Abstract
The keratins are the typical intermediate filament proteins of epithelia, showing an outstanding degree of molecular diversity. Heteropolymeric filaments are formed by pairing of type I and type II molecules. In humans 54 functional keratin genes exist. They are expressed in highly specific patterns related to the epithelial type and stage of cellular differentiation. About half of all keratins—including numerous keratins characterized only recently—are restricted to the various compartments of hair follicles. As part of the epithelial cytoskeleton, keratins are important for the mechanical stability and integrity of epithelial cells and tissues. Moreover, some keratins also have regulatory functions and are involved in intracellular signaling pathways, e.g. protection from stress, wound healing, and apoptosis. Applying the new consensus nomenclature, this article summarizes, for all human keratins, their cell type and tissue distribution and their functional significance in relation to transgenic mouse models and human hereditary keratin diseases. Furthermore, since keratins also exhibit characteristic expression patterns in human tumors, several of them (notably K5, K7, K8/K18, K19, and K20) have great importance in immunohistochemical tumor diagnosis of carcinomas, in particular of unclear metastases and in precise classification and subtyping. Future research might open further fields of clinical application for this remarkable protein family.
Keywords: Keratins, Differentiation, Cytoskeleton, Tumor markers
Introduction
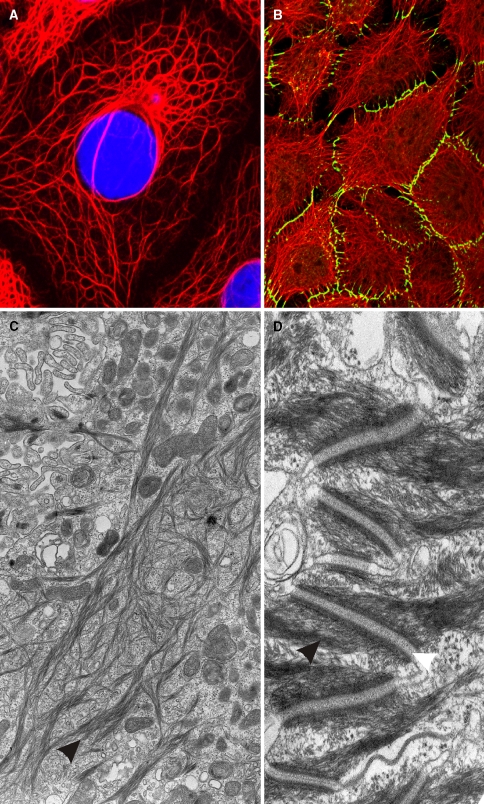
Most eukaryotic cells contain in their cytoplasm a more or less elaborated cytoskeletal system consisting of intermediate filaments (IF), which are chemically very stable long and unbranched filaments of ~10 nm in diameter. Among the various families and subfamilies of IF proteins, that of the keratins is outstanding due to its high molecular diversity. The keratin gene family consists of the highest number of members in humans with 54 distinct functional genes. IF proteins are expressed in a highly cell type-specific manner, and herein keratins represent the typical IF category of epithelial cells. In some but not all epithelia, keratin filaments are conspicuously bundled as tonofilaments. Figure 1 shows these keratin filament bundles at the light microscopical (Fig. 1a, b) and the electron microscopical level (Fig. 1c, d). Inside the cell they braid the nucleus (Fig. 1a), span through the cytoplasm and are attached to the cytoplasmic plaques of the typical epithelial cell–cell junctions, the desmosomes (Fig. 1b, d; for a recent review, see Waschke 2008). This feature already suggests that keratins play a major functional role in the integrity and mechanical stability of both the single epithelial cells and, via cell–cell contacts, of that of the epithelial tissues. Consequently, they are inherent part of the continuum of stability from the single cell to the tissue formation. Evidence for this main function of keratin filaments has been amply provided by the recognition of various hereditary keratin diseases and transgenic mouse models. In addition, however, various regulatory functions have been discovered more recently (for recent reviews, see Magin et al. 2007; Oshima 2007; Uitto et al. 2007; McLean and Irvine 2007).
Fig. 1.
Cytoskeleton of epithelial cells. a Immunofluorescence staining of keratin K18 (red, nuclei stained in blue by DAPI) in PLC (liver carcinoma) cells in vitro. b Keratin filaments (in red) and the desmosomal component desmoplakin (in green) are labeled in cultured keratinocytes of line HaCaT. c Electron microscopic image of tonofilament (keratin) bundles (arrowhead) of HaCaT keratinocytes. d Keratin intermediate filaments (black arrowhead) insert at desmosomes (white arrowhead) at cell–cell contact sites of keratinocytes of the epidermal stratum spinosum (electron microscopy)
Historically, keratin research started with studies of sheep hair (wool) keratins (Crick 1952; Powell and Rogers 1986; Oshima 2007). Several important discoveries were made in the 1970s of the last century. One was the finding of the spontaneous self-assembly and polymerization of keratin filaments from denatured, soluble keratin proteins by dialysis in vitro (Steinert et al. 1976). Further milestones were the findings that antibodies against keratins from epidermis-type epithelia such as the bovine muzzle (“prekeratin”) react with tonofilaments in various epithelial cells including non-stratified “simple” epithelia of inner organs (Franke et al. 1978), and that keratins of various mammalian species exhibit a high degree of molecular diversity with differentiation-specific expression (Franke et al. 1981). Systematic protein biochemical analyses of human cells and tissues by one- and two-dimensional gel electrophoresis, Western blotting and peptide mapping disclosed the diversity of human (cyto)keratin polypeptides (Moll et al. 1982b; Tseng et al. 1982; Wu et al. 1982). From these data, in 1982, the catalog of human cytokeratins including 19 members was proposed (Moll et al. 1982b) which, although intended as provisional, has been widely accepted and used. Along with these studies and subsequently, the principle of separation of these proteins into type I (“acidic”) and type II (“basic to neutral”) keratins (see below) also emerged. Another unique property of the keratins is that in contrast to the other IF proteins they only can constitute their filamentous stage by heteropolymeric pair formation of type I and type II (1:1) molecules. Later on, several new keratins were identified and added to the cytokeratin catalog, the most notable of these being the simple-epithelial keratin 20 (K20; Moll et al. 1990, 1992) and several keratins specific for distinct epithelia such as keratin K2e in the upper epidermis (appendix “e”; now K2), K2p in the upper hard palate epithelium (similar to K2e but with appendix “p” for palate; now K76) (Collin et al. 1992a, b), or several keratin K6 (K6a–h) isoforms (Takahashi et al. 1995). Simultaneously, informations about the keratin gene sequences were revealed.
Moreover, within the last 10 years a large number of hair follicle-specific epithelial keratins were discovered. This series started with K6hf (appendix “hf” stands for “hair follicle expression”, now K75), which was expressed in the hair follicle companion layer (Winter et al. 1998). K75 was the first epithelial keratin specifically expressed in the hair follicle. Surprisingly, there were much more epithelial keratins with hair follicle specificity, namely the type II keratins K6irs1, K6irs2, K6irs3 and K6irs4 (now K71–K74) and type I keratins K25irs1, K25irs2, K25irs3 and K25irs4 (now K25–K28), all of them specifically expressed in and closely restricted to the various compartments of the hair follicle inner root sheath (Langbein et al. 2002, 2003, 2006; for review see Langbein and Schweizer 2005). Besides the variety of epithelial (“soft” or “cyto-”) keratins, hairs and nails are built up from a somewhat separate subfamily of “hard” or “trichocytic” keratins, commonly designated as hair keratins (Heid et al. 1988a; Langbein et al. 1999, 2001, 2004; Langbein and Schweizer 2005; Schweizer et al. 2007). They differ from the epithelial keratins by their considerably higher sulfur content in their non-α-helical head and tail domains, which is mainly responsible for the extraordinary high degree of filamentous cross-linking by keratin-associated proteins (KAPs) (for review, see Rogers et al. 2006).
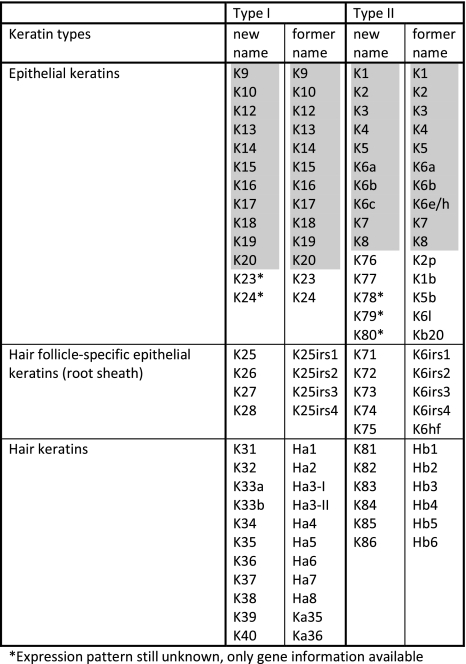
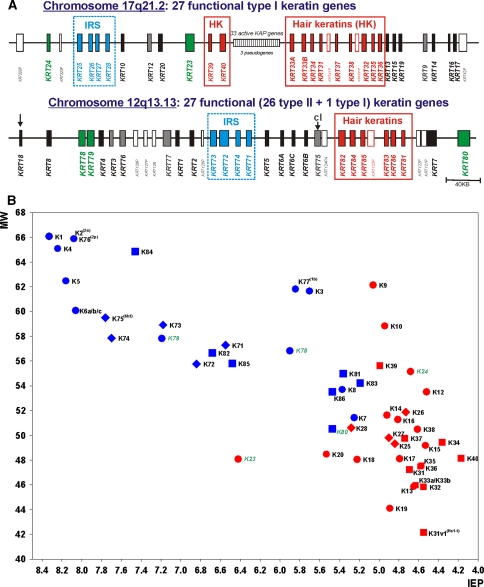
Very recently, the “Keratin Nomenclature Committee” established the novel consensus nomenclature for mammalian keratin genes and proteins (Schweizer et al. 2006), relying upon and systematically extending the aforementioned 1982 catalog. This nomenclature is now in accordance with the nomenclature of the Human Genome Organization (HUGO) for both the gene and protein names. Following the unified new principles, several parts of the former nomenclature were implemented; the hair keratins (e.g. “Ha” and “Hb”) and the special epithelial keratin designations (e.g. K2p, K6hf, K6irs, K25irs) were equally integrated (see Table 1), and the nomenclature system—although now complete for humans—is open to application in other mammalian species by following the same principles. Among human keratins, the new consensus nomenclature (Table 1) comprises the type I keratins K9–K10, K12–K28, and K31–K40 (including K33a and K33b) and the type II keratins K1–K8 (including K6a, K6b and K6c) and K71–K86. Thus, there are 28 type I keratin genes (17 epithelial keratins and 11 hair keratins) and 26 type II keratin genes (20 epithelial keratins and 6 hair keratins). All in all, out of the 54 human keratin genes, at least 26 (~50%) are specifically expressed in the hair follicle. In the human genome, the keratin genes are clustered at two different chromosomal sites: chromosome 17q21.2 (type I keratins, except K18) and chromosome 12q13.13 (type II keratins including K18). The keratin genes are designated as KRT1, KRT2, KRT3, etc. (Schweizer et al. 2006; Fig. 2a).
Table 1.
The new human keratin nomenclature (Schweizer et al. 2006)
Fig. 2.
Human keratins. a Organization of the human keratin genes in the genome. The type I and type II keratin gene subdomains are located on chromosomes 17 and 12, respectively. The type I keratin K18 is located in the type II cluster on chromosome 17 (arrow). b Two-dimensional catalog of the human keratin proteins according to molecular weights (MW) and isoelectric points (IEP) as calculated from amino acid sequences. The keratin genes are designated according to the new keratin nomenclature (Schweizer et al. 2006)
All these keratins belong to the family of IF proteins and therefore share common protein-structural characteristics. They contain a central rod domain of ~310 amino acids with α-helical conformation flanked by non-helical head and tail domains of variable length. The head domain consists of subdomains V1 and H1. The central α-helical rod domain is composed of subdomains 1A, 1B, 2A, and 2B connected by the linkers L1, L12, and L2. The tail domain then consists of subdomains H2 and V2 (Lane and McLean 2004; Parry et al. 2007; Geisler and Weber 1982). The molecular weight of human keratins ranges from ~44 to ~66 kDa (Fig. 2b). A unique feature of keratins, including the hair keratins, is their pairing, i.e. the obligate formation of heterodimers between one type I keratin and one type II keratin. This occurs by association of the corresponding rod domains in α-helical coiled-coil conformation. The resulting heterodimers and -tetramers form the basic building units of the keratin filaments. Single keratin proteins deviating from equimolar type I/type II amounts are rapidly degraded (Lu and Lane 1990).
As keratin filaments are important structural stabilizers of epithelial cells, there is unabatedly high interest in keratins in biology, embryology, pathology, and dermatology. Notably, this main cytoskeletal function transcends the single cell level. Typically, keratin filaments insert at desmosomes (Fig. 1b, d) and hemidesmosomes. Thus, they contribute not only to the stability between epithelial cells itself but also to basement membrane attachment and insofar to the connective tissue compartment of a given epithelium. In the non-stratified (simple) epithelia of internal parenchymatous organs, which experience little mechanical stress, only very few keratin members form sparse and loosely distributed keratin filaments in the cytoplasm. Otherwise, considerably more members take part on the IF cytoskeletal composition of squamous epithelia which increases in the cornified stratified epithelia such as in the epidermis lining the outer body surface where they are abundant and densely bundled as tonofilaments. The loose filaments in the former case are composed of “simple-epithelial keratins” like K8/K18 (and K19), while the bundled filaments (tonofilaments) in the latter case (Fig. 1c, d) are built up from keratinocyte-type keratins such as K5/K14 in the basal layer and—with even more pronounced bundling—K1/K10 in the suprabasal layers and K2/K10 in the uppermost ones. The “rule” that the “stronger/harder” the epithelial structure the more keratin members are involved culminates in the hair fiber where 17 keratins are sequentially expressed. This clearly underscores the importance of the keratins for the tissue integrity and the relevance of the molecular diversity of keratin proteins.
The important mechanical function of stratified epithelial- and epidermis-type keratins is evident and proven not only through knock-out mouse models but also through various human hereditary keratin diseases. Thus, point mutations of distinct keratin genes now widely explain the pathogenesis of several autosomal-dominant familial diseases, many of which are blistering skin diseases. The most well known of these inherited skin fragility disorders is epidermolysis bullosa simplex (EBS), the various variants of which are caused by a spectrum of point mutations of K5 or K14 (Lane and McLean 2004; McLean and Irvine 2007; Uitto et al. 2007). Nineteen different keratin genes including hair keratins and hair follicle-specific epithelial keratins have up to now been identified as being involved in pathogenic keratin mutations (Lane and McLean 2004; Schweizer et al. 2007); they will be discussed in the descriptions of the individual keratins below. Updated details may be retrieved from an Internet database (Human Intermediate Filament Database; http://www.interfil.org). Notably, the knock-out experiments and the genetic diseases demonstrated that mutations in keratin genes often (depending on the locus of mutation within the keratin molecule) cause more severe defects than the complete loss of a keratin gene whose failure might be compensated—if available at this site—by another/other keratin/s.
Moreover, it has been recognized that keratins are not simply static intracellular skeletal structures but rather are highly dynamic. Along with this view, besides their mechanical function new functional roles of keratins have been defined and still emerge under special physiological conditions (Magin et al. 2007; Oshima 2007). These include the protection of the placental and trophoblast barrier function (K8/K18/K19: Jaquemar et al. 2003; Hesse et al. 2000), the protection from apoptosis (K8: Caulin et al. 2000; Ku et al. 2003b; K17: Tong and Coulombe 2006), the protection of the liver against stress and from injury (K8/K18: Zatloukal et al. 2000; Ku et al. 2003a), and the regulation of protein synthesis and cell size during wound healing involving intracellular signaling pathways (K17: Kim et al. 2006). Keratins may also play a role in epithelial polarity and membrane traffic (Oriolo et al. 2007). Thus, keratins obviously exert widely varying signaling functions beyond their mechanical roles.
Beyond their biological functions, keratin expression patterns not only characterize cells as “epithelial”, they are also characteristic for distinct—including the terminal—stages during cellular epithelial differentiation from embryonal to adult or of the internal maturation program during development. Epithelial tumors—including metastases—most widely retain the keratin patterns of their (normal) epithelial origin; thus, the determination of the keratin patterns of tumors are being widely exploited for cell and tumor typing. Therefore, keratins have evolved to be one of the most potent epithelial differentiation and tumor markers in cell biology, embryology, and surgical pathology. Specific antibodies against several keratins are routinely used world wide in pathology laboratories for immunohistochemical typing of carcinomas in tumor diagnostics. Numerous papers published since 1980 deal with the application of keratins as marker proteins in tumor pathology (Oshima 2007), and several previous review articles (e.g. Lane and Alexander 1990; Nagle 1994; Schaafsma and Ramaekers 1994; Moll 1998; Chu and Weiss 2002b) cover this field of application, which also will be especially considered in this review.
Another clinical application is the detection of soluble keratin protein fragments derived from K8, K18, and K19 in the circulation of cancer patients; such fragments—released by carcinoma cells—are increasingly used to monitor tumor load and disease progression in the case of certain carcinomas such as non-small cell lung cancer (Barak et al. 2004; Linder 2007). Through analysis of different K18 fragments in the serum it is also possible to assess the type of chemotherapy-induced tumor cell death and distinguish between apoptosis and necrosis, due to the fact that K18 is cleaved at specific sites during apoptosis and a monoclonal antibody (M30) specific for caspase-cleaved forms of K8 is available (Leers et al. 1999; Linder et al. 2004; Linder 2007).
Human keratins and their expression patterns
In the following, the different human keratins and keratin pairs will be discussed together with their cell type and tissue distribution (summarized in Table 2) and their functional significance in relation to transgenic mouse models and human hereditary keratin diseases. Furthermore, characteristic expression patterns in human tumors (summarized in Table 3) and their possible diagnostic relevance will be considered.
Table 2.
Characteristic expression patterns of typical keratins in selected human normal epithelial tissues
| Keratins of simple epithelia | Keratins of stratified epithelia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type II keratins: | K8 | K7 | K5 | K6 | K1 | K2 | K4 | |||
| Type I keratins: | K18 | K19 | K10 | K14 | K15 | K16 | K17 | K10 | K9 | K13 |
| Parenchymatous epitheliaa | All cells | |||||||||
| Ductal epithelia of parenchymatous organsb | All cells | K7 and K19 in all cells | Sparse cells | Sparse cells | K4 heterogeneously in pancreatic ducts | |||||
| Gastrointestinal epithelia | All cells | K19 in all cells | Gastric foveolar epithelium, intestinal epitheliumc | K4 heterogeneously in luminal cells; K13 in sparse luminal cells | ||||||
| Respiratory epithelium | Predominantly luminal cells | K7 in luminal cells; K19 in all cells | Predominantly basal cells | K6 in basal cells | Predominantly basal cells | |||||
| Urothelium | All cells | K7 and K19 in all cells | Luminal (umbrella) cells | Basal cells | Few basal cells | K13 in all basal and intermediate cells | ||||
| Non-keratinizing stratified squamous epithelia | Some basal cells | K19 in many basal cells | Basal cell layer (predominantly) | Basal cells layer | Suprabasal compartment | At some sites focal expression in suprabasal cells | Suprabasal compartment | |||
| Epidermis | Predominantly basal cell layer | Basal cell layer | Suprabasal compartment | K2 in upper spinous and granular layer; K9 in palmoplantar epidermisd | ||||||
Not included are the corneal keratins K3/K12, the gingival/hard palate keratin K76, the eccrine sweat gland-specific keratin K77, and the hair follicle-specific epithelial and hair keratins (for keratin expression in eccrine sweat glands and in the hair follicle, see the schematic drawings in Figs. 5 and 6)
aIncluding hepatocytes, acinar cells of pancreas, proximal tubular cells of kidney
bBile ducts, pancreatic ducts, renal collecting ducts
cMost villus- and surface-lining cells; scattered cells in crypts
dHeterogeneous expression in the suprabasal compartment
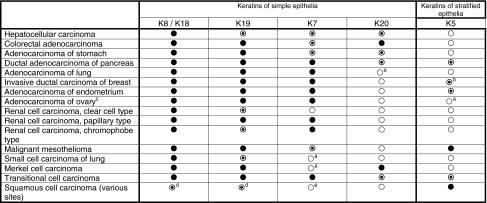
Table 3.
Characteristic expression patterns of typical keratins in selected human carcinomas
Only diagnostically relevant data as detectable by monoclonal antibodies widely established in clinical pathology are presented (for references, see Moll 1998; Chu and Weiss 2002b; and text). Explanation of symbols: filled circle, extended staining of most tumor cases; open dotted circle, focal/heterogeneous staining of some but not all cases; open circle, no staining
aIn rare cases focal staining may be observed
bFocal or extended staining in a subpopulation of tumor cases, corresponding to the basal-like phenotype
cNon-mucinous types
dPreferentially/more extended in poorly differentiated cases
eIn rare cases focal staining may be observed; however, squamous cell carcinomas of the cervix uteri may express K7 extendedly
Simple (one-layered) epithelia
K8/K18: primary keratins of simple epithelial cells
The keratins K8 and K18 typically are co-expressed and constitute the primary keratin pair of simple epithelial cells, including various parenchymatous epithelia (Franke et al. 1981; Moll et al. 1982b; Owens and Lane 2003). They are the first keratins to appear in embryogenesis, as early as in pre-implantation embryos (Jackson et al. 1980), and also seem to be the oldest keratins during phylogenesis (Blumenberg 1988). In some epithelial cell types, K8 and K18 are the sole keratins present. The classical example is the liver, with K8/K18 representing the characteristic and only keratin pair of normal hepatocytes. The same is true for other highly specialized parenchymatous epithelia such as acinar cells of the pancreas, proximal tubular epithelial cells of the kidney, and certain endocrine cells such as pancreatic islet cells. Ultrastructurally, keratin filaments of this simple composition are loosely distributed within the cytoplasm and show little bundling. In other simple, one-layered epithelia such as duct-lining cells, intestinal cells, and mesothelial cells, additional simple-epithelial keratins (K7, K19, and/or K20; see below) are present in addition to the primary pair K8/K18. Furthermore, K8/K18 occur—together with other keratins—in various pseudostratified (e.g. respiratory) and complex (e.g. glandular) epithelia and in the urothelium; in these composite epithelial tissues, K8 and K18 are often most prominent in the lumen-lining cells. Even in non-keratinizing stratified squamous epithelia, K8 and K18 may be focally expressed in the basal cell layer, together with K19 and the constitutive stratified-epithelial keratins (Bosch et al. 1988; Moll 1993). Thus, K8 and K18 are widely distributed among normal epithelial tissues although they are absent in differentiating keratinocytes. It should be noted that K8 and K18 are not strictly epithelium-specific since expression of K8 and K18 may occur in rare mesenchymal cells (more frequently in fetal stages) such as certain smooth muscle cells and fibroblastic reticulum cells of lymph nodes as well as various mesenchymal tumors including rhabdo- and leiomyosarcomas (Huitfeldt and Brandtzaeg 1985; Franke and Moll 1987; van Muijen et al. 1987; Jahn and Franke 1989; Knapp and Franke 1989; Knapp et al. 1989; Jahn et al. 1993; Gould et al. 1995; Kuruc and Franke 1988; Langbein et al. 1989), where they are co-expressed with other intermediate filament types, notably vimentin and desmin.
While highly specialized parenchymatous epithelial cells in their normal state, such as proximal tubular cells of the kidney, only express K8 and K18, this may change in reactive conditions. Upon various types of injury such as inflammation or atrophy these cells may additionally switch on K7 and K19, sometimes also K17 (as well as vimentin) and thus express four to five instead of two keratins (Moll et al. 1991). This increased keratin expression appears to parallel the reduction in the degree of differentiation. Thus, the keratin pattern of a given epithelial tissue may be modulated to some extent in the course of reactive changes, frequently resulting in higher complexity of keratin composition.
Already the tissue distribution of K8/K18—mainly in internal epithelia—suggests that structural and mechanical functions are not their key roles, although their absence or dysfunction may be associated with hepatocyte and trophoblast fragility (for references, see Magin et al. 2007). Instead, genetic knock-out experiments have revealed distinct regulatory functions of these keratins (Magin et al. 2007; Oshima 2007). They play a role in protecting the placental barrier function (K8: Jaquemar et al. 2003) and protecting cells—in particular liver cells—from apoptosis (K8: Caulin et al. 2000; Ku et al. 2003b), against stress, and from injury (K8/K18: Zatloukal et al. 2000; Ku et al. 2003a), possibly by functioning as a phosphate “sponge” for stress-activated kinases (K8: Ku and Omary 2006). Interestingly, K8 and K18 may play a role in the regulation of the cell cycle, whereby phosphorylation of these keratins and binding of 14-3-3 adaptor proteins seem to be involved (Toivola et al. 2001; Ku et al. 2002; Margolis et al. 2006; Galarneau et al. 2007; Magin et al. 2007). In human pathology, defects in K8 and K18 may predispose to liver diseases, in particular cryptogenic liver cirrhosis (Ku et al. 2003a; Zatloukal et al. 2004), as well as to chronic pancreatitis and inflammatory bowel disease (for references, see Owens and Lane 2004). Altered K8 and K18 proteins, together with several stress proteins, in particular ubiquitin and p62, constitute the hyaline protein aggregates of hepatocytes of several (e.g. alcoholic) liver diseases now known as Mallory–Denk bodies (Zatloukal et al. 2007).
In regard to malignant tumors, K8 and K18 are expressed in most carcinomas except for some differentiated squamous cell carcinomas. Therefore, K8 and K18 antibodies strongly stain most adenocarcinomas, hepatocellular carcinomas, renal cell carcinomas, and neuroendocrine carcinomas. Since highly sensitive monoclonal antibodies against these keratins are available, such as the classical mouse monoclonal CAM5.2 clone against K8 (Makin et al. 1984) and clone Ks18.04 against K18 (Bártek et al. 1991), these keratins may be helpful in diagnostic immunohistochemistry in cases of carcinomas with low keratin content such as small-cell lung cancer, to prove their epithelial nature. Regarding carcinoma subtyping, negative or weak/focal immunostaining for K8 and K18 may indicate squamous cell differentiation, although strong expression of these keratins can occur particularly in poorly differentiated squamous cell carcinomas (see below, chapter “Keratins as diagnostic markers in tumor pathology”). In the case of breast carcinomas, certain publications have reported a correlation between the level of K8 or K18 immunostaining and a favorable prognosis for the patients (see below, chapter “Keratins as diagnostic markers in tumor pathology”).
Another clinical application of K8/K18 is the monitoring of fragments of these keratins in the serum as serological tumor markers to monitor cancer load, cancer progression, and response to therapy. Among the oldest of these markers are tissue polypeptide antigen (TPA) and tissue polypeptide-specific antigen (TPS) which have been recognized to correspond to a mixture of K8, K18, and K19 (Weber et al. 1984) and to K18 (Rydlander et al. 1996), respectively (for review, see Linder 2007). More recently, an apoptosis-specific fragment of K18 as detected by monoclonal antibody M30 (Leers et al. 1999) has become of increasing interest for distinguishing between necrosis and apoptosis and for the evaluation of the chemotherapy response of carcinomas by investigation of cancer patient serum, e.g. in prostate, breast, and lung cancer (Linder et al. 2004; Linder 2007).
K7/K19: secondary keratins of simple epithelial cells
Apart from K8/K18, keratins K7 and K19 are “additional” (secondary) and also widely distributed simple-epithelial keratins which are frequently but not always co-expressed. They typically occur as a keratin pair in simple ductal epithelia such as bile and pancreatic ducts (“ductal-type” keratins). However, in several epithelia lacking K7 such as intestinal epithelium, the type I keratin K19 must form a pair with the sole type II keratin K8.
The type I keratin K19 is the smallest keratin and is exceptional since it widely lacks the non-α-helical tail domain typical for all other keratins (Bader et al. 1986). It may have evolved from keratinocyte keratins (Stasiak et al. 1989). As detectable by several specific and well-tested monoclonal antibodies (Karsten et al. 1985; Bártek et al. 1986; Nagle et al. 1986), K19 exhibits a rather broad tissue distribution. It is expressed in most simple epithelia (excluding parenchymatous cells such as hepatocytes, pancreatic acinar cells, and renal proximal tubular cells), notably in various ductal epithelia, in small and large intestinal epithelium, in gastric foveolar epithelium, and in mesothelium. Furthermore, it is present in most cells of pseudostratified epithelia and urothelium as well as in basal cells of non-keratinizing stratified squamous epithelia.
Functionally, keratin K19 is dispensable since K19 knock-out mice were viable, fertile, and appeared normal (Harada et al. 1999). This is apparently due to functional compensation by K18, since only mice, double deficient for K18 and K19 exhibited a severe phenotype with trophoblast fragility and early embryonic lethality (Hesse et al. 2000). No mutation of the human K19 gene causing a disease has yet been found (Owens and Lane 2004).
The expression of K19 may be induced in certain epithelia that normally lack this keratin by pathological alterations. One example is damage to renal proximal tubular epithelia by various types of injury as discussed above (Moll et al. 1991). K19 induction is also observed in suprabasal stratified squamous epithelial cells of oral mucosa with epithelial dysplasia (Lindberg and Rheinwald 1989; for further references, see Moll 1998), but also with inflammation (Bosch et al. 1989; Moll 1993), so that K19 cannot be used as a specific marker for dysplasia in oral mucosa. In carcinomas, K19 is widely expressed in both adenocarcinomas and squamous cell carcinomas and therefore is not extensively used as an immunohistochemical marker for carcinoma subtyping. One example for such application may be, in liver tumors, the distinction of hepatocellular carcinomas, which show little expression of K19, from cholangiocarcinomas and adenocarcinoma metastases, which strongly stain for this keratin (Balaton et al. 1988; Goldstein and Bosler 2006; see Table 3). The detection of soluble K19 fragments in the serum released by carcinoma cells by the CYFRA 21-1 assay has found broad clinical application as a marker to monitor treatment and evaluate response to therapy and has proven particularly useful in the case of squamous cell carcinomas of the lung (for review, see Barak et al. 2004, Gu and Coulombe 2007).
The type II keratin K7, another “ductal-type” keratin, has a basically similar but somewhat more restricted tissue distribution as compared to K19 (Moll et al. 1982b; Ramaekers et al. 1990). Like K19, it is expressed in several simple ductal epithelia, in mesothelium, in pseudostratified epithelia (preferentially in luminal cells), and in urothelium but absent in parenchymatous cells such as hepatocytes. However, K7 is sparsely expressed or absent in gastric foveolar epithelium, intestinal epithelium, and stratified squamous epithelia. Human and mouse K7 genes have been characterized (Glass and Fuchs 1988; Smith et al. 2002), but mutations or disease associations have not yet been reported (Owens and Lane 2004).
Several monoclonal antibodies against K7 have been described, some of which (e.g. Ks7.18, OV-TL12/30) are well reactive with formalin-fixed, paraffin-embedded tissues. Of these, clone OV-TL12/30 (van Niekerk et al. 1991) appears to elicit the broadest span of immunoreactivity and has found wide application in diagnostic tumor pathology, especially in cases where primary tumors or metastases are uncertain. Since the majority of carcinomas are K7 positive, negative reactions are of particular diagnostic significance. One main point of diagnostic utility of this keratin is the negative (or weak/focal) K7 immunostaining in colorectal adenocarcinomas (see Fig. 3c), in contrast to the strong staining in most other adenocarcinomas (see Fig. 3e; Moll et al. 1992, 1993b; Chu et al. 2000; Chu and Weiss 2002b; Tot 2002). This is especially valuable for classifying adenocarcinoma metastases with regard to their possible primary tumor. Low K7 expression (negative or weak immunostaining, or staining of a minor proportion of tumor cells) is also a characteristic feature of conventional (clear-cell) renal cell carcinomas (as opposed to papillary and chromophobe carcinomas) (Moll 1998; Skinnider et al. 2005) and of (non-cervical) squamous cell carcinomas. The validity in predicting the primary tumor in cases of unclear metastases is significantly increased when K7 is used in combination with K20 (see below) since many carcinomas exhibit characteristic K7/K20 phenotypes (Moll et al. 1992, Moll et al. 1993b; Chu et al. 2000; Chu and Weiss 2002b; Tot 2002; Dabbs 2006).
Fig. 3.
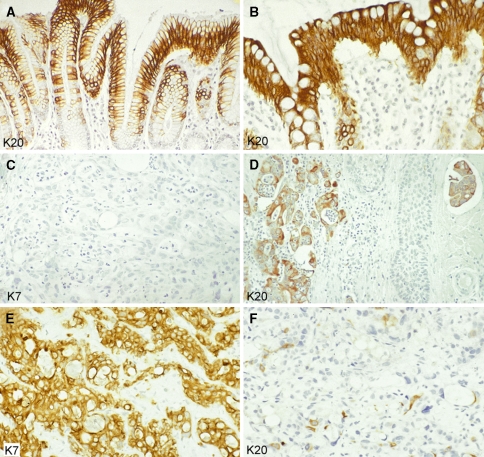
Keratins in simple epithelia and adenocarcinomas (paraffin sections of human tissues; avidin–biotin complex peroxidase staining). Keratin K20 is a characteristic and prominent keratin of the foveolar epithelium of the gastric (a) and colorectal (b) mucosa. The K7−/K20+ phenotype of the normal mucosa is mostly maintained in primary and metastatic colorectal adenocarcinomas. This is shown here for a skin metastasis (on the head) of a poorly differentiated adenocarcinoma: the tumor cells are negative for K7 (c) but positive for K20 (d, left portion of the figure), even including a tumor cell cluster that has invaded a lymphatic capillary (d, right upper corner). This phenotype is strongly suggestive of colorectal origin. A primary tumor in the right colon was detected later. Liver metastasis of a ductal adenocarcinoma of the pancreas with typical keratin pattern, showing extended expression of K7 (e) and staining of scattered tumor cells for K20 (f). Magnifications: a, d ×80; b, c, e, f ×140
K20: keratin of gastrointestinal epithelium, urothelium, and Merkel cells
K20 is the simple-epithelial keratin with the most restricted expression pattern. Although it appeared in our early cytoskeletal preparations of intestinal epithelial cells as a quite prominent protein spot of ~46 kDa, where we tentatively designated it as “IT protein” (from intestinal; Moll et al. 1982b), we succeeded rather late in identifying it as a type I keratin (Moll et al. 1990). For pair formation, its type II partner usually appears to be K8. The remarkable expression spectrum of K20 among normal tissues comprises gastric foveolar epithelium (Fig. 3a) and small and large intestinal epithelium (Fig. 3b; “gastrointestinal-type” keratin) and, in addition, the urothelium and certain neuroendocrine cells, in particular Merkel cells of the skin. In human embryogenesis, it appears in the small intestinal epithelium at embryonic week 8 (Moll et al. 1993b). Within the gastrointestinal and urothelial epithelial tissues, K20 is absent from the stem cell compartment and appears to be switched on during terminal differentiation and thus is most prominent in small intestinal villus-lining and large intestinal surface-lining epithelia (Fig. 3b) and in urothelial umbrella cells. Although K20 is a keratin typically expressed in simple epithelia it is also found in the lone basally located Merkel cells of the epidermis and hair follicle outer root sheath (Moll et al. 1992, 1995).
Our knowledge about the function of K20 still is limited. Mutations of human K20 or associated diseases have not yet been described (Owens and Lane 2004). Transgenic experiments suggest a role for K20 in maintaining keratin filaments in intestinal epithelia (Zhou et al. 2003). In mouse small intestinal epithelium, K20 phosphorylation on serine 13 is induced during apoptosis and tissue injury and thus may serve as stress marker (Zhou et al. 2006).
Among the K20-specific monoclonal antibodies available, clone Ks20.8 reacts well on routine paraffin sections (Moll et al. 1992) while clone Ks20.10 reacts with the K20 homologue of rodents (Moll 1993). K20 is a potent immunohistochemical marker in tumor pathology since its peculiar expression spectrum is essentially maintained in the corresponding primary and metastatic carcinomas (Moll et al. 1992), and the K20 clone Ks20.8 has become part of the routine antibody panel in most pathology laboratories. Most colorectal adenocarcinomas (Fig. 3d), the majority of gastric adenocarcinomas, the majority of transitional cell carcinomas, as well as most Merkel cell carcinomas are K20-positive (Moll et al. 1992, 1993b; Miettinen 1995; Chu et al. 2000; Chu and Weiss 2002b; Goldstein and Bosler 2006). In a few other carcinoma types, variable and focal K20 expression is seen, notably in ductal adenocarcinomas of the pancreas (Fig. 3f) and in adenocarcinomas of the biliary tract including cholangiocarcinomas of the liver (Moll et al. 1992; Miettinen 1995; Chu et al. 2000). Among ovarian carcinomas, K20 is mainly detected in the mucinous type. Most other carcinomas, including adenocarcinomas, irrespective of their morphology, are essentially negative for K20. Thus, significant K20 positivity of a metastatic adenocarcinoma is predictive of a primary tumor in the gastrointestinal or pancreaticobiliary tract. It should be noted that the diagnostic value is increased when the markers K20 and K7, are applied in combination. For example, a K7−/K20+ phenotype of an adenocarcinoma metastasis (Fig. 3c, d) strongly favors a colorectal origin. Some colorectal adenocarcinomas co-express K7 in addition to K20, but as a general rule, the level of K20 immunostaining exceeds that of K7 (see below, chapter “Keratins as diagnostic markers in tumor pathology”).
Using RT-PCR analyses, K20 mRNA can be detected in cell preparations from bone marrow and peripheral blood from some colorectal cancer patients, indicating the presence of disseminated tumor cells that maintain K20 expression, and a number of clinical studies have shown that this is correlated with a worse prognosis (Soeth et al. 1996; Funaki et al. 1998; Wyld et al. 1998; Koch et al. 2005; Katsumata et al. 2006; Friederichs et al. 2007). In addition to patients with metastatic colorectal carcinoma, K20 expression in the peripheral blood was also detected in patients with metastatic gastric and pancreatic adenocarcinoma but hardly in patients with metastatic lung carcinoma (Chausovsky et al. 1999), underlining the tumor type-specific expression of this keratin.
Stratified epithelia
K5/K14: major keratins of basal keratinocytes
The type-II keratin K5 and the type-I keratin K14 form the primary keratin pair of the keratinocytes of stratified squamous epithelia, including the epidermis as well as mucosal non-keratinizing stratified squamous epithelia (Moll et al. 1982b). They are strongly expressed in the undifferentiated basal cell layer containing the stem cells and are down-regulated in the differentiating suprabasal cell layers (Fig. 4a; Fuchs and Green 1980). Otherwise, in the widely well stratified follicular outer root sheath, K5 and K14 are uniformly expressed throughout all layers. Moreover, the follicular companion layer, which is directly adjacent to the outer root sheath (and formerly considered as “innermost layer of the outer root sheath”), is completely negative for both of these keratins and expresses an own special keratin, K75 (see below). Ultrastructurally, K5/K14 keratin filaments are bundled as tonofilaments and attached to desmosomes and hemidesmosomes. The mouse K5 promoter is frequently used in transgenic experiments to promote epidermis-specific expression of transgenes (e.g. Oki-Idouchi and Lorenzo 2007). In addition to their occurrence in keratinocytes, K5 and K14 are expressed in basal and myoepithelial cells of complex and glandular epithelial tissues (Purkis et al. 1990). On the other hand, these keratins are absent from most simple/one-layered epithelia, with very few exceptions, notably the mesothelium lining serous cavities (Moll et al. 1989) and the amnion epithelium.
Fig. 4.
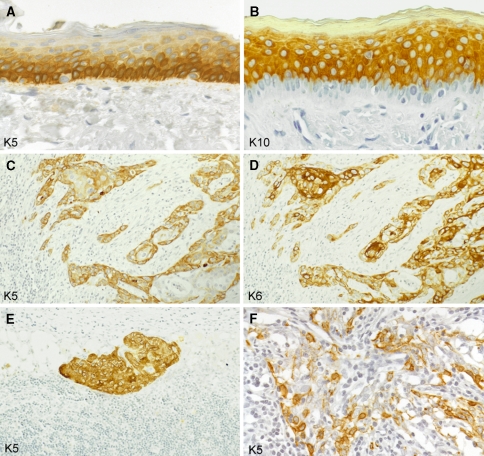
Keratins in stratified squamous epithelia and squamous cell carcinomas (paraffin sections of human tissues; avidin–biotin complex peroxidase staining). In the epidermis as an example of a normal stratified squamous epithelium, the basal cell layer contains abundant keratin K5 (a) whereas the differentiating suprabasal compartment strongly stains for K10 (b; note the negative basal cell layer). Lymph node metastasis of a squamous cell carcinoma of the head and neck region, expressing K5 (c; more intensely in the peripheral tumor cell layers) as well as K6 (d; particularly strongly in central tumor cells) as signs of their keratinocyte origin. Keratin K5 is also maintained in a lymph node micrometastasis of a squamous cell carcinoma of the head and neck region (e) and in a lymph node metastasis of an undifferentiated nasopharyngeal carcinoma with dissociated growth pattern of the tumor cells (f), in these examples being a diagnostically helpful feature. Magnifications: a, b ×160; c–e ×80; f ×140
Several monoclonal antibodies (MAbs) specific for K5 and K14 have been described that helped to reveal their exact tissue distribution. These antibodies include MAb AE14 (Moll et al. 1989) against K5 and MAbs LL001 and LL002 against K14 (Purkis et al. 1990). The best performance on paraffin sections is displayed by MAb D5/16B4 (Lobeck et al. 1989; Demirkesen et al. 1995) which—although being often regarded as “K5/K6 antibody”—specifically recognizes K5 (Böcker et al. 2002).
The functional importance of K5 and K14 for the physical stability of the epidermis has become clearly evident by the recognition that dominant-negative mutations of the K5 or the K14 gene cause the hereditary blistering skin disease epidermolysis bullosa simplex (EBS) (for reviews, see Omary et al. 2004; Lane and McLean 2004). Most of these mutations are missense or small in-frame deletion mutations, primarily affecting the keratin rod domain. The presence of mutated K5 or K14 results in increased fragility of the basal keratinocytes so that even mild physical trauma leads to intraepidermal cytolysis of basal cells and the formation of fluid-filled blisters. These patient-related findings were preceded by the experimental demonstration that expression of mutant K14 in transgenic mice causes abnormalities similar to EBS (Vassar et al. 1991).
The expression spectrum of K5 and K14 in tumors corresponds well to the patterns in normal epithelia. Thus, most squamous cell carcinomas (Fig. 4c, e) as well as malignant mesotheliomas strongly express these keratins whereas little, focal, or no expression is found in adenocarcinomas (Moll et al. 1982b, 1989; Moll 1998; Chu and Weiss 2002a, b). Hence, these keratins, in particular K5, have found several lines of diagnostic application in pathology, which has been aided by the availability of a highly sensitive and specific, robust, paraffin-suited MAb (D5/16B4; see above). Pertinent examples are the recognition and diagnosis of poorly differentiated squamous cell carcinomas, including micrometastases in lymph nodes (Fig. 4e), of undifferentiated nasopharyngeal carcinomas which may be diagnostically difficult due to their dissociated growth pattern (Fig. 4f), and of malignant mesotheliomas. Thus, K5 immunostaining allows the distinction of the small cell type of squamous cell carcinoma of the lung, which is K5+, from a small cell carcinoma or a poorly differentiated adenocarcinoma, both of which are K5− (Moll 1998; Chu and Weiss 2002a), and the distinction of a malignant mesothelioma of the pleura (K5+) from a pulmonary adenocarcinoma with pleural involvement (K5−) (Moll et al. 1989; Ordonez 1998; Yaziji et al. 2006). In well and moderately differentiated squamous cell carcinomas, K5 is preferentially localized in the peripheral layers of the tumor cell formations (Fig. 4c), corresponding to the K5 expression in the basal cell layer of normal stratified squamous epithelia. Focal K5 expression may be observed in certain adenocarcinoma types, notably in adenocarcinomas of the endometrium, the ovary, and the pancreas, which seems to be related to their potency for focal squamous differentiation (Moll 1998; Chu and Weiss 2002a, 2002b). Much interest has evolved regarding the role of K5 in breast pathology in several aspects, including the identification of myoepithelial cells, the classification of proliferative lesions (Otterbach et al. 2000), and the recognition of a certain subtype of invasive ductal breast carcinoma (see below, chapter “Keratins as diagnostic markers in tumor pathology”). In prostate pathology, the diagnosis of prostatic adenocarcinoma is supported by the immunohistochemical demonstration of absence of K5-positive basal cells (Abrahams et al. 2002, 2003).
K15: basal keratinocyte keratin and hair follicle stem cell “marker”
K15 was first identified as a minor keratin of human epidermis, by gel electrophoresis of cytoskeletal preparations (Moll et al. 1982b, c). Its sequence demonstrated its assignment to the type I keratins (Leube et al. 1988). Only later its cellular distribution was recognized, and it was uncovered that K15 is a specific basal cell component of the epidermis (Moll et al. 1993a; Lloyd et al. 1995) and other stratified squamous epithelia (Waseem et al. 1999) (see below for its significance in the hair follicle). Frequently, K5 and K14 can also be detected in the lower suprabasal cell layers (see above and Fig. 4a). Whereas the mRNA synthesis of these keratins is restricted to the basal layer, the K5 and K14 proteins remain integrated in the complex keratin cytoskeleton for some time when cells leave the basal compartment. Thus, they may be stained by immunohistochemistry in more or less suprabasal layers depending on the epitope of the antibody used. In comparison, K15 seems completely restricted to the basal cell layer of stratified squamous epithelia (Lloyd et al. 1995; Waseem et al. 1999) where it can form heteropolymeric filaments with K5 (Lloyd et al. 1995).
MAbs recognizing K15 include clone LHK15 (Waseem et al. 1999) and clone C8/144B (Lyle et al. 1998, 1999). The latter in fact is a surprising and exciting MAb originally made against the lymphocyte antigen CD8 which cross-reacts with K15 especially in the basal keratinocytes of the hair follicle bulge region, where the respective stem cells are assumed. Also polyclonal K15 antibodies have furthermore been described (Moll et al. 1993a; Kurzen et al. 2001, Langbein et al. 2008). K15 expression in basal cells is downregulated in activated epidermal keratinocytes such as in organotypic cultures and in hyperproliferation (Waseem et al. 1999) or upon wounding (Porter et al. 2000). Human mutations or knock-out mice for K15 have not yet been described.
As to the occurrence of K15 in tumors, the literature is inconclusive as to whether there is differential expression of K15 among cutaneous tumors (benign follicular tumors versus basal cell carcinomas), possibly due to the use of different antibodies1 (see also above), and thus a putative diagnostic application of K15 immunohistochemistry in dermatopathology is still open (Kanitakis et al. 1999; Jih et al. 1999; Porter et al. 2000; Kurzen et al. 2001). Currently, the most interesting feature with K15 is the discovery that within the hair follicle at least one antibody against this keratin detects putative stem cells residing in the hair follicle bulge and thus might be used as a stem cell marker in hair follicle biology (Lyle et al. 1999).
K6/K16: keratins of hyperproliferative keratinocytes inducible in “activated” epidermis
By gel electrophoresis, the type-II keratin K6 and the type-I keratin K16 have been identified in epidermis only in samples from plantar glabrous skin while in hairy skin these keratins appeared to be absent in interfollicular epidermis but were clearly present in hair follicle outer root sheath (Moll et al. 1982b, c; Langbein and Schweizer 2005) and companion layer (Winter et al. 1998; Langbein and Schweizer 2005; Gu and Coulombe 2007). In nail epithelia, K6 and K16 are constitutive components (Heid et al. 1988b; Lane and McLean 2004; Perrin et al. 2004). K6 and K16 are also consistently expressed in non-keratinizing stratified squamous epithelia (Moll et al. 1982b). They are often but not always co-expressed as a keratin pair (Moll et al. 1982b; Langbein et al. 2005). Molecular genetic studies have revealed that in humans three isoforms of K6 exist, K6a, K6b, and K6c, encoded by distinct genes (Rogers et al. 2005; Schweizer et al. 2006).
MAb KA12 is an antibody which most probably stains at least keratin K6a isoform and reacts well with paraffin sections (Demirkesen et al. 1995; Langbein et al. 2003; Schmelz et al. 2005) although isoform-specific antibodies against K6a, K6b or K6c will hardly be made because of their extremely high peptide sequence homology. Using this MAb, plantar epidermis shows extended albeit heterogeneous expression of K6a, while interfollicular epidermis is negative or exhibits only some positive suprabasal cell groups (Demirkesen et al. 1995; Swensson et al. 1998). Non-keratinizing stratified squamous epithelia express K6 uniformly in all suprabasal cell layers. A MAb against K16 has also been described (Leigh et al. 1995).
In early immunohistochemical studies using keratin group-specific MAbs, Weiss et al. (1984) demonstrated the induction of K6 (56-kd keratin) and K16 (48-kd keratin) in various hyperproliferative epidermal disorders, suggesting that these keratins may be molecular markers for hyperproliferative keratinocytes. More recent experimental studies showed that after skin wounding, K6 and K16 are rapidly induced within 6 h in human keratinocytes at the wound edge, before migration and regeneration begins (Paladini et al. 1996). The particular cell biological properties of these keratins may confer to activated keratinocytes on the one hand a moderate level of mechanical scaffolding and on the other hand sufficient plasticity required during migration and re-epithelialization (Wawersik et al. 2001). K6a knockout mice showed delayed re-epithelialization after skin wounding (Wojcik et al. 2000). Upon K6a/K6b double knock-out, mice exhibit epithelial disintegration and white plaques in the dorsal tongue epithelium (Wong et al. 2000; Wojcik et al. 2001). The absence of a hair and nail phenotype in these mice—as might be expected from the occurrence of K6 in these appendages—has been shown to be due to the presence and compensatory function of other K6-related keratins in hair follicles and nails, such as mK6hf (Wojcik et al. 2001; now mK75) or mK6irs1—mK6irs4 (now mK71–mK74; cf. Langbein et al. 2002, 2003).
In man, mutations in K6a or K16 have been proven to give rise to the hereditary disorder pachyonychia congenita type 1 (Jadassohn–Lewandowsky form) that manifests with thickened nails, palmoplantar hyperkeratosis, and oral leukoplakias (McLean et al. 1995; for further references, see Lane and McLean 2004). Notably, the pathologic changes affect those tissues that constitutively express K6a and K16 (see above) but not the interfollicular epidermis.
Thus, K6/K16 are constitutive keratins of stratified epithelia built up by keratinocytes of relatively high proliferative state such as mucosal tissues, palmoplantar epidermis, and certain skin appendages. On the other hand, they are “stress-inducible” keratins in interfollicular epidermis, being rapidly switched on e.g. after injury and UV-irradiation or being present also in inflammation and in hyperproliferative disorders.
Expression of these keratins is not restricted to stratified squamous epithelia but may also be observed in certain glandular structures. Thus, K6 (most probably K6a) and K16 are expressed in ductal luminal cells as well as in some secretory cells of human eccrine sweat glands (Fig. 5; Demirkesen et al. 1995; Langbein et al. 2005). K6 has been detected in subpopulations of luminal cells of the mouse mammary gland (Grimm et al. 2006) and the human mammary gland; in the latter, also in ductal myoepithelial cells (Hesse 2003; Langbein et al. 2005). Recently, a population of K6-positive cells in the prostate gland with high potential for proliferation and differentiation has been described (Schmelz et al. 2005).
Fig. 5.
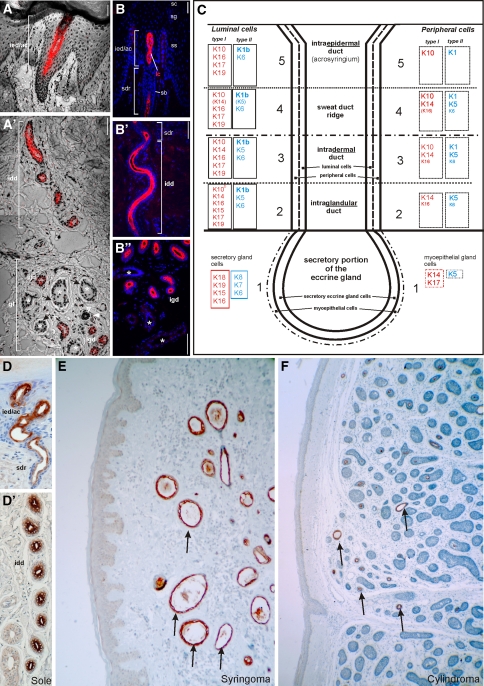
Keratin K77 in eccrine sweat glands and adnexal tumors. K77 mRNA and protein by in situ hybridization (ISH, a, a′) and indirect immunofluorescence (IIF, b-b′′) microscopy. This keratin is specifically expressed in the luminal cells (lc) of the intraglandular (igd), the intradermal (idd), the sweat duct ridge (sdr) and the intraepidermal/acrosyringial (ied/ac) duct of eccrine sweat glands of plantar skin. Peripheral duct cells are negative. The secretory portions (asterisks) are K77-negative. sb str. basale, ss str. spinosum, sg str. granulosum, sc str. corneum, gl glandular region with secretory and intraglandular duct portions. c Expression scheme of keratins of the eccrine sweat gland. d-d′′ Immunoperoxidase staining of K77 in the luminal cells of the eccrine sweat gland duct of the foot sole epidermis. K77 is also expressed in the tubular structures (arrows) of eccrine tumors such as syringoma (e) and cylindroma (f). Bars 100 μm
In the pseudostratified epithelia of respiratory mucosal tissues, K6 and K16 are highly upregulated in squamous metaplasia (Leube and Rustad 1991; Stosiek et al. 1992). Among tumors, K6 and K16 are typically and strongly expressed in squamous cell carcinomas of different sites (Moll et al. 1982b; Moll 1998), preferentially in inner, maturing layers of the tumor cell nests (Fig. 4d). Although low expression of these keratins may be found in adenocarcinomas such as occasionally in adenocarcinomas of the uterine cervix (Smedts et al. 1993) and in less than 20% of invasive breast carcinomas (Wetzels et al. 1991), K6 as detected by MAb KA12 may be suitable—in addition to K5—as another immunohistochemical marker of squamous differentiation in poorly differentiated squamous cell carcinomas (Moll 1998).
K17: keratin of basal/myoepithelial cells and inducible in “activated” keratinocytes
The type I keratin K17 was identified in our early gel electrophoretic studies as a major keratin of basal cell carcinomas of the skin which was also present in the normal pilosebaceous tract but not in normal epidermis (Moll et al. 1982c). Further protein analyses showed its presence in squamous cell carcinomas of various origins as well as in normal glandular tissues (such as sweat glands and breast) but its apparent absence also from non-keratinizing stratified squamous epithelia (Moll et al. 1982b, 1983). The unique cell type distribution of this keratin became apparent after establishment of a specific MAb, clone E3 (Troyanovsky et al. 1989). Broad tissue screening revealed its selective expression in basal and myoepithelial cells of complex tissues, including various glands, respiratory epithelium, and urothelium (Troyanovsky et al. 1989, 1992). Thus, K17 may be regarded as a “basal-/myoepithelial cell keratin”. In the hair follicle, confirming and extending previous biochemical findings (Moll et al. 1982c), K17 has been localized as a prominent component of the suprabasal cell layers of the outer follicular root sheath (Winter et al. 1998; Langbein and Schweizer 2005; Langbein et al. 2006; Tong and Coulombe 2006). It is also present in nail bed and nail matrix epithelia (McGowan and Coulombe 2000; Perrin et al. 2004). Immunohistochemistry also confirmed the essential absence of K17 from adult interfollicular epidermis, but, interestingly, revealed its specific expression in the specialized epidermal keratinocytes of the sensory Merkel cell-associated “haarscheiben” organs (Moll et al. 1993a); this keratin thus may be applied as a sensitive haarscheiben marker in studies of cutaneous neurobiology. In contrast to the adult, K17 is a prominent component of fetal epidermis (Moll et al. 1982a) as well as of cultured epidermal cells (Weiss et al. 1984).
Another interesting feature of K17 is its inducibility after skin injury: after K6/K16 (see above), K17 is switched on in regenerating and migrating epidermal keratinocytes upon wound healing (Paladini et al. 1996). Its functional importance in wound healing is suggested by the observation that K17 knockout mouse embryos show a delay in the closure of surface ectoderm wounds (Mazzalupo et al. 2003). Recent transgenic experiments have shown that K17 can bind to the adaptor protein 14-3-3σ and influences cell growth and size of mouse keratinocytes by regulating protein synthesis (Kim et al. 2006).
The aforementioned expression of K17 in the pilosebaceous tract (in the follicular outer root sheath, companion layer, medulla and sebaceous gland; for review, see Langbein and Schweizer 2005) has also proven to be functionally relevant. K17 null mice develop transient severe alopecia in early postnatal life, correlating with hair fragility and apoptosis in hair matrix cells (McGowan et al. 2002). The same group later showed that K17 modulates hair follicle cycling by delaying apoptosis, whereby K17 is functionally linked with TNFα signaling (Tong and Coulombe 2006).
Hereditary human diseases due to K17 mutations have been identified (for references, see Lane and McLean 2004), most notably pachyonychia congenita type 2 (Jackson–Lawler form). The phenotype of this genodermatosis includes thickened nails and pilosebaceous cysts. Another condition related to K17 mutations is steatocystoma multiplex, in which patients present with multiple hair follicle-associated cysts. These genodermatoses obviously are related to the expression and functional importance of K17 in pilosebaceous and nail (Perrin et al. 2004; Langbein and Schweizer 2005) epithelia.
Since in keratinocytes K17 is—like K6 and K16 (see above)—an inducible keratin upon stress, injury, or inflammation, it is not surprising that squamous cell carcinomas consistently express these three keratins (Moll et al. 1982b, 1983; Chu and Weiss 2002b). As most normal stratified squamous epithelia lack K17, its presence in the corresponding tumors may be regarded as neo-expression during tumorigenesis. In the uterine cervix, K17 is expressed in cervical intraepithelial neoplasia but since it is already present in endocervical reserve cells (Weikel et al. 1987) and immature squamous metaplasia, it has not yet become a routine diagnostic marker (Martens et al. 1999). Among adenocarcinomas, focal K17 expression seems to be particularly characteristic of pancreatic ductal adenocarcinomas (Real et al. 1993; Moll 1998), which may become useful for their distinction from other adenocarcinomas (Chu and Weiss 2002b). In ductal breast carcinomas, K17 is expressed in a minor subset of tumor cases (Malzahn et al. 1998), now recognized to correspond to the basal-like subtype as defined by global gene expression data (see below, chapter “Keratins as diagnostic markers in tumor pathology”).
K1/K10: major keratins of keratinocyte differentiation and keratinization
In the epidermis, the transition of keratinocytes from the proliferative basal cell layer to the postmitotic suprabasal spinous cell layers in the process of terminal differentiation and keratinization is characterized by a profound change in keratin expression. This involves a switch from expression of the basal cell keratins (K5, K14, K15) to the suprabasal epidermal keratins, the type II keratin K1 and subsequently the type I keratin K10 (Fig. 4b; Fuchs and Green 1980; Moll et al. 1982b; Tseng et al. 1982, Weiss et al. 1984; Roop 1987, Stoler et al. 1988). This is one of the classical examples for the carefully regulated differentiation-specific expression of keratin proteins. Ultrastructurally, keratin filaments composed of the pair K1/K10 form particularly dense bundles which are so characteristic of suprabasal epidermal keratinocytes (Fig. 1d). Clearly, this imparts mechanical integrity to the cells and the whole epidermis. In addition, however, there seem to exist further functional roles, as experimental data have demonstrated that K10 specifically inhibits proliferation and cell cycle progression of keratinocytes (Paramio et al. 1999; Koch and Roop 2004) and loss of K10 leads to increased keratinocyte turnover (Reichelt et al. 2004; for review, see Magin et al. 2007).
The importance for epidermal integrity is underscored by the fact that point mutations in K1 and K10 are associated with the blistering disorder epidermolytic hyperkeratosis/bullous congenital ichthyosiform erythroderma (BCIE), initially presenting with skin blisters but later with thickened ichthyotic skin (for reviews, see Lane and McLean 2004; Omary et al. 2004). As expected, the suprabasal cells become fragmented easily and, in addition, the epidermis becomes hyperproliferative and hyperkeratotic. K1 mutations are notably heterogeneous and may result in diverse overlapping, often relatively mild phenotypes (Lane and McLean 2004). In transgenic mouse experiments, mutation or knock-out of K10 results in a phenotype similar to human epidermolytic hyperkeratosis (Fuchs et al. 1992; Porter et al. 1996).
Despite their association with terminal epidermal differentiation and keratinization, K1 and K10 may be focally expressed in suprabasal cells of internal noncornifying stratified squamous epithelia (for references, see Moll 1998). They are also a typical component of cells of eccrine sweat gland ducts (Fig. 5; Langbein et al. 2005). Surprisingly, although the differentiated parts of the hair follicle, such as suprabasal outer root sheath, upper companion layer, upper inner root sheath or the hair fiber, are heavily keratinized structures, all of them are free of K1/K10. Both typical epidermal keratins are completely lost in the infundibulum.
Among various antibodies against K1 and K10 described in the literature, MAb DE-K10 against K10 is particularly suitable for application with paraffin-embedded tissues (Ivanyi et al. 1989). In squamous cell carcinomas focal expression of K1 and K10, usually in relation to maturation and keratinization, can be observed regardless of whether they are derived from the skin or from internal organs (for references, see Moll 1998). They are more sparse in poorly differentiated tumors but may still be detected in about 50% of cases of oral and pharyngeal squamous cell carcinomas (Moll 1998). However, quantitatively, squamous cell carcinomas rather embark on an alternative maturation pathway characterized by abundant expression of K6 and K16 (see above; Fig. 4d). Overall, K1 and K10 can be regarded as “keratinization markers” of keratinocytes. These keratins have not yet been routinely applied to tumor diagnosis except for some special aspects of skin tumors (Yuspa et al. 1991).
K9: palmoplantar epidermal differentiation keratin
The type I keratin K9 is a highly specific keratin of terminally differentiating keratinocytes of palmoplantar epidermis where it is abundantly, albeit heterogeneously expressed (Moll et al. 1987; Langbein et al. 1993). At other body sites, there may be extremely sparse and focal expression in upper epidermal layers (Moll et al. 1987). Thus K9, forming a pair with K1, appears to reflect a special program of keratinocyte differentiation associated with particular mechanical reinforcement (Swensson et al. 1998). Therefore it is not surprising that mutations in the K9 gene are associated with a disorder of the skin of the palms and soles, epidermolytic palmoplantar keratoderma, which manifests itself as cytolysis and epidermal thickening (Reis et al. 1994; Torchard et al. 1994; for review, see Lane and McLean 2004). In its sequence, K9 is most closely related to K10 (Langbein et al. 1993). MAbs against K9 (clones Ks9.70, Ks9.216; PROGEN, Heidelberg, Germany) are available (Langbein et al. 2005, 2006, 2008). Immunostaining for K9 has significance for characterization of palmoplantar keratinocyte direction of transplants (Compton et al. 1998; Stoner and Wood 1999) and of special genodermatoses (McLean and Irvine 2007) but there is no relevance of K9 in tumor diagnosis. It should be noted that the occurrence of keratin K9 (and K1 and K10) may give rise to problems in biochemical practice as biochemicals or buffers are sometimes contaminated with these keratins derived from abraded “horny particles”, i.e. terminally differentiated skin keratinocytes (“laboratory dust”; Clark et al. 1971; Fox et al. 2008).
K2: keratin of highly differentiated, advanced epidermal keratinocytes
K2 (formerly K2e, see before and Table 1) is another keratin specific for the advanced terminal differentiation process of epidermal keratinocytes. Being widely distributed over most body sites, this type II keratin is expressed late, at an advanced stage of differentiation, in the uppermost epidermal layers (upper stratum spinosum, stratum granulosum) to a variable extent (Collin et al. 1992a). K2 is not expressed in follicular skin adnexal structures like the late compartments of outer or inner root sheath. Correspondingly, mutations in K2 have been associated with ichthyosis bullosa of Siemens, a blistering disease showing cytolysis in superficial epidermal layers (for references, see Lane and McLean 2004). MAbs specific for K2 (clones Ks2.342.7.1, Ks2.398.3.1; PROGEN, Heidelberg, Germany) have been described (Langbein et al. 2005, 2006, 2008).
K3/K12: keratins of the corneal epithelium
The K3 (type II)/K12 (type I) pair is the cell type-specific and differentiation-related keratin pair of the corneal epithelium. These keratins are expressed in all corneal epithelial cell layers, whereas in the limbus corneae only suprabasal cells are positive and the basally located corneal stem cells are K3/K12 negative (Schermer et al. 1986; Pitz and Moll 2002). Mutations in these keratins give rise to Meesmann’s corneal dystrophy characterized by intraepithelial microcysts in the corneal epithelium (Irvine et al. 1997; for further references, see Lane and McLean 2004). K12 knock-out mice have a mechanically fragile, easily detachable corneal epithelium (Kao et al. 1996). Antibodies against these corneal keratins described include MAb AE5 (PROGEN, Heidelberg, Germany) which reacts with K3 and additionally with the related K76 (formerly K2p; see below; Collin et al. 1992b) and MAb AK12 which recognizes K12 (Chaloin-Dufau et al. 1993).
K4/K13: keratins of mucosal stratified squamous epithelial cells
In internal stratified squamous epithelia which mostly are non-keratinizing, a highly characteristic keratin pair indicates the mucosal path of keratinocyte differentiation, i.e. the type II keratin K4 and the type I keratin K13 (Moll et al. 1982b; Cooper et al. 1985). Immunohistochemical studies using specific MAbs—such as MAb 6B10 against K4 (van Muijen et al. 1986) and MAbs 1C7, 2D7 (van Muijen et al. 1986) and Ks13.1 (Moll et al. 1988b) against K13—revealed the presence of K4 and K13 in the entire suprabasal compartment of mucosal stratified squamous epithelia, whereas the basal compartment is positive for K5/K14. Interestingly, K4/K13 is completely absent in the epidermis and adnexal structures. Keratin K13 is also expressed in the urothelium as a major component of the basal and intermediate cells whereas it is lost in the superficial umbrella cells (that switch on K20 expression; see above). Keratin K4 is also—heterogeneously—expressed in columnar luminal cells of the pseudostratified respiratory epithelium and in the non-stratified simple epithelial cells of e.g. pancreatic ducts (van Muijen et al. 1986; Moll 1993).
Functionally, K4 and K13 appear to be important particularly as components of mucosal stratified squamous epithelia. Mutations in these keratins, lying in the helix initiation or termination motifs (HIM or HTM, respectively), have been shown to cause the hereditary disorder white sponge nevus of Cannon (for references see Lane and McLean 2004). This mucosal disorder presents with white plaques mainly on the buccal mucosa, histologically showing thickened spongy epithelium with hydropic swelling of suprabasal epithelial cells. Here again, the clinical manifestation of pathological alterations of keratins well reflects their tissue distribution.
It is not surprising that squamous cell carcinomas derived from the epidermis essentially lack K4 and K13 (Kuruc et al. 1989; for further references see Moll 1998). However, in contrast to what might be expected, they are not major components of, but are only focally and variably expressed in squamous cell carcinomas of the head and neck, with more pronounced expression in poorly differentiated cases (Moll 1998). Instead, the predominant maturation-associated keratins expressed by these tumors are the hyperproliferative keratins K6 and 16 (see below, chapter “Keratins as diagnostic markers in tumor pathology”). Corresponding to its characteristic expression in normal urothelium, K13 is maintained—at least focally—in most transitional cell carcinomas of the urinary tract (Moll et al. 1988b; for further references, see Moll 1998). K13 may be part of a panel of markers (which also includes K20) useful in the histological diagnosis of metastatic transitional cell carcinomas (see below, chapter “Keratins as diagnostic markers in tumor pathology”). As to adenocarcinomas, it is noteworthy to mention the frequent expression of K4 in ductal adenocarcinomas of the pancreas (Schüssler et al. 1992; Real et al. 1993; Moll 1998) and in a subpopulation of poorly differentiated invasive ductal breast carcinomas (Malzahn et al. 1998).
K76, K77: keratins with very special expression sites
K76 (previously designated K2p) is specifically expressed in suprabasal cell layers of oral masticatory epithelium, i.e. the slightly orthokeratinized stratified squamous epithelium lining the gingiva and the hard palate (Collin et al. 1992b). Because of the failure of specific antibodies for long time, which are now available (PROGEN, Heidelberg, made by L.L.), no tumor studies have been done yet.
The expression pattern of keratin K77 (previously designated K1b) was very surprising and extremely restricted. This keratin is exclusively expressed in and restricted to the luminal cells of eccrine sweat gland ducts (Fig. 5a–d). All other epithelia and glands tested so far, including apocrine sweat gland, were negative (Langbein et al. 2005). Based on the extensive investigation of the keratin pattern, new aspects of eccrine sweat gland differentiation could be achieved (Langbein et al. 2005 and supplemental data therein). The high specificity of expression makes this keratin recommendable for using as an “eccrine duct marker” in tumor diagnostics. In a first study, this assumption could be confirmed by the investigation of “eccrine” adnexal tumors such as syringoma (Fig. 5e), poroma (Langbein et al. 2008) and cylindroma (Fig. 5f).
K25, K26, K27, K28, K71, K72, K73, K74, K75: hair follicle-specific epithelial keratins
Only recently it has become clear that some of the epithelial root sheaths of the hair follicle, the inner root sheath and the companion layer, are unique by their expression of a number of very special keratins (for review, see Langbein and Schweizer 2005; Langbein et al. 2006; Schweizer et al. 2007). Over the years, these keratins were not detected before by biochemical methods because of their quantitative “under-representation” when compared to the masses of hair or epidermal keratins in the tissue. The first of these new special keratins described was K75. This keratin was originally called K6hf, with “hf” indicating its expression site in the “hair follicle”, not knowing that this aspect of designation would be unfeasible later. K75 (K6hf) is a type II keratin and was, although closely related in its peptide sequence to K5, following the principles of the former keratin designation (cf. Collin et al. 1992a, b) by using the electrophoretic properties, designated as a “K6” keratin (Winter et al. 1998). K75 is specifically expressed in the companion layer of the hair follicle (Fig. 6a, h, i), a thin layer between the outer and the inner epithelial root sheath (Winter et al. 1998). As the expression of this keratin as monitored by its mRNA synthesis using in situ hybridization starts from the bulbar matricial compartment of the hair follicle and the protein is still existent in the upper differentiated part where the K75 mRNA is no longer synthesized, it was one first but doubtless indication that this structure is an own individual compartment of the hair follicle and not the “innermost layer of the outer root sheath” (see Winter et al. 1998; Langbein and Schweizer 2005). The only further structures in which K75 has been detected are the hair medulla of, e.g. beard hairs, the nail bed, and fungiform papillae of the tongue (Wang et al. 2003; Perrin 2007; Langbein and Schweizer 2005). A mutation in K75 appears to predispose to the common hair disorder pseudofolliculitis barbae, which is characterized by ingrown beard hairs with inflammation, induced by shaving (Winter et al. 2004; Schweizer et al. 2007), and to the loose anagen hair syndrome (Chapalain et al. 2002). Immunostaining for K75 has been reported in trichoblastomas and basal cell carcinomas (Kurzen et al. 2001) and has recently been observed in some squamoid cells of pilomatricomas (M. Divo, L. Langbein and R. Moll, in preparation), indicating special focal differentiation in these diverse cutaneous tumors. We have recently found sparse and focal expression of K75 in certain squamous cell carcinomas of inner organs (M. Divo, L. Langbein and R. Moll, in preparation).
Fig. 6.
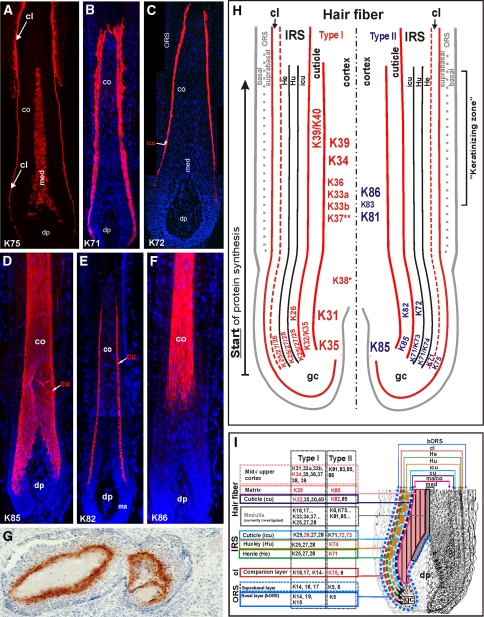
Immunofluorescence labeling of hair follicle-specific and hair keratins. The hair follicle-specific epithelial keratin K75 (a) is specifically found in the hair companion layer (cl) and in the medulla (med) of sexual (e.g. beard) hairs. K71 (b) is expressed in all compartments and K72 (c) in the cuticle (icu) of the hair inner root sheath (IRS). The hair keratin K85 (d) expression is found from the hair matrix to the upper cortex and the hair cuticle (cu), whereas K82 (e) is restricted to the hair cuticle. K86 (f) is an example for hair keratins expressed in the mid-to-upper hair cortex (co). g Hair keratin K81 is also expressed in the upper transitional cells of pilomatricoma. co cortex, dp dermal papilla, ORS outer root sheath. h, i Summary schemes of the expression of all hair and hair follicle-specific keratins in the human hair follicle. **K37 is found in the cortex of vellus hairs and medulla of sexual hairs. *K38 is heterogeneously expressed in the cortex. The keratin genes are designated according to the new keratin nomenclature (Schweizer et al. 2006)
A set of four type I keratins (K25–K28; previous designations K25irs1–K25irs4, see Table 1) and four type II keratins (K71–K74; previous designations K6irs1–K6irs4, see Table 1) is highly specific for the inner root sheath (IRS) of the hair follicle (Fig. 6h, i). These IRS keratins are differentially and partially sequentially expressed in the various IRS compartments, the Henle layer, the Huxley layer, and the IRS cuticle. The keratinocytes of all three compartments synthesize the IRS keratins K71 (Fig. 6b, h, i), K25, K27 and K28. K74 is restricted to the Huxley layer, whereas K73, K72 (Fig. 6c, h, i) and K28 are sequentially expressed in the IRS cuticle (Langbein et al. 2002, 2003, 2006; Langbein and Schweizer 2005; Fig. 6h, i). Otherwise, no “classical” epithelial keratins were evidenced without any doubts in the IRS and earlier reports most probably showed (at that time not expected) cross-reaction of antibodies with at least one of these IRS keratins (cf. Langbein et al. 2006). Some of the IRS keratins—together with many others—have also been found in the hair medulla (see Schweizer et al. 2007 and Langbein et al., in preparation). In mice, spontaneous hair disorders due to mutations in K71 have been identified (for references, see Schweizer et al. 2007). Human hair disorders related to the IRS keratins have not yet been discovered. Monospecific antisera against all of these keratins are available (PROGEN, Heidelberg, Germany; Langbein et al. 2004, 2006).
K31, K32, K33a, K33b, K34, K35, K36, K37, K38, K39, K40, K81, K82, K83, K84, K85, K86: keratins of the hair fiber (hair keratins)
It is long known since the early period of keratin research that in the (hard) material of hairs, wool, nails, claws and feathers, the tremendous masses of keratin filaments (selection of the early literature: Odland 1953; Fraser et al. 1959; Rogers and Clarke 1965; Orfanos and Ruska 1968) are embedded in a matrix of cross-linking specialized keratin associated proteins (KAPs) with more than 85 genes in humans (for review, see Rogers et al. 2006). The special, more sulfur-rich keratin proteins constituting these filaments are the “hard” or “trichocytic” keratins. Originally, eight “major” (type I: Ha1-4, type II: Hb1-4) and two “minor” (Hax, Hbx) keratins were distinguished (Heid et al. 1986, 1988a, b). In the last 10 years many more, namely 17 members of this keratin subfamily have been identified first at their gene level (Rogers et al. 2004, 2005 and references therein) which are now generally referred to as the hair keratins (Langbein et al. 1999, 2001, 2007; Langbein and Schweizer 2005; Schweizer et al. 2006; see also Table 1). The conspicuous abundance of these proteins comprises eleven type I hair keratins (K31–K40; previous designations Ha1–Ha8, Ka35, Ka36; Langbein et al. 1999, 2001, 2007, Langbein and Schweizer 2005; Schweizer et al. 2006; see also Table 1) and six type II hair keratins (K81–K86; previous designations Hb1–Hb6; Langbein et al. 1999, 2001; Schweizer et al. 2006; see also Table 1; Fig. 6h, i). In the hair, they exhibit differential, complex and in many cases sequential expression patterns within the cuticle and the cortex (Langbein et al. 1999, 2001, 2007; Langbein and Schweizer 2005; Schweizer et al. 2007; for medulla: Langbein et al., in preparation). K35 and K85 (Fig. 6d, h, i) are already expressed in the hair-forming matrix of the cortex and the hair cuticle. The other hair keratins [type I: K31, K33a, K33b, K34, K36, K38 (focally) and K39 (including hair cuticle); type II: K81 and K86] are sequentially switched on upon differentiation in the lower hair cortex and in particular the large “bulk” of hair keratins are expressed in the middle cortex (“keratinizing zone”) of the ascending hair fiber (Fig. 6f, h, i). Furthermore, K32, K83, K82 (Fig. 6e, h, i) and K40 are sequentially expressed and restricted in the hair cuticle (Langbein and Schweizer 2005; Langbein et al. 2007). As exceptions, K37 was only found in the cortex of vellus hairs and K84, although a typical hair keratin was not detected in the hairs but specifically in the filiform papillae of the tongue (Langbein and Schweizer 2005).
One has to keep in mind that out of the 54 human keratins at least 26 (~50%) are expressed in the hair follicle. Therefore, it is most amazing that this extraordinary complex structure—built up by a stratified outer root sheath, the companion layer, the inner root sheath comprising Henle, Huxley and IRS-cuticle layers, the hair cuticle, cortex, and sometimes a medulla—differentiate from cells of an undifferentiated and pluripotent “germinative cell pool” (Fig. 6a, h, i). There must exist an incredible “fine-tuning” in the regulation of gene expression when e.g. one cell differentiates into the IRS-cuticle and the directly neighboring cell builds up the hair cuticle, both structures being only one cell wide. Unfortunately, up to now, our knowledge on this most complex regulation of hair keratin gene expression is rather fragmentary.
Hair keratins are also prominently expressed in the nail matrix and nail bed and contribute to the formation of the hard tissue of the nail plate (Perrin et al. 2004; Perrin 2007). Moreover, in the 1980s hair keratins have been additionally detected by immunohistochemistry in filiform papillae of the tongue and, intriguingly, in reticulum cells and Hassall’s corpuscles of the thymus (Heid et al. 1988b).
Some human mutations affecting hair keratin genes have been recognized. The most well-known of these is the congenital hair disease monilethrix, characterized by deformed hair shafts with a beaded appearance. Causative mutations for this disorder have been identified in the hair keratins K86, K81, and rarely in K83 (Winter et al. 1997; for further references, see Schweizer et al. 2007), all of which are expressed in the cortex of hair shafts and thus indicating that monilethrix is a disease of the hair cortex. A further, rare disease recently found to be related to a distinct mutation in the type II hair keratin K85 is ectodermal dysplasia of hair and nail type, characterized by total alopecia and severe nail dystrophy (Naeem et al. 2006). The expression of K85 already in the hair matrix from which hair fiber formation starts (Schweizer et al. 2007) may explain the severe hair phenotype of these patients.
As to tumors, hair keratins have—up to now—only been detected in pilomatricomas (Moll et al. 1988a; Régnier et al. 1997) which are regarded as originating from hair matrix and undergoing true hair differentiation. The complex hair keratin expression in benign and malignant pilomatricoma (Cribier et al. 2001, 2004, 2006) confirms this notion at the molecular level. Monospecific antisera against all of these keratins are available (PROGEN, Heidelberg, Germany; Langbein et al. 2004, 2006).
K23, K24, K78, K79, K80: keratins with still unknown expression pattern
These five, very different keratins complete the family of human keratin proteins. In principle, mainly the gene and cDNA sequences for the type I keratins K23 (Zhang et al. 2001) and K24 and the type II keratins K78 (formerly K5b), K79 (formerly K6l), and K80 (formerly Kb20) (Rogers et al. 2004, 2005) are known up to now. Unfortunately only very vague and preliminary (or no) expression data are available from Northern blot analyses, suggesting their expression in tongue (K78, K80) and skin (K79), respectively (Rogers et al. 2005). As they were obviously “overlooked” in former studies, their expression might be restricted to very special epithelia, distinct cellular differentiation stages or even transient expression phases. The investigation of the detailed expression pattern of these keratins will be one of the most urgent studies in this field.
Keratins as diagnostic markers in tumor pathology
One of the important fields of “application” of keratins (making use of the knowledge of their high number of gene family members combined with their special and characteristic expression patterns in distinct cell types, differentiation stages or functional states) is their use—aided by specific antibodies—as immunohistochemical markers in diagnostic tumor pathology. Epithelial tumors maintain—at least widely—specific features of the keratin expression patterns of the respective cell type of origin. Thus, in cases which on the basis of clinical data and conventional histopathology remain unclear, keratin typing may help to correctly identify and classify the tumor entity present. Keratin profiling is especially valuable for carcinomas of poorly differentiated histology, for carcinomas spreading over several organs, and in particular for metastases of an unknown primary tumor. Of the 54 human keratins, only a relatively small panel has attained diagnostic importance up to now, which might grow further with time and respective studies by including the keratins described within the last years. The carefully selected use of diagnostically relevant keratin antibodies—if appropriate, as part of a panel together with other tumor type markers—has become diagnostic standard in state-of-the-art clinical pathology (for recent overviews, see Chu and Weiss 2002b; Dabbs 2006). In the previous chapter on the individual keratins, some data on their expression patterns in tumors have already been presented. In the following, key diagnostic features of keratin typing concerning important tumor entities will be briefly summarized.
Adenocarcinomas
Adenocarcinomas are one of the largest groups of human malignant tumors and may arise in many organs and tissues. They comprise more than one-half of cases of cancer with unknown primary tumor. The identification of the specific origin—e.g. colon, ovary, pancreas, or lung—has become therapeutically important since effective chemotherapy schedules may be vastly different. As a group, adenocarcinomas are characterized by the predominance of simple-epithelial keratins, notably K8, K18 and K19, whereas K7 and K20 are variably expressed. It is this variability which may be diagnostically exploited, and thus staining for both K7 and K20 is the current practice resulting in different K7/K20 phenotypes (Table 3). The highest diagnostic significance of keratin typing is true for colorectal adenocarcinomas which—like the normal mucosa—almost always remain K20-positive (Fig. 3d). Most cases, including metastases, exhibit a K7−/K20+ phenotype (Fig. 3c, d) or express K7 at lower level as compared to K20 (Moll et al. 1992; Miettinen 1995; for further references, see Moll 1998, Chu and Weiss 2002b). K7 co-expression has been detected more frequently in advanced colorectal cancers (Hernandez et al. 2005). Although so characteristic of colorectal tumors, K20 may in certain situations be reduced. Thus, reduced expression of K20 has been found in a specific subset of colorectal carcinomas with high levels of microsatellite instability (McGregor et al. 2004); notably, K7 expression remained low in these tumors. Since K20 is a differentiation marker its reduction or even loss may also be due to dedifferentiation. In a large tissue microarray study, reduced expression of K8 and K20 was found to be associated with shorter patients’ survival, possibly on the basis of epithelial-mesenchymal transition (Knösel et al. 2006). Adenocarcinomas of the stomach usually show heterogeneous expression of both K7 and K20, the latter being rather variable but yet expressed in the majority of cases (Moll 1998, Chu and Weiss 2002b). No systematic differences between intestinal and diffuse/signet ring cell carcinomas have been described. In adenocarcinomas of the pancreas and the biliary tract, there is usually a clear predominance of K7 (Fig. 3e) together with variable and focal (often minor) expression of K20 in up to 75% of cases (Fig. 3f, Moll 1998; Chu and Weiss 2002b). Ductal adenocarcinomas of the pancreas in addition often express certain stratified-epithelial keratins, most notably K4 and K17 (Schüssler et al. 1992; Real et al. 1993; Moll 1998). Finally, a K7+/K20− phenotype is characteristic of adenocarcinomas of the ovary (except for the mucinous type), the endometrium and the lung. Endometrial adenocarcinomas often focally co-express certain stratified-epithelial keratins including K5, reflecting the potential of the tumor cells for stratification to develop squamous metaplasia.
Like other adenocarcinomas, most breast carcinomas constitutively express K8, K18 and K19 (Altmannsberger et al. 1986; Malzahn et al. 1998). In some studies, however, a high level of K8 or K18 immunostaining, as detected by certain monoclonal antibodies, has been correlated with favorable prognosis and reduced or absent staining was associated with unfavorable outcome (Takei et al. 1995; Schaller et al. 1996; Woelfle et al. 2004). Microarray-based expression profiling of breast carcinomas has led to the definition of distinct subgroups. One of these has been designated as basal-like group (Sørlie et al. 2001) which is characterized by relatively poor prognosis. Interestingly, the typical expression profile of the basal-like cancers includes the basal cell-typical keratins K5, K14 and K17. Several older reports have already described the presence of such keratins in a subpopulation of breast carcinomas (Moll et al. 1983; Wetzels et al. 1991), and some did already point to a negative prognostic significance of basal cell keratin expression in invasive ductal breast cancers (Dairkee et al. 1987; Malzahn et al.1998). This has now been confirmed and extended by the growing body of recent microarray data, allowing to define a subgroup of sporadic breast cancers which exhibit bad prognosis and association with BRCA1 mutation or dysfunction (Gusterson et al. 2005; Diaz et al. 2007). For the recognition of this subgroup, immunostaining for keratins such as K5 may achieve importance (van de Rijn et al. 2002; for further references, see Gusterson et al. 2005).
The different subtypes of renal cell carcinomas (RCCs) exhibit some characteristic features in keratin expression which may support the precise classification of these tumors (Thoenes et al. 1988; Moll 1993; Chu and Weiss 2002b; Skinnider et al. 2005; Liu et al. 2007). Thus, conventional (clear cell) RCCs express a simple keratin pattern of mainly K8/K18 together with variable, mostly minor expression of K19. In contrast, the papillary subtype of RCC is characterized by strong K19 expression as well as K7 expression in addition to the basic pair K8/K18. Chromophobe RCCs, on the other hand, typically express K7 (but little K19) in addition to K8/K18 (Thoenes et al. 1988). The benign oncocytomas, which histologically may resemble chromophobe RCC, are mostly K7-negative (Liu et al. 2007). The peculiar co-expression, together with keratins, of the mesenchymal intermediate filament protein vimentin in conventional (clear cell) and papillary RCCs but not in chromophobe RCCs and oncocytomas is another feature valuable in differential diagnosis. Another tumor category with characteristic co-expression of keratins and vimentin are malignant mesotheliomas. The epithelial type may be difficult to distinguish histologically from adenocarcinomas (e.g. pleural involvement by pulmonary adenocarcinomas or pleural/peritoneal metastases of adenocarcinomas of various origins). In contrast to most adenocarcinomas, epithelial mesotheliomas consistently express keratinocyte-type keratins, notably K5, in addition to keratins K8, K18, K19, and K7 typical for simple epithelia (Moll et al. 1989; Ordonez 1998; Chu and Weiss 2002a; Yaziji et al. 2006). Thus, in recent years, K5 has been included in the battery of useful mesothelioma markers.
Neuroendocrine tumors
Neuroendocrine tumors, a large and heterogeneous tumor group, are generally characterized by the expression of keratins typical of simple epithelia (notably K8, K18, and—more variably—K19) and the complete lack of keratins K5/K14 typically found in stratified epithelia. Particularly interesting in a diagnostic respect is the well-proven value of K20 as a consistent marker of Merkel cell carcinomas of the skin, allowing their delineation from metastatic small cell neuroendocrine carcinomas arising at other sites such as small cell lung carcinoma which—although morphologically similar—consistently lack K20 (Moll et al. 1992; Cheuk et al. 2001). Another interesting recent issue concerns endocrine tumors of the pancreas. Several studies suggest that the expression of K19 in these tumors may be correlated with a poor prognosis (Schmitt et al. 2007; La Rosa et al. 2007).
Transitional cell carcinomas
The urothelium exhibits a unique, complex pattern of keratin expression. K8, K18, K19, and K7 are expressed in all cell layers. K5 and K17 are restricted to the basal cell layer. Particularly characteristic are K13 expressed in the basal and intermediate cell layers and K20 specific for the superficial (umbrella) cell layer. This urothelial keratin pattern is relatively well conserved in noninvasive and invasive transitional cell carcinomas (TCCs) (for detailed reviews, see Moll 1998; Southgate et al. 1999). K20 has been found to be retained in ~80% of TCCs (Moll et al. 1992). Indeed, the combined presence of keratins K8/K18 (as well as K7 and K19) typical for simple epithelia together with K13 and K20 in a tumor is characteristic of urothelial origin, although K13 may be reduced or lost in poorly differentiated TCCs. Squamous metaplasia may modify the keratin expression pattern. Particularly interesting are the recent, prognostically relevant findings that noninvasive papillary TCCs may exhibit a normal K20 pattern (predominantly superficial) or an abnormal K20 pattern (all cell layers or negative) and that the normal pattern is predictive of tumor non-recurrence (Southgate et al. 1999).
Squamous cell carcinomas
Squamous cell carcinomas of different sites of origin are generally characterized by a predominance of stratified-epithelial/keratinocyte-type keratins but may co-express certain simple-epithelial keratins (for details, see Moll 1998). Most of these tumors strongly express the keratins K5 (Fig. 4c, e), K14 and K17 normally found in the basal layer as well as the keratins K6 (Fig. 4d) and K16 characteristic for hyperproliferative keratinocytes. Focally, there may be expression of K1/K10 (particularly in higher differentiated tumor cells which can end in the formation of horn pearls), and—to a lesser extent—K4 and K13. The co-expression of simple epithelia-typical keratins comprises K8, K18, and K19, and different studies have suggested that this co-expression seems to be more pronounced in poorly differentiated squamous cell carcinomas (for references, see Moll 1998). Recently it has been demonstrated that in squamous cell carcinomas of the oral cavity the expression of K8 and K18 is an independent prognostic marker and indicates a decreased overall and progression-free survival (Fillies et al. 2006).
In summary, stratified-epithelial keratins, in particular K5 and K6, are useful as general markers for squamous cell carcinomas in histologically uncertain, poorly differentiated, or metastatic tumor cases. Although certain differences between the keratin expression patterns of squamous cell carcinomas from different sites of origin have been noted, it is not yet possible to use keratins as specific site markers in cases of unclear metastases (Moll 1998).
Perspectives
In the past 10 years, our knowledge on keratins has tremendously increased, regarding their molecular and cell biology as well as their application as markers in pathological diagnosis. The introduction of a new consensus nomenclature (Schweizer et al. 2006), which for the common (non-hair follicle specific) epithelial keratins widely conserves the older names established in the literature, makes this complex field much clearer than before and will facilitate future research. Today much is known about the structural functions of keratins, as proven by a wealth of human hereditary keratin diseases and transgenic mouse models. However, numerous questions still need to be answered, particularly concerning the regulatory functions of keratins. As reviewed in this article, recent experimental studies have pointed to newly recognized roles of certain keratins in apoptosis, cell growth, tissue polarity, wound response, and tissue remodeling. First, specific signaling pathways have already been described which seem to be associated with distinct keratins. The question of whether keratins might play a role in malignant transformation is particularly exciting, but at present a causal role in tumorigenesis has not been established for any keratin (for review, see Magin et al. 2007). However, keratins may in fact be linked to the cell cycle machinery (Margolis et al. 2006). Future research will hopefully answer the question why the human organism actually needs 54 different keratin proteins, all of which in the first instance form the seemingly “primitive”, uniform structure of a 10 nm intermediate filament—although with peculiarities in the head and tail domains of their single molecular components.
One may also expect for the future that the diagnostic application of keratins, although already established in tumor pathology, will be further refined and extended. Examples of very recent new developments in tumor classification are the recognition of the basal-like subtype of breast cancer on the basis of the expression of K5 and the prognostic relevance of K19 in endocrine pancreatic tumors. At the moment, the panel of keratins introduced into routine diagnosis as histopathological tumor markers is distinctly small, essentially consisting of K5, K7, K8/K18, K19, and K20. Furthermore, the expression patterns of a battery of special keratins were detected only in the last few years and these keratins are just started to be involved into tumor diagnostic studies. Not to forget that at the time 5 out of the 54 human keratins are still uncharacterized in their expression sites and patterns and possibly, their expression in neoplasia might have a potential as diagnostic markers. Since all keratins have an exquisite cell type- and differentiation stage-specific regulation and expression pattern, it might well be that upon future research further keratins will be introduced as markers for certain diagnostic questions in pathology to widen and to refine the diagnostic potential of keratins.
Acknowledgments
We are grateful to Dr. Bernard Cribier (Dermatology, University of Strasbourg, France) for his help with images E/F and G in Figs. 5 and 6, respectively. We also thank Renate Baumann (Marburg) and Silke Praetzel-Wunder (Heidelberg) for their expert technical support, Patrick Lebok (Marburg) for help with the figure plates, and Dr. Melissa Stöppler (San Francisco) for correcting the English language.
Footnotes
One principle comment has to be stated. Besides the problem, that “on market” many antibodies (not only against keratins) sometimes are only barely “characterized” and insofar of questionable “specificity” once must keep in mind that also some of the heavily used antibodies were never tested under the viewpoint of the today’s knowledge of all human keratins.
Contributor Information
Roland Moll, Phone: +49-6421-2862270, FAX: +49-6421-2865640, Email: mollr@med.uni-marburg.de.
Markus Divo, Email: divo@med.uni-marburg.de.
Lutz Langbein, Email: langbein@dkfz-heidelberg.de.
References
- Abrahams NA, Ormsby AH, Brainard J (2002) Validation of cytokeratin 5/6 as an effective substitute for keratin 903 in the differentiation of benign from malignant glands in prostate needle biopsies. Histopathology 41(1):35–41 [DOI] [PubMed]
- Abrahams NA, Bostwick DG, Ormsby AH, Qian J, Brainard JA (2003) Distinguishing atrophy and high-grade prostatic intraepithelial neoplasia from prostatic adenocarcinoma with and without previous adjuvant hormone therapy with the aid of cytokeratin 5/6. Am J Clin Pathol 120(3):368–376 [DOI] [PubMed]
- Altmannsberger M, Dirk T, Droese M, Weber K, Osborn M (1986) Keratin polypeptide distribution in benign and malignant breast tumors: subdivision of ductal carcinomas using monoclonal antibodies. Virchows Arch B Cell Pathol Incl Mol Pathol 51(3):265–275 [DOI] [PubMed]
- Bader BL, Magin TM, Hatzfeld M, Franke WW (1986) Amino acid sequence and gene organization of cytokeratin no. 19, an exceptional tail-less intermediate filament protein. EMBO J 5(8):1865–1875 [DOI] [PMC free article] [PubMed]
- Balaton AJ, Nehama-Sibony M, Gotheil C, Callard P, Baviera EE (1988) Distinction between hepatocellular carcinoma, cholangiocarcinoma, and metastatic carcinoma based on immunohistochemical staining for carcinoembryonic antigen and for cytokeratin 19 on paraffin sections. J Pathol 156(4):305–310 [DOI] [PubMed]
- Barak V, Goike H, Panaretakis KW, Einarsson R (2004) Clinical utility of cytokeratins as tumor markers. Clin Biochem 37(7):529–540 [DOI] [PubMed]
- Bártek J, Bartkova J, Taylor-Papadimitriou J, Rejthar A, Kovarik J, Lukas Z, Vojtesek B (1986) Differential expression of keratin 19 in normal human epithelial tissues revealed by monospecific monoclonal antibodies. Histochem J 18(10):565–575 [DOI] [PubMed]
- Bártek J, Vojtĕsek B, Stasková Z, Bártková J, Kerekés Z, Rejthar A, Kovarík J (1991) A series of 14 new monoclonal antibodies to keratins: characterization and value in diagnostic histopathology. J Pathol 164(3):215-224 [DOI] [PubMed]
- Blumenberg M. (1988) Concerted gene duplications in the two keratin gene families. J Mol Evol 27(3):203–211 [DOI] [PubMed]
- Böcker W, Moll R, Poremba C, Holland R, Van Diest PJ, Dervan P, Burger H, Wai D, Ina DR, Brandt B, Herbst H, Schmidt A, Lerch MM, Buchwallow IB (2002) Common adult stem cells in the human breast give rise to glandular and myoepithelial cell lineages: a new cell biological concept. Lab Invest 82(6):737–746 [DOI] [PubMed]
- Bosch FX, Leube RE, Achtstätter T, Moll R, Franke WW (1988) Expression of simple epithelial type cytokeratins in stratified epithelia as detected by immunolocalization and hybridization in situ. J Cell Biol 106(5):1635–1648 [DOI] [PMC free article] [PubMed]
- Bosch FX, Ouhayoun JP, Bader BL, Collin C, Grund C, Lee I, Franke WW (1989) Extensive changes in cytokeratin expression patterns in pathologically affected human gingiva. Virchows Arch B Cell Pathol Incl Mol Pathol 58(1):59–77 [DOI] [PubMed]
- Caulin C, Ware CF, Magin TM, Oshima RG (2000) Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis. J Cell Biol 149(1):17–22 [DOI] [PMC free article] [PubMed]
- Chaloin-Dufau C, Pavitt I, Delorme P, Dhouailly D (1993) Identification of keratins 3 and 12 in corneal epithelium of vertebrates. Epithelial Cell Biol 2(3):120–125 [PubMed]
- Chapalain V, Winter H, Langbein L, LeRoy JM, Labreze C, Nikolic M, Schweizer J, Taieb A (2002) Is the loose anagen hair syndrome a keratin disorder? A clinical and molecular study. Arch Dermatol 138:501–506 [DOI] [PubMed]
- Chausovsky G, Luchansky M, Figer A, Shapira J, Gottfried M, Novis B, Bogelman G, Zemer R, Zimlichman S, Klein A (1999) Expression of cytokeratin 20 in the blood of patients with disseminated carcinoma of the pancreas, colon, stomach, and lung. Cancer 86(11):2398–2405 [PubMed]
- Cheuk W, Kwan MY, Suster S, Chan JK (2001) Immunostaining for thyroid transcription factor 1 and cytokeratin 20 aids the distinction of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch Pathol Lab Med 125(2):228–231 [DOI] [PubMed]
- Chu PG, Weiss LM (2002a) Expression of cytokeratin 5/6 in epithelial neoplasms: an immunohistochemical study of 509 cases. Mod Pathol 15(1):6–10 [DOI] [PubMed]
- Chu PG, Weiss LM (2002b) Keratin expression in human tissues and neoplasms. Histopathology 40(5):403–439 [DOI] [PubMed]
- Chu P, Wu E, Weiss LM (2000) Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol 13(9):962–972 [DOI] [PubMed]
- Clark RP, Cox RN, Lewis HE (1971) Deposition and dispersion of particles from the human micro-environment. J Physiol 216(1):19–20 [PubMed]
- Collin C, Moll R, Kubicka S, Ouhayoun JP, Franke WW (1992a) Characterization of human cytokeratin 2, an epidermal cytoskeletal protein synthesized late during differentiation. Exp Cell Res 1:132–141 [DOI] [PubMed]
- Collin C, Ouhayoun JP, Grund C, Franke WW (1992b) Suprabasal marker proteins distinguishing keratinizing squamous epithelia: cytokeratin 2 polypeptides of oral masticatory epithelium and epidermis are different. Differentiation 51(2):137–148 [DOI] [PubMed]
- Compton CC, Nadire KB, Regauer S, Simon M, Warland G, O’Connor NE, Gallico GG, Landry DB (1998) Cultured human sole-derived keratinocyte grafts re-express site-specific differentiation after transplantation. Differentiation 64(1):45–53 [DOI] [PubMed]
- Cooper D, Schermer A, Sun TT (1985) Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest 52(3):243–256 [PubMed]
- Cribier B, Peltre B, Langbein L, Winter H, Schweizer J, Grosshans E (2001) Expression of type I hair keratins follicular tumors. Br J Dermatol 144:977–982 [DOI] [PubMed]
- Cribier B, Peltre B, Grosshans E, Langbein L, Schweizer J (2004) On the regulation of hair keratin expression: lessons from studies in pilomatricomas. J Invest Dermatol 122(5):1078–1083 [DOI] [PubMed]
- Cribier B, Worret WI, Braun-Falco M, Peltre B, Langbein L, Schweizer J (2006) Expression patterns of hair and epithelial keratins and transcription factors HOXC13, LEF1, and β-catenin in a malignant pilomatricoma: a histological and immunohistochemical study. J Cutan Pathol 33:1–9 [DOI] [PubMed]
- Crick FH (1952) Is alpha-keratin a coiled coil? Nature 170(4334):882–883 [DOI] [PubMed]
- Dabbs DJ (2006) Immunohistology of metastatic carcinoma of unknown primary. In: Dabbs DJ (ed) Diagnostic immunohistochemistry. Churchill Livingstone, Elsevier, Philadelphia, pp 180–226
- Dairkee SH, Mayall BH, Smith HS, Hackett AJ (1987) Monoclonal marker that predicts early recurrence of breast cancer. Lancet 1(8531):514 [DOI] [PubMed]
- Demirkesen C, Hoede N, Moll R (1995) Epithelial markers and differentiation in adnexal neoplasms of the skin: an immunohistochemical study including individual cytokeratins. J Cutan Pathol 22(6):518–535 [DOI] [PubMed]
- Diaz LK, Cryns VL, Symmans WF, Sneige N (2007) Triple negative breast carcinoma and the basal phenotype: from expression profiling to clinical practice. Adv Anat Pathol 14(6):419–430 [DOI] [PubMed]
- Fillies T, Werkmeister R, Packeisen J, Brandt B, Morin P, Weingart D, Joos U, Buerger H (2006) Cytokeratin 8/18 expression indicates a poor prognosis in squamous cell carcinomas of the oral cavity. BMC Cancer 6:10 [DOI] [PMC free article] [PubMed]
- Fox K, Castanha E, Fox A, Feigley C, Salzberg D (2008) Human K10 epithelial keratin is the most abundant protein in airborne dust of both occupied and unoccupied school rooms. J Environ Monit 10(1):55–59 [DOI] [PubMed]
- Franke WW, Weber K, Osborn M, Schmid E, Freudenstein C (1978) Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res 116(2):429–445 [DOI] [PubMed]
- Franke WW, Schiller DL, Moll R, Winter S, Schmid E, Engelbrecht I, Denk H, Krepler R, Platzer B (1981) Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol 153(4):933–959 [DOI] [PubMed]
- Franke WW, Moll R (1987) Cytoskeletal components of lymphoid organs. I. Synthesis of cytokeratins 8 and 18 and desmin in subpopulations of extrafollicular reticulum cells of human lymph nodes, tonsils, and spleen. Differentiation 36(2):145–163 [DOI] [PubMed]
- Fraser RD, Macrae TP, Rogers GE (1959) Structure of alpha-keratin. Nature 183(4661):592–594 [DOI] [PubMed]
- Friederichs J, Gertler R, Rosenberg R, Dahm M, Nekarda H, Holzmann B, Siewert JR (2007) Correlation of CK-20-positive cells in peripheral venous blood with serum CEA levels in patients with colorectal carcinoma. World J Surg 31(12):2329–2334 [DOI] [PubMed]
- Fuchs E, Green H (1980) Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 19(4):1033–1042 [DOI] [PubMed]
- Fuchs E, Esteves RA, Coulombe PA (1992) Transgenic mice expressing a mutant keratin 10 gene reveal the likely genetic basis for epidermolytic hyperkeratosis. Proc Natl Acad Sci USA 89(15):6906–6910 [DOI] [PMC free article] [PubMed]
- Funaki NO, Tanaka J, Ohshio G, Onodera H, Maetani S, Imamura M (1998) Cytokeratin 20 mRNA in peripheral venous blood of colorectal carcinoma patients. Br J Cancer 77(8):1327–1332 [DOI] [PMC free article] [PubMed]
- Galarneau L, Loranger A, Gilbert S, Marceau N (2007) Keratins modulate hepatic cell adhesion, size and G1/S transition. Exp Cell Res 313(1):179–194 [DOI] [PubMed]
- Geisler N, Weber K (1982) The amino acid sequence of chicken muscle desmin provides a common structural model for intermediate filament proteins. EMBO J 1(12):1649–1656 [DOI] [PMC free article] [PubMed]
- Glass C, Fuchs E (1988) Isolation, sequence, and differential expression of a human K7 gene in simple epithelial cells. J Cell Biol 107(4):1337–1350 [DOI] [PMC free article] [PubMed]
- Goldstein NS, Bosler DS (2006) Immunohistochemistry of the gastrointestinal tract, pancreas, bile ducts, gallbladder and liver. In: Dabbs DJ (ed) Diagnostic immunohistochemistry. Churchill Livingstone, Elsevier, Philadelphia, pp 442–508
- Gould VE, Bloom KJ, Franke WW, Warren WH, Moll R (1995) Increased numbers of cytokeratin-positive interstitial reticulum cells (CIRC) in reactive, inflammatory and neoplastic lymphadenopathies: hyperplasia or induced expression? Virchows Arch 425(6):617–629 [DOI] [PubMed]
- Grimm SL, Bu W, Longley MA, Roop DR, Li Y, Rosen JM (2006) Keratin 6 is not essential for mammary gland development. Breast Cancer Res 8(3):R29 [DOI] [PMC free article] [PubMed]
- Gu LH, Coulombe PA (2007) Keratin expression provides novel insight into the morphogenesis and function of the companion layer in hair follicles. J Invest Dermatol 127(5):1061–1073 [DOI] [PubMed]
- Gusterson BA, Ross DT, Heath VJ, Stein T (2005) Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res 7(4):143–148 [DOI] [PMC free article] [PubMed]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM (1999) Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J 18(21):5931–5942 [DOI] [PMC free article] [PubMed]
- Heid HW, Werner E, Franke WW (1986) The complement of native alpha-keratin polypeptides of hair-forming cells: a subset of eight polypeptides that differ from epithelial cytokeratins. Differentiation 32(2):101–119 [DOI] [PubMed]
- Heid HW, Moll I, Franke WW (1988a) Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. I. Human and bovine hair follicles. Differentiation 37(2):137–157 [DOI] [PubMed]
- Heid HW, Moll I, Franke WW (1988b) Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. II. Concomitant and mutually exclusive synthesis of trichocytic and epithelial cytokeratins in diverse human and bovine tissues (hair follicle, nail bed and matrix, lingual papilla, thymic reticulum). Differentiation 37(3):215–230 [DOI] [PubMed]
- Hernandez BY, Frierson HF, Moskaluk CA, Li YJ, Clegg L, Cote TR, McCusker ME, Hankey BF, Edwards BK, Goodman MT (2005) CK20 and CK7 protein expression in colorectal cancer: demonstration of the utility of a population-based tissue microarray. Hum Pathol 36(3):275–281 [DOI] [PubMed]
- Hesse M, Franz T, Tamai Y, Taketo MM, Magin TM (2000) Targeted deletion of keratins 18 and 19 leads to trophoblast fragility and early embryonic lethality. EMBO J 19(19):5060–5070 [DOI] [PMC free article] [PubMed]
- Hesse U (2003) Expressionsmuster von plattenepithelialen Zytokeratinen – insbesondere von Zytokeratin 6 – in nicht-neoplastischen Epithelien, oropharyngealen Plattenepithelkarzinomen und Mammakarzinomen. M.D. thesis, University of Mainz, pp 1–195
- Huitfeldt HS, Brandtzaeg P (1985) Various keratin antibodies produce immunohistochemical staining of human myocardium and myometrium. Histochemistry 83(5):381–389 [DOI] [PubMed]
- Irvine AD, Corden LD, Swensson O, Swensson B, Moore JE, Frazer DG, Smith FJ, Knowlton RG, Christophers E, Rochels R, Uitto J, McLean WH (1997) Mutations in cornea-specific keratin K3 or K12 genes cause Meesmann’s corneal dystrophy. Nat Genet 16(2):184–187 [DOI] [PubMed]
- Ivanyi D, Ansink A, Groeneveld E, Hageman PC, Mooi WJ, Heintz AP (1989) New monoclonal antibodies recognizing epidermal differentiation-associated keratins in formalin-fixed, paraffin-embedded tissue. Keratin 10 expression in carcinoma of the vulva. J Pathol 159(1):7–12 [DOI] [PubMed]
- Jackson BW, Grund C, Schmid E, Burki K, Franke WW, Illmensee K (1980) Formation of cytoskeletal elements during mouse embryogenesis. Intermediate filaments of the cytokeratin type and desmosomes in preimplantation embryos. Differentiation 17(3):161–179 [DOI] [PubMed]
- Jahn L, Franke WW (1989) High frequency of cytokeratin-producing smooth muscle cells in human atherosclerotic plaques. Differentiation 40(1):55–62 [DOI] [PubMed]
- Jahn L, Kreuzer J, von Hodenberg E, Kübler W, Franke WW, Allenberg J, Izumo S (1993) Cytokeratins 8 and 18 in smooth muscle cells. Detection in human coronary artery, peripheral vascular, and vein graft disease and in transplantation-associated arteriosclerosis. Arterioscler Thromb 133(11):1631–1639 [DOI] [PubMed]
- Jaquemar D, Kupriyanov S, Wankell M, Avis J, Benirschke K, Baribault H, Oshima RG (2003) Keratin 8 protection of placental barrier function. J Cell Biol 161(4):749–756 [DOI] [PMC free article] [PubMed]
- Jih DM, Lyle S, Elenitsas R, Elder DE, Cotsarelis G (1999) Cytokeratin 15 expression in trichoepitheliomas and a subset of basal cell carcinomas suggests they originate from hair follicle stem cells. J Cutan Pathol 26(3):113–118 [DOI] [PubMed]
- Kanitakis J, Bourchany D, Faure M, Claudy A (1999) Expression of the hair stem cell-specific keratin 15 in pilar tumors of the skin. Eur J Dermatol 9(5):363–365 [PubMed]
- Kao WW, Liu CY, Converse RL, Shiraishi A, Kao CW, Ishizaki M, Doetschman T, Duffy J (1996) Keratin 12-deficient mice have fragile corneal epithelia. Invest Ophthalmol Vis Sci 37(13):2572–2584 [PubMed]
- Karsten U, Papsdorf G, Roloff G, Stolley P, Abel H, Walther I, Weiss H (1985) Monoclonal anti-cytokeratin antibody from a hybridoma clone generated by electrofusion. Eur J Cancer Clin Oncol 21(6):733–740 [DOI] [PubMed]
- Katsumata K, Sumi T, Mori Y, Hisada M, Tsuchida A, Aoki T (2006) Detection and evaluation of epithelial cells in the blood of colon cancer patients using RT-PCR. Int J Clin Oncol 11(5):385–389 [DOI] [PubMed]
- Kim S, Wong P, Coulombe PA (2006) A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature 441(7091):362–365 [DOI] [PubMed]
- Knapp AC, Franke WW (1989) Spontaneous losses of control of cytokeratin gene expression in transformed, non-epithelial human cells occurring at different levels of regulation. Cell 59(1):67–79 [DOI] [PubMed]
- Knapp AC, Bosch FX, Hergt M, Kuhn C, Winter-Simanowski S, Schmid E, Regauer S, Bartek J, Franke WW (1989) Cytokeratins and cytokeratin filaments in subpopulations of cultured human and rodent cells of nonepithelial origin: modes and patterns of formation. Differentiation 42(2):81–102 [DOI] [PubMed]
- Knösel T, Emde V, Schluns K, Schlag PM, Dietel M, Petersen I (2006) Cytokeratin profiles identify diagnostic signatures in colorectal cancer using multiplex analysis of tissue microarrays. Cell Oncol 28(4):167–175 [DOI] [PMC free article] [PubMed]
- Koch PJ, Roop DR (2004) The role of keratins in epidermal development and homeostasis–going beyond the obvious. J Invest Dermatol 123(5):x–xi [DOI] [PubMed]
- Koch M, Kienle P, Hinz U, Antolovic D, Schmidt J, Herfarth C, von Knebel Doeberitz M, Weitz J (2005) Detection of hematogenous tumor cell dissemination predicts tumor relapse in patients undergoing surgical resection of colorectal liver metastases. Ann Surg 241(2):199–205 [DOI] [PMC free article] [PubMed]
- Ku NO, Omary MB (2006) A disease- and phosphorylation-related nonmechanical function for keratin 8. J Cell Biol 174(1):115–125 [DOI] [PMC free article] [PubMed]
- Ku NO, Michie S, Resurreccion EZ, Broome RL, Omary MB (2002) Keratin binding to 14–3-3 proteins modulates keratin filaments and hepatocyte mitotic progression. Proc Natl Acad Sci USA 99(7):4373–4378 [DOI] [PMC free article] [PubMed]
- Ku NO, Darling JM, Krams SM, Esquivel CO, Keeffe EB, Sibley RK, Lee YM, Wright TL, Omary MB (2003a) Keratin 8 and 18 mutations are risk factors for developing liver disease of multiple etiologies. Proc Natl Acad Sci USA 100(10):6063–6068 [DOI] [PMC free article] [PubMed]
- Ku NO, Soetikno RM, Omary MB (2003b) Keratin mutation in transgenic mice predisposes to Fas but not TNF-induced apoptosis and massive liver injury. Hepatology 37(5):1006–1014 [DOI] [PubMed]
- Kuruc N, Franke WW (1988) Transient coexpression of desmin and cytokeratins 8 and 18 in developing myocardial cells of some vertebrate species. Differentiation 38(3):177–193 [DOI] [PubMed]
- Kuruc N, Leube RE, Moll I, Bader BL, Franke WW (1989) Synthesis of cytokeratin 13, a component characteristic of internal stratified epithelia, is not induced in human epidermal tumors. Differentiation 42(2):111–123 [DOI] [PubMed]
- Kurzen H, Esposito L, Langbein L, Hartschuh W (2001) Cytokeratins as markers of follicular differentiation: an immunohistochemical study of trichoblastoma and basal cell carcinoma. Am J Dermatopathol 23(6):501–509 [DOI] [PubMed]
- La Rosa S, Rigoli E, Uccella S, Novario R, Capella C (2007) Prognostic and biological significance of cytokeratin 19 in pancreatic endocrine tumours. Histopathology 50(5):597–606 [DOI] [PubMed]
- Lane EB, Alexander CM (1990) Use of keratin antibodies in tumor diagnosis. Semin Cancer Biol 1(3):165–179 [PubMed]
- Lane EB, McLean WH (2004) Keratins and skin disorders. J Pathol 204(4):355–366 [DOI] [PubMed]
- Langbein L, Schweizer J (2005) Keratins of the human hair follicle. Int Rev Cytol 243:1–78 [DOI] [PubMed]
- Langbein L, Kosmehl H, Kiss F, Katenkamp D, Neupert G (1989) Cytokeratin expression in experimental murine rhabdomyosarcomas. Intermediate filament pattern in original tumors, allotransplants, cell culture and re-established tumors from cell culture. Exp Pathol 36(1):23–36 [DOI] [PubMed]
- Langbein L, Heid HW, Moll I, Franke WW (1993) Molecular characterization of the body site-specific human epidermal cytokeratin 9: cDNA cloning, amino acid sequence, and tissue specificity of gene expression. Differentiation 55(1):57–71 [DOI] [PubMed]
- Langbein L, Rogers MA, Winter H, Praetzel S, Beckhaus U, Rackwitz HR, Schweizer J (1999) The catalog of human hair keratins: I. Expression of the nine type I members in the hair follicle. J Biol Chem 274:19874–19893 [DOI] [PubMed]
- Langbein L, Rogers MA, Winter H, Praetzel S, Schweizer J (2001) The catalog of human hair keratins: II. Expression of the six type II members in the hair follicle and the combined catalog of human type I and type II keratins. J Biol Chem 276:35123–35132 [DOI] [PubMed]
- Langbein L, Rogers MA, Praetzel S, Aoki N, Winter H, Schweizer J (2002) A novel epithelial keratin, K6irs1, is expressed differentially in all layers of the inner root sheath, including specialized huxley cells (Flügelzellen) of the human hair follicle. J Invest Dermatol 118(5):789–799 [DOI] [PubMed]
- Langbein L, Rogers MA, Praetzel S, Winter H, Schweizer J (2003) K6irs1, K6irs2, K6irs3, and K6irs4 represent the inner-root-sheath-specific type II epithelial keratins of the human hair follicle. J Invest Dermatol 120(4):512–522 [DOI] [PubMed]
- Langbein L, Spring H, Rogers MA, Praetzel S, Schweizer J (2004) Hair keratins and hair follicle-specific epithelial keratins. Methods Cell Biol 78:413–451 [PubMed]
- Langbein L, Rogers MA, Praetzel S, Cribier B, Peltre B, Gassler N, Schweizer J (2005) Characterization of a novel human type II epithelial keratin K1b, specifically expressed in eccrine sweat glands. J Invest Dermatol 125(3):428–444 [DOI] [PubMed]
- Langbein L, Rogers MA, Praetzel-Wunder S, Helmke B, Schirmacher P, Schweizer J (2006) K25 (K25irs1), K26 (K25irs2), K27 (K25irs3), and K28 (K25irs4) represent the type I inner root sheath keratins of the human hair follicle. J Invest Dermatol 126(11):2377–2386 [DOI] [PubMed]
- Langbein L, Rogers MA, Praetzel-Wunder S, Boeckler D, Schirmacher P, Schweizer J (2007) The novel keratins K39 and K40 are the latest expressed type II hair keratins and complete the human hair keratin family. J Invest Dermatol 127:1532–1535 [DOI] [PubMed]
- Langbein L, Cribier B, Schirmacher P, Praetzel-Wunder S, Peltre B, Schweizer J (2008) New concepts on the histogenesis of eccrine neoplasia from keratin expression in normal eccrine gland, syringoma and poroma. Br J Dermatol (in press) [DOI] [PubMed]
- Leers MP, Kölgen W, Björklund V, Bergman T, Tribbick G, Persson B, Björklund P, Ramaekers FC, Björklund B, Nap M, Jörnvall H, Schütte B (1999) Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol 187(5):567–572 [DOI] [PubMed]
- Leigh IM, Navsaria H, Purkis PE, McKay IA, Bowden PE, Riddle PN (1995) Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br J Dermatol 133(4):501–511 [DOI] [PubMed]
- Leube RE, Rustad TJ (1991) Squamous cell metaplasia in the human lung: molecular characteristics of epithelial stratification. Virchows Arch B Cell Pathol Incl Mol Pathol 61(4):227–253 [DOI] [PubMed]
- Leube RE, Bader BL, Bosch FX, Zimbelmann R, Achtstaetter T, Franke WW (1988) Molecular characterization and expression of the stratification-related cytokeratins 4 and 15. J Cell Biol 106(4):1249–1261 [DOI] [PMC free article] [PubMed]
- Lindberg K, Rheinwald JG (1989) Suprabasal 40 kd keratin (K19) expression as an immunohistologic marker of premalignancy in oral epithelium. Am J Pathol 134(1):89–98 [PMC free article] [PubMed]
- Linder S (2007) Cytokeratin markers come of age. Tumour Biol 28(4):189–95 [DOI] [PubMed]
- Linder S, Havelka AM, Ueno T, Shoshan MC (2004) Determining tumor apoptosis and necrosis in patient serum using cytokeratin 18 as a biomarker. Cancer Lett 214(1):1–9 [DOI] [PubMed]
- Liu L, Qian J, Singh H, Meiers I, Zhou X, Bostwick DG (2007) Immunohistochemical analysis of chromophobe renal cell carcinoma, renal oncocytoma, and clear cell carcinoma: an optimal and practical panel for differential diagnosis. Arch Pathol Lab Med 131(8):1290–1297 [DOI] [PubMed]
- Lloyd C, Yu QC, Cheng J, Turksen K, Degenstein L, Hutton E, Fuchs E (1995) The basal keratin network of stratified squamous epithelia: defining K15 function in the absence of K14. J Cell Biol 129(5):1329–1344 [DOI] [PMC free article] [PubMed]
- Lobeck H, Bartke I, Naujoks K, Müller D, Bornhöft G, Mischke D, Wild G (1989) Verteilungsmuster der Zytokeratinpolypeptide 4 und 5 im normalen und neoplastischen Epithel unter Verwendung neuer paraffingängiger monoklonaler Antikörper (eine immunhistochemische Untersuchung). Verh Dtsch Ges Path 73:645
- Lu X, Lane EB (1990) Retrovirus-mediated transgenic keratin expression in cultured fibroblasts: specific domain functions in keratin stabilization and filament formation. Cell 62(4):681–696 [DOI] [PubMed]
- Lyle S, Christofidou-Solomidou M, Liu Y, Elder DE, Albelda S, Cotsarelis G (1998) The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J Cell Sci 111(Pt 21):3179–3188 [DOI] [PubMed]
- Lyle S, Christofidou-Solomidou M, Liu Y, Elder DE, Albelda S, Cotsarelis G (1999) Human hair follicle bulge cells are biochemically distinct and possess an epithelial stem cell phenotype. J Investig Dermatol Symp Proc 4(3):296–301 [DOI] [PubMed]
- Magin TM, Vijayaraj P, Leube RE (2007) Structural and regulatory functions of keratins. Exp Cell Res 313(10):2021–2032 [DOI] [PubMed]
- Makin CA, Bobrow LG, Bodmer WF (1984) Monoclonal antibody to cytokeratin for use in routine histopathology. J Clin Pathol 37(9):975–983 [DOI] [PMC free article] [PubMed]
- Malzahn K, Mitze M, Thoenes M, Moll R (1998) Biological and prognostic significance of stratified epithelial cytokeratins in infiltrating ductal breast carcinomas. Virchows Arch 433(2):119–129 [DOI] [PubMed]
- Margolis SS, Perry JA, Forester CM, Nutt LK, Guo Y, Jardim MJ, Thomenius MJ, Freel CD, Darbandi R, Ahn JH, Arroyo JD, Wang XF, Shenolikar S, Nairn AC, Dunphy WG, Hahn WC, Virshup DM, Kornbluth S (2006) Role for the PP2A/B56delta phosphatase in regulating 14-3-3 release from Cdc25 to control mitosis. Cell 127(4):759–773 [DOI] [PMC free article] [PubMed]
- Martens J, Baars J, Smedts F, Holterheus M, Kok MJ, Vooijs P, Ramaekers F (1999) Can keratin 8 and 17 immunohistochemistry be of diagnostic value in cervical cytology? A feasibility study. Cancer 87(2):87–92 [DOI] [PubMed]
- Mazzalupo S, Wong P, Martin P, Coulombe PA (2003) Role for keratins 6 and 17 during wound closure in embryonic mouse skin. Dev Dyn 226(2):356–365 [DOI] [PubMed]
- McGowan KM, Coulombe PA (2000) Keratin 17 expression in the hard epithelial context of the hair and nail, and its relevance for the pachyonychia congenita phenotype. J Invest Dermatol 114(6):1101–1107 [DOI] [PubMed]
- McGowan KM, Tong X, Colucci-Guyon E, Langa F, Babinet C, Coulombe PA (2002) Keratin 17 null mice exhibit age- and strain-dependent alopecia. Genes Dev 16(11):1412–1422 [DOI] [PMC free article] [PubMed]
- McGregor DK, Wu TT, Rashid A, Luthra R, Hamilton SR (2004) Reduced expression of cytokeratin 20 in colorectal carcinomas with high levels of microsatellite instability. Am J Surg Pathol 28(6):712–718 [DOI] [PubMed]
- McLean WH, Irvine AD (2007) Disorders of keratinisation: from rare to common genetic diseases of skin and other epithelial tissues. Ulster Med J 76(2):72–82 [PMC free article] [PubMed]
- McLean WH, Rugg EL, Lunny DP, Morley SM, Lane EB, Swensson O, Dopping-Hepenstal PJ, Griffiths WA, Eady RA, Higgins C (1995) Keratin 16 and keratin 17 mutations cause pachyonychia congenita. Nat Genet 9(3):273–278 [DOI] [PubMed]
- Miettinen M (1995) Keratin 20: immunohistochemical marker for gastrointestinal, urothelial, and Merkel cell carcinomas. Mod Pathol 8(4):384–388 [PubMed]
- Moll R (1993) Cytokeratins as markers of differentiation: expression profiles in epithelia and epithelial tumors. Progress in pathology (series), vol 142. Gustav Fischer, Stuttgart, pp 1–197 [PubMed]
- Moll R (1998) Cytokeratins as markers of differentiation in the diagnosis of epithelial tumors. Subcell Biochem 31:205–262 [PubMed]
- Moll R, Moll I, Wiest W (1982a) Changes in the pattern of cytokeratin polypeptides in epidermis and hair follicles during skin development in human fetuses. Differentiation 23(2):170–178 [DOI] [PubMed]
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R (1982b) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31(1):11–24 [DOI] [PubMed]
- Moll R, Franke WW, Volc-Platzer B, Krepler R (1982c) Different keratin polypeptides in epidermis and other epithelia of human skin: a specific cytokeratin of molecular weight 46,000 in epithelia of the pilosebaceous tract and basal cell epitheliomas. J Cell Biol 95(1):285–295 [DOI] [PMC free article] [PubMed]
- Moll R, Krepler R, Franke WW (1983) Complex cytokeratin polypeptide patterns observed in certain human carcinomas. Differentiation 23(3):256–269 [DOI] [PubMed]
- Moll I, Heid H, Franke WW, Moll R (1987) Distribution of a special subset of keratinocytes characterized by the expression of cytokeratin 9 in adult and fetal human epidermis of various body sites. Differentiation 33(3):254–265 [DOI] [PubMed]
- Moll I, Heid H, Moll R (1988a) Cytokeratin analysis of pilomatrixoma: changes in cytokeratin-type expression during differentiation. J Invest Dermatol 91(3):251–257 [DOI] [PubMed]
- Moll R, Achtstätter T, Becht E, Balcarova-Ständer J, Ittensohn M, Franke WW (1988b) Cytokeratins in normal and malignant transitional epithelium. Maintenance of expression of urothelial differentiation features in transitional cell carcinomas and bladder carcinoma cell culture lines. Am J Pathol 132(1):123–144 [PMC free article] [PubMed]
- Moll R, Dhouailly D, Sun TT (1989) Expression of keratin 5 as a distinctive feature of epithelial and biphasic mesotheliomas. An immunohistochemical study using monoclonal antibody AE14. Virchows Arch B Cell Pathol Incl Mol Pathol 58(2):129–145 [DOI] [PubMed]
- Moll R, Schiller DL, Franke WW (1990) Identification of protein IT of the intestinal cytoskeleton as a novel type I cytokeratin with unusual properties and expression patterns. J Cell Biol 111(2):567–580 [DOI] [PMC free article] [PubMed]
- Moll R, Hage C, Thoenes W (1991) Expression of intermediate filament proteins in fetal and adult human kidney: modulations of intermediate filament patterns during development and in damaged tissue. Lab Invest 65(1):74–86 [PubMed]
- Moll R, Löwe A, Laufer J, Franke WW (1992) Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol 140(2):427–447 [PMC free article] [PubMed]
- Moll I, Troyanovsky SM, Moll R (1993a) Special program of differentiation expressed in keratinocytes of human haarscheiben: an analysis of individual cytokeratin polypeptides. J Invest Dermatol 100(1):69–76 [DOI] [PubMed]
- Moll R, Zimbelmann R, Goldschmidt MD, Keith M, Laufer J, Kasper M, Koch PJ, Franke WW (1993b) The human gene encoding cytokeratin 20 and its expression during fetal development and in gastrointestinal carcinomas. Differentiation 53(2):75–93 [DOI] [PubMed]
- Moll I, Kuhn C, Moll R (1995) Cytokeratin 20 is a general marker of cutaneous Merkel cells while certain neuronal proteins are absent. J Invest Dermatol 104(6):910–915 [DOI] [PubMed]
- Naeem M, Wajid M, Lee K, Leal SM, Ahmad W (2006) A mutation in the hair matrix and cuticle keratin KRTHB5 gene causes ectodermal dysplasia of hair and nail type. J Med Genet 43(3):274–279 [DOI] [PMC free article] [PubMed]
- Nagle RB (1994) A review of intermediate filament biology and their use in pathologic diagnosis. Mol Biol Rep 19(1):3–21 [DOI] [PubMed]
- Nagle RB, Böcker W, Davis JR, Heid HW, Kaufmann M, Lucas DO, Jarasch ED (1986) Characterization of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. J Histochem Cytochem 34(7):869–881 [DOI] [PubMed]
- Odland GF (1953) Some microscopic studies of the keratinization of human hair. J Invest Dermatol 21(5):305–12 [DOI] [PubMed]
- Oki-Idouchi CE, Lorenzo PS (2007) Transgenic overexpression of RasGRP1 in mouse epidermis results in spontaneous tumors of the skin. Cancer Res 67(1):276–80 [DOI] [PMC free article] [PubMed]
- Omary MB, Coulombe PA, McLean WH. (2004) Intermediate filament proteins and their associated diseases. N Engl J Med 351(20):2087–2100 [DOI] [PubMed]
- Ordonez NG (1998) Role of immunohistochemistry in distinguishing epithelial peritoneal mesotheliomas from peritoneal and ovarian serous carcinomas. Am J Surg Pathol 22(10):1203–1214 [DOI] [PubMed]
- Orfanos C, Ruska H (1968) Fine structure of human hair. II. Hair-cortex. Arch Klin Exp Dermatol 231(3):264–278 [PubMed]
- Oriolo AS, Wald FA, Ramsauer VP, Salas PJ (2007) Intermediate filaments: a role in epithelial polarity. Exp Cell Res 313(10):2255–2264 [DOI] [PMC free article] [PubMed]
- Oshima RG (2007) Intermediate filaments: a historical perspective. Exp Cell Res 313(10):1981–1994 [DOI] [PMC free article] [PubMed]
- Otterbach F, Bankfalvi A, Bergner S, Decker T, Krech R, Boecker W (2000) Cytokeratin 5/6 immunohistochemistry assists the differential diagnosis of atypical proliferations of the breast. Histopathology 37(3):232–240 [DOI] [PubMed]
- Owens DW, Lane EB (2003) The quest for the function of simple epithelial keratins. Bioessays 25(8):748–758 [DOI] [PubMed]
- Owens DW, Lane EB (2004) Keratin mutations and intestinal pathology. J Pathol 204(4):377–385 [DOI] [PubMed]
- Paladini RD, Takahashi K, Bravo NS, Coulombe PA (1996) Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16. J Cell Biol 132(3):381–397 [DOI] [PMC free article] [PubMed]
- Paramio JM, Casanova ML, Segrelles C, Mittnacht S, Lane EB, Jorcano JL (1999) Modulation of cell proliferation by cytokeratins K10 and K16. Mol Cell Biol 19(4):3086–3094 [DOI] [PMC free article] [PubMed]
- Parry DA, Strelkov SV, Burkhard P, Aebi U, Herrmann H (2007) Towards a molecular description of intermediate filament structure and assembly. Exp Cell Res 313(10):2204–2216 [DOI] [PubMed]
- Perrin C (2007) Expression of follicular sheath keratins in the normal nail with special reference to the morphological analysis of the distal nail unit. Am J Dermatopathol 29(6):543–550 [DOI] [PubMed]
- Perrin C, Langbein L, Schweizer J (2004) Expression of hair keratins in the adult nail unit: An immunohistochemical analysis of the onychogenesis in the proximal nail fold, matrix and nail bed. Br J Dermatol 151:362–371 [DOI] [PubMed]
- Pitz S, Moll R (2002) Intermediate-filament expression in ocular tissue. Prog Retin Eye Res 21(2):241–262 [DOI] [PubMed]
- Porter RM, Leitgeb S, Melton DW, Swensson O, Eady RA, Magin TM (1996) Gene targeting at the mouse cytokeratin 10 locus: severe skin fragility and changes of cytokeratin expression in the epidermis. J Cell Biol 132(5):925–936 [DOI] [PMC free article] [PubMed]
- Porter RM, Lunny DP, Ogden PH, Morley SM, McLean WH, Evans A, Harrison DL, Rugg EL, Lane EB (2000) K15 expression implies lateral differentiation within stratified epithelial basal cells. Lab Invest 80(11):1701–1710 [DOI] [PubMed]
- Powell BC, Rogers GE (1986) Hair keratin: composition, structure and biogenesis. In: Bereiter-Hahn J, Matoltsy AG, Richards KS (eds) Biology of the integument, vol 2. Springer, Berlin, pp 695–721
- Purkis PE, Steel JB, Mackenzie IC, Nathrath WB, Leigh IM, Lane EB (1990) Antibody markers of basal cells in complex epithelia. J Cell Sci 97(Pt 1):39–50 [DOI] [PubMed]
- Ramaekers F, van NC, Poels L, Schaafsma E, Huijsmans A, Robben H, Schaart G, Vooijs P (1990) Use of monoclonal antibodies to keratin 7 in the differential diagnosis of adenocarcinomas. Am J Pathol 136(3):641–655 [PMC free article] [PubMed]
- Real FX, Vila MR, Skoudy A, Ramaekers FC, Corominas JM (1993) Intermediate filaments as differentiation markers of exocrine pancreas. II. Expression of cytokeratins of complex and stratified epithelia in normal pancreas and in pancreas cancer. Int J Cancer 54(5):720–727 [DOI] [PubMed]
- Régnier CH, Asch PH, Grosshans E, Rio MC (1997) Expression pattern of human hair keratin basic 1 (hHb1) in hair follicle and pilomatricoma. Exp Dermatol 6(2):87–90 [DOI] [PubMed]
- Reichelt J, Fürstenberger G, Magin TM (2004) Loss of keratin 10 leads to mitogen-activated protein kinase (MAPK) activation, increased keratinocyte turnover, and decreased tumor formation in mice. J Invest Dermatol 123(5):973–981 [DOI] [PubMed]
- Reis A, Hennies HC, Langbein L, Digweed M, Mischke D, Drechsler M, Schröck E, Royer-Pokora B, Franke WW, Sperling K, Küster W (1994) Keratin 9 gene mutations in Epidermolytic Palmoplantar Keratoderma (EPPK). Nat Genet 6:174–179 [DOI] [PubMed]
- Rogers GE, Clarke RM (1965) Keratin protofilaments and ribosomes from hair follicles. Nature 205:77–78 [DOI] [PubMed]
- Rogers MA, Winter H, Langbein L, Bleiler R, Schweizer J (2004) The human type I keratin gene family: Characterization of new hair follicle specific members and evaluation of the chromosome 17q21.2 gene domain. Differentiation 72:527–540 [DOI] [PubMed]
- Rogers MA, Edler L, Winter H, Langbein L, Beckmann I, Schweizer J (2005) Characterization of new members of the human type II keratin gene family and a general evaluation of the keratin gene domain on chromosome 12q13.13. J Invest Dermatol 124(3):536–544 [DOI] [PubMed]
- Rogers MA, Langbein L, Praetzel-Wunder S, Winter H, Schweizer J (2006) Human hair keratin associated proteins (KAPs). Int Rev Cytol 251:209–263 [DOI] [PubMed]
- Roop DR (1987) Regulation of keratin gene expression during differentiation of epidermal and vaginal epithelial cells. Curr Top Dev Biol 22:195–207 [DOI] [PubMed]
- Rydlander L, Ziegler E, Bergman T, Schoberl E, Steiner G, Bergman AC, Zetterberg A, Marberger M, Bjorklund P, Skern T, Einarsson R, Jornvall H (1996) Molecular characterization of a tissue-polypeptide-specific-antigen epitope and its relationship to human cytokeratin 18. Eur J Biochem 241(2):309–314 [DOI] [PubMed]
- Schaafsma HE, Ramaekers FC (1994) Cytokeratin subtyping in normal and neoplastic epithelium: basic principles and diagnostic applications. Pathol Annu 29(Pt 1):21–62 [PubMed]
- Schaller G, Fuchs I, Pritze W, Ebert A, Herbst H, Pantel K, Weitzel H, Lengyel E (1996) Elevated keratin 18 protein expression indicates a favorable prognosis in patients with breast cancer. Clin Cancer Res 2(11):1879–1885 [PubMed]
- Schermer A, Galvin S, Sun TT (1986) Differentiation-related expression of a major 64 K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol 103(1):49–62 [DOI] [PMC free article] [PubMed]
- Schmelz M, Moll R, Hesse U, Prasad AR, Gandolfi JA, Hasan SR, Bartholdi M, Cress AE (2005) Identification of a stem cell candidate in the normal human prostate gland. Eur J Cell Biol 84(2–3):341–354 [DOI] [PMC free article] [PubMed]
- Schmitt AM, Anlauf M, Rousson V, Schmid S, Kofler A, Riniker F, Bauersfeld J, Barghorn A, Probst-Hensch NM, Moch H, Heitz PU, Kloeppel G, Komminoth P, Perren A (2007) WHO 2004 criteria and CK19 are reliable prognostic markers in pancreatic endocrine tumors. Am J Surg Pathol 31(11):1677–1682 [DOI] [PubMed]
- Schüssler MH, Skoudy A, Ramaekers F, Real FX (1992) Intermediate filaments as differentiation markers of normal pancreas and pancreas cancer. Am J Pathol 140(3):559–568 [PMC free article] [PubMed]
- Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DA, Rogers MA, Wright MW (2006) New consensus nomenclature for mammalian keratins. J Cell Biol 174(2):169–174 [DOI] [PMC free article] [PubMed]
- Schweizer J, Langbein L, Rogers MA, Winter H (2007) Hair follicle-specific keratins and their diseases. Exp Cell Res 313(10):2010–2020 [DOI] [PubMed]
- Skinnider BF, Folpe AL, Hennigar RA, Lim SD, Cohen C, Tamboli P, Young A, Peralta-Venturina M, Amin MB (2005) Distribution of cytokeratins and vimentin in adult renal neoplasms and normal renal tissue: potential utility of a cytokeratin antibody panel in the differential diagnosis of renal tumors. Am J Surg Pathol 29(6):747–754 [DOI] [PubMed]
- Smedts F, Ramaekers F, Leube RE, Keijser K, Link M, Vooijs P (1993) Expression of keratins 1, 6, 15, 16, and 20 in normal cervical epithelium, squamous metaplasia, cervical intraepithelial neoplasia, and cervical carcinoma. Am J Pathol 142(2):403–412 [PMC free article] [PubMed]
- Smith FJ, Porter RM, Corden LD, Lunny DP, Lane EB, McLean WH (2002) Cloning of human, murine, and marsupial keratin 7 and a survey of K7 expression in the mouse. Biochem Biophys Res Commun 297(4):818–827 [DOI] [PubMed]
- Soeth E, Roder C, Juhl H, Kruger U, Kremer B, Kalthoff H (1996) The detection of disseminated tumor cells in bone marrow from colorectal-cancer patients by a cytokeratin–20-specific nested reverse-transcriptase-polymerase-chain reaction is related to the stage of disease. Int J Cancer 69(4):278–282 [DOI] [PubMed]
- Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de RM, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein LP, Borresen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98(19):10869–10874 [DOI] [PMC free article] [PubMed]
- Southgate J, Harnden P, Trejdosiewicz LK (1999) Cytokeratin expression patterns in normal and malignant urothelium: a review of the biological and diagnostic implications. Histol Histopathol 14(2):657–664 [DOI] [PubMed]
- Stasiak PC, Purkis PE, Leigh IM, Lane EB (1989) Keratin 19: predicted amino acid sequence and broad tissue distribution suggest it evolved from keratinocyte keratins. J Invest Dermatol 92(5):707–716 [DOI] [PubMed]
- Steinert PM, Idler WW, Zimmerman SB (1976) Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol 108(3):547–567 [DOI] [PubMed]
- Stoler A, Kopan R, Duvic M, Fuchs E (1988) Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J Cell Biol 107(2):427–446 [DOI] [PMC free article] [PubMed]
- Stoner ML, Wood FM (1999) Cultured epithelial autograft “take” confirmed by the presence of cytokeratin 9. J Invest Dermatol 112(3):391–392 [DOI] [PubMed]
- Stosiek P, Kasper M, Moll R (1992) Changes in cytokeratin expression accompany squamous metaplasia of the human respiratory epithelium. Virchows Arch A Pathol Anat Histopathol 421(2):133–141 [DOI] [PubMed]
- Swensson O, Langbein L, McMillan JR, Stevens HP, Leigh IM, McLean WH, Lane EB, Eady RA (1998) Specialized keratin expression pattern in human ridged skin as an adaptation to high physical stress. Br J Dermatol 139(5):767–775 [DOI] [PubMed]
- Takahashi K, Paladini RD, Coulombe PA (1995) Cloning and characterization of multiple human genes and cDNAs encoding highly related type II keratin 6 isoforms. J Biol Chem 270(31):18581–18592 [DOI] [PubMed]
- Takei H, Iino Y, Horiguchi J, Kanoh T, Takao Y, Oyama T, Morishita Y (1995) Immunohistochemical analysis of cytokeratin #8 as a prognostic factor in invasive breast carcinoma. Anticancer Res 15(3):1101–1105 [PubMed]
- Thoenes W, Störkel S, Rumpelt HJ, Moll R, Baum HP, Werner S (1988) Chromophobe cell renal carcinoma and its variants—a report on 32 cases. J Pathol 155(4):277–287 [DOI] [PubMed]
- Toivola DM, Nieminen MI, Hesse M, He T, Baribault H, Magin TM, Omary MB, Eriksson JE (2001) Disturbances in hepatic cell-cycle regulation in mice with assembly-deficient keratins 8/18. Hepatology 34(6):1174–1183 [DOI] [PubMed]
- Tong X, Coulombe PA (2006) Keratin 17 modulates hair follicle cycling in a TNFalpha-dependent fashion. Genes Dev 20(10):1353–1364 [DOI] [PMC free article] [PubMed]
- Torchard D, Blanchet-Bardon C, Serova O, Langbein L, Narod S, Janin N, Goguel AF, Bernheim A, Franke WW, Lenoir GM (1994) Epidermolytic palmoplantar keratoderma cosegrates with a keratin 9 mutation in a pedigree with breast and ovarian cancer. Nat Genet 6(1):106–110 [DOI] [PubMed]
- Tot T (2002) Cytokeratins 20 and 7 as biomarkers: usefulness in discriminating primary from metastatic adenocarcinoma. Eur J Cancer 38(6):758–763 [DOI] [PubMed]
- Troyanovsky SM, Guelstein VI, Tchipysheva TA, Krutovskikh VA, Bannikov GA (1989) Patterns of expression of keratin 17 in human epithelia: dependency on cell position. J Cell Sci 93(Pt 3):419–426 [DOI] [PubMed]
- Troyanovsky SM, Leube RE, Franke WW (1992) Characterization of the human gene encoding cytokeratin 17 and its expression pattern. Eur J Cell Biol 59(1):127–137 [PubMed]
- Tseng SC, Jarvinen MJ, Nelson WG, Huang JW, Woodcock-Mitchell J, Sun TT (1982) Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell 30(2):361–372 [DOI] [PubMed]
- Uitto J, Richard G, McGrath JA (2007) Diseases of epidermal keratins and their linker proteins. Exp Cell Res 313(10):1995–2009 [DOI] [PMC free article] [PubMed]
- van de Rijn RM, Perou CM, Tibshirani R, Haas P, Kallioniemi O, Kononen J, Torhorst J, Sauter G, Zuber M, Kochli OR, Mross F, Dieterich H, Seitz R, Ross D, Botstein D, Brown P (2002) Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol 161(6):1991–1996 [DOI] [PMC free article] [PubMed]
- van Muijen GN, Ruiter DJ, Franke WW, Achtstätter T, Haasnoot WH, Ponec M, Warnaar SO (1986) Cell type heterogeneity of cytokeratin expression in complex epithelia and carcinomas as demonstrated by monoclonal antibodies specific for cytokeratins nos. 4 and 13. Exp Cell Res 162(1):97–113 [DOI] [PubMed]
- van Muijen GN, Ruiter DJ, Warnaar SO (1987) Coexpression of intermediate filament polypeptides in human fetal and adult tissues. Lab Invest 57(4):359–369 [PubMed]
- van Niekerk CC, Jap PH, Ramaekers FC, van de Molengraft F, Poels LG (1991) Immunohistochemical demonstration of keratin 7 in routinely fixed paraffin-embedded human tissues. J Pathol 165(2):145–152 [DOI] [PubMed]
- Vassar R, Coulombe PA, Degenstein L, Albers K, Fuchs E (1991) Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell 64(2):365–380 [DOI] [PubMed]
- Wang Z, Wong P, Langbein L, Schweizer J, Coulombe PA (2003) The type II epithelial keratin 6hf (K6hf) is expressed in the companion layer, matrix and medulla in anagen-stage hair follicles. J Invest Dermatol 121:1276–1282 [DOI] [PubMed]
- Waschke J (2008) The desmosome and pemphigus. Histochem Cell Biol 129. doi:10.1007/s00418-008-0420-0 [DOI] [PMC free article] [PubMed]
- Waseem A, Dogan B, Tidman N, Alam Y, Purkis P, Jackson S, Lalli A, Machesney M, Leigh IM (1999) Keratin 15 expression in stratified epithelia: downregulation in activated keratinocytes. J Invest Dermatol 112(3):362–369 [DOI] [PubMed]
- Wawersik MJ, Mazzalupo S, Nguyen D, Coulombe PA (2001) Increased levels of keratin 16 alter epithelialization potential of mouse skin keratinocytes in vivo and ex vivo. Mol Biol Cell 12(11):3439–3450 [DOI] [PMC free article] [PubMed]
- Weber K, Osborn M, Moll R, Wiklund B, Luning B (1984) Tissue polypeptide antigen (TPA) is related to the non-epidermal keratins 8, 18 and 19 typical of simple and non-squamous epithelia: re-evaluation of a human tumor marker. EMBO J 3(11):2707–2714 [DOI] [PMC free article] [PubMed]
- Weikel W, Wagner R, Moll R (1987) Characterization of subcolumnar reserve cells and other epithelia of human uterine cervix. Demonstration of diverse cytokeratin polypeptides in reserve cells. Virchows Arch B Cell Pathol Incl Mol Pathol 54(2):98–110 [DOI] [PubMed]
- Weiss RA, Eichner R, Sun TT (1984) Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol 98(4):1397–1406 [DOI] [PMC free article] [PubMed]
- Wetzels RH, Kuijpers HJ, Lane EB, Leigh IM, Troyanovsky SM, Holland R, van Haelst UJ, Ramaekers FC (1991) Basal cell-specific and hyperproliferation-related keratins in human breast cancer. Am J Pathol 138(3):751–763 [PMC free article] [PubMed]
- Winter H, Rogers MA, Langbein L, Stevens HP, Leigh IM, Labreze L, Roul S, Taieb A, Krieg T, Schweizer J (1997) Mutations in the hair cortex keratin hHb6 cause the inherited hair disease Monilethrix. Nat Genet 16:372–374 [DOI] [PubMed]
- Winter H, Langbein L, Praetzel S, Jacobs M, Rogers MA, Leigh IM, Tidman N, Schweizer J (1998) A novel human type II cytokeratin, K6hf, specifically expressed in the companion layer of the hair follicle. J Invest Dermatol 111(6):955–962 [DOI] [PubMed]
- Winter H, Schissel D, Parry DA, Smith TA, Liovic M, Birgitte LE, Edler L, Langbein L, Jave-Suarez LF, Rogers MA, Wilde J, Peters G, Schweizer J (2004) An unusual Ala12Thr polymorphism in the 1A alpha-helical segment of the companion layer-specific keratin K6hf: evidence for a risk factor in the etiology of the common hair disorder pseudofolliculitis barbae. J Invest Dermatol 122(3):652–657 [DOI] [PubMed]
- Woelfle U, Sauter G, Santjer S, Brakenhoff R, Pantel K (2004) Down-regulated expression of cytokeratin 18 promotes progression of human breast cancer. Clin Cancer Res 10(8):2670–2674 [DOI] [PubMed]
- Wojcik SM, Bundman DS, Roop DR (2000) Delayed wound healing in keratin 6a knockout mice. Mol Cell Biol 20(14):5248–5255 [DOI] [PMC free article] [PubMed]
- Wojcik SM, Longley MA, Roop DR (2001) Discovery of a novel murine keratin 6 (K6) isoform explains the absence of hair and nail defects in mice deficient for K6a and K6b. J Cell Biol 154(3):619–630 [DOI] [PMC free article] [PubMed]
- Wong P, Colucci-Guyon E, Takahashi K, Gu C, Babinet C, Coulombe PA (2000) Introducing a null mutation in the mouse K6alpha and K6beta genes reveals their essential structural role in the oral mucosa. J Cell Biol 150(4):921–928 [DOI] [PMC free article] [PubMed]
- Wu YJ, Parker LM, Binder NE, Beckett MA, Sinard JH, Griffiths CT, Rheinwald JG (1982) The mesothelial keratins: a new family of cytoskeletal proteins identified in cultured mesothelial cells and nonkeratinizing epithelia. Cell 31(3 Pt 2):693–703 [DOI] [PubMed]
- Wyld DK, Selby P, Perren TJ, Jonas SK, len-Mersh TG, Wheeldon J, Burchill SA (1998) Detection of colorectal cancer cells in peripheral blood by reverse-transcriptase polymerase chain reaction for cytokeratin 20. Int J Cancer 79(3):288–293 [DOI] [PubMed]
- Yaziji H, Battifora H, Barry TS, Hwang HC, Bacchi CE, McIntosh MW, Kussick SJ, Gown AM (2006) Evaluation of 12 antibodies for distinguishing epithelioid mesothelioma from adenocarcinoma: identification of a three-antibody immunohistochemical panel with maximal sensitivity and specificity. Mod Pathol 19(4):514–523 [DOI] [PubMed]
- Yuspa SH, Kilkenny A, Cheng C, Roop D, Hennings H, Kruszewski F, Lee E, Strickland J, Greenhalgh DA (1991) Alterations in epidermal biochemistry as a consequence of stage-specific genetic changes in skin carcinogenesis. Environ Health Perspect 93:3–10 [DOI] [PMC free article] [PubMed]
- Zatloukal K, Stumptner C, Lehner M, Denk H, Baribault H, Eshkind LG, Franke WW (2000) Cytokeratin 8 protects from hepatotoxicity, and its ratio to cytokeratin 18 determines the ability of hepatocytes to form Mallory bodies. Am J Pathol 156(4):1263–1274 [DOI] [PMC free article] [PubMed]
- Zatloukal K, Stumptner C, Fuchsbichler A, Fickert P, Lackner C, Trauner M, Denk H (2004) The keratin cytoskeleton in liver diseases. J Pathol 204(4):367–376 [DOI] [PubMed]
- Zatloukal K, French SW, Stumptner C, Strnad P, Harada M, Toivola DM, Cadrin M, Omary MB (2007) From Mallory to Mallory-Denk bodies: what, how and why? Exp Cell Res 313(10):2033–2049 [DOI] [PubMed]
- Zhang JS, Wang L, Huang H, Nelson M, Smith DI (2001) Keratin 23 (K23), a novel acidic keratin, is highly induced by histone deacetylase inhibitors during differentiation of pancreatic cancer cells. Genes Chromosomes Cancer 30(2):123–135 [PubMed]
- Zhou Q, Toivola DM, Feng N, Greenberg HB, Franke WW, Omary MB (2003) Keratin 20 helps maintain intermediate filament organization in intestinal epithelia. Mol Biol Cell 14(7):2959–2971 [DOI] [PMC free article] [PubMed]
- Zhou Q, Cadrin M, Herrmann H, Chen CH, Chalkley RJ, Burlingame AL, Omary MB (2006) Keratin 20 serine 13 phosphorylation is a stress and intestinal goblet cell marker. J Biol Chem 281(24):16453–16461 [DOI] [PubMed]