Abstract
BACKGROUND:
Inherited polymorphisms of XPD and XPC genes may contribute to subtle variations in NER DNA repair capacity and genetic susceptibility to development of urological cancer such as prostate and bladder cancer.
MATERIALS AND METHODS:
We genotyped four Single Nucleotide Polymorphs (SNPs) of the DNA repair gene XPD and XPC in 195 prostate cancer (PCa) and 212 bladder cancer (BC) patients and 250 healthy controls from the same area. XPD Exon 10 (G>A) by amplification refractory mutation system and Exon 23 (A>C), XPC Intron 9 (Ins/Del) and Exon 15 (A>C) were genotyped by PCR-RFLP.
RESULTS:
Variant genotype of XPC demonstrated association with PCa as well as in BC (P, 0.013; P, 0.003). Combined genotype (GA+AA) revealed association with PCa and in BC (P, 0.012, P, 0.002). Variant allele also demonstrated risk in both the cancer. Diplotype of XPD and XPC was associated with a significant increase in PCa and BC risk. Variant (+/+) genotype of XPC intron 9 shown increased risk with PCa and in BC (P, 0.012; P, 0.032). CC genotype of XPC exon 15 revealed increase risk (P, 0.047) with PCa not in BC. In clinopathological grade variant allele of XPC intron 9 and 15 demonstrated risk with high grade of tumor and bone metastasis of PCa. In BC variant allele of XPD exon 10 and 15 also shown association with tumor grade. XPC intron 9 influences the risk of BC in former tobacco users in BC.
CONCLUSIONS:
Our result support that SNPs in XPD and XPC gene may reduce NER repair capacity and play a major role for PCa and BC in North India.
Keywords: Bladder cancer, Prostate cancer, diplotype, NER genes, polymorphism
Introduction
Cancer is a multifactorial disease that results from complex interactions between the genetic background and environmental factors. Tumors of the urinary tract contribute significantly to the overall human cancer burden.[1] The incidence rate of prostate and bladder cancer in India is low compared to the Western countries, and is the sixth most commonly diagnosed cancer.[2] A wide variability in the incidence of urinary cancer reflects its multi-factorial etiology involving genetic and ethnic backgrounds, which further complexes by the effect of environmental factors.
Carcinogenesis is a multistep, polygenic, and multicausal process. Tobacco smoke and environmental and occupational activities involve several potent chemical carcinogens, which have shown to increase DNA adducts levels and to be associated with many cancers. An accumulation of genetic abnormalities and a decline in DNA repair may lead to carcinogenesis.[3]
Nucleotide excision repair (NER) is one of the most important pathways and eliminates a wide variety of DNA damage, including UV photoproducts by involving the protein products of >30 genes.[4] Mutations and single nucleotide polymorphisms (SNPs) in NER genes may contribute to deficient NER capacity and human cancer risk.[5,6] Among the many genes the XPD and XPC gene play a major role in NER pathway.
The XPD also called ERCC2 gene, is located at chromosome 19q13.3 The XPD protein is a component of the core transcription factor IIH, involved in repair of DNA by opening DNA around the damage. it also acts in the initiation of RNA transcription by RNA polymerase II by anchoring the cyclin-dependent kinase activating kinase complex to the core transcription factor IIH complex.[7] Mutations in the XPD gene can completely prevent DNA opening and dual incision, steps that lead to the repair of DNA adducts.[8] The two (XPD) polymorphic loci that have been of particular interest in molecular epidemiological studies are exon 10 (G>A) (Asp312Asn) and exon 23 (A>T) (Lys751Gln) polymorphisms.[9] These polymorphisms are associated with lower DNA repair capacity (DRC) and a higher level of DNA adducts.[10] Studies have reported significant associations between the Asp312Asn (G>A) or Lys751Gln (A>C) variants and predisposition to many types of cancers.
The XPC gene is located on chromosome 3p25 and encodes a 940-amino acid protein involved in DNA damage recognition during the early steps of the NER process. Sequence variants of the XPC gene may alter NER capacity and modulate cancer risk. An intronic biallelic poly (AT) insertion/deletion polymorphism (PAT) in intron 9 and non-synonymous SNPs, Lys939Gln (an A>C transversion) in exon 15 of XPC have been identified. Although the variant alleles of the intron 9 polymorphism and of exon 15 have been associated with reduced DRC.[11,12] XPC-PAT polymorphism has been reported to be in linkage disequilibrium with XPC exon 15 that causes an amino acid change Lys939Gln (A33512C). The effects of all three of these XPC polymorphisms on cancer risk have been extensively studied, but with inconsistent results. In some studies, the three polymorphisms were found to significantly modify cancer risk, but other studies showed a lack of association between the polymorphisms and cancer risk.
We believe that in addition to the base excision repair and mismatch repair pathways, NER should also be evaluated for its role in urothelial cancer such as prostate cancer (PCa) and bladder cancer (BC) risk. To test this hypothesis, we investigate a possible association between NER repair gene polymorphisms (XPD and XPC) and their diplotypes with PCa and BC risk in north Indian population.
Materials and Methods
Study subjects
The study subjects were enrolled in the Department of Urology (Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow) between January 2006 and February 2009. The participants in this study were unrelated individuals of similar ethnicity from Lucknow and other adjoining cities of North India. A total histologically confirmed 195 (prostate) and 212 (bladder) cancer patients were enrolled for the study. Tumor grade was evaluated in PCa samples by the Gleason scoring system[13] and BC has been classified as per the American Joint Committee on Cancer's TNM staging system.[14] A total of 250 cancer free, unrelated, age- and sex-matched healthy control individuals of similar ethnicity, randomly from general population of Lucknow and adjoining areas, were recruited as controls. PSA was done in all the control individuals. Individuals with total PSA ≥4 ng/ml and/or any irregularity in DRE or with history of cancer were excluded from the PCa study. Serum PSA was assayed by sandwich ELISA using CanAg PSA kit, Sweden. The study was approved by the Ethical Committee Review Board of our Institute and informed written consent was obtained from each participant.
DNA extraction and genotype analysis
Five milliliters of peripheral blood samples was collected in ethylenediaminetetraacetic acid vials from both the patients as well as controls. Genomic DNA was extracted from the stored peripheral blood by salting out method.[15] Genotyping was performed using amplification refractory mutation-specific polymerase chain reaction (PCR) methodology for XPD exon 10 (G>A).[16] PCR was used to amplify regions of XPC-PAT.[17] XPD exon 23 (A>C) and XPC exon 15 was genotyped by PCR–restriction fragment length polymorphism methods using PstI and PVUII (New England Biolabs, Beverly, MA) restriction enzyme.[17,18] All the genes polymorphisms were successfully genotyped in both the patients and controls.
Quality control procedures
Precise quality control procedures were applied during the genotyping process. As a negative control, PCR mix without DNA sample was used to ensure contamination-free PCR product. Samples that failed to genotype were scored as missing and subjected to repetition. Ten percent of samples from patients and controls were repeated to evaluate the quality of genotyping, which showed 100% concordance. Genotyping was performed without knowledge of the case or control status.
Statistical analyses
The sample size was calculated and was found to be adequate using QUANTO software, version 1.0 (http://hydra.usc.edu/gxe) for the genetic marker. Sample size achieved 80% of the statistical power. A two-tailed P value <0.05 was considered statistically significant. Student's t-test was used to assess the significance of differences between the case and control subjects’ Chi-square (χ2) analysis was used to assess deviation from Hardy–Weinberg's equilibrium and to compare the genotype/allele/haplotype frequency between patients and controls. Odds ratios (ORs) were obtained by unconditional logistic regression analysis and adjusted for age and smoking as a continuous variable. All the statistical analyses were conducted using the SPSS software, version 11.5 (SPSS, Chicago, IL, USA). All the statistical tests were two-sided. Values of P < 0.05 were considered statistically significant.
Diplotype analysis
Diplotypes were constructed and their frequencies assessed using the maximum-likelihood method, using an expectation–maximization algorithm by performing 100,000 permutations through software Arlequin (Version 2.0). OR was calculated using unconditional logistic regression for risk haplotypes taking the wild-type haplotype as reference.
Results
Demographical and clinical details of PCa and BC and controls
There was no statistical difference between ages of the PCa and BC patients and healthy controls. The average age in the control group was 64.7 ± 5.71 years as compared to 66.0 ± 5.46 years for PCa patients. In case of BC the average age in the control group was 58.8 ±10.8 years as compared BC patients 59.6 ± 12.4 years. The demographical characteristics of study subjects have been summarized in Tables 1 and 2.
Table 1.
Demographical characteristics of controls and PCa study subjects
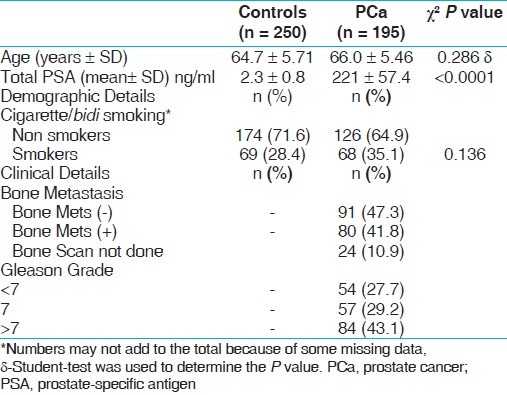
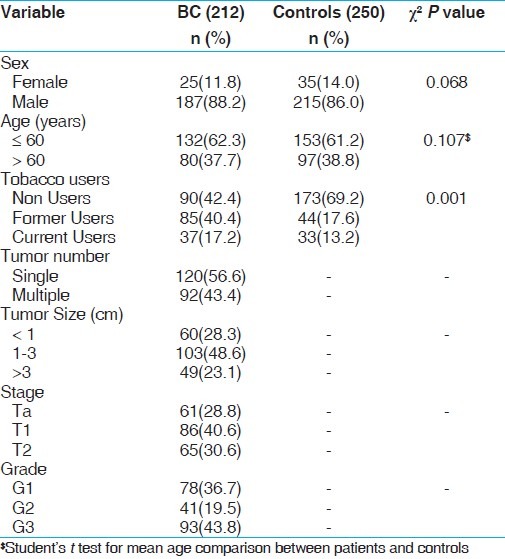
Table 2.
Demographical characteristics of controls and BC study subjects
Association of XPD genotype variants with PCa and BC risk
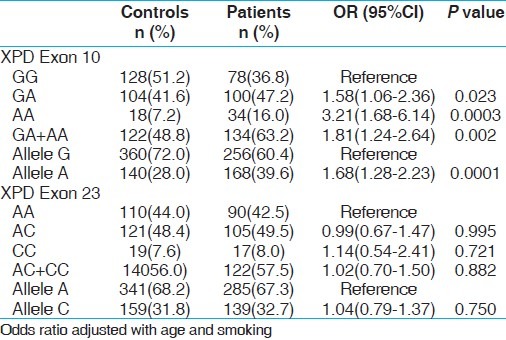
The genotypic distributions of XPD gene polymorphisms in the controls were in Hardy–Weinberg equilibrium (HWE). The genotypic frequency distribution between cases and controls is given in Tables 3 and 4, respectively. The variant allele frequency (A) of XPD exon 10 was significantly higher in both cases as compared to controls and exhibited 1.54 folds risk (P, 0.013) with PCa and 1.68 folds risk (P, 0.0003) for BC. Subsequent variant genotype (AA) demonstrated significant risk associated with PCa and BC (P, 0.013 and P, 0.0003, respectively). Variant allele carrier (GA+AA) also showed increased risk of PCa and BC with significant P value (P, 0.012). In contrast, XPD exon 23 was not differently distributed in the PCa and BC and controls.
Table 3.
Genotype frequency distribution of XPD gene polymorphisms and association in controls and PCa cases
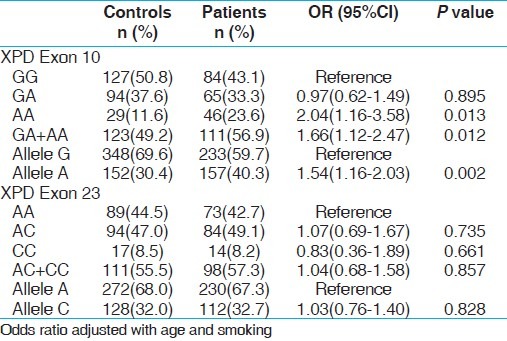
Table 4.
Genotype frequency distribution of XPD gene polymorphisms and association in controls and BC cases
Association of XPC genotype variants with PCa and BC risk
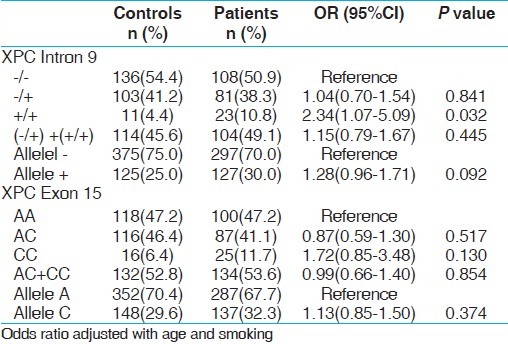
XPC intron 9 homozygous variant (+/+) demonstrated 2.57 folds statistically significant risk with PCa (P, 0.012) and 2.34 folds increased risk with BC (P, 0.032). Interestingly variant genotype CC of XPC exon 15 revealed 1.99 fold increased risk for PCa only (P, 0.047) not with BC [Tables 5 and 6].
Table 5.
Genotype frequency distribution of XPC gene polymorphisms and association in controls and PCa cases
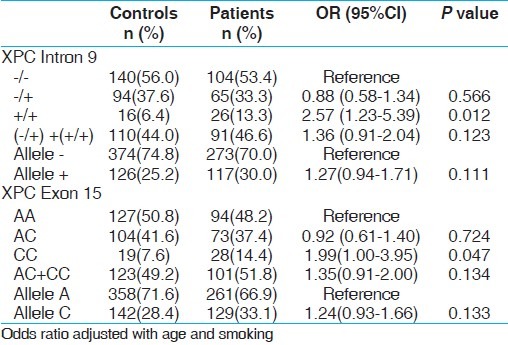
Table 6.
Genotype frequency distribution of XPC gene polymorphisms and association in controls and BC cases
Association of XPD and XPC diplotypes with PCa and BC risk
To elucidate the combined influence of these polymorphisms, we constructed diplotypes of XPD and XPC [Figures 1–4]. Diplotype analysis of these two polymorphisms revealed significant P values. The diplotype containing wild type were considered as reference. The two diplotype GC and AA (XPC exon 10, 23) showed 3.44 and 4.96 folds increase risk for PCa (Bonferroni corrected P value (Pc) - 0.004). Whereas in BC, only one diplotype AC demonstrated 2.06 folds significant risk (Pc- 0.004). Regarding XPC diplotypes, A+ (exon 15 and Intron 9) diplotype shown association with PCa (Pc-0.044). In BC diplotype, C+ revealed 1.8 fold increased risk (Pc-0.008).
Figure 1.
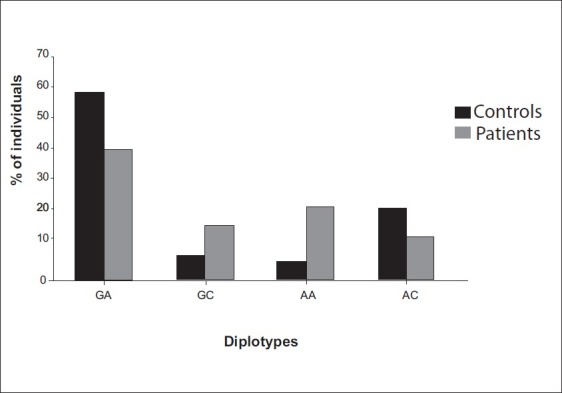
Association of XPD diplotypes with PCa susceptibility GC-pc,0.004; OR, 3.44(2.15-5.51), AA-pc,0.004; OR, 4.96(3.08-7.98)
Figure 4.
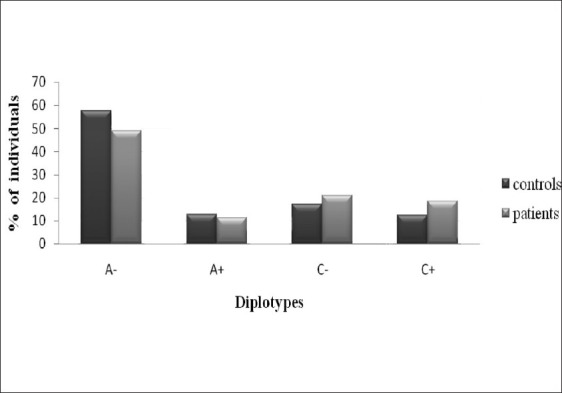
Diplotypes analysis of XPC (Exon 15 and Intron 9) diplotypes with BC susceptibility C+, pc, 0.008; OR, 1.79(1.23-2.62)
Figure 2.
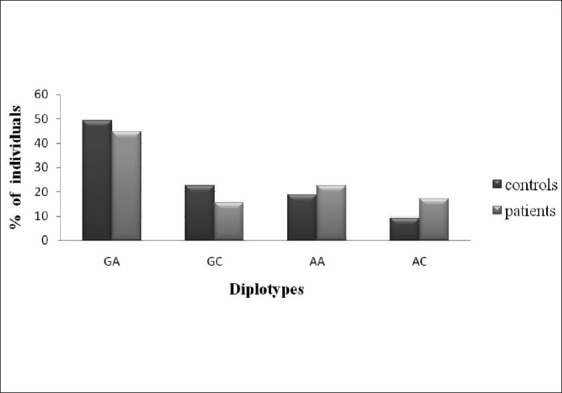
Association of XPD diplotypes with BC susceptibility AC-pc, 0.004; OR, 2.06 (1.36-3.12)
Figure 3.
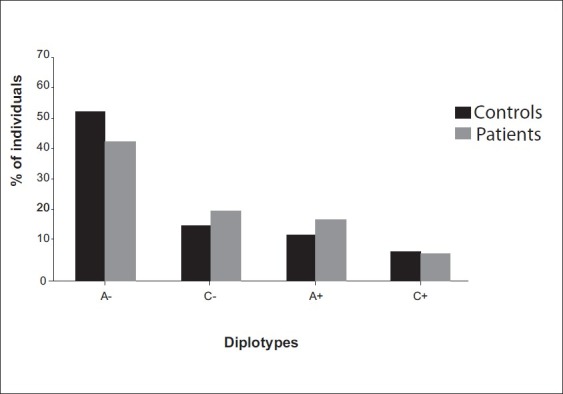
Association of XPC (Exon 15 and Intron 9) diplotypes with PCa susceptibility A+, pc, 0.044; OR, 1.60(1.11-2.30)
Analysis of XPD and XPC genes polymorphisms with risk for tumor grades in PCa and BC
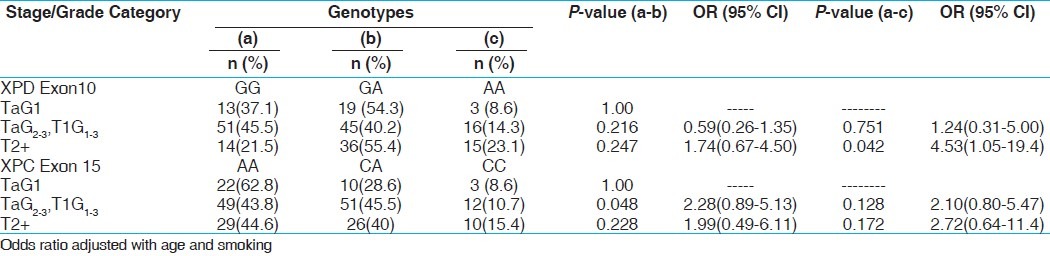
For genotypic comparison, we have divided the PCa patients with different Gleason grades into three groups (low grade <7, intermediate grade 7, and high grade >7) based on the degree of differentiation between the cells. Similarly in BC, we stratified the patients into three categories according to stage/grade (TaG1 (low risk NMIBT), TaG2,3 + T1G1–3 (high risk NMIBT), and T2+ (muscle invasive). The OGG1 GG genotype showed an increased risk in the TaG2–3 + T1G1–3 tumor stage (P, 0.027). Heterozygous (-/+) and variant (+/+) genotype of XPC intron 9 shows association with high grade of tumor in PCa (P 0.042, 0.004). Variant allele containing genotype (-/+/+/+) also showed increase risk with high gleason grade of PCa (P, 0.003). Similarly variant homozygous genotype (TT) of XPC exon 15 demonstrated 5.29 folds significant risk with high gleason grade of PCa (P, 0.012). Among BC, XPD exon 10 variant genotype AA showed 4.53 folds significant increased risk in the muscle invasive stage (P, 0.042). XPC exon 15 heterozygous AC genotype was observed to be associated significantly with elevated risk with TaG2,3+T1G1-3 stage of BC (P, 0.048). Overall statistically no significant association was observed in case of XPD exon 23 genotype with any of the grade of PCa and BC [Tables 7 and 8].
Table 7.
Influence of XPC gene polymorphisms with tumour grade of PCa patients
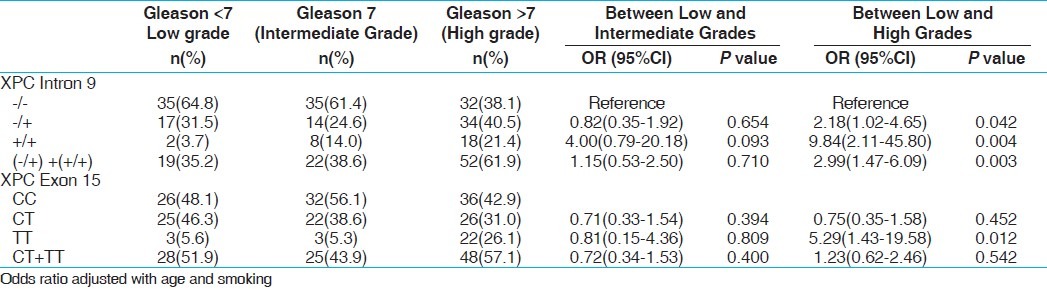
Table 8.
Influence of XPD and XPC gene polymorphisms with tumor stage/grade categories of BC patients
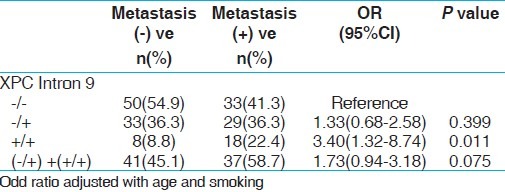
Analysis of XPD and XPC genes polymorphism with risk for bone metastasis with PCa
We also studied XPD and XPC gene variants and their risk associated with bone metastasis. The PCa patients were divided into two groups; one with bone metastasis and the other with non-metastasis. When these two groups were analyzed for risk of susceptibility for bone metastasis only variant genotype (+/+) of XPC intron 9 demonstrated increased risk with PCa bone metastasis (P, 0.011) [Table 9]. We did not find any significant risk with other SNPs of these two genes.
Table 9.
Frequency distribution of XPC gene polymorphisms with risk of bone metastasis in PCa patients
Interaction of XPD and XPC genes polymorphisms with smoking habit in PCa and BC
We evaluated the gene-smoking interaction to study the modulation of PCa risk with respect to these genes polymorphisms. We divided the PCa patients into two groups; one non smoker (never smoked) and the other as smokers (smoking more than 5 years). On analyzing these polymorphisms, we did not observe any association with PCa risk (data not shown). Among BC, we stratified the patients into non-users of tobacco and users of tobacco. Tobacco use of an individual was measured as former (smokers and smokeless) tobacco users and current (smokers and smokeless) tobacco users. The non-users were considered as reference and risk of BC was calculated for tobacco users. Since the numbers in the heterozygous and homozygous genotype group were low, we merged the two groups as the variant allele carrier. We observed that XPC intron 9 was associated with increased risk in former tobacco users (P, 0.05) [Figure 5].
Figure 5.
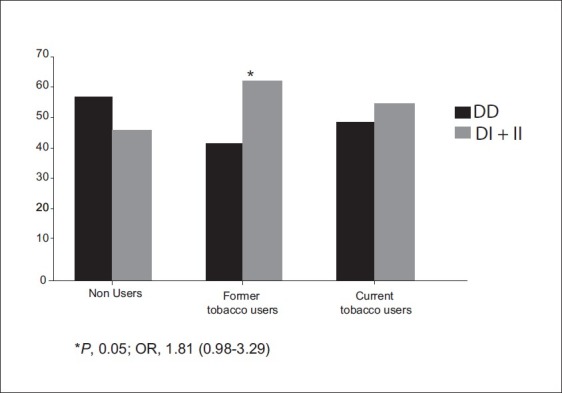
Influence of XPC Intron 9 gene polymorphisms with BC susceptibility in tobacco users
Discussion
The NER pathway has particular importance, because this system removes complex bulky adducts. Several polymorphisms in NER genes were found to alter DNA repair, including variants in XPD and XPC. We therefore assessed whether polymorphisms in XPD exon 10, 23 and XPC Intron 9 and exon 15 had a clinically relevant impact on PCa and BC susceptibility in North Indian population. Our results showed XPD exon 10 G>A to be associated with increased risk of PCa and BC. Same result obtained from Rybicki et al., 2004 and Bau et al., 2007 in PCa.[16,19] Wu et al., 2006 also reported that only carriers of the variant A allele of XPD, Asp312Asn showed evidence of association with BC.[20] Concerning the XPD exon 10 (G>A) Asp312Asn polymorphism, most of the reported data indicate a higher level of DNA adducts in Asn individuals than in Asp individuals, which is interpreted as lower repair efficiency for the XPD Asn allele.[21] Further, the other polymorphic site of XPD exon 23 did not show any association with PCa and BC. The XPC gene product contributes to the global genome repair (GGR) pathway, is a member of the NER pathway, and is tightly associated with one of the two human homologues of Saccharomyces cerevisiae RAD23 protein (HR23B). Interestingly, we found significant association with variant genotype of XPC intron 9 of PCa and BC. This polymorphism has been shown to be associated with increased risks of skin,[22] and breast cancer.[23] Qiao et al., 2002 reported that, individuals with the PAT Ins/Ins polymorphism had a virtual reduction in NER capacity of 23.4% compared with the PAT -/- individuals, and has been recently proposed as a useful biomarker to identify individuals at increased risk for developing cancer.[11]
Regarding association with XPC Exon 15 A>C SNP with PCa. Our observation is compatible with the findings from Gangwar et al., 2010 in cervical cancer, Blankenburg et al., 2005 in melanoma.[22,24] The lack of associations was found in XPC (A>C) CC gene polymorphism with BC in our study; this finding was consistent with the results of a previous study in BC.[25]
Moreover, we examined the diplotype of XPD exon 10, 23 and XPC Intron 9 and 15 in PCa and BC. Interestingly the frequency of G-C and A-A (XPD exon 10 and 23) was significantly increased in PCa and one diplotype AC showed risk with BC. Diplotype (A+) and (C+) of XPC demonstrated statistically increased risk with PCa and BC. The finding demonstrated that the combined genotype was associated with PCa and BC. When we analyzed these genes polymorphisms with clinical grades of the both the disease, we found that variant allele of XPC intron 9 and exon 15 were significantly higher in high Gleason grade of PCa. Similarly XPD exon 10 variant allele is associated with muscle invasive stage and XPC exon 15 heterozygous AC genotype was observed to be increase risk with TaG2,3+T1G1-3 stage of BC. XPC Intron 9 variants genotype revealed increased risk with bone metastasis with PCa. This clearly suggested that these gene variants may be associated with initiation of as well as progression of PCa and BC.
We also analyzed the interaction of XPD and XPC genotypes with tobacco exposure to investigate the modulation of risk in a case-only analysis. Case-only approaches are believed to be better than case-control studies; Tobacco carcinogen exposure has a strong association with BC. In the present study, only XPC intron 9 elevated risks was observed in former tobacco users of BC patients. None of the others SNPs shows significant association with PCa and BC.
In summary, we found increased risk of PCa and BC in individuals with two copies of the XPD exon 10A allele and XPC intron 9 + allele. We also observed that XPC exon 15 A>C genotype was associated with PCa risk. The combined genotype of XPD exons 10 and 23 and XPC intron 9 and exon 15 was found to be associated with PCa and BC risk. Though our study achieved sufficient (>80%) power due to the low incidence rate of PCa and BC in India nevertheless a higher number of sample size can warrant more modest results. These results suggest that genetic variants in NER repair pathways may be involved in PCa and BC etiology.
Acknowledgement
The study was funded by a grant from UP Council of Science Technology, Lucknow (Uttar Pradesh) and Department of Science and Technology, New Delhi Govt. of India. R. K. M is thankful to the Council of Scientific and Industrial Research, New Delhi, for Senior Research Fellowship respectively. We are also thankful to the urologists in the department for providing the details of the clinical samples.
Footnotes
Source of Support: Grant from UP Council of Science Technology, Lucknow (Uttar Pradesh) and Department of Science and Technology, New Delhi Govt. of India
Conflict of Interest: None declared.
References
- 1.Parkin DM. The global burden of urinary bladder cancer. Scand J Urol Nephrol Suppl. 2008;218:12–20. doi: 10.1080/03008880802285032. [DOI] [PubMed] [Google Scholar]
- 2.Sinha R, Anderson DE, McDonald SS, Greenwald P. Cancer risk and diet in India. J Postgrad Med. 2003;49:222–8. [PubMed] [Google Scholar]
- 3.Malins DC, Johnson PM, Wheeler TM, Barker EA, Polissar NL, Vinson MA. Age-related radical-induced DNA damage is linked to prostate cancer. Cancer Res. 2001;61:6025–8. [PubMed] [Google Scholar]
- 4.Wood RD, Mitchell M, Sgouros J, Lindahl T. Human DNA repair genes. Science. 2001;291:1284–9. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- 5.Pastorelli R, Cerri A, Mezzetti M, Consonni E, Airoldi L. Effect of DNA repair gene polymorphisms on BPDE-DNA adducts in human lymphocytes. Int J Cancer. 2002;100:9–13. doi: 10.1002/ijc.10463. [DOI] [PubMed] [Google Scholar]
- 6.Hou SM, Falt S, Angelini S, Yang K, Nyberg F, Lambert B, et al. The XPD variant alleles are associated with increased aromatic DNA adduct level and lung cancer risk. Carcinogenesis. 2002;23:599–603. doi: 10.1093/carcin/23.4.599. [DOI] [PubMed] [Google Scholar]
- 7.Tirode F, Busso D, Coin F, Egly JM. Reconstitution of the transcription factor TFIIH: Assignment of functions for the three enzymatic subunits, XPB, XPD, and cdk7. Mol Cell. 1999;3:87–95. doi: 10.1016/s1097-2765(00)80177-x. [DOI] [PubMed] [Google Scholar]
- 8.Evans E, Moggs J, Hwang G, JR, Egly JM, Wood RD. Mechanism of open complex and dual incision formation by human nucleotide excision repair factors. EMBO J. 1997;16:6559–73. doi: 10.1093/emboj/16.21.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Gu D, Zhang Z, Zhou J, Zhang Z. XPD polymorphisms, cigarette smoking, and bladder cancer risk: A meta-analysis. J Toxicol Environ Health. 2009;72:698–705. doi: 10.1080/15287390902841029. [DOI] [PubMed] [Google Scholar]
- 10.Lunn RM, Helzlsouer KJ, Parshad R, Umbach DM, Harris EL, Sanford KK, et al. XPD polymorphisms: Effects on DNA repair proficiency. Carcinogenesis. 2000;21:551–5. doi: 10.1093/carcin/21.4.551. [DOI] [PubMed] [Google Scholar]
- 11.Qiao Y, Spitz MR, Guo Z, Mohammad H, Lawrence G, Kenneth KH, et al. Rapid assessment of repair of ultraviolet DNA damage with a modified host-cell reactivation assay using a luciferase reporter gene and correlation with polymorphisms of DNA repair genes in normal human lymphocytes. Mutat Res. 2002;509:165–74. doi: 10.1016/s0027-5107(02)00219-1. [DOI] [PubMed] [Google Scholar]
- 12.Khan SG, Muniz-Medina V, Shahlavi T, Baker CC, Inui H, Ueda T, et al. The human XPC DNA repair gene: arrangement, splice site information content and influence of a single nucleotide polymorphism in a splice acceptor site on alternative splicing and function. Nucleic Acids Res. 2002;30:3624–31. doi: 10.1093/nar/gkf469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 14.Colombel M, Soloway M, Akaza H. Epidemiology, staging, grading and risk stratification of bladder cancer. Eur Urol Suppl. 2008;7:618–26. [Google Scholar]
- 15.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rybicki BA, Conti DV, Moreira A, Cicek M, Casev G, Witte JS. DNA repair gene XRCC1 and XPD polymorphisms and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2004;1:23–9. doi: 10.1158/1055-9965.epi-03-0053. [DOI] [PubMed] [Google Scholar]
- 17.López-Cima MF, González-Arriaga P, García-Castro L, Pascual T, Marrón MG, Puente XS. Polymorphisms in XPC, XPD, XRCC1, and XRCC3 DNA repair genes and lung cancer risk in a population of northern Spain. BMC Cancer. 2007;7:162. doi: 10.1186/1471-2407-7-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanyal S, Festa F, Sakano S, Zhang Z, Steineck G, Norming U, et al. Polymorphisms in DNA repair and metabolic genes in bladder cancer. Carcinogenesis. 2004;25:729–34. doi: 10.1093/carcin/bgh058. [DOI] [PubMed] [Google Scholar]
- 19.Bau DT, Wu HC, Chiu CF, Lin CC, Hsu CM, Wang CL, et al. Association of XPD polymorphisms with prostate cancer in Taiwanese patients. Anticancer Res. 2007;27:2893–6. [PubMed] [Google Scholar]
- 20.Wu X, Gu J, Grossman HB, Amos CI, Etzel C, Huang M, et al. Bladder cancer predisposition: A multigenic approach to DNA repair and cell cycle-control genes. Am J Hum Genet. 2006;78:464–79. doi: 10.1086/500848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benhamou S, Sarasin A. Variability in nucleotide excision repair and cancer risk: A Review. Mutat Res. 2000;462:149–58. doi: 10.1016/s1383-5742(00)00032-6. [DOI] [PubMed] [Google Scholar]
- 22.Blankenburg S, Konig IR, Moessner R, Laspe P, Thoms KM, Krueger U, et al. Assessment of 3 xeroderma pigmentosum group C gene polymorphisms and risk of cutaneous melanoma: A case-control study. Carcinogenesis. 2005;26:1085–90. doi: 10.1093/carcin/bgi055. [DOI] [PubMed] [Google Scholar]
- 23.Shore RE, Zeleniuch-Jacquotte A, Currie D, Mohrenweiser H, Afanasyeva Y, Koenig KL, et al. Polymorphisms in XPC and ERCC2 genes, smoking and breast cancer risk. Int J Cancer. 2008;122:2101–5. doi: 10.1002/ijc.23361. [DOI] [PubMed] [Google Scholar]
- 24.Gangwar R, Mittal B, Srivastava S, Singh H, Mittal RD. Genetic variants of DNA repair gene XPC modulating susceptibility to cervical cancer in North India. Oncol Res. 2010;18:329–35. doi: 10.3727/096504010x12626118080028. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Lai M, Yang H, Lin J, Huang M, Grossman HB, et al. Genotypes, haplotypes and diplotypes of XPC and risk of bladder cancer. Carcinogenesis. 2007;28:698–703. doi: 10.1093/carcin/bgl201. [DOI] [PubMed] [Google Scholar]



