Abstract
Photoperiodic flowering has been extensively studied in the annual short-day and long-day plants rice (Oryza sativa) and Arabidopsis (Arabidopsis thaliana), whereas less is known about the control of flowering in perennials. In the perennial wild strawberry, Fragaria vesca (Rosaceae), short-day and perpetual flowering long-day accessions occur. Genetic analyses showed that differences in their flowering responses are caused by a single gene, SEASONAL FLOWERING LOCUS, which may encode the F. vesca homolog of TERMINAL FLOWER1 (FvTFL1). We show through high-resolution mapping and transgenic approaches that FvTFL1 is the basis of this change in flowering behavior and demonstrate that FvTFL1 acts as a photoperiodically regulated repressor. In short-day F. vesca, long photoperiods activate FvTFL1 mRNA expression and short days suppress it, promoting flower induction. These seasonal cycles in FvTFL1 mRNA level confer seasonal cycling of vegetative and reproductive development. Mutations in FvTFL1 prevent long-day suppression of flowering, and the early flowering that then occurs under long days is dependent on the F. vesca homolog of FLOWERING LOCUS T. This photoperiodic response mechanism differs from those described in model annual plants. We suggest that this mechanism controls flowering within the perennial growth cycle in F. vesca and demonstrate that a change in a single gene reverses the photoperiodic requirements for flowering.
Plants must time their vegetative and reproductive growth phases accurately in order to ensure their own survival as well as the survival of their progeny. In addition to endogenous cues such as their developmental stage and hormone levels, plants are able to monitor environmental signals, most importantly photoperiod and temperature, for developmental timing (Simpson, 2004; Samach and Wigge, 2005; Turck et al., 2008; Kim et al., 2009; Mutasa-Göttgens and Hedden, 2009). Appropriate developmental timing is especially important for perennial species, which may live for many years, undergoing repeated cycles of vegetative and reproductive (flowering) development, which are regulated by changes in the seasons through the year. Photoperiod is the major environmental cue for developmental timing in several perennial species (Cooper and Calder, 1964; Böhlenius et al., 2006; Gyllenstrand et al., 2007; Heide and Sønsteby, 2007), because it is the only constant environmental signal that undergoes the same cyclical pattern every year, with increasing amplitude toward higher latitudes.
Many perennial plants are economically important crops. Therefore, knowledge of the molecular regulation of flowering and developmental cycling in these species will enhance their cultivation as well as breeding. The woodland strawberry, Fragaria vesca, which belongs to the most important family of fruit crops, the Rosaceae, serves as a convenient perennial model (Shulaev et al., 2008, 2011). The presence of different accessions with opposite photoperiodic responses (Heide and Sønsteby, 2007; Sønsteby and Heide, 2008; Mouhu et al., 2009) makes this species a particularly useful model for photoperiodic research among perennials. Short-day (SD) F. vesca has a perennial life cycle characteristic of the Rosaceae; flower initiation occurs at the apical meristem under SD and low temperatures during autumn, and flowers emerge the following spring. Under the long days (LDs) of summer, new shoots that have emerged in the uppermost nodes remain vegetative, then as the days become sufficiently short and temperature declines, flower primordia are again produced (Battey et al., 1998; Battey, 2000; Heide and Sønsteby, 2007). Detailed physiological analyses in SD F. vesca demonstrated that a short photoperiod is obligatory for flower induction at intermediate temperatures; at temperatures above 20°C, floral development is inhibited; and cool temperatures of about 10°C strongly induce flowering in both SD and LD conditions (Heide and Sønsteby, 2007). Similar to SD F. vesca, the photoperiod sensitivity of several cultivars of octoploid garden strawberry (Fragaria × ananassa) is strongly dependent on temperature (Heide, 1977; Bradford et al., 2010), suggesting that diploid F. vesca may be used as a model for more complex cultivated species.
In contrast to the seasonal flowering SD F. vesca, perpetual flowering accessions of the species (F. vesca f. semperflorens) flower and bear fruits from early summer until late autumn (Brown and Wareing, 1965). In three perpetual flowering accessions, reversed environmental responses compared with SD accessions have been reported. They are LD plants, which flower rapidly under LDs and high temperature, as opposed to SDs and low temperature, which repress flowering (Sønsteby and Heide, 2008; Mouhu et al., 2009). Classical genetic studies have shown that perpetual flowering is caused by recessive alleles of a single repressor gene called SEASONAL FLOWERING LOCUS (SFL; Brown and Wareing, 1965; Albani et al., 2004). Perpetual flowering cultivars (often called remontant or everbearing) are known also in cultivated strawberry. These cultivars have been considered as day-neutral or temperature-dependent LD plants in different studies (Sønsteby and Heide, 2007; Weebadde et al., 2008; Bradford et al., 2010; Stewart and Folta, 2010).
Photoperiodic flowering has been explained by the coincidence of CONSTANS (CO) diurnal expression rhythm and external light signals in both SD and LD model annuals, rice (Oryza sativa) and Arabidopsis (Arabidopsis thaliana), respectively (Suárez-López et al., 2001; Hayama et al., 2003). In both species, CO controls flowering through the CETS (for CEN, TFL1, and FT) family protein FLOWERING LOCUS T (FT), which is thought to be a universal flowering signal (Suárez-López et al., 2001; Hayama et al., 2003; Komiya et al., 2009; Turnbull, 2011). In the LD plant Arabidopsis, CO protein accumulates in the leaf phloem companion cells and activates FT expression only in LDs when the light period coincides with the CO expression peak in the late afternoon (An et al., 2004; Valverde et al., 2004; Corbesier et al., 2007). In contrast, in the SD plant rice, an FT homolog, Heading date3a, is activated when the CO homolog Heading date1 peaks after dusk in SDs (Hayama et al., 2003). After the activation of FT expression, the FT protein moves through the phloem to the shoot apex (Corbesier et al., 2007; Jaeger and Wigge, 2007; Tamaki et al., 2007), where 14-3-3 proteins bridge its interaction with a bZIP transcription factor, FLOWERING LOCUS D (FD; Taoka et al., 2011). The FT/14-3-3/FD complex, in turn, induces flowering by up-regulating the floral meristem identity genes APETALA1 (AP1) and FRUITFULL (FUL; Abe et al., 2005; Wigge et al., 2005). Another CETS family protein, the floral repressor TERMINAL FLOWER1 (TFL1), also binds to FD and suppresses the expression of LEAFY, AP1, and FUL (Ratcliffe et al., 1999; Hanano and Goto, 2011). In Arabidopsis, TFL1 is developmentally regulated and has not been linked to the photoperiodic pathway. At the vegetative stage, TFL1 mRNA is weakly expressed in the lower part of the apical meristem, whereas the protein can move short distances to repress flowering in the main apex. After flower induction, TFL1 is strongly up-regulated to maintain the indeterminate inflorescence meristem (Shannon and Meeks-Wagner, 1991; Bradley et al., 1997; Ratcliffe et al., 1999; Conti and Bradley, 2007).
The functions of key flowering genes seem to be at least partially conserved between annual and perennial species, but their regulation may vary in the perennial context (Albani and Coupland, 2010). FT homologs have been shown to promote flowering in many perennial species (Endo et al., 2005; Böhlenius et al., 2006; Hsu et al., 2006; Kotoda et al., 2010), but at least in Populus trichocarpa, FT is also involved in the control of growth cessation (Böhlenius et al., 2006). Detailed analyses of two Populus FT paralogs have shown that seasonal changes in their expression control the cycling of reproductive and vegetative growth. Winter cold (vernalization) activates FT1, which promotes flowering, whereas LD and high temperature activate FT2, which promotes vegetative growth and prevents bud set (Hsu et al., 2011). In Arabis alpina, the return to vegetative development after flowering is regulated by transient silencing of the FLOWERING LOCUS C (FLC) ortholog PERPETUAL FLOWERING1 (PEP1) by vernalization (Wang et al., 2009). pep1-1 mutants show reduced vegetative growth but also flower perpetually, which is a trait reported in other perennials such as rose (Rosa sp.) and F. vesca (Brown and Wareing, 1965; Iwata et al., 2012). Furthermore, TFL1 homologs have been shown to repress flowering and to control the length of the juvenile phase in apple (Malus domestica), Populus, and A. alpina (Kotoda et al., 2006; Mohamed et al., 2010; Wang et al., 2011).
Recently, Iwata et al. (2012) identified a F. vesca homolog of TFL1 (FvTFL1) as a candidate gene for SFL. They showed that a 2-bp deletion in FvTFL1 is associated with perpetual flowering in F. vesca, but functional validation was lacking (Iwata et al., 2012). Here, we provide functional evidence for FvTFL1 being SFL. Our results further indicate that FvTFL1 is the key component of the perennial photoperiodic pathway in F. vesca: it confers an SD requirement for flowering and controls cycling between vegetative and reproductive phases. We also demonstrate how a 2-bp deletion in FvTFL1 changes the regulation of seasonal life cycles and leads to FvFT-dependent LD flowering.
RESULTS
Mutation in the Floral Repressor FvTFL1 Reverses the Photoperiodic Requirement for Flower Induction
SFL, which causes seasonal flowering in F. vesca (Brown and Wareing, 1965; Albani et al., 2004), was initially mapped to F. vesca linkage group 6 (Sargent et al., 2004). In a recent study, association was found between perpetual flowering habit and a 2-bp deletion in the first exon of F. vesca TFL1 homolog, and the gene was roughly mapped between two markers with a physical distance of approximately 11 Mb in the same linkage group (Iwata et al., 2012). We narrowed down the location of SFL within the mapping window of 248 kb, and our extensive linkage analysis in two populations fully supports the association found by Iwata et al. (2012; Supplemental Figs. S1 and S2).
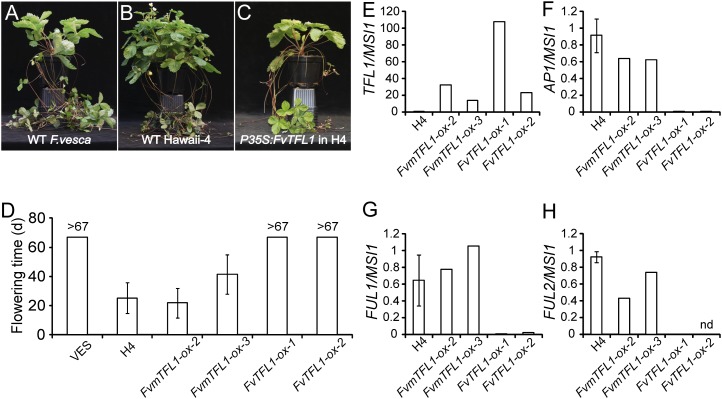
To explore the function of FvTFL1, we overexpressed the SD F. vesca (PI551792 [National Clonal Germplasm Repository]; abbreviated to VES in the figures) FvTFL1 and the allele with a 2-bp deletion under the control of the cauliflower mosaic virus P35S promoter in the LD accession Hawaii-4 (PI551572; abbreviated to H4 in the figures). Primary transgenic lines overexpressing the wild-type FvTFL1 allele from SD F. vesca (P35S:FvTFL1) remained vegetative for at least 10 months under inductive LD conditions, whereas plants overexpressing the mutated FvTFL1 (P35S:FvmTFL1) flowered continuously (Fig. 1, A–C; Supplemental Fig. S3). We further analyzed the flowering time by growing the plants propagated from single-leafed runner cuttings under LD. Both nontransgenic Hawaii-4 and P35S:FvmTFL1 lines flowered early, whereas P35S:FvTFL1 lines and SD F. vesca were still vegetative after about 10 weeks (Fig. 1D). These data indicate that FvTFL1 represses flowering under LD and that the 2-bp deletion in FvTFL1 leads to a nonfunctional FvTFL1 protein.
Figure 1.
FvTFL1 is the floral repressor SFL. A to C, Phenotypes of SD F. vesca (A), LD accession Hawaii-4 (H4; B), and a transgenic line overexpressing functional FvTFL1 (P35S:FvTFL1) in the H4 background (C). Plants were grown under LD for 15 weeks. WT, Wild type. D, Flowering time of SD F. vesca (VES), H4, and transgenic lines overexpressing functional and nonfunctional FvTFL1 (FvTFL1-ox and FvmTFL1-ox, respectively) under the control of the P35S promoter. Plants were propagated from runner cuttings. n = 3 (VES), n = 7 (H4), n = 8 (FvmTFL1-ox2), n = 2 (FvmTFL1-ox3), and n = 6 (FvTFL1-ox1 and FvTFL1-ox2). E to H, Expression of FvTFL1 (E), FvAP1 (F), FvFUL1 (G), and FvFUL2 (H) in the primary shoot apices of H4 and transgenic lines grown in LDs. n = 3 for H4 and n = 1 for each transgenic line. Results for two independent transgenic lines are shown as biological replicates. nd, Not detected.
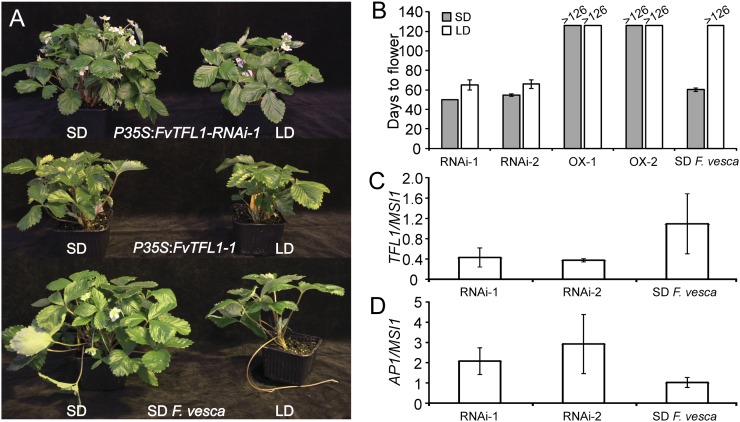
In order to further test the hypothesis that FvTFL1 is SFL, we produced transgenic lines in the SD F. vesca background and analyzed their flowering phenotypes in LD or after a strong flower induction treatment (4 weeks of SD at 11°C). FvTFL1 RNA interference (RNAi) silencing lines flowered almost at the same time in both treatments (Fig. 2, A and B). In contrast, nontransgenic control plants remained vegetative in LD and required SD induction treatment for flowering, whereas P35S:FvTFL1 lines stayed vegetative in both treatments (Fig. 2, A and B; Supplemental Fig. S4). In line with the role of Arabidopsis TFL1 in the transcriptional repression of floral meristem identity genes (Liljegren et al., 1999; Ratcliffe et al., 1999; Hanano and Goto, 2011), the expression of putative F. vesca AP1/FUL homologs correlated negatively with the expression of functional FvTFL1 in the transgenic F. vesca lines (Figs. 1, E–H, and 2, C and D). In conclusion, our results demonstrate that FvTFL1 is the major floral repressor SFL, which prevents the activation of floral meristem identity genes and flowering under LD conditions in SD F. vesca. Moreover, the absence of functional FvTFL1 reverses the photoperiodic requirements for flower induction.
Figure 2.
Silencing of FvTFL1 leads to daylength-independent flowering. A, Phenotypes of FvTFL1 RNAi silencing and overexpression lines in the SD F. vesca background. Clonally propagated plants (runner cuttings) of SD F. vesca and P35S:FvTFL1-RNAi-1 and P35S:FvTFL1-1 lines were subjected to SD induction treatment for 4 weeks followed by LDs (left) or grown continuously under LDs (right). B, Flowering time of SD F. vesca and P35S:FvTFL1-RNAi and P35S:FvTFL1 plants (RNAi and OX, respectively) in SDs and LDs. Flowering time is indicated as days to anthesis from the beginning of the treatments. Treatments and plant materials were as described in A. Values indicate means ± sd. n = 4 (OX-1), n = 5 (RNAi-2), n = 6 (RNAi-1 and OX-2), and n = 7 (SD F. vesca). C and D, Expression of FvTFL1 (C) and FvAP1 (D) in the apices of two independent P35S:FvTFL1-RNAi (RNAi) lines. Values indicate means ± sd. n = 3 (RNAi-1 and SD F. vesca) or n = 2 (RNAi-2).
We also expressed a P35S:FvTFL1 overexpression construct in the Arabidopsis tfl1-2 mutant, which flowers early especially under SD and produces a determinate inflorescence with a few flowers (Shannon and Meeks-Wagner, 1991; Supplemental Fig. S5B). We analyzed three independent P35S:FvTFL1 lines in the tfl1-2 mutant background and showed that FvTFL1 fully complemented the inflorescence defects and the early-flowering phenotype of the mutant in both SDs and LDs (Supplemental Fig. S5), indicating that FvTFL1 is an ortholog of TFL1.
FvTFL1 Is Not Involved in the Photoperiodic Control of Vegetative Development
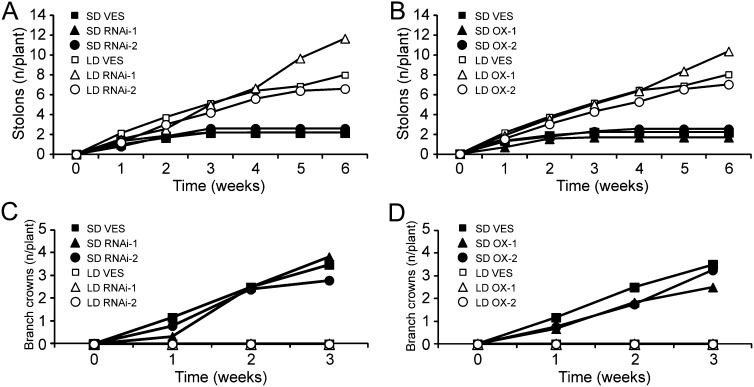
Under LDs, strawberry vegetative development typically involves the formation of a single leaf rosette and the differentiation of axillary buds to stolons (also called runners), whereas flower inductive conditions inhibit the emergence of stolons and activate the production of branch crowns (i.e. axillary leaf rosettes; Hytönen et al., 2004, 2009; Heide and Sønsteby, 2007). We analyzed the role of FvTFL1 in the control of vegetative development by using transgenic lines in a SD F. vesca background. Similar to wild-type plants, both FvTFL1 overexpression and RNAi lines continuously produced runners under LD, whereas 3 weeks of SD was enough to stop stolon formation in all lines (Fig. 3, A and B). After the cessation of stolon production in SD, the number of branch crowns started to increase in both wild-type plants and transgenic lines, whereas under LD no branch crowns were formed (Fig. 3, C and D). These results suggest that although the photoperiodic control of vegetative and floral development is tightly connected in strawberries (for review, see Hytönen and Elomaa, 2011), FvTFL1 does not affect vegetative development and is only involved in the photoperiodic control of floral initiation.
Figure 3.
FvTFL1 is not involved in the photoperiodic control of vegetative development. A and B, The cumulative number of stolons formed from the axillary buds during photoperiodic treatments in the FvTFL1 RNAi (A) and overexpression (B) lines in comparison with SD F. vesca (VES). C and D, The cumulative number of branch crowns (axillary leaf rosettes) formed after the photoperiodic treatments in the FvTFL1 RNAi (C) and overexpression (D) lines in comparison with SD F. vesca. Experimental conditions and the number of plants used for each line are described in Figure 2.
Photoperiodic Repression of FvTFL1 Expression Correlates with Floral Initiation
In Arabidopsis, TFL1 is weakly expressed in the lower part of the vegetative apical meristem, but its expression is activated in the inflorescence meristem after flower induction (Bradley et al., 1997; Ratcliffe et al., 1999). We analyzed the relative expression of FvTFL1 normalized against the stable control gene FvMSI1 MULTICOPY SUPPRESSOR OF IRA1 (Mouhu et al., 2009). FvTFL1 mRNA was detected in all tissues tested (Supplemental Fig. S6A). Similar to TFL1, it was highly expressed in the shoot apex compared with leaves in SD F. vesca grown under LDs (Fig. 4A), but a more detailed analysis revealed a divergent expression pattern. According to in situ hybridization, under LDs FvTFL1 mRNA was localized in the whole apical meristem, young leaf initials, and vascular tissues (Fig. 4, B and C). Furthermore, strong photoperiodic regulation of FvTFL1 was detected in the shoot apices but not in the leaves of SD F. vesca seedlings (Fig. 4D; Supplemental Fig. S6B). Under LDs, FvTFL1 was highly expressed in the shoot apex, whereas only weak expression was found in plants grown under SDs for 3 weeks (Fig. 4D). However, floral meristem identity genes were not yet activated in SD-grown SD F. vesca at this time (Supplemental Fig. S7), indicating that the plants were still vegetative. Because several Norwegian F. vesca accessions have been shown to require more than 1 month of SD treatment for flower induction (Heide and Sønsteby, 2007), we grew our SD F. vesca plants in SDs for 45 d followed by flowering-time analysis under LDs. We observed flower buds about 1 month after the SD treatment in all plants of SD F. vesca, whereas all LD-grown plants remained vegetative (Fig. 4E).
Figure 4.
The expression of FvTFL1 correlates with photoperiodic flowering. A, Expression of FvTFL1 in leaves and shoot apices of SD F. vesca grown under LD. Values indicate means ± sd (n = 4). B, Localization of the FvTFL1 mRNA in the shoot apex of SD F. vesca grown under LDs. C, FvTFL1 sense probe control for B. Bars = 100 µm. D, Expression of FvTFL1 in primary shoot apices of LD- and SD-grown SD F. vesca (VES) and Hawaii-4 (H4) seedlings at the three-leaf stage. Values indicate means ± sd (n = 4). E, Flowering time of seedlings of H4 and SD F. vesca shown as time to visible flower bud. Plants were grown either under continuous LD or for 45 d under SD followed by LDs. F, Flowering time of H4 seedlings shown as the number of leaves in the main shoot before terminal inflorescence under continuous SDs and LDs.
In contrast to SD F. vesca, LD-grown Hawaii-4 seedlings flowered rapidly; seedlings raised under SDs for 45 d flowered late, and the plants grown continuously under SDs remained vegetative for several months (Fig. 4, E and F), indicating that Hawaii-4 is a LD plant. In contrast to seedlings, plants propagated from the stolons of LD-grown mother plants were day neutral and flowered continuously under both LDs and SDs (Supplemental Fig. S8). In Hawaii-4, FvTFL1 mRNA expression was low in both photoperiods (Fig. 4D), most likely because of nonsense-mediated mRNA decay, a mechanism that prevents the accumulation of mRNA containing premature stop codons (Conti and Izaurralde, 2005). In association with flowering, the putative floral meristem identity genes FvAP1 and FvFUL1 were up-regulated in Hawaii-4 seedlings grown under LDs (Supplemental Fig. S6). Taken together, our data indicate that the SD requirement for flower induction in SD F. vesca is based on the photoperiodic regulation of FvTFL1, and the lack of FvTFL1 function leads to the rapid activation of flowering in LD-grown Hawaii-4.
Regulation of the Perennial Growth Cycle by FvTFL1
We further analyzed the expression patterns of FvTFL1 as well as FvAP1 and FvFUL1 in SD F. vesca plants grown under SDs followed by LDs and observed the flowering cycle of the plants. Our data showed that FvTFL1 was gradually down-regulated in the apices of the primary shoots grown under SDs, with a concomitant up-regulation of floral meristem identity genes 4 to 6 weeks after the beginning of the SD treatment (Fig. 5, A–C). When SD-grown plants were returned to LDs after 45 SDs, high FvTFL1 and low FvAP1 and FvFUL1 mRNA levels were again detected in the apices of axillary shoots that emerged after the end of the SD treatment. The observed gene expression patterns were clearly related to flowering, which started about 5 weeks after the end of the SD treatment and continued for about 1 month before declining again (Fig. 5D). In contrast to SD F. vesca, Hawaii-4 continuously produced new inflorescences only under LDs (Fig. 5D). The observed expression pattern of FvTFL1 in SD F. vesca indicates that the photoperiodic regulation of FvTFL1 mRNA expression is crucial for cycling between vegetative and reproductive phases in SD F. vesca.
Figure 5.
Photoperiod regulates the perennial growth cycle through FvTFL1. A to C, Time-course analysis of FvTFL1 (A), FvAP1 (B), and FvFUL1 (C) expression in the primary shoot apices of SD F. vesca. LD-grown plants were subjected to SDs for 6 weeks (wk 0–6) and returned to LDs for 5 weeks (+5 LD). Values indicate means ± se (n = 3). D, Cumulative number of inflorescences in clonally propagated plants (runner cuttings) of Hawaii-4 (H4) and SD F. vesca (VES). Plants were grown under flower-inductive (6 weeks of LD/SD starting from week 0 for H4/VES followed by LD) or noninductive (SD/LD for H4/VES) conditions.
FvFT1 Promotes Flowering under LDs in the F. vesca LD Accession
Functional analysis of FT in several plant species indicates its role as a florigen, a general floral activator (Turck et al., 2008; Turnbull, 2011). To establish the role of FT relative to that of SFL, we analyzed the expression patterns of F. vesca FT-like genes. As demonstrated in Figure 6, A and B, FvFT1 and FvFT2 showed contrasting spatial expression patterns in SD F. vesca. FvFT1 had the highest expression level in old leaves, and no expression or weak expression was observed in other tissues, whereas FvFT2 was expressed almost exclusively in flower buds. The tissue-specific expression pattern of FvFT1 follows the consensus that FT is a mobile signal originating in photosynthetically active leaves (Corbesier et al., 2007; Tamaki et al., 2007). Furthermore, genomic synteny is conserved only around Arabidopsis FT and FvFT1 (Shulaev et al., 2011), suggesting that FvFT1 is a likely ortholog of FT. The comparison of FvFT1 expression levels in seedlings of SD F. vesca and Hawaii-4 in different photoperiods revealed that FvFT1 was expressed under LD in both genotypes, whereas no FvFT1 expression was detected in SD-grown seedlings (Fig. 6C). The finding that FvFT1 mRNA levels did not correlate positively with flowering in the SD F. vesca contrasted with the situation in other SD plants (Hayama et al., 2003, 2007; Kong et al., 2010) and prompted us to perform diurnal rhythm and time-course analyses in older plants of SD F. vesca. As shown in Figure 6D, FvFT1 showed a clear LD-specific expression peak during the night. Moreover, FvFT1 was strongly down-regulated before flower induction under short photoperiods (Fig. 6E). Thus, the activation of FvFT1 mRNA expression correlated positively with flower induction only in the LD accession of F. vesca.
Figure 6.
Spatial and temporal expression of F. vesca FT genes. A and B, FvFT1 (A) is expressed in old leaves and FvFT2 (B) in flower buds in SD F. vesca. Plants were grown under LD after flower induction. Values indicate means ± sd (n = 2). YL, Young unopened leaf; OL, opened leaf; YP, young petiole; OP, petiole of the opened leaf; CR, crown (stem including meristems); RT, runner tip; FB, flower bud; FL, open flower; RO, root. C, Expression of FvFT1 in leaves of SD- and LD-grown seedlings of F. vesca SD accession (VES) and LD accession Hawaii-4 (H4) at the three-leaf stage. Values indicate means ± sd (n = 4). D, Diurnal rhythm of FvFT1 expression is present only under LDs in the SD F. vesca. Runner-propagated plants were either grown under LDs or transferred to SDs 8 d before sampling. Values indicate means ± se (n = 3). ZT, Zeitgeiber time. E, Time-course analysis of FvFT1 in leaves of SD F. vesca plants. Plants were moved to flower-inductive SDs at week 0. Values indicate means ± se (n = 3).
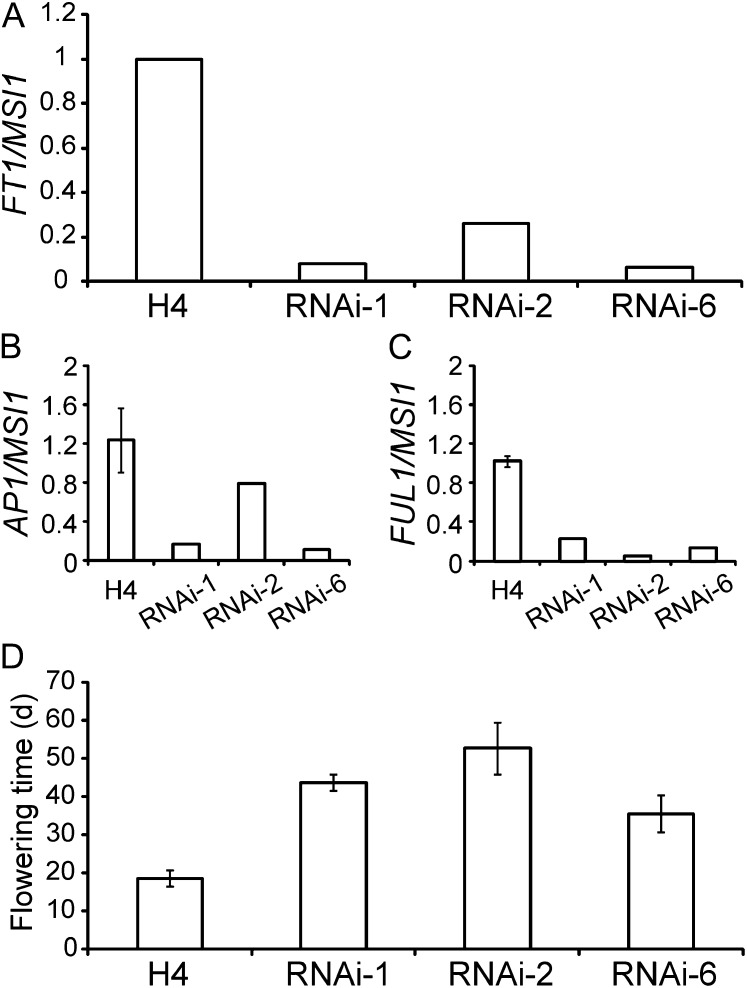
To explore the role of FT in the LD flowering response, we produced FvFT1 RNAi silencing (P35S:FvFT1-RNAi) plants in the perpetual flowering LD accession Hawaii-4. We found down-regulation of floral meristem identity genes in the shoot apex of three independent transgenic lines grown under LD (Fig. 7, A–C; Supplemental Fig. S7). Furthermore, all of these RNAi lines were clearly late flowering under LD (Fig. 7D). These results suggest that in LD-grown Hawaii-4, FvFT1 is required for the normal up-regulation of floral meristem identity genes, which marks the beginning of floral initiation at the apex (Mandel and Yanofsky, 1995; Ferrándiz et al., 2000; Mouhu et al., 2009).
Figure 7.
FvFT1 is a LD-specific activator of flowering. A, Expression of FvFT1 in the leaves of P35S:FvFT1-RNAi lines in the Hawaii-4 (H4) background and in the H4 LD accession grown under LDs. Samples were collected 16 h after dawn (n = 1). The same lines also were analyzed 4 h after dawn (Supplemental Fig. S9). B and C, Expression of FvAP1 (B) and FvFUL1 (C) in the primary shoot apices of P35S:FvFT1-RNAi lines in the H4 background and in the H4 LD accession grown under LDs. n = 3 for H4 and n = 1 for each transgenic line. Results for three independent transgenic lines are shown as biological replicates. D, Flowering time of P35S:FvFT1-RNAi lines in the H4 background and in the H4 LD accession grown under LDs. Plants were propagated from runner cuttings with a single leaf. Values indicate means ± sd (n = 6–11).
DISCUSSION
Early physiological studies indicated the existence of photoperiodically controlled flowering activating and inhibitory signals in strawberries (Hartmann, 1947; Guttridge, 1959; Vince-Prue and Guttridge, 1973). Our results confirm that both signals are present in F. vesca and that SFL is a major switch controlling photoperiodic responses. We provide functional evidence that SFL encodes a Fragaria homolog of the floral repressor TFL1 and demonstrate that FvTFL1 is photoperiodically regulated. Our results suggest that in the SD accessions of F. vesca, down-regulation of FvTFL1 under SD allows flower induction to occur only in the autumn, which leads to seasonal flowering the next spring. In contrast, a mutation in FvTFL1 causes rapid FT-dependent LD flowering and continuous initiation of inflorescences in the perpetual flowering LD accessions.
TFL1 Homologs Are Major Floral Repressors in Rosaceae
A previous study by Iwata et al. (2012) and our data show that perpetual flowering is associated with a 2-bp deletion in F. vesca TFL1 homolog (Supplemental Figs. S1 and S2). We conducted functional analysis of FvTFL1 by overexpression and RNAi approaches. FvTFL1 strongly represses flowering in F. vesca, because the introduction of a P35S:FvTFL1 construct containing functional FvTFL1 into a perpetual flowering LD accession prevented the activation of putative floral meristem identity genes (FvAP1/FUL) and flower initiation under LD. Furthermore, overexpression of the mutated version of the gene did not change the flowering phenotype, indicating that it does not encode a functional protein. As constitutive overexpression of TFL1 homologs from different species has been shown to cause pleiotropic effects (Böhlenius et al., 2006; Imamura et al., 2011), we used RNAi silencing of FvTFL1 in SD F. vesca to provide further evidence that FvTFL1 is SFL. Silencing of FvTFL1 in SD F. vesca removed the SD requirement for flower induction and changed the plants to LD flowering. As TFL1 homologs prevent LD flowering and cause the seasonal flowering habit in F. vesca (Figs. 1 and 2), control the length of the juvenile phase in apple (Kotoda et al., 2006), and may also cause seasonal flowering in roses (Iwata et al., 2012), they are possibly major floral repressors and regulators of the perennial growth cycle in the Rosaceae family.
Earlier studies have shown that photoperiod acts in an opposite manner to control flower initiation and vegetative reproduction through stolons (Hytönen et al., 2004; Heide and Sønsteby, 2007). The finding that neither overexpression nor silencing of FvTFL1 affected the photoperiodic control of stolon/branch crown formation from axillary buds showed that FvTFL1 is not directly involved in the regulation of vegetative development of the strawberry shoot. This is consistent with the genetic evidence that two separate single loci, SFL and RUNNERING LOCUS, respectively, control flowering habit and the formation of stolons in F. vesca (Brown and Wareing, 1965). However, FvTFL1 may indirectly affect vegetative growth, because the development of a terminal inflorescence promotes the outgrowth of the uppermost axillary buds as branch crowns by reducing apical dominance (Arney, 1953).
Regulation of FvTFL1 mRNA Expression Controls Photoperiodic Flowering and Seasonal Growth Cycles
In Arabidopsis, TFL1 is developmentally regulated (Bradley et al., 1997; Ratcliffe et al., 1999), and to our knowledge, no photoperiodic control of TFL1 homologs has been previously reported. Our results suggest that the photoperiodic control of FvTFL1 mRNA level in the shoot apex is a primary mechanism to control photoperiodic flowering in SD F. vesca. In SD-grown plants, FvTFL1 was strongly down-regulated before the activation of floral meristem identity genes and flower initiation. The functionality of transcriptional regulation was supported by the phenotype of FvTFL1 RNAi silencing lines in the SD F. vesca background. In two independent lines, the reduction of FvTFL1 mRNA levels was enough to induce flowering under noninductive LD conditions. In contrast, none of the P35S:FvTFL1 lines flowered under flower-inductive conditions in either SD or LD F. vesca backgrounds, indicating that plants cannot overcome constitutive overexpression of FvTFL1 mRNA. In conclusion, we have identified FvTFL1 as a component of the perennial photoperiodic pathway in F. vesca. We propose that down-regulation of FvTFL1 mRNA expression in the shoot apex of SD F. vesca under SD is required for flower induction and the activation of FvAP1/FUL genes. In contrast, the lack of functional FvTFL1 in LD accessions reverses the photoperiodic requirement for flowering.
The finding that nonfunctional FvTFL1 causes perpetual flowering of LD accessions suggests that in the SD F. vesca, the regulation of FvTFL1 contributes to the cycling of vegetative and reproductive phases, and our gene expression results support this hypothesis. Whereas FvTFL1 was down-regulated and FvAP1/FUL was up-regulated in the apex of the primary shoot under SD, subsequent LD conditions restored high FvTFL1 and low FvAP1/FUL mRNA levels in the apices of new vegetative axillary shoots. Earlier studies have shown that strawberries are able to initiate flowers at the apex of the primary shoot and axillary shoots, which have reached competence to flower (Arney, 1953; Hytönen et al., 2004; Hytönen and Elomaa, 2011). Therefore, we hypothesize that the down-regulation of FvTFL1 under SD in the autumn allows flower induction to occur in the apex of the primary shoot and older axillary shoots. However, its up-regulation in the LDs of spring is crucial for perennialism, because it prevents further floral initiation in the newly emerged axillary shoots until SD conditions return. Our results on FvTFL1 and earlier findings that seasonal changes in the expression of two Populus FT-like genes regulate the perennial growth cycle in Populus (Hsu et al., 2011) highlight the importance of CETS proteins in the control of perennialism. Also, in A. alpina, TFL1 contributes to perennialism by regulating the age-dependent sensitivity of meristems to vernalization; but transient silencing of the FLC ortholog, PEP1, by vernalization has a major role in the cycling between vegetative and generative phases in this species (Wang et al., 2009, 2011).
In contrast to FvTFL1, which is broadly expressed in the shoot apex (Fig. 4B), Arabidopsis TFL1 is weakly expressed in the axillary meristems and lower part of the apical meristem during the vegetative phase and is activated in the inflorescence meristem (Bradley et al., 1997; Ratcliffe et al., 1999). This intriguing difference is probably associated with differences in inflorescence development. In Arabidopsis, TFL1 is activated in the inflorescence meristem in order to allow indeterminate growth of the raceme (Bradley et al., 1997; Ratcliffe et al., 1999), whereas the production of a cymose inflorescence with a terminal flower in strawberry (Jahn and Dana, 1970) would be expected to require the silencing of FvTFL1 in the apical meristem.
FvTFL1 Overcomes the Function of the LD-Specific Floral Activator FvFT1
The CO/FT module controls photoperiodic flowering in both annual and perennial plants (Suárez-López et al., 2001; Hayama et al., 2003; Böhlenius et al., 2006), and FT is thought to be a universal flowering signal (Turck et al., 2008; Turnbull, 2011; Wigge, 2011). In concordance with studies in other plants, we observed that the expression of FvFT1, the likely ortholog of FT, correlated with flowering in the LD accession Hawaii-4. Furthermore, strong reduction in the expression of FvAP1/FUL genes in FvFT1 RNAi plants as well as their late-flowering phenotype suggested that FvFT1 controls flower induction in F. vesca LD accessions (Fig. 7). However, in the SD F. vesca, FvFT1 was highly expressed in noninductive LD, which contrasts with findings in other SD plants (Hayama et al., 2003, 2007; Kong et al., 2010). Our data suggest that in this genotype, FvTFL1 expression in the shoot apex may overcome the function of FvFT1 as an activator of flowering. FT and TFL1 are closely related proteins whose opposite function is caused by differences in the external loop (Ahn et al., 2006). A recent study showed that FT forms a so-called “florigen activation complex” together with FD and 14-3-3 proteins (Taoka et al., 2011). Because TFL1 homologs in different species can also bind FD and 14-3-3 (Pnueli et al., 2001; Purwestri et al., 2009; Hanano and Goto, 2011), FvTFL1 may inhibit flowering by competing for binding partners with FvFT1. This is consistent with the regulation of flowering in tomato (Solanum lycopersicum), in which the balance between the expression of FT and TFL1 homologs controls flowering (Shalit et al., 2009). However, in SD F. vesca, both FvFT1 and FvTFL1 are down-regulated under flower-inductive conditions. Therefore, we hypothesize that flower induction in SD F. vesca takes place via an FvFT1-independent mechanism, whereas in Hawaii-4, FvFT1 functions as a LD-specific floral promoter in the absence of functional FvTFL1. On the other hand, we cannot exclude the possibility that FT is involved in the LD activation of FvTFL1, which is photoperiodically regulated only in the shoot apex (Fig. 4D; Supplemental Fig. S6B). As the perception of photoperiod is thought to occur in the leaves (An et al., 2004; Corbesier et al., 2007), a systemic signal may be required for the photoperiodic control of FvTFL1. FT is a good candidate for such a signal, because it is emerging as a general photoperiodic signaling molecule in diverse plant species (Wigge, 2011). Furthermore, Hecht et al. (2011) have shown evidence that leaf-expressed FT may mediate the photoperiodic control of another CETS family gene in the pea (Pisum sativum) shoot apex. Further studies are needed to resolve the function of FvFT1 in SD F. vesca and to establish which mechanism activates floral meristem identity genes after the down-regulation of FvTFL1.
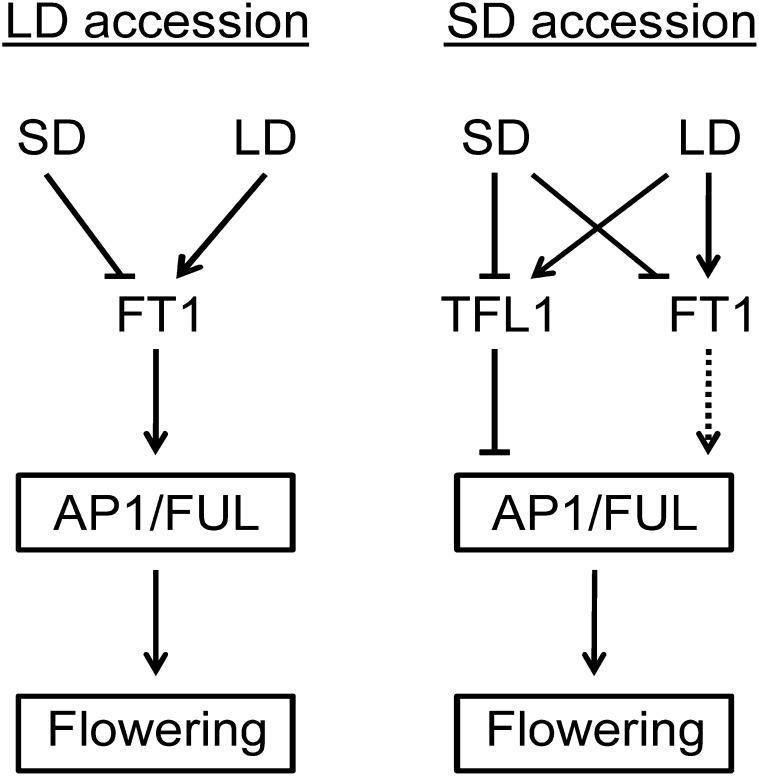
A Model of the Regulation of Flowering and Growth Cycles by F. vesca TFL1 and FT Homologs
Based on our studies, we propose a model (Fig. 8) in which the flowering of SD F. vesca accessions is repressed through LD-activated FvTFL1 expression, which overrides the floral activator function of FvFT1. When photoperiod drops below a critical level during autumn, FvTFL1 mRNA levels decrease, floral meristem identity genes are up-regulated, probably through an FvFT1-independent pathway, and floral development begins. In the following spring and summer, FvTFL1 is activated again in new axillary shoots and keeps them vegetative until inductive conditions return. In contrast, the absence of functional FvTFL1 in LD accessions leads to FvFT1-mediated flower induction and continuous flowering under LDs. More studies are needed to explore how this model generated in the diploid Fragaria can be translated to the more complex octoploid cultivated strawberry, which shows similarities in the environmental control of flowering with F. vesca (Heide, 1977; Heide and Sønsteby, 2007; Sønsteby and Heide, 2007, 2008; Bradford et al., 2010).
Figure 8.
Model showing the photoperiodic control of flowering in F. vesca SD and LD accessions. Arrows indicate activation, and bars indicate repression. The regulation of FvAP1/FUL by FvFT1 in SD F. vesca is shown by a dashed line because the strong repressor FvTFL1 overrides the floral activator function of FvFT1.
The timing and duration of flowering season are traits of importance to crop production because they are major factors contributing to yield potential and the length of the harvest season. Natural variation in TFL1 homologs has been important for the domestication and crop improvement of several annual crops (Turnbull, 2011), and our study shows the importance of TFL1 in the perennial context. As TFL1 homologs exist in all angiosperms (Karlgren et al., 2011) and their function as floral repressors is conserved between plant families (Shannon and Meeks-Wagner, 1991; Foucher et al., 2003; Kotoda et al., 2006; Mohamed et al., 2010; Imamura et al., 2011), our findings are of general significance for understanding the regulation of flowering- and perennial-specific traits in a wide range of economically important crops.
MATERIALS AND METHODS
Plant Material
Fragaria vesca accession (PI551792) and F. vesca f. semperflorens Hawaii-4 (PI551572) were obtained from the National Clonal Germplasm Repository. The Arabidopsis (Arabidopsis thaliana) tfl1-2 mutant (Nottingham Arabidopsis Stock Centre identifier N3091) in the Landsberg erecta background was used in the genetic complementation test.
Growth Conditions and Phenotyping
F. vesca plants were grown in greenhouse rooms equipped with darkening curtains. Standard LD growth conditions were 18 h of high-pressure sodium light (approximately 150 µmol m−2 s−1) at 18°C. In photoperiodic experiments at 18°C, 12 h of high-pressure sodium light (approximately 150 µmol m−2 s−1) was provided for all plants (SD), and under LD, 6 h of day extension was given as low-intensity incandescent light (approximately 8 µmol m−2 s−1). Arabidopsis plants were grown under LD (16 h of light at 22°C) or SD (10 h of light at 22°C) in a growth chamber. Light was provided by high-pressure sodium lamps (approximately 180 µmol m−2 s−1). Flowering time was measured either as the number of leaves initiated before the first inflorescence or the number of days from sowing to the first open flower.
Statistics
The χ2 goodness-of-fit test was performed on the flowering data from the F2 and BC1 mapping populations. The critical value at α = 0.05 was 3.841 for all χ2 tests.
Genotyping and Mapping
DNA extraction was done using a previously described protocol (Albani et al., 2004). The markers used for mapping are listed in Supplemental Table S1. Linkage maps were constructed as described earlier (Sargent et al., 2004). The F2 mapping population was characterized for FvTFL1 using TFL1-6FAM primers (Supplemental Table S1).
Identification of Single-Nucleotide Polymorphism Markers by Genome Resequencing
DNA of SD F. vesca (PI551792) was extracted (Albani et al., 2004) and treated with RNase A. A genomic DNA library was generated using the Genomic DNA Sample Prep Kit (Illumina), and 30-bp reads were sequenced using the Illumina GAIIx flow cell. Image analysis and base calling were done by OLB version 1.6.0 software and sequence alignment by CASAVA GERALD version 1.6.0. A default chastity threshold of 0.6 was used for purity filtering. All sequence reads with two or fewer mismatches were aligned with 801,610 bp of the genomic reference sequence of Hawaii-4 between markers V8p98 and V8p278 (Supplemental Fig. S1) using Bowtie version 0.12.2 and Readaligner version 2010_4rc2 software, and the resulting .sam files (sequence alignment/map files) were viewed by Tablet version 1.10.05.21 in order to find single-nucleotide polymorphisms. Resequencing and related bioinformatics were carried out at Biomedicum Genomics, University of Helsinki.
Expression Analysis
Total RNA was extracted, cDNA was synthesized, and real-time PCR was performed as described earlier (Mouhu et al., 2009). FvMSI1 was used as a stable control gene (Mouhu et al., 2009) for normalization. The number of biological replicates is indicated in each Figure legend. Three technical replicates were performed for each sample. Real-time PCR primers are listed in Supplemental Table S1.
Plasmid Constructs
Plasmid constructs for overexpressing FvTFL1 or FvmTFL1 were created according to Gateway Technology with Clonase II (Invitrogen). For overexpression of TFL1, the primers used to amplify cDNA from SD F. vesca (PI551792) and sfl mutant Baron Solemacher (PI551507) were 5′-AAAAAGCAGGCTCTGTACAACCTTTTCTCTTCTCCCTCT-3′ (attB1) and 5′-AGAAAGCTGGGTCCTCCCTGCAAGGTGCCTA-3′ (attB2). For the double-stranded FvTFL1 RNAi construct, the fragment was amplified from SD F. vesca cDNA with primers 5′-AAAAAGCAGGCTTGTTTGGCCTTGGCATCTCG-3′ (attB1) and 5′-AGAAAGCTGGGTTCTGCAGTCACCGCCAAACC-3′ (attB2). For creating FvFT1 RNAi constructs, cDNA from SD F. vesca (PI551792) was amplified with primers 5′-AAAAAGCAGGCTTGTTTGGCCTTGGCATCTCG-3′ (attB1) and 5′-AGAAAGCTGGGTTCTGCAGTCACCGCCAAACC-3′ (attB2). The destination vectors were p7WG2D for overexpression and PK7GWIWG2(II) for RNAi (Karimi et al., 2002).
Transformation
Arabidopsis plants were transformed with Agrobacterium tumefaciens strain GV3101 by the floral dip method (Zhang et al., 2006). Strawberry transformations were performed as described earlier (Oosumi et al., 2006). Plants of the T1 or T0 generation were analyzed in Arabidopsis and F. vesca, respectively.
In Situ Hybridization
In situ hybridization was performed on the apex of the main shoot as described previously (Kurokura et al., 2006). The probe template fragment was amplified by reverse transciption-PCR using the primers shown in Supplemental Table S1 and cloned into pDrive cloning vector (Qiagen). Sense and antisense probes were synthesized from T7 or SP6 promoters.
Sequence data from this article can be found in the GenBank data library under accession numbers JN172097 and JN172098.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Fine-mapping of SFL.
Supplemental Figure S2. Deletion in FvTFL1 cosegregates with SFL in the SD F. vesca × F. vesca f. semperflorens BC1 population.
Supplemental Figure S3. Phenotypes of 10-month-old LD-grown Hawaii-4 and P35S:FvTFL1 plants.
Supplemental Figure S4. FvTFL1 expression in P35S:FvTFL1 transgenic lines.
Supplemental Figure S5. FvTFL1 complements the Arabidopsis tfl1-2 mutant phenotype.
Supplemental Figure S6. Expression analysis of FvTFL1.
Supplemental Figure S7. Effect of photoperiod on the expression of floral meristem identity genes.
Supplemental Figure S8. F. vesca LD accession Hawaii-4 flowers continuously after LD induction in both SD and LD conditions.
Supplemental Figure S9. Expression of FvFT1 in LD-grown P35S:FvFT1-RNAi lines.
Supplemental Table S1. Primers used in this study.
Supplementary Material
Acknowledgments
We thank L. Sarelainen for technical assistance in genetic transformation and J.P.T. Valkonen, O. Junttila, and M. Mattsson for critical reading of the manuscript.
Glossary
- SD
short day
- LD
long day
- RNAi
RNA interference
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056 [DOI] [PubMed] [Google Scholar]
- Ahn JH, Miller D, Winter VJ, Banfield MJ, Lee JH, Yoo SY, Henz SR, Brady RL, Weigel D. (2006) A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J 25: 605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albani MC, Battey NH, Wilkinson MJ. (2004) The development of ISSR-derived SCAR markers around the SEASONAL FLOWERING LOCUS (SFL) in Fragaria vesca. Theor Appl Genet 109: 571–579 [DOI] [PubMed] [Google Scholar]
- Albani MC, Coupland G. (2010) Comparative analysis of flowering in annual and perennial plants. Curr Top Dev Biol 91: 323–348 [DOI] [PubMed] [Google Scholar]
- An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Piñeiro M, Hepworth S, Mouradov A, Justin S, Turnbull C, et al (2004) CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131: 3615–3626 [DOI] [PubMed] [Google Scholar]
- Arney SE. (1953) Studies of the growth and development of the genus Fragaria. II. The initiation, growth and emergence of leaf primordia in Fragaria. Ann Bot (Lond) 17: 477–492 [Google Scholar]
- Battey NH. (2000) Aspects of seasonality. J Exp Bot 51: 1769–1780 [DOI] [PubMed] [Google Scholar]
- Battey NH, Le Mière P, Tehranifar A, Cekic C, Taylor S, Shrives KJ, Hadley P, Greenland AJ, Darby J, Wilkinson MJ. (1998) Genetic and environmental control of flowering in strawberry. In KE Cockshull, D Gray, GB Seymour, B Thomas, eds, Genetic and Environmental Manipulation of Horticultural Crops. CABI, Wallingford, UK, pp 111–131 [Google Scholar]
- Böhlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O. (2006) CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312: 1040–1043 [DOI] [PubMed] [Google Scholar]
- Bradford E, Hancock JF, Warner RM. (2010) Interactions of temperature and photoperiod determine expression of repeat flowering in strawberry. J Am Soc Hortic Sci 135: 102–107 [Google Scholar]
- Bradley D, Ratcliffe OJ, Vincent C, Carpenter R, Coen E. (1997) Inflorescence commitment and architecture in Arabidopsis. Science 275: 80–83 [DOI] [PubMed] [Google Scholar]
- Brown T, Wareing P. (1965) Genetical control of everbearing habit and 3 other characters in varieties of Fragaria vesca. Euphytica 14: 97–112 [Google Scholar]
- Conti L, Bradley D. (2007) TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture. Plant Cell 19: 767–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti E, Izaurralde E. (2005) Nonsense-mediated mRNA decay: molecular insights and mechanistic variations across species. Curr Opin Cell Biol 17: 316–325 [DOI] [PubMed] [Google Scholar]
- Cooper JP, Calder DM. (1964) The inductive requirements for flowering of some temperate grasses. Grass Forage Sci 19: 6–14 [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033 [DOI] [PubMed] [Google Scholar]
- Endo T, Shimada T, Fujii H, Kobayashi Y, Araki T, Omura M. (2005) Ectopic expression of an FT homolog from citrus confers an early flowering phenotype on trifoliate orange (Poncirus trifoliata L. Raf.). Transgenic Res 14: 703–712 [DOI] [PubMed] [Google Scholar]
- Ferrándiz C, Gu Q, Martienssen R, Yanofsky MF. (2000) Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development 127: 725–734 [DOI] [PubMed] [Google Scholar]
- Foucher F, Morin J, Courtiade J, Cadioux S, Ellis N, Banfield MJ, Rameau C. (2003) DETERMINATE and LATE FLOWERING are two TERMINAL FLOWER1/CENTRORADIALIS homologs that control two distinct phases of flowering initiation and development in pea. Plant Cell 15: 2742–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttridge CG. (1959) Further evidence for a growth-promoting and flower-inhibiting hormone in strawberry. Ann Bot (Lond) 23: 612–621 [Google Scholar]
- Gyllenstrand N, Clapham D, Källman T, Lagercrantz U. (2007) A Norway spruce FLOWERING LOCUS T homolog is implicated in control of growth rhythm in conifers. Plant Physiol 144: 248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanano S, Goto K. (2011) Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. Plant Cell 23: 3172–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann HT. (1947) Some effects of temperature and photoperiod on flower formation and runner production in the strawberry. Plant Physiol 22: 407–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama R, Agashe B, Luley E, King R, Coupland G. (2007) A circadian rhythm set by dusk determines the expression of FT homologs and the short-day photoperiodic flowering response in Pharbitis. Plant Cell 19: 2988–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama R, Yokoi S, Tamaki S, Yano M, Shimamoto K. (2003) Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 422: 719–722 [DOI] [PubMed] [Google Scholar]
- Hecht V, Laurie RE, Vander Schoor JK, Ridge S, Knowles CL, Liew LC, Sussmilch FC, Murfet IC, Macknight RC, Weller JL. (2011) The pea GIGAS gene is a FLOWERING LOCUS T homolog necessary for graft-transmissible specification of flowering but not for responsiveness to photoperiod. Plant Cell 23: 147–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heide OM. (1977) Photoperiod and temperature interactions in growth and flowering of strawberry. Physiol Plant 40: 21–26 [Google Scholar]
- Heide OM, Sønsteby A. (2007) Interactions of temperature and photoperiod in the control of flowering of latitudinal and altitudinal populations of wild strawberry (Fragaria vesca). Physiol Plant 130: 280–286 [Google Scholar]
- Hsu C-Y, Adams JP, Kim H, No K, Ma C, Strauss SH, Drnevich J, Vandervelde L, Ellis JD, Rice BM, et al. (2011) FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proc Natl Acad Sci USA 108: 10756–10761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C-Y, Liu YX, Luthe DS, Yuceer C. (2006) Poplar FT2 shortens the juvenile phase and promotes seasonal flowering. Plant Cell 18: 1846–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hytönen T, Elomaa P. (2011) Genetic and environmental regulation of flowering and runnering in strawberries. Genes Genomes Genomics 5: 56–64 [Google Scholar]
- Hytönen T, Elomaa P, Moritz T, Junttila O. (2009) Gibberellin mediates daylength-controlled differentiation of vegetative meristems in strawberry (Fragaria × ananassa Duch). BMC Plant Biol 9: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hytönen T, Palonen P, Mouhu K, Junttila O. (2004) Crown branching and cropping potential in strawberry (Fragaria × ananassa, Duch.) can be enhanced by daylength treatments. J Hortic Sci Biotechnol 79: 466–471 [Google Scholar]
- Imamura T, Nakatsuka T, Higuchi A, Nishihara M, Takahashi H. (2011) The gentian orthologs of the FT/TFL1 gene family control floral initiation in Gentiana. Plant Cell Physiol 52: 1031–1041 [DOI] [PubMed] [Google Scholar]
- Iwata H, Gaston A, Remay A, Thouroude T, Jeauffre J, Kawamura K, Oyant LH, Araki T, Denoyes B, Foucher F. (2012) The TFL1 homologue KSN is a regulator of continuous flowering in rose and strawberry. Plant J 69: 116–125 [DOI] [PubMed] [Google Scholar]
- Jaeger KE, Wigge PA. (2007) FT protein acts as a long-range signal in Arabidopsis. Curr Biol 17: 1050–1054 [DOI] [PubMed] [Google Scholar]
- Jahn OL, Dana MN. (1970) Crown and inflorescence development in the strawberry, Fragaria ananassa. Am J Bot 57: 607–612 [Google Scholar]
- Komiya R, Yokoi S, Shimamoto K. (2009) A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development 136: 3443–3450 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A. (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Karlgren A, Gyllenstrand N, Källman T, Sundström JF, Moore D, Lascoux M, Lagercrantz U. (2011) Evolution of the PEBP gene family in plants: functional diversification in seed plant evolution. Plant Physiol 156: 1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Doyle MR, Sung S, Amasino RM. (2009) Vernalization: winter and the timing of flowering in plants. Annu Rev Cell Dev Biol 25: 277–299 [DOI] [PubMed] [Google Scholar]
- Kong F, Liu B, Xia Z, Sato S, Kim BM, Watanabe S, Yamada T, Tabata S, Kanazawa A, Harada K, et al (2010) Two coordinately regulated homologs of FLOWERING LOCUS T are involved in the control of photoperiodic flowering in soybean. Plant Physiol 154: 1220–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotoda N, Hayashi H, Suzuki M, Igarashi M, Hatsuyama Y, Kidou S, Igasaki T, Nishiguchi M, Yano K, Shimizu T, et al (2010) Molecular characterization of FLOWERING LOCUS T-like genes of apple (Malus × domestica Borkh.). Plant Cell Physiol 51: 561–575 [DOI] [PubMed] [Google Scholar]
- Kotoda N, Iwanami H, Takahashi S, Abe K. (2006) Antisense expression of MdTFL1, a TFL1-like gene, reduces the juvenile phase in apple. J Am Soc Hortic Sci 131: 74–81 [Google Scholar]
- Kurokura T, Inaba Y, Sugiyama N. (2006) Histone H4 gene expression and morphological changes on shoot apices of strawberry (Fragaria × ananassa Duch.) during floral induction. Sci Hortic (Amsterdam) 110: 192–197 [Google Scholar]
- Liljegren SJ, Gustafson-Brown C, Pinyopich A, Ditta GS, Yanofsky MF. (1999) Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. Plant Cell 11: 1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel MA, Yanofsky MF. (1995) A gene triggering flower formation in Arabidopsis. Nature 377: 522–524 [DOI] [PubMed] [Google Scholar]
- Mohamed R, Wang C-T, Ma C, Shevchenko O, Dye SJ, Puzey JR, Etherington E, Sheng X, Meilan R, Strauss SH, et al (2010) Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identity and dormancy release in Populus. Plant J 62: 674–688 [DOI] [PubMed] [Google Scholar]
- Mouhu K, Hytönen T, Folta K, Rantanen M, Paulin L, Auvinen P, Elomaa P. (2009) Identification of flowering genes in strawberry, a perennial SD plant. BMC Plant Biol 9: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutasa-Göttgens E, Hedden P. (2009) Gibberellin as a factor in floral regulatory networks. J Exp Bot 60: 1979–1989 [DOI] [PubMed] [Google Scholar]
- Oosumi T, Gruszewski HA, Blischak LA, Baxter AJ, Wadl PA, Shuman JL, Veilleux RE, Shulaev V. (2006) High-efficiency transformation of the diploid strawberry (Fragaria vesca) for functional genomics. Planta 223: 1219–1230 [DOI] [PubMed] [Google Scholar]
- Pnueli L, Gutfinger T, Hareven D, Ben-Naim O, Ron N, Adir N, Lifschitz E. (2001) Tomato SP-interacting proteins define a conserved signaling system that regulates shoot architecture and flowering. Plant Cell 13: 2687–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwestri YA, Ogaki Y, Tamaki S, Tsuji H, Shimamoto K. (2009) The 14-3-3 protein GF14c acts as a negative regulator of flowering in rice by interacting with the florigen Hd3a. Plant Cell Physiol 50: 429–438 [DOI] [PubMed] [Google Scholar]
- Ratcliffe OJ, Bradley DJ, Coen ES. (1999) Separation of shoot and floral identity in Arabidopsis. Development 126: 1109–1120 [DOI] [PubMed] [Google Scholar]
- Samach A, Wigge PA. (2005) Ambient temperature perception in plants. Curr Opin Plant Biol 8: 483–486 [DOI] [PubMed] [Google Scholar]
- Sargent DJ, Davis TM, Tobutt KR, Wilkinson MJ, Battey NH, Simpson DW. (2004) A genetic linkage map of microsatellite, gene-specific and morphological markers in diploid Fragaria. Theor Appl Genet 109: 1385–1391 [DOI] [PubMed] [Google Scholar]
- Shalit A, Rozman A, Goldshmidt A, Alvarez JP, Bowman JL, Eshed Y, Lifschitz E. (2009) The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proc Natl Acad Sci USA 106: 8392–8397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon S, Meeks-Wagner DR. (1991) A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell 3: 877–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulaev V, Korban SS, Sosinski B, Abbott AG, Aldwinckle HS, Folta KM, Iezzoni A, Main D, Arús P, Dandekar AM, et al. (2008) Multiple models for Rosaceae genomics. Plant Physiol 147: 985–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulaev V, Sargent DJ, Crowhurst RN, Mockler TC, Folkerts O, Delcher AL, Jaiswal P, Mockaitis K, Liston A, Mane SP, et al. (2011) The genome of woodland strawberry (Fragaria vesca). Nat Genet 43: 109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG. (2004) The autonomous pathway: epigenetic and post-transcriptional gene regulation in the control of Arabidopsis flowering time. Curr Opin Plant Biol 7: 570–574 [DOI] [PubMed] [Google Scholar]
- Sønsteby A, Heide OM. (2007) Long-day control of flowering in everbearing strawberries. J Hortic Sci Biotechnol 82: 875–884 [Google Scholar]
- Sønsteby A, Heide OM. (2008) Long-day rather than autonomous control of flowering in the diploid everbearing strawberry Fragaria vesca ssp semperflorens. J Hortic Sci Biotechnol 83: 360–366 [Google Scholar]
- Stewart PJ, Folta KM. (2010) A review of photoperiodic flowering research in strawberry (Fragaria spp.). Crit Rev Plant Sci 29: 1–13 [Google Scholar]
- Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410: 1116–1120 [DOI] [PubMed] [Google Scholar]
- Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. (2007) Hd3a protein is a mobile flowering signal in rice. Science 316: 1033–1036 [DOI] [PubMed] [Google Scholar]
- Taoka K, Ohki I, Tsuji H, Furuita K, Hayashi K, Yanase T, Yamaguchi M, Nakashima C, Purwestri YA, Tamaki S, et al. (2011) 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 476: 332–335 [DOI] [PubMed] [Google Scholar]
- Turck F, Fornara F, Coupland G. (2008) Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 59: 573–594 [DOI] [PubMed] [Google Scholar]
- Turnbull C. (2011) Long-distance regulation of flowering time. J Exp Bot 62: 4399–4413 [DOI] [PubMed] [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303: 1003–1006 [DOI] [PubMed] [Google Scholar]
- Vince-Prue D, Guttridge CG. (1973) Floral initiation in strawberry: spectral evidence for the regulation of flowering by long-day inhibition. Planta 110: 165–172 [DOI] [PubMed] [Google Scholar]
- Wang R, Albani MC, Vincent C, Bergonzi S, Luan M, Bai Y, Kiefer C, Castillo R, Coupland G. (2011) Aa TFL1 confers an age-dependent response to vernalization in perennial Arabis alpina. Plant Cell 23: 1307–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Farrona S, Vincent C, Joecker A, Schoof H, Turck F, Alonso-Blanco C, Coupland G, Albani MC. (2009) PEP1 regulates perennial flowering in Arabis alpina. Nature 459: 423–427 [DOI] [PubMed] [Google Scholar]
- Weebadde CK, Wang D, Finn CE, Lewers KS, Luby JJ, Bushacra JJ, Sjulin TM, Hancock JF. (2008) Using a linkage mapping approach to identify QTL for day-neutrality in the octoploid strawberry. Plant Breed 127: 94–101 [Google Scholar]
- Wigge PA. (2011) FT, a mobile developmental signal in plants. Curr Biol 21: R374–R378 [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D. (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309: 1056–1059 [DOI] [PubMed] [Google Scholar]
- Zhang XR, Henriques R, Lin SS, Niu QW, Chua NH. (2006) Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 1: 641–646 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.