Abstract
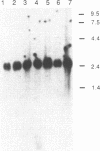
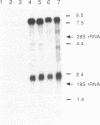
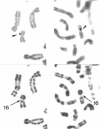
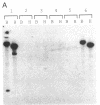
We describe the isolation and characterization of cDNA clones encoding human leukosialin, a major sialoglycoprotein of human leukocytes. Leukosialin is very closely related or identical to the sialophorin molecule, which is involved in T-cell proliferation and whose expression is altered in Wiskott-Aldrich syndrome (WAS), an X chromosome-linked immunodeficiency disease. Using a rabbit anti-serum to leukosialin, a cDNA clone was isolated from a lambda gt11 cDNA library constructed from human peripheral blood cells. This lambda gt11 clone was used to isolate longer cDNA clones that correspond to the entire coding sequence of leukosialin. DNA sequence analysis reveals three domains in the predicted mature protein. The extracellular domain is enriched for Ser, Thr, and Pro and contains four contiguous 18-amino acid repeats. The transmembrane and intracellular domains of the human leukosialin molecule are highly homologous to the rat W3/13 molecule. RNA gel blot analysis reveals two polyadenylylated species of 2.3 and 8 kilobases. Southern blot analysis suggests that human leukosialin is a single-copy gene. Analysis of monochromosomal cell hybrids indicates that the leukosialin gene is not X chromosome linked and in situ hybridization shows leukosialin is located on chromosome 16. These findings demonstrate that the primary mutation in WAS is not a defect in the structural gene for leukosialin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsson B., Hammarström S., Finne J., Perlmann P. The large sialoglycoprotein of human lymphocytes. II. Biochemical features. Eur J Immunol. 1985 May;15(5):427–433. doi: 10.1002/eji.1830150503. [DOI] [PubMed] [Google Scholar]
- Badley J. E., Bishop G. A., St John T., Frelinger J. A. A simple, rapid method for the purification of poly A+ RNA. Biotechniques. 1988 Feb;6(2):114–116. [PubMed] [Google Scholar]
- Baecher C. M., Infante A. J., Semcheski K. L., Frelinger J. G. Identification and characterization of a mouse cell surface antigen with alternative molecular forms. Immunogenetics. 1988;28(5):295–302. doi: 10.1007/BF00364226. [DOI] [PubMed] [Google Scholar]
- Barclay A. N., Jackson D. I., Willis A. C., Williams A. F. Lymphocyte specific heterogeneity in the rat leucocyte common antigen (T200) is due to differences in polypeptide sequences near the NH2-terminus. EMBO J. 1987 May;6(5):1259–1264. doi: 10.1002/j.1460-2075.1987.tb02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bettaieb A., Farace F., Mitjavila M. T., Mishal Z., Dokhelar M. C., Tursz T., Breton-Gorius J., Vainchenker W., Kieffer N. Use of a monoclonal antibody (GA3) to demonstrate lineage restricted O-glycosylation on leukosialin during terminal erythroid differentiation. Blood. 1988 May;71(5):1226–1233. [PubMed] [Google Scholar]
- Carlsson S. R., Fukuda M. Isolation and characterization of leukosialin, a major sialoglycoprotein on human leukocytes. J Biol Chem. 1986 Sep 25;261(27):12779–12786. [PubMed] [Google Scholar]
- Carlsson S. R., Sasaki H., Fukuda M. Structural variations of O-linked oligosaccharides present in leukosialin isolated from erythroid, myeloid, and T-lymphoid cell lines. J Biol Chem. 1986 Sep 25;261(27):12787–12795. [PubMed] [Google Scholar]
- Cohen P. The coordinated control of metabolic pathways by broad-specificity protein kinases and phosphatases. Curr Top Cell Regul. 1985;27:23–37. doi: 10.1016/b978-0-12-152827-0.50010-4. [DOI] [PubMed] [Google Scholar]
- Concannon P., Pickering L. A., Kung P., Hood L. Diversity and structure of human T-cell receptor beta-chain variable region genes. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6598–6602. doi: 10.1073/pnas.83.17.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Chae H. P., Lowman J. T., Krivit W., Good R. A. Wiskott-Aldrich syndrome. An immunologic deficiency disease involving the afferent limb of immunity. Am J Med. 1968 Apr;44(4):499–513. doi: 10.1016/0002-9343(68)90051-x. [DOI] [PubMed] [Google Scholar]
- Corbi A. L., Larson R. S., Kishimoto T. K., Springer T. A., Morton C. C. Chromosomal location of the genes encoding the leukocyte adhesion receptors LFA-1, Mac-1 and p150,95. Identification of a gene cluster involved in cell adhesion. J Exp Med. 1988 May 1;167(5):1597–1607. doi: 10.1084/jem.167.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human brain-granulocyte-T lymphocyte antigen probably homologous to the W 3/13 antigen of the rat. Eur J Immunol. 1980 Oct;10(10):745–749. doi: 10.1002/eji.1830101004. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fournier R. E., Frelinger J. A. Construction of microcell hybrid clones containing specific mouse chromosomes: application to autosomes 8 and 17. Mol Cell Biol. 1982 May;2(5):526–534. doi: 10.1128/mcb.2.5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977 Aug 25;252(16):5888–5894. [PubMed] [Google Scholar]
- Gitschier J., Drayna D., Tuddenham E. G., White R. L., Lawn R. M. Genetic mapping and diagnosis of haemophilia A achieved through a BclI polymorphism in the factor VIII gene. 1985 Apr 25-May 1Nature. 314(6013):738–740. doi: 10.1038/314738a0. [DOI] [PubMed] [Google Scholar]
- Gulley M. L., Ogata L. C., Thorson J. A., Dailey M. O., Kemp J. D. Identification of a murine pan-T cell antigen which is also expressed during the terminal phases of B cell differentiation. J Immunol. 1988 Jun 1;140(11):3751–3757. [PubMed] [Google Scholar]
- Harp J. A., Davis B. S., Ewald S. J. Inhibition of T cell responses to alloantigens and polyclonal mitogens by Ly-5 antisera. J Immunol. 1984 Jul;133(1):10–15. [PubMed] [Google Scholar]
- Kehry M., Ewald S., Douglas R., Sibley C., Raschke W., Fambrough D., Hood L. The immunoglobulin mu chains of membrane-bound and secreted IgM molecules differ in their C-terminal segments. Cell. 1980 Sep;21(2):393–406. doi: 10.1016/0092-8674(80)90476-6. [DOI] [PubMed] [Google Scholar]
- Killeen N., Barclay A. N., Willis A. C., Williams A. F. The sequence of rat leukosialin (W3/13 antigen) reveals a molecule with O-linked glycosylation of one third of its extracellular amino acids. EMBO J. 1987 Dec 20;6(13):4029–4034. doi: 10.1002/j.1460-2075.1987.tb02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima K., Inoue Y., Inoue S. Polysialoglycoproteins of Salmonidae fish eggs. Complete structure of 200-kDa polysialoglycoprotein from the unfertilized eggs of rainbow trout (Salmo gairdneri). J Biol Chem. 1986 Apr 25;261(12):5262–5269. [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky K., Kingsley D., Krieger M. Use of a mutant cell line to study the kinetics and function of O-linked glycosylation of low density lipoprotein receptors. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4335–4339. doi: 10.1073/pnas.85.12.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman D. R., Newton M., Crommie D., Ang S. L., Seidman J. G., Gettner S. N., Weiss A. Characterization of an expressed CD3-associated Ti gamma-chain reveals C gamma domain polymorphism. Nature. 1987 Mar 5;326(6108):85–88. doi: 10.1038/326085a0. [DOI] [PubMed] [Google Scholar]
- Mattei M. G., Philip N., Passage E., Moisan J. P., Mandel J. L., Mattei J. F. DNA probe localization at 18p113 band by in situ hybridization and identification of a small supernumerary chromosome. Hum Genet. 1985;69(3):268–271. doi: 10.1007/BF00293038. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Remold-O'Donnell E., Crimmins M. A., Bierer B. E., Rosen F. S., Burakoff S. J. Sialophorin, a surface sialoglycoprotein defective in the Wiskott-Aldrich syndrome, is involved in human T lymphocyte proliferation. J Exp Med. 1987 May 1;165(5):1383–1392. doi: 10.1084/jem.165.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Parkman R., Remold-O'Donnell E., Kenney D. M., Perrine S., Rosen F. S. Surface protein abnormalities in lymphocytes and platelets from patients with Wiskott-Aldrich syndrome. Lancet. 1981 Dec 19;2(8260-61):1387–1389. doi: 10.1016/s0140-6736(81)92802-6. [DOI] [PubMed] [Google Scholar]
- Peterson T. C., Killary A. M., Fournier R. E. Chromosomal assignment and trans regulation of the tyrosine aminotransferase structural gene in hepatoma hybrid cells. Mol Cell Biol. 1985 Sep;5(9):2491–2494. doi: 10.1128/mcb.5.9.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph S. J., Thomas M. L., Morton C. C., Trowbridge I. S. Structural variants of human T200 glycoprotein (leukocyte-common antigen). EMBO J. 1987 May;6(5):1251–1257. doi: 10.1002/j.1460-2075.1987.tb02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeck G. R., Fisher L. A statistical analysis of the amino acid compositions of proteins. Int J Pept Protein Res. 1973;5(2):109–117. doi: 10.1111/j.1399-3011.1973.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Davis A. E., 3rd, Kenney D., Bhaskar K. R., Rosen F. S. Purification and chemical composition of gpL115, the human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J Biol Chem. 1986 Jun 5;261(16):7526–7530. [PubMed] [Google Scholar]
- Remold-O'Donnell E., Kenney D. M., Parkman R., Cairns L., Savage B., Rosen F. S. Characterization of a human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J Exp Med. 1984 Jun 1;159(6):1705–1723. doi: 10.1084/jem.159.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Kenney D., Rosen F. S. Biosynthesis of human sialophorins and analysis of the polypeptide core. Biochemistry. 1987 Jun 30;26(13):3908–3913. doi: 10.1021/bi00387a025. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Zimmerman C., Kenney D., Rosen F. S. Expression on blood cells of sialophorin, the surface glycoprotein that is defective in Wiskott-Aldrich syndrome. Blood. 1987 Jul;70(1):104–109. [PubMed] [Google Scholar]
- Saga Y., Tung J. S., Shen F. W., Boyse E. A. Sequences of Ly-5 cDNA: isoform-related diversity of Ly-5 mRNA. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6940–6944. doi: 10.1073/pnas.83.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpte C. S., Eckhardt A. E., Abernethy J. L., Hill R. L. Porcine submaxillary gland apomucin contains tandemly repeated, identical sequences of 81 residues. J Biol Chem. 1988 Jan 15;263(2):1081–1088. [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]