Abstract
The orphan transporter Slc6a18 (XT2) is highly expressed at the luminal membrane of kidney proximal tubules and displays ∼50% identity with Slc6a19 (B0AT1), which is the main neutral amino acid transporter in both kidney and small intestine. As yet, the amino acid transport function of XT2 has only been experimentally supported by the urinary glycine loss observed in xt2 null mice. We report here that in Xenopus laevis oocytes, co-expressed ACE2 (angiotensin-converting enzyme 2) associates with XT2 and reveals its function as a Na+- and Cl−-de pend ent neutral amino acid transporter. In contrast to its association with ACE2 observed in Xenopus laevis oocytes, our experiments with ace2 and collectrin null mice demonstrate that in vivo it is Collectrin, a smaller homologue of ACE2, that is required for functional expression of XT2 in kidney. To assess the function of XT2 in vivo, we reanalyzed its knock-out mouse model after more than 10 generations of backcrossing into C57BL/6 background. In addition to the previously published glycinuria, we observed a urinary loss of several other amino acids, in particular β-branched and small neutral ones. Using telemetry, we confirmed the previously described link of XT2 absence with hypertension but only in physically restrained animals. Taken together, our data indicate that the formerly orphan transporter XT2 functions as a sodium and chloride-de pend ent neutral amino acid transporter that we propose to rename B0AT3.
The SLC6 family is composed of a variety of transporters that transfer small organic solutes, such as neurotransmitters and amino acids, across the plasma membrane. These substrates are co-transported with sodium and in many cases also with chloride. The substrates transported by most of the different family members have been identified and include neurotransmitters ((nor)epinephrine, dopamine, serotonin, and γ-aminobutyric acid), osmolytes (taurine and creatinine), and amino acids. Despite this extensive characterization, no data directly demonstrating the function of XT2 (Slc6a18) in an expression system have been reported. In particular, the expression of XT2 in Xenopus laevis oocytes and cultured mammalian cells failed to result in uptake of a variety of tested substrates (1, 2).
However, XT2 exhibits ∼50% identity to B0AT1 (Slc6a19), the Na+ cotransporter of neutral amino acids the defect of which causes Hartnup disorder. The XT2 gene is arranged in tandem with that of B0AT1 (Slc6a19) on chromosome 5 in humans and chromosome 13 in mice and thus presumably arose by gene duplication. A cloning study identified, besides the A12 isoform that resembles most the other Slc6 family members, five shorter transcripts, the physiological significance of which is not established (1). In a localization study performed using mouse tissues, we have shown that XT2 is mainly expressed in the kidney, where it localizes to the brush border membrane of the late proximal tubule (S2, S3) in a complementary fashion to B0AT1 that localizes to the early proximal tubule (S1) (3).
A xt2 null mouse model was generated and analyzed previously (2). The high amounts of glycine found in the urine of these mice supported the hypothesis that the orphan gene product XT2 functions as an amino acid transporter. Measurements of uptake in brush border membrane vesicles additionally demonstrated its function as a high affinity transport system of glycine. Surprisingly, the xt2 null mice displayed a systolic blood pressure that was 15–20 mm Hg higher than that of their wild-type littermates, a difference that was abolished upon glycine supplementation in drinking water. Such an impact of XT2 on blood pressure was, however, not confirmed in humans, where a single-nucleotide polymorphism within the SLC6A18 gene present in 46.7% of a general Japanese population corresponds to a nonsense mutation (Y319X) presumably leading to a loss of function and is not associated with hypertension (4).
We have recently demonstrated that in the proximal kidney tubule, the expression of Slc6 B0 cluster amino acid transporters requires their association with Collectrin (Tmem27, Coll), a membrane protein homologous to the membrane anchor domain of the renin angiotensin system enzyme ACE2 (5). Furthermore, we have shown that the enzyme ACE2, highly expressed in kidney and intestine, plays the role of associated protein in small intestine for luminal Slc6 amino acid transporters (6).
The first aim of the present study was therefore to retest the function of XT2 in the X. laevis expression system using co-expression of both the kidney and the small intestine-associated proteins Coll and ACE2 and, second, to retest the impact of the absence of XT2 in the xt2 null mouse model after backcrossing it more than 10 times into the C57BL/6 background. It appears, based on telemetric measurements, that XT2 is involved in blood pressure control only under stress conditions in the C57BL/6 background. We show in this study that the product of Slc6a18 called XT2 is a Na+- and Cl−-dependent neutral amino acid transporter and displays, compared with B0AT1, a lower K0.5 and a different substrate selectivity. Because of its broad transport selectivity for neutral amino acids, we suggest that it be renamed B0AT3.
EXPERIMENTAL PROCEDURES
cRNA Preparation of Mouse XT2, Collectrin, and ACE2
The constructs of the A12 isoform of mouse XT2-KSM, Collectrin-pcDNA3.1Hygro, and ACE2-pcDNA3 were linearized and used as template for RNA synthesis (mMESSAGE mMACHINE; Ambion, Austin, TX).
Transport Studies in X. laevis Oocytes
Expression studies and uptake measurements in X. laevis oocytes were performed as described previously (7). Ten-minute uptakes were performed with buffer containing 0.1 mm of the corresponding l-amino acid (2 μCi of 14C-l-amino acid/ml or 20 μCi of 3H-l-amino acid/ml). All experiments were done in buffer containing NaCl (100 mm) or NMDG-Cl (100 mm) for ion dependence experiments. Chloride was substituted by corresponding gluconate salts. Kinetics experiments were performed with increasing concentrations of l-Ile (0.01, 0.03, 0.1, 0.3, and 1 mm) or l-Gly (0.01, 0.1, 0.3, 1, and 3 mm) in the presence of NaCl. Data are expressed in pmol/h/oocyte, and values obtained for non-injected oocytes are subtracted. Multiple comparisons within groups were performed by repeated measures one-way analysis of variance, followed by Tukey post test.
Animals
The xt2, ace2, and collectrin wild-type and knock-out mice were housed in standard conditions and fed a standard diet. Generation of the knock-out mice was described elsewhere (2, 5, 8). All procedures for mice handling were according to the Swiss Animal Welfare laws and approved by the Kantonales Veterinäramt Zürich.
Metabolic Cages
Animals were adapted to metabolic cages (Tecniplast, Buguggiate, Italy) for 3 days before data collection, where they had free access to standard mouse diet (18.5% crude protein, Kliba-Nafag, Kaiseraugst, Switzerland) and drinking water. After a first day of data collection in standard conditions, either the diet was switched to low protein (<0.5% crude protein; Kliba-Nafag) for 2 days, or water access was removed for 24 h. Daily food/water intake, urine/feces output, and body weights were measured. Urinary pH was measured using a pH microelectrode (691 pH-meter; Metrohm). Urinary creatinine was measured by the Jaffe method (9). Urinary electrolytes (Na+, K+, Ca2+, Mg2+, Cl−, SO42−) were measured by ion chromatography (Metrohm ion chromatograph; Herisau, Switzerland).
Amino Acid Analysis
Mouse blood was collected by decapitation, and 1 μl of heparin-Na+ (25,000 international units/5 ml) (B. Braun (Melsungen, Germany)) was added. Plasma was collected after centrifugation at 5900 × g and 4 °C. Ice-cold methanol deproteinization of the plasma was performed as described elsewhere (10). One hundred microliters of deproteinized sample was dried and resuspended in 45 μl of borate buffer. Samples were then derivatized using AccQ Tag (Waters, Milford, MA) and analyzed on an Acquity UPLC (Waters) according to the manufacturer's instructions by the Functional Genomics Center Zurich (FGCZ) (11).
One microliter of mouse urine collected over 24 h was diluted up to 100 μl by 50 mm HCl containing the internal standards norvaline and sarcosine in a concentration of 50 pmol/μl. The solutions were centrifuged and transferred into a new hydrolyzing tube. One microliter was injected for precolumn derivatization with ortho-phthaldialdehyde and analyzed on an Amino Quant amino acid analyzer (Agilent Technologies GmbH, Böblingen, Germany) at the Functional Genomics Center Zurich.
RNA and Real Time Quantitative Reverse Transcription-PCR
RNA was extracted from frozen kidneys, and real time quantitative reverse transcription-PCR was performed as previously described (3). Three samples per mouse and genotype were run, and the abundance of the target mRNAs was calculated relative to hypoxanthine guanine phosphoribosyltransferase or glyceraldehyde 3-phosphate dehydrogenase as a reference. Relative expression ratios were calculated as r = 2(Ct(reference) − Ct(test)), where Ct represents cycle number at the threshold, and “test” represents tested mRNAs. Primers and probes were chosen as described elsewhere for B0AT1 (3), XT3 (3), SIT1 (3), XT2 (3), ACE2 (12), Coll (5), glyceraldehyde 3-phosphate dehydrogenase (3), and hypoxanthine guanine phosphoribosyltransferase (13).
Protein Preparation and Western Blot Analysis
Oocyte homogenization and protein preparation was done as described previously (14). Surface labeling of oocytes expressing XT2 alone or co-expressed with ACE2 or Collectrin using MTSEA-Biotin (Sigma) and streptavidin precipitation were performed as previously described (15). For immunoprecipitation, the lysate equivalent to eight oocytes was coupled to 25 μl of anti-XT2 serum (3) in EBC solution (20 mm Tris-HCl, pH 8.0, 120 mm NaCl, 0.5% Nonidet P-40) for 8 h at 4 °C on a rotator. The immunocomplexes were coupled to immobilized Protein A/G beads (Pierce) overnight at 4 °C on a rotator. The beads were washed six times with NET-N solution (20 mm Tris-HCl, pH 8.0, 100 mm NaCl, 0.5% Nonidet P-40, 1 mm EDTA), and the immunoprecipitate was eluted, denatured, and reduced by heating at 95 °C for 5 min in Laemmli buffer. The equivalent of 2.4 oocytes was analyzed by Western blotting. Brush border membrane vesicles were prepared from whole mouse kidney using the Mg2+ precipitation technique, as described elsewhere (15). Immunoblotting of the equivalent of one oocyte or 20 μg of brush border membrane vesicles was performed as described elsewhere (3). Primary antibodies were diluted 1:2000 for rabbit affinity-purified (AP)4 anti-B0AT1 (3)/anti-XT2 (3)/anti-XT3 (3), (1:2000) for guinea pig serum anti-Coll (antigen peptide: NH2-VQSAIRKNRNRINSAC-CONH2 as in Ref. 16) (Pineda, Berlin, Germany), 1:1000 for AP goat anti-mACE2 (R&D Systems, Minneapolis, MI), and 1:10,000 for mouse anti-β-actin (Sigma). Secondary antibodies were diluted 1:5000 for ECLTM anti-rabbit IgG horseradish peroxidase-linked fragment from donkey (Amersham Biosciences), 1:10,000 for anti-guinea pig IgG horseradish peroxidase (Sigma), and 1:10,000 for anti-mouse IgG alkaline phosphatase conjugate (Promega). Antibody binding was detected with Immobilon Western Chemiluminescent horseradish peroxidase or alkaline phosphatase substrate (Millipore, Billerica, MA). Chemiluminescence was detected with a DIANA III camera (Raytest, Dietikon, Switzerland). For quantification relative to β-actin, signal intensity was quantified with the AIDA image analyzer (Raytest).
Organ Fixation
Mice were anesthetized with ketamine and xylazine (90 mg/kg body weight, Narketan 10; Vétoquinol, Lure, France) and Xylazine (10 mg/kg body weight, Xylazin; Streuli, Uznach, Switzerland) intraperitoneally and perfused through the left cardiac ventricle with phosphate-buffered saline (0.9% NaCl in 10 mm phosphate buffer, pH 7.4), followed by a buffered paraformaldehyde solution (4%, pH 7), as previously described (17). Kidneys were then harvested, incubated overnight in paraformaldehyde solution, washed several times with phosphate-buffered saline, and stored in phosphate-buffered saline, 0.02% sodium azide at 4 °C. Tissues were then mounted with Kryostat OCT (Medite, Nunningen, Switzerland), frozen in liquid propane, and stored at −80 °C.
Immunofluorescence
Immunofluorescence was performed as previously described (3). Primary antibodies were diluted 1:200 for rabbit AP anti-mXT2 (3), 1:100 for AP goat anti-mACE2 (R&D Systems), and 1:1000 for rabbit serum anti-mCollectrin (antigen peptide: NH2-CDPLDMKGGHINDGFLT-CONH2 as in Ref. 16) (Pineda). Secondary antibodies were diluted (1:500) for Alexa Fluor 594 donkey anti-rabbit IgG and Alexa Fluor 488 donkey anti-goat IgG (Invitrogen). For actin staining, Texas Red-X phalloidin (Invitrogen) was diluted (1:500). Digital images were viewed using a Nikon Eclipse TE300 epifluorescence microscope (Nikon Instruments Inc., Melville, NY) equipped with a DS-5 M Standard CCD camera (Nikon) and acquired with NIS-Elements (Nikon).
Telemetry Measurement of Blood Pressure
Eight-week-old mice were anesthetized (see “Organ Fixation”) and implanted with a PA-C10 transmitter (Data Sciences International (DSI), St. Paul, MI) through the carotid artery as previously described and following manufacturer information to place the pressure-sensitive tip in the aortic arch (18, 19). For analgesia purposes, 100 μl of bupivacain hydrochloride 0.25% (Bucain, DeltaSelect GmbH, Pfullingen, Germany) was injected directly in the wound at the end of the procedure, and carprofenum (5 mg/kg; Rimadyl; Pfizer, New York) was injected subcutaneously every 12 h for 2 days. Twenty-four-hour continuous measurements were recorded after 2 weeks of recovery with Dataquest ART version 3.1 and RespiRate (DSI). Systolic, diastolic, and mean blood pressure, heart frequency, activity, and respiratory rate were analyzed separately for the light versus dark phases and activity = 0 versus activity > 0. To mimic tail cuff manometry stress, mice were restrained for 20 min in a perforated 50-ml Falcon tube, when the data were continuously measured.
RESULTS
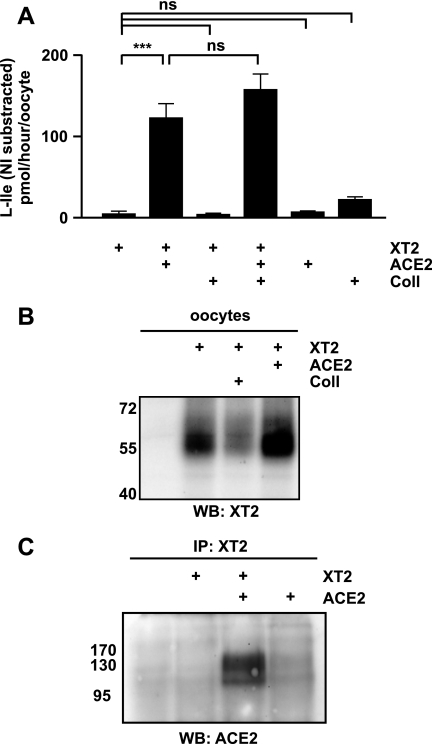
Since XT2 has been shown to be associated in kidney proximal tubule with Coll (5), we tested the possibility that XT2 would be expressed functionally in X. laevis oocytes in the presence of this associated protein. XT2 was therefore co-expressed in X. laevis oocytes with Coll and/or its homologue ACE2, and the uptake of l-isoleucine was tested. Unexpectedly, uptake of l-isoleucine was observed only when XT2 was co-expressed with ACE2, whereas no transport was observed when XT2 was expressed alone or together with Coll (Fig. 1A). Furthermore, co-expression of ACE2 and Coll with XT2 did not further increase the transport rate compared with XT2 plus ACE2.
FIGURE 1.
XT2 and ACE2 functional interaction in vitro. A, co-expression of ACE2 but not of Coll supports the function of XT2 in X. laevis oocytes. X. laevis oocytes were injected with 10 ng of each cRNA and incubated for 6 days. Na+-dependent uptake of l-Ile (0.1 mm) (10 min) was assayed. The data represent 16–24 oocytes from 2–4 independent experiments. Bars, mean value ± S.E. ***, p < 0.001. B, surface-biotinylated proteins of X. laevis oocytes injected with XT2 alone, co-expressed with Coll or with ACE2, were analyzed by Western blot (WB) using anti-XT2 antibody. C, total membranes of X. laevis oocytes were used for immunoprecipitation (IP) with an anti-XT2 antibody, and immunocomplexes were analyzed by Western blot with anti-ACE2 antibody.
The l-isoleucine transport induction upon ACE2 co-expression is accompanied by a slight increase of XT2 surface expression of 1.64 ± 0.39-fold when compared with XT2 alone (Fig. 1B). Furthermore, co-immunoprecipitation of ACE2 from oocyte membranes using XT2 antibody shows that ACE2 interacts with XT2 (Fig. 1C). These results suggest that ACE2 acts as a chaperone for XT2, slightly increasing its surface expression and more strongly its function in X. laevis oocytes. This co-expression renders the measurement of XT2 transport function possible in an expression system for the first time.
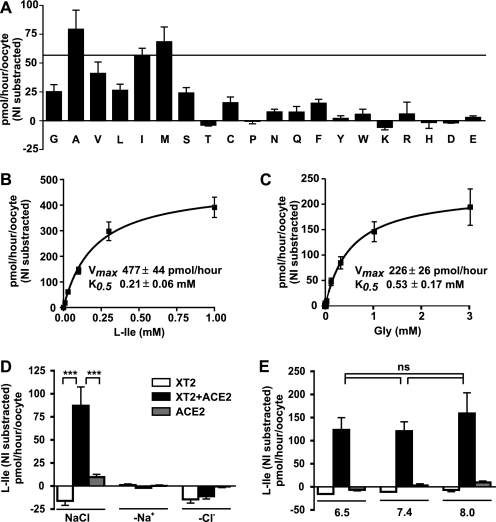
XT2 co-expression with ACE2 allowed the characterization of its transport selectivity, showing the transport of a broad range of neutral amino acids (Ala, Met, Val, Ile > Gly, Ser, Leu) (Fig. 2A). Concentration dependence experiments with l-Ile and Gly revealed a K0.5 of XT2 for these substrates (0.21 and 0.53 mm, respectively) that is lower than that of B0AT1 (Fig. 2, B and C) (20). In contrast to B0AT1 but similarly to other members of the Slc6 family, such as the γ-aminobutyric acid transporter GAT1 (Slc6a1), the transport of l-Ile by XT2 is not only Na+- but also Cl−-dependent as well as pH-independent (Fig. 2, D and E). The Na+ dependence of Gly transport is sigmoidal, with a Hill coefficient that is higher than 1, suggesting that more than one sodium molecule is involved (data not shown). The precise ion transport stoichiometry of XT2 could not be investigated by two-electrode voltage clamp due to the low substrate transport rates.
FIGURE 2.
Transport selectivity and kinetics of XT2 co-expressed with ACE2. A, substrate selectivity of XT2 co-expressed with ACE2 in X. laevis oocytes. Na+-Cl−-dependent uptake selectivity of l-amino acids (0.1 mm) was assayed for 10 min. Amino acids are abbreviated with single-letter codes. B and C, concentration dependence of the XT2-ACE2-mediated transport in oocytes. Curves corresponding to Michaelis-Menten kinetics were fitted to Na+-Cl−-dependent uptake of l-Ile (B) and Gly (C). Data represent means of 18–39 oocytes ± S.E. from 3–5 independent experiments. D, transport induced by XT2 co-expressed with ACE2 is Na+- and Cl−-dependent. Data are means of 13–16 oocytes ± S.E. from two independent experiments. E, transport of l-Ile was not influenced by pH. The transport of l-Ile was assayed in the presence of Na+ and Cl− at different pH values. Shown are means from eight oocytes ± S.E. The open bars show transport rate of XT2-expressing, black bars of XT2 + ACE2-expressing, and gray bars of ACE2-expressing oocytes (D and E). ns, not significant.
To verify and examine the role of XT2 as an amino acid transporter in more detail, we reanalyzed the xt2 null mouse model (2) after more than 10 generations of backcrossing in C57BL/6 background. To evaluate whether the lack of XT2 induces compensatory changes, we examined the expression of the Slc6 family members B0AT1 and XT3 and of the associated proteins ACE2 and Coll at the mRNA and protein levels in xt2−/− mice but observed no difference with wild-type littermate controls (supplemental Fig. 1, A and B).
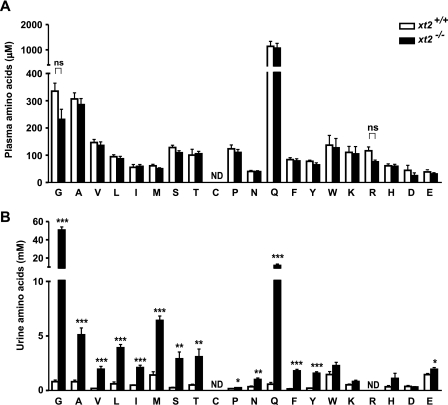
To evaluate the impact of the loss of XT2 on the whole animal, we monitored food and water intake as well as urine and feces output in metabolic cages, but there were no differences in the measured parameters between the xt2+/+ and xt2−/− mice (supplemental Table 1). Analysis of plasma amino acids only suggested a slight but statistically not significant decrease of glycine and l-arginine in the xt2−/− mice as compared with the xt2+/+ mice (Fig. 3A). A massive loss of glycine in the urine of the xt2−/− mice was confirmed, and the loss of other neutral amino acids, notably l-glutamine, was observed (Fig. 3B). To compare the reabsorption of amino acids, we estimated their fractional excretion. This is the proportion of the amino acids filtered by the kidney glomeruli that ends up in the urine (values of glomerular filtration rate were from Ref. 21). Whereas the fractional excretion of l-glutamine was only slightly augmented to 2.94% in xt2 null mice, those of glycine and l-methionine were strongly elevated to 51.37 and 33.41%, respectively (Table 1). These results show that despite aminoaciduria, these mice do not exhibit any compensatory mechanisms, implying that the normal diet readily provides excessive amounts of amino acids. The urinary peak corresponding to l-arginine unfortunately could not be distinguished from that of taurine, preventing the evaluation of l-arginine loss in xt2 null mice. Challenging xt2 null animals with a low protein diet (<0.5% crude protein) did not induce any additional difference in the metabolic cage parameters measured between xt2 null and wild-type mice (supplemental Table 1).
FIGURE 3.
The xt2 null mice have increased levels of neutral amino acids in urine and a slight but not significant decrease of plasma glycine and l-arginine. Plasma (A) and urine (B) of xt2+/+ and xt2−/− mice were deproteinized and analyzed by HPLC. Amino acids are shown in single-letter code. Bars, mean values ± S.E.; n = 7 (A) and n = 6 (B) mice/genotype. *, p < 0.05; **, p < 0.01; ***, p < 0.001. ns, not significant; ND, not determined.
TABLE 1.
Fractional urinary amino acid excretion
| Amino acid | Fractionala excretion |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| xt2+/+b | xt2−/− | Change | ace2+/yc | ace2−1y | Change | coll+/yd | coll−y | Change | |
| % | % | -fold | % | % | -fold | % | % | -fold | |
| Gly | 0.53 | 51.37 | 96.25 | 0.73 | 0.51 | 0.69 | 1.95 | 82.13 | 42.19 |
| Ala | 0.59 | 4.49 | 7.60 | 0.43 | 0.69 | 1.61 | 0.60 | 56.42 | 94.47 |
| Val | 0.33 | 3.50 | 10.75 | 0.13 | 0.07 | 0.51 | 0.21 | 26.36 | 125.00 |
| Leu | 1.23 | 11.71 | 9.50 | 0.45 | 0.25 | 0.56 | 1.55 | 54.56 | 35.22 |
| Ile | 2.30 | 8.97 | 3.90 | 0.39 | 0.15 | 0.37 | 0.41 | 36.45 | 88.31 |
| Met | 5.00 | 33.41 | 6.68 | 1.61 | 1.19 | 0.74 | 6.20 | 58.52 | 9.43 |
| Ser | 0.47 | 6.66 | 14.07 | 2.48 | 1.77 | 0.72 | 1.99 | 77.69 | 39.00 |
| Thr | 1.04 | 7.33 | 7.06 | 0.57 | 0.35 | 0.61 | 1.55 | 114.24 | 73.78 |
| Cys | NDe | ND | ND | ND | ND | ND | ND | ND | ND |
| Pro | 0.33 | 0.59 | 1.79 | 1.71 | 1.13 | 0.66 | 0.46 | 65.93 | 144.59 |
| Asn | 2.91 | 6.76 | 2.33 | ND | ND | ND | ND | ND | ND |
| Gln | 0.11 | 2.94 | 26.72 | 0.32 | 0.32 | 1.00 | 0.36 | 63.69 | 177.61 |
| Phe | 0.39 | 5.87 | 15.21 | 0.87 | 0.67 | 0.77 | 0.52 | 21.25 | 40.83 |
| Tyr | 0.34 | 2.14 | 6.38 | 0.61 | 0.49 | 0.80 | 1.70 | 16.45 | 9.70 |
| Trp | 1.97 | 4.24 | 2.16 | 0.30 | 0.39 | 1.30 | 10.99 | 22.78 | 2.07 |
| Lys | 1.58 | 2.98 | 1.88 | 0.10 | 0.09 | 0.88 | 2.46 | 4.50 | 1.83 |
| Arg | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| His | 1.74 | 9.66 | 5.56 | 1.25 | 1.11 | 0.89 | ND | ND | ND |
| Asp | 2.62 | 4.12 | 1.57 | ND | ND | ND | ND | ND | ND |
| Glu | 11.46 | 16.03 | 1.40 | 4.05 | 3.61 | 0.89 | 2.17 | 6.52 | 3.01 |
a Fractional excretion was calculated using an estimate of the glomerular filtration rate of 150 μl/min in females and 240 μl/min in males (21), the measured amino acid concentrations in plasma and urine, and the volume of urine excreted over 24 h.
b Males, n = 3; females, n = 3.
c Males, n = 5.
d Males, n = 3.
e ND, not determined.
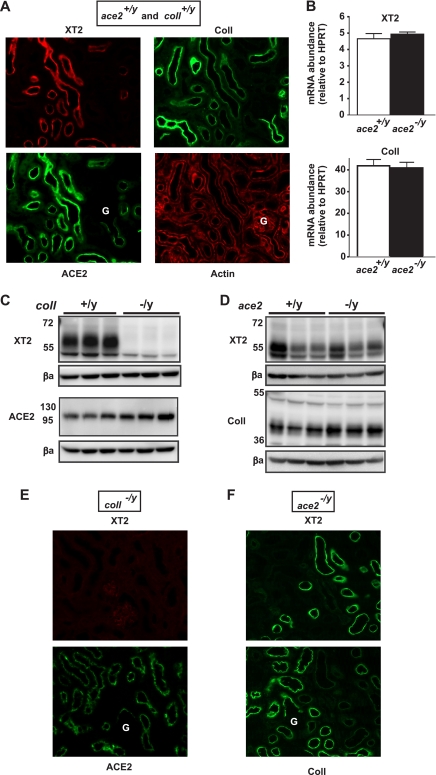
To assess the situation of the different partners of XT2 in vivo, we first checked the localization of XT2, Coll, and ACE2 in mouse kidney. As previously shown, XT2 localizes to the apical membrane in distal segments of the proximal tubule (S2, S3) and is not present in the initial S1 segment that begins at the glomerulus (3) (Fig. 4A). In contrast, ACE2 and Coll are both localized along the entire proximal tubule but with opposed gradients increasing toward the distal and proximal tubular ends, respectively (Fig. 4A and supplemental) Table 2. This shows that the main site of ACE2 expression coincides with the localization of XT2, suggesting that XT2 may interact with ACE2 in the proximal tubule in addition to its known interaction with Coll (5).
FIGURE 4.
Lack of XT2 in coll−/y but not in ace2−/y mouse kidney. A, immunofluorescence of wild-type kidney co-labeled with XT2 and ACE2 and on a consecutive section co-labeled with Coll and actin. G, glomerulus. B, real time reverse transcription-PCR of XT2 and Coll on ace2+/y and ace2−/y total kidney RNA. Bars, mean value ± S.E.; n = 3 mice per genotype. C and D, kidney brush border membrane vesicles (BBMV) (20 μg) from coll+/y and coll−/y mice (C) and ace2+/y and ace2−/y mice (D) were analyzed by Western blot using antibodies against XT2, Coll (with a specific signal at 42 kDa; see supplemental material), ACE2, and β-actin (βa). Each lane corresponds to material prepared from one mouse. E and F, immunofluorescence of coll−/y kidney co-labeled with XT2 and ACE2 (E) and ace2−/y kidney labeled with XT2 and on a consecutive section with Coll (F). Immunofluorescence experiments were performed at least three times with similar results for each antibody. Representative images are shown. As shown also by Western blot in C and D, XT2 expression is lacking in kidney of coll null mice, whereas it is expressed in ace2 null mice.
To test the role of these two potential associated proteins on XT2 expression in vivo, we used ace2 and coll null mice. At the mRNA level in the kidney, XT2 expression was unchanged in coll null mice, as shown previously (5), and in ace2 null mice (Fig. 4B). However, Western blot analysis of kidney brush border membrane proteins showed that XT2 was absent in the coll−/y mice, in contrast to ace2−/y and wild-type mice (Fig. 4, C and D). Immunofluorescence performed on kidney sections confirmed that XT2 is lacking in coll−/y mice proximal tubule, whereas it is normally expressed in ace2−/y as in wild-type control mice (Figs. 4, E and F). Amino acid fractional excretions of ace2−/y and ace2+/y mice were compared to assess the impact of ACE2 on XT2 function (Table 1). The results indicate that amino acid handling was not significantly different in the kidney of ace2 null mice compared with wild-type littermates. In contrast, aminoaciduria was very high in coll null mice, as reported previously (5). Note, however, that in these mice, several other amino acid transporters are affected that contribute to the aminoaciduria. These results confirm that the partner of XT2 in mouse kidney is indeed Coll and not ACE2, despite the fact that the expression gradient of Coll along the proximal tubule is opposed to that of both XT2 and ACE2 and that Coll unlike ACE2 does not allow XT2 function in X. laevis oocytes.
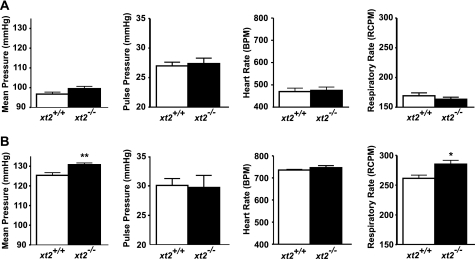
In the original study, xt2 null mice displayed, compared with wild-type littermates, a surprising elevation of blood pressure, as measured by tail cuff manometry. This difference was then abolished when drinking water was supplemented with glycine (0.1 g/ml) (2). The blood pressure of xt2 null mice was now retested using mice backcrossed more than 10 times into C57BL/6 background and using telemetry (Datasciences). The measurements showed no differences in blood pressure between the xt2−/− and xt2+/+ mice during the light phase when mice were not active (Fig. 5A). Similar results were obtained during the light and dark phases, whether the mice were active or not (data not shown). To test whether the previously measured increase in blood pressure was provoked by the stress of the tail cuff manometry method, telemetry measurements were repeated while physically restraining the mice. Submitting the animals to this stress provoked a higher mean blood pressure in xt2−/− mice than in wild-type littermates (130.8 ± 0.89 versus 125.3 ± 1.40 mm Hg) and also a higher respiratory rate (Fig. 5B). This suggests an increased stress-induced reaction possibly involving a dysregulation of the sympathetic nervous system in xt2−/− mice.
FIGURE 5.
The xt2 null mice display a stress-induced increase of blood pressure and respiratory rate. The xt2+/+ and xt2−/− mice were implanted with blood pressure telemetry sensors (DSI). Shown are mean pressure, pulse pressure, heart rate (beats/min (BPM)), and respiratory rate (respiratory cycles/minute (RCPM)) of xt2+/+ and xt2−/− mice during the light period, when the mice were not active (A), or during a 20-min physical restraint stress (B). Bars, mean values ± S.E.; n = 7 mice/genotype. *, p < 0.05; **, p < 0.01.
DISCUSSION
The first aim of this study was to directly characterize the function of the orphan transporter XT2 in the X. laevis oocyte expression system using its in vivo associated protein Coll. However, not Coll but only its homologue ACE2 supported the functional expression of XT2 in X. laevis oocytes such that its transport function of neutral amino acids expected from its homology with B0AT1 could be measured. This raised the suggestion that ACE2 might interact with XT2 in mouse kidney in addition to Coll. However, our experiments with the ace2 and coll null mice confirmed that the in vivo partner of XT2 is Coll and not ACE2. Indeed, XT2 expression and localization is normal in the proximal kidney tubule of ace2 null mice and absent in coll null mice. Furthermore, the absence of ACE2 does not affect the reabsorption of amino acids, whereas that of Coll leads to a strong aminoaciduria that is due to the lack of expression of XT2 and of other proximal tubule amino acid transporters (5). These observations suggest that during evolution, Coll took over the role of ACE2 in amino acid transport due to the increasing involvement of the latter as a key player in the renin-angiotensin system. It is, however, not clear why the restriction of functional XT2 association with ACE2 or Coll is just opposite in X. laevis oocytes as compared with the in vivo situation in kidney. Surprisingly, it appears that the difference observed in X. laevis oocytes is species-specific. Indeed, as suggested to us,5 co-expression of the human Coll ortholog with mouse XT2 generates an uptake similar to that with mouse ACE2 (data not shown). Further experiments will be required to identify potential additional cell-specific players that account for these differences.
The functional expression of XT2 together with ACE2 in oocytes allowed the long sought characterization of the function of this orphan transporter. Interestingly, its broad transport selectivity for neutral amino acids is clearly different from that of B0AT1. Particularly, whereas β-branched amino acids are very good substrates for XT2, as is the case for B0AT1, aromatic amino acids are apparently less efficiently transported. Importantly, l-Ala, l-Met, Gly, l-Ser, and l-Cys are also efficiently transported.
In contrast to B0AT1 that displays a Na+-dependent electrogenic neutral amino acid transport, XT2 is shown here to display a transport mode that is not only Na+-dependent but also Cl−-dependent with a K0.5 for its preferred substrates that is lower than that of B0AT1. In that sense, the axial arrangement of XT2 that follows B0AT1 along the proximal tubule is similar to the sequential arrangement of low and high affinity glucose and peptide transporters SGLT2 and -1 and Pept1 and -2, respectively.
To correlate the measured in vitro function of XT2 with its in vivo function, we reanalyzed the xt2 null mouse model. Since the knock-out mice did not display compensatory changes of other amino acid transporters of the SLC6 cluster or of associated proteins, we hypothesize that the urinary phenotype of xt2 null mice is essentially due to the absence of XT2. In accordance with the in vitro results, we measured a broad aminoaciduria. Strikingly, there was not only a substantial loss of glycine (60-fold higher than in xt2+/+) but also of l-glutamine (20-fold) and of all other neutral amino acids (Fig. 3). XT2 thus appears to be required for reabsorbing the leftover tubular amino acids that have not been reabsorbed in the early proximal kidney tubule segments by B0AT1 or the amino acids that would eventually backleak into the late proximal tubule segments through the paracellular route (22). Additionally, XT2 might also be required for the reabsorption of neutral amino acids that the luminal heterodimeric cystine and cationic amino acid transporter b0,+AT-rBAT releases in exchange for its uptake substrates in the tubular lumen. Thereby, XT2 would interfere with l-arginine metabolism and nitric oxide synthesis (23). This latter hypothesis could be an explanation for the slightly decreased l-arginine plasma level and the tendency for elevated blood pressure observed in xt2 null mice.
Interestingly, XT2 had originally been identified in rat kidney as a renal osmotic stress-induced transcript (24). However, the experiments that we performed in mice could not verify such a regulation.6 This is either due to a species difference or to the less drastic conditions that we used, which might not have been sufficient to trigger this regulation.
In their original description, xt2 null mice displayed a significantly elevated blood pressure (2). This was, however, not observed in the present study after backcrossing these mice 10 times in a C57BL/6 background and using a telemetry system for the blood pressure measurements. Previous reports have already evidenced discrepancies between tail cuff manometry and telemetric blood pressure measurements and also highlighted the importance of the genetic background. In particular, the original xt2 null mouse had an Sv129 genetic background, a strain that contains two renin genes and the renal blood flow of which was suggested to be more sensitive toward nitric oxide than that of C57BL/6 mice (25). We could, however, observe in the present study a stress-sensitive hypertension and a stress-induced increase in respiratory rate in xt2 null mice, suggesting a possible impact of the lack of this transporter on regulatory functions of the sympathetic nervous system. Interestingly, plasma l-arginine of xt2−/− mice was slightly lower than in the xt2+/+ mice, and l-arginine is the precursor of nitric oxide, which, when released by endothelial cells, leads to vasodilation. Slightly lower circulating and renal levels of l-arginine might therefore induce susceptibility to an increase in blood pressure (26).
The role of XT2 in human seems to be limited since SLC6A18 has not been found associated with iminoglycinuria.7 Furthermore, a frequent single-nucleotide polymorphism that leads to a stop codon in SLC6A18 has not been linked to increased blood pressure (4). However, it is not excluded that the individual variability in stress-induced blood pressure increase could be linked to the function of this renal neutral amino acid transporter.
Supplementary Material
Acknowledgment
We thank Robert Kleta for interesting discussion.
This project was supported by Swiss National Science Foundation Grant 31-59141.02 (to F. V.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2 and Fig. 1.
S. Bröer, personal communication.
E. Romeo and F. Verrey, unpublished results.
R. Kleta, personal communication.
- AP
- affinity-purified.
REFERENCES
- 1.Nash S. R., Giros B., Kingsmore S. F., Kim K. M., el-Mestikawy S., Dong Q., Fumagalli F., Seldin M. F., Caron M. G. (1998) Receptors Channels 6, 113–128 [PubMed] [Google Scholar]
- 2.Quan H., Athirakul K., Wetsel W. C., Torres G. E., Stevens R., Chen Y. T., Coffman T. M., Caron M. G. (2004) Mol. Cell. Biol. 24, 4166–4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romeo E., Dave M. H., Bacic D., Ristic Z., Camargo S. M., Loffing J., Wagner C. A., Verrey F. (2006) Am. J. Physiol. Renal Physiol 290, F376–F383 [DOI] [PubMed] [Google Scholar]
- 4.Eslami B., Kinboshi M., Inoue S., Harada K., Inoue K., Koizumi A. (2006) Tohoku J. Exp. Med. 208, 25–31 [DOI] [PubMed] [Google Scholar]
- 5.Danilczyk U., Sarao R., Remy C., Benabbas C., Stange G., Richter A., Arya S., Pospisilik J. A., Singer D., Camargo S. M., Makrides V., Ramadan T., Verrey F., Wagner C. A., Penninger J. M. (2006) Nature 444, 1088–1091 [DOI] [PubMed] [Google Scholar]
- 6.Camargo S. M., Singer D., Makrides V., Huggel K., Pos K. M., Wagner C. A., Kuba K., Danilczyk U., Skovby F., Kleta R., Penninger J. M., Verrey F. (2009) Gastroenterology 136, 872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ristic Z., Camargo S. M., Romeo E., Bodoy S., Bertran J., Palacin M., Makrides V., Furrer E. M., Verrey F. (2006) Am. J. Physiol. Renal Physiol. 290, F880–F887 [DOI] [PubMed] [Google Scholar]
- 8.Crackower M. A., Sarao R., Oudit G. Y., Yagil C., Kozieradzki I., Scanga S. E., Oliveira-dos-Santos A. J., da Costa J., Zhang L., Pei Y., Scholey J., Ferrario C. M., Manoukian A. S., Chappell M. C., Backx P. H., Yagil Y., Penninger J. M. (2002) Nature 417, 822–828 [DOI] [PubMed] [Google Scholar]
- 9.Seaton B., Ali A. (1984) Med. Lab. Sci. 41, 327–336 [PubMed] [Google Scholar]
- 10.Suresh Babu S. V., Shareef M. M., Pavan Kumar Shetty A., Taranath Shetty K. (2002) Indian J. Clin. Biochem. 17, 7–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen S. A., De Antonis K. M. (1994) J. Chromatogr. A 661, 25–34 [DOI] [PubMed] [Google Scholar]
- 12.Wysocki J., Ye M., Soler M. J., Gurley S. B., Xiao H. D., Bernstein K. E., Coffman T. M., Chen S., Batlle D. (2006) Diabetes 55, 2132–2139 [DOI] [PubMed] [Google Scholar]
- 13.Van Abel M., Hoenderop J. G., Dardenne O., St. Arnaud R., Van Os C. H., Van Leeuwen H. J., Bindels R. J. (2002) J. Am. Soc. Nephrol. 13, 2102–2109 [DOI] [PubMed] [Google Scholar]
- 14.Zecevic M., Heitzmann D., Camargo S. M., Verrey F. (2004) Pflugers Arch. 448, 29–35 [DOI] [PubMed] [Google Scholar]
- 15.Ehnes C., Forster I. C., Kohler K., Bacconi A., Stange G., Biber J., Murer H. (2004) J. Gen. Physiol. 124, 475–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akpinar P., Kuwajima S., Krützfeldt J., Stoffel M. (2005) Cell Metab. 2, 385–397 [DOI] [PubMed] [Google Scholar]
- 17.Paunescu T. G., Da Silva N., Marshansky V., McKee M., Breton S., Brown D. (2004) Am. J. Physiol. Cell Physiol. 287, C149–C162 [DOI] [PubMed] [Google Scholar]
- 18.Butz G. M., Davisson R. L. (2001) Physiol. Genomics 5, 89–97 [DOI] [PubMed] [Google Scholar]
- 19.Mills P. A., Huetteman D. A., Brockway B. P., Zwiers L. M., Gelsema A. J., Schwartz R. S., Kramer K. (2000) J. Appl. Physiol. 88, 1537–1544 [DOI] [PubMed] [Google Scholar]
- 20.Camargo S. M., Makrides V., Virkki L. V., Forster I. C., Verrey F. (2005) Pflugers Arch. 451, 338–348 [DOI] [PubMed] [Google Scholar]
- 21.Qi Z., Whitt I., Mehta A., Jin J., Zhao M., Harris R. C., Fogo A. B., Breyer M. D. (2004) Am. J. Physiol. Renal Physiol. 286, F590–F596 [DOI] [PubMed] [Google Scholar]
- 22.Parks L. D., Barfuss D. W. (2002) Am. J. Physiol. Renal Physiol. 283, F1208–F1215 [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer R., Loffing J., Rossier G., Bauch C., Meier C., Eggermann T., Loffing-Cueni D., Kühn L. C., Verrey F. (1999) Mol. Biol. Cell 10, 4135–4147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasserman J. C., Delpire E., Tonidandel W., Kojima R., Gullans S. R. (1994) Am. J. Physiol. 267, F688–F694 [DOI] [PubMed] [Google Scholar]
- 25.Lum C., Shesely E. G., Potter D. L., Beierwaltes W. H. (2004) Hypertension 43, 79–86 [DOI] [PubMed] [Google Scholar]
- 26.Van Vliet B. N., Chafe L. L., Montani J. P. (2003) J. Physiol. 549, 313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.