Significance
Plasma membrane ion channels composed of pore-forming and auxiliary subunits regulate physiological functions in virtually all cell types. A conventional view is that ion channels assemble with their auxiliary subunits prior to surface trafficking of the multiprotein complex. Arterial myocytes express large-conductance Ca2+-activated potassium (BK) channel α and auxiliary β1 subunits that modulate contractility and blood pressure and flow. The data here show that although most BKα subunits are plasma membrane-resident, β1 subunits are primarily intracellular in arterial myocytes. Nitric oxide, an important vasodilator, stimulates rapid surface trafficking of β1 subunits, which associate with, and activate, BK channels, leading to vasodilation. Thus, we show that rapid auxiliary subunit trafficking is a unique mechanism to control functional surface ion channel activity.
Keywords: artery, protein trafficking
Abstract
Ion channels composed of pore-forming and auxiliary subunits control physiological functions in virtually all cell types. A conventional view is that channels assemble with their auxiliary subunits before anterograde plasma membrane trafficking of the protein complex. Whether the multisubunit composition of surface channels is fixed following protein synthesis or flexible and open to acute and, potentially, rapid modulation to control activity and cellular excitability is unclear. Arterial smooth muscle cells (myocytes) express large-conductance Ca2+-activated potassium (BK) channel α and auxiliary β1 subunits that are functionally significant modulators of arterial contractility. Here, we show that native BKα subunits are primarily (∼95%) plasma membrane-localized in human and rat arterial myocytes. In contrast, only a small fraction (∼10%) of total β1 subunits are located at the cell surface. Immunofluorescence resonance energy transfer microscopy demonstrated that intracellular β1 subunits are stored within Rab11A-postive recycling endosomes. Nitric oxide (NO), acting via cGMP-dependent protein kinase, and cAMP-dependent pathways stimulated rapid (≤1 min) anterograde trafficking of β1 subunit-containing recycling endosomes, which increased surface β1 almost threefold. These β1 subunits associated with surface-resident BKα proteins, elevating channel Ca2+ sensitivity and activity. Our data also show that rapid β1 subunit anterograde trafficking is the primary mechanism by which NO activates myocyte BK channels and induces vasodilation. In summary, we show that rapid β1 subunit surface trafficking controls functional BK channel activity in arterial myocytes and vascular contractility. Conceivably, regulated auxiliary subunit trafficking may control ion channel activity in a wide variety of cell types.
Ion channels control physiological functions in virtually all cell types (1). Current (I) generated by an ion channel population is a product of the number of channels (N), open probability (Po), and single-channel current (i), such that I = N⋅Po⋅i. Previous studies have focused on identifying physiological and pathological mechanisms that regulate the Po of plasma membrane-resident ion channels. In contrast, mechanisms that control the number of functional plasma membrane ion channels (N) and their regulatory auxiliary subunits in native cell types are unclear. A conventional view is that ion channels assemble with their auxiliary subunits before anterograde trafficking and plasma membrane insertion of the protein complex. Auxiliary subunits can modulate ion channel surface expression and activity, but it is unclear whether the multisubunit composition of surface channels is fixed following protein synthesis or flexible and open to acute and rapid modulation (2–4). Some recombinant ion channel proteins, including large-conductance calcium (Ca2+)-activated potassium (BK) α subunits, can traffic to the plasma membrane in the absence of their regulatory auxiliary subunits (5–9). This stimulated us to investigate the hypothesis that physiological stimuli rapidly modulate surface levels of auxiliary subunits to control functional ion channel activity. Such a mechanism would fine tune ion channel activity to control cell functionality. To test this hypothesis, we studied trafficking and surface expression of native BK channel subunits in smooth muscle cells (myocytes) of small arteries that regulate regional organ blood flow and systemic blood pressure (10).
BK channels are expressed in a wide variety of mammalian cell types and regulate functions including arterial contractility, neurotransmission, and endocrine secretion (11). BK channels are heterotetramers of pore-forming α subunits that can also contain auxiliary β subunits, of which four are known (β1 to -4) (12, 13). β subunits are expressed in a tissue-specific manner and modulate BK channel Ca2+ sensitivity and gating properties to customize cellular functionality (14). Arterial myocytes express β1 subunits, which elevate BK channel Ca2+ sensitivity into a concentration range that permits functional coupling to local micromolar intracellular Ca2+ transients termed Ca2+ sparks (15). β1 subunits are essential for BK channels to control arterial myocyte membrane potential and contractility and to modulate systemic blood pressure (16, 17). Ca2+ spark to BK channel coupling is weak in β1 subunit knockout mice, leading to membrane depolarization, vasoconstriction, and systemic hypertension (14, 15). Pathological alterations in BK channel β1 subunit expression and function are also associated with cardiovascular diseases, including atherosclerosis, stroke, and hypertension (18). Whether physiological signaling mechanisms can control the α-to-β subunit ratio in BK channels to modulate channel activity and myocyte contractility is unclear.
Results
β1 Subunits Are Primarily Intracellular and Can Be Stimulated to Rapidly Traffic to the Plasma Membrane in Arterial Myocytes.
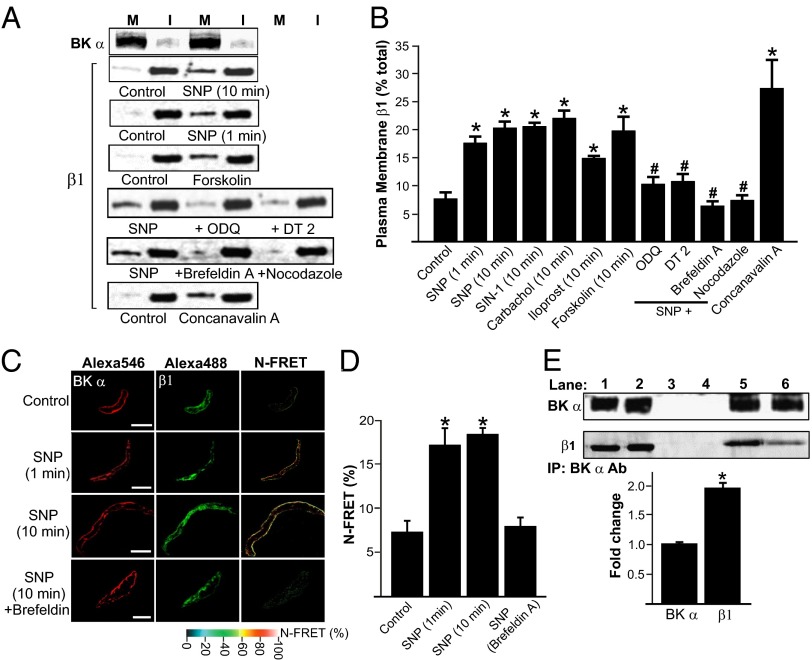
Arterial surface biotinylation was used to measure the cellular distribution of BKα and β1 subunits in myocytes of intact resistance-size (∼200-μm-diameter) arteries that control regional organ blood flow and systemic blood pressure. In rat cerebral arteries, ∼95.2% of BKα subunits were plasma membrane-localized (Fig. 1A and Fig. S1C), whereas only ∼8.1% of β1 subunits were present at the cell surface (Fig. 1 A and B). A similar distribution of BKα and β1 subunits was observed in rat small mesenteric arteries (Fig. S1B) and in cerebral arteries from an 18-y-old human male collected under an institutional review board-approved protocol (Fig. S2). These data indicate that most BKα subunits are located in the arterial myocyte plasma membrane. In contrast, and contrary to expectations, only a small fraction of total β1 subunits are located at the myocyte surface.
Fig. 1.
BKα subunits are plasma membrane-localized, whereas β1 subunits are intracellular, in arterial myocytes. (A) Representative Western blots illustrating plasma membrane (M) and intracellular (I) BKα and β1 proteins in rat cerebral arteries obtained using arterial surface biotinylation. Lanes are contiguous in each blot. All reagents were used at a final concentration of 10 µM, except Con A, which was used at 250 µg/mL. (B) Mean data for surface biotinylation experiments (n = 6–8 for each). Final working dilution of all reagents was 10 µM, except Con A, which was used at 250 µg/mL. *P < 0.05 vs. control; #P < 0.05 vs. SNP. (C) Immunofluorescence and immuno-FRET images of BKα and β1 in arterial myocytes. (D) Mean data for BKα and β1 immuno-FRET (control, n = 18; SNP 1 min, n = 8; SNP 10 min, n = 15; SNP 10 min plus brefeldin A, n = 14). SNP was 10 µM. *P < 0.05 compared with control; #P < 0.05 vs. SNP. (Scale bar, 10 µm.) (E) Original Western blots and bar graph (representative of three experiments; 18 rats total) of co-IP experiments indicating that SNP (10 μM, 10 min) elevates β1 protein associated with BKα in cerebral arteries. Lane 1, SNP positive control; lane 2, untreated positive control; lane 3, SNP negative control; lane 4, untreated negative control; lane 5, SNP co-IP; lane 6, untreated co-IP. *P < 0.05 compared with untreated control.
We tested the hypothesis that β1 subunits are mobile proteins and that physiological stimuli can control β1 trafficking to regulate BK channel activity. BK channels are activated by cAMP- and cGMP-dependent protein kinases (PKA and PKG), leading to vasodilation, but involvement of β-subunit trafficking in this response is unclear (17). Surface biotinylation revealed that sodium nitroprusside (SNP) or SIN-1, NO donors, increased mean arterial surface β1 protein ∼2.7- and ∼2.5-fold, respectively, in cerebral arteries (Fig. 1 A and B). Immunofluorescence, Förster resonance energy transfer (FRET) imaging, and coimmunoprecipitation (co-IP) were used to examine BKα and β1 cellular distribution, colocalization, and spatial proximity. The mean Normalized-Förster resonance energy transfer (N-FRET) between BKα- and β1-bound secondary antibodies was ∼7.3% in control myocytes (Fig. 1 C and D). SNP (10 min) increased N-FRET to ∼18.4%, or ∼2.5-fold (Fig. 1 C and D). The Förster coefficient for the Alexa488-Alexa546 FRET pair used is ∼6.3 nm (19), suggesting that surface-trafficked β1 subunits associate with BKα. Co-IP was performed to test the hypothesis that SNP can elevate the macromolecular subunit ratio of β1 to BKα in arterial myocytes. Because of the small size of the resistance-size vessels used in this study, arteries collected from ∼six rats were required for each co-IP experiment. The BKα antibody coimmunoprecipitated β1 protein from arterial lysate (Fig. 1E). SNP increased β1 protein that coimmunoprecipitated with BKα 1.97 ± 0.08-fold (n = 3, 18 rats total; Fig. 1E). These data indicate that surface-trafficked β1 subunits associate with plasma-resident BKα channels in arterial myocytes.
NO-induced plasma membrane trafficking of β1 was rapid, being maximal within 1 min, the shortest time point investigated (Fig. 1 A and B). Carbachol, a muscarinic receptor agonist, which stimulates endothelial cell NO generation, and iloprost, a PGI2 analog that activates myocyte PKA, both increased surface β1 protein in endothelium-intact arteries (Fig. 1B). Similar data were obtained when experiments were performed at either room temperature or 37 °C (Fig. S1A). BKα and β1 were similarly distributed and SNP stimulated β1 subunit surface expression in myocytes of rat mesenteric and human cerebral arteries (Figs. S1B and S2). ODQ, a soluble guanylyl cyclase (sGC) inhibitor, DT-2, a protein kinase G (PKG) inhibitor, or l-NNA, a NOS inhibitor, alone did not alter β1 cellular distribution, but ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one), L-NNA (Nω-Nitro-L-arginine) or DT-2 blocked the SNP-induced elevation in membrane β1 (Fig. 1 A and B and Fig. S1D). Forskolin, an adenylyl cyclase and thus PKA activator, increased β1 surface expression similarly to NO donors (Fig. 1 A and B). These data indicate that both NO-induced, sGC-mediated PKG activation, and adenylyl cyclase-mediated PKA activation stimulate rapid β1 surface expression in arterial myocytes.
β1 Subunits Are Located in Mobile Rab11A-Positive Recycling Endosomes.
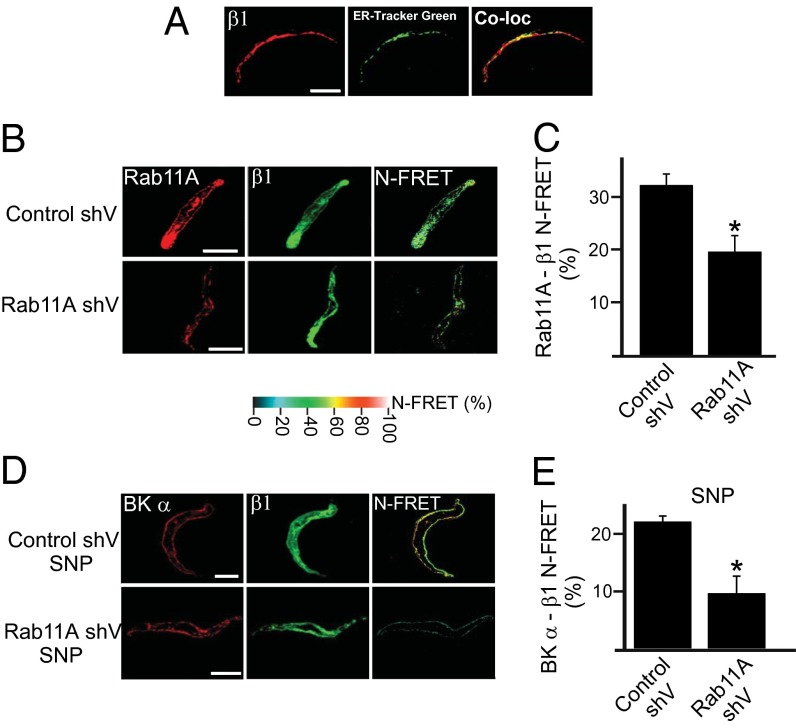
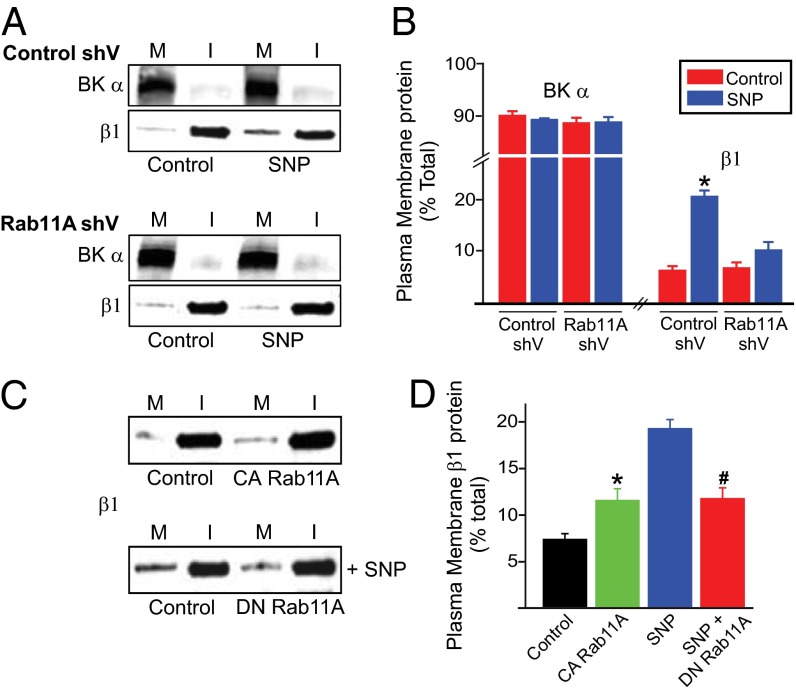
Trafficking pathways that control β1 surface expression were investigated in arterial myocytes. Brefeldin A, an endoplasmic reticulum (ER)-to-Golgi trafficking inhibitor, and nocodazole, a Golgi disruptor, did not alter β1 distribution when applied alone (Fig. S1D). In contrast, concanavalin A (Con A), an endocytosis inhibitor, increased membrane β1 protein ∼3.6-fold (Fig. 1 A and B). Brefeldin A and nocodazole each abolished the SNP-induced increase in surface β1 (Fig. 1 A and B). Brefeldin A also inhibited the SNP-induced elevation in BKα to β1 N-FRET (Fig. 1 C and D). Immunofluorescence indicated that only ∼35% of β1 colocalized with ER-Tracker Green, an ER label, suggesting that β1 subunits reside in an intracellular compartment other than the ER or Golgi (Fig. 2A). Given that β1 surface expression was rapid (≤1 min), we tested the hypothesis that recycling endosomes control β1 trafficking. Rab GTPases modulate endosomal trafficking with either Rab11A or Rab11B expressed on recycling endosomes (20–26). Physiological functions of Rab11 in native cell types, including arterial myocytes, are unclear. Immunofluorescence experiments demonstrated that β1- and Rab11A-bound antibodies generated a mean N-FRET of ∼32.5% in myocytes, indicating close spatial proximity (Fig. 2 B and C). Vectors encoding Rab11A shRNA (Rab11A shV) reduced arterial Rab11A protein by ∼50% but did not alter BKα or β1 expression (Fig. S3 B and C). Rab11A knockdown reduced the mean N-FRET signal between rab11A and β1 from ∼32% in scrambled controls to ∼19.7% (Fig. 2 B and C). Rab11A knockdown also blocked both the SNP-induced elevation in surface β1 and the N-FRET signal between BKα and β1-bound fluorescent antibodies in myocytes (Figs. 2 D and E and 3 A and B). Overexpression of a constitutively active Rab11A mutant (Rab11AQ70L) increased surface β1 ∼1.6 fold, whereas a dominant negative Rab11A mutant (Rab11AS25N) inhibited SNP-induced β1 surface trafficking in arteries (Fig. 3 C and D). In contrast, Rab11B knockdown (by ∼70%; Fig. S4 A and B) did not alter total or surface-localized BKα or β1 protein in control or SNP-treated arteries (Fig. S4 C and D). Immunofluorescence resonance energy transfer (immuno-FRET) experiments indicated that BKα and Rab11A were not localized (Fig. S3A), in stark contrast to the close spatial proximity of β1 and Rab11A. Furthermore, NO, forskolin, brefeldin A or Rab11A knockdown did not alter BKα total protein or cellular distribution (Figs. S1 C and D and S3 B and C). These data indicate that NO stimulates rapid Rab11A-mediated anterograde trafficking of β1 subunits in arterial myocytes. In contrast, BKα is trafficked by a Rab11A- and Rab11B-independent mechanism. These data raised the possibility that regulated anterograde trafficking of β1 subunits is a functional mechanism to control surface BK channel activity in arterial myocytes.
Fig. 2.
β1 and Rab11A are located in close spatial proximity in arterial myocytes. (A) Immunofluorescence images illustrating β1 (Alexa 546) and ER-Tracker Green colocalization (representative of six cells). (B) Immunofluorescence and immuno-FRET images of Rab11A and β1 in control shV or rab11A-knockdown myocytes. (C) Mean data (n = 6 for each). *P < 0.05 vs. control shV. (D) Immunofluorescence and N-FRET images for BKα and β1 in control shV- or Rab11A shV-treated cells in SNP. (E) Mean N-FRET data (n = 6 for each). *P < 0.05 vs. control shV. (Scale bar, 10 μm.)
Fig. 3.
Rab11A knockdown inhibits SNP-induced β1 subunit plasma membrane trafficking in arterial myocytes. (A) Representative Western blots illustrating plasma membrane (M) and intracellular (I) BKα and β1 protein in cerebral arteries transfected with control or rab11A shV and modulation by SNP (10 µM). (B) Mean data for surface BKα and β1 (n = 6 for each). *P < 0.05 vs. control. (C) Representative Western blots of β1 in control arteries and those expressing constitutively active (CA) or dominant-negative (DN) rab11A and regulation by SNP (n = 6 for each). (D) Mean data (n = 6 for each). *P < 0.05 vs. empty vector.
Stimulated Surface Trafficking of β1 Subunits Elevates BK Channel Ca2+ Sensitivity.
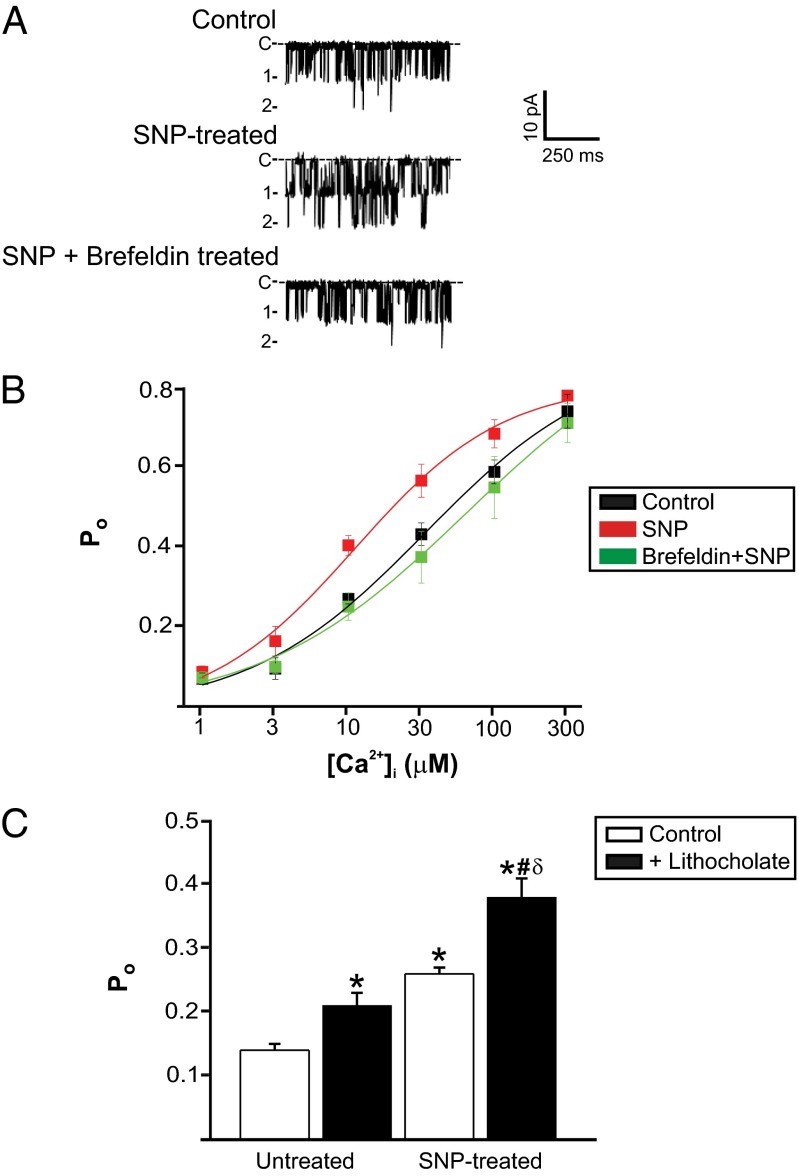
β1 subunits increase BK channel Ca2+ sensitivity (14, 27). Patch-clamp electrophysiology was used to examine the regulation of BK channels by an elevation in surface β1 subunits in arterial myocytes. Inside-out patches were pulled from myocytes that had been exposed to a bath solution either without (control) or with SNP (10 μM, 10 min). BK channel activity was then measured in membrane patches in the absence of SNP at −40 mV, a physiological voltage. The mean apparent dissociation constant (Kd) for Ca2+ of BK channels from control myocytes was ∼25 µM, with a maximum Po of ∼0.80 (Fig. 4B). In patches pulled from SNP-treated myocytes, BK channel mean Kd for Ca2+ was ∼11 µM or 2.3-fold higher, without an alteration in maximal Po. Brefeldin A treatment of myocytes blocked the SNP-induced elevation in BK channel Ca2+ sensitivity (Fig. 4B). These data indicate that SNP stimulates surface trafficking of β1 subunits that associate with BKα subunits and increase Ca2+ sensitivity.
Fig. 4.
Stimulated β1 subunit surface expression activates BKCa channels. (A) Representative traces of BKCa channel activity recorded at 10 μM [Ca2+]i in patches pulled from control myocytes or myocytes exposed to either SNP (10 µM, 10 min) or SNP (10 µM, 10 min) plus brefeldin (10 µM, 1 h). (B) Mean data (n = 6 for each). Minimal and maximal Po values for control, SNP, and brefeldin plus SNP were 0.03, 0.01, and 0.05 and 0.75, 0.77, and 0.75, respectively. (C) Mean BKCa channel Po in control and lithocholate (150 mM) from untreated and SNP-treated myocytes (n = 15 paired experiments for each group). *P < 0.05 vs. control untreated myocytes; #P < 0.05 vs. untreated myocytes plus lithocholate; δP < 0.05 vs. control SNP-treated myocytes.
BK channel regulation by lithocholate, a selective activator of β1 subunit-containing BK channels, was also studied (28). We tested the hypothesis that lithocholate would be a more effective activator of BK channels that contain additional SNP-trafficked β1 subunits. Lithocholate increased BK channel mean Po in inside-out patches pulled from both control cells and SNP-treated myocytes (Fig. 4C). Importantly, lithocholate elevated mean Po 0.07 ± 0.01 in control myocytes and 0.12 ± 0.02 in SNP-treated myocytes, or 1.71-fold more, consistent with the hypothesis being tested (Fig. 4C). These data indicate that NO-stimulated surface trafficking of β1 subunits elevates BK channel Ca2+ sensitivity and BK channel activation by a β1 ligand. When combined with other data in this study, these data indicate that NO increases the amount of functional β1 subunits associated with BK channels.
β1 Surface Trafficking in Arterial Myocytes Dilates Cerebral Arteries.
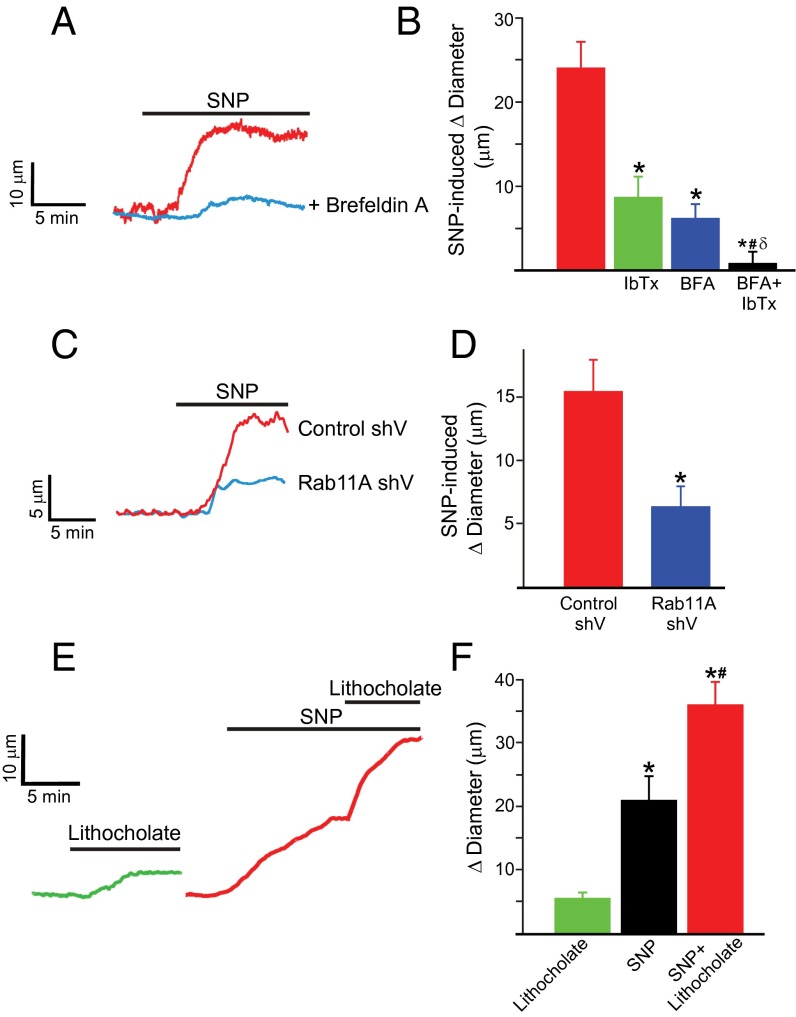
The physiological function of regulated β1 subunit trafficking was studied using pressurized (60 mmHg) endothelium-denuded cerebral arteries. SNP (10 µM) dilated arteries on average by ∼23 µm (Fig. 5 A and B). Iberiotoxin alone constricted arteries by ∼21 µm and reduced SNP-induced vasodilation by ∼65%, indicating that BK channel activation is the primary mechanism underlying NO-induced vasodilation (Fig. 5B). Brefeldin A alone did not modify myogenic tone or iberiotoxin- or 60 mM K+-induced vasoconstriction but reduced SNP-induced vasodilation by ∼74% (Fig. S5C and Fig. 5 A and B). Consistent with these findings, brefeldin A reduced carbachol-induced vasodilation in endothelium-intact arteries by ∼50% (Fig. S5B). Rab11A knockdown did not alter myogenic tone but reduced SNP-induced vasodilation by ∼60%, compared with scrambled controls (Fig. 5 C and D). Lithocholate dilated control arteries on average by ∼6 μm (Fig. 5 E and F). SNP increased mean lithocholate-induced vasodilation to ∼24 μm, or fourfold (Fig. 5 E and F). These data demonstrate that NO stimulates rapid Rab11A-mediated β1 subunit surface trafficking, leading to BK channel activation and vasodilation.
Fig. 5.
SNP-induced vasodilation occurs due to Rab11A-mediated BK channel activation. (A) Original recordings from the same artery illustrating dilation to SNP (10 µM) in control (red trace) and after treatment with brefeldin A (10 µM, 1 h; blue trace). (B) Mean data (experimental number, n = 6–8 for each). *P < 0.05 compared with control; #P < 0.05 vs. brefeldin; δP < 0.05 vs. iberiotoxin. (C) Original recordings illustrating dilation to SNP (10 µM) in arteries transfected with control shV (red) and rab11A shV (blue). (D) Mean data (n = 6 for each). *P < 0.05 vs. control shV. (E) Dilation to lithocholate (45 µM) in control (green) and in in the presence of SNP (red). (F) Mean data (n = 6 for each). *P < 0.05 vs. untreated control; #P < 0.05 compared with SNP.
Discussion
Whether the subunit composition of ion channels is fixed following protein synthesis and shuttling to the plasma membrane or is flexible and open to physiological modulation to control ion channel activity and tissue function was unclear. Here, we demonstrate that distinct pathways control BK channel α and β1 subunit trafficking in arterial myocytes. We also show that BKα subunits are primarily plasma membrane-localized, whereas a large proportion of β1 subunits reside in highly mobile Rab11A-positive recycling endosomes in arterial myocytes. PKG activation stimulates rapid anterograde trafficking of β1 subunits, which associate with surface BK channels, leading to channel activation and vasodilation. These data describe a unique mechanism by which ion channel activity is controlled in cells. We show that rapid and regulated anterograde trafficking of β1, a BK channel auxiliary subunit, controls BK channel activity and myocyte contractility. This study raises the possibility that regulated and rapid trafficking of auxiliary subunits may be a common mechanism to control the activity of many different ion channels and the physiological functions of a multitude of cell types.
In the absence of exogenous stimuli, a very small proportion of total β1 is surface-localized in arterial myocytes. l-NNA, brefeldin, nocodazole, Rab11A knockdown, and Rab11A mutants alone did not alter basal surface β1 levels, suggesting that endothelial cell-mediated NO release and a sGC-, PKG-, and Rab11A-independent mechanism controls basal surface expression of β1 protein. Carbachol, iloprost, and PKG and PKA (based on data with forskolin) activation increased surface β1 protein. An elevation in surface β1 may have occurred because of stimulation of anterograde trafficking and/or inhibition of subunit internalization. Data with brefeldin, nocodazole, Rab11A knockdown, and Rab11A mutants indicate that stimulated anterograde trafficking is the primary mechanism elevating surface β1. These experiments also suggest that β1-containing Rab11A-positive recycling endosomes directly bud from the ER−Golgi complex in arterial myocytes. shRNA reduced Rab11A protein by half, which abolished SNP-induced β1 surface trafficking but did not ablate the FRET signal between Rab11A and β1. These data suggest that partial Rab11A suppression does not prevent the formation of some β1-containing, Rab11A-positive recycling endosomes but blocks PKG-mediated stimulation of anterograde trafficking. BK channel activation was the primary mechanism mediating NO-induced vasodilation. Iberiotoxin and Rab11A knockdown did not abolish NO-induced vasodilation, supporting evidence that BK channel-independent mechanisms, including the activation of other K+ channel types and a reduction in contractile apparatus Ca2+ sensitivity also contribute (29). Downstream targets of PKG/PKA activation that stimulate recycling endosome-mediated β1 subunit trafficking were not determined here. PKG and PKA phosphorylate a wide variety of proteins in arterial myocytes (30). Phosphorylation targets may include Rab11A or other regulatory protein(s) that stimulate this anterograde trafficking pathway. In support of this conclusion, NO stimulated β1 trafficking more than the constitutively active Rab11A mutant (Rab11AQ70L), suggesting that PKG acts via proteins other than Rab11A, which amplify β1 anterograde trafficking. Given the number of potential targets for these kinases, regulatory proteins involved were not identified here. In the absence of exogenous stimuli, Con A elevated surface β1, suggesting that β1 recycles. To test the hypothesis that PKA and PKG also elevate surface β1 by inhibiting retrograde trafficking would require identification of the pathway that internalizes this protein in arterial myocytes. Given that the Rab family alone contains more than 50 members, such an investigation was beyond the scope of the current study. Future studies should aim to identify the β1 subunit internalization pathway.
Previous studies have investigated the composition of BK channels formed from recombinant BKα and β1 subunits overexpressed in immortalized cells (31). These experiments suggested that a recombinant BK channel tetramer can contain one to four β1 subunits and that BKα can associate with β1 in a 1:1 ratio (17, 32). These studies also indicated that the α:β1-tetramer ratio can shift BK channel voltage dependence and Ca2+ dependence (31). One focus of our study was to measure whether stimulated anterograde β1 subunit trafficking shifts BK channel properties consistent with an increase in channel-associated β1, leading to a functional response. The data indicate that native BK channel subunit composition is not rigid, but dynamic, and can be rapidly modulated by physiological vasoregulatory stimuli to control activity in arterial myocytes. In myocytes, BK channels are activated by Ca2+ sparks, with β1 subunit knockout attenuating coupling to these local Ca2+ transients (15, 33, 34). Our data indicate that only a small fraction of total β1 is plasma membrane-localized under basal conditions. It is likely that BK channels within the vicinity of Ca2+ sparks contain β1 subunits, although the subunit ratio of these coupled channels is unclear and remains to be determined. Conceivably, other intracellular signals or Rabs may promote basal β1 trafficking, which then provides a baseline level of BK channel coupling to Ca2+ sparks. SNP and forskolin elevate both Ca2+ spark frequency and transient BK current frequency and amplitude in arterial myocytes, inducing vasodilation (35). This kinase-mediated vasodilation is dependent upon BK channel activation, which we show is blocked by inhibition of β1 subunit trafficking. Thus, β1 subunit anterograde trafficking is likely to be a major contributor to the NO-induced, PKG-stimulated increase in transient BK current frequency and amplitude.
Four BK channel β subunit isoforms exist that exhibit tissue-specific expression in cells including those in the brain, kidney, immune system, and adrenal gland (17). Our findings suggest that the composition of BK channels containing other β subunit isoforms may also be dynamic and subject to rapid and precise regulation to control activity and physiological functions. In addition, many other ion channel pore-forming subunits can traffic to the plasma membrane independently of their auxiliary subunits, including KV (6), KATP (7), GABA (8), and epithelial sodium channel (9). The data here raise the possibility that the subunit composition of many ion channels may be dynamically regulated to alter activity and function in a wide variety of cell types. In summary, our study describes a unique mechanism that controls ion channel activity. We show that regulated and rapid recycling endosome-mediated β1 subunit anterograde trafficking controls BK channel subunit composition and activity in arterial myocytes and vascular contractility.
Materials and Methods
Expanded information can be found in SI Materials and Methods.
Tissue Preparation.
Animal protocols were reviewed and approved by the Animal Care and Use Committee at the University of Tennessee Health Science Center. Adult male Sprague–Dawley rats (7–9 wk of age) were used for all experiments on rodents. Small (<200-μm-diameter) cerebral and mesenteric arteries were dissected and cleaned of connective tissue. Myocytes were isolated as previously described (36). A human brain sample, from which small (<200-μm-diameter) cerebral arteries were obtained, was obtained after institutional review board approval, written informed consent, and in accordance with the guidelines of the Declaration of Helsinki.
Surface Biotinylation of Intact Arteries.
Biotinylation of intact arteries was performed as previously described (2). Arteries were incubated in a mixture each of EZ-Link Sulfo-NHS-LC-LC-Biotin and Maleimide-PEG2-Biotin reagents (Thermo Fisher Scientific) for 1 h. Arteries were washed with 100 mM glycine in PBS for 15 min to remove any unbound biotin. Arteries were then washed in PBS, placed in ice-cold lysis buffer, and homogenized in lysis buffer. Cellular debris was removed by centrifugation. Total protein was then determined to allow normalization for avidin pull down of biotinylated surface proteins. Following pull-down, the supernatant comprised of the nonbiotinylated (intracellular) protein fraction. Biotinylated surface proteins were eluted from the avidin beads.
Western Blotting.
Surface and intracellular proteins were analyzed using Western blotting [7.5% (wt/vol) SDS polyacrylamide gels] and probed with either mouse monoclonal anti-BKα (1:500; NeuroMab, University of California), rabbit polyclonal anti-BKβ1 (1:500; Abcam), or mouse monoclonal anti-rab11A (1:500; Abcam) antibodies. Blots were cut at ∼50 kDa to allow simultaneous probing for both BKα and β1 subunits. Proteins were visualized using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). Band intensities were analyzed using Quantity One software (Bio-Rad).
Confocal Imaging and Immunofluorescence Resonance Energy Transfer Microscopy.
Fixed, permeabilized myocytes were incubated with the same anti-BKα, anti-β1, or anti-rab11A antibodies used for Western blotting experiments or ER-Tracker Green (Life Technologies). Alexa 488- or 546-conjugated secondary antibodies were used. Fluorescence images were acquired using a laser-scanning confocal microscope (LSM5 Pascal; Carl Zeiss). Percentage of weighted colocalization was calculated using Pascal system-embedded software program. For N-FRET analysis, images were background-subtracted and N-FRET was calculated on a pixel-by-pixel basis for the entire image and in regions of interest using the Xia method (37) and Zeiss LSM FRET Macro tool Version 2.5, as previously described (38).
Co-IP.
For each experiment, lysate was harvested from cerebral arteries pooled from six rats using ice-cold radioimmunoprecipitation buffer. Co-IP was performed using the Catch and Release V2.0 Coimmunoprecipitation kit (Millipore) according to the manufacturer’s instructions. Briefly, arterial lysate was incubated with control mouse IgG or BKα mouse monoclonal antibody, antibody-affinity ligand, and capture resin in the column provided. The column was centrifuged and washed, and bound proteins were released using denaturing buffer. Boiled eluate was run on a SDS/PAGE gel, and protein samples were analyzed by Western blotting using mouse monoclonal anti-BKα (NeuroMab) or rabbit polyclonal anti-BKβ1 (Abcam) and horseradish peroxidase-conjugated secondary antibodies.
Rab11A shRNA and Mutant Constructs.
A commercial Rab11A shRNA kit was obtained from OriGene Technologies. Rab11A dominant-negative (Rab11AQ70L) and constitutively active (Rab11AS25N) mutants were generated from rat rab11A cDNA obtained from a clone library (GenScript).
Transfection of Intact Cerebral Arteries.
shRNA, mutant construct, or empty vectors were transfected into arteries using either reverse permeabilization or electroporation (CUY21Vivo-SQ electroporator; Bex), as previously described (39).
Patch-Clamp Electrophysiology.
Single BK channel currents were recorded in inside-out patches from isolated cerebral artery myocytes. The pipette and bath solution both contained: 130 mM KCl, 10 mM Hepes (4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid), 1 mM MgCl2, 5 mM EGTA, 1.6 mM HEDTA (N-(2-Hydroxyethyl)ethylenediamine-N,N′,N′-triacetic acid, pH 7.2 with KOH). Free Ca2+ concentration was adjusted to between 1 and 300 µM by the addition of CaCl2, with free Mg2+ maintained at 1 mM by altering MgCl2. Free Ca2+ concentration in solutions was measured using Ca2+-sensitive (no. 476041; Corning) and reference (no. 476370; Corning) electrodes. Inside-out patch experiments were performed at a membrane voltage of −40 mV. BK currents were filtered at 1 kHz and digitized at 5 kHz. Analysis was performed offline using Clampfit 9.2 (MDS Analytical Technologies). Ca2+-sensitivity data were fit with an unconstrained single Boltzmann function.
Pressurized Artery Diameter Measurements.
Experiments were performed using endothelium-denuded rat cerebral arteries cannulated at each end in a perfusion chamber (Living Systems Instrumentation). Arterial diameter was measured at 1 Hz using a CCD camera. Myogenic tone (percentage) was calculated as follows: 100 × (1 – Dactive/Dpassive), where Dactive is active arterial diameter, and Dpassive is the diameter determined in the presence of Ca2+-free physiological saline solution supplemented with 5 mmol/L EGTA.
Statistical Analysis.
Values are expressed as means ± SEM. Student t test was used for comparing paired and unpaired data from two populations, and ANOVA with Student–Newman–Keuls post hoc test used for multiple group comparisons. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Drs. Alejandro Dopico and Simon Bulley for critical reading of the manuscript. This research was supported by National Institutes of Health Grants R01 HL67071, HL110347, and HL094378 (to J.H.J.) and an American Heart Association postdoctoral fellowship (to M.D.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317527111/-/DCSupplemental.
References
- 1.Niemeyer BA, et al. Ion channels in health and disease. 83rd Boehringer Ingelheim Fonds International Titisee Conference. EMBO Rep. 2001;2(7):568–573. doi: 10.1093/embo-reports/kve145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannister JP, et al. Smooth muscle cell α2δ-1 subunits are essential for vasoregulation by CaV1.2 channels. Circ Res. 2009;105(10):948–955. doi: 10.1161/CIRCRESAHA.109.203620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathie A, Rees KA, El Hachmane MF, Veale EL. Trafficking of neuronal two pore domain potassium channels. Curr Neuropharmacol. 2010;8(3):276–286. doi: 10.2174/157015910792246146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandenberghe W, Nicoll RA, Bredt DS. Stargazin is an AMPA receptor auxiliary subunit. Proc Natl Acad Sci USA. 2005;102(2):485–490. doi: 10.1073/pnas.0408269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu YH, Alvarez-Baron C, Kim EY, Dryer SE. Dominant-negative regulation of cell surface expression by a pentapeptide motif at the extreme COOH terminus of an Slo1 calcium-activated potassium channel splice variant. Mol Pharmacol. 2010;77(4):497–507. doi: 10.1124/mol.109.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutsch E, et al. Kv2.1 cell surface clusters are insertion platforms for ion channel delivery to the plasma membrane. Mol Biol Cell. 2012;23(15):2917–2929. doi: 10.1091/mbc.E12-01-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makhina EN, Nichols CG. Independent trafficking of KATP channel subunits to the plasma membrane. J Biol Chem. 1998;273(6):3369–3374. doi: 10.1074/jbc.273.6.3369. [DOI] [PubMed] [Google Scholar]
- 8.Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABA(A) receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev. 2011;91(3):1009–1022. doi: 10.1152/physrev.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wesch D, et al. Differential N termini in epithelial Na+ channel δ-subunit isoforms modulate channel trafficking to the membrane. Am J Physiol Cell Physiol. 2012;302(6):C868–C879. doi: 10.1152/ajpcell.00255.2011. [DOI] [PubMed] [Google Scholar]
- 10.Mulvany MJ, Aalkjaer C. Structure and function of small arteries. Physiol Rev. 1990;70(4):921–961. doi: 10.1152/physrev.1990.70.4.921. [DOI] [PubMed] [Google Scholar]
- 11.Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci. 2009;66(5):852–875. doi: 10.1007/s00018-008-8609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee US, Cui J. BK channel activation: Structural and functional insights. Trends Neurosci. 2010;33(9):415–423. doi: 10.1016/j.tins.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu R, et al. MaxiK channel partners: Physiological impact. J Physiol. 2006;570(Pt 1):65–72. doi: 10.1113/jphysiol.2005.098913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- 15.Brenner R, et al. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 2000;407(6806):870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 16.Patterson AJ, Henrie-Olson J, Brenner R. Vasoregulation at the molecular level: A role for the beta1 subunit of the calcium-activated potassium (BK) channel. Trends Cardiovasc Med. 2002;12(2):78–82. doi: 10.1016/s1050-1738(01)00146-3. [DOI] [PubMed] [Google Scholar]
- 17.Wu RS, Marx SO. The BK potassium channel in the vascular smooth muscle and kidney: α- and β-subunits. Kidney Int. 2010;78(10):963–974. doi: 10.1038/ki.2010.325. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho-de-Souza JL, Varanda WA, Tostes RC, Chignalia AZ. BK channels in cardiovascular diseases and aging. Aging Dis. 2013;4(1):38–49. [PMC free article] [PubMed] [Google Scholar]
- 19.Berney C, Danuser G. FRET or no FRET: A quantitative comparison. Biophys J. 2003;84(6):3992–4010. doi: 10.1016/S0006-3495(03)75126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chardin P, McCormick F. Brefeldin A: The advantage of being uncompetitive. Cell. 1999;97(2):153–155. doi: 10.1016/s0092-8674(00)80724-2. [DOI] [PubMed] [Google Scholar]
- 21.Lapierre LA, et al. Rab11b resides in a vesicular compartment distinct from Rab11a in parietal cells and other epithelial cells. Exp Cell Res. 2003;290(2):322–331. doi: 10.1016/s0014-4827(03)00340-9. [DOI] [PubMed] [Google Scholar]
- 22.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2(2):107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 23.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 24.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5(2):121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 25.Sönnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149(4):901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butterworth MB, et al. Rab11b regulates the trafficking and recycling of the epithelial sodium channel (ENaC) Am J Physiol Renal Physiol. 2012;302(5):F581–F590. doi: 10.1152/ajprenal.00304.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox DH, Aldrich RW. Role of the beta1 subunit in large-conductance Ca(2+)-activated K(+) channel gating energetics. Mechanisms of enhanced Ca(2+) sensitivity. J Gen Physiol. 2000;116(3):411–432. doi: 10.1085/jgp.116.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bukiya AN, Vaithianathan T, Toro L, Dopico AM. Channel beta2-4 subunits fail to substitute for beta1 in sensitizing BK channels to lithocholate. Biochem Biophys Res Commun. 2009;390(3):995–1000. doi: 10.1016/j.bbrc.2009.10.091. [DOI] [PubMed] [Google Scholar]
- 29.Morgado M, Cairrão E, Santos-Silva AJ, Verde I. Cyclic nucleotide-dependent relaxation pathways in vascular smooth muscle. Cell Mol Life Sci. 2012;69(2):247–266. doi: 10.1007/s00018-011-0815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62(3):525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang YW, Ding JP, Xia XM, Lingle CJ. Consequences of the stoichiometry of Slo1 alpha and auxiliary beta subunits on functional properties of large-conductance Ca2+-activated K+ channels. J Neurosci. 2002;22(5):1550–1561. doi: 10.1523/JNEUROSCI.22-05-01550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knaus HG, Garcia-Calvo M, Kaczorowski GJ, Garcia ML. Subunit composition of the high conductance calcium-activated potassium channel from smooth muscle, a representative of the mSlo and slowpoke family of potassium channels. J Biol Chem. 1994;269(6):3921–3924. [PubMed] [Google Scholar]
- 33.Nelson MT, et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270(5236):633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 34.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278(2):C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 35.Porter VA, et al. Frequency modulation of Ca2+ sparks is involved in regulation of arterial diameter by cyclic nucleotides. Am J Physiol. 1998;274(5 Pt 1):C1346–C1355. doi: 10.1152/ajpcell.1998.274.5.C1346. [DOI] [PubMed] [Google Scholar]
- 36.Jaggar JH. Intravascular pressure regulates local and global Ca(2+) signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2001;281(2):C439–C448. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- 37.Xia Z, Liu Y. Reliable and global measurement of fluorescence resonance energy transfer using fluorescence microscopes. Biophys J. 2001;81(4):2395–2402. doi: 10.1016/S0006-3495(01)75886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao G, et al. Type 1 IP3 receptors activate BKCa channels via local molecular coupling in arterial smooth muscle cells. J Gen Physiol. 2010;136(3):283–291. doi: 10.1085/jgp.201010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narayanan D, et al. Smooth muscle cell transient receptor potential polycystin-2 (TRPP2) channels contribute to the myogenic response in cerebral arteries. J Physiol. 2013;591(Pt 20):5031–5046. doi: 10.1113/jphysiol.2013.258319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.