Abstract
Taste bud cells are epithelial cells with neuronal properties. Voltage-dependent ion channels have been physiologically described in these cells. Here, we report the molecular identification and functional characterization of a voltage-gated chloride channel (ClC-4) and its novel splice variant (ClC-4A) from taste bud cells. ClC-4A skipped an exon near its 5′-end, incurring the loss of 60 amino acids at the N terminus. In situ hybridization and immunohistochemistry localized these two channels’ transcripts and proteins to a subset of taste bud cells. Electrophysiological recordings of the heterologously expressed channels in Xenopus oocytes showed that ClC-4 and ClC-4A have opposite sensitivity to pH and unique ion selectivity. The chloride channel blockers niflumic acid and 5-nitro-2-(3-phenylpropylamino)benzoic acid had a slight or no inhibitory effect on the conductance of ClC-4, but both blockers inhibited ClC-4A, suggesting that ClC-4A is a candidate channel for an acid-induced 5-nitro-2-(3-phenylpropylamino)benzoic acid-sensitive current. Furthermore, these two channels may play a role in bitter-, sweet-, and umami-mediated taste transmission by regulating transmitter uptake into synaptic vesicles.
Most mammals, including humans, are believed to possess at least five taste qualities: sour, salty, bitter, sweet, and umami (the latter being the taste for monosodium glutamate and certain 5′-ribonucleotides in humans while being a taste for a more diverse group of l-amino acids in several other species). Interactions between sapid molecules in foodstuffs and specific receptor cells in taste buds of the oral cavity initiate taste sensation. Bitter, sweet, and umami compounds stimulate G protein-coupled seven-transmembrane receptors (GPCRs),2 whereas ionic stimuli such as protons in acids and Na+ in salts either permeate or activate ion channels on the surface of taste receptor cells (1, 2).
Two families of taste GPCRs, T1Rs and T2Rs (3-16), and truncated versions of metabotropic glutamate receptor-4 (17) and -1, along with some of their downstream components, including G protein subunits α-gustducin, Gβ1, Gβ3, and Gγ13 (18-23); phospholipase Cβ2 (24, 25); inositol 1,4,5-triphosphate receptor-3 (IP3R3) (26); and the transient receptor ion channel TRPM5 (27-31), have been identified. Activation of these taste GPCRs presumably leads to changes in intracellular calcium activity and influx of ions through TRPM5. Opening of the TRPM5 channel is critical to the transmission of gustatory signals to the peripheral nerves. However, an increase in [Ca2+]i alone is not sufficient to trigger the full taste response because TRPM5-null animals in which phospholipase Cβ2 and IP3R3 are intact have markedly reduced behavioral and integrated nerve responses to sweet, bitter, and umami stimuli. TRPM5-mediated ion influx is very likely a major contributor to taste receptor cell membrane depolarization; however, the underlying mechanisms remain to be uncovered.
Sour and salty tastes apparently use several ion channel types to initiate their transduction, including amiloride-sensitive and -insensitive epithelial sodium channels (32), acid-sensing ion channels (33-35), hyperpolarization- and cyclic nucleotide-gated channels (36), and proton-gated potassium channels (37). In addition, acid-induced chloride currents (38) and intracellular acidification (39, 40) have been recorded for taste bud cells. Like taste GPCR-mediated transduction, stimulation of channel receptors by ionic taste stimuli also triggers changes in membrane potential. It remains to be understood how receptor potentials encode and transmit the identity of gustatory stimuli to afferent neural fibers.
Taste bud cells are epithelial cells with neuronal properties. A curious and not well understood property of these cells is their electrical excitability (41, 42). The passing of current or chemical stimulation by appropriate taste substances evokes action potentials in these cells, but not in surrounding non-gustatory lingual epithelial cells. Electrophysiological studies have characterized various voltage-gated currents, including tetrodotoxin-sensitive Na+ currents, tetraethylammonium-sensitive transient and sustained K+ currents, inward rectifier K+ currents, outward rectifier Cl− currents, and low/high voltage-activated Ca2+ currents (43-48). The distribution of these currents across cells within a taste bud is heterogeneous. However, little is known about these channels’ molecular identities and their expression and interaction with other taste signaling molecules. Two types of action potentials have been recorded: 1) fast action potentials with shorter duration and larger inward and outward currents and 2) slow action potentials with longer duration and smaller inward and outward currents. It is unclear how these two types of action potentials may contribute to gustatory signal transmission and peripheral coding. Recently, analyses of the action potential activity of a population of taste buds in response to taste stimuli using an artificial neural network suggested that patterns of action potentials may be the source of taste quality coding (49).
In an attempt to identify signaling molecules in taste bud cells and to gain insight into taste transduction and peripheral coding in the taste end organ, we have employed a single cell strategy and bioinformatics tools to isolate cell type-specific genes that are involved in defining particular physiological functions of each taste bud cell type. This approach has resulted in the identification of several key taste signaling molecules, including the taste receptor T1R3; G protein subunits Gβ1, Gβ3, and Gγ13; and the ion channel TRPM5 (7, 20, 27). In this study, we describe a voltage-gated, pH-sensitive chloride channel (ClC-4) and its novel splice variant (ClC-4A); their coexpression with other taste GPCR-mediated signal transduction components; and their unique pharmacological properties, pH sensitivities, and ion selectivity.
Although the specific functions and activities of taste cell-associated chloride channels are still not well understood at this point, chloride currents have been previously recorded from taste bud cells. Some of these currents are modulated by adrenergic agonists, activated by hypo-osmotic stimuli, or inhibited by the chloride channel blocker 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) (38, 48, 50). During mouse postnatal development, Cl− currents appear in a subset of excitable taste bud cells only after postnatal day 8, which coincides with the maturation of taste bud cells (51). Molecular identification of these chloride channels is important for eventual determination of their contributions to action potential waveforms, firing properties, and ultimately to the strength of transduction. The data from existing literature combined with the characterization studies of taste ClC-4 and ClC-4A presented here suggest that these two channels may play a role in the taste stimulus-induced depolarization of membrane potential and signal transmission.
MATERIALS AND METHODS
Isolation of ClC-4 and Splice Variant ClC-4A cDNAs from Mouse Taste Bud Cells
Isolation of ClC-4 and ClC-4A cDNAs from taste bud cells was accomplished in two steps. 1) A 3′-end cDNA fragment was isolated from a single taste cell cDNA library by differential screening with cDNAs from gustatory and non-gustatory lingual epithelia. Construction and differential screening of single taste cell cDNA libraries were described previously (20, 27). Briefly, taste papillae were separated from the rest of a mouse tongue and enzymatically dissociated into individual cells. Taste bud cells were identified by their characteristic bipolar shape and transferred individually into Eppendorf tubes. First-strand cDNAs were synthesized from single cells with oligo(dT) primers, tailed with dATP and terminal transferase, and amplified by PCR. The PCR products were ligated into the λZapII vector to construct single taste cell cDNA libraries. Individual phage plaques were picked, and their insert cDNAs were amplified by PCR with vector-specific primers. The amplified products were size-fractionated by electrophoresis, transferred onto a nylon membrane, and screened with 32P-labeled cDNAs prepared from non-gustatory lingual epithelium devoid of taste buds. Insert cDNAs that were not hybridized with the non-gustatory probe were presumed to be expressed selectively in taste cells, and their sequences were analyzed and searched against genome and expressed sequence tag data bases. One of the clones matched the 3′-end of human ClC-4. 2) Isolation of full-length cDNAs was carried out with mouse taste tissue cDNA. PCR primers were designed to encompass the entire mouse ClC-4 coding region (sense, 5′-AGGAGGATGATCTAGGACGCTGTC-3′; and antisense, 5′-TCTCAAAATAATGCCCATCTTATTGCT-3′). Two fragments were obtained and subcloned into the pCR-BluntII-TOPO vector. DNA sequence analysis and sequence alignment showed that the long fragment was the same as mouse ClC-4 (GenBank™ accession number XM_193014), whereas the short fragment was a splice variant of ClC-4, which we designated ClC-4A (GenBank™ accession number DQ186662). To determine whether these two isoforms are expressed in all three types of gustatory lingual papillae, a new set of PCR primers (sense, 5′-AGGAGGATGATCTAGGACGCTGTC-3′; and antisense, 5′-CATGCACCAGTGGGCCCTCTTTG-3′) was designed and synthesized to encompass the differentially spliced region, and PCRs were performed with first-strand cDNAs from circumvallate, foliate, and fungiform papillae and non-gustatory lingual epithelium.
In Situ Hybridization
Digoxigenin-labeled RNA probes (ClC-4, 2.2 kb) were used for in situ hybridization on fresh frozen sections (14 μm) as described (52). An alkaline phosphatase-conjugated anti-digoxigenin antibody in the presence of nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate was used for detection.
Immunohistochemistry
Polyclonal antiserum against a keyhole limpet hemocyanin-conjugated 13-amino acid peptide near the C termini of mouse, rat, and human ClC-4 and ClC-4A was raised in rabbits (Alpha Diagnostic International, Inc.). Frozen sections (10 μm) of murine lingual tissue (previously fixed in 4% paraformaldehyde and cryoprotected in 20% sucrose) were blocked in 3% bovine serum albumin, 0.3% Triton X-100, 2% goat serum, and 0.1% sodium azide in phosphate-buffered saline for 1 h at room temperature and then incubated overnight at 4 °C with the polyclonal antiserum (1:1000 dilution). The secondary antibody used was Cy3-conjugated goat anti-rabbit Ig (Jackson ImmunoResearch Laboratories, Inc.).
To determine the type of cells expressing ClC-4/ClC-4A, double immunostaining was carried out on taste sections with the rabbit polyclonal antibody against ClC-4/ClC-4A and the mouse monoclonal antibodies against IP3R3 (1:50 dilution; BD Biosciences) and SNAP-25 (1:1000 dilution; Sternberger Monoclonals Inc., Lutherville, MD). The secondary antibodies used were fluorescein isothiocyanate-conjugated anti-mouse and Cy3-conjugated anti-rabbit antibodies. Fluorescent signals were viewed under a Leica confocal microscope.
Heterologous Expression in Xenopus Oocytes and Electrophysiological Recordings
Murine ClC-4 and ClC-4A cDNAs and human ClC-4 (GenBank ™ accession number AB019432) were subcloned into the pCR-BluntII-TOPO expression vector. Capped sense cRNA was synthesized from the linearized expression constructs by T7 RNA polymerase using the mMessage mMachine in vitro transcription kit and tailed with a poly(A) tailing kit (Ambion Inc.). The synthesized cRNA products were phenol-extracted, ethyl alcohol-precipitated, and then dissolved in nuclease-free water at ~0.5 ng/nl for injection. Dumont stage V or VI oocytes were obtained from adult female laboratory-bred Xenopus laevis, and their follicles were removed by collagenase digestion. Oocytes were injected with 50 nl of 0.5 ng/nl cRNA and maintained at 18 °C for 4–6 days in modified Barth’s solution supplemented with 5 mm sodium pyruvate (53). Membrane currents were recorded using the two-electrode voltage-clamp technique in ND96 solution (96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, and 5 mm NaHEPES (pH 7.4)). To determine anionic selectivity, 80 mm Cl− was substituted with equivalent amounts of Br−, I−, or . In the study on the effect of pH on channel conductance, 5 mm HEPES (for pH 7.4) was replaced with 5 mm MES (for pH <7.0). For pharmacological analyses, inhibitors of niflumic acid (0.3 mm) or NPPB (at 0.1 mm) were dissolved in ND96 solution.
Recording and current-passing micropipettes with tip resistances <5 megaohms when filled with 3 m KCl were pulled from glass capillaries (A-M Systems, Inc., Carlsborg, WA) using a horizontal puller (Sutter P-80/PC). Currents were recorded with a GeneClamp 500 amplifier, digitized with a Digidata 1200 A/DD/A system, and stored on a computer running pCLAMP 6 software (all from Axon Instruments, Foster City, CA). Currents were low pass-filtered at 2 kHz and are shown without subtraction of leakage currents.
RESULTS
Isolation of ClC-4 cDNAs
To identify genes that are selectively expressed in taste bud cells, we isolated individual taste bud cells and constructed single cell cDNA libraries with the λZapII vector (20, 27). Individual phage plaques were picked, and their insert cDNAs were amplified by PCR with vector-specific primers. The amplified products were size-fractionated by electrophoresis and transferred onto a nylon membrane. Southern hybridization of the nylon membranes showed that many inserts from the single cell cDNA libraries could hybridize with the radiolabeled cDNAs prepared from non-gustatory lingual epithelium devoid of taste buds. The few that were not hybridized were considered taste bud-specific (Fig. 1); insert DNAs of these clones were sequenced and bioinformatically analyzed. Among ~200 taste bud-specific clones analyzed, one clone (GA5508) with a 906-bp insert cDNA was 83% identical to the 3′-end sequence of the human voltage-gated, pH-sensitive chloride channel ClC-4 cDNA (GenBank™ accession number NM_001830.2).
FIGURE 1. Hybridization of PCR-amplified insert DNAs from a single taste bud cell cDNA library with 32P-labeled non-gustatory epithelial cDNAs.
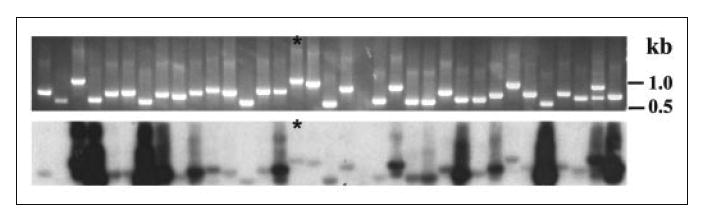
Clones that were not hybridized to the non-gustatory probes were isolated and sequenced. Upper panel, DNA-agarose gel; lower panel, x-ray film of the Southern hybridization. Clone GA5508, which had a significant similarity to human ClC-4 cDNA, is indicated by asterisks.
A subsequent search against the mouse genome data bases with the GA5508 insert sequence and the human ClC-4 cDNA sequence identified a corresponding mouse genomic sequence. Its putative cDNA sequence was predicted using GenScan software, encompassing partial mouse ClC-4 cDNA sequences deposited in the GenBank™ Data Bank. PCR primers were designed to cover the entire coding region of mouse ClC-4.
Surprisingly, PCR amplification with taste circumvallate papilla cDNA yielded two fragments of 2.4 and 2.23 kb. DNA sequencing showed that the former is the same as the previously described mouse ClC-4 chloride channel with 747 amino acid residues (GenBank™ accession number XM_193014). However, the 2.23-kb fragment is a novel splice variant, lacking 155 bp near the 5′-end of ClC-4, including the presumed ClC-4 start codon (ATG). The amino acid sequence of the short form was deduced from the next in-frame start codon, which is probably the true start codon, there being no other start codon. A stop codon is present ~140 bp upstream. Thus, ClC-4A putatively encodes a protein of 687 amino acid residues, lacking the N-terminal 60 amino acid residues of ClC-4. Sequence analysis indicated that the deletion of 155 bp is a result of exon skipping and that the new N terminus of the ClC-4A protein starts within a presumed helix domain of ClC-4 (helix A), which may be expected to significantly affect the channel’s functions (Fig. 2). To determine whether these two isoforms are expressed in all three types of gustatory lingual papillae, a new set of PCR primers was designed and synthesized specifically for amplification of the differentially spliced region of the cDNAs. Reverse transcription-PCR detected both forms of the cDNA in all three types of taste papillae (circumvallate, foliate, and fungiform), but not in non-gustatory lingual epithelium (Fig. 3).
FIGURE 2. Schematic illustration of the ClC-4/ClC-4A gene, mRNA, and protein structures.
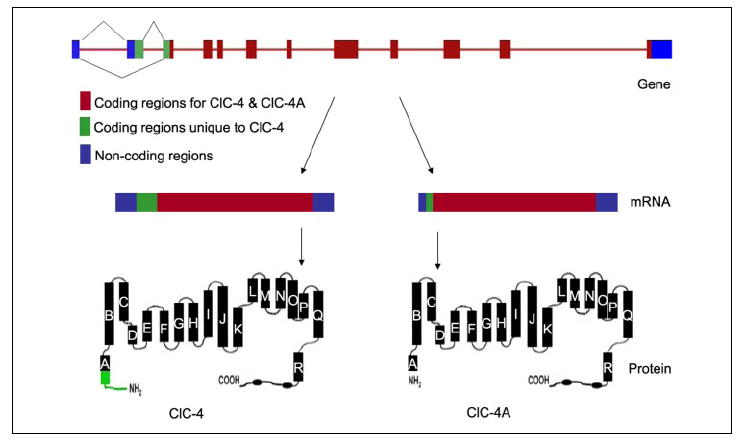
Alternative splicing generates two transcripts that predict unique protein structures: ClC-4 (lower left) and ClC-4A (lower right). Skipping an exon near the 5′-end results in the deletion of 60 amino acid residues at the N terminus of ClC-4A.
FIGURE 3. Expression of the chloride channels in three types of taste papillae.
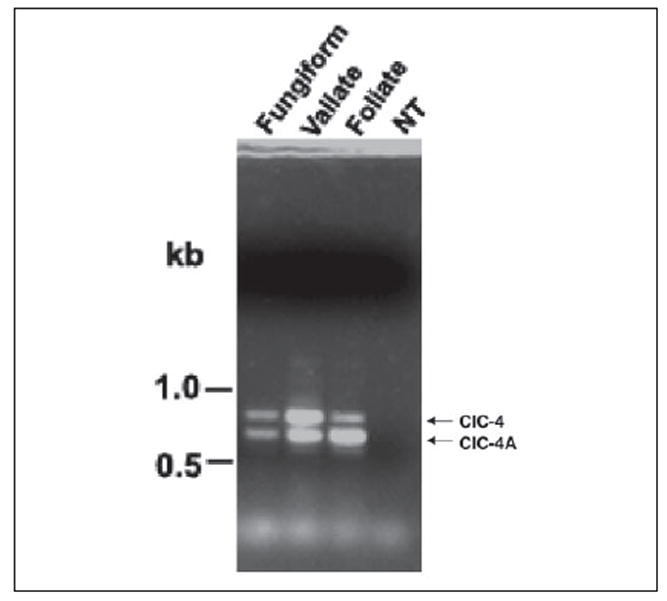
Both ClC-4 and ClC-4A were detected in all three types of taste papillae (fungiform, circumvallate (Vallate), and foliate), but not in non-taste lingual epithelium (NT). PCR amplification was carried out with primers covering the differentially spliced region and first-strand cDNAs prepared from taste and non-taste tissues. The expected PCR products for ClC-4 and ClC-4A are indicated.
Localization of the ClC-4/ClC-4A RNA Transcripts and Proteins to Taste Bud Cells
To localize the RNA transcripts to taste bud cells, in situ hybridization was carried out with a 2.2-kb probe common to both ClC-4 and ClC-4A. The results demonstrated that the ClC-4/ClC-4A transcripts were selectively expressed in taste bud cells, but were absent from the surrounding non-gustatory lingual epithelial cells (Fig. 4). Sense probe controls showed no nonspecific hybridization to lingual tissue.
FIGURE 4. Localization of ClC-4/ClC-4A mRNAs to taste bud cells.
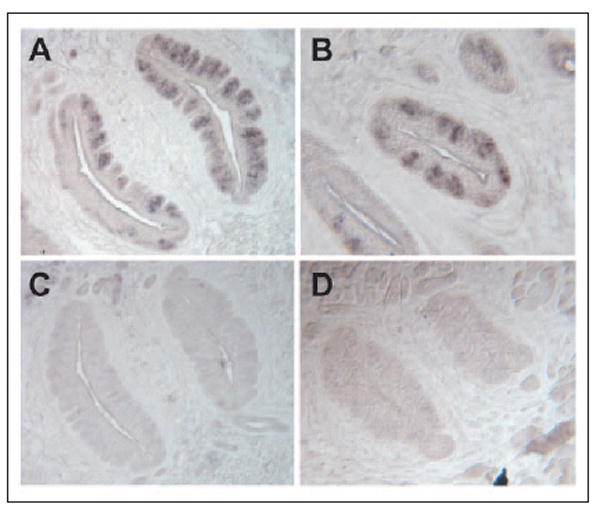
In situ hybridization with antisense probes of ClC-4/ClC-4A detected the expression of ClC-4/ClC-4A in mouse circumvallate (A) and foliate (B) papillae, whereas sense probes showed no specific signal in circumvallate (C) and foliate (D) papillae.
To determine the expression of the ClC-4/ClC-4A proteins in taste receptor cells, we used immunohistochemistry with antiserum to a peptide near the C termini of the ClC-4/ClC-4A proteins on sections of murine lingual tissue. This antibody was able to recognize both ClC-4 and ClC-4A. The immunostaining results indicated that the ClC-4/ClC-4A proteins were present on the cytoplasmic membrane. Some spotty staining seen in cells within the body of the bud suggested that the proteins could also be present in vesicles such as endosomes and synaptic vesicles (Fig. 5).
FIGURE 5. Expression of the ClC-4/ClC-4A proteins in taste bud cells.
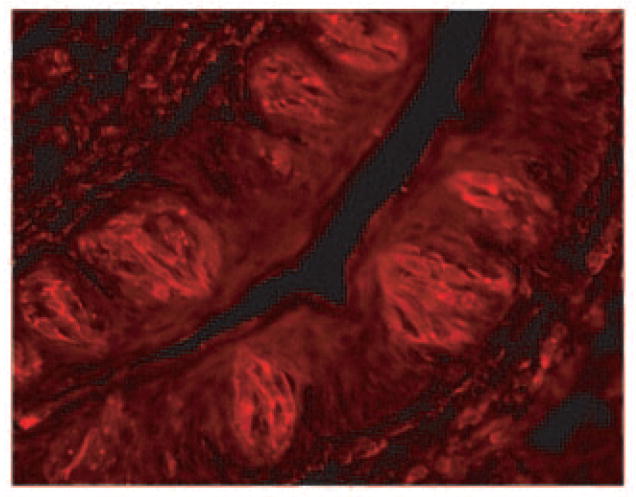
Immunohistochemistry of mouse taste tissue with an antibody against a C-terminal peptide sequence of ClC-4/ClC-4A indicated the presence of the proteins in taste bud cells. Some spotty staining suggested that the proteins may also be enriched in some intracellular vesicles such as endosomes and synaptic vesicles.
To determine the type of cells expressing ClC-4/ClC-4A, double immunostaining was carried out on taste bud-containing sections with the anti-ClC-4/ClC-4A antibody (produced from rabbit) and mouse monoclonal antibodies against IP3R3 and SNAP-25, which are cellular markers for Type II and III taste bud cells, respectively. Confocal laser scanning microscope images showed that nearly all IP3R3-expressing cells also expressed ClC-4/ClC-4A (Fig. 6). Interestingly, many, if not all, SNAP-25-expressing cells also expressed these ClC channels. Because Type II cells are taste receptor cells and because Type III cells make synapses with afferent neurons, the expression of ClC-4/ClC-4A in these two types of cells suggests that these chloride channels may play a major role in bitter, sweet, and umami taste signal transduction, modulation, and transmission.
FIGURE 6. Coexpression of ClC-4/ClC-4A with IP3R3 and SNAP-25 in taste bud cells.
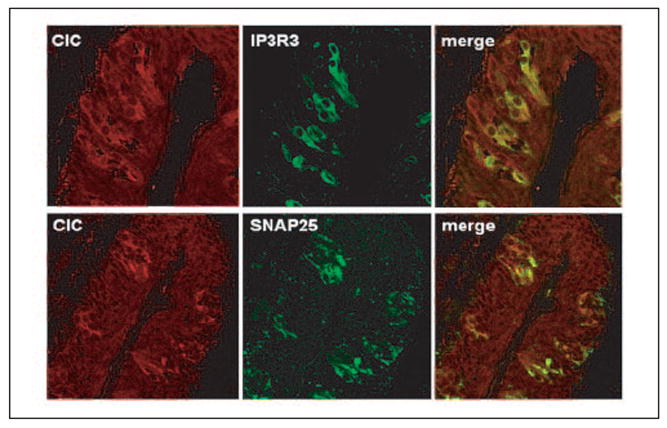
Double immunostaining was carried out with antibodies against ClC-4/ClC-4A, IP3R3, and SNAP-25. Nearly all IP3R3-expressing taste bud cells expressed ClC-4 and/or ClC-4A. In contrast, only partial overlap with SNAP-25-expressing cells was observed.
Functional Characterization of the ClC-4 and ClC-4A Channels
To characterize the function of ClC-4 and ClC-4A, we subcloned their cDNAs into the pCR-BluntII-TOPO expression vector, synthesized the capped sense cRNA with in vitro transcription, and tailed the cRNA with poly(A). The cRNA was then purified and injected into Xenopus oocytes. Membrane currents were recorded using the two-electrode voltage-clamp technique 4–6 days after injection. Strong outward currents (which were absent in the control oocytes) were recorded in the ClC-4 cRNA-injected oocytes.
The regulation of human ClC-4 activity by external pH has been reported, although the results appear contradictory to each other (54, 55). To examine the effect of external pH on these chloride channels in our system and to determine whether pH exerts any differential effect on mouse ClC-4 and ClC-4A, we perfused the oocytes with the bathing solutions buffered to different pH values. Extracellular acidification markedly reduced ClC-4-mediated currents (Fig. 7, left panel), indicating that mouse ClC-4 channels were open in the pH range of 7.5 to 6.5 and began to close as the pH fell from 6.5. In contrast, currents in mouse ClC-4A-injected oocytes at pH 7.5 and 6.5 were close to the basal level as recorded from the control oocytes, and strong outward currents were recorded at pH 6.0 and 5.5, indicating that ClC-4A was closed at pH 7.5 and 6.5, but open at the lower pH (Fig. 7, middle panel). We could not reliably record the chloride currents below pH 5.5 or 5.0 because the endogenous currents of the Xenopus oocytes became more active in these more acidic media.
FIGURE 7. Differential effects of external pH on heterologously expressed ClC-4 and ClC-4A activity.
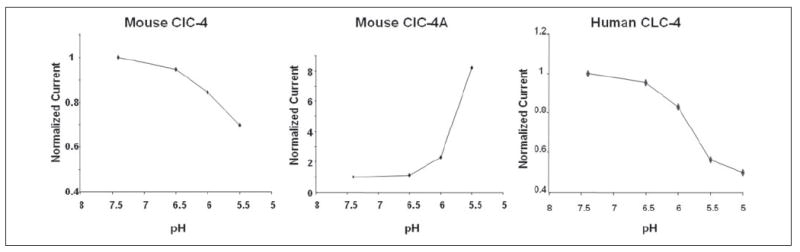
Currents measured at +80 mV were normalized for the current value at pH 7.4 and plotted as a function of pH. Mouse and human ClC-4 channels had maximal conductance at pH 7.5 to 6.5 and began to close at lower pH (left and right panels, respectively). In contrast, the splice variant ClC-4A was closed at neutral pH and open at acidic pH (middle panel).
Our mouse ClC-4 pH sensitivity was in full agreement with previously reported human ClC-4 results from two groups (55, 56), but not with the results from a third group (54). To confirm that this is not due to experimental variation among different laboratories, we expressed the human ClC-4 cDNA isolated from a human taste cDNA library and found that its characteristics are identical to those reported by the first two groups (55, 56): open at neutral pH and closed at acidic pH (Fig. 7, right panel).
To determine the ion selectivity of ClC-4 and ClC-4A, chloride was replaced with other anions (Fig. 8). The conductance sequences of murine ClC-4 and ClC-4A were I− = > Br− > Cl− (Fig. 8A) and I− ≫ Br− = Cl− > (Fig. 8 B), respectively. These ion selectivities are quite different from those reported for human ClC-4 (55, 56). To confirm our recording system, we expressed and recorded from human ClC-4 and found that the conductance sequence we measured was nearly identical to that reported by the two groups (55, 56): > Cl− > Br− > I− (Fig. 8C). These data indicate that all three chloride channels have unique ion selectivities and that the 2% difference in amino acid sequence between human and mouse ClC-4 is critical in conferring anion selectivity.
FIGURE 8. Unique ion selectivity of mouse ClC-4 and ClC-4A and human ClC-4.
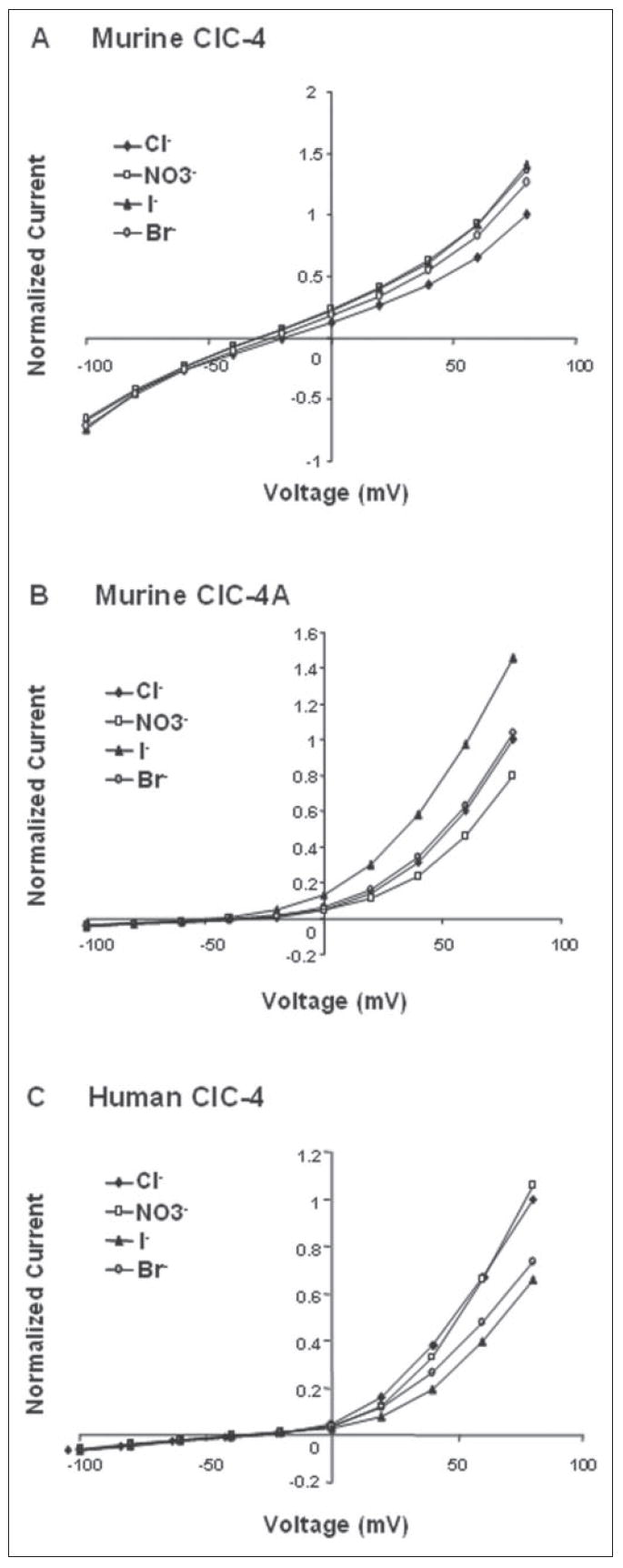
The ion selectivity was measured for Cl−, Br−, I−, and . Currents were recorded at pH 7.4 for mouse (A) and human ClC-4 (C) and at pH 5.5 for mouse ClC-4A (B). Currents were normalized to the current of Cl− at +80 mV.
To pharmacologically characterize these ion channels, we tested the effect of the chloride channel inhibitors niflumic acid and NPPB at the optimal pH for each channel. The results indicated that niflumic acid had only a slight inhibitory effect on human and mouse ClC-4 at pH 7.4 (Fig. 9, left and middle panels), but significantly inhibited the conductance of ClC-4A at pH 5.5 (right panels). NPPB had no apparent effect on the conductance of human and mouse ClC-4 channels, but did slightly inhibit ClC-4A (Fig. 10).
FIGURE 9. Niflumic acid inhibition of ion channel conductance.
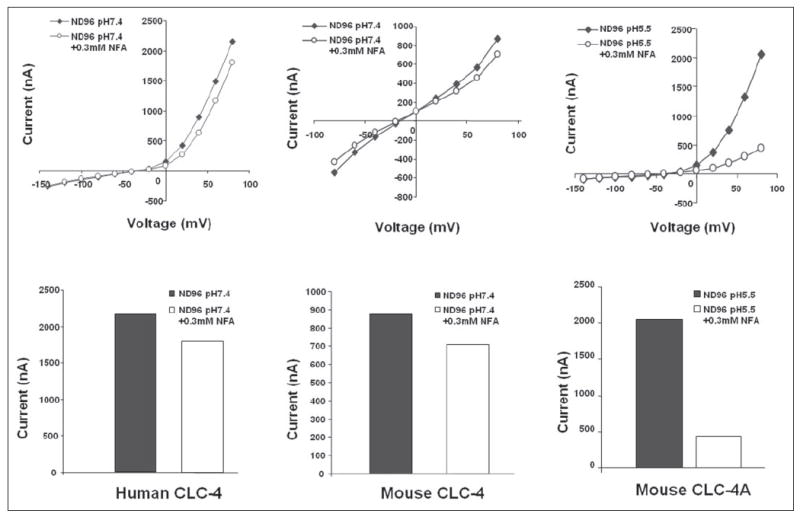
Upper panels, current-voltage curves; lower panels, corresponding bar graphs at maximal conductance. 0.3 mm niflumic acid had a slight effect on human and mouse ClC-4 conductance, whereas it greatly inhibited ClC-4A.
FIGURE 10. NPPB inhibition of ion channel conductance.
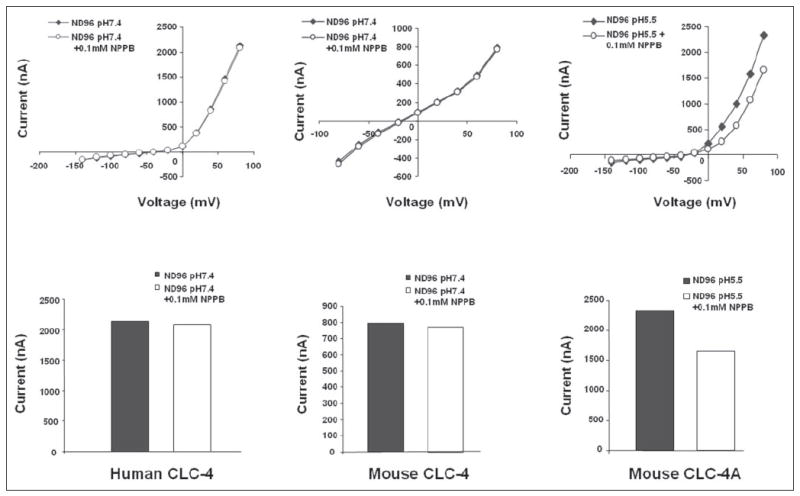
Upper panels, current-voltage curves; lower panels, corresponding bar graphs at maximal conductance. 0.1 mm NPPB had no effect on human and mouse ClC-4 conductance, but inhibited mouse ClC-4A.
DISCUSSION
Anion channels are usually referred to as chloride channels because chloride is the most abundant anion in all organisms. Chloride channels include the voltage-dependent ClC family, the cAMP-activated cystic fibrosis transmembrane conductance regulator, Ca2+-activated Cl− channels, and volume-regulated anion channels. In this study, we used a single cell method to isolate taste bud cell-type selective genes and identified a voltage-gated chloride channel (ClC-4) and its novel splice variant (ClC-4A). To the best of our knowledge, this is the first report showing their occurrence in taste bud cells.
Previous studies have shown that the human ClC-4 channel is sensitive to external pH (54, 55). However, the results reported by two groups, Kawasaki et al. (54) and Jentsch and co-workers (55), were contradictory to each other regarding pH sensitivity and ion selectivity. These discrepancies were thought to be variations from the different expression systems used in these experiments. However, a subsequent report showed that consistent results were obtained from three different systems (56). In this study, we expressed, in Xenopus oocytes, the same cDNA (GenBank™ accession number AB019432) as that used by Jentsch and co-workers, and the pH sensitivity and ion conductance we observed was nearly identical to their results, strongly suggesting that the discrepancies were caused by the different cDNAs used by the two groups. Indeed, as previously noticed (55), there are four amino acid differences between the deduced protein sequences from cDNAs used by the two groups. The variations of the four amino acid residues could arise from polymorphisms, which have been observed in other ion channels (57). Interestingly, substitutions at these four positions significantly shift pH sensitivity and ion selectivity, indicating their importance to the function of this anion channel.
Our data showed that murine ClC-4 was open at neutral pH and closed at acidic pH, similar to our human ClC-4 data, but in conflict with those data reported by the Kawasaki et al. (54). However, the ion selectivity of murine ClC-4 (I− = > Br− > Cl−) is largely in agreement with that observed by Kawasaki et al. (54), i.e. I− >Cl−, but different from our human ClC-4 data. Our mouse ClC-4 cDNA (GenBank™ accession number XM_193014) has 11 amino acid residues replaced and is 13 residues shorter at the N terminus in comparison with our human ClC-4 cDNA (GenBank™ accession number AB019432). These changes did not affect its pH sensitivity, but did reverse ion conductance sequences for I− and Cl−.
Murine ClC-4A is a novel isoform of ClC-4 that skipped an exon near the 5′-end, resulting in the deletion of the N-terminal 60 amino acid residues. Compared with full-length ClC-4, this new truncated isoform displayed the reversed pH sensitivity: open at low pH, suggesting that the N-terminal segment is involved in pH dependence.
The crystal structure of bacterial chloride channels indicates that, in the dimeric, “double-barreled” structure, the N-terminal helix A of one subunit is close to and possibly interacts with helix R of the other subunit. Part of helix R contributes to the formation of the pore region, and one of its amino acid residues directly interacts with the chloride ion (58-61). We speculate that, by interacting with helix R, helix A may affect anion selectivity and gating, and elimination of the N-terminal 60 amino acids of helix A shifts ion conductivity and reverses pH sensitivity. These changes are consistent with the notion that the N terminus of ClC channels is important in channel gating (62).
Alternative splicing and exon skipping have also been observed in other ClC family chloride channels, including ClC-1 (63, 64), ClC-2 (65-67), ClC-3 (68, 69), ClC-5 (70), and ClC-6 (71). Some of these variations were discovered in patients suffering from myotonic diseases (63, 64). Skipping of an exon equivalent to that seen here generating ClC-4A has been reported to also generate two ClC-3 isoforms with different N termini (69). Like the ClC-4 variants, long and short forms of ClC-3 display distinct ion selectivity but the same preference of I− over Cl−. The pH sensitivity of the ClC-3 channels was not examined. It is possible that these isoforms with different functional properties may form hetero- or homodimers and participate in different cellular processes.
Taste bud cells can generate action potentials in response to either passing current or taste stimuli, suggesting that action potentials may be important to taste signal transduction and transmission (41, 44, 72-74). Physiological studies suggest that taste bud cells may possess several types of chloride channels (48, 75). Most of these channels appear after postnatal day 8, a time coincident with the functional maturation of taste bud cells (51). Voltage-gated chloride channels may not be involved in the initiation of action potentials, but instead may contribute to the setting of the resting potentials and electrical excitability and to the form of action potentials.
Immunohistochemical studies using an antibody against a peptide sequence common to both ClC-4 and ClC-4A showed that these channels were expressed on both the plasma membrane and intracellular membranes, consistent with previous reports showing that ClC-4 is expressed on the plasma membrane as well as the endosomes of intestinal epithelial cells (76, 77). Double immunostaining showed that nearly all IP3R3-expressing cells were also immunoreactive to this anti-chloride channel antibody. Double immunostaining also demonstrated that most of the SNAP-25-expressing cells also expressed the ClC channels. The overlap with SNAP-25 suggests that either one or both isoforms of this chloride channel may be involved in synaptic activity. Recent studies on ClC-3, one of the ClC family members most similar to ClC-4, demonstrated that ClC-3 is expressed in synaptic vesicles in neurons and may play a permissive role by dissipating the membrane potential created by H+ buildup in the intracellular organelles and provide an electrical shunt for vesicular acidification by vacuolar ATPase activity. Knockout of ClC-3 leads to disruption of uptake of neurotransmitters by synaptic vesicles (78). In addition, a chloride channel blocker reduces neurotransmitter uptake into synaptic vesicles at the neuromuscular junction (79). By analogy, it is possible that ClC-4 or its ClC-4A variant may also play a role in neurotransmitter uptake.
Several mechanisms have been postulated for sour taste, including an NPPB-sensitive chloride channel and intracellular acidification (38-40). Our results show that human and murine ClC-4 were only slightly inhibited by 0.3 mm niflumic acid, whereas this agent greatly reduced ClC-4A conductance. Furthermore, 0.1 mm NPPB had no significant effect on ClC-4 activity at the optimal pH (pH 7.4), yet this same blocker could significantly reduce the ClC-4A currents at its optimal pH of 5.5, although the NPPB blocking effect may have been underestimated because this chemical is not readily dissolvable in acidic solutions, as also observed by others (35). These data suggest that ClC-4A may be a candidate chloride channel for acid transduction. Like other ClC channels, ClC-4 contributes to acidification of intracellular organelles (77, 80, 81). Therefore, the two hypotheses of sour taste could be combined into one: sour stimuli reduce intracellular pH, which closes the ClC-4 channel, but opens the NPPB-sensitive ClC-4A channel, changing membrane potential and leading to synaptic release.
The Clcn4 gene is located on the X chromosome in the Mediterranean mouse (Mus spretus), but it resides on chromosome 7 in the laboratory mouse (Mus musculus). Back-crossing of these two closely related species has generated (by mendelian segregation) animals with zero, to three alleles of Clcn4 (82, 83). To further define the role of this channel in taste sensation, experiments to examine the phenotypes of animals with loss or overexpression of this gene have begun.
Acknowledgments
We thank Dr. John Teeter for advice on electrophysiological recordings.
Footnotes
This work was supported by National Institutes of Health Grant DC05154 (to L. H.), a grant from the United States Department of Veterans Affairs (to J. G. B.), and National Science Foundation Equipment Grant DBI-0216310.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBank™/EBI Data Bank with accession number(s) DQ186662.
The abbreviations used are: GPCRs, G protein-coupled seven-transmembrane receptors; IP3R3, inositol 1,4,5-triphosphate receptor-3; ClC, chloride channel; NPPB, 5-nitro-2-(3-phenylpropylamino)benzoic acid; SNAP, soluble N-ethylmaleimide-sensitive factor attachment protein; MES, 4-morpholineethanesulfonic acid.
References
- 1.Lindemann B. Physiol Rev. 1996;76:718–766. doi: 10.1152/physrev.1996.76.3.719. [DOI] [PubMed] [Google Scholar]
- 2.Gilbertson TA, Damak S, Margolskee RF. Curr Opin Neurobiol. 2000;10:519–527. doi: 10.1016/s0959-4388(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 3.Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Cell. 1999;96:541–551. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- 4.Matsunami H, Montmayeur JP, Buck LB. Nature. 2000;404:601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- 5.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 6.Montmayeur JP, Liberles SD, Matsunami H, Buck LB. Nat Neurosci. 2001;4:492–498. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- 7.Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Nat Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- 8.Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Biochem Biophys Res Commun. 2001;283:236–242. doi: 10.1006/bbrc.2001.4760. [DOI] [PubMed] [Google Scholar]
- 9.Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Chem Senses. 2001;26:925–933. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sainz E, Korley JN, Battey JF, Sullivan SL. J Neurochem. 2001;77:896–903. doi: 10.1046/j.1471-4159.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- 11.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 12.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Proc Natl Acad Sci U S A. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 15.Bufe B, Hofmann T, Krautwurst D, Raguse JD, Meyerhof W. Nat Genet. 2002;32:397–401. doi: 10.1038/ng1014. [DOI] [PubMed] [Google Scholar]
- 16.Behrens M, Brockhoff A, Kuhn C, Bufe B, Winnig M, Meyerhof W. Biochem Biophys Res Commun. 2004;319:479–485. doi: 10.1016/j.bbrc.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhari N, Roper SD. Ann N Y Acad Sci. 1998;855:398–406. doi: 10.1111/j.1749-6632.1998.tb10598.x. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin SK, McKinnon PJ, Spickofsky N, Danho W, Margolskee RF. Physiol Behav. 1994;56:1157–1164. doi: 10.1016/0031-9384(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 19.Wong GT, Gannon KS, Margolskee RF. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. Nat Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- 21.Rossler P, Boekhoff I, Tareilus E, Beck S, Breer H, Freitag J. Chem Senses. 2000;25:413–421. doi: 10.1093/chemse/25.4.413. [DOI] [PubMed] [Google Scholar]
- 22.Caicedo A, Pereira E, Margolskee RF, Roper SD. J Neurosci. 2003;23:9947–9952. doi: 10.1523/JNEUROSCI.23-30-09947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz CJ, Wray K, Delay E, Margolskee RF, Kinnamon SC. Chem Senses. 2003;28:573–579. doi: 10.1093/chemse/bjg049. [DOI] [PubMed] [Google Scholar]
- 24.Yan W, Sunavala G, Rosenzweig S, Dasso M, Brand JG, Spielman AI. Am J Physiol. 2001;280:C742–C751. doi: 10.1152/ajpcell.2001.280.4.C742. [DOI] [PubMed] [Google Scholar]
- 25.Rossler P, Kroner C, Freitag J, Noe J, Breer H. Eur J Cell Biol. 1998;77:253–261. doi: 10.1016/s0171-9335(98)80114-3. [DOI] [PubMed] [Google Scholar]
- 26.Clapp TR, Stone LM, Margolskee RF, Kinnamon SC. BMC Neuroscience. 2001 doi: 10.1186/1471-2202-2-6. http://www.biomedcentral.com/1471-2202/2/6. [DOI] [PMC free article] [PubMed]
- 27.Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 29.Liu D, Liman ER. Proc Natl Acad Sci U S A. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R. Proc Natl Acad Sci U S A. 2003;100:15166–15171. doi: 10.1073/pnas.2334624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofmann T, Chubanov V, Gudermann T, Montell C. Curr Biol. 2003;13:1153–1158. doi: 10.1016/s0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- 32.Lin W, Finger TE, Rossier BC, Kinnamon SC. J Comp Neurol. 1999;405:406–420. doi: 10.1002/(sici)1096-9861(19990315)405:3<406::aid-cne10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 33.Ugawa S, Minami Y, Guo W, Saishin Y, Takatsuji K, Yamamoto T, Tohyama M, Shimada S. Nature. 1998;395:555–556. doi: 10.1038/26882. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, Simon SA. Brain Res. 2001;923:58–70. doi: 10.1016/s0006-8993(01)03190-0. [DOI] [PubMed] [Google Scholar]
- 35.Lin W, Ogura T, Kinnamon SC. J Neurophysiol. 2002;88:133–141. doi: 10.1152/jn.2002.88.1.133. [DOI] [PubMed] [Google Scholar]
- 36.Stevens DR, Seifert R, Bufe B, Muller F, Kremmer E, Gauss R, Meyerhof W, Kaupp UB, Lindemann B. Nature. 2001;413:631–635. doi: 10.1038/35098087. [DOI] [PubMed] [Google Scholar]
- 37.Kinnamon SC, Dionne VE, Beam KG. Proc Natl Acad Sci U S A. 1988;85:7023–7027. doi: 10.1073/pnas.85.18.7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyamoto T, Fujiyama R, Okada Y, Sato T. J Neurophysiol. 1998;80:1852–1859. doi: 10.1152/jn.1998.80.4.1852. [DOI] [PubMed] [Google Scholar]
- 39.Lyall V, Alam RI, Phan TH, Phan DQ, Heck GL, DeSimone JA. J Neurophysiol. 2002;87:399–408. doi: 10.1152/jn.00331.2001. [DOI] [PubMed] [Google Scholar]
- 40.Lyall V, Alam RI, Phan DQ, Ereso GL, Phan TH, Malik SA, Montrose MH, Chu S, Heck GL, Feldman GM, DeSimone JA. Am J Physiol. 2001;281:C1005–C1013. doi: 10.1152/ajpcell.2001.281.3.C1005. [DOI] [PubMed] [Google Scholar]
- 41.Roper S. Science. 1983;220:1311–1312. doi: 10.1126/science.6857254. [DOI] [PubMed] [Google Scholar]
- 42.Kashiwayanagi M, Miyake M, Kurihara K. Am J Physiol. 1983;244:C82–C88. doi: 10.1152/ajpcell.1983.244.1.C82. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Sun XD, Herness S. J Neurophysiol. 1996;75:820–831. doi: 10.1152/jn.1996.75.2.820. [DOI] [PubMed] [Google Scholar]
- 44.Behe P, DeSimone JA, Avenet P, Lindemann B. J Gen Physiol. 1990;96:1061–1084. doi: 10.1085/jgp.96.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herness MS, Sun XD. J Membr Biol. 1995;146:73–84. doi: 10.1007/BF00232681. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Herness MS. Pfluegers Arch Eur J Physiol. 1997;434:215–226. doi: 10.1007/s004240050388. [DOI] [PubMed] [Google Scholar]
- 47.Sun XD, Herness MS. Am J Physiol. 1996;271:C1221–C1232. doi: 10.1152/ajpcell.1996.271.4.C1221. [DOI] [PubMed] [Google Scholar]
- 48.Herness MS, Sun XD. J Neurophysiol. 1999;82:260–271. doi: 10.1152/jn.1999.82.1.260. [DOI] [PubMed] [Google Scholar]
- 49.Varkevisser B, Peterson D, Ogura T, Kinnamon SC. Chem Senses. 2001;26:499–505. doi: 10.1093/chemse/26.5.499. [DOI] [PubMed] [Google Scholar]
- 50.Gilbertson TA. Chem Senses. 2002;27:383–394. doi: 10.1093/chemse/27.4.383. [DOI] [PubMed] [Google Scholar]
- 51.Ghiaroni V, Fieni F, Pietra P, Bigiani A. Chem Senses. 2003;28:827–833. doi: 10.1093/chemse/bjg076. [DOI] [PubMed] [Google Scholar]
- 52.Schaeren-Wiemers N, Gerfin-Moser A. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- 53.Goldin AL, Sumikawa K. Methods Enzymol. 1992;207:279–297. doi: 10.1016/0076-6879(92)07018-j. [DOI] [PubMed] [Google Scholar]
- 54.Kawasaki M, Fukuma T, Yamauchi K, Sakamoto H, Marumo F, Sasaki S. Am J Physiol. 1999;277:C948–C954. doi: 10.1152/ajpcell.1999.277.5.C948. [DOI] [PubMed] [Google Scholar]
- 55.Friedrich T, Breiderhoff T, Jentsch TJ. J Biol Chem. 1999;274:896–902. doi: 10.1074/jbc.274.2.896. [DOI] [PubMed] [Google Scholar]
- 56.Vanoye CG, George AL., Jr J Physiol (Lond) 2002;539:373–383. doi: 10.1113/jphysiol.2001.013115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campos-Xavier AB, Saraiva JM, Ribeiro LM, Munnich A, Cormier-Daire V. Hum Genet. 2003;112:186–189. doi: 10.1007/s00439-002-0861-9. [DOI] [PubMed] [Google Scholar]
- 58.Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- 59.Middleton RE, Pheasant DJ, Miller C. Nature. 1996;383:337–340. doi: 10.1038/383337a0. [DOI] [PubMed] [Google Scholar]
- 60.Pusch M, Ludewig U, Rehfeldt A, Jentsch TJ. Nature. 1995;373:527–531. doi: 10.1038/373527a0. [DOI] [PubMed] [Google Scholar]
- 61.Ludewig U, Pusch M, Jentsch TJ. Nature. 1996;383:340–343. doi: 10.1038/383340a0. [DOI] [PubMed] [Google Scholar]
- 62.Robinson NC, Huang P, Kaetzel MA, Lamb FS, Nelson DJ. J Physiol (Lond) 2004;556:353–368. doi: 10.1113/jphysiol.2003.058032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen L, Schaerer M, Lu ZH, Lang D, Joncourt F, Weis J, Fritschi J, Kappeler L, Gallati S, Sigel E, Burgunder JM. Muscle Nerve. 2004;29:670–676. doi: 10.1002/mus.20005. [DOI] [PubMed] [Google Scholar]
- 64.Charlet BN, Savkur RS, Singh G, Philips AV, Grice EA, Cooper TA. Mol Cell. 2002;10:45–53. doi: 10.1016/s1097-2765(02)00572-5. [DOI] [PubMed] [Google Scholar]
- 65.Chu S, Murray CB, Liu MM, Zeitlin PL. Nucleic Acids Res. 1996;24:3453–3457. doi: 10.1093/nar/24.17.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chu S, Zeitlin PL. Nucleic Acids Res. 1997;25:4153–4159. doi: 10.1093/nar/25.20.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loewen ME, MacDonald DW, Gaspar KJ, Forsyth GW. Biochim Biophys Acta. 2000;1493:284–288. doi: 10.1016/s0167-4781(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 68.Ogura T, Furukawa T, Toyozaki T, Yamada K, Zheng YJ, Katayama Y, Nakaya H, Inagaki N. FASEB J. 2002;16:863–865. doi: 10.1096/fj.01-0845fje. [DOI] [PubMed] [Google Scholar]
- 69.Shimada K, Li X, Xu G, Nowak DE, Showalter LA, Weinman SA. Am J Physiol. 2000;279:G268–G276. doi: 10.1152/ajpgi.2000.279.2.G268. [DOI] [PubMed] [Google Scholar]
- 70.Forino M, Graziotto R, Tosetto E, Gambaro G, D’Angelo A, Anglani F. J Hum Genet. 2004;49:53–60. doi: 10.1007/s10038-003-0108-1. [DOI] [PubMed] [Google Scholar]
- 71.Eggermont J, Buyse G, Voets T, Tytgat J, De Smedt H, Droogmans G, Nilius B. Biochem J. 1997;325:269–276. doi: 10.1042/bj3250269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cummings TA, Powell J, Kinnamon SC. J Neurophysiol. 1993;70:2326–2336. doi: 10.1152/jn.1993.70.6.2326. [DOI] [PubMed] [Google Scholar]
- 73.Gilbertson TA, Avenet P, Kinnamon SC, Roper SD. J Gen Physiol. 1992;100:803–824. doi: 10.1085/jgp.100.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Avenet P, Lindemann B. J Membr Biol. 1991;124:33–41. doi: 10.1007/BF01871362. [DOI] [PubMed] [Google Scholar]
- 75.Wladkowski SL, Lin W, McPheeters M, Kinnamon SC, Mierson S. J Membr Biol. 1998;164:91–101. doi: 10.1007/s002329900396. [DOI] [PubMed] [Google Scholar]
- 76.Mohammad-Panah R, Ackerley C, Rommens J, Choudhury M, Wang Y, Bear CE. J Biol Chem. 2002;277:566–574. doi: 10.1074/jbc.M106968200. [DOI] [PubMed] [Google Scholar]
- 77.Mohammad-Panah R, Harrison R, Dhani S, Ackerley C, Huan LJ, Wang Y, Bear CE. J Biol Chem. 2003;278:29267–29277. doi: 10.1074/jbc.M304357200. [DOI] [PubMed] [Google Scholar]
- 78.Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bosl MR, Ruether K, Jahn H, Draguhn A, Jahn R, Jentsch TJ. Neuron. 2001;29:185–196. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 79.Van der Kloot W. Brain Res. 2003;961:287–289. doi: 10.1016/s0006-8993(02)03954-9. [DOI] [PubMed] [Google Scholar]
- 80.Hara-Chikuma M, Wang Y, Guggino SE, Guggino WB, Verkman AS. Biochem Biophys Res Commun. 2005;329:941–946. doi: 10.1016/j.bbrc.2005.02.060. [DOI] [PubMed] [Google Scholar]
- 81.Mellman I, Fuchs R, Helenius A. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 82.Adler DA, Rugarli EI, Lingenfelter PA, Tsuchiya K, Poslinski D, Liggitt HD, Chapman VM, Elliott RW, Ballabio A, Disteche CM. Proc Natl Acad Sci U S A. 1997;94:9244–9248. doi: 10.1073/pnas.94.17.9244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rugarli EI, Adler DA, Borsani G, Tsuchiya K, Franco B, Hauge X, Disteche C, Chapman V, Ballabio A. Nat Genet. 1995;10:466–471. doi: 10.1038/ng0895-466. [DOI] [PubMed] [Google Scholar]



