Abstract
Adult neurogenesis, a developmental process encompassing the birth of new neurons from adult neural stem cells and their integration into the existing neuronal circuitry, highlights the plasticity and regenerative capacity of the adult mammalian brain. Substantial evidence suggests essential roles of newborn neurons in specific brain functions; yet it remains unclear how these new neurons make their unique contribution. Recently, a series of studies have delineated the basic steps of the adult neurogenesis process and shown that many of the distinct steps are dynamically regulated by the activity of the existing circuitry. Here we review recent findings on the synaptic integration and plasticity of newborn neurons in the adult hippocampus, including the basic biological process, unique characteristics, critical periods, and activity-dependent regulation by the neurotransmitters GABA and glutamate. We propose that adult neurogenesis represents not merely a replacement mechanism for lost neurons, but also an ongoing developmental process in the adult brain that offers an expanded capacity for plasticity for shaping the existing circuitry in response to experience throughout life.
Since the time of Cajal, it was generally believed that the mature central nervous system lacks regenerative capacity, including the inability to re-grow injured axons and to generate new neurons. The dogma that no new neurons are generated after birth in mammals was overturned during the last decade by clear demonstrations of newly generated neurons in discrete regions of adult mammalian brains, including humans (Eriksson et al. 1998). Adult neurogenesis represents a developmental process that encompasses the proliferation and fate specification of adult neural stem cells along with their differentiation, maturation, migration and incorporation into the existing neural circuitry of their progeny in the mature nervous system (Ming & Song, 2005). A variety of physiological, pathological and pharmacological stimuli have been shown to regulate distinct steps of adult neurogenesis (Ming & Song, 2005). Rapidly accumulating evidence also suggests essential roles of adult-born neurons in specific brain functions, such as learning, memory and mood regulation (Kempermann et al. 2004b; Kitabatake et al. 2007; Zhao et al. 2008). Aided by new methodologies for birth-dating and tracking new neurons in the adult brain, recent studies have elucidated the basic process of adult neurogenesis (Alvarez-Buylla & Lim, 2004; Ming & Song, 2005; Lledo et al. 2006; Zhao et al. 2008). In particular, studies using bromodeoxyuridine (BrdU)-based birth-dating and immunohistochemistry of cell type specific markers have identified a series of intermediate developmental stages during adult neurogenesis (Kempermann et al. 2004a). In addition, electrophysiology and imaging studies of new neurons labelled with retroviruses and in transgenic reporter mice have characterized the maturation and integration of new neurons in the adult brain (Duan et al. 2008). Furthermore, genetic manipulation of adult neural stem cells and their local environment, or ‘neurogenic niche’, is starting to reveal key molecular mechanisms underlying adult neurogenesis and activity-dependent regulation (Ge et al. 2006; Tashiro et al. 2006; Duan et al. 2007). Here we review recent findings on the synaptic integration and plasticity of newborn dentate granule cells in the adult hippocampus. We discuss activity-dependent regulation of adult hippocampal neurogenesis involving γ-aminobutyric acid (GABA) and glutamate, two major neurotransmitters in the adult brain. We also discuss how new neurons may contribute to specific functions of the adult brain.
Synaptic integration of adult-born dentate granule cells into the existing circuitry
Functional incorporation of new neurons into the adult circuitry was first demonstrated over two decades ago in a landmark study where 1 month after adult canaries were injected with [3H]thymidine to label new cells, four randomly recorded neurons (out of a total of 74 neurons) within a vocal control nucleus in the telencephalon exhibited electrical responses to auditory stimulation and were positive for [3H]thymidine labelling (Paton & Nottebohm, 1984). Over the past few years engineered oncoretrovirus (van Praag et al. 2002) and transgenic reporter mice (Overstreet et al. 2004) have greatly facilitated functional analysis of new neurons in vivo. Studies using these tools have revealed a stereotypic integration process in establishing connections by newborn granule cells in the adult hippocampus (Fig. 1), from initial tonic activation by ambient GABA, to dendritic GABAergic synaptic inputs, followed by dendritic glutamatergic inputs and finally perisomatic GABAergic inputs (Esposito et al. 2005; Overstreet Wadiche et al. 2005, 2006; Tozuka et al. 2005; Wang et al. 2005; Ge et al. 2006; Karten et al. 2006). After a prolonged maturation phase, adult-born neurons exhibit electrical and synaptic properties that are indistinguishable from those of neighbouring mature granule cells when examined by electrophysiology at the single-cell level (Laplagne et al. 2006; Ge et al. 2007b; Laplagne et al. 2007). Alternative approaches with imaging of dendritic spines (Zhao et al. 2006) and analysis of immediate early gene expression (Jessberger & Kempermann, 2003) in adult-born granule cells suggest a similar prolonged time course of synaptic integration and maturation of adult-born neurons. These studies revealed interesting similarities and differences between adult neurogenesis and embryonic/early postnatal neurogenesis. First, incorporation of new neurons into the neuronal circuitry follows a conserved sequence in forming different types of synaptic connections throughout life. Second, the tempo of synaptic integration and maturation is significantly slower during adult neurogenesis than during embryonic and early postnatal neurogenesis.
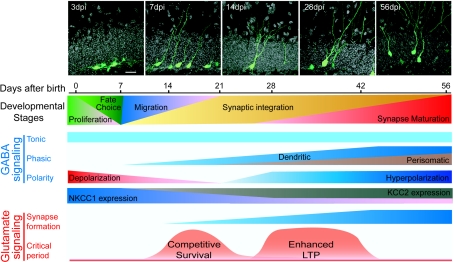
Figure 1. Synaptic integration of new granule cells in the dentate gyrus of the adult mouse hippocampus.
Shown is a schematic summary of the synaptic integration and maturation process of newborn dentate granule cells in the adult mouse hippocampus. Adult neurogenesis is a developmental process consisting of proliferation and fate specification of adult neural progenitors, and migration and integration of new neurons into the existing circuitry. New neurons also follow a stereotypic process to establish different types of synaptic inputs. For GABA signalling, tonic GABA activation occurs first, followed by formation of GABAergic dendritic synaptic inputs and finally perisomatic GABAergic inputs. New neurons exhibit a gradual change in the expression of different chloride transporters. As a consequence, new neurons are initially depolarized by GABA and gradually become hyperpolarized by GABA during their maturation. For glutamate signalling, glutamatergic synapse formation starts after initial synaptogenesis of GABAergic dendritic synaptic inputs and before synaptogenesis of perisomatic GABAergic synaptic inputs. During the maturation stages, there are two critical periods when new neurons are particularly sensitive to glutamatergic signalling, the first involving NR1-dependent competitive survival of new neurons and the second involving NR2B-dependent enhanced synaptic plasticity. The images shown in the top panel were adapted from Ge et al. (2006, 2007b).
The full integration of new neurons requires the establishment of both synaptic inputs and synaptic outputs within the neuronal circuitry. While axons of adult-born granule cells have been shown to extend into the CA3 subfield of the hippocampus (Hastings & Gould, 1999; Markakis & Gage, 1999; Zhao et al. 2006), little is known about properties of their synaptic outputs due to technical hurdles. Electron microscopy reconstruction of the mossy fibre synaptic boutons of retroviral-labelled adult-born neurons suggests that their synaptic outputs exhibit a similar time course of synaptogenesis and maturation to their synaptic inputs (authors' unpublished observations). Future development of novel tools, such as selective optical or chemical stimulation and transneuronal tracers, may facilitate the characterization of the development and properties of synaptic outputs of newborn neurons in the adult brain.
Role of GABA in the integration of newborn dentate granule cells in the adult brain
One hallmark of adult neurogenesis is its regulation by the activity of the existing neuronal circuitry. GABA and glutamate are the major inhibitory and excitatory neurotransmitters, respectively, for mature neurons in the adult brain. These neurotransmitters activate neurons not only locally within synaptic clefts (phasic activation), but also at a distance after diffusion out of synapses (tonic activation) (Farrant & Nusser, 2005; Ge et al. 2007a). GABAARs are chloride (Cl−) permeable channels and the polarity of GABA action depends on the Cl− gradient across the membrane, which in turn is determined by the developmentally regulated expression of C1− transporters (Owens et al. 1996; Ben-Ari, 2002; Tozuka et al. 2005; Ge et al. 2006). Adult hippocampal neural progenitors and immature neurons express functional ionotropic GABA receptors (GABAARs) (Tozuka et al. 2005; Wang et al. 2005; Ge et al. 2006). Newborn granule cells exhibit a gradual decrease in the expression of NKCC1, a Cl− importer, with a concurrent increase in the expression of KCC2, a Cl− exporter, during their maturation process (Fig. 1) (Ge et al. 2006). As a consequence, GABA initially depolarizes immature new neurons because of their high Cl− content and such depolarization is gradually converted to hyperpolarization over a period of 2–3 weeks. Interestingly, retrovirus-mediated expression of short-hairpin RNA against nkcc1 in newborn granule cells abolishes GABA-induced depolarization and leads to significant defects in their dendritic growth and formation of both GABAergic and glutamatergic synaptic inputs. These studies provide the first in vivo evidence for an essential role of GABA-induced depolarization in neuronal development. A similar role of GABA has been demonstrated in the development of mouse embryonic cortical neurons (Cancedda et al. 2007; Wang & Kriegstein, 2008) and Xenopus tectum neurons (Akerman & Cline, 2006) in vivo.
There are several standing questions about the activity-dependent regulation of neuronal development through excitatory GABA signalling. First, it remains to be directly demonstrated that GABA, instead of Cl−, is essential for neuronal development. Second, what is the relative contribution of tonic versus phasic GABA-induced depolarization to neuronal development? Given the extensive recurrent connections between principal neurons and interneurons within the dentate gyrus, the level of ambient GABA may serve as a general indicator of the dynamic neuronal network activity, while GABAergic phasic activation encodes input specific information. Third, how do GABAergic and glutamatergic signalling interact to regulate neuronal development? It is possible that GABAAR signalling cooperates with NMDAR activation to regulate development of new neurons, as recently reported for glutamatergic synapse formation of embryonic cortical neurons (Wang & Kriegstein, 2008). Fourth, what is the downstream signalling mechanism that mediates depolarizing GABA effects in different aspects of neuronal development?
Critical periods of glutamate signalling during new neuron integration in the adult brain
Glutamate signalling has long been implicated in regulating adult hippocampus neurogenesis. Injection of NMDA rapidly decreases cell proliferation in the adult rat dentate gyrus, whereas injection of an NMDAR antagonist exhibits the opposite effect (Cameron et al. 1995; Nacher et al. 2001). On the other hand, induction of long-term potentiation at the glutamatergic medial perforant path–granule cell synapses promotes the proliferation of adult neural progenitors and survival of newborn neurons in an NMDAR-dependent fashion (Bruel-Jungerman et al. 2006; Chun et al. 2006). These findings highlight the complexity of glutamate signalling in regulating adult neurogenesis, which is likely to involve both cell autonomous effects in immature neurons and non-cell autonomous effects through modulation of existing neuronal circuits. Electrophysiological analysis has shown the expression of ionotropic glutamate receptors in immature neurons (Ambrogini et al. 2004; Overstreet Wadiche et al. 2005; Tozuka et al. 2005). Immunohistochemistry studies suggest that the NR1 and NR2B subunits are expressed at early stages during adult neurogenesis (Nacher et al. 2007). Genetic deletion of NR1 in proliferating adult neural progenitors reduces the survival of their neuronal progeny exclusively between 2 and 3 weeks after they are born in the adult brain (Tashiro et al. 2006). Interestingly, injection of an NMDAR antagonist, (+/−)-3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP), to diminish differences in NMDAR signalling in all new neurons promotes survival of these NR1 null neurons. These findings suggest a critical period for NMDAR-dependent competitive survival of newborn neurons in the adult brain (Fig. 1). Interestingly, such a critical period is coincident with a transition from excitatory to inhibitory GABA signalling. Whether GABA cooperates with glutamate signalling in regulating the survival of new neurons during this critical period remains to be determined.
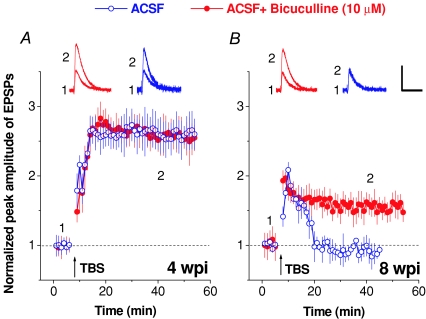
Analysis of the plasticity of glutamatergic synaptic inputs onto newborn granule cells during their maturation process has identified another critical period exhibiting enhanced long-term potentiation (LTP; Fig. 1) (Schmidt-Hieber et al. 2004; Ge et al. 2007b). New neurons within 4–6 weeks after they are born exhibit both reduced induction threshold and increased amplitudes of LTP in response to a physiological pattern of stimulation (Ge et al. 2007b). Interestingly, such a critical period is associated with a developmentally regulated prominent synaptic contribution of NR2B-containing NMDARs in adult-born neurons, since pharmacological inhibition of NR2B-containing NMDARs completely abolished LTP in these neurons, but not in mature neurons. In these initial LTP experiments, bicuculline was added to remove the influence of GABAergic synaptic transmission. We repeated some of these experiments in the absence of bicuculline. Interestingly, 4-week-old adult-born granule cells exhibit enhanced LTP similar to that in the presence of bicculline (10 μm; Fig. 2A). In contrast, no significant LTP was induced by the same theta burst stimulation (TBS) in 8-week-old adult-born granule cells without the presence of bicuculline (Fig. 2A), a result consistent with previous field recordings (Snyder et al. 2001). Thus, the critical period for enhanced synaptic plasticity for these adult-born neurons would be even more striking with intact GABA signalling in the adult brain. The bicuculline insensitivity in LTP expression is not related to differences in the polarity of the GABA response, since the conversion of GABA-induced depolarization to hyperpolarization occurs between two and three weeks after the new neurons are born (Ge et al. 2006). Instead, the interaction between GABA signalling and LTP induction may involve the delayed establishment of perisomatic GABAergic innervations onto new neurons (Fig. 1), which exhibit powerful inhibition of neuronal excitation due to their localization.
Figure 2. Differential modulation of glutamateric synaptic plasticity of adult-born dentate granule cells by GABA.
LTP recorded from GFP+ adult-born dentate granule cells in acute slices prepared at 4 weeks (A) or 8 weeks (B) after retrovirus-mediated birth-dating and expression of GFP as in Ge et al. (2007b). Evoked postsynaptic potentials (EPSPs) were recorded from GFP+ dentate granule cells under the whole-cell current-clamp at 32–34°C and LTP was induced with a physiologically relevant theta burst stimulation (TBS) protocol consisting of four repeated episodes (at 0.1 Hz) of 10 stimuli at 100 Hz, repeated 10 times at 5 Hz and paired with a 100 pA postsynaptic current injection (as in Ge et al. 2007b). Based on the intracellular/extracellular solutions for cells recorded at both time points ([Cl−]i= 19 mm; [Cl−]o= 134.1 mm), the expected chloride reversal potential is −51 mV. Shown in the top row is an example of LTP of EPSPs recorded under the whole-cell current-clamp. Representative EPSPs averaged from five consecutive stimuli were taken before and after LTP induction by a physiologically relevant TBS (arrow) at the time points (1 and 2) indicated in the graph. Scale bars: 5 mV and 50 ms. Shown at the bottom are the summaries of LTP recorded in the presence or absence of bicuculline (10 μm) in the recording bath (artificial cerebrospinal fluid; ACSF). Normalized EPSP amplitudes are shown. Values represent means ±s.e.m. The summary plot for neurons recorded in the presence of bicucculline was adapted from Ge et al. (2007b).
Unique properties and potential functions of newborn neurons in the adult brain
What is the evolutionary pressure for maintaining neurogenesis in specific brain regions? One hypothesis is that because of their unique physiological properties, developing adult-born neurons transiently serve as major mediators for experience-driven plasticity and become selectively integrated as special units within the adult circuitry where they contribute to specific brain functions over the long term.
Adult-born granule cells exhibit a number of unique properties transiently during their integration and maturation process that are distinct from the existing mature neurons, including (i) depolarization by GABA (< 3 weeks old) (Ge et al. 2007b), (ii) enhanced excitability and low LTP induction threshold (∼2–4 weeks old) (Schmidt-Hieber et al. 2004; Ge et al. 2007b), (iii) larger LTP amplitude (∼4–6 weeks old) (Ge et al. 2007b), and (iv) delayed formation of perisomatic GABAergic innervations (> 4 weeks old) (Esposito et al. 2005) (Figs 1 and 2). It is likely that synaptic outputs of new neurons also transiently exhibit unique properties during their maturation. In addition, there are also two critical periods when adult-born neurons are subjected to activity-dependent selective integration into the existing neuronal circuitry, including (i) NMDAR-dependent competitive survival among cohorts of new neurons (∼2–3 weeks old), and (ii) NR2B-dependent enhanced synaptic plasticity of new neurons (∼4–6 weeks old) (Fig. 1). Indeed, a number of studies based on the neuronal expression of the immediate early genes c-Fos and Arc suggest a selective incorporation of adult-born neurons into behaviourally relevant neuronal circuitry and preferential reactivation of these neurons in response to the same stimulation (Ramirez-Amaya et al. 2006; Kee et al. 2007; Tashiro et al. 2007).
Taken together, current evidence demonstrates that adult-born neurons exhibit unique properties during their maturation stages and suggests that a strategic integration of adult-born neurons into the existing circuitry may be the functional basis of their specific contribution to brain functions. We propose that adult neurogenesis represents not merely a basic replacement mechanism for lost neurons, but also is an ongoing developmental process that offers an expanded capacity of plasticity for shaping the existing circuitry in response to experience throughout life. Such a hypothesis can be directly tested in the near future using genetically modified animals with various behavioural tests, such as specific deletion of NR2B-containing NMDARs in new neurons to remove their enhanced plasticity during critical periods.
Summary
Continuous neurogenesis in the adult mammalian brain has attracted both basic and clinical research interests. On one hand, adult hippocampal neurogenesis provides a unique experimental model system to understand basic principles of neuronal development in the mature brain environment with a number of advantages, including a prolonged time course of neuronal development, a single defined neuronal subtype, and established genetic approaches to specifically manipulating new neurons. In addition, the complete neuronal developmental process proceeds within an active mature circuitry, thus offering a system to explore underlying activity-dependent mechanisms. On the other hand, adult neurogenesis highlights the regenerative capacity of the mature brain, not only in generating functional new neurons, but also in providing a permissive environment to guide functional integration. Understanding this unique regenerative process in the adult brain may shed light on strategies to promote functional regeneration after injury or degenerative neurological diseases.
Acknowledgments
The research in the authors' laboratories was supported by the National Institutes of Health, McKnight Scholar Award, the Rett Syndrome Research Foundation, Simons Research Foundation, Maryland Stem Cell Research Fund to H.S., and the National Institutes of Health, Maryland Stem Cell Research Fund, Adelson Medical Research Foundation, and March of Dimes to G.M.S.G. was partially supported by a postdoctoral fellowship from the American Heart Association.
References
- Akerman CJ, Cline HT. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J Neurosci. 2006;26:5117–5130. doi: 10.1523/JNEUROSCI.0319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 2004;1017:21–31. doi: 10.1016/j.brainres.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Davis S, Rampon C, Laroche S. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci. 2006;26:5888–5893. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda L, Fiumelli H, Chen K, Poo MM. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J Neurosci. 2007;27:5224–5235. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun SK, Sun W, Park JJ, Jung MW. Enhanced proliferation of progenitor cells following long-term potentiation induction in the rat dentate gyrus. Neurobiol Learn Mem. 2006;86:322–329. doi: 10.1016/j.nlm.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Kang E, Liu C, Ming GL, Song H-j. Development of neural stem cells in the adult brain. Curr Opin Neurobiol. 2008 doi: 10.1016/j.conb.2008.04.001. DOI 10.101b/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007a;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007b;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- Karten YJ, Jones MA, Jeurling SI, Cameron HA. GABAergic signaling in young granule cells in the adult rat and mouse dentate gyrus. Hippocampus. 2006;16:312–320. doi: 10.1002/hipo.20165. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004a;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004b;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kitabatake Y, Sailor KA, Ming GL, Song H. Adult neurogenesis and hippocampal memory function: new cells, more plasticity, new memories. Neurosurg Clin N Am. 2007;18:105–113. doi: 10.1016/j.nec.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, Schinder AF. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4:e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplagne DA, Kamienkowski JE, Esposito MS, Piatti VC, Zhao C, Gage FH, Schinder AF. Similar GABAergic inputs in dentate granule cells born during embryonic and adult neurogenesis. Eur J Neurosci. 2007;25:2973–2981. doi: 10.1111/j.1460-9568.2007.05549.x. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Nacher J, Rosell DR, Alonso-Llosa G, McEwen BS. NMDA receptor antagonist treatment induces a long-lasting increase in the number of proliferating cells, PSA-NCAM-immunoreactive granule neurons and radial glia in the adult rat dentate gyrus. Eur J Neurosci. 2001;13:512–520. doi: 10.1046/j.0953-816x.2000.01424.x. [DOI] [PubMed] [Google Scholar]
- Nacher J, Varea E, Miguel Blasco-Ibanez J, Gomez-Climent MA, Castillo-Gomez E, Crespo C, Martinez-Guijarro FJ, McEwen BS. N-methyl-d-aspartate receptor expression during adult neurogenesis in the rat dentate gyrus. Neuroscience. 2007;144:855–864. doi: 10.1016/j.neuroscience.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Hentges ST, Bumaschny VF, de Souza FS, Smart JL, Santangelo AM, Low MJ, Westbrook GL, Rubinstein M. A transgenic marker for newly born granule cells in dentate gyrus. J Neurosci. 2004;24:3251–3259. doi: 10.1523/JNEUROSCI.5173-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL. Seizures accelerate functional integration of adult-generated granule cells. J Neurosci. 2006;26:4095–4103. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JA, Nottebohm FN. Neurons generated in the adult brain are recruited into functional circuits. Science. 1984;225:1046–1048. doi: 10.1126/science.6474166. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makion H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Kempermann G, Kettenmann H. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci. 2005;29:181–189. doi: 10.1016/j.mcn.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Wang DD, Kriegstein AR. GABA regulates excitatory synapse formation in the neocortex via NMDA receptor activation. J Neurosci. 2008;28:5547–5558. doi: 10.1523/JNEUROSCI.5599-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]