, Noda T, Satomi K, Suyama K, Kurita T, Aihara N, Kamakura S, Ohe T, Shimizu W. Acute and chronic management in patients with Brugada syndrome associated with electrical storm of ventricular fibrillation. Heart Rhythm. 2007;4:695–700. doi: 10.1016/j.hrthm.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 231.Belhassen B, Viskin S, Fish R, Glick A, Setbon I, Eldar M. Effects of electrophysiologic-guided therapy with Class IA antiarrhythmic drugs on the long-term outcome of patients with idiopathic ventricular fibrillation with or without the Brugada syndrome. J Cardiovasc Electrophysiol. 1999;10:1301–1312. doi: 10.1111/j.1540-8167.1999.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 232.Hermida JS, Denjoy I, Clerc J, Extramiana F, Jarry G, Milliez P, Guicheney P, Di Fusco S, Rey JL, Cauchemez B, Leenhardt A. Hydroquinidine therapy in Brugada syndrome. J Am Coll Cardiol. 2004;43:1853–1860. doi: 10.1016/j.jacc.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 233.Mok NS, Chan NY, Chi-Suen CA. Successful use of quinidine in treatment of electrical storm in Brugada syndrome. Pacing Clin Electrophysiol. 2004;27:821–823. doi: 10.1111/j.1540-8159.2004.00537.x. [DOI] [PubMed] [Google Scholar]
- 234.Bouzeman A, Traulle S, Messali A, Extramiana F, Denjoy I, Narayanan K, Marijon E, Hermida JS, Leenhardt A. Long-term follow-up of asymptomatic Brugada patients with inducible ventricular fibrillation under hydroquinidine. Europace. 2014;16:572–7. doi: 10.1093/europace/eut279. [DOI] [PubMed] [Google Scholar]
- 235.Tsuchiya T, Ashikaga K, Honda T, Arita M. Prevention of ventricular fibrillation by cilostazol, an oral phosphodiesterase inhibitor, in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2002;13:698–701. doi: 10.1046/j.1540-8167.2002.00698.x. [DOI] [PubMed] [Google Scholar]
- 236.Kyriazis K, Bahlmann E, van der SH, Kuck KH. Electrical storm in Brugada syndrome successfully treated with orciprenaline; effect of low-dose quinidine on the electrocardiogram. Europace. 2009;11:665–666. doi: 10.1093/europace/eup070. [DOI] [PubMed] [Google Scholar]
- 237.Maury P, Couderc P, Delay M, Boveda S, Brugada J. Electrical storm in Brugada syndrome successfully treated using isoprenaline. Europace. 2004;6:130–133. doi: 10.1016/j.eupc.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 238.Maury P, Hocini M, Haissaguerre M. Electrical storms in Brugada syndrome: review of pharmacologic and ablative therapeutic options. Indian Pacing Electrophysiol J. 2005;5:25–34. [PMC free article] [PubMed] [Google Scholar]
- 239.Jongman JK, Jepkes-Bruin N, Ramdat Misier AR, Beukema WP, Delnoy PP, Oude LH, Dambrink JH, Hoorntje JC, Elvan A. Electrical storms in Brugada syndrome successfully treated with isoproterenol infusion and quinidine orally. Neth Heart J. 2007;15:151–155. doi: 10.1007/BF03085972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Watanabe A, Fukushima KK, Morita H, Miura D, Sumida W, Hiramatsu S, Banba K, Nishii N, Nagase S, Nakamura K, Sakuragi S, Ohe T. Low-dose isoproterenol for repetitive ventricular arrhythmia in patients with Brugada syndrome. Eur Heart J. 2006;27:1579–1583. doi: 10.1093/eurheartj/ehl060. [DOI] [PubMed] [Google Scholar]
- 241.Furniss G. Isoprenaline and quinidine to calm Brugada VF storm. BMJ CaseRep. 2012 doi: 10.1136/bcr.04.2011.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Suzuki H, Torigoe K, Numata O, Yazaki S. Infant case with a malignant form of Brugada syndrome. J Cardiovasc Electrophysiol. 2000;11:1277–1280. doi: 10.1046/j.1540-8167.2000.01277.x. [DOI] [PubMed] [Google Scholar]
- 243.Tanaka H, Kinoshita O, Uchikawa S, Kasai H, Nakamura M, Izawa A, Yokoseki O, Kitabayashi H, Takahashi W, Yazaki Y, Watanabe N, Imamura H, Kubo K. Successful prevention of recurrent ventricular fibrillation by intravenous isoproterenol in a patient with Brugada syndrome. Pacing Clin Electrophysiol. 2001;24:1293–1294. doi: 10.1046/j.1460-9592.2001.01293.x. [DOI] [PubMed] [Google Scholar]
- 244.Shimizu W, Kamakura S. Catecholamines in children with congenital long QT syndrome and Brugada syndrome. J Electrocardiol. 2001;34 (Suppl):173–175. doi: 10.1054/jelc.2001.28864. [DOI] [PubMed] [Google Scholar]
- 245.Sharif-Kazemi MB, Emkanjoo Z, Tavoosi A, Kafi M, Kheirkhah J, Alizadeh A, Sadr-Ameli MA. Electrical storm in Brugada syndrome during pregnancy. Pacing Clin Electrophysiol. 2011;34:e18–e21. doi: 10.1111/j.1540-8159.2010.02740.x. [DOI] [PubMed] [Google Scholar]
- 246.Roten L, Derval N, Sacher F, Pascale P, Scherr D, Komatsu Y, Ramoul K, Daly M, Denis A, Shah AJ, Hocini M, Jais P, Haissaguerre M. Heterogeneous response of J wave syndromes to beta-adrenergic stimulation. Heart Rhythm. 2012;9:1970–1976. doi: 10.1016/j.hrthm.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 247.Kaneko Y, Horie M, Niwano S, Kusano K, Takatsuki S, Kurita T, Mitsuhashi T, Nakajima T, Irie T, Hasegawa K, Noda T, Kamakura S, Aizawa Y, Yasuoka R, Torigoe K, Suzuki H, Ohe T, Shimizu A, Fukuda K, Kurabayashi M, Aizawa Y. Electrical storm in patients with Brugada syndrome is associated with early repolarization. Circ ArrhythmElectrophysiol. 2014 doi: 10.1161/CIRCEP.114.001806. [DOI] [PubMed] [Google Scholar]
- 248.Agac MT, Erkan H, Korkmaz L. Conversion of Brugada type I to type III and successful control of recurrent ventricular arrhythmia with cilostazol. ArchCardiovasc Dis. 2013 doi: 10.1016/j.acvd.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 249.Kanlop N, Chattipakorn S, Chattipakorn N. Effects of cilostazol in the heart. J Cardiovasc Med (Hagerstown) 2011;12:88–95. doi: 10.2459/JCM.0b013e3283439746. [DOI] [PubMed] [Google Scholar]
- 250.Bai Y, Muqier, Murakami H, Iwasa M, Sumi S, Yamada Y, Ushikoshi H, Aoyama T, Nishigaki K, Takemura G, Uno B, Minatoguchi S. Cilostazol protects the heart against ischaemia reperfusion injury in a rabbit model of myocardial infarction: focus on adenosine, nitric oxide and mitochondrial ATP-sensitive potassium channels. Clin Exp Pharmacol Physiol. 2011;38:658–665. doi: 10.1111/j.1440-1681.2011.05550.x. [DOI] [PubMed] [Google Scholar]
- 251.Shinohara T, Ebata Y, Ayabe R, Fukui A, Okada N, Yufu K, Nakagawa M, Takahashi N. Combination therapy of cilostazol and bepridil suppresses recurrent ventricular fibrillation related to J-wave syndromes. Heart Rhythm. 2014;11:1441–1445. doi: 10.1016/j.hrthm.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 252.Abud A, Bagattin D, Goyeneche R, Becker C. Failure of cilostazol in the prevention of ventricular fibrillation in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2006;17:210–212. doi: 10.1111/j.1540-8167.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 253.Szel T, Koncz I, Antzelevitch C. Cellular mechanisms underlying the effects of milrinone and cilostazol to supress arrhythmogenesis associated with Brugada syndrome. Heart Rhythm. 2013;10:1720–1727. doi: 10.1016/j.hrthm.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Burashnikov A, Petroski A, Hu D, Barajas-Martinez H, Antzelevitch C. Atrial-selective inhibition of sodium channel current by Wenxin Keli is effective in suppressing atrial fibrillation. Heart Rhythm. 2012;9:125–131. doi: 10.1016/j.hrthm.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wang X, Wang X, Gu Y, Wang T, Huang C. Wenxin Keli attenuates ischemia-induced ventricular arrhythmias in rats: Involvement of Ltype calcium and transient outward potassium currents. Mol Med Report. 2013;7:519–524. doi: 10.3892/mmr.2012.1195. [DOI] [PubMed] [Google Scholar]
- 256.Sugao M, Fujiki A, Nishida K, Sakabe M, Tsuneda T, Iwamoto J, Mizumaki K, Inoue H. Repolarization dynamics in patients with idiopathic ventricular fibrillation: pharmacological therapy with bepridil and disopyramide. Journal of Cardiovascular Pharmacology. 2005;45:545–549. doi: 10.1097/01.fjc.0000159660.16793.84. [DOI] [PubMed] [Google Scholar]
- 257.Murakami M, Nakamura K, Kusano KF, Morita H, Nakagawa K, Tanaka M, Tada T, Toh N, Nishii N, Nagase S, Hata Y, Kohno K, Miura D, Ohe T, Ito H. Efficacy of low-dose bepridil for prevention of ventricular fibrillation in patients with Brugada syndrome with and without SCN5A mutation. J Cardiovasc Pharmacol. 2010;56:389–395. doi: 10.1097/FJC.0b013e3181f03c2f. [DOI] [PubMed] [Google Scholar]
- 258.Aizawa Y, Yamakawa H, Takatsuki S, Katsumata Y, Nishiyama T, Kimura T, Nishiyama N, Fukumoto K, Tanimoto Y, Tanimoto K, Mitamura H, Ogawa S, Fukuda K. Efficacy and safety of bepridil for prevention of ICD shocks in patients with Brugada syndrome and idiopathic ventricular fibrillation. Int J Cardiol. 2013;168:5083–5085. doi: 10.1016/j.ijcard.2013.07.187. [DOI] [PubMed] [Google Scholar]
- 259.Kang L, Zheng MQ, Morishima M, Wang Y, Kaku T, Ono K. Bepridil up-regulates cardiac Na+ channels as a long-term effect by blunting proteasome signals through inhibition of calmodulin activity. Br J Pharmacol. 2009;157:404–414. doi: 10.1111/j.1476-5381.2009.00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Fish JM, Welchons DR, Kim YS, Lee SH, Ho WK, Antzelevitch C. Dimethyl lithospermate B, an extract of danshen, suppresses arrhythmogenesis associated with the Brugada syndrome. Circulation. 2006;113:1393–1400. doi: 10.1161/CIRCULATIONAHA.105.601690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Antzelevitch C. The Brugada syndrome: diagnostic criteria and cellular mechanisms. Eur Heart J. 2001;22:356–363. doi: 10.1053/euhj.2000.2461. [DOI] [PubMed] [Google Scholar]
- 262.Krishnan SC, Josephson ME. ST segment elevation induced by class IC antiarrhythmic agents: underlying electrophysiologic mechanisms and insights into drug-induced proarrhythmia. Journal of Cardiovascular Electrophysiology. 1998;9:1167–1172. doi: 10.1111/j.1540-8167.1998.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 263.Fujiki A, Usui M, Nagasawa H, Mizumaki K, Hayashi H, Inoue H. ST segment elevation in the right precordial leads induced with class IC antiarrhythmic drugs: insight into the mechanism of Brugada syndrome. J Cardiovasc Electrophysiol. 1999;10:214–218. doi: 10.1111/j.1540-8167.1999.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 264.Gasparini M, Priori SG, Mantica M, Napolitano C, Galimberti P, Ceriotti C, Simonini S. Flecainide test in Brugada syndrome: a reproducible but risky tool. Pacing Clin Electrophysiol. 2003;26:338–341. doi: 10.1046/j.1460-9592.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 265.Takenaka S, Emori T, Koyama S, Morita H, Fukushima K, Ohe T. Asymptomatic form of Brugada syndrome. Pacing Clin Electrophysiol. 1999;22:1261–1263. doi: 10.1111/j.1540-8159.1999.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 266.Shimizu W, Aiba T, Kurita T, Kamakura S. Paradoxic abbreviation of repolarization in epicardium of the right ventricular outflow tract during augmentation of Brugada-type ST segment elevation. J Cardiovasc Electrophysiol. 2001;12:1418–1421. doi: 10.1046/j.1540-8167.2001.01418.x. [DOI] [PubMed] [Google Scholar]
- 267.Matana A, Goldner V, Stanic K, Mavric Z, Zaputovic L, Matana Z. Unmasking effect of propafenone on the concealed form of the Brugada phenomenon. Pacing Clin Electrophysiol. 2000;23:416–418. doi: 10.1111/j.1540-8159.2000.tb06774.x. [DOI] [PubMed] [Google Scholar]
- 268.Rolf S, Bruns HJ, Wichter T, Kirchhof P, Ribbing M, Wasmer K, Paul M, Breithardt G, Haverkamp W, Eckardt L. The ajmaline challenge in Brugada syndrome: diagnostic impact, safety, and recommended protocol. Eur Heart J. 2003;24:1104–1112. doi: 10.1016/s0195-668x(03)00195-7. [DOI] [PubMed] [Google Scholar]
- 269.Tada H, Nogami A, Shimizu W, Naito S, Nakatsugawa M, Oshima S, Taniguchi K. ST segment and T wave alternans in a patient with Brugada syndrome. Pacing Clin Electrophysiol. 2000;23:413–415. doi: 10.1111/j.1540-8159.2000.tb06773.x. [DOI] [PubMed] [Google Scholar]
- 270.Matsuo K, Shimizu W, Kurita T, Inagaki M, Aihara N, Kamakura S. Dynamic changes of 12-lead electrocardiograms in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 1998;9:508–512. doi: 10.1111/j.1540-8167.1998.tb01843.x. [DOI] [PubMed] [Google Scholar]
- 271.Bolognesi R, Tsialtas D, Vasini P, Conti M, Manca C. Abnormal ventricular repolarization mimicking myocardial infarction after heterocyclic antidepressant overdose. Am J Cardiol. 1997;79:242–245. doi: 10.1016/s0002-9149(96)00727-8. [DOI] [PubMed] [Google Scholar]
- 272.Rouleau F, Asfar P, Boulet S, Dube L, Dupuis JM, Alquier P, Victor J. Transient ST segment elevation in right precordial leads induced by psychotropic drugs: relationship to the Brugada syndrome. J Cardiovasc Electrophysiol. 2001;12:61–65. doi: 10.1046/j.1540-8167.2001.00061.x. [DOI] [PubMed] [Google Scholar]
- 273.El-Menyar A, Khan M, Al SJ, Eljerjawy E, Asaad N. Oxcarbazepine-induced resistant ventricular fibrillation in an apparently healthy young man. Am J Emerg Med. 2011;29:693–693. doi: 10.1016/j.ajem.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 274.Vernooy K, Sicouri S, Dumaine R, Hong K, Oliva A, Burashnikov E, Timmermans C, Delhaas T, Crijns HJ, Antzelevitch C, Rodriguez LM, Brugada R. Genetic and biophysical basis for bupivacaine-induced ST segment elevation and VT/VF. Anesthesia unmasked Brugada syndrome. Heart Rhythm. 2006;3:1074–1078. doi: 10.1016/j.hrthm.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Phillips N, Priestley M, Denniss AR, Uther JB. Brugada-type electrocardiographic pattern induced by epidural bupivacaine. Anesth Analg. 2003;97:264–7. doi: 10.1213/01.ane.0000067410.32384.3a. table. [DOI] [PubMed] [Google Scholar]
- 276.Arumugam D, Atherton JJ, Martin PT. A lethal injection? Lancet. 2012;379:492. doi: 10.1016/S0140-6736(11)61711-X. [DOI] [PubMed] [Google Scholar]
- 277.Inamura M, Okamoto H, Kuroiwa M, Hoka S. General anesthesia for patients with Brugada syndrome. A report of six cases. Can J Anaesth. 2005;52:409–412. doi: 10.1007/BF03016285. [DOI] [PubMed] [Google Scholar]
- 278.Robinson JD, Melman Y, Walsh EP. Cardiac conduction disturbances and ventricular tachycardia after prolonged propofol infusion in an infant. Pacing Clin Electrophysiol. 2008;31:1070–1073. doi: 10.1111/j.1540-8159.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- 279.Vernooy K, Delhaas T, Cremer OL, Di Diego JM, Oliva A, Timmermans C, Volders PG, Prinzen FW, Crijns HJ, Antzelevitch C, Kalkman CJ, Rodriguez LM, Brugada R. Electrocardiographic changes predicting sudden death in propofol-related infusion syndrome. Heart Rhythm. 2006;3:131–137. doi: 10.1016/j.hrthm.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Littmann L, Monroe MH, Svenson RH. Brugada-type electrocardiographic pattern induced by cocaine. Mayo Clin Proc. 2000;75:845–849. doi: 10.4065/75.8.845. [DOI] [PubMed] [Google Scholar]
- 281.Romero-Puche AJ, Trigueros-Ruiz N, Cerdan-Sanchez MC, Perez-Lorente F, Roldan D, Vicente-Vera T. Brugada electrocardiogram pattern induced by cannabis. Rev Esp Cardiol (EnglEd) 2012;65:856–858. doi: 10.1016/j.recesp.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 282.Brugada J, Brugada R, Brugada P. Right bundle-branch block and ST-segment elevation in leads V1 through V3. A marker for sudden death in patients without demonstrable structural heart disease. Circulation. 1998;97:457–460. doi: 10.1161/01.cir.97.5.457. [DOI] [PubMed] [Google Scholar]
- 283.Fish JM, Extramiana F, Antzelevitch C. Tedisamil abolishes the arrhythmogenic substrate responsible for VT/VF in an experimental model of the Brugada syndrome. Heart Rhythm. 2004;1(1S):S158. [Google Scholar]
- 284.Fish JM, Extramiana F, Antzelevitch C. AVE0118, an Ito and IKur blocker, suppresses VT/VF in an experimental model of the Brugada syndrome. Circulation. 2004;110(17):III–193. [Google Scholar]
- 285.Gurabi Z, Koncz I, Patocskai B, Nesterenko VV, Antzelevitch C. Cellular mechanism underlying hypothermia-induced ventricular tachycardia/ventricular fibrillation in the setting of early repolarization and the protective effect of quinidine, cilostazol, and milrinone. Circ Arrhythm Electrophysiol. 2014;7:134–42. doi: 10.1161/CIRCEP.113.000919. [DOI] [PMC free article] [PubMed] [Google Scholar]