Abstract
Cross-species transplantation (xenotransplantation) offers the prospect of an unlimited supply of organs and cells for clinical transplantation, thus resolving the critical shortage of human tissues that currently prohibits a majority of patients on the waiting list from receiving transplants. Between the 17th and 20th centuries, blood was transfused from various animal species into patients with a variety of pathological conditions. Skin grafts were carried out in the 19th century from a variety of animals, with frogs being the most popular. In the 1920s, Voronoff advocated the transplantation of slices of chimpanzee testis into aged men whose “zest for life” was deteriorating, believing that the hormones produced by the testis would rejuvenate his patients. Following the pioneering surgical work of Carrel, who developed the technique of blood vessel anastomosis, numerous attempts at nonhuman primate organ transplantation in patients were carried out in the 20th century. In 1963–1964, when human organs were not available and chronic dialysis was not yet in use, Reemtsma transplanted chimpanzee kidneys into 13 patients, one of whom returned to work for almost 9 months before suddenly dying from what was believed to be an electrolyte disturbance. The first heart transplant in a human ever performed was by Hardy in 1964, using a chimpanzee heart, but the patient died within 2 hours. Starzl carried out the first chimpanzee-to-human liver transplantation in 1966; in 1992, he obtained patient survival for 70 days following a baboon liver transplant. With the advent of genetic engineering and cloning technologies, pigs are currently available with a number of different manipulations that protect their tissues from the human immune response, resulting in increasing pig graft survival in nonhuman primate models. Genetically modified pigs offer hope of a limitless supply of organs and cells for those in need of a transplant.
The increasing demand for organs, tissues, and cells for purposes of clinical transplantation, and the relative lack of improvement in the number of deceased human organs that become available each year, have increased interest in the possibility of using organs and cells from an animal species (1, 2). The concept of cross-species transplantation (or xenotransplantation) is not new, and there has been a surprisingly large number of clinical attempts during the past 300 years or more (1, 3, 4). The barriers to xenotransplantation are considerable but are steadily being overcome, largely by our ability to genetically engineer pigs to make their tissues more resistant to the human immune response.
XENOTRANSPLANTATION IN MYTHOLOGY
A review of Greek mythology and of religious tracts—particularly, for example, from the Hindu religion—draws attention to the fact that humans have been interested in the possibility of merging physical features from various animal species for hundreds of years. For example, the chimera has been used to represent the allotransplantation of organs and cells (transplantation between members of the same species), and the lamassu (Figure 1) has been selected as the mythological figure to represent the International Xenotransplantation Association and its official scientific journal, Xenotransplantation.
Figure 1.
The lamassu. This mythological beast has been adopted for the logo of the International Xenotransplantation Association and its official journal, Xenotransplantation. Image courtesy of IXA.
The late Keith Reemtsma pointed out that possibly one of the earliest examples of xenotransplantation was the attempt by Daedalus and his son, Icarus, to fly across the sea from Crete to mainland Greece with the help of bird wings attached to their arms (5). Icarus failed in the attempt, and Reemtsma put this forward as a possible case of hyperacute rejection (very rapid rejection of the graft), though he thought it was more likely to be related to failure of a thermolabile adhesive. However, Daedalus successfully made the journey, providing this pair with an enviable 50% success rate.
BLOOD XENOTRANSFUSION
If we look beyond the realm of mythology and legend, we come to the 17th century, when Jean Baptiste Denis (Figure 2) began the clinical practice of blood transfusion from animals to humans (6). Perhaps not surprisingly, the results were mixed. As a result, xenotransfusion was banned in France for a number of years. Today, with the increasing risk of transfer of infectious agents with human blood transfusions, a strong case could be made for using an animal such as a pig (housed under ideal “clean” conditions and monitored at intervals to ensure that no infectious agent would be transferred) as the source of blood cells and blood products in the future (6). In fact, this approach has recently been explored again by several groups (7).
Figure 2.

Jean-Baptiste Denis (c. 1635–1704). Image courtesy of Musée d'Histoire de la Médecine, Faculté de Médecine de Paris.
SKIN XENOTRANSPLANTATION
In the 19th century, skin grafts became relatively popular between various animal species and humans (8, 9). The grafts were either free skin grafts or pedicle skin grafts. Pedicle grafts were complicated because they required the donor, for example, a sheep, to be strapped immobile to the patient for several days, during which time the graft would reputedly be vascularized by the recipient. If this occurred, the graft could be disconnected from the donor. It is almost certain that none of these grafts was in any way successful, although some “successes” were reported.
The fact that many of the species used as donors—sheep, rabbits, dogs, cats, rats, chickens, and pigeons—had hair, feathers, or fur growing from the skin did not appear to disconcert the surgeons involved, but the trend was to use animal species in which these accoutrements were not present. The ideal graft would appear to have been from frogs, which were sometimes “skinned alive.” It is possible that some of these grafts were “successful” in that, when used to cover a skin ulcer, they provided protection, at least for a number of days, while the ulcer healed beneath the graft. However, probably none of the grafts actually became permanent.
CORNEAL XENOTRANSPLANTATION
Remarkably, in 1838 the first corneal xenotransplantation (from a pig) was performed in a patient, whereas the first corneal allograft (human-to-human) was not carried out until more than 65 years later, in 1905. The field of corneal xenotransplantation has recently been reviewed by Hara and Cooper (10, 11).
ALEXIS CARREL AND BLOOD VESSEL ANASTOMOSIS
More scientific efforts had to wait until the 20th century, when the French experimental surgeon, Alexis Carrel (Figure 3), working first in France and subsequently in North America, developed surgical techniques for anastomosing blood vessels, which enabled organ transplantation to be carried out successfully for the first time. For this work he was awarded the Nobel Prize in 1912. He developed an interest in cross-species transplantation, at least from an experimental perspective, and in 1907 wrote these prophetic words:
The ideal method would be to transplant in man organs of animals easy to secure and operate on, such as hogs, for instance. But it would in all probability be necessary to immunize organs of the hog against the human serum. The future of transplantation of organs for therapeutic purposes depends on the feasibility of hetero [xeno] transplantation.
Figure 3.

Alexis Carrel (1873–1944), photographed by Huggins. From Images from the History of Medicine (record UI 101411640).
It is remarkable that, more than 100 years ago, Carrel indicated what we are now trying to do, which is to genetically modify pigs to make their tissues resistant to the human immune response. Carrel was clearly a man of vision.
SERGE VORONOFF AND “REJUVENATION” BY CELL XENOTRANSPLANTATION
A few years later, Serge Voronoff (Figure 4), a Russian émigré working in Paris, developed the concept of transplanting cells that produced a hormone in which the recipient was deficient. This is another example of a visionary scientist who was ahead of his time. Today we are doing exactly what he envisaged, namely transplanting human pancreatic islets that produce insulin in patients with severe type 1 diabetes. In view of the limited number of human pancreases that become available each year, there is a growing interest in using pig islets for this purpose (see below).
Figure 4.

Serge Voronoff (1866–1951). Image from the U.S. Library of Congress (record LC-B2- 6197-1).
Voronoff's main interest, however, was in reversing the effects of aging in elderly men who had lost their “zest for life.” He carried out a significant number of chimpanzee or baboon testicular transplants in male human recipients (12, 13). His technique was to slice up the animal testicle and insert the slices into the recipient's testicle. (It can be looked upon as the Viagra of the 1920s.) The procedure became popular on both sides of the Atlantic, and several hundred of these operations were performed. It is inconceivable that any of them had any beneficial effect whatsoever except psychological, but there were reports of remarkable “rejuvenation” of men who reported much increased energy after the operation. The complications of the operations must have been significant because presumably on occasions the slices of donor testicle would have necrosed and set up inflammatory or infectious complications. Surprisingly, reports of such complications appear to have been uncommon.
Voronoff was certainly a man ahead of his time because he also applied to the authorities in Paris to carry out what would have been the first kidney allotransplant, using the kidneys from a criminal who was to be guillotined. His request was refused, and this allowed his Russian compatriot, Yurii Voronoy, to become the first surgeon to perform kidney allotransplantation in 1933 (14).
The concept of transplanting glandular tissue to produce hormones that would benefit the recipient was continued in the United States by a much less scientific doctor, John Brinkley, whose work was carried out largely in Kansas and Texas (15). His chosen donor was the goat, as he had been convinced by a local farmer of its sexual potency. It would appear that Brinkley was a charlatan rather than a serious transplant surgeon, and, although it made him a fortune, his work fell into serious disrepute, and he was eventually driven out of business by the American Medical Association.
Nevertheless, this concept of cell xenotransplantation has been sustained until the present time, with the establishment of several clinics, particularly in Europe, in which animal tissue or serum is injected into patients for a variety of conditions. The results have been controversial (16).
KEITH REEMTSMA AND CHIMPANZEE KIDNEY XENOTRANSPLANTATION
By the 1960s, Keith Reemtsma (Figure 5)—at that time at Tulane University in Louisiana—hypothesized that nonhuman primate kidneys might function in human recipients and thus be a successful treatment for renal failure. At that time, the concept of kidney transplantation had been established largely by French and American surgeons, but the availability of kidneys from deceased humans was extremely limited and chronic dialysis was not yet being undertaken. In Reemtsma's opinion, therefore, there was little alternative to death for the patient unless organs could be made available from nonhuman species. He selected the chimpanzee as the source of organs because of its close evolutionary relationship to humans. He carried out 13 of these transplants, on each occasion transplanting both kidneys from the chimpanzee (which generally weighs significantly less than an adult human) into the recipient (17).
Figure 5.

Keith Reemtsma (1925–2000). Image courtesy the late Keith Reemtsma.
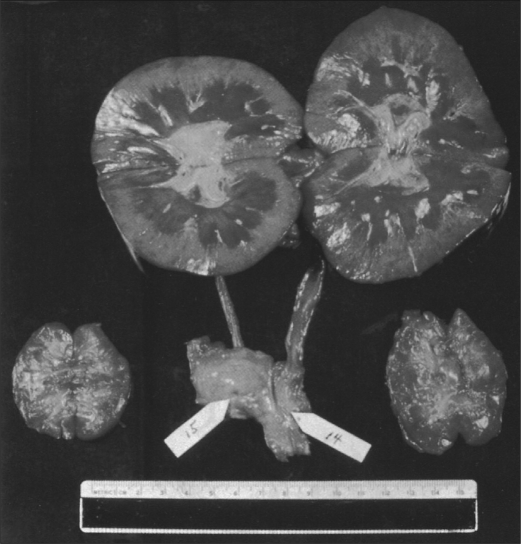
Most of the transplants failed within 4 to 8 weeks, either from rejection (because of the limited immunosuppressive agents available at the time) or from an infectious complication (because of the overadministration of these agents). Nevertheless, one of Reemtsma's patients lived for 9 months, returning to work as a schoolteacher and evidently remaining in good health until she suddenly collapsed and died. At autopsy, the chimpanzee kidneys appeared normal and showed no signs of acute or chronic rejection (Figure 6). It was suggested that she had died from an acute electrolyte disturbance. This is possible since the transplantation of nonhuman primate kidneys into patients was frequently associated with an immense diuresis in the early posttransplantation period, often exceeding 20 liters in 24 hours, and there could have been a late electrolyte imbalance.
Figure 6.
Normal macroscopic appearance at autopsy of chimpanzee kidneys (top) that had functioned well for a period of almost 9 months in a 23-year-old woman who had undergone renal xenotransplantation in 1963. This operation was one of a small series of kidney xenotransplants performed by Keith Reemtsma and his colleagues at Tulane University in New Orleans. Image courtesy of the late Keith Reemtsma.
The concept of using nonhuman primates as kidney donors was expanded by several surgeons, particularly by Tom Starzl (Figure 7), who used baboons as donors in Colorado (18); his results were similar to those of Reemtsma, except that he did not achieve any relatively long-term survivors. Others in the US and in France also had small experiences (3).
Figure 7.
Tom Starzl (born 1926). Image courtesy of Thomas E. Starzl.
JAMES HARDY AND THE FIRST HEART XENOTRANSPLANT
James Hardy (Figure 8), who had carried out the first human lung allotransplant in 1963, visited Reemtsma and was impressed by the health of some of the patients with chimpanzee kidney transplants. In 1964, Hardy was determined to carry out the first clinical heart transplantation and decided to acquire some chimpanzees as potential “donors” in case he could not identify a deceased human donor. He had a less-than-ideal patient who would not be accepted for heart transplantation today, as he had widespread atheromatous vascular disease throughout his body—for which he had undergone amputations of both legs—and was in a semicomatose state at the time the transplant was undertaken. However, as the patient was rapidly dying, Hardy was stimulated to transplant a chimpanzee heart (19). The chimpanzee heart was not large enough to support the circulation and failed within a couple of hours.
Figure 8.

James Hardy (1918–2003). Image courtesy of the late James Hardy.
In contrast to the response to the attempted lung allotransplantation, the public and medical professional response to the heart xenotransplantation was adverse and dissuaded Hardy and his colleagues from carrying out any further attempts. The procedure of cardiac allotransplantation was later established by Barnard and his colleagues in 1967 (20), who later also carried out two cardiac xenotransplants (21).
It is of interest to note that the consent form for Hardy's operation—which, in view of the patient's semicomatose condition, was signed by a close relative—stipulated that no heart transplant had ever been performed, but made no mention of the fact that an animal heart might be used for the procedure. Such was the medicolegal situation at that time that this “informed” consent was not considered in any way inadequate.
LEONARD BAILEY AND “BABY FAE”
Perhaps the best known clinical cardiac xenotransplantation since Hardy's attempt was that by Leonard Bailey (Figure 9), who transplanted a baboon heart into an infant girl, known as Baby Fae, in 1983. At that time, it was almost impossible to obtain human organs from infants, particularly those with anencephaly, for transplantation into infants with life-threatening congenital heart disease. The surgical procedure in Baby Fae was technically successful, but the graft underwent acute rejection and the patient died 20 days later (22). As the graft was necessarily taken from a baboon that was ABO-incompatible with the recipient—as the O blood type is essentially not found in baboons—this might have added to the severity of rejection. Even though cyclosporine had become available by this time, the immunosuppressive therapy was not sufficient to prevent xenograft rejection.
Figure 9.

Leonard Bailey (born 1942). Image courtesy of Leonard Bailey.
This procedure did little to advance progress in xenotransplantation, but it did draw public and medical attention to the dearth of deceased human organs available for infants in need of a transplant. Following the procedure, particularly with the immense publicity associated with it, the situation with regard to donation of organs from infants became very much improved, and Bailey went on to develop an extremely successful cardiac allotransplantation program in infants and children at Loma Linda University.
THOMAS STARZL AND LIVER XENOTRANSPLANTATION
Tom Starzl (Figure 7), who is one of the greatest pioneers in the field of kidney and liver allotransplantation, performed a handful of liver transplants between nonhuman primates and young patients in Colorado in the 1960s without lasting success (23–26). When the addition of tacrolimus had improved the immunosuppressive armamentarium, he and his team in Pittsburgh performed two liver transplants from baboons in adult patients in the 1990s, with one patient surviving for 70 days (27). The results, however, were not successful enough to warrant continuing this experimental clinical trial.
Most of the early attempts at clinical organ xenotransplantation used nonhuman primate species as sources of the organ, although there have been a few attempts using the pig (28) and other nonprimate mammals, but without significant success (3).
CARL-GUSTAV GROTH AND THE FIRST ISLET XENOTRANSPLANTATION
There are an estimated 2 to 3 million patients with type 1 diabetes in the USA alone. As pig insulin differs from human insulin by only one amino acid, and pig insulin was administered successfully for the treatment of diabetic patients for decades until recombinant human insulin became available, it is reasonable to anticipate that successful pig islet transplantation will result in normoglycemia. The Swedish group headed by Carl Groth (Figure 10) was the first to attempt pig islet transplantation in patients with diabetes in 1993 (29). Although porcine C-peptide was documented in the blood of some of the patients, indicating that some islets survived, no clinical benefit was obtained.
Figure 10.

Carl-Gustav Groth (born 1933). Image courtesy of Carl-Gustav Groth.
In recent years, there have been encouraging results from islet allotransplantation in patients with type 1 diabetes, but with such large numbers of patients suffering from the disease, the number of human pancreata that become available will never be sufficient to treat all of the potential patients, particularly as often two (or even three) human pancreata are required to provide enough islets to render a single recipient normoglycemic.
XENOTRANSPLANTATION USING PIGS AS SOURCES OF ORGANS AND CELLS
The advantages of xenotransplantation, particularly if we could use a readily available animal source, such as the pig, would be numerous (Table 1) (30). First, there would be an unlimited supply of “donor” organs, which would resolve the current increasing and severe shortage of human organs.
Table 1.
The advantages and disadvantages of the pig vs baboon as a potential source of organs and cells for humans
| Pig | Baboon | |
| Availability | Unlimited | Limited |
| Breeding potential | Good | Poor |
| Period to reproductive maturity | 4–8 months | 3–5 years |
| Length of pregnancy | 114 ± 2 days | 173–193 days |
| Number of offspring | 5–12 | 1–2 |
| Growth | Rapid (adult human size within 6 months)∗ | Slow (9 years to reach maximum size) |
| Size of adult organs | Adequate | Inadequate∗ |
| Cost of maintenance | Significantly lower | High |
| Anatomical similarity to humans | Moderately close | Close |
| Physiological similarity to humans | Moderately close | Close |
| Relationship of immune system to humans | Distant | Close |
| Knowledge of tissue typing | Considerable (in selected herds) | Limited |
| Necessity for blood type compatibility with humans | Probably unimportant | Important |
| Experience with genetic engineering | Considerable | None |
| Risk of transfer of infection (xenozoonosis) | Low | High |
| Availability of specific pathogen-free animals | Yes | Yes |
| Public opinion | More in favor | Mixed |
∗Breeds of miniature swine are approximately 50% of the weight of domestic pigs at birth and sexual maturity and reach a maximum weight of approximately 30% of standard breeds. At full size, miniature swine are easier to house and to handle. Furthermore, inbred herds are available, though cloning of any pig can result in inbred herds, if needed. Although MHC-identical miniature swine may have some specific immunologic advantage, the disadvantage is that they cannot be cross-bred with other pig strains in which a genetic modification has been introduced; if cross-breeding is carried out, clearly MHC identity is lost.
Second, these organs would be available electively whenever required, which is an equally important point. Currently, a patient may wait several months in an intensive care unit or with a left ventricular assist device while awaiting a heart transplant, or even several years on chronic dialysis awaiting a kidney transplant. If transplantation could be carried out as soon as the patient experiences irreversible organ failure, then immediate transplantation would almost certainly result in significantly improved survival.
Third—a point that is generally overlooked—brain death has numerous adverse effects on the donor organs, particularly the heart, that may lead to primary graft nonfunction or other injury (31, 32). In the case of the xenotransplantation of pig organs, this would be avoided as organs would be excised from a healthy pig under anesthesia.
Fourth, almost no year passes without a novel microorganism being transferred from the donor to the recipient with the graft. In recent years, West Nile virus, rabies, and other microorganisms have been transferred with fatal results. While there has been some concern that a porcine microorganism might be transferred with a pig organ (33–35), the pig herd for transplantation will be housed under ideal conditions and be monitored at regular intervals for infectious agents, providing a much greater chance that the donor animal would be free of all known pathogenic organisms than the average deceased human donor.
In several countries, there are cultural barriers to deceased organ donation, e.g., Japan, and yet there are no barriers to xenotransplantation; thus, the number of transplants performed would be vastly increased. The lack of deceased human donors, particularly with regard to liver transplantation, has popularized living donor liver transplants, in which almost two thirds of the donor liver is transplanted in an adult, and there have been a number of donor deaths after these procedures (estimated donor mortality, 0.1% to 0.8%). These tragedies would be avoided if pig organs could be used for transplantation.
The immunological and pathophysiological problems associated with pig xenotransplantation, however, are significant and probably reflect the fact that it has been 80 million years since the pig and human diverged on the evolutionary scale. Therefore, in the words of Claus Hammer, what we are trying to do is to “outwit evolution.”
OVERCOMING THE PROBLEM OF THE GAL ANTIGEN
Nevertheless, very significant progress has been made since we began to develop the ability to genetically modify large animals, particularly the pig. The “creation” of “Dolly” the sheep, the first cloned mammal, opened the gates to the possibility of rendering pig tissues resistant to the human immune response. It is only through this route that we will overcome the remaining barriers. Many of the barriers have been identified, and some have been overcome.
For example, whereas the human vascular endothelium expresses the ABH blood group antigens, the pig's vascular endothelium expresses a galactose oligosaccharide, Galα1,3Gal (Gal) (36). The presence of Gal in the pig and its absence in humans, who thus generate anti-Gal antibodies, has proved a major challenge (37–40). When a pig organ or cells are transplanted into a human, these antibodies immediately bind to the cells of the graft and activate complement, resulting in hyperacute rejection that occurs within minutes or hours. This problem has largely been overcome by the development of pigs homozygous for α1,3-galactosyltransferase gene-knockout (GTKO), which no longer express Gal epitopes (41–43).
A second way of negating the problem of human antibody binding to pig antigens is to provide the pig with increased resistance to human complement-mediated injury. This has been achieved by inserting into the pig genes one or more human complement-regulatory proteins, such as CD55 or CD46 (44, 45). The combination of GTKO and expression of CD46 and/or CD55 has made hyperacute rejection a rarity in experimental organ xenotransplantation studies (46).
CURRENT RESULTS OF EXPERIMENTAL PIG ORGAN OR CELL XENOTRANSPLANTATION
The experimental results of cell xenotransplantation, e.g., islet or neuronal cells, are currently significantly better than those of pig organ xenotransplantation. For example, pig islets have continued to function effectively in immunosuppressed nonhuman primates for periods of more than a year (47–50). Indeed, a clinical trial of encapsulated pig islet transplantation is under way in diabetic patients in New Zealand (51).
There are an estimated 8 million patients in the US with a neurogenerative disease, such as Parkinson's disease. Human embryonic neural precursor cells can restore local motor activity in patients with Parkinson's disease, but the use of human embryos is largely precluded on ethical grounds or on logistic grounds as too few become available. Genetically engineered pig embryos might provide an alternative source. Indeed, a European group has reported encouraging improvement for >1 year in locomotor function in monkeys with a Parkinson-like condition after the transplantation of genetically modified pig dopamine-producing cells into the brain (52–54).
There is also a great need for corneas, particularly in Asia and Africa; for example, it is estimated that 4 million patients need corneal transplantation in China alone (11). Experimental corneal xenotransplantation has made significant progress in recent years; pig lamellar grafts have survived in monkeys for periods of more than 1 year, with the recipient receiving only corticosteroid injections into the eyes (55). Partly because the risk to the recipient would be small, it is likely that corneas will be the first xenotransplants to be carried out as a clinical trial, perhaps followed soon after by neuronal cell or islet cell transplantation.
With regard to pig organ xenotransplantation in nonhuman primates, we have been able to achieve pig heterotopic cardiac graft survival up to 8 months (56–59) (i.e., non–life-supporting grafts in which the graft is placed in the abdomen and, by being supplied with recipient blood, beats, thus allowing monitoring for rejection) and life-supporting pig kidney survival up to almost 3 months (60, 61). Transplantation of pig livers (62, 63) and lungs (64, 65) in nonhuman primates has been significantly less successful, with grafts functioning for only days. Pig hearts and livers may initially be used as a bridge while the patient is awaiting a human graft; this will give us experience with organ xenografts in humans. Because dialysis maintains life for a number of years in many patients with renal failure, clinical pig kidney transplantation will probably be delayed.
SAFETY AND REGULATORY ASPECTS OF XENOTRANSPLANTATION
The major concern of national regulatory authorities is whether pig organ or cell xenotransplantation will prove safe from the perspective of the transfer of porcine microorganisms with the graft to the recipient and perhaps into the general population (33–35). As mentioned earlier, to prevent this, pigs will be housed under strict barrier conditions and will be screened for potentially pathogenic microorganisms at regular intervals (34). Organs and cells from these pigs should, therefore, be safer from this perspective than an allograft taken from a recently brain-dead human, where there has been insufficient time to monitor for all potential infectious agents.
With regard to xenotransplantation, most concern has related to endogenous retroviruses that are present in the genome of every porcine cell (33, 35). These will inevitably be transferred with the donor tissues. This potential risk gave considerable concern some years ago, but it is now generally believed that these are weak viruses and are unlikely to be problematic, even in an immunosuppressed recipient. There has been no documentation of transfer of these viruses in humans or nonhuman primates exposed to pig tissues. Strict monitoring for infectious complications in the recipient and archiving of tissues from the source pig will be required by the regulatory authorities for a prolonged period of time (66).
PREDICTING FUTURE PROGRESS
In 1969, Sir Peter Medawar, the British scientist who won the Nobel Prize for medicine in 1960 and is considered the father of transplant immunology, stated, “We should solve the problem [of organ transplantation] by using heterografts [xenografts] one day if we try hard enough, and maybe in less than 15 years.” This indicates that even Nobel Prize winners can get their prophecies wrong. In contrast, in 1995, Sir Roy Calne, another great pioneer in organ transplantation, stated that xenotransplantation “is just around the corner, but it may be a very long corner.” He has been proved correct. At least he and Sir Peter Medawar were optimistic about the development of xenotransplantation, whereas Norman Shumway, the pioneer of heart transplantation, stated rather pessimistically that “xenotransplantation is the future of transplantation, and always will be.”
Nevertheless, there is clear evidence that xenotransplantation will become successful in the relatively near future. There is a Native American proverb, “Timing has a lot to do with the success of a rain dance.” With the increasing variety of genetically engineered pigs now becoming available, it is likely that the remaining problems will be resolved and the timing for xenotransplantation will be right. For example, in Pittsburgh, we have available to us (through our colleagues at Revivicor Inc., of Blacksburg, VA) pigs with no fewer than nine different genetic manipulations, of which at least five have been combined in a single pig (Table 2). With interbreeding between these various pigs—and with new genetic modifications being introduced—it is likely that the problems of rejection and coagulation dysfunction (which is the present major barrier [67]) will soon be overcome. Although there are relatively few hard data at present, the current evidence is that the function of pig organs and cells in humans may be adequate (68).
Table 2.
Genetically modified pigs currently available for xenotransplantation research∗
| Gal antigen deletion or “masking” |
| • a1,3-galactosyltransferase gene-knockout (GTKO) |
| • Human H-transferase gene expression (expression of blood type O antigen) |
| • Endo-beta-galactosidase C (reduction of Gal antigen expression) |
| Complement regulation by human complement-regulatory gene expression |
| • CD55 (decay-accelerating factor) |
| • CD46 (membrane cofactor protein) |
| • CD59 (protectin or membrane inhibitor of reactive lysis) |
| Anticoagulation and antiinflammatory gene expression or deletion |
| • Human tissue factor pathway inhibitor (TFPI) |
| • Human thrombomodulin |
| • Human CD39 (ectonucleoside triphosphate diphosphohydrolase-1)† |
| • Von Willebrand factor–deficient (natural mutant) |
| Suppression of cellular immune response by gene expression or downregulation |
| • Porcine CTLA4-Ig (cytotoxic T-lymphocyte antigen 4 or CD152) |
| • LEA29Y (inhibition of the B7/CD28 costimulatory pathway of T-cell activation) |
| • CIITA-DN (MHC class II transactivator knockdown, resulting in swine leukocyte antigen class II knockdown) |
| • Human TRAIL (tumor necrosis factor-alpha–related apoptosis-inducing ligand) |
| • HLA-E/human β2-microglobulin (inhibits human natural killer cell cytotoxicity) |
| • Human CD47 (for species-specific CD47-SIRPalpha natural interaction on macrophages) |
| • Human FAS ligand (CD95L) |
| • Human GnT-III (N-acetylglucosaminyltransferase III) gene |
| Anticoagulation, antiinflammatory, and antiapoptotic gene expression |
| • Human A20 (tumor necrosis factor-alpha–induced protein 3) |
| • Human heme oxygenase-1 (HO-1) |
| • Human TNFRI-Fc (tumor necrosis factor-alpha receptor I-Fc) |
| Prevention of porcine endogenous retrovirus (PERV) activation |
| • PERV siRNA |
∗Kindly collated by Burcin Ekser, MD. Pigs with combinations of genetic modification, e.g., GTKO with added transgenes, are available.
†Personal communication from Drs. S. Robson and P. Cowan.
The words of George Orwell in Animal Farm will be apposite to pig organ transplantation in humans. “The creatures outside looked from pig to man, and from man to pig, and from pig to man again; but already it was impossible to say which was which.” I believe the same will one day be said for the doctor examining a patient with an organ transplant—the doctor will not be able to determine whether the organ is an allograft or a xenograft. Eventually, allotransplantation will be of historic interest only.
Acknowledgments
The author thanks Burcin Ekser, MD, for preparing Table 2. Work on xenotransplantation in the author's laboratory in the Thomas E. Starzl Transplantation Institute at the University of Pittsburgh has been supported in part by National Institutes of Health grants U01 AI068642, R21 AI074844, and U19 AI090959, and by sponsored research agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA.
References
- 1.Cooper DKC, Lanza RP. Xeno—The Promise of Transplanting Animal Organs into Humans. New York: Oxford University Press; 2000. pp. 1–274. [Google Scholar]
- 2.Ekser B, Ezzelarab M, Hara H, van der Windt DJ, Wijkstrom M, Bottino R, Trucco M, Cooper DKC. Clinical xenotransplantation—the next great medical revolution? Lancet, in press. [DOI] [PubMed]
- 3.Taniguchi S, Cooper DKC. Clinical xenotransplantation: past, present and future. Ann R Coll Surg Engl. 1997;79(1):13–19. [PMC free article] [PubMed] [Google Scholar]
- 4.Deschamps JY, Roux FA, Saï P, Gouin E. History of xenotransplantation. Xenotransplantation. 2005;12(2):91–109. doi: 10.1111/j.1399-3089.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 5.Reemtsma K. Xenotransplantation—a brief history of clinical experience: 1900–1965. In: Cooper DKC, Kemp E, Reemtsma K, White DJG, editors. Xenotransplantation: The Transplantation of Organs and Tissues Between Species. 1st ed. Heidelberg: Springer; 1991. pp. 9–22. [Google Scholar]
- 6.Roux FA, Saï P, Deschamps JY. Xenotransfusions, past and present. Xenotransplantation. 2007;14(3):208–216. doi: 10.1111/j.1399-3089.2007.00404.x. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DKC, Hara H, Yazer M. Genetically engineered pigs as a source for clinical red blood cell transfusion. Clin Lab Med. 2010;30(2):365–380. doi: 10.1016/j.cll.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Gibson T. Zoografting: a curious chapter in the history of plastic surgery. Br J Plast Surg. 1955;8(3):234–242. doi: 10.1016/s0007-1226(55)80040-9. [DOI] [PubMed] [Google Scholar]
- 9.Cooper DKC. Xenografting: the early, early years. Xeno. 1997;5:21–22. [Google Scholar]
- 10.Hara H, Cooper DKC. The immunology of corneal xenotransplantation: a review of the literature. Xenotransplantation. 2010;17(5):338–349. doi: 10.1111/j.1399-3089.2010.00608.x. [DOI] [PubMed] [Google Scholar]
- 11.Hara H, Cooper DKC. Xenotransplantation-the future of corneal transplantation? Cornea. 2011;30(4):371–378. doi: 10.1097/ICO.0b013e3181f237ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton D. The Monkey Gland Affair. London: Chatto and Windus; 1986. [Google Scholar]
- 13.Real J. Voronoff [French] Paris: Editors Stock; 2001. [Google Scholar]
- 14.Matevossian E, Kern H, Hüser N, Doll D, Snopok Y, Nährig J, Altomonte J, Sinicina I, Friess H, Thorban S. Surgeon Yurii Voronoy (1895–1961)—a pioneer in the history of clinical transplantation: in memoriam at the 75th anniversary of the first human kidney transplantation. Transpl Int. 2009;22(12):1132–1139. doi: 10.1111/j.1432-2277.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee RA. The Bizarre Careers of John R. Brinkley. Lexington, KY: University Press of Kentucky; 2002. [Google Scholar]
- 16.Sgroi A, Bühler LH, Morel P, Sykes M, Noel L. International human xenotransplantation inventory. Transplantation. 2010;90(6):597–603. doi: 10.1097/TP.0b013e3181eb2e8c. [DOI] [PubMed] [Google Scholar]
- 17.Reemtsma K, McCracken BH, Schlegel JU, Pearl MA, Pearce CW, DeWitt CV, Smith PE, Hewitt RL, Flinner RL, Creech O., Jr Renal heterotransplantation in man. Ann Surg. 1964;160:384–410. doi: 10.1097/00000658-196409000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starzl TE, Marchioro TL, Peters GN, Kirkpatrick CH, Wilson WE, Porter KA, Rifkind D, Ogden DA, Hitchcock CR, Waddell WR. Renal heterotransplantation from baboon to man: experience with 6 cases. Transplantation. 1964;2:752–776. doi: 10.1097/00007890-196411000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy JD, Kurrus FD, Chavez CM, Neely WA, Eraslan S, Turner MD, Fabian LW, Labecki TD. Heart transplantation in man: developmental studies and report of a case. JAMA. 1964;188:1132–1140. [PubMed] [Google Scholar]
- 20.Barnard CN. The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J. 1967;41(48):1271–1274. [PubMed] [Google Scholar]
- 21.Barnard CN, Wolpowitz A, Losman JG. Heterotopic cardiac transplantation with a xenograft for assistance of the left heart in cardiogenic shock after cardiopulmonary bypass. S Afr Med J. 1977;52(26):1035–1038. [PubMed] [Google Scholar]
- 22.Bailey LL, Nehlsen-Cannarella SL, Concepcion W, Jolley WB. Baboon-to-human cardiac xenotransplantation in a neonate. JAMA. 1985;254(23):3321–3329. [PubMed] [Google Scholar]
- 23.Starzl TE, Marchioro TL, Faris TD, McCardle RJ, Iwaski Y. Avenues of future research in homotransplantation of the liver with particular reference to hepatic supportive procedures, antilymphocyte serum, and tissue typing. Am J Surg. 1966;112(3):391–400. doi: 10.1016/0002-9610(66)90209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starzl TE. Orthotopic heterotransplantation. In: Starzl TE, editor. Experience in Hepatic Transplantation. Philadelphia: WB Saunders; 1969. p. 408. [Google Scholar]
- 25.Starzl TE, Ishikawa M, Putnam CW, Porter KA, Picache R, Husberg BS, Halgrimson CG, Schroter G. Progress in and deterrents to orthotopic liver transplantation, with special reference to survival, resistance to hyperacute rejection, and biliary duct reconstruction. Transplant Proc. 1974;6(4 Suppl 1):129–139. [PMC free article] [PubMed] [Google Scholar]
- 26.Giles GR, Boehmig HJ, Amemiya H, Halgrimson CG, Starzl TE. Clinical heterotransplantation of the liver. Transplant Proc. 1970;2(4):506–512. [PMC free article] [PubMed] [Google Scholar]
- 27.Starzl TE, Fung J, Tzakis A, Todo S, Demetris AJ, Marino IR, Doyle H, Zeevi A, Warty V, Michaels M, et al. Baboon-to-human liver transplantation. Lancet. 1993;341(8837):65–71. doi: 10.1016/0140-6736(93)92553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makowka L, Wu GD, Hoffman A, Podesta L, Sher L, Tuso PJ, Breda M, Chapman FA, Cosenza C, Yasunaga C, et al. Immunohistopathologic lesions associated with the rejection of a pig-to-human liver xenograft. Transplant Proc. 1994;26(3):1074–1075. [PubMed] [Google Scholar]
- 29.Groth CG, Korsgren O, Tibell A, Tollemar J, Möller E, Bolinder J, Ostman J, Reinholt FP, Hellerström C, Andersson A. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344(8934):1402–1404. doi: 10.1016/s0140-6736(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 30.Cooper DKC, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002;53:133–147. doi: 10.1146/annurev.med.53.082901.103900. [DOI] [PubMed] [Google Scholar]
- 31.Cooper DKC, Novitzky D, Wicomb WN. The pathophysiological effects of brain death on potential donor organs, with particular reference to the heart. Ann R Coll Surg Engl. 1989;71(4):261–266. [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper DKC, Novitzky D, Wicomb WN, Basker M, Rosendale JD, Myron Kauffman H. A review of studies relating to thyroid hormone therapy in brain-dead organ donors. Front Biosci. 2009;14:3750–3770. doi: 10.2741/3486. [DOI] [PubMed] [Google Scholar]
- 33.Patience C, Takeuchi Y, Weiss RA. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997;3(3):282–286. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- 34.Onions D, Cooper DKC, Alexander TJ, Brown C, Claassen E, Foweraker JE, Harris DL, Mahy BW, Minor PD, Osterhaus AD, Pastoret PP, Yamanouchi K. An approach to the control of disease transmission in pig-to-human xenotransplantation. Xenotransplantation. 2000;7(2):143–155. doi: 10.1034/j.1399-3089.2000.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeuchi Y, Fishman J. Long life with or without PERV. Xenotransplantation. 2010;17(6):429–430. doi: 10.1111/j.1399-3089.2010.00614.x. [DOI] [PubMed] [Google Scholar]
- 36.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263(33):17755–17562. [PubMed] [Google Scholar]
- 37.Cooper DKC. Depletion of natural antibodies in non-human primates—a step towards successful discordant xenografting in humans. Clin Transplant. 1992;6(3 part 1):178–183. [PubMed] [Google Scholar]
- 38.Cooper DKC, Good AH, Koren E, Oriol R, Malcolm AJ, Ippolito RM, Neethling FA, Ye Y, Romano E, Zuhdi N. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1993;1(3):198–205. doi: 10.1016/0966-3274(93)90047-c. [DOI] [PubMed] [Google Scholar]
- 39.Good AH, Cooper DKC, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, Ye Y, Zuhdi N, Lamontagne LR. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc. 1992;24(2):559–562. [PubMed] [Google Scholar]
- 40.Kobayashi T, Cooper DKC. Anti-Gal, alpha-Gal epitopes, and xenotransplantation. Subcell Biochem. 1999;32:229–257. doi: 10.1007/978-1-4615-4771-6_10. [DOI] [PubMed] [Google Scholar]
- 41.Cooper DKC, Koren E, Oriol R. Genetically engineered pigs. Lancet. 1993;342(8872):682–683. doi: 10.1016/0140-6736(93)91791-j. [DOI] [PubMed] [Google Scholar]
- 42.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, Sharma SB, Lamborn AE, Garst AS, Moore M, Demetris AJ, Rudert WA, Bottino R, Bertera S, Trucco M, Starzl TE, Dai Y, Ayares DL. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, Betthauser J, Carter DB, Greenstein JL, Hao Y, Im GS, Liu Z, Mell GD, Murphy CN, Park KW, Rieke A, Ryan DJ, Sachs DH, Forsberg EJ, Prather RS, Hawley RJ. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004;101(19):7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995;1(9):964–966. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 45.Loveland BE, Milland J, Kyriakou P, Thorley BR, Christiansen D, Lanteri MB, Regensburg M, Duffield M, French AJ, Williams L, Baker L, Brandon MR, Xing PX, Kahn D, McKenzie IF. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation. 2004;11(2):171–183. doi: 10.1046/j.1399-3089.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 46.Azimzadeh A, Kelishadi S, Ezzelarab M, Singh AK, Stoddard T, Zhang T, Burdorf L, Avon C, Laaris A, Cheng X, Ayares D, Horvath KA, Corcoran PC, Mohiuddin MM, Cooper DKC, Barth RN, Pierson RN. Early graft failure of GTKO pigs organs in baboons is reduced by hCPRP expression [Abstract IXA-O-2.2] Xenotransplantation. 2009;16(5):356. doi: 10.1111/xen.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hering BJ, Wijkstrom M, Graham ML, Hårdstedt M, Aasheim TC, Jie T, Ansite JD, Nakano M, Cheng J, Li W, Moran K, Christians U, Finnegan C, Mills CD, Sutherland DE, Bansal-Pakala P, Murtaugh MP, Kirchhof N, Schuurman HJ. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 48.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, Bello-Laborn H, Hacquoil B, Strobert E, Gangappa S, Weber CJ, Pearson TC, Rajotte RV, Larsen CP. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12(3):304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 49.van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, Murase N, Hara H, Ball S, Loveland BE, Ayares D, Lakkis FG, Cooper DKC, Trucco M. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9(12):2716–2726. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 50.Dufrane D, Goebbels RM, Gianello P. Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation. 2010;90(10):1054–1062. doi: 10.1097/TP.0b013e3181f6e267. [DOI] [PubMed] [Google Scholar]
- 51.Elliott RB, Living Cell Technologies Towards xenotransplantation of pig islets in the clinic. Curr Opin Organ Transplant. 2011;16(2):195–200. doi: 10.1097/MOT.0b013e3283449dec. [DOI] [PubMed] [Google Scholar]
- 52.Martin C, Plat M, Nerriére-Daguin V, Coulon F, Uzbekova S, Venturi E, Condé F, Hermel JM, Hantraye P, Tesson L, Anegon I, Melchior B, Peschanski M, Le Mauff B, Boeffard F, Sergent-Tanguy S, Neveu I, Naveilhan P, Soulillou JP, Terqui M, Brachet P, Vanhove B. Transgenic expression of CTLA4-Ig by fetal pig neurons for xenotransplantation. Transgenic Res. 2005;14(4):373–384. doi: 10.1007/s11248-004-7268-4. [DOI] [PubMed] [Google Scholar]
- 53.Badin RA, Padoan A, Vadori M, Boldrin M, Cavicchioli L, De Benedictis G, Fante F, Seveso M, Sgarabotto D, Jan C, Daguin V, Naveilhan P, Neveu I, Soulillou J, Vanhove B, Plat M, Bottè F, Eric V, Denaro L, Manara R, Zampieri P, D'avella D, Rubello D, Ancona E, Hantraye P, Cozzi E. Long-term clinical recovery in Parkinsonian monkey recipients of CTLA4-Ig transgenic porcine neural precursors. Transplantation. 2010;90(Suppl 2):47. (Abstract LB-3288) [Google Scholar]
- 54.Lévêque X, Cozzi E, Naveilhan P, Neveu I. Intracerebral xenotransplantation: recent findings and perspectives for local immunosuppression. Curr Opin Organ Transplant. 2011;16(2):190–194. doi: 10.1097/MOT.0b013e32834494b5. [DOI] [PubMed] [Google Scholar]
- 55.Kim MK, Wee WR, Park CG, Kim SJ. Xenocorneal transplantation. Curr Opin Organ Transplant. 2011;16(2):231–236. doi: 10.1097/MOT.0b013e328344870c. [DOI] [PubMed] [Google Scholar]
- 56.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, Lancos CJ, Prabharasuth DD, Cheng J, Moran K, Hisashi Y, Mueller N, Yamada K, Greenstein JL, Hawley RJ, Patience C, Awwad M, Fishman JA, Robson SC, Schuurman HJ, Sachs DH, Cooper DK. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11(1):29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 57.Tseng YL, Kuwaki K, Dor FJ, Shimizu A, Houser S, Hisashi Y, Yamada K, Robson SC, Awwad M, Schuurman HJ, Sachs DH, Cooper DKC. α 1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005;80(10):1493–1500. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 58.McGregor CG, Davies WR, Oi K, Teotia SS, Schirmer JM, Risdahl JM, Tazelaar HD, Kremers WK, Walker RC, Byrne GW, Logan JS. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg. 2005;130(3):844–851. doi: 10.1016/j.jtcvs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 59.Mohiuddin MM, Corcoran PC, Singh AK, Azimzadeh AM, Hoyt RF, Thomas MP, Eckhaus MA, Ayares D, Pierson RN, III, Horvath KA. Over six month survival of cardiac xenograft is achievable but heterotopic placement of the graft may limit consistent prolonged survival (Abstract TTS-1383) Transplantation. 2010;90(Suppl):325. [Google Scholar]
- 60.Cozzi E, Vial C, Ostlie D, Farah B, Chavez G, Smith KG, Bradley JR, Thiru S, Davies HF, Wallwork J, White DJ, Goddard M, Friend PJ. Maintenance triple immunosuppression with cyclosporin A, mycophenolate sodium and steroids allows prolonged survival of primate recipients of hDAF porcine renal xenografts. Xenotransplantation. 2003;10(4):300–310. doi: 10.1034/j.1399-3089.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- 61.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, O'Malley P, Nobori S, Vagefi PA, Patience C, Fishman J, Cooper DK, Hawley RJ, Greenstein J, Schuurman HJ, Awwad M, Sykes M, Sachs DH. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 62.Ekser B, Echeverri GJ, Hassett AC, Yazer MH, Long C, Meyer M, Ezzelarab M, Lin CC, Hara H, van der Windt DJ, Dons EM, Phelps C, Ayares D, Cooper DKC, Gridelli B. Hepatic function after genetically engineered pig liver transplantation in baboons. Transplantation. 2010;90(5):483–493. doi: 10.1097/TP.0b013e3181e98d51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ekser B, Long C, Echeverri GJ, Hara H, Ezzelarab M, Lin CC, de Vera ME, Wagner R, Klein E, Wolf RF, Ayares D, Cooper DKC, Gridelli B. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10(2):273–285. doi: 10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 64.Cantu E, Balsara KR, Li B, Lau C, Gibson S, Wyse A, Baig K, Gaca J, Gonzalez-Stawinski GV, Nichols T, Parker W, Davis RD. Prolonged function of macrophage, von Willebrand factor-deficient porcine pulmonary xenografts. Am J Transplant. 2007;7(1):66–75. doi: 10.1111/j.1600-6143.2006.01603.x. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen BN, Azimzadeh AM, Zhang T, Wu G, Schuurman HJ, Sachs DH, Ayares D, Allan JS, Pierson RN., 3rd Life-supporting function of genetically modified swine lungs in baboons. J Thorac Cardiovasc Surg. 2007;133(5):1354–1363. doi: 10.1016/j.jtcvs.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 66.Schuurman HJ. Regulatory aspects of pig-to-human islet transplantation. Xenotransplantation. 2008;15(2):116–120. doi: 10.1111/j.1399-3089.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- 67.Cowan PJ, Robson SC, d'Apice AJ. Controlling coagulation dysregulation in xenotransplantation. Curr Opin Organ Transplant. 2011;16(2):214–221. doi: 10.1097/MOT.0b013e3283446c65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DKC. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006;13(6):488–499. doi: 10.1111/j.1399-3089.2006.00346.x. [DOI] [PubMed] [Google Scholar]