Abstract
The endogenous ligands for the LT, lipoxin (LX) and oxoeicosanoid receptors are bioactive products produced by the action of the lipoxygenase family of enzymes. The LT receptors BLT1 and BLT2, are activated by LTB4 and the CysLT1 and CysLT2 receptors are activated by the cysteinyl-LTs, whereas oxoeicosanoids exert their action through the OXE receptor. In contrast to these pro-inflammatory mediators, LXA4 transduces responses associated with the resolution of inflammation through the receptor FPR2/ALX (ALX/FPR2). The aim of the present review is to give a state of the field on these receptors, with focus on recent important findings. For example, BLT1 receptor signalling in cancer and the dual role of the BLT2 receptor in pro- and anti-inflammatory actions have added more complexity to lipid mediator signalling. Furthermore, a cross-talk between the CysLT and P2Y receptor systems has been described, and also the presence of novel receptors for cysteinyl-LTs, such as GPR17 and GPR99. Finally, lipoxygenase metabolites derived from ω-3 essential polyunsaturated acids, the resolvins, activate the receptors GPR32 and ChemR23. In conclusion, the receptors for the lipoxygenase products make up a sophisticated and tightly controlled system of endogenous pro- and anti-inflammatory signalling in physiology and pathology.
Keywords: cancer, cardiovascular, eicosanoids, inflammation, LTs, lipoxins, lipoxygenase, respiratory, oxoeicosanoids
Links to online information in the IUPHAR/BPS Guide to PHARMACOLOGY and the BJP's ‘Concise Guide to Pharmacology 2013/14
| Targets | Ligands | |
|---|---|---|
| 5-lipoxygenase (5-LOX) | 5-(6-chloro-2-hexyl-1H-indol-1-yl)-5-oxo-valeric acid | IL-1β |
| 12-lipoxygenase (12-LOX) | 5-Oxo-ETE | IL-2 |
| 15-lipoxygenase (15-LOX) | 5S-HETE | IL-4 |
| adenylyl cyclase (AC) | 12-epi LTB | IL-5 |
| Akt | 12-hydroxyheptadecatrienoic acid (12-HHT) | IL-6 |
| Aquaporin 4 (AQP4) | 12S-HETE | IL-8 (CXCL8) |
| BLT1 receptor | 15S-HETE | leukotriene A (LTA) |
| BLT2 receptor | acetylsalicylic acid (aspirin) | leukotriene B (LTB) |
| C5a receptors | ADP | leukotriene C (LTC) |
| CB receptor | all-trans-retinoic acid (ATRA) | leukotriene D (LTD) |
| Chemerin receptor (ChemR23) | anandamide | leukotriene E (LTE) |
| cyclooxygenase-2 (COX-2) | annexin I-(2-26) (Ac2-26) | lipoxin A (LXA) |
| CysLT1 receptor | arachidonic acid | LL-37 |
| CysLT2 receptor | aspirin-triggered lipoxin A (15-epi-LXA, ATL) | LY255283 |
| ERK | aspirin-triggered RvD1 | montelukast |
| FPR2/ALX (ALX/FPR2) | BAY u9773 | N-methyl LTC |
| GPR17 | BayCysLT2 | PACAP |
| GPR32 | cAMP | PDGF |
| c-Jun N-terminal kinase (JNK) | carbachol | pobilukast |
| Leukotriene A (LTA) hydrolase | CCL26 (eotaxin-3) | pranlukast |
| MMP-9 | CGEN-855A | resolvin D1 (RvD1) |
| OXE receptor | chemerin | resolvin E1 (RvE1) |
| Oxoglutarate receptor (GPR99) | docosahexaenoic acid (DHA) | resolvins |
| P2Y receptors | eicosapentaenoic acid (EPA) | rosuvastatin |
| P2Y12 receptor | fluticasone | serum amyloid A (SAA) |
| p38 MAP kinase | forskolin | SHAAG |
| phospholipase A (PLA) | G-CSF | TGF-β |
| phospholipase C (PLC) | glutathione | thromboxane A |
| PI3K | GM-CSF | U75302 |
| PPARγ | Gue1654 | UDP |
| TNFα | HAMI3379 | UDP-galactose |
| hydroxyproline | UDP-glucose | |
| IFN-γ | WKYMVM | |
| IL-10 | WRW4 | |
| IL-13 | zafirlukast |
This table lists protein targets and ligands that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013a,b).
Introduction
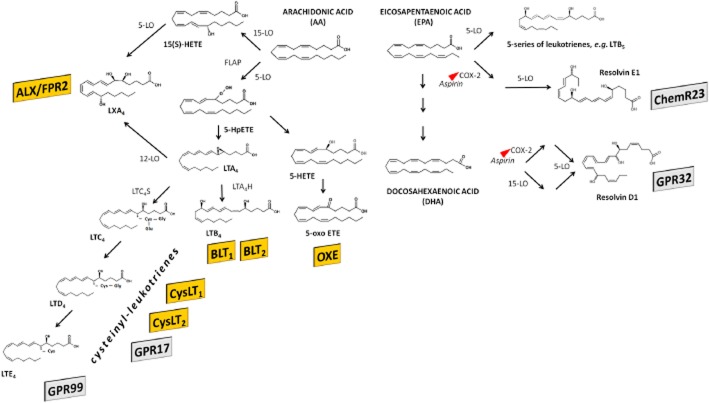
The endogenous ligands for the LT, lipoxin (LX) and oxoeicosanoid receptors are bioactive products produced by the action of the lipoxygenase family of enzymes shown in Figure 1 (Brink et al., 2003; Brink et al., 2004; Chiang et al., 2006; Bäck et al., 2011). The metabolism of arachidonic acid by 5-lipoxygenase yields the epoxide intermediate LTA4, which serves as precursor for the LT receptor agonists (Figure 1). Subsequent metabolism through the enzyme LTA4 hydrolase leads to formation of the dihydroxy-LT LTB4, which is the ligand for the BLT receptors (Figure 1). Alternatively, conjugation of LTA4 with glutathione yields the cysteinyl-LTs acting on the two CysLT receptor (CysLTR) subtypes, CysLT1 and CysLT2 (Figure 1). There is also evidence in the literature for additional CysLT receptor subtypes, derived from functional in vitro studies (Lee et al., 1984; Snyder and Krell, 1984; Bäck et al., 2000; Sakata and Bäck, 2002; Walch et al., 2002), radioligand binding (Capra et al., 1998; Ravasi et al., 2000; 2002) and mice lacking both CysLT1 and CysLT2 receptors (Maekawa et al., 2008). LTE4 has, for example, been suggested to signal through P2Y12 receptors in some studies (Nonaka et al., 2005; Paruchuri et al., 2009; Fredman et al., 2010), although not replicated in all settings (Foster et al., 2013). In support of common receptors mediating purinergic and LT signalling, the orphan GPR17 (Figure 1) has been postulated to be activated by both cysteinyl-LTs and nucleotides (Ciana et al., 2006); this will be further discussed below. In addition, recent evidence point to yet another receptor for cysteinyl-LTs, namely, GPR99 (Kanaoka et al., 2013) as indicated in Figure 1.
Figure 1.
Members of the LT receptor family are depicted in yellow, whereas shaded rectangles indicate related receptors, for which formal ligand pairing is yet to be agreed. ETE, eicosatetraenoic acid; FLAP, 5-lipoxygenase activating protein; GPR, G protein-coupled receptor; HETE, hydroxyeicosatetraenoic acid; HpETE, hydroperoxyeicosatetraenoic acid; LO, lipoxygenase; LTC4S, LT C4 synthase; LTA4H, LTA4 hydrolase; LX, lipoxin.
Oxoeicosanoids are another family of biologically active arachidonic acid derivatives that have been intimately associated with cellular migration (Powell et al., 1995). 5-Oxo-ETE, formed by the oxidation of 5S-HETE by 5-hydroxyeicosanoid dehydrogenase (Figure 1) is a potent chemoattractant for human granulocytes and monocytes by means of the OXE receptor (Brink et al., 2004).
The dual lipoxygenation of arachidonic acid by either the 15- and 5-lipoxygenase or the 5- and 12-lipoxygenase produces eicosanoids known as lipoxins (LXs), as indicated in Figure 1 (Chiang et al., 2006). These eicosanoids are inhibitory or anti-inflammatory mediators, which act as a ‘stop signal’ during inflammatory reactions (Serhan, 2007; Capra et al., 2013) through a receptor with high sequence homology (70%) to the formyl peptide receptors (FPR). However, although a number of peptides activate this receptor, LXA4 is the most potent native endogenous ligand, and the nomenclature recommended for this receptor is FPR2/ALX. The term ALX/FPR2 for the same receptor is suggested when the lipoxin-binding property is of primary concern (Ye et al., 2009).
Besides the 20:4, n-6 fatty acid arachidonic acid, also ω-3 essential polyunsaturated fatty acids, such as eicosapentaenoic acid (EPA; 20:5, n-3) and docosahexaenoic acid (DHA; 22:6, n-3), are metabolized by lipoxygenases in human cells (Figure 1). For example, when metabolized by 5-lipoxygenase, EPA generates LTs of the 5-series (e.g. LTB5, see Figure 1), which are less biologically active and compete with LT binding to the LT receptors, suggesting that lipoxygenase metabolites of ω-3 fatty acids may act as inhibitors of inflammation (Stanke-Labesque et al., 2008). Furthermore, EPA and DHA can enter into the lipoxygenase metabolism and lead to the biosynthesis of either E-series (for EPA-derived), or D-series (for DHA-derived) of resolvins (Rv). These ω-3-derived mediators have been characterized as mediators of inflammation resolution by means of signalling through two GPCRs, GPR32 and ChemR23, as will be further discussed below.
The LT, LX and oxoeicosanoid receptor cloning, ligand affinity, expression and functional significance have been reviewed in previous IUPHAR reports (Brink et al., 2003; Brink et al., 2004; Chiang et al., 2006; Bäck et al., 2011) and are summarized in Tables 1–6. The aim of the present review is to give a state of the field on these receptors, with focus on recent important findings.
Table 1.
The BLT1 receptor
| Agonists | LTB4 (full agonist) | Affinity | 9.2–9.8 (pKd); 9.4 (pKi) | |
| 20-OH-LTB4 (full agonist) | 8.1 (pKi) | |||
| 12(R)-HETE (full agonist) | 7.5 (pKi) | |||
| Antagonists | BIIL-260 | Affinity | 8.5 (pIC50), 8.8 (pKi) | |
| LY-293111 | 6.6 (pKi) | |||
| CP-195543 | 8.6 (pIC50), 8.2 (pKi) | |||
| ONO-4057 | 8.4 (pKi) | |||
| LY-255283 | 6.6 (pKi) | |||
| Transduction mechanisms | ||||
| Transducer | Gi/0 family; Gq/G11; Gα16 | |||
| Effector/response | Adenylate cyclase inhibition; PLC stimulation, MAKP activation | |||
| Receptor distribution | ||||
| Human granulocytes, monocytes, dendritic cells, T-lymphocytes, B-lymphocytes | ||||
| Human coronary artery smooth muscle cells and bronchial smooth muscle cells | ||||
| Human umbilical cord endothelial cells | ||||
| Human atherosclerotic lesions, human abdominal aortic aneurysms | ||||
| Synovial tissues derived from patients with rheumatoid arthritis | ||||
| Pancreatic and colon cancers | ||||
| Examples of functional assays | ||||
| CHO cells transfected with human BLT1: chemotaxis, increase in intracellular calcium | ||||
| Retinoic acid-differentiated HL-60 cells: increase in intracellular calcium | ||||
| Human coronary artery smooth muscle cells: increase in whole cell currents | ||||
| Murine RAW264.7 cells and bronchial smooth muscle cells: MAPK activation | ||||
| Rodent RBL-2H3 cells: PI3K activation | ||||
| Example of physiological functions | ||||
| Human granulocytes: chemotaxis and release of lysosomal enzymes | ||||
| Murine macrophages: phagocytosis | ||||
| Murine T-lymphocytes: Il-2 production | ||||
| Guinea pig pulmonary artery: vasoconstriction | ||||
| Examples of pathophysiological functions (confirmed in BLT1-deficient mice) | ||||
| Bronchial asthma/airway hyperresponsiveness | ||||
| Rheumatoid arthritis (confirmed in both BLT1-null and BLT1/BLT2-null mice) | ||||
| Atherosclerosis | ||||
| Osteoporosis (regulation of osteoclast function) | ||||
| Multiple sclerosis | ||||
| Atopic dermatitis | ||||
| Tumour | ||||
Full information and references available in the IUPHAR/BPS Guide to PHARMACOLOGY, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=267&familyId=35&familyType=GPCR.
Table 6.
The ALX/FPR2 receptor
| Agonists | Lipid mediators: | Affinity |
| LXA4 and ATL (full agonist) | 12 (pEC50); 8.3–9.3 (pKd) | |
| RvD1 and AT-RvD1 (full agonist) | 11.9 (pEC50) | |
| Formyl peptides | ||
| PSMα3 (full agonist) | 8.7 (pEC50) | |
| Host-derived non-amyloidogenic peptides | ||
| Annexin A1 (full agonist) | 5.8–6.1 (pEC50); 6.5 (pKd) | |
| SHAAGtide (full agonist) | 7.7 (pEC50) | |
| LL-37 (full agonist) | 6.0 (pEC50) | |
| Host-derived amyloidogenic peptides | ||
| SAA (full agonist) | 6.6 (pEC50) | |
| Peptides identified from library screen | ||
| WKYMVm (full agonist) | 9.0–10.1 (pEC50) | |
| Antagonists | compound 1754-31 | 7.1 (pIC50) |
| WRWWWW | 6.6 (pIC50) | |
| t-Boc-FLFLF | 4.3–6.0 (pIC50) | |
| Transduction mechanisms | ||
| Transducer | Gi/0 family | |
| Effector/response | PLC, PLA2 and PLD stimulation | |
| Examples of receptor distribution | ||
| Peripheral blood leukocytes | ||
| Synovial fibroblasts | ||
| Intestinal epithelial cells | ||
| Lung, kidney, spleen and placenta | ||
| Examples of functional assays | ||
| PMN, HL-60 cells or CHO cells overexpressing human ALX/FPR2: PLD activation, arachidonic acid release, PSDP increase | ||
| Human macrophages: phagocytosis | ||
| THP-1 cells: calcium mobilization, adherence, chemotaxis | ||
| Human T-cells. ERK activation | ||
| Examples of physiological functions | ||
| LXA4 and ATL induce anti-inflammatory signals such as reducing CD11b/CD18, expression, blocking ROS production, NF-κB activation, pro-inflammatory cytokines/chemokines. | ||
| LXA4 and ATL give pro-resolving signals, stimulating non-phlogistic monocyte activation (calcium mobilization, adherence and chemotaxis), and macrophage phagocytosis of apoptotic PMN | ||
| Examples of pathophysiological functions (confirmed in either Fpr2 or Fpr2/3-deficient mice) | ||
| Mesenteric ischaemia reperfusion | ||
| Carrageenan-induced paw oedema | ||
| K/BxN serum-induced arthritis | ||
| Allergic airway inflammation | ||
Full information and references available in the IUPHAR/BPS Guide to PHARMACOLOGY, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=223&familyId=35&familyType=GPCR.
ATL, aspirin-triggered lipoxin (15-epi-LXA4); SAA, serum amyloid A; SHAAGtide, 18 amino acids from the N-terminal of human CCL23.
Table 2.
The BLT2 receptor
| Agonists | 12-HHT | Affinity | 7.72 (pEC50) |
| CAY10583 | 7.7 (pEC50) | ||
| LTB4 | 7.64 (pKd) | ||
| LTB4 | 7.6 (pIC50) | ||
| 12-epi LTB4 | 7.52 (pEC50) | ||
| 12(S)-HETE | 7.52 (pEC50) | ||
| 15(S)-HETE | 7.52 (pEC50) | ||
| Antagonists | ZK158252 | Affinity | 6.0–7.1 (pIC50) |
| CP195543 | 6.0 (pIC50) | ||
| LY255283 | 6.0 (pIC50) | ||
| Transduction mechanisms | |||
| Transducer | Gi/0 family; Gq/11 family | ||
| Effector/response | Adenylate cyclase inhibition; PLC stimulation | ||
| Examples of receptor distribution | |||
| Human spleen, liver, ovary and leukocytes | |||
| Human atherosclerotic lesions, human abdominal aortic aneurysms | |||
| Synovial tissues derived from patients with rheumatoid arthritis | |||
| Murine small intestine and skin | |||
| Examples of functional assays | |||
| CHO cells transfected with the human BLT2: chemotaxis, increase in intracellular calcium | |||
| MDCK cells trasnfected with human BLT2: increase in transendothelial resistance. | |||
| Guinea pig lung parenchyma: contraction | |||
| Examples of physiological functions | |||
| Chemotaxis | |||
| Angiogenesis | |||
| Examples of pathophysiological functions (confirmed in BLT2-deficient mice) | |||
| Rheumatoid arthritis (in BLT1/BLT2-null mice) | |||
| Endothelial function | |||
| Protection against colitis | |||
| Attenuated allergic airway eosinophilia | |||
| Tumour | |||
Full information and references available in the IUPHAR/BPS Guide to PHARMACOLOGY, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=268&familyId=35&familyType=GPCR. Currently, LY255283 is used as a BLT2 receptor-specific antagonist, but this compound also inhibits BLT1 in a non-competitive manner. 12-HHT, 12-hydroxyheptadecatrienoic acid.
Table 4.
The CysLT2 receptor
| Agonists | LTC4 (full agonist) | Affinity | 7.0–8.6 (pEC50); 8.4–8.5 (pIC50); 7.0–10.8 (pKd) |
| LTD4 (full agonist) | 6.8-8.6 (pEC50); 7.2–8.2 (pIC50); 7.3–9.4 (pKd) | ||
| LTE4 (partial agonist) | 5.6–7.1 (pEC50); 5.7-6.2 (pIC50); 6.5 (pKi) | ||
| N-methyl-LTC4 (full agonist) | 6.9–8.1 (pEC50) | ||
| BAYu9773 (partial agonist) | 7.0–7.2 (pEC50); 6.2–6.4 (pIC50) | ||
| Antagonists | BAYu9773 | Affinity | 6.8-7.7 (pA2); 6.5–6.7 (pKB); 5.3–7.7 (pIC50) |
| BayCysLT2 | 8.3–8.4 (pA2); 6.6–7.3 (pIC50) | ||
| HAMI3379 | 7.4–8.4 (pIC50) | ||
| Transduction mechanisms | |||
| Transducer | Gq/11 family; Gi/0 family | ||
| Effector/response | PLC stimulation; p38 activation | ||
| Examples of receptor distribution | |||
| Human saphenous vein, human coronary artery smooth muscle cells | |||
| Human umbilical vein endothelial cells | |||
| Heart (atria, left ventricle, pericardium) | |||
| Peripheral blood leukocytes, human platelets | |||
| Brain and spinal cord | |||
| Nasal polyps, nasal mucosa | |||
| Colorectal cancer tissue | |||
| Spleen, adrenals and placenta | |||
| Colorectal carcinoma cells | |||
| Examples of functional assays | |||
| HEK293 or COS-7 cells transfected with human CysLT2: [Ca2+]i increase | |||
| Vascular smooth muscle and endothelial cells: [Ca2+]i increase | |||
| Mast cells and umbilical vein endothelial cells: Increased IL-8 secretion, P38 activation | |||
| C2C12 myofibroblasts transfected with the human CysLT2 receptor: β-arrestin binding | |||
| Examples of physiological functions | |||
| Secretion of von Willebrand factor and P-selectin expression in endothelial cells | |||
| Vasoconstriction and endothelium-dependent relaxation | |||
| Up-regulation of early response genes | |||
| Examples of pathophysiological functions (confirmed in CysLT2-deficient mice) | |||
| Skin fibrosis | |||
| Pulmonary inflammation and fibrosis | |||
| Increased vascular permeability | |||
| Colitis | |||
| Retinal oedema | |||
Full information and references available in the IUPHAR/BPS Guide to PHARMACOLOGY, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=270&familyId=35&familyType=GPCR.
Table 5.
The OXE receptor
| Agonists | 5-oxo-ETE (full agonist) | Affinity | 8.3–8.5 (pEC50); 8.4 (pKd) |
| 5-oxo-C20:3 | 8.0 (pEC50) | ||
| 5-oxo-ODE | 8.0 (pEC50) | ||
| 5-oxo-15-HETE | 7.7 (pEC50) | ||
| 5S-HpETE | 6.2–7.5 (pEC50) | ||
| Antagonists | 5-(6-chloro-2-hexyl-1H-indol-1-yl)-5-oxo-valeric acid | 6.4 (pIC50) | |
| 5-oxo-12-HETE | 6.3 (pIC50) | ||
| Transduction mechanisms | |||
| Transducer | Gi/0 family | ||
| Effector/response | PLA2 and PLC stimulation, adenylate cyclase inhibition, stimulation of PI3K, ERK and p38 MAPK. | ||
| Examples of receptor distribution | |||
| Peripheral blood leukocytes, macrophages | |||
| Prostate tumour tissue | |||
| Liver, kidney, lung, spleen, placenta, small intestine, colon, skeletal muscle, heart | |||
| H295R adrenocortical cells | |||
| Cancer cell lines | |||
| Examples of functional assays | |||
| Human granulocytes: increased cytosolic calcium levels, formation of F-actin, shape change, surface expression of CD11b | |||
| Human eosinophil granulocytes: increased surface expression of CD69 and loss of L-selectin from the cell surface | |||
| Human eosinophil granulocytes: release of eosinophil peroxidase and arylsulfatase human neutrophil granulocytes: release of β-glucuronidase and lysozyme | |||
| Examples of physiological function | |||
| Stimulation of the respiratory burst (superoxide production) | |||
| Chemotaxis | |||
| Transendothelial migration | |||
| GM-CSF release | |||
| Cancer cell proliferation | |||
| Steroidogenesis in adrenocortical cells | |||
Full information and references available in the IUPHAR/BPS Guide to PHARMACOLOGY, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=271&familyId=35&familyType=GPCR. As there is no orthologue of OXER1 in mice, gene knockout studies cannot be done in this species.
BLT receptors
The dihydroxy-LT, LTB4 stimulates neutrophil chemotaxis and secretion but may also affect immunomodulation through the activation of several leukocyte populations (Bäck et al., 2011; Nakamura and Shimizu, 2011). In addition, receptors for LTB4 are expressed on non-myeloid cells, such as vascular smooth muscle and endothelial cells (Bäck et al., 2005). Chemotaxis, one of the principal effects of LTB4, occurs via activation of the BLT1 receptor subtype (Yokomizo et al., 1997), which is the high-affinity LTB4 receptor. The open reading frame of the gene encoding a second subtype of BLT receptor was identified during the analysis of the BLT1 promoter (Yokomizo et al., 2000). This receptor was named BLT2 and either HEK or CHO cells transfected with BLT2 cDNA exhibited low affinity to LTB4 and in addition responded to various other hydroxy fatty acids including 12-epi LTB4, 12S-HETE and 15S-HETE (Yokomizo et al., 2000). Search for endogenous high-affinity ligands for BLT2 receptors resulted in the identification of 12-HHT (12(S)-hydroxyheptadeca-5Z, 8E, 10E-trienoic acid), previously known as a by-product of TxA2 biosynthesis, as a high-affinity BLT2 receptor ligand (Okuno et al., 2008). Pro-inflammatory LTB4 signalling through the BLT1 and BLT2 receptors has been implicated in several diseases (Bäck et al., 2011; Nakamura and Shimizu, 2011), such as bronchial asthma (Miyahara et al., 2005; Terawaki et al., 2005), rheumatoid arthritis (Kim et al., 2006; Chou et al., 2010), atherosclerosis (Bäck and Hansson, 2006), abdominal aortic aneurysms (Houard et al., 2009), bone metabolism (Hikiji et al., 2009), multiple sclerosis (Kihara et al., 2010) and cancer (Yokota et al., 2012).
BLT1 receptor
Structure–function relationships
Of the receptors addressed in the present review, the human BLT1 receptor is the best characterized in terms of structure–function relationships. Like several rhodopsin family GPCRs, the BLT1 receptor bears an 8th helix (H8) domain consisting of Val298-Gly-Phe-Val-Ala-Lys-Leu-Leu-Glu-Gly307 (Okuno et al., 2003). Two aromatic residues, Tyr285 and Phe300, may stabilize the inactive form of the BLT1 receptor by holding H8 at an almost right angle from the C-terminus of the seventh transmembrane region (Okuno et al., 2003). Thus, H8-deficient BLT1 receptor mutants exhibit a prolonged intracellular signalling after LTB4 stimulation (Okuno et al., 2003). Hydrophobic amino acid residues in the H8, Val301, Leu304 and Leu305, may act as anchors to the plasma membrane, whereas Thr308 located after the H8 is one of the ligand-induced phosphorylation sites mediated by the GPCR kinase GRK6, and involved in BLT1 receptor inactivation (Gaudreau et al., 2002). Recently, Aratake et al. reported an inhibitory role of the H8 on the LTB4-elicited internalization of the BLT1 receptor (Aratake et al., 2012). The human BLT1 receptor with the mutations of Leu304 and Leu305 in the H8 exhibited an augmentation of LTB4-induced internalization, whereas the wild-type (WT) receptor exhibited minimal internalization. Furthermore, phosphorylations of 5 Ser and Thr residues between 308 and 319 were important for this enhanced internalization of the mutant BLT1 receptor. Therefore, the H8 of BLT1 may repress LTB4-induced internalization by suppressing excessive phosphorylations.
BLT1 receptors in inflammation
The generation of BLT1 receptor-deficient mice confirmed the loss of responsiveness to LTB4 in BLT1 receptor null leukocytes (Haribabu et al., 2000; Tager et al., 2000) and the suppression of several inflammation disease models (Bäck et al., 2011). In contrast, transgenic mice overexpressing the human BLT1 receptor exhibited enhanced responsiveness of leukocytes in acute dermal inflammation (Chiang et al., 1999). Recently, Monterio et al. reported that macrophages, but not mast cells, are involved in the migration of neutrophils, by generating LTB4 in haem-induced neutrophilic inflammation, for example, malaria and sickle cell disease (Monteiro et al., 2011). BLT1 receptor antagonists, CP-105696 and LY-292476, significantly impaired the haem-induced peritoneal neutrophilia, demonstrating further involvement of the BLT1 receptor in this inflammatory response (Monteiro et al., 2011).
BLT1 receptors in cardiovascular disease (CVD)
Recurring nocturnal episodes of airway obstruction, known as obstructive sleep apnea syndrome, causes intermittent hypoxia, which is a detrimental stimuli for the cardiovascular system associated with, for example, early atherosclerosis and an increased cardiovascular risk (Stanke-Labesque et al., 2014). Neutrophil granulocytes derived from patients with obstructive sleep apnea exhibit increased LTB4 production in response to calcium ionophore stimulation, compared with cells derived from healthy subjects (Stanke-Labesque et al., 2012). In addition, expression of LT-synthesizing enzymes in neutrophils correlates with measures of subclinical atherosclerosis and vascular remodelling in these patients (Stanke-Labesque et al., 2012). In support of a role for the LTB4-BLT1 pathway in sleep apnea-associated atherosclerosis, mice deficient in both apolipoprotein E and the BLT1 receptor are protected from the accelerated atherosclerosis observed after subjecting apolipoprotein E-deficient mice to intermittent hypoxia in vivo (Li et al., 2011).
Hypertension is associated with increased levels of LTB4 measured in the saliva (Labat et al., 2013). In addition, cerebral LTB4 levels are increased in spontaneously hypertensive rats compared with Wistar-Kyoto rats (Waki et al., 2013). In the latter study, microinjection of the BLT1 receptor antagonist U75302 into the solitary nucleus of spontaneously hypertensive rats lowered arterial pressure, suggesting that LTB4-BLT1 receptor inflammatory circuits in the brain stem may be associated with neurogenic hypertension (Waki et al., 2013).
BLT1 receptors in rheumatoid arthritis
Several studies have revealed that BLT1 receptor-deficient mice are protected from the development of arthritis using different models, associated with decreased articular neutrophil recruitment (Kim et al., 2006) resulting in reduced production of IL-1 and chemokines in the joint (Chou et al., 2010). In this disease, the recruitment of neutrophils is orchestrated via two pathways, C5a receptor signalling-induced LTB4 release followed by BLT1 receptor activation, and Fcγ receptor signalling-elicited IL-1β release (Sadik et al., 2012).
BLT1 receptors in other diseases
Atopic dermatitis is an inflammatory skin disease. Although LTB4 concentration is elevated in skin lesions of patients with atopic dermatitis, little is known about the role of LTB4 in this disease. Recently, Oyoshi et al. reported an essential role of the LTB4-BLT1 receptor pathway in neutrophils for allergic skin inflammation (Oyoshi et al., 2012b). In the latter study, allergic skin inflammation was significantly decreased in BLT1-deficient mice, and it was demonstrated that both LTB4 production and BLT1 receptor expression in neutrophils were important for the development of this disease (Oyoshi et al., 2012b).
Recently, the role of the BLT1 receptor signalling in tumour immunology has been investigated. Yokota et al. examined the effect of the BLT1-deficiency on the anti-tumour memory responses elicited by s.c. administration of GM-CSF gene-transduced WEHI3B (WGM) leukaemia cells using BLT1 receptor-deficient mice (Yokota et al., 2012). They found that the BLT1 receptor deficiency resulted in reduced tumour-infiltrating myeloid-derived suppressor cells, increase in matured dendritic cells (DCs) in tumour tissues and augmentation of CD4+ T-lymphocyte stimulation capacity during GM-CSF-triggered tumour regression, demonstrating that the lack of the LTB4-BLT1 receptor pathway induced long-term anti-tumour memory responses after immunization with WGM leukaemia cells. In another study however, BLT1 receptor-deficient mice exhibited accelerated tumour growth and reduced survival compared with WT mice in a cervical cancer model (Sharma et al., 2013). These findings were associated with a decreased number of CD8+ T-lymphocytes and NK cells in tumours derived from BLT1 receptor-deficient mice, and decreased IFN-γ and IL-2 expression (Sharma et al., 2013). Taken together, those studies suggest that BLT1 receptor signalling on both suppressor and effector cells of the adaptive immune system may be involved in tumour progression and regression.
BLT2 receptors
Whereas BLT2 receptor-deficient mice exhibit reduced severity in arthritis models (Mathis et al., 2010), this deletion induces more severe colitis induced by dextran sulfate, possibly due to the loss of intestinal barrier function maintained by BLT2 receptors (Iizuka et al., 2010). In line with the latter findings, BLT2 receptor-deficient mice also exhibit more severe eosinophilic inflammation induced by sensitization and elicitation by ovalbumin accompanied by the reduced accumulation of IL-13 in the allergic airway (Matsunaga et al., 2013). Taken together, these findings indicate a protective role of the BLT2 receptor in intestinal and airway inflammation.
Pharmacological treatment of apolipoprotein E-deficient mice fed a high-fat diet using the BLT2 receptor antagonist LY255283 did not alter atherosclerotic lesion size (Hoyer et al., 2012). However, aortic segments derived from LY255283-treated mice exhibited lower levels of reactive oxygen species (ROS), and increased carbachol-induced endothelium-dependent relaxations compared with untreated mice (Hoyer et al., 2012), suggesting that BLT2 receptor signalling may be involved in endothelial dysfunction. However, it should be taken into consideration that although LY255283 has been used as a BLT2 receptor-specific antagonist, this compound was recently shown to inhibit also the BLT1 receptor in a non-competitive manner (Matsunaga et al., 2013).
Screening a human thymus cDNA to identify BLT2 receptor-interacting proteins recently identified that RanBPM, a member of the Ran-GTPase-binding protein family, which can bind at the C-terminal of the BLT2 receptor in the absence of LTB4, whereas the co-localization of these proteins was abolished in the presence of LTB4 (Wei et al., 2013). RanBPM overexpression attenuated, whereas knock-down promoted, BLT2 receptor-mediated motility and generation of ROS in response to either LTB4 or 12HHT (Wei et al., 2013), suggesting that RanBPM may act as a negative regulator of BLT2 receptor signalling in cell motility. Finally, the BLT2 receptor dissociation from RanBPM was dependent on phosphorylation of BLT2 receptors at Thr355, a site previously identified by the same investigators as critical for LTB4-induced BLT2 receptor-mediated chemotaxis through PI3K-Akt signalling (Wei et al., 2011).
Several recent in vitro studies on BLT2 receptor signalling have focused on different cancer cells. For example, human ovarian and prostate cancer cells express BLT2 receptors coupled to activation of NAD(P)H oxidase-4 (NOX4) and subsequent generation of ROS and MMP expression (Lee et al., 2012; Seo et al., 2012), suggesting a BLT2 receptor-dependent pathway in cancer growth, invasiveness and metastasis. In support of the latter, LY255283 inhibits peritoneal metastasis formation 35 days after injection of ovarian cancer cells into athymic mice (Seo et al., 2012).
CysLT receptors
Ever since the identification of cysteinyl-LTs chemical structure and their association with inflammation (Samuelsson, 1983), the pathophysiological role of cysteinyl-LTs has been mainly focused on their potent bronchoconstrictive effects and asthma (Drazen, 2003; Capra et al., 2007; Hallstrand and Henderson, 2010; Laidlaw and Boyce, 2012). However, the cloning of the second CysLT receptor, expressed by cardiovascular and cerebral tissues (Brink et al., 2003; Bäck et al., 2011; see also Table 3) has fostered the research for new functions of these lipid mediators in other physiological and pathological conditions, particularly in CVDs. Indeed, CysLT receptor signalling is emerging as a crucial component in vascular inflammation (Bäck, 2007) and an increasing number of data suggest a major role for cysteinyl-LTs in the pathogenesis and progression of several CVDs (Capra et al., 2013), such as atherosclerosis (Bäck and Hansson, 2006), myocardial infarction, stroke (Ingelsson et al., 2012), aortic stenosis (Nagy et al., 2011) and intimal hyperplasia.
Table 3.
The CysLT1 receptor
| Agonists | LTD4 (full agonist) | Affinity | 7.3–9.4 (pEC50); 8.1 (pIC50); 8.6–10.6 (pKd) |
| LTC4 (full agonist) | 7.4–7.7 (pEC50); 6.4–6.5 (pIC50); 7.0–8.1 (pKi) | ||
| LTE4 (partial agonist) | 6.4–7.2 (pEC50); 6.6–6.97 (pIC50) | ||
| N-methyl-LTC4 (partial agonist) | 5.7 (pEC50) | ||
| Antagonists | Montelukast | Affinity | 8.6 (pKi); 8.3–8.6 (pIC50) |
| Zafirlukast | 8.9 (pKi); 7.7–9.6 (pIC50) | ||
| Pranlukast | 7.1–8.8 (pKi); 8.1–10.0 (pIC50) | ||
| Pobilukast | 7.1 (pKi); 7.5–8.2 (pIC50) | ||
| Iralukast | 7.8 (pKi) | ||
| Verlukast | 8.0 (pIC50) | ||
| BAYu9773 | 5.3–6.4 (pIC50) | ||
| Transduction mechanisms | |||
| Transducer | Gq/11 family; Gi/0 family | ||
| Effector/response | PI turnover and Ca2+ mobilization; PLC stimulation | ||
| Examples of receptor distribution | |||
| Lung, bronchus, bronchiole smooth muscle, airway mucosa | |||
| Nasal polyps | |||
| Peripheral blood leukocytes, macrophages | |||
| Human saphenous vein, human coronary artery smooth muscle cells | |||
| Aortic valves | |||
| Spleen, small intestine and placenta | |||
| Colorectal carcinoma cells | |||
| Examples of functional assays | |||
| Several cell types: activation of MAPK | |||
| X. laevis melanophores transfected with human CysLT1: pigment dispersion | |||
| X. laevis oocyte infected with human CysLT1: Cl current | |||
| HEK293 or COS-7 cells transfected with human CysLT1: [Ca2+]i increase | |||
| Examples of physiological functions | |||
| Bronchoconstriction | |||
| Cell proliferation | |||
| Chemotactic activity and migration | |||
| Actin reorganization | |||
| Release of inflammatory mediators and cytokines | |||
| Cell adhesion | |||
| Activation of transcription factors | |||
| Examples of pathophysiological functions (confirmed in CysLT1-deficient mice) | |||
| Bleomycin-induced pulmonary inflammation | |||
| Zymosan-induced peritonitis | |||
| Cutaneous anaphylaxis | |||
Full information and references available in the IUPHAR/BPS Guide to PHARMACOLOGY, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=269&familyId=35&familyType=GPCR.
CysLT1 receptor
CysLT1 receptors in respiratory diseases
The role of CysLT1 receptor signalling in bronchial asthma depends both on the bronchoconstrictive and pro-inflammatory effects of the cysteinyl-LTs (Bäck et al., 2011). Furthermore, bronchial fibroblasts derived from asthmatic subjects express more CysLT1 receptor mRNA compared with bronchial fibroblasts derived from non-asthmatic subjects (Eap et al., 2012). These authors also showed that activation of the asthmatic bronchial fibroblast CysLT1 receptor by cysteinyl-LTs resulted in increased TGF-β1 which in turn increased pro-collagen (Eap et al., 2012). In airway epithelial cells, IL-13 up-regulates the expression of the CysLT1 receptor, which is associated with an increased release of CCL26 (eotaxin-3), a potent eosinophil chemoattractant (Provost et al., 2012). Taken together, those studies suggest that CysLT1 receptor activation on bronchial fibroblasts and epithelial cells further contribute to the cysteinyl-LTs-induced bronchial narrowing and eosinophil recruitment in asthma. Indeed, cells proliferation and bronchial narrowing are hallmarks of chronic asthma. In this regard, an intriguing study (Capra and Rovati, 2014) has recently demonstrated that rosuvastatin, the latest agent of this lipid-lowering class to be introduced on the market, dose-dependently inhibited LTD4-induced human airway smooth muscle cells growth. The letter effect was exerted by means of inhibited prenylation of signalling proteins, most likely small G proteins such as Ras that are activated in a CysLT1 receptor-dependent manner (McMahon et al., 2002; Capra et al., 2003; 2004; Ravasi et al., 2006; Poulin et al., 2011).
Cysteinyl-LTs are increased in children following infection with respiratory syncytial virus (RSV), associated with a potential subsequent development of asthma-like symptoms. In a mouse model of primary and secondary RSV-infection of newborn mice, pretreatment with montelukast, a selective CysLT1 receptor antagonist, decreased RSV-induced airway hyperresponsiveness, airway inflammation and increased IFN-γ production in primary, but not secondary, infected neonate mice (Han et al., 2010).
In addition to asthma, CysLT1 receptor signalling has also been implicated in chronic obstructive pulmonary disease (COPD). Bronchial mucosa samples from patients with COPD exacerbations of increased CysLT1 receptor protein and mRNA expression was demonstrated on inflammatory cells, particularly mast cells and monocytes/macrophages (Zhu et al., 2012).
In line with an activation of the 5-lipoxygenase pathway, urinary LTE4 has been associated with the degree of obstructive sleep apnea in adults (Stanke-Labesque et al., 2009) and children (Shen et al., 2011). In addition, increased cysteinyl-LTs and CysLT1 receptor expression have been shown in tonsillar tissues derived from children with sleep apnea in China (Shen et al., 2012) and in Greece (Tsaoussoglou et al., 2012), suggesting that CysLT1 receptor signalling may contribute to local proliferative and inflammatory pathways within tonsils in paediatric sleep-disordered breathing (Stanke-Labesque et al., 2014). In support of the latter notion, a recent randomized double-blind study of 46 children showed that a 12 week treatment with daily, oral montelukast reduced the severity of obstructive sleep apnea and the underlying adenoidal hypertrophy (Goldbart et al., 2012).
A role for activation of CysLT1 receptors by cysteinyl-LTs in experimental pulmonary fibrosis has been supported by montelukast treatment in several mouse models. In a bleomycin-induced pulmonary fibrosis model in mice, montelukast decreased expression of IL-6, IL-13, IL-10 and TGF-β and attenuated lung fibrosis and hydroxyproline content (Shimbori et al., 2011). In a GATA-3 transcription factor overexpression model, montelukast-treated mice exhibited less airway inflammation in response to ovalbumin challenge, as demonstrated by decreased levels of TH2 cytokines and TGF-β and decreased smooth muscle cell hyperplasia (Kiwamoto et al., 2011).
CysLT1 receptor expression increases during TH2 cell differentiation (Parmentier et al., 2012). Human TH2 cells selectively express the CysLT1 receptor, whereas CysLT2 receptor, GPR17 and P2Y12 (other receptors that can respond to cysteinyl-LT; see below) are undetectable. The TH2 cell CysLT1 receptor couples through both Gαq and GαI to transduce a chemotactic response. In addition, the dectin-2-cysteinyl-LT pathway is essential for TH2 predominant immunity in mice in response to house dust mite, in part through the CysLT1 receptor (Barrett et al., 2011).
CysLT1 receptors in CVDs
Cysteinyl-LTs are locally produced in coronary atherosclerotic plaques and contribute to vascular inflammation. Although previous studies have shown a dominant CysLT2 receptor expression in vascular smooth muscle cells, lipopolysaccharide stimulation induces CysLT1 receptor expression in human coronary artery vascular smooth muscle cells (Eaton et al., 2012). Interestingly, the CysLT1 receptor exhibited a perinuclear expression in those cells, and its activation was coupled to a predominant nuclear calcium signalling and up-regulation of pro-atherosclerotic genes such as PAI-2 (Eaton et al., 2012). A nuclear membrane localization of the CysLT1 receptor expression was initially reported in intestinal epithelial cells (Nielsen et al., 2005), and perinuclear expression of the CysLT1 receptor has subsequently been demonstrated in human aortic valve myofibroblasts (Nagy et al., 2011). In the latter cells, the LTC4-induced rise in intracellular calcium was most pronounced in the nuclear and perinuclear region of the cell, associated with mitochondrial permeability transition, changes in cell morphology, increased ROS production and an up-regulation of mRNA encoding bone morphogenic proteins (Nagy et al., 2011), all important pathophysiological processes in the calcification of the aortic valve observed in patients with calcific aortic stenosis.
The clinical use of the CysLT1 receptor antagonists has allowed testing the hypothesis of their beneficial role in CVD in observational studies. In a pharmacoepidemiological cohort of approximately 7 million subjects, montelukast exposure was associated with a lower risk for recurrent stroke and with a lower risk for recurrent myocardial infarction in male subjects (Ingelsson et al., 2012).
CysLT1 receptors in other diseases
In a mouse model of experimental autoimmune encephalitis (EAE) expression of the CysLT1 receptor mRNA was up-regulated in spleen and lymph node tissue and in the spinal cord, and cysteinyl-LT concentrations in the blood and CSF were higher than in normal mice (Wang et al., 2011). In this EAE mouse model, treatment with CysLT1 receptor-selective antagonists, either montelukast or zafirlukast, reduced CNS CD4+ T-lymphocyte influx and demyelination, associated with decreased EAE disease scores.
A role for CysLT1 receptor signalling in various cancers has also been demonstrated. LTD4-induced CysLT1 receptor activation on chronic lymphocytic leukaemia cells and normal B-lymphocytes increases calcium and promote chemotaxis. CysLT1 receptor antagonists induced apoptosis and reduced viability suggesting a potential therapeutic treatment (Drost et al., 2012). In line with the latter suggestion, high CysLT1 and low CysLT2 receptor protein expression in breast cancer tissue is associated with increased cancer-related mortality (Magnusson et al., 2011).
Clinical use of CysLT1 receptor antagonists
It is worth noting here that LT modifiers (including LT receptor antagonists and 5-lipoxygenase inhibitors) are a class of drugs that may be used as an add-on treatment for adult patient with mild persistent asthma not satisfactorily controlled with inhaled glucocorticosteroids, or as alternative treatment particularly in patients suffering from aspirin-sensitive asthma or concomitant allergic rhinitis (Scott and Peters-Golden, 2013). They are generally well tolerated and present few, if any, class-related side effects. LT modifiers can also be safely used in children at all level of asthma severity, particularly against exercise-induced bronchoconstriction, considering virtually the absence of safety concerns (from the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2012. Available from http://www.ginasthma.org/). However, LT modifiers are generally less effective than inhaled glucocorticosteroids when used alone as controller (Chauhan and Ducharme, 2012), while long-acting β2-adrenoceptor agonists are modestly superior to LT receptor antagonists in reducing oral corticosteroid-treated exacerbations (Chauhan and Ducharme, 2014).
A number of clinical studies have been published from 2011 to 2013 using the selective CysLT1 receptor antagonists montelukast or pranlukast in asthmatics. For example, montelukast pretreatment decreased hypertonic saline induced bronchoconstriction in asthmatics (Kazani et al., 2011), and provided disease control, similar to that of fluticasone. in actively smoking asthmatics (Price et al., 2013). Furthermore, in an allergen challenge model, pranlukast pretreatment inhibited nasal obstruction and nasal eosinophil cationic protein in allergic Japanese children (Gotoh et al., 2012). Finally, a pilot study indicated that montelukast prevented inflammatory cell responses in patients with persistent rhinitis, with particular emphasis on macrophages and neutrophils (Braido et al., 2012).
LT receptor antagonists have also been studied outside their respiratory indications. A prospective study with zafirlukast in female patients with long-standing mild/severe capsular contracture after surgical procedure for breast prosthesis. The results show a significant decrease in breast compliance values after 6 months of treatment, followed by a substantial increase 1 year after the end of drug intake, suggesting that the control of inflammation is crucial to prevent this multifactorial process (Mazzocchi et al., 2012). Finally, a retrospective study in children with food allergies suggested that montelukast can prevent food-induced adverse allergic reactions such as abdominal pain, which occur during oral immunotherapy (Takahashi et al., 2014).
CysLT2 receptor
CysLT2 receptors in CVDs
A number of studies have reported the involvement of the CysLT2 receptor in the inflammatory process subsequent to brain vascular insults, such as vascular ischaemia or oxygen-glucose deprivation (OGD; Bäck et al., 2011). In a model of focal cerebral ischaemia in the rat, a spatiotemporal up-regulation of the CysLT2 receptor was associated with neuronal and glial cell activation (Zhao et al., 2011). The same authors also demonstrated that the mechanism underlying CysLT2-mediated ischaemic astrocyte injury induced by OGD involves increased expression of the CysLT2 receptor and of the water channel aquaporin 4 (AQP4). The latter was supported by reduced cell injury and AQP4 up-regulation by the CysLT2 receptor antagonist BayCysLT2, (also known as CysLT2cpd; Carnini et al., 2011), but not by the highly selective CysLT1 receptor antagonist montelukast (Qi et al., 2011). Furthermore, intracerebroventricular injection of HAMI3379, another even more selective CysLT2 receptor antagonist (Wunder et al., 2010), before focal cerebral ischaemia in rats protects against acute brain injury attenuating the neurological deficits and reducing infarct volume, brain oedema, IgG exudation, neuronal degeneration and neuronal loss (Shi et al., 2012). Of note, the protective effect induced by CysLT2 receptor antagonism was similar to that of pranlukast. In the latter context, it should be considered that pranlukast, zafirlukast and pobilukast have been found to be partially active also at the CysLT2 receptor (Heise et al., 2000; Wunder et al., 2010; Capra et al., 2011).
In further support of neuronal effects of cysteinyl-LTs mainly being CysLT2 receptor-dependent, HAMI3379 inhibited OGD/recovery, as well as LTD4 and N-methyl-LTC4-induced cell injury and neuronal loss in mixed cultures of cortical cells (Zhang et al., 2013). Although no effect of HAMI3379 was observed on OGD/recovery-induced neuronal injury in primary neurons, this antagonist inhibited neuronal loss and necrosis in neuron-microglial co-cultures, indicating that microglial activation may be crucial in this signalling. Similar effects were obtained by CysLT2 receptor knock-down by shRNA, further supporting that these neuronal effects might indeed be CysLT2 receptor-dependent (Zhang et al., 2013)
As mentioned above, the CysLT2 receptor is highly expressed in endothelial cells of some vascular beds, and has been implicated in a variety of cardiovascular functions. BayCysLT2 administered either before or after ischaemia/reperfusion in transgenic mice overexpressing endothelium specific human CysLT2 receptors attenuated the increased myocardial infarction damage, while this CysLT2 receptor antagonist decreased neutrophil infiltration and leukocyte adhesion molecule (L-selectin) expression (Ni et al., 2011).
CysLT2 receptors in other diseases
Using a loss-of-function murine model (CysLT2R-LacZ), CysLT2 receptor expression has been identified in neurons of the myenteric and submucosal plexus in the small intestine, colonic myenteric plexus, dorsal root ganglia and inferior ganglion of the vagal nerve (Barajas-Espinosa et al., 2011). In this model, LTC4/D4 stimulation of colonic submucosal venules elicited a reduced permeability response in CysLT2 receptor knockout (CysLT2−/−) mice compared with WT mice, while basal neuronal activity of colonic-projecting nociceptive neurons from dorsal root ganglia showed significantly higher excitability. These data suggest that CysLT2 receptor signalling in the murine colonic myenteric plexus may be involved in colitis disease progression, controlling inflammation-associated tissue oedema, and in the increased neuronal sensitivity to nociceptive stimuli (Barajas-Espinosa et al., 2011).
Sensitization and challenge using the dust mite Dermatophagoides farinae induces a marked augmentation of eosinophilic pulmonary inflammation, serum IgE, and TH2 cytokines in CysLT2 receptor-deficient compared with WT mice (Barrett et al., 2012). These observations could be replicated in WT mice sensitized by adoptive transfer of D. farina-pulsed CysLT2 receptor-deficient bone marrow-derived DCs. Those results, taken together with a previous observation of a counter-regulatory role of CysLT2 with respect to CysLT1 receptor activity by dimerization (Jiang et al., 2007), suggest that the CysLT2 receptor negatively regulates the development of cysteinyl-LT–dependent TH2 pulmonary inflammation by inhibiting both CysLT1 receptor signalling and D. farinae-induced LTC4 synthase-dependent cell surface expression of CysLT1 receptors on DCs (Barrett et al., 2012).
Proliferative diabetic retinopathy is associated with an increased synthesis of LTs (Talahalli et al., 2010) and oxygen-induced retinopathy (OIR) in mice induces CysLT2 receptor up-regulation. CysLT2 receptor knockout mice exhibit decreased vascular leakage and retinal oedema, but, surprisingly, increased tissue damage (vaso-obliteration/vasoproliferation) compared with WT mice. In addition, only PGs and hydroxyeicosatetraenoic acids, but not LTs, were detected in A23187-treated retina preparations (Barajas-Espinosa et al., 2012). Taken together, these data point to a confusing role of CysLT2 receptor signalling in OIR progression that could be interpreted as either beneficial or detrimental to retinal health.
As mentioned above, atopic dermatitis is a chronic, relapsing, inflammatory skin disease characterized by dermal thickening, eosinophil infiltration and increased levels of LTE4 in the urine. Although the role of cysteinyl-LTs in the inflammation associated with atopic dermatitis is unclear, there are reports suggesting improvements in atopic dermatitis with the use of LT receptor antagonists at the doses generally recommended for asthma treatment (see Capra et al., 2006 and Bäck et al., 2011 for more details). Recently, it has been reported that skin thickening and collagen deposition were significantly reduced in ovalbumin-sensitized skin of CysLT2 receptor knockout mice. In addition, LTC4 stimulation caused increased collagen synthesis by human skin fibroblasts, which, in turn, secreted factors that elicited keratinocyte proliferation. These effects were blocked by the dual CysLT1/CysLT2 receptor antagonist BAY u9773 (Oyoshi et al., 2012a).
Since the discovery that activation of CysLT1 receptors could induce MAPK phosphorylation and thus, proliferation, survival and migration in a variety of cells, a number of papers have reported CysLT1 receptor expression in brain tumours, as well as in prostate and breast cancer cells (Bäck et al., 2011). Interestingly, while low expression of CysLT1 and high expression of CysLT2 receptors correlated with high differentiation in epithelial colon cancer cells (Magnusson et al., 2007) and mediate good prognosis in colon (Magnusson et al., 2010) and breast cancer patients (Magnusson et al., 2011), very recently, the same group also demonstrated that all-trans retinoic acid (ATRA) induced CysLT2 receptor and LTC4 synthase mRNA expression (without affecting CysLT1 receptor expression) and differentiation of colorectal cancer cells. This latter effect was inhibited by the CysLT2 receptor-specific antagonist BayCysLT2, suggesting that ATRA can have anti-tumorigenic effects through the cysteinyl-LT pathway (Bengtsson et al., 2013).
OXE receptor
5-Oxo-ETE is formed by the oxidation of 5S-HETE by 5-hydroxyeicosanoid dehydrogenase, a highly selective NADP+-dependent enzyme (Powell et al., 1992) found in most inflammatory cells as well as a variety of structural cells (Grant et al., 2009). Soon after the discovery of this pathway, 5-oxo-ETE was found to be a potent chemoattractant for human neutrophils (Powell et al., 1993) and subsequently also for eosinophils (Powell et al., 1995; Schwenk and Schroder, 1995), monocytes (Sozzani et al., 1996) and basophils (Iikura et al., 2005; Sturm et al., 2005). Consistent with this, it promotes migration of eosinophils through basement membrane and endothelial cells (Dallaire et al., 2003), probably mediated by both its chemoattractant effects as well as by stimulation of MMP-9 secretion (Langlois et al., 2006). 5-Oxo-ETE also induces a variety of other responses in eosinophils and neutrophils, including calcium mobilization, actin polymerization, CD11b expression and shedding of L-selectin (Grant et al., 2009). Although it is less effective in eliciting degranulation and the respiratory burst in resting granulocytes, once these cells have been primed with cytokines, including TNFα, G-CSF or GM-CSF, their responsiveness to 5-oxo-ETE is dramatically increased (O'Flaherty et al., 1996a,b; Czech et al., 1997). Interestingly, 5-oxo-ETE induces the release of GM-CSF from monocytes, which has been shown to prolong eosinophil survival (Stamatiou et al., 2004) and could also potentially enhance the responsiveness of leukocytes to this substance.
Biological responses to 5-oxo-ETE are mediated by the OXE receptor, which is encoded by the OXER1 gene (Brink et al., 2004). This receptor was identified independently by three groups and was previously known as TG1019 (Hosoi et al., 2002), R527 (Jones et al., 2003) and GPCR48 (Takeda et al., 2002). Consistent with the biological activities of 5-oxo-ETE, OXE receptors are highly expressed in human eosinophils ≈ basophils > neutrophils > macrophages (Hosoi et al., 2002; Iikura et al., 2005) and is also expressed in a variety of cancer cell lines (O'Flaherty et al., 2005; Sundaram and Ghosh, 2006) as well as an adrenocortical cell line (Cooke et al., 2013). Although OXE receptor orthologues exist in a variety of species, including non-human primates, cows, dogs, cats, ferrets and elephants, as well as various fish species, including zebrafish, neither mice nor rats possess an OXE orthologue. The lack of OXER1 in mice has been a significant impediment to progress in our understanding of the pathophysiological role of 5-oxo-ETE and the OXE receptor. In contrast to rodents, zebrafish possess an OXER1 orthologue that plays an important role in leukocyte infiltration (Enyedi et al., 2013). Down-regulation of the zebrafish OXE receptor blocks infiltration of leukocytes in response to both 5-oxo-ETE and injury, suggesting that 5-oxo-ETE is a key regulator of this process.
The OXE receptor is coupled to Gi/o, as responses to 5-oxo-ETE can be blocked by Pertussis toxin (Powell et al., 1996; Czech et al., 1997; O'Flaherty et al., 2000; Hosoi et al., 2002). Activation of this receptor results in stimulation of a number of second messenger pathways, including PLC, PI3K and ERK and p38 MAPK, as well as inhibition of AC (Norgauer et al., 1996; O'Flaherty et al., 1996b; Hosoi et al., 2005; Langlois et al., 2009). MAPK-mediated activation of cPLA2 has also been observed (O'Flaherty et al., 1996b). With the exception of inhibition of AC, which is mediated by αi, OXE receptor signalling is mediated by release of the βγ subunits from Gαiβγ (Blattermann et al., 2012). The benzobisthiazole derivative Gue1654 has been reported to selectively block βγ-mediated OXE receptor signalling without affecting αi-mediated inhibition of AC (Blattermann et al., 2012).
As a major target of 5-oxo-ETE is the eosinophil, the OXE receptor could play an important role in eosinophilic disorders such as asthma and allergic rhinitis. Consistent with this, intradermal injection of 5-oxo-ETE results in accumulation of eosinophils in human skin, which is more pronounced in asthmatic subjects compared with controls (Muro et al., 2003). The availability of OXE receptor antagonists such as the recently reported indole derivative 5-(6-chloro-2-hexyl-1H-indol-1-yl)-5-oxo-valeric acid (Gore et al., 2013) should make it possible to investigate this in animal models that possess OXER1 orthologues. OXE receptor antagonists could be an important future addition to asthma therapy, administered either alone or along with CysLT1 receptor antagonists or corticosteroids. The OXE receptor could also be an important drug target in cancer, as it appears to play a role in cancer cell proliferation and its down-regulation with siRNA has an antiproliferative effect (Sundaram and Ghosh, 2006).
ALX/FPR2 receptor
Lipoxin A4 (LXA4) is generated from arachidonic acid through either human 15- and 5-lipoxygenase or the 5- and 12-lipoxygenase (Figure 1). In addition, acetylation of the PG-synthesizing enzyme COX-2 by acetylsalicylic acid (aspirin) induces the biosynthesis of carbon-15 epimers of lipoxins (15-epi-lipoxins), also referred to as aspirin-triggered lipoxins. LXA4 and 15-epi-LXA4, as well as the DHA-derived resolvin RvD1 and aspirin-triggered RvD1 signal through the FPR2/ALX receptor (Table 6), referred to as ALX/FPR2 when the lipoxin-binding property is of primary concern (Ye et al., 2009). This receptor has the property of responding both to these lipid mediators and to a number of peptide/protein agonists, as indicated in Table 6. These peptide ligands are associated with both pro-inflammatory signalling (e.g. SAA) and pro-resolution (e.g. annexin A1) signalling pathways (Ye et al., 2009; Brancaleone et al., 2013).
Several lines of evidence have convincingly demonstrated LXA4 and 15-epi-LXA4 as mediators of the resolution of inflammation by means of limiting neutrophil infiltration/activation and promoting non-phlogistic activation of monocytes (Chiang et al., 2006) as well as stimulating a pro-solving phenotype NK cells with potential relevance for asthma pathobiology (Barnig et al., 2013; Peebles, 2013). More recently, RvD1 was identified as another lipid mediator of resolution (Serhan et al., 2002), which could activate human ALX/FPR2 (Krishnamoorthy et al., 2010) recently confirmed in vivo in ALX/FPR2 deficient mice (Norling et al., 2012).
LXA4 and ALX/FPR2 receptors in vitro
Functional in vitro studies of cells with endogenous and recombinant ALX/FPR2 expression have generated contradictory results in terms of lipoxin signalling. For example, HEK cells tagged with an ALX/FPR2-β-arrestin-coupled system have revealed dose-dependent interactions of β-arrestin and the ALX/FPR2 receptor for both LXA4 (Krishnamoorthy et al., 2010) and 15-epi-LXA4 (Dalli et al., 2013a). In contrast, other investigators reported no β-arrestin translocation induced by LXA4 in cells expressing the recombinant human ALX/FPR2 receptors (Forsman et al., 2011), while another study using a fusion protein consisting of β-arrestin-2 and EGFP did not observe any apparent translocation of cytosolic β-arrestin in the presence of LXA4 (Hanson et al., 2013). Furthermore, whereas the ALX/FPR2 receptor agonist WKYMVM inhibited forskolin-induced cAMP levels in either CHO or HEK293 cells transfected with ALX/FPR2 cDNA, LXA4 and 15-epi-LXA4 were inactive (Hanson et al., 2013; Planaguma et al., 2013). Likewise, WKYMVM, but not LXA4, increased ERK phosphorylation in human neutrophils (Bae et al., 2003) and in HEK293 cells transfected with ALX/FPR2 cDNA (Hanson et al., 2013). However, it is difficult to assess these negative findings with LXA4, whose potent bioactions are now documented by many laboratories, and interpretation with caution may be recommended. First, the use of commercial LXA4 in studies reporting lack of actions for LXA4, without validation of the physical integrity of this molecule just prior to testing in these systems, may be delicate. Second, several of the above studies lacked a positive control for LXA4-induced responses (Hanson et al., 2013) hence making negative observations in ALX/FPR2 receptor expressing cells somewhat difficult to interpret.
There are, in addition, studies supporting differential signalling pathways for different ALX/FPR2 receptor agonists. In ALX/FPR2-transfected CHO cells, WKYMVM, but not LXA4, increased intracellular calcium concentrations. Interestingly, also peptide ALX/FPR2 receptor agonists exhibited a differential response in the latter cells, which responded to SHAAG and PACAP, but were unresponsive to the glucocorticoid-derived annexin A1 peptide, Ac2–26 (Hanson et al., 2013). In contrast, both LXA4 and Ac2–26 stimulated receptor internalization in HeLa cells transiently expressing HA-tagged FPR2/ALX receptors (Maderna et al., 2010), suggesting similar signalling pathways for these ligands. Furthermore, a previous study has demonstrated that LXA4 mediates the Ca2+ release-activated Ca2+ current ICRAC in K562 erythroleukaemia cells (Li et al., 2008). Interestingly, the latter response was mimicked by annexin and the ALX/FPR2 receptor agonist BML-111, and inhibited by interference RNA against ALX/FPR receptors (Li et al., 2008), further supporting specific signalling pathways through ALX/FPR2 receptors for pro-resolution mediators of both protein and lipid structure.
Taken together, the divergent results obtained for different ligands in cells expressing recombinant human ALX/FPR2 receptors may hence be an indication of biased agonism at this receptor. Interestingly, a recent study revealed constitutive dimerization of ALX/FPR2 receptors in transfected HEK293 cells, either as homodimers or heterodimers with FPR1 receptors (Cooray et al., 2013). The homodimer was activated by LXA4 and Annexin A1 (but not by either SAA or LL-37) and associated with p38/MAPK and heat shock protein 27 signalling leading to IL-10 release. On the other hand, the ALX/FPR2 and FPR1 receptor heterodimers elicited JNK responses. Although different agonists were not tested against the heterodimer, those results provide an initial suggestion that agonist-biased ALX/FPR2 receptor dimerization can distinguish between agonists with distinct downstream signalling (Cooray et al., 2013).
In human airway epithelial cells, LXA4 induces an increase in intracellular calcium, which is inhibited by the ALX/FPR2 receptor antagonist BOC-2 (Verriere et al., 2012). Furthermore, human lung type II alveolar A549 epithelial cells, which do not express ALX/FPR2 receptors, are unresponsive to LXA4 (Bonnans et al., 2003). When the latter cells are transfected to constitutively express full-length recombinant human ALX/FPR2 receptors, LXA4 and 15-epi-LXA4 suppress IL-6 release induced by acid injury, and IL-8 release induced by either TNF or SAA, whereas the cytokine release induced by those stimuli are unaltered by lipoxin stimulation in untransfected A549 cells (Bonnans et al., 2006; Bozinovski et al., 2012). Although both SAA and lipoxins may signal through ALX/FPR2 receptors, lipoxins induced not only a rightward shift but also a depressed Emax of the SAA-induced IL-8 release, arguing against simple competitive antagonism at a single binding site as a mechanism of inhibition. Applying a Schild analysis to the interaction of lipoxin and SAA yielded a regression slope less than unity and those authors concluded that LXA4 and 15-epi-LXA4 may suppress the response to SAA by means of an allosteric type of non-competitive interaction at ALX/FPR2 receptors (Bozinovski et al., 2012). The notion of LXA4 as an allosteric modulator may however not be selective for only the ALX/FPR2 receptor interactions with LXA4, since LXA4 also has recently been reported to allosterically enhance anandamide-induced activation of CB1 receptors within the brain in an FPR2/ALX receptor-independent manner to mediate protective effect against β-amyloid-induced spatial memory impairment in mice (Pamplona et al., 2012).
The uptake of apoptotic neutrophils by macrophages, known as efferocytosis is one of the cardinal signs of resolution of inflammation (Serhan, 2011). Efferocytosis is stimulated by either LXA4 or Ac2–26 in bone marrow-derived macrophages (BMDM), whereas BMDMs derived from Fpr2/Fpr3 knockout mice (cf. below) did not increase efferocytosis in response to these agonists (Maderna et al., 2010). Furthermore, NK cells were recently shown to express the ALX/FPR receptor, and LXA4 significantly increased NK cell-mediated apoptosis of granulocytes, an effect that was inhibited by the ALX/FPR2 receptor antagonist WRW4 (Barnig et al., 2013). Taken together, these results support a role of LXA4 signalling through ALX/FPR receptors in granulocyte turnover during the resolution of inflammation.
LXA4 and ALX/FPR2 receptors in vivo
The generation of mice with a genetically targeted ALX/FPR2 receptor orthologue has allowed the exploration of ALX/FPR2 signalling in different disease models. The murine FPR gene family consists of at least eight members as opposed to only three in humans, and both the proteins encoded by the mFpr2 and mFpr3 (mFpr-rs1) genes share the lipoxin binding capacity of the human ALX/FPR2 receptor (Ye et al., 2009; He et al., 2013). There is however also evidence of LXA4-induced responses in mFpr-rs4-expressing HEK293 cells (Riviere et al., 2009).
In the first report of Fpr2 knockout, the gene cassette and a GFP reporter was inserted in reverse orientation into intron 1 of Fpr2, which prevented transcriptional read-through of the Fpr2 as well as Fpr3 genes (Dufton et al., 2010). These mice (which were later termed Fpr2/Fpr3 knockout mice) exhibit an increased number of adherent and emigrated leukocytes after mesentery ischaemia-reperfusion, an increased carrageenan-induced paw oedema and also an exacerbation and prolongation of K/BxN serum-induced arthritis (Dufton et al., 2010; Brancaleone et al., 2013), consistent with the pro-resolution signalling through these murine receptors. Importantly, LXA4 inhibited cell recruitment into dorsal air pouches inflamed with IL-1β selectively in WT mice but not in Fpr2/Fpr3 receptor knockouts, supporting that LXA4 exerts its action through the murine Fpr2 and/or Fpr3 receptors in vivo (Dufton et al., 2010).
In the second report, selective Fpr2 receptor deletion was obtained through a recombinase approach (Chen et al., 2010). Those Fpr2 knockout mice exhibit reduced ovalbumin/alum-induced allergic airway inflammation, associated with lower levels of IL-4, IL-5 and IL-13 in BAL and a reduced recruitment of DCs to draining lymph nodes (Chen et al., 2010). Studies of dextran sulphate-induced colitis in this strain revealed that, whereas Fpr2 receptor knockout conferred protection in terms of body weight loss and disease scores in the acute phase, these mice exhibited a delayed healing and increased mortality compared with WT mice after dextran sulphate withdrawal (Chen et al., 2013). In addition, Fpr2 receptor knockout mice display increased susceptibility to chronic inflammation-associated colon tumours (Chen et al., 2013) and exacerbated infection and mortality in response to Listeria monocytogenesis infection. Whether the opposing and time-dependent phenotypes in terms of exacerbated inflammation and protection in those studies are related to the different disease models or the different Fpr gene targeting remains to be established.
Further in vivo evidence for lipoxin signalling through ALX/FPR2 receptors has been provided using the ALX/FPR2 receptor antagonist BOC-2. In a murine model of pneumosepsis, LXA4 treatment 24 h after Klebsiella pneumoniae inoculation improved the survival rate of septic mice, an effect that was abolished by the ALX/FPR2 antagonist BOC-2 (Sordi et al., 2013). In addition, LXA4 induces a significant reduction of cerebral infarct size and neurological score when administered before 2 h middle cerebral artery occlusion followed by 24 h reperfusion in rats, an effect that is only partially blocked by the ALX/FPR2 receptor antagonist BOC-2 (Wu et al., 2013), while another study suggested PPARγ may also be involved in the neuroprotective effects of LXA4 in experimental stroke (Sobrado et al., 2009). Finally, using the peptide ALX/FPR2 receptor agonist CGEN-855A, Hecht and co-workers demonstrated inhibition of neutrophil recruitment to inflamed air pouch and protection against myocardial ischaemia-reperfusion injury (Hecht et al., 2009)
Other related receptors
GPR17
The orphan GPR17 (Figure 1), phylogenetically located at an intermediate position between P2Y and CysLT receptors, has been originally postulated to be activated by cysteinyl-LTs, nucleotides and sugar-nucleotides (UDP, UDP-glucose, UDP-galactose), leading to both AC inhibition and intracellular calcium increases (Ciana et al., 2006). Subsequently, a different group confirmed the activation of GPR17 by uracil nucleotides, but was unable to demonstrate activation or binding by cysteinyl-LTs, while demonstrating that both short and long isoforms were constitutively active through Gαi (Benned-Jensen and Rosenkilde, 2010). A very recent paper, however, examining the activation of GPR17 for four independent downstream signalling responses in a broad range of cell types, indicated that GPR17 was not activated or internalized by either uracil nucleotides, or cysteinyl-LTs (Qi et al., 2013). Moreover, data from the same report are consistent with GPR17 acting as a negative regulator for CysLT1 receptor-mediated responses, as previously hypothesized by Maekawa and collaborators both in vitro and in vivo (Maekawa et al., 2009; 2010). Thus, the formal pairing of GPR17 as a dual receptor for cysteinyl-LTs and nucleotides is yet to be agreed (Davenport et al., 2013).
Oxoglutarate receptor (formally GPR99)
GPR99, a close relative of GPR91, originally described as an orphan GPCR with homology to the P2Y nucleotide receptor subfamily (Wittenberger et al., 2002), was initially recognized as an oxoglutarate receptor based on its binding of and activation by 2-oxoglutarate (α-ketoglutarate) with potency in the high micromolar range via a Gq-mediated pathway (He et al., 2004). This pairing has been replicated in a β-arrestin assay (Southern et al., 2013), again with high potency values and eventually named the oxoglutarate receptor in a Receptor Nomenclature and Drug Classification (NC-IUPHAR) committee pairing paper (Davenport et al., 2013). However, recent evidence points to GPR99 as an additional receptor for LTE4. Cells transfected with the human GPR99 exhibit both functional and binding responses to LTE4 with an affinity in the low nanomolar range, while confirming an apparent affinity for oxoglutarate in the high micromolar range. In addition, GPR99 deletion in mice prevents LTE4-induced vascular leakage, but not that induced by LTC4 or LTD4 (Kanaoka et al., 2013). These results, particularly the potencies and affinities values, suggest that GPR99 might have a preference for LTE4 and qualify it as a candidate for the elusive LTE4 receptor, although further investigations on binding and signalling mechanisms and independent confirmations by a different group are necessary to distinguish the preferred endogenous ligands for GPR99.
Chemerin receptor (ChemR23)
ChemR23, an orphan GPCR related to chemokine receptors, was initially described as one of three GPCRs activated by the chemotactic protein chemerin (Wittamer et al., 2003; 2004) and therefore, classified as a pro-inflammatory receptor. However, several subsequent studies using ChemR23 knockout mice in different disease models, such as zymosan-induced peritonitis (Cash et al., 2008), LPS-induced lung injury (Luangsay et al., 2009), viral pneumonia (Bondue et al., 2011b) and cigarette smoke exposure (Demoor et al., 2011) have pointed to an anti-inflammatory role.
Indeed, ChemR23 was in addition identified as a high-affinity RvE1 receptor through screening of the ability of RvE1 to inhibit TNFα-induced NF-κB activation in HEK293 cells after transfection with candidate GPCRs (Arita et al., 2005). Later, RvE1 was also shown to bind to the human BLT1 receptor, albeit with lower affinity (Arita et al., 2007). Radioligand studies demonstrated concentration-dependent RvE1 binding to CHO cells expressing ChemR23, but not to mock-transfected CHO cells, associated with Akt phosphorylation also demonstrable in human macrophages during phagocytosis (Ohira et al., 2010). In addition, RvE1 displayed nanomolar potency using a β-arrestin assay in ChemR23-overexpressing cells (Krishnamoorthy et al., 2010) Further studies supported RvE1 signalling also through endogenously expressed ChemR23. For example, RvE1 enhanced phagocytosis in human monocyte-derived macrophages, which was inhibited by a ChemR23 antibody (Ohira et al., 2010). ChemR23 is also expressed on human platelets and RvE1 inhibited ADP-induced platelet activation (Dona et al., 2008). In addition, RvE1 inhibited ADP-induced activation in P2Y12 receptor expressing CHO cells transfected with ChemR23, but not in mock transfected cells, supporting a ChemR23-dependent effect of RvE1 (Fredman et al., 2010). Finally, RvE1 inhibits PDGF-BB-induced proliferation in primary mouse fibroblasts, an effect that is abolished after siRNA-based knock-down of ChemR23 (Qu et al., 2000). RvE1 signalling through ChemR23 has also received support from in vivo studies. Transgenic mice overexpressing ChemR23 under the CD11b promoter exhibit decreased number of leukocytes in peritoneal exudate after zymosan-induced peritonitis, and decreased alveolar bone loss after molar ligation (Gao et al., 2013). In addition, the RvE1-induced leukocyte clearance was enhanced in ChemR23 transgenic mice in the peritonitis model (Gao et al., 2013), supporting the notion of ChemR23 as a pro-resolution receptor.
Although some authors have questioned the evidence of RvE1 signalling through ChemR23 (Bondue et al., 2011a; Davenport et al., 2013), only unpublished data were cited in those reviews. These discrepancies, might, at least in part, be explained In light of the recent identified possibility for ChemR23 to form heterodimers with other chemokine receptors (de Poorter et al., 2013).
GPR32
Screening systems to identify receptors for RvD1 revealed two candidates for this lipid mediator, namely ALX/FPR2 and the orphan receptor GPR32 (Krishnamoorthy et al., 2010). This RvD1–GPR32 interaction in human macrophages stimulated miRNA involved in resolution of inflammatory signals (Recchiuti et al., 2011; Recchiuti and Serhan, 2012). Two subsequent studies confirmed this ligand receptor interaction, and extended the observation to show that two other resolvins, RvD5 and RvD3, also activated GPR32 with a similar concentration-response relation (Chiang et al., 2012; Dalli et al., 2013b). Given the relationship between the structures of RvD1, RvD3 and RvD5 and the fact that they are biosynthetically related, this functional mimicry is understandable. However, in an evaluation of 10 500 candidate ligands screened using a β-arrestin assay for 82 GPCRs, RvD1 was not listed to pick out activation of GPR32-expressing cells. Importantly, is should be noted that neither the ligand concentration(s) nor conditions tested were specified in that report and the commercial RvD1 was not validated for structural integrity (Southern et al., 2013) as may be required to fairly assess the potential of these ligand receptor interactions. In further support of biological function associated with the activation of GPR32 by the D-series resolvins, macrophages transfected to express the human GPR32 exhibit an increased phagocytosis of fluorescent Escherichia coli in response to either RvD1 or RvD5 (Chiang et al., 2012). Recently, these observations were extended to show that impedance changes in GPR32-expressing CHO cells were increased upon binding RvD3 and the aspirin-triggered resolvin (AT-RvD3).
Summary and conclusions
Lipid mediators, in particular metabolites of the arachidonic acid cascade, are important signalling molecules for maintenance of homeostasis and development of disease processes. In particular, LTs have proved to be powerful inflammatory and immune regulating mediators in many inflammatory processes, whereas mediators of the lipoxin and resolvin pro-resolution families, activated during host defence, counter-regulate inflammation and promote its resolution. However, as we tried to highlight in this updated report, and despite the many progress in this field of research due to the efforts of a large number of scientists and to the tumultuous progress in technology, there are many issues that still need to be clarified. For example, BLT1 receptor signalling in cancer and the dual role of the BLT2 receptor in pro- and anti-inflammation. Furthermore, the role of cysteinyl-LTs in physiological and pathological conditions other than asthma, particularly in CVDs (Bäck and Hansson, 2006; Bäck, 2007; Nagy et al., 2011; Ingelsson et al., 2012; Capra et al., 2013), or the very interesting issue of the cross-talk between the CysLT and P2Y receptor systems at different levels, agonists/antagonists (Mamedova et al., 2005; Nonaka et al., 2005; Paruchuri et al., 2009; Fredman et al., 2010; Woszczek et al., 2010; Foster et al., 2013) or function/regulation (Capra et al., 2005; Jiang et al., 2009). Likewise, the subcellular localization of functional CysLT1 receptors in the perinuclear region (Nielsen et al., 2005; Nagy et al., 2011; Eaton et al., 2012) opens up for novel signalling pathways for cysteinyl-LTs. Another fascinating notion is the presence of novel receptors for cysteinyl-LTs, such as GPR17 (Ciana et al., 2006; Maekawa et al., 2009; 2010; Benned-Jensen and Rosenkilde, 2010; Qi et al., 2013) or GPR99 (Kanaoka et al., 2013) and resolvin, such as GPR32 (Krishnamoorthy et al., 2010; 2012; Chiang et al., 2012; Southern et al., 2013) and Chem23 (Arita et al., 2005; 2007; Fredman et al., 2010; Bondue et al., 2011a). In conclusion, more comprehensive investigations with in vitro and in vivo models are certainly needed to shed new light on the ever growing roles of this sophisticated and tightly controlled system of endogenous mediators in physiology and pathology.
Acknowledgments
The authors thank the following colleagues for their valuable contributions in the preparation of this review: Dr. Nan Chiang (Boston, MA, USA), Dr. Motonao Nakamura (Tokyo, Japan) and Dr. Joshua Rokach (Melbourne, FL, USA).
Glossary
- AQP4
aquaporin 4
- ATRA
all-trans retinoic acid
- AT-RvD3
aspirin-triggered resolvin
- BMDM
bone marrow-derived macrophages
- COPD
chronic obstructive pulmonary disease
- CVD
cardiovascular disease
- DC
dendritic cell
- EAE
experimental autoimmune encephalitis
- GRK
GPCR kinase
- OGD
oxygen-glucose deprivation
- LX
lipoxin
- OIR
oxygen-induced retinopathy
- RSV
respiratory syncytial virus
- Rv
resolvin
Conflicts of interest
None.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aratake Y, Okuno T, Matsunobu T, Saeki K, Takayanagi R, Furuya S, et al. Helix 8 of leukotriene B4 receptor 1 inhibits ligand-induced internalization. FASEB J. 2012;26:4068–4078. doi: 10.1096/fj.12-212050. [DOI] [PubMed] [Google Scholar]
- Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- Bae YS, Park JC, He R, Ye RD, Kwak JY, Suh PG, et al. Differential signaling of formyl peptide receptor-like 1 by Trp-Lys-Tyr-Met-Val-Met-CONH2 or lipoxin A4 in human neutrophils. Mol Pharmacol. 2003;64:721–730. doi: 10.1124/mol.64.3.721. [DOI] [PubMed] [Google Scholar]
- Barajas-Espinosa A, Ochoa-Cortes F, Moos MP, Ramirez FD, Vanner SJ, Funk CD. Characterization of the cysteinyl leukotriene 2 receptor in novel expression sites of the gastrointestinal tract. Am J Pathol. 2011;178:2682–2689. doi: 10.1016/j.ajpath.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajas-Espinosa A, Ni NC, Yan D, Zarini S, Murphy RC, Funk CD. The cysteinyl leukotriene 2 receptor mediates retinal edema and pathological neovascularization in a murine model of oxygen-induced retinopathy. FASEB J. 2012;26:1100–1109. doi: 10.1096/fj.11-195792. [DOI] [PubMed] [Google Scholar]
- Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5:174ra126. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett NA, Rahman OM, Fernandez JM, Parsons MW, Xing W, Austen KF, et al. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J Exp Med. 2011;208:593–604. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett NA, Fernandez JM, Maekawa A, Xing W, Li L, Parsons MW, et al. Cysteinyl leukotriene 2 receptor on dendritic cells negatively regulates ligand-dependent allergic pulmonary inflammation. J Immunol. 2012;189:4556–4565. doi: 10.4049/jimmunol.1201865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäck M. Leukotriene receptors: crucial components in vascular inflammation. Scientificworldjournal. 2007;7:1422–1439. doi: 10.1100/tsw.2007.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäck M, Hansson GK. Leukotriene receptors in atherosclerosis. Ann Med. 2006;38:493–502. doi: 10.1080/07853890600982737. [DOI] [PubMed] [Google Scholar]
- Bäck M, Norel X, Walch L, Gascard J, Mazmanian G, Dahlén S, et al. Antagonist resistant contractions of the porcine pulmonary artery by cysteinyl-leukotrienes. Eur J Pharmacol. 2000;401:381–388. doi: 10.1016/s0014-2999(00)00452-0. [DOI] [PubMed] [Google Scholar]
- Bäck M, Bu DX, Branstrom R, Sheikine Y, Yan ZQ, Hansson GK. Leukotriene B4 signaling through NF-kappaB-dependent BLT1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia. Proc Natl Acad Sci U S A. 2005;102:17501–17506. doi: 10.1073/pnas.0505845102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäck M, Dahlen SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, et al. International Union of Basic and Clinical Pharmacology. LXXXIV: leukotriene receptor nomenclature, distribution, and pathophysiological functions. Pharmacol Rev. 2011;63:539–584. doi: 10.1124/pr.110.004184. [DOI] [PubMed] [Google Scholar]
- Bengtsson AM, Jonsson G, Magnusson C, Salim T, Axelsson C, Sjolander A. The cysteinyl leukotriene 2 receptor contributes to all-trans retinoic acid-induced differentiation of colon cancer cells. BMC Cancer. 2013;13:336. doi: 10.1186/1471-2407-13-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benned-Jensen T, Rosenkilde M. Distinct expression and ligand-binding profiles of two constitutively active GPR17 splice variants. Br J Pharmacol. 2010;159:1092–1105. doi: 10.1111/j.1476-5381.2009.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattermann S, Peters L, Ottersbach PA, Bock A, Konya V, Weaver CD, et al. A biased ligand for OXE-R uncouples Galpha and Gbetagamma signaling within a heterotrimer. Nat Chem Biol. 2012;8:631–638. doi: 10.1038/nchembio.962. [DOI] [PubMed] [Google Scholar]
- Bondue B, Wittamer V, Parmentier M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011a;22:331–338. doi: 10.1016/j.cytogfr.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Bondue B, Vosters O, de Nadai P, Glineur S, De Henau O, Luangsay S, et al. ChemR23 dampens lung inflammation and enhances anti-viral immunity in a mouse model of acute viral pneumonia. PLoS Pathog. 2011b;7:e1002358. doi: 10.1371/journal.ppat.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnans C, Mainprice B, Chanez P, Bousquet J, Urbach V. Lipoxin A4 stimulates a cytosolic Ca2+ increase in human bronchial epithelium. J Biol Chem. 2003;278:10879–10884. doi: 10.1074/jbc.M210294200. [DOI] [PubMed] [Google Scholar]
- Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am J Pathol. 2006;168:1064–1072. doi: 10.2353/ajpath.2006.051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozinovski S, Uddin M, Vlahos R, Thompson M, McQualter JL, Merritt AS, et al. Serum amyloid A opposes lipoxin A(4) to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 2012;109:935–940. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braido F, Riccio AM, Rogkakou A, Massacane P, Guerra L, Fumagalli F, et al. Montelukast effects on inflammation in allergic rhinitis: a double blind placebo controlled pilot study. Eur Ann Allergy Clin Immunol. 2012;44:48–53. [PubMed] [Google Scholar]
- Brancaleone V, Gobbetti T, Cenac N, le Faouder P, Colom B, Flower RJ, et al. A vasculo-protective circuit centered on lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 operative in murine microcirculation. Blood. 2013;122:608–617. doi: 10.1182/blood-2013-04-496661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink C, Dahlén SE, Drazen J, Evans JF, Hay DW, Nicosia S, et al. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol Rev. 2003;55:195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- Brink C, Dahlén SE, Drazen J, Evans JF, Hay DW, Rovati GE, et al. International Union of Pharmacology XLIV. Nomenclature for the oxoeicosanoid receptor. Pharmacol Rev. 2004;56:149–157. doi: 10.1124/pr.56.1.4. [DOI] [PubMed] [Google Scholar]
- Capra V, Rovati GE. Rosuvastatin inhibits human airway smooth muscle cells mitogenic response to eicosanoid contractile agents. Pulm Pharmacol Ther. 2014;27:10–16. doi: 10.1016/j.pupt.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Capra V, Nicosia S, Ragnini D, Mezzetti M, Keppler D, Rovati GE. Identification and characterization of two cysteinyl-leukotriene high affinity binding sites with receptor characteristics in human lung parenchyma. Mol Pharmacol. 1998;53:750–758. doi: 10.1124/mol.53.4.750. [DOI] [PubMed] [Google Scholar]
- Capra V, Accomazzo MR, Ravasi S, Parenti M, Macchia M, Nicosia S, et al. Involvement of prenylated proteins in calcium signaling induced by LTD4 in differentiated U937 cells. Prostaglandins Other Lipid Mediat. 2003;71:235–251. doi: 10.1016/s1098-8823(03)00045-5. [DOI] [PubMed] [Google Scholar]
- Capra V, Ravasi S, Accomazzo MR, Parenti M, Rovati GE. CysLT1 signal transduction in differentiated U937 cells involves the activation of the small GTP-binding protein Ras. Biochem Pharmacol. 2004;67:1569–1577. doi: 10.1016/j.bcp.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Capra V, Ravasi S, Accomazzo MR, Citro S, Grimoldi M, Abbracchio MP, et al. CysLT1 receptor is a target for extracellular nucleotide-induced heterologous desensitization: a possible feedback mechanism in inflammation. J Cell Sci. 2005;118((Pt 23)):5625–5636. doi: 10.1242/jcs.02668. [DOI] [PubMed] [Google Scholar]
- Capra V, Ambrosio M, Riccioni G, Rovati GE. Cysteinyl-leukotriene receptor antagonists: present situation and future opportunities. Curr Med Chem. 2006;13:3213–3226. doi: 10.2174/092986706778742963. [DOI] [PubMed] [Google Scholar]
- Capra V, Thompson MD, Sala A, Cole DE, Folco G, Rovati GE. Cysteinyl-leukotrienes and their receptors in asthma and other inflammatory diseases: critical update and emerging trends. Med Res Rev. 2007;27:469–527. doi: 10.1002/med.20071. [DOI] [PubMed] [Google Scholar]
- Capra V, Back M, Barbieri SS, Camera M, Tremoli E, Rovati GE. Eicosanoids and their drugs in cardiovascular diseases: focus on atherosclerosis and stroke. Med Res Rev. 2013;33:364–438. doi: 10.1002/med.21251. [DOI] [PubMed] [Google Scholar]
- Capra V, Carnini C, Accomazzo MR, Di Gennaro A, Fiumicelli M, Borroni E, et al. Autocrine activity of cysteinyl leukotrienes in human vascular endothelial cells: signaling through the CysLT2 receptor. J Lipid Res. 2014 doi: 10.1016/j.prostaglandins.2015.03.007. (in press) [DOI] [PubMed] [Google Scholar]
- Carnini C, Accomazzo MR, Borroni E, Vitellaro-Zuccarello L, Durand T, Folco G, et al. Synthesis of cysteinyl leukotrienes in human endothelial cells: subcellular localization and autocrine signaling through the CysLT2 receptor. FASEB J. 2011;25:3519–3528. doi: 10.1096/fj.10-177030. [DOI] [PubMed] [Google Scholar]
- Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, et al. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med. 2008;205:767–775. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5) doi: 10.1002/14651858.CD002314.pub3. CD002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2014;(1) doi: 10.1002/14651858.CD003137.pub5. CD003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Le Y, Liu Y, Gong W, Ying G, Huang J, et al. A critical role for the g protein-coupled receptor mFPR2 in airway inflammation and immune responses. J Immunol. 2010;184:3331–3335. doi: 10.4049/jimmunol.0903022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Liu M, Liu Y, Yoshimura T, Shen W, Le Y, et al. Formylpeptide receptor-2 contributes to colonic epithelial homeostasis, inflammation, and tumorigenesis. J Clin Invest. 2013;123:1694–1704. doi: 10.1172/JCI65569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Gronert K, Clish CB, O'Brien JA, Freeman MW, Serhan CN. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J Clin Invest. 1999;104:309–316. doi: 10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou RC, Kim ND, Sadik CD, Seung E, Lan Y, Byrne MH, et al. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, et al. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke M, Di Consoli H, Maloberti P, Cornejo Maciel F. Expression and function of OXE receptor, an eicosanoid receptor, in steroidogenic cells. Mol Cell Endocrinol. 2013;371:71–78. doi: 10.1016/j.mce.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, et al. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A. 2013;110:18232–18237. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech W, Barbisch M, Tenscher K, Schopf E, Schroder JM, Norgauer J. Chemotactic 5-oxo-eicosatetraenoic acids induce oxygen radical production, Ca2+-mobilization, and actin reorganization in human eosinophils via a pertussis toxin-sensitive G-protein. J Invest Dermatol. 1997;108:108–112. doi: 10.1111/1523-1747.ep12285653. [DOI] [PubMed] [Google Scholar]
- Dallaire MJ, Ferland C, Page N, Lavigne S, Davoine F, Laviolette M. Endothelial cells modulate eosinophil surface markers and mediator release. Eur Respir J. 2003;21:918–924. doi: 10.1183/09031936.03.00102002. [DOI] [PubMed] [Google Scholar]
- Dalli J, Consalvo AP, Ray V, Di Filippo C, D'Amico M, Mehta N, et al. Proresolving and tissue-protective actions of annexin A1-based cleavage-resistant peptides are mediated by formyl peptide receptor 2/lipoxin A4 receptor. J Immunol. 2013a;190:6478–6487. doi: 10.4049/jimmunol.1203000. [DOI] [PubMed] [Google Scholar]
- Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, et al. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. 2013b;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport AP, Alexander SP, Sharman JL, Pawson AJ, Benson HE, Monaghan AE, et al. International union of basic and clinical pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol Rev. 2013;65:967–986. doi: 10.1124/pr.112.007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoor T, Bracke KR, Dupont LL, Plantinga M, Bondue B, Roy MO, et al. The role of ChemR23 in the induction and resolution of cigarette smoke-induced inflammation. J Immunol. 2011;186:5457–5467. doi: 10.4049/jimmunol.1003862. [DOI] [PubMed] [Google Scholar]
- Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazen JM. Leukotrienes in asthma. Adv Exp Med Biol. 2003;525:1–5. doi: 10.1007/978-1-4419-9194-2_1. [DOI] [PubMed] [Google Scholar]
- Drost AC, Seitz G, Boehmler A, Funk M, Norz KP, Zipfel A, et al. The G-protein-coupled receptor CysLT1 mediates chemokine-like effects and prolongs survival in chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53:665–673. doi: 10.3109/10428194.2011.625578. [DOI] [PubMed] [Google Scholar]
- Dufton N, Hannon R, Brancaleone V, Dalli J, Patel HB, Gray M, et al. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol. 2010;184:2611–2619. doi: 10.4049/jimmunol.0903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eap R, Jacques E, Semlali A, Plante S, Chakir J. Cysteinyl leukotrienes regulate TGF-beta(1) and collagen production by bronchial fibroblasts obtained from asthmatic subjects. Prostaglandins Leukot Essent Fatty Acids. 2012;86:127–133. doi: 10.1016/j.plefa.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Eaton A, Nagy E, Pacault M, Fauconnier J, Back M. Cysteinyl leukotriene signaling through perinuclear CysLT(1) receptors on vascular smooth muscle cells transduces nuclear calcium signaling and alterations of gene expression. J Mol Med (Berl) 2012;90:1223–1231. doi: 10.1007/s00109-012-0904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi B, Kala S, Nikolich-Zugich T, Niethammer P. Tissue damage detection by osmotic surveillance. Nat Cell Biol. 2013;15:1123–1130. doi: 10.1038/ncb2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman H, Onnheim K, Andreasson E, Dahlgren C. What formyl peptide receptors, if any, are triggered by compound 43 and lipoxin A4? Scand J Immunol. 2011;74:227–234. doi: 10.1111/j.1365-3083.2011.02570.x. [DOI] [PubMed] [Google Scholar]
- Foster HR, Fuerst E, Lee TH, Cousins DJ, Woszczek G. Characterisation of P2Y(12) receptor responsiveness to cysteinyl leukotrienes. PLoS ONE. 2013;8:e58305. doi: 10.1371/journal.pone.0058305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman G, Van Dyke TE, Serhan CN. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler Thromb Vasc Biol. 2010;30:2005–2013. doi: 10.1161/ATVBAHA.110.209908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Faibish D, Fredman G, Herrera BS, Chiang N, Serhan CN, et al. Resolvin E1 and chemokine-like receptor 1 mediate bone preservation. J Immunol. 2013;190:689–694. doi: 10.4049/jimmunol.1103688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreau R, Le Gouill C, Venne MH, Stankova J, Rola-Pleszczynski M. Threonine 308 within a putative casein kinase 2 site of the cytoplasmic tail of leukotriene B(4) receptor (BLT1) is crucial for ligand-induced, G-protein-coupled receptor-specific kinase 6-mediated desensitization. J Biol Chem. 2002;277:31567–31576. doi: 10.1074/jbc.M202723200. [DOI] [PubMed] [Google Scholar]
- Goldbart AD, Greenberg-Dotan S, Tal A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130:e575–e580. doi: 10.1542/peds.2012-0310. [DOI] [PubMed] [Google Scholar]
- Gore V, Patel P, Chang CT, Sivendran S, Kang N, Ouedraogo YP, et al. 5-Oxo-ETE receptor antagonists. J Med Chem. 2013;56:3725–3732. doi: 10.1021/jm400480j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh M, Okubo K, Hashiguchi K, Wakabayashi K, Kanzaki S, Tanaka N, et al. Noninvasive biological evaluation of response to pranlukast treatment in pediatric patients with Japanese cedar pollinosis. Allergy Asthma Proc. 2012;33:459–466. doi: 10.2500/aap.2012.33.3615. [DOI] [PubMed] [Google Scholar]
- Grant GE, Rokach J, Powell WS. 5-Oxo-ETE and the OXE receptor. Prostaglandins Other Lipid Mediat. 2009;89:98–104. doi: 10.1016/j.prostaglandins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallstrand TS, Henderson WR., Jr An update on the role of leukotrienes in asthma. Curr Opin Allergy Clin Immunol. 2010;10:60–66. doi: 10.1097/ACI.0b013e32833489c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Jia Y, Takeda K, Shiraishi Y, Okamoto M, Dakhama A, et al. Montelukast during primary infection prevents airway hyperresponsiveness and inflammation after reinfection with respiratory syncytial virus. Am J Resp Crit Care Med. 2010;182:455–463. doi: 10.1164/rccm.200912-1811OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J, Ferreiros N, Pirotte B, Geisslinger G, Offermanns S. Heterologously expressed formyl peptide receptor 2 (FPR2/ALX) does not respond to lipoxin A(4) Biochem Pharmacol. 2013;85:1795–1802. doi: 10.1016/j.bcp.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Haribabu B, Verghese MW, Steeber DA, Sellars DD, Bock CB, Snyderman R. Targeted disruption of the leukotriene B(4) receptor in mice reveals its role in inflammation and platelet-activating factor-induced anaphylaxis. J Exp Med. 2000;192:433–438. doi: 10.1084/jem.192.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HQ, Liao D, Wang ZG, Wang ZL, Zhou HC, Wang MW, et al. Functional characterization of three mouse formyl peptide receptors. Mol Pharmacol. 2013;83:389–398. doi: 10.1124/mol.112.081315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- Hecht I, Rong J, Sampaio AL, Hermesh C, Rutledge C, Shemesh R, et al. A novel peptide agonist of formyl-peptide receptor-like 1 (ALX) displays anti-inflammatory and cardioprotective effects. J Pharmacol Exp Ther. 2009;328:426–434. doi: 10.1124/jpet.108.145821. [DOI] [PubMed] [Google Scholar]
- Heise CE, O'Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im D-S, et al. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- Hikiji H, Ishii S, Yokomizo T, Takato T, Shimizu T. A distinctive role of the leukotriene B4 receptor BLT1 in osteoclastic activity during bone loss. Proc Natl Acad Sci U S A. 2009;106:21294–21299. doi: 10.1073/pnas.0905209106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi T, Koguchi Y, Sugikawa E, Chikada A, Ogawa K, Tsuda N, et al. Identification of a novel human eicosanoid receptor coupled to G(i/o) J Biol Chem. 2002;277:31459–31465. doi: 10.1074/jbc.M203194200. [DOI] [PubMed] [Google Scholar]
- Hosoi T, Sugikawa E, Chikada A, Koguchi Y, Ohnuki T. TG1019/OXE, a Galpha(i/o)-protein-coupled receptor, mediates 5-oxo-eicosatetraenoic acid-induced chemotaxis. Biochem Biophys Res Commun. 2005;334:987–995. doi: 10.1016/j.bbrc.2005.06.191. [DOI] [PubMed] [Google Scholar]
- Houard X, Ollivier V, Louedec L, Michel JB, Bäck M. Differential inflammatory activity across human abdominal aortic aneurysms reveals neutrophil-derived leukotriene B4 as a major chemotactic factor released from the intraluminal thrombus. FASEB J. 2009;23:1376–1383. doi: 10.1096/fj.08-116202. [DOI] [PubMed] [Google Scholar]
- Hoyer FF, Albrecht L, Nickenig G, Muller C. Selective inhibition of leukotriene receptor BLT-2 reduces vascular oxidative stress and improves endothelial function in ApoE-/- mice. Mol Cell Biochem. 2012;359:25–31. doi: 10.1007/s11010-011-0995-y. [DOI] [PubMed] [Google Scholar]
- Iikura M, Suzukawa M, Yamaguchi M, Sekiya T, Komiya A, Yoshimura-Uchiyama C, et al. 5-Lipoxygenase products regulate basophil functions: 5-Oxo-ETE elicits migration, and leukotriene B(4) induces degranulation. J Allergy Clin Immunol. 2005;116:578–585. doi: 10.1016/j.jaci.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Iizuka Y, Okuno T, Saeki K, Uozaki H, Okada S, Misaka T, et al. Protective role of the leukotriene B4 receptor BLT2 in murine inflammatory colitis. FASEB J. 2010;24:4678–4690. doi: 10.1096/fj.10-165050. [DOI] [PubMed] [Google Scholar]
- Ingelsson E, Yin L, Bäck M. Nationwide cohort study of the leukotriene receptor antagonist montelukast and incident or recurrent cardiovascular disease. J Allergy Clin Immunol. 2012;129:702–707 e702. doi: 10.1016/j.jaci.2011.11.052. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Borrelli LA, Kanaoka Y, Bacskai BJ, Boyce JA. CysLT2 receptors interact with CysLT1 receptors and down-modulate cysteinyl leukotriene-dependent mitogenic responses of mast cells. Blood. 2007;110:3263–3270. doi: 10.1182/blood-2007-07-100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Borrelli L, Bacskai BJ, Kanaoka Y, Boyce JA. P2Y6 receptors require an intact cysteinyl leukotriene synthetic and signaling system to induce survival and activation of mast cells. J Immunol. 2009;182:1129–1137. doi: 10.4049/jimmunol.182.2.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Holden S, Tenaillon L, Bhatia U, Seuwen K, Tranter P, et al. Expression and characterization of a 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid receptor highly expressed on human eosinophils and neutrophils. Mol Pharmacol. 2003;63:471–477. doi: 10.1124/mol.63.3.471. [DOI] [PubMed] [Google Scholar]
- Kanaoka Y, Maekawa A, Austen KF. Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J Biol Chem. 2013;288:10967–10972. doi: 10.1074/jbc.C113.453704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazani S, Sadeh J, Bunga S, Wechsler ME, Israel E. Cysteinyl leukotriene antagonism inhibits bronchoconstriction in response to hypertonic saline inhalation in asthma. Respir Med. 2011;105:667–673. doi: 10.1016/j.rmed.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara Y, Yokomizo T, Kunita A, Morishita Y, Fukayama M, Ishii S, et al. The leukotriene B4 receptor, BLT1, is required for the induction of experimental autoimmune encephalomyelitis. Biochem Biophys Res Commun. 2010;394:673–678. doi: 10.1016/j.bbrc.2010.03.049. [DOI] [PubMed] [Google Scholar]
- Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med. 2006;203:829–835. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiwamoto T, Ishii Y, Morishima Y, Yoh K, Kikuchi N, Haraguchi N, et al. Blockade of cysteinyl leukotriene-1 receptors suppresses airway remodelling in mice overexpressing GATA-3. Clin Exp Allergy. 2011;41:116–128. doi: 10.1111/j.1365-2222.2010.03571.x. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol. 2012;180:2018–2027. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labat C, Temmar M, Nagy E, Bean K, Brink C, Benetos A, et al. Inflammatory mediators in saliva associated with arterial stiffness and subclinical atherosclerosis. J Hypertens. 2013;31:2251–2258. doi: 10.1097/HJH.0b013e328363dccc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw TM, Boyce JA. Cysteinyl leukotriene receptors, old and new; implications for asthma. Clin Exp Allergy. 2012;42:1313–1320. doi: 10.1111/j.1365-2222.2012.03982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois A, Ferland C, Tremblay GM, Laviolette M. Montelukast regulates eosinophil protease activity through a leukotriene-independent mechanism. J Allergy Clin Immunol. 2006;118:113–119. doi: 10.1016/j.jaci.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Langlois A, Chouinard F, Flamand N, Ferland C, Rola-Pleszczynski M, Laviolette M. Crucial implication of protein kinase C (PKC)-delta, PKC-zeta, ERK-1/2, and p38 MAPK in migration of human asthmatic eosinophils. J Leukoc Biol. 2009;85:656–663. doi: 10.1189/jlb.0808492. [DOI] [PubMed] [Google Scholar]
- Lee JW, Kim GY, Kim JH. Androgen receptor is up-regulated by a BLT2-linked pathway to contribute to prostate cancer progression. Biochem Biophys Res Commun. 2012;420:428–433. doi: 10.1016/j.bbrc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Lee TH, Austen KF, Corey EJ, Drazen JM. Leukotriene E4-induced airway hyperresponsiveness of guinea pig tracheal smooth muscle to histamine and evidence for three separate sulfidopeptide leukotriene receptors. Proc Natl Acad Sci U S A. 1984;81:4922–4925. doi: 10.1073/pnas.81.15.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RC, Haribabu B, Mathis SP, Kim J, Gozal D. Leukotriene B4 receptor-1 mediates intermittent hypoxia-induced atherogenesis. Am J Respir Crit Care Med. 2011;184((1)):124–131. doi: 10.1164/rccm.201012-2039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YS, Wu P, Zhou XY, Chen JG, Cai L, Wang F, et al. Formyl-peptide receptor like 1: a potent mediator of the Ca2+ release-activated Ca2+ current ICRAC. Arch Biochem Biophys. 2008;478:110–118. doi: 10.1016/j.abb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Luangsay S, Wittamer V, Bondue B, De Henau O, Rouger L, Brait M, et al. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J Immunol. 2009;183:6489–6499. doi: 10.4049/jimmunol.0901037. [DOI] [PubMed] [Google Scholar]
- Maderna P, Cottell DC, Toivonen T, Dufton N, Dalli J, Perretti M, et al. FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J. 2010;24:4240–4249. doi: 10.1096/fj.10-159913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa A, Kanaoka Y, Xing W, Austen KF. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc Natl Acad Sci U S A. 2008;105:16695–16700. doi: 10.1073/pnas.0808993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa A, Balestrieri B, Austen KF, Kanaoka Y. GPR17 is a negative regulator of the cysteinyl leukotriene 1 receptor response to leukotriene D4. Proc Natl Acad Sci U S A. 2009;106:11685–11690. doi: 10.1073/pnas.0905364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa A, Xing W, Austen KF, Kanaoka Y. GPR17 regulates immune pulmonary inflammation induced by house dust mites. J Immunol. 2010;185:1846–1854. doi: 10.4049/jimmunol.1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson C, Ehrnstrom R, Olsen J, Sjolander A. An increased expression of cysteinyl leukotriene 2 receptor in colorectal adenocarcinomas correlates with high differentiation. Cancer Res. 2007;67:9190–9198. doi: 10.1158/0008-5472.CAN-07-0771. [DOI] [PubMed] [Google Scholar]
- Magnusson C, Mezhybovska M, Lorinc E, Fernebro E, Nilbert M, Sjolander A. Low expression of CysLT1R and high expression of CysLT2R mediate good prognosis in colorectal cancer. Eur J Cancer. 2010;46:826–835. doi: 10.1016/j.ejca.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Magnusson C, Liu J, Ehrnstrom R, Manjer J, Jirstrom K, Andersson T, et al. Cysteinyl leukotriene receptor expression pattern affects migration of breast cancer cells and survival of breast cancer patients. Int J Cancer. 2011;129:9–22. doi: 10.1002/ijc.25648. [DOI] [PubMed] [Google Scholar]
- Mamedova L, Capra V, Accomazzo MR, Gao ZG, Ferrario S, Fumagalli M, et al. CysLT(1) leukotriene receptor antagonists inhibit the effects of nucleotides acting at P2Y receptors. Biochem Pharmacol. 2005;71:115–125. doi: 10.1016/j.bcp.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis SP, Jala VR, Lee DM, Haribabu B. Nonredundant roles for leukotriene B4 receptors BLT1 and BLT2 in inflammatory arthritis. J Immunol. 2010;185:3049–3056. doi: 10.4049/jimmunol.1001031. [DOI] [PubMed] [Google Scholar]
- Matsunaga Y, Fukuyama S, Okuno T, Sasaki F, Matsunobu T, Asai Y, et al. Leukotriene B4 receptor BLT2 negatively regulates allergic airway eosinophilia. FASEB J. 2013;27:3306–3314. doi: 10.1096/fj.12-217000. [DOI] [PubMed] [Google Scholar]
- Mazzocchi M, Dessy LA, Alfano C, Scuderi N. Effects of zafirlukast on capsular contracture: long-term results. Int J Immunopathol Pharmacol. 2012;25:935–944. doi: 10.1177/039463201202500411. [DOI] [PubMed] [Google Scholar]
- McMahon B, Mitchell D, Shattock R, Martin F, Brady HR, Godson C. Lipoxin, leukotriene, and PDGF receptors cross-talk to regulate mesangial cell proliferation. FASEB J. 2002;16:1817–1819. doi: 10.1096/fj.02-0416fje. [DOI] [PubMed] [Google Scholar]
- Miyahara N, Takeda K, Miyahara S, Matsubara S, Koya T, Joetham A, et al. Requirement for leukotriene B4 receptor 1 in allergen-induced airway hyperresponsiveness. Am J Respir Crit Care Med. 2005;172:161–167. doi: 10.1164/rccm.200502-205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro AP, Pinheiro CS, Luna-Gomes T, Alves LR, Maya-Monteiro CM, Porto BN, et al. Leukotriene B4 mediates neutrophil migration induced by heme. J Immunol. 2011;186:6562–6567. doi: 10.4049/jimmunol.1002400. [DOI] [PubMed] [Google Scholar]
- Muro S, Hamid Q, Olivenstein R, Taha R, Rokach J, Powell WS. 5-oxo-6,8,11,14-eicosatetraenoic acid induces the infiltration of granulocytes into human skin. J Allergy Clin Immunol. 2003;112:768–774. doi: 10.1016/s0091-6749(03)01888-8. [DOI] [PubMed] [Google Scholar]
- Nagy E, Andersson DC, Caidahl K, Eriksson MJ, Eriksson P, Franco-Cereceda A, et al. Upregulation of the 5-lipoxygenase pathway in human aortic valves correlates with severity of stenosis and leads to leukotriene-induced effects on valvular myofibroblasts. Circulation. 2011;123:1316–1325. doi: 10.1161/CIRCULATIONAHA.110.966846. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Shimizu T. Leukotriene receptors. Chem Rev. 2011;111:6231–6298. doi: 10.1021/cr100392s. [DOI] [PubMed] [Google Scholar]
- Ni NC, Yan D, Ballantyne LL, Barajas-Espinosa A, St Amand T, Pratt DA, et al. A selective cysteinyl leukotriene receptor 2 antagonist blocks myocardial ischemia/reperfusion injury and vascular permeability in mice. J Pharmacol Exp Ther. 2011;339:768–778. doi: 10.1124/jpet.111.186031. [DOI] [PubMed] [Google Scholar]
- Nielsen CK, Campbell JI, Ohd JF, Morgelin M, Riesbeck K, Landberg G, et al. A novel localization of the G-protein-coupled CysLT1 receptor in the nucleus of colorectal adenocarcinoma cells. Cancer Res. 2005;65:732–742. [PubMed] [Google Scholar]
- Nonaka Y, Hiramoto T, Fujita N. Identification of endogenous surrogate ligands for human P2Y12 receptors by in silico and in vitro methods. Biochem Biophys Res Commun. 2005;337:281–288. doi: 10.1016/j.bbrc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- Norgauer J, Barbisch M, Czech W, Pareigis J, Schwenk U, Schroder JM. Chemotactic 5-oxo-icosatetraenoic acids activate a unique pattern of neutrophil responses. Analysis of phospholipid metabolism, intracellular Ca2+ transients, actin reorganization, superoxide-anion production and receptor up-regulation. Eur J Biochem. 1996;236:1003–1009. doi: 10.1111/j.1432-1033.1996.01003.x. [DOI] [PubMed] [Google Scholar]
- Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arterioscler Thromb Vasc Biol. 2012;32:1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty JT, Kuroki M, Nixon AB, Wijkander J, Yee E, Lee SL, et al. 5-Oxo-eicosanoids and hematopoietic cytokines cooperate in stimulating neutrophil function and the mitogen-activated protein kinase pathway. J Biol Chem. 1996a;271:17821–17828. doi: 10.1074/jbc.271.30.17821. [DOI] [PubMed] [Google Scholar]
- O'Flaherty JT, Kuroki M, Nixon AB, Wijkander J, Yee E, Lee SL, et al. 5-Oxo-eicosatetraenoate is a broadly active, eosinophil-selective stimulus for human granulocytes. J Immunol. 1996b;157:336–342. [PubMed] [Google Scholar]
- O'Flaherty JT, Taylor JS, Kuroki M. The coupling of 5-oxo-eicosanoid receptors to heterotrimeric G proteins. J Immunol. 2000;164:3345–3352. doi: 10.4049/jimmunol.164.6.3345. [DOI] [PubMed] [Google Scholar]
- O'Flaherty JT, Rogers LC, Paumi CM, Hantgan RR, Thomas LR, Clay CE, et al. 5-Oxo-ETE analogs and the proliferation of cancer cells. Biochim Biophys Acta. 2005;1736:228–236. doi: 10.1016/j.bbalip.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2010;285:3451–3461. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T, Ago H, Terawaki K, Miyano M, Shimizu T, Yokomizo T. Helix 8 of the leukotriene B4 receptor is required for the conformational change to the low affinity state after G-protein activation. J Biol Chem. 2003;278:41500–41509. doi: 10.1074/jbc.M307335200. [DOI] [PubMed] [Google Scholar]
- Okuno T, Iizuka Y, Okazaki H, Yokomizo T, Taguchi R, Shimizu T. 12(S)-Hydroxyheptadeca-5Z, 8E, 10E-trienoic acid is a natural ligand for leukotriene B4 receptor 2. J Exp Med. 2008;205:759–766. doi: 10.1084/jem.20072329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyoshi MK, He R, Kanaoka Y, ElKhal A, Kawamoto S, Lewis CN, et al. Eosinophil-derived leukotriene C4 signals via type 2 cysteinyl leukotriene receptor to promote skin fibrosis in a mouse model of atopic dermatitis. Proc Natl Acad Sci U S A. 2012a;109:4992–4997. doi: 10.1073/pnas.1203127109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyoshi MK, He R, Li Y, Mondal S, Yoon J, Afshar R, et al. Leukotriene B4-driven neutrophil recruitment to the skin is essential for allergic skin inflammation. Immunity. 2012b;37:747–758. doi: 10.1016/j.immuni.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Ferreira J, Menezes de Lima O, Jr, Duarte FS, Bento AF, Forner S, et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc Natl Acad Sci U S A. 2012;109:21134–21139. doi: 10.1073/pnas.1202906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier CN, Fuerst E, McDonald J, Bowen H, Lee TH, Pease JE, et al. Human T(H)2 cells respond to cysteinyl leukotrienes through selective expression of cysteinyl leukotriene receptor 1. J Allergy Clin Immunol. 2012;129:1136–1142. doi: 10.1016/j.jaci.2012.01.057. [DOI] [PubMed] [Google Scholar]
- Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, et al. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med. 2009;206:2543–2555. doi: 10.1084/jem.20091240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucleic Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. PMID: 24234439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles RS., Jr A new horizon in asthma: inhibiting ILC function. Sci Transl Med. 2013;5:174fs177. doi: 10.1126/scitranslmed.3005881. [DOI] [PubMed] [Google Scholar]
- Planaguma A, Domenech T, Jover I, Ramos I, Sentellas S, Malhotra R, et al. Lack of activity of 15-epi-lipoxin A4 on FPR2/ALX and CysLT1 receptors in interleukin-8-driven human neutrophil function. Clin Exp Immunol. 2013;173:298–309. doi: 10.1111/cei.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Poorter C, Baertsoen K, Lannoy V, Parmentier M, Springael JY. Consequences of ChemR23 heteromerization with the chemokine receptors CXCR4 and CCR7. PLoS ONE. 2013;8:e58075. doi: 10.1371/journal.pone.0058075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin S, Thompson C, Thivierge M, Veronneau S, McMahon S, Dubois CM, et al. Cysteinyl-leukotrienes induce vascular endothelial growth factor production in human monocytes and bronchial smooth muscle cells. Clin Exp Allergy. 2011;41:204–217. doi: 10.1111/j.1365-2222.2010.03653.x. [DOI] [PubMed] [Google Scholar]
- Powell WS, Gravelle F, Gravel S. Metabolism of 5(S)-hydroxy-6,8,11,14-eicosatetraenoic acid and other 5(S)-hydroxyeicosanoids by a specific dehydrogenase in human polymorphonuclear leukocytes. J Biol Chem. 1992;267:19233–19241. [PubMed] [Google Scholar]
- Powell WS, Gravel S, MacLeod RJ, Mills E, Hashefi M. Stimulation of human neutrophils by 5-oxo-6,8,11,14-eicosatetraenoic acid by a mechanism independent of the leukotriene B4 receptor. J Biol Chem. 1993;268:9280–9286. [PubMed] [Google Scholar]
- Powell WS, Chung D, Gravel S. 5-Oxo-6,8,11,14-eicosatetraenoic acid is a potent stimulator of human eosinophil migration. J Immunol. 1995;154:4123–4132. [PubMed] [Google Scholar]
- Powell WS, MacLeod RJ, Gravel S, Gravelle F, Bhakar A. Metabolism and biologic effects of 5-oxoeicosanoids on human neutrophils. J Immunol. 1996;156:336–342. [PubMed] [Google Scholar]
- Price D, Popov TA, Bjermer L, Lu S, Petrovic R, Vandormael K, et al. Effect of montelukast for treatment of asthma in cigarette smokers. J Allergy Clin Immunol. 2013;131:763–771. doi: 10.1016/j.jaci.2012.12.673. [DOI] [PubMed] [Google Scholar]
- Provost V, Langlois A, Chouinard F, Rola-Pleszczynski M, Chakir J, Flamand N, et al. Leukotriene D4 and interleukin-13 cooperate to increase the release of eotaxin-3 by airway epithelial cells. PLoS ONE. 2012;7:e43544. doi: 10.1371/journal.pone.0043544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi AD, Harden TK, Nicholas RA. Is GPR17 a P2Y/Leukotriene receptor? Examination of uracil nucleotides, nucleotide-sugars, and cysteinyl-leukotrienes as agonists of GPR17. J Pharmacol Exp Ther. 2013;347:38–46. doi: 10.1124/jpet.113.207647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi LL, Fang SH, Shi WZ, Huang XQ, Zhang XY, Lu YB, et al. CysLT2 receptor-mediated AQP4 up-regulation is involved in ischemic-like injury through activation of ERK and p38 MAPK in rat astrocytes. Life Sci. 2011;88:50–56. doi: 10.1016/j.lfs.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Qu X, Zhang X, Yao J, Song J, Nikolic-Paterson DJ, Li J. Resolvins E1 and D1 inhibit interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation. J Pathol. 2012 doi: 10.1002/path.4050. doi: 10.1002/path.4050. [DOI] [PubMed] [Google Scholar]
- Ravasi S, Capra V, Mezzetti M, Nicosia S, Rovati GE. A kinetic binding study to evaluate the pharmacological profile of a specific leukotriene C(4) binding site not coupled to contraction in human lung parenchyma. Mol Pharmacol. 2000;57:1182–1189. [PubMed] [Google Scholar]
- Ravasi S, Capra V, Panigalli T, Rovati GE, Nicosia S. Pharmacological differences among CysLT(1) receptor antagonists with respect to LTC(4) and LTD(4) in human lung parenchyma. Biochem Pharmacol. 2002;63:1537–1546. doi: 10.1016/s0006-2952(02)00889-4. [DOI] [PubMed] [Google Scholar]
- Ravasi S, Citro S, Viviani B, Capra V, Rovati GE. CysLT1 receptor-induced human airway smooth muscle cells proliferation requires ROS generation, EGF receptor transactivation and ERK1/2 phosphorylation. Respir Res. 2006;7:42. doi: 10.1186/1465-9921-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchiuti A, Serhan CN. Pro-resolving lipid mediators (SPMs) and their actions in regulating miRNA in novel resolution circuits in inflammation. Front Immunol. 2012;3:298. doi: 10.3389/fimmu.2012.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, Serhan CN. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 2011;25:544–560. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere S, Challet L, Fluegge D, Spehr M, Rodriguez I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature. 2009;459(7246):574–577. doi: 10.1038/nature08029. [DOI] [PubMed] [Google Scholar]
- Sadik CD, Kim ND, Iwakura Y, Luster AD. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcgammaR signaling. Proc Natl Acad Sci U S A. 2012;109:E3177–E3185. doi: 10.1073/pnas.1213797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata K, Bäck M. Receptor preferences of cysteinyl-leukotrienes in the guinea pig lung parenchyma. Eur J Pharmacol. 2002;436:119–126. doi: 10.1016/s0014-2999(01)01594-1. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Schwenk U, Schroder JM. 5-Oxo-eicosanoids are potent eosinophil chemotactic factors. Functional characterization and structural requirements. J Biol Chem. 1995;270:15029–15036. doi: 10.1074/jbc.270.25.15029. [DOI] [PubMed] [Google Scholar]
- Scott JP, Peters-Golden M. Antileukotriene agents for the treatment of lung disease. Am J Resp Crit Care Med. 2013;188:538–544. doi: 10.1164/rccm.201301-0023PP. [DOI] [PubMed] [Google Scholar]
- Seo JM, Park S, Kim JH. Leukotriene B4 receptor-2 promotes invasiveness and metastasis of ovarian cancer cells through signal transducer and activator of transcription 3 (STAT3)-dependent up-regulation of matrix metalloproteinase 2. J Biol Chem. 2012;287:13840–13849. doi: 10.1074/jbc.M111.317131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J. 2011;25:1441–1448. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RK, Chheda Z, Jala VR, Haribabu B. Expression of leukotriene B4 receptor-1 on CD8+ T cells is required for their migration into tumors to elicit effective antitumor immunity. J Immunol. 2013;191:3462–3470. doi: 10.4049/jimmunol.1300967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Xu Z, Shen K. Urinary leukotriene E4, obesity, and adenotonsillar hypertrophy in Chinese children with sleep disordered breathing. Sleep. 2011;34:1135–1141. doi: 10.5665/SLEEP.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Xu Z, Huang Z, Xu J, Qin Q, Shen K. Increased cysteinyl leukotriene concentration and receptor expression in tonsillar tissues of Chinese children with sleep-disordered breathing. Int Immunopharmacol. 2012;13:371–376. doi: 10.1016/j.intimp.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Shi QJ, Xiao L, Zhao B, Zhang XY, Wang XR, Xu DM, et al. Intracerebroventricular injection of HAMI 3379, a selective cysteinyl leukotriene receptor 2 antagonist, protects against acute brain injury after focal cerebral ischemia in rats. Brain Res. 2012;1484:57–67. doi: 10.1016/j.brainres.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Shimbori C, Shiota N, Okunishi H. Effects of montelukast, a cysteinyl-leukotriene type 1 receptor antagonist, on the pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Eur J Pharmacol. 2011;650:424–430. doi: 10.1016/j.ejphar.2010.09.084. [DOI] [PubMed] [Google Scholar]
- Snyder DW, Krell RD. Pharmacological evidence for a distinct leukotriene C4 receptor in guinea-pig trachea. J Pharmacol Exp Ther. 1984;231:616–622. [PubMed] [Google Scholar]
- Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernandez-Lopez D, Pradillo JM, et al. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci. 2009;29:3875–3884. doi: 10.1523/JNEUROSCI.5529-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordi R, Menezes-de-Lima O, Jr, Horewicz V, Scheschowitsch K, Santos LF, Assreuy J. Dual role of lipoxin A in pneumosepsis pathogenesis. Int Immunopharmacol. 2013;17:283–292. doi: 10.1016/j.intimp.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Southern C, Cook JM, Neetoo-Isseljee Z, Taylor DL, Kettleborough CA, Merritt A, et al. Screening beta-arrestin recruitment for the identification of natural ligands for orphan G-protein-coupled receptors. J Biomol Screen. 2013;18:599–609. doi: 10.1177/1087057113475480. [DOI] [PubMed] [Google Scholar]
- Sozzani S, Zhou D, Locati M, Bernasconi S, Luini W, Mantovani A, et al. Stimulating properties of 5-oxo-eicosanoids for human monocytes: synergism with monocyte chemotactic protein-1 and −3. J Immunol. 1996;157:4664–4671. [PubMed] [Google Scholar]
- Stamatiou PB, Chan CC, Monneret G, Ethier D, Rokach J, Powell WS. 5-oxo-6,8,11,14-eicosatetraenoic acid stimulates the release of the eosinophil survival factor granulocyte/macrophage colony-stimulating factor from monocytes. J Biol Chem. 2004;279:28159–28164. doi: 10.1074/jbc.M401537200. [DOI] [PubMed] [Google Scholar]
- Stanke-Labesque F, Moliere P, Bessard J, Laville M, Vericel E, Lagarde M. Effect of dietary supplementation with increasing doses of docosahexaenoic acid on neutrophil lipid composition and leukotriene production in human healthy volunteers. Br J Nutr. 2008;100:829–833. doi: 10.1017/S0007114508923692. [DOI] [PubMed] [Google Scholar]
- Stanke-Labesque F, Bäck M, Lefebvre B, Tamisier R, Baguet JP, Arnol N, et al. Increased urinary leukotriene E4 excretion in obstructive sleep apnea: effects of obesity and hypoxia. J Allergy Clin Immunol. 2009;124:364–370. doi: 10.1016/j.jaci.2009.05.033. 370 e361–362. [DOI] [PubMed] [Google Scholar]
- Stanke-Labesque F, Pepin JL, de Jouvencel T, Arnaud C, Baguet JP, Petri MH, et al. Leukotriene B4 pathway activation and atherosclerosis in obstructive sleep apnea. J Lipid Res. 2012;53:1944–1951. doi: 10.1194/jlr.P022814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke-Labesque F, Pepin JL, Gautier-Veyret E, Levy P, Bäck M. Leukotrienes as a molecular link between obstructive sleep apnoea and atherosclerosis. Cardiovasc Res. 2014;101:187–193. doi: 10.1093/cvr/cvt247. [DOI] [PubMed] [Google Scholar]
- Sturm GJ, Schuligoi R, Sturm EM, Royer JF, Lang-Loidolt D, Stammberger H, et al. 5-Oxo-6,8,11,14-eicosatetraenoic acid is a potent chemoattractant for human basophils. J Allergy Clin Immunol. 2005;116:1014–1019. doi: 10.1016/j.jaci.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Sundaram S, Ghosh J. Expression of 5-oxoETE receptor in prostate cancer cells: critical role in survival. Biochem Biophys Res Commun. 2006;339:93–98. doi: 10.1016/j.bbrc.2005.10.189. [DOI] [PubMed] [Google Scholar]
- Tager AM, Dufour JH, Goodarzi K, Bercury SD, von Andrian UH, Luster AD. BLTR mediates leukotriene B(4)-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J Exp Med. 2000;192:439–446. doi: 10.1084/jem.192.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Taniuchi S, Soejima K, Sudo K, Hatano Y, Kaneko K. New efficacy of LTRAs (montelukast sodium): it possibly prevents food-induced abdominal symptoms during oral immunotherapy. Allergy Asthma Clin Immunol. 2014;10:3. doi: 10.1186/1710-1492-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Yamamoto A, Haga T. Identification of a G protein-coupled receptor for 5-oxo-eicosatetraenoic acid. Biomed Res. 2002;23:101–108. [Google Scholar]
- Talahalli R, Zarini S, Sheibani N, Murphy RC, Gubitosi-Klug RA. Increased synthesis of leukotrienes in the mouse model of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51:1699–1708. doi: 10.1167/iovs.09-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki K, Yokomizo T, Nagase T, Toda A, Taniguchi M, Hashizume K, et al. Absence of leukotriene B4 receptor 1 confers resistance to airway hyperresponsiveness and Th2-type immune responses. J Immunol. 2005;175:4217–4225. doi: 10.4049/jimmunol.175.7.4217. [DOI] [PubMed] [Google Scholar]
- Tsaoussoglou M, Lianou L, Maragozidis P, Hatzinikolaou S, Mavromati M, Orologas N, et al. Cysteinyl leukotriene receptors in tonsillar B- and T-lymphocytes from children with obstructive sleep apnea. Sleep Med. 2012;13:879–885. doi: 10.1016/j.sleep.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Verriere V, Higgins G, Al-Alawi M, Costello RW, McNally P, Chiron R, et al. Lipoxin A4 stimulates calcium-activated chloride currents and increases airway surface liquid height in normal and cystic fibrosis airway epithelia. PLoS ONE. 2012;7:e37746. doi: 10.1371/journal.pone.0037746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki H, Hendy EB, Hindmarch CC, Gouraud S, Toward M, Kasparov S, et al. Excessive leukotriene B4 in nucleus tractus solitarii is prohypertensive in spontaneously hypertensive rats. Hypertension. 2013;61:194–201. doi: 10.1161/HYPERTENSIONAHA.112.192252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch L, Norel X, Bäck M, Gascard JP, Dahlen SE, Brink C. Pharmacological evidence for a novel cysteinyl-leukotriene receptor subtype in human pulmonary artery smooth muscle. Br J Pharmacol. 2002;137:1339–1345. doi: 10.1038/sj.bjp.0704991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Du C, Lv J, Wei W, Cui Y, Xie X. Antiasthmatic drugs targeting the cysteinyl leukotriene receptor 1 alleviate central nervous system inflammatory cell infiltration and pathogenesis of experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2336–2345. doi: 10.4049/jimmunol.1100333. [DOI] [PubMed] [Google Scholar]
- Wei JD, Kim JY, Kim JH. BLT2 phosphorylation at Thr355 by Akt is necessary for BLT2-mediated chemotaxis. FEBS Lett. 2011;585:3501–3506. doi: 10.1016/j.febslet.2011.10.037. [DOI] [PubMed] [Google Scholar]
- Wei JD, Kim JY, Kim AK, Jang SK, Kim JH. RanBPM acts as a negative regulator of BLT2 to attenuate BLT2-mediated cell motility. J Biol Chem. 2013;288:26753–26763. doi: 10.1074/jbc.M113.470260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittamer V, Gregoire F, Robberecht P, Vassart G, Communi D, Parmentier M. The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J Biol Chem. 2004;279:9956–9962. doi: 10.1074/jbc.M313016200. [DOI] [PubMed] [Google Scholar]
- Wittenberger T, Hellebrand S, Munck A, Kreienkamp HJ, Schaller HC, Hampe W. GPR99, a new G protein-coupled receptor with homology to a new subgroup of nucleotide receptors. BMC Genomics. 2002;3:17. doi: 10.1186/1471-2164-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woszczek G, Chen LY, Alsaaty S, Nagineni S, Shelhamer JH. Concentration-dependent noncysteinyl leukotriene type 1 receptor-mediated inhibitory activity of leukotriene receptor antagonists. J Immunol. 2010;184:2219–2225. doi: 10.4049/jimmunol.0900071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Liu ZJ, Miao S, Zou LB, Cai L, Wu P, et al. Lipoxin A ameliorates cerebral ischaemia/reperfusion injury through upregulation of nuclear factor erythroid 2-related factor 2. Neurol Res. 2013;35:968–975. doi: 10.1179/1743132813Y.0000000242. [DOI] [PubMed] [Google Scholar]
- Wunder F, Tinel H, Kast R, Geerts A, Becker EM, Kolkhof P, et al. Pharmacological characterization of the first potent and selective antagonist at the cysteinyl leukotriene 2 (CysLT(2)) receptor. Br J Pharmacol. 2010;160:399–409. doi: 10.1111/j.1476-5381.2010.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, et al. International union of basic and clinical pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387(6633):620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med. 2000;192:421–432. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Inoue H, Matsumura Y, Nabeta H, Narusawa M, Watanabe A, et al. Absence of LTB4/BLT1 axis facilitates generation of mouse GM-CSF-induced long-lasting antitumor immunologic memory by enhancing innate and adaptive immune systems. Blood. 2012;120:3444–3454. doi: 10.1182/blood-2011-10-383240. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Wang XR, Xu DM, Yu SY, Shi QJ, Zhang LH, et al. HAMI 3379, a CysLT2 receptor antagonist, attenuates ischemia-like neuronal injury by inhibiting microglial activation. J Pharmacol Exp Ther. 2013;346:328–341. doi: 10.1124/jpet.113.203604. [DOI] [PubMed] [Google Scholar]
- Zhao C, Zhao B, Zhang X, Huang X, Shi W, Liu H, et al. Cysteinyl leukotriene receptor 2 is spatiotemporally involved in neuron injury, astrocytosis and microgliosis after focal cerebral ischemia in rats. Neuroscience. 2011;189:1–11. doi: 10.1016/j.neuroscience.2011.05.066. [DOI] [PubMed] [Google Scholar]
- Zhu J, Bandi V, Qiu S, Figueroa DJ, Evans JF, Barnes N, et al. Cysteinyl leukotriene 1 receptor expression associated with bronchial inflammation in severe exacerbations of COPD. Chest. 2012;142:347–357. doi: 10.1378/chest.11-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]