Abstract
Despite being an everyday sensory experience, the nature of astringency perception is not clear. In this issue of Chemical Senses, Schöbel et al. demonstrate that astringency is a trigeminal sensation in human, and astringents trigger a G protein-coupled pathway in trigeminal ganglion cells in the mouse.
Key words: astringency, G protein, taste, trigeminal sensation
If you accidentally bite into an unripe banana or persimmon, the drying and puckering feeling on your tongue and oral cavity is astringency. Astringency is a frequent sensory experience that can be caused by consumption of various food and beverages, including unripe fruit, nut skin, tea, and red wine (Figure 1). The major astringents from these sources are tannins, the naturally occurring plant polyphenols that are usually found in plant leaves, seeds, and fruit skins. Besides polyphenols, metal salts, acids, and dehydrating agents can also cause astringency (Green 1993). Though astringency is primarily perceived as a disagreeable sensation, under certain circumstances astringency is desirable: it adds flavors to red wines and extends the finish, characteristics described using the term “smooth” by wine writers.
Figure 1.

A collection of food and beverages that cause astringency sensation.
Astringency is typically discussed in the context of taste as it is an oral feeling and is usually caused by food. In fact, it was considered a basic taste modality in ancient Indian culture (Lochan 2011). Nowadays, astringency is excluded from the widely accepted 5 basic taste modalities: salty, sour, umami, sweet, and bitter. These basic taste modalities are sensed by taste buds on the tongue, which relay sensory information to the brain through taste nerves, the chorda tympani and the glossopharyngeal nerve. In addition to the neural basis, we understand many aspects of the molecular basis of these basic tastes. Umami-, sweet-, and bitter-tasting compounds are detected by G protein-coupled receptors (GPCRs): T1R1/T1R3 heteromer for umami (Nelson et al. 2002), T1R2/T1R3 heteromer for sweet (Nelson et al. 2001), and T2Rs for bitter (Chandrashekar et al. 2000; Matsunami et al. 2000). Epithelial sodium channels (ENaC) are partly responsible for salt sensation (Chandrashekar et al. 2010). In contrast, both the neural and molecular bases of the astringency sensation remain elusive. It is debated whether astringency is a taste sensation, or a trigeminal sensation analogous to the spicy taste of hot chili peppers. It is unclear whether astringents trigger mechanosensation, chemosensation, or a combination of both. Furthermore, we do not know which receptor(s) recognize astringents, if there are any, or the signaling cascades downstream.
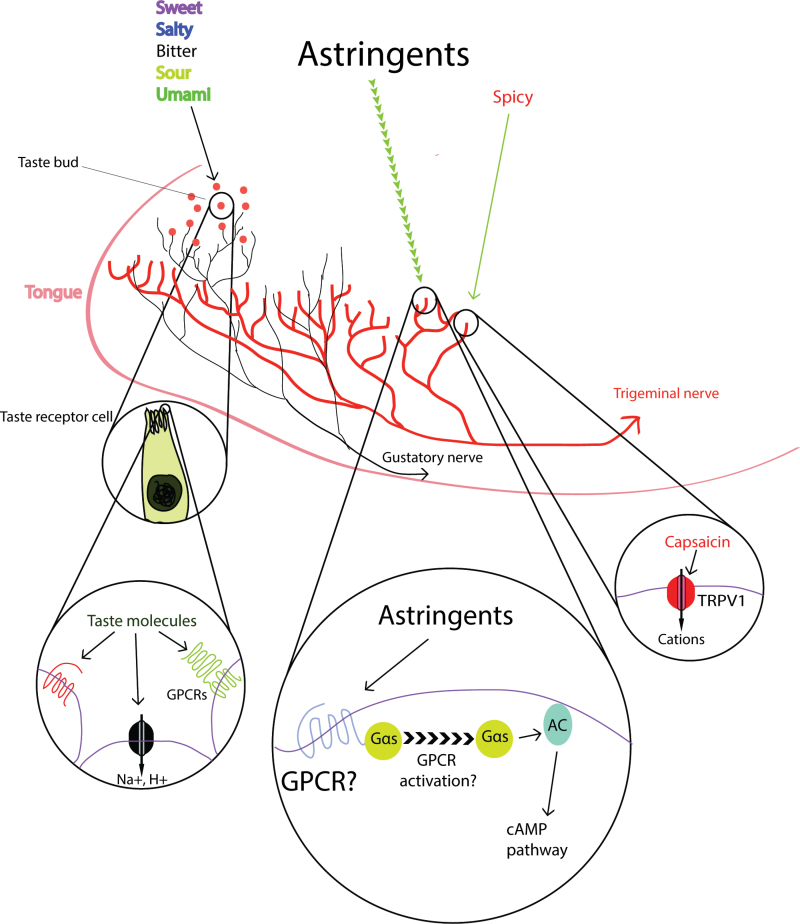
In this issue of Chemical Senses, Schöbel et al. took a step forward in unveiling the mysterious identity of astringency sensation and demonstrated that human astringency perception is likely a trigeminal rather than a taste sensation. In line with this, they showed that isolated mouse trigeminal ganglion (TG) cells responded to various astringents, including red wine fractions. The authors further dissected the downstream signaling underlying this response and demonstrated that a Gs-coupled pathway seems to be involved (Figure 2).
Figure 2.
Astringency and taste sensation. Basic tastes are detected by taste receptor cells in the taste buds. These cells express taste receptors including GPCRs and ion channels. Gustatory nerves receive input from these receptor cells and transmit the taste information to the brain. The hot chili pepper ingredient capsaicin activates trigeminal nerves though TRPV1 (transient receptor potential cation channel, subfamily V, member 1) channels. Astringency perception is mediated by trigeminal nerves through the activation of Gs and adenylyl cyclase.
Astringent chemicals were reported to activate taste nerves in rodents (Schiffman et al. 1992). However, it is not clear whether astringency sensation is associated with taste nerve activation. In this study, Schöbel et al. directly addressed this question with human subjects. Strikingly, astringency sensation was intact when the subjects’ taste nerves were blocked by either nerve transection or local anesthesia, which almost completely abolished salty, sweet, sour, and bitter tastes. Only when the trigeminal and taste nerves were both blocked did the subjects lose their astringency sensation. Although it has yet to be demonstrated whether only blocking the trigeminal nerve would lead to the loss of astringency sensation, the available evidence argues that astringency is a trigeminal sensation in humans.
After demonstrating that astringency is more likely a trigeminal sensation for humans, Schöbel et al. went on to investigate the relevant signaling pathways. The traditional view on astringency focuses on mechanosensation: the astringents react with salivary proteins, especially proline-rich proteins, causing them to precipitate, and the resulting loss of lubricity leads to the tactile feeling associated with astringency in the mouth (Baxter et al. 1997). However, it is less clear if astringents can be directly detected by chemosensors. In line with the involvement of trigeminal chemical senses in astringency sensation, Schöbel et al. showed that isolated mouse TG cells displayed calcium responses to a variety of astringents, though not to all the tested astringents. Using this system, Schöbel et al. discovered that the activation of TG neurons by an astringent, epigallocatechin gallate (EGCG), is not dependent on transient receptor potential (TRP) channels that respond to traditional trigeminal stimuli such as temperature and capsaicin. Instead, the EGCG-induced TG neuron activation requires a G protein-coupled signaling, in particular the Gs-adenylyl cyclase–cyclic nucleotide-gated (CNG) channel cascade, reminiscent of the olfactory pathway. These results obtained under the ex vivo settings do not disprove the traditional salivary protein precipitation theory. However, they do provide an attractive alternative or additional hypothesis for further investigation using genetic and molecular biological approaches.
The study by Schöbel et al. also raises several interesting questions. For instance, it is well established that oral movement facilitates the astringency sensation. How do chemosensors for astringents fit in this scenario? Moreover, given that a Gs-coupled pathway is involved, what could be the receptors for astringents? Transcriptome analysis in TG cells detected many GPCRs, some of which are orphan receptors specific for the TG (Manteniotis et al. 2013). Given the diverse nature of astringents, it is possible that astringency is a more complex sensation and that astringents are detected by multiple sensory mechanisms (Lee et al. 2012). If so, caution should be taken when interpreting the results obtained from one astringent to another. Nevertheless, the study by Schöbel et al. represents an important step forward toward understanding this enigmatic sensation.
Funding
This work was supported by the National Institute of Health [award numbers DC010857 and DC012095] .
References
- Baxter NJ, Lilley TH, Haslam E, Williamson MP. 1997. Multiple interactions between polyphenols and a salivary proline-rich protein repeat result in complexation and precipitation. Biochemistry. 36(18):5566–5577 [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. 2010. The cells and peripheral representation of sodium taste in mice. Nature. 464(7286):297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. 2000. T2Rs function as bitter taste receptors. Cell. 100(6):703–711 [DOI] [PubMed] [Google Scholar]
- Green BG. 1993. Oral astringency: a tactile component of flavor. Acta Psychol (Amst). 84(1):119–125 [DOI] [PubMed] [Google Scholar]
- Lee CA, Ismail B, Vickers ZM. 2012. The role of salivary proteins in the mechanism of astringency. J Food Sci. 77(4):C381–C387 [DOI] [PubMed] [Google Scholar]
- Lochan K. 2011. Encyclopedic dictionary of ayurveda. New Delhi (India): Chaukhambha Publications [Google Scholar]
- Manteniotis S, Lehmann R, Flegel C, Vogel F, Hofreuter A, Schreiner BS, Altmüller J, Becker C, Schöbel N, Hatt H, et al. 2013. Comprehensive RNA-Seq expression analysis of sensory ganglia with a focus on ion channels and GPCRs in Trigeminal ganglia. PLoS One. 8(11):e79523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami H, Montmayeur JP, Buck LB. 2000. A family of candidate taste receptors in human and mouse. Nature. 404(6778):601–604 [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. 2002. An amino-acid taste receptor. Nature. 416(6877):199–202 [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. 2001. Mammalian sweet taste receptors. Cell. 106(3):381–390 [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Suggs MS, Sostman AL, Simon SA. 1992. Chorda tympani and lingual nerve responses to astringent compounds in rodents. Physiol Behav. 51(1):55–63 [DOI] [PubMed] [Google Scholar]