Abstract
Background
The world is facing a novel H1N1 pandemic. A pandemic scare with a similar virus in 1976 resulted in the vaccination of nearly 45 million persons. We hypothesized that prior receipt of the 1976 “swine flu” vaccine would enhance immune responses to the 2009 novel H1N1 strain.
Methods
A prospective, volunteer sample of employees 55 years of age and older at a children’s cancer hospital in August of 2009 was assessed for antibody responses to the 2009 pandemic H1N1 influenza virus and the 2008-2009 seasonal H1N1 influenza virus.
Results
Antibody responses by hemagglutination-inhibition assay were high against both the seasonal (89.7% had a titer considered seroprotective) and pandemic (88.8% had a seroprotective titer) H1N1 viruses. These antibodies were effective at neutralizing the seasonal H1N1 virus in 68.1% of participants (titer ≥ 40), but only 18.1% had detectable neutralizing titers against the pandemic H1N1. Of 116 participants, 46 (39.7%) received the 1976 “swine flu” vaccine. Receipt of this vaccine significantly enhanced neutralization responses as 8 of 46 (17.4%) vaccine recipients had titers ≥ 160 compared to only 3 of 70 (4.3%) who did not receive the vaccine (P = 0.018 by chi-squared test).
Conclusions
In this cohort, persons 55 years and older had evidence of robust immunity to the 2008-2009 seasonal H1N1 virus. These antibodies were cross-reactive but non-neutralizing against the 2009 pandemic H1N1 strain. Receipt of a vaccine to a related virus significantly enhanced the neutralization capacity of these responses, suggesting homologous vaccination against the 2009 pandemic H1N1 would have a similar effect.
Background
The world is facing a new influenza pandemic for the first time in more than 40 years [1]. A triple reassortant influenza virus of the H1N1 subtype emerged from an animal reservoir in early 2009 and has spread worldwide. This strain’s H1 hemagglutinin (HA), the surface protein against which the majority of our neutralizing antibody responses are directed, is derived from the “classic” swine lineage [2]. These “classic” H1N1 viruses are endemic in pigs and are derived from a progenitor strain that entered the swine population in 1918, the same virus that caused a human pandemic resulting in more than 40 million deaths [3].
The epidemiology of this nascent pandemic has been different than recent seasonal epidemics, with the majority of cases and hospitalizations being identified in children and young adults. Severe illness has frequently been seen in this age demographic in persons with no underlying chronic medical conditions [4,5]. However, unlike in seasonal influenza where the majority of hospitalizations and deaths are in the elderly [6], less than 5% of hospitalizations for the pandemic H1N1 have been in those 65 years of age and older, primarily in those with underlying chronic medical conditions [7]. The reason for this relative sparing of the elderly is unclear, but is likely related to cross-reactive antibody responses providing some measure of immunity [8]. Whether this cross-reactive antibody is from prior infection with a specific, related virus, or is due to the accumulation of exposures to unrelated viruses that share epitopes with the pandemic H1N1 [9], is not known at this time.
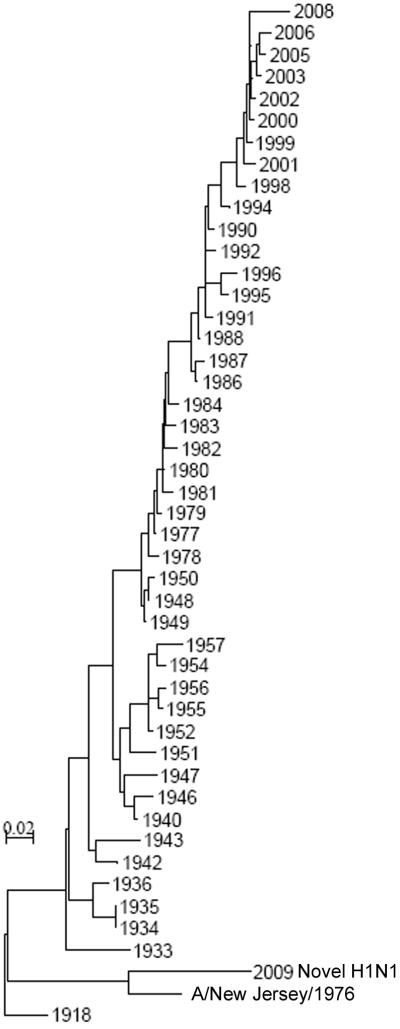
In 1976 an influenza outbreak with an H1N1 influenza virus of the “classic” swine lineage caused a pandemic scare. More than 200 military recruits at Fort Dix, New Jersey, were infected, but the virus did not spread beyond the military base [10]. Fear70 s over a repeat of the disastrous 1918 pandemic, however, prompted a rapid and massive immunization campaign resulting in the vaccination of 45 million persons, nearly a quarter of the population of the United States [11]. Phylogenetic analysis of the HA of H1N1 influenza viruses that have spread in humans in the last century demonstrates that the HAs of viruses which circulated in humans in the 1930s and 1940s are more closely related to the 2009 H1N1 pandemic strain than are recent seasonal strains, suggesting that exposure to these viruses in early childhood might provide some cross-protective immunity and help explain the age distribution (Figure 1). However, the A/New Jersey/76 strain that caused the Fort Dix outbreak is the most closely related human virus to the 2009 H1N1 pandemic strain. Thus, receipt of vaccine in 1976 might provide some current benefit to vaccinees exposed to the 2009 pandemic H1N1. We undertook this study to define the influenza specific antibody response in older persons and determine whether receipt of the 1976 “swine flu” vaccine influences those responses.
Figure 1.
Phylogenetic tree (distance method [22]) of the HA1 region of HAs from representative human epidemic and pandemic H1N1 influenza viruses from 1918 to the present.
Methods
Study Design and Participants
Employees of St. Jude Children’s Research Hospital and their spouses were eligible for recruitment if they were 55 years of age or older. Of 250 randomly selected employees contacted for this study, 110 (44%) employees elected to participate and 6 spouses volunteered. The mean age of those contacted who did not volunteer to participate (59.6 years) was not different from that of participants. This study population was chosen partly for convenience and partly because the 1976 “swine flu” vaccine was administered to hospital employees as part of a clinical trial in 1976, and many of these vaccinated persons still work at the hospital. Enrollment took place in late July and early August 2009, prior to the widespread circulation of the 2009 pandemic H1N1 virus in Memphis. Demographics and elements of history including age, gender, history of chronic medical conditions predisposing to influenza hospitalization as defined by the Advisory Committee on Immunization Practices [12], and receipt of influenza vaccine in 2008 (seasonal vaccine) or 1976 (“swine flu” vaccine) were collected in a standardized manner under an Institutional Review Board approved protocol. Discard sera collected anonymously at Le Bonheur Children’s Medical Center during a 2001 study of sero-responses were used as controls as these children could not have been exposed to any of the viruses studied.
Ascertainment of Outcomes
The main outcomes to be studied were hemagglutination-inhibition (HI) and microneutralization (MN) titers stratified by age and by prior receipt of the 1976 “swine flu” vaccine. Sera were treated with receptor destroying enzyme (Accurate Chemical & Scientific Corp., Westbury, NY) and heat-inactivated prior to analysis for influenza-specific antibody using a standard HI titer assay [13]. For determination of HI titers, individual H1N1 virus stocks expressing HA from A/Brisbane/59/07 or A/California/7/09 were adjusted to 4 HA units and incubated with diluted sera for 1 h at 4°C. Chicken red blood cells (0.5%) were added to the plates, and HI titers, reported as the reciprocal of the final serum dilution that inhibits hemagglutination, were recorded 30 minutes later.
For determination of MN titers, sera diluted in infection media were incubated with individual viral stocks (2000 TCID50 mL-1) expressing HAs from A/Brisbane/59/07 or A/California/7/09 for 2h. Confluent MDCK monolayers (3 × 105 cells mL-1) were rinsed with PBS and exposed to serum:virus mixtures for 18h. Inoculum was removed and cells were incubated for 18h in infection media supplemented with 2 μg mL-1 TPCK-trypsin. Cells were fixed with 80% acetone and influenza virus nucleoprotein was detected using monoclonal antibodies to NP (Millipore Fisher catalog number MAB8251) at a dilution of 1:2000 as described [14]. MN titers are reported as the reciprocal of the final dilution that neutralizes virus to a neutralization endpoint defined as described [8,15].
Statistical Analyses
Data from all participants were included in the analyses. Geometric mean titers were calculated for both HA and MN. In cases where all titers were below the level of detection (< 1:10), a value of “5” was used for comparisons. For HA, a geometric mean titer of ≥ 40 was considered “protective”; for MN, a titer of ≥ 160 was used as a correlate of seroprotection. [8]. Categorical variables were compared using Chi-square tests wit133 h Yates correction, and the Mann-Whitney U test was used for continuous variables. The 95% confidence intervals were computed when relevant. P-values less than 0.05 were considered statistically significant. SigmaStat for Windows (SysStat Software, Inc., V 3.11) was utilized for all statistical analyses.
Results
Subjects
This volunteer sample of 116 persons was comprised of 81 women (69.8%) and 35 men and had an average age of 60.1 years (range 55 to 73). Chronic medical conditions predisposing to complications of influenza were present in 44 subjects (37.9%). Because the sample was derived mainly from employees of a children’s cancer hospital, this was a highly vaccinated population with 106 (94.1%) of subjects having received the 2008-2009 seasonal vaccine the prior year, and 46 (39.7%) having received the 1976 “swine flu” vaccine during the national campaign in 1976.
Antibody responses to seasonal and pandemic H1N1 viruses
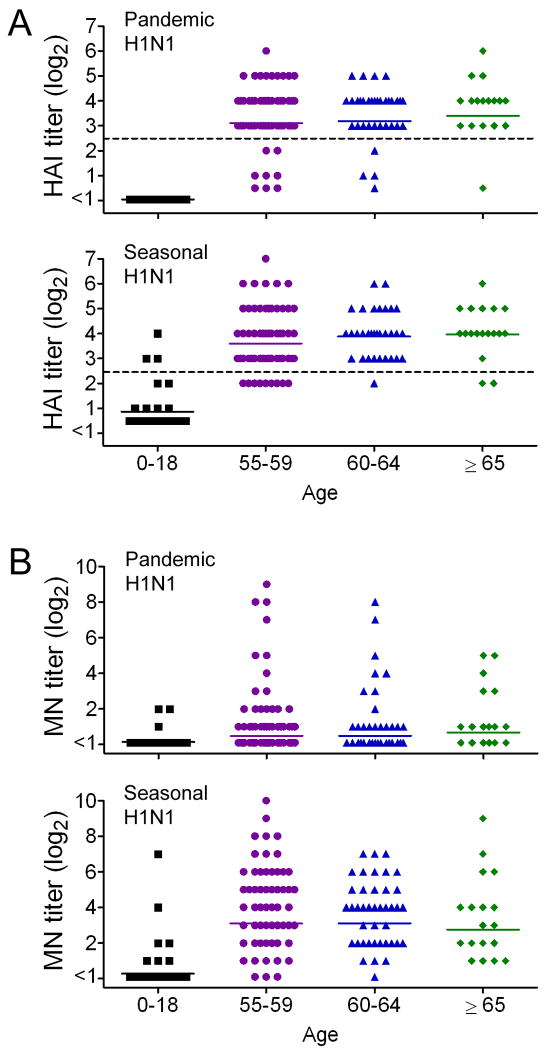
The participants had high levels of influenza specific antibody by HI assay against the 2008-2009 seasonal H1N1 influenza strain (Table 1). The geometric mean titer (GMT) trended up with increasing age, and 89.7% of participants had a titer ≥ 40, which is considered to be seroprotective (Figure 2A). This was specific for this older population and was not an artifact of the assay utilized, as the children included as controls had negligible antibody titers with only 3 reaching seroprotective titers. Similar levels of antibody were detected against the novel pandemic H1N1 strain, with 88.8% achieving a seroprotective titer (Figure 2A). No differences in any age group could be determined between the HI responses to seasonal and pandemic H1N1 strains by comparison of the GMTs (Table 1). The control sera from children had no detectable antibody to the pandemic H1N1. Because the study was conducted prior to the availability of the monovalent H1N1 vaccine or widespread circulation of the pandemic H1N1 in Memphis, the immune responses to the 2009 H1N1 likely represent cross-reactive antibody responses from prior infection or vaccination.
Table 1.
Geometric mean anti-influenza titers by age group.
| Age, y (n) | GMT to 2008-2009 Seasonal H1N1 (95% CI) | GMT to 2009 Pandemic H1N1 (95% CI) | P-value |
|---|---|---|---|
| 0-18 (n=20) | |||
| HAI | 9.3 (8.2 – 10.4) | 5a | 0.015 b |
| MN | 9.3 (8.1 – 10.5) | 5.9 (4.9 – 7.0) | 0.27 |
| 55-59 (n=62) | |||
| HAI | 70.0 (57.5 – 82.4) | 54.1 (41.7 – 66.4) | 0.35 |
| MN | 84.1 (69.3-98.8) | 12.8 (−1.3 – 26.9) | < 0.001b |
| 60-64 (n=36) | |||
| HAI | 80.0 (67.6 – 92.4) | 54.4 (41.7 – 67.2) | 0.081 |
| MN | 59.9 (45.0 – 74.9) | 12.6 (−2.6 – 27.8) | < 0.001 b |
| ≥ 65 (n=18) | |||
| HAI | 86.4 (72.2 – 100.6) | 63.5 (48.5 – 66.4) | 0.21 |
| MN | 54.4 (36.4 – 72.5) | 14.1 (−3.0 – 31.3) | 0.005 b |
GMT = geometric mean titer, HAI – hemagglutination-inhibition assay, MN = microneutralization
all titers were below the limit of detection (10)
a statistically significant difference in titer (P < 0.05) is present by the Mann-Whitney rank sum test
Figure 2.
Antibody titers by A) hemagglutination-inhibition (HI) and B) microneutralization (MN) methods against the 2008-2009 seasonal H1N1 and the 2009 pandemic H1N1 influenza viruses, stratified by age. The dotted line in (A) represents the breakpoint for presumed seroprotection.
Neutralizing antibodies against the seasonal H1N1, as measured by the MN assay, were detected at similar levels as hemagglutinating antibodies were detected in the HI assay (Figure 2B). GMTs did not differ significantly as measured by HI vs. MN for any age group (P = 0.549 for 55-59, P = 0.272 for 60-64, and P = 0.103 for ≥ 65 years). However, MN responses to the pandemic H1N1 were significantly lower than corresponding titers to the seasonal H1N1 (Figure 2; Table 1), and were also significantly lower than titers as measured by HI for all age groups (P < 0.001 for 55-59, P < 0.001 for 60-64, and P = 0.001 for ≥ 65). Overall, 68.1% of participants had MN titers ≥ 1:40 to the seasonal H1N1 strain, compared to only 18.1% to the pandemic H1N1 strain (P < 0.001). We conclude from these data that persons over 55 years of age have high levels of influenza specific antibodies that can recognize both seasonal and pandemic H1N1 strains, but these responses differ in quality as they are poorly neutralizing against the 2009 pandemic H1N1 strain.
Effect of vaccination against the 1976 “swine flu”
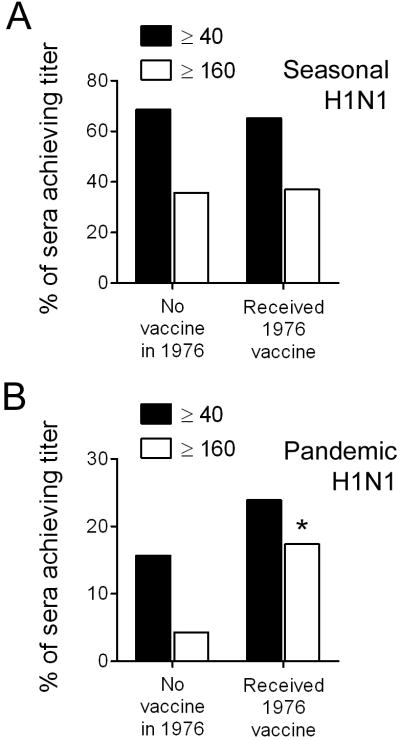
We next assessed the effect of vaccination in 1976 against A/New Jersey/76 (H1N1) on both total virus specific antibody responses as well as on neutralizing responses. Of this volunteer sample, 44 of 116 (37.9%) subjects received the “swine flu” vaccine in 1976. Recipients did not differ in age, gender, or frequency of chronic medical conditions from those who did not receive this vaccine (Table 2). However, recipients were more likely to have been vaccinated in 2008 with the seasonal vaccine. Despite this higher rate of vaccine uptake in 2008, antibody levels against the seasonal H1N1 strain contained in the vaccine did not differ between the groups by HI (GMT of recipients 81.2 (95% CI, 68.8-93.6) vs. no vaccine 71.7 (95% CI, 59.7-83.8), P = 0.482). Similarly, the percentage of participants reaching a titer of 40 or 160 by MN assay did not differ by 1976 vaccination status (Figure 3A).
Table 2.
Characteristics of Study Population by 1976 vaccination status.
| Received 1976 vaccine (n = 46) | No 1976 vaccine (n = 70) | P-value | |
|---|---|---|---|
| Age (SD), y | 60.8 (3.8) | 59.7 (5.5) | 0.56 |
| Women, No. (%) | 29 (41.3) | 52 (25.7) | 0.08 |
| Chronic medical condition, No. (%) | 17 (37.0) | 27 (38.6) | 0.86 |
| 2008 Seasonal vaccine, No. (%) | 45 (97.8) | 61 (87.1) | 0.044 a |
p < 0.05 by Chi-squared test
Figure 3.
Microneutralization titers against the 2008-2009 seasonal H1N1 and the 2009 pandemic H1N1 influenza viruses, stratified by receipt of “swine flu” vaccine in 1976.
When responses against the 2009 H1N1 were assessed, they were similar by HI between recipients of the 1976 vaccine and those who did not receive vaccine (GMT of recipients 59.2 (95% CI, 46.5-71.9) vs. no vaccine 53.3 (95% CI, 41.2-65.4), P = 0.924). However, neutralizing antibody levels against the pandemic H1N1 were higher by MN assay in recipients of the 1976 vaccine, with 11 of 46 achieving a titer of 40 or better, compared to 11 of 70 who did not receive vaccine, and 8 of 46 achieving a titer of 160 or better, compared to 3 of 70 (Figure 3B). Only the comparison of titers at 160 or better was statistically significant (P = 0.018). We conclude from these data that receipt of the 1976 “swine flu” vaccine results in enhanced neutralization responses against the 2009 pandemic H1N1 strain.
Discussion
In this study we have demonstrated that a cohort of older adults has a significant amount of antibody that cross-reacts with the 2009 pandemic H1N1 strain. However, these antibodies are generally non-neutralizing. Vaccination against a related virus, A/New Jersey/1976, enhanced these neutralizing responses. Since neutralizing responses were very low in adults who did not receive the “swine flu” vaccine in 1976 (GMT of 11.3, 95% CI -1.9-24.4, and only 3 of 70 subjects had a titer ≥ 160), it is unlikely that virus neutralization accounts for the low clinical attack rate and relatively low hospitalization rate observed in elderly persons without chronic medical conditions. Indeed, the epidemiology of pandemic H1N1 from many countries where the 1976 “swine flu” vaccine was never used is similar to that in the United States [16]. However, our data suggest that vaccination against a homologous or even closely related strain is likely to significantly boost neutralizing responses to the pandemic H1N1. The limited data published so far on vaccination with the monovalent H1N1 vaccine show good responses by HI in the elderly [17,18], but only limited data on neutralizing responses have been reported [19].
Our study differs in several important ways from previously published data on pre-existing immune responses to the 2009 pandemic H1N1 and the effect of 1976 “swine flu” vaccination [8,20]. The current study was prospective, and measured antibody responses in persons in 2009, while the only other study which examined responses to the 1976 vaccine utilized stored sera from prior studies in prior years, primarily influenza vaccine studies [8]. Immunity to the 1976 “swine flu” vaccine was thus assessed immediately after vaccination against the virus, rather than now after more than 30 years have passed. In that study, Hancock et al. found that more than 30% of ser225 a from persons born in the 1940s showed neutralizing responses ≥ 40 to the 2009 pandemic H1N1, and the 1976 “swine flu” vaccine boosted these responses such that more than 60% had an MN titer against the 2009 H1N1 of ≥ 160 [8]. In our population, only 18.1% of the overall cohort had MN titers of ≥ 40 against pandemic H1N1, and of the sub-group who received the 1976 vaccine, only 17.4% retained MN titers ≥ 160 to the present day. Two other studies which reported neutralization titers to the 2009 pandemic H1N1 showed either no responses (retrospective study of stored sera from April, 2009 from hospital employees and patients) [20], or responses similar to ours (pre-vaccination titers in a monovalent H1N1 vaccine study) [19], but neither assessed the impact of the 1976 vaccine.
A major difference from previous studies appears to be that our population was highly vaccinated; 91.4% overall received the seasonal vaccine in 2008-2009, and most of the cohort has had repeated annual immunization because of their status as health-care workers [21]. Thus, overall HI titers against seasonal H1N1 were very high in comparison to other published data [8,19,20], and cross-reactive responses against the pandemic H1N1 were similar to those against the seasonal H1N1. Hancock et al. showed very low post-vaccination GMTs against the 2009 H1N1 (GMT 10-11 by HI, 95% CI 7- 14) in their cohorts of older adults (> 60y) receiving recent seasonal influenza vaccines, and only saw predicted seroprotective responses (≥ 40) in 12-13% of subjects [8]. Greenberg et al. saw low pre-vaccination baseline GMTs against the 2009 H1N1 (GMT 15.0 and 13.8 by HI in two vaccine groups, 95% CI 11.4-19.6 and 8.4-14.3, respectively) in their cohort of older adults (50-64y), and only 27.4% and 13.8% in the two groups had predicted seroprotective responses [19]. Thus, their populations, s, which were selected for inclusion in vaccine trials and had low baseline titers to influenza virus, are likely different from our population who are routinely immunized annually and in whom higher GMTs and more frequent seroprotective responses were identified.
This study has several important limitations that must be considered to best understand the data. First, this was a highly vaccinated population, so probably represents the upper end of the spectrum in terms of antibody responses. This is partly due to the study design which was a convenience sampling of employees and partly due to a likely selection bias in that persons who volunteered were aware that we were studying responses to the 1976 “swine flu” vaccine and nearly 40% of all participants had received the vaccine previously. We did not attempt to assess other groups that might be more representative of the general population as we were focused on the central hypothesis that 1976 vaccine would enhance responses to the current 2009 pandemic strain. Therefore, generalization of these results to other groups should be done cautiously. Finally, the assays in use are subject to some variation between laboratories, although we attempted to minimize the effect of this by utilizing the same methods and definitions as those in the previously reported study [8].
In summary, we present the first prospective data analyzing antibody responses to the 2009 pandemic H1N1 influenza virus in relation to the 1976 “swine flu” vaccine. Our findings are notable in that little neutralizing activity is seen in our highly vaccinated cohort of older adults despite high titers of cross-reactive antibody by HI. Prior receipt of the 1976 vaccine, however, enhanced these neutralizing responses. These results suggest that neutralizing immunity against the 2009 pandemic H1N1 strain is unlikely to account for the low morbidity seen in the elderly during the current pandemic, and the results of vaccine trials that report only HI data need to be interpreted cautiously.
Acknowledgments
This study was funded by NIAID award to JAM N01-AI-70005-CEIRS H1N1 P8 and ALSAC. We would like to thank Ms. Amy Iverson for excellent technical support.
Footnotes
All authors declare no competing interests.
JAM conceived of the study; JAM, KJA, KCB, RJW, and PJF designed the study, JAM and RJW acquired funding, KJA and LAV acquired the data, KCB provided administrative support, JAM analyzed and interpreted the data including the statistical analyses and drafted the manuscript, and all authors reviewed and had input into the final manuscript.
References
- 1.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quinones- Falconi F, et al. Pneumonia and Respiratory Failure from Swine-Origin Influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 2.Peiris JS, Poon LL, Guan Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J Clin Virol. 2009;45:169–173. doi: 10.1016/j.jcv.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potter CW. Chronicle of influenza pandemics. In: Nicholson KG, Webster RG, Hay AJ, editors. Textbook of Influenza. London: Blackwell Scientific Publications; 1998. pp. 3–18. [Google Scholar]
- 4.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 5.Dominguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 6.Simonsen L, Fukuda K, Schonberger LB, Cox NJ. The impact of influenza epidemics on hospitalizations. J Infect Dis. 2000;181:831–837. doi: 10.1086/315320. [DOI] [PubMed] [Google Scholar]
- 7.Reed C, Angulo FJ, Swerdlow DL, Lipsitch M, Meltzer MI, et al. Estimates of the prevalence of pandemic (H1N1) 2009, United States, April-July 2009. Emerg Infect Dis. 2009;15:2004–2007. doi: 10.3201/eid1512.091413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, et al. Cross-reactiv334 e antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 9.Greenbaum JA, Kotturi MF, Kim Y, Oseroff C, Vaughan K, et al. Pre338 existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc Natl Acad Sci U S A. 2009;106:20365–20370. doi: 10.1073/pnas.0911580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaydos JC, Top FH, Jr, Hodder RA, Russell PK. Swine influenza A outbreak, Fort Dix, New Jersey, 1976. Emerg Infect Dis. 2006;12:23–28. doi: 10.3201/eid1201.050965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sencer DJ, Millar JD. Reflections on the 1976 swine flu vaccination program. Emerg Infect Dis. 2006;12:29–33. doi: 10.3201/eid1201.051007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58:1– 52. [PubMed] [Google Scholar]
- 13.Huber VC, Thomas PG, McCullers JA. A multi-valent vaccine approach that elicits broad immunity within an influenza subtype. Vaccine. 2009;27:1192–1200. doi: 10.1016/j.vaccine.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber VC, McCullers JA. Live attenuated influenza vaccine is safe and immunogenic in immunocompromised ferrets. J Infect Dis. 2006;193:677–684. doi: 10.1086/500247. [DOI] [PubMed] [Google Scholar]
- 15.Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichert T, Chowell G, Nishiura H, Christensen RA, McCullers JA. Does glycosylation as a modifier of Original Antigenic Sin explain the case age distribution and unusual toxicity in pandemic novel H1N1 influenza? BMC Infect Dis. 2010;10:5. doi: 10.1186/1471-2334-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control. Update on influenza A (H1N1) 2009 monovalent vaccines. MMWR Morb Mortal Wkly Rep. 2009;58:1100–1101. [PubMed] [Google Scholar]
- 18.Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, et al. A Novel Influenza A (H1N1) Vaccine in Various Age Groups. N Engl J Med. 2009;361:2414–2423. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–2413. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 20.Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, et al. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021– 1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCullers JA, Speck KM, Williams BF, Liang H, Mirro J., Jr Increased influenza vaccination of healthcare workers at a pediatric cancer hospital: results of a comprehensive influenza vaccination campaign. Infect Control Hosp Epidemiol. 2006;27:77–79. doi: 10.1086/500003. [DOI] [PubMed] [Google Scholar]
- 22.McCullers JA, Saito T, Iverson AR. Multiple genotypes of influenza B viru372 s circulated between 1979 and 2003. J Virol. 2004;78:12817–12828. doi: 10.1128/JVI.78.23.12817-12828.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]