SUMMARY
Transcription of highly expressed genes has been shown to occur in stochastic bursts. But the origin of such ubiquitous phenomenon has not been understood. Here we present the mechanism in bacteria. We developed a high-throughput in vitro single-molecule assay to follow transcription on individual DNA templates in real time. We showed that positive supercoiling buildup on a DNA segment by transcription slows down transcription elongation and eventually stops transcription initiation. Transcription can be resumed upon gyrase binding to the DNA segment. Furthermore, using single-cell mRNA counting fluorescence in situ hybridization (FISH), we found the extent of transcriptional bursting depends on the intracellular gyrase concentration. Together, these findings prove that transcriptional bursting of highly expressed genes in bacteria is primarily caused by reversible gyrase dissociation from and rebinding to a DNA segment, changing the supercoiling level of the segment.
INTRODUCTION
Essential for all cell functions, transcription, the synthesis of mRNAs from DNA carried out by RNA polymerase (RNApol), is the first step in gene expression. Many recent experiments have shown the general phenomenon that transcription of highly expressed genes occurs in stochastic bursts in bacteria (Golding et al., 2005; So et al., 2011; Taniguchi et al., 2010; Zong et al., 2010) and eukaryotic cells (Suter et al., 2011). A major source of gene expression noise, transcriptional bursting results in cellular diversity of an isogenic population, possibly enhances survival of the population in the face of environmental uncertainty (Kussell and Leibler, 2005; Thattai and van Oudenaarden, 2004; Wolf et al., 2005). Golding and coworkers directly observed transcriptional bursting in real time by using MS2 loops to monitor mRNA production in E. coli (Golding et al., 2005). Our group reported a high-throughput single-molecule FISH assay to measure the cellular copy number distribution of a particular mRNA for a large population of isogenic E. coli cells (Taniguchi et al., 2010). When mRNAs are generated with a constant flux, one expects a Poisson distribution of mRNAs across the population. Bursting transcription would lead to nonPoissonian distributions. For all the highly expressed E. coli genes, we found that the distributions are not Poissonian, with the Fano factor (variance divided by the mean of a given distribution) larger than 1. This indicates the ubiquity of transcriptional bursting in bacteria.
However, the origin of bacterial transcriptional bursting is still unknown. Its stochasticity implies it is a single-molecule behavior: there is only one copy of the gene in the cell. Its universality implies that it cannot be attributed to a specific gene or protein factor. Rather, it must originate from a fundamental and general mechanism pertinent to the chromosomal DNA structure and its influence on transcription regulation.
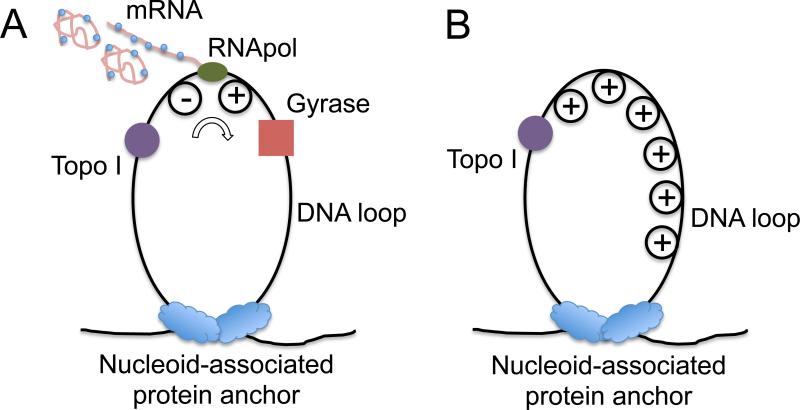
It has been shown that E. coli chromosomal DNA is segregated to ~400 topologically constrained loops with an average size of 10k base pairs (Hardy and Cozzarelli, 2005; Postow et al., 2004). Recent work discussed that E. coli nucleoid-associated proteins such as H-NS and Fis can serve as anchoring points of the loops based on both chromosome conformation capture (3C) and super-resolution optical imaging experiments (Wang et al., 2011). Such chromosome structure provides us a clue to explain the transcriptional bursting phenomenon (Figure 1). In such a DNA loop, transcription generates positive supercoiling ahead of the RNApol, and negative supercoiling behind the RNApol (Deng et al., 2004; Liu and Wang, 1987; Samul and Leng, 2007; Tsao et al., 1989; Wu et al., 1988). There exist two major topoisomerases in E. coli cells, gyrase and topoisomerase I (Topo I), which release positive and negative supercoiling respectively (Drlica, 1992). It is known that negative supercoiling formed during transcription elongation is rapidly removed by Topo I (Cheng et al., 2003). This is necessary because accumulation of negative supercoiling could lead to the formation of detrimental R-loops, an RNA-DNA hybrid (Drolet, 2006). The activity of gyrase, on the other hand, is not as sufficient to keep up with transcription (Guptasarma, 1996), leading to positive supercoiling accumulation on the DNA loops containing highly transcribed operons (El Hanafi and Bossi, 2000).
Figure 1. Transcription on topologically isolated chromosomal DNA loops.
(A) Gyrase releases positive supercoiling generated by transcription on a DNA loop, and RNApol keeps transcribing the gene. (B) In the absence of gyrase, active transcription on a DNA loop leads to positive supercoiling accumulation, which inhibits further transcription on the particular DNA loop.
It has been found that there are ~500 gyrase molecules per E. coli cell (Baker et al., 1987; Higgins et al., 1978; Liu and Wang, 1987), which happens to be roughly the number of topologically constrained DNA loops per chromosome. On average there is one gyrase molecule per DNA loop. When a gyrase molecule reacts on the DNA loop, positive supercoiling is released, and RNApol can keep transcribing the gene (“on” state, Figure 1A). In the absence of gyrase, positive supercoiling is built up by transcription, possibly slowing down transcription elongation and stopping transcription initiation (“off” state, Figure 1B).
In this study, through a series of in vitro single-molecule and live-cell experiments, we prove that transcriptional bursting of highly expressed genes in bacteria is primarily caused by gyrase dissociation from and reversible binding to a DNA segment, such as a chromosomal loop, which changes the supercoiling level of the DNA segment.
RESULTS
A new in vitro single-molecule assay allows real-time monitoring of transcription on individual DNA templates
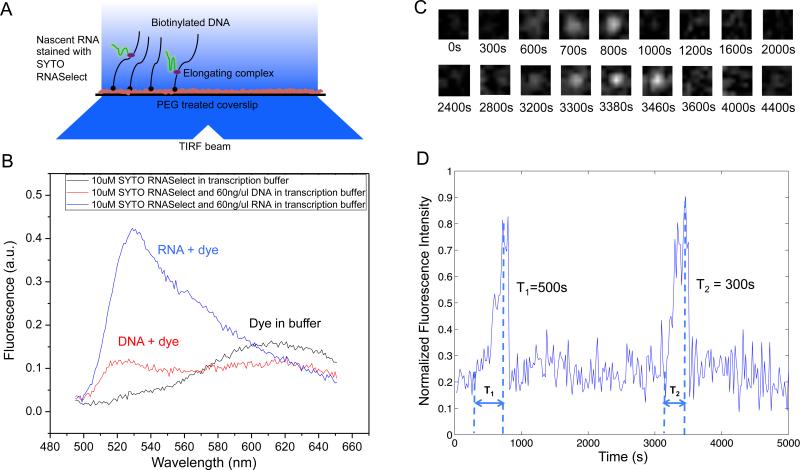
We developed an in vitro single-molecule assay to monitor repetitive stochastic transcription events in real time on individual DNA templates with controlled supercoiling levels (Figure 2A). We used a nucleic acid stain, SYTO RNASelect (Life Technologies), that is nonfluorescent at 530 nm but becomes fluorescent upon binding to RNA (Figure 2B). It has been used to detect RNA in the presence of DNA (Kannemeier et al., 2007). An Argon laser line at 488 nm was used to excite SYTO RNASelect in a total internal reflection fluorescence (TIRF) microscope. We collected fluorescence at 530 nm and recorded time-lapse movies with a CCD camera. In the presence of the dye, a single nascent mRNA becomes visible, and its fluorescence intensity increases with the mRNA length. Therefore we were able to track transcription elongation in real time as the nascent mRNA being produced on a surface-tethered DNA template. Transcription activities on up to hundreds of templates in one field of view can be monitored simultaneously.
Figure 2. In vitro single-molecule assay to monitor real-time transcription on individual DNA templates using SYTO RNASelect stain.
(A) Schematic representation of the experimental arrangement (not drawn to scale). In the presence of 250 nM SYTO RNASelect, nascent RNAs are fluorescent under TIRF excitation at 488 nm. With an excitation power density of 0.22 W/cm2 and an image acquisition time of 5 s, a transcript of 2.3k nucleotides yields a SNR of 1. (B) Fluorescence emission spectra of SYTO RNASelect solution under 488 nm excitation. The dye selectively stains RNA and emits fluorescence with a peak at 530 nm. In the absence of nucleic acids, the dye is not fluorescent at 530 nm. (C) Time-lapse images of 1.1 μm×1.1 μm sub-field-of-view to monitor T7 transcription on one 12 kb-long template. (D) Intensity versus time trajectory of the DNA template shown in (C). Full transcripts are produced repetitively on the template, with transcription elongation time T1 = 500 s and T2 = 300 s, respectively. See also Figure S1 and S2.
As a control, we examined the effect of SYTO RNASelect on the activities of enzymes involved in our system, including T7 RNApol, E. coli RNApol, E. coli gyrase, and E. coli Topo I. None of them were found to be affected by the stain (Figure S1A-H). With sufficiently low laser power and the presence of a fresh oxygen scavenger system, photobleaching of the dye and photocleavage of nucleic acids were negligible (Figure S2).
In our single-molecule assay, DNA templates containing a promoter were tethered on the passivated surface of the flow cell through biotin-streptavidin linkage. After we flowed RNApol and NTPs into the flow cell, the fluorescence intensity of some spots in the field of view linearly ramped up due to transcription elongation, followed by abrupt disappearance upon transcription termination (Figure 2C). “Blinking” of fluorescence occurred when multiple transcripts were produced. As a control, no fluorescence intensity increase was observed under any of the following conditions: 1) no RNApol in the solution, 2) no NTPs in the solution, 3) no promoter in the DNA template. Full-length transcripts (>12 kb) were generated as confirmed by RNA gel electrophoresis (Figure S1G-I).
By recording fluorescent movies, we were able to measure intensity versus time for a field of view containing hundreds of individual DNA templates, from which we could monitor how individual transcripts were generated (Figure 2D). This in vitro single-molecule assay allows us to investigate the effects of supercoiling on transcription initiation and elongation in a clean and controlled system.
Positive supercoiling buildup by transcription slows down transcription elongation
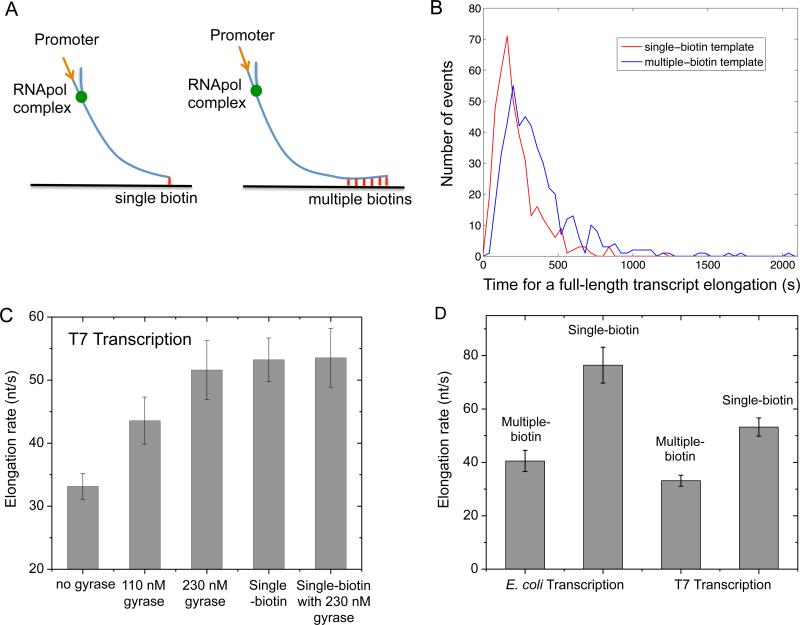
We examined the effect of positive supercoiling buildup on transcription elongation in vitro. We designed 12 kb-long linear DNA templates with T7 or E. coli promoter on the 5’ end, and single or multiple biotinylated nucleotides on the 3’ end (Figure 3A).
Figure 3. Supercoiling dependence of transcription elongation rate.
(A) In vitro transcription template design containing a T7 or T7A1 promoter and a 12 kb transcribing sequence. The template is anchored to the flow cell surface via either a single or multiple biotin-streptavidin linkages. (B) Histogram of T7 transcription elongation time on the templates anchored with single (red curve) or multiple biotin-streptavidin linkages (blue curve). The average elongation time for the multiple-biotin template is 60% longer. (C) Titration of T7 transcription elongation rate (23 °C) on the multiple-biotin template (the three bars on the left) with gyrase. The elongation rate increases with the gyrase concentration till it gets as high as that on the single-biotin template (the 4th bar). The elongation rate on the single-biotin template does not change in the presence of a saturating concentration of gyrase (the bar on the right). (D) E. coli transcription elongation rate (37 °C) and T7 transcription elongation rate (23 °C) on the multiple-biotin template are slower than on the single-biotin template. See also Figure S1 and S2.
When the DNA duplex is tethered to the surface with a single biotin-streptavidin linkage, we found the average T7 transcription elongation rate is 53.2±3.4 nt/s (0.3 mM each NTP, 23 °C), which is consistent with previously reported rates (Skinner et al., 2004). This result further proved that transcription was not affected by the SYTO RNASelect dye. In this case, supercoiling cannot accumulate since DNA can rotate around its single linkage to the surface.
On the other hand, DNA with multiple biotinylated nucleotides cannot rotate around its multiple linkages to the surface. Positive supercoiling would accumulate downstream of the elongation complex when the free spiral of the bulky complex around the DNA is hindered by the frictional drag on the complex. Interestingly, we found T7 transcription elongation was slowed down by 38% as positive supercoiling accumulated on the multiple-biotin DNA template (Figure 3B).
There is a concern that supercoiling might arise from immobilization of the elongation complex to the surface. Special care was taken to minimize interactions of the elongation complex with the surface in our experiment. Besides, we note our result is consistent with the earlier in vitro report that the frictional drag on a sizable nascent transcript is enough to lead to DNA supercoiling (Tsao et al., 1989) even in aqueous solution and free of surface perturbation. Moreover, the measured elongation rate with the single linkage did not seem to be perturbed by the surface interaction with the elongation complex, if any.
Interestingly, the elongation rate on the multiple-biotin template was recovered when gyrase was added into the system. Figure 3C shows the elongation rate as a function of gyrase concentration, reaching the value of the single-biotin template at a saturating gyrase concentration. As a control, we found that gyrase did not affect the elongation rate on the single-biotin template (Figure 3C), indicating that gyrase play no other role than releasing positive supercoiling.
Similarly, with E. coli RNApol, we found positive supercoiling accumulation on the multiple-biotin template also slowed down transcription elongation by 47% (Figure 3D), which is consistent with a recent report based on mechanical manipulation (Ma et al., 2013).
Dissociation constant of gyrase-DNA complex is determined from gyrase concentration dependence of transcription elongation rates
Gyrase-DNA binding can be described by two steps (Gore et al., 2006). First, DNA and gyrase form a complex with limited protein-DNA-binding interface, which is prone to rapid dissociation. Second, a chiral DNA wrap is formed around gyrase, which in the presence of ATP generates negative DNA supercoils. Here we discuss the binding stability and kinetics of the DNA wrapping state, which are relevant to transcription dynamics.
By titrating the elongation rate on the multiple-biotin template with gyrase (Figure 3C), we determined the gyrase-DNA dissociation constant Kd from the gyrase concentration at which the increase of the T7 transcription elongation rate reaches half of its saturation value, that is Kd ≈ 100 nM (Extended Experimental Procedures). This Kd is larger than previously reported 0.2~0.5 nM (Higgins and Cozzarelli, 1982; Maxwell and Gellert, 1984), where specific gyrase binding sequences were used (Morrison and Cozzarelli, 1981; Rau et al., 1987). Strong gyrase binding sites comparable to these sequences are sparsely distributed on the E. coli chromosome with a frequency of only one per 100 kb (Snyder and Drlica, 1979). The nuoB-N DNA sequence (~12 kb) we used in our in vitro assay better represents a chromosomal DNA loop (~10 kb) that binds to gyrase at multiple weak binding sites (Franco and Drlica, 1988; Reece and Maxwell, 1991).
Positive supercoiling buildup by transcription essentially stops transcription initiation
Next we examined the effect of positive supercoiling on transcription initiation. In order to mimic topologically isolated DNA loops in the bacterial chromosome, we designed a circular template (Figure 4A) and tethered it to the surface with multiple biotin-streptavidin linkages. The circular template consists of a T7 or E. coli promoter, a 12 kb-long transcribing sequence and a T7 or E. coli terminator. Due to the low circularization efficiency, a significant fraction of the purified DNAs remained to be linear, which are also tethered on the flow cell surface and transcribed. We picked the circular templates for analysis by staining the DNA molecules with SYTOX Orange and imaging them under flow after recording transcription movies (Figure S3).
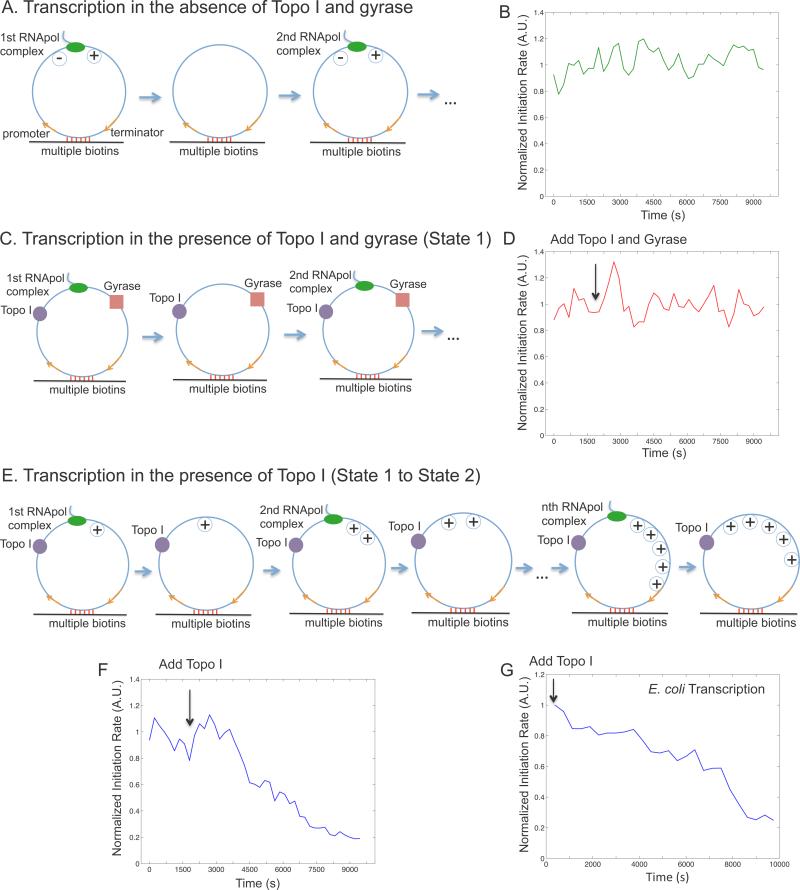
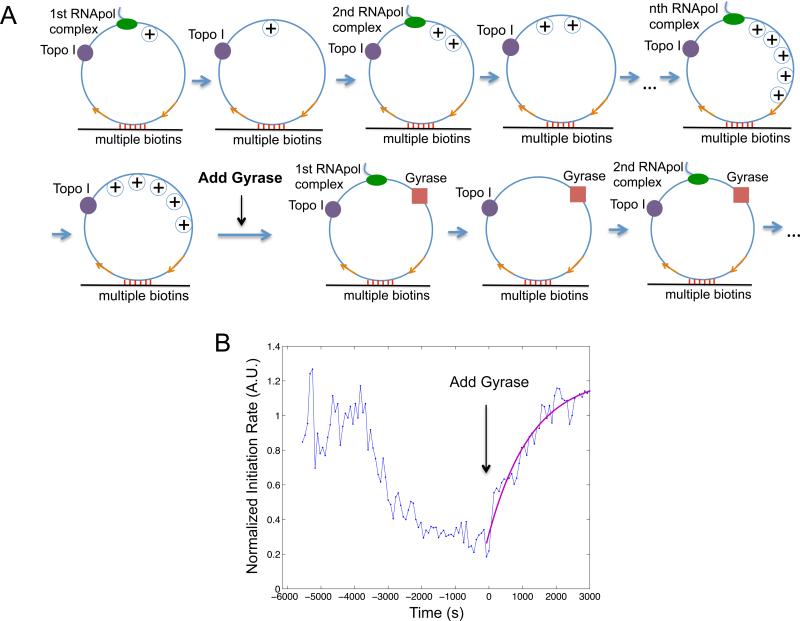
Figure 4. Supercoiling dependence of transcription initiation rate.
(A) Schematic of transcription on a circular template in the absence of topoisomerases. Positive and negative supercoiling annihilate each other after RNApol completes transcription and dissociates from the template. (B) Time dependence of T7 transcription initiation rate under the condition of (A). (C) Schematic of transcription on the circular template in the presence of 41 nM Topo I and 0.1 μM gyrase (same as State 1 in Figure 7A). (D) Time dependence of T7 transcription initiation rate under the condition of (C). The arrow shows the time when the topoisomerases were added into the system. (E) Schematic of transcription on the circular template in the presence of 41 nM Topo I and absence of gyrase. Positive supercoiling is built up as transcripts are produced. (F) Time dependence of T7 transcription initiation rate under the condition of (E). B, D&F are the total intensity versus time from 160 circular templates under respective conditions normalized to the same fluorescence intensity. (G) Time dependence of E. coli transcription initiation rate in the presence of 62 nM Topo I and absence of gyrase. This is the intensity averaged from 106 circular templates at each time point normalized to that from 209 linear templates (Extended Experimental Procedures). See also Figure S1, S2, S3, S4 and S5.
For a single template under steady-state condition, transcription initiation rate is the number of initiation events over a fixed period of time (frequency of “spikes” in the intensity trajectory from a template, Figure S4A). According to the ergodic principle, the initiation rate is the sum of initiated events from a population of templates at a specific time point. We measured the total intensity of the circular templates, which is proportional to the initiation rate.
We examined the first steady-state condition, in which T7 transcription occurs on the circular templates in the absence of Topo I and gyrase. A bulky elongation complex generates positive supercoiling ahead of it and negative supercoiling behind it, which annihilate each other when the elongation complex dissociates from the template upon transcription termination (Figure 4A). We found the initiation rate was indeed constant over time because of repetitive annihilation of supercoiling (Figure 4B).
We then examined the second steady-state condition, in which T7 transcription occurs on the circular templates in the presence of both Topo I and gyrase (Figure 4C). Because both positive and negative supercoiling on the DNA template is continuously removed, the initiation rate remained constant over time under this condition (Figure 4D), which is the same as that in the first steady-state condition (Figure S4C&D).
We now examine how positive supercoiling buildup would hinder transcription initiation. After introduction of Topo I, negative supercoiling is rapidly removed, and positive supercoiling is expected to accumulate on the circular template as multiple transcripts are made (Figure 4E). Indeed we observed the initiation rate decreased over time (Figure 4F). Interestingly, the final intensity has dropped to under 20% of its initial value, indicating transcription initiation was essentially stopped by the buildup of positive supercoiling. This final state corresponds to the gene “off” state. We found that it takes ~9 individuals T7 transcripts to be made in order to build up sufficient positive supercoiling that inhibits transcription initiation on a single template in vitro (Figure S4B, Extended Experimental Procedures).
Similar to T7 transcription, we found transcription initiation rate of E. coli RNApol dropped to ~25% after ~5 transcripts were produced from a circular template of the same length (12 kb) in the presence of Topo I (Figure 4E&G, S5, Extended Experimental Procedures). We note that fewer than 5 rounds of transcription might be sufficient to generate the same level of supercoiling in a live cell, where the environment is more viscous and the elongation complex is bulkier due to transcription-translation coupling (Lynch and Wang, 1993).
With regards to why supercoiling stops transcription initiation, earlier magnetic tweezer experiments have shown that DNA positive supercoiling leads to significantly slower and less stable formation of E. coli RNApol-promoter open complex (Revyakin et al., 2004). Therefore we conclude that the observed inhibition of transcription initiation arises from hindered formation of RNApol-promoter open complex due to positive supercoiling accumulation.
Gyrase binding to positively supercoiled DNA restarts transcription
We now prove that gyrase binding on the positively supercoiled DNA restarts transcription. We started with T7 transcription on the circular templates in the presence of Topo I, generating the gene “off” state (Figure 5). Upon addition of gyrase into the system, transcription initiation rate started to increase and reached a plateau at the initial value of the relaxed templates (Figure 5B), indicating that transcription initiation was fully recovered when positive supercoiling was released by gyrase.
Figure 5. Transition from gene “off” to “on” state.
(A) Schematic of transcription on the circular template first in the presence of 41 nM Topo I only (same as Figure 4E), and then both 41 nM Topo I and 0.1 μM gyrase (same as Figure 4C). (B) Time dependence of T7 transcription initiation rate (blue) under the condition of (A). This is the intensity averaged from 160 circular templates at each time point normalized to that from 120 linear templates. Gyrase was added into the system at T = 0, when transcription initiation was essentially stopped by positive supercoiling accumulation. The trajectory after T = 0 is fitted with a single exponential function (magenta). See also Figure S1, S2 and S3.
The initiation rate versus time after the introduction of gyrase can be fitted well with a single exponential rise (Figure 5B), suggesting a single step is rate-limiting for the transition. The rate constant is determined to be 0.78×10−3 s−1, comparable to the pseudo-first-order gyrase-DNA binding rate constant ~10−3 s−1, which is the product between the bimolecular binding rate constant kon ≈ 104 M−1 s−1 under our salt concentration (Higgins and Cozzarelli, 1982) and the gyrase concentration used in our in vitro assay 0.1 μM. Such consistency suggests that gyrase binding to the DNA template is the rate-limiting step to restart transcription in vitro.
The Kd and kon of gyrase-DNA binding determined in vitro allow us to estimate the time it takes gyrase to dissociate from and rebind to a specific chromosomal DNA loop in an E. coli cell. Since there are comparable number of gyrase molecules and chromosomal DNA loops per E. coli cell, many gyrase molecules are trapped on DNA loops. According to Kd, the intracellular concentration of unbound gyrase [G] is ~0.3 μM (Extended Experimental Procedures). Since kon of gyrase-DNA binding is ~104 M−1 s−1 as determined in vitro, the in vivo pseudo-first-order rate constant for gyrase-DNA binding kon•[G] is ~3 × 10−3 s−1. Therefore the average gyrase rebinding time is 1/(kon•[G]) ≈ 6 min. Since the dissociation rate constant of gyrase-DNA complex is koff = Kdβkon ≈ 10−3 s−1, the average gyrase dissociation time is 1/koff ≈ 17 min. The gyrase rebinding and dissociation time is in the same order of magnitude with the “off” and “on” periods of transcriptional bursting observed in live E. coli cells (Golding et al., 2005).
In summary, the in vitro experiments demonstrated that DNA positive supercoiling generated by transcription slows down both transcription initiation and elongation, and eventually stops initiation. Inhibited transcription initiation and elongation can be recovered upon gyrase binding to DNA. Therefore, accumulation and removal of positive supercoiling of a chromosomal DNA loop containing a highly expressed gene can switch the gene off and on. Next we performed live-cell experiments to further support this mechanism.
Live-cell experiments confirm that positive supercoiling buildup slows down transcription elongation
We examined whether chromosomal supercoiling level affects transcription elongation in live E. coli cells. Using quantitative RT-PCR, we measured the steady-state abundance of different segments of fully induced lac operon mRNA under gyrase inhibition by novobiocin or norfloxacin. No difference in the abundance was observed throughout the transcript (Figure 6A). This result suggests that the elongation complex does not stop or dissociate from the DNA template in the middle of one round of transcription more often when the DNA template is more positively supercoiled. We note that an early in vitro experiment found that stable norfloxacin-gyrase-DNA complex could form at a strong gyrase binding site and block transcription elongation (Willmott et al., 1994). This effect was not observed in our live-cell assay, likely due to a low intracellular norfloxacin concentration and the lack of strong gyrase binding sites in the probed region.
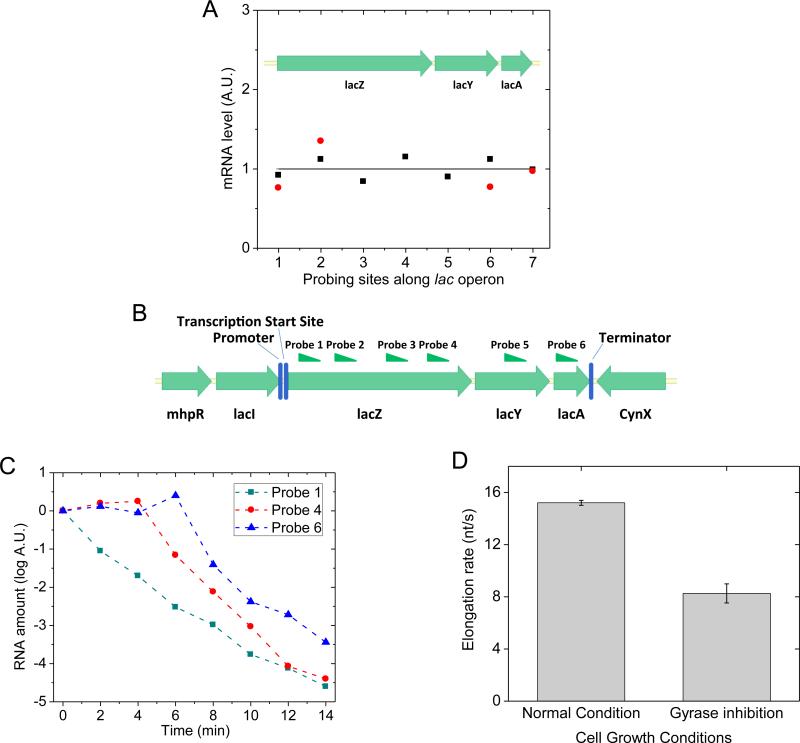
Figure 6. Transcription processivity and elongation rate upon gyrase inhibition in live E. coli cells.
(A) Quantitative RT-PCR measurement of the abundance of different parts of lac operon mRNA under fully induced condition. x-axis: the position of probing sites along lac operon; y-axis: mRNA abundance. Black squares: gyrase partial inhibition by 50 ng/μL novobiocin; Red dots: gyrase complete inhibition by 10 ng/μL norfloxacin. The result indicates non-stop transcription elongation upon positive supercoiling buildup on the DNA. The abundance of each mRNA part is normalized to its abundance under wild type condition, which is plotted as the flat curve. (B) Six sites on lac operon mRNA that were probed in the measurement of transcription elongation rate. (C) 500 ng/μL rifampicin was added into the cell culture at time zero to stop transcription initiation but not elongation. The abundance of different positions on the lac operon mRNA was probed by quantitative RT-PCR at multiple time points. (D) Transcription elongation rate decreased upon gyrase inhibition by 10 ng/μL norfloxacin in live E. coli cells. See also Figure S6.
Next we measured transcription elongation rate in live E. coli cells using transcription initiation inhibitor rifampicin (Epshtein and Nudler, 2003) and quantitative RT-PCR (Chen, H., Shiroguchi, K., Ge, H. and Xie, X.S., unpublished data). We added rifampicin to the cell culture at time zero, and measured the mRNA abundance in multiple regions (Figure 6B) along the transcript at multiple time points afterwards. While the mRNA abundance at the 5’ end decreased immediately upon the addition of rifampicin, the mRNA abundance downstream started to decrease after a time delay (Figure 6C). The distance between the two probes on the transcript divided by the time delay was the elongation rate. Gyrase inhibition was achieved by norfloxacin treatment where most cells were viable through the 14-min long rifampicin assay (Figure S6A). We found that the elongation rate of fully induced lac operon decreased by 46% upon gyrase inhibition (Figure 6D), similar to the result previously reported by Higgins group on Salmonella enterica (Rovinskiy et al., 2012).
A two-state model describes transcriptional bursting
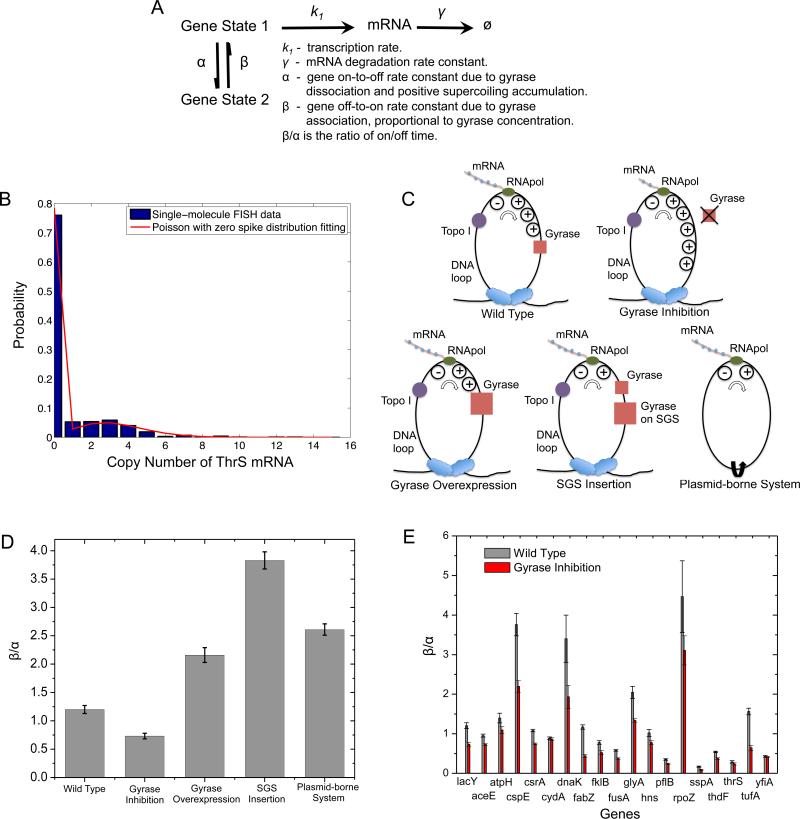
Transcriptional bursting has been described with a two-state model, but the origin of the two states was not understood (Golding et al., 2005; Munsky et al., 2012; So et al., 2011). We now understand the mechanism of bacterial transcriptional bursting (Figure 7A): the gene stochastically switches between on and off states due to release and accumulation of positive supercoiling. The “on” state (State 1) generates mRNAs with an average transcription rate k1, and the mRNAs degrade with rate constant γ. The “off” state (State 2) does not generate any mRNA. The interconversion rate constants between the two states are α and β. α is the gene on-to-off transition rate constant due to gyrase dissociation from the DNA loop and positive supercoiling accumulation. For simplicity, we assume positive supercoiling accumulation is fast, and gyrase dissociation is rate-limiting. β corresponds □ to the pseudo-first-order rate constant of □ □ □ □ □ □-DNA binding, which is also rate-limiting in the gene off-to-on transition and proportional to the effective intracellular gyrase concentration. The lower the effective gyrase concentration is, the longer the gene stays in the “off” state and the smaller the on/off duty cycle ratio (β/α), which should result in a higher extent of bursting reflected by a larger Fano factor and a larger fraction of cells that contain zero copy of mRNA at a given time point.
Figure 7. Dependence of on/off duty cycle ratio (β/α) on effective intracellular gyrase concentration.
(A) Kinetic scheme of the two-state model with relevant rate constants. (B) Fitting of cellular ThrS mRNA copy number distribution with “Poisson with zero spike” distribution. (C) Schematics of interactions between effective gyrase concentration and DNA supercoiling generated by transcription under different conditions. Upon gyrase inhibition, positive supercoiling accumulates on the chromosomal DNA loop to a higher extent than wild type. Gyrase overexpression or SGS insertion is the opposite. In a plasmid-borne expression module, positive and negative supercoiling annihilate each other due to the lack of topological barriers. (D) β/α of fully induced lac operon decreases upon gyrase partial inhibition by 50 ng/μL novobiocin treatment, increases upon gyrase overexpression, SGS insertion, and in a plasmid-borne system. (E) β/α of fully induced lac operon and other 18 highly transcribed E. coli genes. β/α of all the 19 genes decrease upon gyrase partial inhibition by 50 ng/μL novobiocin treatment. See also Figure S6, S7 and Table S1.
Transcription bursts of highly transcribed genes are reflected by the nonPoissonian mRNA copy number distribution. Under the condition that α and β are significantly smaller than k1 and γ □ □ previously observed (Golding et al., 2005), the steady-state mRNA copy number distribution for a population of cells is bimodal (Munsky et al., 2012), and can be approximated with a “Poisson with zero spike” distribution (Eq. 1). Based on the two-state model, the Fano factor (F) can be derived as Eq. 2 (Extended Experimental Procedures).
| (Eq. 1) |
| (Eq. 2) |
We note these results hold only under the condition that gyrase dissociation and rebinding are rate-limiting, longer than the time scales of positive supercoiling accumulation and release. Although this is a simplified model, it captures the origin of transcriptional bursting, i.e., gyrase dissociation, and establishes the gyrase concentration dependence of β, which can now be subject to in vivo experimental tests.
The dependence of transcriptional bursting on effective gyrase concentration revealed by single-molecule mRNA FISH assay
We now experimentally verify this model by measuring the steady-state mRNA copy number distribution in a population of isogenic E. coli cells under gyrase inhibition and overexpression conditions. We performed mRNA FISH assay with single-molecule sensitivity, using a single Atto 594-labeled 20-oligomer nucleotide probing the yfp sequence in an E. coli strain with the target gene fused to yfp sequence endogenously. By measuring the intensity of each fluorescent spot and counting the number of spots per cell, we determined cellular mRNA copy number for thousands of E. coli cells. The efficiency of our single-molecule mRNA FISH assay is ~90% (Taniguchi et al., 2010).
We measured the cellular mRNA copy number distribution of the fully induced lac operon and 18 highly transcribed genes from the YFP library that our group has constructed (Taniguchi et al., 2010). Partial gyrase inhibition was achieved by novobiocin treatment at low concentration without affecting normal bacterial growth and morphology (Figure S6B). We found that the cellular mRNA copy number distribution can be fitted well by the “Poisson with zero spike” distribution for the 19 genes with excellent coefficients of determination (Figure 7B, Table S1). The fitting allows estimation of the on/off duty cycle ratio of transcriptional bursting (β/α□ , with an error bar obtained by the bootstrapping method (Brad Efron, 1993).
For fully induced lac operon, β/α was 1.20 for wild type, decreased to 0.73 upon gyrase inhibition, increased to 2.16 upon gyrase overexpression, and increased to 3.83 when a strong gyrase site (SGS) was inserted next to the lac operon (Figure 7C&D). This result indicates that transcriptional bursting is sensitively dependent on the availability of gyrase to remove positive supercoiling accumulated during active transcription.
If lac operon is on a plasmid that lacks topological constraint, positive and negative supercoiling generated by transcription could diffuse along the circular DNA in opposite directions and annihilate each other (Figure 7C). As a critical control, a plasmid-borne system in E. coli indeed showed an even higher β/α□ than that of gyrase overexpression (Figure 7D).
One would expect β/α□ to be infinitely large if the gene is always “on” in the complete absence of positive supercoiling accumulation. Yet it was not the case for the plasmid-borne system since there could be weak and transient topological barriers on the plasmid DNA due to transient protein binding (Leng et al., 2011). To confirm this point, we performed control experiments on the same plasmid-borne system under gyrase inhibition and overexpression conditions. We found that β/α□ changed in the same direction as the chromosomal gene, but to a smaller extent. Under the same conditions, β/α□ of the plasmid-borne system was always higher than the chromosomal counterpart, since the plasmid has much lower topological barriers and thus more efficient removal of positive supercoiling during active transcription (Figure S6C).
Intriguingly, similar to the scenario of fully induced lac operon, all the other 18 genes showed a decreased β/α□ (Figure 7E) upon gyrase inhibition. In addition, most of the genes showed an increased Fano factor (Figure S7A) and an increased fraction of cells containing zero copy of mRNA (Figure S7B) upon gyrase inhibition. These findings are consistent with the prediction based on our model and demonstrate the ubiquitous effect of gyrase concentration on transcriptional bursting.
DISCUSSION
Mechanism of transcriptional bursting under induced condition revealed
Pertinent to the fact that there is only one (or two) copy of the gene in a cell, gene expression is stochastic. In recent years, stochastic gene expression has stimulated wide interest (Blake et al., 2003; Elowitz and Leibler, 2000; Elowitz et al., 2002; Ozbudak et al., 2002; Pedraza and Paulsson, 2008). Such stochasticity, or noise, causes phenotypic variability among genetically identical cells and organisms despite identical histories of environmental exposure (Choi et al., 2008; Maamar et al., 2007), and arises from the fact that DNA, mRNA and gene regulatory proteins can be present and active at only a few copies per cell. Due to the small copy numbers and the fact that stochastic gene expression cannot be synchronized among different cells, quantitative studies of gene expression at the single-cell level necessitates single-molecule sensitivity for mRNA and protein detection.
The stochastic gene expression dynamics of repressed genes have already been well studied and understood to date (Li and Xie, 2011). For highly expressed genes in both prokaryotic and eukaryotic organisms, bursting transcription has been demonstrated by a number of techniques, including single-molecule FISH assay that counts cellular mRNA copy number (Raj et al., 2006; Raj et al., 2010; Taniguchi et al., 2010; Zong et al., 2010), MS2 or PP7 technique that visualizes single mRNA production in real time (Chubb et al., 2006; Golding et al., 2005; Hocine et al., 2013; Larson et al., 2011; Lionnet et al., 2011; Muramoto et al., 2012), and fluorescent protein (Singh et al., 2010) or luciferase (Suter et al., 2011) as gene expression reporter in live cells. Nevertheless, the mechanism of this ubiquitous phenomenon under induced condition is not understood.
We note that the transcriptional bursting phenomenon studied in this paper is different from transcriptional pausing in prokaryotic and eukaryotic cells (Core et al., 2008; Landick, 2006; Weixlbaumer et al., 2013), which has been studied by recent single-molecule manipulation (Davenport et al., 2000; Herbert et al., 2006; Hodges et al., 2009; Ma et al., 2013; Shundrovsky et al., 2004) and RNA sequencing experiments (Churchman and Weissman, 2011; Core et al., 2008). While pausing describes intermittent elongation of a transcript, bursting describes discontinuous production of many transcripts over a much longer time scale and involves inhibition of both transcription initiation and elongation.
We have revealed the origin of stochastic transcriptional bursts in bacteria under induced conditions by conducting a series of in vitro and live-cell experiments, and demonstrated that reversible switching between different chromosomal supercoiling levels via gyrase dissociation from and rebinding to a DNA loop gives rise to bursting transcription. We note this is a fundamental mechanism pertinent to the chromosome structure and should be applicable to all the highly expressed genes in prokaryotic cells and even eukaryotic cells. However, the situation of eukaryotic cells is more complex than that of bacteria due to more complicated transcription regulation and the existence of nucleosomes (Raser and O'Shea, 2004).
A new role of DNA supercoiling in gene expression regulation
The interaction between DNA supercoiling and gene expression in bacteria has been investigated for decades. Our knowledge comes primarily from ensemble studies on the relationship between the global DNA supercoiling level and the gene expression level. On one hand, bacterial DNA supercoiling level affects the expression of a few E. coli genes called supercoiling-sensitive genes (Peter et al., 2004), as well as transcription elongation rate (Rovinskiy et al., 2012) due to a combined effect of torsional and bending stress sustained by the supercoiled DNA at the transcription site (Lionberger and Meyhofer, 2010; ten Heggeler-Bordier et al., 1992). On the other hand, local DNA supercoiling is generated by transcription, according to the “twin-domain model” developed by Wang and Liu groups in late 1980s (Deng et al., 2005; Leng et al., 2011; Lim et al., 2003; Liu and Wang, 1987; Samul and Leng, 2007; Tsao et al., 1989; Wu et al., 1988). Here we report a new role of DNA supercoiling in gene expression regulation that can only be revealed by single-molecule and single-cell approaches: transient DNA supercoiling generated locally during active transcription gives rise to transcriptional bursting, which is a major source of gene expression noise that causes cell-to-cell variability in an isogenic population. Although earlier work proposed DNA supercoiling can be involved in bursting transcription (Mitarai et al., 2008; So et al., 2011), we have experimentally proved that supercoiling dynamics is the primary origin of transcriptional bursting.
Novel in vitro single-molecule transcription assay
In order to investigate the effect of positive supercoiling buildup on transcription elongation and initiation in a clean and controlled fashion, we developed an in vitro single-molecule assay that could monitor real-time transcription on individual DNA templates. We note our assay is different from other existing in vitro transcription assays using single-molecule manipulation (Abbondanzieri et al., 2005; Bai et al., 2006; Billingsley et al., 2012; Bustamante et al., 2011; Herbert et al., 2008) or single-molecule fluorescence imaging (Chakraborty et al., 2012; Friedman and Gelles, 2012; Kapanidis et al., 2006; Revyakin et al., 2012; Tang et al., 2009; Zhang et al., 2014). Our assay uses RNA staining so that the elongation process on templates with any sequence can be easily monitored for multiple rounds of transcription on each template. This is a high-throughput measurement because hundreds of templates in one field of view can be monitored simultaneously. The new assay will be generally useful for studying other questions in transcription, such as pausing and termination kinetics.
Other possible mechanisms of transcriptional bursting
The current report proves that stochastic changes of supercoiling level in DNA segments due to gyrase dissociation and rebinding is a main mechanism that gives rise to bursting transcription of highly expressed genes in bacteria. However, we note there could be other possible causes of bacterial transcriptional bursting, such as the change of chromosomal looping structure due to the dissociation and rebinding of nucleoid-associated proteins, as well as facilitated transcription reinitiation due to dynamical gene looping, where an operon DNA places its promoter and terminator in spatial proximity (Hebenstreit, 2013). Although they might cause transcription rate fluctuations in addition to the supercoiling effect that we observed, none of these alternative mechanisms have been experimentally proved to switch genes on and off.
EXPERIMENTAL PROCEDURES
In Vitro Single-Molecule Transcription Assay
To measure transcription elongation rates, T7 RNApol (New England Biolabs) or E. coli RNApol (Epicentre), NTPs, 250 nM SYTO RNASelect and an oxygen scavenger system were added to transcription buffer. After infusing the mixture into the flow cell containing immobilized DNA templates, a fluorescent movie was recorded under 488 nm laser excitation at 0.22 W/cm2. Images were taken every 20 seconds for 60-80 minutes and the acquisition time of each image was 5 seconds.
To measure transcription initiation rates, the reaction mixture was the same as that for elongation rate measurements except that higher concentrations of RNApol and NTPs were used. The excitation power density was 0.15 W/cm2. Images were taken every 75 seconds with 5 second of image acquisition time.
DNA Staining Assay
In order to locate the linear and circular templates in the field of view, 100 nM SYTOX Orange (Life Technologies) in 50 mM Tris-HCl buffer (pH 8.0) was used to stain the immobilized DNAs after transcription movies were recorded. Fluorescent movies were recorded under 532 nm laser excitation with a power density above 4 W/cm2. The image acquisition time was 0.3 second. The imaging buffer was kept flowing at 8 mL/hr by a syringe pump (PhD 2000, Harvard Apparatus) during the movie recording.
Single-Molecule mRNA FISH Assay
The BW25993 E. coli cells were grown in M9 medium with 0.4% glycerol, amino acids and vitamins, together with antibiotics and saturating amount of IPTG if necessary. The cells were subsequently inoculated 1:500 into the same medium and incubated for ~7 hrs at 37°C with 250 rpm shaking till OD600nm reached 0.2~0.3. 50 ng/μL novobiocin (Sigma) was added into the medium and incubated for another 2 hrs before harvest. 2 hrs was long enough (several cell cycles) to allow all the cells to enter steady state, and thus minimized potential cell-to-cell variation due to different transition kinetics in response to the drug treatment.
The YFP library strains were grown in LB medium with chloramphenicol at 30 °C. The cells were subsequently inoculated 1:400 into M9 medium with 0.4% glucose, amino acids and vitamins, and incubated for 11 hrs at 30°C with 250 rpm shaking till OD600nm reached 0.2~0.3. 50 ng/μL novobiocin was added and incubated for another 2 hrs before harvest.
Single-molecule mRNA FISH assay was performed as previously described (Taniguchi et al., 2010) using Venus495r mRNA FISH probe covalently linked to a dye molecule Atto 594 (Sigma-Aldrich). The images were taken under epi-illumination by a fiber laser at 580 nm and phase contrast illumination by a halogen lamp.
Supplementary Material
ACKNOWLEDGMENTS
We thank Xiaowei Zhuang for the collaboration on bacterial chromosomal structure study which prompted us to conduct the current study; N. Patrick Higgins for providing the plasmid containing strong gyrase site sequence; Gene-Wei Li for development of FISH protocol; Minbiao Ji for help with microscope construction; Rahul Roy for advice on data analysis; and James Wang, Long Cai, Gene-Wei Li, and Paul Choi for critical reading of the manuscript. This work was supported by NIH Pioneer Award ( DP1 OD0000277, X.S.X.), NIH grant TR01 (5R01GM096450-02, X.S.X.), National Science Foundation of China (21373021, H.G.), and the Foundation for the Author of National Excellent Doctoral Dissertation of China (201119, H.G.).
Footnotes
AUTHOR CONTRIBUTIONS
X.S.X. conceived the project and supervised the experiments. S.C. developed the in vitro single-molecule transcription assay. S.C. performed the in vitro imaging experiments, data analysis and biophysical calculations based on the in vitro data. S.C. and C.C. performed the control of the enzyme activities in the in vitro single-molecule transcription assay. C.C. performed the single-molecule mRNA FISH assay, data analysis and live-cell experiments based on quantitative RTPCR. C.C. made the DNA constructs for the in vitro single-molecule assay and the FISH assay. H.G. built the mathematical model. H.G., C.C. and S.C fitted the model to the single-molecule FISH data. S.C., C.C. and X.S.X. designed the experiments and wrote the manuscript.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Experimental Procedures, seven figures and one table and can be found with this article online.
References
- Abbondanzieri EA, Greenleaf WJ, Shaevitz JW, Landick R, Block SM. Direct observation of base-pair stepping by RNA polymerase. Nature. 2005;438:460–465. doi: 10.1038/nature04268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Santangelo TJ, Wang MD. Single-molecule analysis of RNA polymerase transcription. Annu Rev Biophys Biomol Struct. 2006;35:343–360. doi: 10.1146/annurev.biophys.35.010406.150153. [DOI] [PubMed] [Google Scholar]
- Baker TA, Funnell BE, Kornberg A. Helicase action of dnaB protein during replication from the Escherichia coli chromosomal origin in vitro. J Biol Chem. 1987;262:6877–6885. [PubMed] [Google Scholar]
- Billingsley DJ, Bonass WA, Crampton N, Kirkham J, Thomson NH. Single-molecule studies of DNA transcription using atomic force microscopy. Phys Biol. 2012;9:021001. doi: 10.1088/1478-3975/9/2/021001. [DOI] [PubMed] [Google Scholar]
- Blake WJ, M KA, Cantor CR, Collins JJ. Noise in eukaryotic gene expression. Nature. 2003;422:633–637. doi: 10.1038/nature01546. [DOI] [PubMed] [Google Scholar]
- Brad Efron RT. An Introduction to the Bootstrap. CRC Press; 1993. [Google Scholar]
- Bustamante C, Cheng W, Meija YX. Revisiting the central dogma one molecule at a time. Cell. 2011;144:480–497. doi: 10.1016/j.cell.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, Wang D, Ebright YW, Korlann Y, Kortkhonjia E, Kim T, Chowdhury S, Wigneshweraraj S, Irschik H, Jansen R, et al. Opening and closing of the bacterial RNA polymerase clamp. Science. 2012;337:591–595. doi: 10.1126/science.1218716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng BK, Zhu CX, Ji CL, Ahumada A, Tse-Dinh YC. Direct interaction between Escherichia coli RNA polymerase and the zinc ribbon domains of DNA topoisomerase I. J Biol Chem. 2003;278:30705–30710. doi: 10.1074/jbc.M303403200. [DOI] [PubMed] [Google Scholar]
- Choi PJ, Cai L, Frieda K, Xie XS. A stochastic single-molecule event triggers phenotype switching of a bacterial cell. Science. 2008;322:442–446. doi: 10.1126/science.1161427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb JR, Trcek T, Shenoy SM, Singer RH. Transcriptional pulsing of a developmental gene. Curr Biol. 2006;16:1018–1025. doi: 10.1016/j.cub.2006.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchman LS, Weissman JS. Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature. 2011;469:368–373. doi: 10.1038/nature09652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322:1845–1848. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport RJ, Wuite GJ, Landick R, Bustamante C. Single-molecule study of transcriptional pausing and arrest by E. coli RNA polymerase. Science. 2000;287:2497–2500. doi: 10.1126/science.287.5462.2497. [DOI] [PubMed] [Google Scholar]
- Deng S, Stein RA, Higgins NP. Transcription-induced barriers to supercoil diffusion in the Salmonella typhimurium chromosome. Proc Natl Acad Sci USA. 2004;101:3398–3403. doi: 10.1073/pnas.0307550101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S, Stein RA, Higgins NP. Organization of supercoil domains and their reorganization by transcription. Mol Microbiol. 2005;57:1511–1521. doi: 10.1111/j.1365-2958.2005.04796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K. Control of bacterial DNA supercoiling. Mol Microbiol. 1992;6:425–433. doi: 10.1111/j.1365-2958.1992.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Drolet M. Growth inhibition mediated by excess negative supercoiling: the interplay between transcription elongation, R-loop formation and DNA topology. Mol Microbiol. 2006;59:723–730. doi: 10.1111/j.1365-2958.2005.05006.x. [DOI] [PubMed] [Google Scholar]
- El Hanafi D, Bossi L. Activation and silencing of leu-500 promoter by transcription-induced DNA supercoiling in the Salmonella chromosome. Mol Microbiol. 2000;37:583–594. doi: 10.1046/j.1365-2958.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- Elowitz MB, Leibler S. A synthetic oscillatory network of transcriptional regulators. Nature. 2000;403:335–338. doi: 10.1038/35002125. [DOI] [PubMed] [Google Scholar]
- Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- Epshtein V, Nudler E. Cooperation between RNA polymerase molecules in transcription elongation. Science. 2003;300:801–805. doi: 10.1126/science.1083219. [DOI] [PubMed] [Google Scholar]
- Franco RJ, Drlica K. DNA Gyrase on the Bacterial Chromosome - Oxolinic Acid-Induced DNA Cleavage in the Dnaa-Gyrb Region. J Mol Biol. 1988;201:229–233. doi: 10.1016/0022-2836(88)90449-4. [DOI] [PubMed] [Google Scholar]
- Friedman LJ, Gelles J. Mechanism of transcription initiation at an activator-dependent promoter defined by single-molecule observation. Cell. 2012;148:679–689. doi: 10.1016/j.cell.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding I, Paulsson J, Zawilski SM, Cox EC. Real-time kinetics of gene activity in individual bacteria. Cell. 2005;123:1025–1036. doi: 10.1016/j.cell.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Gore J, Bryant Z, Stone MD, Nollmann M, Cozzarelli NR, Bustamante C. Mechanochemical analysis of DNA gyrase using rotor bead tracking. Nature. 2006;439:100–104. doi: 10.1038/nature04319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guptasarma P. Cooperative relaxation of supercoils and periodic transcriptional initiation within polymerase batteries. Bioessays. 1996;18:325–332. doi: 10.1002/bies.950180411. [DOI] [PubMed] [Google Scholar]
- Hardy CD, Cozzarelli NR. A genetic selection for supercoiling mutants of Escherichia coli reveals proteins implicated in chromosome structure. Mol Microbiol. 2005;57:1636–1652. doi: 10.1111/j.1365-2958.2005.04799.x. [DOI] [PubMed] [Google Scholar]
- Hebenstreit D. Are gene loops the cause of transcriptional noise? Trends Genet. 2013;29:333–338. doi: 10.1016/j.tig.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Herbert KM, Greenleaf WJ, Block SM. Single-molecule studies of RNA polymerase: motoring along. Annu Rev Biochem. 2008;77:149–176. doi: 10.1146/annurev.biochem.77.073106.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert KM, La Porta A, Wong BJ, Mooney RA, Neuman KC, Landick R, Block SM. Sequence-resolved detection of pausing by single RNA polymerase molecules. Cell. 2006;125:1083–1094. doi: 10.1016/j.cell.2006.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins NP, Cozzarelli NR. The binding of gyrase to DNA: analysis by retention by nitrocellulose filters. Nucleic Acids Res. 1982;10:6833–6847. doi: 10.1093/nar/10.21.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins NP, Peebles CL, Sugino A, Cozzarelli NR. Purification of subunits of Escherichia coli DNA gyrase and reconstitution of enzymatic activity. Proc Natl Acad Sci U S A. 1978;75:1773–1777. doi: 10.1073/pnas.75.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocine S, Raymond P, Zenklusen D, Chao JA, Singer RH. Single-molecule analysis of gene expression using two-color RNA labeling in live yeast. Nat Methods. 2013;10:119–121. doi: 10.1038/nmeth.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges C, Bintu L, Lubkowska L, Kashlev M, Bustamante C. Nucleosomal fluctuations govern the transcription dynamics of RNA polymerase II. Science. 2009;325:626–628. doi: 10.1126/science.1172926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannemeier C, Shibamiya A, Nakazawa F, Trusheim H, Ruppert C, Markart P, Song Y, Tzima E, Kennerknecht E, Niepmann M, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci USA. 2007;104:6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapanidis AN, Margeat E, Ho SO, Kortkhonjia E, Weiss S, Ebright RH. Initial transcription by RNA polymerase proceeds through a DNA-scrunching mechanism. Science. 2006;314:1144–1147. doi: 10.1126/science.1131399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kussell E, Leibler S. Phenotypic diversity, population growth, and information in fluctuating environments. Science. 2005;309:2075–2078. doi: 10.1126/science.1114383. [DOI] [PubMed] [Google Scholar]
- Landick R. The regulatory roles and mechanism of transcriptional pausing. Biochemical Society transactions. 2006;34:1062–1066. doi: 10.1042/BST0341062. [DOI] [PubMed] [Google Scholar]
- Larson DR, Zenklusen D, Wu B, Chao JA, Singer RH. Real-time observation of transcription initiation and elongation on an endogenous yeast gene. Science. 2011;332:475–478. doi: 10.1126/science.1202142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng F, Chen B, Dunlap DD. Dividing a supercoiled DNA molecule into two independent topological domains. Proc Natl Acad Sci USA. 2011;108:19973–19978. doi: 10.1073/pnas.1109854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GW, Xie XS. Central dogma at the single-molecule level in living cells. Nature. 2011;475:308–315. doi: 10.1038/nature10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HM, Lewis DE, Lee HJ, Liu M, Adhya S. Effect of varying the supercoiling of DNA on transcription and its regulation. Biochemistry. 2003;42:10718–10725. doi: 10.1021/bi030110t. [DOI] [PubMed] [Google Scholar]
- Lionberger TA, Meyhofer E. Bending the rules of transcriptional repression: tightly looped DNA directly represses T7 RNA polymerase. Biophys J. 2010;99:1139–1148. doi: 10.1016/j.bpj.2010.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionnet T, Czaplinski K, Darzacq X, Shav-Tal Y, Wells AL, Chao JA, Park HY, de Turris V, Lopez-Jones M, Singer RH. A transgenic mouse for in vivo detection of endogenous labeled mRNA. Nat Methods. 2011;8:165–170. doi: 10.1038/nmeth.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LF, Wang JC. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci USA. 1987;84:7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AS, Wang JC. Anchoring of DNA to the bacterial cytoplasmic membrane through cotranscriptional synthesis of polypeptides encoding membrane proteins or proteins for export: a mechanism of plasmid hypernegative supercoiling in mutants deficient in DNA topoisomerase I. J Bacteriol. 1993;175:1645–1655. doi: 10.1128/jb.175.6.1645-1655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Bai L, Wang MD. Transcription under torsion. Science. 2013;340:1580–1583. doi: 10.1126/science.1235441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maamar H, Raj A, Dubnau D. Noise in gene expression determines cell fate in Bacillus subtilis. Science. 2007;317:526–529. doi: 10.1126/science.1140818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell A, Gellert M. The DNA Dependence of the Atpase Activity of DNA Gyrase. J Biol Chem. 1984;259:4472–4480. [PubMed] [Google Scholar]
- Mitarai N, Dodd IB, Crooks MT, Sneppen K. The Generation of Promoter-Mediated Transcriptional Noise in Bacteria. PLoS Comput Biol. 2008;4:e1000109. doi: 10.1371/journal.pcbi.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A, Cozzarelli NR. Contacts between DNA Gyrase and Its Binding-Site on DNA - Features of Symmetry and Asymmetry Revealed by Protection from Nucleases. Proc Natl Acad Sci USA. 1981;78:1416–1420. doi: 10.1073/pnas.78.3.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsky B, Neuert G, van Oudenaarden A. Using Gene Expression Noise to Understand Gene Regulation. Science. 2012;336:183–187. doi: 10.1126/science.1216379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto T, Cannon D, Gierlinski M, Corrigan A, Barton GJ, Chubb JR. Live imaging of nascent RNA dynamics reveals distinct types of transcriptional pulse regulation. Proc Natl Acad Sci U S A. 2012;109:7350–7355. doi: 10.1073/pnas.1117603109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbudak EM, Thattai M, Kurtser I, Grossman AD, van Oudenaarden A. Regulation of noise in the expression of a single gene. Nat Genet. 2002;31:69–73. doi: 10.1038/ng869. [DOI] [PubMed] [Google Scholar]
- Pedraza JM, Paulsson J. Effects of molecular memory and bursting on fluctuations in gene expression. Science. 2008;319:339–343. doi: 10.1126/science.1144331. [DOI] [PubMed] [Google Scholar]
- Peter BJ, Arsuaga J, Breier AM, Khodursky AB, Brown PO, Cozzarelli NR. Genomic transcriptional response to loss of chromosomal supercoiling in Escherichia coli. Genome Biol. 2004;5:R87. doi: 10.1186/gb-2004-5-11-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow L, Hardy CD, Arsuaga J, Cozzarelli NR. Topological domain structure of the Escherichia coli chromosome. Gene Dev. 2004;18:1766–1779. doi: 10.1101/gad.1207504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, Peskin CS, Tranchina D, Vargas DY, Tyagi S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 2006;4:e309. doi: 10.1371/journal.pbio.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, Rifkin SA, Andersen E, van Oudenaarden A. Variability in gene expression underlies incomplete penetrance. Nature. 2010;463:913–918. doi: 10.1038/nature08781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raser JM, O'Shea EK. Control of stochasticity in eukaryotic gene expression. Science. 2004;304:1811–1814. doi: 10.1126/science.1098641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau DC, Gellert M, Thoma F, Maxwell A. Structure of the DNA Gyrase DNA Complex as Revealed by Transient Electric Dichroism. J Mol Biol. 1987;193:555–569. doi: 10.1016/0022-2836(87)90266-x. [DOI] [PubMed] [Google Scholar]
- Reece RJ, Maxwell A. DNA Gyrase - Structure and Function. Crit Rev Biochem Mol. 1991;26:335–375. doi: 10.3109/10409239109114072. [DOI] [PubMed] [Google Scholar]
- Revyakin A, Ebright RH, Strick TR. Promoter unwinding and promoter clearance by RNA polymerase: detection by single-molecule DNA nanomanipulation. Proc Natl Acad Sci USA. 2004;101:4776–4780. doi: 10.1073/pnas.0307241101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revyakin A, Zhang ZJ, Coleman RA, Li Y, Inouye C, Lucas JK, Park SR, Chu S, Tjian R. Transcription initiation by human RNA polymerase II visualized at single-molecule resolution. Gene Dev. 2012;26:1691–1702. doi: 10.1101/gad.194936.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovinskiy N, Agbleke AA, Chesnokova O, Pang Z, Higgins NP. Rates of gyrase supercoiling and transcription elongation control supercoil density in a bacterial chromosome. PLoS Genet. 2012;8:e1002845. doi: 10.1371/journal.pgen.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samul R, Leng F. Transcription-coupled hypernegative supercoiling of plasmid DNA by T7 RNA polymerase in Escherichia coli topoisomerase I-deficient strains. J Mol Biol. 2007;374:925–935. doi: 10.1016/j.jmb.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shundrovsky A, Santangelo TJ, Roberts JW, Wang MD. A single-molecule technique to study sequence-dependent transcription pausing. Biophys J. 2004;87:3945–3953. doi: 10.1529/biophysj.104.044081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Razooky B, Cox CD, Simpson ML, Weinberger LS. Transcriptional bursting from the HIV-1 promoter is a significant source of stochastic noise in HIV-1 gene expression. Biophys J. 2010;98:L32–34. doi: 10.1016/j.bpj.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner GM, Baumann CG, Quinn DM, Molloy JE, Hoggett JG. Promoter binding, initiation, and elongation by bacteriophage T7 RNA polymerase. A single-molecule view of the transcription cycle. J Biol Chem. 2004;279:3239–3244. doi: 10.1074/jbc.M310471200. [DOI] [PubMed] [Google Scholar]
- Snyder M, Drlica K. DNA Gyrase on the Bacterial Chromosome - DNA Cleavage Induced by Oxolinic Acid. J Mol Biol. 1979;131:287–302. doi: 10.1016/0022-2836(79)90077-9. [DOI] [PubMed] [Google Scholar]
- So LH, Ghosh A, Zong C, Sepulveda LA, Segev R, Golding I. General properties of transcriptional time series in Escherichia coli. Nat Genet. 2011;43:554–560. doi: 10.1038/ng.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter DM, Molina N, Gatfield D, Schneider K, Schibler U, Naef F. Mammalian genes are transcribed with widely different bursting kinetics. Science. 2011;332:472–474. doi: 10.1126/science.1198817. [DOI] [PubMed] [Google Scholar]
- Tang GQ, Roy R, Bandwar RP, Ha T, Patel SS. Real-time observation of the transition from transcription initiation to elongation of the RNA polymerase. Proc Natl Acad Sci U S A. 2009;106:22175–22180. doi: 10.1073/pnas.0906979106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi Y, Choi PJ, Li GW, Chen H, Babu M, Hearn J, Emili A, Xie XS. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science. 2010;329:533–538. doi: 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Heggeler-Bordier B, Wahli W, Adrian M, Stasiak A, Dubochet J. The apical localization of transcribing RNA polymerases on supercoiled DNA prevents their rotation around the template. Embo J. 1992;11:667–672. doi: 10.1002/j.1460-2075.1992.tb05098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thattai M, van Oudenaarden A. Stochastic gene expression in fluctuating environments. Genetics. 2004;167:523–530. doi: 10.1534/genetics.167.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao YP, Wu HY, Liu LF. Transcription-driven supercoiling of DNA: direct biochemical evidence from in vitro studies. Cell. 1989;56:111–118. doi: 10.1016/0092-8674(89)90989-6. [DOI] [PubMed] [Google Scholar]
- Wang W, Li GW, Chen C, Xie XS, Zhuang X. Chromosome organization by a nucleoid-associated protein in live bacteria. Science. 2011;333:1445–1449. doi: 10.1126/science.1204697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weixlbaumer A, Leon K, Landick R, Darst SA. Structural basis of transcriptional pausing in bacteria. Cell. 2013;152:431–441. doi: 10.1016/j.cell.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmott CJ, Critchlow SE, Eperon IC, Maxwell A. The complex of DNA gyrase and quinolone drugs with DNA forms a barrier to transcription by RNA polymerase. J Mol Biol. 1994;242:351–363. doi: 10.1006/jmbi.1994.1586. [DOI] [PubMed] [Google Scholar]
- Wolf DM, Vazirani VV, Arkin AP. Diversity in times of adversity: probabilistic strategies in microbial survival games. J Theor Biol. 2005;234:227–253. doi: 10.1016/j.jtbi.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Wu HY, Shyy SH, Wang JC, Liu LF. Transcription generates positively and negatively supercoiled domains in the template. Cell. 1988;53:433–440. doi: 10.1016/0092-8674(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Revyakin A, Grimm JB, Lavis LD, Tjian R, Kadonaga JT. Single-molecule tracking of the transcription cycle by sub-second RNA detection. eLife. 2014;3:e01775. doi: 10.7554/eLife.01775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong C, So LH, Sepulveda LA, Skinner SO, Golding I. Lysogen stability is determined by the frequency of activity bursts from the fate-determining gene. Mol Syst Biol. 2010;6:440. doi: 10.1038/msb.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.