Abstract
Morphine and related compounds are the first line of therapy in the treatment of moderate to severe pain. Over time, individuals taking opioids can develop an increasing sensitivity to noxious stimuli, even evolving into a painful response to previously non-noxious stimuli (opioid-induced hyperalgesia; OIH). The mechanism underlying OIH is not well understood although complex intracellular neural mechanisms, including opioid receptor desensitization and down-regulation, are believed to be major mechanisms underlying OIH. However, OIH may also be associated with changes in gene expression. A growing body of evidence suggests that cellular exposure to mu agonists upregulate chemokines/receptors and recent work from our lab implicates chemokine upregulation in a variety of neuropathic pain behaviors. Here we characterized the degree to which chemokines/receptors signaling is increased in primary afferent neurons of the dorsal root ganglion (DRG) following chronic morphine sulphate treatment and correlated these changes with tactile hyperalgesic behavior in rodents. We demonstrate that mRNA expression of the chemokine, stromal-derived factor-1 (SDF1/CXCL12) is upregulated following morphine treatment in sensory neurons of the rat. The release of SDF1 was found to be constitutive when compared with the activity dependent release of the C-C chemokine, monocyte chemoattractant protein-1 (MCP1/CCL2) in a line of F-11 neuroblastoma-sensory neuron hybrid cells. We further determined that there is pronounced CXCR4 expression in satellite glial cells and following morphine treatment, increased functional CXCR4 expression in sensory neurons of the DRG. Moreover, intraperitoneal administration of the specific CXCR4 antagonist, AMD3100, completely reversed OIH in the rat. Taken together; the data suggest that opioid-induced SDF1/CXCR4 signaling is central to the development of long lasting OIH and that receptor antagonists represent a promising novel approach to the management of the side effects associated with the use of opioids for chronic pain management.
Introduction
Opioids such as morphine currently represent the best option for the management of moderate to severe trauma induced, perioperative and cancer pain. Opioid compounds are also increasingly being used for non cancer associated chronic pathological pain. However, prolonged administration of opioids is associated with significant problems including the development of anti-nociceptive tolerance, wherein higher doses of the drug are required over time to elicit the same degree of analgesia. Repeated administration of higher doses of morphine or fentanyl also results in increasing pain sensitivity, a syndrome clinically known as opioid-induced hyperalgesia (OIH) (Angst et al., 2003; Arner et al., 1988; Singla et al., 2007). This increased pain is usually experienced at different locations from the original site of injury (Ossipov et al., 2004).
While it is thought that opioids modulate tactile hyperalgesia solely by acting at neuronal opioid receptors, administration of chronic morphine is also known to induce a rapid increase in the expression of the proinflammatory cytokines such as TNFα, IL1β and IL-6 in a number of cell types within the nervous system (Johnston et al., 2004). These proinflammatory cytokines are powerful pain enhancing proteins that may, in turn, suppress acute opioid analgesia and contribute to the apparent loss of opioid analgesia upon repeated opioid administration (“tolerance”) (Hutchinson et al., 2008). The family of pro-nociceptive cytokines includes chemotactic cytokines (chemokines). Proalgesic effects of chemokines have been implicated in both acute and chronic tactile hyperalgesic behavior (Abbadie et al., 2003; Bhangoo et al., 2007a; Bhangoo et al., 2007b; Johnston et al., 2004; Jung et al., 2009; Menetski et al., 2007; Milligan et al., 2004; Oh et al., 2001; Wang et al., 2008; White et al., 2005; Xie et al., 2006). However, the degree to which chronic morphine treatment alters gene expression of chemokines and their receptors, and whether this contributes to syndromes such as OIH is unknown.
Effects of opioids on chemokine receptor expression are potentially important determinants of HIV-1 infection rates among intravenous drug users as the chemokine receptors CCR5 and CXCR4 are co-receptors for the HIV-1 virus coat protein, gp120. To this end a number of studies using chronic morphine or the selective μ opioid agonist, [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) produce increased expression of monocyte chemoattractant protein-1 (MCP1/CCL2), regulated upon activation normal T-cell expressed and secreted (RANTES/CCL5), and their respective receptors, CCR2 and CCR5, in astrocytes and neurons via largely unknown mechanisms (Avdoshina et al., 2010; Mahajan et al., 2005; Rock et al., 2006). A similar study demonstrated that DAMGO substantially increased the expression of both CCR5 and CXCR4 in leukocytes (Steele et al., 2003). Taken together, these observations raise the possibility that repeated exposure to opioids and subsequent increases in chemokine receptor signaling might also be central to OIH.
We now demonstrate that many nociceptive neurons express functional receptors for a number of chemokines following systemic injection of morphine. Chemokine receptor signaling via the CXCR4 receptor may be central to OIH as the administration of the specific CXCR4 receptor antagonist, AMD3100, transiently reversed OIH in rats. Collectively, the data suggest that chemokine receptor antagonists represent a promising novel approach to the management of the side effects associated with long term opioids for chronic pain control.
Methods
Animals
Pathogen-free, adult female Sprague-Dawley rats (150–200 g; Harlan Laboratories, Madison, WI) were housed in temperature (23 ± 3°C) and light (12-hlight: 12-h dark cycle; lights on at 07:00 h) controlled rooms with standard rodent chow and water available ad libitum. Experiments were performed during the light cycle. These experiments were approved by the Institutional Animal Care and Use Committee of Loyola University, Chicago and Indiana University/Purdue University in Indianapolis. All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health and the ethical guidelines of the International Association for the Study of Pain. All animals were randomly assigned to either treatment or control groups.
Drugs and method of administration
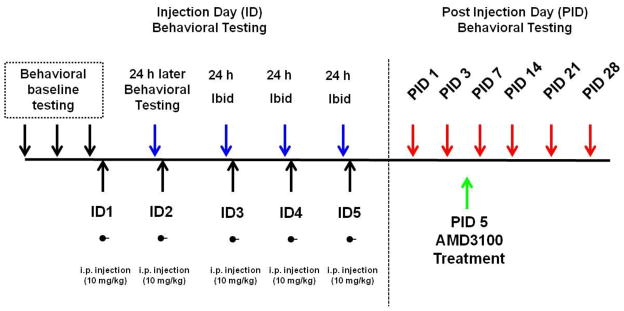
The drugs, morphine sulfate salt and the bicyclam, AMD3100, were employed in this study. Morphine sulfate salt and AMD3100 were purchased from NIDA Drug Supply Program (Rockville, MD) and Sigma-Aldrich (St. Louis, MO), respectively. All drugs were freshly prepared in saline on the day of the experiment. Morphine sulfate- and vehicle-treated groups were given intraperitoneal (i.p.) injections once daily for 5 days of 10 mg/kg or saline (vehicle). After tactile hyperalgesia was established, animals were given an i.p. injection of AMD3100 (10 mg/kg) (Fig. 1). Previous nociceptive behavioral studies from our lab using AMD3100 at doses of (1,5,10, and 25 mg/kg) observed: no reversal of effect with 1 mg/kg, partial inconsistent effect with 5 mg/kg, reversal with 10 mg/kg, and reversal with side effects at 25 mg/kg (unpublished observations). Therefore, 10 mg/kg AMD3100 was selected for these studies.
Figure 1.
Repeated morphine treatment paradigm. Animals underwent baseline testing for 2–3 days prior to the start of injections. Rats received once daily i.p. injections of morphine (10 mg/kg) for 5 days. Behavioral testing that occurred during the 5 days of injections (ID) were carried out 24 hours after the last morphine injection. Following the injection period, behavior was conducted on animals for up to 28 days following the last morphine injection (PID). Behavioral testing with the CXCR4 antagonist, AMD3100, occurred five days after the last morphine injection (PID5).
Behavioral assessment
Von Frey filaments were used to test mechanical sensitivity before, during and after cessation of morphine sulfate administration. Prior to initial von Frey tactile testing, all rodents were habituated to testing chambers for at least two days. Animals were tested for baseline responses (BL) at least two times before undergoing the repeated morphine sulfate treatment (10 mg/kg, i.p. daily). Mechanical testing with von Frey filaments during the morphine sulfate dosing paradigm was limited to injection day (ID) 3. Behavioral assessment on ID3 occurred 18–20 hours after the ID2 morphine administration and before ID3 morphine or vehicle treatment (Fig. 1). Additional behavioral assessment following drug or vehicle administration occurred on post-injection day (PID) 1, 2, 3, 7, 14, 21, and 28. All behavioral testing was performed by laboratory assistants who were blinded to the experimental conditions and unfamiliar with the experimental aims.
The incidence of foot withdrawal in response to mechanical indentation of the plantar surface of each hindpaw was measured with a Von Frey filament capable of exerting forces of 10, 20, 40, 60, 80 and 120 mN. These probes exhibit a uniform tip diameter (0.2 mm) and were applied to 6 designated loci distributed over the plantar surface of the foot (Ma et al., 2003). These 6 spots are representative of the distal nerve distributions of saphenous, tibial and sural nerves (medial to lateral) in the glabrous hindpaw. During each test, the rodent was placed in a transparent plastic cage with a floor of wire with ~1×1 cm openings. The cage is elevated so that stimulation can be applied to each hind foot from beneath the rodent. The filaments were applied in order of ascending force. Each filament was applied alternately to each foot and to each locus. The duration of each stimulus was approximately 1 s and the inter-stimulus interval was approximately 10–15 s. The incidence of foot withdrawal is expressed as a percentage of the 6 applications of each stimulus and the percentage of withdrawals was then plotted as a function of force (Bhangoo et al., 2007a; Ma et al., 2003). The von Frey withdrawal threshold was defined as the force that evoked a minimum detectable withdrawal observed on 50% of the tests given at the same force level. For cases in which none of the specific filaments used evoked withdrawals on exactly 50% of the tests, linear interpolation was used to define the threshold.
Tissue processing and immunocytochemistry for neural tissue
Morphine or control treatments rats’ lumbar (L3–L6) DRG tissue was collected after animals were sacrificed and transcardially-perfused with saline followed by fixative. Fixed tissue was then embedded for sectioning and processed using immunocytochemical methodologies commonly used in this lab (Bhangoo et al., 2007a). Tissue sections from L4 and L5 were used in immunocytochemical experiments. Primary antisera used was the anti-CXCR4 rat monoclonal antibody, 2B11 (1:20,000 dilution; BD Biosciences, San Jose, CA) which binds to both human and mouse CXCR4 (Forster et al., 1998; Schabath et al., 1999). After primary incubation, slides were incubated in secondary antibodies (anti-rat made in horse conjugated to CY3, Jackson ImmunoResearch, West Grove, PA).
Preparation of acutely dissociated dorsal root ganglion neurons
The L1–L6 DRGs were acutely dissociated using methods described by Ma and LaMotte (Ma and LaMotte, 2005). Briefly, L1–L6 DRGs were removed from naive or morphine-treated animals four to six days following the last morphine injection. The DRGs were treated with collagenase A and collagenase D in HBSS for 20 minutes (1 mg/ml; Roche Applied Science, Indianapolis, IN), followed by treatment with papain (30 units/ml, Worthington Biochemical, Lakewood, NJ) in HBSS containing .5 mM EDTA and cysteine at 35°C. The cells were then dissociated via mechanical trituration in culture media containing 1 mg/ml bovine serum albumin and trypsin inhibitor (1 mg/ml, Sigma, St. Louis MO). The culture media was Ham’s F12 mixture, supplemented with 10% fetal bovine serum, penicillin and streptomycin (100 ug/ml and 100 U/ml) and N2 (Life Technologies). The cells were then plated on coverslips coated with poly-L lysine and laminin (1 mg/ml) and incubated for 2–3 hours before more culture media was added to the wells. The cells were then allowed to sit undisturbed for 12–15 hours to adhere at 37°C (with 5% CO2).
Intracellular Ca2+ imaging
The dissociated DRG cells were loaded with fura-2 AM (3 uM, Molecular Probes/Invitrogen Corporation, Carlsbad CA) for 25 minutes at room temperature in a balanced sterile salt solution (BSS) [NaCl (140 mM), Hepes (10 mM), CaCl2 (2 mM), MgCl2 (1 mM), Glucose (10 mM), KCl (5 mM)]. The cells were rinsed with the BSS and mounted onto a chamber that was placed onto the inverted microscope. Intracellular calcium was measured by digital video microfluorometry with an intensified CCD camera coupled to a microscope and MetaFluor software (Molecular Devices Corporation, Downington, PA). Cells were illuminated with a 150 W xenon arc lamp, and the excitation wavelengths of the fura-2 (340/380 nm) were selected by a filter changer. Sterile solution was applied to cells prior to chemokine application, any cells that responded to buffer alone were not used in chemokine responsive counts. Chemokines were applied directly into the coverslip bathing solution. If no response was seen within 1 minute, the chemokine was washed out. For all experiments, MCP1, SDF1, regulated upon activation, normal T cell expressed and secreted (RANTES/CCL5), and interferon-gamma-induced protein (IP10/CXCL10) were added to the cells in random order, after which capsaicin (3nM), high K+ (50μM) and ATP (3nM) were added. The chemokines used were purchased from R & D Systems (Minneapolis, MN; <1.0 endotoxin per 1 μg of the protein by the LAL method), and all were used at a concentration of 100 nM to ensure maximal activation (Bhangoo et al 2007a; Bhangoo et al 2007b). Chemokines were reconstituted in sterile 0.1%BSA/PBS, and aliquots were stored at −20°C. Calcium imaging traces were analyzed by two independent analyzers and only responses that were in agreement between two individuals were used in the counts.
In situ hybridization
In situ hybridization histochemistry for chemokine receptors was performed using digoxigenin-labeled riboprobes. Treated and non-treated rodents were sacrificed using carbon monoxide. Lumbar DRGs from the injected and control animals were rapidly removed, embedded in OCT compound (Tissue Tek, Ted Pella, Inc., Redding, CA) and frozen. L4 and L5 DRG sections were cut serially at 12 μm. The SDF1 probes were generated as described previously (Lu et al., 2002). Signals were visualized by using NBT/BCIP reagents (Roche Applied Science, Indianapolis, IN) in the dark for 2–20 h depending upon the abundance of the RNA. The in situ image was captured using a Retiga EX charge-coupled device camera (Q-imaging, Burnaby, BC).
Plasmid construction
To make chemokine-fluorescent protein fusion constructs, MCP1 and SDF1-alpha protein coding sequence was cloned into pEGFP-N1 or pmCherry-N1 (Clontech).
F11 Culture Conditions
F11 cells (a mouse N18TG2 neuroblastoma X rat DRG sensory neuron hybrid cell line) were grown as monolayers either in 100-mm plastic dishes under 5% CO2 in Ham’s F-12 medium supplemented with 20% fetal bovine serum (Hyclone), 100 pM hypoxanthine/1 pM aminopterin/l2 pA4 thymidine, and 50 IU/ml of penicillin/streptomycin. Cells were fed every other day for several days preceding an experiment with Ham’s F- I2 medium supplemented with 1% fetal bovine serum, 50 ng/ml of NGF, 2 pM retinoic acid, 0.5 mM dibutyryl cyclic AMP, 10 pM3-isobutyl-I-methylxanthine (IBMX), a 1:500 dilution of 2.5 mg/ml of bovine insulin, a 1:100 dilution of 10 mg/ml of transferrin, and 50 IU/ml of penicillin/streptomycin.
Enzyme-linked immunosorbant assay (ELISA)
Constitutive and regulated release of MCP1-RFP (mCherry) and SDF1-RFP (mCherry) was measured by sandwich ELISA. F11 DRG neuronal cells were transfected with MCP1-RFP or SDF1-RFP. 24 h after the transfection, cells were placed under differentiating conditions and allowed to differentiate for 48 h. When cells were fully differentiated, culture medium was replaced with balanced salt solution (BSS) containing either 5 mM (normal) or 50 mM KCl (depolarizing). Normal BSS (145 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2) and depolarizing BSS (100 mM NaCl, 50 mM KCl, 2mM CaCl2, 1mM MgCl2) had the same osmolarity. After 30 min, released MCP1-RFP or SDF1-RFP was measured from supernatant by sandwich ELISA. A polyclonal anti-RFP antibody (Abcam ab34771) was used as the capture antibody (1:50,000). Chemokine-specific antibodies were used as the detecting antibodies: for SDF1, a mouse monoclonal anti-SDF1 antibody (Santa Cruz sc-74271); for MCP1, a goat polyclonal antibody (Santa Cruz sc-1785).
Statistics
Data for sandwich ELISA were presented as mean ± SEM and analyzed by one way ANOVA followed by Newman-Keuls multiple comparison tests. Prism 5 (GraphPad, LaJolla, CA) was used to determine the statistical significance of differences in the mean threshold forces for foot withdrawal to punctate indentation as a function of time and between experimental groups by means of repeated measures analyses of variance (RMANOVA) followed by post hoc pairwise comparisons (Tukey method). Statistical significance was set at p < 0.05. GraphPad Software (LaJolla, CA) was used to determine the statistical significance of differences in calcium response among naïve and treatment groups using Chi-square test with Yates correction with p<0.05 set as statistical significance.
RESULTS
Repeated morphine treatment leads to tactile hyperalgesia
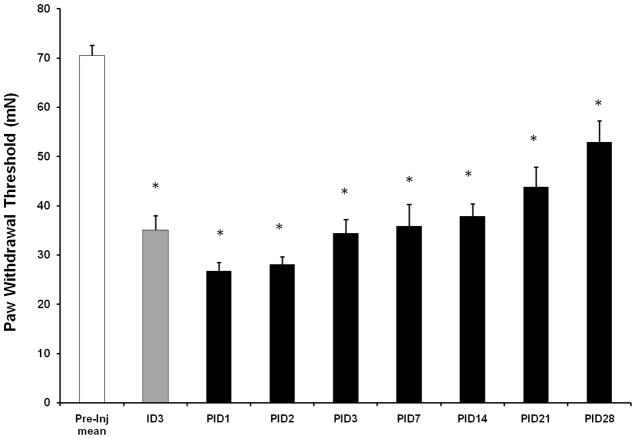
Tactile hyperalgesia as measured by von Frey filaments is a characteristic behavioral response that develops in rodents following repeated administration of morphine (Celerier et al., 2000; Gardell et al., 2002). Our behavioral assessment of tactile hyperalgesia was performed prior to the start of the injection paradigm, during the 5 day dosing regimen, and for 28 days following the repeated morphine treatment paradigm (Fig. 1). In our experiments, the mean paw withdrawal threshold (PWT) of the tested hind paws exhibited a decrease after only two daily morphine injections relative to pre-injection baseline PWT (ID3; 70.5±2.1 mN to 35.1±2.9 mN; n=12; p<0.0001). Statistically significant decreases in PWT were maintained until at least PID28 (Fig. 2). The changes in PWT observed at ID3 suggest that this nociceptive behavior is unaffiliated with morphine withdrawal signs such as jumping or wet dog shakes. Opioid withdrawal behaviors were observed between 24–48 hours following the last morphine injection (data not shown). Alterations in the paw withdrawal latency evoked by thermal stimulation were not observed with this dosing paradigm (data not shown).
Figure 2.
Repeated morphine treatment (10 mg/kg for 5 days) results in the development of tactile hyperalgesia as measured by von Frey filaments. Tactile hyperalgesic behavior persists for at least 28 days following the last morphine injection. ID, injection day, PID, post injection day (One-way ANOVA; *p<0.05, significant difference from baseline).
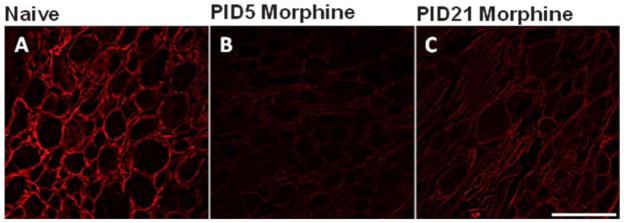
Repeated morphine treatment decreases CXCR4 immunoreactivity in the rat DRG
The CXCR4 antibody binds the N-glycosylation site g1 of human CXCR4. Although this site does not influence HIV-1 coreceptor function (Huskens et al., 2007), this antibody is an effective neutralizing antibody in tumor formation and angiogenesis (Katoh et al., 2010). In the adult rat dorsal root ganglia (DRG), CXCR4 expression is largely limited to presumptive nonmyelinating satellite glial cells (SGCs) of the DRG based on anatomical locale (Fig. 3A). In addition to the SGCs, an occasional neuron was also observed to express the CXCR4. This expression pattern in the DRG coincides with CXCR4 mRNA expression pattern seen previously (Bhangoo et al 2007). By comparison, very few CXCR4-immunoreactive, nonmyelinating SGCs were evident following repeated morphine treatment at PID5 (Fig. 3B). By PID21, CXCR4 immunoreactivity (-IR) in the nonmyelinating SGCs of the morphine treated rats was again evident (Fig. 3C). Concurrent with return of CXCR4-IR, SGCs in the DRG at PID21 was the gradual return of PWT to pre-treatment BL thresholds (Fig. 2).
Figure 3.
Repeated morphine injections reduces CXCR4-immunoreactivity (-IR) in satellite glial cells of rat lumbar DRG sections. Animals received repeated morphine injections (10 mg/kg for 5 days) and tissue was collected at 5 days (PID5) and 21 days (PID21) after the last morphine injection. A) CXCR4-IR (red) is largely restricted to satellite glial cells in the naïve rodent DRG. B) Following repeated morphine treatment, CXCR4-IR is reduced at PID5. C) By PID21 CXCR4-IR begins to return to naïve levels. Scale bar is 100 μm (n = 7 for day 5 and n = 7 for each day 21).
SDF1 mRNA is increased in sensory neurons following repeated morphine treatment and the protein is tonically released
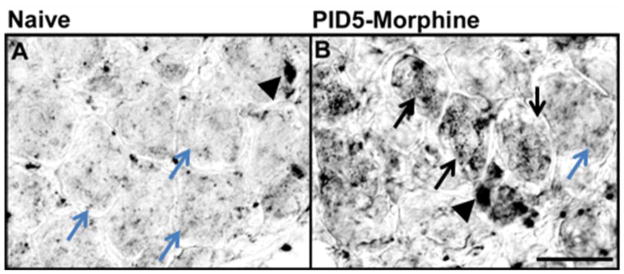
Given the apparent decline of CXCR4-IR in the DRG following the repeated morphine treatment paradigm, we determined whether SDF1 mRNA expression in the lumbar DRG was also altered by the dosing paradigm. We observed cellular expression patterns of SDF1 mRNA transcripts by in situ hybridization using digoxigenin-labeled riboprobes in relatively few cells of the saline-treated DRG (Fig. 4A). Following repeated morphine treatment both non-neuronal cells and numerous sensory neurons exhibited SDF1 mRNA transcripts (Fig. 4B).
Figure 4.
SDF1 mRNA expression is increased in the lumbar DRG following repeated morphine exposure. In situ hybridization was used to assess the expression pattern of SDF1 mRNA. A) High power photomicrograph of basal expression of SDF1 mRNA was observed in the lumbar DRG from saline injected rats in non-neuronal cells (black arrowhead indicates SDF1 mRNA transcripts in non-neuronal cell). Teal arrows indicate a lack of neuronal SDF1 mRNA transcripts. After a repeated morphine exposure, the level of SDF1 mRNA expression increased by post-injection day (PID) -5. SDF1 mRNA expression appears in both neuronal and non-neuronal cells. (Black arrows indicate neurons positive for SDF1 mRNA transcripts; arrowheads, presumptive glial cells positive for SDF1 mRNA transcripts). Scale bar A and B is 50 μm (n = 5 for each condition).
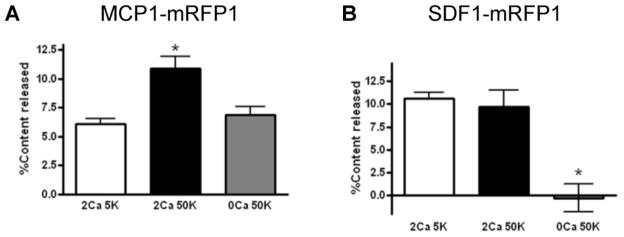
The observation that SDF1 is expressed in DRG neurons following repeated morphine treatment raises the possibility that the ligand may be tonically released from these cells. We therefore examined the release of SDF1 using the F11 cell line. This cell line was derived from DRG neurons and maintains many of the differentiated properties of these cells (Platika et al., 1985). We compared the characteristics of SDF1 release to those of MCP1/CCL2, another chemokine that has been shown to be expressed and released by DRG neurons (Jung et al., 2008). Following the expression of chemokine fluorescent fusion proteins (SDF1-RFP and MCP1-EGFP) we noted that the two chemokines localized to different sets of secretory vesicles (Supplementary Figure 1). We measured constitutive and K+ depolarization induced release of each chemokine from F11 cells using a sandwich ELISA, and observed that the patterns of release for the two chemokines were also different. Release of MCP1-RFP from differentiated F11 cells was significantly increased by depolarizing medium containing high K+ (Fig 5A). However, the release of SDF1-RFP was quite apparent under non depolarizing conditions and was not increased further by K+ depolarization (Fig 5B), suggesting that most SDF1 release was constitutive whereas significant portion of MCP1 release was regulated by neuronal depolarization. This result implies that once the expression of SDF1 has been increased in DRG neurons (see Fig 4), it will be constitutively released from these cells.
Figure 5.
Chemokine specific release from transfected F11 cells is by regulated (MCP1) or constitutive release mechanisms (SDF1). F11 DRG neurons were differentiated and then depolarized by high K stimulation (50 mM; 50K), either with or without extracellular Ca (2 mM or 0 mM; 2Ca or 0CA). The amount of MCP1-RFP released into the culture medium was measured by sandwich ELISA. The release of SDF1-mRFP1 was examined in the same manner as MCP1-mRFP1. Baseline levels of MCP1-RFP (A) or SDF1-RFP (B) in F11 cells prior to addition of 50mM K were 6.3 ± 0.7 or 10 ± 1.1% of total media, respectively (A sandwich ELISA; *p<0.01 vs. any other group, Newman-Keuls Multiple Comparison Test).
Repeated morphine treatment increases functional chemokine receptor expression by capsaicin sensitive DRG neurons
To further investigate the status of functional CXCR4 receptor expression in the DRG following repeated morphine treatment, we utilized Ca2+ imaging studies in acutely dissociated DRGs derived from animals subjected to repeated morphine conditions and naive controls. The time points used corresponded to time points used for immunohistochemical and behavioral assessment (Fig 2). The acutely dissociated DRG preparations were categorized into three neuronal and non-neuronal cell types: non-capsaicin sensitive neurons (high K and ATP responsive), capsaicin sensitive neurons (capsaicin, high K, and ATP responsive), and glia (ATP responsive only). These cell response criteria were chosen strictly as an indicator of the types of cells that may be affected by the repeated morphine treatment paradigm. The tested chemokines were selected so as to activate a wide spectrum of chemokine receptors known to be expressed by neurons and non-neuronal cells (CXCR4-SDF1/CXCL12, CXCR3-IP10/CXCL10, CCR2-MCP1/CCL2, CCR5-RANTES/CCL5). The chemokine concentration used for these experiments were based on their maximally effective concentrations using our previous observations on acutely dissociated DRGs (Bhangoo et al., 2007a; Bhangoo et al., 2007b).
Application of all tested chemokines produced [Ca2+]i changes in small numbers of neuronal and non-neuronal populations of cells derived from control DRGs (Table 1). Following exposure to repeated morphine treatment, we observed a significant increase in the chemokine responsiveness of non-capsaicin sensitive and capsaicin sensitive neurons. This included a robust increase in SDF1 responsive capsaicin sensitive neurons (p<0.0001). Hence it appears that DRG nociceptive neurons express more functional chemokine receptors, including CXCR4 receptors, following the repeated morphine treatment paradigm (Table 1).
Table 1.
Repeated morphine treatment increases the percentage of nociceptive neurons that respond to chemokine administration as indicated by a change in intracellular calcium. Daily morphine injections (10 mg/kg for 5 days) were administered to animals. Lumbar DRG were acutely dissociated from these animals 4–6 days following the last morphine injection. The most significant increase in chemokine-induced calcium responsiveness occurred in nociceptive neurons.
| Naïve | Morphine-Treated | |||||
|---|---|---|---|---|---|---|
| Non-capsaicin sensitive neurons | Capsaicin- sensitive neurons | Glia | Non-capsaicin sensitive neurons | Capsaicin- sensitive neurons | Glia | |
| SDF1 | 7% (6/85) | 7% (5/72) | 21% (9/44) | 15% (17/112) | 34% (25/73)** | 27% (13/49) |
| IP-10 | 4% (3/85) | 0% (0/72) | 2% (1/44) | 13% (14/112)* | 25% (18/73)** | 8% (4/49) |
| MCP1 | 4% (3/85) | 6% (4/72) | 9% (4/44) | 16% (18/112)** | 29% (21/73)** | 25% (12/49) |
| RANTES | 14% (12/85) | 4% (6/72) | 21% (9/44) | 17% (19/112) | 29% (21/73)** | 12% (6/49) |
p<0.001,
p<0.05, Chi-square with Yates correction
Reduced CXCR4 expression following repeated morphine treatment is abolished by AMD3100 treatment
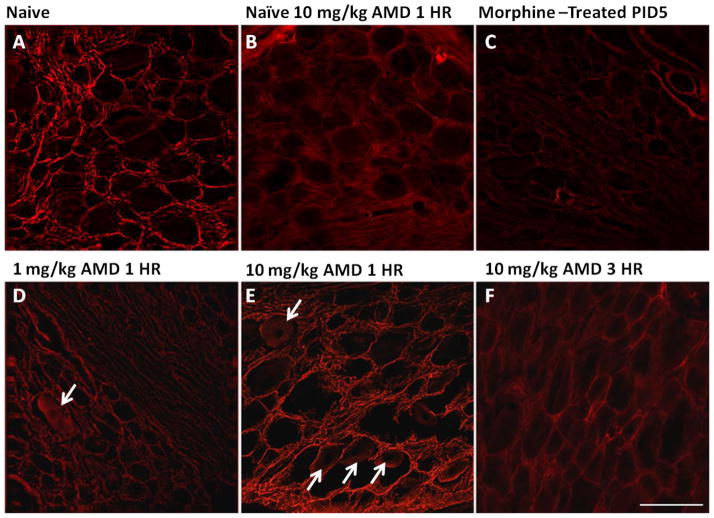
Given that tonic activation of CXCR4 by SDF1 leads to internalization of both chemokine and receptor (Burger and Kipps, 2006), it is entirely possible that the constitutive release of neuronal SDF1 and subsequent neuronal signaling via CXCR4 may result in diminished evidence of CXCR4-IR in the sensory ganglia of morphine treated rats (Fig 6C). This event would not be unlike previous reports in the dentate gyrus (Bhattacharyya et al., 2008; Kolodziej et al., 2008). Additional studies have shown that the binding capabilities of the CXCR4 antibody utilized for these studies can compete with SDF1 binding sites (Dubeykovskaya et al., 2009). To test this possibility we intraperitoneally administered the CXCR4 antagonist, AMD3100, at doses of 1 and 10 mg/kg and sacrificed the animals one hour later. Overall increases in CXCR4-IR binding were observed following AMD3100 administration in morphine treated animals at 10 mg/kg (Fig. 6E), but not 1 mg/kg (Fig. 6D). As evidence of the de novo SDF1 signaling via CXCR4, CXCR4-IR was also observed in numerous neurons (Fig. 6E). This data provides further support for the increased functional CXCR4 receptors observed in neurons (see Table 1). Three hours after AMD3100 administration, the CXCR4-IR was again qualitatively decreased in the DRG (Fig. 6F). Following administration of AMD3100 in the naïve animal (Fig. 6B), there was a noticeable decrease in CXCR4-IR when compared with the naïve animal (Fig. 6A). This is likely attributed to the competition that exists between AMD3100 and CXCR4 antibody for available receptor binding sites.
Figure 6.
The CXCR4 antagonist AMD3100 reverses loss of CXCR4 immunoreactivity in DRG derived from repeated morphine treated rats. Untreated rats (A), naive animal administered AMD3100 1 hour before sacrifice (B), repeated morphine treatment alone (i.p., 10 mg/kg, once daily for 5 days) (C) repeated morphine treatment in combination with different doses of AMD3100 1 hour before sacrifice (D, E) and 3 hours before sacrifice (F). Treatment with AMD3100 reverses repeated morphine treatment-induced loss of CXCR4 immunoreactivity at PID5 (C) in a dose-dependent manner (D, E). CXCR4-immunoreactivity (red label) in rat DRG sections following 10 mg/kg (E), but not 1 mg/kg (D) dose of AMD3100 returns CXCR4 immunoreactivity to levels observed in untreated control rats (A). White arrows indicate the presence of CXCR4-immunopositive neurons following AMD3100 treatment. Scale bar is 100 μm.
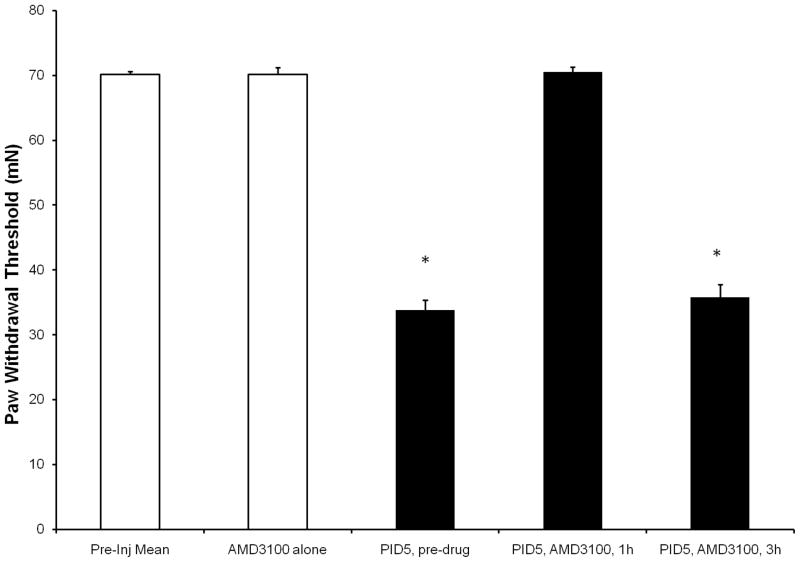
Decreased tactile hyperalgesia following intraperitoneal injection of CXCR4 antagonist, AMD3100
To determine whether SDF1/CXCR4 signaling was involved in OIH, we administered a single systemic dose of either vehicle or 10 mg/kg AMD3100 i.p. at PID5. Systemic injection of AMD3100 (10 mg/kg) in naïve rodents did not alter baseline PWT (70.5±2.1 mN; Fig 7). Vehicle injections in morphine treated rats did not alter PWT (data not shown). However, 1 hour after AMD3100 was administered to morphine treated rats, PWT returned to baseline (69.25±2.75 mN; p<0.01). The rapid onset of AMD3100 was short lived as PWTs returned to predosing levels by three hours (35.25±4.59 mN) (Fig 7). The return of morphine-induced behavior 3 hours post AMD3100 injection coincides with the returned loss of CXCR4-ir observed 3 hours post AMD3100 injection in rat DRG sections (Fig 6F).
Figure 7.
Morphine-induced tactile hyperalgesia in rodents is transiently reversed with CXCR4 antagonist, AMD3100 treatment. Five days following the last morphine injection (PID5) rats received an i.p. injection of AMD3100 (10 mg/kg) and tactile behavior was measured by von Frey filaments 1 and 3 hours post injection. AMD3100 treatment completely reversed morphine-induced tactile hyperalgesia by one hour post injection. Tactile hyperalgesia returned 3 hours following injection of AMD3100. *Significant difference from baseline; one-way ANOVA; p<0.01).
DISCUSSION
The experiments reported here demonstrate that following repeated morphine exposure, rodents exhibited a prolonged tactile hyperalgesia. This change in paw withdrawal threshold was maintained at least until PID28. Importantly, morphine-induced tactile hyperalgesia could be transiently reversed by a chemokine receptor antagonist that is specific for CXCR4 receptors. These results provide the first demonstration that morphine induced tactile hyperalgesic behavior in the rodent appears to be dependent on activation of CXCR4 receptors. Thus, morphine induced SDF1 signaling via CXCR4 receptors appears to change the balance between opioid analgesia and hyperalgesia.
OIH has been observed both clinically (Angst et al., 2003; Arner et al., 1988; Singla et al., 2007) and experimentally (Laulin et al., 1999; Woolf, 1981). Many explanations for this phenomenon have been suggested which have centered largely on changes within the central nervous system. These potential mechanisms include enhanced production/release of glutamate and neuropeptides in the spinal cord (Belanger et al., 2002; Ibuki et al., 2003; Mao et al., 2002), protein kinase C γ-induced signaling (Lim et al., 2005), spinal Ca2+/calmodulin-dependent protein kinase II alpha activity (Chen et al., 2010), enhanced descending facilitation of nociceptive pathways from the rostral ventromedial medulla (Vanderah et al., 2001) and activation of non-classical opioid receptors (Lewis et al., 2010). Alternative peripheral mechanisms also include sensitization of peripheral nociceptors (Aley and Levine, 1997; Liang et al., 2008).
The mechanisms responsible for morphine induced SDF1/CXCR4 signaling in primary sensory neurons of the DRG are largely unknown. Thus, the effects we have observed might be downstream of morphine’s interactions with μ-opioid receptors or possibly through interactions with TLR4 as has been recently suggested (Hutchinson et al., 2010). A number studies have been conducted demonstrating the ability of chemokines and opioid agonists administration to induce heterologous desensitization to their respective systems (Szabo et al, 2002, Chen et al, 2007). These studies have clearly shown that there is a relationship between the chemokine and opioid signaling pathways during acute administration. However, our results expand on these studies by suggesting that following the chronic administration of morphine produces an alteration in sensory neuron SDF1/CXCR4 signaling that lasts for at least 5 days after the last opioid injection.
With respect to morphine induced changes in CXCR4 activation, there appear to be two important changes in the status of SDF1/CXCR4 signaling within the DRG. First, it appears that greater CXCR4 signaling occurs following the dosing regimen. This is indicated by the fact that during OIH there is an AMD3100 reversible decline in CXCR4-IR in the DRG, presumably resulting from SDF1 activation of CXCR4 receptors followed by their internalization and recycling mechanisms, as previously observed in the dentate gyrus (Bhattacharyya et al., 2008; Kolodziej et al., 2008). Secondly, the increased SDF1/CXCR4 activation could result from enhanced tonic release of neuronal SDF1 whose expression was upregulated under these conditions, or some postsynaptic effect of morphine which produces enhanced CXCR4 desensitization in response to tonically released SDF1. In particular, the Ca2+ imaging experiments and the immunohistochemistry studies following AMD3100 administration clearly demonstrate that morphine treatment results in considerable degree of upregulated expression of CXCR4 by DRG nociceptors. Our results highlight the rapid timecourse of CXCR4 downregulation and recycling that occurs in the DRG and other cell types. Numerous studies have demonstrated that the time course and extent of CXCR4 recycling in different cell types is subject to a very large number of factors that can interact with the receptor and regulate the different stages of endocytosis, and recycling or degradation (Tarasova et al., 1998; Zhang et al., 2004). Thus it is becoming clear that regulating the levels of CXCR4 cell surface expression is one important mechanism of adjusting the signaling possibilities through this pathway. As activation of chemokine receptors expressed by DRG neurons produces excitation (White et al., 2007), it is likely the activation of these receptors by SDF1 contributes to the ectopic excitability of these neurons and produces AMD3100 reversible tactile hyperalgesia. The transient effect of AMD3100 may be explained by the short half life of 0.9 hours following a single administration (Hendrix et al., 2000). To this end, our observations support the growing body of literature that chemokines can act as neurotransmitters under some circumstances (White et al., 2007).
In the case of SDF1, its release mechanism may be unusual as it does not seem to require a depolarization induced increase in Ca2+, in contrast to the depolarization dependent release of MCP1. The different release mechanisms may be due to the observation that the two chemokines appear to be stored in separate subcellular compartments. Thus, it is possible that the SDF1 storage vesicles may be released by lower levels of Ca2+ or by low Ca2+ in cooperation with some other signaling mechanism. We demonstrated that morphine will increase the expression of SDF1 within DRG neurons. According to our data increased concentrations of SDF1 within DRG neurons should result in increased tonic release of the chemokine. The fact that appreciable levels of SDF1 may be tonically released both in the DRG (data herein) and the dentate gyrus suggest that SDF1 may generally be secreted in this way when utilized in the nervous system (Bhattacharyya et al., 2008; Kolodziej et al., 2008). Until recently, CXCR4 was known to be the only receptor for SDF1. This idea was challenged when the chemokine receptor, CXCR7, was shown to bind SDF1 (Balabanian et al., 2005). Initially described as a scavenger receptor, more recent interactions describe CXCR7 as possibly moderating the response of CXCR4 to SDF1 by internalizing the ligand (Zabel et al., 2009). Whether SDF1/CXCR7 activation serves to modulate OIH is unknown. However, we have observed that CXCR7 is expressed in the DRG of adult mice (unpublished observations) and so this remains a possibility.
In conclusion, ongoing SDF1/CXCR4 signaling within sensory neurons provides a mechanistic basis for understanding OIH modifications within the nervous system. Beyond its signaling relevance in the sensory neuron, the relationship between neuronal expression of SDF1/CXCR4 and tactile hyperalgesia in the rodent may imply that chemokine-sensitized sensory neurons may serve as an excitatory signal central to OIH. Thus, OIH represents another example of chronic pain behavior where chemokine signaling in DRG neurons has been observed to be upregulated (White et al., 2009), further highlighting the potential role of chemokine signaling in the generation of chronic pain.
Supplementary Material
MCP1 and SDF1 are sorted into different pools of vesicles in F11 cells. (A) MCP1-RFP (left panel), SDF1-RFP (right panel), or RFP alone was transfected into F11 cells. After 2 days, MCP1-RFP or SDF1-RFP was detected from the cell lysate by Western blot analyses using antibodies against MCP1, SDF1, and RFP. The precursor form (**) as well as the mature form, (*) in which the signal peptide has been cleaved, could be detected both by the RFP antibody and the chemokine antibodies (MCP1 or SDF1), indicating that the fusion of RFP to the C-termini of MCP1 and SDF1 does not alter their processing into the secretory pathway. (B) MCP1-EGFP was cotransfected with RFP alone (top panels), MCP1-RFP (middle panels), or SDF1-RFP (bottom panels). Unlike RFP alone which diffusively localized throughout the cell including the nucleus, MCP1-RFP and SDF1-RFP both exhibited perinuclear localization and punctate subcellular localization reminiscent of secretory vesicles. MCP1-EGFP and SDF1-RFP did not colocalize (bottom panels) unlike MCP1-EGFP and MCP1-RFP (middle panels), indicating that MCP1 and SDF1 are packaged into different pools of secretory vesicles.
Acknowledgments
This work was supported by grants from the National Institutes of Health (FAW - DA026040, NS049136; RJM - NS043095, DA013141) and the National Science Foundation (NMW - NSF Graduate STEM Fellows in K-12 Education).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Natalie M. Wilson, Department of Pharmacology, Loyola University, Chicago, Maywood, Illinois 60153
Hosung Jung, Department of Molecular Pharmacology and Biological Chemistry, Northwestern University, Chicago, IL 60611.
Matthew S. Ripsch, Department of Anesthesia, Indiana University, Indianapolis, IN 46208
Richard J. Miller, Department of Molecular Pharmacology and Biological Chemistry, Northwestern University, Chicago, IL 60611
Fletcher A. White, Department of Anesthesia, Program in Medical Neurosciences, Stark Neurosciences Research Institute, Indiana University, Indianapolis, IN 46208
References
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Dissociation of tolerance and dependence for opioid peripheral antinociception in rats. J Neurosci. 1997;17:3907–3912. doi: 10.1523/JNEUROSCI.17-10-03907.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57. doi: 10.1016/s0304-3959(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Arner S, Rawal N, Gustafsson LL. Clinical experience of long-term treatment with epidural and intrathecal opioids--a nationwide survey. Acta Anaesthesiol Scand. 1988;32:253–259. doi: 10.1111/j.1399-6576.1988.tb02725.x. [DOI] [PubMed] [Google Scholar]
- Avdoshina V, Biggio F, Palchik G, Campbell LA, Mocchetti I. Morphine induces the release of CCL5 from astrocytes: potential neuroprotective mechanism against the HIV protein gp120. Glia. 2010;58:1630–1639. doi: 10.1002/glia.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- Belanger S, Ma W, Chabot JG, Quirion R. Expression of calcitonin gene-related peptide, substance P and protein kinase C in cultured dorsal root ganglion neurons following chronic exposure to mu, delta and kappa opiates. Neuroscience. 2002;115:441–453. doi: 10.1016/s0306-4522(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, Li B, Monahan PE, Chan DM, Ripsch MS, White FA. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain. 2007a;3:38. doi: 10.1186/1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhangoo SK, Ren D, Miller RJ, Chan DM, Ripsch MS, Weiss C, McGinnis C, White FA. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav Immun. 2007b;21:581–591. doi: 10.1016/j.bbi.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya BJ, Banisadr G, Jung H, Ren D, Cronshaw DG, Zou Y, Miller RJ. The chemokine stromal cell-derived factor-1 regulates GABAergic inputs to neural progenitors in the postnatal dentate gyrus. J Neurosci. 2008;28:6720–6730. doi: 10.1523/JNEUROSCI.1677-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92:465–472. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang C, Wang ZJ. Ca2+/calmodulin-dependent protein kinase IIalpha is required for the initiation and maintenance of opioid-induced hyperalgesia. J Neurosci. 2010;30:38–46. doi: 10.1523/JNEUROSCI.4346-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Kremmer E, Schubel A, Breitfeld D, Kleinschmidt A, Nerl C, Bernhardt G, Lipp M. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J Immunol. 1998;160:1522–1531. [PubMed] [Google Scholar]
- Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP, Jr, Lai J, Porreca F. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci. 2002;22:6747–6755. doi: 10.1523/JNEUROSCI.22-15-06747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix CW, Flexner C, MacFarland RT, Giandomenico C, Fuchs EJ, Redpath E, Bridger G, Henson GW. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44:1667–1673. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskens D, Princen K, Schreiber M, Schols D. The role of N-glycosylation sites on the CXCR4 receptor for CXCL-12 binding and signaling and X4 HIV-1 viral infectivity. Virology. 2007;363:280–287. doi: 10.1016/j.virol.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008;22:1178–1189. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Rezvani N, Zhang Y, Wieseler JL, Somogyi AA, Yin H, Maier SF, Rice KC, Watkins LR. Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience. 2010;167:880–893. doi: 10.1016/j.neuroscience.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibuki T, Marsala M, Masuyama T, Yaksh TL. Spinal amino acid release and repeated withdrawal in spinal morphine tolerant rats. Br J Pharmacol. 2003;138:689–697. doi: 10.1038/sj.bjp.0705102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Bhangoo S, Banisadr G, Freitag C, Ren D, White FA, Miller RJ. Visualization of chemokine receptor activation in transgenic mice reveals peripheral activation of CCR2 receptors in states of neuropathic pain. J Neurosci. 2009;29:8051–8062. doi: 10.1523/JNEUROSCI.0485-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej A, Schulz S, Guyon A, Wu DF, Pfeiffer M, Odemis V, Hollt V, Stumm R. Tonic activation of CXC chemokine receptor 4 in immature granule cells supports neurogenesis in the adult dentate gyrus. J Neurosci. 2008;28:4488–4500. doi: 10.1523/JNEUROSCI.4721-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laulin JP, Celerier E, Larcher A, Le Moal M, Simonnet G. Opiate tolerance to daily heroin administration: an apparent phenomenon associated with enhanced pain sensitivity. Neuroscience. 1999;89:631–636. doi: 10.1016/s0306-4522(98)00652-6. [DOI] [PubMed] [Google Scholar]
- Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, Rice KC, Watkins LR. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience. 2010;165:569–583. doi: 10.1016/j.neuroscience.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang DY, Shi X, Qiao Y, Angst MS, Yeomans DC, Clark JD. Chronic morphine administration enhances nociceptive sensitivity and local cytokine production after incision. Mol Pain. 2008;4:7. doi: 10.1186/1744-8069-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G, Wang S, Zeng Q, Sung B, Yang L, Mao J. Expression of spinal NMDA receptor and PKCgamma after chronic morphine is regulated by spinal glucocorticoid receptor. J Neurosci. 2005;25:11145–11154. doi: 10.1523/JNEUROSCI.3768-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. PNAS. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, LaMotte RH. Enhanced excitability of dissociated primary sensory neurons after chronic compression of the dorsal root ganglion in the rat. Pain. 2005;113:106–112. doi: 10.1016/j.pain.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar Electrophysiological Changes in Axotomized and Neighboring Intact Dorsal Root Ganglion Neurons. J Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Aalinkeel R, Chawda RP, Sykes DE, Nair MP. Morphine modulates chemokine gene regulation in normal human astrocytes. Clin Immunol. 2005;115:323–332. doi: 10.1016/j.clim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, Demartino JA, Macintyre DE, Abbadie C. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience. 2007;149:706–714. doi: 10.1016/j.neuroscience.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O’Connor KA, Verge GM, Chapman G, Green P, Foster AC, Naeve GS, Maier SF, Watkins LR. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004;20:2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and Glycoprotein120 Produce Pain Hypersensitivity by Directly Exciting Primary Nociceptive Neurons. J Neurosci. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, King T, Vanderah TW, Malan TP, Jr, Hruby VJ, Porreca F. Antinociceptive and nociceptive actions of opioids. J Neurobiol. 2004;61:126–148. doi: 10.1002/neu.20091. [DOI] [PubMed] [Google Scholar]
- Platika D, Boulos MH, Baizer L, Fishman MC. Neuronal traits of clonal cell lines derived by fusion of dorsal root ganglia neurons with neuroblastoma cells. Proc Natl Acad Sci U S A. 1985;82:3499–3503. doi: 10.1073/pnas.82.10.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock RB, Hu S, Sheng WS, Peterson PK. Morphine stimulates CCL2 production by human neurons. J Neuroinflammation. 2006;3:32. doi: 10.1186/1742-2094-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabath R, Muller G, Schubel A, Kremmer E, Lipp M, Forster R. The murine chemokine receptor CXCR4 is tightly regulated during T cell development and activation. J Leukoc Biol. 1999;66:996–1004. doi: 10.1002/jlb.66.6.996. [DOI] [PubMed] [Google Scholar]
- Singla A, Stojanovic MP, Chen L, Mao J. A differential diagnosis of hyperalgesia, toxicity, and withdrawal from intrathecal morphine infusion. Anesth Analg. 2007;105:1816–1819. doi: 10.1213/01.ane.0000290338.39037.38. table of contents. [DOI] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309:99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Tarasova NI, Stauber RH, Michejda CJ. Spontaneous and ligand-induced trafficking of CXC-chemokine receptor 4. J Biol Chem. 1998;273:15883–15886. doi: 10.1074/jbc.273.26.15883. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Suenaga NM, Ossipov MH, Malan TP, Jr, Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci. 2001;21:279–286. doi: 10.1523/JNEUROSCI.21-01-00279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JG, Strong JA, Xie W, Yang RH, Coyle DE, Wick DM, Dorsey ED, Zhang JM. The chemokine CXCL1/growth related oncogene increases sodium currents and neuronal excitability in small diameter sensory neurons. Mol Pain. 2008;4:38. doi: 10.1186/1744-8069-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Feldman P, Miller RJ. Chemokine signaling and the management of neuropathic pain. Mol Interv. 2009;9:188–195. doi: 10.1124/mi.9.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:20151–20158. doi: 10.1073/pnas.0709250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, Lamotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. Intrathecal high dose morphine produces hyperalgesia in the rat. Brain Res. 1981;209:491–495. doi: 10.1016/0006-8993(81)90176-1. [DOI] [PubMed] [Google Scholar]
- Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142(3):809–822. 809–822. doi: 10.1016/j.neuroscience.2006.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel BA, Wang Y, Lewen S, Berahovich RD, Penfold ME, Zhang P, Powers J, Summers BC, Miao Z, Zhao B, Jalili A, Janowska-Wieczorek A, Jaen JC, Schall TJ. Elucidation of CXCR7-mediated signaling events and inhibition of CXCR4-mediated tumor cell transendothelial migration by CXCR7 ligands. J Immunol. 2009;183:3204–3211. doi: 10.4049/jimmunol.0900269. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Foudi A, Geay JF, Berthebaud M, Buet D, Jarrier P, Jalil A, Vainchenker W, Louache F. Intracellular localization and constitutive endocytosis of CXCR4 in human CD34+ hematopoietic progenitor cells. Stem Cells. 2004;22:1015–1029. doi: 10.1634/stemcells.22-6-1015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MCP1 and SDF1 are sorted into different pools of vesicles in F11 cells. (A) MCP1-RFP (left panel), SDF1-RFP (right panel), or RFP alone was transfected into F11 cells. After 2 days, MCP1-RFP or SDF1-RFP was detected from the cell lysate by Western blot analyses using antibodies against MCP1, SDF1, and RFP. The precursor form (**) as well as the mature form, (*) in which the signal peptide has been cleaved, could be detected both by the RFP antibody and the chemokine antibodies (MCP1 or SDF1), indicating that the fusion of RFP to the C-termini of MCP1 and SDF1 does not alter their processing into the secretory pathway. (B) MCP1-EGFP was cotransfected with RFP alone (top panels), MCP1-RFP (middle panels), or SDF1-RFP (bottom panels). Unlike RFP alone which diffusively localized throughout the cell including the nucleus, MCP1-RFP and SDF1-RFP both exhibited perinuclear localization and punctate subcellular localization reminiscent of secretory vesicles. MCP1-EGFP and SDF1-RFP did not colocalize (bottom panels) unlike MCP1-EGFP and MCP1-RFP (middle panels), indicating that MCP1 and SDF1 are packaged into different pools of secretory vesicles.