On the historic occasion of the 122nd shattuck lecture and the 200th anniversary of the New England Journal of Medicine, we chose to address a topic that is at once scientific and personally historic. In recent debates over legalizing marijuana, from all-out acceptance in Colorado to narrow decriminalization in Maryland, the scientific question of the role of marijuana as a gateway drug (i.e., a drug that lowers the threshold for addiction to other agents) has loomed large. Both opponents and proponents of legalization have distorted what science does and does not tell us — and both sides have overlooked the importance of nicotine as a gateway drug.
Epidemiologic studies have shown that nicotine use is a gateway to the use of marijuana and cocaine in human populations. What has not been clear is how nicotine accomplishes this. In this article, we describe how our personal collaboration allowed us to bring the techniques of molecular biology to bear on this question and to reveal the action of nicotine in the brain of mice. We then apply our conclusions to the public health concerns that are being raised as the popularity of electronic cigarettes (e-cigarettes) has soared. In the process, we show the potential benefits to society of translating epidemiologic findings into public health policy.
GATEWAY HYPOTHESIS AND THE COMMON LIABILITY MODEL
The gateway hypothesis was developed by Denise Kandel, who observed that young people become involved in drugs in stages and sequences.1 She found that in the general population of the United States and other Western societies, a well-defined developmental sequence of drug use occurs that starts with a legal drug and proceeds to illegal drugs. Specifically, the use of tobacco or alcohol precedes the use of marijuana, which in turn precedes the use of cocaine and other illicit drugs.1–6 Thus, in 2012, among U.S. adults 18 to 34 years of age who had ever used cocaine, 87.9% had smoked cigarettes before using cocaine, 5.7% began using cigarettes and cocaine at the same time, 3.5% used cocaine first, and 2.9% had never smoked cigarettes.
An alternative to the gateway hypothesis has been proposed on the basis of the idea that the use of multiple drugs reflects a common liability for drug use and that addiction, rather than the use of a particular drug, increases the risk of progressing to the use of another drug.2,7–10 Population studies have shown both generalized risk across substances and substance-specific risk — in particular, risk attributable to tobacco use.11
Although epidemiologic studies can establish the sequence in which different substances are used and can specify their associations, such studies cannot determine what causes the progression from one drug to the next, nor can they identify on a molecular level the mechanisms underlying the progression. Testing the validity of the gateway hypothesis in biologic and molecular terms requires an animal model, in which investigators administer one drug and observe how it influences the reaction of the animal to a second drug. Investigators can change the order of drug exposures and observe the effect on outcomes.
DRUG USE AND ADDICTION AS A FORM OF MEMORY
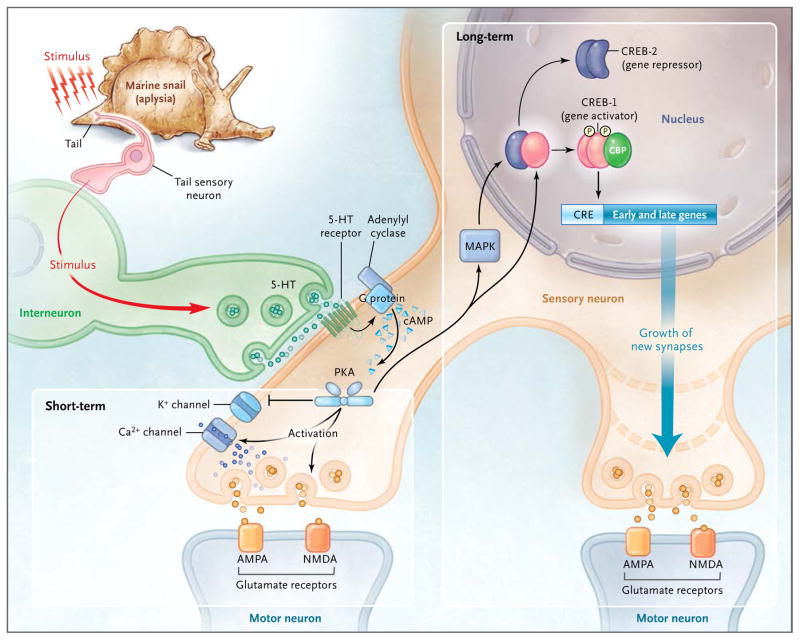
Early psychological studies involving humans suggested that addiction is a form of learning and that relapse is a persistent memory of the drug experience.12 To test this idea, investigators needed some insight into the cell-biologic nature of memory in general and of addiction in particular. Initial insights into the nature of memory were provided by Eric Kandel and colleagues, who found that the gene transcription factor cyclic AMP response-element–binding protein (CREB) acts as a switch, converting short-term memory to long-term memory (Fig. 1). This conversion involves a CREB-mediated modification in chromatin (Fig. 2).13
Figure 1. Cellular and Molecular Mechanisms Underlying the Switch from Short-Term to Long-Term Memory in a Simple Animal Model.
The sensitization of the gill-withdrawal reflex is shown in the marine snail aplysia. In short-term memory, an external stimulus, such as an electric shock to the tail of the animal, causes the sensory neuron to send information in the form of chemical signals across the synapse to a motor neuron. The structure of neither cell is changed. In long-term memory, repeated stimuli recruit the gene transcription factor cyclic AMP (cAMP) response-element–binding protein (CREB), which activates downstream genes and results in the growth of new synapses in the sensory cell, thus strengthening the connection between neurons (i.e., long-term potentiation). 5-HT denotes 5-hydroxytryptamine, AMPA α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid, CRE cyclic adenosine monophosphate response element, G guanine nucleotide–binding, MAPK mitogen-activated protein kinase, NMDA anti–N-methyl-D-aspartate, and PKA protein kinase A.
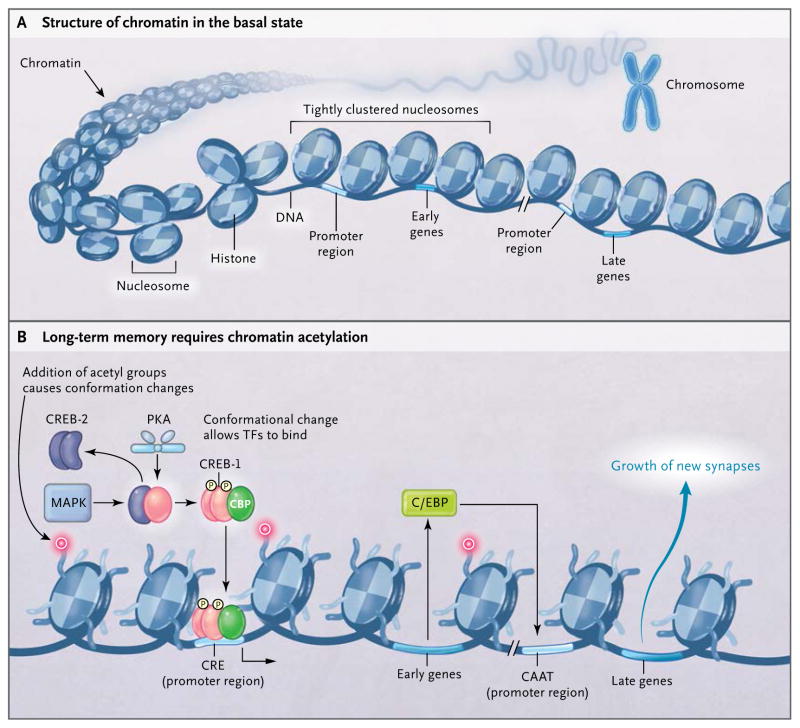
Figure 2. Long-Term Memory and Changes in the Structure of Chromatin.
Genes associated with long-term memory have two regions: a regulatory (promoter) region and a coding (early genes and late genes) region. Early genes refer to genes that are turned on early in the process of switching on long-term memory and in turn lead to the activation of late genes, which encode for proteins essential for the growth of new synaptic connections. The DNA of genes is wrapped around nucleosomes (octamers of histones). The combination of DNA and nucleosomes is called chromatin. The nucleosomes cluster around the promoter region and need to be modified (acetylated) for the transcription factors to bind to the promoter regions so that memory is stored. This modification is referred to as the acetylation of chromatin structure. C/EBP denotes CAAT/enhancer–binding protein, and TF transcription factor.
Hyma, Malenka, and Nestler14–16 explored the learning mechanisms underlying addiction in the striatum, a critical brain area that is targeted by drugs of abuse. They found that the same molecular steps Kandel had delineated as underlying memory also play a major role in addiction17: activation of CREB and the transcription of downstream target genes such as FOSB and its isoform ΔFosB. The accumulation of ΔFosB in the striatum is a crucial step in establishing addiction to most drugs of abuse and has been used as a molecular marker for these processes. Levine et al.17 and Alibhai et al.18 found that structural changes occur in the chromatin of FosB in response also to cocaine.
TEST OF THE GATEWAY HYPOTHESIS IN MICE
In collaboration with Amir Levine, we developed a mouse model that would enable us to explore the behavioral, electrophysiological, and molecular genetic effects of particular drug-use sequences. We examined two addiction-related behaviors, locomotor sensitization and conditioned place preference, and the physiological and molecular markers of the priming effects of one drug on another in the nucleus accumbens, a region of the striatum that is critical for reward and addiction.19
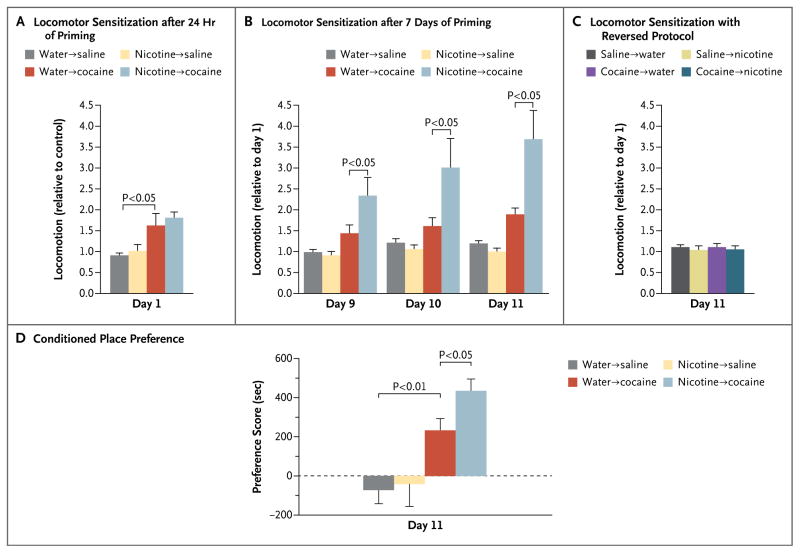
Locomotor sensitization showed that priming mice with nicotine can enhance the effect of cocaine. Mice given nicotine in their drinking water were no more active than control mice given plain water. Mice given only cocaine were 58% more active than controls (Fig. 3A); mice given nicotine for 1 day, followed by 4 days of nicotine and cocaine, showed no increase in locomotor response, but mice given nicotine for 7 days, followed by 4 days of nicotine and cocaine, were significantly (98%) more active than controls (Fig. 3A and 3B). Activity did not increase when the protocol was reversed (7 days of cocaine, followed by 4 days of concurrent cocaine and nicotine) (Fig. 3C).
Figure 3. Effects of Priming with Nicotine on Cocaine-Induced Locomotor Sensitization and Conditioned Place Preference in Mice.
For sensitization, we gave the mice nicotine (50 μg per milliliter) in their drinking water for either 24 hours (Panel A) or 7 days (Panel B). For the subsequent 4 days, we gave the mice a single injection of cocaine per day (20 mg per kilogram of body weight), along with the same amount of nicotine in their drinking water as received previously (10 to 15 mice per group). In Panel B, data are expressed as the total distance traveled on days 9 through 11, as compared with day 1. Panel A shows the lack of effect of 24 hours of nicotine treatment on cocaine-induced locomotion, as compared with the water and saline control, and Panel B the significant effect of 7 days of nicotine treatment on cocaine-induced locomotion on days 9 through 11. Panel C shows the lack of effect of 7 days of cocaine treatment on nicotine-induced locomotion on day 11. Similarly, for conditioned place preference (Panel D), we gave the mice nicotine for 7 days, followed by 4 days of cocaine and nicotine; Panel D shows the data for conditioned preference for the cocaine chamber on day 11. Preference scores were calculated by subtracting the time spent in the cocaine-paired side after conditioning from the time spent before conditioning (8 mice per group). In all panels, data are means ±SE. Data are from Levine et al.19
Conditioned place preference is a more naturalistic model of addictive behavior than sensitization. It measures the preference of an animal for a particular place in its environment as that place becomes associated with a reward and assumes some of the pleasurable effects of the reward. As with sensitization, mice primed with 7 days of nicotine and then given both nicotine and cocaine for 4 days had a 78% greater preference for the chamber associated with cocaine than were mice given only water and then cocaine (Fig. 3D).
We next examined synaptic plasticity, as measured by changes in long-term potentiation, in the core of the nucleus accumbens, a region of the ventral striatum that integrates rewarding input from dopamine-producing neurons in the ventral tegmental area with excitatory input from glutamate-producing neurons in the amygdala and the prefrontal cortex. Reducing excitatory input to the nucleus accumbens is thought to decrease inhibitory output from the nucleus accumbens to the ventral tegmental area and thereby to contribute, by means of disinhibition, to enhanced reward with drugs of abuse. This disinhibition results in the production of more dopamine and contributes to an enhanced rewarding effect of drugs of abuse.
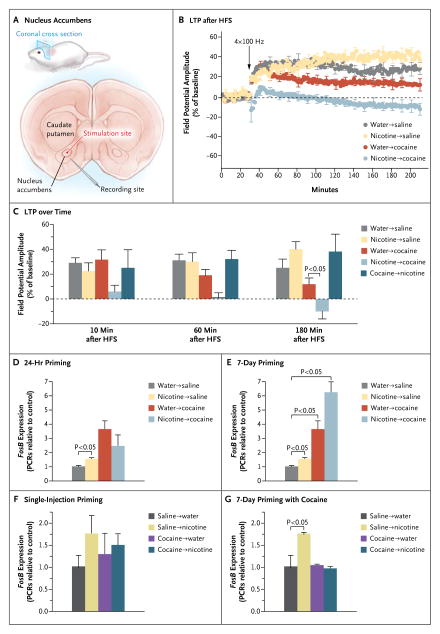
Since we knew that the repeated administration of cocaine resulted in reduced long-term potentiation in the excitatory synapses of the nucleus accumbens in the mouse, we stimulated those synapses and measured long-term potentiation (Fig. 4A). We found that just one injection of cocaine in a mouse given nicotine for 7 days led to a marked reduction in long-term potentiation that started immediately after stimulation and persisted for up to 180 minutes. Nicotine alone, cocaine alone for 7 days, or 7 days of cocaine followed by 24 hours of nicotine did not alter long-term potentiation (Fig. 4B and 4C).
Figure 4. Effects of Priming with Nicotine and Cocaine-Induced Synaptic Plasticity and FosB Expression in Mice.
Panel A shows a schematic illustration of the stimulation and recording sites in a coronal slice of the nucleus accumbens of the mouse. Panel B shows long-term potentiation (LTP) measured 180 minutes after high-frequency stimulation (HFS) applied at 30 minutes (arrow). Experimental groups included six control mice given water followed by saline, six mice given nicotine for 7 days in drinking water, six mice given a single injection of cocaine, and nine mice given nicotine for 7 days followed by a single injection of cocaine. Panel C shows the change in long-term potentiation over time in all the groups. An additional experimental group (five mice given cocaine for 7 days, followed by 24 hours of nicotine) was included. Panel D shows the effect of 24 hours of nicotine followed by cocaine (nine mice per group) on real-time FosB expression, measured in polymerase chain reactions (PCRs; control [water followed by saline] normalized to 1 in Panels D through G). Panel E shows the effects of 7 days of nicotine followed by cocaine (nine mice per group). Panel F shows the results of a single injection of cocaine followed by 24 hours of nicotine (five mice per group). Panel G shows the results of 7 days of cocaine followed by 24 hours of nicotine (seven mice per group). In Panels B through G, data are means ±SE. Data are from Levine et al.19
As in the behavioral experiments, priming with nicotine enhanced the effects of cocaine — in this case, priming changed synaptic plasticity (i.e., decreased long-term potentiation) in the nucleus accumbens. Priming with nicotine appeared to increase the rewarding properties of cocaine by further disinhibiting dopaminergic neurons in the ventral tegmental area.
Previous studies have shown that an important step in the sequence of molecular events leading to addictive behavior in mice is the increased expression of FosB in the striatum. Colby et al.20 found that the targeted expression of ΔFosB in the nucleus accumbens enhanced cocaine-induced behavior. We therefore asked whether the effects of nicotine on behavior that we had observed (cocaine-induced changes in sensitization and conditioned place preference) and changes in synaptic strength (long-term potentiation) correlated with changes in FosB expression in the striatum. We found that giving mice nicotine in their drinking water for 24 hours and for 7 days caused increases in FosB expression of 50% and 61%, respectively (Fig. 4D and 4E). A single injection of cocaine after 7 days of nicotine led to a further 74% increase in FosB expression (Fig. 4E), as compared with 7 days of exposure to cocaine alone (Fig. 4F). As in behavioral and physiological experiments, our genetic study showed that mice given nicotine for 24 hours did not respond to cocaine as dramatically as mice given nicotine for 7 days before being given cocaine (Fig. 4D and 4E). Moreover, nicotine given after cocaine did not increase gene expression (Fig. 4F and 4G).
We next wanted to determine whether nicotine enhances FosB expression in the striatum by altering chromatin structure at the FosB promoter and thereby magnifying the effect of cocaine. We examined the acetylation of histones H3 and H4 at the FosB promoter.19 After 7 days of nicotine, the acetylation of histones H3 and H4 had increased. Cocaine alone increased the acetylation of histone H4 only; moreover, a single cocaine injection after 7 days of nicotine did not increase the acetylation of histone H4 further.
The ability of nicotine to produce robust acetylation at the FosB promoter suggested that nicotine-induced enhancement of acetylation could be occurring on a widespread scale, at the promoters of other genes expressed in the striatum. Using immunoblotting, we found that after 7 days of nicotine, the acetylation of histones H3 and H4 increased by 32% and 61%, respectively, everywhere in the striatum. These increases were similar to those we found at the FosB promoter. By contrast, 7 days of cocaine alone did not increase the acetylation of histones H3 and H4 in the striatum.19
Is the hyperacetylation produced by nicotine the result of the activation of one or more acetylases or the inhibition of deacetylases? To address this question, we assayed histone deacetylase (HDAC) activity directly in the nuclear fraction of striatum cells and found a 28% reduction in mice given nicotine for 7 to 10 days; by contrast, the mice given cocaine for 7 days had no decrease in HDAC activity.19 The increased histone acetylation in mice given nicotine seemed to result from reduced HDAC activity in the striatum.
The finding that nicotine inhibited HDAC activity in the striatum, thus inducing global changes in histone acetylation in the nucleus accumbens — changes that are known to alter the transcription of genes other than FosB when cocaine is administered — suggested that nicotine enhanced the transcription of FosB in response to cocaine. As an independent test of the finding that nicotine produces its effect on cocaine responses by inhibiting HDAC activity, we simulated the effect of nicotine using the HDAC inhibitor suberoylanilide hydroxamic acid (SAHA),19 which enhances the response to cocaine in conditioned place-preference experiments.21 We gave mice SAHA 2 hours before giving them cocaine and observed a 71% increase in FosB expression, as compared with mice given cocaine alone.19
We then asked whether substituting SAHA for nicotine would produce similar effects on cocaine-induced synaptic plasticity. We found that SAHA fully simulated nicotine, inducing a greater reduction in long-term potentiation in the core of the nucleus accumbens than cocaine alone. This is consistent with the idea that increased histone acetylation in the striatum is responsible for the reduction of long-term potentiation after 7 days of nicotine. Moreover, like nicotine alone (Fig. 4B and 4C), SAHA alone did not cause a drop in long-term potentiation. Overall, the effects of SAHA and nicotine were quantitatively and qualitatively similar. However, although SAHA, like nicotine, enhances the behavioral effects of cocaine,21 its electrophysiological effects are unknown. These results support our experimental finding that nicotine inhibits HDAC activity.
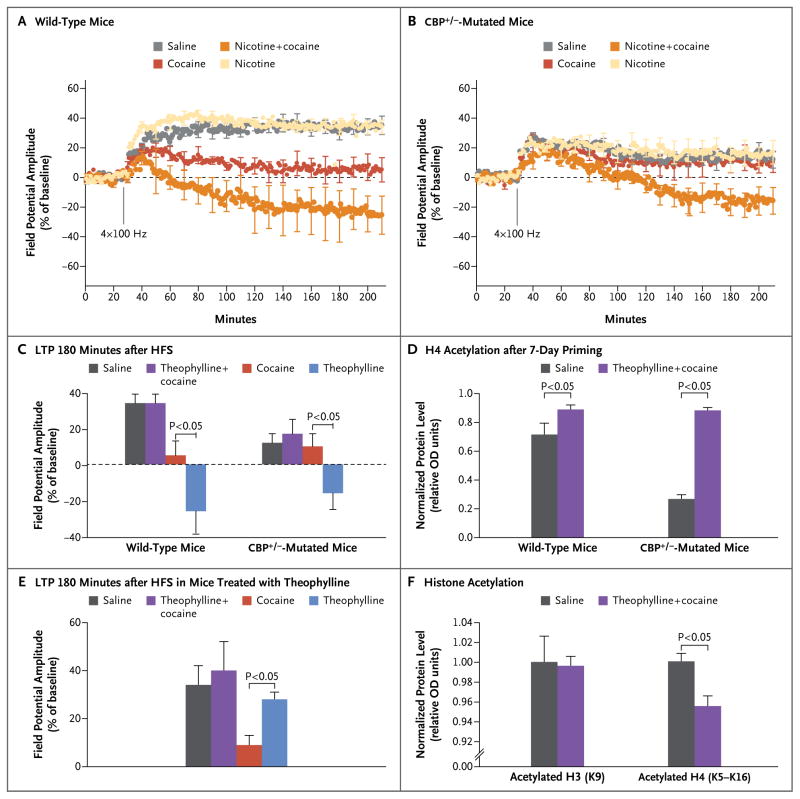
To test further the idea that histone acetylation and deacetylation are key molecular mechanisms of the effect of nicotine on the murine response to cocaine, we conducted genetic and pharmacologic experiments. We studied genetically modified mice with the Rubinstein–Taybi syndrome that lack one functional allele of the gene for the CREB binding protein (CBP) governing histone acetylation. The lack of this allele results in hypoacetylation (abnormally low histone acetylation) in the striatum. The mutant mice had impaired long-term potentiation, as compared with nonengineered (wild-type) controls (Fig. 5A and 5B). After being given nicotine for 7 days, these mice had reduced long-term potentiation in response to cocaine (Fig. 5C). Using immunoblots, we found that the mutant mice had roughly a 49% reduction in histone H4 acetylation in the striatum, as compared with the control mice. After 7 days of nicotine, histone H4 acetylation in the mutant mice had increased to values that were similar to those in wild-type mice exposed to nicotine for 7 days (Fig. 5D).
Figure 5. Priming Effect of Nicotine on Cocaine-Induced Changes in Wild-Type Mice and Genetically Engineered Mice and in Wild-Type Mice Given Theophylline.
Panels A and B show long-term potentiation in wild-type mice and in genetically engineered littermates with mutations in the CREB-binding protein CBP (CBP+/−), respectively. The mice were given saline as a control, nicotine for 7 days, a single injection of cocaine, or 7 days of nicotine followed by an injection of cocaine (5 to 8 mice per group). Panel C shows changes in the long-term potentiation amplitude 180 minutes after HFS in the same groups of mice. Panel D shows histone H4 (K5 to K16) acetylation in the tail domain of histone proteins in striatal lysates of CBP+/− mice and wild-type littermates after 7 days of nicotine (4 mice per group). In Panels D and F, values are normalized protein levels, with β-tubulin as a loading control. The Western raw data are produced by measuring the optical densities (ODs) of the different bands. These values are then transformed by first normalizing with β-tubulin as a loading control and then dividing by the values of the control group. Panel E shows changes in the long-term potentiation amplitude 180 minutes after HFS in mice treated with theophylline for 7 days, mice treated with theophylline followed by a single cocaine injection, mice treated with cocaine alone, and controls (6 to 10 mice in each group). Panel F shows graphs of immunoblots of striatal lysates from mice given saline or theophylline (200 mg per liter) in their drinking water for 7 days and then probed with antibodies against acetylated histone H3 (K9) and acetylated histone H4 (K5 to K16). Data are from Levine et al.19
We hypothesized that hypoacetylation would weaken the effect of cocaine on wild-type mice and produce the opposite effect of SAHA and nicotine. To spur HDAC activity and create a hypoacetylated state, we gave mice low doses of theophylline, an HDAC stimulator. After 7 days, there was no difference in long-term potentiation between mice given theophylline and control mice given plain water: the two groups had a similar increase in long-term potentiation. However, in the mice given theophylline, long-term potentiation did not decrease as much in response to cocaine as it did in the controls (Fig. 5E and 5F). Moreover, the mice given theophylline had less acetylated histone H4 (Fig. 5E); specifically, less K12-acetylated H4 and K16-acetylated H4 (Fig. 5F).
These data support the idea that a hypoacetylated state, whether caused genetically or pharmacologically, reduces FosB expression and the depression of long-term potentiation in response to cocaine. This is consistent with the earlier finding of Hiroi et al. that the inactivation of FosB lessened addictive behavior.22 Nicotine reduced HDAC activity, thereby increasing histone acetylation and creating an environment conducive to FosB expression. In this way, nicotine promotes greater FosB expression in response to cocaine than cocaine alone does. Moreover, this gene expression cannot be rapidly reversed, because HDAC activity is inhibited (Fig. 6).
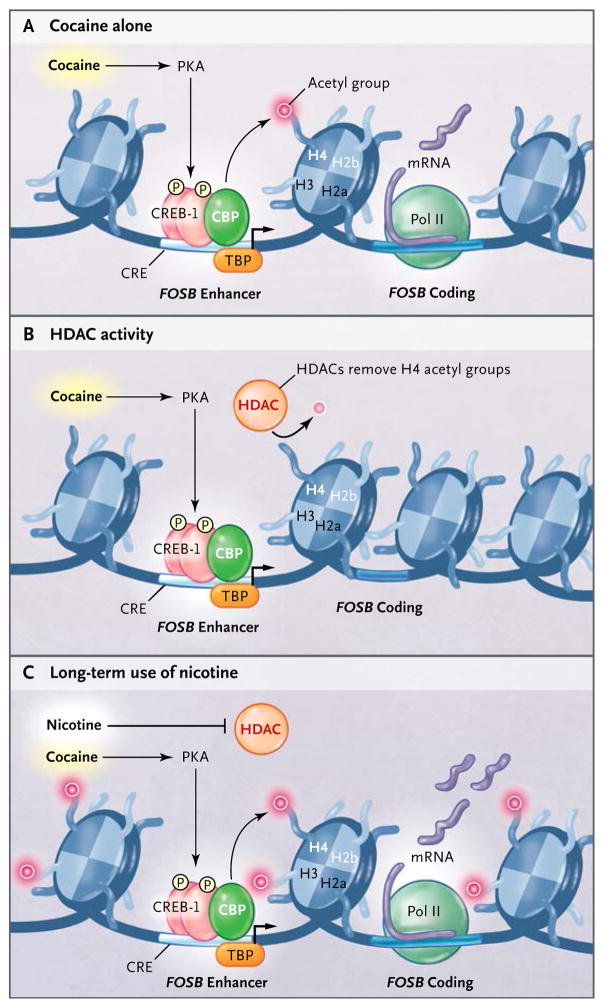
Figure 6. Proposed Model of Nicotine as a Gateway Drug.
Panel A shows the action of cocaine alone. Cocaine leads to the activation of PKA, which phosphorylates CREB-1 and leads to the recruitment of the CREB-binding protein CBP, which acetylates histone H4. CRE denotes cAMP responsive element, mRNA messenger RNA, Pol polymerase, and TBP TATA-box–binding protein. Panel B shows that the action of cocaine alone is rapidly reversed by histone deacetylase (HDAC) activity. Panel C shows that nicotine inhibits HDAC activity, thus increasing histone acetylation throughout the striatum and creating an environment conducive to expression of FOSB.19
To investigate the duration of the priming effect of nicotine, we repeated some of our studies, with one variation: after giving the mice nicotine for 7 days, we waited 14 days before giving them cocaine. We found that the locomotor effect of cocaine was not enhanced — unlike the increase we observed when we gave the mice nicotine for 7 days and then gave them both nicotine and cocaine without a delay. Similarly, the depression of long-term potentiation and FosB expression in response to cocaine were the same in mice given nicotine 14 days previously and in mice given no nicotine. These findings indicate that the priming effect of nicotine does not occur unless nicotine is given repeatedly and in close conjunction with cocaine. We have not defined the duration of the priming effect and suspect that it is influenced by the intensity and duration of nicotine exposure.
BEYOND THE STRIATUM — AMYGDALA AND HIPPOCAMPUS
Given that nicotine enhanced the changes in synaptic plasticity in the striatum induced by cocaine, we next asked whether the gateway effect also applied to the amygdala, the region of the brain that orchestrates emotion and is critical for drug addiction. We found that nicotine enhanced long-term potentiation in the amygdala in response to cocaine and that the effect was unidirectional. Moreover, as in the striatum, SAHA simulated the priming effects of nicotine.23
Finally, we asked whether the dopamine D1/D5 receptor (which is important in reward reinforcement) and histone acetylation played a role in the dentate gyrus of the hippocampus, a brain area that is critical for spatial memory and thus for behaviors related to drug addiction that are commonly cued to the spatial context in which the addictive drug is acquired and consumed. We found that priming with nicotine substantially enhanced the long-term potentiation produced by cocaine in the dentate gyrus, and again, the priming effect was unidirectional (i.e., nicotine primed cocaine but cocaine did not prime nicotine). Moreover, the facilitation induced by nicotine and cocaine was blocked by some receptor antagonists that act on the D1/D5 receptor and enhanced by others. Finally, SAHA simulated the priming effect of nicotine but was blocked in the genetically modified mice that had reduced histone acetylation.24
These results extend the evidence that the priming effect of nicotine is achieved, at least partially, by means of histone acetylation and show that the amygdala and the hippocampus are important in processing the effects of nicotine and cocaine. If similar changes in chromatin acetylation and FOSB expression occur in people after nicotine exposure, and if the magnitude of the changes is sufficient to alter human addictive behavior, these experiments suggest new approaches to the treatment of addiction.
ANIMAL MODEL–BASED PREDICTIONS FOR TESTS IN HUMANS
Our findings that more than 1 day of nicotine exposure was required to prime cocaine responses in mice and that the first exposure to cocaine had to occur while the mice were being exposed to nicotine prompted us to return to human populations and ask the following questions: What is the smoking status of cocaine users when they start using cocaine? Does beginning cocaine use while actively smoking enhance the effects of cocaine and result in higher rates of cocaine addiction?
To address these questions, we reexamined existing data from a small group of students followed from 15.7 to 34.2 years of age.25 The majority of cocaine users (75.2%) were smoking during the month they began using cocaine. Furthermore, in a large, longitudinal national sample,26 we found that the rate of cocaine dependence (addiction as measured in the population) was highest (20.2%) among users who started using cocaine after having smoked cigarettes. Dependence was much lower among persons who had begun using cocaine before they started smoking (6.3%) and among those who had never smoked more than 100 cigarettes (10.2%) (P<0.001).
CONCLUSIONS
GATEWAY EFFECT OF NICOTINE AS A CONSEQUENCE OF GLOBAL ACETYLATION IN THE STRIATUM
The results we obtained by combining epidemiologic and biologic studies suggest a model (Fig. 6) in which nicotine exerts its priming effect on cocaine by means of HDAC inhibition and provide a molecular explanation of the unidirectional sequence of drug use observed in mice and in human populations. Nicotine acts as a gateway drug and exerts a priming effect on cocaine in the sequence of drug use through global acetylation in the striatum, creating an environment primed for the induction of gene expression. Long-term potentiation in the nucleus accumbens is blocked when long-term exposure to nicotine is followed by cocaine use, which presumably lessens constraints on dopaminergic neurons in the ventral tegmental area and leads to the enhanced release of dopamine.19 For all the measures we studied — locomotor sensitization, conditioned place preference, long-term potentiation, and FosB expression — reversing the order of nicotine and cocaine exposure was ineffective: cocaine did not enhance the effect of nicotine. The priming effect of nicotine depended on its being given for 7 days before cocaine. Priming did not occur when nicotine was given for only 24 hours before cocaine.
These results provide a biologic basis and a molecular mechanism for the sequence of drug use observed in people. One drug affects the circuitry of the brain in a manner that potentiates the effects of a subsequent drug.
Moreover, we observed the priming effect of nicotine only when mice were given cocaine at the same time as nicotine, which suggests that HDAC inhibition by nicotine depends on the continuous intake of nicotine. This observation is consistent with epidemiologic data that show that most people start using cocaine while using nicotine, a state that may enhance the physiological effects of cocaine. We found evidence that there is a specific biologic mechanism that explains the sequence from cigarettes to cocaine in the population.
Thus, we believe that the gateway hypothesis and the common liability model are complementary. Common factors will explain the use of drugs in general, and specific factors will explain why young people use specific drugs and do so in a particular sequence. We can now ask whether the hyperacetylation produced by nicotine also accounts for the gateway effects of alcohol and marijuana. Is there a single mechanism for all gateway sequences, or does each sequence rely on a distinct mechanism?
HDAC activators might be of some use in treating addiction because they could decrease FOSB expression in response to cocaine. Modifying HDAC activators to target the striatum would be particularly desirable, since systemic treatments with HDAC activators or histone acetyltransferase inhibitors probably have deleterious effects on cognitive and other functioning.
IMPLICATIONS FOR E-CIGARETTES
Our findings also provide initial biologic insights that may help inform the current debate about electronic e-cigarettes,27 which have been promoted as a tool to stop smoking and reduce the harmful effects of combustible tobacco use in the population.28 Although e-cigarettes eliminate some of the morbidity associated with combustible tobacco, they and related products are pure nicotine-delivery devices. They have the same effects on the brain as those reported here for nicotine, such as the acetylation of the FOSB promoter and the inhibition of HDAC, and they pose the same risk of addiction to other drugs and experiences.
Although the typical e-cigarette user has been described as a long-term smoker who is unable to stop smoking,28 the use of e-cigarettes is increasing exponentially among adolescents and young adults.29 Our society needs to be concerned about the effect of e-cigarettes on the brain, especially in young people, and the potential for creating a new generation of persons addicted to nicotine.29,30 The effects we found in adult mice are likely to be even stronger in adolescent animals. Priming with nicotine has been shown to lead to enhanced cocaine-induced locomotor activity and increased initial self-administration of cocaine among adolescent, but not adult, rats.31,32 Whether e-cigarettes will prove to be a gateway to the use of combustible cigarettes and illicit drugs is uncertain, but it is clearly a possibility.
Nicotine acts as a gateway drug on the brain, and this effect is likely to occur whether the exposure is from smoking tobacco, passive tobacco smoke, or e-cigarettes. More effective prevention programs need to be developed for all the products that contain nicotine, especially those targeting young people. Our data suggest that effective interventions would not only prevent smoking and its negative health consequences but also decrease the risk of progressing to illicit drug use and addiction.
Acknowledgments
Supported by grants from the Howard Hughes Medical Institute (to Dr. E. Kandel) and the National Institutes of Health (R01 DA024001, to Drs. E. Kandel and D. Kandel) and the National Institute on Drug Abuse (K5 DA00081, to Dr. E. Kandel).
We thank our collaborators Amir Levine, YanYou Huang, Edmund Griffin, and Luca Colnaghi for contributions to this article and for helpful comments on an earlier draft of the manuscript. Parts of this article draw on the study first reported by Levine et al.19
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Kandel DB. Stages in adolescent involvement in drug use. Science. 1975;190:912–4. doi: 10.1126/science.1188374. [DOI] [PubMed] [Google Scholar]
- 2.Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J Stud Alcohol. 1992;53:447–57. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- 3.Wagner FA, Anthony JC. Into the world of illegal drug use: exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana, and cocaine. Am J Epidemiol. 2002;155:918–25. doi: 10.1093/aje/155.10.918. [DOI] [PubMed] [Google Scholar]
- 4.Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge: United Kingdom: Cambridge University Press; 2002. [Google Scholar]
- 5.Huizink AC, Levälahti E, Korhonen T, et al. Tobacco, cannabis, and other illicit drug use among Finnish adolescent twins: causal relationship or correlated liabilities? J Stud Alcohol Drugs. 2010;71:5–14. doi: 10.15288/jsad.2010.71.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degenhardt L, Dierker L, Chiu WT, et al. Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend. 2010;108:84–97. doi: 10.1016/j.drugalcdep.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60:1256–64. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- 8.Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry. 2007;64:1313–20. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- 9.Vanyukov MM, Tarter RE, Kirillova GP, et al. Common liability to addiction and “gateway hypothesis”: theoretical, empirical and evolutionary perspective. Drug Alcohol Depend. 2012;123(Suppl 1):S3–S17. doi: 10.1016/j.drugalcdep.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer RHC, Young SE, Hopfer CJ, et al. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: evidence of generalized risk. Drug Alcohol Depend. 2009;102:78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Results from the 2012 National Survey on Drug Use and Health: summary of national findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- 12.Wikler A. On the nature of addiction and habituation. Br J Addict Alcohol Other Drugs. 1961;57:73–9. [Google Scholar]
- 13.Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–86. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 15.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 16.Nestler EJ, Malenka RC. The addicted brain. Sci Am. 2004;290:78–85. doi: 10.1038/scientificamerican0304-78. [DOI] [PubMed] [Google Scholar]
- 17.Levine AA, Guan Z, Barco A, Xu S, Kandel ER, Schwartz JH. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci U S A. 2005;102:19186–91. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alibhai IN, Green TA, Potashkin JA, Nestler EJ. Regulation of fosB and Delta-fosB mRNA expression: in vivo and in vitro studies. Brain Res. 2007;1143:22–33. doi: 10.1016/j.brainres.2007.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine AA, Huang YY, Drisaldi B, et al. Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci Transl Med. 2011;3:107ra109. doi: 10.1126/scitranslmed.3003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. Striatal cell type-specific over-expression of DeltaFosB enhances incentive for cocaine. J Neurosci. 2003;23:2488–93. doi: 10.1523/JNEUROSCI.23-06-02488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renthal W, Maze I, Krishnan V, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–29. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 22.Hiroi N, Brown JR, Haile CN, Ye H, Greenberg ME, Nestler EJ. FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine’s psychomotor and rewarding effects. Proc Natl Acad Sci U S A. 1997;94:10397–402. doi: 10.1073/pnas.94.19.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang YY, Kandel DB, Kandel ER, Levine AA. Nicotine primes the effect of cocaine on the induction of LTP in the amygdala. Neuropharmacology. 2013;74:126–34. doi: 10.1016/j.neuropharm.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 24.Huang YY, Levine A, Kandel DB, et al. D1/D5 receptors and histone deacetylation mediate the gateway effect of LTP in hippocampal dentate gyrus. Learn Mem. 2014;21:153–60. doi: 10.1101/lm.032292.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kandel DB, Logan JA. Patterns of drug use from adolescence to young adulthood: I. Periods of risk for initiation, continued use, and discontinuation. Am J Public Health. 1984;74:660–6. doi: 10.2105/ajph.74.7.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psych. 2004;61:1107–15. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 27.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972–86. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caponnetto P, Campagna D, Papale G, Russo C, Polosa R. The emerging phenomenon of electronic cigarettes. Expert Rev Respir Med. 2012;6:63–74. doi: 10.1586/ers.11.92. [DOI] [PubMed] [Google Scholar]
- 29.Frieden T. E-cigarette use more than doubles among US middle and high school students from 2011–2012. Atlanta: Centers for Disease Control and Prevention; 2013. ( http://www.cdc.gov/media/releases/2013/p0905-ecigarette-use.html) [Google Scholar]
- 30.Stanbrook MB. Regulate e-cigarettes as drug-delivery devices. CMAJ. 2013;185:1379. doi: 10.1503/cmaj.131469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol Teratol. 2007;29:66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McQuown SC, Dao JM, Belluzzi JD, Leslie FM. Age-dependent effects of low-dose nicotine treatment on cocaine- induced behavioral plasticity in rats. Psychopharmacology (Berl) 2009;207:143–52. doi: 10.1007/s00213-009-1642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]