Abstract
Objective
To investigate whether resting body temperature is elevated and linked to fatigue in patients with relapsing-remitting multiple sclerosis (RRMS).
Design
Cross-sectional study investigating (a) differences in resting body temperature across RRMS, SPMS, and healthy groups, and (b) the relationship between body temperature and fatigue in RRMS patients.
Setting
Climate-controlled laboratory (~22°C) within a non-profit medical rehabilitation research center.
Participants
Fifty patients with RRMS, 40 matched healthy controls, and 22 patients with secondary-progressive MS (SPMS).
Intervention
None.
Main Outcome Measure(s)
Body temperature was measured with an aural infrared thermometer (normal body temperature for this thermometer is 36.75°C), and differences were compared across RRMS, SPMS, and healthy persons. RRMS patients completed measures of general fatigue (Fatigue Severity Scale; FSS), as well as physical and cognitive fatigue (Modified Fatigue Impact Scale; MFIS).
Results
There was a large effect of group (p<.001, ηp2=.132) whereby body temperature was higher in RRMS patients (37.04°C±0.27) relative to healthy controls (36.83 ± 0.33; p = .009) and SPMS patients (36.75°C±0.39; p=.001). Warmer body temperature in RRMS patients was associated with worse general fatigue (FSS; rp=.315, p=.028) and physical fatigue (pMFIS; rp=.318, p=.026), but not cognitive fatigue (cMIFS; rp=−.017, p=.909).
Conclusions
These are the first-ever demonstrations that body temperature is elevated endogenously in RRMS patients, and linked to worse fatigue. We discuss these findings in the context of failed treatments for fatigue in RRMS, including several failed randomized controlled trials (RCTs) of stimulants (modafinil). In contrast, our findings may help explain how RCTs of cooling garments and antipyretics (aspirin) have effectively reduced MS fatigue, and encourage further research on cooling/antipyretic treatments of fatigue in RRMS.
Keywords: Multiple sclerosis, relapsing-remitting multiple sclerosis, fatigue, body temperature, inflammation, aspirin
Fatigue is among the most prevalent, debilitating, and difficult to treat symptoms of relapsing-remitting multiple sclerosis (RRMS),1 a chronic autoimmune disease characterized by inflammatory lesions within the central nervous system. Fatigue in RRMS patients is temporarily worsened when body temperature is elevated experimentally through heat exposure.2 Elevated body temperature has been recognized as a trigger of RRMS symptoms since 1889, when Wilhelm Uhthoff first observed worsened vision in patients after warm baths and exercise. In the intervening 120+ years, studies have confirmed Uhthoff’s Phenomenon by experimentally raising body temperature in MS patients (e.g., hot baths, steaming saunas) and observing worsened symptoms,2–4 including fatigue.2 These experimental studies have established a causal link between heat and MS fatigue; however, no one has investigated whether body temperature is elevated endogenously (without heat exposure) and linked to fatigue in RRMS. Elevated temperature and fatigue are common consequences of systemic inflammation generally (i.e., sickness behavior5) and may also result from the inflammatory processes of RRMS. Here, we investigate whether body temperature is (a) elevated in RRMS patients relative to healthy controls, and (b) correlated with fatigue.
Many patients with RRMS eventually convert to a secondary-progressive phase of the disease (SPMS) characterized by an abatement of disease-related inflammatory processes, resulting in the cessation/reduction of clinical exacerbations and the absence/reduction of inflammatory lesions.6 In addition to comparing body temperature between RRMS patients and healthy persons, we also examined temperature in SPMS patients. Inclusion of SPMS patients provides a clinical control condition similar to RRMS in many ways (i.e., both have relapse-onset MS), except that disease-related inflammatory processes have abated in SPMS patients. As such, if elevated temperature is related to inflammatory processes, then body temperature should be (a) higher among RRMS patients relative to both healthy persons and SPMS patients, and (b) similar between SPMS patients and healthy persons.
METHODS
Subject enrollment
Subjects were 50 RRMS patients7 (46 women) without an exacerbation in the last six weeks, no current corticosteroid or antipyretic use, and no history of other neurologic or inflammatory disease. Mean age was 47.8±8.9 years, with mean disease duration of 12.8±8.0 years. Forty healthy controls were also recruited as a comparison group (age: 46.0±11.2 years; 37 women), with no differences in age (t[88] = 0.85, p = .400) or sex (χ2 = .01, p = .930). A second comparison group consisted of 22 SPMS patients (age: 53.8±7.4; 14 women; disease duration: 17.6 ± 7.4) also participated, and met all aforementioned inclusion criteria (e.g., no antipyretic use). Consistent with SPMS versus RRMS generally,8 our SPMS sample was older (t[70] = 2.76, p = .007), had a higher proportion of men (χ2 = 8.85, p = .003), and had a longer disease course (t[70] = 2.38, p = .020) than our RRMS sample.
MS patients were recruited from local clinical MS centers within the New York Metropolitan Area, and healthy controls were recruited from the local community. This study was approved by the Kessler Foundation Institutional Review Board, and all patients provided written informed consent prior to enrollment.
Setting
All subjects were seated within a climate-controlled research laboratory (~22.0°C) for at least thirty-minutes prior to participating to minimize any immediate effects of physical exercise or outdoor temperature on their body temperature or fatigue.
Core Body Temperature
Core body temperature was recorded aurally with an infrared thermometer.a Consistent with previous reports that normal body temperature (including infrared temperature) is actually below 37.0°C (98.6°F),9–10 normal temperature for persons aged 16 to 65 years reported by the manufacturer for the Braun IRT range from 35.9°C (96.6°F) to 37.6°C (99.7°F).10 Test-retest reliability of thermometer measurements was high within our sample (r =. 78). We used the first recording for subsequent analyses.
Fatigue
Fatigue was assessed in RRMS patients with two widely used measures of fatigue: the Fatigue Severity Scale (FSS)11 and the Modified Fatigue Impact Scale (MFIS).12 The FSS consists of nine fatigue symptoms endorsed on a scale from 1 (strongly disagree) to 7 (strongly agree). The FSS is the most commonly used scale of fatigue in MS, and yields one total score (mean endorsement across items; higher scores indicate worse fatigue). The MFIS consists of 21 fatigue symptoms endorsed on a scale from 0 (never) to 4 (almost always). The MFIS provides separate scores for cognitive fatigue (possible range = 0 to 40) and physical fatigue (possible range = 0 to 36), which allows us to investigate whether temperature is related more to one type of fatigue. (There is also a psychosocial fatigue composite which we do not use, with a possible range of 0 to 8). Both the FSS and MFIS demonstrate adequate reliability in MS patients.13 Self-reported fatigue correlates with depression,1 likely due to over-reporting of fatigue symptoms by patients with lower mood. As such, we also administered the Beck Depression Inventory, Second Edition (BDI-II) to control for the mood-related aspect of self-reported fatigue.
Primary Statistical Analyses
All analyses were performed with the IBM SPSS software, version 21 (www.ibm.com/software/analytics/spss).
Core body temperature
We first performed an analysis of variance (ANOVA) to investigate differences in body temperature (dependent variable) across groups (RRMS, healthy controls, SPMS). Pairwise comparisons between groups were controlled for multiple comparisons using the Bonferroni method. Next, we performed three one-sample t-tests to investigate whether groups differed from normal body temperature readings for the IR aural thermometer used in this study (normal range for persons aged 11–65 years: 35.9 – 37.6 °C; test value for our analyses: 36.75°C).
Fatigue in RRMS patients
We first performed one sample t-tests to investigate whether RRMS patients reported more general fatigue (FSS), physical fatigue (pMFIS), and cognitive fatigue (cMFIS) relative to published normative data.12 Next, we performed two-tailed partial correlations between body temperature and FSS, pMFIS, and cMFIS, controlling for depressive symptomology (BDI-II). We controlled for multiple comparisons by performing two-tailed analyses despite our a priori hypothesis that higher temperature would be associated with higher (worse) fatigue.
RESULTS
Core Body Temperature
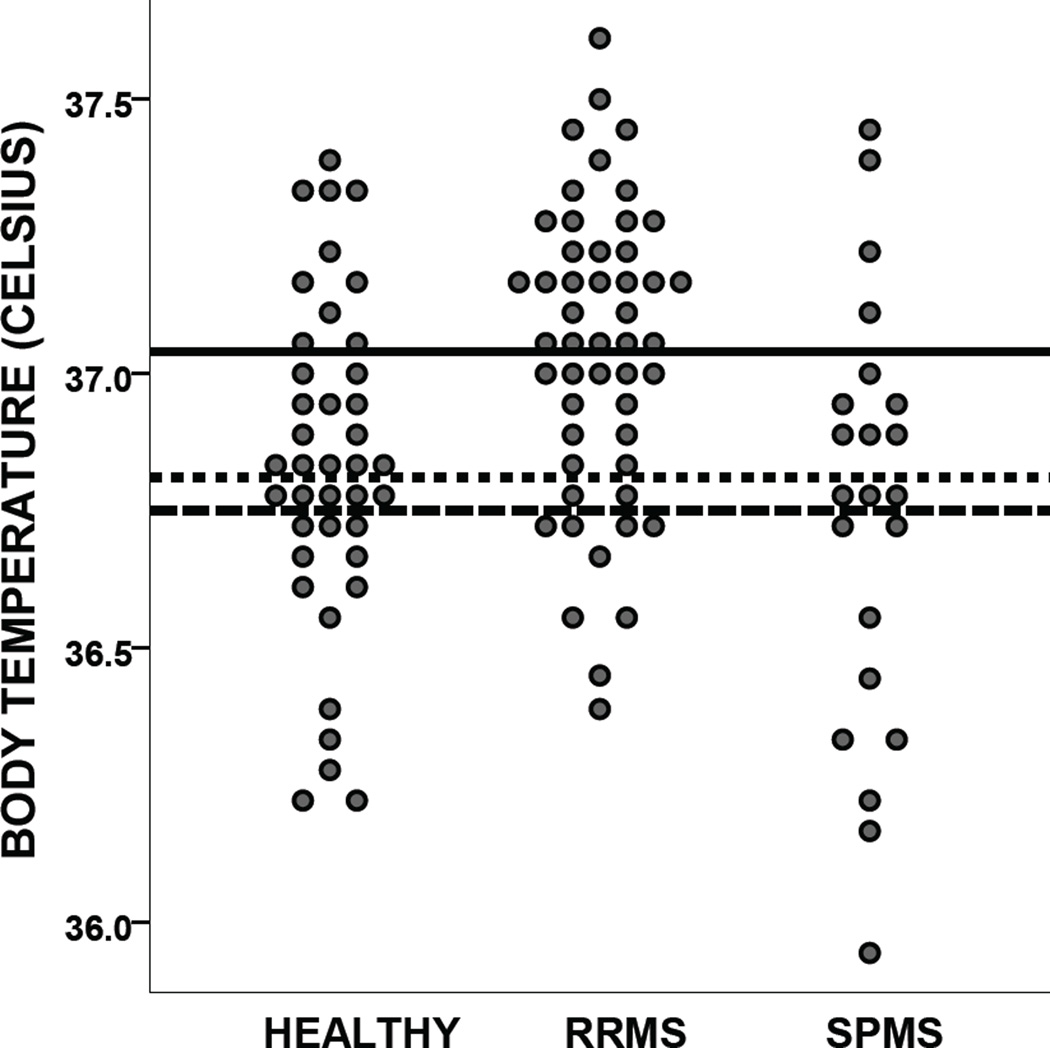
Analysis of variance indicated a large main effect of group on body temperature (F[2, 109] = 8.31, p < .001, ηp2 = .132). Pairwise comparisons revealed higher body temperature among RRMS patients (mean ± standard deviation: 37.04 ± 0.27) relative to healthy controls (36.83 ± 0.33; p = .009) and SPMS patients (36.75 ± 0.39; p = .001). There was no difference between healthy controls and SPMS patients (p = .840).As illustrated in Figure 1, the distribution of body temperatures is shifted upward among RRMS patients relative to healthy persons and SPMS patients.
Figure 1.
Body temperature in healthy persons, RRMS patients, and SPMS patients . Lines indicate the mean body temperature for healthy controls (dotted), RRMS patients (solid), and SPMS patients (dashed). Note that the published normative temperature for the thermometer used in the study is 36.75°C.
Body temperature was higher in RRMS patients (t[49]=7.42, p<.001) but not healthy controls (t[39]=1.88, p=.068) or SPMS patients (t[21]=0.00, p = 1.00) relative to normative data for the thermometer (36.75°C).
Supplemental analysis
Body temperature tends to be higher among women relative to men,9–10 and higher among younger relative to older persons.10 Our RRMS and healthy control groups were very well-matched for age and sex, but the SPMS group was older with proportionately more men than the RRMS group. As such, we performed an analysis of covariance to investigate differences in body temperature across groups while controlling for age and sex. The large main effect of group on body temperature remained (F[4, 108] = 6.62, p = .002, ηp2 = .110), with RRMS patients still warmer than both healthy persons (p = .008) and SPMS patients (p = ..016). Body temperature of SPMS patients remained comparable to healthy persons (p = 1.00).
Fatigue in RRMS patients
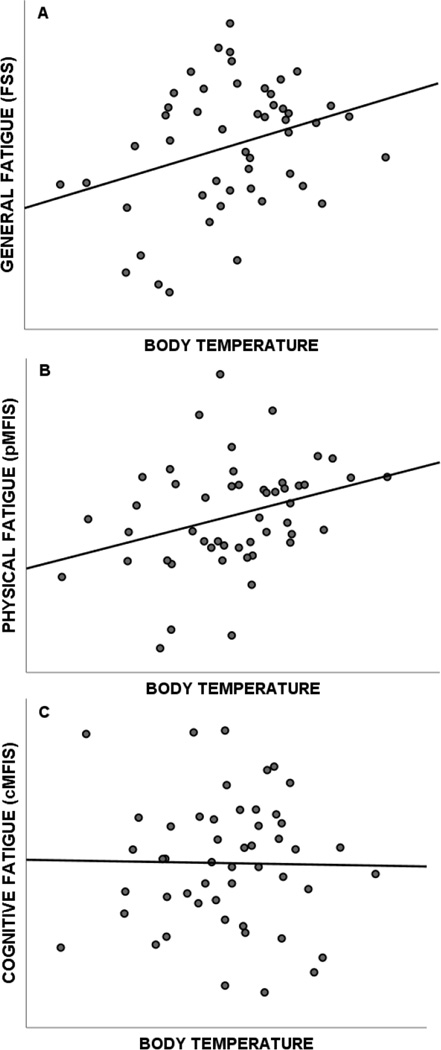
RRMS patients reported elevated levels of general fatigue on the FSS (4.37±1.66; t[49]=4.55, p<.001), as well as elevated levels of physical fatigue (17.16 ± 8.06; t[49] = 10.67, p < .001) and cognitive fatigue (16.96±9.11; t[49]=9.29, p<.001) on the MFIS. As shown in Figure 2, warmer core body temperature within RRMS patients was associated with higher self-reported general fatigue on the FSS (rp=.315, p=.028) and physical fatigue on the MFIS (rp=.318, p=.026), but not cognitive fatigue on the MFIS (rp= −.017, p=.909). That is, body temperature accounted for approximately 10% of the variance in self-reported general fatigue and physical fatigue in RRMS patients.
Figure 2.
Correlation between body temperature in RRMS patients and general fatigue (A), physical fatigue (B), and cognitive fatigue (C).
DISCUSSION
We have shown for the first time that body temperature is elevated in RRMS patients relative to healthy controls (and SPMS patients), and that higher body temperature is linked to worse fatigue in RRMS patients. Note that body temperature among RRMS patients within our sample was elevated during rest, even without experimental manipulation (e.g., hot baths). Elevated temperature may have evaded clinical and empirical attention to date because (a) body temperature among RRMS patients remains within the normal range, and (b) empirical attention has been focused on experimental manipulations of body temperature rather than endogenous body temperature (e.g., 2–4). Despite being within the normal range, the effect size of this RRMS-related temperature elevation relative to healthy persons is large (d=0.70, see Figure 1) and clinically meaningful, as indicated by the correlations between body temperature and fatigue, specifically physical fatigue. RRMS patients also had higher body temperature relative to SPMS patients (large effect: d=.086, see Figure 1), which supports our notion that body temperature elevations among RRMS patients are due to disease-related inflammatory processes, which are greatly reduced/abated in SPMS patients.6 Note, however, that the possible link between body temperature and disease-related inflammatory processes in RRMS patients requires more direct/thorough validation.
Fatigue in RRMS patients is notoriously difficult to treat, perhaps due to an underdeveloped understanding of fatigue’s etiology. Illustrative of this is the fact that despite several failed randomized controlled trials (RCTs), 14–16, RRMS patients are still prescribed modafinil (a stimulant), likely based on a conceptualization of MS fatigue as stemming from dysfunctional arousal (e.g., 17). More promising may be exploration of an inflammatory etiology for MS fatigue, which is supported by a link between fatigue and inflammatory markers (tumor necrosis factor-alpha and interferon-gamma)18, and by the link between fatigue and elevated body temperature presented herein. Consistent with our observation linking MS fatigue and elevated body temperature, RCTs of cooling garments19–20 and antipyretic medication (aspirin) 21 have been effective in treating MS fatigue. Our findings linking warmer body temperature and MS fatigue help explain the efficacy of cooling/antipyretic treatments, and encourage further research on such treatments for MS fatigue.
The relationship between elevated body temperature and worse fatigue was observed on the FSS and the physical fatigue subscale of the MFIS, but not the cognitive fatigue subscale of the MFIS. Note that the FSS is more a measure of physical than cognitive fatigue, as indicated by (a) examination of the scale’s items, (b) a stronger correlation between the FSS and the MFIS physical fatigue subscale (r = .75) than the cognitive fatigue subscale (r = .44) in a large normative sample.12 The FSS was also more related to MFIS physical fatigue (rp = .53, p < .001) than MFIS cognitive fatigue (rp = .36, p = .361) in the current sample, controlling for depression.
Limitations
The current investigation is limited by a relatively small sample size, although this is mitigated somewhat by the large effect sizes of the temperature elevation in RRMS patients relative to healthy controls and SPMS patients (Ds=0.70–0.86). We acknowledge that a multitude of factors influence body temperature, such as circadian rhythms (time of day) and menstrual cycles. That is, body temperature fluctuates within persons throughout the day, with lower body temperatures in the early morning and late evening/night.9–10 All of our subjects were seen between 8am and 6pm, when body temperatures are more stable.9–10 There was no relationship between body temperature and time-of-day within our sample (r = −.024, p > .8), likely because time-related fluctuations in body temperature are less common during this period of the day. Also, there were no differences across groups in the time of temperature recordings (Ps > .9; RRMS mean = 12:16, median = 11:49; SPMS mean = 12:06, median = 11:30; healthy mean = 12:00, median = 11:30). As such, higher body temperature among RRMS patients relative to SPMS patients and healthy persons was not due to differences in the time-of-day that temperatures were recorded. (Some evidence suggests that temperature may continue to increase from early morning to 10am before reaching a plateau, but proportions of subjects seen before 10am were similar across groups.) Note also that there was no relationship between time-of-day and self-reported fatigue on the FSS (r = −.012, p = .935), nor physical fatigue on the MFIS (r = −.153, p = .288) or cognitive fatigue on the MFIS (r = −.167, p = .245) among RRMS patients. As such, RRMS patients with more severe fatigue were equally likely to be seen earlier or later in the day (from 8am to 6pm). Importantly, although such factors as time of day or menstrual cycles may introduce error, this error should affect RRMS patients, SPMS patients, and healthy persons equally, and is therefore not systematic (i.e., cannot account for our finding, as random error would only serve to obfuscate or diminish our finding). Similarly, regarding the matter of measurement error, any unreliability of temperature or fatigue measurement represents a source of random error that would lessen our effect rather than inflate it (Type II error). As such, despite potential sources of random error, we have observed elevated body temperature among RRMS patients relative to healthy controls and SPMS patients, with reliable links to worse fatigue.
The temperature elevation among RRMS patients may align with the disease-related inflammatory processes that characterize this disease stage, and is supported by our finding of non-elevated body temperature in SPMS patients (in whom disease-related inflammatory processes abate6). Also, the link between body temperature and fatigue is consistent with previous work linking fatigue to circulating inflammatory markers.18 Future research is needed to more directly investigate the interrelationships among body temperature, fatigue, and disease-related inflammation in persons with RRMS.
CONCLUSIONS
Fatigue is among the most prevalent and debilitating symptoms of RRMS. In the current study, body temperature was elevated among RRMS patients, and warmer body temperature was linked to worse fatigue. To date, fatigue in MS patients has been notoriously difficult to treat, and many patients are still prescribed stimulants (modafinal) despite several failed RCTs.14–16 In contrast, RCTs of cooling garments19–20 and antipyretics (aspirin)21 have effectively reduced fatigue in MS patients. Our observation of elevated body temperature linked to worse fatigue in RRMS patients helps explain the effectiveness of previous cooling/antipyretic treatments, and encourages further research on cooling/antipyretic treatments of fatigue in RRMS.
Acknowledgements
Funding for this project was provided in part by the National Institutes of Health (R00HD060765 to JFS) and the Kessler Foundation Research Center. JFS and VML have no conflicts of interest to report.
Abbreviations
- RRMS
Relapsing-remitting multiple sclerosis
- SPMS
secondary-progressive multiple sclerosis
- FSS
Fatigue Severity Scale
- MFIS
Modified Fatigue Impact Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Braun ThermoScan® IRT 4520, Consumer Relations, Kaz USA, Inc., 250 Turnpike Road, Southborough, MA 01772, USA, www.kaz.com/braun
REFERENCES
- 1.Krupp LB, Serafin DJ, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother. 2010 Sep;10(9):1437–1447. doi: 10.1586/ern.10.99. [DOI] [PubMed] [Google Scholar]
- 2.White AT, Vanhaitsma TA, Vener J, Davis SL. Effect of passive whole-body heating on central conduction and cortical excitability in multiple sclerosis patients and healthy controls. J Appl Physiol. 2013 Apr 18; doi: 10.1152/japplphysiol.01119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis SL, Frohman TC, Crandall CG, et al. Modeling Uhthoff's phenomenon in MS patients with internuclear ophthalmoparesis. Neurology. 2008 Mar 25;70(13 Pt 2):1098–1106. doi: 10.1212/01.wnl.0000291009.69226.4d. [DOI] [PubMed] [Google Scholar]
- 4.Hamalainen P, Ikonen A, Romberg A, Helenius H, Ruutiainen J. The effects of heat stress on cognition in persons with multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England) 2012 Apr;18(4):489–497. doi: 10.1177/1352458511422926. [DOI] [PubMed] [Google Scholar]
- 5.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008 Jan;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kappos L, Moeri D, Radue EW, et al. Predictive value of gadolinium-enhanced magnetic resonance imaging for relapse rate and changes in disability or impairment in multiple sclerosis: a meta-analysis. Gadolinium MRI Meta-analysis Group. Lancet. 1999 Mar 20;353(9157):964–969. doi: 10.1016/s0140-6736(98)03053-0. [DOI] [PubMed] [Google Scholar]
- 7.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011 Feb;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koch M, Kingwell E, Rieckmann P, Tremlett H. The natural history of secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2010 Sep;81(9):1039–1043. doi: 10.1136/jnnp.2010.208173. [DOI] [PubMed] [Google Scholar]
- 9.Mackowiak PA, Wasserman SS, Levine MM. A critical appraisal of 98.6 degrees F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA. 1992 Sep 23–30;268(12):1578–1580. [PubMed] [Google Scholar]
- 10.Chamberlain JM, Terndrup TE, Alexander DT, et al. Determination of normal ear temperature with an infrared emission detection thermometer. Ann Emerg Med. 1995 Jan;25(1):15–20. doi: 10.1016/s0196-0644(95)70349-7. [DOI] [PubMed] [Google Scholar]
- 11.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989 Oct;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 12.Tellez N, Rio J, Tintore M, Nos C, Galan I, Montalban X. Does the Modified Fatigue Impact Scale offer a more comprehensive assessment of fatigue in MS? Mult Scler. 2005 Apr;11(2):198–202. doi: 10.1191/1352458505ms1148oa. [DOI] [PubMed] [Google Scholar]
- 13.Learmonth YC, Dlugonski D, Pilutti LA, Sandroff BM, Klaren R, Motl RW. Psychometric properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J Neurol Sci. 2013 Aug 15;331(1–2):102–107. doi: 10.1016/j.jns.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Peuckmann V, Elsner F, Krumm N, Trottenberg P, Radbruch L. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2010;(11):CD006788. doi: 10.1002/14651858.CD006788.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Moller F, Poettgen J, Broemel F, Neuhaus A, Daumer M, Heesen C. HAGIL (Hamburg Vigil Study): a randomized placebo-controlled double-blind study with modafinil for treatment of fatigue in patients with multiple sclerosis. Mult Scler. 2011 Aug;17(8):1002–1009. doi: 10.1177/1352458511402410. [DOI] [PubMed] [Google Scholar]
- 16.Stankoff B, Waubant E, Confavreux C, et al. Modafinil for fatigue in MS: a randomized placebo-controlled double-blind study. Neurology. 2005 Apr 12;64(7):1139–1143. doi: 10.1212/01.WNL.0000158272.27070.6A. [DOI] [PubMed] [Google Scholar]
- 17.Niepel G, Bibani RH, Vilisaar J, et al. Association of a deficit of arousal with fatigue in multiple sclerosis: effect of modafinil. Neuropharmacology. 2013 Jan;64:380–388. doi: 10.1016/j.neuropharm.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 18.Heesen C, Nawrath L, Reich C, Bauer N, Schulz KH, Gold SM. Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry. 2006 Jan;77(1):34–39. doi: 10.1136/jnnp.2005.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beenakker EA, Oparina TI, Hartgring A, Teelken A, Arutjunyan AV, De Keyser J. Cooling garment treatment in MS: clinical improvement and decrease in leukocyte NO production. Neurology. 2001 Sep 11;57(5):892–894. doi: 10.1212/wnl.57.5.892. [DOI] [PubMed] [Google Scholar]
- 20.Schwid SR, Petrie MD, Murray R, et al. A randomized controlled study of the acute and chronic effects of cooling therapy for MS. Neurology. 2003 Jun 24;60(12):1955–1960. doi: 10.1212/01.wnl.0000070183.30517.2f. [DOI] [PubMed] [Google Scholar]
- 21.Wingerchuk DM, Benarroch EE, O'Brien PC, et al. A randomized controlled crossover trial of aspirin for fatigue in multiple sclerosis. Neurology. 2005 Apr 12;64(7):1267–1269. doi: 10.1212/01.WNL.0000156803.23698.9A. [DOI] [PubMed] [Google Scholar]