Abstract
Prescribing of antidepressant treatment (ADT) for major depressive disorder (MDD) has increased in quantity and popularity over the last two decades. This is likely due to the approval of safer medications, better education of clinicians and their patients, direct-to-consumer marketing practices, and less stigma associated with those taking ADT. This trend has also been met with some controversy, however, as the ongoing safety and effectiveness of these treatments have at times been called into question. This paper discusses the differing levels of evidence that support the use of ADT based on (A) Food and Drug Administration approvals, (B) data from randomized controlled trials or meta-analyses and, where these are not available, the authors discuss and apply, (C) theoretical pharmacodynamic principles to justify antidepressant choice in the treatment of MDD patients. The final section discusses standard psychopharmacology guideline approaches to better alert the reader as to which practices are commonplace compared with those which are more outside of the standard of care.
Keywords: antidepressants, pharmacotherapy, psychotropics, efficacy, depression, pharmacodynamics, safety, effectiveness
Introduction
The use of pharmacotherapy for major depressive disorder (MDD) has been a mainstay of treatment ever since the first “classical” antidepressants, iproniazid and imipramine, were clinically introduced in the 1950s. That decade’s serendipitous discovery that depletion of monoaminergic neurotransmitters in the brain could lead to depressive symptoms forced a rapid paradigm shift, Freudian to biological, in the understanding of mood disorders generally and of MDD in particular. This ultimately incentivized pharmaceutical companies to develop antidepressant treatments (ADTs) specifically targeted to correct these neurochemical imbalances. The initial wave of such drugs included the monoamine oxidase inhibitors (MAOIs) and the tricyclic antidepressants (TCAs), yet concerns over their relative safety continued to fuel the search for a “better pill” well into the 1980s, when a safer ADT class called selective serotonin reuptake inhibitors (SSRIs) revolutionized the field of psychopharmacology. Their unprecedented success and popularity heralded prescription therapy as a clear standard of care for MDD of any severity; by 2008, ADTs were the third-most prescribed medication in the USA [1].
As scientific knowledge of the neurobiological basis for MDD continues to increase, so too, in apparent lockstep, does the arsenal of drugs used to treat it. In addition to the wide array of ADT classes available on the market today, each modulating one or more of the relevant biogenic amines (serotonin, norepinephrine, dopamine), there exists a panoply of diverse compounds per class, all with distinct pharmacokinetic and pharmacodynamic properties affecting drug potency and efficacy. The complex interplay of these intrinsic pharmacologic properties with countless clinical, environmental, and genetic factors (age, sex, comorbid conditions, diet, smoking habits, genetic polymorphisms related to drug metabolism, hepatic/ renal function, neurotransmission, etc.) underlies the immense variability seen in ADT safety, response, and effectiveness at the individual level.
The abundance of pharmaceutical alternatives over the years has engendered both enthusiasm and skepticism from those in the psychiatric community. On the one hand, a mixed bag of options means greater opportunity for individualization (i.e., stepwise treatment switching in order to “find the right fit”) as well as for more aggressive therapeutic strategies like augmentation and combining ADT together. On the other hand, the preponderance of psychopharmacotherapy has been denounced for instituting an overprescribed, “pill-happy” culture. The fact that one in ten citizens is currently prescribed an ADT may reflect overtreatment of patients with subsyndromal MDD or adjustment disorders [2]. However, data also suggest that only half of the MDD patient population is accurately diagnosed and that only half of the accurately diagnosed MDD population is adequately prescribed [3]. Thus, two distinct problems—overprescribing and inadequate prescribing—need to be addressed, in part by increasing awareness of the proper ADT treatment guidelines. Other critics instead have questioned the clinical effectiveness of ADT monotherapy as a whole, unconvinced of the “serotonin-hypothesis” [4]. While our understanding of MDD etiopathogenesis has long surpassed the naïve notion that the clinically depressed brain is merely a bland neurochemical “soup” in need of enrichment, monoaminergic modulation at the synaptic level remains a fundamental part of the explanation. What poses perhaps a bigger problem to the advancement of MDD treatment is failure to recognize the importance of illness heterogeneity no less than ADT heterogeneity. As part of a fluid spectrum of mood disorders, MDD may manifest very differently in some patients than in others, defying nosological convention or straightforward syndromal classification. As an example, melancholic depression presents very differently from an atypical depression or a seasonal one. The variable amenability of MDD to ADT reflects similar neurobiological subtleties at the individual patient level (some better known than others), a fact which undermines the all-too-common assumption that ADTs are “one-size-fits-all”: equally effective for all MDD patients. This paper seeks not only to challenge this assumption and emphasize the need for personalized MDD treatment, but to offer a brief, synthesized compendium of information on available ADT that may guide the general clinician through the lesser-known subtleties and complexities of psychopharmacological practice.
Examining the evidence: randomized controlled trials
To better understand how the “one-size-fits-all” assumption of ADT has arisen over the past two decades, a brief look at the ADT drug development and approval process is needed. In clinical research, randomized controlled trials (RCTs) represent the gold standard by which the overall quality of drugs, treatments, and other therapeutic interventions can be assessed and compared. The whole process is multiphasic and strictly regulated by the US Food and Drug Administration (FDA). Schwartz et al. provide a thorough review of the antidepressant approval process and it is summarized in part below [5]. RCTs have been a standard for evaluating medical therapies, including ADT, for over 60 years. Based on the results of these RCTs, it has been suggested for decades that all FDA-approved ADTs are roughly equal in achieving a “reasonable” response outcome in MDD patients, defined by the FDA as a 50% reduction in depressive symptoms. In clinical practice, where prescribers strive for complete remission (in which the patient is functionally well again and free of depression), this 50% reduction benchmark appears arbitrary and, perhaps, too modest [6–9]. Furthermore, to judge ADT efficacy based largely on this arbitrary benchmark, set across all MDD trials, would appear to assume homogeneity of the MDD patient population with regard to symptom type and severity. This assumption, however, rarely holds, and, if taken for granted, can lead to underpowered RCTs and to the inaccurate interpretation that all antidepressants are equal. Clinical practice, which often renders apparent those subtle patient differences obscured in clinical trials, is a constant rebuke to that interpretation [10,11].
Statistically, ADTs are expected to deliver a response rate of approximately 67% compared with a placebo response rate of about only 33%. The advantage of an active ADT study drug in this case could be expressed as an absolute value of only +34%, as a relative benefit (+100% or a twofold advantage), or as an odds ratio (OR; computed as: 0.67/0.33 divided by 0.33/0.67=2 divided by 0.5=+4.0). This is often measured as a difference in pre-/post-treatment scores on a standard depression scale, such as the Hamilton Rating Scale for Depression (HAM-D) or the Montgomery–Asberg Depression Rating Scale [12,13]. Typically, a 33% advantage in a response rate would correspond to a 6-point lessening on a HAM-D. Such a difference can be expressed with the standardized term effect size (referred to as d). In this case, the effect size (d) would equal 0.6. This would be considered a large effect size, suggesting a high likelihood that the ADT is very effective in treating MDD. An investigation planned to detect such a difference would need to enroll only 30–50 patients per arm in order to obtain the 80% statistical power required to interpret valid results [14]. If smaller treatment differences (d) are to be observed, then RCT sample sizes would need to be greatly increased to ensure good statistical power. This approach, or this type of large study, is relatively unheard of in psychiatric trials and may explain why no clear RCT literature exists to definitively show superiority of one ADT over another. This often forces clinicians to rely on the “in-the-trenches art” of psychotropic prescribing, based on the clinician’s knowledge of drug pharmacodynamics, functional neuroanatomy, and clinical experience treating many different patients, each of whom may benefit from slightly different pharmaceutical regimens.
Type 2 bias errors in antidepressant clinical trials have increased over time as well [10]. Intent-to-treat principles in analyzing study results, decisions to conduct longer trials, and completing trials in less severe patients and ambulatory settings all have resulted in higher attrition rates [8,15]. Increases in placebo response rates are often elevated up to 40% and are widespread when compared with the initial TCA trials conducted in the 1950s and 1960s [16]. These older trials often included more severe, more chronic, and more melancholic MDD patients as well. Accounting for these statistical biases in modern-day comparative ADT studies would necessitate enrollment of 500 patients or more per arm to yield 80% statistical power. Again, this is typically not the case in FDA RCTs, nor independent National Institute of Mental Health RCTs [17,18]. The net result is to have all ADTs appear to be equally effective.
Meta-analyses are also used to better delineate ADT efficacy differences. There are two types of meta-analyses: one that uses summary data extracted from published papers existing in the literature, and another that pools the raw data (using the actual rating scale data) from each participant in each RCT. The former method is more widely used, as it is easier to obtain these data through literature searches than it is to obtain raw rating scale data from many different corporate trials. Meta-analyses may be biased depending on which studies are included or excluded and what data are utilized or discarded, and are often open to greater controversy post hoc than RCT. Meta-analyses are often met with controversies that result in conflicting editorials in journals and lay press about how to interpret the initial results.
ADT: comparing classes and compounds
Now that the authors have established how standard RCTs and meta-analyses are conducted, this next section will provide a brief review and comparison of the major classes of ADT, starting with the older and more established TCA and MAOI agents—which are still part of standard care and are sometimes employed in resistant and comorbid cases—and then moving toward those drugs which are more widely prescribed today as first-line treatment, including the popular SSRIs as well as several “atypical” compounds that have complex mechanisms of action and thus cannot be neatly categorized. Furthermore, to enhance the general clinician’s understanding of how ADTs exert their effects on the brain, the discussion of SSRIs will include a section outlining the basic neurobiology behind how a typical, first-line SSRI monotherapy may result in gradual improvement of depressive symptoms.
The classic ADTs
TCAs and MAOIs
These two classes of compounds comprise the earliest specific ADT. Their clinical introduction in the 1950s following fortuitous discovery of their antidepressant properties marked the advent of psychopharmacotherapy as an indispensable tool in the treatment of MDD and spurred the first monoaminergic theories in the etiopathogenesis of depression.
TCAs, which take their name from the basic three-ring chemical structure common to them, act primarily by elevating serotonin and norepinephrine levels via uptake inhibition (similar to the later developed serotonin norepinephrine reuptake inhibitors [SNRIs] mechanistically). However, as they also antagonize muscarinic acetylcholine receptors, they are prone to anticholinergic side effects (e.g., dry mouth, blurry vision, constipation, urinary retention), which often limit their utility. In addition, TCAs are known to cause prominent weight gain and sedation and can block cardiac sodium channels, which in the case of overdose may lead to sudden cardiac death [19]. In a meta-analytic review of TCA compared with SSRI side effects, Montgomery et al. found that patients on TCAs discontinued treatment 27% of the time compared with 19% for those on SSRIs [20]. In elderly MDD patients, the rates were 33% and 16%, respectively [21].
MAOIs, in contrast, act by inhibiting the activity of the enzyme monoamine oxidase, thereby preventing the breakdown of monoamine neurotransmitters. Two enzyme isoforms exist, MAO-A and MAO-B, which preferentially degrade different amines. The early nonselective MAOIs, like the TCAs, were often limited in their use due to adverse events. In the case of MAOIs, this included dangerous and potentially lethal interactions with food, particularly foods rich in tyramine (e.g., aged cheese), and with other medications. Fatal serotonin syndromes or hypertensive crises may develop, respectively, by inappropriate use of these agents. In fact, MAOIs should not be used in ADT augmentation with SSRIs due to a potentially lethal increase in serotonin, known as “serotonin syndrome.” MAOIs are also known to promote weight gain, as well as fatigue and hypotension. Consequently, they are often the last pharmacologic alternative after all other ADT options have failed to yield remission. Some newer MAOIs, however, such as selegiline and the reversible MAOI moclobemide, have proven safer and may be considered for use earlier in treatment perhaps.
Thase et al. conducted a meta-analysis of all published reports comparing TCA and MAOI agents [22]. This team found that, although MAOIs outperformed placebo in treating hospitalized, severely depressed patients, they were significantly less effective than TCAs in this patient subgroup. In contrast, other studies have found that MAOIs are more effective than TCAs in treating outpatients who were less depressed and exhibited more atypical MDD features [23]. Such findings suggest that these two classes of ADTs may have a niche, or specific patient subtype, for which they are most effective, a fact which further underlines the importance of considering inpatient–outpatient status and depressive severity in treatment algorithms. RCT data equating these two ADT classes in the absence of stratified analysis should therefore be heavily scrutinized by the prescriber. Meta-analyses comparing these classic ADTs with the more novel (post-1980s) ADTs have also been published. According to these data, the advantage of TCAs in the more melancholic and severely depressed inpatient setting persists not only over MAOIs but also over the well-prescribed, modern SSRIs [18,22,24–27]. The TCA agents imipramine and amitriptyline faired the best. Considering the mechanism of action of the TCAs, it is postulated that serotonergic facilitation ameliorates limbic dysfunction, whereas more norepinephrine promotes better function in the dorsolateral prefrontal cortex (DLPFC).
While these two older classes have largely fallen out of favor, there continues to be wide-scale use of TCAs for insomnia, pain, and resistant MDD, and of MAOIs for resistant MDD and social anxiety disorder. With regard to the MAOIs, there is some new research to indicate that the risk and severity of adverse dietary interactions may be much less alarming than previously thought, especially in the setting of proper monitoring and adherence [28]. Nevertheless, misconceptions and fears of the drug’s risks can lead to patients never starting or later discontinuing potentially helpful treatment. This tends to promote a conservative prescribing practice. It is, therefore, important that clinicians address and discuss with their patients any fears or concerns about medication side effects in order to keep all therapeutic avenues open.
The modern ADTs
SSRIs
The SSRI class of ADT is the most widely utilized in the modern era of MDD treatment. It is comprised of five agents: fluoxetine, sertraline, paroxetine, citalopram, and escitalopram. These agents use the serotonin reuptake inhibition property found in a majority of the TCA class, but are selective for this one mechanism alone, and thus avoid many of the anticholinergic and cardiac side effects of the TCAs. Additionally, they do not require dietary and drug-related restrictions as the MAOIs do. The enhanced safety of SSRIs drove a veritable revolution in the treatment of depression during the 1990s, allowing more patients to be treated by prescription than ever before. During the previous era, MDD patients had to suffer severe MDD or melancholia to warrant the side-effect risk associated with these ADTs. With the development and introduction of safer drugs on the market, and the risk of pharmacotherapy greatly reduced, ADT could now be prescribed for MDD patients who were mild or moderately symptomatic, possibly even subsyndromal [29].
Having now introduced the most commonly prescribed first-line treatment for MDD, it will be useful here to provide the reader with a basic neurobiological picture of how a typical SSRI may start to relieve depressive symptoms in a patient with moderate, uncomplicated MDD. As mentioned, a typical SSRI blocks serotonin reuptake pumps (more often called serotonin transporters in the literature), acutely raising this transmitter in neuronal synapses. Despite this relatively immediate effect, MDD symptoms usually begin to resolve a few weeks later [30], suggesting that an acute rise in the level of neurotransmitter is necessary but insufficient to effect a change in phenotypic, exhibited depressive symptoms. For observable symptom improvement to arise, there must be a gradual shift in brain homeostasis and neurofunctioning over time, a shift away from a current depressive baseline, involving many different neuroanatomical areas and structures in the brain (e.g., receptors and enzymes). In this process, elevated serotonin may be, at first, biologically “interpreted” by neurons as locally toxic, causing certain cells to undergo stress-related changes in order to accommodate, or adapt to, higher-than-baseline serotonin levels. Subsequently, the activity of certain neuronal enzymes (i.e., those involved in serotonin metabolism) will increase, while that of others will decrease; similarly, a subset of receptors will start to become desensitized or downregulated, while other receptors (i.e., those responsive to serotonin) will be more heavily synthesized and shuttled to the plasma membrane. This degree of selective gene activation with associated protein formation may take 4–6 weeks, thus correlating with the time it takes an antidepressant to reach full effect [30].
According to this view, then, antidepressants create a new environment in the central nervous system (CNS)—a favorable yet initially stressful chemical milieu—that prompts certain genes to be activated, and new proteins to be synthesized, that may better accommodate the perceived neurochemical stress. However, once these changes have taken place, sustained increases in serotonin associated with SSRI adherence will start to reestablish a new, elevated baseline, one which will not only allow a return to normal functioning but also provide the neurotrophic substrate necessary for building new adaptive neural pathways. In fact, many antidepressants and mood stabilizers, regardless of mechanism of action, promote greater amounts and activity of brain neurotrophic factors, which may reverse MDD-induced brain atrophy, increase synaptic plasticity, and perhaps restore balance between limbic and cortical activities to alleviate depression [31–33]. This ability to increase gene activity to promote better synaptic levels of neurotrophic proteins may indeed start with the excessive “toxic” serotonin initially provided, most commonly, by SSRI monotherapy.
Among the SSRI class, there are some subtle yet notable pharmacologic differences. For example, the therapeutic effects of fluoxetine may emerge more slowly than those of some other SSRIs. Escitalopram may be more effective than its parent drug citalopram [34]. While all of the mentioned SSRIs are approved to treat MDD, some are also specifically approved to treat panic disorder, social anxiety disorder, obsessive– compulsive disorder, post-traumatic stress disorder (PTSD), bulimia nervosa, and/or premenstrual dysphoric disorder. To be FDA approved for a particular disorder, an SSRI must have at least two positive RCTs demonstrating their efficacy against that disease. Sertraline and paroxetine have the most FDA approvals and indications to treat a myriad of psychiatric disorders. An initial SSRI monotherapy should be chosen based on the patient’s diagnosis utilizing knowledge of these FDA approvals [23].
There are also subtle side-effect differences among the SSRIs. All may cause headaches, gastrointestinal disturbance, insomnia, fatigue, initial anxiety, and so on. Paroxetine eventually may allow more weight gain. This and citalopram may be the most sedating. Sertraline may have more adverse gastrointestinal effects. This and paroxetine more commonly cause withdrawal symptoms upon abrupt cessation due to their relatively short half-lives. Paroxetine and fluoxetine have more drug–drug interactions via CYP 4502D6 hepatic enzyme inhibition. These subtle diagnostic considerations, differences in efficacy, and adverse effects are usually weighed by the prescriber prior to choosing the ADT on a per-patient basis. Each SSRI is felt to be unique in clinical application despite being considered essentially equal per current regulatory processes.
SNRIs
Other modern ADTs exist that are not SSRI based. These include the SNRIs—chiefly venlafaxine, desvenlafaxine, duloxetine, and levomilnacipran—which dually inhibit serotonin and norepinephrine reuptake pumps comparably, allowing treatment of a wide range of depressive symptoms. This kind of dual reuptake inhibition is similar to the TCAs, but like the SSRI class, SNRIs are associated with less serious side effects. Some of these side effects include initial increases in anxiety, insomnia, and restlessness, and possible sexual dysfunction and headaches as well. Compared with the SSRI class, the SNRI class tends to induce more nausea, insomnia, dry mouth, and in rare cases elevated blood pressure [23].
With regard to comparative efficacy, Thase and colleagues [35] conducted a pooled analysis using the more stringent depressive remission benchmark over the usual FDA 50% response benchmark. They found that 45% of venlafaxine-treated patients gained remission compared with 35% on SSRI and 25% on placebo [35]. This was largely replicated by Nemeroff et al. in his team’s meta-analysis and also by a smaller study by Smith et al., all observing superior response rates for SNRIs [36,37]. A pooled analysis of another SNRI, duloxetine, utilizing less RCT data, found no statistical differences with SSRI [38] despite better response rates and higher HAM-D scale scores. It is currently unclear, therefore, whether SNRIs globally achieve a greater effect in MDD treatment or whether the effect is drug or dose dependent.
Desvenlafaxine, synthesized from the primary active metabolite of venlafaxine, is a relatively new SNRI developed to improve upon the strength of its parent drug. In addition to in vitro studies indicating its more potent inhibition of norepinephrine transporters, noninferiority RCTs have compared the efficacy (symptom reduction) of desvenlafaxine favorably with venlafaxine and SSRIs. This is evidenced by a similar magnitude of change among experimental groups from baseline to endpoint on mean HAM-D17 scores compared with placebo [39,40]. Treatment outcome (effectiveness) data have also been positive, with observed remission rates similar to those reported by Thase and colleagues from placebo-controlled venlafaxine studies [41].
The fourth member of the SNRI class, levomilnacipran, differs from the other SNRIs in being a more potent and selective inhibitor of norepinephrine than serotonin, especially at low doses [42]. In the absence of modern selective norepinephrine reuptake inhibitors, this characteristic may represent a novel therapeutic contribution to MDD patients, especially among those with symptoms of DLPFC hypofunction (e.g., fatigue, poor concentration, executive dysfunction) who have not been able to tolerate other SNRIs.
The “atypical” ADTs
Mirtazapine, trazodone, and bupropion
Among the ADTs arsenal prescribers have at their disposal, some drugs have been labeled “atypical” in the sense that they do not fit neatly—whether structurally or mechanistically—into any of the previous classes. Mirtazapine is one such agent, named colloquially one of the “sedating antidepressants,” as it tends to promote sleep or drowsiness. Unfortunately, its daytime sedation rates are high, and it is a weight gain-prone ADT. It has a low rate of sexual dysfunction [23]. It does not utilize a typical SNRI mechanism, but rather, in a more complicated manner, inhibits norepinephrine alpha-2 autoreceptors, allowing more norepinephrine to be released from nerve terminals. It also blocks 5-HT2A/2C receptors, thus allowing more serotonin, dopamine, and norepinephrine modulation in the cortex. It may be described in more specific terms as a noradrenergic antagonist-specific serotonin antagonist (NaSSA). As such, it achieves greater neurotransmitter levels via a different mechanism of action than the SNRI or SSRI, perhaps also explaining its different side-effect profile. Quitkin’s pooled analysis showed a faster onset of effect with mirtazapine yet no ultimate significant difference in effectiveness between mirtazapine and the SSRI [43]. Further meta-analysis has confirmed the accelerant property of mirtazapine monotherapy, suggesting faster onset of efficacy but equal efficacy by the end of 8-week acute trials [44].
Like the SSRIs, trazodone was an early “second generation” ADT whose low side effect and toxicity profile made it a popular alternative to MAOIs and TCAs in the early 1980s [45]. As an atypical agent, its mechanism of action is complex and multifaceted. Marketed as a mild serotonin reuptake inhibitor, trazodone, in fact, can be viewed as a mixed serotonergic agonist–antagonist and is more widely referred to by clinicians as a serotonin antagonist and reuptake inhibitor (SARI). The drug increases serotonin levels in the CNS through a combined effect on serotonin reuptake pumps and 5-HT2A/2C receptors via both receptor agonist and antagonist activities [46,47]. It is considered, like mirtazapine, a sedating ADT, and marked sedation often limits its use. Trazodone is available in generic form and may be the preferred treatment in select cases of MDD depending on the patient’s unique profile. Its anxiolytic and sedative effects, for instance, may be advantageous to MDD patients with concomitant generalized anxiety disorder or insomnia [45]. Other psychotherapeutic contexts in which trazodone has been investigated include PTSD, bulimia nervosa, and adjustment disorders, among others [48–50]. More recently, a slow release preparation has been marketed that lowers plasma levels and tends to minimize sedating side effects. It should be mentioned that nefazodone is also a SARI-type ADT but largely has fallen out of use due to fear of hepatotoxicity.
Bupropion is an antidepressant that was initially released as an ADT and removed from the market due to induction of seizures in MDD patients. It was remarketed after its lower doses were noted to be safer and rereleased after the blockbuster SSRI fluoxetine went on the market. Bupropion might have been an initial blockbuster ADT as well if it had had a better safety profile. While many clinicians classify it as “atypical” because of the fact that, unlike most ADTs, it has no effect on serotonin, some prefer the specific designation of norepinephrine– dopamine reuptake inhibitor (NDRI), due to its dual mechanism that raises dopamine and norepinephrine levels instead. This gives it a unique side-effect profile characterized by no sexual dysfunction or weight gain. In fact, as it promotes weight loss, it is contraindicated in patients with eating disorders. It is also more activating with regard to insomnia and anxiety. It is frequently prescribed as part of combined ADT polypharmacy, often added to an initially, partially effective SSRI [23,51]. Meta-analysis has revealed comparable efficacy between bupropion and the SSRIs [52].
The newest ADTs
Vilazodone and vortioxetine
Vilazodone and vortioxetine are two of the most recently approved drugs for MDD. Both may be considered as “SSRI Plus” agents as their core mechanism is serotonin reuptake inhibition, but both also manipulate serotonin receptors. Vilazodone is considered SSRI-like with additionally strong 5-HT1A receptor partial agonist properties. This is somewhat similar to the mechanism of buspirone, an anxiolytic, in treating generalized anxiety disorder. Vilazodone, however, with both pre- and post-synaptic 5-HT1A agonism, is considered to be more potent in this respect. Due to the fact that this drug uses two proserotonergic mechanisms, it is sometimes termed a serotonin partial agonist reuptake inhibitor (SPARI) [53]. In animal models, there was promise of faster antidepressant effects, but this has not been replicated as yet in human trials. As a newer agent, no meta-analyses exist like those discussed previously for SSRI, SNRI, TCA, mirtazapine, or bupropion. However, it appears to have a lower risk of weight gain and sexual side effects than the SSRI or SNRI, based on various noncomparative FDA trials. A 1-year, open-label multicenter study assessing the long-term safety of vilazodone found the drug to be safe and well tolerated by MDD adults, with no clinically important changes in physical examinations, electrocardiograms, or clinical chemistries [54].
Like vilazodone, vortioxetine possesses multimodal activity and is often classified as a serotonin modulator and stimulator (SMS). In addition to its serotonin reuptake-blocking (i.e., SRI) properties, it appears to have mixed agonist–antagonist effects on a variety of serotonin receptors [55]. For example, it agonizes 5-HT1A receptors and antagonizes the 5-HT1B/D, 5-HT3, and 5-HT7 receptors, making it a unique ADT. A 2014 meta-analysis not only concluded that vortioxetine achieved significant reductions in depression scores compared with placebo in 6 of 10 RCTs, but ranked this drug above escitalopram, vilazodone, and sertraline for both efficacy and tolerability [56]. Indeed, one potential advantage of this drug over comparable ADTs is a potentially low risk of sexual side effects, weight gain, and sedation [55]. An additional advantage, as cited by McIntyre and colleagues in a recent study, may lie in its potential to improve cognitive function in adults with recurrent MDD, effects which the researchers determined to be independent of depressive symptom relief and may be due to the drug’s unique 5-HT7 receptor antagonism [57].
Of note, a large meta-analysis has been conducted to evaluate most of the modern-day antidepressants with regard to effectiveness and tolerability [58]. Specific findings here suggest that mirtazapine, escitalopram (SSRI), venlafaxine (SNRI), and sertraline (SSRI) are significantly more efficacious than duloxetine (SNRI), fluoxetine (SSRI), or paroxetine (SSRI). These mixed findings would suggest no definitive superiority of any ADT class, but rather individual drug superiority. Additionally, two SSRIs (escitalopram and sertraline) showed the best profiles of patient acceptability and thus the lowest rates of discontinuation, in support of previous data showing the SSRI class to be the best tolerated. Finally, the authors determined that sertraline for moderate-to-severe MDD may offer the best balance of effectiveness, acceptability, and cost. Of note, the newer levomilnacipran, vilazodone, and vortioxetine were not included in this meta-analysis.
ADT: the take-home message
Thus far, this paper has sought to correct the perception, inadvertently fueled perhaps by generalized drug approval and marketing procedures, that ADTs are equally appropriate and effective for all depressed individuals. This “one-size-fits-all” belief is as inaccurate as it is potentially harmful. The clinical reality is that there is often a particular ADT class, if not single drug, that is best suited for each patient. Selecting ADT among the wide array of options depends on the prescriber’s astute appraisal of symptoms, comorbid conditions, tolerability, and other patient-centered factors. Table 1 concisely summarizes the specific drugs, side effects, and important prescribing considerations associated with each ADT class. While it is true that a psychiatrist’s expertise can only come from years of clinical experience and may appear out of reach to those lacking requisite training, there are nonetheless several rules of thumb that the health professional may find helpful to follow. Many of these have been detailed here. For example, patients with symptoms suggestive of DLPFC hypofunction, such as fatigue, poor concentration, and executive problems, may benefit from SNRIs, NaSSAs, and TCAs, whose noradrenergic activity specifically targets and modulates DLPFC neurochemistry. On the other hand, patients with symptoms suggestive of limbic dysfunction, such as agitation, worry, insomnia, and suicidality, may benefit from the more serotonergically active SSRIs, SARIs, SPARIs, or SMSs. For adequate monitoring of drug response and possible dose escalation, knowledge of common side effects is fundamental. In addition, prescribers should be aware of the major ADTs that have been indicated or shown clinical promise in more than one psychiatric condition.
Table 1.
Classes, SEs, and prescribing considerations for ADT.
| Class | Drugs | SE | Considerations |
|---|---|---|---|
| TCA | Imipramine Amitriptyline Doxepin Desipramine Nortriptyline |
Weight gain, sedation, dry mouth, nausea, blurred vision, constipation, tachycardia | Generally not first-line therapy due to increased anticholinergic and cardiotoxic SE |
|
| |||
| MAOI | Isocarboxazid Phenelzine Tranylcypromine Selegiline |
Weight gain, fatigue, sexual dysfunction, hypotension | Generally not first-line therapy due to serotonin syndrome and hypertensive crises |
|
| |||
| SSRI | Fluoxetine Paroxetine Sertraline Citalopram Escitalopram |
Headaches, GI distress, insomnia, fatigue, anxiety, sexual dysfunction, weight gain | Often first-line treatment due to safer SE profile. Subtle SE differences must be weighed by the prescriber |
|
| |||
| SNRI | Venlafaxine Desvenlafaxine Duloxetine Levomilnacipran |
Nausea, insomnia, dry mouth, headache, increased blood pressure, sexual dysfunction, weight gain | SEs are similar to but may be slightly more frequent than with SSRI |
|
| |||
| Atypical | Bupropion | Headache, agitation, insomnia, loss of appetite, weight loss, sweating | Increased seizure risk in eating disorder and epilepsy patients. No sexual dysfunction or weight gain. May also help to quit smoking |
| Mirtazapine | Sedation, increased appetite, weight gain | Sedation may be less with higher dose. Much reduced nausea and sexual dysfunction compared with SSRI/SNRI. Some risk of reduced white blood cell count | |
| Trazodone | Sedation, nausea, priapism (rare) | Lower risk of weight gain and sexual dysfunction, but may cause priapism. Often used to induce sleep as a positive effect | |
| Vilazodone | Nausea, diarrhea, insomnia | Better SE profile than most ADTs with lower risk of sexual dysfunction or weight gain | |
| Vortioxetine | Nausea, diarrhea, dizziness | Similar SE profile to the SSRI. May have precognitivebenefits in adults with MDD | |
ADT, antidepressant treatment; MAOI, monoamine oxidase inhibitor; MDD, major depressive disorder; SE, side effect; SNRI, serotonin norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
MDD is increasingly being acknowledged as part of a fluid spectrum of mood disorders, and trends in research suggest that treatment success is often greatest by applying augmentation, polypharmacy, or treatment-switching strategies that are sensitive to the patient’s dynamic course and fluctuating symptoms [59]. In some cases, a medication switch within ADT class may be as efficacious as a switch between drug classes, reflecting the importance of subtle pharmacokinetic and pharmacodynamic differences [60]. The very concept of treatment “success,” in fact, has evolved over the decades. It is clear today that aggressive, early therapy focused on achieving complete remission (i.e., total amelioration of symptoms and return to normal functioning) far surpasses the FDA’s benchmark of a 50% reduction in depressive symptoms or severity as the best prognosticator of long-term outcome, especially in the context of extraordinarily high MDD recurrence rates [61]. In light of these paradigm shifts in the understanding of MDD, it is incumbent upon all clinicians to remain abreast of the latest literature and informed about the properties, mechanisms of action, and specific indications of commonly prescribed ADT.
Treatment guidelines for the general practitioner
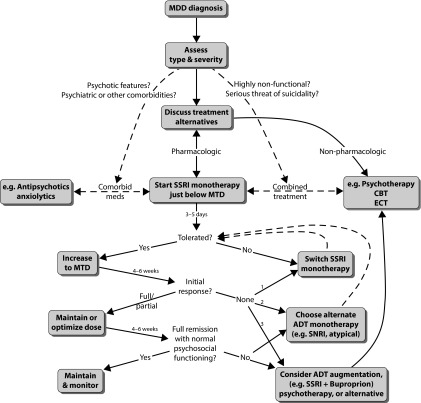
Figure 1 illustrates a basic algorithm psychiatrists often follow in the initial treatment of MDD. This can serve as an invaluable reference for other clinicians who are also in the care of a patient with MDD. After the initial diagnosis, the type and severity of MDD should be meticulously assessed, since the presence or absence of particular symptoms or features, as well as the existence of comorbid conditions, will dictate effective first-line treatment selection. A patient with severe MDD, for example, may benefit from starting immediate combined treatment, comprised of an ADT trial with concurrent psychotherapy; patients with a history of MDD in whom previous treatment has failed may strongly consider electroconvulsive therapy, especially if there is serious threat of suicidality. MDD with psychotic features, on the other hand, will require concomitant comorbid medication; therefore, an ADT monotherapy must be appropriately selected that avoids dangerous interactions with other psychotropic substances. While it is important to tailor treatment recommendations to the patient’s unique health condition, it is no less crucial to discuss, as always, all possible treatment alternatives, both pharmacologic and nonpharmacologic, such that the patient can choose a regimen that they feel comfortable with and can adhere to confidently.
Figure 1.
Treatment algorithm for MDD.
ADT, antidepressant treatment; CBT, cognitive behavioural therapy; ECT, electroconvulsive therapy; GI, gastrointestinal; MDD, major depressive disorder; MTD, minimum therapeutic dose; SNRI, serotonin norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
In most uncomplicated cases, the first-line treatment is usually monotherapy with an established SSRI, such as sertraline (Zoloft). An SSRI approach is often seen in clinical practice and is supported by many guidelines and reviews [62,63]. However, some of the newer SNRIs (e.g., duloxetine [Cymbalta] or desvenlafaxine [Pristiq]) are occasionally used if the patient’s symptoms suggest norepinephrine imbalance or there are strong preferences. Discrepancies in the selection of initial SSRI monotherapy are usually due to slight differences in side-effect profiles rather than effectiveness. The prescriber will start the patient just below the minimum therapeutic dose (MTD), that is, the threshold, previously established by regulatory trials, at which the ADT becomes statistically efficacious in treating MDD. The MTD for sertraline, for example, is 50 mg. A sub-MTD approach is employed to keep plasma levels, and thus the risk of potential adverse side effects, low at ADT initiation [64]. This should build the patient’s trust in the medication, strengthen rapport, and ultimately improve adherence to daily ADT use, all of which lead to better outcomes [65]. After 3–5 days of subtherapeutic dosing, the SSRI is increased to the MTD and the patient maintained on daily SSRI until reassessment at approximately 4–6 weeks. A phone communication should be set up earlier, however, around 2 weeks to monitor acute side effects, including suicidal ideation [66]. If there is a full initial response (notable symptom alleviation) at this first reassessment, the dose should remain at the MTD for another 4–6 weeks, and a new appointment is scheduled at this time to determine whether complete remission exists. In the case of a partial response (minimal symptom alleviation) at the first reassessment, the dose should be steadily and appropriately increased (optimized), with a reevaluation scheduled at the 4- to 6-week time point. If there is no appreciable response with an initial SSRI at MTD after the first reassessment, a dose increase may be warranted, or the ineffective SSRI may instead be abandoned, and a new SSRI monotherapy trial may begin. If there is not clear and full remission of symptoms after 8–12 weeks of treatment, prescribers often fully maximize SSRI dosing, switch to a different ADT class monotherapy, or add an approved or evidence-based augmentation agent (e.g., SSRI + Bupropion) or psychotherapy trial, depending on the clinician’s comfort level [62,61,67]. With regard to switching ADT class monotherapies, large comparative studies have yet to show a clear advantage of any one particular strategy, despite several smaller studies slightly favoring SNRIs [59]. Today, most experts agree that a class switch is warranted if a patient has not first received major relief from a previous, fully dosed SSRI. The rationale is that if maximum serotonergic facilitation has not provided relief, the pathogenesis of the depression may not be entirely serotonin-based, and a different neuromodulatory approach (e.g., a cross-titration onto an SNRI, NDRI, NASSA, or SPARI) may prove more fruitful [64,68,69].
Leaving a patient in a partial response state may increase the risk of future MDD relapse, recurrence, suicide, social and occupational discord, or dysfunction [70]. Clinicians often measure MDD symptoms again by checking each DSM-5 symptom verbally or by utilizing a rating scale measure. Sometimes, however, patients may exhibit a fair degree of symptom resolution (even obtaining statistical remission according to rating scales) yet may not be truly well again. For example, symptoms may subside despite the patient being unable to work successfully, improve grades at school, or return to interpersonal activities (e.g., attend church, interact with grandchildren, and manage finance). Along with diligent monitoring of symptoms, clinicians should establish psychosocial markers with the patient [64,71]. These markers are often behaviors that the MDD patient can relate to, which would indicate that they are socially well, remitted, and recovered. Patients might consider themselves back to normal again if, for example, they returned to their volunteer work, paid their bills accurately and on time, or began working out or dating again. Full symptom remission and full return to baseline psychosocial functioning are the ultimate goals of MDD treatment. A psychotherapist performing psychotherapy in parallel with a clinician prescribing ADT may be invaluable for assessing whether psychosocial wellness or remission has occurred, as typically a therapist will have more face-to-face time to determine whether there has been a full return to baseline functioning.
Abbreviations:
- ADT
antidepressant treatment;
- DLPFC
dorsolateral prefrontal cortex;
- ECT
electroconvulsive therapy;
- FDA
Food and Drug Administration;
- GI
gastrointestinal;
- HAM-D
Hamilton Rating Scale for Depression;
- ITT
intent-to-treat;
- MADRS
Montgomery–Asberg Depression Rating Scale;
- MAOI
monoamine oxidase inhibitor;
- MDD
major depressive disorder;
- MTD
minimum therapeutic dose;
- NaSSA
noradrenergic antagonist-specific serotonin antagonist;
- NDRI
norepinephrine–dopamine reuptake inhibitor;
- RCT
randomized controlled trial;
- SARI
serotonin antagonist and reuptake inhibitor;
- SMS
serotonin modulator and stimulator;
- SNRI
serotonin norepinephrine reuptake inhibitor;
- SPARI
serotonin partial agonist reuptake inhibitor;
- SSRI
selective serotonin reuptake inhibitor;
- TCA
tricyclic antidepressant.
References
- 1.Murkherjee S. Post-prozac nation: the science and history of treating depression. The New York Times (Sunday Magazine) 2012 Apr 19;:MM48. [Google Scholar]
- 2.Pratt LA, Brody DJ, Gu Q. Antidepressant use in persons aged 12 and over: United States, 2005–2008. Hyattsville, MD: National Center for Health Statistics; 2011. NCHS data brief, no 76. [Google Scholar]
- 3.Sheehan DV. Depression: underdiagnosed, undertreated, underappreciated. Manag Care. 2004;13(6):6–8. [PubMed] [Google Scholar]
- 4.Kirsch I. The emperor’s new drugs: exploding the antidepressant myth. Philadelphia, PA: Basic Books; 2010. [Google Scholar]
- 5.Schwartz TL, Stormon L, Thase M. Treatment outcomes with acute pharmacotherapy/psychotherapy. In: Schwartz T, Petersen T, editors. Depression: treatment strategies and management. 1st ed. New York: Taylor and Francis; 2006. [Google Scholar]
- 6.Morris JB, Beck AT. The efficacy of antidepressant drugs: a review of research (1958–1972) Arch Gen Psychiatry. 1974;30:667–74. doi: 10.1001/archpsyc.1974.01760110083010. http://dx.doi.org/10.1001/archpsyc.1974.01760110083010. [DOI] [PubMed] [Google Scholar]
- 7.Klein DF, Gittelman R, Quitkin F, Rifkin A. Diagnosis and drug treatment of psychiatric disorders: adult and children. 2nd ed. Baltimore, MD: Williams & Wilkins; 1980. [Google Scholar]
- 8.Depression Guideline Panel . Clinical Practice Guideline Number 5. Rockville, MD: US Depart of Health and Human Services Agency for Health Care Policy and Research; 1993. Depression in primary care, Volume 2: treatment of major depression. AHCPR publication no. 93-0551. [Google Scholar]
- 9.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Press; 2000. text rev. [Google Scholar]
- 10.Thase ME. Studying new antidepressants: if there was a light at the end of the tunnel could we see it? J Clin Psychiatry. 2002;63(2):24–8. [PubMed] [Google Scholar]
- 11.Thase ME. Comparing the methods used to compare antidepressants. Psychopharmacol Bull. 2002;36(1):4–17. [PubMed] [Google Scholar]
- 12.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press; 1977. [Google Scholar]
- 15.Kirsch I, Moore TJ, Scoboria A, Nicholls SS. The emperor’s new drugs: an analysis of antidepressant medication data submitted to the U.S. Food and Drug Administration. Prev Treat. 2002;5 Article 23. http://journals.apa.org/prevention/volume5/pre0050023a.html [Last accessed: September 22, 2015]. [Google Scholar]
- 16.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287(14):1840–7. doi: 10.1001/jama.287.14.1840. http://dx.doi.org/10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 17.Khan A, Warner HA, Brown WA. Symptom reduction and suicide risk in patients treated with placebo in antidepressant clinical trials: an analysis of the Food and Drug Administration database. Arch GenPsychiatry. 2000;57:311–7. doi: 10.1001/archpsyc.57.4.311. http://dx.doi.org/10.1001/archpsyc.57.4.311. [DOI] [PubMed] [Google Scholar]
- 18.Stahl SM, Entsuah R, Rudolph RL. Comparative efficacy between venlafaxine and SSRIs: a pooled analysis of patients with depression. Biol Psychiatry. 2002;52(12):1166–74. doi: 10.1016/s0006-3223(02)01425-7. http://dx.doi.org/10.1016/S0006-3223(02)01425-7. [DOI] [PubMed] [Google Scholar]
- 19.Stahl SM. The 7 habits of highly effective psychopharmacologists, part 3: sharpen the saw with selective choices of continuing medical education programs. J Clin Psychiatry. 2000;61(6):401–2. doi: 10.4088/jcp.v61n0601. [DOI] [PubMed] [Google Scholar]
- 20.Montgomery SA, Henry J, McDonald G, Dinan T, Lader M, Hindmarch I, Clare A, Nutt D. Selective serotonin reuptake inhibitors: meta-analysis of discontinuation rates. Int Clin Psychopharmacol. 1994;9:47–53. doi: 10.1097/00004850-199400910-00008. http://dx.doi.org/10.1097/00004850-199400910-00008. [DOI] [PubMed] [Google Scholar]
- 21.Mulsant BH, Pollack BG, Nebes R, Miller MD, Sweet RA, Stack J, Houch PR, Bensasi S, Mazumdar S, Reynolds CF., 3rd A twelve-week, double-blind, randomized comparison of nortriptyline and paroxetine in older depressed inpatients and outpatients. Am J Ger Psychiatry. 2001;9(4):406–14. http://dx.doi.org/10.1097/00019442-200111000-00009. [PubMed] [Google Scholar]
- 22.Thase ME, Trivedi MH, Rush AJ. MAOIs in the contemporary treatment of depression. Neuropsychopharmacology. 1995;12(3):185–219. doi: 10.1016/0893-133X(94)00058-8. http://dx.doi.org/10.1016/0893-133X(94)00058-8. [DOI] [PubMed] [Google Scholar]
- 23.Stewart JS, McGrath PJ, Rabkin JG, Quitkin FM. Atypical depression: a valid clinical entity? Psychiatr Clin North Am. 1993;16(3):479–95. [PubMed] [Google Scholar]
- 24.American Psychiatric Association Practice guideline for the treatment of patients with major depressive disorder (revision) Am J Psychiatry. 2000;157(4):1–45. [PubMed] [Google Scholar]
- 25.Anderson IM, Tomenson BM. The efficacy of selective serotonin re-uptake inhibitors in depression: a meta-analysis of studies against tricyclic antidepressants. J Psychopharmacol. 1994;8:238–49. doi: 10.1177/026988119400800407. http://dx.doi.org/10.1177/026988119400800407. [DOI] [PubMed] [Google Scholar]
- 26.Anderson IM. SSRIS versus tricyclic antidepressants in depressed inpatients: a meta-analysis of efficacy and tolerability. Depress Anxiety. 1998;7(1):11–7. [PubMed] [Google Scholar]
- 27.Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–5. doi: 10.1001/archpsyc.1991.01810330075011. http://dx.doi.org/10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 28.Grady MM, Stahl SM. Practical guide for prescribing MAOIs: debunking myths and removing barriers. CNS Spectrums. 2012;17(1):2–10. doi: 10.1017/S109285291200003X. http://dx.doi.org/10.1017/S109285291200003X. [DOI] [PubMed] [Google Scholar]
- 29.Hameed U, Schwartz TL, Malhotra K. Antidepressant treatment in the primary care office: outcomes for adjustment disorder versus major depression? Ann Clin Psychiatry. 2005;17(2):1–5. doi: 10.1080/10401230590932344. http://dx.doi.org/10.1080/10401230590932344. [DOI] [PubMed] [Google Scholar]
- 30.Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical application. 4th ed. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- 31.Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48(8):732–9. doi: 10.1016/s0006-3223(00)00935-5. http://dx.doi.org/10.1016/S0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- 32.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57(10):925–35. doi: 10.1001/archpsyc.57.10.925. http://dx.doi.org/10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 33.Williams RS, Cheng L, Mudge AW, Harwood AJ. A common mechanism of action for three mood-stabilizing drugs. Nature. 2002;417(6886):292–5. doi: 10.1038/417292a. http://dx.doi.org/10.1038/417292a. [DOI] [PubMed] [Google Scholar]
- 34.Gorman JM, Korotzer A, Su G. Efficacy comparison of escitalopram and citalopram in the treatment of major depressive disorder: pooled analysis of placebo-controlled trials. CNS Spectr. 2002;7(1):40–4. doi: 10.1017/s1092852900028595. http://dx.doi.org/10.1017/S1092852900028595. [DOI] [PubMed] [Google Scholar]
- 35.Thase ME, Entsuah AR, Rudolph RI. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J Psychiatry. 2001;178:234–41. doi: 10.1192/bjp.178.3.234. http://dx.doi.org/10.1192/bjp.178.3.234. [DOI] [PubMed] [Google Scholar]
- 36.Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP, Jr, Weiss PM, Dunner DL, Rotherbaum BO, Kornstein S, Keitner G, Keller MB. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci. 2003;100:14293–6. doi: 10.1073/pnas.2336126100. http://dx.doi.org/10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith D, Dempster C, Glanville J, Freemantle N, Anderson I. Efficacy and tolerability of venlafaxine compared with selective serotonin reuptake inhibitors and other antidepressants: a meta-analysis. Br J Psychiatry. 2002;180:396–404. doi: 10.1192/bjp.180.5.396. http://dx.doi.org/10.1192/bjp.180.5.396. [DOI] [PubMed] [Google Scholar]
- 38.Thase M, Lu YI, Joliat M, Detke M. Remission rates in double-blind, placebo-controlled clinical trials of duloxetine with SSRI as a comparator. Eur Neuropsychopharmacol. 2003;13(4):S215. http://dx.doi.org/10.1016/S0924-977X(03)91806-2. [Google Scholar]
- 39.Deecher DC, Beyer CE, Johnston G, Bray J, Shah S, Abou-Gharbia M, Andree TH. Desvenlafaxine succinate: a new serotonin and norepinephrine reuptake inhibitor. J pharmacol Exp Ther. 2006;318(2):657–65. doi: 10.1124/jpet.106.103382. http://dx.doi.org/10.1124/jpet.106.103382. [DOI] [PubMed] [Google Scholar]
- 40.Cohen LJ. Desvenlafaxine: frequently asked questions. Primary Psychiatry. 2009;16(12):1–8. [Google Scholar]
- 41.Boyer P, Montgomery S, Lepola U, Germain JM, Brisard C, Ganguly R, Padmanabhan SK, Tourian KA. Efficacy, safety, and tolerability of fixed-dose desvenlafaxine 50 and 100 mg/day for major depressive disorder in a placebo-controlled trial. Int Clin Psychopharmacol. 2008;23(5):243–53. doi: 10.1097/YIC.0b013e32830cebed. http://dx.doi.org/10.1097/YIC.0b013e32830cebed. [DOI] [PubMed] [Google Scholar]
- 42.Sansone RA, Sansone LA. Serotonin norepinephrine reuptake inhibitors: a pharmacological comparison. Innov Clin Neurosci. 2014;11(3–4):37–42. [PMC free article] [PubMed] [Google Scholar]
- 43.Quitkin FM, Taylor BP, Kremer C. Does mirtazapine have a more rapid onset than SSRIs? J Clin Psychiatry. 2001;62(5):358–61. doi: 10.4088/jcp.v62n0509. http://dx.doi.org/10.4088/JCP.v62n0509. [DOI] [PubMed] [Google Scholar]
- 44.Thase M, Schutte AJ, Van der flier S, Heukels A. Remission with mirtazapine versus SSRIs: a meta-analysis on data of more than 2500 depressed patients treated in randomized controlled trials. J Affect Disord. 2004;78(1):S136. [Google Scholar]
- 45.Golden RN, Dawkins K, Nicholas L. Trazodone and nefazodone. In: Schatzberg AF, Nemeroff CB, editors. The American Psychiatric Publishing textbook of psychopharmacology. 4th ed. Arlington, VA: American Psychiatric Publishing; 2009. [Google Scholar]
- 46.Pazzagli M, Giovannini MG, Pepeu G. Trazodone increase extracellular serotonin levels in the frontal cortex of rats. Eur J Pharmacol. 1999;383(3):249–57. doi: 10.1016/s0014-2999(99)00644-5. http://dx.doi.org/10.1016/S0014-2999(99)00644-5. [DOI] [PubMed] [Google Scholar]
- 47.Haria M, Fitton A, McTavish D. Trazodone: a review of its pharmacology, therapeutic use in depression and therapeutic potential in other disorders. Drugs Aging. 1994;4(4):331–55. doi: 10.2165/00002512-199404040-00006. [DOI] [PubMed] [Google Scholar]
- 48.Warner MD, Dorn MR, Peabody CA. Survey on the usefulness of trazodone in patients with PTSD with insomnia or nightmares. Pharmacopsychiatry. 2001;34(4):128–31. doi: 10.1055/s-2001-15871. http://dx.doi.org/10.1055/s-2001-15871. [DOI] [PubMed] [Google Scholar]
- 49.Hudson JI, Pope HG, Jr, Keck PE, Jr, McElroy SL. Treatment of bulimia nervosa with trazodone: short-term response and long-term follow-up. Clin Neuropharmacol. 1989;12(Suppl 1):S38–S46. doi: 10.1097/00002826-198901001-00007. Discussion S47–S49. http://dx.doi.org/10.1097/00002826-198901001-00007. [DOI] [PubMed] [Google Scholar]
- 50.Rasavi D, Kormoss N, Collard A, Farvacques C, Delvaux N. Comparative study of the efficacy and safety of trazodone versus clorazepate in the treatment of adjustment disorders in cancer patients: a pilot study. J Int Med Res. 1999;27(6):264–72. doi: 10.1177/030006059902700602. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz TL, Rashid A. Augmentation and combination pharmacotherapy trends in major depressive disorder: results of a brief survey of psychiatrists. P&T. 2007;32(1):28–31. [Google Scholar]
- 52.Thase ME, Wang Y, Richard N, Mitton M, Haight B, Goodale E. Remission rates following therapy with bupropion or selective serotonin reuptake inhibitors. Eur Neuropsychopharmacol. 2003;13(4):S259. http://dx.doi.org/10.4088/JCP.v66n0803. [Google Scholar]
- 53.Schwartz TL, Stahl SM. Vilazodone: a brief pharmacologic and clinical review of the novel SPARI (serotonin partial agonist and reuptake inhibitor) Ther Adv Psychopharmacol. 2011;1(3):81–7. doi: 10.1177/2045125311409486. http://dx.doi.org/10.1177/2045125311409486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson DS, Kajdasz DK, Gallipoli S, Whalen H, Wamil A, Reed CR. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31(5):643–6. doi: 10.1097/JCP.0b013e31822c6741. http://dx.doi.org/10.1097/JCP.0b013e31822c6741. [DOI] [PubMed] [Google Scholar]
- 55.Schatzberg AF, DeBattista C. Manual of clinical psychopharmacology. 8th ed. Arlington, VA: American Psychiatric Publishing; 2015. [Google Scholar]
- 56.Llorca PM, Lancon C, Brignone M, Rive B, Salah S, Ereshefsky L, Francois C. Relative efficacy and tolerability of vortioxetine versus selected antidepressants by indirect comparisons of similar clinical studies. Curr Med Res Opin. 2014;30:2589–606. doi: 10.1185/03007995.2014.969566. http://dx.doi.org/10.1185/03007995.2014.969566. [DOI] [PubMed] [Google Scholar]
- 57.McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2004;17:1557–67. doi: 10.1017/S1461145714000546. http://dx.doi.org/10.1017/S1461145714000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746–58. doi: 10.1016/S0140-6736(09)60046-5. http://dx.doi.org/10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- 59.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupher DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. http://dx.doi.org/10.1176/appi.ajp.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 60.Bresee C, Gotto J, Rapaport MH. Treatment of depression. In: Schatzberg AF, Nemeroff CB, editors. The American Psychiatric Publishing textbook of psychopharmacology. 4th ed. Arlington, VA: American Psychiatric Publishing; 2009. [Google Scholar]
- 61.Kupfer DJ. Long-term treatment of depression. J Clin Psychiatry. 1991;52(Suppl):28–34. [PubMed] [Google Scholar]
- 62.American Psychiatric Association . Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. Arlington, VA: American Psychiatric Association; 2010. [Google Scholar]
- 63.Topel ME, Zajecka JM, Goldstein CN, Siddiqui UA, Schwartz TL. Using what we have: combining medications to achieve remission. Clin Neuropsychiatry. 2011;8(1):4–27. [Google Scholar]
- 64.Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 3rd ed. New York: Cambridge University Press; 2008. pp. 511–666. [Google Scholar]
- 65.Schwartz TL. Psychopharmacology today: where are we and where do we go from here? Mens Sana Monogr. 2010;8(1):6–16. doi: 10.4103/0973-1229.58816. http://dx.doi.org/10.4103/0973-1229.58816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weihs K, Wert JM. A primary care focus on the treatment of patients with major depressive disorder. Am J Med Sci. 2011;342(4):324–30. doi: 10.1097/MAJ.0b013e318210ff56. http://dx.doi.org/10.1097/MAJ.0b013e318210ff56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwartz TL. Introduction to the special issue focused on the future of psychopharmacological practice. Clin Neuropsychiatry. 2011;8(1):3. http://dx.doi.org/10.1002/pits.20419. [Google Scholar]
- 68.Zajecka JM, Goldstein C. Combining medication to achieve remission. In: Schwartz TL, Petersen T, editors. Depression: treatment strategies and management. 2nd ed. New York: Taylor & Francis; 2009. [Google Scholar]
- 69.Stahl SM. Essential psychopharmacology: the prescriber’s guide. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- 70.Thase ME. Defining remission in patients treated with antidepressants. J Clin Psychiatry. 1999;60(22):3–6. [PubMed] [Google Scholar]
- 71.Stahl SM. Essential psychopharmacology: the prescriber’s guide. 4th ed. Cambridge: Cambridge University Press; 2011. [Google Scholar]