Abstract
Following the discovery of interleukin (IL)-17 producing T helper (Th17) cells as a distinct lineage of CD4+ T helper cells it became clear that these cells play an important role in the host defense against extracellular fungal and bacterial pathogens and participate in the pathogenesis of multiple inflammatory and autoimmune disorders. Depending on the microenvironment, Th17 cells can alter their differentiation programme ultimately giving rise to either protective or pro-inflammatory pathogenic cells. We found that besides the conventional in vitro protocol for Th17 differentiation by transforming growth factor-beta (TGF-β) plus IL-6 cytokines, a combination of IL-23 plus IL-6 can also induce Th17 cells. The Th17 cells induced by IL-23 plus IL-6 (termed as effector Th17, Teff17 cells) are pathogenic upon adoptive transfer into non-obese diabetic (NOD) mice contributing to the development of type 1 diabetes (T1D) while cells induced by TGF-β plus IL-6 (termed as regulatory T cells, Treg17 cells) are non pathogenic and regulatory, and suppressed the pathogenic T cells in T1D. These cells differentially expressed a number of cytokines where Teff17 cells exhibited an increase in granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-22 whereas Treg17 cells demonstrated increased expression of IL-21 and immunosuppressive cytokine IL-10. Differentiation of Th17 cells is controlled by a transcription factor, RORγT although these cells also express variable levels of T-bet and FoxP3 transcription factors. This points to a dual functional role of Th17 subsets in autoimmune diseases particularly T1D. We suggest that similar to conventional regulatory T cells (Treg), induction of regulatory Treg17 cells could play an important role in modulating and preventing certain autoimmune diseases.
Keywords: Autoimmunity, cytokines, immunoregulation, helper T cell plasticity, regulatory T cells, Th17 cells, Type 1 diabetes
Introduction
Type 1 diabetes (T1D) is a T cell mediated autoimmune disease in which insulin producing β cells are selectively destroyed in the pancreatic islets of Langerhans. Both CD4 and CD8 T cells have been implicated in T1D and the disease pathogenesis is primarily driven by CD4+ Th1 (T helper type 1) cells1. Several studies including our own have shown that CD4+CD25+ regulatory T cells (Treg) induced by various immunotherapy approaches are strongly protective in the NOD (non-obese diabetic) mouse model of T1D and in human subjects2,3,4. The pathogenic role of Th17 cells has been implicated in many autoimmune diseases but direct evidence supporting their involvement in T1D pathogenesis is lacking5,6,7. Th17 cells infiltrating the pancreas of NOD mice showed plasticity and could alter their phenotype in vivo8. Moreover, interleukin (IL)-17 the signature cytokine produced by Th17 cells, is dispensable in the pathogenesis of autoimmune diabetes and NOD mice lacking IL-17 still develop T1D9.
Pathogenic and protective Th17 cells in type 1 diabetes
In vitro treatment of naive CD4+ T cells with transforming growth factor (TGF)-β alone induces Treg cells, while TGF-β plus IL-6 generates Th17 cells10. It is not clear what drives the maturation of Th17 in vivo cells but is likely that commensal microbiota-derived metabolites may be involved in the induction of Th17 cells and also in mediating defense against pathogens11. Several studies point to the fact that in vitro generated Th17 cells can have a pathogenic and non-pathogenic phenotype. Th17 cells that have been matured with TGF-β and IL-6 have high expression of the genes encoding the transcription factor RORγT and cytokines IL-17A, IL-17F and IL-21 and IL-10. These can express both RORγT and FoxP3 transcription factors and have been shown to be capable of immunoregulatory functions12,13. FoxP3 is a marker of highly immunosuppressive Treg cells14,15 and Th17 cells that express both transcription factors have the capacity for immunoregulatory functions. The discovery of human IL-17-producing RORγT+ Foxp3+ T cells that retain their ability to suppress effector lymphocytes supports the dichotomous nature of Th17 regulatory cells12,16,17,18,19. These studies suggest that cells co-expressing RORγt and Foxp3 exist in vivo and are not simply an intermediate stage in the differentiation of inducible Treg or Th17 cells20. We have previously reported that NOD mice immunized with mycobacterial adjuvants, complete Freund's adjuvant (CFA) or BCG vaccine leads to the induction of similar regulatory Th17 cells. These cells produce IL-17, IL-22, IL-10, and interferon (IFN)-γ and support the idea of regulatory Th17 cell existence in vivo in T1D prone NOD mice21.
Depending on the microenvironment, Th17 cells can alter their differentiation programme, ultimately giving rise to either protective or proinflammatory pathogenic cells. Besides the conventional protocol for Th17 differentiation by TGF-β plus IL-6 cytokines, we found that a combination of IL-23 plus IL-6 can induce Th17 cells. The population of Th17 cells induced by IL-23 plus IL-6 and [termed as effector T (Teff17) cells] were pathogenic upon adoptive transfer into NOD mice causing type 1 diabetes while cells induced by TGF-β plus IL-6 (termed as Treg17 cells) were protective and suppressed the pathogenic T cells. The Teff17 cells differentially secreted granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-22 while Treg17 cells produced increased levels of IL-10 and IL-21 (Fig.). The identification of two distinct Th17 populations one with pathogenic properties (Teff17) and another with regulatory and protective properties (Treg17) may explain the controversial nature of Th17 cells in autoimmunity particularly T1D.
Fig.
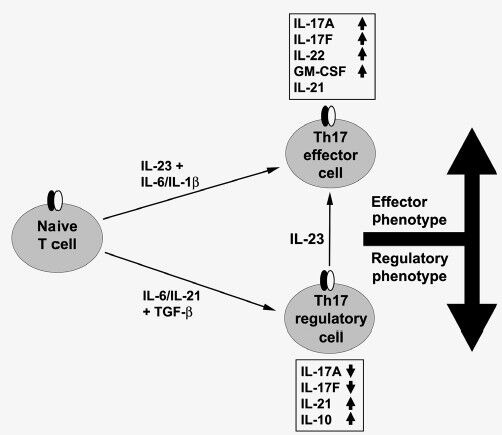
Functionally distinct Th17 cell subsets are induced by different cytokines. Transforming growth factor-beta (TGF)-β plus interleukin (IL)-6 promote the maturation of regulatory (Treg17) cells and IL-23 drives the pathogenic (Teff17) cells. These cells differentially produce increased (↑) or reduced (↓) levels of pro-inflammatory and/or regulatory cytokines.
Plasticity of Th17 cells
To determine the adoptive transfer capacity of Th17 cells in T1D two different approaches were used. The TGF-β plus IL-6 polarized Th17 cells when transferred into wild type NOD mice promoted pancreatic inflammation but no overt diabetes6,7,8. In lymphopenic NODSCID (severe combined immunodeficiency) recipients Th17 cells mediated transfer of T1D but this was due to the plasticity of Th17 cells. It was found that Th17 cells converted into IFN-γ producing Th1 cells in the lymphopenic environment of NOD.SCID mice21. Th17 cells generated in vivo have been found to co-produce IL-17 and IFN-γ and these cells express Th1 transcription factor T-bet in addition to RORγT. The expression of IFN-γ by Th17 cells is dependent on IL-2322.
IL23 promotes effector pathogenic Th17 cells
IL-23 appears to be the key player in the terminal differentiation of Th17 cells and generation of pathogenic Th17 cells (Fig.)12,23. Treg17 cells can be converted to Teff17 cells by culturing with IL-23 in vitro. Several investigators have shown that TGF-β plus IL-6 induced Th17 cells produce IL-17 but do not transfer autoimmunity. IL-23 is required for these cells to acquire a pathogenic phenotype10,12,24. This supports the results that IL-23 and IL-23R deficient mice are highly resistant to autoimmune diseases24. IL-23 has emerged as the key factor in determining if Th17 cells can become pathogenic during the differentiation process. This also influences the expression of pathogenic cytokines particularly GM-CSF. This effector phenotype is a result of the network of transcription factors that include RORγT, T-bet and FoxP3. Treg17 cells produce variable amounts of IL-10 and IL-23 which diminish IL-10 production by Th17 cells while TGF-β promotes the production of IL-1025,26. GM-CSF is important for the pathogenicity of Th17 cells and TGF-β suppresses its production27,28. The proinflammatory cytokines IL-1β and IL-23 drive the production of pathogenic cytokine GM-CSF. A recent study has confirmed that IL-23-induced GM-CSF is essential for the pathogenicity of Th17 cells in the autoimmune model of multiple sclerosis (MS) namely experimental autoimmune encephalomyelitis (EAE)24. Moreover, Th17 cells that lack the ability to produce GM-CSF do not transfer autoimmune disease28. Thus the effector function of Teff17 cells is driven by GM-CSF via IL-23, and IL-10 drives the effector function of Treg17 cells via TGF-β. In summary, high TGF-β levels contribute to the maturation of Treg17 cells and IL-23 supports induction of Teff17 cells in autoimmunity.
Conclusion
The initial focus of Th17 studies was to determine their pathogenic role in inflammatory and autoimmune diseases. In the case of T1D, the published work has provided conflicting results either supporting their role in the destruction of islet β cells or having no effect or protecting animals from adoptive transfer of disease. Recent studies support the regulatory function of Th17 cells induced by mycobacterial adjuvants i.e. BCG and CFA. The in vitro conditions used for the generation of the Th17 cells for these studies contribute to the functional differences observed by various investigators. The Th17 subsets induced by TGF-β and IL-6 and/or IL-23 produce immunosuppressive to proinflammatory cytokines. In addition, the plasticity of Th17 cells has been discovered where these can give rise to Th1 cells in vivo. Immunization with adjuvants suggests that it is possible to change the functional phenotype of Th17 cells from pathogenic to protective by altering the cytokine microenvironment. This may also be feasible by immunization with autoantigens to modulate autoimmune diseases. In summary, the role of Th17 cells in autoimmune diseases is not simply to be proinflammatory and cause tissue damage but these can also act in an immunoregulatory fashion leading to prevention of autoimmunity by inhibiting pathogenic T cells.
Acknowledgment
The work carried out in authors’ laboratory was supported by grants from the Canadian Institutes of Health Research. Authors thank Edwin Lee-Chan and Olga Krougly for their help in the studies carried out in their laboratory.
References
- 1.Delovitch TL, Singh B. Autoimmune diabetes in the nonobese diabetic mouse: Immune dysregulation gets the NOD. Immunity. 1997;7:727–38. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee R, Chaturvedi P, Qin HY, Singh B. CD4+CD25+ Regulatory T cells generated in response to insulin B:9-23 peptide prevent adoptive transfer of diabetes by diabetogenic T cells. J Autoimmun. 2003;21:221–37. doi: 10.1016/s0896-8411(03)00114-8. [DOI] [PubMed] [Google Scholar]
- 3.Nikoopour E, Schwartz JA, Singh B. Therapeutic benefits of regulating inflammation in autoimmunity. Inflamm Allergy Drug Targets. 2008;7:203–10. doi: 10.2174/187152808785748155. [DOI] [PubMed] [Google Scholar]
- 4.Penaranda C, Tang Q, Bluestone JA. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J Immunol. 2011;187:2015–22. doi: 10.4049/jimmunol.1100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, et al. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58:1302–11. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–72. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–24. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lexberg MH, Taubner A, Albrecht I, Lepenies I, Richter A, Kamradt T, et al0. IFN-γ and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. Eur J Immunol. 2010;40:3017–27. doi: 10.1002/eji.201040539. [DOI] [PubMed] [Google Scholar]
- 9.Joseph J, Bittner S, Kaiser FM, Wiendl H, Kissler S. IL-17 silencing does not protect nonobese diabetic mice from autoimmune diabetes. J Immunol. 2012;188:216–21. doi: 10.4049/jimmunol.1101215. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 11.Huber S, Gagliani N, Flavell RA. Life, death, and miracles: Th17 cells in the intestine. Eur J Immunol. 2012;42:2238–45. doi: 10.1002/eji.201242619. [DOI] [PubMed] [Google Scholar]
- 12.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–7. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 13.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 14.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 16.Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–9. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voo KS, Wang Y, Santori FR, Boggiano CY, Wang Y, Arima K, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793–8. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORγt. Proc Natl Acad Sci USA. 2009;106:8635–40. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tartar DM, VanMorlan AM, Wan X, Guloglu FB, Jain R, Haymaker CL, et al. FoxP3+RORgammat+ T helper intermediates display suppressive function against autoimmune diabetes. J Immunol. 2010;184:3377–85. doi: 10.4049/jimmunol.0903324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikoopour E, Schwartz JA, Huszarik K, Sandrock C, Krougly O, Lee-Chan E, et al. Th17 polarized cells from NOD mice following mycobacterial adjuvant immunotherapy delay type I diabetes development. J Immunol. 2010;184:4779–88. doi: 10.4049/jimmunol.0902822. [DOI] [PubMed] [Google Scholar]
- 22.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–71. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Croxford AL, Mair F, Becher B. IL-23: One cytokine in control of autoimmunity. Eur J Immunol. 2012;42:2263–73. doi: 10.1002/eji.201242598. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Zielinski CE, Lanzavecchia A. Human Th17 subsets. Eur J Immunol. 2012;42:2215–20. doi: 10.1002/eji.201242741. [DOI] [PubMed] [Google Scholar]
- 26.Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–7. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–75. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–7. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]



