Abstract
A previously uncharacterized putative ion channel, NALCN (sodium leak channel, non-selective), has been recently shown to be responsible for the tetrodotoxin (TTX)-resistant sodium leak current implicated in the regulation of neuronal excitability. Here, we show that NALCN encodes a current that is activated by M3 muscarinic receptors (M3R) in a pancreatic β-cell line. This current is primarily permeant to sodium ions, independent of intracellular calcium stores and G proteins but dependent on Src activation, and resistant to TTX. The current is recapitulated by co-expression of NALCN and M3R in human embryonic kidney-293 cells and in Xenopus oocytes. We also show that NALCN and M3R belong to the same protein complex, involving the intracellular I–II loop of NALCN and the intracellular i3 loop of M3R. Taken together, our data show the molecular basis of a muscarinic-activated inward sodium current that is independent of G-protein activation, and provide new insights into the properties of NALCN channels.
Keywords: ion channel, G proteins, M3 muscarinic receptor, NALCN, Src
Introduction
Ion channels are crucial components for cell excitability. They are involved in numerous physiological processes, as well as in pathologies referred to as channelopathies, and are drug targets of interest (Ashcroft, 2006; Kaczorowski et al, 2008). In 1999, Lee et al (1999) reported the cloning of a new putative ion channel mainly expressed in the brain and, to a lesser extent, in the heart and pancreas (supplementary Fig S1A,B online). The corresponding protein, NALCN (sodium leak channel, non-selective), is highly homologous to the voltage-gated sodium channel-α and calcium channel-α1 pore-forming subunits (Snutch & Monteil, 2007). Recently, Lu et al (2007) provided evidence that NALCN is responsible for a tetrodotoxin (TTX)-resistant sodium leak current implicated in neuronal excitability by generating knockout mice and overexpressing the corresponding complementary DNA (cDNA) in human embryonic kidney (HEK)-293 cells. In addition, the same group also reported a modulation of NALCN by substance P and neurotensin through a G-protein-independent and Src family of tyrosine kinases (SFKs)-dependent pathway (Lu et al, 2009).
We independently set out to characterize NALCN channel activity. In this study, we used both overexpression and knock-down approaches in the MIN6 pancreatic β-cell line, which endogenously expresses NALCN. After initial experiments, we did not detect any evidence of leak activity mediated by NALCN in this cellular model (supplementary Fig S2 online). We then investigated the possibility that NALCN could encode another type of current in these cells. Here, we report that in MIN6 cells, NALCN encodes an inward cationic channel activated by acetylcholine through the activation of M3 muscarinic receptors (M3R). Similar to the leak current observed by Lu et al (2007), the M3R-activated NALCN current observed in MIN6 cells is primarily mediated by sodium ions, is resistant to TTX and is sensitive to gadolinium ions. This current was recapitulated in the HEK-293 cell line as well as in Xenopus oocytes, and involves the inclusion of both NALCN and M3R in a protein complex. Thus, this study provides new insights into the complexity of NALCN gating properties.
Results
Knockdown and overexpression of NALCN in MIN6 cells
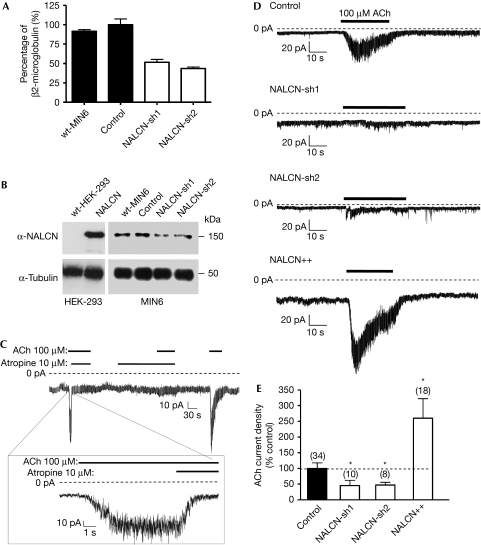
To facilitate the study of NALCN, we screened pancreatic β-cell lines for its expression using reverse transcription quantitative PCR (RT–qPCR) and western blot analysis because expression of the corresponding messenger RNA (mRNA) was detected in islets of Langerhans (supplementary Fig S1B online). We observed robust expression in the MIN6 pancreatic β-cell line (Fig 1A,B). Owing to NALCN's apparent voltage independence and the lack of any leak activity in our experimental conditions, we decided to investigate ‘orphan' currents—that is, a current of unknown molecular identity—activated by means other than changes in membrane potential in pancreatic β-cells and related cell lines. Primary pancreatic β-cells have been reported to have an inward sodium conductance of unknown molecular origin activated by muscarinic acetylcholine receptors (MRs), without G-protein involvement (Rolland et al, 2002). In keeping with these observations, application of acetylcholine (100 μM) to MIN6 pancreatic β-cells held at −80 mV in a standard whole-cell voltage-clamp configuration activated an atropine-sensitive inward current with a mean current density of −7.7±0.9 pA/pF (N=34; Fig 1C). This current was found to show some desensitization during acetylcholine application (Fig 1C,D). We therefore used both NALCN knockdown and overexpression strategies to investigate the hypothesis that NALCN encodes the observed β-cell MR-activated sodium conductance. Using two different short hairpin RNA (shRNA) sequences, we were able to substantially reduce the expression of endogenous NALCN, as confirmed by RT–qPCR and western blotting (∼50% at the mRNA level, with a transduction rate of ∼80%; Fig 1A,B). Acetylcholine-activated current density in MIN6 cells was significantly reduced by NALCN-shRNA knockdown (NALCN-sh1: 44.8±17.1% of control, N=10, P=0.03; NALCN-sh2: 43.1±9.3% of control, N=8, P=0.02; Fig 1D,E), and was increased by NALCN overexpression (260.2±62.1% of control, N=18, P=0.02; Fig 1D,E). Taken together, these results suggest that application of acetylcholine induces an inward cationic current through activation of NALCN.
Figure 1.
Knockdown and overexpression of NALCN in MIN6 cells alter an atropine-sensitive acetylcholine-activated current. (A) Endogenous NALCN expression in MIN6 cells assessed by RT–qPCR (data are presented as a percentage of expression compared with the β2M level) and (B) western blot analysis in the absence and presence of shRNAs. (C) Representative sample of acetylcholine (ACh) activated current on a MIN6 pancreatic β-cell held at −80 mV in a standard whole-cell voltage-clamp configuration. (D) This current is substantially reduced with shRNA targeted against NALCN and increased with NALCN overexpression. (E) Summary of the effects of NALCN shRNA knockdown and overexpression on acetylcholine current density, expressed as a percentage of mean control-cell current. NALCN, sodium leak channel, non-selective; RT–qPCR, reverse transcription quantitative PCR; shRNA, short hairpin RNA.
Permeation and gating properties in MIN6 cells
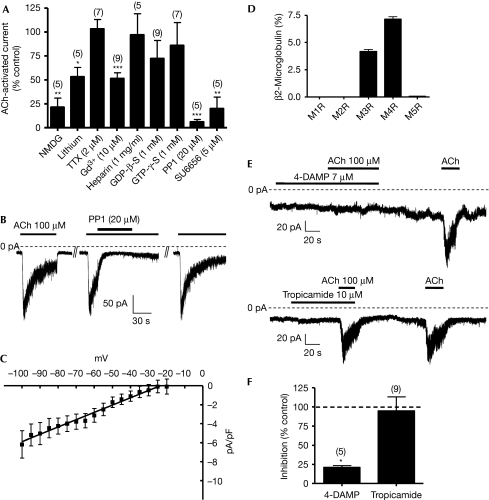
We then examined some of the ion permeation and gating properties of the acetylcholine-activated current in MIN6 cells. Substitution of sodium ions by N-methyl-D-glucamine or lithium in the extracellular solution had a robust inhibitory effect, suggesting that sodium is the main ion species contributing to the NALCN inward conductance (21.4±9.4% of control, N=5, P=0.001 and 53.2±9.7% of control, N=5, P=0.008, respectively; Fig 2A). The current was also insensitive to TTX (2 μM; 103.3±9.8% of control, N=7; Fig 2A), but was partly blocked by gadolinium (10 μM; 51.4±6.1% of control, N=9, P<0.0001; Fig 2A). Addition of heparin (1 mg/ml) to the patch pipette did not affect the current density, indicating that the current was independent of intracellular calcium stores mobilized through activation of IP3 receptors (97.1±22.2% of control, N=12, P=0.89; Fig 2A). Replacement of GTP with either GDP-β-S (1 mM) or GTP-γ-S (1 mM) in the pipette solution did not significantly change the current density (72.3±18.7% of control, N=9, P=0.178, and 86±23.8% of control, N=7, P=0.579, respectively; Fig 2A). Even the use of a pipette solution with no GTP did not have any effect on the current (data not shown). It has been shown that some G-protein-coupled receptors (GPCRs) can signal directly through SFKs (McGarrigle & Huang, 2007). Thus, we also examined the possibility that NALCN could be activated through an SFK-dependent pathway by using the SFK inhibitor 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine (PP1) (Fig 2A,B). Bath application of PP1 (20 μM) almost fully and reversibly abolished the acetylcholine-activated current (6.1±2.5% of control, N=5, P<0.0001; Fig 2A,B). A strong inhibition was also observed with the SFK inhibitor SU6656 (20.1±11.9% of control, N=5, P=0.001; Fig 2A and supplementary Fig S3 online). The current–voltage (I–V) relationship of the acetylcholine-activated current was determined by using a voltage ramp protocol in the absence and presence of acetylcholine (N=8; Fig 2C). We found the relationship to be linear in the range of −110 to −20 mV, with a slope conductance of 76.8±2.5 pS/pF, and a reversal potential Erev of approximately −23 mV. As the activation of the current by acetylcholine was associated with an increase in membrane noise, we also carried out a noise analysis to estimate the single-channel amplitude and found a value of 1.6±0.1 pA at −80 mV with an estimated single-channel conductance of ∼27 pS (supplementary Fig S4 online). To determine further which MRs were involved in the modulation of NALCN, we first carried out an analysis by using RT–qPCR in MIN6 cells (Fig 2D). Our results indicated that M3R and M4R are the muscarinic receptor subtypes present in MIN6 cells (Fig 2D). We therefore used specific antagonists of M3R (7 μM 4-diphenylacetoxy-N-methylpiperidine methiodide) and M4R (10 μM tropicamide) to determine the MR subtype responsible for current activation (Fig 2E,F). Our results suggest that M3R was responsible for current activation (4-diphenylacetoxy-N-methylpiperidine methiodide: 20.9±2.6% of control, N=5, P=0.0002; tropicamide: 94.8±18.3% of control, N=9; Fig 2F). These data suggest that M3R activation induces an inward, primarily sodium, conductance through NALCN channels that is independent of intracellular calcium stores and G proteins, dependent on SFK activation, resistant to TTX and sensitive to gadolinium inhibition.
Figure 2.
Permeation and gating properties of the acetylcholine-activated current in MIN6 cells. (A) Replacement of sodium ions by NMDG or lithium in the extracellular solution greatly diminishes the acetylcholine (ACh)-induced inward current. The current is also resistant to 2 μM TTX and was partly blocked by 10 μM Gd3+. Inclusion of heparin (1 mg/ml), GTP-γ-S (1 mM) or GDP-β-S (1 mM) in the pipette solution does not significantly affect the current, whereas the SFK inhibitors PP1 (20 μM) and SU6656 (5 μM) have a strong inhibitory effect. (B) Representative traces showing the effects of an SFK inhibitor (PP1, 20 μM) on the acetylcholine-activated inward current in MIN6 cells. (C) A voltage-ramp protocol (−100 to 100 mV over 0.2 s) was used to determine the current–voltage relationship of the acetylcholine-activated current. Values obtained in the absence of acetylcholine were subtracted from values obtained in the presence of acetylcholine to determine the I–V curve attributable to acetylcholine. Currents obtained at voltages greater than −20 mV were too variable for meaningful analysis and were therefore excluded. (D) RT–qPCR analysis shows that only mRNA from M3R and M4R can be detected in MIN6 cells. (E) Representative traces showing the effects of an M3R-specific antagonist (4-DAMP, 7 μM) and an M4R-specific antagonist (tropicamide, 10 μM) on the acetylcholine-activated inward current in MIN6 cells. (F) Summary of the effects of 4-DAMP and tropicamide on the acetylcholine-activated current, expressed as a percentage of control current. 4-DAMP, 4-diphenylacetoxy-N-methylpiperidine methiodide; Gd3+, gadolinium; MR, muscarinic receptor; NMDG, N-methyl-D-glucamine; mRNA, messenger RNA; PP1, 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine; RT–qPCR, reverse transcription quantitative PCR; SFK, Src family of tyrosine kinase; TTX, tetrodotoxin.
Heterologous co-expression of NALCN and M3R
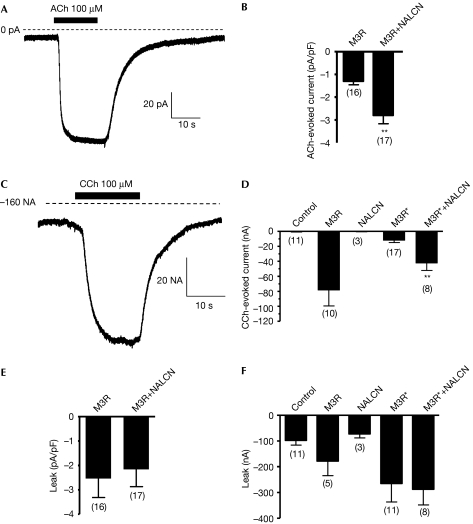
Next we sought to address whether NALCN and M3R are sufficient to reconstitute an acetylcholine-activated current in two commonly used heterologous expression systems. Expression of NALCN in HEK-293 cells stably expressing M3R, in which NALCN is not endogenously expressed (Fig 1B and supplementary Fig S6A online), resulted in a significant acetylcholine-activated current with a similar profile to but of a smaller density than that observed in MIN6 cells (NALCN+M3R −2.7±0.3 pA/pF, N=17, P=0.001; M3R −1.2±0.1 pA/pF, N=16; Fig 3A,B). This result shows that M3R and NALCN are two components necessary for recapitulating the current of interest, and suggests that additional proteins probably modulate the channel activity and/or the channel level at the plasma membrane in native systems. We next examined whether the current could be recapitulated even in Xenopus oocytes by expressing NALCN and an M3R mutant (M3R*) lacking a stretch of 10 amino acids (YKETEKRTKE) in the i3 loop involved in G-protein activation in this cellular model (Lechleiter et al, 1990). As expected, agonist stimulation of M3R* did not result in the activation of a strong endogenous Ca2+-activated Cl− current as did M3R (Fig 3C,D). When NALCN and M3R* were co-expressed, carbachol (100 μM) application resulted in the activation of an inward current (M3R*+NALCN −42.3±9.9 nA, N=8, P=0.008; Fig 3C,D), as this current was abolished by the substitution of sodium ions using N-methyl-D-glucamine (supplementary Fig S5 online). Interestingly, contrary to our data for MIN6 cells, the NALCN-generated current did not show any desensitization in HEK-293 cells or Xenopus oocytes. In our study, the desensitization process was not found to be agonist dependent (data not shown), suggesting that it is likely to be cell-type dependent. We did not observe any significant effect resulting from the expression of NALCN on the leak activity in HEK-293 cells (NALCN+M3R −2.1±0.7 pA/pF, N=17; M3R −2.5±0.7 pA/pF, N=16; Fig 3E) or in Xenopus oocytes (control: −97.7±18.3 nA, N=11; NALCN: −72.3±15.6 nA, N=3; M3R: −178.6±55.9 nA, N=5; M3R*: −264.9±72 nA, N=17; and M3R*+NALCN: −287.5±60.6 nA, N=8; Fig 3F). Thus, we conclude that, under our experimental conditions, NALCN did not encode for a leak channel but for an inward cationic channel activated by acetylcholine through M3R.
Figure 3.
Co-expression of NALCN and M3R in the HEK-293 cell line and in Xenopus oocytes results in the expression of an M3R agonist-activated current. (A) Co-expression of NALCN and M3R cDNAs in HEK-293 cells results in the expression of an acetylcholine (ACh)-activated inward current with the same profile as in the MIN6 cells. (B) Mean acetylcholine-activated current density in HEK-293 cells expressing NALCN and M3R. (C) Co-expression of NALCN and an M3R deletion mutant (M3R*) that is deficient in the activation of G proteins in Xenopus oocytes results in the expression of a carbachol (CCh)-activated inward cationic current. (D) Mean carbachol-activated current density in Xenopus oocytes, mock-injected or expressing M3R, NALCN, M3R* or NALCN+M3R*. (E) Leak activity in HEK-293 cells and (F) in Xenopus oocytes is not significantly altered by NALCN overexpression. cDNA, complementary DNA; HEK, human embryonic kidney; M3R, M3 muscarinic receptor; NALCN, sodium leak channel, non-selective.
NALCN and M3R belong to the same protein complex
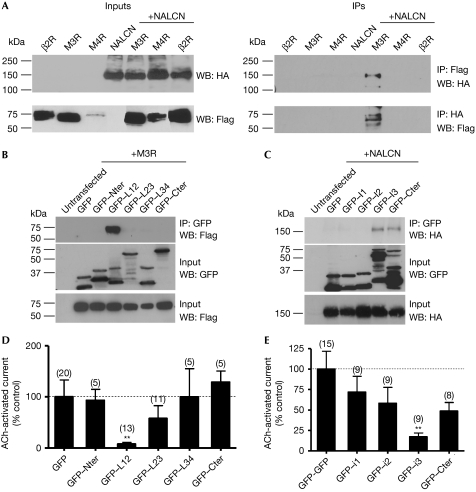
Considering that NALCN was activated by M3R but not by M4R, whereas it has been suggested that both muscarinic receptors can activate SFKs (Rosenblum et al, 2000), we next investigated the hypothesis that NALCN could associate with M3R in the same complex, thus involving a spatially localized signalling event. Owing to the lack of available antibodies to perform immunoprecipitation experiments from native systems, we carried out co-immunoprecipitation experiments with haemagglutinin-tagged NALCN and Flag-tagged M3R that were heterologously expressed in HEK-293 cells (Fig 4A). Our results indicated that NALCN and M3R associate with one another, whereas this is not the case with M4R and with the β2-adrenergic receptor used as controls (Fig 4A). It remains to be determined whether this is through a direct interaction or through co-inclusion in a larger protein complex. Intracellular portions of ion channels and GPCRs are known to be involved in protein–protein interactions in some protein complexes (Bockaert et al, 2004; Levitan, 2006). Thus, we next examined whether intracellular fragments of NALCN and M3R were involved in their interaction by using GFP fusion proteins (Fig 4B,C). We found that M3R co-immunoprecipitates with the I–II loop of NALCN and that NALCN co-immunoprecipitates with the i3 loop and the carboxy-terminal portion of M3R (Fig 4B,C). Overexpression of these intracellular fragments in MIN6 cells showed that the I–II loop of NALCN and the i3 loop of M3R significantly inhibited the native acetylcholine-activated inward current, probably through uncoupling of NALCN and M3R (loop I–II, 7.8±3.2% of control, N=13, P<0.01; loop i3, 17.3±4.6% of control, N=9, P<0.01; Fig 4D,E). These data suggest that NALCN and M3R coexist in the same protein complex and that this requires molecular determinants present in the I–II loop of NALCN and the i3 loop of M3R.
Figure 4.
NALCN and M3R belong to the same protein complex in HEK-293 cells. (A) NALCN and M3R co-immunoprecipitate (IP) when co-expressed in the HEK-293 cell line. (B) M3R co-immunoprecipitates with the NALCN's GFP-tagged intracellular I–II loop. (C) NALCN co-immunoprecipitates with the M3R's GFP-tagged intracellular segment i3 and the carboxy-terminus (Cter) part. (D) Overexpression of the NALCN's GFP-tagged intracellular loop I–II and (E) M3R's GFP-tagged intracellular segment i3 in the MIN6 cell line results in a strong inhibition of the acetylcholine (ACh)-activated inward current. GFP, green fluorescent protein; HA, haemagglutinin; HEK, human embryonic kidney; M3R, M3 muscarinic receptors; NALCN, sodium leak channel, non-selective; WB, western blot.
Discussion
Our data show that NALCN channels can be activated by M3R receptors without the involvement of G proteins but through an SFK-dependent pathway, and involves the inclusion of the two molecules in the same protein complex. We have shown that the resulting current is endogenous to the MIN6 pancreatic β-cell line, and can be reconstituted in recombinant systems. The M3R-mediated activation of the NALCN current possibly occurs through a direct interaction between these proteins or through interactions resulting from their co-assembly into a larger protein complex. Activation of inward cationic currents similar to those observed in this study and that do not require G-protein activation has been previously described in native pancreatic β-cells (Rolland et al, 2002), CA3 pyramidal neurons (Guerineau et al, 1995) and cardiac myocytes (Shirayama et al, 1993). Further studies are needed to determine the physiological roles of NALCN in these cell types.
Our results show that M3R and NALCN are necessary for recapitulating the native current in recombinant systems, but also suggest that further proteins probably modulate channel activity and/or the level of channel expression at the plasma membrane in native systems. With respect to this idea, it is known that most ion channels exist as heteromultimeric protein complexes in which binding partners modulate the transport and the biophysical properties of the pore-forming subunit (Levitan, 2006). Further work is needed to determine the proteins that might be included in any complex together with NALCN and M3R. One clue about such proteins is provided by genetic evidence suggesting a functional interaction between NALCN orthologues in invertebrates and two proteins named UNC-79 and UNC-80 (Humphrey et al, 2007; Jospin et al, 2007; Yeh et al, 2008). The corresponding mRNAs are not expressed in HEK-293 cells but are detected in MIN6 cells and in the islets of Langerhans (supplementary Fig S5A,B online). In addition, while this paper was under consideration, a report by Lu et al showed that UNC-80 is required to observe a strong modulation of NALCN activity by substance P through neurokinin 1 receptors in HEK-293 cells (Lu et al, 2009). Whether UNC-80 and/or UNC-79 is required in the NALCN–M3R complex remains to be established.
It should be noted that under our experimental conditions, we have been unable to detect any NALCN-induced leak activity in MIN6 cells, as well as in recombinant expression systems such as HEK-293 cells and Xenopus oocytes. Instead, we observed that NALCN is activated by M3R in our cellular models. It is known that GPCRs can show constitutive activity that is modulated by factors such as binding partners (Ango et al, 2001; Milligan, 2003). Thus, one hypothesis to explain the leak activity described by Lu et al (2007) in neurons is that it results from constitutive activation of NALCN by one or more GPCRs. Lu et al (2007) were also able to record a leak current after expression of NALCN in HEK-293 cells. We, and others, have been unable to observe any leak activity in this cell type (M. Biel, personal communication). However, this cell line is known to show a variation in the repertoire of expressed genes depending on the culture procedure, passage number and sub-clone types (Thomas & Smart, 2005), thus providing a possible explanation for this discrepancy.
In conclusion, our study indicates that NALCN is activated by M3Rs in the MIN6 cell line and might behave as a leak channel depending on the cellular environment. Our study provides new insights into the properties of NALCN channels.
Methods
Reagents. Human complementary DNAs (cDNAs) encoding wild-type M3R, Flag-tagged M3R and Flag-tagged β2-adrenergic receptor, as well as the pLVTHM vector were provided by Drs E. Lutz, M. Hosey and D. Trono, respectively. All drugs were purchased from Sigma (Saint-Quentin, Fallavier, France), with the exception of PP1 and SU6656 (Calbiochem, Nottingham, UK).
Cloning of human NALCN and antibody generation. Human NALCN cDNA was amplified from human brain RNA using RT–PCR (Clontech, Palo Alto, CA, USA) and cloned into pcDNA5FRT (Invitrogen, Cergy Pontoise, France). The NALCN rabbit polyclonal antibody was generated with a glutathione-S-transferase fusion protein of the last 288 amino acids of NALCN and affinity-purified.
Other constructs. Haemagglutinin-tagged human NALCN, Flag-tagged M4R (amino terminus) and M3R* were generated by PCR and cloned into pcDNA5FRT (Invitrogen).
Lentivirus-mediated RNA interference. The shRNAs used in this study target sequences encoding for the III–IV linker (no alternative splicing events detected; shRNA-1: AAGAUCGCACAGCCUCUUCAU; shRNA-2: AAUGUAUGACAUAACCCAGC; and control: GCUCAGUACGAUCAUACUCAC). Corresponding pairs of oligonucleotides were annealed and cloned in the pLVTHM lentiviral vector. Cells were transduced at a multiplicity of infection of 10.
Quantitative RT–PCR. DNase-treated (Ambion, Austin, TX, USA) RNAs were reverse-transcribed using random hexameric oligonucleotides and MoMuLV-RT (Invitrogen). Real-time PCR was carried out in duplicate (7500 System; Applied Biosystems, Courtaboeuf, France), according to the manufacturer's instructions. Target RNA expression level was normalized to β2-microglobulin, according to the 2−ΔCt method (2−(Ct(X)−Ct(B2M)), where Ct is the threshold cycle for the target RNA (Ct(X)) and β2-microglobulin (Ct(B2M)).
Electrophysiology. Patch-clamp measurements were carried out in whole-cell voltage-clamp configuration (Axopatch 200B patch clamp amplifier, Clampex9.2 software (Axon Instruments, Molecular Devices, Wokingham, UK)). Pipettes were pulled from borosilicate glass to give 4−6 MΩ resistance. Acetylcholine-activated currents were measured in cells held at −80 mV at room temperature (20–25°C). The extracellular solution consisted of NaCl 119 mM, KCl 4 mM, KH2PO4 1.2 mM, MgSO4 1.2 mM, CaCl2 2.5 mM, Na2CO3 0.42 g/l, HEPES 20 mM and glucose 10 mM (pH 7.2 with NaOH; osmolarity approximately 290 mOsm). The pipette solution consisted of K-aspartate 100 mM, KCl 25 mM, NaCl 10 mM, Mg–ATP 2 mM, Na–GTP 0.1 mM, creatine phosphate 5 mM, HEPES 5 mM, CaCl2 0.04 mM and MgCl2 1 mM (pH 7.2 with KOH; osmolarity ∼290 mOsm). Alterations to solutions are noted in the text and figure legends wherever appropriate. Current amplitude was defined as peak-current value during acetylcholine application compared with baseline. Nuclear injection and recording of macroscopic currents in Xenopus oocytes were carried out as reported previously (Altier et al, 2001).
Data analysis. Data are presented as mean±s.e.m. Statistical significance was evaluated using Student's t-test for paired or unpaired data (significance level 0.05). Multiple comparisons were carried out by analysis of variance followed by Tukey's multiple comparison test. *P<0.05; **P<0.01; ***P<0.001.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank E. Lutz, M. Hosey and D. Trono for kindly providing some of the constructs used in this study, C. Barrère and I. Bidaud for technical assistance, and T.P. Snutch and J. Bockaert for comments on the paper. L.A.S. was supported by a Marie Curie Incoming International Postdoctoral Fellowship. This work was supported by the Programme National de Recherche sur le Diabète de l' Institut National de la Santé et de la Recherche Médicale, as well as by the Association de Recherche sur le Diabète.
Footnotes
The authors declare that they have no conflict of interest.
References
- Altier C, Spaetgens RL, Nargeot J, Bourinet E, Zamponi GW (2001) Multiple structural elements contribute to voltage-dependent facilitation of neuronal alpha 1C (CaV1.2) L-type calcium channels. Neuropharmacol 40: 1050–1057 [DOI] [PubMed] [Google Scholar]
- Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L (2001) Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature 411: 962–965 [DOI] [PubMed] [Google Scholar]
- Ashcroft FM (2006) From molecule to malady. Nature 440: 440–447 [DOI] [PubMed] [Google Scholar]
- Bockaert J, Fagni L, Dumuis A, Marin P (2004) GPCR interacting proteins (GIP). Pharmacol Ther 103: 203–221 [DOI] [PubMed] [Google Scholar]
- Guerineau NC, Bossu JL, Gahwiler BH, Gerber U (1995) Activation of a nonselective cationic conductance by metabotropic glutamatergic and muscarinic agonists in CA3 pyramidal neurons of the rat hippocampus. J Neurosci 15: 4395–4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JA, Hamming KS, Thacker CM, Scott RL, Sedensky MM, Snutch TP, Morgan PG, Nash HA (2007) A putative cation channel and its novel regulator: cross-species conservation of effects on general anesthesia. Curr Biol 17: 624–629 [DOI] [PubMed] [Google Scholar]
- Jospin M, Watanabe S, Joshi D, Young S, Hamming K, Thacker C, Snutch TP, Jorgensen EM, Schuske K (2007) UNC-80 and the NCA ion channels contribute to endocytosis defects in synaptojanin mutants. Curr Biol 17: 1595–1600 [DOI] [PubMed] [Google Scholar]
- Kaczorowski GJ, McManus OB, Priest BT, Garcia ML (2008) Ion channels as drug targets: the next GPCRs. J Gen Physiol 131: 399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J, Hellmiss R, Duerson K, Ennulat D, David N, Clapham D, Peralta E (1990) Distinct sequence elements control the specificity of G protein activation by muscarinic acetylcholine receptor subtypes. EMBO J 9: 4381–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Cribbs LL, Perez-Reyes E (1999) Cloning of a novel four repeat protein related to voltage-gated sodium and calcium channels. FEBS Lett 445: 231–236 [DOI] [PubMed] [Google Scholar]
- Levitan IB (2006) Signaling protein complexes associated with neuronal ion channels. Nat Neurosci 9: 305–310 [DOI] [PubMed] [Google Scholar]
- Lu B, Su Y, Das S, Liu J, Xia J, Ren D (2007) The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell 129: 371–383 [DOI] [PubMed] [Google Scholar]
- Lu B, Su Y, Das S, Wang H, Wang Y, Liu J, Ren D (2009) Peptide neurotransmitters activate a cation channel complex of NALCN and UNC-80. Nature 457: 741–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarrigle D, Huang XY (2007) GPCRs signaling directly through Src-family kinases. Sci STKE 2007: pe35. [DOI] [PubMed] [Google Scholar]
- Milligan G (2003) Constitutive activity and inverse agonists of G protein-coupled receptors: a current perspective. Mol Pharmacol 64: 1271–1276 [DOI] [PubMed] [Google Scholar]
- Rolland JF, Henquin JC, Gilon P (2002) G protein-independent activation of an inward Na(+) current by muscarinic receptors in mouse pancreatic beta-cells. J Biol Chem 277: 38373–38380 [DOI] [PubMed] [Google Scholar]
- Rosenblum K, Futter M, Jones M, Hulme EC, Bliss TV (2000) ERKI/II regulation by the muscarinic acetylcholine receptors in neurons. J Neurosci 20: 977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama T, Matsumoto K, Pappano AJ (1993) Carbachol-induced sodium current in guinea pig ventricular myocytes is not regulated by guanine nucleotides. J Pharmacol Exp Ther 265: 641–648 [PubMed] [Google Scholar]
- Snutch TP, Monteil A (2007) The sodium ‘leak' has finally been plugged. Neuron 54: 505–507 [DOI] [PubMed] [Google Scholar]
- Thomas P, Smart TG (2005) HEK293 cell line: a vehicle for the expression of recombinant proteins. J Pharmacol Toxicol Methods 51: 187–200 [DOI] [PubMed] [Google Scholar]
- Yeh E et al. (2008) A putative cation channel, NCA-1, and a novel protein, UNC-80, transmit neuronal activity in C. elegans. PLoS Biol 6: e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information