Abstract
The exploitation of parental care is common in avian and insect ‘cuckoos’ and these species engage in a coevolutionary arms race. Caterpillars of the lycaenid butterfly Niphanda fusca develop as parasites inside the nests of host ants (Camponotus japonicus) where they grow by feeding on the worker trophallaxis. We hypothesized that N. fusca caterpillars chemically mimic host larvae, or some particular castes of the host ant, so that the caterpillars are accepted and cared for by the host workers. Behaviourally, it was observed that the host workers enthusiastically tended glass dummies coated with the cuticular chemicals of larvae or males and those of N. fusca caterpillars living together. Cuticular chemical analyses revealed that N. fusca caterpillars grown in a host ant nest acquired a colony-specific blend of cuticular hydrocarbons (CHCs). Furthermore, the CHC profiles of the N. fusca caterpillars were particularly close to those of the males rather than those of the host larvae and the others. We suggest that N. fusca caterpillars exploit worker care by matching their cuticular profile to that of the host males, since the males are fed by trophallaxis with workers in their natal nests for approximately ten months.
Keywords: lycaenidae, ants, social parasite, cuticular hydrocarbons, chemical mimicry, brood parasite
1. Introduction
The exploitation of parental or maternal care is common in avian and insect brood parasites (Wilson 1971; Davies et al. 1989). Such ‘cuckoo’ species and their host engage in a coevolutionary arms race (Dawkins & Krebs 1979; Davies et al. 1989), which involves parasitic adaptations for deceiving host, and host defences as counter adaptations (Soler & Møller 1990; Lotem et al. 1995; Foitzik et al. 2001; Nash et al. 2008). This arms race escalates through the exaggeration of obvious traits for deceive and defence, and parasites often use sophisticated strategies to be tolerated and fed by the host species. For example, female birds of avian common cuckoo, Cuculus canorus lay striking mimetic eggs to avoid rejection by the host (Brooke & Davies 1988). After hatching, their brood uses the visual signal of gape and exaggerated vocal stimulus to induce feeding by its host foster parents (Davies et al. 1998; Kilner et al. 1999).
Insect brood parasitism is well known in the parasites of social insects (Schmid-Hempel 1998; Thomas et al. 2005). They penetrate and inhabit an insect society to exploit any resource that is valued and protected by the host species. Therefore, like avian cuckoos, they use manipulative signals to avoid rejection and to induce care by host species. The signals used so that they are tolerated by host species have been well studied, especially in parasites of ants and wasps. These signals are principally cuticular hydrocarbons (CHCs), which convey important information in insect societies, such as colony, age, caste and task (reviewed in Howard & Blomquist 2005). By wearing either unnoticeable CHCs (chemical insignificance; Lenoir et al. 2001a) or host-like CHCs (chemical mimicry and camouflage; Dettner & Liepert 1994), the parasites gain access to the host society (Akino et al. 1999; Lenoir et al. 2001a; Sledge et al. 2001). Even after successful accession, the parasites and their brood demand care and food supply from the host workers (Wilson 1971). These requirements are communicated by mechanical and chemical signals (Hölldobler & Wilson 1990; Zimmerli & Mori 1993), although there are only few studies on signals used to get care from the host species in insect cuckoos (Cammaerts 1992; Cervo et al. 2004).
Insect cuckoo species are also found, for example, lycaenid butterflies. These include approximately 6000 species, 200 of which are suspected to be ant parasites (Pierce et al. 2002). After the parasitic lycaenid caterpillars are adopted by the restricted host ant species, they either prey on host ant larvae (predatory) or feed primarily on regurgitations of the ant workers (cuckoos) (Thomas & Elmes 1998). The cuckoo Maculinea rebeli caterpillars are adopted by their host Myrmica ants, owing to their mimicking of ant larva chemicals (Elmes et al. 1991; Fiedler et al. 1996; Akino et al. 1999). Cuckoo caterpillars are preferentially reared by Myrmica ant workers rather than their own ant larvae inside the ant nest (Thomas et al. 1998), as is the case for avian cuckoo species (Soler et al. 1995). This preferential care could stem from additional chemical signals, for example, hydrocarbons, and these chemicals could act as ‘markers’ for a high caste of host ant (Schönrogge et al. 2004).
Here, we examined chemical mimicry in the parasitic lycaenid butterfly, Niphanda fusca. It is univoltine, and the newly hatched caterpillars initially feed on excretions of the aphids tended by the Japanese monogynous carpenter ant Camponotus japonicus. The caterpillars are carried by C. japonicus workers into their nest when they reach the third instar. They are fed by ant worker trophallaxis to grow (figure S1 in the electronic supplementary material) and pupate inside the ant nest after hibernation. As the pupae eclose in the next late spring, they spend approximately 10 months in the host ant nest. The caterpillars survive only when associated with C. japonicus (Nagayama 1950), so it is crucial for them to avoid host attacks, as well as to receive worker care.
We hypothesized that the adopted N. fusca caterpillars have host colony-specific CHC profiles, because CHC profiles serve as the nest-mate recognition pheromone in C. japonicus (Ozaki et al. 2005). Furthermore, since cuticular chemical profiles are also used for recognition of typical caste and tasks in the societies, we hypothesized that N. fusca caterpillars induce worker care by matching their cuticular chemical profiles to the typical castes that are fed by worker ants. To test the above hypotheses, we conducted behavioural assays and comparative gas chromatograph analyses on the cuticular chemicals.
2. Material and methods
(a) Collection and rearing of study animals
We collected approximately 50 eggs of N. fusca with aphids (species not identified) feeding on the Japanese pampas grass Miscanthus sinensis and a tending C. japonicus colony with workers, broods, males and female sexuals (unmated alates), but no mature queen, at Fujinomiya in Shizuoka prefecture, Japan in 2003. We also collected final (fifth or sixth) instar caterpillars with C. japonicus workers, broods, males and female sexuals in 2003, 2004 and 2006 at the same site in Shizuoka, Japan. In the laboratory, the newly hatched caterpillars were reared with the aphids, because they initially feed on the honeydew of aphids. When the caterpillars grew to third instars, they were moved to the foraging arena of the C. japonicus colony to be adopted by the workers. The third and final instar caterpillars were reared with C. japonicus at room temperature in a plastic box (350×250×60 mm) serving as a foraging arena, in which a two nest-boxes (110×75×30 mm) had been placed. The nest-box was covered by a glass plate (120×90×3 mm) to allow for observations and removal of individuals. Mealworms and 10 per cent sucrose aqueous solution were provided as food in the arena box twice in a week.
(b) Behavioural bioassays
Immediately before the experiments, one of the nest-boxes was emptied, and then 20 workers were randomly chosen and returned to the nest-box. The nest entrance was sealed with cotton and was later removed from the nest entrance. The workers settled down for 10 min by covering the box with a glass plate. Each test specimen was quietly placed in the centre of the foraging box so as not to disturb the ant workers. Ten minutes after placing the specimen, we recorded all events occurring between the workers and the specimen by a video camera (IXY DV M5; Canon, Japan) for 5 min. All records were reviewed by a single observer for comparative analyses.
To test the ant response to living broods, both C. japonicus kin larvae and final instar caterpillars of N. fusca were picked up from a single C. japonicus colony, and individually tested as specimens. The experiment was conducted on two colonies.
In the same manner, glass bead dummies (3.0 mm in diameter) were tested as specimens after being coated with 0.1 individual equivalents of the corresponding extracts from one of C. japonicus worker, male and female sexuals, as well as the N. fusca final instar caterpillar. Each was immersed in approximately 2 ml of n-hexane for 5 min, and the extracted solutions were individually concentrated to 10 μl for applying them to the dummies. Concerning the ant larvae, one larva equivalent of extract was used to treat the glass dummies, because the amounts of larval CHCs were approximately 10 times lower than those of adult CHCs in our preliminary analyses. As a blank control, solvent-treated glass dummies were also tested. The experiment was conducted on four colonies. The observer was not aware of the treated materials.
(c) Solvent extraction and SPME
Five second and three final instar caterpillars were immersed in approximately 2 ml of n-hexane for 5 min respectively. No second instar caterpillars had ever been exposed to any ants in the laboratory, while all of the final instars had been cared for by C. japonicus. The respective extracts were concentrated and chromatographed on approximately 0.2 g of silica gel (230–400 mesh ASTM, 0.040–0.063 mm, Merck Ltd., Germany), and the hydrocarbons were eluted with 2 ml of n-hexane.
Separately, a solid-phase microextraction (SPME) technique was applied for comparison of the cuticular chemical profiles in living ant workers, males, female sexuals, larvae and parasite caterpillars. Immobilized individuals were rubbed 100 times with a SPME fibre coated with a 7 μm PDMS film (SUPELCO, USA). Extraction was conducted on 30 individuals of N. fusca (15 third instar and 15 final instar) and 70 C. japonicus (34 workers, 8 larvae, 15 males and 13 alate queens) from four colonies. The third instar caterpillars had been adopted by host workers for at least one week and their CHC profiles were not statistically separated from final instar (Wilks' λ=0.892, Χ2=2.982, p=0.561).
(d) Chemical analyses
The SPME fibre was directly inserted into the injection port of a gas chromatograph (GC-14A; Shimadzu, Japan) equipped with a non-polar capillary column DB-1 (30 m×0.25 mm×0.25 μm; J & W Scientific, USA) and a flame ionization detector. Injection was made in the splitless mode for 1 min at 300°C, and the detector was at 300°C. The column temperature was maintained at 80°C for 1 min, programmed to 230°C at a rate of 30°C min−1 and from 230°C to 300°C at 5°C min−1. Helium was used as a carrier gas, and the column head pressure was 50 kPa.
GC-MS analyses were achieved using an HP5890-II GC (Hewlett Packard, USA) interfaced to a JEOL SX102A double focusing magnetic sector mass spectrometer (JEOL Ltd., Japan) in EI mode with 70 eV, and operated by an HP model 715/64 computer. The GC was fitted with a DB-1HT column (15 m×0.25 mm×0.15 μm; J &W Scientific, USA), programmed from 50°C for 5 min, 20°C min−1 to 380°C and held for 5 min. Helium was used as a carrier gas and the column head pressure was 60 kPa. Injections were made directly onto the capillary column through the cool on-column injector at 53°C and injection temperature was programmed to be oven temperature plus 3°C thereafter. The MS interface temperature was 380°C. Although different temperature profile was used for the GC and GC-MS, the two sets of peaks could be matched up easily, and the relative peak areas were the same in the two analyses.
(e) Statistical analysis
All statistical analyses were performed using SPSS v. 15.0 (SPSS, Inc. 2006). In both behavioural experiments, the workers showed attending behaviour (resting on a specimen) towards ant larvae, parasite caterpillars and glass dummies coated with extracts. During the attending, a licking behaviour was occasionally observed, but in reviewing videotapes, licking time was difficult to discriminate from attending. Therefore, licking time was included in attending time. Aggressive behaviours (for example, bites, opening mandibles or bending abdomen) were not observed. The attending time was normally distributed in both behavioural experiments (one sample Kolmogorov–Smirnov test for normal distribution; broods assay, p=0.856; extracts assay, p=0.092). To compare the attending time between treatments, a t-test was used in the broods assay. In the extracts assay, attending time were compared using general linear models (GLM) with extracts and colony nested within extracts as fixed factors. Tukey's HSD test was used for subsequent multiple comparisons.
Multivariate analysis was performed to determine whether the predefined groups could be discriminated on the basis of the 18 shared CHC peaks of the individuals constituting the groups. Peak areas were transformed according to Zij=ln[Yij/g(Yj)], where Yij is the peak area i for individual j and g(Yj) is the geometric mean of all peak areas for individual j (Reyment 1988). To investigate whether hydrocarbon profiles of parasitic caterpillars were assigned to the host colony, we first performed principal component analysis (PCA), and only factors with eigenvalues greater than 1 were used in the subsequent discriminant analysis (DA). Four principal components were extracted and were then used as variables in DA by assigning them to the origin of colony. In this analysis, we excluded data on C. japonicus larvae because nest-mate recognition ability of social insects has been principally studied in adult workers and usually non-kin larvae are accepted by workers (Hölldobler & Wilson 1990). We also performed multivariate analysis to determine whether the five castes (N. fusca caterpillars were included as one caste) could be discriminated on the basis of CHC profiles regardless of their colony of origin. In this analysis, three principal components were extracted and then used as variables in DA.
Chemical distances between individual N. fusca caterpillars and each caste of their host ants were calculated as Mahalanobis distances with 18 of the transformed peak areas. Distances were calculated relative to the centroids of each of five combinations (C. japonicus workers, larvae, female sexuals, males and N. fusca caterpillars). Kruskal–Wallis analysis following Mann–Whitney U-test coupled with Bonferroni correction was performed to compare the chemical distances. We also compared the relative proportions of three classes (n-alkane, n-alkene and branched alkane) of 18 shared CHCs. Each composition was compared among four castes of C. japonicus and N. fusca caterpillars using Mann–Whitney U-test coupled with Bonferroni correction.
3. Results
(a) Behavioural experiments
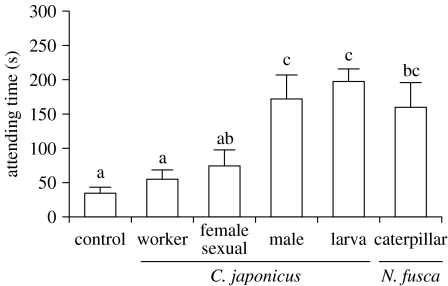
The attending times of workers for N. fusca caterpillars did not differ significantly from that for C. japonicus larvae (mean s±s.e.: C. japonicus larvae 214.9±25.8, n=11; N. fusca caterpillars 183.0±23.1, n=11; t=−0.917, d.f.=20, p=0.370). The ant attending times to extracted chemicals were significantly affected by the kind of extract (GLM; extract, F5,46=14.001, p<0.001), regardless of the ant colonies tested (colony nested with in extract, F12,46=1.152, p=0.344). The extracts of the ant larvae, males and parasite caterpillars made the ant attending times significantly longer than those for the solvent, whereas the attending time for the workers and the female sexuals extracts were not different from those for the solvent (figure 1; Tukey's post hoc test, p<0.05).
Figure 1.
Attending behaviour of workers towards glass bead dummies coated with a cuticular compound of C. japonicus workers (n=14), female sexuals (n=8), males (n=8), larvae (n=14) and N. fusca caterpillars (n=6). Dummies coated with solvent were used as controls (n=14). Significant differences between columns are indicated by different letters (Tukey–Kramer HSD, p<0.05).
(b) Chemical analyses
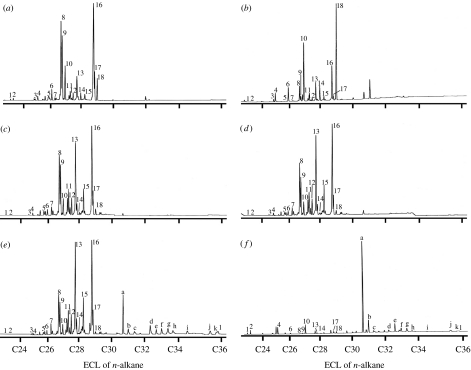
All cuticular chemical compounds were estimated on the basis of the respective retention indices and mass spectra. The cuticular chemicals of C. japonicus workers mainly consisted of hydrocarbons, which gave a total of 18 peaks in the GC profile (figure 2a). Compounds of the 18 peaks were identified as n-alkenes, n-alkanes, and methyl-branched alkanes that contained a total of 20–30 carbons in the molecules. This identification agrees with the report by Ozaki et al. (2005). The larvae, male and female sexuals, as well as workers, had these 18 peaks of the same hydrocarbon compounds (figure 2b–d).
Figure 2.
Gas chromatograms of cuticular compounds from a C. japonicus (a) worker, (b) larva, (c) female sexual, (d) male, and N. fusca (e) final- and (f) second instar caterpillar. Compounds were extracted by SPME methods, except for the N. fusca second-instar caterpillar (f), which was extracted by n-hexane. Peaks were tentatively identified as follows: Peaks: 1, n-C23: 1; 2, n-C23; 3, n-C25: 1; 4, n-C25; 5, n-C26: 1; 6, n-C26; 7, 5,7,12- and 7,9,12-trimeC25; 8, n-C27: 1(9); 9, n-C27: 1(7); 10, n-C27; 11, 13-meC27; 12, 5-meC27; 13, 7,15- dimeC27; 14, n-C28; 15, 5,7,12-trimeC27; 16, n-C29: 1(9); 17, n-C29: 1(7); 18, n-C29; peaks: a, 4-meC30; b, n-C31; c, 13-meC31; d, dimeC31; e, 4-meC32; f, dimeC32; g,13-meC33; h, dimeC33; I, C35: 1; j, 13-meC35; k, 13,17-dimeC35.
The cuticular chemicals of N. fusca caterpillars mainly consisted of hydrocarbons as well (figure 2e,f). However, the hydrocarbon profiles substantially differed before and after the adoption of the caterpillars by C. japonicus. The pre-adoption caterpillars at the second instar had at least 23 hydrocarbons, 11 of which (peaks 1,2,4,6,8–10,13,14,17 and 18) were common to the hydrocarbons of C. japonicus (Ozaki et al. 2005), but the other 12 hydrocarbons were much longer carbon-chained ones that consisted of n-alkanes and methyl-branched alkanes with a total of 31 to 37 carbons in the molecules (peak a-l). The caterpillars possessed these long-chained hydrocarbons in much larger amounts than C. japonicus-like hydrocarbons. The post-adoption caterpillars at the third and final instars possessed both complete sets of C. japonicus-like hydrocarbons and their specific hydrocarbons. However, their C. japonicus-like hydrocarbons were much larger in amounts than their specific hydrocarbons, and their profiles closely resembled those of the C. japonicus workers, larvae, males and female sexuals.
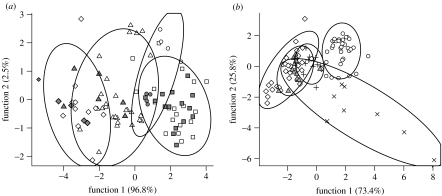
DA, performed by assigning each colony using 18 shared hydrocarbons, suggested that C. japonicus has colony-specific CHC profiles (Wilks' λ=0.121, Χ2=120.552, p<0.001; 82.3 per cent of all the individuals are correctly classified), and it correctly assigned the N. fusca caterpillars to their host colonies (figure 3a; Wilks' λ=0.160, Χ2=159.484, p<0.001; 83.7% of all the individuals are correctly classified). This demonstrates that the CHC profiles of the N. fusca caterpillars were quantitatively similar to the colony they had parasitized.
Figure 3.
(a) DA of C. japonicus adults and N. fusca caterpillars based on four principal components extracted by PCA of 18 shared hydrocarbons. Different symbols indicate four different colonies; open and closed symbols indicate C. japonicus adult and N. fusca caterpillar individuals, respectively. (b) DA of C. japonicus workers, larvae, males, female sexuals and N. fusca caterpillars based on three principal components extracted by PCA of 18 shared hydrocarbons. Ellipses are 90% confidence limit lines. Open circles, workers; crosses, larvae; pluses, female sexuals; triangles, male; diamonds, N. fusca.
By contrast, DA performed by assigning N. fusca caterpillars and individual caste of C. japonicus (figure 3b) indicates that the CHC profiles differed considerably among C. japonicus workers, larvae, males, female sexuals and N. fusca caterpillars (Wilks' λ=0.101, Χ2=218.171, p<0.001; 71.0% of all individuals are correctly classified). Although all categories were statistically separated, C. japonicus larvae were distinguished from C. japonicus adults by discriminant function 2 (figure 3b). By contrast, discriminant function 2 was almost the same among C. japonicus adults, but sexuals and workers were separated by discriminant function 1. Discriminant function 2 of N. fusca caterpillars was almost the same as that for adult C. japonicus. Furthermore, the parasite caterpillars and C. japonicus males were clustered together by both discriminant function 1 and 2.
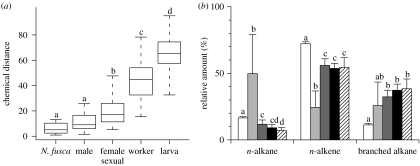
Chemical distances between pairs of N. fusca caterpillars and C. japonicus (figure 4a) were significantly different among the ant castes (Kruskal–Wallis, Χ42=115.191, p<0.001). The chemical distance between N. fusca caterpillars and C. japonicus workers was significantly larger than that of inter individuals among N. fusca caterpillars (Mann–Whitney U-test with Bonferroni correction, p<0.05). This was also true for the distances between the caterpillars and ant larvae, and between the caterpillars and female sexuals (p<0.05), but not between N. fusca caterpillars and ant males (p=0.108). This indicates that CHC profiles were not quantitatively different between N. fusca caterpillars and male ants. The relative proportions of the three chemical classes of CHCs (figure 4b) also differed among groups. Camponotus japonicus larvae had relatively large amounts of n-alkanes and lower amounts of n-alkene than workers (Mann–Whitney U-test with Bonferroni correction, p<0.05). By contrast, C. japonicus males, female sexuals and N. fusca caterpillars had relatively large amounts of methyl-branched alkanes and lower amounts of n-alkane and n-alkene than workers (p<0.05).
Figure 4.
(a) Chemical distances between N. fusca caterpillars from each of four castes (workers, female sexuals, males and larvae) of C. japonicus and N. fusca caterpillars themselves. Box plots shows 75th and 25th percentiles as the box, the median as the line in the box and the extremes as the vertical lines. (b) Variations in the relative percentages of n-alkanes, n-alkenes and methyl-branched alkanes in the CHCs of the four castes of the C. japonicus and the N. fusca caterpillars (mean+s.e.m). Significant differences between columns are indicated by different letters (p<0.05, Mann–Whitney U-test with Bonferroni correction). Open bars, workers; light grey bars, larvae; dark grey bars, female sexuals; black bars, males; hatched bars, N. fusca.
4. Discussion
This study demonstrates that C. japonicus workers care for N. fusca caterpillars as often as their kin larvae, and that they attended to glass dummies treated with the respective cuticular extracts, as well as the ant male cuticular extract (figure 1). These results suggest that the cuticular chemicals of the caterpillars, ant larvae and males induce such attending behaviour of the workers. We also confirmed that the cuticular chemicals of N. fusca caterpillars were drastically changed before and after ant adoption, and the levels of host ant-like CHCs were increased after adoption. The profiles of such increasing hydrocarbons were shared among colony members, and distinguished from those of foreign colonies, as is often pointed out (Sledge et al. 2001; D'Ettorre et al. 2002; Elgar & Allan 2004). When the CHC profiles were compared among the parasites and the four castes of host ant, the post-adoption caterpillars resembled the males, whereas the CHC profiles of ant larvae were quite different. These results suggest that the post-adoption caterpillars exploit worker care by matching their cuticular profiles to host ant males rather than ant larvae.
Since social insects show aggressive behaviour towards non-nest-mates based on differences in the colony odours, the parasites of social insects have the ability to mimic host colony odour (Dettner & Liepert 1994; Lenoir et al. 2001a). Because C. japonicus workers show aggressive behaviour against non-nest-mate CHCs but not nest-mate CHCs (Ozaki et al. 2005), N. fusca caterpillars can avoid aggression of the host workers by wearing colony-specific CHC profiles after adoption. However, CHC profile is not fixed, but changes over time and season in each colony (Nielsen et al. 1999; Liu et al. 2001). Therefore, N. fusca caterpillars probably have the ability to keep adjusting their CHCs to the host colony-specific hydrocarbon profiles. In the host ant nest, N. fusca caterpillars were frequently attended by the workers, and they actively contacted the worker ants. Such physical and social contacts with the host workers will enable parasites to acquire and renew the host colony odour (D'Ettorre et al. 2002), because social interactions, such as allogrooming and trophallaxis, play a key part in the formation of colony odour (Lenoir et al. 2001b).
The CHC profiles of N. fusca caterpillars significantly differed from that of the workers (figure 3b); N. fusca caterpillars had large amounts of methyl-branched hydrocarbons on their cuticles compared with the workers (figure 4b). Because C. japonicus sexuals also have a large amount of methyl-branched hydrocarbons, our results are consistent with the hypothesis that increased hydrocarbons are related to the position of N. fusca caterpillars in the colony's hierarchy and markers for high caste of the host ant (Schönrogge et al. 2004). A previous study reported that the European parasitic lycaenid butterfly M. rebeli biosynthesizes six typical hydrocarbons after intruding into a host Myrmica ant colony (Schönrogge et al. 2004). In N. fusca, since the caste-specific CHCs are increased after adoption, the simplest explanation of our results is that the parasitic caterpillars passively acquire the host-caste-specific CHCs profile (namely, chemical camouflage). In the artificial nest, however, N. fusca caterpillars were mainly attended by the workers and they not actively contact with the host males. Therefore, there is a possibility that N. fusca caterpillars also actively biosynthesize typical compounds, such as methyl-branched hydrocarbons (namely, chemical mimicry) and further study is needed to clarify this.
Cooperative brood care is a key feature of eusociality (Wilson 1971). Therefore, if the larvae of social parasites mimic the host larvae, they are able to receive effective care by the host workers. Our present study does not support this, as the chemical signature of parasite caterpillars was quite different from that of the host larvae. One possible explanation for our result is that parasite caterpillars avoid elimination from workers by mimicking ant males. In social Hymenoptera, since the female broods can develop into an adult queen or worker, there are potential conflicts over caste determination between broods and adult workers (Bourke & Ratnieks 1999). In such conflicts, the workers have opportunities to manipulate developing females by controlling larval nutrition or eliminating larger larvae. In fact, inquiline parasitic ants, which produce no workers but only sexuals, miniaturize their larval size to avoid elimination (Nonacs & Tobin 1992; Aron et al. 1999; but see Aron et al. 2004). In the case of the parasitic lycaenid butterfly, however, their caterpillars develop obviously larger than the host larvae (figure S2 in the electronic supplementary material). Therefore, if the parasite caterpillars chemically mimic the ant larvae, they would be exposed to the risk of elimination as is suggested in Maculinea arion (Thomas & Wardlaw 1990). Males of social Hymenoptera are also at high risk of being eliminated by workers over sex allocation (Trivers & Hare 1976) and worker reproduction (Ratnieks 1988), but male elimination basically occurred during the brood stage in ants of the genus Camponotus (Nonacs & Carlin 1990; Endler et al. 2004). On the other hand, adult males not only contribute nothing to any social performance, but are also highly competitive in begging for food from female colony members (Wilson 1971). Furthermore, in two Camponotus species, males live for approximately ten months in their natal nests and exchange food through trophallaxis with workers (Hölldobler 1966). This is also the case for C. japonicus. Both males and female sexuals of C. japonicus appear in late summer and rest in the colony until the next spring, often undergoing trophallaxis with workers (Wang & Liu 1998; M. K. Hojo 2005–2006, personal observation). This sexual production cycle largely corresponds to the parasite cycle of N. fusca caterpillars, which are adopted in summer and pupate the next spring. Therefore, during the course of growing inside the ant nest, N. fusca caterpillars receive effective care by mimicking host males. To date, much of the work on chemical strategy of parasitic lycaenid butterfly has been done on Maculinea butterflies. Because genus Niphanda (Niphanditi) and Maculinea (Polyommatiti) belong to different subtribes, the chemical strategy of N. fusca probably evolved independently from that of Maculinea butterflies.
Although some studies showed that larvae of social parasites are able to obtain more food than host larvae (Hölldobler 1967; Thomas et al. 1998; Beekman et al. 2000; Cervo et al. 2004), it is not well studied to date how they induce host worker care. Our results demonstrate a novel chemical strategy by the parasitic lycaenid butterfly to induce worker care. Future work in comparative analyses of worker behaviour towards ant larvae, males and N. fusca will clarify how such chemical strategies enable parasites to be preferentially cared for by the host workers.
Because the n-hexane crude extracts were used for behavioural assays, our results show that workers were able to discriminate other castes on the basis of cuticular compounds, but the role of hydrocarbons is controversial. The hexane extracts will probably contain not only hydrocarbons but also other polar lipids. However, for many ant species, there is robust evidence that the hydrocarbon fraction alone is sufficient for the recognition of nest-mates (Lahav et al. 1999; Akino et al. 2004), tasks (Greene & Gordon 2003) and fertility (Dietemann et al. 2003). A previous study showed that a worker of C. japonicus could specifically detect non-nest-mate CHC profiles using a particular chemosensillum on their antennae, although there are slight differences in CHC profiles among nest-mates and non-nest-mates (Ozaki et al. 2005). Therefore, the C. japonicus workers might also use the differences in CHC profiles for caste recognition. Further purification of cuticular chemicals for behavioural and electrophysiological studies is needed to confirm which compounds are responsible for the worker attending behaviour.
The cuticular chemicals of N. fusca caterpillars also contained their own specific hydrocarbons, but these compounds will not induce nursing behaviour in workers. These compounds were also detected from pre-adoption second-instar caterpillars of N. fusca, which induce neither adoption nor nursing behaviour by the host workers. Therefore, we suggest that principal cuticular components involved in the exploitation of the worker care are common compounds between the parasites and the host ants, most of which are acquired after adoption. Nevertheless, pre-adoption second-instar caterpillars have some C. japonicus-like hydrocarbons. In M. rebeli, pre-adoption caterpillars have many compounds similar to those found in host Myrmica larvae (Akino et al. 1999). These host-like compounds induce carrying behaviour by host workers, but this has not been investigated in N. fusca. More studies are needed to clarify whether host-like compounds also induce adoption behaviour in N. fusca caterpillars.
Acknowledgments
We are grateful to David Nash of the University of Copenhagen and two anonymous referees for their valuable comments on this manuscript, and also thank to our collaborators, Takeshi Takeda, Yuji Satoji, Daisuke Imaeda, Hayato Inui and Daisuke Umemoto for their help in the fieldwork.
Supplementary Material
Final instar caterpillar of the N. fusca that is fed by workers via trophallaxis
Niphanda fusca final instar caterpillar with workers, larvae and pupae of the host ant
References
- Akino T., Knapp J.J., Thomas J.A., Elmes G.W. Chemical mimicry and host specificity in the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc. R. Soc. B. 1999;266:1419–1426. doi:10.1098/rspb.1999.0796 [Google Scholar]
- Akino T., Yamamura K., Wakamura S., Yamaoka R. Direct behavioral evidence for hydrocarbons as nestmate recognition cues in Formica japonica (Hymenoptera: Formicidae) Appl. Entomol. Zool. 2004;39:381–387. doi:10.1303/aez.2004.381 [Google Scholar]
- Aron S., Passera L., Keller L. Evolution of social parasitism in ants: size of sexuals, sex ratio and mechanisms of caste determination. Proc. R. Soc. B. 1999;266:173–177. doi:10.1098/rspb.1999.0618 [Google Scholar]
- Aron S., Passera L., Keller L. Evolution of miniaturisation in inquiline parasitic ants: timing of male elimination in Plagiolepis pygmaea, the host of Plagiolepis xene. Insect Soc. 2004;51:395–399. doi:10.1007/s00040-004-0758-9 [Google Scholar]
- Beekman M., Calis J.N.M., Boot W.J. Parasitic honeybees get royal treatment. Nature. 2000;404:723. doi: 10.1038/35008148. doi:10.1038/35008148 [DOI] [PubMed] [Google Scholar]
- Bourke A.F.G., Ratnieks F.L.W. Kin conflict over caste determination in social Hymenoptera. Behav. Ecol. Sociobiol. 1999;46:287–297. doi:10.1007/s002650050622 [Google Scholar]
- Brooke M.D.L., Davies N.B. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature. 1988;335:630–632. doi:10.1038/335630a0 [Google Scholar]
- Cammaerts R. Stimuli inducing the regurgitation of the workers of Lasius flavus (Formicidae) upon the myrmecophilous beetle Claviger testaceus (Pselaphidae) Behav. Process. 1992;28:81–95. doi: 10.1016/0376-6357(92)90051-E. doi:10.1016/0376-6357(92)90051-E [DOI] [PubMed] [Google Scholar]
- Cervo R., Macinai V., Dechigi F., Turillazzi S. Fast growth of immature brood in a social parasite wasp: a convergent evolution between avian and insect cuckoos. Am. Nat. 2004;164:814–820. doi: 10.1086/425987. doi:10.1086/425987 [DOI] [PubMed] [Google Scholar]
- D'Ettorre P., Mondy N., Lenoir A., Errard C. Blending in with the crowd: social parasites integrate into their host colonies using a flexible chemical signature. Proc. R. Soc. B. 2002;269:1911–1918. doi: 10.1098/rspb.2002.2110. doi:10.1098/rspb.2002.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.B., Bourke A.F.G., Brooke M.D.L. Cuckoos and parasitic ants: interspecific brood parasitism as an evolutionary arms race. Trends Ecol. Evol. 1989;4:274–278. doi: 10.1016/0169-5347(89)90202-4. doi:10.1016/0169-5347(89)90202-4 [DOI] [PubMed] [Google Scholar]
- Davies N.B., Kilner R.M., Noble D.G. Nestling cuckoos, Cuculus canorus, exploit hosts with begging calls that mimic a brood. Proc. R. Soc. B. 1998;265:673–678. doi:10.1098/rspb.1998.0346 [Google Scholar]
- Dawkins R., Krebs J.R. Arms races between and within species. Proc. R. Soc. B. 1979;205:489–511. doi: 10.1098/rspb.1979.0081. doi:10.1098/rspb.1979.0081 [DOI] [PubMed] [Google Scholar]
- Dettner K., Liepert C. Chemical mimicry and camouflage. Annu. Rev. Entomol. 1994;39:129–154. doi:10.1146/annurev.en.39.010194.001021 [Google Scholar]
- Dietemann V., Peeters C., Liebig J., Thivet V., Hölldobler B. Cuticular hydrocarbons mediate discrimination of reproductives and nonreproductives in the ant Myrmecia gulosa. Proc. Natl Acad. Sci. USA. 2003;100:10 341–10 346. doi: 10.1073/pnas.1834281100. doi:10.1073/pnas.1834281100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgar M.A., Allan R.A. Predatory spider mimics acquire colony-specific cuticular hydrocarbons from their ant model prey. Naturwissenschaften. 2004;91:143–147. doi: 10.1007/s00114-004-0507-y. doi:10.1007/s00114-004-0507-y [DOI] [PubMed] [Google Scholar]
- Elmes G.W., Thomas J.A., Wardlaw J.C. Larvae of Maculinea rebeli, a large-blue butterfly, and their Myrmica host ants: wild adoption and behaviour in ant-nests. J. Zool. 1991;223:447–460. [Google Scholar]
- Endler A., Liebig J., Schmitt T., Parker J.E., Jones G.R., Schreier P., Hölldobler B. Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc. Natl Acad. Sci. USA. 2004;101:2945–2950. doi: 10.1073/pnas.0308447101. doi:10.1073/pnas.0308447101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K., Hölldobler B., Seufert P. Butterflies and ants: the communicative domain. Experientia. 1996;52:14–24. doi:10.1007/BF01922410 [Google Scholar]
- Foitzik S., DeHeer C.J., Hunjan D.N., Herbers J.M. Coevolution in host–parasite systems: behavioural strategies of slave-making ants and their hosts. Proc. R. Soc. B. 2001;268:1139–1146. doi: 10.1098/rspb.2001.1627. doi:10.1098/rspb.2001.1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M.J., Gordon D.M. Cuticular hydrocarbons inform task decisions. Nature. 2003;423:32. doi: 10.1038/423032a. doi:10.1038/423032a [DOI] [PubMed] [Google Scholar]
- Howard R.W., Blomquist G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005;50:371–393. doi: 10.1146/annurev.ento.50.071803.130359. doi:10.1146/annurev.ento.50.071803.130359 [DOI] [PubMed] [Google Scholar]
- Hölldobler B. Futterverteilung durch Männchen im Ameisenstaat. J. Comp. Physiol. A. 1966;52:430–455. doi:10.1007/BF00302295 [Google Scholar]
- Hölldobler B. Zur Physiologie der Gast-Wirt-Beziehungen (Myrmecophilie) bei Ameisen I. Das Gastverhältnis der Atemeles- und Lomechusa-Larven (Col. Staphylinidae) zu Formica (Hym. Formicidae) J. Comp. Physiol. A. 1967;56:1–21. doi:10.1007/BF00333561 [Google Scholar]
- Hölldobler B., Wilson E.O. The Belknap Press of Harvard University Press; Cambridge, MA: 1990. The ants. [Google Scholar]
- Kilner R.M., Noble D.G., Davies N.B. Signals of need in parent–offspring communication and their exploitation by the common cuckoo. Nature. 1999;397:667–672. doi:10.1038/17746 [Google Scholar]
- Lahav S., Soroker V., Hefetz A., Meer R.K.V. Direct behavioral evidence for hydrocarbons as ant recognition discriminators. Naturwissenschaften. 1999;86:246–249. doi:10.1007/s001140050609 [Google Scholar]
- Lenoir A., D'Ettorre P., Errard C., Hefetz A. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 2001a;46:573–599. doi: 10.1146/annurev.ento.46.1.573. doi:10.1146/annurev.ento.46.1.573 [DOI] [PubMed] [Google Scholar]
- Lenoir A., Hefetz A., Simon T., Soroker V. Comparative dynamics of gestalt odour formation in two ant species Camponotus fellah and Aphaenogaster senilis (Hymenoptera: Formicidae) Physiol. Entomol. 2001b;26:275–283. doi:10.1046/j.0307-6962.2001.00244.x [Google Scholar]
- Liu Z.B., Bagnères A.G., Yamane S., Wang Q.C., Kojima J. Intra-colony, inter-colony and seasonal variations of cuticular hydrocarbon profiles in Formica japonica (Hymenoptera, Formicidae) Insect Soc. 2001;48:342–346. doi:10.1007/PL00001787 [Google Scholar]
- Lotem A., Nakamura H., Zahavi A. Constraints on egg discrimination and cuckoo–host co-evolution. Anim. Behav. 1995;49:1185–1209. doi:10.1006/anbe.1995.0152 [Google Scholar]
- Nagayama H. Life history of Niphanda fusca Bremer et Grey. Insect Ecol. 1950;3:9–18. [Google Scholar]
- Nash D.R., Als T.D., Maile R., Jones G.R., Boomsma J.J. A mosaic of chemical coevolution in a large blue butterfly. Science. 2008;319:88–90. doi: 10.1126/science.1149180. doi:10.1126/science.1149180 [DOI] [PubMed] [Google Scholar]
- Nielsen J., Boomsma J.J., Oldham N.J., Petersen H.C., Morgan E.D. Colony-level and season-specific variation in cuticular hydrocarbon profiles of individual workers in the ant Formica truncorum. Insect Soc. 1999;46:58–65. doi:10.1007/s000400050113 [Google Scholar]
- Nonacs P., Carlin N.F. When can ants discriminate the sex of brood? A new aspect of queen–worker conflict. Proc. Natl Acad. Sci. USA. 1990;87:9670–9673. doi: 10.1073/pnas.87.24.9670. doi:10.1073/pnas.87.24.9670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonacs P., Tobin J.E. Selfish larvae: development and the evolution of parasitic behavior in the Hymenoptera. Evolution. 1992;46:1605–1620. doi: 10.1111/j.1558-5646.1992.tb01157.x. doi:10.2307/2410019 [DOI] [PubMed] [Google Scholar]
- Ozaki M., Wada-Katsumata A., Fujikawa K., Iwasaki M., Yokohari F., Satoji Y., Nisimura T., Yamaoka R. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science. 2005;309:311–314. doi: 10.1126/science.1105244. doi:10.1126/science.1105244 [DOI] [PubMed] [Google Scholar]
- Pierce N.E., Braby M.F., Heath A., Lohman D.J., Mathew J., Rand D.B., Travassos M.A. The ecology and evolution of ant association in the Lycaenidae (Lepidoptera) Annu. Rev. Entomol. 2002;47:733–771. doi: 10.1146/annurev.ento.47.091201.145257. doi:10.1146/annurev.ento.47.091201.145257 [DOI] [PubMed] [Google Scholar]
- Ratnieks F.L.W. Reproductive harmony via mutual policing by workers in eusocial Hymenoptera. Am. Nat. 1988;132:217–236. doi:10.1086/284846 [Google Scholar]
- Reyment R.A. Compositional data analysis. Terra Nova. 1988;1:29–34. doi:10.1111/j.1365-3121.1989.tb00322.x [Google Scholar]
- Schmid-Hempel P. Monographs in Behavior and Ecology. Princeton University Press; Princeton, NJ: 1998. Parasites in social insects. [Google Scholar]
- Schönrogge K., Wardlaw J.C., Peters A.J., Everett S., Thomas J.A., Elmes G.W. Changes in chemical signature and host specificity from larval retrieval to full social integration in the myrmecophilous butterfly Maculinea rebeli. J. Chem. Ecol. 2004;30:91–107. doi: 10.1023/b:joec.0000013184.18176.a9. doi:10.1023/B:JOEC.0000013184.18176.a9 [DOI] [PubMed] [Google Scholar]
- Sledge M.F., Dani F.R., Cervo R., Dapporto L., Turillazzi S. Recognition of social parasites as nest-mates: adoption of colony-specific host cuticular odours by the paper wasp parasite Polistes sulcifer. Proc. R. Soc. B. 2001;268:2253–2260. doi: 10.1098/rspb.2001.1799. doi:10.1098/rspb.2001.1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler M., Møller A.P. Duration of sympatry and coevolution between the great spotted cuckoo and its magpie host. Nature. 1990;343:748–750. doi:10.1038/343748a0 [Google Scholar]
- Soler M., Martinez J.G., Soler J.J., Møller A.P. Preferential allocation of food by magpies Pica pica to great spotted cuckoo Clamator glandarius chicks. Behav. Ecol. Sociobiol. 1995;37:7–13. doi:10.1007/s002650050167 [Google Scholar]
- Thomas J.A., Elmes G.W. Higher productivity at the cost of increased host-specificity when Maculinea butterfly larvae expoit ant colonies through trophallaxis rather than by predation. Ecol. Entomol. 1998;23:457–464. doi:10.1046/j.1365-2311.1998.00153.x [Google Scholar]
- Thomas J.A., Wardlaw J.C. The effect of queen ants on the survival on Maculinea arion larvae in Myrmica ant nests. Oecologia. 1990;85:87–91. doi: 10.1007/BF00317347. doi:10.1007/BF00317347 [DOI] [PubMed] [Google Scholar]
- Thomas J.A., Elmes G.W., Wardlaw J.C. Polymorphic growth in larvae of the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc. R. Soc. B. 1998;265:1895–1901. doi:10.1098/rspb.1998.0517 [Google Scholar]
- Thomas J.A., Schönrogge K., Elmes G.W. Specializations and host associations of social parasites of ants. In: Fellowes M.D.E., Holloway G.J., Rolff J., editors. Insect evolutionary ecology. CABI Publishing; Wallingford, UK: 2005. pp. 479–518. [Google Scholar]
- Trivers R.L., Hare H. Haplodiploidy and the evolution of the social insect. Science. 1976;191:249–263. doi: 10.1126/science.1108197. doi:10.1126/science.1108197 [DOI] [PubMed] [Google Scholar]
- Wang Z.J., Liu Z.B. The nest architecture, frequency distribution of head width and daily activity rhythm of the carpenter ant Camponotus japonicus Mayr. Acta Entomol. Sin. 1998;41:56–60. [Google Scholar]
- Wilson E.O. The Belknap Press of Harvard University Press; Cambridge, MA: 1971. The insect societies. [Google Scholar]
- Zimmerli E.J., Mori A. The role of an attractive brood pheromone in the obligatory, slavemaking ant, Polyergus breviceps (Hymenoptera: Formicidae) J. Insect Behav. 1993;6:761–770. doi:10.1007/BF01201675 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Final instar caterpillar of the N. fusca that is fed by workers via trophallaxis
Niphanda fusca final instar caterpillar with workers, larvae and pupae of the host ant