Abstract
This contribution reviews the evidence that has resolved the branching structure of the higher primate part of the tree of life and the substantial body of fossil evidence for human evolution. It considers some of the problems faced by those who try to interpret the taxonomy and systematics of the human fossil record. How do you to tell an early human taxon from one in a closely related clade? How do you determine the number of taxa represented in the human clade? How can homoplasy be recognized and factored into attempts to recover phylogeny?
Keywords: history, hominin
This contribution begins by considering two achievements relevant to reconstructing human evolution: resolving the branching structure of the higher primate part of the tree of life and the recovery of a substantial body of fossil evidence for human evolution (Fig. 1). The second part considers some of the challenges faced by those who try to interpret the taxonomy and systematics of the human fossil record. How do you to tell an early human taxon from one in a closely related clade? How many taxa are represented in the human clade? How to recognize and cope with homoplasy in and around the human clade? The third part of this contribution suggests how new ways of gathering morphologic data may help researchers overcome some of the challenges referred to above.
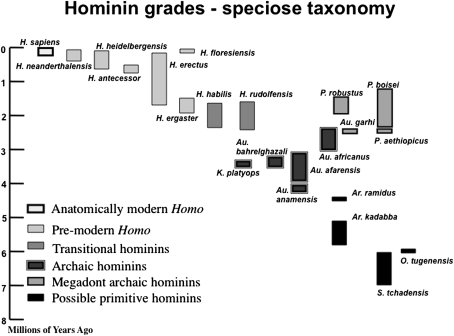
Fig. 1.
Taxa recognized in a typical speciose hominin taxonomy. Note that the height of the columns reflects either uncertainties about the temporal age of a taxon, or in cases where there are well-dated horizons at several sites it reflects current evidence about the earliest (called the first appearance datum, or FAD) and the most recent (called the last appearance datum, or LAD) fossil evidence of any particular hominin taxon. However, the time between the FAD and the LAD is likely to be represent the minimum time span of a taxon, because it is highly unlikely that the fossil record of a taxon, and particularly the relatively sparse fossil records of early hominin taxa, include the earliest and most recent fossil evidence of a taxon. The newest archaic hominin taxon, the ca.1.9 Ma Australopithecus sediba, would occupy the space just above the box for Au. africanus.
Achievements
Resolving the Branching Structure of the Higher Primate Part of the Tree of Life.
The first systematic investigation of the relationships among the living great ape taxa was in 1863 by Thomas Henry Huxley. In the second of the three essays in his Evidence as to Man’s Place in Nature (1) Huxley addresses “the place which Man occupies in nature and of his relations to the universe of things” (p. 57). After reviewing the evidence Huxley concluded that “the structural differences which separate Man from the Gorilla and the Chimpanzee are not so great as those which separate the Gorilla from the lower apes” (p. 103). The next significant advance in our understanding of the relationships among the great apes came when developments in biochemistry and immunology made in the first half of the 20th century allowed the focus of the search for evidence to be expanded beyond traditional gross morphologic evidence to the properties of molecules (2–4), to the structure of proteins (5), and most recently to the composition of the genome (6, 7). A recent molecular supermatrix analysis based on 15 mitochondrial and 43 nuclear genes (8) provides strong support for modern humans being more closely related to chimpanzees and bonobos than to any other living great ape. Gorillas are more distantly related to modern humans than to chimpanzees and bonobos, and a recent report notwithstanding (9), the orangutan is the great ape most distantly related to modern humans; these relationships can also be expressed in the form [Pongo, (Gorilla, (Pan, Homo))]. This recent molecular supermatrix analysis effectively removes any reasonable doubt that extant Pan species are more closely related to modern humans than they are to extant Gorilla taxa. This is an important advance in our understanding of human evolution because, in combination with the principle of parsimony, it enables researchers to generate hypotheses about character evolution within the great ape clade. These hypotheses can then be used as the equivalent of a null hypothesis when considering where to place newly discovered fossil great ape taxa.
The Human Fossil Record.
The fossil record of the human clade consists of fossil evidence for modern humans plus that of all extinct taxa that are hypothesized to be more closely related to modern humans than to any other living taxon. Not so long ago nearly all researchers were comfortable with according the human clade the status of a family, the Hominidae, with the nonhuman extant great apes (i.e., chimpanzees, bonobos, gorillas, and orangutans) placed in a separate family, the Pongidae. But given the abundant evidence for a closer relationship between Pan and Homo than between Pan and Gorilla (see above), many researchers have concluded that the human clade should be distinguished beneath the level of the family in the Linnaean hierarchy. These researchers now use the family Hominidae for all of the extant great apes (including modern humans), and they use the subfamily Homininae either for Gorilla, Pan, and Homo (e.g., ref. 10) or for just Pan and Homo. Some of the researchers who opt for the former, more inclusive, solution use the tribe Hominini for both the chimpanzee/bonobo and the human clades and treat the human clade as a subtribe, the Hominina (so individuals and taxa within it are referred to as “homininans”). Other researchers use the tribe Hominini to refer to just the human clade. Thus, in this scheme the taxa within the human clade are referred to as “hominin” taxa, and the individual fossils in those taxa are called “hominin” fossils. In the first, more inclusive, scheme taxa in the chimpanzee/bonobo clade are referred to as “paninans”, whereas in the second scheme they are referred to as “panins”. In this review we use the second scheme and its “hominin/panin” terminology.
Classifying Hominins.
Whereas clades reflect the process of evolutionary history, the grade concept (11) is based on assessing the outcome of evolutionary history. Taxa in the same grade eat the same sorts of foods and share the same posture and mode(s) of locomotion; no store is set by how they came by those behaviors. The judgment about how different two diets or two locomotor strategies have to be before the taxa concerned are considered to belong to different grades is still a subjective one, but until we can be sure we are generating reliable hypotheses about the relationships among hominin taxa the grade concept helps sort taxa into broad functional categories, albeit sometimes frustratingly “fuzzy” (e.g., where to place Homo floresiensis) ones. The grades used in this review are “Anatomically modern Homo”, “Premodern Homo”, “Transitional hominins”, “Archaic hominins”, “Megadont archaic hominins”, and “Possible hominins”. We use a relatively speciose taxonomic hypothesis (Table 1) and present the species within each grade in the historical order the taxa were recognized, not in their temporal order.
Table 1.
Hominin species in a speciose taxonomy sorted into six grade groupings
| Grade | Species included in a splitting taxonomy |
| Possible hominins | Ar. ramidus* |
| O. tugenensis | |
| S. tchadensis | |
| Ar. kadabba | |
| Archaic hominins | Au. africanus* |
| Au. afarensis* | |
| Au. bahrelgazali | |
| Au. anamensis | |
| Au. garhi | |
| K. platyops | |
| Au. sediba | |
| Megadont archaic hominins | P. robustus* |
| P. boisei | |
| P. aethiopicus | |
| Transitional hominins | H. habilis* |
| H. rudolfensis | |
| Premodern Homo | H. erectus* |
| H. neanderthalensis | |
| H. heidelbergensis | |
| H. ergaster | |
| H. antecessor | |
| H. floresiensis | |
| Anatomically modern Homo | H. sapiens* |
*A lumping taxonomy might only recognize these species.
Discovering Fossil Hominins.
The earliest discoveries of fossil hominins were chance events at isolated sites. The circumstances of the first hominin fossil to be discovered, at Goat’s Hole Cave in Paviland on the Gower Peninsula in South Wales, was typical. Local people interested in natural history were exploring coastal caves when they found animal fossils and later a burial of a fossil hominin. In some cases individuals have taken advantage of what otherwise were not auspicious circumstances to look for fossils. Captain Brome was an ardent fossil collector, so when he was posted to the Rock of Gibraltar as the Governor of the Military Prison he thought it more sensible to put the prisoners to work excavating rather than just breaking rocks, and it was during excavations at Forbe’s Quarry using the labor of military prisoners that the Gibraltar Neanderthal cranium was recovered. Its discovery was announced at a meeting of the Gibraltar Scientific Society in 1848, and the records of scientific and natural history societies (e.g., the East Africa and Uganda Natural History Society) have proved to be a rich source of information about possible hominin fossil sites.
The first researcher to deliberately travel to another continent in search of hominin fossils was Eugène Dubois. Dubois’ interest in human evolution came from reading Charles Darwin and especially Ernst Haeckel, who was convinced that our ancestors had emerged in the jungles of Asia. The discovery of primate fossils in the Siwalik Hills of India by Theobald in 1878 (and their description by Lydekker in 1879) encouraged Dubois’ conviction that the creatures Haeckel had referred to as the Pithecanthropi in the History of Creation might be found in the Dutch East Indies. After resigning his university post in 1887 Dubois enlisted as a medical officer in the Royal Dutch East Indies Army and began his search for the evolutionary link between apes and modern humans. He found a piece of hominin lower jaw at Kedung Brubus, Java, in November 1890, and in 1891 Dubois began excavating along the banks of the Solo River near the village of Trinil. In September of that year a hominin molar was discovered, and in October Dubois’ team of excavators found the hominin skullcap that was to become the type specimen of Pithecanthropus erectus, later designated as Homo erectus.
The first important hominin fossil discoveries in Africa, the cranium found at Broken Hill (now Kabwe) in 1921 and the Taung child’s skull recovered in 1924, were both chance discoveries, and it took more than 50 years for the search for hominin sites in Africa to become more systematic. In the late 1980s The Paleoanthropological Inventory of Ethiopia (12) successfully located potential hominin fossil sites on a regional scale. Led by Berhane Asfaw, the inventory used Landsat thematic mapping (TM) and large-format camera high-resolution images. The former measures the intensity of reflected sunlight in seven wavebands, and the resulting color images were used to identify the distinctive ash layers, or tephra, that are typically found in the types of strata that contain Plio-Pleistocene fossils. The two sets of data were used to identify promising sedimentary basins, which were explored by vehicle and on foot to verify the presence of potential sites. At least two sources of hominin fossils in the Ethiopian Rift Valley, the site complex within the Kesem-Kebena basin in the north and the site of Fejej in the south, were located this way.
Anatomically Modern Homo.
This grade includes hominin fossil evidence that is indistinguishable from the morphology found in at least one regional population of modern humans. Modern humans belong to the species Homo sapiens Linnaeus 1758, and the earliest H. sapiens fossils are dated to just less than 200 ka. Since the initial discovery of a fossil modern human in 1822–1823 in Goat’s Hole Cave in Wales, fossil evidence of H. sapiens has been recovered from sites on all continents except Antarctica. Many H. sapiens fossils are burials, so the fossil evidence is abundant and generally in good condition. The earliest evidence of anatomically modern human morphology in the fossil record comes from Omo Kibish in Ethiopia (13), and it is also in Africa that we find evidence of crania that are generally more robust and archaic-looking than those of anatomically modern humans, yet they are not archaic or derived enough to justify being allocated to Homo heidelbergensis or to Homo neanderthalensis (see below). Specimens in this category include Jebel Irhoud from North Africa, Laetoli 18 from East Africa, and Florisbad and the Cave of Hearths from southern Africa. There is undoubtedly a gradation in morphology that makes it difficult to set the boundary between anatomically modern humans and H. heidelbergensis, but the variation in the later Homo fossil record is too great to be accommodated in a single taxon. Researchers who wish to make a distinction between fossils such as Florisbad and Laetoli 18 and subrecent and living modern humans either do so taxonomically by referring the former specimens to a separate species, Homo helmei Dreyer 1935, or they distinguish them informally as “archaic Homo sapiens”.
Premodern Homo.
This grade grouping includes Pleistocene Homo taxa that lack the derived and distinctive size and shape of the modern human cranium and postcranial skeleton. Some individuals in these taxa possessed only medium-sized brains, yet they exhibit modern human-like body proportions. The first fossil taxon to be recognized in this grade is H. neanderthalensis King 1864, whose temporal range is ca. 200–28 ka (but if the Sima de los Huesos material is included then it is ca. >450–28 ka). The first example of H. neanderthalensis to be discovered was a child’s cranium recovered in 1829 from a cave in Belgium called Engis, but the type specimen, the Neanderthal 1 skeleton, was found in 1856 at the Kleine Feldhofer Grotte in Elberfield, Germany. Fossil evidence for H. neanderthalensis has since been found in Europe as well as in the Near East, the Levant, and Western Asia. The distinctive features of the cranium of H. neanderthalensis include thick, double-arched brow ridges, a face that projects anteriorly in the midline, a large nose, laterally projecting and rounded parietal bones, and a rounded, posteriorly projecting occipital bone. Mandibular and dental features include a retromolar space, distinctively high incidences of some nonmetrical dental traits, and thinner tooth enamel than in modern humans. The average endocranial volume of H. neanderthalensis was the same as that of contemporary H. sapiens, but it is larger than that of living modern humans. Postcranially, H. neanderthalensis individuals were stout with a broad rib cage, a long clavicle, a wide pelvis, and limb bones that are generally robust with well-developed muscle insertions. The distal extremities tend to be short compared with most modern H. sapiens, but H. neanderthalensis was evidently an obligate biped. The generally well-marked muscle attachments and the relative thickness of long bone shafts point to a strenuous lifestyle. For some researchers the H. neanderthalensis hypodigm is restricted to fossils from Europe and the Near East that used to be referred to as “Classic” Neanderthals. Others interpret the taxon more inclusively and include fossil evidence that is generally older and less distinctive (e.g., Steinheim, Swanscombe, and from the Sima de los Huesos). The first DNA recovered from a fossil hominin was from the type specimen of H. neanderthalensis (14), and recently Green et al. (15) sequenced the complete mtDNA of a specimen from Vindija. Briggs et al. (16) reported the mtDNA sequences of five individuals and concluded that genetic diversity within H. neanderthalensis was substantially lower than that in modern humans.
The next fossil hominin taxon in this grade to be discovered was H. erectus (Dubois 1893) Weidenreich 1940. Its temporal range is ca. 1.8 Ma to ca. 30 ka. The initial discovery at Kedung Brubus was made in 1890, but the type specimen was recovered in 1891 from Trinil. H. erectus is known from sites in Indonesia (e.g., Trinil, Sangiran, and Sambungmachan), China (e.g., Zhoukoudian and Lantian), and Africa (e.g., Olduvai Gorge and Melka Kunturé). The hypodigm of H. erectus is dominated by cranial remains; there is some postcranial evidence but very few hand and foot fossils. Crania belonging to H. erectus have a low vault, a substantial more-or-less continuous torus above the orbits, and a sharply angulated occipital region, and the inner and outer tables of the cranial vault are thick. The body of the mandible is more robust than that of H. sapiens, it lacks a chin, and the mandibular tooth crowns are generally larger and the premolar roots more complicated than those of modern humans. The limb proportions of H. erectus are modern human-like, but the shafts of the long bones are robust and those of the lower limb are flattened (the femur from front to back and the tibia from side to side) relative to those of modern humans. Overall, the cortical bone of H. erectus is thicker than is the case in modern humans. All of the dental and cranial evidence points to a modern human-like diet for H. erectus, and the postcranial elements are consistent with an upright posture and obligate bipedalism.
The next taxon recognized within the genus Homo was H. heidelbergensis Schoetensack 1908. The initial discovery and the type specimen, the Mauer 1 adult mandible, was found in 1907 in a sand quarry near Heidelberg, Germany. Other evidence included in the taxon comes from sites in Europe (e.g., Petralona), the Near East (e.g., Zuttiyeh), Africa (e.g., Kabwe and Bodo), China (e.g., Dali, Jinniushan, Xujiayao, and Yunxian), possibly India (Hathnora), and depending on how inclusively H. neanderthalensis is interpreted, from the Sima de los Huesos at Atapuerca, Spain. The temporal range of H. heidelbergensis is ca. 600–100 ka. What sets this material apart from H. sapiens and H. neanderthalensis is the morphology of the cranium and the robusticity of the postcranial skeleton. Some H. heidelbergensis have endocranial volumes as large as those of some modern humans, but they are always more robustly built, with a thickened occipital region and a projecting face and with large separate ridges above the orbits. Compared with H. erectus the parietals are expanded, the occipital is more rounded, and the frontal bone is broader. H. heidelbergensis is the earliest hominin to have a brain as large as that of some anatomically modern Homo, and its postcranial skeleton suggests that its robust long bones and large lower limb joints were well suited to long-distance travel. Researchers who see the African part of this hypodigm as distinctive refer it to a separate species, Homo rhodesiensis. Those who see the European component of the H. heidelbergensis hypodigm (e.g., Sima de los Huesos) as already showing signs of H. neanderthalensis autapomorphies would sink it into the latter taxon.
Those who support Homo ergaster Groves and Mazák 1975 as a separate species point to features that are more primitive than H. erectus (e.g., mandibular premolar root and crown morphology) and those that are less derived than H. erectus (e.g., vault and cranial base morphology) (17). However, many researchers are unconvinced there are sufficient consistent differences between the hypodigms of H. ergaster and H. erectus (18) to justify the former being a separate species. The taxon Homo antecessor Bermúdez de Castro et al. 1997 was introduced for hominins recovered from the Gran Dolina site at Atapuerca, Spain. The researchers who found the remains claim the combination of a modern human-like facial morphology with large and relatively primitive tooth crowns and roots is not seen in H. heidelbergensis (see below), and they see H. antecessor and not H. heidelbergensis as the likely recent common ancestor of H. neanderthalensis and H. sapiens.
The most recent taxon to be added to the genus Homo is H. floresiensis Brown et al. 2004. It is only known from Liang Bua, a cave in Flores, and its temporal range is ca. 74–17 ka. The initial discovery and type specimen is LB1 an associated partial adult skeleton, but a second associated skeleton and close to 100 separate fossils representing up to 10 individuals have subsequently been recovered. This hominin displays a unique combination of early Homo-like cranial and dental morphology, a hitherto unknown suite of pelvic and femoral features, a small brain (ca. 417 cm3), a small body mass (25–30 kg), and small stature (1 m). When it was first described researchers interpreted it as a H. erectus, or H. erectus-like, taxon that had undergone endemic dwarfing, but more recently researchers have suggested it could be a dwarfed Homo habilis-like transitional grade taxon (19, 20).
Transitional Hominins.
For the purposes of this review H. habilis and Homo rudolfensis are retained within Homo, but they are treated separately from the premodern Homo grade (21). The taxon H. habilis Leakey, Tobias, and Napier 1964 was introduced for fossils recovered from Olduvai Gorge, Tanzania. The rest of the H. habilis hypodigm consists of other fossils found at Olduvai Gorge and of fossils from Ethiopia (Omo Shungura and Hadar) and Kenya (Koobi Fora and perhaps Chemeron). Some have claimed that there is also evidence of H. habilis in southern Africa at Sterkfontein, Swartkrans, and Drimolen. The H. habilis hypodigm consists of mostly cranial and dental evidence; only a few postcranial bones can be confidently assigned to that taxon (see below). The endocranial volume of H. habilis ranges from ca. 500 cm3 to ca. 700 cm3, but most commentators opt for an upper limit closer to 600 cm3. All of the crania are wider at the base than across the vault, but the face is broadest in its upper part. The only postcranial fossils that can be assigned to H. habilis with confidence are the postcranial bones associated with the type specimen, OH 7 and the associated skeleton, OH 62: isolated postcranial bones from Olduvai Gorge assigned to H. habilis (e.g., OH 10) could also belong to P. boisei (see below). If OH 62 is representative of H. habilis the skeletal evidence suggests that its limb proportions and locomotion (22) and carpal bones (23) were archaic hominin-like, and the curvature and well-developed muscle markings on the phalanges of OH 7 indicate that H. habilis was capable of powerful grasping. The inference that H. habilis used spoken language was based on links between endocranial morphology and language comprehension and production that are no longer supported by comparative evidence.
Some researchers suggest the transitional hominin grade contains a second taxon, H. rudolfensis (Alexeev 1986) sensu Wood 1992 (17), but not all researchers are convinced the scale and nature of the variation within early Homo justifies the recognition of two taxa (24, 25). Its temporal range would be ca. 2.4–1.6 Ma, and aside from the lectotype KNM-ER 1470 from Koobi Fora, Kenya, the members of the proposed hypodigm include other fossils recovered from Koobi Fora and those from Chemeron, Kenya and Uraha, Malawi. Compared with H. habilis the absolute size of the brain case in H. rudolfensis is greater, and its face is widest in its midpart whereas the face of H. habilis is widest superiorly. Despite the absolute size of the H. rudolfensis brain (ca. 725 cm3), when it is related to estimates of body mass based on orbit size the brain is not substantially larger than those of the archaic hominins. The distinctive face of H. rudolfensis is combined with a robust mandibular corpus and mandibular postcanine teeth with larger, broader, crowns and more complex premolar root systems than those of H. habilis. At present no postcranial remains can be reliably linked with H. rudolfensis. The size of the mandible and postcanine teeth suggest that its diet made similar mechanical demands as those of the archaic hominins (see below).
Archaic Hominins.
This grade includes all of the remaining unambiguously hominin taxa not conventionally included in Homo and Paranthropus (see below). The first taxon to be recognized in this grade was Australopithecus africanus Dart 1925. The type specimen, Taung 1, a juvenile skull with a partial natural endocast, was recovered in 1924 from the limeworks at Taung (formerly Taungs), now in South Africa. Most of the other fossil evidence for Au. africanus comes from two caves, Sterkfontein and Makapansgat, with other evidence coming from the Gladysvale cave. Unless the associated skeleton StW 573 from Mb 2 (26) and 12 hominin fossils recovered from the Jacovec Cavern (27) expands it, the temporal range of Au. africanus is ca. 3–2.4 Ma. The cranium, mandible, and the dentition are well sampled; the postcranial skeleton, and particularly the axial skeleton, is less well represented, but there is at least one specimen of each of the long bones, but many of the fossils have been crushed and deformed by rocks falling on the bones before they were fully fossilized. The picture that has emerged from morphologic and functional analyses suggests that although Au. africanus was capable of walking bipedally it was probably more arboreally adapted (i.e., it was a facultative and not an obligate biped) than other archaic hominin taxa, such as Australopithecus afarensis. It had relatively large chewing teeth, and apart from the reduced canines the skull is relatively ape-like. Its mean endocranial volume is ca. 460 cm3. The Sterkfontein evidence suggests that males and females of Au. africanus differed substantially in body size but probably not to the degree they did in Au. afarensis.
The taxon Au. afarensis Johanson, White, and Coppens 1978 is only known from East African sites. The type specimen is an adult mandible, LH 4, recovered in 1974 from Laetoli, Tanzania. The largest contribution to the Au. afarensis hypodigm comes from Hadar, but other sites in Ethiopia (Belohdelie, Brown Sands, Dikika, Fejej, Maka, and White Sands) and sites in Kenya (Allia Bay, Koobi Fora, Tabarin, and West Turkana) have contributed to it. The temporal range of Au. afarensis is ca. 3.7–3 Ma (ca. 4–3 Ma if the presence of Au. afarensis is confirmed at Belohdelie and Fejej). The Au. afarensis hypodigm includes a well-preserved skull, partial and fragmented crania, many lower jaws, sufficient limb bones to be able to estimate stature and body mass (28), and a specimen, A.L.-288, that preserves ca. 25% of the skeleton of an adult female. Most body mass estimates range from ca. 30–45 kg, and the endocranial volume of Au. afarensis is estimated to be between 400 and 550 cm3. It has smaller incisors than those of extant chimps/bonobos, but its premolars and molars are relatively larger. Comparative evidence suggests that the hind limbs of A.L.-288 are substantially shorter than those of a modern human of similar stature. The appearance of the pelvis and the relatively short lower limb suggests that although Au. afarensis was capable of bipedal walking it was not adapted for long-range bipedalism. This indirect evidence for the locomotion of Au. afarensis is complemented by the discovery at Laetoli of several trails of fossil footprints. These provide very graphic direct evidence that at least one contemporary hominin, presumably Au. afarensis, but possibly Kenyanthropus platyops (see below), was capable of bipedal locomotion, but the Laetoli prints are less modern human-like than the 1.5-Ma footprints from Koobi Fora presumed to be of premodern Homo (29). The upper limb, especially the hand (23) and the shoulder girdle, of Au. afarensis retains morphology that most likely reflects a significant element of arboreal locomotion. Although a recent study argues that sexual dimorphism in this taxon is relatively poorly developed, most researchers interpret it as showing substantial sexual dimorphism (e.g., 28).
The taxon Australopithecus anamensis Leakey, Feibel, McDougall, and Walker 1995 is also presently restricted to East Africa. The type specimen, KNM-KP 29281, was recovered in 1994 from Kanapoi, Kenya. Other sites contributing to the hypodigm are Allia Bay, also in Kenya, and the Middle Awash study area, Ethiopia. The temporal range of Au. anamensis is ca. 4.2–3.9 Ma. The fossil evidence consists of jaws, teeth, and postcranial elements from the upper and lower limbs. Most of the differences between Au. anamensis and Au. afarensis relate to details of the dentition. In some respects the teeth of Au. anamensis are more primitive than those of Au. afarensis (e.g., the asymmetry of the premolar crowns and the relatively simple crowns of the deciduous first mandibular molars), but in others (e.g., the low cross-sectional profiles and bulging sides of the molar crowns) they show some similarities to Paranthropus (see below). The upper limb remains are similar to those of Au. afarensis, and a tibia attributed to Au. anamensis has features associated with bipedality. Researchers familiar with the fossil evidence have suggested that Au. anamensis and Au. afarensis are most likely time successive taxa within a single lineage (30), with the Laetoli hypodigm of the former taxon intermediate between Au. anamensis and the Hadar hypodigm of Au. afarensis. The taxon Australopithecus bahrelghazali Brunet et al. 1996 is most likely a regional variant of Au. afarensis (28). But the Chad discovery substantially extended the geographic range of early hominins and reminds us that important events in human evolution (e.g., speciation, extinction) may have been taking place well away from the very small (relative to the size of the African continent) regions sampled by the existing early hominin sites.
The most recently recognized taxon in this grade is Kenyanthropus platyops Leakey et al. 2001. The type specimen, KNM-WT 40000, a ca. 3.5–3.3-Ma relatively complete but distorted cranium, was found in 1999 at Lomekwi, West Turkana, Kenya. The main reasons Leakey et al. (31) did not assign this material to Au. afarensis are its reduced subnasal prognathism, anteriorly situated zygomatic root, flat and vertically orientated malar region, relatively small but thick-enameled molars, and the unusually small M1 compared with the size of the P4 and M3. Despite this unique combination of facial and dental morphology, White (32) claims the new taxon is not justified because the cranium could be a distorted Au. afarensis cranium, but this explanation is not consistent with the small size of the postcanine teeth.
Megadont Archaic Hominins.
This grade includes hominin taxa conventionally included in the genus Paranthropus and one Australopithecus species, Australopithecus garhi. The genus Paranthropus, into which Zinjanthropus and Paraustralopithecus are subsumed, was reintroduced when cladistic analyses suggested that the first three species discussed in this section most likely formed a clade. The term megadontia refers to both the absolute size of the postcanine teeth, as well as their relative size when compared with the length of the anterior tooth row.
The taxon Paranthropus robustus Broom 1938 was established to accommodate TM 1517, an associated skeleton recovered in 1938 from the southern African site of Kromdraai B. Other sites that contribute to the P. robustus hypodigm are Swartkrans, Gondolin, Drimolen, and Cooper’s caves, all situated in the Blauuwbank Valley near Johannesburg, South Africa. The dentition is well represented in the hypodigm of P. robustus, but although some of the cranial remains are well preserved most are crushed or distorted and the postcranial skeleton is not well represented. Research at Drimolen was only initiated in 1992 yet already more than 80 hominin specimens (many of them otherwise rare juvenile specimens) have been recovered and it promises to be a rich source of evidence about P. robustus. The temporal range of the taxon is ca. 2.0–1.5 Ma. The brain, face, and chewing teeth of P. robustus are on average larger than those of Au. africanus, yet the incisor teeth are smaller. The morphology of the pelvis and the hip joint is much like that of Au. africanus; Paranthropus robustus was most likely capable of bipedal walking, but it was probably not an obligate biped. It has been suggested that the thumb of P. robustus would have been capable of the type of grip necessary for the manufacture of simple stone tool, but this claim has not been accepted by all researchers. A second southern African taxon, Paranthropus crassidens, was proposed for the part of the P. robustus hypodigm that comes from Swartkrans, but almost all researchers consider that taxon to be a junior synonym of P. robustus.
In 1959 Louis Leakey suggested that a new genus and species, Zinjanthropus boisei Leakey 1959, was needed to accommodate OH 5, a subadult cranium recovered in 1959 from Bed I, Olduvai Gorge, Tanzania. A year later John Robinson suggested that Z. boisei be subsumed into the genus Paranthropus as Paranthropus boisei, and in 1967 Phillip Tobias suggested it should be subsumed into Australopithecus, as Australopithecus boisei; in this review it is referred to as Paranthropus boisei (Leakey 1959) Robinson 1960. Additional fossils from Olduvai Gorge have subsequently been added to the hypodigm, as well as fossil evidence from the East African sites of Peninj, Omo Shungura, Konso, Koobi Fora, Chesowanja, and West Turkana. The temporal range of the taxon is ca. 2.3–1.4 Ma. P. boisei has a comprehensive craniodental fossil record, comprising several skulls and well-preserved crania, many mandibles, and isolated teeth. There is evidence of both large- and small-bodied individuals, and the range of the size difference suggests a substantial degree of body size sexual dimorphism despite its modest canine sexual dimorphism. P. boisei is the only hominin to combine a wide, flat, face, massive premolars and molars, small anterior teeth, and a modest endocranial volume (ca. 480 cm3). The face of P. boisei is larger and wider than that of P. robustus, yet their brain volumes are similar. The mandible of P. boisei has a larger and wider body or corpus than any other hominin (see Paranthropus aethiopicus below) and the tooth crowns apparently grow at a faster rate than has been recorded for any other early hominin. There is no postcranial evidence that can with certainty be attributed to P. boisei (33), but some of the postcranial fossils from Bed I at Olduvai Gorge currently attributed to H. habilis may belong to P. boisei. The fossil record of P. boisei extends across approximately 1 million years, during which there is little evidence of any substantial change in the size or shape of the components of the cranium, mandible, and dentition (34).
The taxon Paranthropus aethiopicus (Arambourg and Coppens, 1968) Chamberlain and Wood 1985 was introduced as Paraustralopithecus aethiopicus to accommodate Omo 18.18 (or 18.1967.18), an edentulous adult mandible recovered in 1967 from Omo Shungura in Ethiopia. Other contributions to the hypodigm of this taxon have come from West Turkana and Kenya and probably also from Melema, Malawi, and Laetoli, Tanzania. The hypodigm is small, but it includes a well-preserved adult cranium from West Turkana (KNM-WT 17000) together with mandibles (e.g., KNM-WT 16005) and isolated teeth from Omo Shungura (some also assign the Omo 338y-6 cranium to this taxon). No published postcranial fossils have been assigned to P. aethiopicus, but a proximal tibia from Laetoli may belong to P. aethiopicus. The temporal range of P. aethiopicus is ca. 2.5–2.3 Ma. P. aethiopicus is similar to P. boisei (see above) except that the face is more prognathic, the cranial base is less flexed, the incisors are larger, and the postcanine teeth are not so large or morphologically specialized.
The most recent addition to the megadont archaic hominin hypodigm is Australopithecus garhi Asfaw et al. 1999 (35). It was introduced to accommodate specimens recovered in 1997 from Aramis in the Middle Awash study area, Ethiopia. The hypodigm is presently restricted to fossils recovered from the Hata Member in the Middle Awash study area, Ethiopia. The type specimen, the ca. 2.5-Ma BOU-VP-12/130, combines a primitive cranium with large-crowned postcanine teeth. However, unlike P. boisei (see above), the incisors and canines are large and the enamel apparently lacks the extreme thickness seen in the latter taxon. A partial skeleton with a long femur and forearm was found nearby but is not associated with the type cranium, and it has not been formerly assigned to Au. garhi. If the type specimen of P. aethiopicus (Omo 18.18) belongs to the same hypodigm as the mandibles that seem to match the Au. garhi cranium, then P. aethiopicus would have priority as the name for the hypodigm presently attributed to Au. garhi.
Possible Hominins.
This group includes taxa that may belong to the human clade. However, most of the taxonomic assignments reviewed below take little or no account of the possibility that cranial and dental features assumed to be diagnostic of the human clade (e.g., foramen magnum position and canine size and shape) may be homoplasies (see below). Thus, for the reasons set out in the next section rather than assume these taxa are hominins, the prudent course is to consider them as candidates for being early members of the human clade.
The type specimen, ARA-VP-6/1, of the taxon now called Ardipithecus ramidus (White, Suwa, and Asfaw 1994) White, Suwa, and Asfaw 1995 (36, 37) was recovered in 1993 from Aramis, in the Middle Awash study area, Ethiopia. All of the hypodigm comes from the sites of Aramis, Kuseralee Dora, and Sagantole in the Central Awash Complex, Middle Awash study area or from sites in the Gona study area, also in Ethiopia. Its temporal range is ca. 4.5–4.3 Ma. The published evidence consists of two associated skeletons, one of which (ARA-VP-6/500) includes a partial skull and especially good preservation of the hands and feet, a piece of the base of the cranium, mandibles, associated dentitions, isolated teeth, two vertebrae, a first rib, fragments of long bones, and other isolated postcranial fossils. The remains attributed to Ar. ramidus share some features in common with living species of Pan, others that are shared with the African apes in general, and several dental and cranial features that it is claimed are shared only with later hominins, such as Au. afarensis. Thus, the discoverers have suggested that the taxon belongs within the human clade (38). The body mass of the presumed female partial skeleton has been estimated to be ca. 50 kg, the canines are claimed to be less projecting than those of common chimpanzees, and the degree of functional honing is modest. The postcanine teeth are relatively small, and the thin enamel covering on the teeth suggests that the diet of Ar. ramidus may have been closer to that of chimps/bonobos than to later hominins. Despite having ape-like hands and feet, the position of the foramen magnum and the reconstruction of the poorly preserved pelvic bone have been interpreted as confirmation that Ar. ramidus was an upright biped.
The type specimen of the taxon Orrorin tugenensis Senut et al. 2001 is BAR 1000’00, a fragmentary mandible, recovered in 2000 from the locality called Kapsomin at Baringo in the Tugen Hills, Kenya. The 13 specimens in the hypodigm all come from four ca. 6-Ma localities in the Lukeino Formation. The morphology of three femoral fragments has been interpreted as suggesting that O. tugenensis is an obligate biped (39, 40), but other researchers interpret the radiographs and CT scans of the femoral neck as indicating a mix of bipedal and nonbipedal locomotion (41). Otherwise, the discoverers admit that much of the critical dental morphology is “ape-like” (39).
Sahelanthropus tchadensis Brunet et al. 2002 is the taxon name given to fossils recovered in 2001 from the ca. 7 Ma Anthrocotheriid Unit at Toros-Menalla, Chad. The type specimen is TM266-01-060-1, a plastically deformed adult cranium, and the rest of the small hypodigm consists of mandibles and some teeth; there is no published postcranial evidence. S. tchadensis is a chimp/bonobo-sized animal displaying a novel combination of primitive and derived features. Much about the base and vault of the cranium is chimp/bonobo-like, but the relatively anterior placement of the foramen magnum is hominin-like. The supraorbital torus, lack of a muzzle, apically worn canines, low, rounded, molar cusps, relatively thick tooth enamel, and relatively thick mandibular corpus all suggest that S. tchadensis does not belong in the Pan clade (42).
The most recently recognized taxon in the “possible hominin” grade category is Ardipithecus kadabba Haile-Selassie, Suwa, and White 2004 (43, 44). The new species was established to accommodate cranial and postcranial remains announced in 2001 and six new dental specimens announced in 2004. All of the hypodigm was recovered from five ca. 5.8-5.2-Ma localities in the Middle Awash study area, Ethiopia. The main differences between Ar. kadabba and Ar. ramidus are that the apical crests of the upper canine crown of the former taxon are longer and the P3 crown outline of Ar. kadabba is more asymmetrical than that of Ar. ramidus. Haile-Selassie et al. (2004) suggest that there is a morphocline in upper canine morphology, with Ar. kadabba exhibiting the most ape-like morphology and Ar. ramidus and Au. afarensis interpreted as becoming progressively more like the lower and more asymmetric crowns of later hominins. The proximal foot phalanx (AME-VP-1/71) combines an ape-like curvature with a proximal joint surface that is like that of Au. afarensis (43). These four taxa could be primitive hominins, but they could also belong to separate clades of apes that share homoplasies with the human clade.
Challenges
Differences Between an Early-Hominin Taxon and a Taxon in a Closely Related Clade.
The differences between the skeletons of living modern humans and their closest living relatives, common chimpanzees and bonobos, are particularly marked in the brain case, dentition, face and base of the cranium, and in the hand, pelvis, knee, and the foot. But the differences between the first, or stem, hominins and the first, or stem, panins were likely to have been much more subtle. In what ways would the earliest hominins have differed from the last common ancestor (LCA) of chimps/bonobos and modern humans, and from the earliest panins? Compared with panins they would most likely have had smaller canine teeth, larger chewing teeth, and thicker lower jaws. There would also have been some changes in the skull, axial skeleton, and the limbs linked with more time spent upright and with a greater dependence on the hind limbs for bipedal locomotion. These changes would have included, among other things, a forward shift in the foramen magnum, adjustments to the pelvis, habitually more extended knees, and a more stable foot.
But all this assumes there is no homoplasy (see below) and that the only options for a 8–5-Ma African higher primate are being the LCA of modern humans and chimps/bonobos, a primitive hominin, or a primitive panin. It is, however, also possible that such a creature may belong to an extinct clade (e.g., a sister taxon of the LCA of modern humans and chimps/bonobos, or the sister taxon of the earliest hominins or panins).
Species Recognition in the Hominin Clade.
It is difficult to apply process-related species definitions to the fossil record (45). Most paleoanthropologists use one version or other, of one of the species concepts in the pattern-related subcategory [i.e., the phenetic species concept (PeSC), the phylogenetic species concept (PySC), or the monophyletic species concept (MSC)]. These concepts all focus on an organism’s hard-tissue phenotype (thus they are sometimes referred to as morphospecies concepts), but each of the concepts emphasizes a different aspect of the phenotype. The PeSC as interpreted by Sokal and Crovello (46) gives equal weight to all aspects of the phenotype. It is based on a matrix that records the expression of each phenotypic character for each specimen, and then multivariate analysis is used to detect clusters of individual specimens that share the same, or similar, character expressions. In contrast, the version of the PySC introduced by Cracraft (47) emphasizes the unique suite of derived and primitive characters that defines each species. According to Nixon and Wheeler (48) in such a scheme a species is “the smallest aggregation of populations diagnosable by a unique combination of character states”. The problem with the third species concept in the pattern-related subcategory, the MSC, is that it assumes researchers know which characters are uniquely derived. But to know this you must have performed a cladistic analysis (see below), and to do that you must have already decided on the taxa to include in the analysis.
In practice most paleoanthropologists use one or other version of the PySC. They search for the smallest cluster of individual organisms that is “diagnosable” on the basis of the preserved morphology, and then they seek to recognize taxa that embrace the levels of variation that are seen in living taxa. So why do competent researchers disagree about how many species should be recognized within the hominin fossil record? Researchers who favor a more anagenetic (or gradualistic) interpretation of the fossil record tend to stress the importance of continuities in the fossil record and opt for fewer species, whereas researchers who favor a more cladogenetic (or punctuated equilibrium) interpretation of the fossil record tend to stress the importance of discontinuities within the fossil record and opt for more speciose taxonomic hypotheses. These latter interpretations are referred to as taxic because they stress the importance of taxonomy for the interpretation of evolutionary history. But when all is said and done a taxonomy is just a hypothesis; it is not written on stone tablets.
Recognizing and Coping with Homoplasy in and Around the Hominin Clade.
Homoplasy, that is shared characters not inherited from the most recent common ancestor of the taxa that express them, complicates attempts to reconstruct phylogenetic relationships because homoplasies give the impression that two taxa are more closely related than they really are. There are many aspects of morphology that might represent homoplasy in the hominin clade. The genus Paranthropus is based primarily on craniofacial morphology that suggests an adaptation to feeding on hard or abrasive foods. These features include postcanine megadontia, thick enamel, and changes to the zygomatic and other cranial bones that result in an improved mechanical advantage for chewing on the postcanine tooth crowns. If these adaptations of the megadont archaic hominins were inherited from a recent common ancestor then a separate Paranthropus genus is justified; however, if they occurred independently in the P. aethiopicus and P. boisei lineage in East Africa and in the P. robustus lineage in southern Africa, and thus were examples of homoplasy, then a separate genus would not be justified. Locomotor and postural adaptations of the postcranial skeleton are another possible source of homoplasy. It is generally assumed that bipedal locomotion, and the morphological changes it entails, arose only once during the course of hominin evolution. But there is no logical reason to exclude the hypothesis that bipedality arose more than once in the hominin clade (49), indeed the evidence that there may have been more than one pattern of limb proportions among the taxa within the archaic hominin grade (50) lends support to at least some aspects the hypothesis that bipedalism may be homoplasic within the hominin clade. Moreover, there is no a priori reason to conclude that facultative bipedalism was confined to the hominin clade.
What should the null hypothesis be with respect to homoplasy in the great ape part of the tree of life? Should similar characters be considered homologous until proven otherwise? Or is the possibility of homoplasy sufficiently likely that a more prudent null hypothesis would be that all similarites are considered at least as likely to be homoplasies as homologies? The extent of homoplasy in other mammalian lineages as well as in other primate groups suggests that homoplasy should be given more consideration than it has when developing taxonomic hypotheses about new great ape taxa.
Opportunities
Advances in Data Capture.
Obviously new fossil discoveries provide additional evidence about human evolution, but additional evidence can also be extracted from the existing fossil record. Ionizing radiation has been harnessed to provide images of the internal structure of fossil hominins for more than 70 years, but recently clinical imaging techniques in the form of computed tomography (CT) have been used to access hitherto unavailable morphology (51). Techniques such as microCT (52), confocal microscopy (53), and synchroton radiation microtomography (SR-μCT) (54) have been used to image the internal macro- and microstructure of higher primates and hominin fossils (55). MicroCT provides better images of small structures such as teeth than regular CT, and it is now being used to capture the detailed morphology of the enamel–dentine junction (EDJ) (56, 57). This has a 2-fold advantage. First, it provides morphologic information in 3D about the EDJ, a structure that was hitherto inaccessible without destructively sectioning a tooth crown, and second, by focusing on the morphology of EDJ it means that worn teeth, which may preserve very little in the way of detailed outer enamel surface morphology, can be used to generate information about the range of intraspecific variation in hominin fossil taxa (58).
All three of these imaging techniques have, and will, prove to be especially useful for helping to sort homoplasies from homologies. For example, what may superficially look like a dental homology (e.g., the possession of an apparently similar shared nonmetrical trait in two taxa) at the outer enamel surface may turn out to be a homoplasy if by using microCT it can be shown that it has significantly different manifestations at the EDJ (59). Information about the ontogeny of dental enamel (e.g., enamel secretion rates, extension rates, the lifespan of ameloblasts) at the cellular level has been obtained from naturally or deliberately sectioned fossil teeth (60), and now both confocal microscopy (53) and SR-μCT (54, 55) can be used to investigate the dental microstructure of fossil teeth nondestructively. This means, for example, that it is possible to investigate whether the thick enamel shared by two hominin taxa has the same developmental basis (61). If its ontogeny is the same, then it is not possible to refute the hypothesis that the shared enamel thickness is a homology, but if the pattern of cellular activity involved in the ontogeny of the thick enamel is different in the two taxa, then the hypothesis that thick enamel is a homology can be refuted.
Conclusions
In the third essay in his Evidence as to Man’s Place in Nature (1) Huxley discusses just two hominin fossils, the child’s cranium from Engis and the adult cranium from the Kleine Feldhofer Grotte. Huxley’s analysis of the two fossil crania is perceptive and prescient. He suggests that even though the Neanderthal remains are “the most pithecoid of known human skulls”, he goes on to write that “in no sense … can the Neanderthal bones be regarded as the remains of a human being intermediate between Man and Apes”, and he notes that if we want to seek “the fossilized bones of an Ape more anthropoid, or a Man more pithecoid” than the Neanderthal cranium, then researchers need to look “in still older strata” (p. 159).
Since 1863 much progress has been made in both the accumulation of fossil evidence germane to human evolution, in the techniques used to capture morphologic information from that fossil evidence, and in the methods used to analyze those data. To better understand our evolutionary history these three enterprises—the acquisition of new fossil evidence (62), the extraction of data from that evidence, and its analysis—must all advance. Effective techniques for data acquisition and analysis in the absence of fossils and an abundance of fossil evidence in the absence of effective data acquisition and analytical techniques are of little value. We trust that when the time comes to celebrate the 150th anniversary of the publication of Darwin’s Descent of Man in 2021, significant progress will have been made in all three of these endeavors.
Acknowledgments
Thanks to the George Washington University Vice President for Academic Affairs and to the George Washington University Signature Program for support. Research by graduate students funded by National Science Foundation Integrative Graduate Education and Research Traineeship Program Grants DGE-0801634 and 9987590 has been cited in this review.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution IV: The Human Condition,” held December 10–12, 2009, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/SACKLER_Human_Condition.
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Huxley TH. Evidence as to Man’s place in Nature. London: Williams and Norgate; 1863. [Google Scholar]
- 2.Goodman M. Man’s place in the phylogeny of the primates as reflected in serum Proteins. In: Washburn SL, editor. Classification and Human Evolution. Chicago: Aldine; 1963. pp. 204–234. [Google Scholar]
- 3.Zuckerkandl E. Perspectives in molecular anthropology. In: Washburn SL, editor. Classification and Human Evolution. Chicago: Aldine; 1963. pp. 243–272. [Google Scholar]
- 4.Sarich V, Wilson AC. Immunological time scale for hominid evolution. Science. 1967;158:1200–1203. doi: 10.1126/science.158.3805.1200. [DOI] [PubMed] [Google Scholar]
- 5.King MC, Wilson AC. Evolution in two levels in humans and chimpanzees. Science. 1975;188:107–116. doi: 10.1126/science.1090005. [DOI] [PubMed] [Google Scholar]
- 6.Ruvolo M. Molecular phylogeny of the hominoids: Inferences from multiple independent DNA sequence data sets. Mol Biol Evol. 1997;14:248–265. doi: 10.1093/oxfordjournals.molbev.a025761. [DOI] [PubMed] [Google Scholar]
- 7.Bradley B. Reconstructing phylogenies and phenotypes: A molecular view of human evolution. J Anat. 2008;212:337–353. doi: 10.1111/j.1469-7580.2007.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabre PH, Rodrigues A, Douzery EJP. Patterns of macroevolution among Primates inferred from a supermatrix of mitochondrial and nuclear DNA. Mol Phylogenet Evol. 2009;53:808–825. doi: 10.1016/j.ympev.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Grehan JR, Schwartz JH. Evolution of the second orangutan: Phylogeny and biogeography of hominid origins. J Biogeogr. 2009;31:1263–1266. [Google Scholar]
- 10.Harrison T. Apes among the tangled branches of human origins. Science. 2010;327:532–534. doi: 10.1126/science.1184703. [DOI] [PubMed] [Google Scholar]
- 11.Huxley JS. Evolutionary processes and taxonomy with special reference to grades. Upps Univ Arssks. 1958:21–38. [Google Scholar]
- 12.Asfaw B, Ebinger C, Harding D, White T, WoldeGabriel W. Space-based imagery in paleoanthropological research: An Ethiopian example. Natl Geogr Res. 1990;6:418–434. [Google Scholar]
- 13.McDougall I, Brown FH, Fleagle JG. Stratigraphic placement and age of modern humans from Kibish, Ethiopia. Nature. 2005;433:733–736. doi: 10.1038/nature03258. [DOI] [PubMed] [Google Scholar]
- 14.Krings M, et al. Neandertal DNA sequences and the origin of modern humans. Cell. 1997;90:19–30. doi: 10.1016/s0092-8674(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 15.Green R, et al. A complete neandertal mitochondrial genome sequence determined by high-throughput sequencing. Cell. 2008;134:416–426. doi: 10.1016/j.cell.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briggs AW, et al. Targeted retrieval and analysis of five Neandertal mtDNA genomes. Science. 2009;325:318–321. doi: 10.1126/science.1174462. [DOI] [PubMed] [Google Scholar]
- 17.Wood B. Vol 4. Oxford: Clarendon Press; 1994. Hominid Cranial Remains, Koobi Fora Research Project; pp. 1–492. [Google Scholar]
- 18.Spoor F, et al. Implications of new early Homo fossils from Ileret, east of Lake Turkana, Kenya. Nature. 2007;448:688–691. doi: 10.1038/nature05986. [DOI] [PubMed] [Google Scholar]
- 19.Brown P, Moeda T. Liang Bua Homo floresiensis mandibles and mandibular teeth: A contribution to the comparative morphology of a new hominin species. J Hum Evol. 2009;57:571–596. doi: 10.1016/j.jhevol.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Morwood MJ, Jungers WL. Conclusions: Implications of the Liang Bua excavations for hominin evolution and biogeography. J Hum Evol. 2009;57:640–648. doi: 10.1016/j.jhevol.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Wood BA, Collard M. The human genus. Science. 1999;284:65–71. doi: 10.1126/science.284.5411.65. [DOI] [PubMed] [Google Scholar]
- 22.Ruff C. Relative limb strength and locomotion in Homo habilis. Am J Phys Anthropol. 2009;138:90–100. doi: 10.1002/ajpa.20907. [DOI] [PubMed] [Google Scholar]
- 23.Tocheri MW, Orr CM, Larson SG. The primitive wrist of Homo floresiensis and its implications for hominin evolution. Science. 2007;317:1743–1745. doi: 10.1126/science.1147143. [DOI] [PubMed] [Google Scholar]
- 24.Suwa G, White TD, Howell FC. Mandibular postcanine dentition from the Shungura Formation, Ethiopia: Crown morphology, taxonomic allocations, and Plio-Pleistocene hominid evolution. Am J Phys Anthropol. 1996;101:247–282. doi: 10.1002/(SICI)1096-8644(199610)101:2<247::AID-AJPA9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 25.Tobias PV. Olduvai Gorge. Vol. 4. Cambridge, UK: Cambridge Univ Press; 1991. The skulls, endocasts and teeth of Homo habilis; pp. 1–921. [Google Scholar]
- 26.Clarke RJ. Latest information on Sterkfontein’s Australopithecus skeleton and a new look at Australopithecus. S Afr J Sci. 2008;104:443–449. [Google Scholar]
- 27.Partridge TC, Granger DE, Caffee MW, Clarke RJ. Lower Pliocene hominid remains from Sterkfontein. Science. 2003;300:607–612. doi: 10.1126/science.1081651. [DOI] [PubMed] [Google Scholar]
- 28.Kimbel WH, Delezene LK. “Lucy” redux: A review of research on Australopithecus afarensis. Am J Phys Anthropol. 2009;52:2–48. doi: 10.1002/ajpa.21183. [DOI] [PubMed] [Google Scholar]
- 29.Bennett MR. Early hominin foot morphology based on 1.5-million-year-old footprints from Ileret, Kenya. Science. 2009;323:1197–1201. doi: 10.1126/science.1168132. [DOI] [PubMed] [Google Scholar]
- 30.Kimbel WH, et al. Was Australopithecus anamenis ancestral to Australopithecus afarensis? A case of anagenesis in the hominin fossil record. J Hum Evol. 2006;51:134–152. doi: 10.1016/j.jhevol.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Leakey MG, et al. New hominin genus from eastern Africa shows diverse middle Pliocene lineages. Nature. 2001;410:433–440. doi: 10.1038/35068500. [DOI] [PubMed] [Google Scholar]
- 32.White TD. Early hominids—diversity or distortion? Science. 2003;299:1994–1997. doi: 10.1126/science.1078294. [DOI] [PubMed] [Google Scholar]
- 33.Wood B, Constantino P. Paranthropus boisei: Fifty years of evidence and analysis. Am J Phys Anthropol. 2009;50:106–132. doi: 10.1002/ajpa.20732. [DOI] [PubMed] [Google Scholar]
- 34.Wood BA, Wood CW, Konigsberg LW. Paranthropus boisei—an example of evolutionary stasis? Am J Phys Anthropol. 1994;95:117–136. doi: 10.1002/ajpa.1330950202. [DOI] [PubMed] [Google Scholar]
- 35.Asfaw B, et al. Australopithecus garhi: A new species of early hominid from Ethiopia. Science. 1999;284:629–635. doi: 10.1126/science.284.5414.629. [DOI] [PubMed] [Google Scholar]
- 36.White TD, Suwa G, Asfaw B. Australopithecus ramidus, a new species of early hominid from Aramis, Ethiopia. Nature. 1994;371:306–312. doi: 10.1038/371306a0. [DOI] [PubMed] [Google Scholar]
- 37.White TD, Suwa G, Asfaw B. Australopithecus ramidus, a new species of early hominid from Aramis, Ethiopia— a corrigendum. Nature. 1995;375:88. doi: 10.1038/375088a0. [DOI] [PubMed] [Google Scholar]
- 38.White TD, et al. Ardipithecus ramidus and the paleobiology of early hominins. Science. 2009;326:75–86. [PubMed] [Google Scholar]
- 39.Senut B, Pickford M, Gommery D, Mein P, Cheboi K, Coppens Y. First hominid from the Miocene (Lukeino Formation, Kenya) Comptes Rendus de l’Academie des Sciences. 2001;332:137–144. [Google Scholar]
- 40.Richmond BG, Jungers WL. Orrorin tugenensis femoral morphology and the evolution of hominin bipedalism. Science. 2008;319:1662–1665. doi: 10.1126/science.1154197. [DOI] [PubMed] [Google Scholar]
- 41.Ohman JC, Lovejoy CO, White T. Questions about Orrorin tugenensis. Science. 2005;307:845. doi: 10.1126/science.307.5711.845b. [DOI] [PubMed] [Google Scholar]
- 42.Brunet M, et al. A new hominid from the Upper Miocene of Chad, Central Africa. Nature. 2002;418:145–151. doi: 10.1038/nature00879. [DOI] [PubMed] [Google Scholar]
- 43.Haile-Selassie Y. Late Miocene hominids from the Middle Awash, Ethiopia. Nature. 2001;412:178–181. doi: 10.1038/35084063. [DOI] [PubMed] [Google Scholar]
- 44.Haile-Selassie Y, Asfaw B, White TD. Hominid cranial remains from Upper Pleistocene deposits at Aduma, Middle Awash, Ethiopia. Am J Phys Anthropol. 2004;123:1–10. doi: 10.1002/ajpa.10330. [DOI] [PubMed] [Google Scholar]
- 45.Smith AB. Systematics and the Fossil Record: Documenting Evolutionary Patterns. Oxford: Blackwell; 1994. [Google Scholar]
- 46.Sokal RR, Crovello TJ. The biological species concept: A critical evaluation. Am Nat. 1970;104:127–153. [Google Scholar]
- 47.Cracraft J. Species concepts and speciation analysis. In: Johnson RF, editor. Current Ornithology. New York: Plenum Press; 1983. [Google Scholar]
- 48.Nixon KC, Wheeler QD. An amplification of the phylogenetic species concept. Cladistics. 1990;6:211–233. [Google Scholar]
- 49.Wood B. Investigating human evolutionary history. J Anat. 2000;197:1–17. doi: 10.1046/j.1469-7580.2000.19710003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green DJ, Gordon AD, Richmond BG. Limb-size proportions in Australopithecus afarensis and Australopithecus africanus. J Hum Evol. 2007;52:187–200. doi: 10.1016/j.jhevol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Spoor F, Jeffery N, Zonneveld F. Using diagnostic radiology in human evolutionary studies. J Anat. 2000;197:61–76. doi: 10.1046/j.1469-7580.2000.19710061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kono R. Molar enamel thickness and distribution patterns in extant primates and humans: New insights based on a 3-dimensional whole crown perspective. Anthropol Sci. 2004;112:121–146. [Google Scholar]
- 53.Bromage TG, Perez-Ochoa A, Boyde A. Portable confocal microscope reveals fossil hominid microstructure. Microscop Anal. 2005;19:5–7. [Google Scholar]
- 54.Tafforeau P, Smith TM. Nondestructive imaging of hominoid dental microstructure using phase contrast X-ray synchrotron microtomography. J Hum Evol. 2007;54:272–278. doi: 10.1016/j.jhevol.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 55.Smith T, Tafforeau P. New visions of dental tissue research: tooth development, chemistry, and structure. Evol Anthropol. 2008;17:213–226. [Google Scholar]
- 56.Skinner M, et al. Dental trait expression at the enamel-dentine junction of lower molars in extant and fossil hominoids. J Hum Evol. 2008;54:173–186. doi: 10.1016/j.jhevol.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 57.Braga J, et al. The enamel-dentine junction in the postcanine dentition of Australopithecus africanus: Intra-individual metameric and antimreric variation. J Anat. 2010;216:62–79. doi: 10.1111/j.1469-7580.2009.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skinner M, Wood BA, Hublin J-JH. Enamel-dentine junction (EDJ) morphology distinguishes the lower molars of Australopithecus africanus and Paranthropus robustus. J Hum Evol. 2008;55:979–988. doi: 10.1016/j.jhevol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 59.Skinner M, Wood BA, Hublin J-JH. Protostylid expression at the enamel-dentine junction and enamel surface of mandibular molars of Paranthropus robustus and Australopithecus africanus. J Hum Evol. 2009;56:76–85. doi: 10.1016/j.jhevol.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 60.Dean C. Progress in understanding hominoid dental development. J Anat. 2000;197:77–101. doi: 10.1046/j.1469-7580.2000.19710077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lacruz RS, Dean MC, Ramirez-Rossi F, Bromage TG. Megadontia, striae periodicity and patterns of enamel secretion in Plio-Pleistocene fossil hominins. J Anat. 2008;213:148–158. doi: 10.1111/j.1469-7580.2008.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berger LR, et al. Australopithecus sediba: A new species of Homo-like australopith from South Africa. Science. 2010;328:195–204. doi: 10.1126/science.1184944. [DOI] [PubMed] [Google Scholar]