Abstract
Pollination by sexual deception is arguably one of the most unusual liaisons linking plants and insects, and perhaps the most illustrative example of extreme floral specialization in angiosperms. While considerable progress has been made in understanding the floral traits involved in sexual deception, less is known about how this remarkable mimicry system might have arisen, the role of pre-adaptations in promoting its evolution and its extent as a pollination mechanism outside the few groups of plants (primarily orchids) where it has been described to date. In the Euro-Mediterranean region, pollination by sexual deception is traditionally considered to be the hallmark of the orchid genus Ophrys. Here, we introduce two new cases outside of Ophrys, in plant groups dominated by generalized, shelter-mimicking species. On the basis of phylogenetic reconstructions of ancestral pollination strategies, we provide evidence for independent and bidirectional evolutionary transitions between generalized (shelter mimicry) and specialized (sexual deception) pollination strategies in three groups of flowering plants, and suggest that pseudocopulation has evolved from pre-adaptations (floral colours, shapes and odour bouquets) that selectively attract male pollinators through shelter mimicry. These findings, along with comparative analyses of floral traits (colours and scents), shed light on particular phenotypic changes that might have fuelled the parallel evolution of these extraordinary pollination strategies. Collectively, our results provide the first substantive insights into how pollination sexual deception might have evolved in the Euro-Mediterranean region, and demonstrate that even the most extreme cases of pollinator specialization can reverse to more generalized interactions, breaking ‘Cope's rule of specialization’.
Keywords: floral scent, evolutionary transitions, mimicry, floral evolution
1. Introduction
Although a considerable proportion of flowering plants attract many pollinators of disparate phylogenetic affiliations by providing nectar, pollen or other floral products [1–7], some groups of plants have specialized upon specific (functional groups of) pollinators (reviewed by Fenster et al. [8]). From an evolutionary perspective, floral specialization has been put forward as a driving force in plant reproductive isolation [9,10] and diversification [11–15]. Why some groups of plants have shifted away from a more ‘generalized’ condition to exploit particular pollinators, which pre-adaptations might have promoted this evolutionary pathway and to what extent this evolutionary trend towards floral specialization is reversible or leads to increasing levels of specialization over time represent major issues in our understanding of the diversification of Angiosperms, and the floral morphologies, colours and scents of their flowers [16–20]. Traditionally, pollination ecologists have referred to ‘Cope's rule of specialization’ [21], which postulates that, at a macroevolutionary scale, organisms gradually develop higher degrees of specialization over time, and generally descend from generalized (or less specialized) ancestors. This hypothesis therefore assumes that specialization is a unidirectional and irreversible phenomenon. Yet reversals from specialized to generalized pollination systems that contradict Cope's rule of specialization have also been reported, highlighting that floral specialization is not necessarily an evolutionary dead end (see [19–22] and references therein).
Pollination by sexual deception is one of the most unusual and highly specialized liaisons between orchids and their pollinators. In this mimicry system, male insects are attracted to the insectiform flowers by blends of chemical compounds also found in the females' sex pheromones (cuticular hydrocarbons, in particular n-alkenes, as well as oxygenated acids, aldehydes, alcohols, fatty acids and corresponding esters) [23–27] and pollinate the flowers during attempted copulations (so-called pseudocopulation) [28–30]. These interactions are usually almost species-specific, each orchid species attracting only males of one or (more rarely) a couple of closely related insect species [25,31–34]. Despite considerable progress made in our understanding of the floral traits involved in pollination by sexual deception, little is known about the evolutionary origin of this remarkable mimicry system, the role of pre-adaptations in promoting its evolution and its extent as a pollination mechanism in flowering plants [35]. Pollination by male insects through sexual deception was described for the first time in the early twentieth century in the orchid genus Ophrys (subtribe Orchidinae). To date, this pollination strategy is thought to be the hallmark of the genus Ophrys in the Euro-Mediterranean region [28–30,36]. The exclusive attraction of male solitary bees and wasps is rare among flowering plants [37], yet species in the orchid genus Serapias (subtribe Orchidinae) and in section Oncocyclus of the genus Iris (Iridaceae) have also evolved such specialized interactions. The tunnel-like flowers of these species are generally dark red, lack UV reflection (see the electronic supplementary material, figure S1) and provide protective shelters (i.e. a non-nutritive form of reward) used by male solitary bees belonging to different families, particularly by eucerine bees (Apidae, tribe Eucerini; electronic supplementary material, table S1). The pollinators typically ignore the flowers during daytime but pollinate them late in the afternoon when searching for an overnight shelter, or during other times of the day under overcast conditions, when they can also be found sheltering in rock crevices, under flat stones or in hollow wood stems [38–40] (N. J. Vereecken 2008–2012, personal observations). Shelter-mimicking species usually attract a wide taxonomic range of pollinators (see the electronic supplementary material, table S1), and we therefore consider that they have evolved more generalized interactions with their pollen vectors than sexually deceptive orchids [41].
To date, these two pollination strategies involving the exploitation of male insects are considered to be restricted to different groups of plants—shelter mimicry is traditionally associated with all outcrossing Serapias and Oncocyclus species [38–40], and sexual deception with all outcrossing Ophrys species [25,30]. In this study, we introduce two new cases of pollination by sexual deception outside the genus Ophrys, one in the orchid genus Serapias and the other in section Oncocyclus of the genus Iris. Furthermore, our behavioural observations also confirm that Ophrys helenae, a little-known and narrow endemic species from the remote parts of Albania and northern Greece, is pollinated by shelter mimicry rather than by sexual deception. On the basis of a multidisciplinary approach that combines quantitative analyses of floral traits (floral scents and colours) and pollinator behaviour, as well as phylogenetic reconstructions and character mapping, we show for the first time (i) that these two pollination strategies are intimately linked to one another within the same groups of plants; (ii) that particular pre-adaptations in floral colour, scent and morphology have facilitated the shifts between floral shelters and sexual mimicry; and (iii) that the evolutionary transitions between sexual deception, a highly specialized mimicry system and floral shelters occurred in both directions, indicating that even the most extreme cases of floral specialization do not necessarily lead to an evolutionary dead end.
2. Material and methods
(a). Behavioural observations of pollinators
Males of Ceratina cucurbitina (Hym. Apidae) were observed, filmed and photographed over 4 successive years (2008–2011) while pseudocopulating on the floral callus of Serapias lingua. This behaviour was observed at multiple locations throughout the plant's native range—for example, in Villanova Monteleone (8 May 2009, Sardinia, Italy), Le Clapier (24 May 2007, southern France), Saint-Léons (21 May 2010, southern France).
Males of Xylocopa valga (Hym. Apidae) were observed, filmed and photographed while pseudocopulating on the reduced, velvety petals of Iris paradoxa both in the plant's native range (1 June 2007, Leriksky area, near Gosmaljan, Azerbaijan) and in cultivation (13 May 2008, Pyatigorsk, near the Georgian border, Russia).
Sheltering males of Eucera vulpes and Synhalonia rufa (Hym. Apidae) were observed sleeping on flowers of O. helenae and withdrawing the orchid's pollen masses in the process at several locations within the orchid's native range (18–30 April 2011 at Mesovouni, Zagori, and 19 April to 1 May 2011 at Paliouri, Evrymenoi, northern Greece). No other insect, including the above-mentioned species, approached individual plants of O. helenae during sunny days, suggesting that the species has evolved a pollination mechanism based on shelter mimicry.
The pollinator records of the other shelter-mimicking species presented in the electronic supplementary material, table S1 have been collected over the years (2008–2011) during field experiments carried out in France, Sardinia (Italy), Greece, Israel and Spain. Other records are drawn from the literature.
(b). Floral scent analyses
Fresh, unpollinated flowers were extracted in n-hexane (HPLC grade); we used 2 ml of solvent to extract flowers of Iris atropurpurea (n = 14, collected at Yaqum, Israel, in March 2010) and I. paradoxa (n = 10, collected from cultivated plants originating from the Lake Van district, E-Turkey, in April 2011), and 400 μl of solvent to extract flowers of Serapias cordigera (n = 10, collected at Roquebrune-sur-Argens, France, in May 2010), S. lingua (n = 13, collected at Villanova Monteleone, Sardinia, Italy, in May 2009), O. helenae (n = 10, collected at Mesovounion, northern Greece, in May 2011) and O. mammosa (n = 10, collected at Paliouri, northern Greece, in May 2011). The quantification and identification methods follow Vereecken & Schiestl [42,43], and the double bond positions in mono-unsaturated compounds were assigned by dimethyl disulphide derivatization [44,45]. We used Mann–Whitney U-tests with a Bonferroni correction (α = 0.05 divided by the number of comparisons) to compare the relative amounts of n-alkenes and n-alkanes between night-sheltering and sexually deceptive species. All these statistical tests were performed with the software SPSS v. 17.0 [46].
(c). Spectral measurements of flowers
We used a portable spectrophotometer (AVASPEC-2048-USB2-UA, Avantes, Eerbeek, The Netherlands) equipped with a Xenon light source (AVALIGHT-XE, Avantes) to measure the relative reflectance (in %, 300–700 nm) of 20 flowers, picked randomly in natural populations, of each shelter-mimicking species. The spectrophotometer was calibrated with a white standard (WS-2, Avantes). The spectral sensitivity functions of the honeybee (Apis mellifera) are drawn from Chittka & Kevan [47]; the measurements used are representative of a wide taxonomic spectrum of higher Hymenoptera, and they are largely consistent within the Apoidea (bees sensu lato) [47].
(d). Phylogenetic analyses and ancestral state reconstructions
(i). Phylogenetic analysis of Serapias orchids
We used the tree typology and bootstrap values from a recent molecular phylogeny [48] based on four non-coding regions of chloroplast DNA to determine the evolution of pollination strategies in this genus of Mediterranean orchids. Posterior probability (PP) values were obtained using sequences deposited in GenBank from Bellusci et al. [48] that were aligned in Geneious Pro v. 4.7.6 (Biomatters Ltd., New Zealand) and analysed with MrBayes v. 3.1.2 [49], and are reported when more than 0.70. Four replicates of Bayesian inference (BI) were performed using MrBayes v. 3.1.2 [49] with the number of substitution rates set to six, the number of nucleotide frequencies set to four, the gamma distribution was approximated using four rate categories and a proportion of the nucleotide sites were allowed to be invariable. The BI analyses were run for 1.1 million generations, each with six chains, and sampled every 100 generations. The final average standard deviations of split frequencies were less than 0.001. The first 1000 trees from each run were excluded from the final tree set that was used to determine the PP distribution. The parameters used in this Bayesian analysis were used in subsequent analyses of Ophrys and Iris datasets reported below.
(ii). Phylogenetic analysis of Ophrys orchids
Although DNA sequences for several regions are available for O. helenae in GenBank [50], no comprehensive phylogeny showing its position relative to other Ophrys species was available in the literature. We therefore estimated its phylogenetic position from DNA sequences downloaded from Genbank. Sequences of the nuclear ribosomal internal-transcribed spacer 1 and chloroplast tRNA–Leu (trnL) DNA regions were downloaded for O. helenae (accession numbers: AY014514 and AY014552), 10 other Ophrys species (AY699950, AY364875, AY699953, AY014541, AY699973, AY699976, AY014540, AY014537, AY014539, AY014521, AY014569, AY014574, AY014573, AY014553, AY014559, AY014578, AY014575, AY014577, AY014561, AY014579) and two outgroups from the subtribe Orchidinae (AY364873, AY014546, AY014587, AY014584). A recent molecular phylogeny [51] guided our species selection to ensure that representative taxa of all sections of Ophrys were included. Sequences were aligned using the Muscle algorithm [52] in the software Aligner (CodonCode Corporation). A maximum-likelihood (ML) tree was inferred for each locus separately and combined with PhyML v. 3.0 [53]. GTR + G was identified for both DNA gene regions and the combined dataset as the most appropriate model of nucleotide substitution using the Akaike information criterion with the program jModeltest v. 0.1.1 [54]. Clade support was evaluated with a bootstrap analysis (1000 replicates) in PhyML, and values are reported when more than 50. Tree topologies agreed, and the combined dataset phylogeny is shown. PP values were obtained by analysing the combined dataset with MrBayes v. 3.1.2 [49] and are reported when more than 0.70.
(iii). Phylogenetic analysis of Oncocyclus irises
Genomic DNA was isolated from silica-dried leaf materials of 13 Iris section Oncocyclus species and four outgroup species from the same subgenus using protocols modified from the cetyl trimethyl ammonium (CTAB) method [55]. Modifications from this procedure included RNase treatment and an ethanol precipitation with ammonium acetate following the initial isopropanol precipitation. Sequence data of the matK gene, trnK introns, and petL–psbE, psbM–trnD and trnL–F regions were obtained for each of the 17 Iris species using protocols previously successful in Iris [56]. Reaction conditions for all amplifications were: 97°C for 1 min; 40 cycles of 97°C for 10 s, 48°–50°C for 1 min, 72°C for 20 s; 72°C for 4 min. Amplification products were purified using polyethylene glycol precipitation. The matK gene and flanking trnK introns were amplified in two reactions. The 5′ segment was amplified using the primer pairs 3914m and 1235r [57] or 1360ir [58]. The 3′ end was amplified using 1176i [59] and trnK2r [57]. The trnL–F region was amplified using the primer pair trnc [60] and trnf-10 [61], the psbM–trnD interspacer region using psbM-F and trnD-R, and the petL–psbE region using petL-F and psbE-R [62]. Purified PCR products were processed using a BigDye Terminating (Applied Biosystems, Foster City, CA, USA) cycle sequencing reaction following the manufacturer's instructions except that 5 per cent DMSO was added to the reaction mix. Cycle sequencing products were purified using Sephadex columns (Amersham Biosciences, Piscataway, NJ). Sequencing primers used for the matK gene and trnK introns were the amplification primers and internal primers 07i, 8i and 8ir from [59], 5 and 5r from [63], and 5i, 510i and 780ir from [58]. The amplification primers were used to sequencing the trnL–F, psbM–trnD and petL–psbE regions. Products were sequenced at the Rancho Santa Ana Botanic Garden on an automated sequencer (3130, Applied Biosystems).
Sequences were edited, assembled and aligned in Geneious Pro v. 4.7.6 resulting in a 5602 base pair dataset. An ML tree was inferred with PhyML v. 3.0 [53]. Clade support was evaluated with a bootstrap analysis (1000 replicates) in PhyML, and values are reported when more than 50. BI analyses were performed using MrBayes v. 3.1.2 [49], and PP values more than 0.70 are reported. Summary statistics for each dataset are given in the electronic supplementary material, table S3. Information on vouchers and GenBank numbers is given in the electronic supplementary material, appendix.
(iv). Ancestral state reconstructions
The evolution of pollination strategies for Serapias, Ophrys and Iris section Oncocyclus species was estimated using Mesquite v. 2.74 [64]. The pollination strategy history for each species group was traced on trees imported from PhyML analyses. The likelihood method was used with the Mk1 model (‘Markov k-state 1 parameter model’) to optimize ancestral states and shifts in pollination strategy within the one Iris and two orchid trees. There were zero length branches for some terminal nodes in the Serapias and Ophrys ML trees. To facilitate the analyses, lengths for these branches were set to 0.1 × 10−9.
3. Results and discussion
(a). Linking shelter mimicry and sexual deception
Our parallel investigations into the pollination biology of species in the orchid genus Serapias and in the Oncocyclus irises reveal that two species within these groups, namely S. lingua and I. paradoxa, attract their pollinators by sexual deception through pseudocopulation rather than by shelter mimicry. In these species, pollination occurs during the day and species-specifically by male solitary bees (C. cucurbitina and X. valga, respectively; figure 1; see electronic supplementary material, movies S1 and S2). These findings are, to the best of our knowledge, the first conclusive reports of pollination by sexual deception through pseudocopulation outside the genus Ophrys in the Euro-Mediterranean region and its surrounding countries, as well as the first account of this pollination strategy in the family Iridaceae. Until recently, pollination by sexual deception was considered restricted to orchids (see Vereecken & McNeil [27] for a review), but Ellis & Johnson [65] have reported the first example of this strategy outside the family Orchidaceae in Gorteria diffusa, a South African daisy (Asteraceae). The evolution of pollination by sexual deception in the Oncocyclus irises therefore represents the second confirmed case outside the orchid family, and suggests that this extraordinary reproductive strategy might be more widespread in flowering plants than previously thought.
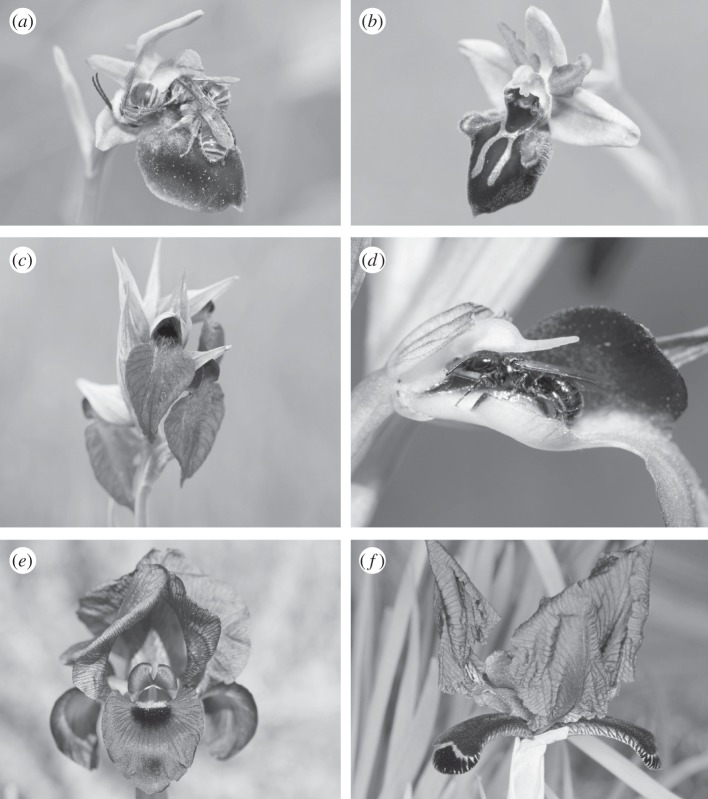
Figure 1.
Floral detail of irises and orchids pollinated (a,c,e) by sheltering mimicry and (b,d,f) by sexual deception. (a) Ophrys helenae with sleeping males of Eucera vulpes (Hym. Apidae); (b) Ophrys mammosa, a sister species of O. helenae; (c) Serapias cordigera; (d) Ceratina cucurbitina male (Hym. Apidae) attempting copulation on the floral callus of Serapias lingua (the tunnel-like flower was opened artificially); (e) Iris atropurpurea; (f) Iris paradoxa. All photographs courtesy of N. J. Vereecken except (f) by M. Streinzer.
Although shelter mimicry and sexual deception were considered to be restricted to different groups of plants, our investigations into the pollination biology of O. helenae have confirmed an earlier report [66] that it is the only species in the genus pollinated by shelter mimicry rather than by sexual deception. Our results presented here, coupled with the pollinator records of the other shelter-mimicking species, show that O. helenae exploits male eucerine bees through a mechanism common to most Serapias orchids and Oncocyclus irises [38–40] (figure 1a; electronic supplementary material, table S1), and that the two pollination strategies are therefore intimately linked to one another, irrespective of their phylogenetic origin.
(b). Convergent evolution in pollination strategies
The parallel evolution of dark red floral colours, tunnel-like (protective) shapes and floral scent bouquets dominated by n-alkanes and n-alkenes in orchids and irises pollinated by sheltering male eucerine bees suggest that these pollinators were the main driving force behind the evolutionary convergence in floral traits among these phylogenetically independent lineages of flowering plants (see figure 1; electronic supplementary material, table S1 and figure S2). Likewise, the evolutionary convergence in form (insectiform flowers with floral scents dominated by higher proportions of n-alkenes) and function (male bees as pollinators) among sexually deceptive species also illustrates that floral traits of sexually deceptive species respond similarly to selection pressures exerted by male solitary bees. These remarkable instances of evolutionary convergences can be regarded as new forms of pollination syndromes (i.e. floral traits that correlate with one another across independent evolutionary events and that adapt distantly related plants for the exploitation of a specific functional group of pollinators [8]).
(c). Directionality of evolutionary transitions
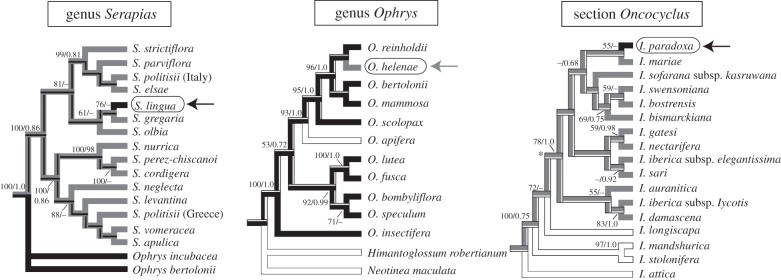
Our reconstructions of ancestral character states based on molecular phylogenies indicate that the transitions between shelter mimicry and sexual deception have occurred in all three groups and in a bidirectional mode (figure 3). For example, our results show that O. helenae appears to have derived from a stock of sexually deceptive species (the ancestral condition in the genus Ophrys; figure 3), whereas S. lingua and I. paradoxa are derived from shelter mimics, a condition ancestral in Serapias and in Iris section Oncocyclus (figure 3). The insectiform sepals of I. paradoxa are unique within the Oncocyclus species and also the genus Iris.
Figure 3.
The evolution tree of pollination by shelter mimicry and sexual deception in orchids (Serapias and Ophrys) and irises (section Oncocyclus of the genus Iris). Grey branches indicate species pollinated by shelter mimicry; black branches indicate species pollinated by sexual deception and white branches indicate species with another pollination strategy. The arrows indicate species with an alternative pollination strategy (sexual deception in Serapias lingua and Iris paradoxa; shelter mimicry in Ophrys helenae). Most likely reconstruction of ancestral pollination strategies are shown on a most parsimonious tree of Serapias, adapted from Chittka & Kevan [47], a maximum-likelihood (ML) tree of Ophrys and an ML tree of Iris focusing on the section Oncocyclus (branch leading to Oncocyclus species indicated with an asterisk). The relative likelihoods of alternative ancestral pollination strategies are indicated on backbone branches. In each of the three examples, the likelihood of the dominant colour exceeded 0.90. Bootstrap values >50 and posterior probabilities >0.70 are indicated above branches.
The irreversibility of ecological specialization, whether in pollination systems or in other fields of plant–insect interactions and biological sciences, has been challenged on very few occasions [22,67–75]. Consequently, it is generally acknowledged that ecological specialists only rarely revert to a generalist condition and that Cope's rule is therefore valid in the majority of cases. Here, we showed that the floral traits associated with shelter mimicry in Serapias and section Oncocyclus served as important pre-adaptations for the evolution of pollination by sexual deception, and, in turn, that minor changes in floral colour/ornamentation and scent triggered a departure from the typical sexually deceptive condition to shelter mimicry in Ophrys orchids. Collectively, our results therefore provide evidence against Cope's rule of specialization by showing that sexual deception, perhaps the most extreme case of floral (pollinator) specialization, has reverted to shelter mimicry, a comparatively more generalized pollination strategy.
(d). Phenotypic adaptations to sexual deception and shelter mimicry
We hypothesize that the large amounts of n-alkanes and n-alkenes (straight chain saturated and unsaturated hydrocarbons, respectively) emitted in the floral scents of our study species (figure 2; see electronic supplementary material, table S2 and figure S3) have contributed to the recurrent evolutionary transitions between these two pollination strategies. These compounds are components of plant and insect cuticular waxes, and serve the primary physiological function of reducing water loss from tissues, and have been proposed as pre-adaptations for the attraction of male hymenopterans [76] because they are known as female sex pheromone constituents in several groups of solitary bees [27,37,77], and also as key pollinator attractants in the floral scent of most Ophrys species investigated so far [25,27,37].
Figure 2.
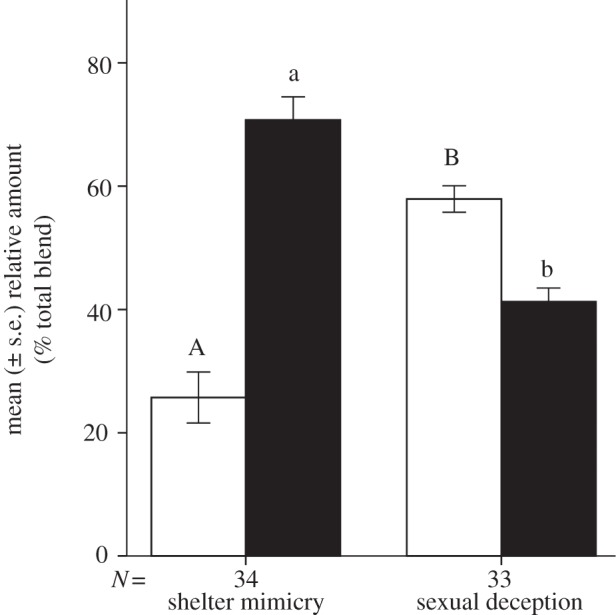
Comparative floral scent chemistry between shelter-mimicking and sexually deceptive species. Mean (± s.e.) relative amount (in % of the total blend) of n-alkenes (white bars) and n-alkanes (black bars) in the floral scent of all shelter-mimicking species (Iris atropurpurea, Ophrys helenae and Serapias cordigera) versus all sexually deceptive species (I. paradoxa, O. mammosa and S. lingua) investigated in this study. Different letters on top of error bars indicate significant differences (Mann–Whitney U-test, p < 0.05). See electronic supplementary material, figure S3 and table S2 for details on the floral scent chemistry.
The completion of the shift from shelter mimicry to sexual deception in the Serapias orchids and in the Oncocyclus irises required the combined acquisition of two additional traits: (i) a significant increase in the mean n-alkene:n-alkane ratio (% of the total blend) of the floral scent (figure 2; see electronic supplementary material, table S2), which presumably implies regulatory genetic changes at only a modest number of loci with major phenotypic effects [77], followed by pollinator selection that will act on standing biochemical pathways to gradually produce more subtle sex-pheromone-mimicking blends of compounds [42]; and (ii) the development of morphological structures that would trigger copulation attempts by the male bees in the appropriate position for the uptake of pollen. Within these two genera, such unique insect-mimicking floral structures are found only in S. lingua (the ‘tongue-like’, three-dimensional callus inside the floral tube; figure 1d) and I. paradoxa (the reduced, sub-horizontal and velvety sepals; figure 1f and electronic supplementary material, figure S2). These ‘bogus female’ structures are an important floral component to trigger the full pre-copulatory routine or copulation attempt of male insects upon contact with the flowers in all cases of pollination by sexual deception described so far [30,65,78–80].
The evolutionary transition from sexual deception to shelter mimicry in O. helenae required primarily minor changes in the labellum ornamentation. In particular, the loss of brightly coloured and complex labellum patterns (figure 1a,b) occurred without significant changes in the relative proportions of n-alkenes compared with its extant sexually deceptive congeners. Our results show that individual flowers of O. helenae emit significantly higher relative amounts of n-alkenes compared with the shelter-mimicking species in the genus Serapias and in the section Oncocyclus of the genus Iris (see the electronic supplementary material, figure S3), which presumably indicates a historical phenotypic signature (i.e. the retention of shared floral scent chemistry between O. helenae and its sexually deceptive ancestors in the genus Ophrys).
Several authors have discussed the role of biochemical pre-adaptations in the evolution of specialized pollination systems. For example, Armbruster et al. have shown that the production of herbivore-deterring resins has facilitated the evolution of a specialized pollination system involving resin-collecting bees in Dalechampia vines [81]; likewise, plant defence traits have been reported to act as pre-adaptations for pollinator shifts in Hakeas (Proteaceae) [82]. The results of our study suggest that the production of n-alkenes in the floral scent of Serapias orchids and Oncocyclus irises has been an important pre-adaptation that has set the stage for multiple phylogenetically independent evolutionary transition to sexual deception.
(e). Drivers of evolutionary transitions
We suggest that the transition from shelter mimicry to sexual deception may have evolved by selection for increased pollination efficiency, because the attraction of a narrower taxonomic range of pollinators of only one sex generally enhances the precision of pollen transfer [5,66,74]. Increased pollinator specialization can potentially drive the rapid evolution of reproductive isolation, and may thus directly affect both micro- and macro-evolutionary processes (e.g. pollinator sharing among species, patterns of gene flow within and among populations, species diversification, hybridization) [34,83]. By contrast, the evolved shift out of sexual deception and into shelter mimicry in O. helenae shows that extreme floral and pollinator specialization is not irreversible (i.e. it does not affect the lineage's potential for adapting to new conditions), as shown by the fact that generalists (shelter-mimicking species) can evolve from extremely specialized (sexually deceptive) ancestors (figure 3; see also [84,85]). One possible scenario is that external ecological factors such as pollinator limitation might have shifted this lineage away from extreme pollinator specialization by selection for increased reproductive success [5]. The evolutionary transitions between these two pollination strategies could therefore have been driven by fitness trade-offs between pollinator specialization and pollination efficiency. More detailed studies on the phylogenetic patterns of floral scents in groups of shelter-mimicking and sexually deceptive species, as well as experiments on odour-guided behaviour with male eucerine bees, will offer unprecedented opportunities to uncover the floral art of manipulating solitary bees as pollinators in these fascinating groups of flowering plants.
Acknowledgements
N.J.V. designed the study, collected samples, analysed the floral scents, performed the statistical analyses and led the writing; C.A.W. performed the phylogenetic analyses of the Oncocyclus irises; S.H. and S.S. helped identifying the compounds in the floral scent extracts; S.A.B. collected samples and performed behavioural experiments; P.M. performed the phylogenetic analyses of Ophrys orchids; P.M. and C.A.W. performed the reconstructions of ancestral character states. All authors contributed to the preparation of the manuscript and approved its final version. We are grateful to R. D. Dominguez and M. Streinzer for sharing their material to illustrate this study. Thanks are also due to J. N. McNeil, J.-C. de Biseau, J.-M. Lassance, M. A. Liénard, J. M. Pasteels, J. Spaethe, M. Streinzer, A. Dafni, Y. Sapir, A. Dorchin, S. Watts, P. Cortis, P. Niolu and T. D. Wyatt for their comments on an earlier version of the manuscript, as well as for their help in collecting samples. We are grateful to W. S. Armbruster, S. Renner and an anonymous referee for their helpful comments on an earlier version of the manuscript. N.J.V. received financial support for this study through a Belgian FRS-FNRS post-doctoral grant (2008–2012) and a Jean-Marie Delwart Foundation travel grant (2011). C.A.W. received support from the NSF:DEB-1020826 and the American Iris Society Foundation. P.M. is a research associate at the Belgian National Fund for Scientific Research (FRS-FNRS).
References
- 1.Simpson B. B., Neff J. L. 1981. Floral rewards: alternatives to pollen and nectar. Ann. Miss. Bot. Gard. 68, 301–322 10.2307/2398800 (doi:10.2307/2398800) [DOI] [Google Scholar]
- 2.Herrera C. M. 1996. Floral traits and plant adaptation to insect pollinators: a devil's advocate approach. In Floral biology: studies on floral evolution in animal-pollinated plants (eds Lloyd D. G., Barrett S. C. H.), pp. 65–87 New York, NY: Chapman and Hall [Google Scholar]
- 3.Herrera C. M. 2005. Plant generalization on pollinators: species property or local phenomenon? Am. J. Bot. 92, 13–20 10.3732/ajb.92.1.13 (doi:10.3732/ajb.92.1.13) [DOI] [PubMed] [Google Scholar]
- 4.Ollerton J. 1996. Reconciling ecological processes with phylogenetic patterns: the apparent paradox of plant–pollinator systems. J. Ecol. 84, 767–769 10.2307/2261338 (doi:10.2307/2261338) [DOI] [Google Scholar]
- 5.Waser N. M., Chittka L., Price M. V., Williams N. M., Ollerton J. 1996. Generalization in pollination systems, and why it matters. Ecology 77, 1043–1060 10.2307/2265575 (doi:10.2307/2265575) [DOI] [Google Scholar]
- 6.Johnson S. D., Steiner K. E. 2000. Generalization versus specialization in plant pollination systems. Trends Ecol. Evol. 15, 140–143 10.1016/S0169-5347(99)01811-X (doi:10.1016/S0169-5347(99)01811-X) [DOI] [PubMed] [Google Scholar]
- 7.Bascompte J., Jordano P., Melián C. J., Olesen J. M. 2003. The nested assembly of plant–animal mutualistic networks. Proc. Natl Acad. Sci. USA 100, 9383–9387 10.1073/pnas.1633576100 (doi:10.1073/pnas.1633576100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenster C. B., Armbruster W. S., Wilson P., Dudash M. R., Thomson J. D. 2004. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 35, 375–403 10.1146/annurev.ecolsys.34.011802.132347 (doi:10.1146/annurev.ecolsys.34.011802.132347) [DOI] [Google Scholar]
- 9.Gegear R. J., Burns J. G. 2007. The birds, the bees, and the virtual flowers: can pollinator behavior drive ecological speciation in flowering plants? Am. Nat. 170, 551–566 10.1086/521230 (doi:10.1086/521230) [DOI] [PubMed] [Google Scholar]
- 10.Schiestl F. P., Schlüter P. M. 2009. Floral isolation, specialized pollination, and pollinator behavior in orchids. Annu. Rev. Entomol. 54, 425–446 10.1146/annurev.ento.54.110807.090603 (doi:10.1146/annurev.ento.54.110807.090603) [DOI] [PubMed] [Google Scholar]
- 11.Kay K. M., Sargent R. D. 2009. The role of animal pollination in plant speciation: integrating ecology, geography, and genetics. Annu. Rev. Ecol. Evol. Syst. 40, 637–656 10.1146/annurev.ecolsys.110308.120310 (doi:10.1146/annurev.ecolsys.110308.120310) [DOI] [Google Scholar]
- 12.Knapp S. 2010. On ‘various contrivances’: pollination, phylogeny and flower form in the Solanaceae. Phil. Trans. R. Soc. B 365, 449–460 10.1098/rstb.2009.0236 (doi:10.1098/rstb.2009.0236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson O., Bremer B. 1992. Pollination systems, dispersal modes, life forms, and diversification rates in angiosperm families. Evolution 46, 258–266 10.2307/2409820 (doi:10.2307/2409820) [DOI] [PubMed] [Google Scholar]
- 14.Dodd M. E., Silvertown J., Chase M. W. 1999. Phylogenetic analysis of trait evolution and species diversity variation among angiosperm families. Evolution 53, 732–744 10.2307/2640713 (doi:10.2307/2640713) [DOI] [PubMed] [Google Scholar]
- 15.Cozzolino S., Widmer A. 2005. Orchid diversity: an evolutionary consequence of deception? Trends Ecol. Evol. 20, 487–494 10.1016/j.tree.2005.06.004 (doi:10.1016/j.tree.2005.06.004) [DOI] [PubMed] [Google Scholar]
- 16.Grant V., Grant K. A. 1965. Flower pollination in the Phlox family. New York, NY: Columbia University Press [Google Scholar]
- 17.Faegri K., Van Der Pijl L. 1966. The principles of pollination ecology. Oxford, UK: Pergamon Press [Google Scholar]
- 18.Stebbins G. L. 1970. Adaptive radiation of reproductive characteristics in angiosperms, I: pollination mechanisms. Annu. Rev. Ecol. Syst. 1, 307–326 10.1146/annurev.es.01.110170.001515 (doi:10.1146/annurev.es.01.110170.001515) [DOI] [Google Scholar]
- 19.Futuyma D. J., Moreno G. 1988. The evolution of ecological specialization. Annu. Rev. Ecol. Syst. 19, 207–233 10.1146/annurev.es.19.110188.001231 (doi:10.1146/annurev.es.19.110188.001231) [DOI] [Google Scholar]
- 20.Tripp E. A., Manos P. S. 2009. Is floral specialization an evolutionary dead-end? Pollination systems transitions in Ruellia (Acanthaceae). Evolution 62, 1712–1737 10.1111/j.1558-5646.2008.00398.x (doi:10.1111/j.1558-5646.2008.00398.x) [DOI] [PubMed] [Google Scholar]
- 21.Cope E. D. 1896. The primary factors of organic evolution. Chicago, IL: Open Court Publishing [Google Scholar]
- 22.Armbruster W. S. 2006. Evolutionary and ecological aspects of specialized pollination: views from the Arctic to the Tropics. In Plant–pollinator interactions: from specialization to generalization (eds Waser N. M., Ollerton J.), pp. 260–282 Chicago, IL: University of Chicago Press [Google Scholar]
- 23.Schiestl F. P., Ayasse M., Paulus H. F., Löfstedt C., Hansson B. S., Ibarra F., Francke W. 1999. Orchid pollination by sexual swindle. Nature 399, 421–422 10.1038/20829 (doi:10.1038/20829) [DOI] [Google Scholar]
- 24.Schiestl F. P., Peakall R., Mant J. G., Ibarra F., Schulz C., Franke S., Francke W. 2003. The chemistry of sexual deception in an orchid–wasp pollination system. Science 302, 437–438 10.1126/science.1087835 (doi:10.1126/science.1087835) [DOI] [PubMed] [Google Scholar]
- 25.Ayasse M., Stökl J., Francke W. 2011. Chemical ecology and speciation in sexually deceptive orchids. Phytochemistry 72, 1667–1677 10.1016/j.phytochem.2011.03.023 (doi:10.1016/j.phytochem.2011.03.023) [DOI] [PubMed] [Google Scholar]
- 26.Peakall R., Ebert D., Poldy J., Barrow R. A., Francke W., Bower C. C., Schiestl F. P. 2010. Pollinator specificity, floral odour chemistry and the phylogeny of Australian sexually deceptive Chiloglottis orchids: implications for pollinator-driven speciation. New Phytol. 188, 437–450 10.1111/j.1469-8137.2010.03308.x (doi:10.1111/j.1469-8137.2010.03308.x) [DOI] [PubMed] [Google Scholar]
- 27.Vereecken N. J., McNeil J. N. 2010. Cheaters and liars: chemical mimicry at its finest. Can. J. Zool. 88, 725–752 10.1139/Z10-040 (doi:10.1139/Z10-040) [DOI] [Google Scholar]
- 28.Correvon H., Pouyanne M. 1916. Un curieux cas de mimétisme chez les ophrydées. J. Soc. Nat. Hortic. Fr. 4, 29–47 [Google Scholar]
- 29.Correvon H., Pouyanne M. 1923. Nouvelles observations sur le mimétisme et la fécondation chez les Ophrys speculum et lutea. J. Soc. Nat. Hortic. Fr. 4, 372–377 [Google Scholar]
- 30.Kullenberg B. 1961. Studies in Ophrys pollination. Zool. Bidrag från Uppsala 34, 1–340 [Google Scholar]
- 31.Borg-Karlson A.-K. 1990. Chemical and ethological studies of pollination in the genus Ophrys (Orchidaceae). Phytochemistry 29, 1359–1387 10.1016/0031-9422(90)80086-V (doi:10.1016/0031-9422(90)80086-V) [DOI] [Google Scholar]
- 32.Paulus H. F., Gack C. 1990. Pollinators as prepollinating isolation factors: evolution and speciation in Ophrys (Orchidaceae). Isr. J. Bot. 39, 43–79 [Google Scholar]
- 33.Vereecken N. J. 2009. Deceptive behavior in plants. I. Pollination by sexual deception in orchids: a host–parasite perspective. In Plant–enviroment interactions (ed. Baluska F.), pp. 203–222 Berlin, Germany: Springer [Google Scholar]
- 34.Vereecken N. J., Cozzolino S., Schiestl F. P. 2010. Hybrid floral novelty drives pollinator shift in sexually deceptive orchids. BMC Evol. Biol. 10, 103. 10.1186/1471-2148-10-103 (doi:10.1186/1471-2148-10-103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiestl F. P. 2010. Pollination: sexual mimicry abounds. Curr. Biol. 20, R1020–R1022 10.1016/j.cub.2010.10.019 (doi:10.1016/j.cub.2010.10.019) [DOI] [PubMed] [Google Scholar]
- 36.Pouyanne M. 1917. La fécondation des Ophrys par les Insectes. Bull. Soc. Hist. Nat. Afr. Nord. 43, 53–62 [Google Scholar]
- 37.Dötterl S., Vereecken N. J. 2010. The chemical ecology and evolution of bee-flower interactions: a review and perspectives. Can. J. Zool. 88, 668–697 10.1139/Z10-031 (doi:10.1139/Z10-031) [DOI] [Google Scholar]
- 38.Dafni A., Ivri Y., Brantjes N. B. M. 1981. Pollination of Serapias vomeracea Briq. (Orchidaceae) by imitation of holes for sleeping solitary male bees (Hymenoptera). Acta Bot. Neerl. 30, 69–73 [Google Scholar]
- 39.Sapir Y., Shmida A., Ne'eman G. 2005. Pollination of the Oncocyclus irises (Iris: Iridaceae) by night-sheltering male bees. Plant Biol. 7, 417–424 10.1055/s-2005-837709 (doi:10.1055/s-2005-837709) [DOI] [PubMed] [Google Scholar]
- 40.Monty A., Saad L., Mahy G. 2006. Bimodal pollination system in rare endemic Oncocyclus irises (Iridaceae) of Lebanon. Can. J. Bot. 84, 1327–1338 10.1139/B06-081 (doi:10.1139/B06-081) [DOI] [Google Scholar]
- 41.Futuyma D. J. 2001. Ecological specialization and generalization. In Evolutionary ecology (eds Fox C. W., Roff D. A., Fairbairn D. J.), pp. 177–192 Oxford, UK: Oxford University Press [Google Scholar]
- 42.Vereecken N. J., Schiestl F. P. 2008. The evolution of imperfect floral mimicry. Proc. Natl Acad. Sci. USA 105, 7484–7488 10.1073/pnas.0800194105 (doi:10.1073/pnas.0800194105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vereecken N. J., Schiestl F. P. 2009. On the roles of colour and scent in a specialized floral mimicry system. Ann. Bot. 104, 1077–1084 10.1093/aob/mcp208 (doi:10.1093/aob/mcp208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buser H. R., Arn H., Guerin P., Rauscher S. 1983. Determination of double bond position in mono-unsaturated acetates by mass spectrometry of dimethyl disulfide adducts. Anal. Chem. 55, 818–822 10.1021/ac00257a003 (doi:10.1021/ac00257a003) [DOI] [Google Scholar]
- 45.Dunkelblum E., Tan S. H., Silk P. J. 1985. Double bond location in monounsaturated fatty acids by dimethyl disulfide derivatization and mass spectrometry: application to analysis of fatty acids in pheromone glands of 4 Lepidoptera . J. Chem. Ecol. 11, 265–277 10.1007/BF01411414 (doi:10.1007/BF01411414) [DOI] [PubMed] [Google Scholar]
- 46.Brosius F. 2002. SPSS version 11. Bonn, Germany: Mitp [Google Scholar]
- 47.Chittka L., Kevan P. G. 2005. Flower colour as advertisement. In Practical pollination biology (eds Dafni A., Kevan P. G., Husband B. C.), pp. 157–196 Cambridge, UK: Enviroquest [Google Scholar]
- 48.Bellusci F., Pellegrino G., Palermo A. M., Musacchio A. 2008. Phylogenetic relationships in the orchid genus Serapias L. based on noncoding regions of the chloroplast genome. Mol. Phylogenet. Evol. 47, 986–991 10.1016/j.ympev.2008.03.019 (doi:10.1016/j.ympev.2008.03.019) [DOI] [PubMed] [Google Scholar]
- 49.Huelsenbeck J. P., Ronquist F. 2001. MrBayes: Bayesian inference of phylogeny. Bioinformatics 19, 1572–1574 10.1093/bioinformatics/17.8.754 (doi:10.1093/bioinformatics/17.8.754) [DOI] [PubMed] [Google Scholar]
- 50.Soliva M., Kocyan A., Widmer A. 2001. Molecular phylogenetics of the sexually deceptive orchid genus Ophrys (Orchidaceae) based on nuclear and chloroplast DNA sequences. Mol. Phylogenet. Evol. 20, 79–88 10.1006/mpev.2001.0953 (doi:10.1006/mpev.2001.0953) [DOI] [PubMed] [Google Scholar]
- 51.Devey D. S., Bateman R. M., Fay M. F., Hawkins J. A. 2008. Friends or relatives? Phylogenetics and species delimitation in the controversial European orchid genus Ophrys. Ann. Bot. 101, 385–402 10.1093/aob/mcm299 (doi:10.1093/aob/mcm299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edgar R. C. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5, 113. 10.1186/1471-2105-5-113 (doi:10.1186/1471-2105-5-113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guindon S., Dufayard J.-F., Lefort V., Anisimova M., Hordijk W., Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML v. 3.0. Syst. Biol. 59, 307–321 10.1093/sysbio/syq010 (doi:10.1093/sysbio/syq010) [DOI] [PubMed] [Google Scholar]
- 54.Posada D. 2008. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 25, 1253–1256 10.1093/molbev/msn083 (doi:10.1093/molbev/msn083) [DOI] [PubMed] [Google Scholar]
- 55.Doyle J. J., Doyle J. L. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15 [Google Scholar]
- 56.Wilson C. A. 2011. Subgeneric classification in Iris re-examined using chloroplast sequence data. Taxon 60, 27–35 [Google Scholar]
- 57.Johnson L. A., Soltis D. E. 1994. matK DNA sequences and phylogenetic reconstruction in Saxifragaceae s. str. Syst. Bot. 19, 143–156 10.2307/2419718 (doi:10.2307/2419718) [DOI] [Google Scholar]
- 58.Wilson C. A. 2009. Phylogenetic relationships among the recognized series in Iris section Limniris. Syst. Bot. 34, 277–284 10.1600/036364409788606316 (doi:10.1600/036364409788606316) [DOI] [Google Scholar]
- 59.Wilson C. A. 2004. Phylogeny of Iris based on chloroplast matK gene and trnK intron sequence data. Mol. Phyl. Evol. 33, 402–412 10.1016/j.ympev.2004.06.013 (doi:10.1016/j.ympev.2004.06.013) [DOI] [PubMed] [Google Scholar]
- 60.Taberlet P., Gielly L., Pautou G., Bouvet J. 1991. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 17, 1105–1109 10.1007/BF00037152 (doi:10.1007/BF00037152) [DOI] [PubMed] [Google Scholar]
- 61.Wilson C. A., Calvin C. L. 2006. An origin of aerial branch parasitism in the mistletoe family, Loranthaceae. Am. J. Bot. 93, 787–796 10.3732/ajb.93.5.787 (doi:10.3732/ajb.93.5.787) [DOI] [PubMed] [Google Scholar]
- 62.Shaw J., et al. 2005. The tortoise and hare II: relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am. J. Bot. 92, 142–166 10.3732/ajb.92.1.142 (doi:10.3732/ajb.92.1.142) [DOI] [PubMed] [Google Scholar]
- 63.Steele K. P., Vilgalys R. 1994. Phylogenetic analysis of Polemoniaceae using nucleotide sequences of the plastid gene matK. Syst. Bot. 19, 126–142 10.2307/2419717 (doi:10.2307/2419717) [DOI] [Google Scholar]
- 64.Maddison W. P., Maddison D. R. 2011. Mesquite: a modular system for evolutionary analysis, v. 2.75. See http://mesquiteproject.org. [Google Scholar]
- 65.Ellis A. G., Johnson S. D. 2010. Floral mimicry enhances pollen export: the evolution of pollination by sexual deceit outside of the orchidaceae. Am. Nat. 176, E143–E151 10.1086/656487 (doi:10.1086/656487) [DOI] [PubMed] [Google Scholar]
- 66.Paulus H. F., Gack C. 1993. Schlafplatzmimikry bei der mediterranen Orchidee Ophrys helenae . Verh. Dt. Zool. Ges. Salzburg 86, 267 [Google Scholar]
- 67.Futuyma D. J. 1991. Evolution of host specificity in herbivorous insects: genetic, ecological and phylogenetic aspects. In Plant–animal interactions: evolutionary ecology in tropical and temperate regions (eds Price P. W., Lewinsohn T. M., Fernandes G. W., Benson W. W.), pp. 431–454 New York, NY: Wiley [Google Scholar]
- 68.Lanyon S. 1992. Interspecific brood parasitism in blackbirds (Icterinae). Science 255, 77–79 10.1126/science.1553533 (doi:10.1126/science.1553533) [DOI] [PubMed] [Google Scholar]
- 69.Thompson J. N. 1994. The co-evolutionary process. Chicago, IL: University of Chicago Press [Google Scholar]
- 70.Kaiser H. E., Boucot A. J. 1996. Specialisation and extinction: Cope's law revisited. Hist. Biol. 11, 247–265 10.1080/10292389609380544 (doi:10.1080/10292389609380544) [DOI] [Google Scholar]
- 71.Armbruster W. S., Baldwin B. G. 1998. Switch from specialized to generalized pollination. Nature 394, 632. 10.1038/29210 (doi:10.1038/29210) [DOI] [Google Scholar]
- 72.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press [Google Scholar]
- 73.Termonia A., Hsiao T. H., Pasteels J. M., Milinkovitch M. C. 2001. Feeding specialization and host-derived chemical defense in Chrysomeline leaf beetles did not lead to an evolutionary dead end. Proc. Natl Acad. Sci. USA 98, 3909–3914 10.1073/pnas.061034598 (doi:10.1073/pnas.061034598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michez D., Patiny S., Rasmont P., Timmermann K., Vereecken N. J. 2008. Phylogeny and host plant evolution in Melittidae s.l. (Hymenoptera: Apoidea). Apidologie 39, 146–162 10.1051/apido:2007048 (doi:10.1051/apido:2007048) [DOI] [Google Scholar]
- 75.Winkler I. S., Mitter C. 2008. The phylogenetic dimension of insect-plant interactions: a review of recent evidence. In Specialization, speciation and radiation: the evolutionary biology of herbivorous insects (ed. Tilmon K. J.), pp. 240–263 Berkeley, CA: University of California Press [Google Scholar]
- 76.Schiestl F. P., Cozzolino S. 2008. Evolution of sexual mimicry in the orchid subtribe orchidinae: the role of preadaptations in the attraction of male bees as pollinators. BMC Evol. Biol. 8, 27. 10.1186/1471-2148-8-27 (doi:10.1186/1471-2148-8-27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ayasse M., Paxton R. J., Tengö J. 2001. Mating behavior and chemical communication in the order Hymenoptera. Annu. Rev. Entomol. 46, 31–78 10.1146/annurev.ento.46.1.31 (doi:10.1146/annurev.ento.46.1.31) [DOI] [PubMed] [Google Scholar]
- 78.Schlueter P. M., Xu S., Gagliardini V., Whittle E., Shanklin J., Grossniklaus U., Schiestl F. P. 2011. Stearoyl-acyl carrier protein desaturases are associated with floral isolation in sexually deceptive orchids. Proc. Natl Acad. Sci. USA 108, 5696–5701 10.1073/pnas.1013313108 (doi:10.1073/pnas.1013313108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gaskett A. C. 2011. Orchid pollination by sexual deception: pollinator perspectives. Biol. Rev. 86, 33–75 10.1111/j.1469-185X.2010.00134.x (doi:10.1111/j.1469-185X.2010.00134.x) [DOI] [PubMed] [Google Scholar]
- 80.Ågren L., Kullenberg B., Sensenbaugh T. 1984. Congruences in pilosity between 3 species of Ophrys Orchidaceae and their hymenopteran pollinators. Nova Acta Reg. Soc Sci. Upsaliensis V C 3, 15–26 [Google Scholar]
- 81.Armbruster W. S., Howard J. J., Clausen T. P., Debevec E. M., Loquvam J. C., Matsuki M., Cerendolo B., Andel F. 1997. Do biochemical exaptations link evolution of plant defense and pollination systems? Historical hypotheses and experimental tests with Dalechampia vines. Am. Nat. 149, 461–484 10.1086/286000 (doi:10.1086/286000) [DOI] [Google Scholar]
- 82.Hanley M. E., Lamont B. B., Armbruster W. S. 2009. Pollination and plant defence traits co-vary in Western Australian Hakeas. New Phytol. 182, 251–260 10.1111/j.1469-8137.2008.02709.x (doi:10.1111/j.1469-8137.2008.02709.x) [DOI] [PubMed] [Google Scholar]
- 83.Scopece G., Cozzolino S., Johnson S. D., Schiestl F. P. 2010. Pollination efficiency and the evolution of specialized deceptive pollination systems. Am. Nat. 175, 98–105 10.1086/648555 (doi:10.1086/648555) [DOI] [PubMed] [Google Scholar]
- 84.Martén-Rodríguez S., Fenster C. B., Agnarsson I., Skog L. E., Zimmer E. A. 2010. Evolutionary breakdown of pollination specialization in a Caribbean plant radiation. New Phytol. 188, 403–417 10.1111/j.1469-8137.2010.03330.x (doi:10.1111/j.1469-8137.2010.03330.x) [DOI] [PubMed] [Google Scholar]
- 85.Aigner P. A. 2001. Optimality modelling and fitness trade-offs: when should plants become pollinator specialists? Oikos 95, 177–184 10.1034/j.1600-0706.2001.950121.x (doi:10.1034/j.1600-0706.2001.950121.x) [DOI] [Google Scholar]