Abstract
Modern medicine faces a growing crisis as demand for organ transplantations continues to far outstrip supply. By stimulating the body’s own repair mechanisms, regenerative medicine aims to reduce demand for organs, while the closely related field of tissue engineering promises to deliver “off-the-self” organs grown from patients’ own stem cells to improve supply. To deliver on these promises, we must have reliable means of generating complex tissues. Thus far, the majority of successful tissue engineering approaches have relied on macroporous scaffolds to provide cells with both mechanical support and differentiative cues. In order to engineer complex tissues, greater attention must be paid to nanoscale cues present in a cell’s microenvironment. As the extracellular matrix is capable of driving complexity during development, it must be understood and reproduced in order to recapitulate complexity in engineered tissues. This review will summarize current progress in engineering complex tissue through the integration of nanocomposites and biomimetic scaffolds.
Keywords: nanotechnology, tissue regeneration, bone, liver, cardiac
Introduction
The ability to regenerate damaged tissues is a common feature across species’. While salamanders may regenerate entire limbs, humans are limited in meaningful regeneration of functional tissue to specialized organs such as the liver.1 The possibilities of harnessing tissue regeneration in human medicine have not been lost on the medical and scientific professions since Réaumuer first described the regeneration of a crayfish claw in 1712.2
Over the past two decades, Anthony Atala, Robert Langer, and others have pioneered the fields of tissue engineering and regenerative medicine. Breakthroughs such as Atala’s neobladder,3 and more recently, a pilot study of tissue-engineered autologous vaginal organs,4 come at a time when the world is facing a looming organ transplantation crisis as demand continues to far outstrip supply.5,6 Continued advances in regenerative medicine promise a means to stimulate the body’s own repair mechanisms to regenerate damaged tissue. Furthermore, the use of patient-derived stem cells to provide “off-the-self” organs grown in vitro offers an alternative to lifelong immunosuppressive drugs for transplant patients in a postantibiotic age.
Strategies for regenerative medicine
Towards regenerative medicine in humans—the process of replacing, engineering, or regenerating human cells, tissues, or organs to restore or establish normal function7—there are currently three broad strategies. Perhaps the most synonymous with regenerative medicine are the stem cell therapies. As stem cells are responsible for engineering an organ’s original complexity during development, it holds that they should be capable of recapitulating complexity after injury. Although recent advances in the field show great promise,8–11 much work is still required before such therapeutic strategies are available to the vast majority of patients.
In animals capable of tissue regeneration, stem and progenitor cells first organize into a blastema at the site of injury.12 One theory holds that the immune system of adult mammals precludes regeneration by inhibiting this arrangement, and instead, shows a strong bias for fast recovery and early return of movement.13 Hence, another prominent strategy in tissue engineering aims to modulate the immune system to allow endogenous regeneration.14 In fact there is a strong correlation between the development of adaptive immunity and the loss of regenerative ability.15 However, this is currently no more than a correlation, and other evidences point to a positive role for the immune system in regeneration. For example, there is strong evidence that the complement protein C5 modulates the release of pro-regenerative Interleukin-6 (IL-6) from Kupffer cells during liver regeneration in mammals16 and C5 is expressed in the newt blastema during limb and lens regeneration,17 pointing to a common role across species.
Despite an incomplete understanding of the processes involved in regeneration, progress has been made in applying the principles of regenerative medicine to humans, and with a more complete understanding of the limited regenerative capacity of humans, new insights will increase the scope of stem cell therapies.8 However, currently these purely cell-based therapies are limited, not the least by the fear of teratoma formation. At present, the majority of successful tissue engineering projects have relied on macroporous scaffolds to facilitate both initial attachment and subsequent differentiation cues for seeded stem cells. While successful for relatively simple tissues such as blood vessels18 and bladders,3 complex metabolic organs such as the liver and kidneys will require more sophisticated biomimetic scaffolds capable of providing multilayer information to differentiating cells.19
To deliver on the promise of “off-the-self” organs and to successfully stimulate in vivo tissue regeneration, the growing field of nanomedicine must be integrated into the tissue engineering approach (Fig. 1). This review aims to summarize some of the key current examples of integrated nanotechnology in regenerative medicine and tissue engineering, as well as highlighting some of the most promising future directions. Specifically, this article will discuss how nanotechnology is integrated into scaffolds for tissue engineering in order to mimic a tissue’s natural extracellular matrix (ECM). Equally important areas of research encompassing both nanotechnology and regenerative medicine, eg, nanoparticle drug delivery, fall out of the scope of this article and will not be discussed.
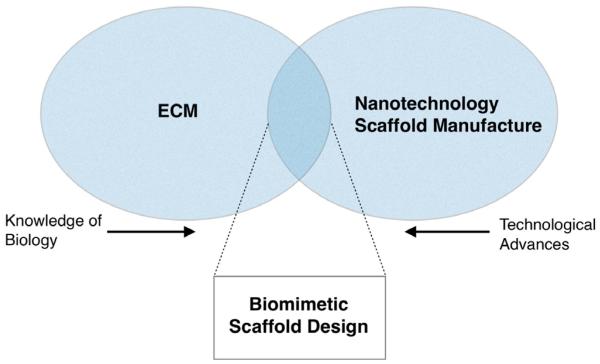
Figure 1.
Venn diagram showing the crossover between tissue-specific ECM and advances in nanotechnology. As manufacturing techniques improve, eg, advances in photolithography, higher resolution, and more biomimetic scaffolds become available. Similarly, as knowledge of the natural tissue ECM improves, currently available techniques can be utilized to better mimic the natural ECM. In the overlap between nanotechnology, manufacturing techniques, and knowledge of the natural ECM lays the possibility of successful biomimetic scaffold design for nanotechnology.
The Extracellular Microenvironment: Driving Complexity
In the body, a cell’s direct microenvironment is composed of an intricate three-dimensional (3D) network of fibrillar proteins, proteoglycans, and glycosaminoglycans (GAGs), collectively termed the extracellular matrix (ECM). Each tissue has a unique ECM composition and topology generated through a dynamic biomechanical and biophysical dialog between various cell lineages and their respective microenvironments.20 Far from simply providing mechanical support, the ECM provides an environment rich in topographical, mechanical, and biochemical cues capable of guiding a heterogeneous population of cells into a functional organ during development.21
Furthermore, it is becoming apparent that both cells and ECM are absolutely required to define a specific tissue. For example, bone and cartilage are primarily composed of ECM, encapsulating a small number of cells. In bone, a mineralized ECM confers the typical rigidity, whereas in soft cartilage, chondrocytes are entrapped in a highly hydrated ECM, which allows movement.22 Likewise, collagens in our ligaments and tendons are secreted along their major axis, conferring resistance to load and strain, whereas collagens in the intestine are arranged in a cylindrical fashion to allow for coordinated peristalsis.23 Often, removal or substitution of the ECM leads not only to the loss of structure but also to the complete loss of function or even viability.24
In a seminal paper presented by Allan Hall25 the role for focal adhesion formation and integrin signaling on cell fate was cemented. Work since then has shown how mesenchymal stem cell (MSC) adhesions can be manipulated to either efficiently form bone26 or maintain multipotency.27 The fact that such diverse cell fates can be controlled by slight surface manipulation highlights the complex relationship between cells and matrix. Through reciprocal action (cells depositing matrix, influencing cell fate, and controlling deposition of new matrix), tissues can exist in dynamic equilibrium, be highly sensitive to slight changes in the local and systemic milieu, and be able to adjust accordingly through growth factor sequestration and the development of morphogen gradients.28 In order to successfully engineer a complex tissue, the microscale and nanoscale features (Fig. 2) of such a matrix must be understood in a tissue-specific context.
Figure 2.
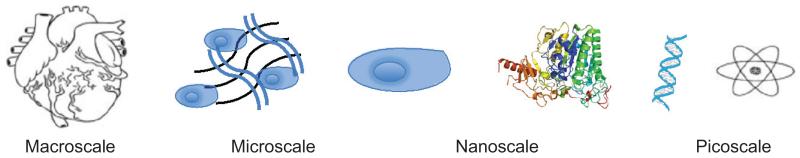
Micro- and nanoscales in nature. Cells are in the order of a hundred nanometers, they interact with proteins and molecules of nano- and picoscale dimensions to form tissues and organs of micro- and macroscale.
Materials to Mirror the ECM
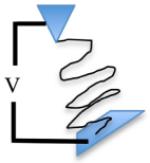
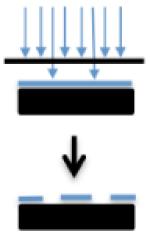
As our understanding of a cell’s interaction with its microenvironment becomes clearer, the need for appropriate nanoscale physiochemical cues to guide cell attachment and differentiation has become obvious. In general, scaffolds should aim to recapitulate specific parameters and components of the tissue-specific ECM while retaining structural and mechanical support for cells and organs. Various manufacturing techniques, primarily under development in the electronics industry, have been employed in the creation of tissue engineering scaffolds. Table 1 provides a brief overview of key techniques, and for a detailed review, see Engel et al.29
Table 1.
Typical scaffold manufacturing techniques.
| ELECTROSPINNING | PHOTOLITHOGRAPHY | DIP-PEN NANO LITHOGRAPHY | MICROCONTACT PRINTING | |
|---|---|---|---|---|
| Maximum Resolution | 5 nm–1 μm | 37 nm | 20–30 nm | 35 nm–1 μm |
| Advantages | Easy set up, versatile materials, high relevance to ECM | Precise control and introduction of defined disorder | Precise control of complex patterns and growth factor gradients | Flexibility of printed materials, print on non-planar surfaces |
| Disadvantages | Poor mechanical properties with most materials | Photosensitive compounds used, potentially toxic and very expensive to set up | Small areas only | Low control over ligand density |
| Schematic |
|
|

|
|
| Reference | 55,88 | 26,27,30 | 89 | 90 |
Perhaps one of the most advanced uses of nanotechnology in tissue engineering is the patterning of implantable surfaces with nanotopographies. Polymer demixing, chemical etching, and colloidal lithography are some of the most relevant techniques for producing random patterns favored by osteoblasts on bone implants,26,30,31 whereas soft-lithography techniques are better suited for producing regular geometries.
As a bridge between tissue engineering and providing the required mechanical support, nanopatterning of orthopedic surfaces has shown great benefit.32 However, engineering complex tissues will require complex 3D scaffolds to become a reality. Some of the most common structures used in experimental tissue engineering aim to mimic the fibrous phase of the ECM, composed of collagens and elastins. Electrospinning has emerged as a versatile technique, capable of creating complex 3D cell scaffolds from synthetic polylactic acid (PLA), polyglycolic acid (PGA), poly(lactic-co-glycolic acid) (PLGA)33 and natural (silk, collagen, and chitosan)34 materials, while requiring a relatively basic set up.35
Numerous techniques are available to further functionalize the 3D microfibrous and nanofibrous scaffolds created by electrospinning (see Tables 1 and 2). For example, to mimic the presence of adhesion proteins such as laminins and fibronectins within the matrix, Arg-Gly-Asp (RGD) peptide sequences can by conjugated to the matrix backbone to promote and control cell adhesion.36,37 Furthermore, isoleucine-lysine-valine-alanine-valine (IKVAV) laminin adhesion sequences have been conjugated to PLA electrospun nanofibers to promote neurite regrowth in models of peripheral nervous repair.38 Beyond conjugation of adhesion motifs, various methods have been established to functionalize the matrix backbone, including sulfating with perlecan or chitosan to control the presentation of growth factors39,40 and adding tissue-specific minerals such as hydroxyapatite to induce osteogenesis and mineralization in bone repair.41,42
Table 2.
Nanocomposites to compensate for matrix limitations.
| NANOCOMPOSITE MATERIAL | EFFECT ON MATRIX | REFERENCE |
|---|---|---|
| Carbon Nanotubes | Increase Young’s modulus and tensile strength, overcoming weak matrix mechanical properties | 46 |
|
| ||
| Increased matrix conductivity | 91 | |
|
| ||
| Synthetic Nanospheres | Allow controlled release of morphogens and biomolecules | 92 |
|
| ||
| Magnetic nanoparticles to aid vascular tissue engineering—control over shape | 93 | |
|
| ||
| Nanotitanite wires | Improve cell matrix interactions and increases cell adhesion | 94 |
|
| ||
| Gold nanowires | Allow precise control over biomolecule localization within a scaffold | 95 |
State of the art
Molecular self-assembly has been one of the most exciting recent developments in the field of biomaterial research. Peptides amphiphiles can produce thermodynamically stable structures with final properties readily tunable by molecular chemistry (altering pH, solvents, temperature, etc.).43 By adjusting the initial monomers (eg, by patterning with IKVAV sequences)44 and the assembly conditions, complex structures can be produced in an entirely free energy–driven process. These structures have the capacity to present multiscale information to cells with exquisite levels of control. A major current limitation is in the lack of mechanical strength; however, there is the possibility of utilizing nanocomposites to compensate for such scaffold limitations (Table 2). For example, carbon nanotubes show viscoelastic behavior similar to that observed in soft-tissue membranes45 and have been used to increase the Young’s modulus and tensile strength of hybrid biomaterials.46 An excellent review of self-assembling peptide amphiphiles has been published by Samuel Stupp and colleagues at the Northwestern University.43
Cardiac Tissue
Clinical need
Heart disease is the leading cause of death worldwide. Although incidence in the developed world is reducing, this is countered by an overall increase in many developing countries. Although not a cure for heart disease, cardiac transplant surgery is a life-saving procedure for the most severe cases of heart failure.47 Of the 3,500 worldwide transplant procedures carried out annually, 2,000 are performed in the United States, pointing to a potential rise in demand as developing countries mature. In 2007, it was estimated that 50,000 people were awaiting heart transplantation worldwide.48 The vast disparity between the number of required procedures and the number performed annually, together with rising demand from developing countries, has driven research into both bioartificial and xenograft alternatives. To date, neither of these approaches is as effective as traditional tissue-typed allograft procedures.49
Cardiac ECM
The heart wall is composed of tightly packed fibrillar collagen and elastin bundles that form a dense, elastic network ranging from 10 to several hundreds of nanometers across. This dense mesh is covered with nanoscale adhesive proteins such as laminin and fibronectin, allowing cells to interact through integrins and cadherins on their surface.50 The high metabolic demand of the heart muscle means that it consumes large amounts of oxygen and cannot tolerate hypoxia. It is therefore highly perfused by a supporting network of vasculature. This dense, but defined network, confers on the heart its unique mechanical and electrical properties as cardiomyocytes are forced to couple with each other in a 3D syncytium of elongated and aligned cell bundles (Fig. 3).51
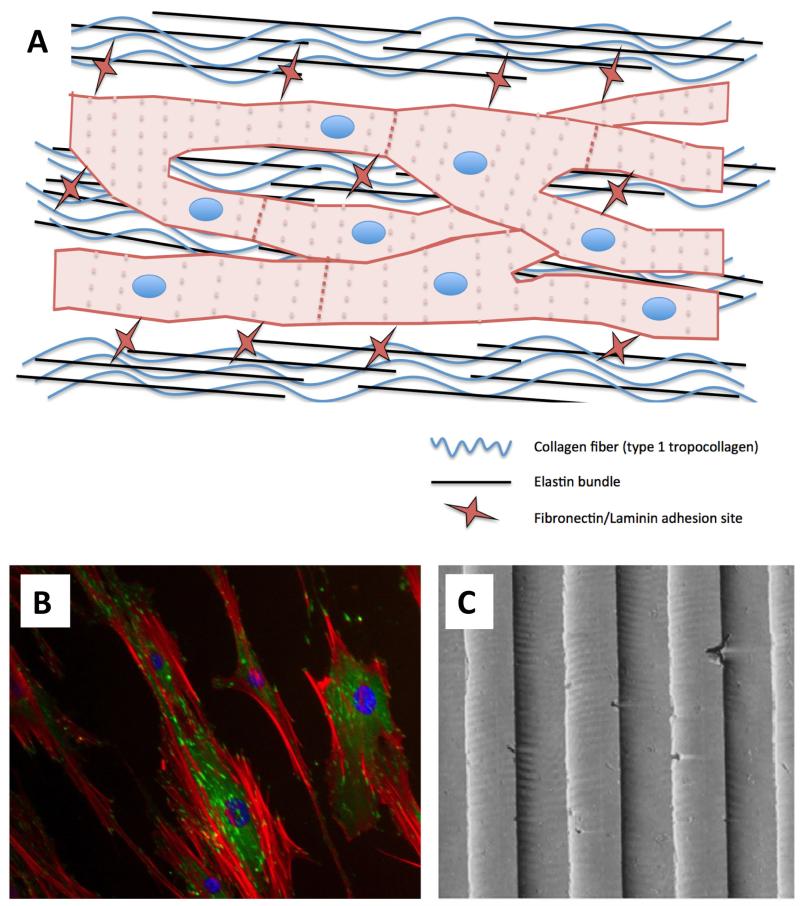
Figure 3.
Schematic of cardiomyocyte interaction with the ECM (A). Tightly wound collagen and elastin bundles force an elongated cell morphology and cell coupling through gap junctions. Similar cellular organization can be seen in vitro by culturing cardiomyocytes on nanometric-grooved topographies. Shown for illustration are endothelial cells (B) exhibiting contact guidance when cultured on 240-nm–grooved arrays (C). Immunocytochemistry: Red is actin, green is vinculin, and blue is DAPI (nuclear).
Tissue engineering approaches
In 2008, Ott and co-workers reported a study emphasizing the importance of the ECM in guiding essential morphological organization and physiological function by engineering a bioartificial rat heart.52 By decellularizing adult rat hearts by coronary perfusion with detergents, the researchers were able to preserve the underlying ECM. When reseeded with cardiac and endothelial cells, the ECM guided self-organization and allowed restoration of pump function. In contrast, when seeded on a flat scaffold, cardiomyocytes lost their elongated morphology and adopted a random distribution, which compromised many of their physiological functions. In an attempt to mimic the in vivo ventricular structure, Kim and colleagues cultured cardiac cells on polyethylene glycol hydrogel–grooved arrays with widths ranging from 50 to 800 nm.53 Guided by the nanoscale mechanical cues, these tissue constructs displayed anisotropic action potential propagation and produced the aligned contractility essential for heart function. Although this paper made important advances, the difficulty of patterning such arrays within 3D scaffolds remains, and hence, the utility of such techniques remains to be proven.
One radically different approach to engineering cardiac tissue involves the creation of stackable cell sheets. Shimizu and colleagues reported a method of culturing rat neonatal ventricular myocytes on temperature-sensitive poly(N-isopropylamide) surfaces.54 At 37°C, these surfaces support normal cell adhesion and growth; however, at 32°C they become hydrophobic and cease to support cell adhesion. Through this method, the researchers were able to remove entire monolayers of electrically coupled cardiomyocytes, without loss of cell–cell contacts through trypsinization. Monolayers could then by stacked and grafted onto defects created in rat hearts in vivo, where they showed coordinated depolarization and contraction in synchrony with the native tissue. Perhaps unsurprisingly from what we know of the highly vascularized nature of cardiac tissues, this process was limited to sheet thicknesses of around 80 μm. A major limitation of nonscaffold techniques such as these is the lack of tissue perfusion; clearly to have clinical relevance for correcting defects in human myocardium, these types of processes must be adapted to promote angiogenesis.
Montero and colleagues recently reported increased sprouting in an in vitro angiogenesis assay when Human Umbilical Vein Endothelial Cells (HUVECs) were seeded onto an electrospun gelatin scaffold with aligned nanoarchitectural features and embedded basic fibroblast growth factor (bFGF).55 Electrospinning techniques can also be employed to coat surfaces in aligned fibers.56 Although yet to be experimentally validated, cardiomyocytes could be seeded in monolayer with nanoscale gelatin fibers and bFGF, potentially creating cell sheets primed for angiogenesis in vivo. Taking this further, cells could be seeded onto a surface containing sacrificial carbohydrate glass channels, and in vivo these channels could be aligned to provide a cell-free conduit for efficient neoangiogenesis driven by bFGF.57
Tissue engineering heart valves
Beyond the regeneration of a functional myocardium, a substantial amount of research is geared toward construction of tissue-engineered heart valves in vitro. Each year in the United States, 85,000 artificial (requiring chronic anticoagulant therapy) or animal-derived (prone to calcification) valves are implanted, representing a substantial clinical need.58 Characterization of the decellularized basement membrane of porcine aortic valves by Brody and colleagues revealed a matrix of fibers and pores with respective diameters of 30 and 22 nm.59 An optimum tissue engineering strategy should therefore comprise a cellularized nanoscale substrate of similar scale.60
Consistent with the limited progress in recapitulating the natural ECM, by far the most advanced clinical studies involving bioartificial heart valves have used decellularized allografts. One early study presented by Dohmen and colleagues used a decellularized pulmonary allograft seeded with autologous endothelial cells and conditioned in a bioreactor to reconstruct the right ventricular outflow tract of adults undergoing the Ross procedure.61 Medium and long-term follow-ups have been published, showing that most valves retain excellent hemodynamic profiles, and all patients were alive, and showing New York Heart Association class I after 10 years.62,63 Clearly, these procedures were life changing for the patients involved, but without detracting from this study’s impact, the ultimate goal remains the creation of completely autologous organs.
Tissue Engineering the Liver
Clinical need
Liver disease and subsequent loss of liver function is currently the 12th most frequent cause of death in the United States.64 Liver transplantation is the only therapy proven to improve mortality, but despite recent advances in surgical techniques (split transplants, living related partial donor procedures), the situation remains acute.65 Beyond global trends in organ transplantation, liver disease in particular suffers from compounding factors including the emergence of new liver diseases such as steatohepatitis and an aging population of hepatitis patients at risk of progression to hepatocellular carcinoma.66 Like the other pathologies discussed, the shortage of donor organs remains acute despite improvements in allocation, suggesting that liver transplantation procedures alone will not be able to meet any increased demand.
Epithelial tissue ECM
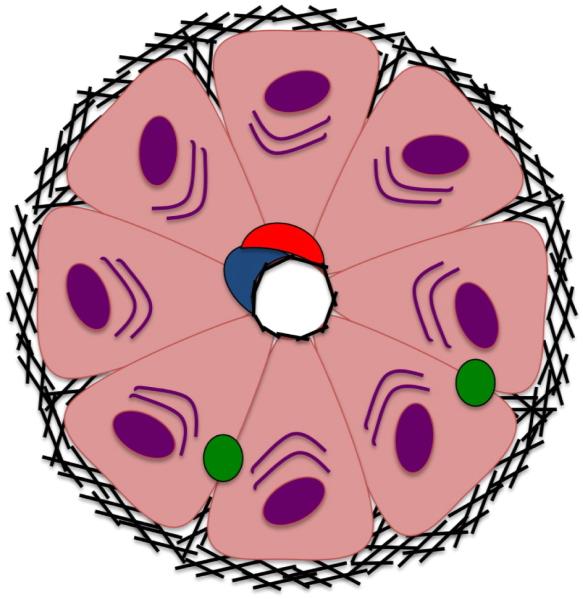
The dense and highly aligned ECM seen in cardiac tissues is not a ubiquitous requirement in the development of complex tissues. In a stark contrast to the cardiac syncytium, epithelial tissues such as those in the liver, kidneys, and pancreas comprise a unique schema of polarized cell contacts. For example, in the liver, each hepatocyte has a basal surface contacting the ECM, an apical surface facing the lumen, and a lateral surface in contact with other cells (Fig. 4). Like the heart, the cellular organization and hierarchy is directly relevant to the organ’s function and completely guided by the ECM.67 Cells lacking luminal contact will be unable to contribute to secretion or absorption into surrounding capillaries and will undergo apoptosis to create a luminal space.68 Hence, scaffolds should aim to recapitulate the nanometric ECM in order to guide cells into a highly perfused columnar structure (Fig. 5).
Figure 4.
Basic structure of a liver lobule, each hepatocyte has a basal surface contacting the ECM, an apical surface facing the lumen, and a lateral surface in contact with other cell. Like the heart, the cellular organization and hierarchy is directly relevant to the organ’s function and completely guided by the ECM.67
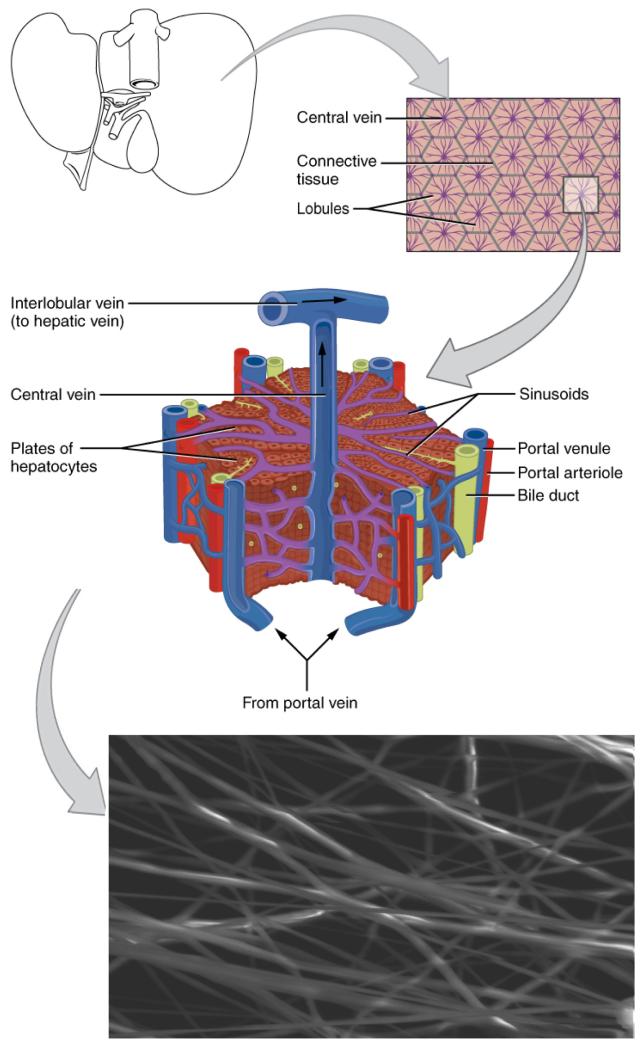
Figure 5.
Schematic showing a tissue engineering strategy for liver cells based on electrospun nanofibers. The high metabolic demand and liver-specific functions (absorption, detoxification, etc.) of hepatocytes requires a highly perfused tissue structure. Electrospun scaffolds (lower panel) allow excellent hepatocyte perfusion and are desirable options for BAL–assisted devices, tissue engineering for liver regeneration, and hepatocyte culture for drug safety analysis. In a series of studies, Feng and colleagues have developed one such method based on chitosan nanofibers in an attempt to mimic the natural liver lobule structure.72,96 Bar is 25 μm. The diagrams in this figure are adapted from OpenStax College, Anatomy & Physiology. OpenStax CNX. Jul 31, 2014. http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@6.27@6.27.
Tissue engineering approaches
In order to create a bioartificial liver (BAL) system for in vivo transplantation, it is necessary to recapitulate the complex 3D nanotopography of the liver ECM using defined or artificial matrices. The complex architecture of the liver ensures proper oxygenation of the metabolically hyperactive hepatocytes alongside efficient nutrient exchange and liver-specific functions (ammonia detoxification, albumin secretion, urea metabolism, etc.).69 BAL systems must promote culture on nonxenogenic surfaces of defined topography, with excellent means of nutrient exchange and blood detoxification.70
To create functional tissues able to undergo efficient diffusion to and from adjacent lumens, Bettinger and colleagues created poly(ester amide) substrates nanopatterned with pillar diameters between 260 and 410 nm.71 In doing so, the researchers were able to mimic the cross-sectional dimensions of collagen fibrils and enhance the attachment and proliferation of hepatocytes in vitro. Crucially, metabolic function was increased in hepatocytes cultured on such substrates. To ensure formation of appropriate lumens, Feng and colleagues took this concept further by culturing hepatocytes on nanofibrous galactosylated chitosan scaffolds and were able to show increased levels of liver-specific functions.72 The rationale behind fibrous hepatocyte scaffolds is shown diagrammatically in Figure 5.
Several attempts have been made to scale up knowledge of hepatocyte culture in two-dimensions (2D) into 3D scaffold systems. Synthetic hydrogels based on poly(ethylene glycol) (PEG) have been studied extensively for tissue engineering approaches73 and have found recent utility in 3D liver platforms. Liu Tsang and colleagues incorporated RGD peptide sequences into photopolymerizable PEG hydrogels, which could be polymerized into a complex 3D architecture in the presence of primary hepatocytes.74 Selective cell adhesion to RGD sequences ensured microscale channels throughout the scaffold, minimizing barriers to nutrient exchange by these highly metabolic cells.
In a similar attempt to preserve liver function in a defined 3D environment, Giri and colleagues have recently reported a clinically relevant bioreactor culture system for primary rat hepatocytes based on a self-assembling peptide nanoscaffold.69 Here the authors sought to mimic the complex nanotopography of a hepatocyte basement membrane while minimizing the use of any xenogenic, nondefined, or toxic materials.
Incentive to create a biomimetic hepatocyte culture system also exists from the pharmaceuticals industry. In an attempt to improve on existing methods of toxicology screening, Lee and colleagues have described a novel hepatocyte culture device based on microfluidic channels etched by photolithography.75,76 Here, liver microsomes are encapsulated in a 3D hydrogel matrix on a PEG diacrylate surface. Nanoscale microfluidic channels are employed to recapitulate the in vivo exchange of nutrients and waste in a liver-on-a-chip format. By modeling cytochrome P450 reaction kinetics, the researchers were able to show the utility of this platform in pharmaceutical toxicology screening. Others have suggested scaling up such a system for use in hepatocyte encapsulation in vivo.77
Bone Regeneration
Clinical need
By 2020, it is expected that 9.3% of adults in the United States will suffer from osteoarthritis.78 The current generation of orthopedic implants is lacking in biofunctionality, and it is imperative that steps are taken to better integrate host and implant. Micromotion caused by nonspecific differentiation of cells at the host–implant boundary limits the lifespan of primary devices and causes damage to the surrounding tissue.79 Additionally, modulus mismatch between titanium implants and much softer bone causes tissue wastage and compounds the need for secondary surgery.80 In order to maximize the lifespan of primary orthopedic implants, cellular differentiation at the host–implant boundary must be controlled while implant modulus must be matched to native tissue to encourage regeneration.
Bone ECM
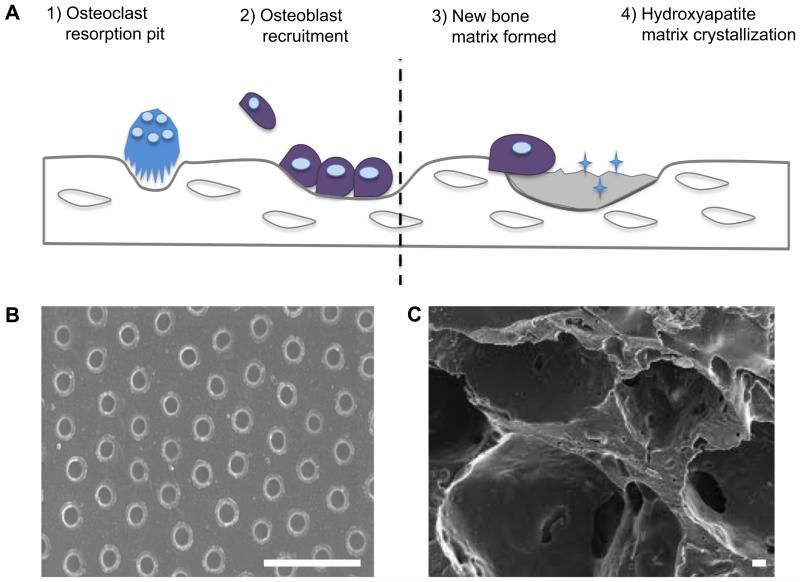
Bone is primarily composed of bone matrix, interspersed by cells and vasculature. Eighty percent of the total bone mass of an adult skeleton is made up of cortical bone, characterized by parallel alignment of type I collagen embedded in GAG gel and arranged into osteons. The remaining 20% by weight is composed of internal cancellous, or spongy, bone characterized by significant porosity and haphazard arrangement of collagen fibers.22 The inorganic composition of bone, responsible for much of its mechanical strength, is composed of carbonated hydroxyapatite. Bone matrix is continually degraded by osteoclasts and remodeled by osteoblasts laying down nonmineralized osteoid. Osteoblasts then secrete vesicles containing alkaline phosphatases, which cleave osteoid terminal phosphates and act as foci for hydroxyapetate crystal formation shown diagrammatically in Figure 6 panel A. During this continuous process of bone remodeling, osteoblasts may become trapped in their own matrix, triggering differentiation into osteocytes of illdefined function.81
Figure 6.
Schematic of bone tissue remodeling. Osteoclasts degrade old mineralized bone creating resorption pits. These pits are thought to recruit osteoblasts, which lay down new unmineralized matrix. Panel B shows nanopits produced in acrylic polymer, thought to reflect resorption pits and known to recapitulate bone formation in vitro.26 Bar is 1 μm. Mature osteoblasts secrete alkaline phosphatase to allow crystallization of hydroxyapatite and mineralization of new matrix. Panel C shows a macroporous poly(glycolic co-lactic) acid scaffold often used in solid bone engineering. Such scaffolds can be functionalized with hydroxyapatite nanocrystals to aid mineralization.41 Bar is 10 μm.
Bone tissue engineering
Tissue engineering of bone tissue presents an interesting situation. Orthopedic implants are routinely used in surgery, and the clinical limitations of such devices are increasingly relevant.80 Thus, a great deal of tissue engineering research has investigated the driving factors behind bone differentiation. For example, it has long been known that MSC differentiation into osteoblast, chondrocyte, or adipocyte lineage can be controlled by focal adhesion size and cell spreading.82 More recent research has shown that MSC differentiation can be directed toward osteogenic lineage through nanoscale disorder26 as well as nanoscale pits and pillars, Figure 6 panel B.83 It is thought that such features mimic the osteoclast resorption pits created during bone remodeling. Hence, there is a great deal of effort into translating this basic research into improving bone formation along an implant’s edge.32 It is thought that techniques such as coating titanium implants in hydroxyapatite could direct MSC differentiation, reduce implant micromovements, and show very real benefits in terms of patient quality of life in the short to medium term.84 However, as an orthopedic implant is designed to be a permanent feature, and not resorbed in the body, by definition it is not tissue engineering.
Various attempts have been made toward tissue engineering of bone; however, by and large these have been limited to correcting small defects without any major load-bearing function. For example, Tarafder and colleagues fabricated an interconnected porous tricalcium phosphate (TCP) scaffold with nanoscale SrO and MgO by direct 3D printing.85 Although this scaffold showed a significant increase in osteoid-like new bone formation and accelerated healing through osteogenesis compared to pure TCP scaffolds, current technology limits pore sizes to the microscale. Such large pores invariably compromise a scaffolds load-bearing capacity repesentative example shown in Figure 6 panel C. Similar attempts have been made with both bioresorbable Bioglass and polymeric scaffolds. In one study, Wu and colleagues introduced ionic cobalt to mesoporous Bioglass scaffolds to mimic hypoxia and increase bone marrow–derived stem cell proliferation and osteogenic differentiation.86 While studies of this nature show promise, current manufacturing techniques limit the translation of insights from 2D osteoblast culture into 3D scaffolds. However, self-assembling peptide nanofiber scaffolds are beginning to see utility for osteointegration of dental implants, and with appropriate improvements to mechanical strength (Table 2), it is possible that such scaffolds could translate into orthopedic implant surgery.87
Future Prospects
As the ECM drives the initial complexity of a developing organ, it is necessary to understand and reproduce this matrix to help recapitulate complexity in tissue engineering. A cell’s extracellular environment is a complex milieu of growth factors, cytokines, morphogens, and biophysical cues. As many of these natural differentiative cues are in the order of several nanometers, synthetic nanoscale materials should be incorporated into tissue engineering scaffolds. Over the past several years, the synthesis of new synthetic nanostructures and their incorporation into existing macro- and microscale scaffolds has led to improvements in our ability to produce a true biomimetic microenvironment. In addition, the utility of nanocomposites in overcoming matrix limitations in current biomaterials is becoming apparent.
However, major challenges still exist in the use nanotechnologies to engineer complex tissues. Foremost is the need for systematic studies into the biocompatibility and biodegradation of newly synthesized nanotechnologies before they can be incorporated into clinical trials.
The interaction of cells with various nanotopographies has been studied extensively, and efforts have been made in the creation of complex monolayers of functional tissues. However, major challenges remain in the scaling up of nanopatterning technologies for use in 3D scaffolds. One possibility is that direct 3D printing technologies will allow nanoscale resolution in the near future. Another, perhaps more exciting, possibility is that self-assembling peptides and hydrogels will be utilized in more applications. Such bottom-up manufacturing techniques allow nanoscale cues to be incorporated into monomers, in the form of adhesion sequences, morphogens, or topographical cues. Monomer self-assembly can then be finely tuned by modifying biochemical and biophysical parameters, allowing precise control over the seeded cells microenvironment. Integrated biomimetic approaches such as these will surely be required to deliver on the promises made by tissue engineering and regenerative medicine.
Acknowledgments
JWC is thankful to MJ Dalby and JN Roberts for valuable discussion and guidance and to BJ Cathcart for criticism of the first draft of the manuscript.
FUNDING: JWC was previously funded by a Scholarship from the university of Glasgow and is now in receipt of a Cancer Research UK Scholarship. The views expressed in this review paper do not necessarily reflect the views or policy of Cancer Research UK. The author confirms that the funder had no influence over the study design, content of the article, or selection of this journal.
Footnotes
COMPETING INTERESTS: Author discloses no potential conflicts of interest.
REFERENCES
- 1.Brockes JP, Gates PB. Mechanisms underlying vertebrate limb regeneration: lessons from the salamander. Biochem Soc Trans. 2014;42(3):625–630. doi: 10.1042/BST20140002. [DOI] [PubMed] [Google Scholar]
- 2.de Reaumuer RAF. Sur les diverses reproductions qui se font dans les écrevisses, les omars, les crabes, etc. et entre autres sur celles de leurs jambes et de leurs écailles. impr. royale. Mem Acad Sci. 1711:109–36. [Google Scholar]
- 3.Kwon TG, Yoo JJ, Atala A. Local and systemic effects of a tissue engineered neobladder in a canine cystoplasty model. J Urol. 2008;179(5):2035–2041. doi: 10.1016/j.juro.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Raya-Rivera AM, Esquiliano D, Fierro-Pastrana R, et al. Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet. 2014;384:329–336. doi: 10.1016/S0140-6736(14)60542-0. [DOI] [PubMed] [Google Scholar]
- 5.Klein AS, Messersmith EE, Ratner LE, Kochik R, Baliga PK, Ojo AO. Organ donation and utilization in the United States, 1999–2008. Am J Transplant. 2010;10(4 pt 2):973–986. doi: 10.1111/j.1600-6143.2009.03008.x. [DOI] [PubMed] [Google Scholar]
- 6.Mason C, Dunnill P. The strong financial case for regenerative medicine and the regen industry. Regen Med. 2008;3(3):351–363. doi: 10.2217/17460751.3.3.351. [DOI] [PubMed] [Google Scholar]
- 7.Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med. 2008;3(1):1–5. doi: 10.2217/17460751.3.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451(7181):937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 9.Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14(2):211–215. doi: 10.1111/j.1756-185X.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 10.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363(2):147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 11.Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209(1):10–17. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science. 2007;318(5851):772–777. doi: 10.1126/science.1147710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godwin JW, Brockes JP. Regeneration, tissue injury and the immune response. J Anat. 2006;209(4):423–432. doi: 10.1111/j.1469-7580.2006.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearl JI, Lee AS, Leveson-Gower DB, et al. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell. 2011;8(3):309–317. doi: 10.1016/j.stem.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nature reviews. Genetics. 2010;11(1):47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol. 2001;166(4):2479–2486. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- 17.Kimura Y, Madhavan M, Call MK, et al. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J Immunol. 2003;170(5):2331–2339. doi: 10.4049/jimmunol.170.5.2331. [DOI] [PubMed] [Google Scholar]
- 18.L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12(1):47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 19.Langer R. Tissue engineering. Mol Ther. 2000;1(1):12–15. doi: 10.1006/mthe.1999.0003. [DOI] [PubMed] [Google Scholar]
- 20.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(pt 24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117(4):1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- 22.Gentili C, Cancedda R. Cartilage and bone extracellular matrix. Curr Pharm Des. 2009;15(12):1334–1348. doi: 10.2174/138161209787846739. [DOI] [PubMed] [Google Scholar]
- 23.Full SM, Delman C, Gluck JM, Abdmaulen R, Shemin RJ, Heydarkhan-Hagvall S. Effect of fiber orientation of collagen-based electrospun meshes on human fibroblasts for ligament tissue engineering applications. J Biomed Mater Res B Appl Biomater. 2014 doi: 10.1002/jbm.b.33153. DOI: 10.1002/jbm.b.33153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guguen-Guillouzo C, Guillouzo A. General review on in vitro hepatocyte models and their applications. Methods Mol Biol. 2010;640:1–40. doi: 10.1007/978-1-60761-688-7_1. [DOI] [PubMed] [Google Scholar]
- 25.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 26.Dalby MJ, Gadegaard N, Tare R, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6(12):997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 27.McMurray RJ, Gadegaard N, Tsimbouri PM, et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater. 2011;10(8):637–644. doi: 10.1038/nmat3058. [DOI] [PubMed] [Google Scholar]
- 28.Vaday GG, Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J Leukoc Biol. 2000;67(2):149–159. doi: 10.1002/jlb.67.2.149. [DOI] [PubMed] [Google Scholar]
- 29.Engel E, Michiardi A, Navarro M, Lacroix D, Planell JA. Nanotechnology in regenerative medicine: the materials side. Trends Biotechnol. 2008;26(1):39–47. doi: 10.1016/j.tibtech.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Cassidy JW, Roberts JN, Smith CA, et al. Osteogenic lineage restriction by osteoprogenitors cultured on nanometric grooved surfaces: the role of focal adhesion maturation. Acta Biomater. 2014;10(2):651–660. doi: 10.1016/j.actbio.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Grater SV, Corbellini F, et al. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 2009;9(3):1111–1116. doi: 10.1021/nl803548b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavenus S, Ricquier JC, Louarn G, Layrolle P. Cell interaction with nanopatterned surface of implants. Nanomedicine. 2010;5(6):937–947. doi: 10.2217/nnm.10.54. [DOI] [PubMed] [Google Scholar]
- 33.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24(12):2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 34.Yilgor P, Tuzlakoglu K, Reis RL, Hasirci N, Hasirci V. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials. 2009;30(21):3551–3559. doi: 10.1016/j.biomaterials.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 35.Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev. 2007;59(14):1413–1433. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 36.Re’em T, Tsur-Gang O, Cohen S. The effect of immobilized RGD peptide in macroporous alginate scaffolds on TGFbeta1-induced chondrogenesis of human mesenchymal stem cells. Biomaterials. 2010;31(26):6746–6755. doi: 10.1016/j.biomaterials.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Jeon O, Bouhadir KH, Mansour JM, Alsberg E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials. 2009;30(14):2724–2734. doi: 10.1016/j.biomaterials.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 38.Leach MK. Biomimetic Electrospun Fibers for Peripheral Nervous System Repair. University of Michigan: Biomedical Engineering University of Michigan; Ann Arbor: 2013. [Google Scholar]
- 39.Casper CL, Yang W, Farach-Carson MC, Rabolt JF. Coating electrospun collagen and gelatin fibers with perlecan domain I for increased growth factor binding. Biomacromolecules. 2007;8(4):1116–1123. doi: 10.1021/bm061003s. [DOI] [PubMed] [Google Scholar]
- 40.Freeman I, Kedem A, Cohen S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials. 2008;29(22):3260–3268. doi: 10.1016/j.biomaterials.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 41.Roohani-Esfahani SI, Nouri-Khorasani S, Lu Z, Appleyard R, Zreiqat H. The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite-PCL composites. Biomaterials. 2010;31(21):5498–5509. doi: 10.1016/j.biomaterials.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Reddy VJ, Wong SY, et al. Enhanced biomineralization in osteoblasts on a novel electrospun biocomposite nanofibrous substrate of hydroxyapatite/collagen/chitosan. Tissue Eng Part A. 2010;16(6):1949–1960. doi: 10.1089/ten.TEA.2009.0221. [DOI] [PubMed] [Google Scholar]
- 43.Cui H, Webber MJ, Stupp SI. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers. 2010;94(1):1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tysseling-Mattiace VM, Sahni V, Niece KL, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28(14):3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suhr J, Victor P, Ci L, et al. Fatigue resistance of aligned carbon nanotube arrays under cyclic compression. Nat Nanotechnol. 2007;2(7):417–421. doi: 10.1038/nnano.2007.186. [DOI] [PubMed] [Google Scholar]
- 46.Wang SF, Shen L, Zhang WD, Tong YJ. Preparation and mechanical properties of chitosan/carbon nanotubes composites. Biomacromolecules. 2005;6(6):3067–3072. doi: 10.1021/bm050378v. [DOI] [PubMed] [Google Scholar]
- 47.Burch M, Aurora P. Current status of paediatric heart, lung, and heart-lung transplantation. Arch Dis Child. 2004;89(4):386–389. doi: 10.1136/adc.2002.017186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor DO, Edwards LB, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report—2007. J Heart Lung Transplant. 2007;26(8):769–781. doi: 10.1016/j.healun.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Michaels PJ, Espejo ML, Kobashigawa J, et al. Humoral rejection in cardiac transplantation: risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J Heart Lung Transplant. 2003;22(1):58–69. doi: 10.1016/s1053-2498(02)00472-2. [DOI] [PubMed] [Google Scholar]
- 50.Andreu I, Luque T, Sancho A, et al. Heterogeneous micromechanical properties of the extracellular matrix in healthy and infarcted hearts. Acta Biomater. 2014;10(7):3235–3242. doi: 10.1016/j.actbio.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 51.Fomovsky GM, Thomopoulos S, Holmes JW. Contribution of extracellular matrix to the mechanical properties of the heart. J Mol Cell Cardiol. 2010;48(3):490–496. doi: 10.1016/j.yjmcc.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ott HC, Matthiesen TS, Goh SK, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 53.Kim DH, Lipke EA, Kim P, et al. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci U S A. 2010;107(2):565–570. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimizu T, Yamato M, Isoi Y, et al. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. 2002;90(3):e40. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 55.Montero RB, Vial X, Nguyen DT, et al. bFGF-containing electrospun gelatin scaffolds with controlled nano-architectural features for directed angiogenesis. Acta Biomater. 2012;8(5):1778–1791. doi: 10.1016/j.actbio.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoo HS, Kim TG, Park TG. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv Drug Deliv Rev. 2009;61(12):1033–1042. doi: 10.1016/j.addr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Forgacs G. Tissue engineering: perfusable vascular networks. Nat Mater. 2012;11(9):746–747. doi: 10.1038/nmat3412. [DOI] [PubMed] [Google Scholar]
- 58.Neidert MR, Tranquillo RT. Tissue-engineered valves with commissural alignment. Tissue Eng. 2006;12(4):891–903. doi: 10.1089/ten.2006.12.891. [DOI] [PubMed] [Google Scholar]
- 59.Brody S, Anilkumar T, Liliensiek S, Last JA, Murphy CJ, Pandit A. Characterizing nanoscale topography of the aortic heart valve basement membrane for tissue engineering heart valve scaffold design. Tissue Eng. 2006;12(2):413–421. doi: 10.1089/ten.2006.12.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sacks MS, Schoen FJ, Mayer JE. Bioengineering challenges for heart valve tissue engineering. Annu Rev Biomed Eng. 2009;11:289–313. doi: 10.1146/annurev-bioeng-061008-124903. [DOI] [PubMed] [Google Scholar]
- 61.Dohmen PM, Ozaki S, Verbeken E, Yperman J, Flameng W, Konertz WF. Tissue engineering of an auto-xenograft pulmonary heart valve. Asian Cardiovasc Thorac Ann. 2002;10(1):25–30. doi: 10.1177/021849230201000107. [DOI] [PubMed] [Google Scholar]
- 62.Dohmen PM, Lembcke A, Holinski S, Pruss A, Konertz W. Ten years of clinical results with a tissue-engineered pulmonary valve. Ann Thorac Surg. 2011;92(4):1308–1314. doi: 10.1016/j.athoracsur.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Dohmen PM, Lembcke A, Holinski S, et al. Mid-term clinical results using a tissue-engineered pulmonary valve to reconstruct the right ventricular outflow tract during the Ross procedure. Ann Thorac Surg. 2007;84(3):729–736. doi: 10.1016/j.athoracsur.2007.04.072. [DOI] [PubMed] [Google Scholar]
- 64.Mann RE, Smart RG, Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res Health. 2003;27(3):209–219. [PMC free article] [PubMed] [Google Scholar]
- 65.Brown KA. Liver transplantation. Curr Opin Gastroenterol. 2005;21(3):331–336. doi: 10.1097/01.mog.0000159830.36793.2b. [DOI] [PubMed] [Google Scholar]
- 66.Kim WR, Brown RS, Jr, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36(1):227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 67.Brill S, Zvibel I, Halpern Z, Oren R. The role of fetal and adult hepatocyte extracellular matrix in the regulation of tissue-specific gene expression in fetal and adult hepatocytes. Eur J Cell Biol. 2002;81(1):43–50. doi: 10.1078/0171-9335-00200. [DOI] [PubMed] [Google Scholar]
- 68.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9(11):887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giri S, Acikgöz A, Pathak P, et al. Three dimensional cultures of rat liver cells using a natural self-assembling nanoscaffold in a clinically relevant bioreactor for bioartificial liver construction. J Cell Physiol. 2012;227(1):313–327. doi: 10.1002/jcp.22738. [DOI] [PubMed] [Google Scholar]
- 70.Bhatia SN, Underhill GH, Zaret KS, Fox IJ. Cell and tissue engineering for liver disease. Sci Transl Med. 2014;6(245):245sr242. doi: 10.1126/scitranslmed.3005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bettinger CJ, Kulig KM, Vacanti JP, Langer R, Borenstein JT. Nanofabricated collagen-inspired synthetic elastomers for primary rat hepatocyte culture. Tissue Eng Part A. 2009;15(6):1321–1329. doi: 10.1089/ten.tea.2008.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng ZQ, Chu X, Huang NP, et al. The effect of nanofibrous galactosylated chitosan scaffolds on the formation of rat primary hepatocyte aggregates and the maintenance of liver function. Biomaterials. 2009;30(14):2753–2763. doi: 10.1016/j.biomaterials.2009.01.053. [DOI] [PubMed] [Google Scholar]
- 73.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54(1):3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 74.Liu Tsang V, Chen AA, Cho LM, et al. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007;21(3):790–801. doi: 10.1096/fj.06-7117com. [DOI] [PubMed] [Google Scholar]
- 75.Lee J, Kim SH, Kim YC, Choi I, Sung JH. Fabrication and characterization of microfluidic liver-on-a-chip using microsomal enzymes. Enzyme Microb Technol. 2013;53(3):159–164. doi: 10.1016/j.enzmictec.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 76.Kwon GH, Choi YY, Park JY, et al. Electrically-driven hydrogel actuators in microfluidic channels: fabrication, characterization, and biological application. Lab Chip. 2010;10(12):1604–1610. doi: 10.1039/b926443d. [DOI] [PubMed] [Google Scholar]
- 77.Tendulkar S, Mirmalek-Sani SH, Childers C, Saul J, Opara EC, Ramasubramanian MK. A three-dimensional microfluidic approach to scaling up microencapsulation of cells. Biomed Microdevices. 2012;14(3):461–469. doi: 10.1007/s10544-011-9623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Navarro M, Michiardi A, Castano O, Planell JA. Biomaterials in orthopaedics. J R Soc Interface. 2008;5(27):1137–1158. doi: 10.1098/rsif.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang BG, Myers DE, Wallace GG, Brandt M, Choong PF. Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings. Int J Mol Sci. 2014;15(7):11878–11921. doi: 10.3390/ijms150711878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thelen S, Barthelat F, Brinson LC. Mechanics considerations for microporous titanium as an orthopedic implant material. J Biomed Mater Res A. 2004;69(4):601–610. doi: 10.1002/jbm.a.20100. [DOI] [PubMed] [Google Scholar]
- 81.Blair HC, Zaidi M, Huang CL, Sun L. The developmental basis of skeletal cell differentiation and the molecular basis of major skeletal defects. Biol Rev Camb Philos Soc. 2008;83(4):401–415. doi: 10.1111/j.1469-185X.2008.00048.x. [DOI] [PubMed] [Google Scholar]
- 82.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 83.Brammer KS, Choi C, Frandsen CJ, Oh S, Jin S. Hydrophobic nanopillars initiate mesenchymal stem cell aggregation and osteo-differentiation. Acta Biomater. 2011;7(2):683–690. doi: 10.1016/j.actbio.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 84.Roy M, Bandyopadhyay A, Bose S. Induction plasma sprayed Sr and Mg doped nano hydroxyapatite coatings on Ti for bone implant. J Biomed Mater Res B Appl Biomater. 2011;99(2):258–265. doi: 10.1002/jbm.b.31893. [DOI] [PubMed] [Google Scholar]
- 85.Tarafder S, Davies NM, Bandyopadhyay A, Bose S. 3D printed tricalcium phosphate scaffolds: effect of SrO and MgO doping on osteogenesis in a rat distal femoral defect model. Biomater Sci. 2013;1(12):1250–1259. doi: 10.1039/C3BM60132C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu C, Zhou Y, Fan W, et al. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials. 2012;33(7):2076–2085. doi: 10.1016/j.biomaterials.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 87.Kohgo T, Yamada Y, Ito K, et al. Bone regeneration with self-assembling peptide nanofiber scaffolds in tissue engineering for osseointegration of dental implants. Int J Periodontics Restorative Dent. 2011;31(4):e9–e16. [PubMed] [Google Scholar]
- 88.Liu X, Liu S, Liu S, Cui W. Evaluation of oriented electrospun fibers for periosteal flap regeneration in biomimetic triphasic osteochondral implant. J Biomed Mater Res B Appl Biomater. 2014;102:1407–1414. doi: 10.1002/jbm.b.33119. [DOI] [PubMed] [Google Scholar]
- 89.Elloumi Hannachi I, Itoga K, Kumashiro Y, Kobayashi J, Yamato M, Okano T. Fabrication of transferable micropatterned-co-cultured cell sheets with micro-contact printing. Biomaterials. 2009;30(29):5427–5432. doi: 10.1016/j.biomaterials.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 90.Shekaran A, Garcia AJ. Nanoscale engineering of extracellular matrix-mimetic bioadhesive surfaces and implants for tissue engineering. Biochim Biophys Acta. 2011;1810(3):350–360. doi: 10.1016/j.bbagen.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gui X, Cao A, Wei J, et al. Soft, highly conductive nanotube sponges and composites with controlled compressibility. ACS Nano. 2010;4(4):2320–2326. doi: 10.1021/nn100114d. [DOI] [PubMed] [Google Scholar]
- 92.Zhang S, Uludag H. Nanoparticulate systems for growth factor delivery. Pharm Res. 2009;26(7):1561–1580. doi: 10.1007/s11095-009-9897-z. [DOI] [PubMed] [Google Scholar]
- 93.Pislaru SV, Harbuzariu A, Agarwal G, et al. Magnetic forces enable rapid endothelialization of synthetic vascular grafts. Circulation. 2006;114(1 suppl):I314–I318. doi: 10.1161/CIRCULATIONAHA.105.001446. [DOI] [PubMed] [Google Scholar]
- 94.Wu S, Liu X, Hu T, et al. A biomimetic hierarchical scaffold: natural growth of nanotitanates on three-dimensional microporous Ti-based metals. Nano Lett. 2008;8(11):3803–3808. doi: 10.1021/nl802145n. [DOI] [PubMed] [Google Scholar]
- 95.Fan D, Yin Z, Cheong R, et al. Subcellular-resolution delivery of a cytokine through precisely manipulated nanowires. Nat Nanotechnol. 2010;5(7):545–551. doi: 10.1038/nnano.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feng ZQ, Leach MK, Chu XH, et al. Electrospun chitosan nanofibers for hepatocyte culture. J Biomed Nanotechnol. 2010;6(6):658–666. doi: 10.1166/jbn.2010.1159. [DOI] [PubMed] [Google Scholar]