Abstract
Background and purpose
In our search for an indirect dopamine agonist as therapy for cocaine addiction, several selective inhibitors of the dopamine transporter (DAT), which are 3-phenyltropane analogues, were assayed for their effect on locomotor activity in mice. Interestingly, several of the compounds showed a poor correlation between stimulation of locomotion and DAT inhibition. One of the compounds, 3β-(4-methylphenyl)-2β-[3-(4-chlorophenyl)isoxazol-5-yl]tropane (RTI-371), was shown to cross the blood-brain barrier, by binding studies in vivo, and block cocaine-induced locomotor stimulation. As poor pharmacokinetics could not explain the behavioural effects of RTI-371, this compound was screened through our functional assays for activity at other CNS receptors. Initial screening identified RTI-371 as a positive allosteric modulator of the human CB1 (hCB1) receptor.
Experimental approach
The effect of RTI-371 and other DAT-selective inhibitors on CP55940-stimulated calcium mobilization was characterized in a calcium mobilization-based functional assay for the hCB1 receptor. Selected compounds were also characterized in a similar assay for human µ opioid receptor activation to assess the specificity of their effects.
Key results
RTI-371 and several other DAT-selective inhibitors with atypical actions on locomotor behaviour increased the efficacy of CP55940 in a concentration-dependent manner.
Conclusions and implications
These results suggest that the lack of correlation between the DAT-binding affinity and locomotor stimulation of RTI-371 could be due at least in part to its activity as a positive modulator of the hCB1 receptor.
Keywords: cannabinoid receptor, allosteric modulation, 3-phenyltropane, calcium mobilization
Introduction
Cocaine addiction continues to be a large problem in the United States with an estimated 1.6 million users of this drug aged 12 or older (Substance Abuse and Mental Health Services Administration, 2006), and the yearly costs associated with health care and lost productivity that accompany cocaine addiction are estimated to be in the billions (Volkow and Li, 2005). Thus, there remains an urgent need to develop effective cocaine addiction pharmacotherapies. Cocaine has been shown to inhibit the reuptake of noradrenaline, 5-HT and dopamine by their respective monoamine transporters, noradrenaline transporter, serotonin transporter and dopamine transporter (DAT) (Koe, 1976; Wise, 1984; Gu et al., 1994). The inhibition of the DAT and subsequent elevation of synaptic dopamine levels are believed to be the biochemical events underlying the reinforcing effects of cocaine (Ritz et al., 1987; Bergman et al., 1989; Madras et al., 1989; Kuhar et al., 1991). Synthetic DAT-selective inhibitors produce cocaine-like behavioural stimulation and substitute for cocaine in drug discrimination experiments (Koetzner et al., 1996; Tamiz et al., 2001; Cook et al., 2002; Katz et al., 2004; Carroll et al., 2006a, Carroll et al., 2006b). These and other data have led to the development of selective DAT inhibitors as indirect agonist pharmacotherapy for the long-term treatment for cocaine addiction (Newman and Kulkarni, 2002; Runyon and Carroll, 2006).
Although most of the high-affinity selective DAT inhibitors developed for this purpose produce behavioural effects similar to those seen with cocaine administration, behavioural testing has identified a few DAT-selective inhibitors that do not stimulate behaviour and do not substitute for cocaine in drug discrimination tests. One such compound, the benztropine N-butyl-3α-[bis(4-fluorophenyl)methoxy]tropane (JHW007) (Figure 1), is a potent inhibitor of [3H]dopamine uptake (Agoston et al., 1997) that also antagonizes the locomotor effects of cocaine (Desai et al., 2005). JHW007 also binds to muscarinic receptors but experiments with muscarinic antagonists suggested that lack of locomotor stimulation was not due to muscarinic receptor inactivation (Tanda et al., 2007). Slow onset of DAT inhibition has been postulated as a possible explanation for the lack of intrinsic activity but it does not fully explain the antagonism of cocaine's in vivo effects, because JHW007 does not cause locomotor stimulation despite high levels of DAT occupancy (Desai et al., 2005). Another highly DAT-selective inhibitor, 3β-(4-methylphenyl)-2β-[3-(4-chlorophenyl)isoxazol-5-yl]tropane (RTI-371), also does not stimulate locomotor activity in mice (Carroll et al, 2004a; Carroll et al, 2006a). This compound crosses the blood-brain barrier, as it was shown to displace the in vivo binding of [125I]RTI-55 in rat caudate, and, similarly to JHW007, it antagonized the locomotor effects of cocaine (Navarro et al., 2005).
Figure 1.
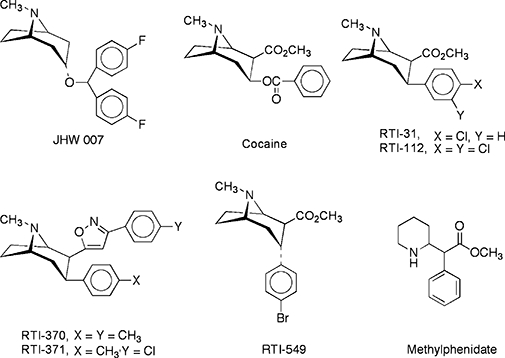
Structures for cocaine, JHW007, RTI-31, RTI-112, RTI-370, RTI-371, RTI-549 and methylphenidate.
Because the endocannabinoid system can affect dopamine neurotransmission (Fernandez-Ruiz et al., 2002) and cause hypolocomotion, we evaluated RTI-371 for intrinsic, antagonist and allosteric modulatory activity at the human CB1 (hCB1) receptor using a functional assay based on calcium mobilization. Here we report that RTI-371 and other DAT inhibitors with a similar in vitro and in vivo pharmacological profile were positive allosteric modulators of the hCB1 receptor.
Methods
Initial in vivo screening
The mice were housed and cared for in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals, and all animal work was conducted under an approved and active Institutional Animal Care and Use Committee protocol. The test compounds were screened for their effect on mouse locomotor activity as part of our general procedures for characterizing DAT-selective compounds. Adult male mice (CD-1; Charles River Laboratories, Raleigh, NC; 6 per dose group) were used in standard locomotor activity tests. Briefly, horizontal locomotion was monitored by photocells in a cage-rack system (San Diego Instruments, San Diego, CA). One mouse was placed in each cage and allowed to habituate for 30 min, after which it was administered (i.p.) test compound (up to a dose of 30 mg·kg−1) or 0.5% methylcellulose (vehicle). Activity was recorded in 10 min bins for 4 h.
Calcium flux assays
Test compounds
The test compounds were stored as 10 mmol·L−1 solutions in 100% DMSO. The CP55 940 [(-)-5-(1,1-dimethylheptyl)-2-[5-hydroxy-2-(3-hydroxypropyl)cyclo-hexyl]-phenol] was stored in 100% ethanol. The final assay concentrations of DMSO and ethanol were 0.5% for test and control samples.
Human CB1 receptor
The cDNA for this receptor was purchased from the UMR cDNA Resource Center (University of Missouri-Rolla, Rolla, MO), and it was stably transfected in the RD-HGA16 cells (CHO cell; Molecular Devices, Sunnyvale, CA). These cells overexpress the promiscuous G protein Gα16 and enable CB1 receptor activation to be coupled to the mobilization of internal calcium. This provides a rapid, robust and non-radioactive homogeneous (no separation) functional assay for the hCB1 receptor. The calcium 3 dye assays (Molecular Devices) were run according to manufacturer's specifications. Briefly, the wells of black clear-bottom 96-well tissue culture-treated plates (Corning, Corning, NY) were seeded with 20 000 cells on the afternoon before assay. On the day of assay, the cells were incubated with the calcium indicator dye (1 h; 37°C; 0.5× suggested concentration). The duplicate samples of test compounds were first evaluated at 10 µmol·L−1 for intrinsic and antagonist activity using a 6 point, log unit, concentration-response curve of test compound or CP55940. For antagonist assays, the test compound was pre-incubated with the cells during the last 15 min of the dye incubation. The assay plate was then placed into a FlexStation384 (Molecular Devices) pre-warmed to 37°C. Basal (unstimulated) fluorescence intensity was recorded from each well for 13 s followed by the addition of test compound (intrinsic activity) or CP55940 (antagonist assay). Fluorescence intensity was recorded for an additional 47 s. The effect of test compound in each well during this period was determined by using the MAX-MIN function in the analysis software. It became clear from the initial screening that some of the test compounds in the antagonist assay appeared to enhance the effect of CP55940. For this reason, the test compounds were assayed for their ability to act as positive allosteric modulators of the hCB1 receptor. For these assays, 10 point, half log unit, concentration-response curves for CP55940 were generated in the presence and absence of a single concentration of test compound (15 min pre-incubation). The assay data from each plate were normalized to the net fluorescence intensity recorded for 1 µmol·L−1 CP55940.
Human µ opioid receptor (hMOR)
This receptor was also purchased from the UMR cDNA Resource Center and stably transfected into the RD-HGA16 cell line to create a calcium flux assay similar to that described for the hCB1. These cell lines were used for negative control assays for RTI-371 and JHW007 to determine if their positive allosteric effects on hCB1 receptor activation were specific for hCB1 receptors or were the result of non-specific effects of the test compounds on RD-HGA16 cell responsiveness in the calcium dye assays. The agonist used for these experiments was d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO), and the assays were run as described above for the evaluation of positive allosteric activity at hCB1 receptors.
Data analysis
A four-parameter logistic equation was fit to the calcium flux concentration response data with Prism (v5 for Macintosh, GraphPad Software; San Diego, CA) and used to calculate the EC50, Emax and Hill slope for CP55940 in the presence and absence of test compound. A global analysis of the EC50, lower asymptote, Emax and Hill slope values was performed by one-way anova for each parameter. The effects of individual compounds on the best fit parameters of the agonist concentration response curve were evaluated with the ‘comparisons’ feature within the non-linear curve fitting analysis in Prism. All the concentration-response data were included in these analyses. The concentration-response data in the figures represent the averaged relative fluorescence ± SEM from all experiments. Statistical significance was assumed at P < 0.05 for main effects.
Materials
The 3-phenyltropanes and JHW007 were synthesized at Research Triangle Institute. Structures are shown in Figure 1. Cocaine, GBR12909 (1-[2-[bis-(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine dihydrochloride), DAMGO, WIN55212-2 and CP55940 were provided by the National Institute on Drug Abuse through its Drug Supply Program. Tissue culture supplies were obtained from the cell culture facility at Duke University (Durham, NC). General laboratory supplies were obtained from Sigma-Aldrich (St. Louis, MO). Nomenclature for drugs and molecular targets conforms with the British Journal of Pharmacology Guide to Receptors and Channels (Alexander et al., 2008).
Results
In vivo screening of RTI compounds
All of the compounds tested were highly selective for the DAT but their locomotor effects were variable (Table 1). RTI-370 and 371 had little or no effect on locomotor activity, whereas RTI-112 and GBR12909 showed less stimulation than cocaine. RTI-31 and RTI-549 displayed stimulation similar to that of cocaine.
Table 1.
Transporter selectivity and locomotor effects of DAT inhibitors (from earlier work)
| Compound | DAT IC50 (nmol·L−1) | NET Ki (nmol·L−1) | SERT Ki (nmol·L−1) | Locomotor activity (% of cocaine stimulation) |
|---|---|---|---|---|
| Cocaine | 89 ± 5 | 523 ± 45a | 1988 ± 150a | 100 ± 22 |
| (Carroll et al., 1995) | ||||
| RTI-31 | 1.12 ± 0.1 | 18.5 ± 1a | 27 ± 1a | 110 ± 18 |
| (Carroll et al., 1995) | ||||
| RTI-112 | 0.81 ± 0.05 | 18 ± 1a | 6.6 ± 0.1a | 74 ± 27 |
| (Carroll et al., 1995) | ||||
| RTI-370 | 13 ± 2 | >100 000 | >100 000 | 1 ± 2 |
| (Carroll et al., 2004a) | ||||
| RTI-371 | 8.7 ± 1.7 | 3990 ± 270 | >100 000 | 5 ± 3 |
| (Carroll et al., 2004a) | ||||
| RTI-549 | 1.7 ± 0.4 | 16 ± 2 | 21 ± 3 | 112 ± 23 |
| (Carroll et al., 2004b) | ||||
The test compounds were all highly selective for the DAT; nevertheless, some produced little or no locomotor stimulation. The effect of the test compounds on locomotor stimulation in male CD-1 mice was normalized to the maximum stimulation observed with 30 mg·kg−1 (i.p.) cocaine. The highest concentration of test compound used in these experiments was 30 mg·kg−1 (i.p.). The data from the transporter binding experiments represent the mean ± SEM from at least three independent experiments.
Ki values estimated from the IC50 values.
Calcium flux assays for hCB1 receptors
The compounds did not have measurable intrinsic activity at 10 µmol·L−1 (data not shown). In keeping with this, the results at the lower end of the concentration-response curves were similar to the CP55940 control (P= 0.99). Global analysis of data indicated a significant effect of test compound on the Emax (P < 0.0001) and EC50 (P < 0.0001) for CP55940, but not on the Hill slope (P= 0.88). Based on this, the effects of individual compounds on the EC50 and Emax for CP55940 were examined (Table 2, Figures 2 and 3). The 3-phenyltropane RTI-371 (Figure 2) at 10 µmol·L−1 but not at 1 µmol·L−1, increased the efficacy (36%; P < 0.0001) and the potency (P < 0.0001) of CP55940. Pre-incubation with 10 µmol·L−1 RTI-370 (Figure 2), which is structurally similar to RTI-371, showed the same concentration-dependent effect on efficacy as RTI-371, with 10 µmol·L−1 causing a 23% elevation in the Emax (P < 0.0001) for CP55 940. Unlike RTI-371, RTI-370 at 1 µmol·L−1 (P= 0.05) but not 10 µmol·L−1 (P= 0.27) increased agonist potency (Table 2). We also tested the benztropine JHW007 (Figure 2), which has high affinity for the DAT but with the same paradoxical lack of stimulatory effect on locomotor activity as RTI-370 and RTI-371. JHW007 was a more potent positive allosteric modulator than either RTI-370 or RTI-371, as 1 µmol·L−1 increased the efficacy (34%; P < 0.0001) and caused a small decrease in EC50 (P < 0.0001). This effect on Emax was concentration-dependent as it was increased by 76% in the presence of 10 µmol·L−1 JHW007 (P < 0.0001) but the EC50 of CP55940 was unaffected (P= 0.16). RTI-370, RTI-371 and JHW007 were the only compounds that had readily apparent positive allosteric effects below the CP55 940 EC50.
Table 2.
Effects of pre-incubation with DAT inhibitors on CP55 940-mediated calcium mobilization
| CP55 940 |
RTI-371 |
RTI-370 |
JHW007 |
RTI-31 |
10 µmol·L−1112 |
RTI-549 |
Cocaine |
GBR12909 |
Methylphenidate |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 µmol·L−1 | 10 µmol·L−1 | 1 µmol·L−1 | 10 µmol·L−1 | 1 µmol·L−1 | 10 µmol·L−1 | 10 µmol·L−1 | 10 µmol·L−1 | 10 µmol·L−1 | 10 µmol·L−1 | 1 µmol·L−1 | 10 µmol·L−1 | 10 µmol·L−1 | ||
| Emax (%) | 100 | 107 | 136* | 105 | 123* | 134* | 176* | 100 | 113* | 114* | 111* | 151* | 165* | 110* |
| 2 | 7 | 5 | 6 | 6 | 6 | 6 | 7 | 7 | 7 | 6 | 11 | 6 | 6 | |
| EC50 (nmol·L−1) | 11.4 | 9.7 | 4.7* | 7.9* | 9.3 | 5.1* | 8.7 | 15.5 | 9.3 | 13.2 | 16.1* | 54.9* | 35.5* | 20* |
| 10–13 | 6–14 | 2–9 | 5–11 | 5–17 | 4–7 | 5–15 | 12–20 | 6–15 | 10–17 | 13–21 | 14–212 | 26–48 | 14–32 | |
| Hill slope | 1.0 | 1.0 | 0.9 | 1.6 | 1.4 | 1.3 | 1.2 | 1.3 | 1.0 | 1.4 | 1.2 | 0.6 | 1.3 | 0.9 |
| 0.1 | 0.4 | 0.2 | 0.5 | 0.3 | 0.2 | 0.2 | 0.4 | 0.3 | 0.3 | 0.3 | 0.2 | 0.1 | 0.2 | |
This Table contains the Emax, EC50 and Hill slope data for the data presented in Figure 2. The data represent the mean ± SEM or 95% confidence intervals from at least three separate experiments for each compound. The 95% confidence interval is given for the EC50 data because the error is not symmetrically distributed around the mean. The asterisk indicates significant difference from the corresponding CP55 940 value.
Figure 2.
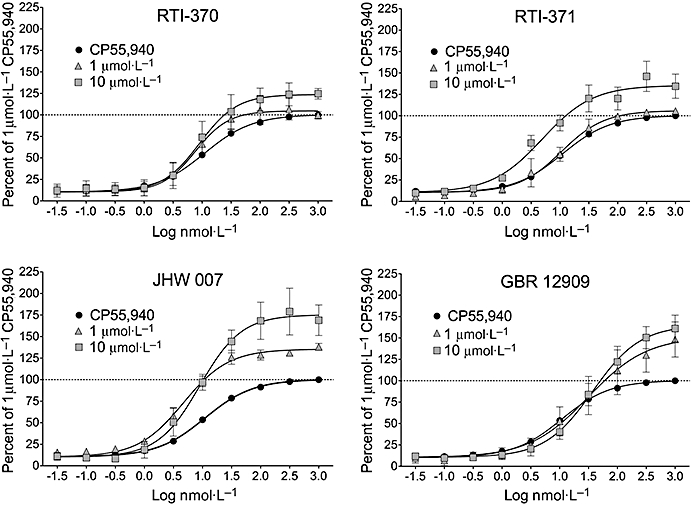
Positive allosteric modulation of hCB1 receptor activation. RTI-370, RTI-371, JHW007 and GBR12909 increased the intrinsic activity of the agonist with variable effects on potency. The data represent the mean ± SEM from at least three independent experiments per compound. The maximum net (MAX-MIN) relative fluorescence units in this assay were typically 4000–5000.
Figure 3.
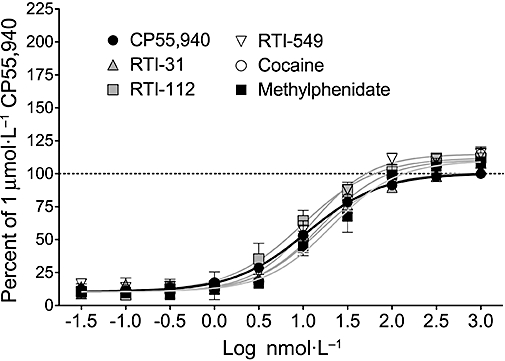
Effects of other 3-phenyltropanes, cocaine and methylphenidate. These compounds produced locomotor behaviour similar to those observed with cocaine and had little or no allosteric modulator activity. The data represent the mean ± SEM from at least three independent experiments per compound.
We also evaluated GBR12909, a relatively selective DAT inhibitor with a slow onset of action (Figure 2). Interestingly, at 10 µmol·L−1 it was the second most effective positive allosteric modulator, increasing the CP55940 Emax by 65% (P < 0.0001); but unlike the other compounds that had no effect or caused a slight leftward shift in CP55940 potency, GBR12909 caused a significant, threefold rightward shift in the agonist EC50 (P < 0.0001). Similar effects on efficacy and potency were observed at 1 µmol·L−1 GBR12909 (Table 2). We also tested at 10 µmol·L−1 cocaine, methylphenidate and several other 3-phenyl tropanes that have high affinity for the DAT but that caused the expected stimulation of rodent locomotor activity based on their affinity for the DAT (Figure 3). All but RTI-31 (P= 0.87) caused a small but significant increase in the Emax for CP55940. For example, methylphenidate (P < 0.02), RTI-112 (P < 0.01), RTI-549 (P < 0.0001) and cocaine (P < 0.02) elevated the CP55940 Emax by 11–15%. Among these compounds, only cocaine (P < 0.05) and methylphenidate (P < 0.0001) displayed a significant rightward shift of the EC50 value.
Calcium flux assays for hMOR
To determine if the positive allosteric effect on hCB1 receptor activation was specific to this receptor or the result of a non-specific effect of the test compounds or solvents on agonist-stimulated calcium mobilization, RTI-371 and JHW007 were evaluated for their ability to alter DAMGO-stimulated calcium mobilization in CHO cells where the hMOR was also coupled to Gq (Figure 4). Global analysis of the data indicated that pre-incubation with 10 µmol·L−1 RTI-371 or 1 or 10 µmol·L−1 JHW007 had no effect on the EC50 (P= 0.32) or Hill slope (P= 0.49) but a significant effect on Emax (P < 0.001) (Table 3). Analysis of the effects of individual compounds showed that the effect was confined to 10 µmol·L−1 JHW007 (P < 0.001) which caused a 30% reduction in the Emax for DAMGO (Figure 4), in contrast to its positive allosteric effect on hCB1 receptor activation. These results indicate the positive allosteric effects of the compounds on the Emax for CP55940 are not secondary to non-specific effects of the compounds or solvents.
Figure 4.
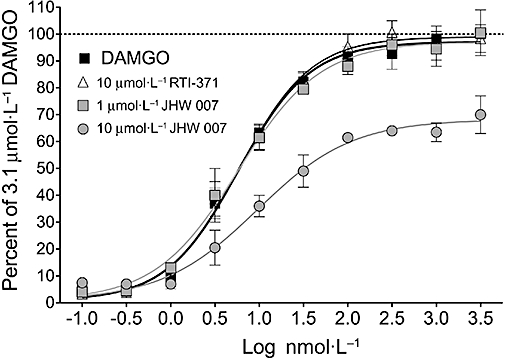
DAMGO-mediated calcium mobilization. The specificity of the positive allosteric effect of the two most active compounds in the hCB1 receptor assays was evaluated in this assay as a control for non-specific effects of the compounds or solvents on calcium mobilization. In contrast to their effects at hCB1 receptors, these compounds at 10 µmol·L−1 had no effect on, or inhibited, the DAMGO-stimulated calcium mobilization. The data represent the mean ± SEM from at least two independent experiments per compound. The maximum net (MAX-MIN) relative fluorescence units in this assay were typically 9000–10 000.
Table 3.
Effects of pre-incubation with DAT inhibitors of DAMGO-mediated calcium mobilization
| DAMGO |
RTI-371 |
JHW007 |
JHW007 |
|
|---|---|---|---|---|
| 10 µmol·L−1 | 1 µmol·L−1 | 10 µmol·L−1 | ||
| Emax (%) | 97 | 99 | 97 | 68* |
| 2 | 2 | 2 | 2 | |
| EC50 (nmol·L−1) | 5.7 | 6 | 5.7 | 9.5 |
| 4.5–7.3 | 4.7–7.5 | 4.2–7.9 | 5.5–16 | |
| Hill slope | 1.1 | 1.1 | 0.9 | 0.8 |
| 0.1 | 0.1 | 0.1 | 0.2 |
This Table contains the Emax, EC50 and Hill slope data for the data presented in Figure 3. The 95% confidence interval is given for the EC50 data because the error is not symmetrically distributed around the mean. The asterisk indicates significant difference from the corresponding DAMGO value.
Discussion
Allosteric modulators are compounds that bind to a receptor and indirectly affect the actions of compounds binding to the orthosteric site. The results of this study indicate that several DAT-selective uptake inhibitors that do not produce the expected locomotor behavioural stimulation in mice are positive allosteric modulators of hCB1 receptors. That is, the binding of these compounds causes a structural change in the hCB1 receptor such that the intrinsic activity of CP55940 is enhanced, perhaps by stabilizing the active conformation of the receptor (Jensen and Spalding, 2004) or a subset of active conformations, increasing receptor effector coupling efficiency, or both. The positive allosteric effect of RTI-370, RTI-371 and JHW007 occurred with no or small increases in potency, whereas the positive allosteric effect of GBR12909 was accompanied by a decrease in agonist potency. This suggests that binding of these modulators can cause a conformational change in the receptor that alters the apparent binding affinity of CP55940. This has been observed for several Organon compounds that increased the affinity of [3H]CP55 940 for the mouse CB1 receptor (Price et al., 2005; Horswill et al., 2007) and with the muscarinic receptor allosteric modulator, heptane-1,7-bis-(dimethyl-3′-phthalimidopropyl) ammonium bromide (Christopoulos et al., 1999). Because different agonists can have varying effects on receptor activation, we also evaluated the allosteric effects of 10 µmol·L−1 RTI-371, JHW007 and GBR12909 on the hCB1 receptor, using WIN55212-2 activation of the receptor. Our preliminary data indicate their effects on intrinsic activity were similar to those seen with CP55940, but in contrast, potency was not affected. This suggests these positive modulators have the potential to differentially affect the actions of agonists. Additional work is needed to determine if these modulators alter agonist affinity for hCB1 receptors, activation of second messenger signalling cascades or both.
That these DAT-selective uptake inhibitors are positive allosteric modulators of the hCB1 receptor in vitro raises the interesting possibility that they are modulating the effects of DAT inhibition in vivo by enhancing endocannabinoid neurotransmission. Δ9-Tetrahydrocannbinol, a psychoactive cannabinoid from marijuana, produces a tetrad of effects that includes reduced locomotor activity (Martin et al., 1991), as do synthetic cannabinoids (Romero et al., 2002). The inhibitory effects of these compounds are mediated by the CB1 receptor (Howlett, 2005). In vivo evidence indicates that endocannabinoids also reduce locomotor activity (Fernandez-Ruiz and Gonzales, 2005). For example, the endocannabinoid anandamide is highly concentrated in the basal ganglia (see De Petrocellis et al., 2004), and rats treated with anandamide show reduced locomotor activity with parallel decreases in nigrostriatal dopaminergic activity (Romero et al., 1995). The release of anandamide in the striatum is linked to the release of dopamine and dopamine D2 receptor activation (Giuffrida et al., 1999; Ferrer et al., 2003), and the cannabinoid receptor antagonist SR141716A enhances the locomotor effects of the D2 receptor agonist quinpirole, suggesting that the endocannabinoid system counters the stimulatory effects of dopamine (Giuffrida et al., 1999). In keeping with this, reductions in levodopa-induced dyskinesias are observed following administration of the CB1 receptor agonist WIN55212-2 (Ferrer et al., 2003). Levodopa also selectively elevates anandamide levels in the basal ganglia, implying involvement of this endocannabinoid in the modulation of dopamine neurotransmission in this brain region (Ferrer et al., 2003). Elevating endocannabinoid levels by inhibiting their reuptake has also been shown to reduce hyperkinetic symptoms in an animals model of Huntington's disease (Lastres-Becker et al., 2002), further supporting the idea that enhancing endocannabinoid neurotransmission has the potential to decrease locomotor activity. How much enhancement of neurotransmission via hCB1 receptors is needed to counteract dopamine-mediated locomotor behaviour is not known, nor is the effect these positive modulators have on receptor activation by endocannabinoids. Selective functional activation of specific receptor conformations is also possible, in which case different in vitro end points will need to be evaluated to better understand the effect these modulators are having on hCB1 receptor activation.
In summary, in a cell-based calcium mobilization assay, we have identified several DAT-selective inhibitors that are positive allosteric modulators of the hCB1 receptor. Enhanced endocannabinoid neurotransmission could contribute to the atypical locomotor effects observed with these compounds. Although more work is necessary, compounds with these dual properties could be useful Parkinson's disease medications, as they would increase dopaminergic neurotransmission but have potentially fewer motor side effects. Studies are currently underway to determine whether these compounds have a similar effect on activation of hCB2 receptors.
Acknowledgments
This research was supported by the National Institute on Drug Abuse, Grant DA 05477. The authors wish to thank Dr Brian F. Thomas of the Research Triangle Institute for providing the hCB1 receptor overexpressing cell line used in our experiments, and Ms Tiffany Langston for her technical assistance.
Glossary
Abbreviations
- CP55940
(-)-5-(1,1-dimethylheptyl)-2-[5-hydroxy-2-(3-hydroxypropyl)cyclo-hexyl]-phenol
- DAMGO
d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- DAT
dopamine transporter
- GBR12909
1-[2-[bis-(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine dihydrochloride
- hCB1
human cannabinoid 1 receptor
- hMOR
human µ opioid receptor
- [125I]RTI-55
[125I]-3β-(4-iodophenyl)-2β-carboxylic acid methyl ester
- JHW007
N-butyl-3α-[bis(4-fluorophenyl)methoxy]tropane
- RTI-371
3β-(4-methylphenyl)-2β-[3-(4-chlorophenyl)isoxazol-5-yl]tropane
- SR141716A
N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide
- WIN55212-2
(R)-(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo-[1,2,3-de]-1,4-benzoxazinyl](1-napthalenyl)methanone mesylate
Conflict of interest
None.
References
- Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH, et al. Novel N-substituted 3 alpha-[bis(4′-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem. 1997;40(26):4329–4339. doi: 10.1021/jm970525a. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd) 2008;153(Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251(1):150–155. [PubMed] [Google Scholar]
- Carroll FI, Kotian P, Dehghani A, Gray JL, Kuzemko MA, Parham KA, et al. Cocaine and 3β-(4′-substituted phenyl)tropane-2 beta-carboxylic acid ester and amide analogues. New high-affinity and selective compounds for the dopamine transporter. J Med Chem. 1995;38(2):379–388. doi: 10.1021/jm00002a020. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Pawlush N, Kuhar MJ, Pollard GT, Howard JL. Synthesis, monoamine transporter binding properties, and behavioral pharmacology of a series of 3beta-(substituted phenyl)-2beta-(3′-substituted isoxazol-5-yl)tropanes. J Med Chem. 2004a;47(2):296–302. doi: 10.1021/jm030453p. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Runyon SP, Abraham P, Navarro H, Kuhar MJ, Pollard GT, et al. Monoamine transporter binding, locomotor activity, and drug discrimination properties of 3-(4-substituted-phenyl)tropane-2-carboxylic acid methyl ester isomers. J Med Chem. 2004b;47(25):6401–6409. doi: 10.1021/jm0401311. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Fox BS, Kuhar MJ, Howard JL, Pollard GT, Schenk S. Effects of dopamine transporter selective 3-phenyltropane analogs on locomotor activity, drug discrimination, and cocaine self-administration after oral administration. Eur J Pharmacol. 2006a;553(1–3):149–156. doi: 10.1016/j.ejphar.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Howard JL, Howell LL, Fox BS, Kuhar MJ. Development of the dopamine transporter selective RTI-336 as a pharmacotherapy for cocaine abuse. AAPS J. 2006b;8(1):E196–E203. doi: 10.1208/aapsj080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos A, Sorman JL, Mitchelson F, El-Fakahany EE. Characterization of the subtype selectivity of the allosteric modulator heptane-1,7-bis-(dimethyl-3′-phthalimidopropyl) ammonium bromide (C7/3-phth) at cloned muscarinic acetylcholine receptors. Biochem Pharmacol. 1999;57(2):171–179. doi: 10.1016/s0006-2952(98)00277-9. [DOI] [PubMed] [Google Scholar]
- Cook CD, Carroll FI, Beardsley PM. RTI 113, a 3-phenyltropane analog, produces long-lasting cocaine-like discriminative stimulus effects in rats and squirrel monkeys. Eur J Pharmacol. 2002;442(1–2):93–98. doi: 10.1016/s0014-2999(02)01501-7. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141(5):765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL. Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci. 2005;25(8):1889–1893. doi: 10.1523/JNEUROSCI.4778-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Gonzales S. Cannabinoid control of motor function at the basal ganglia. Handb Exp Pharmacol. 2005;168:479–507. doi: 10.1007/3-540-26573-2_16. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Lastres-Becker I, Cabranes A, Gonzalez S, Ramos JA. Endocannabinoids and basal ganglia functionality. Prostaglandins Leukot Essent Fatty Acids. 2002;66(2–3):257–267. doi: 10.1054/plef.2001.0350. [DOI] [PubMed] [Google Scholar]
- Ferrer B, Asbrock N, Kathuria S, Piomelli D, Giuffrida A. Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa-induced dyskinesias. Eur J Neurosci. 2003;18(6):1607–1614. doi: 10.1046/j.1460-9568.2003.02896.x. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2(4):358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Gu H, Wall SC, Rudnick G. Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J Biol Chem. 1994;269(10):7124–7130. [PubMed] [Google Scholar]
- Horswill JG, Bali U, Shaaban S, Keily JF, Jeevaratnam P, Babbs AJ, et al. PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB1 receptors with hypophagic effects in rats. Br J Pharmacol. 2007;152(5):805–814. doi: 10.1038/sj.bjp.0707347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005;168:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Spalding TA. Allosteric modulation of G-protein coupled receptors. Eur J Pharm Sci. 2004;21(4):407–420. doi: 10.1016/j.ejps.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Katz JL, Kopajtic TA, Agoston GE, Newman AH. Effects of N-substituted analogs of benztropine: diminished cocaine-like effects in dopamine transporter ligands. J Pharmacol Exp Ther. 2004;309(2):650–660. doi: 10.1124/jpet.103.060525. [DOI] [PubMed] [Google Scholar]
- Koe BK. Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther. 1976;199(3):649–661. [PubMed] [Google Scholar]
- Koetzner L, Riley AL, Glowa JR. Discriminative stimulus effects of dopaminergic agents in rhesus monkeys. Pharmacol Biochem Behav. 1996;54(2):517–523. doi: 10.1016/0091-3057(95)02282-1. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14(7):299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, Hansen HH, Berrendero F, De Miguel R, Perez-Rosado A, Manzanares J, et al. Alleviation of motor hyperactivity and neurochemical deficits by endocannabinoid uptake inhibition in a rat model of Huntington's disease. Synapse. 2002;44(1):23–35. doi: 10.1002/syn.10054. [DOI] [PubMed] [Google Scholar]
- Madras BK, Fahey MA, Bergman J, Canfield DR, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. I. [3H]cocaine binding sites in caudate-putamen. J Pharmacol Exp Ther. 1989;251(1):131–141. [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, et al. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40(3):471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- Navarro H, Howard J, Pollard G, Carroll F. The DAT-selective 3-phenyltropane, RTI-371, antagonizes the in vivo effects of cocaine. 67th Annual Meeting of the College on Problems of Drug Dependence. p. 124.
- Newman AH, Kulkarni S. Probes for the dopamine transporter: new leads toward a cocaine-abuse therapeutic – a focus on analogues of benztropine and rimcazole. Med Res Rev. 2002;22(5):429–464. doi: 10.1002/med.10014. [DOI] [PubMed] [Google Scholar]
- Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, et al. Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol. 2005;68(5):1484–1495. doi: 10.1124/mol.105.016162. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237(4819):1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Romero J, Garcia L, Cebeira M, Zadrozny D, Fernandez-Ruiz JJ, Ramos JA. The endogenous cannabinoid receptor ligand, anandamide, inhibits the motor behavior: role of nigrostriatal dopaminergic neurons. Life Sci. 1995;56(23–24):2033–2040. doi: 10.1016/0024-3205(95)00186-a. [DOI] [PubMed] [Google Scholar]
- Romero J, Lastres-Becker I, de Miguel R, Berrendero F, Ramos JA, Fernandez-Ruiz J. The endogenous cannabinoid system and the basal ganglia: biochemical, pharmacological, and therapeutic aspects. Pharmacol Ther. 2002;95(2):137–152. doi: 10.1016/s0163-7258(02)00253-x. [DOI] [PubMed] [Google Scholar]
- Runyon SP, Carroll FI. Dopamine transporter ligands: recent developments and therapeutic potential. Curr Top Med Chem. 2006;6(17):1825–1843. doi: 10.2174/156802606778249775. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2005 National Survey on Drug Use and Health: National Findings. Rockville, MD: Office of Applied Studies; 2006. NSDUH Series H-30. DHHS Publication No. SMA 06-4194. [Google Scholar]
- Tamiz AP, Bandyopadhyay BC, Zhang J, Flippen-Anderson JL, Zhang M, Wang CZ, et al. Pharmacological and behavioral analysis of the effects of some bivalent ligand-based monoamine reuptake inhibitors. J Med Chem. 2001;44(10):1615–1622. doi: 10.1021/jm000552s. [DOI] [PubMed] [Google Scholar]
- Tanda G, Ebbs AL, Kopajtic TA, Elias LM, Campbell BL, Newman AH, et al. Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. J Pharmacol Exp Ther. 2007;321(1):334–344. doi: 10.1124/jpet.106.118067. [DOI] [PubMed] [Google Scholar]
- Volkow N, Li TK. The neuroscience of addiction. Nat Neurosci. 2005;8(11):1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neural mechanisms of the reinforcing action of cocaine. NIDA Res Monogr. 1984;50:15–33. [PubMed] [Google Scholar]



