Abstract
Context
Aspirin is widely used for relief of pain and for cardio-protective effects. Its use is of concern to ophthalmologists when ocular surgery is being considered and also in the presence of age-related macular degeneration (AMD).
Objective
To examine the association of regular aspirin use with incidence of AMD.
Design, Setting, and Participants
A longitudinal population-based study of age-related eye diseases in Beaver Dam, Wisconsin. Examinations were performed every 5 years over a 20-year period (1988–1990 through 2008–2010). Participants were aged 43–86 years at the baseline examination. At subsequent examinations, study participants were asked if they had regularly used aspirin at least twice a week for more than 3 months.
Main Outcome Measure
The incidence of early AMD, late AMD, and 2 subtypes of late AMD (neovascular AMD and pure geographic atrophy) were assessed in retinal photographs according to the Wisconsin Age-Related Maculopathy Grading System.
Results
The mean duration of follow-up was 14.8 years. There were 512 incident cases of early (of 6243 person-visits at risk) and 117 incident cases of late AMD (of 8675 person-visits at risk) over the course of the study. Regular aspirin use 10 years prior to retinal examination was associated with late AMD (hazard ratio 1.63; 95% CI 1.01–2.63; P=0.05) with estimated incidence of 1.76% (1.17–2.64) in regular users and 1.03% (0.70–1.51) in non-users. For subtypes of late AMD, regular aspirin use 10 years prior to retinal examination was significantly associated with neovascular AMD (reported as hazard ratio 2.20; 95% CI 1.20–4.15; P=0.01) but not pure geographic atrophy (0.66; 0.25–1.95; P=0.45). Aspirin use 5 (0.86; 0.71–1.05; P=0.13) or 10 years prior (0.86; 0.65–1.13; P=0.28) to retinal examination was not associated with incident early AMD.
Conclusions
Among persons aged 43 years and older followed for 20 years, aspirin use 5 years prior to observed incidence was not associated with incident early or late AMD. However, regular aspirin use 10 years prior was associated with a small but statistically significant increase in the risk of incident late and neovascular AMD.
Keywords: aspirin, age-related macular degeneration, epidemiology
Aspirin use in the United States is widespread, with an estimated 19.3% of adults reporting regular consumption, and reported use increases with age.1 Aspirin is used for temporary relief of pain and for arthritic or rheumatologic diseases2 and for its anti-pyretic effects. It is considered a non-steroidal anti-inflammatory drug (NSAID) but it also suppresses thromboxanes by inactivation of cyclooxygenase, thus impairing the clot-enhancing action of platelets. This has made it attractive as a medical intervention for acute myocardial infarction; about half of persons who were told that they have heart disease reported taking aspirin every day or every other day.1
The results of cross-sectional studies of aspirin use and its relation to age-related macular degeneration (AMD) have been inconsistent.3–5 AMD is a potentially blinding condition whose prevalence and incidence is increasing with the increased survival of the population, and regular use of aspirin is common and becoming more widespread in persons in the age range at highest risk for this disease. Therefore, it is imperative to further examine this potential association. The Beaver Dam Eye Study, a longitudinal study of age-related eye diseases, has followed an adult population aged 43–86 years at baseline at 5-year intervals over a 20-year period. This study provided the unique opportunity to investigate the link between AMD and aspirin use in a population which, by virtue of its age distribution and low attrition, permitted examination of the associations of aspirin use 5 and 10 years before observed incidence.
Methods
Participants
A private census of Beaver Dam, Wisconsin, was performed in 1987–1988 to identify all residents eligible for the study.6 Participants were examined at the baseline examination (1988–1990) and every five years thereafter (1993–1995, 1998–2000, 2003–2005, 2008–2010) over a 20-year period. All data were collected with Institutional Review Board approval from the University of Wisconsin-Madison in conformity with all federal and state laws, the work was HIPAA compliant, and the study adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from every participant at each examination.
Participants were examined at the study site, a nursing home, or their home. By design, participants were requested to be seen on or near the anniversary date of their first examination. In this way, examinations occurred at regular 5-year intervals. For all person-visits included in analyses, 86% of visits occurred within 6 months of the target visit date. The same protocols for measurements relevant to this investigation were used at each examination.7 Participants were asked if they regularly used aspirin at least twice per week for more than 3 months. This self-report of regular aspirin use was the main exposure measure of interest in our primary analysis because it was asked at every examination. Additional information concerning frequency of aspirin use (<1 every other day, 1 every other day, 1/day, 2/day, 3–7/day or ≥8/day) and dosage were obtained at the third, fourth, and fifth examinations. These data were used to calculate an estimated dose (in milligrams) per day and were used for auxiliary analyses to examine the potential dosing effect of aspirin on incidence of AMD.
Participants were asked to bring all currently used medications to the examinations. All medications, including NSAIDs and anticoagulants (eg, warfarin), were recorded. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, and/or history of blood pressure medication use. Blood samples were obtained and analyzed for glycosylated hemoglobin A1c and inflammatory factors, eg, leukocyte count and C-reactive protein (CRP). CRP was measured only at the baseline examination, and leukocyte count was measured at the baseline and second examinations. Diabetes was defined as self-report confirmed by use of insulin or diet to control diabetes, self-report with glycosylated hemoglobin A1c level above 6.5%, or no self-report with glycosylated hemoglobin A1c above 7%.8
Photographs of the retina7 were taken after pupillary dilation according to protocol9 and graded in masked fashion by experienced graders using the Wisconsin Age-Related Maculopathy Grading System to assess the presence and severity of lesions associated with AMD.9–11 Grading procedures, lesion descriptions, and detailed definitions of presence and severity appear elsewhere (see eMethods).9
The natural progression of this disease is described by the increase in level of severity. It is generally understood that an eye will have transitioned through each previous level (lower number in the scoring system) when it presents at a given severity level.
Statistical Analysis
We examined the relationship between self-reported regular aspirin use and incidence of early and late AMD in the presence of other known risk variables over 20 years of follow-up. Presence of early or late AMD was analyzed by person, combining the data from both eyes. Person-level variables were calculated at each visit for presence of early, late, neovascular AMD and pure GA. At a given visit, a person was considered free from a given type of AMD if both eyes were gradable and determined to be free of that type of AMD. A person was considered to have a specific type of AMD at a given examination if at least one eye had gradable photos and was determined to be prevalent of the given type of AMD. If information from one eye was missing and the other eye was free from the given type of AMD, the person-level data was considered missing at that examination.
To be eligible for incidence of a specified type of AMD (early, late, neovascular, pure GA), a participant must have been free of the given AMD outcome at the baseline examination and have complete AMD data from consecutive follow-up examinations, until incidence or censoring occurred. Further, to be included in analyses, a participant must have had complete data for self-reported aspirin use, age, sex, education, history of arthritis, and history of CVD.
The two types of late AMD are not mutually exclusive, and both types may appear in the same eye sequentially or simultaneously. Incidence of late AMD was calculated as the first incidence of pure GA or neovascular AMD. We followed the commonly accepted paradigm that once a person develops neovascular AMD they cannot be further classified as having developed pure GA (despite a change in the appearance of the fundus lesion); therefore, while a person who has prevalent or incident pure GA is still at risk of developing neovascular AMD, the converse is not true. In this way, there were more persons at risk of developing neovascular AMD than any late AMD or pure GA. Similarly, participants with early AMD were still at risk for developing late AMD; therefore, there were more participants in the risk set for late AMD than for early AMD.
For preliminary analyses, we calculated the overall percentage of persons incident for each combination of aspirin use 5 and 10 years prior to observed incidence (none at 5 and none at 10, 5 years only, 10 years only, 5 and 10 years). We then calculated the age- and sex-adjusted percentages for incidence for each combination. To explore the potential longitudinal association between aspirin use and AMD, we computed hazard ratios (HRs) for incidence of early and late AMD over 20 years with time-varying covariates updated at each examination. As the first incident cases were observed at the second examination and the main risk factor of interest was aspirin use at baseline, we refer to this as aspirin use “5 years prior”. We also considered the hypothesis that the association between aspirin and development of AMD may not be apparent with exposure at only 5 years prior to incidence. Therefore, for this analysis we accounted for aspirin use at the examination 5 years prior to incidence as well as aspirin use reported at the previous examination, 10 years prior to observed incidence. When examining data which included aspirin use 10 years prior to incidence, those cases incident at the first interval were not included because aspirin use 5 years prior to the baseline examination was unknown. Because of this, the total interval for the longitudinal analysis of 10-year aspirin use is 15 years.
To establish the maximally adjusted statistical models, variables potentially associated with risk of AMD were first analyzed individually in age- and sex-adjusted models. These variables included body mass index, annual income, education, diabetes, systolic and diastolic blood pressure, hypertension, history of cancer, smoking (never, past, current), ever drinking, ever heavy drinking, history of arthritis, and history of CVD. All significant factors in the age- and sex-adjusted models were then included in a maximally adjusted model. The maximally adjusted model for early AMD included age, sex, education level, ever heavy drinking, smoking, and history of arthritis. The maximally adjusted model for late AMD and its subtypes included age, age2, sex, education, heavy drinking history, and smoking. Lastly, non-significant predictors from the maximally adjusted model were removed to establish the most parsimonious model; only these data are presented. This resulted in adjustment for age, arthritis history, and education level in models for early AMD and age, age2, and education level in models for late AMD. Interactions for potential reasons for aspirin use (arthritis and CVD history) with aspirin were tested.
To assess whether the timing of visits was driven by confounding factors, visits were divided into 3 groups: early (at least 6 months before the targeted visit date [the anniversary of the baseline visit]), late (more than 6 months after the targeted visit date), and on time (within 6 months of the targeted visit date). For the sensitivity analysis, observations from early or late visits were censored. The point estimates and confidence intervals (CIs) in the two models were very consistent; therefore the full models are presented.
To explore whether frequency and amount of aspirin used was associated with AMD, we examined the association between self-reported daily dose of aspirin (in milligrams) on the incidence of early and late AMD with available data from the third, fourth, and fifth examinations. We also modeled the effect of inflammatory factors (leukocyte count, interleukin-6, and CRP) on the association between aspirin and incidence of AMD. We then examined the relationship between any NSAID and incidence of AMD, and the relationship between warfarin and incidence of AMD.
All models presented were fitted using the discrete-time hazard model using the complementary log-log link function with time-varying predictors, with P values representing a 2-tailed test of significance with alpha level 0.05.12 In this way, risk variables (eg, use of aspirin 5 and 10 years previously) were updated throughout the course of the study and the model thus captures the change in risk for incidence of AMD, and censoring is accounted for appropriately. SAS software version 9.3 (SAS Institute, Cary, NC) was used for all analyses.
We also conducted a secondary, exploratory analysis to examine whether the data supported the notion that time since first report of regular aspirin use was associated with incidence of late AMD. For these models, the outcome of interest was incidence of AMD between the fourth and fifth examinations. Our exposure variable was first self-reported aspirin use 5, 10, 15, or 20 years prior to observed incidence, which was examined in two ways. First, we included only participants who reported using aspirin consistently at each examination following their first self-reported use, or never reported using aspirin regularly. Next, we included participants who were inconsistent in reporting regular aspirin use following their first self-report of regular aspirin use. Participants with missing aspirin data were excluded from both of these analyses. For these models, logistic regression was used with a two-tailed test of significance at alpha level 0.05.
Results
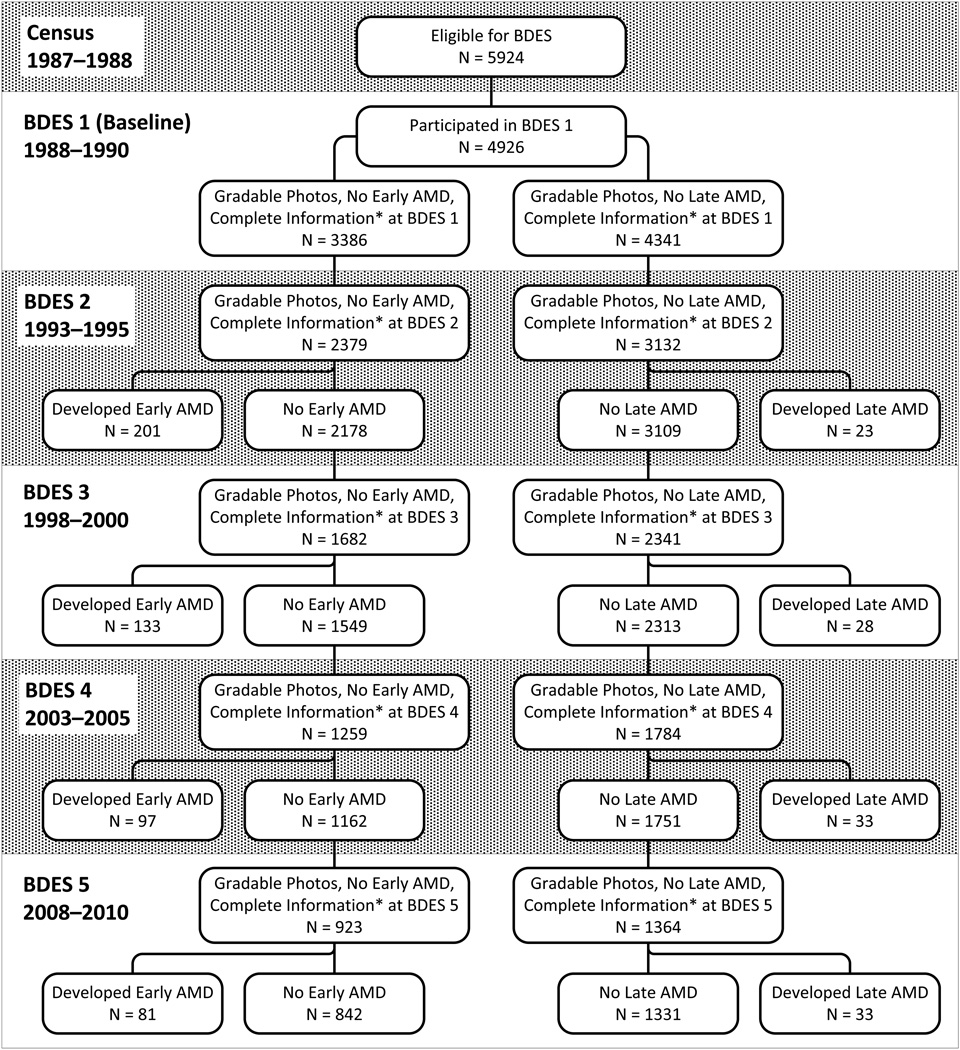
Of the 5924 eligible, 4926 (83%) persons aged 43–86 years participated in the baseline examination in 1988–1990. Ninety-nine percent of the population was white and 56% was female. The cohort was re-examined at 5- (n=3722), 10- (n=2962), 15- (n=2375) and 20-year (n=1913) follow-up examinations. There was greater than 80% participation among survivors at each examination.13–15 The mean duration of follow-up time was 14.8 years, with a median duration of 15.9 years. Participants included in these analyses tended to be younger and have fewer comorbidities at baseline than those excluded (Table 1). For incident early AMD, 2547 persons of the 4926 seen at baseline were excluded from analysis (1008 had prevalent early or late AMD at baseline, 84 persons were missing a covariate, 448 were missing AMD data at baseline, and 1007 did not have data at the first follow-up examination). Overall, there were 2379 participants at risk for early AMD, of which 512 were incident, with a total of 6243 person-visits contributing to the analysis (Figure 1). For incidence of late AMD, 1794 persons of the 4926 seen at baseline were excluded from analysis (74 persons had prevalent late AMD at baseline, 104 were missing a covariate, 407 had missing AMD data at baseline, and 1209 had missing data at the first follow-up examination). There were 3132 participants at risk for developing late AMD, of which 117 were incident, with a total of 8621 person-visits included in analyses (Figure 2). The unadjusted incidence rate per 10 person-years was 0.164 for early AMD and 0.027 for late AMD.
Table 1.
Baseline Characteristics of the Beaver Dam Eye Study Population and Those Included and Excluded from Analyses.
| Whole Population (N=4926) |
Included* (N=3206) |
Excluded* (N=1720) |
||||
|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | Mean | SD |
| Age (years) | 62.0 | 11.2 | 59.3 | 10.0 | 67.2 | 11.6 |
| Body mass index (kg/m2) | 28.8 | 5.4 | 28.8 | 5.3 | 28.7 | 5.5 |
| Systolic BP (mmHg) | 132.1 | 20.5 | 130.1 | 19.2 | 135.9 | 22.2 |
| N | % | N | % | N | % | |
| Sex | ||||||
| Women | 2762 | 56.1 | 1790 | 55.8 | 972 | 56.5 |
| Men | 2164 | 43.9 | 1416 | 44.2 | 748 | 43.5 |
| Annual income (USD) | ||||||
| ≤$9,000 | 760 | 16.3 | 354 | 11.4 | 406 | 25.7 |
| $10–19,000 | 1301 | 27.8 | 768 | 24.8 | 533 | 33.8 |
| $20–29,000 | 946 | 20.2 | 677 | 21.9 | 269 | 17.1 |
| $30–44,000 | 956 | 20.5 | 723 | 23.4 | 233 | 14.8 |
| ≥ $45,000 | 709 | 15.2 | 573 | 18.5 | 136 | 8.6 |
| Education | ||||||
| Less than high school | 1440 | 29.3 | 719 | 22.4 | 721 | 42.1 |
| High school | 2134 | 43.4 | 1480 | 46.2 | 654 | 38.2 |
| College | 701 | 14.2 | 498 | 15.5 | 203 | 11.8 |
| More than college | 645 | 13.1 | 509 | 15.9 | 136 | 7.9 |
| Smoking status | ||||||
| Never | 2204 | 44.8 | 1433 | 44.7 | 771 | 44.9 |
| Past | 1747 | 35.5 | 1148 | 35.8 | 599 | 34.9 |
| Current | 970 | 19.7 | 624 | 19.5 | 346 | 20.2 |
| Diabetes present | ||||||
| No | 4460 | 91.0 | 2984 | 93.4 | 1476 | 86.5 |
| Yes | 441 | 9.0 | 211 | 6.6 | 230 | 13.5 |
| Hypertension† present | ||||||
| No | 2428 | 49.4 | 1723 | 53.8 | 705 | 41.2 |
| Yes | 2489 | 50.6 | 1482 | 46.2 | 1007 | 58.8 |
| History of CVD | ||||||
| No | 4124 | 84.9 | 2843 | 89.3 | 1281 | 76.6 |
| Yes | 731 | 15.1 | 339 | 10.7 | 392 | 23.4 |
| History of heavy drinking | ||||||
| No | 4068 | 82.8 | 2677 | 83.6 | 1391 | 81.4 |
| Yes | 844 | 17.2 | 526 | 16.4 | 318 | 18.6 |
| Using aspirin | ||||||
| No | 3816 | 77.6 | 2513 | 78.4 | 1303 | 76.2 |
| Yes | 1101 | 22.4 | 693 | 21.6 | 408 | 23.8 |
AMD, age-related macular degeneration; BP, blood pressure; CVD, cardiovascular disease; GA, geographic atrophy; SD, standard deviation; USD, United States dollars.
Included = participant data included in ≥1 analysis (incidence of early AMD, late AMD, neovascular AMD and/or pure GA); Excluded = Participant data excluded from all analyses
Defined as systolic BP ≥ 140 mmHg and/or diastolic BP ≥90 mmHg and/or use of antihypertensive medication(s).
Figure.
Numbers of participants at each phase of the Beaver Dam Eye Study included in analyses of incidence of early and late age-related macular degeneration. *Complete information includes complete data on self-reported use of aspirin, age, education, and (for early AMD) history of arthritis.
There was no significant association of self-reported aspirin use 5 years prior to observed incidence of early AMD accumulated over 20 years (HR 0.86; 95% CI 0.71–1.05; P=0.13; age-sex adjusted incidence for users 9.55% [95% CI 8.27–11.01] vs. non-users 10.46% [95% CI 9.46–11.56], Table 2). The incidence of late AMD was greater in persons using aspirin 5 years previously than in non-users (age-sex adjusted incidence 1.4% [95% CI 0.97–1.87] vs. 1.0% [95% CI 0.74–1.39]), although the association was not significant (HR 1.21; 95% CI 0.84–1.74; P=0.31), and there was no significant association for either late AMD subtype (neovascular AMD: HR 1.07, 95% CI 0.68–1.67, P= 0.77; pure GA: HR 1.65, 95% CI 0.91–2.99, P=0.10) for those who reported aspirin use 5 years prior (age-sex adjusted incidence for neovascular AMD: 0.84% [95% CI 0.54–1.29]; pure GA: 0.59% [95% CI 0.36–0.95]) versus those who did not (age-sex adjusted incidence for neovascular AMD: 0.69% [0.48–0.99], pure GA: 0.35% [0.20–0.61], Table 2).
Table 2.
Relationships of Incidence of Age-related Macular Degeneration Outcomes with Self-Reported Regular Aspirin Use 5 Years Prior Over 20 Years in the Beaver Dam Eye Study.
| Person-visits |
||||||
|---|---|---|---|---|---|---|
| Incident AMD Outcome |
Using Aspirin 5 Years Prior to Incidence |
N at Risk |
N Incident Cases |
Age-Sex Adjusted % Incidence (95% CI) |
HR (95% CI) | P value |
| Early AMD* | No | 4398 | 348 | 10.5 (9.5, 11.6) | Referent | 0.13 |
| Yes | 1845 | 164 | 9.6 (8.3, 11.0) | 0.86 (0.71, 1.05) | ||
| Any Late AMD† | No | 5957 | 62 | 1.0 (0.7, 1.4) | Referent | 0.31 |
| Yes | 2664 | 55 | 1.4 (1.0, 1.9) | 1.21 (0.84, 1.74) | ||
| Neovascular AMD† | No | 5994 | 44 | 0.7 (0.5, 1.0) | Referent | 0.77 |
| Yes | 2681 | 34 | 0.8 (0.5, 1.3) | 1.07 (0.68, 1.67) | ||
| Pure GA† | No | 5915 | 20 | 0.4 (0.2, 0.6) | Referent | 0.10 |
| Yes | 2633 | 24 | 0.6 (0.4, 1.0) | 1.65 (0.91, 2.99) | ||
AMD, age-related macular degeneration; CI, confidence interval; GA, geographic atrophy; HR, hazard ratio.
Adjusted for age, arthritis history, and education level.
Adjusted for age, age2, and education level.
Because of the possibility of a lag in effect of first reported regular use of aspirin and AMD, we examined use at both 5 and 10 years prior to observed incidence. These data were combined and modeled as a 4-level non-ordinal categorical variable (Table 3). Only incidence analysis over 15 years can be performed because of the lack of information regarding regular aspirin use prior to the baseline examination. The overall test for association was not significant for any category of aspirin use and incident early AMD (P=0.43), late AMD (P=0.20), neovascular AMD (P=0.07) and pure GA (P=0.20, Table 3). We then tested the main effects of aspirin use 5 and 10 years prior to observed incidence in this model. The main effect of aspirin use 5 years prior showed no significant association with incident early AMD (HR 0.93; 95% CI 0.70–1.23; P=0.60; age-sex adjusted incidence for users 9.0% [95% CI 7.6–10.7] vs. non-users 9.0% [95% CI 7.6–10.6]), late AMD (HR 0.91; 95% CI 0.57–1.46; P=0.69; age-sex adjusted incidence for users 1.3% [95% CI 0.9–1.9] vs. non-users 1.4% [95% CI 1.9–2.1]), neovascular AMD (HR 0.66, 95% CI 0.37–1.19; P=0.17; age-sex adjusted incidence for users 0.8% [95% CI 0.5–1.3] vs. non-users 1.1% [95% CI 0.7–1.6]), or pure GA (HR 2.25; 95% CI 0.75–6.72; P=0.15; age-sex adjusted incidence for users 0.6% [95% CI 0.4–1.1] vs. non-users 0.4% [95% CI 0.2–0.8]). The main effect of aspirin use 10 years prior was significant for predicting the incidence of late AMD (HR 1.63; 95% CI 1.01–2.63; P=0.045; age-sex adjusted incidence for users 1.8% [95% CI 1.2–2.6] vs. non-users 1.0% [95% CI 0.7–1.5]). When examining the relationships by late AMD subtype, neovascular AMD was significantly associated with such use (HR 2.20; 95% CI 1.20–4.15; P=0.01; age-sex adjusted incidence for users 1.4% [95% CI 0.9–2.1] vs. non-users 0.6% [95% CI 0.4–1.0]) but pure GA was not (HR 0.66; 95% CI 0.25–1.95; P=0.46; age-sex adjusted incidence for users 0.5% [95% CI 0.3–1.0] vs. non-users 0.5% [95% CI 0.3–0.9]). Similar analyses for the incidence of early AMD showed no significant associations with use of aspirin 10 years prior (HR 0.86; 95% CI 0.65–1.13; P=0.28; age-sex adjusted incidence for users 8.5% [95% CI 6.9–10.5] vs. non-users 9.5% [95% CI 8.3–10.9]).
Table 3.
Relationships of Incidence of Age-Related Macular Degeneration Outcomes with Self-Reported Regular Use of Aspirin 5 and 10 Years Prior to Observed Incidence Over 15 Years in the Beaver Dam Eye Study.
| Person-visits |
||||||
|---|---|---|---|---|---|---|
| N at Risk for Outcome |
N Incident Cases |
Age-Sex Adjusted % Incidence (95% CI) |
HR (95% CI) | P value | Overall P value |
|
| Early AMD | ||||||
| Aspirin Use | ||||||
| No Use 5 or 10 Years Prior | 2254 | 170 | 9.3 (8.1, 10.7) | Referent | 0.43 | |
| Use 5 Years Prior, No Use 10 Years Prior | 644 | 60 | 10.0 (7.9, 12.6) | 1.03 (0.77, 1.38) | 0.85 | |
| No Use 5 Years Prior, Use 10 Years Prior | 277 | 24 | 9.6 (6.7, 13.7) | 0.95 (0.63, 1.45) | 0.83 | |
| Use 5 and 10 Years Prior | 686 | 57 | 8.1 (6.3, 10.4) | 0.79 (0.58, 1.09) | 0.15 | |
| Test of Main Effects | ||||||
| Use vs. No Use 5 Years Prior | ||||||
| No Use 5 Years Prior | 2531 | 194 | 9.0 (7.6, 10.6) | Referent | ||
| Use 5 Years Prior | 1330 | 117 | 9.0 (7.6, 10.7) | 0.93 (0.70, 1.23) | 0.60 | |
| Use vs. No Use 10 Years Prior | ||||||
| No Use 10 Years Prior | 2898 | 230 | 9.5 (8.3, 10.9) | Referent | ||
| Use 10 Years Prior | 963 | 81 | 8.5 (6.9, 10.5) | 0.86 (0.65, 1.13) | 0.28 | |
| Any Late AMD | ||||||
| Aspirin Use | ||||||
| No Use 5 or 10 Years Prior | 3091 | 38 | 1.1 (0.7, 1.7) | Referent | 0.20 | |
| Use 5 Years Prior, No Use 10 Years Prior | 948 | 13 | 0.9 (0.5, 1.6) | 0.81 (0.44, 1.52) | 0.52 | |
| No Use 5 Years Prior, Use 10 Years Prior | 401 | 10 | 1.7 (0.9, 3.1) | 1.46 (0.73, 2.91) | 0.29 | |
| Use 5 and 10 Years Prior | 1045 | 33 | 1.8 (1.1, 2.7) | 1.48 (0.93, 2.37) | 0.10 | |
| Test of Main Effects | ||||||
| Use vs. No Use 5 Years Prior | ||||||
| No Use 5 Years Prior | 3492 | 48 | 1.4 (0.9, 2.1) | Referent | ||
| Use 5 Years Prior | 1993 | 46 | 1.3 (0.9, 1.9) | 0.91 (0.57, 1.46) | 0.69 | |
| Use vs. No Use 10 Years Prior | ||||||
| No Use 10 Years Prior | 4039 | 51 | 1.0 (0.7, 1.5) | Referent | ||
| Use 10 Years Prior | 1446 | 43 | 1.8 (1.2, 2.6) | 1.63 (1.01, 2.63) | 0.05 | |
| Neovascular AMD | ||||||
| Aspirin Use | ||||||
| No Use 5 or 10 Years Prior | 3111 | 25 | 0.7 (0.5, 1.2) | Referent | 0.07 | |
| Use 5 Years Prior, No Use 10 Years Prior | 954 | 6 | 0.4 (0.2, 1.1) | 0.58 (0.24, 1.40) | 0.23 | |
| No Use 5 Years Prior, Use 10 Years Prior | 408 | 9 | 1.5 (0.7, 2.9) | 1.92 (0.89, 4.13) | 0.10 | |
| Use 5 and 10 Years Prior | 1054 | 21 | 1.2 (0.7, 2.0) | 1.46 (0.81, 2.60) | 0.21 | |
| Test of Main Effects | ||||||
| Use vs. No Use 5 Years Prior | ||||||
| No Use 5 Years Prior | 3519 | 34 | 1.1 (0.7, 1.6) | Referent | ||
| Use 5 Years Prior | 2008 | 27 | 0.8 (0.5, 1.3) | 0.66 (0.37, 1.19) | 0.17 | |
| Use vs. No Use 10 Years Prior | ||||||
| No Use 10 Years Prior | 4065 | 31 | 0.6 (0.4, 1.0) | Referent | ||
| Use 10 Years Prior | 1462 | 30 | 1.4 (0.9, 2.1) | 2.20 (1.20, 4.15) | 0.01 | |
| Pure Geographic Atrophy | ||||||
| Aspirin Use | ||||||
| No Use 5 or 10 Years Prior | 3068 | 15 | 0.5 (0.2, 0.9) | Referent | 0.20 | |
| Use 5 Years Prior, No Use 10 Years Prior | 943 | 8 | 0.6 (0.3, 1.2) | 1.26 (0.54, 2.94) | 0.60 | |
| No Use 5 Years Prior, Use 10 Years Prior | 392 | 1 | 0.2 (0.0, 1.3) | 0.37 (0.05, 2.66) | 0.32 | |
| Use 5 and 10 Years Prior | 1025 | 13 | 0.7 (0.3, 1.3) | 1.49 (0.71, 3.12) | 0.29 | |
| Test of Main Effects | ||||||
| Use vs. No Use 5 Years Prior | ||||||
| No Use 5 Years Prior | 3460 | 16 | 0.4 (0.2, 0.8) | Referent | ||
| Use 5 Years Prior | 1968 | 21 | 0.6 (0.4, 1.1) | 2.25 (0.75, 6.72) | 0.15 | |
| Use vs. No Use 10 Years Prior | ||||||
| No Use 10 Years Prior | 4011 | 23 | 0.5 (0.3, 0.9) | Referent | ||
| Use 10 Years Prior | 1417 | 14 | 0.5 (0.3, 1.0) | 0.66 (0.25, 1.95) | 0.45 | |
AMD, age-related macular degeneration; CI, confidence interval; HR, hazard ratio.
Adjusted for age, arthritis history, and education level.
Adjusted for age, age2, and education level.
History of arthritis and CVD, two common reasons for aspirin use, were analyzed to investigate the possibility of confounding by indication. No significant interactions were found in predicting early AMD between arthritis or CVD and aspirin use 5 years prior to incidence (arthritis P=0.16, CVD P=0.45) or 5 and 10 years prior (arthritis P=0.64, CVD P=0.33). Similarly, no significant interactions were found in predicting incidence of any form of late AMD between aspirin use 5 years prior and history of arthritis (P=0.28) or CVD (P=0.62), or with aspirin use 5 and 10 years prior with arthritis (P=0.16) or CVD (P=0.43).
Milligrams of aspirin per day were calculated for the third, fourth and fifth examination phases. No significant relationship was found between milligrams of aspirin per day taken 5 years prior to observed early AMD (P=0.53) or late AMD (P=0.22). Similarly, no significant relationship was found between milligrams of aspirin reported 5 and 10 years prior to observed incidence of early AMD (P=0.27) or late AMD (P=0.37).
We examined whether the association of aspirin to neovascular AMD was related to use of any NSAID and found no relationship between the use of any NSAID 10 years prior to incidence of neovascular AMD (P=0.33). We also investigated whether warfarin was associated with incidence of late AMD or its subtypes, and found no associations between AMD and warfarin use 5 years prior (late AMD P=0.56; neovascular AMD P=0.88; pure GA P=0.52) or 10 years prior (late AMD P=0.15; neovascular AMD non-estimable; pure GA P=0.89) to observed incidence.
To examine possible effects of systemic inflammation and the possible protective role of aspirin in the presence of evidence of systemic inflammation, we examined the associations of leukocyte count and CRP with incidence of AMD and their effects on the relationship between aspirin use reported 5 years prior and incident AMD. Neither were associated with incidence of early (leukocyte count P=0.13; CRP P=0.21) or late AMD (leukocyte count P=0.56; CRP P=0.29), and neither showed a significant interaction with aspirin use (early AMD: leukocyte count P=0.87, CRP P=0.29; late AMD: leukocyte count P=0.25, CRP P=0.07). Adjusting for leukocyte count and CRP did not alter the associations seen between aspirin use and incident late AMD.
To further explore the finding that time of first reported regular aspirin use was associated with AMD, we examined the data on aspirin use only in participants with complete information on self-reported aspirin use at all study visits from the baseline visit through the fourth visit, who were free from AMD at the fourth visit, and had complete outcome information from the most recent visit (Table 4). There was no apparent relationship between the visit since first regular use of aspirin and incidence of early AMD. Results are similar for those with consistent and inconsistent use.
Table 4.
Relationship of Age-related Macular Degeneration Outcomes to Aspirin Exposure Patterns Prior to the Incidence of Age-related Macular Degeneration.
| Unadjusted | Age-Sex Adjusted | |||||
|---|---|---|---|---|---|---|
| AMD Outcome and Aspirin Exposure Pattern |
N at risk | N Incident | % Incidence (95% CI) |
OR (95% CI) | P value |
Overall P value |
| Early AMD | ||||||
| First consistent exposure | ||||||
| None | 403 | 40 | 9.4 (6.8, 12.8) | Referent | 0.52 | |
| 5 years prior | 169 | 21 | 11.7 (7.6, 17.5) | 1.28 (0.72, 2.26) | 0.41 | |
| 10 years prior | 164 | 12 | 6.3 (3.5, 11.0) | 0.65 (0.33, 1.29) | 0.22 | |
| 15 years prior | 61 | 8 | 9.5 (4.6, 18.6) | 1.01 (0.44, 2.33) | 0.98 | |
| 20 years prior | 64 | 7 | 8.9 (4.1, 18.0) | 0.94 (0.39, 2.26) | 0.89 | |
| None or at visit 4 only | 572 | 61 | 10.1 (7.8, 13.0) | Referent | ||
| 10, 15, or 20 years prior | 289 | 27 | 7.6 (5.1, 11.2) | 0.73 (0.45, 1.20) | 0.22 | |
| First exposure* | ||||||
| None | 403 | 40 | 9.6 (6.8, 12.7) | Referent | 0.48 | |
| 5 years prior | 169 | 21 | 11.7 (7.7, 17.5) | 1.29 (0.73, 2.29) | 0.39 | |
| 10 years prior | 199 | 15 | 6.6 (4.0, 10.9) | 0.69 (0.37, 1.30) | 0.25 | |
| 15 years prior | 115 | 14 | 9.3 (5.4, 15.6) | 1.00 (0.51, 1.94) | 0.99 | |
| 20 years prior | 175 | 22 | 10.6 (6.9, 15.9) | 1.15 (0.65, 2.04) | 0.63 | |
| None or at visit 4 only | 572 | 61 | 10.1 (7.8, 12.9) | Referent | ||
| 10, 15, or 20 years prior | 489 | 51 | 8.7 (6.5, 11.6) | 0.85 (0.57, 1.28) | 0.44 | |
| Any Late AMD | ||||||
| First consistent exposure | ||||||
| None | 514 | 9 | 1.1 (0.5, 2.3) | Referent | 0.53 | |
| 5 years prior | 215 | 3 | 0.9 (0.3, 2.8) | 0.81 (0.21, 3.09) | 0.76 | |
| 10 years prior | 214 | 10 | 2.1 (1.0, 4.7) | 2.02 (0.77, 5.30) | 0.15 | |
| 15 years prior | 95 | 4 | 1.5 (0.5, 4.5) | 1.38 (0.40, 4.79) | 0.62 | |
| 20 years prior | 98 | 5 | 2.0 (0.7, 5.6) | 1.85 (0.56, 6.08) | 0.31 | |
| None or at visit 4 only | 729 | 12 | 1.0 (0.5, 2.0) | Referent | ||
| 10, 15, or 20 years prior | 407 | 19 | 1.9 (1.0, 3.8) | 1.91 (0.88, 4.14) | 0.10 | |
| First exposure* | ||||||
| None | 514 | 9 | 1.1 (0.5, 2.3) | Referent | 0.64 | |
| 5 years prior | 215 | 3 | 0.9 (0.3, 2.9) | 0.81 (0.21, 3.09) | 0.76 | |
| 10 years prior | 268 | 10 | 1.6 (0.8, 3.8) | 1.62 (0.63, 4.20) | 0.32 | |
| 15 years prior | 170 | 9 | 1.9 (0.8, 4.4) | 1.79 (0.67, 4.80) | 0.25 | |
| 20 years prior | 249 | 7 | 1.2 (0.5, 2.9) | 1.11 (0.40, 3.12) | 0.84 | |
| None or at visit 4 only | 729 | 12 | 1.0 (0.5, 2.0) | Referent | ||
| 10, 15, or 20 years prior | 687 | 26 | 1.6 (0.9, 2.9) | 1.57 (0.76, 3.23) | 0.22 | |
| Pure Geographic Atrophy | ||||||
| First consistent exposure | ||||||
| None | 508 | 3 | 0.3 (0.1, 1.2) | † | ||
| 5 years prior | 214 | 2 | 0.5 (0.1, 2.4) | † | ||
| 10 years prior | 206 | 2 | 0.3 (0.1, 2.0) | † | ||
| 15 years prior | 92 | 1 | 0.3 (0.0, 2.8) | † | ||
| 20 years prior | 93 | 0 | † | |||
| None or at visit 4 only | 722 | 5 | 0.3 (0.1, 1.2) | † | ||
| 10, 15, or 20 years prior | 391 | 3 | 0.2 (0.1, 1.2) | † | ||
| First exposure* | ||||||
| None | 508 | 3 | 0.3 (0.1, 1.3) | † | ||
| 5 years prior | 214 | 2 | 0.5 (0.1, 2.5) | † | ||
| 10 years prior | 260 | 2 | 0.3 (0.1, 1.6) | † | ||
| 15 years prior | 163 | 2 | 0.6 (0.1, 2.0) | † | ||
| 20 years prior | 243 | 1 | 0.2 (0.0, 1.3) | † | ||
| None or at visit 4 only | 722 | 5 | 0.4 (0.1, 1.2) | Referent | ||
| 10, 15, or 20 years prior | 666 | 5 | 0.3 (0.1, 1.0) | 0.69 (0.19, 2.50) | 0.57 | |
| Neovascular AMD | ||||||
| First consistent exposure | ||||||
| None | 518 | 6 | 0.8 (0.3, 1.8) | Referent | 0.14 | |
| 5 years prior | 217 | 1 | 0.3 (0.0, 2.2) | 0.41 (0.05, 3.45) | 0.41 | |
| 10 years prior | 214 | 8 | 1.9 (0.8, 4.4) | 2.52 (0.83, 7.63) | 0.10 | |
| 15 years prior | 98 | 4 | 1.6 (0.5, 4.9) | 2.09 (0.56, 7.88) | 0.28 | |
| 20 years prior | 100 | 5 | 2.1 (0.7, 6.1) | 2.87 (0.80, 10.37) | 0.11 | |
| None or at visit 4 only | 735 | 7 | 0.6 (0.3, 1.4) | Referent | ||
| 10, 15, or 20 years prior | 412 | 17 | 1.8 (0.9, 3.8) | 2.99 (1.18, 7.57) | 0.02 | |
| First exposure* | ||||||
| None | 518 | 6 | 0.8 (0.3, 1.9) | Referent | 0.23 | |
| 5 years prior | 217 | 1 | 0.3 (0.0, 2.3) | 0.41 (0.05, 3.44) | 0.41 | |
| 10 years prior | 269 | 8 | 1.5 (0.7, 3.5) | 1.99 (0.66, 5.97) | 0.22 | |
| 15 years prior | 175 | 8 | 1.8 (0.8, 4.4) | 2.41 (0.79, 7.32) | 0.12 | |
| 20 years prior | 254 | 7 | 1.3 (0.5, 3.1) | 1.69 (0.55, 5.24) | 0.36 | |
| None or at visit 4 only | 735 | 7 | 0.6 (0.3, 1.4) | Referent | ||
| 10, 15, or 20 years prior | 698 | 23 | 1.5 (0.8, 2.8) | 2.41 (1.00, 5.81) | 0.05 | |
AMD, age-related macular degeneration.
Includes participants with inconsistent aspirin exposure (a participant reported aspirin use, followed by reporting no aspirin use at a later examination). This category does not include participants with reported aspirin use followed by missing aspirin use data.
Cannot estimate.
For any late AMD, participants with no aspirin use and those only with aspirin use at the visit prior to the incidence of late AMD (use at the fourth visit) had a similar incidence (1.75% and 1.40%, respectively). Those who had reported regular aspirin use 10, 15, or 20 years prior to observed incidence showed a higher incidence than those with no aspirin use or only recent aspirin use (5 years prior to observed incidence). Incidence was similar for 10, 15, and 20 years (4.67%, 4.21%, and 5.10%, respectively) since aspirin use was first consistently reported. The results are similar for those with inconsistent use. It should be noted that for late AMD, there are several cells with very low counts for incident cases.
For pure geographic atrophy, there was no discernible pattern between incidence and years since first self-reported aspirin use. For neovascular AMD, the pattern was similar to what was seen for incidence of any late AMD.
Comment
In our study, aspirin use 10 years prior to observed AMD incidence was associated with the 15-year incidence of neovascular AMD. Our exploratory analyses tend to support the findings of our primary analysis. Our hazard ratio estimate, given in Table 3, for neovascular AMD in those whose first regular use of aspirin was at least 10 years prior to observed AMD is 2.20 (95% CI 1.20–4.15). This is based on our modeling of specific potential risk factors in a Midwestern, primarily white population. While it is possible to estimate an attributable risk, the number of incident cases that our estimate is based on is small and requires corroboration before developing risk algorithms for clinical use. Adjusting for age, age2, education level and aspirin use 5 years prior to observed incidence, the attributable risk of late AMD for aspirin use 10 years prior to observed incidence was 0.77%, with adjusted attributable risk fraction of 53.2%.16 This is in keeping with the finding of a small but significant cross-sectional association between aspirin use and AMD in the EUREYE study and the inference that for a patient, aspirin use for cardio-prevention does not imply a great increase in risk of AMD.17,18 If our finding is borne out in other studies, it suggests that the effect of aspirin on mechanisms leading to AMD may be different, at least partially, from aspirin’s immediate effects on clotting that seem to be responsible for cardio-protection.19 Not all retinal lesions characterizing neovascular AMD involve bleeding that is detectable in photography. Aspirin, aside from its effects on clotting, may enhance choroidal neovascularization.20 Aspirin has been shown to increase vascular density in a laboratory model.21 Thus, it is possible that in the presence of injury, aspirin encourages the growth of aberrant new vessels.
Two studies by Christen and colleagues22,23 describing the experience in 2 large randomized controlled trials for prevention of CVD, one with a 7-year follow-up and the other with 10-year follow-up, found no evidence of a direct association of low-dose aspirin use and late lesions of AMD. Those studies were performed in health professionals who are likely to be more health conscious than general populations. The number of AMD cases was small in both studies, and the definition and method of classification of the endpoint differed from the current study and the European Eye Study,17 which both used photographic documentation and systematic grading of lesions as opposed to self-reported AMD with decreased vision confirmed by medical record. Thus, there are likely to be important differences in exposures and outcomes and ascertainment between studies that may have caused the disparate findings.
Several limitations may have affected our findings. First, there was a lack of detailed information on aspirin exposure at some visits. When the study began, questions on frequency of use and dosage were not initially included, but were added into subsequent examinations to accommodate important clinical therapeutic trends in the community, especially the increasing use of aspirin for CVD. Second, leukocyte count was only measured at the baseline and second examinations; therefore, we could not evaluate potential associations for every study interval. Similar limitations apply to CRP measures, which might have informed our analysis regarding possible effects of systemic inflammation and its potentially modifying effect on the association of AMD with aspirin. Third, the study population is almost entirely white of European ancestry, so the extent to which our results may generalize to other races/ethnicities, particularly groups at elevated risk for CVD, is unknown.
Our findings are consistent with an association between regular aspirin use and incidence of neovascular AMD. Additional replication is required to confirm our observation. If confirmed, defining the causal mechanisms will be important in developing methods to block this effect to prevent or retard the development of neovascular AMD in persons who use aspirin especially to prevent CVD.
Acknowledgments
Funding/Support: This research is supported by National Institutes of Health grant EY06594 (Drs. B. E. K. Klein, R. Klein). The National Eye Institute provided funding for entire study, including collection and analyses of data. Additional support was provided by Senior Scientific Investigator Awards from Research to Prevent Blindness (Drs B. E. K. Klein and R. Klein). Heidi M. G. Christian, BA, and Mary Kay Aprison, BS, of the Department of Ophthalmology and Visual Sciences, University of Wisconsin School of Medicine and Public Health assisted with technical editing and preparation of the manuscript. They did not receive any additional compensation beyond their normal wages as employees of the University of Wisconsin for their assistance.
Role of the Sponsor: Neither organization that provided funding had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Eye Institute or the National Institutes of Health.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. R. Klein reported serving as a consultant to Pfizer. All other authors reported no potential conflicts of interest.
Contributions of the Authors: Conception and design (BEKK, RK), acquisition of data (BEKK, JOD, RK), analysis and interpretation of data (BEKK, KPH, REG, KEL), drafting of the manuscript (BEKK, KPH), critical revision of the manuscript for important intellectual content (BEKK, KPH, REG, JOD, KEL, RK), statistical analysis (KPH, REG, KEL), obtaining funding (BEKK, RK), administrative, technical or material support (BEKK, JOD, RK).
Access to Data Statement: Dr. B. E. K. Klein had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Soni A. Agency for Healthcare Research and Quality. Rockville, MD: 2007. Aspirin Use among the Adult U.S. Noninstitutionalized Population, with and without Indicators of Heart Disease, 2005. Statistical Brief #179. [Google Scholar]
- 2.U.S. Food and Drug Administration. [Accessed January 17, 2012];Aspirin: Questions and Answers. http://www.fda.gov/drugs/resourcesforyou/consumers/questionsanswers/ucm071879.htm.
- 3.el Baba F, Jarrett WH, Harbin TS, Jr, et al. Massive hemorrhage complicating age-related macular degeneration. Clinicopathologic correlation and role of anticoagulants. Ophthalmology. 1986;93(12):1581–1592. doi: 10.1016/s0161-6420(86)33540-1. [DOI] [PubMed] [Google Scholar]
- 4.Kiernan DF, Hariprasad SM, Rusu IM, et al. Epidemiology of the association between anticoagulants and intraocular hemorrhage in patients with neovascular age-related macular degeneration. Retina. 2010;30(10):1573–1578. doi: 10.1097/IAE.0b013e3181e2266d. [DOI] [PubMed] [Google Scholar]
- 5.Tilanus MA, Vaandrager W, Cuypers MH, Verbeek AM, Hoyng CB. Relationship between anticoagulant medication and massive intraocular hemorrhage in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2000;238(6):482–485. doi: 10.1007/pl00007887. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98(8):1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Davis MD, Magli YL, et al. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98(7):1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 8.Sahakyan K, Lee KE, Shankar A, Klein R. Serum cystatin C and the incidence of type 2 diabetes mellitus. Diabetologia. 2011;54(6):1335–1340. doi: 10.1007/s00125-011-2096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein BE, Klein R, Linton KL, Magli YL, Neider MW. Assessment of cataracts from photographs in the Beaver Dam Eye Study. Ophthalmology. 1990;97(11):1428–1433. doi: 10.1016/s0161-6420(90)32391-6. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99(6):933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104(1):7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 12.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford: 2003. pp. 407–467. [Google Scholar]
- 13.Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996;103(8):1169–1178. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BE, Lee KE, Cruickshanks KJ, Chappell RJ. Changes in visual acuity in a population over a 10-year period: The Beaver Dam Eye Study. Ophthalmology. 2001;108(10):1757–1766. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- 15.Klein R, Klein BE, Lee KE, Cruickshanks KJ, Gangnon RE. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142(4):539–549. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;160(4):301–305. doi: 10.1093/aje/kwh221. [DOI] [PubMed] [Google Scholar]
- 17.de Jong PT, Chakravarthy U, Rahu M, et al. Associations between aspirin use and aging macula disorder: the European Eye Study. Ophthalmology. 2012;119(1):112–118. doi: 10.1016/j.ophtha.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Scheck A. [Accessed September 12, 2012];Daily Aspirin Use Associated With AMD. http://commonspot.aao.org/publications/eyenet/201201/news cfm#two.
- 19.Ridker PM, Manson JE, Buring JE, Goldhaber SZ, Hennekens CH. The effect of chronic platelet inhibition with low-dose aspirin on atherosclerotic progression and acute thrombosis: clinical evidence from the Physicians' Health Study. Am Heart J. 1991;122(6):1588–1592. doi: 10.1016/0002-8703(91)90275-m. [DOI] [PubMed] [Google Scholar]
- 20.Battinelli EM, Markens BA, Italiano JE., Jr Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118(5):1359–1369. doi: 10.1182/blood-2011-02-334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goertz O, Ring A, Buschhaus B, et al. Influence of anti-inflammatory and vasoactive drugs on microcirculation and angiogenesis after burn in mice. Burns. 2011;37(4):656–664. doi: 10.1016/j.burns.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Christen WG, Glynn RJ, Ajani UA, et al. Age-related maculopathy in a randomized trial of low-dose aspirin among US physicians. Arch Ophthalmol. 2001;119(8):1143–1149. doi: 10.1001/archopht.119.8.1143. [DOI] [PubMed] [Google Scholar]
- 23.Christen WG, Glynn RJ, Chew EY, Buring JE. Low-dose aspirin and medical record-confirmed age-related macular degeneration in a randomized trial of women. Ophthalmology. 2009;116(12):2386–2392. doi: 10.1016/j.ophtha.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]